Abstract
Iron oxide nanoparticles (IONPs) have transitioned from conventional magnetic resonance imaging (MRI) contrast agents into structurally programmable combined imaging/treatment tools, leveraging their superparamagnetism, catalytic activity, and surface engineering versatility to achieve spatiotemporal control over drug delivery and immune modulation. Advances in nanofabrication now yield size-optimized aggregates with enhanced tumor accumulation through the enhanced permeability and retention (EPR) effect, while clinically approved formulations like ferumoxytol demonstrate intrinsic immunomodulatory functionality, positioning IONPs as pivotal tools for precision oncology. Conversely, cancer immunotherapy remains limited by the immunosuppressive tumor microenvironment (TME), where cellular suppression via M2-polarized macrophages and regulatory T cells (Tregs) synergizes with physical exclusion from dense extracellular matrices and metabolic sabotage through lactate-driven acidosis. These barriers establish “immune-cold” phenotypes characterized by deficient CD8⁺ T-cell infiltration and tertiary lymphoid structure formation, driving checkpoint inhibitor resistance with sub-30% response rates in solid tumors. To overcome these constraints, IONPs orchestrate multimodal immunotherapeutic strategies: they reprogram suppressive niches by polarizing macrophages toward M1 phenotypes, activate STING pathways, and induce immunogenic ferroptosis; enable precision delivery via magnetic lymph node targeting and cancer cell membrane-mediated homologous tumor homing; and facilitate real-time theranostics through MRI/magnetic particle imaging (MPI)-monitored immune cell trafficking. Preclinical validation confirms synergistic efficacy, with combinatorial regimens achieving over 50% complete tumor regression by converting immunologically cold microenvironments into inflamed states. This review systematically explores cutting-edge IONP-based innovations—spanning immune cell engineering, biohybrid systems, and energy-amplified therapies—that bridge localized tumor eradication with systemic antitumor immunity, while critically evaluating translational barriers for clinical implementation.
Graphical abstract
Keywords: Iron oxide nanoparticles (IONPs), Immunotherapy, Tumor microenvironment reprogramming, Multimodal theranostics, Biohybrid nanoplatforms
Introduction
Iron oxide nanoparticles (IONPs) are a promising tool in oncological nanomedicine, characterized by their intrinsic superparamagnetism, biocompatibility, and ability to undergo flexible surface modifications [1–4]. Originally developed as magnetic resonance imaging (MRI) contrast agents—exemplified by clinically established formulations like ferumoxytol—IONPs have evolved into multifunctional combined imaging/treatment tools designed to precisely control where and when treatments act [3–7]. Their unique capabilities include magnetic navigation for enhanced tumor-targeted delivery, stimulus-triggered drug release activated by tumor microenvironment (TME)-related cues, and catalytic generation of cytotoxic species that simultaneously induce immunogenic cell death (ICD) [8–12]. New designs now boost clinical potential significantly: optimized tiny structures improve body distribution patterns while maintaining renal clearance capacity; surface modifications via biomimetic coatings significantly prolong systemic circulation; and enhanced magnetic responsiveness enables efficient energy conversion under clinically relevant conditions [3, 13–18]. Platelet membrane coatings further demonstrate active metastasis suppression through P-selectin-mediated circulating tumor cell capture [19]. Unlike conventional biomimetic coatings, recent IONP designs integrate dual-pathway metastasis interception—simultaneously capturing CTCs and clearing immunosuppressive exosomes—surpassing the single-mechanism targeting of earlier NanoGhosts platforms [20]. These developments collectively address historical challenges in tumor penetration and retention efficiency. Clinically, IONP-based systems demonstrate integrated diagnostic-therapeutic functionality. Ferumoxytol not only corrects iron deficiency but also modulates macrophage polarization in ongoing oncology trials, while blood-brain barrier-penetrating designs show marked efficacy in aggressive glioblastoma models [7, 21–25]. SPION-labeled cellular therapies further exemplify this convergence, enabling non-invasive monitoring of immune cell trafficking dynamics [26–29]. As precision oncology evolves, IONPs have emerged as indispensable biological tools that reconcile nanoscale engineering with therapeutic complexity, delivering superior targeting precision while mitigating systemic toxicity across preclinical and clinical applications [30–33].
The TME plays a critical role in shaping the immune response within solid tumors, orchestrating a complex network of immunosuppressive barriers [34–38]. This network is composed of biological, physical, and metabolic factors that work synergistically to suppress immune function [39–41]. Cellular suppression is predominantly mediated by M2-polarized tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and regulatory T cells (Tregs) [42–48]. These cells secrete immunosuppressive factors such as interleukin-10 (IL-10), transforming growth factor-β (TGF-β), and adenosine, which inhibit the activity of cytotoxic T lymphocytes and prevent dendritic cell maturation [49–54]. Additionally, the upregulation of immune checkpoint molecules such as PD-L1, CTLA-4, and TIM-3 exacerbates T-cell exhaustion, further contributing to immune evasion [35, 36, 55–57]. The TME is also characterized by several physical barriers, including a dense extracellular matrix comprised of components like collagen and hyaluronic acid [34, 58, 59]. These structural elements create interstitial pressures exceeding 40 mmHg, which impede the effective penetration of therapeutic agents and hinder immune cell infiltration [60, 61]. Moreover, abnormal tumor vasculature disrupts perfusion and promotes hypoxia, exacerbating the challenges faced by immune cells and therapeutic agents alike [62–64]. Metabolically, hypoxia-driven acidosis and lactate accumulation suppress the cytotoxic activity of natural killer (NK) and T cells, while nutrient competition further starves effector cells [65–68]. Together, these factors create a poorly immunogenic tumor state, characterized by low CD8+ T-cell infiltration and a lack of tertiary lymphoid structures, leading to response rates below 30% to checkpoint inhibitors in solid tumors [69–71].
IONPs offer a transformative solution to overcoming these barriers by integrating diagnostic, therapeutic, and immune-altering abilities. Their intrinsic ability to reprogram TAMs toward antitumor phenotypes, induce ICD, and deliver immune checkpoint inhibitors directly addresses the immunosuppressive mechanisms within the TME [27, 72, 73]. Furthermore, real-time monitoring via MRI and magnetic particle imaging enables the personalization of therapeutic strategies, allowing for dynamic adjustments based on treatment response [4, 74–76]. This review highlights cutting-edge IONP-based strategies-ranging from engineered immune cells and biomimetic systems to energy-triggered immunomodulation—that bridge localized tumor targeting with the activation of systemic antitumor immunity.
Advancements in ionps for overcoming biological barriers in cancer immunotherapy and precision medicine
Overcoming biological barriers: the role of nanoparticles in precision medicine
Nanoparticles, particularly IONPs, are increasingly recognized for their potential to revolutionize cancer treatment. Their unique properties enable them to overcome numerous biological barriers that traditionally hinder the efficacy of conventional therapies, especially in oncology [77–79]. These barriers are multifaceted, encompassing challenges related to cellular uptake, immune system evasion, and precise tissue-specific delivery [80–82]. Conventional drugs often fail to penetrate cell membranes effectively due to their size and electrostatic repulsion, limiting their ability to target tumors with the required specificity [83, 84]. IONPs, on the other hand, can be designed to overcome these delivery challenges more accurately, enhancing the drug delivery process and improving the therapeutic index [12, 85, 86].
One of the key obstacles in cancer therapy is achieving efficient cellular uptake of therapeutic agents. Traditional drugs face challenges in crossing the cell membrane, due to size limitations and electrostatic repulsion [87, 88]. However, nanoparticles, particularly those modified with surface coatings, can overcome these hurdles. For example, IONPs can be engineered with specific surface modifications that improve cellular binding and facilitate internalization [89–91]. This can occur either passively, through the enhanced permeability and retention (EPR) effect, or actively, using targeting ligands that specifically bind to cell surface receptors [92, 93]. The ability of IONPs to selectively target tumor cells significantly reduces off-target effects, thereby improving the therapeutic outcome and minimizing toxicity to healthy tissues [94–96]. This enhanced targeting capability is particularly crucial in cancer therapy, where maximizing treatment efficacy while reducing side effects is paramount.
In addition to overcoming cellular uptake challenges, nanoparticles like IONPs also address the issue of immune system evasion. The immune system constantly monitors the body for foreign invaders, including therapeutic agents, which it neutralizes through processes such as phagocytosis [97–99]. This poses a significant challenge for the effective delivery of cancer therapies. To circumvent this issue, IONPs can be engineered to evade immune detection. Surface modifications, such as PEGylation, provide a “stealth” layer that prolongs the circulation time of the nanoparticles, reducing their clearance by the immune system [100–102]. This is particularly important for therapeutic nanoparticles that need to remain in circulation long enough to reach their target tissues. This extended circulation time is crucial for immunotherapies, which often require prolonged interaction with the immune system to achieve optimal therapeutic effects.
Moreover, IONPs can significantly improve targeted tissue delivery, a cornerstone of precision medicine [103–105]. One of the most important advantages of nanoparticles is their ability to accumulate preferentially in tumor tissues. This is due to the leaky vasculature typical of tumors, a phenomenon referred to as the EPR effect [106, 107]. However, while the EPR effect facilitates some degree of tumor targeting, it may not be sufficient for precise and effective treatment. Therefore, IONPs can be further engineered with specific targeting ligands that recognize overexpressed tumor markers, such as epidermal growth factor receptors (EGFR) or other cancer-specific antigens [108–110]. By ensuring that the nanoparticles selectively accumulate within the TME, therapeutic agents can be delivered directly to the site of disease, minimizing systemic exposure and reducing side effects. Overall, Fig. 1 illustrates a dual-ring framework summarizing nanomedicine advancements.
Fig. 1.
This schematic illustrates a dual-ring framework summarizing nanomedicine advancements. The inner ring denotes key biological barriers addressable by engineered nanoparticles—including cellular internalization hurdles, immune clearance evasion, and tissue-specific targeting—while the outer ring highlights precision medicine applications enhanced by these strategies. Nanoparticle platforms demonstrate capabilities to overcome these physiological constraints, thereby optimizing therapeutic delivery. Representative applications encompass chimeric antigen receptor (CAR) immunotherapies, epidermal growth factor receptor (EGFR)-targeted interventions, exploitation of the enhanced permeability and retention (EPR) effect, and CRISPR-based delivery of guide RNA (gRNA)/ribonucleoprotein (RNP) complexes. Collectively, these innovations expedite clinical translation of precision therapeutics
Applications of nanoparticles in precision medicine
The integration of nanoparticles into precision medicine holds tremendous promise for improving cancer treatment outcomes. IONPs, in particular, offer unique capabilities that can dramatically enhance the efficacy of various cancer therapies. A prominent application of IONPs is in chimeric antigen receptor (CAR) therapies [111, 112]. CAR-T cell therapy has demonstrated remarkable success in treating hematological cancers by genetically modifying a patient’s T cells to express receptors that target tumor antigens. However, the delivery and activation of CAR-T cells within the body remains a significant challenge. IONPs can be used to deliver genetic material—such as mRNA or DNA encoding for CAR constructs—directly to T cells, ensuring efficient modification and improved anti-tumor activity [113]. Moreover, IONPs can be used to track the distribution of CAR-T cells and monitor their infiltration into tumors using MRI [114]. This provides valuable real-time information on treatment progress and enables dynamic adjustments to therapy, enhancing the overall effectiveness of CAR-T cell therapy.
