Abstract
Background
During pulmonary arterial hypertension (PAH), cardiac cells develop a hypertrophic and apoptosis-resistant phenotype. Mesenchymal stromal cell (MSC) therapy has been shown to mitigate pulmonary vascular remodeling in PAH; however, successful application is limited by low potency and the need for a high number of MSCs. MSCs exposed to hypoxia release more extracellular vesicles (EV)s with different content than normoxia. We aimed to evaluate the proteomic profile and therapeutic effects of EVs derived from normoxia- and hypoxia-preconditioned MSCs on cardiac tissue remodeling in experimental PAH.
Methods
Isolated bone marrow MSCs were subjected to normoxia (N, 21%O2) or hypoxia (H, 1%O2) for 48 h and EVs were collected from the MSCs by ultracentrifugation. Proteomic data of the EVs were reanalyzed using PatternLab for Proteomics 5.0. Thirty-two male Wistar rats were randomly assigned to PAH plus intraperitoneal monocrotaline (60 mg/kg) or control (CTRL) with saline. On day 14, PAH animals received saline (1 mL/kg; PAH-SAL), EV-N (EVs from 1 × 106 MSCs; PAH-EV-N) or EV-H (EVs from 1 × 106 MSCs; PAH-EV-H) by jugular vein. On day 28, right ventricular systolic pressure (RVSP), pulmonary acceleration time/pulmonary ejection time (PAT/PET) ratio, right ventricle (RV) outflow diameter, and right ventricular hypertrophy (RVH) index were evaluated. The heart was harvested for histologic and molecular biology analyses.
Results
Among 695 proteins identified, 203 were present only in EV-H and 51 in EV-N. EV-H was enriched in proteins involved in the negative regulation of mitogen-activated protein kinase and apoptosis pathways. On day 28, both EV-N and EV-H therapies decreased RVSP compared with PAH-SAL (32 ± 5 mmHg and 29 ± 4 mmHg versus 39 ± 2 mmHg; p < 0.01). Only EV-H increased PAT/PET, reduced RV outflow diameter, and the RVH index compared with PAH-SAL. The expressions of c-Myc, a marker of myocardial injury, and p-GSK3β-Ser9, a proliferative marker, were higher in the PAH-SAL group than in the CTRL group. EV-N and EV-H decreased c-Myc expression, but only EV-H significantly reduced p-GSK3β-Ser9.
Conclusion
EV-N and EV-H reduced RVSP, but only EV-H improved RVH and RV outflow diameter, increased the PAT/PET ratio, and downregulated GSK3β protein levels. EVs from hypoxia-preconditioned MSCs demonstrated greater cardioprotective effects than those from normoxia-conditioned MSCs.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13287-025-04604-y.
Keywords: Extracellular vesicles, Mesenchymal stromal cells, Hypoxic pre-conditioning, Cardiac remodeling, Mass spectrometry, Proteomic profile, Ventricle hypertrophy, Monocrotaline
Introduction
Pulmonary arterial hypertension (PAH) is a progressive disease characterized by sustained vasoconstriction and excessive vascular remodeling, leading to a reduced cross-sectional area of the pulmonary arteries and increased mean pulmonary arterial pressure [1]. The resulting right ventricle (RV) afterload increases shear stress on the cardiac wall, inducing cardiomyocyte hypertrophy via intracellular signaling pathways such as mitogen-activated protein kinase and PI3K/Akt [2, 3]. Over time, chronic RV overload and macrophage infiltration contribute to inflammation and apoptosis, driving cardiac tissue remodeling and eventual heart failure [4, 5]. Current pharmacologic treatments focus primarily on vasodilation with limited effects on vascular remodeling [6]. Mesenchymal stromal cells (MSCs) have demonstrated immunomodulatory and tissue repair properties in preclinical models, reducing inflammation, vascular remodeling, and RV hypertrophy [7–10]. However, limitations such as low cell survival, production variability, and the need for high cell doses (potentially increasing the risk of thromboembolism) hinder their clinical application [11–14]. Consequently, extracellular vesicles (EVs) have emerged as a promising cell-free alternative due to their small size, efficient distribution, and potential for long-term cryopreservation [15–17]. Bone marrow-derived mesenchymal stromal cell (BM-MSC) culture, under conventional conditions, does not replicate this physiologic niche, which has low-oxygen tension [18], potentially limiting their therapeutic efficacy [19, 20]. Hypoxic pre-conditioning enhances BM-MSC function by upregulating HIF-1α and modulating the genes involved in metabolism, inflammation, proliferation, and apoptosis [21, 22]. Previous studies have shown that hypoxia (1% O2 for 48 h) increases BM-MSC viability, enhances EV release, and enriches proteins related to immune regulation and extracellular matrix production [23]. Moreover, EVs from hypoxia-preconditioned MSCs have improved cardiac function and reduced infarct size in ischemic models [24].
Despite these findings, the impact of MSC-derived EVs on cardiac remodeling in PAH remains largely unexplored, even though heart failure is the leading cause of mortality in patients with PAH [25]. We hypothesize that EVs derived from hypoxia-preconditioned MSCs may mitigate functional and structural cardiac alterations in experimental PAH. This study aimed to evaluate the proteomic profile and therapeutic effects of EVs derived from normoxia- or hypoxia-preconditioned MSCs on cardiac tissue remodeling in experimental PAH.
Methods
Study approval
The study protocol was approved by the Institutional Animal Care and Use Committee of the Health Sciences Center, Federal University of Rio de Janeiro (CEUA 043/22). All procedures complied with the Principles of Laboratory Animal Care and the US National Academy of Sciences Guide for the Care and Use of Laboratory Animals. The study followed the ARRIVE guidelines [26].
Culture of BM-MSCs and hypoxia challenge
BM-MSCs were obtained from healthy male Wistar rats (220 ± 10 g, 7 weeks) by flushing femurs and tibias with phosphate-buffered saline (PBS, pH 7,0). Samples were centrifuged (300 × g for 5 min) and the pellet was resuspended in Iscove’s modified Dulbecco’s medium (IMDM; Thermo Fisher Scientific, Waltham, MA, USA), supplemented with 1% penicillin/streptomycin (Thermo Fisher Scientific), 10% fetal bovine serum, and 10% horse serum (Thermo Fisher Scientific) [23]. BM-MSCs were cultured at 37 °C in a humidified incubator (5% CO2, 21% O2) and expanded to passage 4.
At passage 4 (confluence < 80%), BM-MSCs were exposed to normoxia (21% O2, 5% CO2, 74% N2) or hypoxia (1% O2, 5% CO2, 94% N2) for 48 h in serum-free IMDM to stimulate EV release. Hypoxic conditions were maintained using a water-jacketed CO2 incubator (Thermo Fisher Scientific). The culture medium was not changed during exposure [23].
Isolation and characterization of EVs
EVs were collected from BM-MSC-conditioned media after 48 h of serum starvation under normoxia (EV-N) and hypoxia (EV-H). The supernatant was centrifuged sequentially (300 × g, 20 min; 2000 × g, 30 min) to remove cells and debris, followed by ultracentrifugation at 100,000 × g for 2 h at 4 °C (Optima XE-90, Beckman Coulter) [27]. The EV pellet was resuspended in PBS (pH 7.0) at a concentration equivalent to 1 × 10⁶ MSCs per 200 µL [23] and EVs from all four biological replicates were combined to achieve a total of 1 × 10⁹ particles per treatment.
Nanoparticle tracking analysis was performed to determine EV size and concentration (Nanosight 3.4, Malvern Panalytical). Each condition (n = 4) was measured 3 times (30 s per measurement with 25 frames per seconds) at 25 °C with a viscosity of 0.9 cP [28].
EV morphology was analyzed using transmission electron microscopy (JEOL 1200) operating at 80 kV. EVs were deposited on Formvar-coated cupper grids for 3 h, fixed in 4% formaldehyde for 30 min, washed in Milli-Q water, and stained with 3% methylcellulose/4% uranyl acetate (9:1) for 10 min on ice [29].
EV surface markers were identified via flow cytometry. EVs (50 µL) were incubated with 25 µL aldehyde/sulfate-latex beads (4 μm; Invitrogen #A37304) for 20 min, followed by overnight incubation with FACS buffer (PBS supplemented with 0.1% BSA and 0.01% NaN3) at 4 °C with shaking. EVs were stained for CD9 (1:200 dilution; APC #124812), CD63 (1:200 dilution; PE #143904), CD81 (1:200 dilution; PE #104905), and CD90 (1:500 dilution; FITC #206106) (BioLegend) for 45 min at 4 °C. Data were acquired on a FACS Canto II and analyzed using Flowing Software 2.5.1 [30].
Proteomic data analysis
Raw proteomic data [23] were obtained from the ProteomeXchange Consortium (ID: PXD027718) and reanalyzed using PatternLab for Proteomics 5.0 (http://www.patternlabforproteomics.org/). Peptide identification was performed with the extracted ion chromatography method, using the UniProt Rattus norvegicus database (ID: UP000002494; taxonomy: 10116 downloaded November 1st, 2024) [31]. Extracted ion chromatograms were assessed using the following search parameters: 2 minimum number of unique peptides, 1 minimum number of peptides, 3 minimum number of MS1 count, up to 360 s retention time, 1000 top-n peptides, and 1.2 labels. A false discovery rate (FDR) of up to 1% was accepted. Sequence lengths shorter than 6 amino acid residues were eliminated. Proteins in the final list were grouped following maximum parsimony criteria. Only proteins identified in at least 2 samples per analysis group were considered. Proteins were classified based on exclusive allocation or upregulation (p < 0.05). Functional enrichment was assessed using Gene Ontology, PANTHER, and Metascape, with pathway analysis performed using KEGG and Reactome [32]. A hypergeometric test and Benjamini-Hochberg correction were applied. Network visualization was done in STRING and Cytoscape, with hierarchical clustering in MetaboAnalyst [33].
Animal preparation and experimental protocol
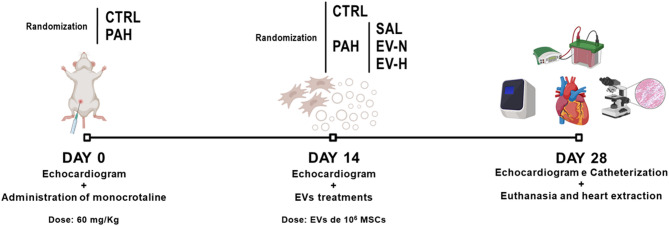
Animals were housed at 23 °C under a 12 h light-dark cycle with access to food and water ad libitum. Thirty-two male Wistar rats (160 ± 200 g, 7 weeks) were randomized to receive an intraperitoneal injection of monocrotaline (MCT, 60 mg/kg; C2401, Sigma-Aldrich, St Louis, MO, USA) (PAH group, n = 24) or saline (CTRL group, n = 8) [34, 35]. On day 14, PAH animals (n = 24) were anesthetized with midazolam (2 mg/kg; Cristália, São Paulo, Brazil) and ketamine (75 mg/kg; Cristália). They were randomly assigned via sealed envelopes to 1 of 3 treatment groups, receiving intravenous injections through the jugular vein: (1) saline (PAH-SAL, n = 8); (2) EVs from MSCs under normoxia (PAH-EV-N, n = 8); and (3) EVs from MSCs under hypoxia (PAH-EV-H, n = 8). The PAH-EV-N and PAH-EV-H groups received EVs derived from 1 × 106 MSCs exposed to normoxia or hypoxia, respectively. On day 28, cardiovascular parameters were assessed, animals were euthanized by exsanguination, and the heart was collected for postmortem analyses (Fig. 1) [34]. The depth of anesthesia was evaluated based on pupil diameter, light response, nictitating membrane position, and reaction to tail stimulation. Experiments commenced when responses to noise (handclap), whisker stimulation, and tail clamp were absent.
Fig. 1.
