Abstract
Background
Root surface biomodification (RSB) enhances tissue attachment by removing the smear layer, facilitating collagen fibril formation, and promoting clot formation and stabilization. This study aimed to evaluate the efficacy of injectable platelet-rich fibrin (i-PRF), an autologous blood product, as a potential adjunct to ethylenediaminetetraacetic acid (EDTA) for RSB in gingival fibroblast attachment and proliferation in vitro.
Methods
Dentin discs (4 mm in diameter) underwent root surface debridement to remove damaged cementum. The discs were treated with 24% EDTA, followed by the application of i-PRF for 1, 3, 5-min. Enamel matrix derivatives (EMD) were used as a positive control (5 min application), while untreated discs served as the negative control. Cell viability was assessed using the XTT assay. Cellular morphology was examined via scanning electron microscopy (SEM), and cytoskeletal organization was analyzed using fluorescence microscopy (FM) analysis. Intracellular and extracellular alkaline phosphatase (iALP/eALP) gene expression levels were evaluated. Data was analyzed using ANOVA.
Results
XTT analysis revealed no statistically significant difference in cell viability between the i-PRF and the EMD groups at different time points. Actin filament organization was evident in the i-PRF 3-min group and became more pronounced in the i-PRF 5-min and the EMD groups. The i-PRF 3-min, i-PRF 5-min, and the EMD groups exhibited a spindle-shaped fibroblast morphology. No statistically significant difference was observed in iALP levels between the i-PRF 5-min and the EMD groups (p > 0.001). The EMD group exhibited the highest eALP level (p < 0.001), while the i-PRF 3-min and 5-min groups demonstrated the second highest levels.
Conclusion
The application of i-PRF for 5 min following EDTA treatment appears to enhance gingival fibroblast proliferation, attachment, and ALP expression. The effectiveness of i-PRF may vary depending on the duration of the application. Therefore, in vitro data from i-PRF followed by EDTA application to dentin surfaces may be useful for further development of in vivo and clinical approaches.
Keywords: EDTA, Fibrin, Fibroblasts, Fluorescence microscopy, Periodontal disease, Platelet rich fibrin, Scanning electron microscopy, Smear layer
Background
Periodontal therapy aims to create a favorable environment for restoring epithelial and connective tissue attachment to root surfaces affected by periodontal disease. Mechanical therapy, which involves for the debridement of periodontitis-affected root surfaces, seeks to eliminate the etiological agents of periodontal disease, restore biological compatibility, and establish conditions conducive to periodontal tissue reattachment [1]. However, complete decontamination of periodontitis-affected root surfaces through mechanical means alone is not feasible. This limitation arises because a smear layer forms on the root surface following manual or ultrasonic instrumentation. The smear layer has been shown to inhibit cell proliferation and differentiation, both of which are critical for periodontal wound healing [2].
To overcome the limitations of mechanical root surface debridement (RSD), chemical root surface modification techniques have been developed. These chemical methods enhance clot stabilization, as well as the migration and proliferation of healing-associated cells in the early stages of periodontal healing by removing the smear layer and eliminating cytotoxic bacterial byproducts [3]. The demineralization process exposes collagen, facilitating matrix synthesis. Studies from the early 1980s demonstrated that removing the smear layer using acidic solutions or calcium chelators significantly promoted fibroblast attachment [4–6].
Various root-conditioning agents have been investigated for root surface biomodification (RSB) to eliminate the smear layer and expose the collagen matrix [7–10]. While agents such as tetracycline hydrochloride and citric acid have been commonly used, excessive root dentin removal may occur when cementum is thin. More recently, laser applications (Nd: YAG, Er: YAG) have been explored as a more controlled alternative [11]. However, studies indicate that laser treatment alone, when used in conjunction with mechanical debridement, does not provide significant advantages over mechanical therapy alone [12]. This has led to an increased focus on agents that not only expose collagen but also actively promote wound healing. In periodontology, EDTA has emerged as a prevalent agent in root surface conditioning procedures. The synergy between EDTA gel application and RSD has been documented to facilitate the dissolution of the smear layer by exposing collagens on the root surface, thereby creating a conducive environment for cellular activity [13]. Following the introduction of EDTA, enamel matrix derivates (EMD) have gained widespread clinical utilization. These proteins serve to provide the structural framework essential for regeneration by promoting cellular adhesion and influencing cell proliferation [14]. EMD, a purified form of acidic material obtained from developing tooth germs originating from pig embryos, is a biological agent that facilitates the remineralization process while increasing cementoblast activity to the exposed collagen fibres. EMD also suppresses epithelial cells and prevents the migration of these cells to the wound site, thus creating the necessary environment for wound regeneration [15, 16].
During the periodontal healing process, the formation and stabilization of the blood clots are essential to preventing epithelial down-growth and ensuring proper tissue regeneration. Platelets play a key role in coagulation and release signaling molecules that regulate healing. Platelet concentrates such as platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) have been used in periodontal regeneration for over three decades due to their ability to enhance tissue repair through autologous sources. These blood-derived products have been shown to stimulate cell proliferation, differentiation, and periodontal tissue regeneration [17].
In recent years, PRF has gained preference over PRP due to the absence of anticoagulants, which have been suggested to negatively affect wound healing [18]. PRF has been widely utilized in bone and soft tissue regeneration processes because it serves as a growth factor delivery system, functions as a barrier membrane, and provides a three-dimensional fibrin scaffold for tissue repair. Its reported benefits include accelerated wound healing, enhanced angiogenesis, lower cost, and immunocompatibility. Advances in centrifugation techniques have led to the development of a liquid form of PRF, known as injectable platelet-rich fibrin (i-PRF), which retains higher concentrations of growth factors compared to traditional PRF formulations processed at higher speeds [19, 20]. Flow cytometry studies have shown that i-PRF contains the highest level of platelets, leukocytes, monocytes, and granulocytes among all PRF-based matrices [21]. While the chelating effect of EDTA on the tooth surface allows the release of collagen fibrils and growth factors from the dental matrix, although factors similar to solid PRF have been shown to be released after 10 days, i-PRF has been shown to have a much more gradual release of growth factors (PDGF-AA, -AB, TGF-B1 and VEGF) over time [22]. Due to its regenerative potential, i-PRF has been widely applied in periodontal soft tissue surgeries [23], gingival recession management [24], periodontitis treatment [25], guided bone regeneration (GBR) in conjunction with implant placement [26, 27], prevention of alveolar ridge atrophy [28] and sinus augmentation procedures [29].
