Abstract
Background
Exploring the prognostic value of systemic inflammatory response index (SIRI) for 3-year disease-free survival (DFS) in stage II colon cancer patients after radical surgery.
Methods
A retrospective analysis was conducted on 87 patients with stage II colon cancer. Patients were divided by 3-year DFS status: poor prognosis group (DFS event; n = 28) vs. good prognosis group (event-free; n = 59). Univariate analysis and multivariate Cox regression analysis were performed to identify the influencing factors on postoperative prognosis of stage II colon cancer patients. Receiver operating characteristic (ROC) curve was used to evaluate the predictive value of various influencing factors on postoperative prognosis of stage II colon cancer patients. Kaplan-Meier method and Log-rank test were used to compare survival differences among patients with different SIRI levels.
Results
One week before surgery, the neutrophil to lymphocyte ratio (NLR) level in the poor prognosis group was significantly lower (P < 0.05). The levels of SIRI, carcinoembryonic antigen (CEA), carbohydrate antigen 19 − 9 (CA19-9), carbohydrate antigen 125 (CA125), and carbohydrate antigen 72 − 4 (CA72-4) in the poor prognosis group were significantly higher (P < 0.05). NLR, SIRI, CEA, CA19-9, CA125, and CA72-4 were all independent risk factors for the prognosis of postoperative patients with stage II colon cancer. The area under the curve (AUC) for predicting patient prognosis using NLR, SIRI, CEA, CA19-9, CA125, and CA72-4 were 0.726, 0.828, 0.693, 0.741, 0.706, and 0.605, respectively. The optimal cutoff value for SIRI was 1.41, with a sensitivity of 0.67 and a specificity of 0.93. There was a significant difference in the 3-year disease-free survival rate between stage II colon cancer patients with SIRI ≥ 1.41 and SIRI < 1.41 after surgery (P < 0.05).
Conclusion
Preoperative SIRI predicts early recurrence risk (3-year DFS) more effectively than traditional biomarkers. DFS was significantly higher in patients with SIRI < 1.41.
Keywords: Colon cancer, Prognosis, Systemic inflammatory response index, Disease-free survival, Predictive value
Introduction
Colon cancer is one of the most common gastrointestinal malignancies and has become the fifth leading cause of cancer death worldwide, as well as the fifth most common malignant tumor [1]. With the change of lifestyle and eating habits, the incidence rate of colon cancer is rising year by year and showing a younger trend [2]. Although the progress of surgical techniques and systemic treatment has improved the overall prognosis of patients with early colon cancer, the disease still has a high mortality, which seriously affects the life and health of patients [3]. Stage II colorectal cancer is a local tumor without lymph node metastasis, and is often treated with radical resection in clinical practice [4]. However, most patients have a high recurrence rate and poor prognosis [5]. At present, TNM staging of colon cancer is the most commonly used indicator for predicting recurrence and survival in clinical practice. However, differences in prognosis can be observed within the same TNM staging, which poses significant challenges for clinical doctors in selecting treatment plans [6]. Therefore, finding effective biomarkers has become the key to accurately predicting the prognosis of colon cancer patients. Research has shown that the interaction between systemic inflammation and local immune response is associated with the onset and progression of colon cancer [7]. Systemic Inflammatory Response Index (SIRI) is a new type of inflammatory index. It can reflect the state of inflammatory reaction more comprehensively based on the count of monocytes, neutrophils and lymphocytes [8]. It has been confirmed to be a prognostic marker for breast cancer and gallbladder cancer [9, 10], yet comprehensive validation in colon cancer remains limited, with only sparse reports in existing literature [11, 12]. This study aims to explore the relationship between SIRI and the prognosis of colon cancer patients from the perspective of inflammation, and analyze its predictive value.
Patients and methods
Clinical data
Retrospective analysis of clinical and pathological data of 87 patients with stage II colon cancer who underwent concurrent radical surgery in the Department of General Surgery of our hospital from July 2019 to June 2021. Among them, there were 48 male patients and 39 female patients; Age ranged from 39 to 83 years old, with an average age of (64.5 ± 8.6) years. The core ethical standard followed in this study is the Helsinki Declaration. This retrospective analysis received approval from the Ethics Committee of Dongying People’s Hospital (Approval number: 20231215) for data collection and analysis. Since this study was a retrospective analysis based on the data obtained from previous clinical diagnosis and treatment, acquiring informed consent was waived by the Ethics Committee of Dongying People’s Hospital specifically for this retrospective analysis of anonymized clinical data. All patient data were de-identified prior to analysis, stored on password-protected hospital servers with restricted access, and used solely for research purposes in compliance with China’s Personal Information Protection Law (PIPL).
Inclusion criteria
(1) Postoperative pathology showed stage II colon cancer; (2) Complete clinical laboratory tests and medical records; (3) Risk-stratified adjuvant chemotherapy per NCCN guidelines (v2.2020): Low-risk: Observation (no T4 lesions, well-differentiated, ≥ 12 lymph nodes examined, no obstruction/perforation, negative margins, no lymphovascular invasion); Intermediate-risk: Fluorouracil monotherapy (capecitabine or 5-FU/LV); High-risk: Combination regimens (FOLFOX or CAPEOX; presence of ≥ 1 high-risk feature: T4 stage, < 12 lymph nodes examined, obstruction/perforation, lymphovascular/perineural invasion, poorly differentiated histology, or positive margins).
