Abstract
Background
Transformation into small-cell lung carcinoma (SCLC) is a common acquired resistance mechanism to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs). Re-tumor biopsy is crucial for identifying the definite tumor resistance mechanism. However, multiple mechanisms may occur simultaneously during TKI treatment. A single biopsy specimen is insufficient to accurately represent all resistance mechanisms at progressive sites.
Case Description
In this case, we present a 58-year-old male with metastatic pulmonary adenocarcinoma (ADC) who had an EGFR exon 19 mutation and received first-line gefitinib and second-line osimertinib. Biopsy results from different progressive sites confirmed the presence of SCLC in pleural metastatic specimens, while the primary tumor had the EGFR exon 19 mutation and mutations ofPhosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) andv-Ki-ras2-Kirsten rat sarcoma viral oncogene homolog (KRAS). We utilized an effective combination therapy of permanent radioactive iodine-125 seed implantation (PRISI) as local consolidative therapy (LCT), along with the standard carboplatin-etoposide regimen for SCLC and continued osimertinib. Extracranial tumors were successfully controlled. The patient succumbed to intracranial disease progression without radiotherapy, with an overall survival (OS) of 15 months after SCLC transformation.
Conclusions
Confirming SCLC transformation from a single site alone through biopsy may not provide a comprehensive understanding of the resistance mechanisms underlying progression at all sites. This highlights the significance of combined treatment strategies, particularly with LCT, for heterogeneous tumors in SCLC transformation.
Keywords: Case report, heterogeneous tyrosine kinase inhibitor-resistance (heterogeneous TKI-resistance), small cell lung cancer transformation, 125I radiotherapy, local consolidative therapy (LCT)
Highlight box.
Key findings
• Genetic and phenotypic variation of tyrosine kinase inhibitor (TKI)-resistant cells cooccurs in advanced non-small cell lung cancer (NSCLC). Histologic transformation from NSCLC to small-cell lung carcinoma (SCLC) is various, including localized transformed SCLC (T-SCLC), systemic T-SCLC, central nervous system (CNS) sanctuary T-SCLC.
What is known and what is new?
• Both targeted therapy and chemotherapy are commonly used to treat T-SCLC.
• Adding local consolidation therapy may reform therapeutic avenue for overcoming tumor heterogeneity in T-SCLC.
What is the implication, and what should change now?
• Multi-site biopsies should be prioritized during epidermal growth factor receptor-TKI progression to identify spatially separated resistance.
• Combined systemic and local therapies may improve outcomes in heterogeneous tumors.
Introduction
Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) have revolutionized the treatment of patients with advanced non-small cell lung cancer (NSCLC) harboring an EGFR mutation (1). However, the emergence of resistance to EGFR-TKIs has become a significant issue, leading to disease relapse or progression (2). This acquired resistance is highly heterogeneous, involving both EGFR-dependent and EGFR-independent mechanisms (3). Another observed mechanism is the histological transformation of EGFR-mutant NSCLC to small-cell lung carcinoma (SCLC), accounting for 1–15% of resistance cases (3-5). Despite recognizing transformed SCLC (T-SCLC), our understanding of the complexity and heterogeneity of TKI-resistance mechanisms remains limited. There has been little focus on the co-occurrence of genetic and phenotypic variation in TKI-resistant cells during disease progression. Consequently, developing an efficient therapy strategy becomes challenging due to the heterogeneity and co-occurrence of multiple resistance mechanisms. In this case, we present a patient who experienced EGFR-TKI-induced transformation from NSCLC to SCLC. Rebiopsy of pleural metastases after Osimertinib treatment revealed SCLC transformation, while concurrent rebiopsy of the right upper lobe showed the presence of ADC with additional mutations of PIK3CA and KRAS. This case demonstrates that different mechanisms of acquired resistance can coexist. Considering the tumor heterogeneity, localized brachytherapy was considered as a strengthened treatment approach. The patient experienced complete regression of extracranial lesions induced by permanent radioactive iodine-125 seed implantation (PRISI), combined with platinum-etoposide and osimertinib. Unfortunately, the patient eventually succumbed to central nervous system (CNS) metastasis progression, with an overall survival of 15 months after SCLC transformation. We present this article in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-24-67/rc).
