Abstract
Objective
Guidelines recommend a multidisciplinary heart team approach for managing complex coronary artery disease (CAD), yet its impact on clinical outcomes and adherence to recommendations is rarely reported.
Methods
Between June 2021 and August 2022, 210 high-risk patients with isolated, complex CAD were evaluated at our institution’s weekly heart team conference for consideration of coronary artery bypass grafting (CABG), percutaneous coronary intervention (PCI), hybrid PCI/CABG, or optimal medical therapy (OMT). Adherence to recommendations and clinical outcomes, including 30-day, 1-year, and 2-year mortality, were assessed.
Results
Overall adherence to heart team recommendations was 92%, with 96% adherence for CABG, 90% for PCI, 87% for OMT, and 75% for hybrid PCI/CABG. CABG was the most frequently recommended treatment (53%) and demonstrated the lowest mortality at 1 year (4%) and 2 years (6%) compared with PCI (1 year, 28%; 2 year, 40%) and OMT (1 year, 10%; 2 year, 20%). CABG patients had a lower-than-expected mortality (observed-to-expected ratio 0.9), while PCI was associated with significantly higher mortality (observed-to-expected ratio 3.0).
Conclusion
This single-center multidisciplinary heart team approach for complex CAD offers a collaborative, patient-centered model that facilitates high adherence rates and favorable patient outcomes. These findings highlight the potential benefits of integrating multidisciplinary evaluation and support its implementation into standard practice for high-risk CAD patients.
Keywords: Coronary artery bypass grafting, coronary artery disease, long-term outcomes, multidisciplinary heart team, percutaneous coronary intervention
Management of complex coronary artery disease (CAD) poses substantial challenges, requiring a comprehensive and collaborative strategy to optimize patient outcomes. Although risk calculators such as The Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) and National Cardiovascular Data Registry (NCDR) risk score aid in the risk stratification and decision-making for patients with CAD, the concept of a multidisciplinary heart team (MHT) has emerged as a foundational component of modern cardiovascular care.1–4 In this model, cardiac surgeons, interventional cardiologists, and other subspecialists collaborate to develop individualized treatment plans tailored to each patient’s clinical scenario. This interdisciplinary approach aims to improve decision-making, enhance diagnostic accuracy, facilitate risk stratification, and establish patient-centered strategies, ultimately leading to improved quality metrics and patient satisfaction.5,6
Due to its potential to enhance decision-making and improve outcomes, the MHT approach is supported by both US and European guidelines with a Class I recommendation.7,8 Landmark trials such as SYNTAX and EXCEL, though not specifically designed to investigate MHT as a primary endpoint, utilized team-based discussions and demonstrated reduced rates of major adverse cardiac events and improved long-term survival.9,10 Nonetheless, evidence supporting the MHT model is largely derived from nonrandomized or observational studies (Level B evidence).11–14 Additionally, while the MHT model is widely accepted, adherence to MHT recommendations and subsequent long-term outcomes are scarcely reported.15,16
This study investigated the influence of an institutional MHT on treatment recommendations, adherence, and clinical outcomes in high-risk patients undergoing surgical evaluation for complex isolated CAD. We aimed to contribute to the growing body of evidence supporting the implementation of the MHT model and provide insights into short- and long-term outcomes based on MHT recommendations.
METHODS
This study was approved by the Baylor Scott & White Research Institute Institutional Review Board. Individual consent was waived due to the retrospective nature of the study.
Multidisciplinary heart team structure and workflow
A formal weekly MHT conference was established at our institution in 2014, consisting of cardiothoracic surgeons and interventional cardiologists. In 2018, this conference was expanded to include heart failure specialists, cardiovascular imaging specialists, critical care intensivists, palliative care specialists, and any other relevant team members to ensure a holistic patient evaluation. Each meeting is moderated by a senior cardiac surgeon and includes at least two additional senior faculty members (≥20 years of experience), who guide a structured discussion facilitating a comprehensive case review and informed decision-making. The primary goal is to formulate individualized treatment plans that consider disease complexity, imaging findings, comorbidities, and patient preferences.
Patients who are considered high-risk for isolated coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI) are presented at the conference. Surgical revascularization candidates with an STS-PROM score ≥4% or with other significant risk factors not captured by the STS-PROM score such as morbid obesity (body mass index >35 kg/m2 in the presence of obesity-related health conditions), critical illness, frailty, poor conduit options, poor quality revascularization targets, and uncommon clinical disease or presentation undergo MHT review. Other patients deemed high risk by the attending surgeon may also be presented on a case-by-case basis.
Patients presenting with high-risk coronary anatomy (e.g., left main disease, severe multivessel disease, heavy calcification), ST-elevation myocardial infarction (STEMI), an NCDR Cath PCI risk score >55 (indicating an estimated procedural mortality of ≥5%), hemodynamic support requirements (e.g., intra-aortic balloon pump, temporary left ventricular assist device such as Impella, extracorporeal membrane oxygenation), or poor candidacy for operative revascularization are evaluated for high-risk PCI.