Despite these advancements, no standardized research, protocol, or guideline exists regarding the use of i-PRF for root surface biomodification following EDTA application to enhance gingival fibroblast attachment. Considering that the effectiveness of i-PRF on the root surface has not been investigated in the literature yet and EMD was also planned to compare with the results obtained in i-PRF groups. This study aimed to evaluate the effects of i-PRF application following EDTA treatment on cell viability and morphology under in vitro conditions, as well as to determine the optimal application duration for maximizing its benefits.
Methods
This study was approved by the Ankara Yıldırım Beyazıt Research Ethics Committee (Approval date: 21/09/2020 Decision No: 2020–259). The flowchart of the study is shown in Fig. 1.
Fig. 1.

Schematic representation of the study design
Sample preparation
Extracted maxillary or mandibular anterior incisors from patients treated at Ankara Gazi University, Faculty of Dentistry were collected following informed consent. The study included incisors without grooves or fractures on the root surface extracted from patients with an indication of extraction aged 18 years and older. Single-rooted teeth were selected due to their smooth and broad root surfaces, which allow easy access and enable multiple samples to be prepared from a single tooth. Endodontically treated, impacted or non-vital teeth were excluded. The collected teeth were immediately cleaned from soft tissue residues and blood. The extracted teeth were stored in 0.5% w/v sodium azide solution at + 4 °C until use.
All specimens underwent root surface debridement (RSD), during which cementum was removed using twenty apicocoronal strokes of a Gracey 5–6 curette (Nordent Manufacturing Inc, Elk Grove Village, IL). To confirm successful root resurfacing, two specimens were analyzed using scanning electron microscopy (SEM) immediately after RSD. The exposure of the root dentin was verified and the specimens were stored in steril saline (%0.9 NaCl) at + 4 °C until further process. Before the disc preparation 70% ethyl alcohol solution was used for 15 min. Samples were washed three times with sterile saline to ensure that no residue remains on the dentin surface and prevents cell adhesion. Dentin discs (ø4mm) were obtained from the specimens using trephine burs under water cooling.
Groups
Samples were divided into five main groups; EDTA, i-PRF 1-min, i-PRF 3-min, i-PRF 5-min, and EMD. In the EDTA group, the dentin discs were only treated with 24% EDTA gel (Straumann® PrefGel®) for two minutes and irrigated with saline for 30 s according to the manufacturer instructions. In the EMD group, the dentin discs were treated with enamel matrix derivative (EMD) (Emdogain®, Straumann, Basel, Switzerland) for 5 min following EDTA treatment. In the i-PRF groups, the dentin discs were treated with i-PRF for 1, 3, and 5 min following EDTA treatment. The EDTA group was used as the negative control and the EMD group was used as the positive control.
i-PRF preparation
A 10 ml blood sample were collected with informed consent from one systemically healthy, non-smoking volunteer donor, and the blood was then collected in an anticoagulant-free PET plastic tube. The sample was centrifugated with slow centrifugation protocol of 700 rpm at 60 × g for 3 min at room temperature (PC-O2, Process for PRF, Nice, France). After centrifugation, the lower layer contained red blood cells, while the upper layer contained i-PRF, which was yellow orange in color. i-PRF was extracted with a disposable syringe and applied to dentin discs immediately upon preparation.
Human gingival fibroblast cells
Human gingival fibroblast (hGF) cells (PCS-201–018) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were stored in liquid nitrogen until use. The cells were thawed in a 37 °C water bath and suspended in 3 ml phosphate-buffered saline (PBS). After centrifugation at 200 × g for 5 min, the supernatant was discarded, and the cell pellet was resuspended in 5 ml Dulbecco’s Modified Eagle Medium (DMEM) (Capricorn Scientific GmbH, Germany) supplemented with 10% fetal bovine serum (FBS) (Biological Industries, Israel) and 1% penicillin–streptomycin (Biowest, France).
Cells were cultured in 25 cm2 polystyrene flasks (Costar, Corning Incorporated, USA) under 5% CO2 atmosphere at 37 °C. The media was refreshed every 48 h until 80% confluency.
Cell viability
Confluent cells were washed with 5 ml PBS and detached using 0.05% trypsin–EDTA (Biological Industries, Israel). The trypsinized cells were neutralized with twice the volume of DMEM and centrifuged at 200 × g for 5 min. The pellet was reconstituted with 1 ml DMEM and cell counting was performed using a TC 20 Automated cell counter (Bio-Rad, CA, USA). The dentin discs were placed into 96-well culture plates (Costar, Corning Incorporated, USA) in eight replicates for each group (EDTA, i-PRF 1-min, i-PRF 3-min, i-PRF 5-min, and EMD). The cells were seeded onto the discs the at 1 × 104 cells/well concentration. After 24 h incubation under 5% CO2 atmosphere and 37 °C, the XTT cell proliferation assay was performed following the kit manufacturer’s protocol (Biological Industries, Beit Haemek, Israel). Absorbance values were measured at 450 nm (test) and 600 nm (background) wavelengths using a multi-plate spectrophotometer (Varioscan Flash, Thermo Scientific, USA). Background absorbance at 600 nm was subtracted from the 450 nm absorbance, and percent cell viability was calculated relative to the negative control group.
Scanning electron microscopy analysis
The cells were seeded onto the dentin discs as described above. After 48 h incubation, the discs were washed with 37 °C PBS and fixed with 4% paraformaldehyde for 5 min. Samples were dehydrated sequentially in 10%, 30%, 60%, 75%, and 100% ethanol solutions (10 min each) and stored at + 4 °C until analysis.
Before imaging, specimens were gold-coated (Leica EM ACE200, Leica Microsystems, Germany) and examined using a Hitachi SU5000 field emission SEM at 10 kV accelerating voltage (Hitachi High Technologies Corp., Japan).
Fluorescence microscopy analysis
FAK100 Actin Cytoskeleton/Focal Adhesion Staining Kit (Sigma-Aldrich, USA) was used to visualize the actin organization in the cells. The cells were seeded onto the dentin discs as described above. After 48 h incubation, the cells were fixed in 4% paraformaldehyde prepared in PBS for 20 min, washed twice with PBS, and permeabilized with 0.1% Triton X-100 for 5 min. The actin filaments were stained with Tetramethylrhodamine (TRITC)-conjugated Phalloidin (1:500 dilution) for 30 min at room temperature. The nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI) (1:1000 dilution) for 5 min. Fluorescence images were obtained using a CKX41 fluorescence microscope (Olympus, Japan).
