Abstract
Background
Maturity-onset diabetes mellitus of the young (MODY) is a form of autosomal dominant inherited diabetes, featuring diverse clinical characteristics due to distinct pathogenic gene mutation sites. Reports on MODY caused by RFX6 mutations are scarce, and it is prone to being misdiagnosed as type 1 or type 2 diabetes in clinical settings. To date, no cases have been reported regarding patients with RFX6 gene mutations accompanied by refractory hyperlipidemia, and the treatment remains undetermined. We present a rare case of a 13-year-old Chinese girl who was admitted to the Third Affiliated Hospital of Soochow University with diabetes combined with refractory hyperlipidemia.
Case Description
A 13-year-old adolescent female presented with persistent dry mouth, polydipsia, and polyuria. The physical examination accidentally found high blood glucose. She had refractory hyperlipidemia in the past and was treated with a variety of lipid-lowering programs, but her lipids were still poorly controlled. Due to the patient’s early age of onset, the special type of diabetes cannot be excluded, and she is recommended to be further examined in our hospital.
Conclusions
We sequenced the MODY-related genes of the patient and her mother. RFX6 heterozygous frame-shifting variants were found in the proband and her mother (NNM_173560:c.1500delT). The patient was eventually diagnosed with MODY. During the hospitalization, we treated the patient with insulin hypoglycemic treatment, and the patient’s blood glucose was stable. Surprisingly, the patient’s blood lipid also decreased significantly, and even without using any lipid-lowering drugs, the blood lipid remained at a low level.
Keywords: Maturity-onset diabetes mellitus of the young (MODY), RFX6, hyperlipidemia, case report
Highlight box.
Key findings
• We have identified a novel RFX6 gene mutation in an adolescent female leading to maturity-onset diabetes mellitus of the young (MODY), and this mutation is highly likely to cause refractory hyperlipidemia.
What is known and what is new?
• This article covers the well-known gene mutations responsible for MODY, previously reported clinical manifestations caused by RFX6 gene mutations, and the established relationship between the RFX6 gene and glucose-lipid metabolism.
• The novel contributions include the identification of a rare and previously unreported RFX6 mutation leading to MODY with refractory hyperlipidemia, along with diagnostic and therapeutic strategies for this specific MODY subtype.
What is the implication, and what should change now?
• This suggests the emergence of a new MODY subtype and provides reference for treatment options, highlighting the importance of proactive genetic testing when encountering similar cases to ensure accurate diagnosis and avoid missed diagnoses. We should select the most appropriate and effective treatment strategies based on distinct genetic phenotypic characteristics, thereby minimizing disease burden for patients.
Introduction
Maturity-onset diabetes mellitus of the young (MODY) is an autosomal dominant monogenic diabetes mellitus (1). Its pathogenesis is related to the dysfunction of pancreatic β cells, and its phenotype overlaps with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM), which makes it easy to be misdiagnosed (2,3). The most common forms of MODY are caused by mutations in HNF4A, GCK, and HNF1A (4). With advances in genetic testing technology, new subtypes of MODY are constantly being discovered. However, there is still a lack of experience with the diagnosis, characteristics and treatment of MODY, which often leads to missed treatment opportunities (5). Therefore, it is of great significance to improve the classification of MODY and clarify the characteristics and treatment experience of different subtypes of MODY to guide clinical diagnosis and treatment.
As an important transcription factor, RFX6 regulates insulin secretion by regulating β-cell voltage-gated Ca2+ channel-related gene expression (6). Down-regulation of RFX6 can lead to decreased activity of type I Ca2+ channels, resulting in decreased insulin secretion (7). Homozygous RFX6 mutations can cause Mitchell-Riley syndrome, which is mainly manifested as neonatal diabetes, intestinal atresia, pancreas-gallbladder dysplasia, etc. (8). Heterozygous RFX6 mutations have been poorly studied. Recently, we found a case of MODY in a 13-year-old adolescent female with RFX6 frame-shift mutation (NNM_173560:c.1500delT), characterized as follows: age of onset <25 years old, family history of diabetes, lack of diabetes autoantibodies, refractory hyperlipidemia, insensitivity to oral hypoglycemic drugs, Glucagon-like peptide-1 receptor agonists respond well (9). Here, we report the diagnosis and 7-month treatment follow-up of this RFX6 heterozygous variant MODY patient. We present this article in accordance with the CARE reporting checklist (available at https://acr.amegroups.com/article/view/10.21037/acr-24-266/rc).