In emergent or time-critical scenarios—such as high-risk STEMI, hemodynamic instability, or cardiogenic shock—an ad hoc MHT conference is convened via teleconference to provide immediate multidisciplinary input on PCI feasibility, alternative revascularization strategies, and possible adjunctive support, without delaying time to intervention. The structure, activation criteria, and workflow of this pathway have been previously described.17
Study population and data collection
Between June 2021 and August 2022, patients with complex or high-risk CAD who met one or more of the above criteria were presented at the weekly MHT conference for consideration of surgical revascularization or high-risk PCI. Patients evaluated for valvular heart disease, aortic dissection or aneurysm, cardiac tumor or thrombus, and other structural heart pathology were excluded from this analysis. Risk stratification was performed by the patient’s primary provider team, including attending physicians, residents/fellows, and advanced care practitioners.
Patient-specific details including presenting symptoms, diagnosis, initial plan of care, and MHT recommendations were recorded during the conference. Additional data including patient demographics, past medical and surgical history, STS-PROM and NCDR scores, procedures performed, and outcomes were collected retrospectively via the electronic medical record. Periprocedural outcomes including stroke, myocardial infarction, renal replacement therapy requirements, and 30-day readmission rates were also collected. Mortality at 30 days, 1 year, and 2 years was calculated from the date of the procedure for those who received intervention or, for those managed medically, from the date of MHT presentation. Follow-up was determined by clinical in-person or virtual visit notes; public records (including obituary searches, state vital records, and the Social Security Death Index) were reviewed if no recent follow-up was available in the electronic medical record.18
Statistical analysis
Descriptive statistics were used to characterize demographics, comorbidities, STS-PROM scores, MHT recommendations, actual treatment received, and clinical outcomes. Categorical variables were presented as frequencies and percentages, while continuous variables were expressed as mean ± standard deviation or median (interquartile range), as appropriate. Kaplan-Meier survival analysis was also performed, with pairwise comparisons of hazard ratios between cohorts. Statistical analysis was performed using Stata/SE 17.0 (StataCorp, College Station, Texas).
RESULTS
Baseline characteristics
Between June 2021 and August 2022, 392 patients were discussed at the MHT conference, and 210 were evaluated for complex coronary revascularization. The median age was 69 [61, 75] years, with a male predominance of 70.5%. Patient demographics, comorbidities, and risk profiles are shown in Table 1. The majority of patients presented with non‐STEMI (51.4%), followed by stable angina (22.4%), unstable angina (19.0%), and select high-risk STEMI (2.9%). Triple-vessel CAD was present in 74.3% of patients, with 21.4% and 4.3% presenting with double- or single-vessel disease, respectively. The median left ventricular ejection fraction was 45% [35%, 60%], as determined by the lowest quantitative measure from the preprocedural echocardiogram. The overall mean STS-PROM score was 2.8% ± 3.9%, with 18.6% of patients presenting with a > 4% risk of 30-day mortality. The mean NCDR score was 39.9 ± 8.9, with a subsequent risk of inpatient mortality of 2.1% ± 1.8%.
Table 1.
Baseline characteristics
| Variable | Overall (N = 210) | |
|---|---|---|
| Age (years) | ||
| Average | 67 ± 12 | |
| Median | 69 [61, 75] | |
| Male sex | 148 (70.5%) | |
| Comorbidities | ||
| Body mass index, kg/m2 | 29.7 ± 7.9 | |
| Hypertension | 194 (92.4%) | |
| Diabetes mellitus | 118 (56.2%) | |
| Chronic obstructive pulmonary disease | 46 (21.9%) | |
| Peripheral arterial disease | 48 (22.9%) | |
| ESKD on dialysis | 24 (11.4%) | |
| Prior cerebrovascular disease | 34 (16.2%) | |
| Prior PCI | 38 (18.1%) | |
| Prior CABG | 27 (12.9%) | |
| History of arrhythmia | 45 (21.4%) | |
| Cancer | 13 (6.2%) | |
| Immunocompromised | 5 (2.4%) | |
| Coronary artery disease presentation | ||
| STEMI | 6 (2.9%) | |
| NSTEMI | 108 (51.4%) | |
| Unstable angina | 40 (19.0%) | |
| Stable angina | 47 (22.4%) | |
| No angina | 9 (4.3%) | |
| Heart failure | 106 (50.5%) | |
| LVEF | 45[35, 60] | |
| STS PROM score | ||
| Average | 2.8 ± 3.9 | |
| Median | 1.5 [0.8, 3.1] | |
| NCDR score | ||
| Average | 39.9 ± 8.9 | |
| Median | 40 [36, 47] | |
| NCDR risk of inpatient mortality | ||
| Average | 2.1% ± 1.8% | |
| Median | 1.4% [0.9%, 2.3%] | |
| Number of diseased vessels | ||
| 1 | 9 (4.3) | |
| 2 | 45 (21.4) | |
| ≥3 | 156 (74.3) | |
| Status at time of meeting | ||
| Inpatient | 131 (62.4%) | |
| Outpatient or transfer | 89 (37.6%) | |
Categorical data are described as n (%). Continuous data are described as mean ± standard deviation or median [interquartile range]. CABG indicates coronary artery bypass graft; ESKD, end-stage kidney disease; LVEF, left ventricular ejection fraction; NCDR, National Cardiovascular Data Registry; NSTEMI, non–ST-elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction; STS-PROM, Society of Thoracic Surgeons predicted risk of mortality for isolated coronary artery bypass score.
