Abstract
As a global public health issue, intracerebral hemorrhage (ICH) is characterized by high morbidity and mortality. Brain injury following ICH is composed of primary and secondary injury, with the latter being more severe and resulting in increased apoptosis. Sox11 (sex-determining region Y-related high-mobility-group 11), a vital member of the Sox gene family, is broadly discovered in the developing nervous system and may have a vital impact on neurogenesis, neuronal survival, and neurite outgrowth. The level and impacts of Sox11 in brain with ICH remain indistinct. The major objective of the current work was to explore the spatiotemporal expression of Sox11 and its roles in secondary brain injury under the ICH impairment. The ICH rat model was established by injecting autologous blood into the right basal ganglia of male Sprague–Dawley rats. It was observed that Sox11 expression was notably elevated in brain tissue after ICH. The enhancement of Sox11 expression through miR-221 reduced neuronal apoptosis and inflammation in the affected rats. Furthermore, overexpression of Sox11 mitigated ICH-induced brain edema, blood-brain barrier disruption, and cognitive impairments. In contrast, Sox11 knockdown resulted in opposing effects. These findings highlight the crucial role of Sox11 in alleviating secondary brain injury following ICH. Thus, upregulating Sox11 presents a promising therapeutic strategy to reduce secondary brain injury in clinical ICH cases.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-15239-7.
Keywords: Sox11, MiR-221, Secondary brain injury, Intracerebral hemorrhage, Neuroinflammation
Subject terms: Neuroscience, Molecular neuroscience, Neuroimmunology
Introduction
Intracerebral hemorrhage (ICH), a common acute central nervous system (CNS) disorder, is associated with high mortality and morbidity rates. Which accounts for approximately 15% of all stroke cases1,2. Only 20% of patients with ICH maintain functional independence six months after the injury3,4. Following ICH, concomitant brain damage includes primary brain injury (PBI) and secondary brain injury (SBI)5. PBI involves the formation and expansion of the intracerebral hematoma, resulting in direct mechanical damage to the brain tissue. SBI, on the other hand, encompasses a series of pathological processes, including the activation of apoptosis, exacerbation of ischemia and edema in the brain tissue surrounding the hematoma, and the toxic effects associated with these conditions6,7. Many studies have indicated that neurological deterioration is primarily caused by SBI, rather than PBI, following ICH. Although there have been many studies and clinical tests for developing promising therapies for ICH, resultant mortality maintained at a high level and there were no efficient treatments have successfully improved the prognosis of patients8–11. Therefore, the integrated interpretation of the molecular mechanisms involved in ICH-induced SBI is crucial for further elucidating and effectively treating ICH.
Over the past thirty years, the family of sex-determining region Y (SRY)-related high-mobility-group (HMG) box (Sox) transcription factors has become the hotspot for its underlying physiological and pathological impacts on cell biology, a common feature of members of the sequence of Sox family is highly homologous with the SRY-HMG DNA-binding domain12,13. The expression of Sox11 is broadly discovered in the developing nervous system, which may affect the neurogenesis, neuronal survival, and neurite outgrowth14. In mature neurons, Sox11 is significantly down-regulated, but emerging evidence has suggested that the expressions of Sox11 in mature central and peripheral neurons were highly induced by axotomy and/or ischemia15–21. Caspases are a group of proteolytic enzymes that play a crucial role in eukaryotic cell apoptosis. They are involved in regulating various cellular processes, including cell growth, differentiation, and apoptosis., it has been determined that there are at least 11 caspase members; caspase-3, caspase-6, and caspase-7 are the performers of apoptosis, and Sox11 is identified as a potential caspase interacting protein, which can significantly reduce the activity of these three caspases22,23. It has been revealed that Sox11 could modulates BDNF expression in an exon promoter-specific manner24. The high expressions of Sox11 are commonly found in most malignant glioblastomas and medulloblastomas, which has excellent prognostic value in epithelial ovarian tumors and high-grade breast cancer tumors25–28. It has been reported that Sox11 was highly expressed in virtually all aggressive Mantle cell lymphomas29.
miRNAs are highly expressed in the mammalian brain; however, their biological roles remain largely unclear in various neurological disorders, including Parkinson’s disease, Alzheimer’s disease, and stroke30. Numerous studies have shown that miRNAs play crucial roles in biological processes like cell growth and proliferation, and that many miRNAs hold promise as therapeutic targets for treating cancers and metabolic disorders31. Multiple studies have identified miR-221 as a potential biomarker for stroke, as its plasma levels are reduced in affected patients. Experimental studies in animals have further demonstrated that miR-221 can confer neuroprotective effects through various mechanisms32. Furthermore, recent studies have revealed a strong association between miR-221 and SOX11, with both implicated in various diseases, including liver cancer and degenerative osteoarthropathy33,34. This naturally raises the question of whether miR-221 and SOX11 may also have a connected role in the context of stroke.
Given the existing evidence, Sox11 has been shown to play a role in neuronal survival, apoptosis, and neurite outgrowth in various CNS disorders. After brain injury, Sox11 may offer protective effects by inhibiting apoptosis. It is hypothesized that there could be a relationship between Sox11 expression and apoptosis in brain tissue affected by ICH. However, the expression levels of Sox11 in rats following ICH have not been thoroughly explored in previous research. Therefore, the present study aims to investigate the spatiotemporal expression of Sox11 in response to ICH and to explore its potential role in secondary brain injury induced by ICH.
Materials and methods
Animals and ethics
All experiments were approved by the Institutional Animal Care Committee of Zhejiang Provincial People’s Hospital and conducted in accordance with the guidelines set forth by the National Institutes of Health for the care and use of animals. Adult male Sprague-Dawley (SD) rats weighing 280–300 g were sourced from the Animal Center of the Chinese Academy of Sciences (Shanghai, China). Euthanasia was performed in compliance with established guidelines, with cervical dislocation following deep anesthesia induced by an intraperitoneal injection of sodium pentobarbital (40 mg/kg). Efforts were made to minimize the number of animals used and to reduce their suffering before euthanasia and perfusion/fixation.