Experimental protocol. CTRL, control; EV-H, extracellular vesicles from mesenchymal stromal cells subjected to hypoxia; EV-N, extracellular vesicles from mesenchymal stromal cells subjected to normoxia; EVs, extracellular vesicles; MCT, monocrotaline; MSCs, mesenchymal stromal cells; PAH, pulmonary arterial hypertension; SAL, saline. Monocrotaline dose, 60 mg/kg; extracellular vesicle dose, originating from 1 × 106 mesenchymal stromal cells
Echocardiography
Echocardiographic assessments were performed on days 1, 14, and 28 to evaluate the progression of PAH (Fig. 2). A blinded expert (NNR) conducted transthoracic echocardiography using a UGEO HM70A system (Samsung, São Paulo, Brazil) with an 8- to 13-MHz linear phased-array probe. Images were acquired from the parasternal long and short axes, and RV outflow diameter, pulmonary arterial acceleration time (PAT) and pulmonary artery ejection time (PET) were measured via pulsed-wave Doppler. The PAT/PET ratio served as an indirect PAH index [36, 37]. Measurements adhered to guidelines from the American Society of Echocardiography and the European Association of Cardiovascular Imaging [38].
Fig. 2.
Echocardiographic representative images and functional parameters on day 28. (A) Upper row shows pulmonary artery Doppler and the lower row displays the diameter of the right ventricle outflow tract obtained in the long axis; (B) PAT/PET ratio; (C) RV outflow diameter measured via echocardiography on day 28 of the experimental protocol; and (D) right ventricular hypertrophy index calculated as the ratio of the right ventricular weight to the combined weight of the left ventricle and septum (RV/LV + S), adjusted for body weight. Yellow outline, peak blood flow in the pulmonary artery; yellow connector, right ventricle outflow diameter. CTRL, control; EV-H, extracellular vesicles from mesenchymal stromal cells subjected to hypoxia; EV-N, extracellular vesicles from mesenchymal stromal cells subjected to normoxia; PAH, pulmonary arterial hypertension; PAT, pulmonary acceleration time; PET, pulmonary ejection time; SAL, saline; n = 8 per group. Comparisons were done using one-way ANOVA followed by the Tukey multiple comparison test (p < 0.05); *significance versus CTRL, #significance versus PAH-SAL, &significance versus PAH-EV-N. Data are presented as means ± standard deviation
Hemodynamic measurements
On day 28, right ventricular systolic pressure (RVSP) was recorded using a heparinized 19G scalp vein winged infusion set connected to a physiologic data acquisition system and analyzed using LabVIEW software (National Instruments, Austin, TX, USA). Anesthetized animals (midazolam 2 mg/kg, ketamine 75 mg/kg) were placed in dorsal recumbency, and a 19G scalp needle was inserted into the RV via thoracotomy [34]. A blinded investigator (RTS) validated real-time systolic pressure curves. Euthanasia was performed via exsanguination through an abdominal aorta transection.
Right ventricular hypertrophy index
After euthanasia, hearts were dissected, and right ventricular hypertrophy (RVH) was assessed using the Fulton index [39], calculated as the RV weight divided by the sum of the left ventricle (LV) and septum (S) weights (RV/LV + S). Weights were normalized to body weight.
Histologic analysis
RV fragments were fixed in 10% formaldehyde, embedded in paraffin, and cut into 4-µm-thick sections. Sections were stained with hematoxylin-eosin [40, 41] and scanned using a MoticEasyScan Pro 6 scanner (Motic, Hong Kong, China). Immune cells were quantified in histologic images, normalized by tissue area, and expressed as cells per unit area. Cellularity was evaluated in 10 random fields per slide using QuPath software (version 0.5.0; GitHub) [42] by a blinded investigator (RTS).
Immunohistochemistry
Paraffin-embedded RV sections were deparaffinized, rehydrated, and treated with 50 mM ammonium chloride to block free aldehyde residues. Endogenous peroxidase activity was inhibited using 3% hydrogen peroxide. Antigen retrieval was performed in 10 mM sodium citrate at 96 °C. Slides were washed in PBS (pH 7,0) with 0.05% Tween-20 (Sigma) and blocked with rat liver homogenate and serum-free protein block (Dako, Carpinteria, CA, USA). Primary antibodies were incubated overnight at 4 °C: CD68 (1:50 dilution; Bio-Rad #MCA341R), mannose receptor (1:100 dilution; Abcam #ab64693), inducible nitric oxide synthase (iNOS; 1:100 dilution; Sigma-Aldrich #ZRB1449), and cleaved caspase-3 (1:100 dilution; Bioss #bsm-33199 M). Secondary antibody Histofine Simple Stain MAX PO Multi (Nichirei Biosciences #414191F) and 3,3′-diaminobenzidine tetrahydrochloride (Dako Omnis #K346811-2) were applied, followed by hematoxylin counterstaining. Whole RV sections were scanned (MoticEasyScan Pro 6), and positive staining was quantified in 10 random fields at 400× magnification using ImagePro Plus software (version 4.0; Media Cybernetics). Data were normalized to tissue area and expressed as a percentage. A blinded investigator (RTS) quantified CD68, iNOS, and cleaved caspase-3 labeling, and another blinded investigator (RGV) quantified mannose receptor labeling.
Western blotting
RV fragments were homogenized in 10 mM HEPES-Tris buffer (Sigma-Aldrich) containing 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and a protease inhibitor cocktail (1:500; Sigma-Aldrich #I3786). Samples were centrifuged at 300 × g for 10 min at 4 °C. Protein concentrations were determined using the Lowry method [43]. Then, 60 µg of protein per sample was resolved via SDS-PAGE for 60 min at 180 V and transferred onto polyvinylidene fluoride membranes in Tris-glycine buffer for 60 min at 380 A. Membranes were blocked with 5% skimmed milk in Tris-saline-Tween 20 buffer for 60 min at room temperature on an orbital shaker (TS-2000ª VDRL Shaker; BiomiXer). Primary antibodies were incubated overnight at 4 °C: p-GSK3β-Ser9 (Cell Signaling #9322), and GSK3β (Invitrogen #MA5-15597). Secondary antibody anti-rabbit IgG (1:1000 dilution; Signaling #7074) were incubated for 60 min. GAPDH (1:1000 dilution; Cell Signaling #2118L) was used as an internal control.
Detection was performed with ECL Plus (G&E Healthcare, Chicago, Illinois, EUA) and images were acquired with an ImageQuant LAS 4000 (G&E Healthcare). Each image was quantified in ImageJ software (version Java 1.8.0_345 64bit), taking as a parameter the pixel density per area. The results were expressed in arbitrary units. In cases of proteins with phosphorylated residues, result was obtained by the ratio of the phosphorylated protein and total protein expression. A blinded investigator (DET) quantified p-GSK3β-Ser9 and GSK3β.
Reverse transcription polymerase chain reaction
Total RNA was extracted from heart tissue using the ReliaPrep RNA Tissue Miniprep System (Promega, Madison, WI, USA). cDNA was synthesized using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Gene expression was quantified using SYBR Green RT-PCR (Promega, Madison, WI, USA) on a QuantStudio 3 Real-Time PCR System (Thermo Fisher Scientific). Expression levels were normalized to the housekeeping gene 36B4 (acidic ribosomal phosphoprotein P0) and calculated using the 2−ΔΔCT method [44]. ΔCt was determined as Ct (target gene) − Ct (reference gene). An investigator (RTS), blinded to the group assignments, performed the analyses. The following primers were obtained from Invitrogen (Carlsbad, CA, USA): c-Myc: forward 5′-ACG GGC AGA CAC TTC TCA CT-3′, reverse 5′-GCT TCA AAT AAC GCG AGG AG-3′, and 36B4: forward 5′-CAA CCC AGC TCT GGA GAA AC-3′, reverse 5′-CAA CCC AGC TCT GGA GAA AC-3′.
Statistical analysis
The sample size was calculated based on a previous study [7] using an effect size of d = 1.60 for differences in RVSP between PAH and PAH-MSC. Assuming a power of 80% (1 − β = 0.8) and α = 0.05, a minimum of 8 animals per group was required. Calculations were performed using G*Power 3.1.9.2 (University of Düsseldorf, Germany).
Data normality was assessed using the Shapiro-Wilk test. The results are presented as means ± standard deviation. A Student’s t test was used to compare particle concentrations between the EV-N and EV-H groups. On days 1 and 14, the PAH-SAL and CTRL groups were compared using two-way ANOVA followed by Tukey’s multiple comparison test (p < 0.05). On day 28, comparisons of the CTRL, PAH-SAL, PAH-EV-H, and PAH-EV-N groups were conducted using one-way ANOVA with Tukey’s post hoc test (p < 0.05). Analyses were performed using GraphPad Prism (version 9.02; La Jolla, CA, USA).
Results
Characterization of EVs
EV surface markers showed that EV-N and EV-H was positive for CD9, CD63, CD81, and CD90 (Supplementary Fig. S1). Nanoparticle tracking analysis revealed a higher particle concentration in EV-H (5.1 × 109 ± 7.2 × 108 particles/mL) compared with EV-N (2.9 × 109 ± 1.5 × 108 particles/mL, p = 0.0278). No significant difference in EV mean size was observed, with 90% of particles measuring up to 340 nm in both groups (Supplementary Fig. S2). Transmission electron microscopy showed that EVs from both normoxia- and hypoxia-exposed MSCs exhibited heterogeneous subpopulations regarding size, morphology, and distribution, including vesicles with intracellular membranes, folds, or smaller luminal vesicles (Supplementary Fig. S3).
Proteomic analysis of EVs in hypoxic and normoxic conditions
Proteomic analysis identified 695 proteins in EVs (excluding contaminants): 203 unique to EV-H, 51 unique to EV-N, and 411 shared between both groups (venn diagram, Supplementary Fig. S4A and Supplementary Table S1). Differential expression analysis using PatternLab for Proteomics 5.0 software revealed 17 upregulated proteins in EV-H and 34 in EV-N (volcano plot, Supplementary Fig. S4B).
Enrichment analysis for biological processes and signaling pathways
Proteins detected in at least 2 samples per group (EV-H, 102; EV-N, 4) and differentially expressed proteins (EV-H, 17; EV-N, 34) were analyzed via Gene Ontology. A total of 119 proteins in EV-H and 38 in EV-N were evaluated (Supplementary Fig. S4C). EV-H was enriched for 146 biological processes, including 27 related to differentially expressed proteins and 72 exclusive to EV-H. EV-N had 131 processes, with 124 linked to differentially expressed proteins (Supplementary Table S2). All processes had an FDR < 0.05. Enrichment analysis via Metascape clustered terms with p < 0.01 and an enrichment factor > 1.5 (Supplementary Fig. S5A, B). Unique biological processes in EV-H included innate immune system, cellular responses to stimuli, cytoplasmic translational initiation, IMP biosynthesis process, complement and coagulation cascades, M phase, glutathione metabolism, cellular homeostasis, response to steroid hormone, and COPI-independent Golgi-to-endoplasmic reticulum retrograde traffic. EV-N-specific processes involved non-integrin membrane-extracellular matrix interactions, cardiac ventricle development, regulation of insulin-like growth factor transport and uptake insulin-like growth factor binding proteins, and translational elongation regulation. Shared processes included collagen biosynthesis and modifying enzimes, homeostasis, response to mechanical stimuli, regulation of actin dynamics in phagocytic cup formation, and RHOF GTPase cycling. A heatmap of the most significant terms per cluster is shown in Supplementary Fig. S5C. Biological processes and pathways related to cardiac hypertrophy and apoptosis were analyzed via KEGG and Reactome (Tables 1 and 2). Interaction network analysis of 20 EV-H proteins revealed 19 interactions (p = 6.63E − 05), and 11 EV-N proteins had 12 interactions (p = 1.36E − 06) (Supplementary Fig. S6). Notable pathways in EV-H included ERK inactivation (RNO-202670), MAPK negative regulation (RNO-5675221), and apoptosis (rno04210, RNO-109581). In EV-N, key pathways involved hypertrophic cardiomyopathy (rno05410) and MAPK2K/MAPK activation (RNO-5674135). Hierarchic clustering of 14 selected proteins showed 19 interactions and differential enrichment between EV-H and EV-N (Supplementary Fig. S7).
Table 1.