Final multidisciplinary heart team recommendations
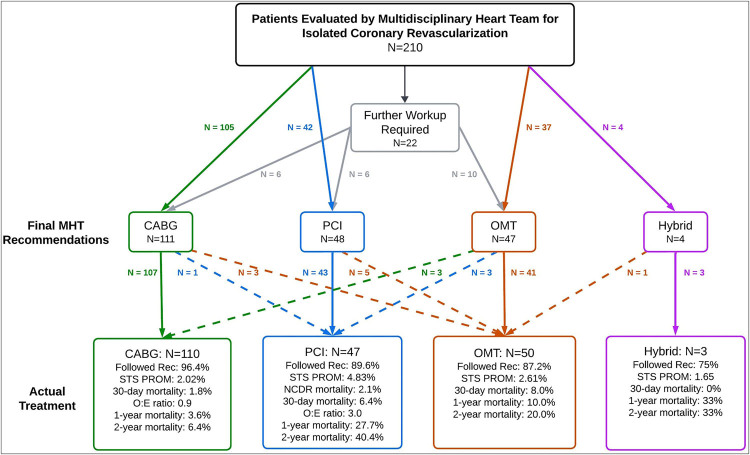
Of the 210 patients, 131 (62.4%) were inpatients at the time of MHT discussion. Twenty-two (10.5%) patients required additional imaging or assessments prior to a final MHT recommendation; ultimately these were allocated to CABG (6), PCI (6), and optimal medical therapy (OMT) (10). In total, the MHT recommended CABG in 111 patients (52.9%), PCI in 48 (22.9%), OMT in 47 (22.4%), and hybrid PCI/CABG in 4 (1.9%) (Figure 1, Central Illustration). Consultation with palliative care specialists was initiated in 45 patients (21.4%) to aid in patient and family education and decision-making to ensure alignment of treatment strategy with the patients’ goals of care.
Figure 1.
Central Illustration. MHT recommendations, patient crossovers, and outcomes. Of 210 patients evaluated, recommendations were made for coronary artery bypass grafting (CABG), percutaneous coronary intervention (PCI), optimal medical therapy (OMT), or hybrid procedures. Dashed lines indicate patient crossover between treatment groups. Outcomes for each group, including 30-day and 1-year mortality rates, adherence to recommendations, and observed-to-expected mortality ratios, are shown based on the actual treatments received.
Adherence to MHT recommendations and actual treatments
Overall adherence to MHT recommendations was 91.5%. Adherence rates were 96.4% for CABG, 89.6% for PCI, 87.2% for OMT, and 75.0% for hybrid revascularization. Sixteen patients (7.6%) received a different treatment from the one initially recommended. The most common deviations involved switching from OMT to an invasive approach (n = 6) or from PCI to OMT (n = 6) (Table 2). Ultimately, 110 patients (52.4%) underwent CABG, 50 (23.8%) received OMT, 47 (22.4%) underwent PCI, and 3 (1.4%) had a hybrid procedure (Table 3).
Table 2.
Reasons for deviation from multidisciplinary heart team recommendations and associated outcomes
| MHT recommended → Actual treatment | Deviation n/N (%) |
30-day mortality |
1-year mortality |
2-year mortality |
Key reasons for deviation |
|---|---|---|---|---|---|
| OMT → CABG/PCI | 6/47 (12.8%) | 0 | 1 | 2 | 6: Clinical improvement warranted reevaluation of the risks/benefits of intervention; the patients/families desired to undergo intervention |
| PCI → OMT | 5/48 (10.4%) | 2 | 2 | 2 | 1: Decompensated shortly after the MHT evaluation prior to intervention; the family elected to withdraw care 1: Impella placed but developed refractory multisystem organ failure; the family elected to withdraw care 1: Attempted PCI but unable to cross CTO; transitioned to OMT with clinical improvement 2: Resolution of symptoms with OMT and thus patient/family desired to pursue OMT with close observation and follow-up |
| CABG → OMT | 3/111 (2.7%) | 0 | 0 | 1 | 1: Chest pain resolved after treatment of lower-extremity gangrene; the patient/family elected OMT with close observation 1: Symptoms resolved following OMT; the patient/family elected close observation and follow-up 1: After discussion of the risks and benefits of the procedure, patient/family elected OMT with close observation and follow-up |
| CABG → PCI | 1/111 (0.9%) | 0 | 0 | 0 | 1: Attempted robotic LIMA to LAD but was aborted due to adhesive disease secondary to mediastinal radiation; PCI was successfully performed |
| Hybrid → OMT | 1/4 (25.0%) | 0 | 0 | 0 | 1: Symptoms improved following treatment of COVID infection; the patient/family elected OMT with close observation and follow-up |
n/N (%) indicates the proportion of patients who deviated out of those originally recommended for each therapy. Mortality figures in each column represent the number of deaths specifically among the patients who deviated from that recommendation. CABG indicates coronary artery bypass graft; CTO, chronic total occlusion; LAD, left anterior descending artery; LIMA, left internal mammary artery; MHT, multidisciplinary heart team; OMT, optimal medical therapy; PCI, percutaneous coronary intervention.