The establishment of ICH rats
The rat ICH model was established based on existing literature using an autologous blood injection method5. In brief, SD rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (40 mg/kg) and secured to a stereotaxic apparatus. The investigators extracted 100 µl of autologous blood from the rat’s femoral artery using a microinjector, and a hole was made about 3.5 mm to the right of the sagittal suture. The microinjector was installed on the stereotactic frame, and the needle was inserted slowly, about 5.5 mm deep. Subsequently, 100 µl of autologous blood was injected into the right basal ganglia with a speed of approximately 20 µl/min tardily, and the needle was removed 5 min after the injection. The hole in the skull was sealed by bone wax. The method of injecting intervention reagents into the lateral ventricle is as follows: Identify the sagittal suture, then move 1.5 mm laterally to the right and 1.2 mm posteriorly. Drill a small cranial hole at this location, insert the needle to a depth of 4.5 mm, and inject mic-miR-221 (Thermo Fischer Scientific, Assay IDs: MC11012) and si-Sox11 (Sequence: 5′-CCAGCAGAGUGUCAAGAAUTT-3′) into the lateral ventricle. The rats in the Sham group were simply injected with the same volume of saline solution with the method just illustrated as ICH rats.
Throughout the surgery, the rats were maintained in a supine position on a heating blanket to maintain their body temperature at approximately 37 ± 0.5 °C. No rats died in the Sham group. There was no significant difference in mortality between the experimental groups. The procedures of execution were carried out in accordance with the euthanasia guidelines, and cervical dislocation was performed after deep anesthesia. In a word, we endeavored to reduce the usage rate and suffering of rats to the greatest extent.
Experimental grouping
The experiments were divided into two main parts: Experiment 1 and Experiment 2. The rats were allocated to different groups using a random assignment method, which involved a researcher who was blind to the group allocations assigning the rats based on a random-number table.
In Experiment 1, following the induction of ICH, the surviving 30 ICH rats were distributed into various ICH groups for this experiment. This part included six distinct groups: a Sham group and five experimental groups categorized by time intervals post-ICH—specifically at 1, 12, 24, 36 and 48 h after ICH onset (with each group consisting of five randomly selected subjects from the surviving population). After treating the ICH condition, deep anesthesia was administered to the rats using sodium pentobarbital at designated times, allowing for collection of relevant brain tissues for further analysis. For each rat subject, two coronal slices of cerebral tissue were taken—one located three millimeters before and another four millimeters after where the coronal injection occurred. The right basal ganglia were then promptly isolated from one slice measuring three millimeters thick for Western blot analysis; meanwhile, another slice measuring four millimeters thick was fixed in a solution of 4% paraformaldehyde before being embedded in paraffin and sectioned into slices that are four micrometers thick for immunofluorescence studies.
Experiment 2 consisted of six additional groups with each containing eighteen randomly selected rats derived from both Sham and ICH subjects as previously described. These included a Sham group, an ICH group, an ICH + negative-control small interfering RNA (siRNA) group (Si-Control), an ICH + Sox11 siRNA group (Si-Sox11), an ICH + Vector control group, and an ICH + mimic-miR-221 treatment group. Subsequently, injections of mimic-miR-221 and siRNA took place twenty-four hours prior to inducing ICH (further details provided below). Following induction of ICH, treatments would be extended to include eighteen surviving rats within each respective experimental subgroup.
Western blot (WB) assay
As indicated in former study, the Western blot assay was commonly applied for the measurement of protein levels35. Brain specimens taken from the brain tissue of the bloodswatted side 3 mm before the needlepoint were used to performed WB, When the expression level of sox11 was the highest, it indicated that sox11 played the strongest role at this time point, at this time point, the brain tissue from the ICH rats was selected for further experiments. The right basal ganglia of each rat were carefully separated, homogenized, and centrifuged at 12,000 g for 10 min at 4 °C. The supernatants were then collected. Protein concentrations were determined using the Enhanced BCA Protein Assay Kit. Equivalent amounts of protein (30 µg per lane) were loaded onto an SDS polyacrylamide gel for electrophoresis. After electrophoresis, the samples were transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 5% nonfat milk for 1 h and then incubated with primary antibodies overnight at 4 °C. Anti-Sox11 and anti-β-tubulin antibodies (Table 1) were used for immunoblotting. The membranes were then washed three times with PBST and incubated with secondary antibodies. Signal acquisition was performed using the specific kit. The relative protein levels were quantified using ImageJ software.
Table 1.
Resource identifiers for antibodies.
| Antibody name | Sox11 antibody | β-tubulin antibody | NeuN antibody | IF secondary antibod |
|---|---|---|---|---|
| Target antigen | Human, rat, mouse | Human, rat, mouse | Human, rat, mouse | Human, rat, mouse |
| Vendor | ABCAM | Santa-cruz | ABCAM | Life Technologies |
| Cat number | Ab234996 | Sc166729 | ab177487 | A-21,208 and A32727 |
| Clonality | Monoclonal anti-body | Polyclonal anti-body | Monoclonal anti-body | Polyclonal anti-body |
| Host organism | Rabbit | mouse | rabbit | Donkey |
| Comments | IHC-P, WB | WB | WB, IF | IF |
| Application dilution |
WB = 1:1000 IF = 1:200 |
WB = 1:3000 | IF = 1:300 | IF = 1:200 |
Immunofluorescence (IF) staining
As previously discussed, the immunofluorescence staining was conducted in this study36. The brain samples were fixed with 4% paraformaldehyde at 4 °C overnight, then embedded in paraffin and sectioned to a thickness of 4 μm. After antigen retrieval and blocking, the sections were incubated with primary antibodies for Sox11 and NeuN overnight at 4 °C. The slices were then washed three times with TBST. Next, the appropriate secondary antibodies were applied. Finally, the sections were mounted with an anti-fading mounting medium containing 4,6-diamino-2-phenylindole (DAPI). 6 microscopic fields with 400 magnifications in the nearest cortex nearby the hematoma in ipsilateral cerebral hemisphere in every slice were at least observed by a researcher unaware of the grouping of rats, the representative photos of which were independently obtained from at least 6 tests with 6 different rats, Image J software was employed for analyzing the relevant fluorescence intensity and counting the positive cells by a researcher unaware of the grouping information.
Quantitative real‑time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from the striata of the rats using the specific Kit. cDNA was synthesized for RT-PCR. Gene expression levels were determined by one-step real-time RT-PCR using Luminaris Color HiGreen qPCR Master Mix. The qPCR reaction mixture consisted of cDNA, 200 nM specific primers, and Maxima SYBR Green/ROX qPCR Master Mix. PCR amplification was performed under the following conditions: 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min, with a final step of 60 °C for 30 s.