Case presentation
A 13-year-old adolescent girl, usually dry mouth, polydipsia, polyuria symptoms are obvious. She was found to have hyperglycaemia during physical examination, so she was checked in the outpatient department of a local hospital for glucose of 18.79 mmol/L, total cholesterol of 3.43 mmo/L, triglyceride of 5.71 mmol/L, high density lipoprotein cholesterol of 0.58 mmol/L, low-density lipoprotein cholesterol of 1.67 mmol/L, and apolipoprotein A1 0.83 g/L. She was diagnosed with diabetes and went to the Nanjing Jinyu Medical Laboratory in China for genetic testing. When the patient came to our hospital, she was 166 cm tall, weighed 55 kg, and BMI 20.0 kg/m2. She had previously suffered from hyperlipidemia and had received lipid-lowering treatment with oral drugs “fenofibrate + ezetimibe + pivastatin”. PSK9 treatment had been initiated in other hospitals, and her lipids were poorly controlled. No obvious abnormality was found in physical examination. In the hospital, the relevant examination was completed. The hemoglobin A1c (HbA1c) was 13.1%; the anti-human insulin antibody and glutamate decarboxylase antibody were negative; C-peptide measurement (fasting, 60, 120, and 180 min): 599.20, 1320.00, 1,351.00, and 1,317.00 pmol/L, respectively; insulin determination (fasting, 60, 120, and 180 min): 46.57, 163.80, 163.40, and 140.60 pmol/L, respectively; the adrenal axis, thyroid axis and gonadal axis were normal. The patient had no diabetic retinopathy or neuropathy, urinary albumin level was 33.81 mg/L, and bone mineral density examination showed bone mass loss. Ultrasound and computed tomography showed no abnormalities in the gallbladder, pancreas, bilateral ureter, bladder, thyroid, uterus and its appendages except for mild fatty liver, splenomegaly and enhanced echo of the bilateral renal cortex.
Four injections of insulin intensive therapy (insulin lispro 10 U for breakfast, 6 U for lunch, 8 U for dinner, recombinant insulin glargine 18 U for all day basis) combined with metformin 1 g twice daily and acarbose 100 mg three times daily for 7 days, the patient’s glucose was controlled smoothly and discharged from hospital. Lipid-lowering drugs were discontinued during hospitalization. As shown in Figure 1, the patient’s lipid levels decreased significantly after treatment. Health education, low fat diabetes diet and exercise are used to achieve disease management. One month after discharge, it was found that the fasting blood glucose was controlled at 6–7 mmol/L and the postprandial blood glucose was controlled at 7–10 mmol/L. Blood lipid decreased significantly, and lipid-lowering drugs were discontinued. The blood lipid level and the application of related drugs are shown in Figure 1. Four months after discharge, the patient returned to the hospital with fasting blood glucose of 6–7 mmol/L and postprandial blood glucose of 7–12 mmol/L, with occasional blood glucose fluctuations. The patients and their families were satisfied with the treatment results. In order to simplify the hypoglycemic regimen, with the consent of the patient and her family, the hypoglycemic regimen was adjusted to “insulin degludec and liraglutide injection 10 U/day, metformin 1.0 g twice daily, acarbose 100 mg three times daily”. After 3 months, the blood glucose control was stable: fasting blood glucose was 6–7 mmol/L, and postprandial blood glucose was 7–10 mmol/L. During the adjustment of the hypoglycemic program, the DPP-4 inhibitor saxagliptin was tried, but the hypoglycemic effect was poor. Due to the patient’s early onset age, family history of diabetes, and secondary hyperlipidemia that was difficult to correct with drugs, we sequenced the MODY-related genes of the patient and her mother. RFX6 heterozygous frame-shifting variants were found in the proband and her mother (NNM_173560:c.1500delT). The gene sequencing was carried out in consultation with clinical geneticists. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration and its subsequent amendments. Written informed consent was obtained from the patient’s legal guardians for the publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Figure 1.