Table 3.
Multidisciplinary heart team meeting data and outcomes
| Variable | CABG | PCI | OMT | Hybrid |
|---|---|---|---|---|
| MHT recommendation | 111 (52.8%) | 48 (22.9%) | 47 (22.4%) | 4 (1.9%) |
| Actual treatment received (including crossover patients) | 110 (52.4%) | 47 (22.4%) | 50 (23.8%) | 3 (1.4%) |
| Adherence to MHT recommendation | 96.4% (107/111) | 89.6% (43/48) | 87.2% (41/47) | 75.0% (3/4) |
| STS-PROM | 2.02 ± 1.6 | 4.83 ± 3.4 | 2.61 ± 2.1 | 1.65 ± 1.3 |
| 30-day outcomes | ||||
| Length of stay (days) | 7 [6, 11] | 6 [4, 10] | 5 [4, 9] | 6 [5, 10] |
| Stroke | 1 (0.9%) | 1 (2.1%) | 1 (2.0%) | 0 (0%) |
| Dialysis requirement | 1 (0.9%) | 1 (2.1%) | 1 (2.0%) | 0 (0%) |
| Myocardial infarction | 0 (0%) | 1 (2.1%) | 0 (0%) | 0 (0%) |
| Readmission | 9 (8.2%) | 10 (21.3%) | 2 (4.0%) | 1 (25%) |
| Mortality | ||||
| 30-day | 2 (1.8%) | 3 (6.4%) | 4 (8.0%) | 0 (0.0) |
| 1 year | 4 (3.6%) | 13 (27.7%) | 5 (10%) | 1 (33.3%) |
| 2 years | 7 (6.4%) | 19 (40.4%) | 10 (20%) | 1 (33.3%) |
Categorical data are described as n (%). Continuous data are described as mean ± standard deviation or median [interquartile range]. CABG indicates coronary artery bypass graft; MHT, multidisciplinary heart team; OMT, optimal medical therapy; PCI, percutaneous coronary intervention; STS-PROM, Society of Thoracic Surgeons isolated coronary artery bypass grafting risk of mortality score.
Interventional details of revascularization procedures
Of the 110 CABG procedures performed, 351 vessels were bypassed, with the left anterior descending artery being treated in all but two cases (98.2%). The internal mammary artery was utilized in 107 cases (97.3%) and the radial artery in 21 cases (19.1%). Dual arterial grafting was performed in 19 cases (17.3%), with the radial artery used alone in two cases (1.8%). Nine procedures (8.2%) were off-pump, and 9 were redo CABG procedures (8.2%). Additional mechanical circulatory support was occasionally required, with the use of an intra-aortic balloon pump in 2 cases (1.8%) and postoperative extracorporeal membrane oxygenation in 1 case (0.9%) (Table 4).
Table 4.
Revascularization strategies
| Percutaneous coronary intervention | Isolated PCI (N = 47) |
|---|---|
| Vessel treated | 71 |
| LMCA | 14 (29.8) |
| LAD | 29 (61.7) |
| LCX | 12 (25.5) |
| Ramus intermedius | 1 (2.1) |
| RCA | 5 (10.6) |
| OM1 | 2 (4.3) |
| OM2 | 2 (4.3) |
| Diagonal 1 | 6 (12.8) |
| Balloon angioplasty | 238 (506.4) |
| No. stents implanted (all DES) | 80 (170.2) |
| IVUS (cases used) | 23 (48.9) |
| Shockwave | 5 (10.6) |
| Rotational atherectomy | 1 (2.1) |
| Thrombectomy | 1 (2.1) |
| Staged | 8 (17.0) |
| Impella-assisted | 6 (12.8) |
| Hybrid |
3 (6.4) |
|
Coronary artery bypass grafting
|
Isolated CABG (N = 110)
|
| Vessel bypassed | 351 |
| LAD | 108 (98.2) |
| LCX | 2 (1.8) |
| Ramus | 26 (23.6) |
| RCA | 29 (26.4) |
| OM | 80 (72.7) |
| Diagonal | 35 (31.8) |
| PDA | 53 (48.2) |
| PLB | 18 (16.4) |
| Bypass conduit | |
| Arterial graft | 128 (116.4) |
| IMA | 107 (97.3) |
| Radial | 21 (19.1) |
| Venous graft | 223 (202.7) |
| Off-pump | 9 (8.2) |
| IABP | 2 (1.8) |
| ECMO (postoperatively) | 1 (0.9) |
| Redo CABG | 9 (8.2) |
Categorical data are described as n (%). CABG indicates coronary artery bypass grafting; DES, drug-eluting stent; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; IMA, internal mammary artery; IVUS, intravascular ultrasound; LAD, left anterior descending artery; LCX, left circumflex artery; LMCA, left main coronary artery; OM, obtuse marginal artery; PCI, percutaneous coronary intervention; PDA, posterior descending artery; PLB, posterolateral branch; RCA, right coronary artery.