Enzyme-linked immunosorbent assay (ELISA)
At 72 h after ICH (but before immediately euthanized), the rat is deeply anesthetized and fixed on the surgical table. With the bevel of the needle facing upward, it is inserted vertically or slightly obliquely into the left ventricle or pericardial cavity (at the point of visible pulsation). Blood is then slowly aspirated, collecting 5–10 mL of whole blood. The CSF samples were collected from the foramen magnum. All blood samples were prepared under the centrifugation at 1000g for 5 min at 4 °C, and CSF samples were under immediate centrifugation at 12,000g for 30 min at 4 °C. Next, the supernatants were gathered for the analyses of the expressions of IL-1β and TNF-α using the specific ELISA kits (Bio-Swamp, China). The above analyses were obtained via the manufacturer’s guidelines.
Reactive oxygen species (ROS) analysis
The levels of cerebral ROS were considered as an indicator of oxidative stress, which were measured by an ROS assay kit (Beyotime, S0035M). The experiment was conducted strictly according to the protocol provided in the instructions. The ROS was evaluated 72 h after ICH.
The quantity of brain water content
As reported37, the wet-dry method was employed to assess the index of cerebral edema. In brief, sodium pentobarbital was i.p. injected to the subjects 72 h post to the ICH impairment, and the intact brain was instantly collected. Each brain was divided into two hemispheres along the midline, and then further dissected into five subregions. The wet weights of the sections were immediately recorded. Following this, the samples were dehydrated at 100 °C for 72 h, after which their dry weights were measured. The formula to calculate the proportion of water quantity was revealed as follows: [(wet weight − dry weight)/wet weight] ×100%.
Neurobehavioral evaluations
Behavioral trials for evaluating neurology were conducted at 1 h before euthanasia. All rats that underwent neurological function assessment were scored according to the seven independent functional experiments shown in Table 238. The Neurobehavioral evaluations was evaluated 72 h after ICH.
Table 2.
Neurobehavioral evaluation: neuroscore scoring criteria for the sub-tests.
| Category | Behavior | Score |
|---|---|---|
| Spontaneous activity (SA) | Animal was akinesitic | 0 |
| Animal moves slowly or minimally | 1 | |
| Animal approached 1–2 walls | 2 | |
| Animal approached at least 3 walls of the cage or raised on hindlimbs to explore the top of the cage | 3 | |
| Vibrissae proprioception (VP) | – | 0 |
| Animal had a unilateral response | 1 | |
| Animal had either a weak bilateral response or weak left response and brisk right response | 2 | |
| Animal had a brisk bilateral response | 3 | |
| Axial senation (AS) | – | 0 |
| Animal had no response on left side | 1 | |
| Animal had either a weak bilateral response or weak left response and brisk right response | 2 | |
| Animal had a brisk bilateral response | 3 | |
| Limb symmetry (LS) | Hemiparesis | 0 |
| Left forelimb or left hindlimb flexed | 1 | |
| Asymmetric extension | 2 | |
| All limbs were extended symmetrically | 3 | |
| Lateral turning (LT) | Animal had no turning at all on one side | 0 |
| Animal had unequal turning | 1 | |
| Animal turned bilaterally less than 45° on both sides | 2 | |
| Animal turned bilaterally at least 45° on both sides | 3 | |
| Forelimb walking (FW) | Animal had a paretic forelimb | 0 |
| Animal walked in circles | 1 | |
| Animal walked asymmetrically or to one side | 2 | |
| Animal briskly walked symmetrically on forepaws | 3 | |
| Climbing (CL) | – | 0 |
| Animal failed to climb or circled instead of climbing | 1 | |
| Animal climbed to the top and had a weak grip or animal climbed but had a strong grip | 2 | |
| Animal climbed to the top and had a strong grip | 3 |
TdT-mediated dUTP nick-end labeling (TUNEL) staining
TUNEL staining was employed for the detection of apoptosis in the brain tissues. First, brain slices were dehydrated for 1 h at 70 °C. Next, 100% dimethylbenzene was applied for the gradual deparaffinate of the sections which were further treated with a series of ethanol solutions (100%, 100%, 95%, 95%, and 80%). Subsequently, these samples were treated with the TUNEL staining reagents (Beyotime, C1088) for 1 h at 37 °C. Eventually, the TUNEL-stained slices were subsequently treated with the NeuN antibody at 4 °C overnight for the identification of neuronal apoptosis. The fluorescent microscope (Nikon, Japan) was employed for the visualization of TUNEL-positive neurons by an experimenter unaware of the grouping of rats.
Fluoro-Jade C (FJC) staining
Neuronal degeneration was investigated based on FJC staining. FJC staining was conducted as mentioned in the existing study39. In brief, brain slices were deparaffinized and rehydrated as described previously. They were then immersed in a 0.06% potassium permanganate solution for 15 min, rinsed in deionized water, and soaked in the FJC working solution (Biosensis, USA; Cat# TR-100-FJC) containing 0.1% acetic acid for 30 min. The slices were then dehydrated in an incubator. Finally, the slices were rinsed with 100% dimethylbenzene for 2 min and mounted with neutral balsam.
Measurement of Evans blue (EB) leakage
The integrity of the blood–brain barrier (BBB) following ICH was quantitatively evaluated through Evans blue (EB) extravasation methodology, in accordance with established protocols40. Experimental animals received retro-orbital intravenous administration of 100 µL 2% EB solution (Sigma-Aldrich #E2129, St. Louis, MO, USA). Following an 18–20-h circulation period, subjects underwent deep anesthesia induction with isoflurane, succeeded by transcardial perfusion with 20 mL ice-cold physiological saline (0.9% NaCl) through the left ventricular apex to eliminate intravascular EB tracer. Cerebral tissues were subsequently harvested, subjected to digital documentation, and systematically dissected into ipsilateral hemorrhagic hemispheres and contralateral non-hemorrhagic control regions. Tissue specimens were mechanically homogenized in 1 mL 50% trichloroacetic acid solution (w/v) followed by centrifugal separation (10,000 × g, 20 min, 4 °C). The resultant supernatant underwent fourfold volumetric dilution with absolute ethanol prior to spectrofluorometric analysis (excitation λ = 620 nm; emission λ = 680 nm; slit width 5 nm). Quantitative EB concentrations were normalized to tissue mass (µg/g wet weight) and expressed as ipsilateral-to-contralateral hemispheric ratios to account for potential inter-individual variability.
Lactate dehydrogenase (LDH) assay
The levels of LDH in CSF were measured based on the specific kit (Beyotime, C0018S). The experiment was conducted strictly according to the protocol provided in the instructions. The LDH was evaluated 72 h after ICH.