Exclusion criteria
(1) Severe complications leading to a survival time of less than 30 days; (2) Simultaneously suffering from other cancers; (3) History of other malignant tumors in the past; (4) The presence of inflammatory diseases affects laboratory test results; (5) There are other diseases that may affect the level of inflammatory markers, such as liver disease, autoimmune diseases, or bone diseases; (6) Poor patient compliance and lack of cooperation with treatment.
Experimental grouping basis
The primary outcome was 3-year disease-free survival (DFS), defined as the time from surgery to the first occurrence of locoregional recurrence, distant metastasis, or cancer-specific death. Non-cancer deaths were censored at the time of occurrence. Patients experiencing any of these DFS event within 3 years were classified as the poor prognosis group (n = 28), while those remaining event-free constituted the good prognosis group (n = 59).
The 3-year DFS endpoint was selected based on peak recurrence patterns in stage II colon cancer and its validated surrogacy for 5-year overall survival. This timeframe optimizes clinical utility while mitigating loss-to-follow-up biases common in single-center retrospective studies.
Chemotherapy regimen
Fluorouracil monotherapy regimen: On the first day of chemotherapy, an indwelling needle venous access is established, and fluorouracil 28-30 mg/KG is intravenously dripped. After the infusion is completed, the NS20ml pulse positive pressure flushing tube is used and then the tube is sealed with heparin solution. From the 2nd to the 8th day of chemotherapy, intravenous infusion of fluorouracil was administered daily, similar to the first day.
FOLFOX regimen (Fluorouracil + Oxaliplatin): The drugs used include oxaliplatin injection, calcium folinate injection, and fluorouracil injection. Oxaliplatin injection and calcium folinate injection are intravenously administered for 2 h on the first day, while fluorouracil injection is continuously intravenously administered for 46–48 h in the first 2 days. After 3–4 weeks of outpatient follow-up evaluation of the patient’s condition, the first chemotherapy can be administered. If there is no obvious discomfort, chemotherapy can be carried out according to this regimen thereafter. Suggest repeating once every 2 weeks for a total of 12 chemotherapy sessions.
CAPEOX regimen (Capecitabine + Oxaliplatin): The drugs used include oxaliplatin injection and capecitabine tablets. The method of use is intravenous infusion of oxaliplatin injection for 2 h on the first day, followed by continuous oral administration of capecitabine tablets for 2 weeks. Then stop using capecitabine tablets for one week, repeating once every three weeks for a total of eight chemotherapy sessions.
Observation index
Collect and organize basic case data and blood indicators within one week before surgery for two groups of patients.
General information mainly includes: age, gender, chemotherapy method, adhesion, number of lymph nodes, occurrence of intestinal obstruction, degree of tumor differentiation, depth of tumor infiltration, presence of > 4 positive lymph nodes, surgery time, etc.
Blood indicators mainly include white blood cell count (WBC), neutrophil percentage (Neut), lymphocyte percentage (Lymph%), neutrophil to lymphocyte ratio (NLR), red blood cell count (RBC), hemoglobin (Hb), hematocrit (Hct), red blood cell volume distribution width (RDW), neutrophil count (NEUT), monocyte count (MONO), and lymphocyte count (LYC).
Common gastrointestinal tumor markers: carcinoembryonic antigen (CEA), carbohydrate antigen 19 − 9 (CA19-9), carbohydrate antigen 125 (CA125), carbohydrate antigen 72 − 4 (CA72-4).
The absolute counts of neutrophils (NEUT), monocytes (MONO), and lymphocytes (LYC) were measured in units of 10⁹/L. SIRI calculation formula: SIRI= (NEUT × MONO)/LYC, where all cell counts are absolute values 10⁹/L.
Laboratory methods and quality control
Complete blood counts (including NEUT, MONO, LYC) were measured using a Sysmex XN-9000 hematology analyzer (Sysmex Corporation, Japan). The laboratory reference ranges were as follows:
Neutrophils (NEUT): 1.8–6.3 × 10⁹/L;
Lymphocytes (LYC): 1.1–3.2 × 10⁹/L;
Monocytes (MONO): 0.1–0.6 × 10⁹/L.
Quality control was ensured through daily calibration, internal quality controls (two levels), and participation in external proficiency testing (National Center for Clinical Laboratories, China). Coefficients of variation (CV) for all parameters were < 5%.