Alkaline phosphatase analysis
To assess mineralization potential, ALP expression was measured using a SensoLyte pNPP Alkaline Phosphatase Assay Kit (Anaspec ATC Group, USA) following the manufacturer’s protocol. The kit utilizes p-Nitrophenyl phosphate (pNPP) as a chromogenic substrate, which turns yellow upon dephosphorylation, allowing detection at 405 nm. The extracellular ALP levels were determined directly in the media collected from the wells. For intracellular ALP measurement, the cells were detached from the dentin discs as described above. After centrifugation at 200 × g for 5 min, the supernatant was discarded, and the cell pellet was resuspended in the assay buffer provided in the kit. The cell suspension was homogenized in an ultrasonic bath with 5 s bursts followed by 30 s cooling interval. The solution was checked under a microscope to confirm the homogenization was complete and centrifuged at 13,000 × g for 3 min. The supernatant was used for measurements.
Statistical analysis
Data were analyzed using IBM SPSS v25 (IBM, USA). Normality was assessed using the Shapiro–Wilk test. Data following normal distribution were presented as mean ± standard deviation (SD). Differences between groups in the XTT cell viability test and the ALP levels were analyzed using one-way ANOVA, followed by Tukey’s post-hoc test for pairwise comparisons. Tukey’s test was used to control for Type I error across multiple comparisons. A total of 10 pairwise comparisons were performed among the five groups in each analysis. Tukey’s test was chosen for its ability to control the family-wise error rate across multiple comparisons. A p-value < 0.05 was considered statistically significant.
Results
Cell viability
The percentage cell viability levels from the XTT method are shown in Fig. 3. One-way ANOVA test revealed a statistically significant difference between the groups (F (5.41) = 8.328 p < 0.05). In the Tukey post hoc test, cell viability was found to be a statistically significant lower in the EDTA group compared to the other groups. No statistically significant difference was observed among the i-PRF 1-min, the i-PRF 3-min, the i-PRF 5-min, and the EMD groups (p > 0.05) (Fig. 2).
Fig. 3.

SEM micrographs of the hGF cells grown on the dentin discs for 48 h (a) EDTA group (500x) (b) EDTA + PRF 1-min group (500x) (c) EDTA + PRF 3-min group (500x) (d) EDTA + PRF 5-min group (500x) (e) EMD group (500x)
Fig. 2.
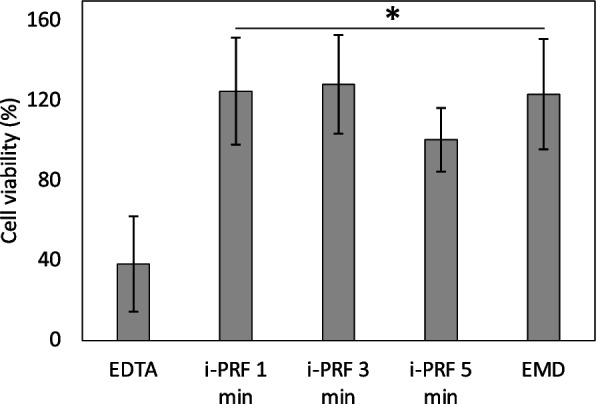
Percentage cell viability levels from the XTT assay: Descriptive statistics of XTT OD (absorbance) values, *: p < 0.05
Scanning electron microscopy
SEM imaging was used to examine the morphological characteristics of GF cells. In the EDTA group, it was observed that the cells retained their spherical morphology and were not well-spread on the surface (Fig. 3a). In the i-PRF 1-min group, the cells appeared to be more spread (Fig. 3b), but pseudopods were not fully elongated, suggesting that adhesion was in its early stages. In the i-PRF 3-min (Fig. 3c), the i-PRF 5-min (Fig. 3d), and the EMD groups (Fig. 3e), the cells have appeared to acquire the characteristic spindle-like fibroblast morphology and have adhered well to the surface.
Fluorescence microscopy
Fluorescence microscopy was used to qualitatively examine the morphological structures of GFs on the applied materials. The cytoskeletal organization of the cells was visualized using double staining with DAPI and TRITC-conjugated Phalloidin. Well-organized actin filaments appeared dense and parallel to each other, signifying strong cellular attachment. The best cytoskeletal organization was observed in the i-PRF 5-min and the EMD groups (Fig. 4d and 4e). In the EDTA and the i-PRF 1-min groups, shorter and less organized fibrils were observed (Fig. 4a and 4b). In the i-PRF 3-min group (Fig. 4c), fibrils appeared to be organized but were less prominent compared to the i-PRF 5-min and the EMD groups (Fig. 4d and 4e).
Fig. 4.
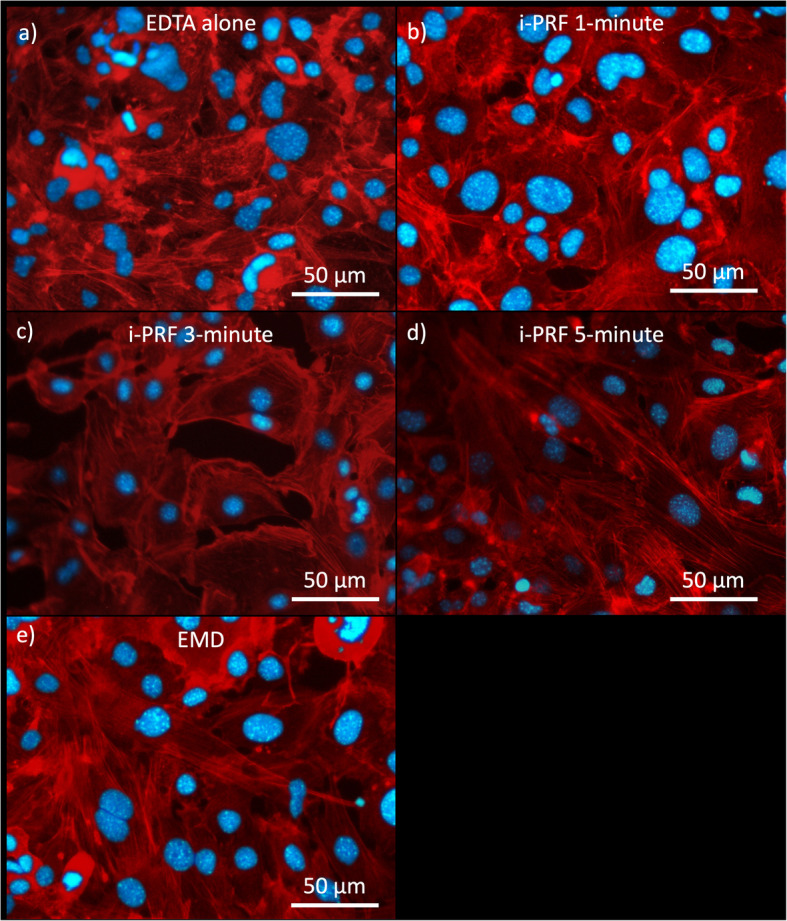
Merged fluorescence images of the hGF cells grown on the dentin discs for 48 h (Blue: DAPI, Red: TRITC) (a) EDTA group (500x) (b) EDTA + PRF 1-min group (500x) (c) EDTA + PRF 3-min group (500x) (d) EDTA + PRF 5-min group (500x) (e) EMD group (500x)
Alkaline phosphatase
ALP levels were assessed based on a calibration curve of ALP standard supplied with the kit. The descriptive statistics for intracellular and extracellular ALP levels are presented in Table 1.