Case presentation
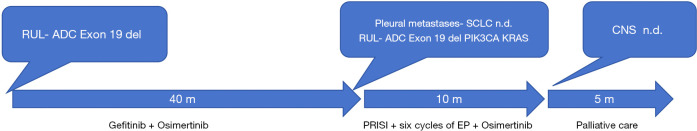
A 58-year-old male with a history of smoking presented with persistent chest distress for over 1 month and sought medical attention at an external facility. During the initial visit, a chest CT scan revealed a 4.2 cm × 4.3 cm lesion in the right upper lobe of the lung with metastases to the mediastinum and right hilum lymph nodes, as well as pleural effusion. A percutaneous lung biopsy confirmed the presence of EGFR 19del mutation, leading to a diagnosis of stage IVA right upper lobe lung ADC with EGFR 19del. The patient was initially treated with gefitinib as first-line therapy, resulting in a partial response (PR) and resolution of pleural effusion. The progression-free survival (PFS) of the first-line therapy was 23 months. Subsequently, the patient developed leptomeningeal metastasis (LM) and genetic testing of blood confirmed EGFR 19del. Therefore, the patient was switched to Osimertinib as second-line therapy, resulting in a PFS of 16 months. The patient presented to our clinic with pain in the right rib area. Imaging examination revealed new pleural metastases involving the ribs (Figure 1A). An enlarged primary right upper lobe mass (Figure 1B), as well as new brain metastases in the right occipital lobe and cerebellum. Laboratory findings showed an increase in serum carcinoembryonic antigen level to 50.59 ng/mL (normal range, 0–5 ng/mL) and an elevated serum neuron-specific enolase (NSE) level of 27.40 ng/mL (normal range, 0–16.2 ng/mL). and additionally pro-gastrin-releasing (ProGRP) peptide of level of 39.6 ng/mL (normal range, 0–50 ng/mL, which was not detected at the time of diagnosis). The patient underwent biopsies and PRISI on both the pleural metastases (Figure 1C) and the primary right upper lobe mass (Figure 1D). The pathology findings of the pleural metastases indicated SCLC (Figure 2A). Immunohistochemical staining confirmed positive results for chromogranin A (CgA), synaptophysin (Syn) (Figure 2B) and CD56 (Figure 2C), and negative results for thyroid transcription factor-1 (TTF-1), CK5/6, and vimentin. On the other hand, the pathology findings of the right upper lobe mass were consistent with primitive ADC (Figure 2D). The mass showed positive results for CK7 (Figure 2E) and TTF1 (Figure 2F), and negative results for CgA, CD56, and Syn. Next-generation sequencing of the right upper lobe mass tissue revealed EGFR 19del mutation, as well as mutations of PIK3CA and KRAS. Unfortunately, molecular analyses on pleural metastases could not be performed due to insufficient material. Following LPRISI, the patient received 6 cycles of platinum-etoposide in combination with continuous osimertinib and tolerated well. This treatment regimen resulted in a progression-free survival (PFS) of 10 months for the three-line therapy. Subsequently, the patient experienced difficulty in moving his right leg. Magnetic resonance imaging (MRI) revealed the progression of CNS metastasis. However, the CT scan indicated that there was a maintenance of complete response (CR) in both pleural metastases (Figure 3A) and the right upper lobe mass (Figure 3B), and no extracranial metastases were detected. Receiving only palliative care, the patient unfortunately passed away 15 months after the transformation into SCLC (Figure 4).
Figure 1.
Chest CT findings at different time points during diagnosis and treatment. (A) The patient experienced progression following osimertinib treatment. Chest CT findings at the time of diagnosis in our department, showing right lower pleural metastasis. (B) Patient's chest CT findings at the time of diagnosis in our department after osimertinib treatment, showing cancer in the upper lobe of the right lung. (C) The patient underwent radioactive seed implantation in the right lower pleural metastasis lesion. (D) Patient underwent radioactive seed implantation in the upper lobe of the right lung. CT, computed tomography.
Figure 2.
Lung adenocarcinoma and small cell lung cancer by biopsy. (A) Right lower pleural metastasis: small cell lung cancer (HE staining, ×40); (B) Syn (+) (immunohistochemical staining, ×40); (C) CD56 (+) (immunohistochemical staining, ×40). (D) The upper lobe of the right: lung adenocarcinoma (HE staining, ×40); (E) TTF-1 (+) (immunohistochemical staining, ×40); (F) CK-7 (+) (immunohistochemical staining, ×40). HE, hematoxylin and eosin.
Figure 3.
Nine months after 125I seed implantation. (A) Response of the right lower pleural metastasis lesion maintained CR. (B) Response of the upper lobe lesions in the right lung maintained CR. CR, complete response.