Morris water maze tests
Cognitive capacity was evaluated by the Morris water maze tests, as indicated in previous research41. In brief, the water maze was divided into four quadrants. The target platform was placed at the center of the third quadrant. In the training and testing processes, the visual reference points around the water maze remained consistent. The subjects were put in the water maze at all quadrants and provided 60 s to search for the target stage in the training process. If the rats failed to arrive at the target platform within 60 s, a stick was applied to help them to swim onto the platform. These rats were permitted to stay at the stage for 10 s to consolidate the corresponding learning and memory after reaching the target platform. This method of training was consecutively conducted for five days, and the ICH impairment was completed on the day after training. Throughout the experimental process, the time from the starting position to the final stage was documented, which was defined as escape latency. The rats were tested by the same methods on days 31, 32, 33, 34 after ICH.
Rotarod performance test
The rotarod test was mainly used to assess the effects of therapeutic intervention on vestibulo motor function42. Briefly, the rats were placed on a rotarod cylinde and the time that each rat remained on the cylinder was recorded. The speed was increased at a constant rate from 4 to 30 rpm within 1 min. The experiment ended when the rat dropped or gripped the cylinder and spun around for two consecutive revolutions. Each rat was trained for 3 days, three times per day, before the ICH model was established. The average time in the test before the onset of ICH was recorded as the baseline value (Pre). The rats were tested by the same methods on days 1, 3, 5, 7, 10, 14, 21, 28, 35 after ICH.
Adhesive removal
The sensorimotor function of contralateral limbs in rats with intracerebral hemorrhage (ICH) was quantitatively assessed using the adhesive tape removal behavioral assay, following established protocols36. Preoperatively, animals underwent a 3-day acclimatization protocol comprising three daily training sessions to establish baseline performance metrics. The mean latency for adhesive removal recorded during these sessions served as the preoperative baseline (Pre-ICH). Synchronized with rotarod testing, longitudinal foot-fault assessments were systematically performed at postoperative days 1, 3, 7, 10, 14, 21, 28, and 35 to evaluate dynamic functional recovery43.
Foot fault
Sensorimotor coordination during spontaneous locomotion was quantitatively evaluated using a standardized grid-walking apparatus. Experimental subjects were video-recorded for 60-s intervals while traversing an elevated grid platform (mesh size: 1 cm2). A researcher blinded to experimental conditions analyzed the recordings using frame-by-frame analysis (Noldus EthoVision XT 15), quantifying both total step counts and contralateral limb foot-faults (ipsilateral to cerebral lesion). Foot-faults were operationally defined as complete limb protrusion through grid apertures during weight-bearing phases of either forelimb or hindlimb movement cycles. Data normalization was performed by calculating fault percentages relative to total limb-usage events (faults/steps × 100). The rats were tested by the same methods on days 1, 3, 5, 7, 10, 14, 21, 28, 35 after ICH.
Statistical analysis
All data were analyzed using GraphPad Prism and are presented as the mean ± SD. Data were analyzed using one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons. For two-group comparisons, Student’s t-test was used. Non-parametric data were analyzed using the Mann–Whitney U test. All statistical analyses were performed using GraphPad Prism 9, and a p-value < 0.05 was considered statistically significant.
Results
Sox11 protein level was significantly up-regulated in the ICH-induced tissues of brain
To investigate the protein expression of Sox11 in the ICH-induced tissues of brain at different times, Western blotting assays were applied. ICH induced a remarkable increase of the protein expression of Sox11 in compared with the Sham group. Under the ICH impairment, levels of Sox11 gradually rose, peaking at 24 h and remaining at high levels as late as 48 h post-ICH (Fig. 1A,B). To clarify the distribution of Sox11 in the cells of ICH-induced brain tissues, double-immunofluorescent staining was also conducted. It was revealed that Sox11 was mainly expressed in neurons and the ICH-induced increase of Sox11 was generally located in neurons (Fig. 1C,D). The results of PCR test showed that the expression level of miR-221 increased after intracerebral hemorrhage, and the trend was consistent with that of Sox11, suggesting that the increased expression of Sox11 may be related to miR-221(Fig. 1E), subsequent experiments investigating Sox11 were performed at 24 h after ICH.
Fig. 1.
The expression level of Sox11 increased after intracerebral hemorrhage. (A) Western blot analysis showed that Sox11 protein levels gradually rise and peak at 24 h after ICH. (B) Quantitative analysis of the protein levels of Sox11 which were normalized to the mean value of that in the Sham group. (C) Double-immunofluorescence analysis was performed with antibodies against Sox11 and NeuN (neuronal marker). The nuclei were fluorescently labeled with DAPI. Arrows point to Sox11-positive neurons. Scale bar = 20 μm. (D) Quantification of the relative fluorescence intensities. (E) qPCR shown that the relative expression level of miR-221 changed after ICH. **P < 0.01 compared with the Sham group. All values are expressed as mean ± SD, n = 6.
mic-miR-221 and siRNA transfection increased and decreased the expression of Sox11 protein level in brain tissues respectively
To further verify whether Sox11 plays a role in the ICH-induced brain damage, targeted siRNA was employed to knockdown Sox11, as well as used miR-221 mimics. The control groups for Si-Sox11 and mic-miR-221 consisted of Si-Control and a plasmid with the expression of an empty vector, respectively. Following Western blot analysis and double-immunofluorescent staining, it could be demonstrated that the Si-Sox11 intervention significantly inhibited the protein expression of Sox11, whereas mic-miR-221 treatment markedly increased protein expression of Sox11 in ICH rats (Fig. 2A–D).
Fig. 2.
mic-miR-221 and siRNA transfection effectively interfered with the expression of Sox11. (A) Western blot analysis showed that Sox11 protein levels are decreased in the Si-Sox11 treatment group but increased in the ICH + mic-miR-221 group. (B) Quantification of protein levels of Sox11 in rats in various groups. (C) Double-immunofluorescence analysis was performed with antibodies for Sox11 and NeuN (neuronal marker, red) and nuclei was fluorescently labeled with DAPI. Arrows point to Sox11-positive neurons. Scale bar = 20 μm. (D) Quantification of the relative fluorescence intensity of Sox11. ICH group compared with the Sham group; ICH + mic-miR-221 group compared with the ICH + Vector group; ICH + Si-Sox11 group compared with the ICH + Si-Control group. ** P < 0.01. All data are expressed as mean ± SD, n = 6.