Biological processes and pathways found in the EV-H group
| Term ID | Term description | Gene count | FDR | Proteins |
|---|---|---|---|---|
| rno04261 | Adrenergic signaling in cardiomyocytes | 6 | 0.0056 | Mapk1, Camk2d, Cacna2d1, Atp2b1, Ppp2r1b, Mapk3 |
| rno04150 | mTOR signaling pathway | 5 | 0.0261 | Mapk1, Atp6v1b2, Atp6v1e1, Slc3a2, Mapk3 |
| RNO-202,670 | ERKs are inactivated | 3 | 0.0035 | Mapk1, Ppp2r1b, Mapk3 |
| RNO-5,673,001 | RAF/MAP kinase cascade | 7 | 0.0177 | Mapk1, Psmc5, Camk2d, Psmd13, Ppp2r1b, Psmd11, Mapk3 |
| RNO-5,674,499 | Negative feedback regulation of the MAPK pathway | 2 | 0.0197 | Mapk1, Mapk3 |
| RNO-5,675,221 | Negative regulation of the MAPK pathway | 3 | 0.0363 | Mapk1, Ppp2r1b, Mapk3 |
| RNO-1,257,604 | PIP3 activates AKT signaling | 6 | 0.0382 | Mapk1, Psmc5, Psmd13, Ppp2rb1, Psmd11, Mapk3 |
| GO:0001906 | Cell killing | 5 | 0.0187 | C9, F2, Gzmb, LOC103689983, Rpl30 |
| rno04210 | Apoptosis | 4 | 0.0485 | Mapk1, Ctsd, Gzmb, Mapk3 |
| RNO-109,581 | Apoptosis | 5 | 0.0093 | Mapk1, Kpnb1, Gsn, Gzmb, Mapk3 |
| RNO-109,606 | Intrinsic pathway for apoptosis | 3 | 0.0325 | Mapk1, Gzmb, Mapk3 |
Table 2.
Biological processes and pathways found in the EV-N group
| Term ID | Term description | Gene count | FDR | Proteins |
|---|---|---|---|---|
| mo05410 | Hypertrophic cardiomyopathy | 4 | 0.00082 | Actc1, Actg1, Actb, Tpm1 |
| mo05414 | Dilated cardiomyopathy | 4 | 0.00086 | Actc1, Actg1, Actb, Tpm1 |
| GO:0007507 | Heart development | 9 | 0.00041 | Col3a1, Actc1, Fn1, Ltbp1, Ap1b1, Cpe, Col11a1, Ap2b1, Tpm1 |
| GO:0003231 | Cardiac ventricle development | 5 | 0.0018 | Ltbp1, Cpe, Col11a1, Ap2b1, Tpm1 |
| GO:0055008 | Cardiac muscle tissue morphogenesis | 3 | 0.0367 | Actc1, Col11a1, Tpm1 |
| RNO-5,674,135 | MAP2K and MAPK activation | 3 | 0.0031 | Fn1, Actg1, Actb |
Echocardiography and hemodynamic measurements
On day 14, compared with day 0, the PAT/PET ratio was lower in the PAH-D14 group (0.31 ± 0.01) than in the PAH-D0 group (0.41 ± 0.01, p < 0.001), indicating PAH development (Supplementary Fig. S8A). RV outflow diameter was higher in the PAH-D14 group (0.33 ± 0.01 cm) compared with the PAH-D0 group (0.31 ± 0.01 cm, p < 0.001), which also serves as a marker for the development of PAH (Supplementary Fig. S8B).
On day 28, all PAH groups (PAH-SAL: 0.25 ± 0.01, PAH-EV-N: 0.27 ± 0.01, PAH-EV-H: 0.30 ± 0.01) had a significantly lower PAT/PET ratio than CTRL animals (0.41 ± 0.01, p < 0.001). However, the PAH-EV-H group (0.30 ± 0.01) had a higher PAT/PET ratio than the PAH-SAL group (0.25 ± 0.01, p < 0.001) and the PAH-EV-N group (0.27 ± 0.01, p = 0.014) (Fig. 2A, B). The RV outflow diameter was increased in the PAH-SAL group (0.35 ± 0.01 cm, p < 0.001) and the PAH-EV-N group (0.35 ± 0.01 cm, p < 0.001) compared with the CTRL group (0.32 ± 0.01 cm). Only the PAH-EV-H group (0.32 ± 0.004) showed a significant reduction compared with the PAH-SAL group (p < 0.001) and the PAH-EV-N group (p = 0.004) (Fig. 2A, C).
On day 28, RVSP was higher in the PAH-SAL group (39 ± 2 mmHg) than in the CTRL group (22 ± 1 mmHg, p < 0.001). The PAH-EV-N (31 ± 1 mmHg, p = 0.011) and PAH-EV-H (29 ± 1 mmHg, p < 0.001) groups had lower RVSP than the PAH-SAL group (Fig. 3).
Fig. 3.
Right ventricular systolic pressure (RVSP) measurements on day 28. (A) Representative curves and (B) graph of RVSP measurements. CTRL, control; EV-H, extracellular vesicles from mesenchymal stromal cells subjected to hypoxia; EV-N, extracellular vesicles from mesenchymal stromal cells subjected to normoxia; PAH, pulmonary arterial hypertension; SAL, saline; n = 8 per group. Statistical comparisons were performed using one-way ANOVA followed by the Tukey multiple comparison test (p < 0.05); *significance versus CTRL, #significance versus PAH-SAL. Data are presented as means ± standard deviation
Right ventricle hypertrophy
On day 28, the RVH index, measured by the RV/LV + S ratio, was higher in the PAH-SAL group (0.56 ± 0.03) than in the CTRL group (0.26 ± 0.01, p < 0.001). The PAH-EV-N group (0.39 ± 0.01) showed reduced RVH versus the PAH-SAL group (p < 0.001). The PAH-EV-H group (0.33 ± 0.01) had the lowest RVH index, significantly lower than the PAH-SAL (p < 0.001) and PAH-EV-N (p = 0.0031) groups (Fig. 2D).
The phosphorylation of glycogen synthase kinase 3 beta (GSK3β) at serine 9 residue (p-GSK3β-Ser9), a key protein in cardiac hypertrophy, was significantly higher in the PAH-SAL group (0.98 ± 0.04 a.u., p = 0.039), the PAH-EV-N group (2.31 ± 0.31 a.u., p < 0.001), and the PAH-EV-H group (1.30 ± 0.21 a.u., p = 0.004) compared with the CTRL group (0.16 ± 0.02 a.u.). The PAH-EV-N group exhibited the highest p-GSK3β-Ser9 level, significantly surpassing that in the PAH-SAL (p = 0.001) and PAH-EV-H (p = 0.009) groups (Fig. 4A, B). The relative expression of c-Myc, a proto-oncogene involved in cardiac hypertrophy, was significantly increased in the PAH-SAL group (3.03 ± 0.44) compared with the CTRL group (1.00 ± 0.07, p = 0.001) (Fig. 4C). Both treatment groups, PAH-EV-N (1.54 ± 0.11) and PAH-EV-H (1.15 ± 0.14), showed reduced c-Myc expression relative to the PAH-SAL group (p = 0.008 and p = 0.001, respectively).
Fig. 4.
GSK3β inhibition and c-Myc expression on day 28. (A) Representative western blot analysis; (B) graph showing densitometric expression of phosphorylated GSK3β (p-GSK3β) at serine 9 (Ser9) relative to total GSK3β. GAPDH are also shown. (C) Real-time polymerase chain reaction analysis of c-Myc expression in cardiac tissue. Relative gene expression was calculated as the ratio of average gene expression levels compared with the reference gene 36B4 and expressed as fold change relative to the CTRL group. CTRL, control; EV-H, MSC-derived extracellular vesicles subjected to hypoxia; EV-N, MSC-derived extracellular vesicles subjected to normoxia; PAH, pulmonary arterial hypertension; SAL, saline; n = 5 per group. Statistical comparisons were performed using one-way ANOVA followed by the Tukey multiple comparison test (p < 0.05); *significance versus CTRL, #significance versus PAH-SAL; &significance versus PAH-EV-N. Data are presented as means ± standard deviation
Inflammation
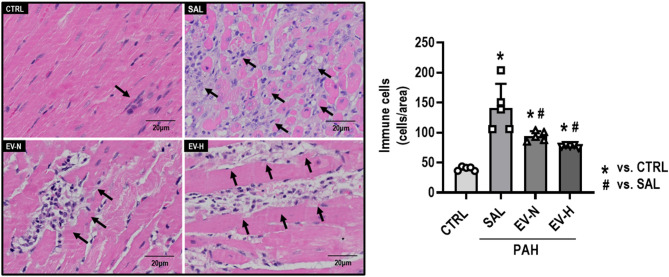
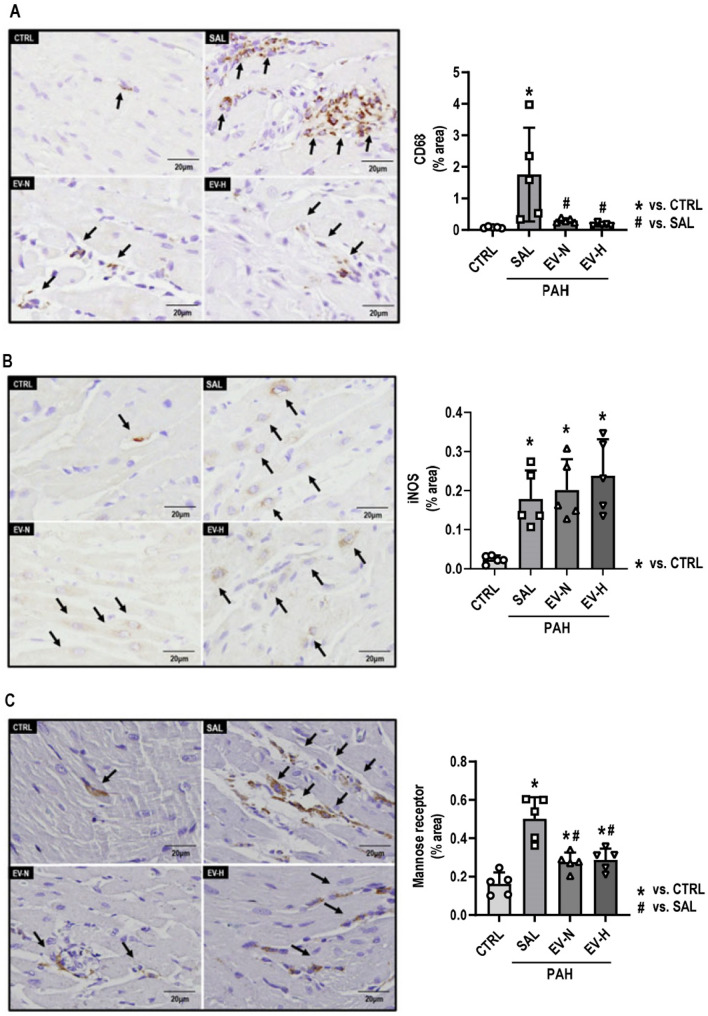
On day 28, immune cell infiltration in the RV was significantly higher in the PAH-SAL group (132.9 ± 16.8) than in the CTRL group (40.8 ± 1.5, p < 0.001). Both treatments reduced immune cell counts (EV-N: 95.8 ± 3.4, p = 0.032; EV-H: 79.6 ± 2.4, p = 0.002) (Fig. 5). CD68, a macrophage marker, was significantly increased in the PAH-SAL group (1.75 ± 0.66%) compared with the CTRL group (0.07 ± 0.01%, p = 0.012). Both therapies reduced CD68 expression (EV-N: 0.29 ± 0.03%, p = 0.032; EV-H: 0.16 ± 0.02%, p = 0.018) relative to PAH-SAL (Fig. 6A). Levels of iNOS, an M1 macrophage marker, were higher in the PAH-SAL (0.17 ± 0.03%), PAH-EV-N (0.20 ± 0.03%), and PAH-EV-H (0.23 ± 0.04%) groups than in the CTRL group (0.02 ± 0.01%; p = 0.016, p = 0.006, p = 0.001, respectively) (Fig. 6B). Levels of mannose receptor, an M2 macrophage marker, were higher in the PAH-SAL group (0.46 ± 0.05%) than in the CTRL group (0.14 ± 0.03%, p < 0.001). Both treatments significantly reduced mannose receptor levels (EV-N: 0.30 ± 0.03%, p = 0.032; EV-H: 0.30 ± 0.02%, p = 0.028) compared with PAH-SAL (Fig. 6C).
Fig. 5.