Figure 4.
Summary of the patient’s treatment from diagnosis to death. ADC, adenocarcinoma; CNS, central nervous system; EP, cisplatin and etoposide; m, months; n.d., not done; PRISI, permanent radioactive iodine-125 seed implantation; RUL, right upper lobe; SCLC, small-cell lung carcinoma.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki and its subsequent amendments. Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made.
Discussion
Our case study demonstrates that localized T-SCLC coexists with acquired new mutations in EGFR-mutant lung ADC after EGFR-TKIs. To effectively address heterogeneous resistance, we implemented a comprehensive treatment model that includes local consolidation brachytherapy, standard chemotherapy, and EGFR-TKIs as maintenance therapy. This treatment approach successfully halted the progression of extracranial tumors. However, it is important to note that the patient did not receive timely local treatments for CNS metastasis, which raises the question of assessing the effectiveness of local consolidative therapy (LCT) in controlling CNS metastasis.
The coexistence of T-SCLC and ADC in EGFR-TKI-refractory lesions
The incidence of T-SCLC has been increasing recently (6). It is believed that the occurrence of localized T-SCLC alongside ADC after EGFR-TKI treatment failure is rare, based on current understanding and reported cases. One potential bias in clinical practice is that more repeat biopsy samples are typically taken from a single site rather than multiple sites. We conducted a systematic evaluation of the literature and reviewed previous case reports of multiple TKI-refractory lesions with T-SCLC potentially present in a portion of the EGFR-TKI-refractory lesions. The relevant case information is presented in Table 1 (7-14). These cases clearly demonstrate distinct mechanisms of resistance in different metastatic lesions within a patient, even within a single organ (12). Histologic transformation from NSCLC to SCLC can occur in various forms, including localized T-SCLC, systemic T-SCLC (15), and CNS sanctuary T-SCLC (7). The lack of multi-point sampling detection at the same time in clinical practice may lead to the possibility of missing the truth of the coexistence of ADC and localized T-SCLC. Ninomaru et al. (16) reported a case in which the pathological diagnosis alternated between ADC and SCLC in EGFR-mutated NSCLC following treatment with Gefitinib and subsequent Osimertinib. However, Niederst et al. (11) suggested that the observed oscillating pattern of ADC and T-SCLC could be attributed to the selection of different clones under the pressure of the applied treatment. The coexistence of ADC and T-SCLC with distinct genetic aberrations in resistant lesions was confirmed through autopsy. When analyzing the pathology of biopsied small samples from a single lesion to make treatment decisions, it is crucial to consider the possibility of inter-tumor spatial heterogeneity, especially when studying TKI resistance mechanisms. The heterogeneity of this spatial separation can occur before treatment (17-19). The cases reveal that most post-treatment lesions transformed into T-SCLC, while the pre-treatment tumors already had the same mutation, indicating that these were not independent new cancers (7,8,12,13). Instead, the original ADC lesions acquired new mutations, supporting the hypothesis of lineage plasticity. This hypothesis is further supported by other studies (20,21). Several reports have indicated that SCLC transformation and EGFR T790M mutation can occur reciprocally in a single patient when developing resistance to EGFR-TKIs. However, it was not observed for SCLC-transformed samples to have the EGFR T790M mutation (8,11,12). Additionally, fewer EGFR T790M mutations were identified among the samples that ultimately transformed into SCLC in EGFR/RB1/TP53-mutant lung cancers (22). These clinical observations support the hypothesis of an early branching event between the SCLC clone and the initially predominant NSCLC clonal population, from which EGFR T790M-positive clones emerge (23).
Table 1. Literature reports of patients who have data for multiple TKI-refractory lesions with T-SCLC.