Statistical analysis
SPSS 23.0 software (IBM Corp.) was used for the statistical analysis of the data. Normally distributed data were expressed as mean ± standard deviation (‾x ± s), and t-test was used to compare the two groups, whereas non-normally distributed data were represented as the median (P25, P75), and the two groups were compared using the Mann-Whitney test. The count data were expressed as percentages (%) and analyzed using the χ2 test. P < 0.05 was considered to indicate a statistically significant difference. All statistical outputs underwent triple verification. Evaluate the predictive value of blood indicators and tumor markers for postoperative patient prognosis using receiver operating characteristic (ROC) curves and area under curve (AUC). Cox regression and Kaplan-Meier analyses evaluated associations between biomarkers and disease-free survival (DFS). To account for potential confounding effects, chemotherapy regimen type (observation/monotherapy/combination) was included as a categorical covariate in all multivariate Cox regression models. Compare the survival differences of different SIRI patients using Kaplan Meier method and Log-rank test. All AUC-related statistics (including 95% CIs) were independently verified by two biostatisticians using R 4.3.0 (pROC package) and SPSS 23.0. Consistency checks confirmed that reported intervals strictly adhered to the inequality Lower ≤ AUC ≤ Upper. As an exploratory study, post-hoc analysis showed 72% power to detect HR = 3.44 (α = 0.05). Although EPV = 4.7 suggests potential overfitting, bootstrap validation (n = 2000) supported model stability.
Results
Results of baseline data comparison
A total of 87 patients with stage II colon cancer who underwent radical resection were included in the study. Three years after surgery, 28 patients experienced DFS events (recurrence, metastasis, or cancer-specific death), while 59 patients remained event-free. The baseline characteristic data of patients showed no statistically significant differences in age, gender, chemotherapy method, adhesion, number of lymph nodes, intestinal obstruction, tumor differentiation degree, tumor infiltration depth, presence of > 4 positive lymph nodes, and surgical time (P > 0.05), Table 1.
Table 1.
Comparison results of patient baseline data [n (%)]
| Variables | Poor prognosis group (n = 28) | Good prognosis group (n = 59) | t/χ2/F | P-value |
|---|---|---|---|---|
| Age (years) | 65.7 ± 8.1 | 63.2 ± 10.6 | 0.315 | 0.563 |
| Gender | 0.513 | 0.474 | ||
| male | 17 (60.71) | 31 (52.54) | ||
| female | 11 (39.29) | 28 (47.46) | ||
| Intestinal obstruction | 0.247 | 0.619 | ||
| yes | 16 (57.14) | 37 (62.71) | ||
| no | 12 (42.86) | 22 (37.29) | ||
| Colonic perforation | 0.329 | 0.566 | ||
| yes | 25 (89.29) | 50 (84.75) | ||
| no | 3 (10.71) | 9 (15.25) | ||
| Adhesion of colon | 0.094 | 0.760 | ||
| yes | 22 (78.57) | 48 (81.36) | ||
| no | 6 (21.43) | 11 (18.64) | ||
| Degree of tumor differentiation | 0.148 | 0.700 | ||
| higher | 13 (46.43) | 30 (50.85) | ||
| lower | 15 (53.57) | 29 (49.15) | ||
| Invasion depth | -- | 0.967 | ||
| T1 | 1 (3.57) | 2 (3.39) | ||
| T2 | 3 (10.71) | 8 (13.56) | ||
| T3 | 20 (71.43) | 40 (67.80) | ||
| T4a | 3 (10.71) | 6 (10.17) | ||
| T4b | 1 (3.57) | 3 (5.08) | ||
| Operative time | 0.024 | 0.878 | ||
| ≤ 180 min | 19 (67.86) | 41 (69.49) | ||
| > 180 min | 9 (32.14) | 18 (30.51) | ||
| > 4 positive lymph nodes | 0.105 | 0.746 | ||
| yes | 18 (64.29) | 40 (67.80) | ||
| no | 10 (35.71) | 19 (32.20) | ||
| Chemotherapy regimen | 1.115 | 0.573 | ||
| observation | 1 (3.57) | 5 (8.47) | ||
| monotherapy chemotherapy | 13 (46.43) | 30 (50.85) | ||
| combined chemotherapy | 14 (50.00) | 24 (40.68) | ||
| Number of lymph nodes | 3.0 (2.0, 6.0) | 2.0 (1.0, 4.0) | 0.529 | 0.121 |
According to AJCC 8th edition standard terminology, the representation method for intrusion depth is as follows: T1: Submucosa, T2: Muscularis propria, T3: Subserosa, T4: Serosa, T5: Adjacent organs
Results of univariate analysis affecting patient prognosis
Comparing the differences in blood indicators between two groups of patients within one week before surgery, as shown in Table 2, there was no statistically significant difference in blood indicators WBC, Neut, Lymph%, RBC, Hb, Hct, and RDW between the two groups of patients within one week before surgery (P > 0.05); Univariate analysis confirmed significantly higher NLR in the poor prognosis group (P < 0.05), supporting its role as a marker of systemic inflammation. The levels of SIRI, CEA, CA19-9, CA125, and CA72-4 in the poor prognosis group were significantly higher than those in the good prognosis group, and the differences between the groups were statistically significant (P < 0.05).
Table 2.