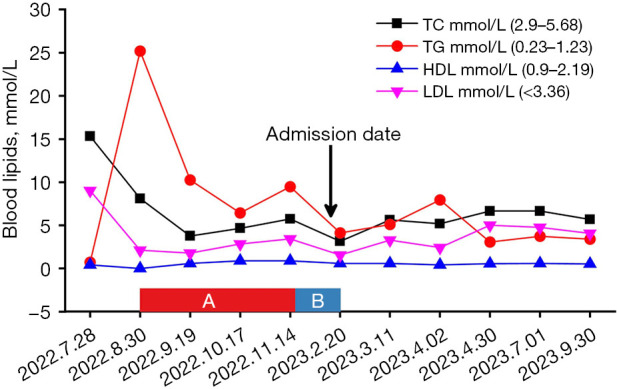
Blood lipid administration and control curve. (A) Pitavastatin 2 mg once daily + ezetimibe 10 mg once nightly + fenofibrate 0.2 g once daily; (B) fenofibrate 0.2 g once daily + evolocumab injection 140 mg once weekly. The black arrow indicates the date of admission. The black curve represents the patient’s TC trend, the red curve shows TG levels, the dark blue curve depicts HDL changes, and the purple curve illustrates LDL variations. HDL, high-density lipoprotein; LDL, low-density lipoprotein; TC, total cholesterol; TG, triglyceride.
Discussion
This patient was accompanied by hyperlipidemia, and the lipid control was still poor under the combination of various lipid-lowering drugs. After standard hypoglycemic treatment, patients’ blood glucose control reached the standard, and blood lipids also decreased, even after stopping lipid-lowering drugs. This indicates that the hyperlipidemia in this case is secondary hyperlipidemia caused by hyperglycemia. In this case, when the patient’s glucose was controlled, the body’s glucose metabolism gradually returned to normal. We found that although patients’ lipids gradually decreased and leveled off, they were never able to reach the target range. Therefore, we speculate that refractory hyperlipidemia in this case may be closely related to RFX6 mutations. Studies have shown that RFX6 not only affects insulin secretion but also may directly cause lipid metabolism disorders by regulating intestinal hormones and liver metabolism (1,3). Many studies have shown that RFX6 has functional interactions with other lipid metabolism-related genes (such as APOE, LPL) to regulate lipid metabolism (1,2,4). This article puts forward this point of view, hoping to attract the attention of peers.
In the pathogenesis of MODY associated with RFX6 heterozygous mutations, the mainstream view suggests that this mutation leads to reduced activity of pancreatic Ca2+ channels, decreased insulin secretion, and disordered glucose metabolism. However, in this case, the patient’s C-peptide levels were not reduced; instead, they indicated excessive insulin secretion, suggesting the possible presence of insulin resistance. This phenotypic difference may be related to the following mechanisms: firstly, pleiotropic effects of the RFX6 gene: RFX6 not only regulates insulin secretion but may also indirectly influence other metabolic pathways, leading to insulin resistance. Studies have shown that RFX6 plays a critical role in pancreatic development and function, but its specific mechanisms are not yet fully understood. Certain RFX6 variants may cause β-cell dysfunction, manifesting as excessive insulin secretion rather than reduced secretion (1,5). Secondly, secondary effects of insulin resistance: the patient’s insulin resistance may have led to compensatory overproduction of insulin by β-cells, thereby masking the reduced insulin secretion caused by the RFX6 mutation. In this scenario, C-peptide levels may be elevated, but the biological effectiveness of insulin is diminished (6). Moreover, environmental or genetic modifying factors: the patient’s unhealthy diet, lifestyle, inflammatory status, or other genetic factors may have influenced the phenotypic expression of the RFX6 mutation, resulting in a phenotype different from that of typical MODY (5,7,8).
Different types of MODY have different sensitivities to drug response. The treatment of patients with RFX6 mutations is still inconclusive. Patients with RFX6 mutations we reported responded well to the GLP-1 receptor agonist liraglutide. Studies have shown that RFX6 defects lead to significant reductions in GLP and GLP-1 levels in mice (7,10-12). By activating the GLP-1 receptor, GLP-1 receptor agonists increase insulin secretion in a glucose concentration-dependent manner, inhibit glucagon secretion, delay gastric emptying, and reduce food intake through central control of appetite, thus achieving the effect of lowering glucose. Therefore, this mechanism may be an important reason why liraglutide significantly improved the blood glucose level in the patient in this case. However, more evidence is needed to confirm whether GLP-1 agonists can be used as the first choice for MODY patients with RFX6 mutations. In addition, we tried to apply DPP-4 inhibitors to hypoglycemia in this case, but the effect was not satisfactory (detailed data were lacking). Although the drug is in the same pathway as GLP-1 agonists, factors such as mode of administration and duration of administration may affect the hypoglycemic effect. At present, the use of calcium channel agonists in MODY patients has not been reported, and their efficacy is still unknown. Clinical studies are lacking on whether Ca2+ channel agonists can be applied to treat hyperglycemia caused by an RFX6 heterozygous mutation, and what the advantages and disadvantages are.