| Study | Year | Sex | Prior treatment | Time (months) | Pathology-location | EGFR mutation | Subsequent SCLC therapy |
|---|---|---|---|---|---|---|---|
| Sequist, et al. (7) | 2011 | F | Erlotinib | M0 | ADC-Cerv LN | L858R | Radiation therapy salvage EP-based chemotherapy |
| M22 | SCLC-lingula lung | L858R | |||||
| Autopsy | SCLC-liver, nodule1,2,3 | L858R | |||||
| SCLC-LN-paratracheal, LUL apex | L858R | ||||||
| SCLC-diaphragm | L858R | ||||||
| ADC-brain metastasis | L858R | ||||||
| Fallet, et al. (8) | 2012 | F | Multi-lines chemotherapy + erlotinib | M0 | ADC-bronchioloalveolar lavage | Exon 19 del | EP + erlotinib |
| M18 | SCLC-RUL | Exon 19 del | |||||
| ADC-bronchial aspirate | Exon 19 delT790M | ||||||
| Van Riel (9) | 2012 | F | Multi-lines chemotherapy + erlotinib | M0 | ADC-RUL | ? | Chemotherapy + palliative radiation |
| M24 | ADC-left lung | Exon 19 del T790M | |||||
| SCLC-supraclavicular LN | Exon 19 del | ||||||
| Norkowski et al. (10) | 2013 | F | Erlotinib | M 0 | ADC-RML | Exon 19 del | Six cycles of EP, one cycle of topotecan |
| M31 | ADC-RML | L858R, T790M | |||||
| SCLC-right adrenal | Exon 21 E872K | ||||||
| Niederst et al. (11) | 2015 | F | Erlotinib | M0 | ADC-NDR | L858R | Chemotherapy + radiation + erlotinib |
| M13 | SCLC-pulmonary nodule | L858R PIK3CA E545K | |||||
| M24+ | ADC-pleural effusion | L858R | |||||
| M30 | SCLC-bone biopsy | L858R PIK3CA E545K | |||||
| ADC-pleural effusion | L858R | ||||||
| M36+ (autopsy) | SCLC-liver and lung | L858R PIK3CA E545K | |||||
| ADC-diaphragm | L858R T790M | ||||||
| Suda et al. (12) | 2015 | F | chemotherapy with concurrent radiation and Gefitinib |
M0 | ADC-right lung | Exon19delTP53 PIK3CA | Gefitinib + palliative radiation |
| M23 (autopsy) | SCLC-left lobe liver | Exon 19 del | |||||
| SCLC-left lung | Exon 19 del | ||||||
| SCLC-Ret-LN 1,2,3 | Exon 19 del | ||||||
| SCLC-IVC | Exon 19 del | ||||||
| SCLC-left AG | Exon 19 del | ||||||
| SCLC-left AG-inv | Exon 19 del | ||||||
| ADC-right lobe liver | Exon 19 del T790M | ||||||
| ADC-peri-panc LN | Exon 19 del T790M | ||||||
| Furugen et al. (13) | 2015 | M | Erlotinib + whole-brain irradiation | M0 | ADC–LUL | Exon 19 deletion | Palliative treatment |
| M10 | ADC + SCLC-left pleural effusion | exon 19 deletion | |||||
| M11 (autopsy) | ADC + SCLC-limited squamous cell-primary lung lesion | n.d. | |||||
| SCLC-pancreas | Exon 19 del T790M(−) | ||||||
| SCLC-left kidney | Exon 19 del T790M(−) | ||||||
| ADC-right kidney | Exon 19 del T790M | ||||||
| ADC + SCLC-small intestine | n.d. | ||||||
| ADC-spleen | n.d. | ||||||
| ADC-spondylus | n.d. | ||||||
| SCLC-mediastinum | n.d. | ||||||
| SCLC-epicardium | n.d. | ||||||
| SCLC-liver | n.d. | ||||||
| SCLC-gall bladder-SCLC | n.d. | ||||||
| SCLC-bone marrow | n.d. | ||||||
| SCLC-para-aortic LN | n.d. | ||||||
| Chen et al. (14) | 2019 | M | Multi-lines chemotherapy + gefitinib + osimertinib | M0 | ADC-LLL | L858R | EC regimen of irinotecan combined with oxaliplatin (IO), abraxane, and apatinib |
| M23 | ADC-right lung | L858R T790M | |||||
| M31 | SCLC-right lung | n.d. | |||||
| M64 | SCLC-LUL | n.d. | |||||
| ADC-LLL | n.d. | ||||||
| Present case | 2023 | M | Gefitinib + osimertinib | M0 | ADC-RUL | Exon 19 del | PRISI + six cycles of EP + osimertinib |
| M40 | SCLC-pleural metastases | n.d. | |||||
| ADC-RUL | Exon 19 del PIK3CA KRAS |
Peri-panc., Ret-LN, IVC, AG, and AG-inv. indicate peri-pancreatic lymph node, retroperitoneum lymph node, tumour embolism in inferior vena cava, adrenal grand, and adrenal grand invading part to retroperitoneum, respectively. RUL/RLL/RML/LUL/LLL: lobes of the lung designated as right or left and upper, middle or lower; Adrenal, adrenal gland; LN, lymph node; n.d., not done; EP, cisplatin and etoposide; NDR, no details recorded; SCLC, small-cell lung carcinoma.