Table 1.
XTT OD (absorbance) and intracellular ALP and extracellular ALP concentration values (Mean ± SD)
| Groups (Mean ± SD) | XTT | iALP (ng/ml) | eALP (ng/ml) |
|---|---|---|---|
| Control | 0,7689 ± 0,12,619 | 0,1484 ± 0,00827 | 0,3276 ± 0,02464 |
| RSD + 24% EDTA (2 min) | 0,2954 ± 0,25,574 | 0,1967 ± 0,00630 | 0,3547 ± 0,00872 |
| RSD + 24% EDTA (2 min) + i-PRF 1-min | 0,9603 ± 0,28,636 | 0,1935 ± 0,00125 | 0,4579 ± 0,00133 |
| RSD + 24% EDTA (2 min) + i-PRF 3-min | 0,9869 ± 0,2635 | 0,2242 ± 0,00241 | 0,6725 ± 0,07734 |
| RSD + 24% EDTA (2 min) + i-PRF 5-min | 0,7731 ± 0,17,249 | 0,2942 ± 0,00666 | 0,6536 ± 0,04517 |
| RSD + 24% EDTA (2 min) + EMD | 0,9487 ± 0,29,514 | 0,3144 ± 0,00249 | 0,8772 ± 0,00407 |
SD Standard deviation, RSD Root surface debridement, i-PRF İnjectable Platelet Rich Fibrin, EMD Emdogain, SEM Scanning electron microscopy, i-ALP Intracellular alkaline phosphatase analysis, e-ALP Extracellular Alkaline phosphatase analysis, XTT Tetrazolium Salt Test
Intracellular ALP concentration was found to be lower than the extracellular ALP concentration (F(4.9) = 324.088 p < 0.001). No statistically significant difference was found in intracellular ALP between the EDTA and i-PRF 1-min groups and these groups were found to be significantly lower compared to the i-PRF 3-min, i-PRF 5-min and EMD groups (p < 0.001). i-PRF 3-min group was found to be significantly lower compared to the i-PRF 5-min and EMD groups (p < 0.001). No significant difference was observed between the i-PRF 5-min and the EMD groups (Fig. 5a).
Fig. 5.
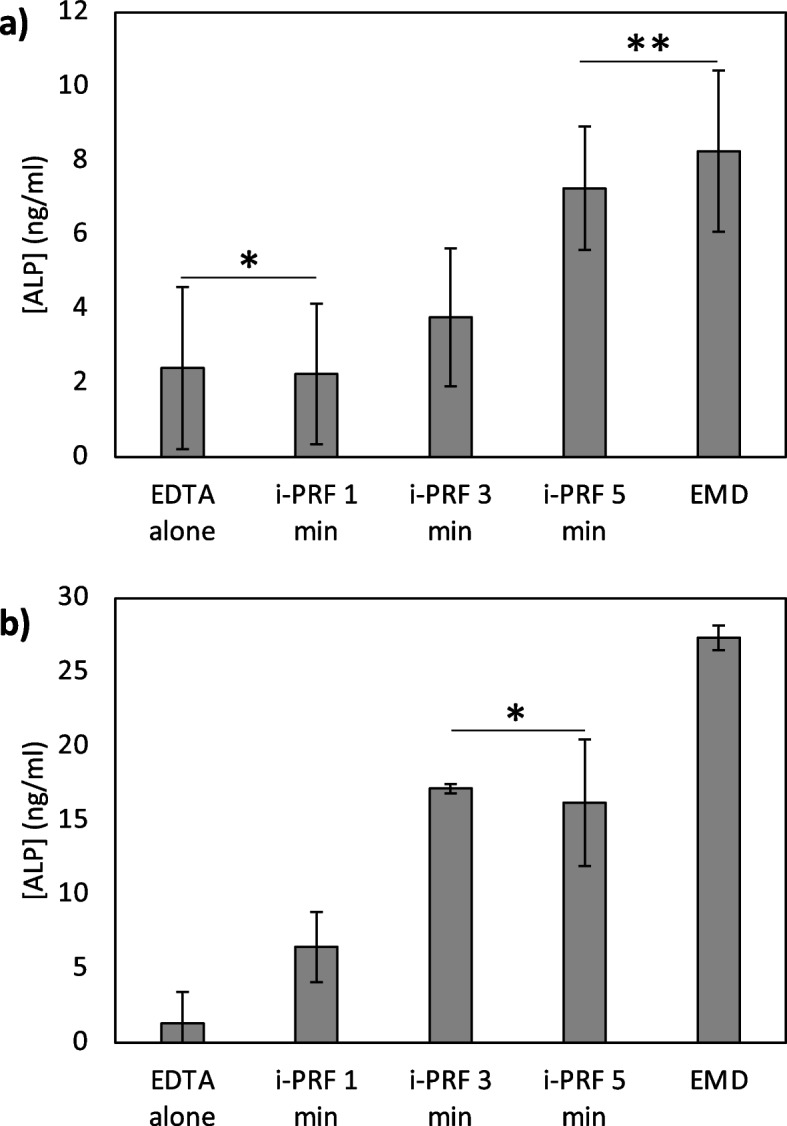
Intracellular (a) and extracellular (b) ALP concentrations (* and ** indicates no statistically significant difference between the groups)
A statistically significant difference in the extracellular ALP levels found among the groups (F (4.9) = 50.910, p < 0.001). No significant difference was observed between the EDTA and i-PRF 1-min groups and these groups were found to be significantly lower compared to the i-PRF 3-min, i-PRF 5-min and EMD groups (p < 0,001). No statistically significant difference was observed between the i-PRF 3-min and i-PRF 5-min groups. EMD group was found to be significantly higher compared to the other groups (Fig. 5b).