Among the 47 PCI procedures, 71 vessels were treated with 80 drug-eluting stents and 238 balloon angioplasties. The left anterior descending artery was the most frequently treated vessel (61.7%), followed by the left main coronary artery (29.8%). Intravascular ultrasound was utilized in 23 cases (48.9%), and additional revascularization techniques including shockwave lithotripsy (5 cases; 10.6%), rotational atherectomy (1 case; 2.1%), and thrombectomy (1 case; 2.1%) were employed as needed. Eight cases (17%) were staged, and 6 (12.8%) required Impella support.
As-treated morbidity and mortality outcomes
The median length of stay was 6 [5, 11] days for the entire cohort, broken down as 7 [6, 11] days for CABG, 6 [4, 10] days for PCI, 6 [5, 10] for hybrid, and 5 [4, 9] for OMT (Table 3). Stroke and new dialysis requirements each occurred in one patient from the CABG, PCI, and OMT cohorts, respectively. One patient in the PCI group experienced a nonfatal myocardial infarction at postprocedure day 19. The 30-day readmission rate was 10.5% overall, with cohort-specific rates of 8.1% (CABG), 20.8% (PCI), 25% (hybrid), and 4.3% (OMT).
Over a median follow-up of 24 [4.7, 33.9] months, 25 patients were lost to follow-up by 30 days and 71 by 1 year. The overall 30-day mortality was 4.3% (9/210), comprising 1.8% (2/110) for CABG, 6.4% (3/47) for PCI, and 8.0% (4/50) for OMT (Table 3). The average STS-PROM scores for patients who died within 30 days were 23.4% ± 11.1% for CABG, 7.1% ± 4.0% for PCI, and 8.2% ± 3.2% for the OMT group, yielding an observed-to-expected (O:E) ratio of 0.9 for CABG. The average NCDR mortality risk was 2.1% in the PCI subgroup, resulting in an O:E ratio of 3.0.
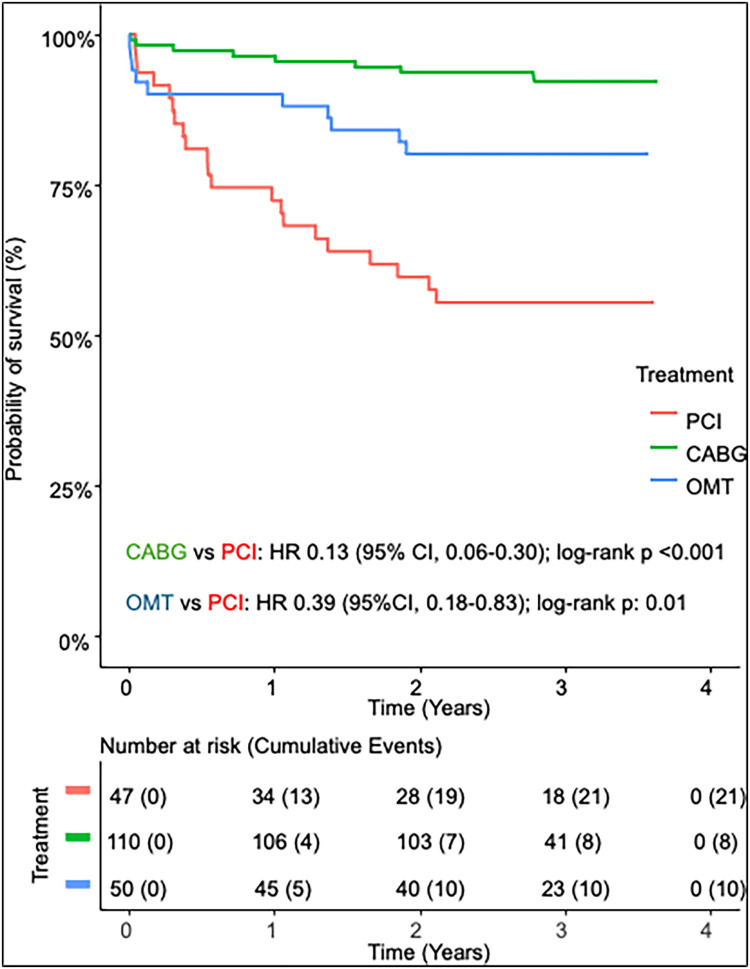
At 1 year, overall mortality rate was 11.0% (23/210), comprising 3.6% (4/110) for CABG, 27.7% (13/47) for PCI, 10.0% (5/50) for OMT, and 33.3% (1/3) for the hybrid group. By 2 years, overall mortality reached 17.6% (37/210), with 6.4% (7/110) for CABG, 40.4% (19/47) for PCI, 20.0% (10/50) for OMT, and 33.3% (1/3) for hybrid (Figure 2). Kaplan-Meier survival analysis indicated a significant survival difference between the CABG and PCI cohorts (hazard ratio 0.13, 95% confidence interval 0.06–0.30, P < 0.001) as well and for OMT compared with PCI (hazard ratio 0.39, 95% confidence interval 0.18–0.83, P = 0.01) in this study population.
Figure 2.
Kaplan-Meier survival analysis for the coronary artery bypass grafting (CABG), percutaneous coronary intervention (PCI), and optimal medical therapy (OMT) cohorts.