Comparison of preoperative blood routine indicators between two groups of patients
| Variables | Poor prognosis group (n = 28) | Good prognosis group (n = 59) | t | P-value |
|---|---|---|---|---|
| WBC(×109/L) | 7.01 ± 0.64 | 6.72 ± 0.53 | 2.031 | 0.082 |
| Neut(×109/L) | 7.93 ± 0.71 | 7.55 ± 0.58 | 0.615 | 0.693 |
| Lymph%(%) | 46.25 ± 5.15 | 48.13 ± 4.93 | −0.391 | 0.756 |
| NLR | 7.53 ± 0.72 | 5.01 ± 0.63 | 3.274 | 0.039* |
| RBC(×1012/L) | 4.71 ± 0.49 | 4.62 ± 0.55 | 1.873 | 0.636 |
| Hb(g/L) | 153.17 ± 15.33 | 159.64 ± 15.07 | −1.982 | 0.141 |
| Hct(%) | 41.54 ± 4.08 | 43.03 ± 4.19 | −2.031 | 0.097 |
| RDW(fL) | 46.37 ± 4.52 | 50.85 ± 4.38 | −2.634 | 0.091 |
| SIRI | 1.76 ± 0.12 | 0.98 ± 0.13 | 4.072 | 0.016* |
| CEA(ng/mL) | 26.84 ± 4.07 | 19.01 ± 3.34 | 3.067 | 0.043* |
| CA19-9(U/mL) | 46.06 ± 3.29 | 40.11 ± 4.35 | 2.894 | 0.047* |
| CA125(U/mL) | 56.91 ± 6.43 | 50.11 ± 5.08 | 3.471 | 0.036* |
| CA72-4(U/mL) | 16.71 ± 2.12 | 11.25 ± 1.52 | 3.542 | 0.033* |
*Indicates statistically significant intergroup difference Exact P-values: NLR = 0.039, SIRI = 0.016, CEA = 0.043, CA19-9 = 0.047, CA125 = 0.036, CA72-4 = 0.033
Results of Cox regression analysis affecting patient prognosis
The Cox regression analysis results of the impact of various indicators on patient prognosis are shown in Table 3. From the data in the table, it can be seen that NLR, SIRI, CEA, CA19-9, CA125, and CA72-4 are all independent risk factors for the prognosis of postoperative patients with stage II colon cancer. Multivariate Cox regression models were adjusted for chemotherapy regimen (observation/monotherapy/combination). No significant association was observed between chemotherapy regimen and DFS outcomes (P = 0.573, Table 1). Despite this adjustment, NLR, SIRI, CEA, CA19-9, CA125, and CA72-4 remained independent risk factors for poor prognosis.
Table 3.
Cox regression analysis results of the impact of various indicators on patient prognosis
| Variables | B | SE | Wald χ² | P-values | OR | 95% CI | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| NLR | 1.235 | 0.358 | 11.894 | <0.001 | 3.438 | 0.358 | 1.704 |
| SIRI | 2.196 | 0.413 | 28.249 | <0.001 | 8.989 | 4.000 | 20.203 |
| CEA | 1.007 | 0.208 | 23.539 | <0.001 | 2.737 | 1.822 | 4.112 |
| CA19-9 | 1.015 | 0.290 | 12.230 | <0.001 | 2.759 | 1.562 | 4.874 |
| CA125 | 1.172 | 0.223 | 27.684 | <0.001 | 3.228 | 2.086 | 4.996 |
| CA72-4 | 1.635 | 0.358 | 20.861 | <0.001 | 5.129 | 2.543 | 10.346 |
B partial regression coefficient, SE, standard error, Wald χ² Wald chi-square statistic, OR odds ratio, 95% CI 95% confidence interval for odds ratio, Lower minimum interval value, Upper maximum interval value
Results of predictive value of various factors on prognosis
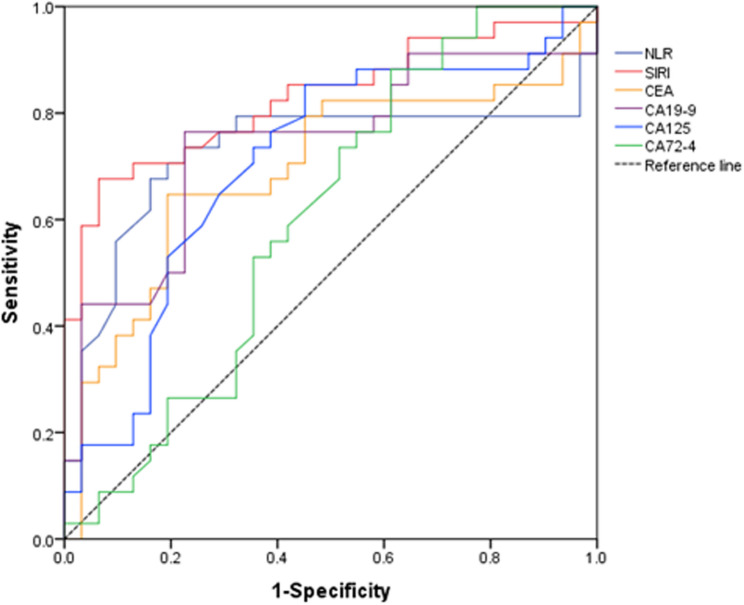
Using Cox regression analysis, ROC curves were plotted for each influencing factor, as shown in Fig. 1; Table 4. The results showed that the AUC of NLR, SIRI, CEA, CA19-9, CA125, and CA72-4 were 0.726, 0.828, 0.693, 0.741, 0.706, and 0.605, respectively. The AUC of SIRI is greater than 0.8, indicating that SIRI has a high predictive value for the prognosis of postoperative patients with stage II colon cancer. According to the ROC curve results, the optimal cutoff value for SIRI is 1.41, with a sensitivity of 0.67 and a specificity of 0.93.