Discussion
The lack of an optimized protocol for root surface biomodification indicates the need for new methods and based on this point, this study was aimed to use the regenerative properties of i-PRF as a root surface biomodifier. This study compared the effects of i-PRF and EMD on cell viability, adhesion, cytoskeletal organization, and alkaline phosphatase (ALP) activity in hGFs and evaluated the effect of treatment duration for i-PRF. The findings demonstrate that treatment with EDTA alone may create a less favorable environment for cellular activity, while i-PRF and EMD treatment following EDTA treatment enhances cell viability, adhesion, and differentiation.
Blömlöf et al. [30] applied EDTA at concentrations of 1.5%, 5%, 15%, and 24% for 2 min and evaluated their efficacy. It was reported that 24% EDTA was the concentration that removed the smear layer and exposed the most collagen fibrils compared to all other concentrations. In the light of this information, we preferred to apply 24% EDTA gel for 2 min after RDS procedure, based on the neutral pH and demineralization ability of EDTA. The experimental studies showing that 24% EDTA effectively removes the smear layer produced by scaling and root planing exposes the collagenous matrix with minimal mineral disturbance from the dentin or cementum surface. In contrast, etching with citric and phosphoric acids appears to remove not only the mineral component but also the collagenous matrix [9, 13, 30–32]. In a recently published study by Tapashetti et al., the effectiveness of MTAD, which contains 4.25% citric acid and 3% doxycycline (an isomer of tetracycline), in combination with i-PRF was evaluated in vitro by SEM surface analysis on the formation and adhesion of fibrin network on treated dentin surfaces. In this study, it was stated that MTAD can be used to biomodify the root surface and that its combination with i-PRF can provide a significant advantage in terms of periodontal regeneration by providing greater adhesion of the fibrin network to the dentin surface [33]. Due to the low pH of other RSBs (e.g. citric acid, tetracycline), EDTA, a neutral pH chelating agent that is commercially available and used in the clinic, was preferred as a root surface biomodifying agent because it is safer in terms of the risk of complications in gingival tissues [22]. Therefore, EDTA was preferred to compare the ability of i-PRF to bind to collagen matrice exposed on demineralized dentin surfaces with EMD.
Miron et al. [19] introduced i-PRF obtained with low centrifugation force and speed (700 rpm, 3 min, 60 g). According to the Miron’s et al. protocol [19], as a result of reducing the centrifugation time, a more porous fibrin network can be obtained, which helps leukocytes adhere more, and growth factors can be released more quickly. In the same study, i-PRF was compared with PRP, and growth factor release, PDGF, TGF-β, and type 1 collagen expression were investigated for 10 days. As a result, it was reported that i-PRF had a higher fibroblast migration than the other groups, and increased expressions of the parameters were determined [19]. Therefore, i-PRF acts as a scaffold biological material for the accumulation of adherent cells at the intervention sites with a 3D fibrin matrix [34]. For example, a recently published systematic review reported that i-PRF injection alone may be clinically effective in increasing gingival thickness and keratinized tissue in patients with thin gingival phenotype with at least 4 applications at 10-day intervals [35]. A recent study published by Gowda et al. revealed that the expression of growth factors was significantly higher when partially demineralized dental matrix was mixed with i-PRF compared to when combined with platelet-rich fibrin [36].
The XTT assay revealed a significant difference in cell viability between the groups, with the EDTA group showing the lowest viability compared to all other groups. The i-PRF and EMD groups revealed higher viability levels, with no significant differences among them. These findings align with previous studies demonstrating that growth factor-rich biomaterials [37, 38], such as i-PRF and EMD, support fibroblast viability by promoting cellular proliferation and matrix interactions [18, 19, 21].
SEM imaging provided further evidence of the differences in cellular adhesion and morphology across the groups. In the EDTA group, cells retained a spherical morphology and showed limited spreading, indicating weak attachment to the surface. The i-PRF 1-min group showed early signs of adhesion, while the i-PRF 3-min, i-PRF 5-min, and EMD groups demonstrated fully elongated, adherent fibroblasts. Similarly, in a study the effect of i-PRF on the cell adhesion, morphological changes and proliferation of gingival mesenchymal stem cells (GMSCs) using pretreated collagen scaffolds was examined. When i-PRF was used alone, the cells showed moderate penetration and rounded morphology. It was stated that i-PRF could be used as a xenofree (animal source-free) alternative in clinical applications, as it provided cell proliferation similar to FBS [39].
Fluorescence microscopy confirmed the SEM findings by providing a qualitative assessment of cytoskeletal organization. The EDTA and i-PRF 1-min groups exhibited sparse, short actin filaments, indicating poor cytoskeletal organization and weak attachment. As exposure time to i-PRF increased, the actin cytoskeleton became more structured, with the most organized filamentous actin observed in the i-PRF 5-min and EMD groups. The dense, parallel actin filaments observed in the i-PRF 5-min and EMD groups are indicative of strong cellular anchorage, which may contribute to improved regenerative outcomes in periodontal applications. The microscopy findings together suggest that longer exposure to i-PRF, particularly 3 to 5-minuteutes, enhances cellular attachment, potentially due to sustained release of bioactive factors that support integrin-mediated adhesion [19, 40].
ALP was assessed as a key marker of early osteogenic differentiation for both intracellular and extracellular fractions. Intracellular ALP levels were found to be significantly lower than extracellular levels across all groups. This is expected as Phosphoethanolamine/phosphocholine phosphatase (PHOSPHO1) is the primary intracellular phosphatase located within matrix vesicles of mineralizing cells [41], while ALP is an extracellular enzyme that hydrolyzes inorganic pyrophosphate, a potent inhibitor of hydroxyapatite formation, thus increasing the availability of inorganic phosphate in the extracellular matrix. This regulation promotes the growth and maturation of hydroxyapatite crystals in the collagenous bone matrix.