Another significant application is the use of IONPs for targeted drug delivery, particularly to cancer cells overexpressing tumor-specific receptors. One of the most extensively studied targets is the EGFR, which is overexpressed in a variety of cancers, including non-small cell lung cancer (NSCLC) and glioblastomas [108, 115, 116]. By utilizing IONPs to deliver chemotherapeutic agents, immunomodulators, or RNA-based therapeutics—such as guide RNA (gRNA) and ribonucleoprotein (RNP) complexes—specifically to these tumors, it is possible to achieve higher local concentrations of the therapeutic agents [117–119]. This targeted delivery ensures that the treatment is highly effective, while minimizing systemic side effects that are often seen with conventional chemotherapy. Additionally, the application of external magnetic fields can further enhance the targeting of IONPs to the tumor site, ensuring greater precision in drug delivery and reducing the likelihood of off-target effects.
Comparative analysis of ionps and alternative nanoplatforms
IONPs exhibit distinctive advantages for cancer immunotherapy that merit rigorous assessment relative to alternative nanotechnologies. IONPs demonstrate superior magnetic responsiveness enabling precise tumor targeting capabilities not achievable with non-magnetic platforms [10]. Their intrinsic catalytic activity facilitates reactive oxygen species (ROS)-dependent therapeutic mechanisms, including ferroptosis induction and chemodynamic modulation, outperforming gold nanoparticles in these fundamental processes [120].
In contrast, gold nanoparticles possess enhanced photothermal conversion properties that render them particularly suitable for photoimmunotherapy applications [121, 122]. Polymeric nanoparticles offer greater payload versatility and more predictable biodegradation kinetics, advantageous for sustained immunomodulator delivery [123, 124]. Lipid-based systems maintain dominance in nucleic acid delivery applications despite limited tumor-targeting specificity [125, 126].
Clinical translation landscapes reveal significant differentiation among platforms. IONPs lead in regulatory approvals for human use, though applications beyond iron supplementation remain investigational [4]. Gold nanoparticles show promise in localized therapies but exhibit delayed progress in systemic immunotherapeutic applications [127]. Polymeric and lipid platforms demonstrate established clinical utility for conventional drug delivery yet face challenges in achieving multifunctional immunotherapeutic integration comparable to IONPs [128, 129].
Therapeutic limitations further distinguish these technologies. IONPs present potential oxidative safety concerns requiring careful dosing optimization [75, 130]. Gold nanoparticles suffer from penetration depth restrictions in deep-seated malignancies [131]. Polymeric carriers may trigger batch-dependent immune reactions, while lipid systems demonstrate suboptimal extrahepatic biodistribution [132]. This technological ecosystem positions IONPs advantageously for magnetically guided immunotherapeutic strategies, with alternative platforms serving complementary roles in precision oncology [133].
The potential roles of ionps in cancer immunotherapy
The potential of IONPs to address these critical challenges in cancer immunotherapy and precision medicine is vast. However, successful clinical translation of these technologies requires overcoming several key hurdles. One of the primary challenges is the scalability and reproducibility of nanoparticle production, which is essential for clinical use. Advances in nanomaterial synthesis, quality control, and manufacturing processes will be crucial to meet the stringent requirements for human use. Additionally, the long-term safety of nanoparticles must be rigorously evaluated, including potential toxicity, immune reactions, and biodistribution.
IONPs: intrinsic activators of antitumor immunity
IONPs naturally stimulate immune responses, positioning them as promising agents for cancer immunotherapy. Intrinsic immune-altering abilities of IONPs were showed in Table 1. A notable example is ferumoxytol, a clinically approved formulation, which has demonstrated therapeutic efficacy in treating early-stage breast cancer and lung metastases. Ferumoxytol exerts its effect by polarizing TAMs toward a pro-inflammatory M1 phenotype. Systematic TAM reprogramming approaches follow in Sect. "Reprogramming TAMs via iron oxide nanoplatforms". In vitro studies have shown that ferumoxytol enhances macrophage production of reactive oxygen species (ROS) and augments cancer cell cytotoxicity. Furthermore, in vivo experiments have reported significant suppression of subcutaneous adenocarcinoma growth in murine models, with effects observed at both high and low concentrations, suggesting a dose-independent response [134]. Importantly, this tumor-suppressive effect appears to be independent of nanoparticle coating, as similar outcomes were observed with dextran-coated ferumoxtran-10 [134].
Table 1.
Intrinsic immune-altering abilities of ionps
| Nanoparticle type | Cancer model | Immune mechanism | Key findings | Therapeutic outcomes | References |
|---|---|---|---|---|---|
| Ferumoxytol | Early-stage breast cancer, lung metastasis | Enhances macrophage ROS production | Coating-independent tumor growth suppression | Inhibits subcutaneous adenocarcinoma growth; potential “off-label” use to prevent metastasis | [134] |
| Induces M1 macrophage polarization | Equivalent efficacy at high/low concentrations | ||||
| Fe₃O₄ NPs | B16-OVA melanoma (subcutaneous/metastatic) | Acts as “nanoimmunoenhancer” | First evidence of Fe₃O₄ immunostimulatory function beyond imaging/drug delivery | Prevents tumor formation; suppresses growth of established tumors | [135] |
| Stimulates DC maturation, T-cell activation, macrophage activation | |||||
| FH-MPLA | Immunotherapy-resistant melanoma | Activates antitumor macrophage phenotype | No chemical modification of nanoparticles/drugs required | Synergizes with α-CD40 mAb to induce tumor necrosis/regression | [29] |
| (Ferumoxytol + MPLA) | Reshapes tumor-immune microenvironment | ||||
| Glycosylated IONPs | Solid tumors | Direct interaction with TLR4-MD2 complex | Pro-inflammatory potency exceeds LPS in specific conditions | Triggers M1 macrophage polarization; enhances antitumor immunity | [136] |
| Activates NF-κB/MAPK/STAT1 pathways | |||||
| IONP-COOH | AML | Complement-dependent phagocytosis of tumor cells | Surface chemistry dictates immune effectiveness | Indirect tumor cell killing; no direct cytotoxicity | [2] |
| (Carboxymethyl dextran-coated) | Activates innate/adaptive immunity | IONP-NH₂/IONP-OH ineffective due to distinct protein corona |
MPLA monophosphoryl lipid A, DC dendritic cells, LPS lipopolysaccharide, AML acute myeloid leukemia, IONP-COOH carboxymethyl dextran-coated iron oxide nanoparticles, IONP-NH₂ amino-modified iron oxide nanoparticles, IONP-OH hydroxyl-modified iron oxide nanoparticles
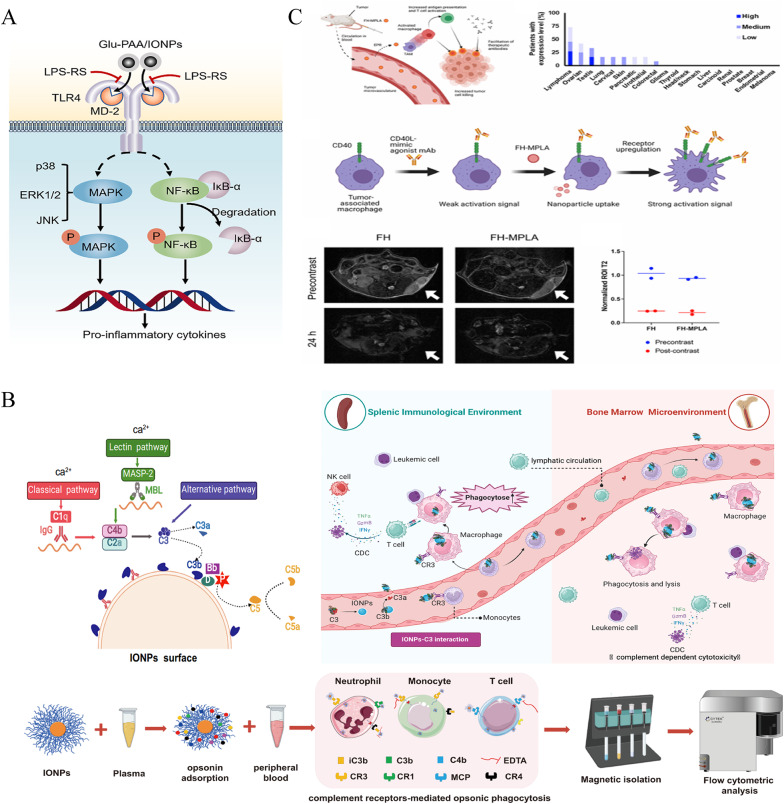
Beyond its clinical formulations, IONPs—especially ultrasmall Fe3O4 nanoparticles—act as potent immune enhancers when conjugated with model antigens like ovalbumin (OVA). The Fe3O4-OVA nanocomposite has been shown to stimulate dendritic cell maturation, promote T-cell activation, and engage macrophages. This composite effectively inhibits both subcutaneous and metastatic B16-OVA tumor growth in therapeutic and prophylactic settings [135]. The immunomodulatory properties of IONPs are further amplified through surface engineering. For instance, glucosylation of IONPs transforms them into powerful agonists of the TLR4-MD2 complex, surpassing lipopolysaccharide (LPS) in their ability to activate the NF-κB, MAPK, and STAT1 signaling pathways, which in turn drive M1 polarization and enhance antitumor immunity [136] (Fig. 2A).
Fig. 2.
Iron oxide nanoparticles: intrinsic activators of antitumor immunity. A Schematic illustration: LPS-RS prevents the interaction of Glu-PAA/IONP with TLR4-MD2 and the activation of downstream signaling.Reproduced with permission [136]. Copyright from Elsevier Ltd, 2024. B Nanoparticles dynamically interact with immune cells via complement corona to exert antileukaemia efficacy.Reproduced with permission [2]. Copyright from Springer Nature, 2024. The interactions occurring at the IONP-complement interface are crucial in shaping the immunological outcomes. Iron oxide nanoparticles (IONPs) can opsonize with complement component C3b, subsequently engaging the C3 receptor (CR3) on circulating monocytes. These activated monocytes then migrate to immune organs and differentiate into resident macrophages. Concurrently, the IONP-C3b complex enhances the phagocytic activity of these resident macrophages against leukemia cells. Moreover, macrophages are capable of presenting tumor antigens to T cells, thereby activating adaptive anti-leukemia immunity. C Therapeutic scheme and imaging of nanoparticle deposition in tumors.Reproduced with permission [29]. Copyright from American Chemical Society, 2023. General framework for FH-MPLA immunotherapy in solid tumors. CD40 expression analyzed by IHC on cancer samples from human patients, archived in the Human Protein Atlas bioinformatics repository. CD40 expression is predominantly observed in lymphomas; most solid tumors exhibit negligible expression. Potential mechanism of FH-MPLA in augmenting α-CD40 therapy: FH-MPLA enhances CD40 expression on antigen-presenting cells, thereby amplifying activation by agonistic α-CD40 monoclonal antibodies. Representative T2-weighted MRI contrast images of mice pre- and post-injection with either unloaded FH or FH-MPLA nanoparticles. Tumor ROI indicated by white arrows. Quantification of T2 signal intensity within the tumor ROI, normalized to CSF signal intensity in pre- and post-contrast scans.