Fig. 1.
ROC curves of various indicators for predicting postoperative prognosis of patients Note: Receiver operating characteristic (ROC) curves for NLR, SIRI, and tumor markers (CEA, CA19-9, CA125, CA72-4) in predicting 3-year DFS after radical surgery for stage II colon cancer. Diagonal line indicates reference (AUC = 0.5). SIRI demonstrates superior predictive accuracy (AUC = 0.828)
Table 4.
The ROC curve data results of various indicators for predicting postoperative prognosis of patients
| Parameters | AUC | SE | 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| NLR | 0.726 | 0.069 | 0.591 | 0.861 |
| SIRI | 0.828 | 0.052 | 0.726 | 0.930 |
| CEA | 0.693 | 0.068 | 0.560 | 0.826 |
| CA19-9 | 0.741 | 0.064 | 0.616 | 0.866 |
| CA125 | 0.706 | 0.067 | 0.575 | 0.837 |
| CA72-4 | 0.605 | 0.072 | 0.464 | 0.746 |
AUC area under curve, SE standard error, 95% CI 95% confidence interval for odds ratio, Lower minimum interval value, Upper maximum interval value. Confidence intervals were recalculated using DeLong’s method for AUC comparison, with verification via 2000 bootstrap resamples to ensure robustness
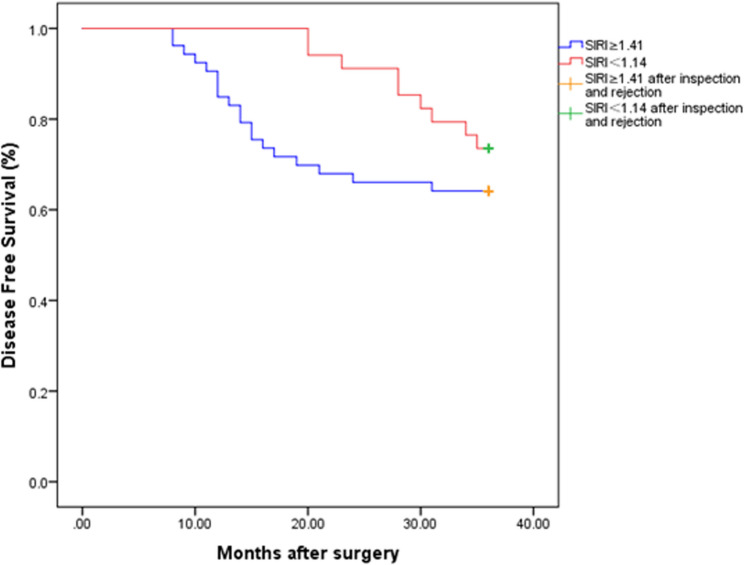
Results of relationship between preoperative SIRI and disease free survival in patients
The 3-year disease-free survival curves of different SIRI patients after surgery are shown in Fig. 2. From the curves in the figure, it can be found that there are differences in the 3-year disease-free survival rates between stage II colon cancer patients with SIRI ≥ 1.41 and SIRI < 1.41. Using the Log rank test analysis, it was found that χ2 = 10.2483, P = 0.0014<0.05, the difference is statistically significant, that is, the postoperative prognosis of stage II colon cancer patients with different SIRIs is not the same, and SIRI has an impact on the prognosis of stage II colon cancer resection patients.
Fig. 2.
Kaplan Meier curve for 3-year disease-free survival (DFS) stratified by preoperative SIRI Note: Kaplan-Meier curves comparing 3-year disease-free survival (DFS) in stage II colon cancer patients stratified by preoperative SIRI (cutoff = 1.41). Patients with SIRI < 1.41 (blue line) show significantly improved survival (log-rank P = 0.0014)
Discussion
Colon cancer is a common malignant tumor in clinical practice, mainly manifested as abdominal pain, bloody stools, weight loss, etc. It is more common in people with a family history and middle-aged alcoholism [13]. Colon cancer has a strong insidious onset and early symptoms are not obvious [14]. As the disease progresses, systemic symptoms become more severe and involve multiple organ systems, endangering life safety. At present, surgical treatment is mainly used for colon cancer in clinical practice. With the advancement of diagnosis and treatment technology, early diagnosis and clinical efficacy have significantly improved. However, the high recurrence rate of stage II colon cancer patients after radical surgery remains a focus of clinical attention [15]. Research has shown that the 5-year survival rate of early colon cancer patients can reach up to 80%, while the survival rate of stage II colon cancer patients is only 70%. Therefore, analyzing the factors that affect prognosis is of great significance for prolonging the survival time of colon cancer patients [16]. While 5-year survival remains the conventional benchmark, recent evidence suggests 3-year disease-free survival (DFS) strongly correlates with long-term outcomes in stage II colon cancer and may serve as an early efficacy indicator for adjuvant therapy. Given that > 80% of recurrences occur within 3 years post-resection, this endpoint balances clinical relevance with feasibility in retrospective cohorts. Finding prognostic markers related to colon cancer and exploring the mechanisms of colon cancer growth, recurrence, and metastasis are currently the focus of research.