The EDTA and i-PRF 1-min groups showed the lowest ALP activity, indicating limited osteogenic potential [42]. The i-PRF 3-min and i-PRF 5-min groups exhibited a progressive increase in ALP activity, with the highest values observed in the EMD group. These findings suggest that prolonged exposure to i-PRF not only enhances fibroblast adhesion but also osteogenic differentiation. Similarly, i-PRF exhibits better biological properties, including the ability of osteoblasts to proliferate, differentiate, and produce mineralized nodules on either tissue-culture plastic or titanium discs, than PRP, A-PRF, L-PRP, and freeze-dried homologous PRP [43–45]. The EMD group demonstrated the highest ALP levels, which is consistent with previous studies highlighting the osteoinductive potential of enamel matrix proteins. These proteins are known to stimulate fibroblast proliferation, extracellular matrix deposition, and osteogenic differentiation [46, 47], making them highly effective in regenerative treatments. In a recent study published by Ramenzoni et al., previously decontaminated titanium (Ti) discs were compared with fibrin (i-PRF), advanced platelet-rich fibrin (A-PRF +) or enamel matrix derivatives (EMDs) with known osteoinducing potential. Similar to our study, osteoblasts seeded onto i-PRF and EMD-treated surfaces were found to have increased adhesion and migration, and significant increases of up to 2.8-fold in ALP, OC, ON, RUNX-2 and COL1a2 mRNA levels [48]. Even though three- and five-minutes treatment with i-PRF yielded lower ALP levels compared to EMD, a considerable increase was obtained compared to the EDTA alone or one-minute treatment with i-PRF. Although the i-PRF 5-min group showed slightly lower ALP activity compared to the EMD group, the difference was not statistically significant. Given that i-PRF is an autologous preparation with significantly lower cost and greater accessibility than EMD, it represents a clinically practical and economically favorable alternative, particularly in resource-limited settings.
The clinical potential of i-PRF extends beyond periodontal regeneration and has shown promising results in broader oral wound healing applications, including the management of medication-related osteonecrosis of the jaws and enhancement of post-extraction healing. Recent studies have highlighted the regenerative benefits of autologous hemoconcentrates such as i-PRF in these contexts, reinforcing the need to refine application protocols to maximize therapeutic benefits across various indications [49]. These findings highlight the translational relevance of i-PRF and its potential in oral tissue regeneration.
The findings of this study have important clinical implications for periodontal regeneration and wound healing. The significant differences in cell viability, adhesion, and differentiation potential among the groups suggest that i-PRF and EMD are superior to EDTA alone in terms of promoting fibroblast function. The time-dependent effect of i-PRF indicates that application protocols can be further optimized to enhance clinical outcomes. It is important to note that while our in vitro model allows for controlled evaluation of fibroblast behavior, it does not fully replicate the complex biological environment encountered in vivo. Key elements such as the presence of inflammatory mediators, microbial biofilms, host immune responses, and mechanical forces are absent in this experimental setting. These factors play a critical role in wound healing and regenerative outcomes and should be considered when interpreting the clinical relevance of our findings. Therefore, further in vivo studies are essential to validate the translational potential of i-PRF in periodontal therapy.
Although this study provides preliminary data, which requires further demonstration, that the use of i-PRF in combination with EDTA may be an alternative to EMD by inducing mineralization of fibroblasts on the dentin surface, the main limitation of this study is that it was performed on a specific cell line under laboratory conditions. The methodology of this study does not fully replicate the complex microenvironment of periodontal tissues, although the study was performed on root surfaces. Further investigations should focus on the molecular pathways underlying the observed effects, in particular the role of specific growth factors and signaling pathways involved in fibroblast adhesion and differentiation tests with quantitative analysis. In addition, the effects of i-PRF on periodontal, bone and gingival tissues are likely to be comparable to those of EMD, taking into account possible recent findings [17, 48, 50], and these data should be complemented by in vivo or clinical studies that can replicate dynamic and variable conditions in living organisms. Future studies should also evaluate the long-term effects of i-PRF and EMD on fibroblast activity to determine their sustained regenerative potential.
A further limitation of this study is that i-PRF was prepared from a single donor. Given the known variability in platelet concentration and growth factor composition across individuals, the results may not be representative of broader patient populations. Future studies should incorporate samples from multiple donors to account for inter-individual differences and enhance the generalizability of the findings.
Conclusion
In conclusion, this study demonstrates that i-PRF and EMD enhance fibroblast viability, adhesion, and differentiation when applied with conjunction to EDTA treatment. The time-dependent effects of i-PRF suggest that exposure duration plays a crucial role in maximizing its regenerative potential. The in vitro findings provide a basis for optimising periodontal regeneration procedures and demonstrate the clinical potential of i-PRF in cases where conventional biomodification agents used for periodontal tissue regeneration have limited efficacy.
Acknowledgements
Not applicable
Abbreviations
- ALP
Alkaline Phosphatase
- EDTA
Ethylene Diamine Tetra Acetic Acid
- EMD
Enamel matrix derivates
- FBS
Fetal Bovine Serum
- GF
Gingival Fibroblast
- i-PRF
Injectable platelet-rich fibrin
- SEM
Scanning Electron Microscope
- PRP
Platelet-Rich Plasma
- PRF
Platelet-Rich Fibrin
- RSB
Root surface biomodification
- RSD
Root surface debridement
- XTT
Tetrazolium Salt Test
Authors’ contributions
All authors contributed substantially to the study's conception and design. CA and MG performed the cell culture, biochemical, and data analysis. CA and SG drafted the manuscript. All authors reviewed the manuscript and approved the final version of the manuscript.
Funding
This study was supported by Gazi University Scientific Research Projects Coordination Unit (BAP) with project number TDH-2021–7120.