Predictors of SCLC transformation
Owing to the limitations of performing biopsies on single lesions and intratumoral genetic heterogeneity, the detection of localized T-SCLC may be missed. Conducting multifocal biopsies is not feasible due to the increased risk and financial burden on the patient. Therefore, it is important to find early clues for potential disease transformation and enhance the diagnostic capacity of SCLC transformation. Some patients have shown increased levels of tumor biomarkers associated with T-SCLC, particularly NSE and Pro-GRP (24,25). Dong et al. suggested that pro-GRP demonstrates higher sensitivity and specificity than NSE in primary SCLC (26). In our case, NSE levels increased when T-SCLC occurred, and the fluctuation of NSE was consistent with the treatment response. On the other hand, Pro-GRP remained within the normal range throughout the course of the disease, which is consistent with previous reports by Chen et al. (14). NSE and Pro-GRP are not routinely tested for NSCLC patients, and further data is needed to explore their predictive value in the pathological transformation from NSCLC to SCLC. Lee et al. (23) provide a novel insight that the IHC assay for Rb and p53 can help predict which tumors are more likely to transform into SCLC. Lung cancers with EGFR/TP53/RB1 mutations are at a unique risk of histologic transformation, with 25% of cases presenting with de novo SCLC or eventually developing SCLC (22). The inactivation of RB1 and P53 is a significant indicator for predicting SCLC transformation. Therefore, It is recommended to regularly monitor NSE and Pro-GRP levels in the serum during TKI treatment. If there is an increase in NSE or Pro-GRP levels, or if RB1 and P53 inactivation is observed, clinicians may consider conducting multifocal biopsies to confirm SCLC transformation in cases of disease progression. Besides, our case study reveals that when SCLC transformation occurred in pleural metastases, the primary tumor of the right upper lobe showed new mutations of PIK3CA and KRAS. Recent data suggest that PI3K/AKT signaling plays a role in lineage plasticity and neuroendocrine transformation (27,28). In Chinese patients who underwent EGFR-TKI treatment and experienced SCLC transformation, the most commonly identified mutations in samples with SCLC transformation were in TP53 (17/25, 68.0%), RB1 (9/25, 36.0%), and PIK3CA (3/25, 12.0%) (29). Therefore, it is important to consider the involvement of PI3K/AKT signaling in SCLC transformation.
Proceed personalized comprehensive treatment for transformed SCLC
SCLC transformed from EGFR mutant NSCLC is associated with a very poor prognosis. Currently, there is no consensus on the optimal treatment approach. Both targeted therapy and chemotherapy are commonly used to treat T-SCLC, but their effectiveness is limited, and the prognosis remains bleak, with a median OS of less than 1 year (21). The presence of the founder EGFR mutation in transformed SCLC lesions indicates that these tumors still retain NSCLC-mutant characteristics, which exhibit an immune-inert phenotype and have a poor response to immune checkpoint inhibitors (30,31). Immunotherapy for T-SCLC has yielded unsatisfactory results. Studies by Nishikawa et al. (32) and Tokaca et al. (33) demonstrated no benefit from nivolumab treatment in transformed EGFR-mutant SCLC, similar to the findings of the retrospective study by Marcoux (21), where patients received treatments with anti-PD1, anti-CTLA4, and anti-PDL1 agents without any clinical improvement. The heterogeneity of spatial separation and molecular aberrations in ADC transformation to SCLC significantly impacts therapeutic effect, indicating that a single approach cannot effectively control T-SCLC. In this report, we propose an effective comprehensive treatment model that combines chemotherapy, targeted therapy, and brachytherapy for managing T-SCLC coexisting with ADC after EGFR-TKI treatment. The clinical benefit of local brachytherapy and chemotherapy has shown significant inhibition of extracranial tumor progression, Additionally, Osimertinib, as maintenance therapy, effectively manages CNS metastasis. Similarly, Pignataro et al. (34) reported a case of a patient with EGFR-mutated lung ADC who