Among the 16 patients whose treatment deviated from the MHT recommendations, 30-day, 1-year, and 2-year mortality was higher than in patients who followed recommendations, although these differences were not statistically significant (12.5% vs 7.4% [P = 0.50], 18.8% vs 21.3% [P = 0.82], and 25% vs 35.1% [P = 0.43], respectively) (Table 2).
DISCUSSION
Although a variety of institutional approaches to heart team implementation have been described, the existing literature largely supports the hypothesis that a collaborative, multidisciplinary approach enhances decision-making and may improve outcomes.11–13,19,20 While the true effect of the MHT model is difficult to quantify due to significant heterogeneity of the high-risk patient populations, their presentation, and unmeasured confounders, this retrospective analysis highlighted several key findings that clarify its clinical impact. First, our data demonstrate a high rate of adherence to MHT recommendations. Second, despite elevated surgical risk profiles, CABG was the most frequently recommended therapy (52.9%) and showed a lower-than-expected 30-day mortality (O:E ratio 0.9), along with superior 1- and 2-year survival compared with PCI and OMT. Third, those who underwent PCI experienced a higher O:E ratio as well as high short- and long-term mortality rates. Fourth, those who received medical therapy alone had high rates of early mortality, which then stabilized in the long term.
Our analysis revealed a high adherence rate of 91.5% to MHT recommendations, with CABG patients exhibiting notably lower-than-expected early mortality (O:E ratio, 0.9). Not only was CABG the most frequently recommended therapy (52.8%), it also showed the lowest 1-year (3.6%) and 2-year (6.4%) mortality among the treatment groups. This suggests that, despite the high-risk nature of these patients, the MHT model likely facilitated improved patient selection as well as preoperative optimization, contributing to improved survival in this subset. These patients also likely experienced a survival benefit due to complete revascularization afforded by CABG, as the majority of patients presented with multivessel disease.21–23 This finding is further contextualized by the 5-year results of the EXCEL and SYNTAX trials, which demonstrated that CABG offers superior event-free survival compared to PCI in left main and multivessel disease.9,10 Moreover, the low incidence of perioperative stroke, dialysis requirements, myocardial infarction, and 30-day readmissions in this cohort highlights the potential benefits of multidisciplinary evaluation, optimization, and management in achieving favorable and durable outcomes in these high-risk patients.
Despite the proposed strengths of the MHT approach, it is not without limitations. Recommendations are inherently influenced by institutional expertise, resource availability, and subjective clinical judgment, which may lead to added variability. Furthermore, while the MHT structure described enhanced evaluation, it cannot fully mitigate residual confounding or predict long-term outcomes in a heterogeneous, high-risk population. This was particularly evident in the high-risk PCI cohort, as noted by the significantly higher mortality rates (27.7% at 1 year, 40.4% at 2 years) compared to the CABG and OMT cohorts. The PCI patients in this study represented a particularly high-risk group, as indicated by their notably higher average STS-PROM scores (4.83%) and NCDR risk of inpatient mortality of 2.1%. As many of these patients were considered inoperable or at prohibitively high risk for surgery, many likely received PCI with a palliative rather than curative intent. This high rate of mortality was also likely secondary to incomplete revascularization and a high residual SYNTAX score.24,25 This may explain why the O:E ratio was higher in the PCI cohort (3.0), reflecting the inherent risk in this patient population rather than an MHT failure. Given the high rate of mortality in this subgroup, even with palliative intent, it may be prudent to reassess the role of PCI in such patients, recognizing that a more liberal application of medical therapy alone could potentially offer a comparable quality of life, minimize procedural risks, and ideally align with patient-centered goals of care in advanced disease settings. While our findings highlight the limitations of PCI in a cohort of high-risk patients, additional investigation is needed to more effectively identify which subsets might still benefit from PCI over medical therapy alone. Well-powered, prospective randomized trials or propensity‐matched analyses may help define the clinical or anatomical predictors of PCI success or futility.
Apart from procedural feasibility and the overall tolerability of the patient, the decision to pursue medical therapy alone versus an invasive approach relied heavily on aligning treatment strategy with patient-centered goals. Critical questions addressed during heart team discussions included: What outcomes are most important to the patient? What specific life activities do they wish to maintain or regain? While life-or-death considerations are paramount, it is important to examine closely the patient’s priorities and goals of care in these discussions. The palliative care specialists played a valuable role in facilitating these challenging conversations with patients and their families. Their involvement augmented the comprehensive approach to care taken by the heart team and its efforts to improve patient satisfaction and minimize interventions inconsistent with the patients’ goals of care.26,27
While the MHT is a valuable tool for guiding initial treatment decisions, its recommendations are dependent on the clinical status at the time of presentation. As such, it is important to remain adaptable and continuously reassess the patient’s evolving clinical course, which may dictate a change in treatment strategy. As noted in this study, the most significant deviation from the recommended therapy was observed among patients initially allocated to medical therapy. As a result of guideline-directed medical therapy, six patients improved sufficiently to undergo PCI (3) or CABG (3). Conversely, two patients scheduled for PCI deteriorated and died prior to performing the planned intervention. Driven in part by the two inpatient mortalities, treatment that differed from MHT recommendations had numerically higher 30-day, 1-year, and 2-year mortality, although these differences were not statistically significant (P = 0.58, 1.0, and NA, respectively).