Data availability
The datasets analysed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Ankara Yıldırım Beyazit Research Ethics Committee (Ethics Committee approval date: 21/09/2020 Decision No: 2020–259). The authors stated that all experiments were performed in accordance with relevant guidelines and regulations. Informed consent was obtained from patients for the collection of isolated teeth.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jepsen S, Deschner J, Braun A, Schwarz F, Eberhard J. Calculus removal and the prevention of its formation. Periodontol 2000. 2011;55(1):167–88. [DOI] [PubMed] [Google Scholar]
- 2.Bosshardt DD, Sculean A. Does periodontal tissue regeneration really work? Periodontol 2000. 2009;51(1):208–19. [DOI] [PubMed] [Google Scholar]
- 3.Mariotti A. Efficacy of chemical root surface modifiers in the treatment of periodontal disease. A systematic review. Ann Periodontol. 2003;8(1):205–26. [DOI] [PubMed] [Google Scholar]
- 4.Polson A, Proye M. Effect of root surface alterations on periodontal healing. II. Citric acid treatment of the denuded root. J Clin Periodontol. 1982;9(6):441–54. [DOI] [PubMed] [Google Scholar]
- 5.Caton J, Polson A, Prato GP, Bartolucci E, Clauser C. Healing after application of tissue-adhesive material to denuded and citric acid-treated root surfaces. J Periodontol. 1986;57(6):385–90. [DOI] [PubMed] [Google Scholar]
- 6.Polson AM, Frederick GT, Ladenheim S, Hanes PJ. The production of a root surface smear layer by instrumentation and its removal by citric acid. J Periodontol. 1984;55(8):443–6. [DOI] [PubMed] [Google Scholar]
- 7.Sterrett J, Bankey T, Murphy H. Dentin demineralization: the effects of citric acid concentration and application time. J Clin Periodontol. 1993;20(5):366–70. [DOI] [PubMed] [Google Scholar]
- 8.Labahn R, Fahrenbach WH, Clark SM, Lie T, Adams DF. Root dentin morphology after different modes of citric acid and tetracycline hydrochloride conditioning. J Periodontol. 1992;63(4):303–9. [DOI] [PubMed] [Google Scholar]
- 9.Blomlöf J, Jansson L, Blomlof L, Lindskog S. Long-time etching at low pH jeopardizes periodontal healing. J Clin Periodontol. 1995;22(6):459–63. [DOI] [PubMed] [Google Scholar]
- 10.Sterrett J, Simmons J, Whitford G, Russell C. Tetracycline demineralization of dentin: the effects of concentration and application time. J Clin Periodontol. 1997;24(7):457–63. [DOI] [PubMed] [Google Scholar]
- 11.Fekrazad R, Lotfi G, Harandi M, Ayremlou S, Kalhori KA. Evaluation of fibroblast attachment in root conditioning with Er, Cr: YSGG laser versus EDTA: a SEM study. Microsc Res Tech. 2015;78(4):317–22. [DOI] [PubMed] [Google Scholar]
- 12.Aoki A, Mizutani K, Taniguchi Y, Lin T, Ohsugi Y, Mikami R, Katagiri S, Meinzer W, Iwata T. Current status of Er: YAG laser in periodontal surgery. Jpn Dent Sci Rev. 2024;60:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blomlöf JP, Blomlöf LB, Lindskog SF. Smear removal and collagen exposure after non-surgical root planing followed by etching with an EDTA gel preparation. J Periodontol. 1996;67(9):841–5. [DOI] [PubMed] [Google Scholar]
- 14.Hammarström L. Enamel matrix, cementum development and regeneration. J Clin Periodontol. 1997;24(9):658–68. [DOI] [PubMed] [Google Scholar]
- 15.Miron R, Dard M, Weinreb M. Enamel matrix derivative, inflammation and soft tissue wound healing. J Periodontal Res. 2015;50(5):555–69. [DOI] [PubMed] [Google Scholar]
- 16.Miron RJ, Sculean A, Cochran DL, Froum S, Zucchelli G, Nemcovsky C, Donos N, Lyngstadaas SP, Deschner J, Dard M. Twenty years of enamel matrix derivative: the past, the present and the future. J Clin Periodontol. 2016;43(8):668–83. [DOI] [PubMed] [Google Scholar]
- 17.Fujioka-Kobayashi M, Katagiri H, Kono M, Schaller B, Zhang Y, Sculean A, Miron RJ. Improved growth factor delivery and cellular activity using concentrated platelet-rich fibrin (C-PRF) when compared with traditional injectable (i-PRF) protocols. Clin Oral Invest. 2020;24:4373–83. [DOI] [PubMed] [Google Scholar]
- 18.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e37–44. [DOI] [PubMed] [Google Scholar]
- 19.Miron RJ, Fujioka-Kobayashi M, Hernandez M, Kandalam U, Zhang Y, Ghanaati S, Choukroun J. Injectable platelet rich fibrin (i-PRF): opportunities in regenerative dentistry? Clin Oral Invest. 2017;21:2619–27. [DOI] [PubMed] [Google Scholar]
- 20.Choukroun J. Advanced PRF, & i-PRF: platelet concentrates or blood concentrates. J Periodontal Med Clin Pract. 2014;1(1):3. [Google Scholar]
- 21.Choukroun J, Ghanaati S. Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients’ own inflammatory cells, platelets and growth factors: the first introduction to the low speed centrifugation concept. Eur J Trauma Emerg Surg. 2018;44:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saleh MHA, Dias DR, Ravida A, Wang H-L: Root surface biomodification in periodontal therapy: Biological rationale and clinical applications. Periodontology 2000 2024, n/a(n/a). [DOI] [PubMed]
- 23.Mourão CFdAB, Valiense H, Melo ER, Mourão NBMF, Maia MD-C: Obtention of injectable platelets rich-fibrin (i-PRF) and its polymerization with bone graft. Revista do Colégio Brasileiro de Cirurgiões 2015, 42:421–423. [DOI] [PubMed]
- 24.İzol BS, Üner DD. A new approach for root surface biomodification using injectable platelet-rich fibrin (I-PRF). Med Sci Monit. 2019;25:4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vučković M, Nikolić N, Milašin J, Đorđević V, Milinković I, Asotić J, Jezdić Z, Janković S, Aleksić Z. The effect of injectable platelet-rich fibrin use in the initial treatment of chronic periodontitis. Srp Arh Celok Lek. 2020;148(5–6):280–5. [Google Scholar]
- 26.Işık G, Özden Yüce M, Koçak-Topbaş N, Günbay T. Guided bone regeneration simultaneous with implant placement using bovine-derived xenograft with and without liquid platelet-rich fibrin: a randomized controlled clinical trial. Clin Oral Invest. 2021;25(9):5563–75. [DOI] [PubMed] [Google Scholar]
- 27.Kurtiş B, Şahin S, Gürbüz S, Yurduseven S, Altay C, Kurtiş B, Ayyıldız S, Barış E. Vertical bone augmentation with customized CAD/CAM titanium mesh for severe alveolar ridge defect in the posterior mandible: a case letter. J Oral Implantol. 2023;49(2):147–56. [DOI] [PubMed] [Google Scholar]