experienced lung lesion progression after gefitinib and osimertinib treatment. The lung lesion was pathologically confirmed as SCLC. The patient received standard limited-disease SCLC therapy of concurrent chemoradiotherapy, followed by resumption of osimertinib to target the putative TKI-sensitive brain clones due to brain progression during chemoradiotherapy. After 8 months of therapy, radiologic evaluations revealed radiation-induced pulmonary fibrosis in the upper left lobe but complete encephalic response. A personalized combination of systemic and local therapy may be a more effective treatment approach for patients with T-SCLC. This model emphasizes the importance of not only systemic drug therapy but also local treatment to eliminate all progressive lesions, similar to LCT in oligometastatic disease states. Radiotherapy, especially stereotactic radiotherapy SBRT, is preferred as a form of LCT, while surgery is considered in select cases. PRISI, a type of brachytherapy, allows for higher radiation doses to be delivered to the target lesion while minimizing exposure to normal tissues. The effectiveness of PRISI has been demonstrated in the treatment of lung cancer (35). In this case, PRISI was chosen to avoid accumulating lung damage caused by long-term TKI application. Previous studies on T-SCLC have highlighted tumor heterogeneity, where different progressive lesions respond differently to serial therapies (36-38). These case series demonstrate that heterogenous cells in T-SCLC are less likely to be eliminated by subsequent single therapies and may contribute to further disease progression. Therefore, it is important to consider the role of LCT in reducing the burden of heterogenous cells in this scenario. Further studies should aim to definitively assess the effectiveness of LCT in overcoming heterogeneity in progressive diseases.
Our study had two limitations. Firstly, we were unable to detect genetic alterations in pleural metastatic lesions of T-SCLC due to tissue depletion. Secondly, we did not perform tissue detection of intracranial progressive lesions, and the patient did not receive whole-brain radiation therapy. These limitations significantly restrict the clinical benefits of inhibiting the progression of extracranial tumors.
Conclusions
This study emphasizes the significance of conducting multi-point sampling biopsies to evaluate spatially separated mechanisms after EGFR TKI resistance. Overcoming the heterogeneity in T-SCLC requires individualized comprehensive treatment strategies, such as combining LCT with multiple systemic treatments. These strategies can expand therapeutic possibilities and serve as a reference for future clinical research.
Supplementary
The article’s supplementary files as
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki and its subsequent amendments. Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made.
Footnotes
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-24-67/rc
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-24-67/coif). The authors have no conflicts of interest to declare.
References
- 1.Hendriks LE, Kerr KM, Menis J, et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2023;34:339-57. 10.1016/j.annonc.2022.12.009 [DOI] [PubMed] [Google Scholar]
- 2.He J, Huang Z, Han L, et al. Mechanisms and management of 3rd‑generation EGFR‑TKI resistance in advanced non‑small cell lung cancer (Review). Int J Oncol 2021;59:90. 10.3892/ijo.2021.5270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piper-Vallillo AJ, Sequist LV, Piotrowska Z. Emerging Treatment Paradigms for EGFR-Mutant Lung Cancers Progressing on Osimertinib: A Review. J Clin Oncol 2020. [Epub ahead of print]. doi: . 10.1200/JCO.19.03123 [DOI] [PubMed] [Google Scholar]
- 4.Schoenfeld AJ, Yu HA. The Evolving Landscape of Resistance to Osimertinib. J Thorac Oncol 2020;15:18-21. 10.1016/j.jtho.2019.11.005 [DOI] [PubMed] [Google Scholar]
- 5.Lee J, Kim HS, Lee B, et al. Genomic landscape of acquired resistance to third-generation EGFR tyrosine kinase inhibitors in EGFR T790M-mutant non-small cell lung cancer. Cancer 2020;126:2704-12. 10.1002/cncr.32809 [DOI] [PubMed] [Google Scholar]