Increasing the expression of Sox11 can help relieve neuroinflammation and oxidative stress
Immunofluorescence verified the change in the proportion of M2-type microglia after intracerebral hemorrhage. We found that when the expression level of Sox11 increased, the number of M2-type microglia increased, suggesting that Sox11 can maintain neuroinflammation response (Fig. 3A,B). To further explore the roles of Sox11 in inflammation, the expressions of pro-inflammatory cytokines (TNF-α and IL-1β) in CSF and serum were measured based on ELISA. Both the expressions of IL-1β and TNF-α were remarkably up-regulated in the ICH-induced rats with the treatment of Si-Sox11, in contrast to those in ICH + Si-Control rats. Comparatively, the expressions of IL-1β and TNF-α were dramatically down-regulated in the ICH + mic-miR221 group in contrast to that of the ICH + Vector group (Fig. 3C–F). while mic-miR-221 elevating the expression level the anti-inflammatory factor IL-13 (Fig. 3G,H). The above data suggested that Sox11 may involve in the inhibition of neuroinflammation in ICH rats.
Fig. 3.
Sox11 effectively inhibits neuroinflammation. (A) Double-immunofluorescence analysis was performed with antibodies for microglia (green) and M2-microglia (red) and nuclei was fluorescently labeled with DAPI. Arrows point to Sox11-positive neurons. Scale bar = 20 μm. (B) Quantification of the percentage of M2-microglia. (C–H) The levels of TNF-α, IL-β and IL-13 in the serum and CSF were tested by ELISA. ICH group compared with the Sham group; ICH + mic-miR-221 group compared with the ICH + Vector group; ICH + Si-Sox11 group compared with the ICH + Si-Control group. ** P < 0.01 and * P < 0.05. All data are expressed as mean ± SD, n = 6.
Knockdown of Sox11 exacerbates, while upregulation of Sox11 ameliorates, ICH-induced cell death
TUNEL was used to evaluate the impact of Sox11 on neuron apoptosis, while FJC staining was performed to investigate neurodegeneration in brain tissues under different interventions. The neurodegeneration ratio and cell death ratio were remarkably increased in the ICH-induced brain tissues in comparison with those in the Sham group. The data of TUNEL staining suggested that the quantity of apoptotic neurons was predominantly up-regulated after the Si-Sox11 treatment, whereas this number was significantly decreased following over-expression of Sox11 (Fig. 4A,C). Similarly, FJC staining indicated ICH-induced neuronal degeneration was significantly exacerbated after Si-Sox11 treatment, whereas this degeneration was significantly ameliorated via over-expression of Sox11 (Fig. 4B,D).
Fig. 4.
Sox alleviates brain damage by inhibiting nerve cell death. (A) Double immunofluorescence analysis for NeuN and TUNEL was performed. Nuclei were fluorescently labeled with DAPI. Arrows indicated apoptotic neurons (TUNEL- and NeuN-positive cells). Scale bar = 20 μm. (B) Fluoro-Jade C (FJC) staining was performed to assess neuronal degeneration in various groups at 72 h after ICH. Scale bar = 20 μm. (C) Percentages of TUNEL-positive neurons were shown. (D) Quantitative analysis of FJC staining in brain sections of each group. FJC-positive cells were counted per × 400 field. ICH group compared with the Sham group; ICH + mic-miR-221 group compared with the ICH + Vector group; ICH + Si-Sox11 group compared with the ICH + Si-Control group; **P < 0.01. All data are expressed as mean ± SD, n = 6.
Effects of Si-Sox11 and Mic-miR221 treatments on cognitive behavior in ICH-induced rats
To ulteriorly determine whether Sox11 involves in brain damage in ICH rats, the Morris water maze, rotarod test, adhesive removal test, foot fault test was applied to evaluate the cognitive function, locomotor impairments, sense and balance function respectively. The differences between the behaviors of ICH rats and those of the ICH rats under different treatments (Si-Control and empty Vector) were statistically non-significant. In contrast, mic-miR-221 treatments ameliorated ICH-induced cognitive deficits and locomotor impairments, whereas Si-Sox11 treatments exacerbated such deficits (Fig. 5A–F). The neurological scores of the ICH + Si-Sox11 group scored lower than those of the ICH + Si-Control group, however the scores were predominantly higher in the ICH + mic-miR-221 group in contrast to those of the ICH + Vector group (Fig. 5G). Collectively, it was indicated that the inhibition of Sox11 exacerbates neurological deficits and locomotor impairments induced by ICH.
Fig. 5.
The protective effect of Sox11 on behavioral/cognitive dysfunction testing in the rat after ICH. (A) Representative images of swimming trajectories on day 34 was shown. (B) Motor learning was assessed by the escape latency (time, s). (C) Spatial learning was assessed by the length of the swimming track of each rat. (D) Motor ability is assessed by time spent on the rotating stick. (E) The assessment of sensory ability was conducted by measuring the time required to remove the sticker. (F) Balance is evaluated based on the ratio of errors made. (G) The neurological scores of rats in each group. ICH group compared with the Sham group; ICH + mic-miR-221 group compared with the ICH + Vector group; ICH + Si-Sox11 group compared with the ICH + Si-Control group; **P < 0.01 and *P < 0.05. All data are expressed as mean ± SD, n = 6.
Sox11 alleviates brain injury after cerebral hemorrhage by maintaining the integrity of the blood-brain barrier and maintaining cerebral edema
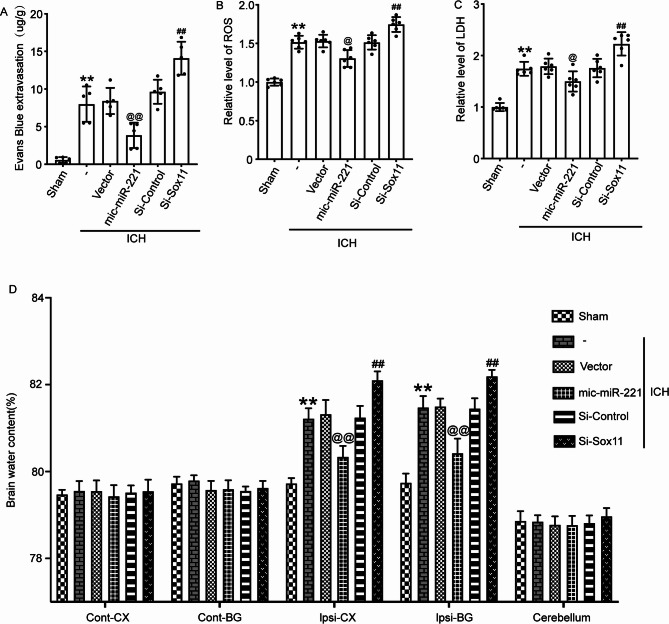
The BBB is an important indicator of SBI. Albumin extravasation was adopted for the assessment of BBB permeability, and Western blot assay was used for the quantification of albumin in the tissues of brain in different experimental groups. Si-Sox11 aggravated the albumin extravasation and BBB impairment in ICH rats, while mic-miR-221 ameliorated these injuries (Fig. 6A). ROS is an index of oxidative stress and LDH is an indicator of cellular necrosis. It was showed that both the concentrations of ROS and LDH were markedly up-regulated in the ICH + Si-Sox11 group in comparison with those in the ICH + Si-Control group. In contrast, the concentrations of ROS and LDH were predominantly downregulated in the ICH + mic-miR-221 group in contrast to those in the ICH + Vector group (Fig. 6B,C). Hence, upregulating Sox11 reduced oxidative stress and cellular necrosis in ICH subjects.