The immune response induced by IONPs is heavily influenced by their surface chemistry. For example, carboxymethyl dextran-coated IONPs (IONP-COOH) have been shown to indirectly inhibit the progression of acute myeloid leukemia (AML) by complement-dependent enhancement of macrophage phagocytosis, which triggers tumor antigen presentation and initiates adaptive immune responses. In contrast, amino or hydroxyl-modified IONPs show limited efficacy, likely due to the divergent protein corona formation on their surfaces, which affects immune recognition [2] (Fig. 2B). This chemical specificity offers a strategic framework for designing nanoplatforms with optimized immunostimulatory capabilities.
In terms of clinical translation, ferumoxytol-loaded monophosphoryl lipid A (FH-MPLA) has been developed to reprogram macrophages toward an antitumor phenotype. When combined with α-CD40 monoclonal antibody therapy, FH-MPLA induces tumor necrosis and regression in melanoma models that are resistant to conventional immunotherapy. This combination strategy demonstrates dual targeting of both adaptive and innate immune systems without requiring chemical modifications, underscoring its translational potential for clinical application [29] (Fig. 2C).
Multifunctional iron oxide platforms for coordinated Immunomodulation
Advanced IONP-based platforms have emerged as sophisticated vehicles for the co-delivery of therapeutic agents, enabling real-time imaging and targeted tumor accumulation. These systems leverage engineered nanostructures to overcome barriers in drug delivery while orchestrating multimodal antitumor responses. Clinically oriented designs often incorporate magnetic navigation to minimize off-target effects. Table 2 summarized the multifunctional iron oxide platforms for coordinated immunomodulation. For instance, IO@FuDex3 nanomedicine, which integrates T-cell activators with anti-PD-L1, has significantly extended median survival in preclinical models, while also reducing adverse events compared to soluble checkpoint inhibitors [137].
Table 2.
Multifunctional iron oxide platforms for coordinated Immunomodulation
| Nanoparticle system | Cancer model | Core components | Delivery strategy | Key mechanisms | Therapeutic outcomes | References |
|---|---|---|---|---|---|---|
| Zn-doped IONPs + TLR agonists | Aggressive melanoma | Zn-doped Fe₃O₄ MNPs | MRI-trackable vaccine delivery | Synergistic T-cell activation | Significant tumor suppression in aggressive melanoma | [141] |
| PolyIC (TLR3 agonist) | Immune checkpoint blockade | Enhanced tumor-specific immunity | ||||
| R837 (TLR7 agonist) | ||||||
| OVA antigen | ||||||
| Anti-PD-L1/PD-1 | ||||||
| IO@FuDex3 | Solid tumors | SPIONs + fucoidan/aldehyde dextran | Magnetic navigation to tumor site | Tumor immune escape inhibition | Adverse events; median survival vs. soluble anti-PD-L1 | [137] |
| Anti-PD-L1 | Minimized off-target effects | TIL reactivation | ||||
| Anti-CD3/CD28 (T-cell activators) | ||||||
| MIRDs | Broad applicability | Fe₃O₄ MPs + ICG (core) | Long circulation | PTT-triggered tumor ablation | Synergistic PTT/immunotherapy inhibits growth/metastasis/recurrence | [142] |
| DPA-PEG + R837 (shell) | MRI-guided magnetic targeting | R837-enhanced immune response | ||||
| SPIO NP@M-P | Lung cancer | SPIO NPs wrapped in H460 membrane | Homologous tumor targeting | PD-L1 blockade | TPP1 half-life 60; suppresses tumor growth in vitro/vivo | [139] |
| TPP1 (PD-L1 inhibitor) | MMP2-triggered release | T-cell reactivation | ||||
| PLGLLG (MMP2 substrate) | ||||||
| Fe₃O₄-ICG@IRM | Ovarian cancer | Fe₃O₄-ICG core | Homologous targeting | PTT-induced antigen release | Effective against primary/metastatic tumors | [140] |
| Hybrid ID8/RBC membrane (IRM) | Prolonged circulation | Enhanced antitumor immunity | ||||
| Fe₃O₄ core NP | Triple-negative breast cancer | Fe₃O₄ core | Tumor-specific targeting | Direct tumor killing | Tumor growth/metastasis; survival in aggressive model | [191] |
| Doxorubicin | DC-mediated immune activation | Innate/adaptive immune activation | ||||
| Poly(I: C) (TLR3 agonist) | ||||||
| Magnetic silica-PLGA hybrid | Broad applicability | pH-sensitive carrier | pH-responsive drug release | PD-L1 gene silencing | Tumor growth vs. monotherapy; tumor cell apoptosis | [143] |
| Paclitaxel (PTX) | Enhanced cellular uptake | CD8⁺ T-cell-mediated cytotoxicity | ||||
| PD-L1 siRNA | ||||||
| GOx@FeNPs | Colorectal cancer | Fe₃O₄ core/PDA shell | Dual tumor targeting | GOx-enhanced ferroptosis | > 90% tumor suppression with anti-PD-L1 synergy | [12] |
| GOx | NIR-triggered PTT | PTT-induced ICD DC/CTL activation | ||||
| PEG/cRGD/AA (dual targeting) |
MNPs magnetic nanoparticles, TIL tumor-infiltrating lymphocytes, PTT photothermal therapy, ICG indocyanine green, RBC red blood cell, cRGD cyclic arginylglycylaspartic acid, AA ascorbic acid, GOx glucose oxidase, ICD immunogenic cell death, DC Dendritic cells, CTL Cytotoxic T lymphocytes
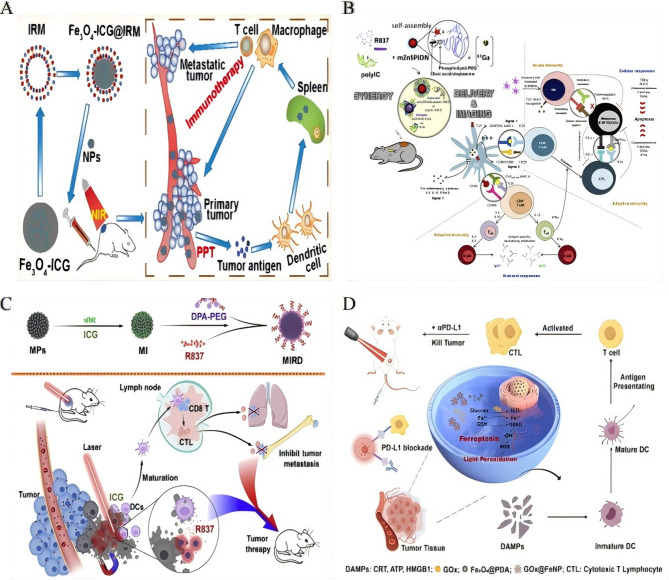
Bioinspired targeting strategies further enhance tumor specificity. Current IONP-lipid hybrid systems achieve > 15% higher drug loading efficiency than mesoporous silica/carbon quantum dots (MSNP-CQD) while maintaining redox-responsive release, enabling real-time MRI-guided chemo-immunotherapy unattainable with static MSNP-CQD platforms [138]. For example, SPIO NP@M-P nanoparticles, cloaked in H460 lung cancer cell membranes and conjugated with a PD-L1-inhibitory peptide, prolonged the peptide half-life by 60-fold compared to free counterparts. This prolonged release was achieved through an MMP2-cleavable substrate, which effectively reactivated T cells and contributed to enhanced therapeutic efficacy [139]. Similarly, Fe₃O₄-ICG@IRM platforms coated with hybrid membranes derived from ID8 ovarian cancer cells and erythrocytes demonstrated prolonged circulation times and homologous targeting. These platforms enabled photothermally triggered antigen release, contributing to suppression of metastatic tumor growth [140] (Fig. 3A).
Fig. 3.
Multifunctional iron oxide platforms for coordinated immunomodulation. A The biomimetic Fe3O4-ICG@IRM nanoparticles showed synergistic photothermal-immunotherapy for ovarian cancer.Reproduced with permission [140]. Copyright from American Chemical Society, 2021. B Self-assembly achieved by simple physical mixture of the vaccine and nanovaccine components promotes effective drug synergy, delivery and imaging. Adjuvant and tumour antigen uptake by DCs at site of injection and/or via direct trafficking to LNs is able to activate potent and longlasting immunity against the B16-F10(OVA) melanoma cells, in particular when the expression of PD-L1 is downregulated.Reproduced with permission [141]. Copyright from Elsevier Ltd, 2018. C Once targeting to the tumor, the MIRDs with the near-infrared (NIR) irradiation caused tumor ablation and resulted in tumorassociated antigens releasing to induce the body's immunological response, which was markedly improved it to attack the tumors with the R837 releasing from the outer DPA-PEG.Reproduced with permission [142]. Copyright from Elsevier Ltd, 2020. D Schematic illustration of Gox@FeNPs-mediated PTT synergizing with ferroptosis by inducing ICD to improve colorectal cancer immunotherapy.Reproduced with permission [12]. Springer Nature, 2024.
Beyond individual modifications, composite systems can synergize immunomodulation with physical energy-based therapies. For instance, zinc-doped IONPs conjugated with TLR agonists co-delivered PolyIC and R837, activating complementary innate immune pathways. When combined with PD-1/PD-L1 blockade, this platform elicited robust tumor-specific T-cell responses against aggressive melanoma [141] (Fig. 3B). Another example, the MIRDs platform, co-loaded indocyanine green (ICG) and R837 into Fe₃O₄@DPA-PEG nanoparticles. Near-infrared (NIR) irradiation induced photothermal ablation and antigen release, while R837 enhanced antitumor immunity, leading to suppression of tumor recurrence [142] (Fig. 3C).
More complex composite systems, such as pH-responsive silica-PLGA hybrids, have been developed to co-encapsulate paclitaxel and PD-L1-targeting siRNA. These systems achieved sustained drug release and enhanced CD8⁺ T-cell-mediated tumor killing, improving overall therapeutic efficacy [143]. For metabolic modulation, GOx@FeNPs, which combine glucose oxidase (GOD)-enhanced ferroptosis with photothermal ICD and anti-PD-L1 blockade, have demonstrated impressive tumor suppression in colorectal cancer models. This outcome was achieved through dendritic cell maturation and cytotoxic T lymphocyte infiltration [12] (Fig. 3D).