- 6.Calabrese F, Pezzuto F, Lunardi F, et al. Morphologic-Molecular Transformation of Oncogene Addicted Non-Small Cell Lung Cancer. Int J Mol Sci 2022;23:4164. 10.3390/ijms23084164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. 10.1126/scitranslmed.3002003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fallet V, Ruppert AM, Poulot V, et al. Secondary resistance to erlotinib: acquired T790M mutation and small-cell lung cancer transformation in the same patient. J Thorac Oncol 2012;7:1061-3. 10.1097/JTO.0b013e31824fea45 [DOI] [PubMed] [Google Scholar]
- 9.van Riel S, Thunnissen E, Heideman D, et al. A patient with simultaneously appearing adenocarcinoma and small-cell lung carcinoma harbouring an identical EGFR exon 19 mutation. Ann Oncol 2012;23:3188-9. 10.1093/annonc/mds525 [DOI] [PubMed] [Google Scholar]
- 10.Norkowski E, Ghigna MR, Lacroix L, et al. Small-cell carcinoma in the setting of pulmonary adenocarcinoma: new insights in the era of molecular pathology. J Thorac Oncol 2013;8:1265-71. 10.1097/JTO.0b013e3182a407fa [DOI] [PubMed] [Google Scholar]
- 11.Niederst MJ, Sequist LV, Poirier JT, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun 2015;6:6377. 10.1038/ncomms7377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suda K, Murakami I, Sakai K, et al. Small cell lung cancer transformation and T790M mutation: complimentary roles in acquired resistance to kinase inhibitors in lung cancer. Sci Rep 2015;5:14447. 10.1038/srep14447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furugen M, Uechi K, Hirai J, et al. An Autopsy Case of Two Distinct, Acquired Drug Resistance Mechanisms in Epidermal Growth Factor Receptor-mutant Lung Adenocarcinoma: Small Cell Carcinoma Transformation and Epidermal Growth Factor Receptor T790M Mutation. Intern Med 2015;54:2491-6. 10.2169/internalmedicine.54.5481 [DOI] [PubMed] [Google Scholar]
- 14.Chen S, He Y, Liu J, et al. Third-Generation TKI Resistance Due to SCLC Transformation: A Case Report and Brief Review. Onco Targets Ther 2019;12:11305-11. 10.2147/OTT.S228301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morinaga R, Okamoto I, Furuta K, et al. Sequential occurrence of non-small cell and small cell lung cancer with the same EGFR mutation. Lung Cancer 2007;58:411-3. 10.1016/j.lungcan.2007.05.014 [DOI] [PubMed] [Google Scholar]
- 16.Ninomaru T, Hata A, Hara S, et al. Heterogeneity or transformation? A whack-a-mole case of EGFR-mutant lung adenocarcinoma and small cell carcinoma: A case report. Thorac Cancer 2022;13:2394-7. 10.1111/1759-7714.14563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batra U, Nathany S, Sharma M, et al. Successful Treatment of EGFR-Mutant Synchronous SCLC and Lung Adenocarcinoma With Osimertinib. JTO Clin Res Rep 2020;2:100098. 10.1016/j.jtocrr.2020.100098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito T, Oi I, Saito Z, et al. De Novo SCLC Transformation From KRAS G12C-Mutated Lung Adenocarcinoma With Excellent Response to Sotorasib: A Case Report. JTO Clin Res Rep 2023;4:100510. 10.1016/j.jtocrr.2023.100510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takuma S, Inoue Y, Karayama M, et al. EGFR-Mutated Lung Adenocarcinoma Successfully Treated With Osimertinib After Spontaneous Transformation to SCLC and Adenocarcinoma With Neuroendocrine Differentiation: Case Report. JTO Clin Res Rep 2021;3:100264. 10.1016/j.jtocrr.2021.100264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrer L, Giaj Levra M, Brevet M, et al. A Brief Report of Transformation From NSCLC to SCLC: Molecular and Therapeutic Characteristics. J Thorac Oncol 2019;14:130-4. 10.1016/j.jtho.2018.08.2028 [DOI] [PubMed] [Google Scholar]
- 21.Marcoux N, Gettinger SN, O'Kane G, et al. EGFR-Mutant Adenocarcinomas That Transform to Small-Cell Lung Cancer and Other Neuroendocrine Carcinomas: Clinical Outcomes. J Clin Oncol 2019;37:278-85. 10.1200/JCO.18.01585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Offin M, Chan JM, Tenet M, et al. Concurrent RB1 and TP53 Alterations Define a Subset of EGFR-Mutant Lung Cancers at risk for Histologic Transformation and Inferior Clinical Outcomes. J Thorac Oncol 2019;14:1784-93. 10.1016/j.jtho.2019.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JK, Lee J, Kim S, et al. Clonal History and Genetic Predictors of Transformation Into Small-Cell Carcinomas From Lung Adenocarcinomas. J Clin Oncol 2017;35:3065-74. 10.1200/JCO.2016.71.9096 [DOI] [PubMed] [Google Scholar]