Studies have confirmed that inflammation and the immune system play important roles in the occurrence and development of malignant tumors, and many preoperative inflammatory indicators have been shown to be closely related to the prognosis of various tumor patients [17]. Research has found that chronic inflammatory colitis (such as ulcerative colitis and Crohn’s disease) is a high-risk factor for colon cancer, and nonsteroidal anti-inflammatory drugs can reduce the risk of colon cancer. Therefore, colon cancer is driven to some extent by the inflammatory microenvironment, and the infiltration of inflammatory cells and mediators in the tumor microenvironment plays a crucial role in the pathogenesis of colon cancer [18]. Adjuvant chemotherapy decisions for stage II colon cancer remain challenging, primarily relying on clinicopathological features such as T4 stage, lymphovascular or perineural invasion, tumor perforation or obstruction, poor differentiation, and inadequate lymph node sampling (< 12). However, significant heterogeneity in outcomes persists even within these established high-risk groups, indicating an unmet need for more refined prognostic biomarkers to better identify patients most likely to benefit from adjuvant therapy.
SIRI is an easily accessible, replicable, and minimally invasive inflammatory response assessment tool that includes monocytes, lymphocytes, and neutrophils. Monocytes and neutrophils belong to inflammatory cells, while lymphocytes belong to immune cells. When the number of inflammatory cells increases and the number of immune cells decreases, cancer progression and recurrence are more likely to occur [19]. SIRI is increasingly used for prognosis analysis of cancer patients. This study identified preoperative SIRI as a predictor of 3-year DFS—a clinically meaningful endpoint capturing > 80% of recurrences while enabling earlier clinical decision-making than 5-year benchmarks. Research shows that high SIRI is related to the increase of clinical stage and poor prognosis of cervical cancer. SIRI is more accurate in predicting the prognosis of cervical cancer patients than neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, lymphocyte and monocyte ratio. SIRI is significantly related to TNM stage, endocrine therapy, and total survival of breast cancer patients, and can independently predict the total survival of breast cancer patients [20, 21]. This study found that an increase in SIRI is closely related to the clinical and pathological characteristics of malignant invasion of colon cancer and low DFS rate. The AUC (Area Under the Curve) for SIRI in predicting colon cancer prognosis was 0.828, which is greater than 0.8, indicating that SIRI can be used as a biomarker for colon cancer prognosis This may be because inflammation directly causes changes in the tumor microenvironment, promoting and enhancing the transformation, invasion, and metastasis of malignant cells Neutrophils are heterogeneous white blood cells that are abundant in human peripheral blood. They can secrete cytokines and chemokines, inhibit T cell activation, reduce the body’s anti-tumor response, and promote tumor progression and metastasis by forming neutrophil extracellular traps [22]. Consistent with established literature [20], univariate and multivariate analyses confirmed that elevated NLR (reflecting neutrophilia and lymphocytopenia) was significantly associated with poorer DFS (OR = 3.438, P < 0.001), underscoring its role as a systemic inflammation marker. This aligns with the biological plausibility of neutrophils promoting tumor progression via cytokine secretion and T-cell suppression [22]. Monocytes infiltrate tumor tissue and differentiate into tumor associated macrophages, inducing apoptosis of anti-tumor CD8 + T cells and secreting cytokines that promote tumor cell invasion, leading to tumor growth, invasion, and metastasis [23]. Lymphocytes are important anti-tumor cells in the body, and a decrease in their quantity can reduce immune surveillance and defense capabilities, promote cancer cell immune escape, and facilitate tumor invasion and growth [24].
Our findings demonstrate that a high preoperative SIRI is a robust and independent predictor of worse OS and DFS in stage II colon cancer patients, even after adjusting for established high-risk features including T stage, lymphovascular invasion, perineural invasion, and tumor perforation/obstruction. This suggests that SIRI captures a distinct biological dimension—namely, the degree of systemic inflammation—which complements the information provided by traditional clinicopathological factors. The added value of SIRI lies in its ability to offer a quantifiable, readily available, and cost-effective assessment of this inflammatory milieu, potentially refining risk stratification beyond anatomical extent. Our findings demonstrate that a high preoperative SIRI is a robust and independent predictor of worse OS and DFS in stage II colon cancer patients, even after adjusting for established high-risk features including T stage, lymphovascular invasion, perineural invasion, and tumor perforation/obstruction. This suggests that SIRI captures a distinct biological dimension—namely, the degree of systemic inflammation—which complements the information provided by traditional clinicopathological factors. The added value of SIRI lies in its ability to offer a quantifiable, readily available, and cost-effective assessment of this inflammatory milieu, potentially refining risk stratification beyond anatomical extent. Notably, the prognostic value of SIRI persisted after adjusting for chemotherapy intensity, suggesting its independence from treatment effects. The SIRI may complement TNM staging by identifying high-risk subsets among conventionally intermediate-risk stage II colon cancer patients (e.g., T3N0 with SIRI ≥ 1.41), where its high specificity (93%) could support adjuvant chemotherapy decisions when traditional pathological features are equivocal, though prospective trials are needed to validate this approach and define optimal integration with existing NCCN high-risk criteria such as lymphovascular invasion or inadequate lymph node harvest.