Engineering immune cells with iron oxide nanoplatforms
The strategic integration of IONPs with immune effector cells enables precise spatiotemporal control over tumor targeting and therapeutic efficacy (Table 3). NK cells, known for their intrinsic antitumor activity, are particularly promising candidates for such engineering approaches. Magnetic delivery systems utilizing Fe₃O₄@PDA nanoparticles have been shown to enhance the recruitment of NK cells to NSCLC tumors under the influence of an external magnetic field. This targeted approach significantly increases tumor cell apoptosis without impairing NK cell functionality [144] (Fig. 4A). In a similar vein, umbilical cord-derived NK cells conjugated with IONPs, referred to as NK: IONP hybrids, maintain their phenotype while demonstrating enhanced cytolytic activity in both 2D and 3D tumor models, showcasing the potential of magnetically guided precision therapy [145] (Fig. 4B).
Table 3.
Engineering immune cells with iron oxide nanoplatforms
| Immune cell type | Nanoparticle system | Engineering strategy | Functional modifications | Key mechanisms | Therapeutic outcomes | References |
|---|---|---|---|---|---|---|
| NK cells | Fe3O4@PDA NPs | Magnetic core-dopamine shell internalization | Enhanced tumor infiltration | Magnetic guidance under external field | Apoptosis of NSCLC cells; tumor growth | [144] |
| (Primary) | Unaltered biological function | |||||
| NK cells | IONP-conjugated | Surface conjugation via biocoupling | Preserved phenotype/function | Magnetic localization | Cytolytic activity in 2D/3D models | [145] |
| (Umbilical cord-derived) | Magnetically navigable | |||||
| Macrophages | HA-coated SPIONs | Internalization of HIONs | Tumor targeting | Reprogramming TAMs to M1 phenotype | Synergistic tumor suppression | [150] |
| (Patient-derived) | (HIONs) | ROS/cytokine production | ||||
| Resistance to immunosuppressive TME | ||||||
| T lymphocytes | SPION Citrate | Surface functionalization | Blood-compatible | Magnetic enrichment in “cold” tumors | T cell attraction by external magnetism | [148] |
| Magnetic responsiveness | ||||||
| NK cells | Fe3O4@SiO2 CMNPs | Cancer cell membrane coating | Activation receptor expression | Tumor antigen presentation | Enhanced antitumor capability of NK cells | [146] |
| Cytotoxic factor secretion | ||||||
| NK-92 cells | Anti-CD56-Fe3O4 conjugates | Antibody-mediated conjugation | Magnetically drivable migration | MRI-trackable targeting | Tumor suppression in solid tumors | [147] |
| Human T cells | SPION-loaded | Surface loading | Unaltered cell mechanics | Magnetic enrichment without functional impairment | Retained tumor-killing ability post-loading | [149] |
| (Incl. CAR-T) | Preserved proliferation/cytotoxicity | |||||
| T cells/CAR-T cells | Ultrasmall IONPs | Surface labeling | Unaffected viability/proliferation | MRI-based spatial distribution monitoring | Predictive of therapy response via tumor infiltration | [114] |
| (Human & murine) | Unimpaired effector function | |||||
| CAR-T cells | SPION-loaded | Surface loading | Cytokine release | Reduced systemic toxicity | Retained specific cytolytic activity; MRI-detectable | [111] |
| Shift from pyroptosis to apoptosis |
PDA polydopamine, NSCLC non-small cell lung cancer, HIONs hyaluronic acid-coated superparamagnetic iron oxide nanoparticles, TME tumor microenvironment, CMNPs cancer cell membrane-coated nanoparticles, CAR-T chimeric antigen receptor T-cell, SPION superparamagnetic iron oxide nanoparticle
Fig. 4.
Engineering immune cells with iron oxide nanoplatforms. A Diagram illustrating the complete 3D molecular model used in the experiment.Reproduced with permission [144]. Copyright from Royal Society of Chemistry 2018. B Schematic illustration: designing magnetically responsive biohybrids composed of cord blood-derived natural killer cells and iron oxide nanoparticles.Reproduced with permission [145]. Copyright from American Chemical Society, 2019. C Schematic illustration: Cell membrane-encapsulated magnetic nanoparticles for enhancing natural killer cell-mediated cancer immunotherapy.Reproduced with permission [146]. Copyright from Elsevier Ltd, 2021. D A diagrammatic representation of the mechanism by which magnetically targeted NK-92 cells, in conjunction with iron oxide nanoparticles, suppress the proliferation of solid tumors. (DMSA: meso-2,3-dimercaptosuccinic acid and aCD56: anti-CD56 antibody).Reproduced with permission [147]. Copyright from Royal Society of Chemistry 2021. E The schematic representation illustrates that the engineered HION@Macs homing to tumors via active chemotaxis and magnetic navigation, secrete pro-inflammatory mediators (including TNF-α, NO, and ROS) to inhibit tumor growth, and reprogram in situ M2 macrophages into a pro-inflammatory M1 phenotype, facilitating a synergistic, cancer-specific therapeutic approach.Reproduced with permission [150]. Copyright from Wiley-VCH GmbH, 2019.
Bioinspired targeting strategies further enhance the therapeutic potential of these engineered cells. For instance, coating Fe₃O₄@SiO₂ nanoparticles with cancer cell membranes (CMNPs) enables the presentation of tumor-specific antigens to NK cells, thereby boosting the expression of activation receptors and enhancing cytotoxic factor secretion [146] (Fig. 4C). In the context of clinical translation, anti-CD56-conjugated Fe₃O₄ nanoparticles have been used to magnetically direct NK-92 cells to solid tumors. This approach not only facilitates targeted tumor suppression but also enables real-time MRI tracking, offering a non-invasive means of monitoring therapeutic outcomes [147] (Fig. 4D).
Similarly, T lymphocytes can benefit from magnetic functionalization, which enhances their targeting and efficacy. SPION-citrate-labeled T cells retain unimpaired viability and can be efficiently guided to immune-cold tumors via external magnetic fields, enhancing the effectiveness of checkpoint inhibitor therapy [148]. Notably, the loading of SPIONs on T cells preserves their proliferative capacity, tumor-killing ability, and functional integrity, which is crucial for the success of CAR-T cell therapies [149]. Ultrasmall IONP-labeled CAR-T cells retain their effector function while enabling MRI-based monitoring of tumor infiltration patterns, thus providing predictive insights into therapy response [114]. Interestingly, SPIONs have been shown to modulate CAR-T cell behavior by shifting the mode of tumor cell death from pyroptosis to apoptosis. This shift reduces the release of inflammatory cytokines and mitigates systemic toxicity, all while preserving cytolytic specificity [111].
Beyond effector cells, macrophages engineered with hyaluronic acid-coated SPIONs (HION@Macs) exhibit enhanced tumor-tropic properties, resistance to immunosuppression, and the ability to reprogram TAMs into an antitumor M1 phenotype. This reprogramming induces synergistic tumor suppression, further highlighting the potential of magnetically enhanced immune cells for comprehensive cancer therapy [150] (Fig. 4E).
Physical energy-amplified immunotherapy via iron oxide nanoplatforms
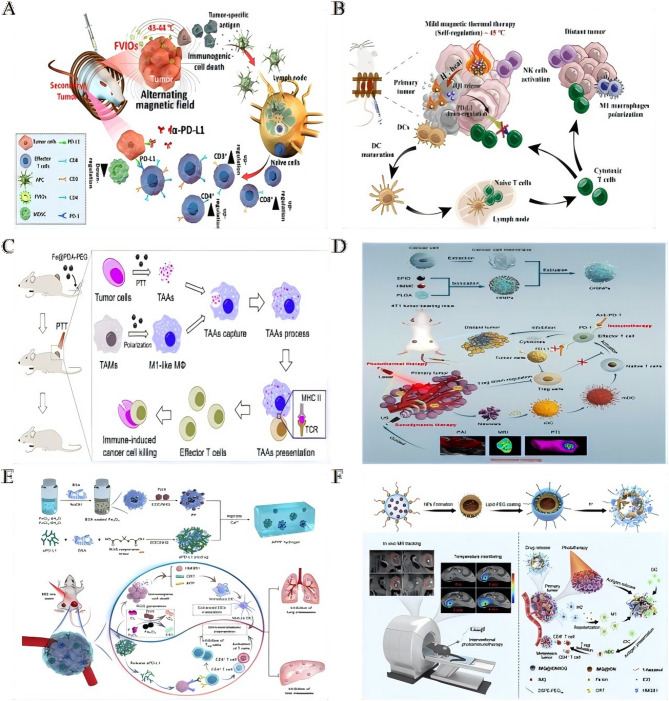
The integration of physical energy modalities with immunotherapeutic agents, via iron oxide-based platforms, enables precise spatiotemporal control and enhances antitumor immune responses (Table 4). Among these, magnetic hyperthermia has emerged as a potent immunogenic trigger. Ferromagnetic vortex-domain iron oxide nanorings (FVIOs) induce mild thermal stress, leading to the exposure of calreticulin (CRT) on tumor cells. This exposure initiates ICD, macrophage polarization, and CD8⁺ T-cell infiltration. When integrated with PD-L1 blockade (initially discussed in Sect. ''Multifunctional iron oxide platforms for coordinated immunomodulation''), FVIO-mediated hyperthermia significantly suppresses tumor recurrence and metastasis [151] (Fig. 5A). Similarly, temperature-responsive iron oxide nanoparticle assemblies (IONAs), loaded with the immunomodulator JQ1, deliver controlled heat and modulator release under alternating magnetic fields. This strategy effectively downregulates PD-L1 expression and eradicates primary tumors while preventing distal tumor growth [152] (Fig. 5B).
Table 4.