Immune cell quantification in right ventricular sections on day 28. Representative photomicrographs and quantification of immune cells in right ventricle sections stained with hematoxylin-eosin at 400× magnification. Arrows indicate immune cells. CTRL, control; EV-H, MSC-derived extracellular vesicles subjected to hypoxia; EV-N, MSC-derived extracellular vesicles subjected to normoxia; PAH, pulmonary arterial hypertension; SAL, saline; n = 5 per group. Scale bar, 20 μm. Statistical comparisons were performed using one-way ANOVA followed by the Tukey multiple comparison test (p < 0.05); *significance versus CTRL, #significance versus PAH-SAL. Data are presented as means ± standard deviation
Fig. 6.
Macrophage infiltration in right ventricular sections on day 28. Representative photomicrographs and quantification of macrophages in right ventricle sections. (A) Total macrophage quantification; (B) phenotypic characterization of M1 macrophages; (C) phenotypic characterization of M2 macrophages. Immunohistochemistry was performed for CD68, iNOS, and mannose receptor (brown) at 400× magnification. Arrows indicate CD68, iNOS, and mannose receptor-positive cells. CTRL, control; EV-H, MSC-derived extracellular vesicles subjected to hypoxia; EV-N, MSC-derived extracellular vesicles subjected to normoxia; PAH, pulmonary arterial hypertension; SAL, saline; n = 5 per group. Scale bar, 20 μm. Statistical comparisons were performed using one-way ANOVA followed by the Tukey multiple comparison test (p < 0.05); *significance versus CTRL, #significance versus PAH-SAL. Data are presented as means ± standard deviation
Programmed cell death
Cleaved caspase-3, an apoptosis marker, was significantly increased in the PAH-SAL group (22.78 ± 1.94%, p = 0.013) and the PAH-EV-H group (22.06 ± 1.15%, p = 0.028) compared with the CTRL group (15.64 ± 0.73%). PAH-EV-N treatment significantly reduced cleaved caspase-3 levels (15.54 ± 1.74%) compared with PAH-SAL (p = 0.012) and PAH-EV-H (p = 0.025) (Fig. 7).
Fig. 7.
Caspase-3-mediated programmed cell death in right ventricular sections on day 28. Representative photomicrographs and quantification of cleaved caspase-3 in right ventricle sections. Immunohistochemistry for cleaved caspase-3 (brown) at 400× magnification. Arrows indicate nuclear cleaved caspase-3 positive cells; arrowheads indicate cytoplasmic cleaved caspase-3 positive cells. CTRL, control; EV-H, MSC-derived extracellular vesicles subjected to hypoxia; EV-N, MSC-derived extracellular vesicles subjected to normoxia; PAH, pulmonary arterial hypertension; SAL, saline; n = 5 per group. Scale bar, 20 μm. Statistical comparisons were performed using one-way ANOVA followed by the Tukey multiple comparison test (p < 0.05); *significance versus CTRL, #significance versus PAH-SAL, &significance versus PAH-EV-N. Data are presented as means ± standard deviation
Discussion
In this study, hypoxia pre-conditioning of MSCs significantly increased EV production compared with normoxic conditions. Post-conditioning, we observed approximately 5 × 109 particles/mL expressing specific markers and displaying a range of sizes; the majority measured up to 340 nm. Proteomic analysis revealed that EVs derived from hypoxia-conditioned MSCs were enriched in biological processes and signaling pathways related to cardiac hypertrophy and programmed cell death. These findings suggest that hypoxia (1% O2 for 48 h) is a safe and effective pre-conditioning strategy that modulates the EV content.
In vivo, both normoxic and hypoxic EV therapies effectively reduced RVSP and the RVH index. EV-N therapy increased phosphorylation of the hypertrophy biomarker GSK3β and reduced cleaved caspase-3-mediated apoptosis. Notably, only EV-H therapy improved the PAT/PET ratio and reduced the RV outflow diameter. Both therapies led to decreased expression of c-Myc, reduced inflammatory cell infiltration, and lower expression of CD68 and mannose receptor. However, iNOS expression remained unchanged.
Various animal models have been developed to induce PAH. The Sugen 5416/hypoxia model is widely used due to its ability to replicate specific PAH lesions, such as plexiform formations. However, this model does not respond to clinically approved PAH treatments such as iloprost [45] and treprostinil [46]. The MCT-induced model, in contrast, promotes intense vascular remodeling, inflammation, and RV failure. Recent studies have demonstrated the presence of plexiform lesions in the MCT model by day 37 of the experimental protocol [47]. The MCT model offers several advantages, including low cost, ease of execution, high reproducibility, and insights into key pathophysiologic mechanisms [48].
The paracrine potential of MSCs contributes to their immunomodulatory and regenerative properties [49]. Consequently, EVs have emerged as promising candidates for cell-free therapies due to their ability to traverse biological membranes and reach distant tissues [16]. In addition, EVs tolerate cryopreservation, facilitating large-scale production and clinical application [50]. The therapeutic effects of EV administration depend on the MSC source. For instance, Blanco et al. [51] demonstrated that BM-MSC-EVs are enriched in anti-inflammatory proteins compared with EVs from other sources. Furthermore, pre-conditioning MSCs via various stimuli, including hypoxia, can enhance their therapeutic efficacy [52]. Hypoxic pre-conditioning simulates the oxygen tension in bone marrow, altering the MSCs’ secretome and augmenting their regenerative potential [18, 23, 49]. Consistently, Braga et al. [23] demonstrated that hypoxia-conditioned BM-MSCs released more EVs enriched in immune-regulatory proteins and extracellular matrix components.
Previous studies have shown that MSC therapy effectively reduces RVSP [53]. In our study, both normoxic and hypoxic EV therapies reduced RVSP compared with the PAH group, likely due to attenuation of vascular remodeling [7, 34, 54] and suppression of inflammation, as shown by decreased of counting inflammatory cells and macrophage (CD68+) infiltration in RV tissue. In PAH, there is an increase in T helper cells, which secrete pro-inflammatory factors and promote vascular remodeling [55]. In contrast, there is a deficiency in regulatory T cells (Treg), which indirectly suppress inflammation [56]. Natural killer cells are decreased in the lungs in PAH, suggesting a role for impaired innate immunity [57]. Macrophages are increased in lungs and RV tissues and secrete inflammatory cytokines and growth factors [58]. Pro-inflammatory cytokines such as IL-6, MIP-2, and TNF-α, known contributors to PAH pathogenesis, were also downregulated in the presence of MAPK pathway inhibitors [59], potentially facilitating vasodilation. The protein content found in EV-H may suggest an indirect action in decreasing these cytokines. Hypoxic pre-conditioning likely activated the HIF-1α pathway, which suppresses adaptive immunity by promoting myeloid-derived suppressor cells and regulatory T cells, leading to an immunosuppressive environment [60]. The total iNOS+ and mannose receptor+ cell counts were low across all groups, likely due to the 28-day gap between induction of PAH and tissue assessment. Macrophage phenotypic modulation typically lasts 7–10 days; M1 peaks at 1–3 days and M2 peaks around 7 days [61, 62]. The reduction observed in M2 macrophages after therapy suggests a potential role in mitigating fibrosis-promoting interactions with resident fibroblasts.
Interestingly, only EV-H therapy improved the PAT/PET ratio, reduced RV outflow diameter, and further decreased RVH. These outcomes may be explained by the proteomic profile of EV-H, which showed enrichment of proteins involved in MAPK/ERK pathway inhibition and negative feedback regulation of MAPK signaling. The MAPK/ERK pathway plays a crucial role in cardiac hypertrophy; MAPK1/3 activation triggers transcription factors such as GATA4, which upregulate pro-hypertrophic genes such as c-Myc [63]. In addition, MAPK1/3 activates kinases such as p90RSK, which phosphorylates GSK3β at Ser9, a modification that promotes hypertrophy. Inhibition of GSK3β occurs through phosphorylation of Ser9 residue, which results in changes in the activities of transcription factors and promotes expressions of proteins related to hypertrophic responses [64, 65]. We found that EV-N therapy increased GSK3β-Ser9 phosphorylation, likely due to MAP2K and MAPK activation pathway enrichment in EV-N proteomics. This may explain the improved hemodynamics observed in the EV-H group compared with the EV-N group, as demonstrated by the echocardiographic parameters and the RV/LV + S ratio.
Moreover, hypoxia pre-conditioning enriched EVs with proteins associated with programmed cell death pathways, including apoptosis. Notably, granzyme B (Gzmb) was exclusively identified in EV-H, potentially sustaining apoptotic signaling at levels comparable to those observed in the PAH group. Gzmb is known to induce apoptosis in cardiac cells by directly activating caspase-3 [66]. Emerging evidence suggests that regulated apoptosis may serve a reparative role in PAH by selectively eliminating dysfunctional or proliferative vascular cells. Our previous data showed that EV-H can preserve endothelial identity and inhibit EndMT, supporting vascular integrity [34]. Moreover, MSC-derived EVs have been shown to activate caspase-mediated efferocytosis, contributing to immunometabolic remodeling [67]. In this context, the presence of apoptosis-related proteins such as Gzmb in EV-H, though variable, may reflect selective activation of repair pathways rather than widespread cytotoxicity. Collectively, these effects may underlie the ability of EV-H to attenuate pulmonary vascular remodeling, reduce RV hypertrophy, and improve functional hemodynamics. In contrast, MAPK/ERK activation observed in the EV-N group is generally linked to cell survival pathways, as it promotes the expression of anti-apoptotic proteins such as Bcl-2 and Bcl-xL [68, 69]. This may account for the reduced number of cleaved caspase-3+ cells in the EV-N group. Interestingly, Pinto et al. demonstrated that cardiac tissue comprises 25–35% cardiomyocytes, 60% endothelial cells, 20% fibroblasts, and 5–10% leukocytes [70]. Therefore, we cannot assure which cell type within heart tissue is under apoptosis. In PAH-SAL, former monocrotaline studies showed extensive and diffuse endothelial cell death [71, 72] and cardiomyocytes damage [73, 74], which may elicit disorganized apoptosis of heart cells. Despite the absence of difference in caspase-3 between PAH-SAL and EV-H, the functional improvement was only observed in EV-H, and we might explain it by selective apoptosis and efferocytosis mechanisms. In line with this, Smits et al. reported that endothelial colony-forming cells (ECFCs) exhibit high proliferative activity in patients with PAH, which correlates with disease severity markers such as reduced right ventricular ejection fraction [75]. These findings are also consistent with the Metabolic Theory of PAH, which posits that endothelial cells acquire a tumor-like phenotype characterized by high proliferation and resistance to apoptosis [76]. Our data suggest that the observed increase in cleaved caspase-3 in the EV-H group may contribute positively to improvements in hemodynamics and cardiac hypertrophy. The differential enrichment of apoptotic and survival pathways indicates that hypoxia pre-conditioning may enhance the therapeutic efficacy of MSC-derived EVs by fine-tuning apoptosis-related signaling mechanisms. Therefore, our data suggest that the presence of cleaved caspase-3 in EV-H group may positively contribute to improvement of hemodynamics and cardiac hypertrophy.
Limitations.
The present study has some limitations. First, the results were obtained in monocrotaline-induced PAH and should not be directly extrapolated to other pre-clinical models and clinical disease. Nevertheless, monocrotaline has confirmed its value and provided several insights about PAH pathophysiology in the last years [48]. Second, there is an urgent call to follow standard production of EVs, as well as properly characterize them [27, 77]. For instance, some inter-individual variation between hypoxic and normoxic MSC-EVs has been observed [78]. Although EVs were pooled to achieve the required treatment dose, variability among individual batches may have attenuated or masked protein-specific effects, such as those involving Gzmb or MAPK signalling. Future studies should evaluate the contribution of individual EV subpopulations to the observed biological outcomes. Third, the use of two different techniques for protein quantification would further strengthen the data presented. However, with the limitation of the RV tissue size and the original experimental design of preserving a large fragment for immunohistochemistry and other small fragment for western blotting and RT-PCR, it compromised the western blotting analysis. Nevertheless, the antibodies used in immunohistochemistry produce results comparable to those obtained by western blotting, reinforcing the reliability of IHC for protein detection in tissues [79]. Furthermore, immunohistochemistry antibodies were properly validated by negative and positive controls during analysis.