Fig. 6.
Increasing the expression level of Sox11 can maintain the integrity of the blood-brain barrier and inhibit brain edema. (A) The level of EB extravasation was increased in the rat brain after ICH, whereas it significantly reduced in mic-miR-221-treated rats. (B) The levels of ROS in the brain tissue of rats in various groups. (C) The levels of LDH in the CSF of rats in each group. (D) Brain water content was partially reduced by overexpression of Sox11. ICH group compared with the Sham group; ICH + mic-miR-221 group compared with the ICH + Vector group; ICH + Si-Sox11 group compared with the ICH + Si-Control group; **P < 0.01. All data are expressed as mean ± SD, n = 6.
To explore the influence of Sox11 on brain edema after the ICH induction, the quantity of brain water was obtained based on the wet/dry weight method. It was revealed that in contrast to that of the ICH + Si-Control group, the quantity of brain water was remarkably deteriorated in the ICH + Si-Sox11 group, while the overexpressed Sox11 reduced brain edema (Fig. 6D).
To sum up, we conclude that Sox11 participates in a variety of important processes of the ICH-induced brain impairment, including neuroinflammation, oxidative stress, neurodegeneration, BBB injury, brain edema, and cell death.
Discussion
Previous research has emphasized the importance of Sox11 in neuronal survival and neurite growth. Moreover, evidence has shown that Sox11 expression is strongly promoted in mature central and peripheral neurons after axotomy and ischemia, suggesting that Sox11 plays a role in responding to neuronal injury15–21.
As a transcription factor, Sox11 has been indicated to affect the cerebral development and regeneration, including neuron survival and neurite growth16. At the same time, changes in Sox11 expression have been linked to malformations of various organs, such as the heart, lung, stomach, and skeletal system44. Over the past 30 years, much more information has become available on the connection between Sox11 and various tumors. Sox11 is considered to be one of the most commonly increased transcripts in various human cancers, such as MCL, epithelial ovarian cancer, breast cancer, gastrointestinal tumors, and nervous system tumors26,27,45,46, which implies that the level of Sox11 may be related to cell proliferation and apoptosis. Additionally, Sox11 exhibits peak expression in immature neurons, which is in turn down-regulated in mature neurons. However, it is interesting that increasing evidence revealed that Sox11 could be re-induced in mature neurons through various stimulation on pathology and physiology. The Sox11 expression is strongly up-regulated in the mature central and peripheral neurons by both axotomy and ischemia, suggesting that Sox11 involves in a response to neuronal injury47. After cerebral ischemia in rats, Sox11 levels are dramatically increased, implying that Sox11 may play an important role in modulating brain damage. In the current study, it was shown that protein expression of Sox11 was significantly increased in rat brain tissue after ICH. Furthermore, inhibition of Sox11 expression exacerbated ICH-induced neuronal death, neuroinflammation, behavioral/cognitive dysfunction, brain edema, and BBB impairment.
As mentioned in the former literature, Sox11 is closely related to neurogenesis and neuronal survival14. The expression of Sox11 in adult dorsal root ganglia neurons is usually low, but it will increase within 24 h in response to peripheral nerve injury; while knocking down Sox11 with SiRNA, neuron death increased and neurite growth decreased20,48. In the adult brain, a variety of neuronal insults involve in the regulation of Sox11, which has been demonstrated to affect the regeneration of axon24. For example, Sox11 expression has been shown to significantly promoted axon regeneration under the optic nerve injury49. Furthermore, the corticospinal tract regeneration after spinal injury could be promoted by the overexpressed Sox11, which improved functional recovery50. In addition, Sox11 can significantly reduce the activity of caspase-3, caspase-6 and caspase-7. The regions where Sox11 function are located at amino acids 117–214 and the C-terminal transactivation domain (TAD). The role of Sox11 in the reduction of caspase activity also has been manifested in its effects on inhibiting apoptosis under toxic insults23. Caspase-6 can mediate axonal degeneration during stroke, and then cause neuron death; after stroke, axonal mutation was reduced and functional outcome was improved in rats with caspase-6 gene knockout51. Caspase-3 is the final link of multiple signaling pathways leading to apoptosis after ICH52. Brain-derived neurotrophic factor (BDNF) is broadly located in the CNS and plays a vital role in the growth, differentiation, and survival of neurons; additionally, BDNF can prevent neuronal death, as well as promote cell regeneration and differentiation53. Recently, several researches have revealed that Sox11 modulates BDNF expression in an exon promoter-specific manner, and the increase of Sox11 could lead to the long-term activation of BDNF transcription24.
In the current work, it was revealed that under the induction of ICH, the expressions of Sox11 peaked at 24 h and remained high for as long as 48 h post-ICH. This implies that Sox11 plays a role in response to ICH and deserves further investigation. The results indicate that miR-221 plays an important role in regulating the expression of Sox11. Our study investigated the effect of Sox11 on neuroinflammation after ICH at the molecular level and found that increased expression of Sox11 could reduce ICH-induced neuroinflammation, suggesting that Sox11 may play a role in controlling the inflammatory response. The role of Sox11 in controlling inflammation can also be further investigated. Neuronal cell death is the direct cause of neurological dysfunction following ICH. Our study found that upregulation of Sox11 increased the number of surviving neurons, with similar results obtained through FJC staining. This suggests that Sox11 helps prevent brain cell death and promotes the recovery of brain function. These findings provide molecular evidence for the neuroprotective role of Sox11. However, beyond this mechanism, brain tissue damage caused by ICH is also associated with brain edema, oxidative stress, and neurobehavioral dysfunction54. The molecular effects of Sox11 were studied further and it was found that increasing the expression of Sox11 reduced BBB impairment, brain edema, and oxidative stress in ICH rats. This indicates that Sox11 also offers protection through other pathways to alleviate brain damage. Through the experiments conducted, we tested the hypothesis on the neuroprotective effect of Sox11 at the molecular level. However, molecular level studies alone cannot fully explain the overall effect of Sox11. Therefore, we also conducted related studies on rat behavior. The results showed that an increase in the expression level of Sox11 can improve the rats’ motor, learning, and perception abilities. This provides strong evidence that Sox11 has a protective effect on the brain. Combined with the existing literature, we believe that Sox11 may also exert anti-inflammatory effects under certain conditions. Sox11 regulates the neuroinflammatory response by promoting the transformation of microglia from the pro-inflammatory M1 type to the anti-inflammatory M2 type and reducing the release of inflammatory factors such as IL-1β, TNF-α, and IL-6. Furthermore, Sox11 can inhibit key signaling pathways including NF-κB, and reduce the expression of downstream inflammatory genes by interfering with the nuclear translocation or transcriptional activity of NF-κB. Sox11 may also promote the expression of anti-inflammatory micrornas and drive the expression pattern of anti-inflammatory genes through epigenetic regulation. Through these mechanisms, Sox11 helps alleviate neuroinflammation and protects neural tissue from damage caused by excessive immune responses. This confirms our hypothesis. However, the specific mechanisms behind Sox11’s ability to bring about these changes are still unknown.