Physical energy-amplified immunotherapy via iron oxide nanoplatforms
| Nanoparticle system | Physical energy | Cancer model | Core components | Key mechanisms | Therapeutic outcomes | References |
|---|---|---|---|---|---|---|
| Ce6/Fe3O4-L | Ultrasound (SDT) | Solid tumors | Fe3O4 NPs (10 nm) | ROS-triggered Fe3O4 release | Eliminates SDT-treated & distant untreated tumors with anti-PD-L1 | [157] |
| Chlorin e6 (Ce6) | ETA capture/transport to LNs | |||||
| FVIOs | Mild magnetic hyperthermia | Metastatic tumors | FVIOs | CRT exposure “eat me” signal | CD8+ T-cell infiltration; recurrence/metastasis with PD-L1 blockade | [151] |
| ICD & macrophage polarization | ||||||
| MINPs | NIR-triggered PTT | Metastatic tumors | SPIO NPs | PA/MR imaging-guided ablation | Attacks residual/distant metastases | [162] |
| CpG ODN | TAAs release systemic immunity | |||||
| Fe@PDA-PEG | NIR-triggered PTT | Colon/breast cancer | Polydopamine-coated iron-chelated NPs | M2→M1 TAM repolarization | Tumor volume; survival | [155] |
| TAA presentation & T-cell infiltration | ||||||
| TSIO | NIR-II-triggered PTT | Metastatic tumors | 2D TiS2 nano-carriers | Magnetic targeting tumor accumulation | Recurrence/metastasis with PD-1 blockade | [153] |
| HCSVs | Circularly polarized MF | Solid tumors | IONCs | MF-triggered Fe2+ release Fenton reaction | CRT exposure antitumor immunity | [154] |
| Ascorbic acid core | ||||||
| Anti-HER2 SPIONs | Immunohyperthermia | HER2+ cancer | Antibody-conjugated SPIONs | Selective induction of apoptosis in HER2+ cells | Targeted elimination of antigen-expressing cells | [160] |
| CHINPs | PTT/SDT | Breast cancer | SPIO NPs | Sequential US/NIR activation complete tumor ablation | Systemic antitumor immunity; metastasis | [156] |
| HMME | ||||||
| Cancer cell membrane | ||||||
| GAPFBD | NIR-triggered PTT | Solid tumors | AuNCs/Fe3O4 | pH-sensitive NO release + IDO inhibition | Long-term antitumor immunity | [218] |
| GSNO/1-M-DT | ||||||
| ROS-responsive hydrogel | PDT/CDT | Metastatic tumors | PpIX-Fe3O4 | Enhanced ROS ICD + aPD-L1 activation | Primary tumor suppression & metastasis prevention | [158] |
| ROS-responsive aPD-L1 | ||||||
| JQ1/IONAs | Mild magnetic hyperthermia | Metastatic tumors | Temp-responsive IONAs | Controlled JQ1 release + PD-L1 downregulation | Eradicates primary tumors; prevents recurrence | [152] |
| IMQ@IONs/ICG | MRI-guided PTT | Pancreatic cancer | IONs/ICG | Tumor penetration ICD | Complete primary tumor elimination; mesenteric metastasis | [161] |
| Imiquimod | ||||||
| IONP-C/O@LP | N/A (Vaccine) | Tumor models | IONPs conjugated to OVAp/CpG | Dual antigen/adjuvant delivery DC activation | Antigen-specific T-cell response; tumor suppression | [159] |
| Lipid vesicles with P30 peptide |
SDT sonodynamic therapy, CRT calreticulin, ODN oligodeoxynucleotide, HMME Hematoporphyrin monomethyl ether, IDO indoleamine 2,3-dioxygenase, aPD-L1 anti-PD-L1 antibody, MF magnetic field, MINPs magnetically responsive immunostimulatory nanoreagents, PTT photothermal therapy, TAAs tumor-associated antigens
Fig. 5.
Physical energy-amplified immunotherapy via iron oxide nanoplatforms. A Ferrimagnetic vortex-domain iron oxide nanoring (FVIO)-mediated mild magnetic hyperthermia can activate the host immune systems and efficiently cooperate with PD-L1 blockade to inhibit the potential metastatic spreading as well as the growth of distant tumors.Reproduced with permission [151]. Copyright from American Chemical Society, 2019. B Schematic illustration of AMF triggered disassembly of JQ1/IONAs for self-regulated magnetic thermal therapy and controlled JQ1 release to improve immunological response.Reproduced with permission [152]. Copyright from Elsevier Ltd, 2023. C Fe@PDA-PEG induced M2-like TAMs to M1 polarization combined with PTT-induced TAAs release altering the functional orientation of the tumorpromoting microenvironment toward an antitumor mode with immune-induced cancer cell killing.Reproduced with permission [155]. Copyright from Elsevier Ltd, 2019. D PTT and SDT are synergistically augmented by a novel multimodal imaging nanoprobe integrated with cancer cell membrane-biomimetic nanoparticles (CHINPs) loaded with superparamagnetic iron oxide (SPIO) and hematoporphyrin monomethyl ether (HMME).Reproduced with permission [156]. Copyright from Springer Nature, 2022. CHINPs exhibit excellent homologous tumor targeting, and are sequentially triggered by ultrasound and near infrared (NIR) light under the guidance of magnetic resonance, photoacoustic and photothermal imaging, leading to complete in situ tumor eradication and systemic anti-tumor immune activation. Further combination of this approach with immune checkpoint blockade therapy is shown to suppress tumor metastasis. E Schematic illustration of the fabrication and applications in synergistic cancer immunotherapy of prodrug hydrogels. The fabrication illustration of prodrug hydrogels and the prodrug hydrogel-mediated synergistic PDT/CDT/immunotherapy to treat primary and distant tumors and prevent lung and liver metastasis.Reproduced with permission [158]. Copyright from Elsevier Ltd, 2022. F Schematic illustration of IMQ@IONs/ICG-mediated interventional photothermal immunotherapy for the treatment of metastatic pancreatic cancer. Schematic diagram of interventional photothermal-immunotherapy with imaging guidance and temperature monitoring by magnetic resonance (MR) technique and mechanism of anti-tumor immune response induced by IMQ@IONs/ICG based interventional photothermal-immunotherapy.Reproduced with permission [161]. Copyright from Elsevier Ltd, 2021.
Photothermal therapy (PTT) has shown robust synergy with immune checkpoint inhibition. Magnetically targeted TiS₂ nanocarriers, when subjected to NIR-II irradiation, generate tumor antigens that enhance the efficacy of PD-1 blockade, thus suppressing metastasis [153]. Additionally, hybrid vesicles (HCSVs) containing ascorbic acid and iron oxide nanocubes use circularly polarized magnetic fields to amplify Fenton reactions, generating ROS that promote ferroptosis-like cell death and CRT-mediated immunity [154]. Polydopamine-coated Fe@PDA-PEG nanoparticles combine PTT with macrophage polarization, enhancing M1 macrophage presentation of tumor antigens and T-cell infiltration, thereby extending survival in colon and breast cancer models [155] (Fig. 5C). Cancer cell membrane-camouflaged nanoplatforms (CHINPs), co-loaded with superparamagnetic iron oxide (SPIO) and sonosensitizers, enable sequential ultrasound-triggered sonodynamic therapy (SDT) and NIR-P-mediated PTT. This strategy achieves complete ablation of primary tumors and elicits systemic antitumor immunity [156] (Fig. 5D). Multimodal energy delivery systems significantly amplify immunostimulation through engineered responsiveness. For example, core-shell Ce6/Fe₃O₄-L nanoparticles, upon ultrasound exposure, release protein-capturing Fe₃O₄, which transports endogenous antigens to lymph nodes. This mechanism synergizes with anti-PD-L1 therapy to eliminate both treated and distant tumors [157] . ROS-responsive alginate hydrogels co-deliver protoporphyrin IX (PpIX)-modified Fe₃O₄ and anti-PD-L1 prodrugs. The resulting enhanced ROS generation induces ICD and activates checkpoint blockade, effectively suppressing both primary tumors and metastases [158] (Fig. 5E). Additionally, the IONP-C/O@LP nanovaccine facilitates dual intracellular delivery of antigen and adjuvant to dendritic cells (DCs) via membrane fusion and endocytosis. By promoting ROS generation, IONPs enhance dendritic cell maturation, thereby eliciting potent T-cell responses [159].
Precision targeting of these platforms minimizes off-tumor toxicity. For instance, antibody-conjugated superparamagnetic iron oxide nanoparticles (SPIONs) targeting HER2 enable “immunohyperthermia” by selectively inducing apoptosis in antigen-expressing cancer cells in co-culture systems [160]. The tumor-microenvironment-sensitive IMQ@IONs/ICG platform improves drug penetration in pancreatic cancer, enabling MRI-guided interventional PTT. This strategy induces ICD, while imiquimod release triggers systemic immunity, leading to the eradication of primary tumors and reduction in metastases [161] (Fig. 5F). Lastly, magnetically responsive immunostimulatory nanoreagents (MINPs), co-loaded with SPIO and CpG oligodeoxynucleotides (ODNs), facilitate field-directed tumor accumulation for PTT. They release antigens that synergize with CpG to target residual and metastatic lesions [162].
Ferroptosis and chemodynamic synergy in iron-enabled cancer immunotherapy
Following introduction of ferroptosis as an immunomodulatory mechanism (Sect. "Multifunctional iron oxide platforms for coordinated immunomodulation" applications), we now detail its biochemical foundations in reprogramming the tumor immune microenvironment (Table 5). Therapeutic exploitation of ferroptosis appears in Sect. "Biohybrid nanoplatforms: nature-inspired tumor immunotherapy" and "Discussion and prospects". Ferroptosis orchestrates T-cell infiltration through multistep mechanochemical signaling. Mechanism I: Ferroptotic cells release DAMPs (HMGB1, ATP) that activate dendritic cells via TLR4/P2 × 7 receptors, triggering CCR7-dependent migration to lymph nodes where they prime tumor-specific T cells. Mechanism II: Lipid peroxidation derivatives (e.g., 4-HNE, oxidized phospholipids) function as chemoattractants that: (i) upregulate endothelial VCAM-1/ICAM-1 to enable T-cell adhesion, (ii) generate CXCL9/CXCL10 gradients through STAT1 activation in stromal cells, and (iii) promote T-cell extravasation via CXCR3 binding. Mechanism III: Iron-catalyzed depletion of immunosuppressive Arg1+ MDSCs and M2-TAMs removes physical barriers and TGF-β/CD47 ‘don’t eat me’ signals, permitting T-cell penetration into tumor cores. These coordinated changes convert immune-excluded phenotypes into T-cell-inflamed microenvironments, potentiating checkpoint responses. Platelet-membrane-coated Fe₃O₄-SAS@PLT nanoparticles exploit immune evasion mechanisms to deliver sulfasalazine (SAS), thereby inducing ferroptosis that reprograms immunosuppressive M2 macrophages into antitumor M1 phenotypes. This reprogramming enhances the efficacy of PD-1 blockade in metastatic breast cancer by triggering tumor-specific immunity [163] (Fig. 6A). Similarly, chemically programmed iron oxide nanovaccines (IONVs) combine catalytic iron delivery with targeted antigen presentation, eradicating aggressive tumors through ferroptosis-driven danger signals and the repolarization of TAMs into M1 phenotypes [164].
Table 5.