In conclusion, our findings demonstrate that hypoxia pre-conditioning of MSCs enhances EV yield and alters their proteomic profile, leading to distinct functional effects in PAH therapy. Both normoxic and hypoxic EV therapies mitigated inflammation and improved RV function, but only EV-H demonstrated additional benefits in hemodynamic parameters and RV remodeling. These results highlight the importance of pre-conditioning strategies in optimizing MSC-EV-based therapies for PAH and warrant further investigation into their translational potential.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Fig. S1: Immunophenotypic analysis of EV-N and EV-H by flow cytometry. This figure presents the immunophenotypic characterization of extracellular vesicles (EVs) from EV-N (normoxia) and EV-H (hypoxia). (A) Flow cytometric analysis of EV-N. (B) Flow cytometric analysis of EV-H. Gating was performed exclusively for particles associated with beads. The blue histograms represent the percentage of the population positive for each specific antibody, and the red histograms show the isotypic controls for the antibodies. The accompanying dot plot illustrates the spectral overlap between PE and FITC fluorophores, with size (FSC) and complexity (SSC) parameters assessed through light scattering. Quadrant designations are as follows: Q1, CD90 + and CD81−; Q2, CD90 + and CD81+; Q3, CD90 − and CD81+; Q4, CD90 − and CD81−. Data are presented as mean percentages, with n = 1 per group. Analyses were conducted using Flowing Software 2.5.1
Supplementary Fig. S2: Nanoparticle tracking analysis of extracellular vesicles. (A) Representative graphs depicting the hydrodynamic size distribution profiles of extracellular vesicles (EVs) measured by nanoparticle tracking analysis. (B) Particle concentration of the fractions per milliliter. (C) Boxplots showing the sizes of 90% of the nanoparticles in the sample measured by diameter. EV-H, extracellular vesicles from mesenchymal stromal cells subjected to hypoxia; EV-N, extracellular vesicles from mesenchymal stromal cells subjected to normoxia; n = 4 per group. Comparisons were made using the unpaired t test (p < 0.05). Data are presented as medians and interquartile range
Supplementary Fig. S3: Electron microscopic analysis of extracellular vesicles. Images show the presence of small and medium/large vesicles (arrows) with intracellular membranes, folds, or smaller vesicles in the lumen (arrowheads) under normoxic (A–C) and hypoxic (D–F) conditions. Some vesicles appear spherical, others are elongated (C, F). EV-H, extracellular vesicles from mesenchymal stromal cells subjected to hypoxia; EV-N, extracellular vesicles from mesenchymal stromal cells subjected to normoxia; n = 1 per group. Scale bars: A, C, D, E, 500 nm; F, 250 nm; C, 1 μm. Images were captured using a JEOL 1200 transmission electron microscope and edited with Adobe Photoshop
Supplementary Fig. 4: Distribution of mapped proteins in EV-H and EV-N. (A) Area-proportional Venn diagram illustrating the overlap of proteins between EV-H and EV-N. A total of 695 non-redundant proteins were identified, with 203 unique to EV-H, 51 unique to EV-N, and 441 shared between both groups. (B) Volcano plot analysis of the intersecting proteins from the Venn diagram. Each dot represents a protein mapped according to its log2(fold change) (x axis) and log2(t test p value) (y axis). Red circles indicate proteins not meeting the fold change cutoff and the false discovery rate (FDR) cutoff (α = 0.05); green circles indicate proteins meeting the fold change cutoff but not the FDR; blue circles show proteins meeting all statistical filters; blue at the top indicates significantly upregulated proteins in EV-H and blue at the bottom indicates downregulated proteins. (C) Descriptive diagram of proteins identified in the proteomic analysis. EV, extracellular vesicle; EV-H, extracellular vesicles from mesenchymal stromal cells subjected to hypoxia; EV-N, extracellular vesicles from mesenchymal stromal cells subjected to normoxia. n = 4 flasks per condition (technical replicates). Data were generated using PatternLab software (version 5.0)
Supplementary Fig. S5: Top 20 enriched terms analysis. (A) Interaction network of the top 20 enriched terms, color-coded by cluster ID. (B) Pie charts displaying the proportion of enriched terms based on experimental groups: red, EV-H; blue, EV-N. (C) Heatmap of the top 20 enriched terms, color-coded by p-values. EV-N: group treated with extracellular vesicles from mesenchymal stromal cells subjected to normoxia; EV-H: group treated with extracellular vesicles from mesenchymal stromal cells subjected to hypoxia. N = 4 flasks per condition (technical replicates). Data were generated using Metascape software
Supplementary Fig. S6: Protein interaction network involved in cardiac hypertrophy and cell apoptosis. (A) Interaction network of proteins involved in cardiac hypertrophy and cell apoptosis for the EV-H group. (B) Interaction network of proteins for the EV-N group. Nodes represent proteins. Edges represent protein-protein associations. Actb, Actin cytoplasmic 1; Actc1, Actin alpha cardiac muscle 1; Actg1, Actin cytoplasmic 2; Ap1b1, AP-1 complex subunit beta-1; Ap2b1, AP-2 complex subunit beta; Atp2b1, Plasma membrane calcium-transporting ATPase 1; Atp6v1b2, V-type proton ATPase subunit B; Atp6v1e1, V-type proton ATPase subunit E1; C9, Complement component C9; Cacna2d1, Voltage-dependent calcium channel subunit alpha-2/delta-1; Camk2d, Calcium/calmodulin-dependent protein kinase type II subunit delta; Col11a1, Collagen alpha-1(XI) chain; Col3a1, Collagen alpha-1(III) chain; Cpe, Carboxypeptidase E; Ctsd, Cathepsin D 12 kDa light chain; EV-H, extracellular vesicles from mesenchymal stromal cells subjected to hypoxia; EV-N:, extracellular vesicles from mesenchymal stromal cells subjected to normoxia; F2, Activation peptide fragment 1; Fn1, Fibronectin; Gsn, Gelsolin; Gzmb, Granzyme B; Kpnb1, Importin subunit beta-1; LOC103689983, Hypoxanthine-guanine phosphoribosyl transferase; Ltbp1, Latent-transforming growth factor beta-binding protein 1; Mapk1, Mitogen-activated protein kinase 1; Mapk3, Mitogen-activated protein kinase 3; Ppp2r1b, Serine/threonine-protein phosphatase 2 A 65 kDa regulatory subunit A beta; Psmc5, 26 S proteasome regulatory subunit 8; Psmd11, 26 S proteasome non-ATPase regulatory subunit 11; Psmd13, 26 S proteasome non-ATPase regulatory subunit 13; Rpl30, 60 S ribosomal protein L30; Slc3a2, 4F2 cell-surface antigen heavy chain; Tpm1, Tropomyosin alpha-1 chain. n = 4 flasks, each condition, technical replicate. The data were generated by STRING software
Supplementary Fig. S7: Proteins related to the MAPK pathway. (A) Interaction network and (B) hierarchical cluster analysis by heat map showing the expression of proteins related to the MAPK pathway in the context of cardiac hypertrophy and cell apoptosis. Nodes represent proteins. Edges represent protein-protein associations. Actb, Actin cytoplasmic 1; Actc1, Actin alpha cardiac muscle 1; Actg1, Actin cytoplasmic 2; Camk2d, Calcium/calmodulin-dependent protein kinase type II subunit delta; Col11a1, Collagen alpha-1(XI) chain; Col3a1, Collagen alpha-1(III) chain; EV-H, extracellular vesicles from mesenchymal stromal cells subjected to hypoxia; EV-N, extracellular vesicles from mesenchymal stromal cells subjected to normoxia; F2, Activation peptide fragment 1; Gsn, Gelsolin; Gzmb, Granzyme B; Mapk1, Mitogen-activated protein kinase 1; Mapk3, Mitogen-activated protein kinase 3; Ppp2r1b, Serine/threonine-protein phosphatase 2 A 65 kDa regulatory subunit A beta; Slc3a2, 4F2 cell-surface antigen heavy chain; Tpm1, Tropomyosin alpha-1 chain. n = 4 flasks, each condition, technical replicate. The data were generated by Cytoscape (version 3.10.3) and MetaboAnalyst (version 6.0) software
Supplementary Fig. S8: Progression of echocardiographic parameters on day 14. Echocardiographic assessments performed on days 0 and 14 of the experimental protocol for diagnosing pulmonary arterial hypertension (PAH), based on (A) the PAT/PET ratio and (B) the diameter of the right ventricular outflow tract. CTRL, control; D0, start of the protocol; D14, 14 days after disease induction; PAH, pulmonary arterial hypertension; PAT, pulmonary acceleration time; PET, pulmonary ejection time. CTRL n = 8, PAH n = 24. Comparisons made by one-way ANOVA, followed by the Tukey multiple comparison test (p < 0.05); *significance versus CTRL-D14, #significance versus PAH-D14. Data are presented as means ± standard deviation
Supplementary Fig. S9: Full Western-Blotting panel. In the left, the molecular weight reference values. In the upper right, p-GSK3b-Ser9 protein bands are shown from 2 independent samples. In the middle right, total GSK3b protein bands are shown from 3 independent samples. In the lower right, GAPDH protein bands are shown depicting similar protein loads
Supplementary Table S1: Proteins associated with cardiac hypertrophy and cell apoptosis in EV-H. This table lists proteins identified in the extracellular vesicles (EVs) from the EV-H group, which received treatment with mesenchymal stromal cell-derived EVs subjected to hypoxia. The proteins are implicated in various biological processes and pathways related to cardiac hypertrophy and cell apoptosis. Atp2b1, Plasma membrane calcium-transporting ATPase 1; Atp6v1b2, V-type proton ATPase subunit B; Atp6v1e1, V-type proton ATPase subunit E1; C9, Complement component C9; Cacna2d1, Voltage-dependent calcium channel subunit alpha-2/delta-1; Camk2d, Calcium/calmodulin-dependent protein kinase type II subunit delta; Ctsd, Cathepsin D 12 kDa light chain; F2, Activation peptide fragment 1; Fn1, Fibronectin; Gsn, Gelsolin; Gzmb, Granzyme B; Kpnb1, Importin subunit beta-1; LOC103689983, Hypoxanthine-guanine phosphoribosyl transferase; Mapk1, Mitogen-activated protein kinase 1; Mapk3, Mitogen-activated protein kinase 3; Ppp2r1b, Serine/threonine-protein phosphatase 2 A 65 kDa regulatory subunit A beta; Psmc5, 26 S proteasome regulatory subunit 8; Psmd11, 26 S proteasome non-ATPase regulatory subunit 11; Psmd13, 26 S proteasome non-ATPase regulatory subunit 13; Rpl30, 60 S ribosomal protein L30; Slc3a2, 4F2 cell-surface antigen heavy chain. n = 4 flasks, each condition, technical replicate. Data were derived from 4 technical replicates for each condition and analyzed using Gene Ontology, KEGG, and Reactome software
Supplementary Table S2: Proteins associated with cardiac hypertrophy and cell apoptosis in the EV-N group. Actb, Actin cytoplasmic 1; Actc1, Actin alpha cardiac muscle 1; Actg1, Actin cytoplasmic 2; Ap1b1, AP-1 complex subunit beta-1; Ap2b1, AP-2 complex subunit beta; Col11a1, Collagen alpha-1(XI) chain; Col3a1, Collagen alpha-1(III) chain; Cpe, Carboxypeptidase E; EV-N, extracellular vesicles from mesenchymal stromal cells subjected to normoxia; Fn1, Fibronectin; Gsn, Gelsolin; Ltbp1, Latent-transforming growth factor beta-binding protein 1; Tpm1, Tropomyosin alpha-1 chain. n = 4 flasks, each condition, technical replicate. Data were obtained from 4 technical replicates for each condition and analyzed using Gene Ontology, KEGG, and Reactome software
Supplementary Table S3: All proteins detected in EVs. n = 4 flasks, each condition, technical replicate. Data were obtained from 4 technical replicates for each condition and analyzed using PatternLab software (version 5.0)
Supplementary Table S4: Proteins in EVs used for proteins interaction. Actb, Actin cytoplasmic 1; Actc1, Actin alpha cardiac muscle 1; Actg1, Actin cytoplasmic 2; Camk2d, Calcium/calmodulin-dependent protein kinase type II subunit delta; Col11a1, Collagen alpha-1(XI) chain; Col3a1, Collagen alpha-1(III) chain; EV-H, extracellular vesicles from mesenchymal stromal cells subjected to hypoxia; EV-N, extracellular vesicles from mesenchymal stromal cells subjected to normoxia; F2, Activation peptide fragment 1; Gsn, Gelsolin; Gzmb, Granzyme B; Mapk1, Mitogen-activated protein kinase 1; Mapk3, Mitogen-activated protein kinase 3; Ppp2r1b, Serine/threonine-protein phosphatase 2 A 65 kDa regulatory subunit A beta; Slc3a2, 4F2 cell-surface antigen heavy chain; Tpm1, Tropomyosin alpha-1 chain. n = 4 flasks, each condition, technical replicate. Data were analyzed by PatternLab software (version 5.0)
Acknowledgements
The authors thank Mr. André Benedito da Silva for support with the experiments and Ms Verônica Cristina dos Santos Lima for animal care, and Ms. Lorna O’Brien for English grammar revision. The authors declare that they have not use AI-generated work in this manuscript.