Conclusions
We sequenced the MODY related genes of the patient and her mother. Detailed genetic testing methods are provided in Appendix 1. RFX6 heterozygous frame-shifting variants were found in the proband and her mother (NNM_173560:c.1500delT). The patient was eventually diagnosed with MODY. During the hospitalization, we treated the patient with insulin hypoglycemic treatment and the patient’s blood glucose was stable. Surprisingly, the patient’s blood lipid also decreased significantly, and even without using any lipid-lowering drugs, the blood lipid remained at a low level. In addition, we found that this patient responded well to glucagon-like peptide-1 receptor agonists. Other family members of the patient have refused genetic testing, making it impossible for us to construct a family pedigree of the gene mutation. We further inquired about the medical history and clinical manifestations of the patient’s mother. The patient’s mother does not have early-onset diabetes or hyperlipidemia. This is also consistent with the clinical heterogeneity observed in MODY patients.
Supplementary
The article’s supplementary files as
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration and its subsequent amendments. Written informed consent was obtained from the patient’s legal guardians for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Footnotes
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://acr.amegroups.com/article/view/10.21037/acr-24-266/rc
Funding: This work was supported by the Changzhou Longcheng Talent Program-Leading Innovative Talent Introduction and Cultivation Project (No. CQ20230116), Changzhou Medical Center, Nanjing Medical University (No. CMCB202419), and Changzhou key Medical Discipline (No. CZXK202201).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://acr.amegroups.com/article/view/10.21037/acr-24-266/coif). The authors have no conflicts of interest to declare.
References
- 1.Bonnefond A, Unnikrishnan R, Doria A, et al. Monogenic diabetes. Nat Rev Dis Primers 2023;9:12. 10.1038/s41572-023-00421-w [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim H, Balboa D, Saarimäki-Vire J, et al. RFX6 haploinsufficiency predisposes to diabetes through impaired beta cell function. Diabetologia 2024;67:1642-62. 10.1007/s00125-024-06163-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winter WE, Nakamura M, House DV. Monogenic diabetes mellitus in youth. The MODY syndromes. Endocrinol Metab Clin North Am 1999;28:765-85. 10.1016/S0889-8529(05)70101-8 [DOI] [PubMed] [Google Scholar]
- 4.Thanabalasingham G, Owen KR. Diagnosis and management of maturity onset diabetes of the young (MODY). BMJ 2011;343:d6044. 10.1136/bmj.d6044 [DOI] [PubMed] [Google Scholar]
- 5.Bishay RH, Greenfield JR. A review of maturity onset diabetes of the young (MODY) and challenges in the management of glucokinase-MODY. Med J Aust 2017;207:223. 10.5694/mja16.01467 [DOI] [PubMed] [Google Scholar]
- 6.Chandra V, Albagli-Curiel O, Hastoy B, et al. RFX6 regulates insulin secretion by modulating Ca2+ homeostasis in human β cells. Cell Rep 2014;9:2206-18. 10.1016/j.celrep.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 7.Piccand J, Strasser P, Hodson DJ, et al. Rfx6 maintains the functional identity of adult pancreatic β cells. Cell Rep. 2014;9:2219-32. 10.1016/j.celrep.2014.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trott J, Alpagu Y, Tan EK, et al. Mitchell-Riley syndrome iPSCs exhibit reduced pancreatic endoderm differentiation due to a mutation in RFX6. Development 2020;147:dev194878. 10.1242/dev.194878 [DOI] [PubMed] [Google Scholar]
- 9.Patel KA, Kettunen J, Laakso M, et al. Heterozygous RFX6 protein truncating variants are associated with MODY with reduced penetrance. Nat Commun 2017;8:888. 10.1038/s41467-017-00895-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith SB, Qu HQ, Taleb N, et al. Rfx6 directs islet formation and insulin production in mice and humans. Nature 2010;463:775-80. 10.1038/nature08748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanchez JG, Rankin S, Paul E, et al. RFX6 regulates human intestinal patterning and function upstream of PDX1. Development 2024;151:dev204379. 10.1242/dev.204379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCauley HA, Matthis AL, Enriquez JR, et al. Enteroendocrine cells couple nutrient sensing to nutrient absorption by regulating ion transport. Nat Commun 2020;11:4791. 10.1038/s41467-020-18536-z [DOI] [PMC free article] [PubMed] [Google Scholar]