Ferroptosis and chemodynamic synergy in iron-enabled cancer immunotherapy
| Nanoparticle system | Cancer model | Key components | Mechanism of action | Combined therapy | Therapeutic outcomes | References |
|---|---|---|---|---|---|---|
| Fe3O4-SAS@PLT | Metastatic 4T1 breast cancer | Fe3O4 | Platelet membrane enables immune evasion/tumor targeting | Anti-PD-1 | Anti-PD-1 efficacy; triggers tumor-specific immunity | [163] |
| SAS | SAS induces ferroptosis ICD | |||||
| Platelet membrane | M2 M1 macrophage repolarization | |||||
| IONVs | Aggressive tumors | Chemically programmed iron oxide core | Catalytic iron delivery ferroptosis | Antibody-mediated TME modulation | Complete tumor eradication; durable antitumor immunity | [164] |
| Targeted antigen delivery via reversible covalent bonds | ||||||
| Reprograms TAMs to antitumor state | ||||||
| IONP-GOD@ART | Solid tumors | Mesoporous IONPs | GOD depletes glucose → H2O2 generation | N/A | “Butterfly effect”: tumor regression & metastasis prevention | [165] |
| GOD | Fe2+/Fe3+ Fenton reaction OH | |||||
| ART | ART-derived ROS → ICD | |||||
| M2→M1 TAM polarization | ||||||
| Fe3O4@Chl/Fe CNPs | Bladder cancer (BC) | Fe3O4 clusters | Intravesical CPBA-targeted delivery | PDT + CDT | Survival; remodels immunosuppressive TME | [170] |
| Chlorin photosensitizer | PDT/CDT synergy ROS/ferroptosis | |||||
| Lp-IO | Broad applicability | PEGylated IONPs embedded in liposomes | Fenton reaction in lipid bilayer → lipid peroxidation | Doxorubicin chemotherapy | Synergistic antitumor effect; systemic toxicity | [120] |
| pH/ROS-responsive drug release | ||||||
| FDPM | Resistant tumors | Fe3O4 | DHJS inhibits Nrf-2 → overcomes ferroptosis resistance | N/A | Enhanced ferroptosis & antitumor efficacy | [166] |
| DHJS (Nrf-2 inhibitor) | Immune modulation in TME | |||||
| Hybrid cell membrane | ||||||
| rPAE@SPIONs | Solid tumors | SPIONs in pomegranate-like NPs | NIR-triggered DOX release | Mild photothermia | Tumor suppression vs. conventional chemotherapy | [219] |
| Reduced poly (β-amino ester)-PEG | Ferroptosis induction | |||||
| M1 macrophage polarization | ||||||
| PEG-Fe3O4@C5aRA | Breast cancer | Hollow Fe3O4 | Blocks C5aR reverses ferroptosis resistance | Metal metabolism regulation | Antitumor efficacy; overcomes ferroptosis resistance | [167] |
| C5a receptor antagonist (C5aRA) | M1 macrophage polarization | |||||
| AZOSH | Solid tumors | Self-catalytic NO nanocomposite | NO-induced Fe2+ overload/GSH depletion ferroptosis | Anti-PD-1 | Significant tumor suppression via ICD-induced immunity | [168] |
| Arg/H2O2 reactants | • Facilitates siRNA endosomal escape | |||||
| Platelet-engineered “Nanofactory" | Hepatocellular carcinoma (HCC) | Erastin | LOX depletes lactate H2O2 | MRI-guided immunotherapy | Synergistic enhancement of immunotherapy | [169] |
| SPIO NPs | Enhanced erastin-induced ferroptosis | |||||
| LOX | Reverses lactate-mediated immunosuppression |
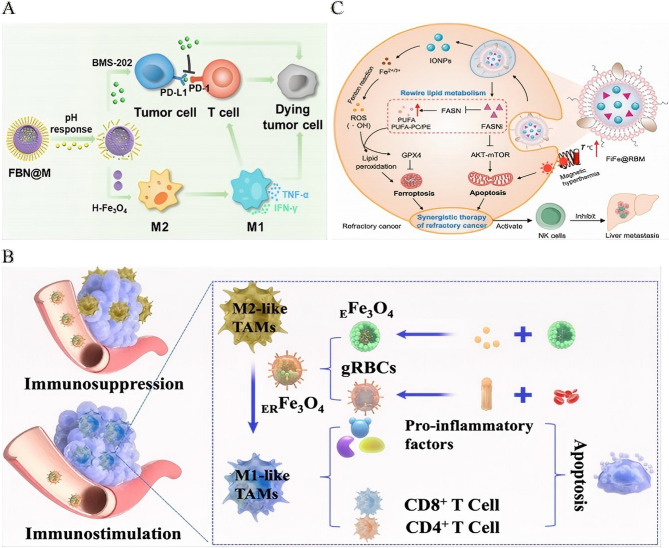
SAS sulfasalazine, GSH glutathione, CNPs chlorin nanoparticles, DOX doxorubicin, NIR near-infrared, LOX lactate oxidase, ICD immunogenic cell death, PDT photodynamic therapy, CDT chemodynamic therapy, TME tumor microenvironment
Fig. 6.
Ferroptosis and chemodynamic synergy in iron-enabled cancer immunotherapy. A Schematic illustration of platelet membrane-camouflaged magnetic nanoparticles for ferroptosis-enhanced cancer immunotherapy. Preparationof Fe3O4-SAS@PLT. Fe3O4-SAS@PLT-induced cell death by ferroptosis. Mechanisms of Fe3O4-SAS@PLT-mediated ferroptosis enhancing immune checkpoint blockade in metastasis tumors.Reproduced with permission [163]. Copyright from Wiley-VCH GmbH, 2020. B Schematic mechanism of IONP-GOD@ART for enhanced immunotherapy.Reproduced with permission [165]. Copyright from Wiley-VCH GmbH, 2021. C Schematic illustration of superior anti-tumor effect of PEG-Fe3O4@C5aRA through ferroptosis and macrophages polarization to M1 phenotype.Reproduced with permission [167]. Copyright from Frontiers Media SA, 2024. D Schematic illustration of the synthesis of Fe3O4@Chl/Fe-CPBA CNPs for targeted delivery into BC cells and demonstration of their antitumor effect and immunoregulatory effect within BC cells after the combination of chemodynamic and photodynamic therapy.Reproduced with permission [170]. Copyright from Springer Nature, 2022.
Metabolic modulation within the TME significantly amplifies therapeutic efficacy. The IONP-GOD@ART cascade platform utilizes GOD to deplete glucose and generate hydrogen peroxide (H₂O₂), fueling Fenton reactions that produce hydroxyl radicals for chemodynamic therapy (CDT). Concurrently, artemisinin (ART)-derived ROS polarize M2 TAMs to M1 states, creating a “butterfly effect” that drives tumor regression and prevents metastasis [165] (Fig. 6B). In a similar approach, Lp-IO liposomes enhance intraliposomal Fenton reactions, promoting lipid peroxidation and ferroptosis while enabling pH/ROS-responsive release of doxorubicin, synergistically increasing cytotoxicity [120].
Resistance to ferroptosis represents a significant challenge, which can be addressed through innovative nanoformulations. FDPM nanoparticles counteract Nrf-2-mediated antioxidant defenses by co-delivering Fe₃O₄ and the Nrf-2 inhibitor DHJS. This combination restores ferroptotic susceptibility and enhances immunomodulation, offering a promising solution to ferroptosis resistance [166]. The PEG-Fe₃O₄@C5aRA system blocks C5a/C5aR signaling to reverse ferroptosis resistance while polarizing macrophages toward M1 phenotypes, further boosting antitumor immune responses [167] (Fig. 6C). Advanced platforms integrate ferroptosis with immune stimulation for enhanced therapeutic outcomes. AZOSH, a self-catalytic nitric oxide (NO) nanocomposite, induces Fe²⁺ overload and glutathione depletion to trigger ferroptosis. This system also facilitates siRNA escape, enhancing gene silencing, and, when combined with anti-PD-1 therapy, induces ICD for potent antitumor immunity [168]. Platelet-engineered “nanofactories” that co-load erastin, SPIONs, and lactate oxidase (LOX) deplete immunosuppressive lactate, amplify ferroptosis, and induce ROS-mediated immune activation, with real-time monitoring via MRI [169].
Notably, localized delivery strategies have demonstrated remarkable clinical translation. Intravesical administration of CPBA-modified Fe₃O₄@Chl/Fe CNPs for bladder cancer combines photodynamic therapy (PDT) and CDT to reshape the immunosuppressive TME, resulting in a substantial increase in survival rates from 0–91.7% [170] (Fig. 6D).
Ferroptosis, triggered by IONPs, elevates T-cell infiltration through precise modulation of the TME via the release of immunostimulatory factors and metabolic reprogramming. Upon induction, ferroptotic tumor cells release damage-associated molecular patterns (DAMPs) such as calreticulin (CRT), ATP, and high-mobility group box 1 (HMGB1), which act as “find-me” and “eat-me” signals to recruit dendritic cells (DCs). These DAMPs activate DCs, enabling them to phagocytose tumor antigens released by ferroptotic cells and migrate to lymph nodes for efficient antigen presentation to naive T cells. Concurrently, ferroptosis-driven reactive oxygen species (ROS) accumulation and lipid peroxidation polarize immunosuppressive M2 TAMs toward the pro-inflammatory M1 phenotype, which secrete cytokines like IL-12 and IFN-γ to chemoattract CD8⁺ T cells into the tumor. This cascade—from DAMP-mediated DC activation to cytokine-driven T-cell recruitment—directly enhances T-cell infiltration, as seen in models like GOx@FeNPs, where ferroptosis synergizes with PD-L1 blockade to boost CTL infiltration and achieve > 90% tumor suppression. By bridging ferroptotic cell death with adaptive immune activation, this mechanism strengthens antitumor responses and improves therapeutic outcomes in preclinical settings.
Reprogramming TAMs via iron oxide nanoplatforms
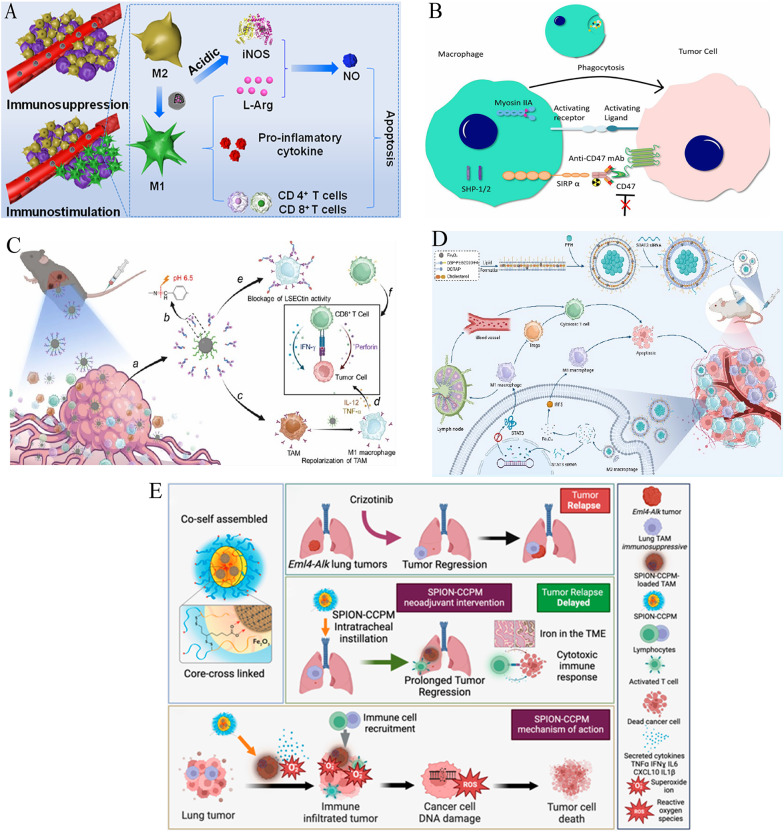
Building upon initial observations of IONP-mediated macrophage polarization (Sect. "IONPs: intrinsic activators of antitumor immunity"), this section systematizes TAM reprogramming strategies in reversing immunosuppressive microenvironments and activating antitumor immunity (Table 6). One such strategy involves pharmacological inhibition through kinase modulators. For instance, mannose-decorated porous hollow IONPs deliver the PI3Kγ inhibitor 3-MA, which upregulates NF-κB p65 and drives the repolarization of TAMs from the immunosuppressive M2 phenotype to the antitumor M1 phenotype. This approach enhances the secretion of immunostimulatory cytokines, promoting antitumor immunity [171].