Abbreviations
- BM-MSC
Bone marrow-derived mesenchymal stromal cell
- EV
Extracellular vesicle
- EV-H
Ev from MSCs subjected to hypoxia
- EV-N
EV from MSCs subjected to normoxia
- FDR
False discovery rate
- GSK3β
Glycogen synthase kinase 3 beta
- Gzmb
Granzyme B
- IMDM
Iscove’s modified Dulbecco’s medium
- iNOS
Inducible nitric oxide synthase
- LV
Left ventricle
- MCT
Monocrotaline
- MSC
Mesenchymal stromal cell
- PAH
Pulmonary arterial hypertension
- PBS
Phosphate-buffered saline
- RV
Right ventricle
- RVH
Right ventricular hypertrophy
- RVSP
Right ventricular systolic pressure
- S
Septum
Author contributions
RTS, PRMR, FFC, and PLS were involved in the conception and design of the study. RTS, CLB, MESFO, CMS, NNR, SSSS, DET, BTM, MGP, MROT, CMT, and PLS were involved in the acquisition of data. RTS, NNR, RGV, DET, FFC, and PLS were involved in the analysis and interpretation of data. RTS, FFC, and PLS were involved in drafting or revising the manuscript. All authors have approved the final article.
Funding
This work received funding from the Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro (FAPERJ, E-26/202.766/2018, E-26/010.001488/2019).
Data availability
The raw proteomics data are available in the ProteomeXchange Consortium via the Proteomics Identification Database with identifier PXD027718. All other data are available within the article and its supplementary material.
Declarations
Competing interest
The authors have no competing interests in the information described in this article.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Galiè N, McLaughlin VV, Rubin LJ, Simonneau G. An overview of the 6th world symposium on pulmonary hypertension. Eur Respir J. 2019;53(1):1802148. 10.1183/13993003.02148-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao X, Fang X, Guo M, Li X, He Y, Xie M, et al. TRB3 mediates vascular remodeling by activating the MAPK signaling pathway in hypoxic pulmonary hypertension. Respir Res. 2021;22(1):312. 10.1186/s12931-021-01908-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lv X, Li J, Wei R, Meng L, Kong X, Wei K, et al. Ethyl pyruvate alleviates pulmonary arterial hypertension via PI3K-Akt signaling. Mol Cell Biochem. 2025;480(2):1045–54. 10.1007/s11010-024-05020-1 [DOI] [PubMed] [Google Scholar]
- 4.Gurbanov E, Shiliang X. The key role of apoptosis in the pathogenesis and treatment of pulmonary hypertension. Eur J Cardiothorac Surg. 2006;30(3):499–507. 10.1016/j.ejcts.2006.05.026 [DOI] [PubMed] [Google Scholar]
- 5.Lafuse WP, Wozniak DJ, Rajaram MVS. Role of cardiac macrophages on cardiac inflammation, fibrosis and tissue repair. Cells. 2020;10(1):51. 10.3390/cells10010051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santos RT, de Sá Freire Onofre ME, de Assis Fernandes Caldeira D, Klein AB, Rocco PRM, Cruz FF, et al. Pharmacological agents and potential new therapies in pulmonary arterial hypertension. Curr Vasc Pharmacol. 2024;22(3):155–70. 10.2174/0115701611266576231211045731 [DOI] [PubMed] [Google Scholar]
- 7.de Mendonça L, Felix NS, Blanco NG, Da Silva JS, Ferreira TP, Abreu SC, et al. Mesenchymal stromal cell therapy reduces lung inflammation and vascular remodeling and improves hemodynamics in experimental pulmonary arterial hypertension. Stem Cell Res Ther. 2017;8(1):220. 10.1186/s13287-017-0669-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JC, Cha CI, Kim D, Choe SY. Therapeutic effects of umbilical cord blood derived mesenchymal stem cell-conditioned medium on pulmonary arterial hypertension in rats. J Anat Soc India. 2016;65(1):15–9. 10.1016/j.jasi.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Luan Y, Zhang X, Qi T-G, Cheng G-H, Sun C, Kong F. Long-term research of stem cells in monocrotaline-induced pulmonary arterial hypertension. Clin Exp Med. 2014;14(4):439–46. 10.1007/s10238-013-0256-3 [DOI] [PubMed] [Google Scholar]
- 10.Silva MMCD, Alencar AKN, Silva JSD, Montagnoli TL, Silva GFD, Rocha BS, et al. Therapeutic benefit of the association of Lodenafil with mesenchymal stem cells on hypoxia-induced pulmonary hypertension in rats. Cells. 2020;9(9):2120. 10.3390/cells9092120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang MG, Tung L, Sekar RB, Chang CY, Cysyk J, Dong P, et al. Proarrhythmic potential of mesenchymal stem cell transplantation revealed in an in vitro coculture model. Circulation. 2006;113(15):1832–41. 10.1161/CIRCULATIONAHA.105.593038 [DOI] [PubMed] [Google Scholar]
- 12.Guo Y, Yu Y, Hu S, Chen Y, Shen Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020;11(5):349. 10.1038/s41419-020-2542-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanki-Horimoto S, Horimoto H, Mieno S, Kishida K, Watanabe F, Furuya E, et al. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation. 2006;114(1 suppl):I181–5. 10.1161/CIRCULATIONAHA.105.001487 [DOI] [PubMed] [Google Scholar]
- 14.Miceli V, Bulati M, Iannolo G, Zito G, Gallo A, Conaldi PG. Therapeutic properties of mesenchymal stromal/stem cells: the need of cell priming for cell-free therapies in regenerative medicine. Int J Mol Sci. 2021;22(2):763. 10.3390/ijms22020763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa-Ferro ZSM, Rocha GV, da Silva KN, Paredes BD, Loiola EC, Silva JD, et al. GMP-compliant extracellular vesicles derived from umbilical cord mesenchymal stromal cells: manufacturing and pre-clinical evaluation in ARDS treatment. Cytotherapy. 2024;26(9):1013–25. 10.1016/j.jcyt.2024.04.074 [DOI] [PubMed] [Google Scholar]
- 16.Kalra H, Drummen G, Mathivanan S. Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci. 2016;17(2):170. 10.3390/ijms17020170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melo MM, Cruz FF, Rocco PRM. Mesenchymal stromal cell therapy for chronic lung diseases: experimental and clinical evidence. Expert Rev Respir Med. 2023;17(3):223–35. 10.1080/17476348.2023.2196015 [DOI] [PubMed] [Google Scholar]
- 18.Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508(7495):269–73. 10.1038/nature13034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abreu SC, Hampton TH, Hoffman E, Dearborn J, Ashare A, Singh Sidhu K, et al. Differential effects of the cystic fibrosis lung inflammatory environment on mesenchymal stromal cells. Am J Physiol Lung Cell Mol Physiol. 2020;319(6):L908–25. 10.1152/ajplung.00218.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosová I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26(8):2173–82. 10.1634/stemcells.2007-1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mas-Bargues C, Sanz-Ros J, Román-Domínguez A, Inglés M, Gimeno-Mallench L, El Alami M, et al. Relevance of oxygen concentration in stem cell culture for regenerative medicine. Int J Mol Sci. 2019;20(5):1195. 10.3390/ijms20051195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pulido-Escribano V, Torrecillas-Baena B, Camacho-Cardenosa M, Dorado G, Gálvez-Moreno MÁ, Casado-Díaz A. Role of hypoxia preconditioning in therapeutic potential of mesenchymal stem-cell-derived extracellular vesicles. World J Stem Cells. 2022;14(7):453–72. 10.4252/wjsc.v14.i7.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braga CL, da Silva LR, Santos RT, de Carvalho LRP, Mandacaru SC, de Oliveira Trugilho MR, et al. Proteomics profile of mesenchymal stromal cells and extracellular vesicles in normoxic and hypoxic conditions. Cytotherapy. 2022;24(12):1211–24. 10.1016/j.jcyt.2022.08.009 [DOI] [PubMed] [Google Scholar]
- 24.Ren W, Hou J, Yang C, Wang H, Wu S, Wu Y, et al. Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote non-small cell lung cancer cell growth and mobility as well as macrophage M2 polarization via miR-21-5p delivery. J Exp Clin Cancer Res. 2019;38(1):62. 10.1186/s13046-019-1027-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoeper MM, Kramer T, Pan Z, Eichstaedt CA, Spiesshoefer J, Benjamin N, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J. 2017;50(2):1700740. 10.1183/13993003.00740-2017 [DOI] [PubMed] [Google Scholar]
- 26.Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Cereb Blood Flow Metab. 2020;40(9):1769–77. 10.1177/0271678X20943823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welsh JA, Goberdhan DCI, O’Driscoll L, Buzas EI, Blenkiron C, Bussolati B, et al. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J Extracell Vesicles. 2024;13(2):e12404. 10.1002/jev2.12404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longjohn MN, Christian SL. Characterizing extracellular vesicles using nanoparticle-tracking analysis. In: Christian SL, editor. Cancer cell biology. Methods in. Volume 2508. New York: Humana; 2022. pp. 353–73. 10.1007/978-1-0716-2376-3_23 [DOI] [PubMed] [Google Scholar]
- 29.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83. 10.1083/jcb.201211138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morales-Kastresana A, Jones JC. Flow cytometric analysis of extracellular vesicles. In: Hill A, editor. Exosomes and microvesicles. Methods in molecular biology. Volume 1545. New York: Humana; 2017. pp. 215–25. 10.1007/978-1-4939-6728-5_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santos MDM, Lima DB, Fischer JSG, Clasen MA, Kurt LU, Camillo-Andrade AC, et al. Simple, efficient and thorough shotgun proteomic analysis with patternlab V. Nat Protoc. 2022;17(7):1553–78. 10.1038/s41596-022-00690-x [DOI] [PubMed] [Google Scholar]
- 32.Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1):1523. 10.1038/s41467-019-09234-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pang Z, Lu Y, Zhou G, Hui F, Xu L, Viau C, et al. MetaboAnalyst 6.0: towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024;2(W1):W398–406. 10.1093/nar/gkae253 [DOI] [PMC free article] [PubMed]
- 34.Braga CL, Santos RT, da Silva CM, de Novaes Rocha N, Felix NS, Medeiros M, et al. Therapeutic effects of hypoxia-preconditioned bone marrow-derived mesenchymal stromal cells and their extracellular vesicles in experimental pulmonary arterial hypertension. Life Sci. 2023;329:121988. 10.1016/j.lfs.2023.121988 [DOI] [PubMed] [Google Scholar]
- 35.Nogueira-Ferreira R, Vitorino R, Ferreira R, Henriques-Coelho T. Exploring the monocrotaline animal model for the study of pulmonary arterial hypertension: a network approach. Pulm Pharmacol Ther. 2015;35:8–16. 10.1016/j.pupt.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 36.Thibault HB, Kurtz B, Raher MJ, Shaik RS, Waxman A, Derumeaux G, et al. Noninvasive assessment of murine pulmonary arterial pressure. Circ Cardiovasc Imaging. 2010;3(2):157–63. 10.1161/CIRCIMAGING.109.887109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trittmann JK, Almazroue H, Nelin LD, Shaffer TA, Celestine CR, Green HW, et al. PATET ratio by doppler echocardiography: noninvasive detection of pediatric pulmonary arterial hypertension. Pediatr Res. 2022;92(3):631–6. 10.1038/s41390-021-01840-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28(1):1–e3914. 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 39.Spyropoulos F, Vitali SH, Touma M, Rose CD, Petty CR, Levy P, et al. Echocardiographic markers of pulmonary hemodynamics and right ventricular hypertrophy in rat models of pulmonary hypertension. Pulm Circ. 2020;10(2):1–10. 10.1177/2045894020910976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basso C, Calabrese F, Angelini A, Carturan E, Thiene G. Classification and histological, immunohistochemical, and molecular diagnosis of inflammatory myocardial disease. Heart Fail Rev. 2013;18(6):673–81. 10.1007/s10741-012-9355-6 [DOI] [PubMed] [Google Scholar]