- 28.Amaral Valladão CA, Freitas Monteiro M, Joly JC. Guided bone regeneration in staged vertical and horizontal bone augmentation using platelet-rich fibrin associated with bone grafts: a retrospective clinical study. Int J Implant Dent. 2020;6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irdem H, Dolanmaz D, Esen A, Ünlükal N, Şimsek S. Evaluation of the effectiveness of liquid platelet-rich fibrin and deproteinized bovine bone mineral mixture on newly formed bone in maxillary sinus augmentation: a split-mouth, histomorphometric study. Niger J Clin Pract. 2021;24(9):1366–72. [DOI] [PubMed] [Google Scholar]
- 30.Blomlöf J, Blomlöf L, Lindskog S. Effect of different concentrations of EDTA on smear removal and collagen exposure in periodontitis-affected root surfaces. J Clin Periodontol. 1997;24(8):534–7. [DOI] [PubMed] [Google Scholar]
- 31.Blornlöf J, Lindskog S. Periodontal tissue-vitality after different etching modalities. J Clin Periodontol. 1995;22(6):464–8. [PubMed] [Google Scholar]
- 32.Sculean A, Berakdar M, Willershausen B, Arweiler NB, Becker J, Schwarz F. Effect of EDTA root conditioning on the healing of intrabony defects treated with an enamel matrix protein derivative. J Periodontol. 2006;77(7):1167–72. [DOI] [PubMed] [Google Scholar]
- 33.Tapashetti R, Bhutani N, Deodurg S, Kulkarni A. A comparative evaluation of efficacy of root surface biomodification using MTAD, MTAD+I-PRF on adhesion of fibrin clot to dentin sem study. J Pharm Bioallied Sci. 2024;16(Suppl 1):S431-s433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adhikary R, Mohan P, Wadhawan A, Tyagi P. Gingival augmentation in the thin phenotype using injectable platelet-rich fibrin and microneedling. Cureus. 2023;15(6): e40435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Idris MI, Burhan AS, Hajeer MY, Sultan K, Nawaya FR. Efficacy of the injectable platelet-rich fibrin (i-PRF) in gingival phenotype modification: a systematic review and meta-analysis of randomized controlled trials. BMC Oral Health. 2024;24(1):1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gowda BHH, Krishna R, Srinivasan S, Astekar M. Comparing the release of growth factors when partially demineralized tooth matrix is mixed with platelet rich fibrin and injectable platelet rich fibrin: an in-vitro observational study. J Oral Maxillofac Pathol. 2024;28(3):415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puig-Herreros C, Sanz JL, García-Bernal D, Rodríguez-Lozano FJ, Murcia L, Forner L, Ghilotti J, Oñate-Sánchez RE, López-García S. Comparative cytotoxicity of menthol and eucalyptol: an in vitro study on human gingival fibroblasts. Pharmaceutics. 2024. 10.3390/pharmaceutics16040521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanz JL, López-García S, Forner L, Rodríguez-Lozano FJ, García-Bernal D, Sánchez-Bautista S, Puig-Herreros C, Rosell-Clari V, Oñate-Sánchez RE. Are endodontic solvents cytotoxic? An in vitro study on human periodontal ligament stem cells. Pharmaceutics. 2022. 10.3390/pharmaceutics14112415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghadge KK, Shetty SK, Kharat A, Kheur S, Kulloli A, Gopalakrishnan D, Bhonde R. Translational implications of a novel combination of iPRF and collagen scaffold for proliferation of gingival mesenchymal stem cells. Sci Rep. 2024;14(1):27789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marrelli M, Tatullo M. Influence of PRF in the healing of bone and gingival tissues. Clinical and histological evaluations. Eur Rev Med Pharmacol Sci. 2013;17(14):1958–62. [PubMed] [Google Scholar]
- 41.Roberts SJ, Stewart AJ, Sadler PJ, Farquharson C. Human PHOSPHO1 exhibits high specific phosphoethanolamine and phosphocholine phosphatase activities. Biochem J. 2004;382(Pt 1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Golub EE, Boesze-Battaglia K. The role of alkaline phosphatase in mineralization. Curr Opin Orthop. 2007;18(5):444–8. [Google Scholar]
- 43.Wang X, Zhang Y, Choukroun J, Ghanaati S, Miron RJ. Effects of an injectable platelet-rich fibrin on osteoblast behavior and bone tissue formation in comparison to platelet-rich plasma. Platelets. 2018;29(1):48–55. [DOI] [PubMed] [Google Scholar]
- 44.Dohle E, El Bagdadi K, Sader R, Choukroun J, James Kirkpatrick C, Ghanaati S. Platelet-rich fibrin-based matrices to improve angiogenesis in an in vitro co-culture model for bone tissue engineering. J Tissue Eng Regen Med. 2018;12(3):598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah R, Thomas R, Gowda TM, Baron TKA, Vemanaradhya GG, Bhagat S. In vitro evaluation of osteoblast response to the effect of injectable platelet-rich fibrin coating on titanium disks. J Contemp Dent Pract. 2021;22(2):107–10. [PubMed] [Google Scholar]
- 46.Wu S-M, Chiu H-C, Chin Y-T, Lin H-Y, Chiang C-Y, Tu H-P, Fu MM, Fu E. Effects of enamel matrix derivative on the proliferation and osteogenic differentiation of human gingival mesenchymal stem cells. Stem Cell Res Ther. 2014;5:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li G, Hu J, Chen H, Chen L, Zhang N, Zhao L, Wen N, Yang Y. Enamel matrix derivative enhances the proliferation and osteogenic differentiation of human periodontal ligament stem cells on the titanium implant surface. Organogenesis. 2017;13(3):103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramenzoni LL, Varghese J, Schmidlin PR, Mehrotra S. Effects of i-PRF, A-PRF+, and EMD on osteogenic potential of osteoblasts on titanium. Clin Implant Dent Relat Res. 2025;27(1): e13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bennardo F, Barone S, Antonelli A, Giudice A. Autologous platelet concentrates as adjuvant in the surgical management of medication-related osteonecrosis of the jaw. Periodontol 2000. 2025;97(1):287–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alsabri GA, van der Horst F, Alkaabi SA, Alavi SA, Forouzanfar T, Helder MN. Evaluating growth-factor release in leukocyte- and platelet-rich fibrin, advanced platelet-rich fibrin, and injectable platelet-rich fibrin protocols: a narrative review. Growth Factors. 2024;42(4):216–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author upon reasonable request.