Additionally, there were still several limitations to the current study. Firstly, only male rats were applied in the study. Secondly, the role of Sox11 in the later stages of ICH-induced brain injury remains unknown and requires further investigation. Thirdly, the expression of Sox11 and its role in human brain damage during ICH have not been examined using clinical samples. Finally, the exact mechanism by which Sox11 protects the brain after ICH is still unclear. In conclusion, our findings suggest that Sox11 may be a potential therapeutic target for treating ICH-induced brain injury.
Conclusion
Our experimental data indicate that Sox11 plays a key role in inhibiting the neuroinflammatory response induced by ICH. Upregulating Sox11 levels through miR-221 effectively reduces neuronal apoptosis, brain edema, blood-brain barrier damage, and neurological dysfunction. This finding is significant for understanding the mechanisms of brain injury caused by ICH and may provide new molecular targets for future treatments, offering a solid theoretical foundation for clinical management and therapeutic outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
All the authors had full access to all the data of the study, who were responsible for data integrity and accuracy. T.L. wrote the manuscript. M.X. worked on study concept and design. T.L. were responsible for data acquisition. X.S., R.L. and Y.L. participated in the administrative, technical, and material support. M.X. worked on supervision.
Data availability
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All the experiments were authorized by the Institutional Animal Care Committee of Zhejiang Provincial people’s hospital and were conducted based on the guidelines of the National Institutes of Health on the care and use of animals. The study is reported in accordance with ARRIVE guidelines.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xianmei Shen, Email: shenxianmeihnh@163.com.
Mingxia Xu, Email: xumingxiaaa@126.com.
References
- 1.Zhang, S. et al. AAV/BBB-Mediated gene transfer of CHIP attenuates brain injury following experimental intracerebral hemorrhage. Transl. Stroke Res.11 (2), 296–309 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Chu, H. et al. Lactate dehydrogenase predicts early hematoma expansion and poor outcomes in intracerebral hemorrhage patients. Transl. Stroke Res.10 (6), 620–629 (2019). [DOI] [PubMed] [Google Scholar]
- 3.van Asch, C. J. et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol.9 (2), 167–176 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Wang, Z. et al. Treatment of secondary brain injury by perturbing postsynaptic density protein-95-NMDA receptor interaction after intracerebral hemorrhage in rats. J. Cereb. Blood Flow. Metab.39 (8), 1588–1601 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang, Z. et al. Melatonin alleviates intracerebral Hemorrhage-Induced secondary brain injury in rats via suppressing apoptosis, inflammation, oxidative stress, DNA damage, and mitochondria injury. Transl. Stroke Res.9 (1), 74–91 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang, P. et al. Exploration of MST1-Mediated secondary brain injury induced by intracerebral hemorrhage in rats via Hippo signaling pathway. Transl. Stroke Res.10 (6), 729–743 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Wu, X. et al. Recombinant adiponectin peptide ameliorates brain injury following intracerebral hemorrhage by suppressing astrocyte-derived inflammation via the inhibition of Drp1-mediated mitochondrial fission. Transl. Stroke Res. (2020). [DOI] [PubMed]
- 8.Pandey, A. S. et al. A combination of deferoxamine mesylate and minimally invasive surgery with hematoma Lysis for evacuation of intracerebral hemorrhage. J. Cereb. Blood Flow. Metab.40 (2), 456–458 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li, H. et al. Overexpression of LH3 reduces the incidence of hypertensive intracerebral hemorrhage in mice. J. Cereb. Blood Flow. Metab.39 (3), 547–561 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang, B. Y. et al. Advances in neuroprotective strategies: potential therapies for intracerebral hemorrhage. Cerebrovasc. Dis.31 (3), 211–222 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behrouz, R. Re-exploring tumor necrosis factor alpha as a target for therapy in intracerebral hemorrhage. Transl. Stroke Res.7 (2), 93–96 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Gubbay, J. et al. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 346 (6281), 245–250 (1990). [DOI] [PubMed] [Google Scholar]
- 13.Sinclair, A. H. et al. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 346 (6281), 240–244 (1990). [DOI] [PubMed] [Google Scholar]
- 14.Bergsland, M. et al. The establishment of neuronal properties is controlled by Sox4 and Sox11. Genes Dev.20 (24), 3475–3486 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hui, S. P. et al. Genome wide expression profiling during spinal cord regeneration identifies comprehensive cellular responses in zebrafish. PLoS One. 9 (1), e84212 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jankowski, M. P. et al. SRY-box containing gene 11 (Sox11) transcription factor is required for neuron survival and neurite growth. Neuroscience. 143 (2), 501–514 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanaan, N. M. et al. The longitudinal transcriptomic response of the substantia Nigra to intrastriatal 6-hydroxydopamine reveals significant upregulation of regeneration-associated genes. PLoS One. 10 (5), e0127768 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim, D. K. et al. Expression of Sox11 and Brn transcription factors during development and following transient forebrain ischemia in the rat. Neurosci. Lett.433 (3), 259–264 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Mu, L. et al. SoxC transcription factors are required for neuronal differentiation in adult hippocampal neurogenesis. J. Neurosci.32 (9), 3067–3080 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanabe, K. et al. Fibroblast growth factor-inducible-14 is induced in axotomized neurons and promotes neurite outgrowth. J. Neurosci.23 (29), 9675–9686 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veldman, M. B. et al. Gene expression analysis of zebrafish retinal ganglion cells during optic nerve regeneration identifies KLF6a and KLF7a as important regulators of axon regeneration. Dev. Biol.312 (2), 596–612 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Fink, K. et al. Prolonged therapeutic window for ischemic brain damage caused by delayed caspase activation. J. Cereb. Blood Flow. Metab.18 (10), 1071–1076 (1998). [DOI] [PubMed] [Google Scholar]