- 41.Hassoun PM. Pulmonary arterial hypertension. N Engl J Med. 2021;385(25):2361–76. 10.1056/NEJMra2000348 [DOI] [PubMed] [Google Scholar]
- 42.Bankhead P, Loughrey MB, Fernández JA, et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7(1):1–7. 10.1038/s41598-017-17204-5 [DOI] [PMC free article] [PubMed]
- 43.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–75. [PubMed] [Google Scholar]
- 44.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3(6):1101–8. 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- 45.Gomez-Arroyo J, Saleem SJ, Mizuno S, Syed AA, Bogaard HJ, Abbate A, et al. A brief overview of mouse models of pulmonary arterial hypertension: problems and prospects. Am J Physiol Lung Cell Mol Physiol. 2012;302(10):L977–91. 10.1152/ajplung.00362.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaudhary KR, Deng Y, Suen CM, Taha M, Petersen TH, Mei SHJ, et al. Efficacy of treprostinil in the SU5416-hypoxia model of severe pulmonary arterial hypertension: haemodynamic benefits are not associated with improvements in arterial remodelling. Br J Pharmacol. 2018;175(20):3976–89. 10.1111/bph.14472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gewehr DM, Salgueiro GR, de Noronha L, Kubrusly FB, Kubrusly LF, et al. Lesões plexiformes Em Modelo experimental de Hipertensão arterial pulmonar Induzida Por monocrotaline. Arq Bras Cardiol. 2020;115(3):480–90. 10.36660/abc.20190306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hill NS, Gillespie MN, McMurtry IF. Fifty years of monocrotaline-induced pulmonary hypertension. Chest. 2017;152(6):1106–8. 10.1016/j.chest.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 49.Noronha NC, Mizukami A, Caliári-Oliveira C, Cominal JG, Rocha JLM, Covas DT, et al. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther. 2019;10(1):131. 10.1186/s13287-019-1224-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yeo RWY, Lai RC, Tan KH, Lim SK. Exosome: a novel and safer therapeutic refinement of mesenchymal stem cells. J Circ Biomark. 2013;1(1). 10.33393/jcb.2013.2038
- 51.Blanco NG, Machado NM, Castro LL, Antunes MA, Takiya CM, Trugilho MRO, et al. Extracellular vesicles from different sources of mesenchymal stromal cells have distinct effects on lung and distal organs in experimental sepsis. Int J Mol Sci. 2023;24(9):8234. 10.3390/ijms24098234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silva LHA, Antunes MA, Dos Santos CC, Weiss DJ, Cruz FF, Rocco PRM. Strategies to improve the therapeutic effects of mesenchymal stromal cells in respiratory diseases. Stem Cell Res Ther. 2018;9(1):45. 10.1186/s13287-018-0802-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suen CM, Stewart DJ, Montroy J, Welsh C, Levac B, Wesch N, et al. Regenerative cell therapy for pulmonary arterial hypertension in animal models: a systematic review. Stem Cell Res Ther. 2019;10(1):75. 10.1186/s13287-019-1172-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sakao S, Taraseviciene-Stewart L, Wood K, Cool CD, Voelkel NF. Apoptosis of pulmonary microvascular endothelial cells stimulates vascular smooth muscle cell growth. Am J Physiol Lung Cell Mol Physiol. 2006;291(3):L362–8. 10.1152/ajplung.00111.2005 [DOI] [PubMed] [Google Scholar]
- 55.Qiu H, He Y, Ouyang F, Jiang P, Guo S, Guo Y. The role of regulatory T cells in pulmonary arterial hypertension. J Am Heart Association. 2019;8(23):e014201. 10.1161/JAHA.119.01420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y, Shi JZ, Jiang R, Liu SF, He YY, van der Vorst EP, et al. Regulatory T cell-related gene indicators in pulmonary hypertension. Front Pharmacol. 2022;13908783. 10.3389/fphar.2022.908783 [DOI] [PMC free article] [PubMed]
- 57.Ormiston ML, Chang C, Long LL, Soon E, Jones D, Machado R, et al. Impaired natural killer cell phenotype and function in idiopathic and heritable pulmonary arterial hypertension. Circulation. 2012;9(126):1099–109. 10.1161/CIRCULATIONAHA.112.110619 [DOI] [PubMed] [Google Scholar]
- 58.Al-Qazazi R, Lima PD, Prisco SZ, Potus F, Dasgupta A, Chen KH, et al. Macrophage–NLRP3 activation promotes right ventricle failure in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2022;5(206):608–24. 10.1164/rccm.202110-2274OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang W, Liu H, Pan Y, Yang H, Lin J, Zhang H. Mechanical stretching of the pulmonary vein mediates pulmonary hypertension due to left heart disease by regulating SAC/MAPK pathway and the expression of IL-6 and TNF-α. J Cardiothorac Surg. 2021;16(1):127. 10.1186/s13019-021-01471-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.LaGory EL, Giaccia AJ. The ever-expanding role of HIF in tumour and stromal biology. Nat Cell Biol. 2016;18(4):356–65. 10.1038/ncb3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mouton AJ, DeLeon-Pennell KY, Rivera Gonzalez OJ, Flynn ER, Freeman TC, Saucerman JJ, et al. Mapping macrophage polarization over the myocardial infarction time continuum. Basic Res Cardiol. 2018;113(4):26. 10.1007/s00395-018-0686-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wculek SK, Dunphy G, Heras-Murillo I, Mastrangelo A, Sancho D. Metabolism of tissue macrophages in homeostasis and pathology. Cell Mol Immunol. 2022;19(3):384–408. 10.1038/s41423-021-00791-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu J, Blenis J, Yuan J. Activation of PI3K/Akt and MAPK pathways regulates Myc-mediated transcription by phosphorylating and promoting the degradation of Mad1. Proc Natl Acad Sci U S A. 2008;105(18):6584–9. 10.1073/pnas.0802785105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ding Q, Xia W, Liu J-C, Yang J-Y, Lee D-F, Xia J, et al. Erk associates with and primes GSK-3β for its inactivation resulting in upregulation of β-catenin. Mol Cell. 2005;19(2):159–70. 10.1016/j.molcel.2005.06.009 [DOI] [PubMed] [Google Scholar]
- 65.Sugden PH, Fuller SJ, Weiss SC, Clerk A. Glycogen synthase kinase 3 (GSK3) in the heart: a point of integration in hypertrophic signalling and a therapeutic target? A critical analysis. Br J Pharmacol. 2008;153(Suppl 1):S137–53. 10.1038/sj.bjp.0707659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goping IS, Barry M, Liston P, Sawchuk T, Constantinescu G, Michalak KM, et al. Granzyme B-induced apoptosis requires both direct caspase activation and relief of caspase Inhibition. Immunity. 2003;18(3):355–65. 10.1016/S1074-7613(03)00032-3 [DOI] [PubMed] [Google Scholar]
- 67.Hoseinzadeh A, Esmaeili SA, Sahebi R, Melak AM, et al. Fate and long-lasting therapeutic effects of mesenchymal stromal/stem-like cells: mechanistic insights. Stem Cell Res Ther. 2025;16(1):33. 10.1186/s13287-025-04158-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karl E, Zhang Z, Dong Z, Neiva KG, Soengas MS, Koch AE, et al. Unidirectional crosstalk between Bcl-xL and Bcl-2 enhances the angiogenic phenotype of endothelial cells. Cell Death Differ. 2007;14(9):1657–66. 10.1038/sj.cdd.4402174 [DOI] [PubMed] [Google Scholar]
- 69.Nakamura M, Keller MA, Fefelova N, Zhai P, Liu T, Tian Y, et al. Ser14 phosphorylation of Bcl-xL mediates compensatory cardiac hypertrophy in male mice. Nat Commun. 2023;14(1):5805. 10.1038/s41467-023-41595-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD. Revisiting cardiac cellular composition. Circul Res. 2016;118(3):400–9. 10.1161/CIRCRESAHA.115.307778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiao R, Su Y, Feng T, Sun M, Liu B, Zhang J, et al. Monocrotaline induces endothelial injury and pulmonary hypertension by targeting the extracellular Calcium-Sensing receptor. J Am Heart Association. 2017;6(4):e004865. 10.1161/JAHA.116.004865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakayama Wong LS, Lamé MW, Jones AD, Wilson DW. Differential cellular responses to protein adducts of naphthoquinone and monocrotaline pyrrole. Chem Res Toxicol. 2010;23(9):1504–13. 10.1021/tx1002436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen L, Gan XT, Haist JV, Feng Q, Lu X, et al. Attenuation of compensatory right ventricular hypertrophy and heart failure following monocrotaline-induced pulmonary vascular injury by the Na+-H + exchange inhibitor cariporide. J Pharmacol Exp Ther. 2001;298(2):469–76. 10.1016/S0022-3565(24)29404-0 [PubMed] [Google Scholar]
- 74.Falcão-Pires I, Gonçalves N, Henriques-Coelho T, Moreira-Gonçalves D, Roncon-Albuquerque R Jr, Leite-Moreira AF. Apelin decreases myocardial injury and improves right ventricular function in monocrotaline-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2009;296(6):H2007–14. 10.1152/ajpheart.00089.2009 [DOI] [PubMed] [Google Scholar]
- 75.Smits J, Tasev D, Andersen S, Szulcek R, Botros L, Ringgaard S, Andersen A, Vonk-Noordegraaf A, Koolwijk P, Bogaard HJ. Blood outgrowth and proliferation of endothelial colony forming cells are related to markers of disease severity in patients with pulmonary arterial hypertension. International journal of molecular sciences. 2018;19(12):3763, 2018. 10.3390/ijms19123763 [DOI] [PMC free article] [PubMed]
- 76.Alencar AKN, Cruz FF, Rocco PRM, Silva PL. Metabolic theory of pulmonary arterial hypertension: connecting mitochondrial roles with disease control. Physiological Mini Reviews. 2022;15(1):1–13. [Google Scholar]
- 77.Bettin BA, Varga Z, Nieuwland R, van der Pol E. Standardization of extracellular vesicle concentration measurements by flow cytometry: the past, present, and future. J Thromb Haemost. 2023;21(8):2032–44. 10.1016/j.jtha.2023.04.042 [DOI] [PubMed] [Google Scholar]
- 78.Peltzer J, Lund K, Goriot ME, Grosbot M, Lataillade JJ, Mauduit P, Banzet S. Interferon-γ and hypoxia priming have limited effect on the MiRNA landscape of human mesenchymal stromal cells-derived extracellular vesicles. Front Cell Dev Biology. 2020;8:581436. 10.3389/fcell.2020.581436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Porcario C, Hall SM, Martucci F, Corona C, Iulini B, Perazzini AZ, Acutis P, Hamir AN, Loiacono CM, Greenlee JJ, Richt JA, Caramelli M, Casalone C. Evaluation of two sets of immunohistochemical and Western blot confirmatory methods in the detection of typical and atypical BSE cases. BMC Res Notes. 2011;4:376. 10.1186/1756-0500-4-376 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. S1: Immunophenotypic analysis of EV-N and EV-H by flow cytometry. This figure presents the immunophenotypic characterization of extracellular vesicles (EVs) from EV-N (normoxia) and EV-H (hypoxia). (A) Flow cytometric analysis of EV-N. (B) Flow cytometric analysis of EV-H. Gating was performed exclusively for particles associated with beads. The blue histograms represent the percentage of the population positive for each specific antibody, and the red histograms show the isotypic controls for the antibodies. The accompanying dot plot illustrates the spectral overlap between PE and FITC fluorophores, with size (FSC) and complexity (SSC) parameters assessed through light scattering. Quadrant designations are as follows: Q1, CD90 + and CD81−; Q2, CD90 + and CD81+; Q3, CD90 − and CD81+; Q4, CD90 − and CD81−. Data are presented as mean percentages, with n = 1 per group. Analyses were conducted using Flowing Software 2.5.1