The MHT model employed at our institution is unique and may not be easily replicated at other institutions due to differences in patient volume, resource availability, or access to subspecialists. Nevertheless, we continue to endorse the implementation of the MHT model, as its principles remain applicable across diverse healthcare settings. Institutions with limited resources can overcome these challenges by implementing streamlined MHT teams, utilizing virtual consultations with subspecialists at affiliated centers, and/or establishing structured referral pathways to high-volume centers, particularly in the setting of complex CAD.
This study’s single-center, retrospective design and absence of a contemporaneous or historical control limits the ability to make definitive inferences regarding the superiority of MHT-guided care over standard decision-making and limits the generalizability to institutions with different heart team configurations. Additionally, while we recognize the potential value of advanced statistical modeling, this study was not designed or powered to support adjusted comparisons between treatment strategies. While the morbidity and mortality outcomes provide meaningful data, they should be interpreted within the intended context, emphasizing the importance of multidisciplinary collaboration in optimizing patient outcomes through individualized, patient-specific care plans. Given the rarity of crossover, the limited number of patients precluded a meaningful comparison of mortality between those who received the recommended therapy and those who, despite the same recommendation, underwent an alternative strategy. Moreover, the observed differences in mortality between groups suggest that an intention-to-treat versus per-protocol (as-treated) analysis would not alter the overall conclusions. Finally, because not all complex CAD patients were captured (due to urgent clinical circumstances or logistical constraints), there is a potential for missing data. Future research, ideally involving larger multicenter cohorts or propensity-matched comparisons, is needed to more definitively characterize the impact of MHT-guided decision-making on long-term outcomes in high-risk CAD.
CONCLUSION
Our single-institution experience demonstrates that a formal MHT approach yields high adherence to treatment recommendations, better-than-expected CABG outcomes, and key insights into high-risk PCI decision-making. These findings reinforce the importance of collaborative, multidisciplinary evaluation, particularly in patients with prohibitively high risk, where procedural choices must be carefully balanced against survival expectations and patient-centered goals.
CONFLICT OF INTEREST
Dr. Potluri: Advisory board member, proctor, and speaker for Medtronic, Boston Scientific, Abbott, and Cordis; proctor and speaker for Edwards Lifesciences, Terumo, and AstraZeneca. Dr. Szerlip: Proctor, speaker, and consultant for Edwards Lifesciences; advisory board member, consultant, and proctor for Abbott Vascular; steering committee member for Medtronic; speaker for Boston Scientific. Dr. Al-Azizi: Proctor and consultant for Edwards Lifesciences; consultant and advisory board member for Medtronic; consultant for Boston Scientific; speaker bureau member. Dr. Harrington: Consultant for Abbott Laboratories and Maquet Cardiovascular; speaker for Artivion, Inc. and Medtronic, Inc.; speaker and advisory board member for Boston Scientific Corporation; speaker and proctor for Edwards Lifesciences Corporation. Dr. Brinkman: Teaching and travel support from Artivion; consultant for Bolton Medical, Inc., Maquet Cardiovascular, Medtronic, Terumo Medical Corporation, and W.L. Gore; advisory board member for Medtronic. Dr. Smith: Grant support recipient from Edwards Lifesciences; speaker honoraria recipient from Edwards Lifesciences, Abbott, and CryoLife. Dr. George: Speaker for Abiomed. Dr. DiMaio: Investment and ownership interests in HeartFlow, Inc. and Spectral MD. The remaining authors have no competing interests to declare.
References
- 1.Shahian DM, Jacobs JP, Badhwar V, et al. The Society of Thoracic Surgeons 2018 adult cardiac surgery risk models: part 1—background, design considerations, and model development. Ann Thorac Surg. 2018;105(5):1411–1418. doi: 10.1016/j.athoracsur.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien SM, Feng L, He X, et al. The Society of Thoracic Surgeons 2018 adult cardiac surgery risk models: part 2—statistical methods and results. Ann Thorac Surg. 2018;105:1419–1428. doi: 10.1016/j.athoracsur.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Castro-Dominguez YS, Wang Y, Minges KE, et al. Predicting in-hospital mortality in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2021;78(3):216–229. doi: 10.1016/j.jacc.2021.04.067. [DOI] [PubMed] [Google Scholar]
- 4.Holmes DR, Rich JB, Zoghbi WA, Mack MJ.. The heart team of cardiovascular care. J Am Coll Cardiol. 2013;61(9):903–907. doi: 10.1016/j.jacc.2012.08.1034. [DOI] [PubMed] [Google Scholar]
- 5.Burlacu A, Covic A, Cinteza M, Lupu PM, Deac R, Tinica G.. Exploring current evidence on the past, the present, and the future of the heart team: a narrative review. Cardiovasc Ther. 2020;9241081–9241088. doi: 10.1155/2020/9241081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batchelor WB, Anwaruddin S, Wang DD, et al. The multidisciplinary heart team in cardiovascular medicine: current role and future challenges. JACC Adv. 2023;2(1):100160. doi: 10.1016/j.jacadv.2022.100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neumann F-J, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 8.Lawton JS, Tamis-Holland JE, Bangalore S, et al. ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;e18–e114. doi: 10.1161/CIR.0000000000001038. [DOI] [PubMed] [Google Scholar]