- 23.Waldron-Roby, E. et al. Sox11 reduces Caspase-6 cleavage and activity. PLoS One. 10 (10), e0141439 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salerno, K. M. et al. Sox11 modulates brain-derived neurotrophic factor expression in an exon promoter-specific manner. J. Neurosci. Res.90 (5), 1011–1019 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee, C. J. et al. Differential expression of SOX4 and SOX11 in Medulloblastoma. J. Neurooncol. 57 (3), 201–214 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Sernbo, S. et al. The tumour suppressor SOX11 is associated with improved survival among high grade epithelial ovarian cancers and is regulated by reversible promoter methylation. BMC Cancer. 11, 405 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shepherd, J. H. et al. The SOX11 transcription factor is a critical regulator of basal-like breast cancer growth, invasion, and basal-like gene expression. Oncotarget. 7 (11), 13106–13121 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weigle, B. et al. Highly specific overexpression of the transcription factor SOX11 in human malignant gliomas. Oncol. Rep.13 (1), 139–144 (2005). [PubMed] [Google Scholar]
- 29.Ek, S. et al. Nuclear expression of the Non B-cell lineage Sox11 transcription factor identifies mantle cell lymphoma. Blood. 111 (2), 800–805 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Dharap, A. et al. Transient focal ischemia induces extensive Temporal changes in rat cerebral MicroRNAome. J. Cereb. Blood Flow. Metab.29 (4), 675–687 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lovat, F. et al. Knockout of both miR-15/16 loci induces acute myeloid leukemia. Proc. Natl. Acad. Sci. U S A. 115 (51), 13069–13074 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shan, Y. et al. miR-221 exerts neuroprotective effects in ischemic stroke by inhibiting the proinflammatory response. J. Stroke Cerebrovasc. Dis.30 (2), 105489 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Li, S. et al. Inhibition of hepatocellular carcinoma growth via modulation of the miR-221/SOX11 axis by Curcumin and Berberine. PeerJ. 11, e16593 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samal, S. et al. Synergistic interaction between polysaccharide-based extracellular matrix and mineralized osteoblast-derived evs promotes bone regeneration via miRNA–mRNA regulatory axis. Biomacromolecules. 25 (7), 4139–4155 (2024). [DOI] [PubMed] [Google Scholar]
- 35.Sun, L. et al. TAR DNA binding protein-43 loss of function induced by phosphorylation at S409/410 blocks autophagic flux and participates in secondary brain injury after intracerebral hemorrhage. Front. Cell. Neurosci.12, 79 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dou, Y. et al. Tumor necrosis factor receptor-associated factor 6 participates in early brain injury after subarachnoid hemorrhage in rats through inhibiting autophagy and promoting oxidative stress. J. Neurochem. 142 (3), 478–492 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Wang, Z. et al. Identification of two phosphorylation sites essential for Annexin A1 in blood-brain barrier protection after experimental intracerebral hemorrhage in rats. J. Cereb. Blood Flow. Metab.37 (7), 2509–2525 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugawara, T. et al. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J. Neurosci. Methods. 167 (2), 327–334 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mo, J. et al. AVE 0991 attenuates oxidative stress and neuronal apoptosis via Mas/PKA/CREB/UCP-2 pathway after subarachnoid hemorrhage in rats. Redox Biol.20, 75–86 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang, T. et al. miRNA506 activates Sphk1 binding with Sirt1 to inhibit brain injury after intracerebral hemorrhage via PI3K/AKT signaling pathway. Mol. Neurobiol. (2024). [DOI] [PubMed]
- 41.Guo, Y. C. et al. The expression and mechanism of BDNF and NGB in perihematomal tissue in rats with intracerebral hemorrhage. Eur. Rev. Med. Pharmacol. Sci.21 (15), 3452–3458 (2017). [PubMed] [Google Scholar]
- 42.Cui, C. M. et al. Chloroquine exerts neuroprotection following traumatic brain injury via suppression of inflammation and neuronal autophagic death. Mol. Med. Rep.12 (2), 2323–2328 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Liang, T. et al. Activating transcription factor 6 alleviates secondary brain injury by increasing cystathionine gamma-lyase expression in a rat model of intracerebral hemorrhage. Aging (Albany NY). 16 (8), 6990–7008 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wurm, A. et al. Anterior segment dysgenesis in the eyes of mice deficient for the high-mobility-group transcription factor Sox11. Exp. Eye Res.86 (6), 895–907 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Wasik, A. M. et al. SOXC transcription factors in mantle cell lymphoma: the role of promoter methylation in SOX11 expression. Sci. Rep.3, 1400 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu, X. et al. Aberrant SOX11 promoter methylation is associated with poor prognosis in gastric cancer. Cell. Oncol. (Dordr). 38 (3), 183–194 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Kavyanifar, A., Turan, S. & Lie, D. C. SoxC transcription factors: multifunctional regulators of neurodevelopment. Cell. Tissue Res.371 (1), 91–103 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Jankowski, M. P. et al. Sox11 transcription factor modulates peripheral nerve regeneration in adult mice. Brain Res.1256, 43–54 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuwajima, T. & Mason, C. Live or die?? Depends on who you are. Neuron. 94 (6), 1043–1046 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Wang, Z. et al. Overexpression of Sox11 promotes corticospinal tract regeneration after spinal injury while interfering with functional recovery. J. Neurosci.35 (7), 3139–3145 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akpan, N. et al. Intranasal delivery of caspase-9 inhibitor reduces caspase-6-dependent axon/neuron loss and improves neurological function after stroke. J. Neurosci.31 (24), 8894–8904 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu, H. et al. GATA-4 regulates neuronal apoptosis after intracerebral hemorrhage via the NF-kappaB/Bax/Caspase-3 pathway both in vivo and in vitro. Exp. Neurol.315, 21–31 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Ozer, A. B. et al. Effect of different anesthesia techniques on the serum brain-derived neurotrophic factor (BDNF) levels. Eur. Rev. Med. Pharmacol. Sci.19 (20), 3886–3894 (2015). [PubMed] [Google Scholar]
- 54.Zheng, H. et al. Mechanism and therapy of brain edema after intracerebral hemorrhage. Cerebrovasc. Dis.42 (3–4), 155–169 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.