This study analyzed SIRI at only a single preoperative time point and did not track its dynamic changes during postoperative or chemotherapy periods. Inflammatory status may fluctuate due to treatment interventions. Future research should validate the prognostic value of SIRI trajectories through multi-timepoint monitoring (e.g., preoperative/postoperative, during adjuvant therapy). Nevertheless, preoperative SIRI—as a stable baseline indicator prior to treatment—retains immediate clinical utility due to its readily available and cost-effective nature.
Cancer associated inflammation is associated with multiple stages of tumor development, including promoting tumor cell survival, proliferation, invasion, and metastasis. At present, the mechanism by which inflammation promotes malignant tumors is not fully understood. There are some limitations to this study that warrant consideration. Firstly, the sample size of 87 patients with 28 events, while adequate for preliminary hypothesis generation, falls below the recommended threshold of 10 events per variable (EPV) for multivariate Cox regression (6 predictors; EPV = 4.7). This increases the risk of overfitting, as evidenced by the wide confidence intervals for some predictors (e.g., SIRI OR: 4.00–20.20). Post-hoc power analysis using G*Power 3.1 indicated only 72% power to detect the observed effect size (HR = 3.44, α = 0.05), suggesting potential Type II error for smaller effects. While bootstrap validation (n = 2000 resamples) supported internal consistency, these findings require confirmation in larger cohorts. Additionally, the small number of individual events (recurrence/metastasis/death) precluded robust subgroup analyses. Future larger studies should validate SIRI’s predictive utility for distinct outcomes. Secondly, our primary endpoint (DFS) combines distinct events (recurrence/metastasis/cancer-death). While clinically relevant for stage II colon cancer, this approach may obscure differential relationships between SIRI and specific event types. Future studies with larger cohorts should analyze these outcomes separately to determine whether SIRI preferentially predicts recurrence versus overall survival. Thirdly, the retrospective, single-center design may introduce selection bias and limit the applicability of our results to broader populations. Fourthly, while 3-year DFS was selected for its established surrogacy and practicality, longer follow-up (e.g., 5 years) would provide complementary data on late recurrences. Future multi-center studies should validate these findings with extended observation periods. Fifthly, comorbidities (e.g., diabetes), nutritional status, and chemotherapy compliance were not analyzed due to incomplete retrospective data. These factors may interact with systemic inflammation and survival outcomes, potentially confounding our results. Future studies should incorporate these variables to refine SIRI’s predictive accuracy. Lastly, this study did not elucidate the detailed underlying mechanism by which preoperative SIRI influences prognosis. Consequently, future validation in larger, prospective, and multi-center studies is essential to confirm the robustness and general applicability of our findings, particularly the proposed SIRI cutoff. In summary, preoperative SIRI as a simple, convenient, and low-cost prognostic indicator for patients undergoing radical surgery for stage II colon cancer is expected to be utilized by clinical workers. It can predict the prognosis of patients to a certain extent, and has certain significance in improving the prognosis of patients by improving their nutrition and protecting their liver function during the perioperative period.
Conclusion
In summary, preoperative SIRI predicts early recurrence risk (3-year DFS) more effectively than traditional biomarkers. DFS was significantly higher in patients with SIRI < 1.41. While longer follow-up is ideal, this metric captures critical prognostic information within a clinically actionable timeframe.
Acknowledgements
Not applicable.
Authors’ contributions
YW and LD conceived and designed the study, and drafted the manuscript. YW, LD, LJ and ZY collected, analyzed and interpreted the data. LD and ZY revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding
No funding was received.
Data availability
De-identified processed data supporting the findings are available from the corresponding author upon reasonable request, pending approval from the Dongying People’s Hospital Ethics Committee. Raw data are not publicly available due to ongoing research.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Dongying People’s Hospital. Patients who participated in the study had complete clinical data. The study was conducted in compliance with the Helsinki Declaration. Since this study was a retrospective analysis based on the data obtained from previous clinical diagnosis and treatment, acquiring the informed consent was exempted by the Ethics Committee of Dongying People’s Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yong Wang and Longlong Ding contributed equally to this work.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 2.Li R, Sun J, Wang T, Huang L, Wang S, Sun P, Yu C. Comparison of secular trends in esophageal Cancer mortality in China and Japan during 1990–2019: an Age-Period-Cohort analysis. Int J Environ Res Public Health. 2022;19:10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bierbaum V, Bobeth C, Roessler M, Gerken M, Tol KK, Reissfelder C, Fürst A, Günster C, Dröge P, Ruhnke T, Klinkhammer-Schalke M, Schmitt J, Schoffer O. Treatment in certified cancer centers is related to better survival in patients with colon and rectal cancer: evidence from a large German cohort study. World J Surg Oncol. 2024;22: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong S, Xu J, Li M, Xiong G, Wang R. Tumor necrosis serves as an important pathological characteristic of stage I-II colon cancer. Indian J Pathol Microbiol. 2024;67:794–800. [DOI] [PubMed] [Google Scholar]