- 9.Serruys PW, Morice M-C, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 10.Stone GW, Kappetein AP, Sabik JF, et al. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med. 2019;381(19):1820–1830. doi: 10.1056/NEJMoa1909406. [DOI] [PubMed] [Google Scholar]
- 11.Bonzel T, Schächinger V, Dörge H.. Description of a heart team approach to coronary revascularization and its beneficial long-term effect on clinical events after PCI. Clin Res Cardiol. 2016;105(5):388–400. doi: 10.1007/s00392-015-0932-2. [DOI] [PubMed] [Google Scholar]
- 12.Chu D, Anastacio MM, Mulukutla SR, et al. Safety and efficacy of implementing a multidisciplinary heart team approach for revascularization in patients with complex coronary artery disease: an observational cohort pilot study. JAMA Surg. 2014;149(11):1109–1112. doi: 10.1001/jamasurg.2014.2059. [DOI] [PubMed] [Google Scholar]
- 13.Leonardi S, Marino M, Crimi G, et al. APpropriAteness of percutaneous Coronary interventions in patients with ischaemic HEart disease in Italy: the APACHE pilot study. BMJ Open. 2017;7(9):e016909. doi: 10.1136/bmjopen-2017-016909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez CE, Dota A, Badhwar V, et al. Revascularization heart team recommendations as an adjunct to appropriate use criteria for coronary revascularization in patients with complex coronary artery disease. Catheter Cardiovasc Interv. 2016;88(4):E103–E112. doi: 10.1002/ccd.26276. [DOI] [PubMed] [Google Scholar]
- 15.Tsang MB, Schwalm JD, Gandhi S, et al. Comparison of heart team vs interventional cardiologist recommendations for the treatment of patients with multivessel coronary artery disease. JAMA Netw Open. 2020;3(8):e2012749. doi: 10.1001/jamanetworkopen.2020.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young MN, Kolte D, Cadigan ME, et al. Multidisciplinary heart team approach for complex coronary artery disease: single center clinical presentation. J Am Heart Assoc. 2020;9(8):e014738. doi: 10.1161/JAHA.119.014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potluri S, Sawhney R, Dorton C, et al. Institution of a formal multidisciplinary heart team for high-risk coronary revascularization. Proc (Bayl Univ Med Cent). 2025;38(1):28–33. doi: 10.1080/08998280.2024.2426925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wooley J, Neatherlin H, Mahoney C, et al. Description of a method to obtain complete one-year follow-up in the Society of Thoracic Surgeons/American College of Cardiology transcatheter valve therapy registry. Am J Cardiol. 2018;121(6):758–761. doi: 10.1016/j.amjcard.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 19.Yamasaki M, Abe K, Horikoshi R, et al. Enhanced outcomes for coronary artery disease obtained by a multidisciplinary heart team approach. Gen Thorac Cardiovasc Surg. 2019;67(10):841–848. doi: 10.1007/s11748-019-01108-4. [DOI] [PubMed] [Google Scholar]
- 20.Patterson T, McConkey HZR, Ahmed-Jushuf F, et al. Long‐term outcomes following heart team revascularization recommendations in complex coronary artery disease. J Am Heart Assoc. 2019;8(8):e011279. doi: 10.1161/JAHA.118.011279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Habib RH, Dimitrova KR, Badour SA, et al. CABG versus PCI. J Am Coll Cardiol. 2015;66(13):1417–1427. doi: 10.1016/j.jacc.2015.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng S, Li M, Fei J, et al. Ten-year outcomes after percutaneous coronary intervention versus coronary artery bypass grafting for multivessel or left main coronary artery disease: a systematic review and meta-analysis. J Cardiothorac Surg. 2023;18(1):54. doi: 10.1186/s13019-023-02101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirov H, Caldonazo T, Riedel LL, et al. Comparing outcomes between coronary artery bypass grafting and percutaneous coronary intervention in octogenarians with left main or multivessel disease. Sci Rep. 2023;13(1):22323. doi: 10.1038/s41598-023-49069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia S, Sandoval Y, Roukoz H, et al. Outcomes after complete versus incomplete revascularization of patients with multivessel coronary artery disease. J Am Coll Cardiol. 2013;62(16):1421–1431. doi: 10.1016/j.jacc.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 25.Ali ZA, Garcia JJ, Karimi Galougahi K, et al. Impact of incomplete revascularization after PCI in left main disease: the EXCEL trial. Circ Cardiovasc Interv. 2024;17(3):e013192. doi: 10.1161/CIRCINTERVENTIONS.123.013192. [DOI] [PubMed] [Google Scholar]
- 26.Robbins-Welty GA, Webb JA, Shalev D, et al. Advancing palliative care integration in hematology: building upon existing evidence. Curr Treat Options Oncol. 2023;24(5):542–564. doi: 10.1007/s11864-023-01084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Remawi BN, Gadoud A, Preston N.. The experiences of patients with advanced heart failure, family carers, and health professionals with palliative care services: a secondary reflexive thematic analysis of longitudinal interview data. BMC Palliat Care. 2023;22(1):115. doi: 10.1186/s12904-023-01241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]