Table 6.
Reprogramming TAMs via iron oxide nanoplatforms
| Nanoparticle system | Cancer model | TAM reprogramming Strategy | Key mechanisms | Validation models | Therapeutic outcomes | References |
|---|---|---|---|---|---|---|
| PHNPs@DPA-S-S-BSA-MA@3-MA | Solid tumors | Mannose-targeted delivery of PI3Kγ inhibitor (3-MA) | Upregulates NF-κB p65 pathway | In vitro & in vivo | Immunosuppressive factors; immunostimulatory factors; tumor growth inhibition | [171] |
| M2→M1 repolarization | ||||||
| LPFe₃O₄ NPs | Solid tumors | pH-sensitive PAA coating | Acidic TME-triggered L-Arg release → iNOS/NO production | In vitro & in vivo | Activates T cells; pro-inflammatory cytokines; synergistic tumor elimination | [172] |
| L-Arg loading in hollow Fe₃O₄ | M2→M1 conversion | |||||
| CMPTR/IONPs | B16F10 melanoma | Carboxymethylated β-D-glucan + IONPs | Activates NF-κB/IRF5 pathways | M2-like BMDMs | M1/M2 ratio; CD4+/CD8+ T-cell infiltration; tumor cell apoptosis | [173] |
| M1 polarization | Mouse melanoma model | |||||
| Pani/γ-Fe₂O₃ NPs | Breast cancer | Polyaniline-coated γ-Fe₂O₃ | CD86+ cells | 2D IL-10-stimulated macrophages | Successful macrophage uptake; M2→M1 repolarization | [174] |
| CD163+ cells | 3D MCTS | |||||
| IOP-enhanced MRI | Osteosarcoma | Clinically translatable IOP nanoparticles | T₂* shortening indicates TAM activation | Rat biodistribution study | Detects CD47 mAb-induced TAM activation | [175] |
| Osteosarcoma model | ||||||
| SPION-CCPMs | ALK + NSCLC | SPION-loaded CCPMs | Reprograms TAMs to pro-inflammatory state | Co-culture with lung cancer cells | Tumor cell proliferation/viability; prevents tumor regrowth after TKI therapy | [27] |
| Mouse tumor model | ||||||
| Ab-decorated IONPs | Subcutaneous/orthotopic tumors | pH-responsive IONP-LSECtin antibody conjugates | Acidic pH-triggered dissociation → IONPs polarize TAMs to M1 | Subcutaneous & orthotopic mouse models | CD8+ T-cell immunosuppression; significant tumor growth suppression | [176] |
| LSECtin blockade on M1 macrophages | ||||||
| FA-liposomal nanobubbles | NSCLC | Folate-targeted PFH liposomes | LIFU-triggered phase transition Fe₃O₄ release activates IRF5 | Not specified in key points | Converts M2→M1 macrophages; T-cell activation/proliferation | [177] |
| Fe₃O₄ NPs + STAT3 siRNA | STAT3 siRNA inhibits JAK-STAT3 |
PHNPs porous hollow nanoparticles, L-Arg L-arginine, BMDMs bone marrow-derived macrophages, MCTS multicellular tumor spheroids, LIFU low-intensity focused ultrasound, TKI tyrosine kinase inhibitor, PAA polyacrylic acid, iNOS inducible nitric oxide synthase, NO nitric oxide
Complementary metabolic strategies exploit the acidic TME to further reprogram TAMs. For example, pH-responsive polyacrylic acid-coated hollow Fe₃O₄ nanoparticles release L-arginine, which is then converted by M1-TAMs into NO, a potent activator of T cells that aids in tumor elimination [172] (Fig. 7A). Additionally, carbohydrate-functionalized IONP systems, such as the CMPTR/IONP complexes, activate NF-κB/IRF5 pathways, which enhance the M1/M2 macrophage ratio in melanoma, surpassing the effects of single-agent treatments and boosting CD4⁺ and CD8⁺ T-cell infiltration [173].
Fig. 7.
Reprogramming tumor-associated macrophages via iron oxide nanoplatforms. A Schematic illustration: Immunomodulation of tumor microenvironment by arginine-loaded iron oxide nanoparticles for gaseous immunotherapy.Reproduced with permission [172].Copyright from American Chemical Society, 2021. B Illustrative diagram depicting the mechanism of CD47 monoclonal antibody (mAb)-induced macrophage activation.Reproduced with permission [175].Copyright from Wolters Kluwer Health, Inc. 2024. The interaction between CD47 on osteosarcoma cell surfaces and SIRPα on macrophages inhibits phagocytosis by delivering a strong "don't eat me" signal. The administration of CD47 mAb interrupts these interactions, thereby promoting phagocytosis and facilitating tumor cell clearance. Activation of macrophages subsequently enhances the phagocytic uptake of iron oxide nanoparticles, which can be detected via MRI. C Schematic illustration: antibody-decorated nanoplatform to reprogram macrophage and block immune checkpoint lsectin for effective cancer immunotherapy.Reproduced with permission [176].Copyright from American Chemical Society, 2024. D Schematic illustration: ultrasound-responsive nanocarriers delivering siRNA and Fe3O4 nanoparticles reprogram macrophages and inhibit M2 polarization for enhanced nsclc immunotherapy.Reproduced with permission [177].Copyright from American Chemical Society, 2024. E Schematic illustration: SPION-CCPMs reprogram tumor-associated macrophages (TAMs) toward M1 phenotypes and disrupt immunosuppressive niches, preventing ALK-positive NSCLC recurrence after crizotinib cessation.Reproduced with permission [27]. Copyright from American Chemical Society, 2024.
In translational models, polyaniline-coated γ-Fe₂O₃ nanoparticles have been shown to increase the proportion of CD86⁺ M1 macrophages in 3D breast cancer spheroids [174]. Moreover, IONPs enhance MRI detection of CD47 monoclonal antibody-induced TAM activation, as evidenced by T₂* shortening in osteosarcoma [175] (Fig. 7B). These findings highlight the potential of IONP-mediated TAM reprogramming for both basic and clinical applications.
Advanced therapeutic platforms integrate dual-pathway targeting for more effective TAM reprogramming. For instance, antibody-conjugated IONPs can release LSECtin-blocking antibodies in the acidic TME, while simultaneously polarizing TAMs to the M1 phenotype, thus alleviating the suppression of CD8⁺ T-cell responses [176] (Fig. 7C). Similarly, folate-targeted lipid nanobubbles co-deliver Fe₃O₄ and STAT3 siRNA. Ultrasound-triggered release of these agents activates IRF5, driving the conversion of M2 to M1 TAMs, while silencing the JAK-STAT3 pathway to block M2 polarization. This dual approach enhances T-cell responses in NSCLC [177] (Fig. 7D).
Additionally, SPION-CCPMs have been demonstrated to reprogram TAMs in ALK-positive lung cancer, delaying tumor growth and preventing recurrence post-therapy [27] (Fig. 7E). These integrated strategies collectively illustrate the potential of IONP-mediated TAM reprogramming as a robust approach to remodel immunosuppressive niches, enhancing the efficacy of conventional immunotherapies.
Biohybrid nanoplatforms: nature-inspired tumor immunotherapy
Neutrophil-platelet hybrid membranes exemplify this evolution, simultaneously clearing immunosuppressive exosomes and circulating tumor cells [178]. IONP-camouflaged nanovesicles exhibit 3-fold deeper tumor penetration than cell-derived optotheranostics, with sustained tumor retention (168 h vs. 24 h) and synergistic ferroptosis/PDT efficacy—overcoming photobleaching limitations of organic dye-based systems [179]. Innovative biohybrid platforms harness natural biological components to enhance the precision and therapeutic efficacy of IONP-based cancer immunotherapy. One such approach involves engineered platelets that co-deliver anti-PD-L1 antibodies and photothermal IONPs. These platelets adhere specifically to surgical sites, where localized PTT induces necrosis of residual tumor cells. This process not only releases tumor-associated antigens but also blocks immunosuppressive PD-L1 signals, significantly inhibiting postoperative breast tumor recurrence and enhancing CD4⁺/CD8⁺ T-cell infiltration [180].
In a similar strategy, supramolecular conjugates, such as FeAMV, integrate live Salmonella VNP20009 bacteria with cancer cell membrane-coated IONPs. This system exploits the bacterial tumor tropism for targeted delivery of ferroptosis inducers, such as Fe₃O₄ and SAS. The resulting ferroptosis (Sect. "Ferroptosis and chemodynamic synergy in iron-enabled cancer immunotherapy") synergizes with bacteria-induced ICD, significantly amplifying antitumor immunity [181].
Hierarchical biomimetic designs further optimize therapeutic release through microenvironment-responsive mechanisms. For instance, FBN@M systems encapsulate PD-1/PD-L1 inhibitor BMS-202 and pH-sensitive NaHCO₃ within macrophage membrane-coated hollow IONPs. In response to the acidic TME, CO₂ is generated, which ruptures the membrane and releases BMS-202, while simultaneously reprogramming M2 TAMs into antitumor M1 phenotypes [182] (Fig. 8A).
Fig. 8.
Biohybrid nanoplatforms: nature-inspired tumor immunotherapy. A Schematic illustration: construction of hierarchically biomimetic iron oxide nanosystems for macrophage repolarization-promoted immune checkpoint blockade of cancer immunotherapy. Reproduced with permission [182]. Copyright from American Chemical Society, 2024. B Schematic illustration: biomimetic iron-based nanoparticles remodel immunosuppressive tumor microenvironment for metabolic immunotherapy.Reproduced with permission [183]. Copyright from Dovepress Taylor & Francis Group, 2024. C Schematic illustration: nanovesicles for lipid metabolism reprogram-enhanced ferroptosis and magnetotherapy of refractory tumors and inhibiting metastasis with activated innate immunity. Reproduced with permission [184]. Copyright from American Chemical Society, 2025.