- 24.Kato Y, Tanaka Y, Hino M, et al. ProGRP as early predictive marker of non-small-cell lung cancer to small-cell lung cancer transformation after EGFR-TKI treatment. Respir Med Case Rep 2019;27:100837. 10.1016/j.rmcr.2019.100837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao X, Liu J, Hu F, et al. Serum NSE is Early Marker of Transformed Neuroendocrine Tumor After EGFR-TKI Treatment of Lung Adenocarcinoma. Cancer Manag Res 2022;14:1293-302. 10.2147/CMAR.S349082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong A, Zhang J, Chen X, et al. Diagnostic value of ProGRP for small cell lung cancer in different stages. J Thorac Dis 2019;11:1182-9. 10.21037/jtd.2019.04.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quintanal-Villalonga A, Taniguchi H, Zhan YA, et al. Multiomic Analysis of Lung Tumors Defines Pathways Activated in Neuroendocrine Transformation. Cancer Discov 2021;11:3028-47. 10.1158/2159-8290.CD-20-1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quintanal-Villalonga Á, Chan JM, Yu HA, et al. Lineage plasticity in cancer: a shared pathway of therapeutic resistance. Nat Rev Clin Oncol 2020;17:360-71. 10.1038/s41571-020-0340-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang W, Xu C, Chen H, et al. Genomic alterations and clinical outcomes in patients with lung adenocarcinoma with transformation to small cell lung cancer after treatment with EGFR tyrosine kinase inhibitors: A multicenter retrospective study. Lung Cancer 2021;155:20-7. 10.1016/j.lungcan.2021.03.006 [DOI] [PubMed] [Google Scholar]
- 30.Lee CK, Man J, Lord S, et al. Clinical and Molecular Characteristics Associated With Survival Among Patients Treated With Checkpoint Inhibitors for Advanced Non-Small Cell Lung Carcinoma: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:210-6. 10.1001/jamaoncol.2017.4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le X, Negrao MV, Reuben A, et al. Characterization of the Immune Landscape of EGFR-Mutant NSCLC Identifies CD73/Adenosine Pathway as a Potential Therapeutic Target. J Thorac Oncol 2021;16:583-600. 10.1016/j.jtho.2020.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishikawa S, Tambo Y, Ninomiya H, et al. A case treated with nivolumab after small cell lung cancer transformation of mutant EGFR non-small cell lung cancer. Ann Oncol 2016;27:2300-2. 10.1093/annonc/mdw431 [DOI] [PubMed] [Google Scholar]
- 33.Tokaca N, Wotherspoon A, Nicholson AG, et al. Lack of response to nivolumab in a patient with EGFR-mutant non-small cell lung cancer adenocarcinoma sub-type transformed to small cell lung cancer. Lung Cancer 2017;111:65-8. 10.1016/j.lungcan.2017.07.012 [DOI] [PubMed] [Google Scholar]
- 34.Pignataro D, Bertaglia V, Bironzo P, et al. Oligoprogressive Disease With SCLC Transformation in EGFR-Mutated NSCLC: How Biology Knowledge Can Change the Game Rules. J Thorac Oncol 2020;15:e170-2. 10.1016/j.jtho.2020.06.020 [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Li J, Li R, et al. Efficacy and safety of iodine-125 radioactive seeds brachytherapy for advanced non-small cell lung cancer-A meta-analysis. Brachytherapy 2018;17:439-48. 10.1016/j.brachy.2017.11.015 [DOI] [PubMed] [Google Scholar]
- 36.Santoni-Rugiu E, Grauslund M, Melchior LC, et al. Heterogeneous resistance mechanisms in an EGFR exon 19-mutated non-small cell lung cancer patient treated with erlotinib: Persistent FGFR3-mutation, localized transformation to EGFR-mutated SCLC, and acquired T790M EGFR-mutation. Lung Cancer 2017;113:14-7. 10.1016/j.lungcan.2017.08.024 [DOI] [PubMed] [Google Scholar]
- 37.Yang MH, Yu J, Cai CL, et al. Small cell lung cancer transformation and tumor heterogeneity after sequential targeted therapy and immunotherapy in EGFR-mutant non-small cell lung cancer: A case report. Front Oncol 2022;12:1029282. 10.3389/fonc.2022.1029282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wysota M, Asiry S, Goldstein Y, et al. Revolving Door of Histologic Transformation-Tumor Heterogeneity Complicating the Management of EGFR-Mutated Lung Adenocarcinoma: A Case of Jekyll and Hyde. JTO Clin Res Rep 2020;2:100128. 10.1016/j.jtocrr.2020.100128 [DOI] [PMC free article] [PubMed] [Google Scholar]