- 5.Tan C, Wang Q, Yao S. Effects of adjuvant chemotherapy on early-onset stage II colon cancer at different tumor sites. Am J Clin Oncol. 2024;47:253–8. [DOI] [PubMed] [Google Scholar]
- 6.Oguz Erdogan AS, Simmer F, Nagtegaal ID. Tumor deposits should not be placed in the M category of TNM: a comparative survival analysis using SEER data. Int J Cancer. 2025;156:274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu C, Shi W, Wu H, Zou S, Li J, Wang D, Liu G, Song Z, Xu X, Hu J, Geng H. Identification of molecular subtypes and a prognostic signature based on Inflammation-Related genes in Colon adenocarcinoma. Front Immunol. 2021;12:769685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu M, Chen L, Kong X, Wang X, Fang Y, Li X, Wang J. The systemic inflammation response index as an independent predictor of survival in breast cancer patients: a retrospective study. Front Mol Biosci. 2022;9: 856064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jinhui F, Qiming W. Correlation of systemic immune-inflammatory response index with clinical data in patients with malignant ovarian tumor. Am J Transl Res. 2023;15:3309–17. [PMC free article] [PubMed] [Google Scholar]
- 10.Sun L, Hu W, Liu M, Chen Y, Jin B, Xu H, Du S, Xu Y, Zhao H, Lu X, Sang X, Zhong S, Yang H, Mao Y. High systemic inflammation response index (SIRI) indicates poor outcome in gallbladder cancer patients with surgical resection: a single institution experience in China. Cancer Res Treat. 2020;52:1199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuk P, Pedersen AK, Lambertsen KL, Mogensen CB, Nielsen MF, Helligsø P, Gögenur I, Ellebæk MB. Systemic inflammatory response in robot-assisted and laparoscopic surgery for colon cancer (SIRIRALS): study protocol of a randomized controlled trial. BMC Surg. 2021;21:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McSorley ST, Watt DG, Horgan PG, McMillan DC. Postoperative systemic inflammatory response, complication severity, and survival following surgery for colorectal cancer. Ann Surg Oncol. 2016;23:2832–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells BJ, Kattan MW, Cooper GS, Jackson L, Koroukian S. Colorectal cancer predicted risk online (CRC-PRO) calculator using data from the multi-ethnic cohort study. J Am Board Fam Med. 2014;27:42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smyth J, Godet J, Choudhary A, Das A, Gkoutos GV, Acharjee A. Microbiome-Based Colon cancer patient stratification and survival analysis. Cancer Med. 2024;13:e70434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grellier N, Severino A, Archilei S, Kim J, Gasbarrini A, Cammarota G, Porcari S, Benech N. Gut microbiota in inflammation and colorectal cancer: a potential toolbox for clinicians. Best Pract Res Clin Gastroenterol. 2024;72: 101942. [DOI] [PubMed] [Google Scholar]
- 16.Li AJ, Jiang HY, Jia YH. Statin exposure and risk of colorectal cancer in patients with inflammatory bowel disease: a systematic review and meta-analysis. Front Med (Lausanne). 2024;11: 1507739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon M, Nigam GB, Sinopoulou V, Al Bakir I, Bateman AC, Din S, Dolwani S, Dhar A, Faiz OD, Hayee B, et al. Update from 2010 (standard operating procedure): protocol for the 2024 British society of gastroenterology guidelines on colorectal surveillance in inflammatory bowel disease. BMJ Open Gastroenterol. 2024;11: e001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chao B, Ju X, Zhang L, Xu X, Zhao Y. A novel prognostic marker systemic inflammation response index (SIRI) for operable cervical cancer patients. Front Oncol. 2020;10: 766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hua X, Long ZQ, Huang X, Deng JP, Wen W, He ZY, Guo L, Zhang WW, Lin HX. The preoperative systemic inflammation response index (SIRI) independently predicts survival in postmenopausal women with breast cancer. Curr Probl Cancer. 2020;44: 100560. [DOI] [PubMed] [Google Scholar]
- 20.Mantilla A, Ramírez-Ramírez ML, Berlanga-Taylor AJ, Vadillo E. Roles emergentes de Los neutrófilos En El Pronóstico Del cáncer colorrectal [Emerging roles of neutrophils in the prognosis of colorectal cancer]. Rev Med Inst Mex Seguro Soc. 2024;62:1–7. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Wu J, Chen W, Liang X. Pretreatment system inflammation response index (SIRI) is a valuable marker for evaluating the efficacy of neoadjuvant therapy in breast cancer patients. Int J Gen Med. 2024;17:4359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao Y, Cong M, Li J, He D, Wu Q, Tian P, Wang Y, Yang S, Liang C, Liang Y, et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell. 2021;39:423–37. [DOI] [PubMed] [Google Scholar]
- 23.Zhang D, Huang H, Gao X, Yu G, Zhang X, Jin H, Xu R, Wang Z, Zhang G. High expression of B7-H3 on monocyte/macrophages in tumor microenvironment promotes lung cancer progression by inhibiting apoptosis. Transl Oncol. 2024;41: 101874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cupp MA, Cariolou M, Tzoulaki I, Aune D, Evangelou E, Berlanga-Taylor AJ. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020;18:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
De-identified processed data supporting the findings are available from the corresponding author upon reasonable request, pending approval from the Dongying People’s Hospital Ethics Committee. Raw data are not publicly available due to ongoing research.