Supplementary Fig. S2: Nanoparticle tracking analysis of extracellular vesicles. (A) Representative graphs depicting the hydrodynamic size distribution profiles of extracellular vesicles (EVs) measured by nanoparticle tracking analysis. (B) Particle concentration of the fractions per milliliter. (C) Boxplots showing the sizes of 90% of the nanoparticles in the sample measured by diameter. EV-H, extracellular vesicles from mesenchymal stromal cells subjected to hypoxia; EV-N, extracellular vesicles from mesenchymal stromal cells subjected to normoxia; n = 4 per group. Comparisons were made using the unpaired t test (p < 0.05). Data are presented as medians and interquartile range
Supplementary Fig. S3: Electron microscopic analysis of extracellular vesicles. Images show the presence of small and medium/large vesicles (arrows) with intracellular membranes, folds, or smaller vesicles in the lumen (arrowheads) under normoxic (A–C) and hypoxic (D–F) conditions. Some vesicles appear spherical, others are elongated (C, F). EV-H, extracellular vesicles from mesenchymal stromal cells subjected to hypoxia; EV-N, extracellular vesicles from mesenchymal stromal cells subjected to normoxia; n = 1 per group. Scale bars: A, C, D, E, 500 nm; F, 250 nm; C, 1 μm. Images were captured using a JEOL 1200 transmission electron microscope and edited with Adobe Photoshop
Supplementary Fig. 4: Distribution of mapped proteins in EV-H and EV-N. (A) Area-proportional Venn diagram illustrating the overlap of proteins between EV-H and EV-N. A total of 695 non-redundant proteins were identified, with 203 unique to EV-H, 51 unique to EV-N, and 441 shared between both groups. (B) Volcano plot analysis of the intersecting proteins from the Venn diagram. Each dot represents a protein mapped according to its log2(fold change) (x axis) and log2(t test p value) (y axis). Red circles indicate proteins not meeting the fold change cutoff and the false discovery rate (FDR) cutoff (α = 0.05); green circles indicate proteins meeting the fold change cutoff but not the FDR; blue circles show proteins meeting all statistical filters; blue at the top indicates significantly upregulated proteins in EV-H and blue at the bottom indicates downregulated proteins. (C) Descriptive diagram of proteins identified in the proteomic analysis. EV, extracellular vesicle; EV-H, extracellular vesicles from mesenchymal stromal cells subjected to hypoxia; EV-N, extracellular vesicles from mesenchymal stromal cells subjected to normoxia. n = 4 flasks per condition (technical replicates). Data were generated using PatternLab software (version 5.0)
Supplementary Fig. S5: Top 20 enriched terms analysis. (A) Interaction network of the top 20 enriched terms, color-coded by cluster ID. (B) Pie charts displaying the proportion of enriched terms based on experimental groups: red, EV-H; blue, EV-N. (C) Heatmap of the top 20 enriched terms, color-coded by p-values. EV-N: group treated with extracellular vesicles from mesenchymal stromal cells subjected to normoxia; EV-H: group treated with extracellular vesicles from mesenchymal stromal cells subjected to hypoxia. N = 4 flasks per condition (technical replicates). Data were generated using Metascape software
Supplementary Fig. S6: Protein interaction network involved in cardiac hypertrophy and cell apoptosis. (A) Interaction network of proteins involved in cardiac hypertrophy and cell apoptosis for the EV-H group. (B) Interaction network of proteins for the EV-N group. Nodes represent proteins. Edges represent protein-protein associations. Actb, Actin cytoplasmic 1; Actc1, Actin alpha cardiac muscle 1; Actg1, Actin cytoplasmic 2; Ap1b1, AP-1 complex subunit beta-1; Ap2b1, AP-2 complex subunit beta; Atp2b1, Plasma membrane calcium-transporting ATPase 1; Atp6v1b2, V-type proton ATPase subunit B; Atp6v1e1, V-type proton ATPase subunit E1; C9, Complement component C9; Cacna2d1, Voltage-dependent calcium channel subunit alpha-2/delta-1; Camk2d, Calcium/calmodulin-dependent protein kinase type II subunit delta; Col11a1, Collagen alpha-1(XI) chain; Col3a1, Collagen alpha-1(III) chain; Cpe, Carboxypeptidase E; Ctsd, Cathepsin D 12 kDa light chain; EV-H, extracellular vesicles from mesenchymal stromal cells subjected to hypoxia; EV-N:, extracellular vesicles from mesenchymal stromal cells subjected to normoxia; F2, Activation peptide fragment 1; Fn1, Fibronectin; Gsn, Gelsolin; Gzmb, Granzyme B; Kpnb1, Importin subunit beta-1; LOC103689983, Hypoxanthine-guanine phosphoribosyl transferase; Ltbp1, Latent-transforming growth factor beta-binding protein 1; Mapk1, Mitogen-activated protein kinase 1; Mapk3, Mitogen-activated protein kinase 3; Ppp2r1b, Serine/threonine-protein phosphatase 2 A 65 kDa regulatory subunit A beta; Psmc5, 26 S proteasome regulatory subunit 8; Psmd11, 26 S proteasome non-ATPase regulatory subunit 11; Psmd13, 26 S proteasome non-ATPase regulatory subunit 13; Rpl30, 60 S ribosomal protein L30; Slc3a2, 4F2 cell-surface antigen heavy chain; Tpm1, Tropomyosin alpha-1 chain. n = 4 flasks, each condition, technical replicate. The data were generated by STRING software
Supplementary Fig. S7: Proteins related to the MAPK pathway. (A) Interaction network and (B) hierarchical cluster analysis by heat map showing the expression of proteins related to the MAPK pathway in the context of cardiac hypertrophy and cell apoptosis. Nodes represent proteins. Edges represent protein-protein associations. Actb, Actin cytoplasmic 1; Actc1, Actin alpha cardiac muscle 1; Actg1, Actin cytoplasmic 2; Camk2d, Calcium/calmodulin-dependent protein kinase type II subunit delta; Col11a1, Collagen alpha-1(XI) chain; Col3a1, Collagen alpha-1(III) chain; EV-H, extracellular vesicles from mesenchymal stromal cells subjected to hypoxia; EV-N, extracellular vesicles from mesenchymal stromal cells subjected to normoxia; F2, Activation peptide fragment 1; Gsn, Gelsolin; Gzmb, Granzyme B; Mapk1, Mitogen-activated protein kinase 1; Mapk3, Mitogen-activated protein kinase 3; Ppp2r1b, Serine/threonine-protein phosphatase 2 A 65 kDa regulatory subunit A beta; Slc3a2, 4F2 cell-surface antigen heavy chain; Tpm1, Tropomyosin alpha-1 chain. n = 4 flasks, each condition, technical replicate. The data were generated by Cytoscape (version 3.10.3) and MetaboAnalyst (version 6.0) software
Supplementary Fig. S8: Progression of echocardiographic parameters on day 14. Echocardiographic assessments performed on days 0 and 14 of the experimental protocol for diagnosing pulmonary arterial hypertension (PAH), based on (A) the PAT/PET ratio and (B) the diameter of the right ventricular outflow tract. CTRL, control; D0, start of the protocol; D14, 14 days after disease induction; PAH, pulmonary arterial hypertension; PAT, pulmonary acceleration time; PET, pulmonary ejection time. CTRL n = 8, PAH n = 24. Comparisons made by one-way ANOVA, followed by the Tukey multiple comparison test (p < 0.05); *significance versus CTRL-D14, #significance versus PAH-D14. Data are presented as means ± standard deviation
Supplementary Fig. S9: Full Western-Blotting panel. In the left, the molecular weight reference values. In the upper right, p-GSK3b-Ser9 protein bands are shown from 2 independent samples. In the middle right, total GSK3b protein bands are shown from 3 independent samples. In the lower right, GAPDH protein bands are shown depicting similar protein loads
Supplementary Table S1: Proteins associated with cardiac hypertrophy and cell apoptosis in EV-H. This table lists proteins identified in the extracellular vesicles (EVs) from the EV-H group, which received treatment with mesenchymal stromal cell-derived EVs subjected to hypoxia. The proteins are implicated in various biological processes and pathways related to cardiac hypertrophy and cell apoptosis. Atp2b1, Plasma membrane calcium-transporting ATPase 1; Atp6v1b2, V-type proton ATPase subunit B; Atp6v1e1, V-type proton ATPase subunit E1; C9, Complement component C9; Cacna2d1, Voltage-dependent calcium channel subunit alpha-2/delta-1; Camk2d, Calcium/calmodulin-dependent protein kinase type II subunit delta; Ctsd, Cathepsin D 12 kDa light chain; F2, Activation peptide fragment 1; Fn1, Fibronectin; Gsn, Gelsolin; Gzmb, Granzyme B; Kpnb1, Importin subunit beta-1; LOC103689983, Hypoxanthine-guanine phosphoribosyl transferase; Mapk1, Mitogen-activated protein kinase 1; Mapk3, Mitogen-activated protein kinase 3; Ppp2r1b, Serine/threonine-protein phosphatase 2 A 65 kDa regulatory subunit A beta; Psmc5, 26 S proteasome regulatory subunit 8; Psmd11, 26 S proteasome non-ATPase regulatory subunit 11; Psmd13, 26 S proteasome non-ATPase regulatory subunit 13; Rpl30, 60 S ribosomal protein L30; Slc3a2, 4F2 cell-surface antigen heavy chain. n = 4 flasks, each condition, technical replicate. Data were derived from 4 technical replicates for each condition and analyzed using Gene Ontology, KEGG, and Reactome software
Supplementary Table S2: Proteins associated with cardiac hypertrophy and cell apoptosis in the EV-N group. Actb, Actin cytoplasmic 1; Actc1, Actin alpha cardiac muscle 1; Actg1, Actin cytoplasmic 2; Ap1b1, AP-1 complex subunit beta-1; Ap2b1, AP-2 complex subunit beta; Col11a1, Collagen alpha-1(XI) chain; Col3a1, Collagen alpha-1(III) chain; Cpe, Carboxypeptidase E; EV-N, extracellular vesicles from mesenchymal stromal cells subjected to normoxia; Fn1, Fibronectin; Gsn, Gelsolin; Ltbp1, Latent-transforming growth factor beta-binding protein 1; Tpm1, Tropomyosin alpha-1 chain. n = 4 flasks, each condition, technical replicate. Data were obtained from 4 technical replicates for each condition and analyzed using Gene Ontology, KEGG, and Reactome software
Supplementary Table S3: All proteins detected in EVs. n = 4 flasks, each condition, technical replicate. Data were obtained from 4 technical replicates for each condition and analyzed using PatternLab software (version 5.0)
Supplementary Table S4: Proteins in EVs used for proteins interaction. Actb, Actin cytoplasmic 1; Actc1, Actin alpha cardiac muscle 1; Actg1, Actin cytoplasmic 2; Camk2d, Calcium/calmodulin-dependent protein kinase type II subunit delta; Col11a1, Collagen alpha-1(XI) chain; Col3a1, Collagen alpha-1(III) chain; EV-H, extracellular vesicles from mesenchymal stromal cells subjected to hypoxia; EV-N, extracellular vesicles from mesenchymal stromal cells subjected to normoxia; F2, Activation peptide fragment 1; Gsn, Gelsolin; Gzmb, Granzyme B; Mapk1, Mitogen-activated protein kinase 1; Mapk3, Mitogen-activated protein kinase 3; Ppp2r1b, Serine/threonine-protein phosphatase 2 A 65 kDa regulatory subunit A beta; Slc3a2, 4F2 cell-surface antigen heavy chain; Tpm1, Tropomyosin alpha-1 chain. n = 4 flasks, each condition, technical replicate. Data were analyzed by PatternLab software (version 5.0)
Data Availability Statement
The raw proteomics data are available in the ProteomeXchange Consortium via the Proteomics Identification Database with identifier PXD027718. All other data are available within the article and its supplementary material.