For metabolic reprogramming, ER Fe₃O₄ nanoparticles employ galactose-modified red blood cell membranes to target M2 TAMs, delivering epiberberine to inhibit lactate production and counteract TME acidosis. Meanwhile, the Fe₃O₄ cores polarize TAMs towards the M1 phenotype, increasing the secretion of pro-inflammatory cytokines (IL-12, IFN-γ, TNF-α), which remodel immunosuppressive niches within the tumor [183] (Fig. 8B).
The precision of these biohybrid and biomimetic systems extends to the suppression of metastasis. FiFe@RBM nanovesicles, which co-encapsulate fatty acid synthase inhibitors and IONPs, selectively accumulate in castration-resistant prostate cancer (CRPC). Here, they induce ROS-mediated mitochondrial dysfunction and inhibit the AKT-mTOR signaling pathway. This dual-action approach promotes both apoptosis and ferroptosis induced by metabolic reprogramming, facilitating MRI-guided magnetothermal eradication of primary tumors. Moreover, the activation of NK cells helps suppress hepatic metastases [184] (Fig. 8C).
Integrated theranostics and monitoring for precision cancer immunotherapy
IONPs have emerged as key tools in real-time visualization of therapeutic processes and in predicting treatment outcomes, thus bridging diagnostic precision with therapeutic monitoring. One promising approach involves molecular imaging probes such as PDL1-SPIO conjugates, which specifically target PD-L1 on temozolomide-resistant glioblastoma cells. Prussian blue staining, coupled with in vivo T2*-weighted MRI, quantitatively confirms the expression of PD-L1, offering a non-invasive diagnostic method for identifying therapy-resistant gliomas [185]. This capability not only facilitates early diagnosis but also provides valuable insights into the effectiveness of therapeutic interventions.
In addition to diagnostic applications, ultrasmall IONPs have been employed to label adoptively transferred T cells and CAR-T cells, with no observed impairment of their functionality. This approach enables MRI-based spatial mapping of tumor-infiltrating lymphocytes, which serves as a predictive biomarker for therapy response. This technique marks an important step towards personalized immunotherapy, where real-time monitoring of immune cell dynamics is critical for optimizing treatment efficacy [114].
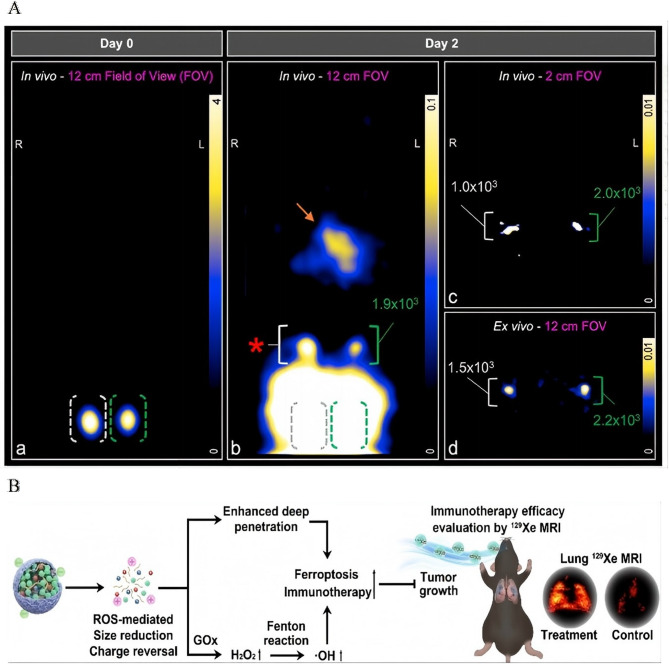
Novel imaging modalities further enhance the capacity for in vivo tracking. MPI addresses the limitations in dynamic range inherent in traditional imaging techniques. By quantifying the migration of SPIO-labeled DCs to lymph nodes in vivo, MPI enables the detection of clinically relevant cell quantities using focused-field scanning. This approach holds significant promise for advancing cellular therapies, providing a more precise and sensitive method for tracking immune cell trafficking during treatment [186] (Fig. 9A).
Fig. 9.
Integrated theranostics and monitoring for precision cancer immunotherapy. A Schematic illustration: In vivo tracking of adenoviral-transduced iron oxide-labeled bone marrow-derived dendritic cells using magnetic particle imaging. Reproduced with permission [186]. Copyright from Springer Nature, 2023. B Schematic illustration: Monitoring ROS Responsive Fe3O4-based Nanoparticle Mediated Ferroptosis and Immunotherapy via 129Xe MRI. Reproduced with permission [187]. Copyright from John Wiley and Sons, 2024.
For therapy-response correlation, ROS-responsive FGTL nanoparticles offer a sophisticated solution for monitoring treatment outcomes. These nanoparticles release GOD and immunostimulatory tuftsin in high-H₂O₂ TMEs, thereby amplifying ferroptosis and promoting the recruitment of effector T cells. This process can be noninvasively monitored using hyperpolarized ¹²⁹Xe MRI, establishing the first radiation-free method for simultaneously inducing ferroptosis and assessing treatment efficacy in metastatic lung cancer. This innovative approach not only enhances therapeutic precision but also provides an essential tool for monitoring dynamic treatment responses [187] (Fig. 9B). Unlike optotheranostic platforms limited by photobleaching and shallow tissue penetration (< 2 cm), IONP-enabled MPI/Xe-MRI overcomes the fundamental trade-off between imaging depth (> 5 cm) and biosafety through radiation-free magnetic detection-a capability unattainable with organic fluorophore-based systems [20].
IONP-engineered vaccine platforms for amplified antitumor immunity
IONPs have emerged as potent platforms for enhancing antigen delivery and immunostimulation in cancer vaccines. One such strategy involves lymph-targeted delivery systems, which leverage magnetic guidance to direct antigen-loaded IONPs to lymph nodes. OVA-loaded IONPs encapsulated in biodegradable gelatin methacrylate microspheres are directed to lymphoid tissues through magnetic navigation. This method enhances both cellular and humoral immunity, resulting in improved antitumor efficacy with a single booster immunization. The sustained release of antigens within the lymph nodes, which are rich in antigen-presenting cells (APCs), promotes a robust immune response [188] (Fig. 10A).
Fig. 10.

IONP-engineered vaccine platforms for amplified antitumor immunity. A Schematic illustration: A Magnetically Driven Biodegradable Microsphere with Mass Production Capability for Subunit Vaccine Delivery and Enhanced Immunotherapy.Reproduced with permission [188]. Copyright from American Chemical Society, 2024. B Schematic illustration: Direct cGAMP Delivery via Iron Oxide Nanoparticles for Enhanced STING Activation and Durable Antitumor Immunity. Reproduced with permission [189]. Copyright from American Chemical Society, 2025.
The activation of the STING pathway is another key focus in IONP-based adjuvant design. Acid-ionized iron nanoadjuvants, such as PEIM, co-assemble IONPs with the STING agonist MSA-2. This combination significantly boosts the production of type I interferons (IFN-I) through ROS-dependent NF-κB activation. Furthermore, PEIM enhances lymphatic delivery, promotes cross-presentation by CD169⁺ APCs, and stimulates CD8⁺ T-cell responses. When loaded with autologous tumor membrane antigens, PEIM@Mem vaccines synergize with anti-PD-L1 therapy to effectively prevent postoperative recurrence and metastasis, underscoring their potential in clinical applications [26].
Advancing this approach, Fe-cGAMP conjugates are designed by chemically tethering the STING agonist cyclic GMP-AMP (cGAMP) directly to the surface of IONPs. This configuration not only enhances cellular uptake and STING activation compared to free cGAMP, but also allows IONPs to independently generate ROS and activate Toll-like receptors, resulting in synergistic immune activation. Combining Fe-cGAMP with checkpoint inhibitors has demonstrated remarkable success in inducing complete tumor regression in more than 50% of treated mice, establishing durable antitumor immunity [189] (Fig. 10B).
Discussion and prospects
IONPs have emerged as versatile and transformative tools in cancer immunotherapy, offering a unique combination of immunomodulation, targeted drug delivery, and theranostic capabilities [133, 190, 191]. Their potential is particularly evident in their ability to reprogram the immunosuppressive TME, a critical challenge in cancer treatment [27, 192, 193]. One of the key features of IONPs is their capacity to influence TAMs, promoting their polarization towards the antitumor M1 phenotype [27, 194, 195]. This shift enhances the immune response against the tumor, providing a promising strategy for overcoming immune evasion by solid tumors. Additionally, IONPs have demonstrated the ability to activate innate immune pathways such as the STING pathway, which plays a crucial role in triggering an effective immune response [26, 189]. These advancements in immune modulation are accompanied by the growing realization of IONPs’ potential to precisely target tumors, improving therapeutic efficacy while reducing off-target effects [94, 95, 196]. Preclinical evidence robustly validates IONPs as transformative immunomodulators. Key successes include: (1) Profound reprogramming of immunosuppressive tumor microenvironments through macrophage polarization and STING pathway activation; (2) Synergistic induction of systemic antitumor immunity when combined with checkpoint inhibitors, achieving complete regression in refractory tumors; (3) Effective metastasis suppression via magnetically targeted immunotherapeutic delivery; and (4) Significant extension of survival across multiple aggressive tumor models through engineered biohybrid platforms. Clinical translation of IONP-based immunotherapies remains predominantly investigational. Current human applications leverage FDA-approved ferumoxytol for diagnostic staging, revealing unique biodistribution patterns in pediatric patients differing from adults: Benign lymph nodes exhibit hypointense hilum and hyperintense parenchyma on T₂-FSE MRI, while malignant nodes show homogeneous hypointensity without discernible hilum. Ferumoxytol accumulation in benign node hila suggests potential as a theranostic bridge for future immune cell priming applications [197].
These preclinical successes are further amplified by the integration of biohybrid designs that leverage cancer cell membranes offer a significant advantage in enhancing the targeting specificity of nanoparticles [198, 199]. By utilizing tumor cell membranes, these biohybrid nanoparticles achieve a level of homologous targeting that allows them to accumulate more effectively in tumor tissues. This approach also significantly extends the systemic circulation time of IONPs, which is crucial for ensuring that the nanoparticles can reach distant tumors before being cleared from the body [80]. Additionally, magnetic navigation has emerged as a powerful tool for enhancing the delivery of therapeutic agents to specific sites, such as lymph nodes, further improving the precision of treatment. The ability of IONPs to combine multiple therapeutic modalities, such as magnetic hyperthermia, PTT, and chemodynamic induction, with immune checkpoint blockade has shown remarkable promise, yielding complete tumor regression in over 50% of preclinical models [104, 133, 152, 189, 200–202]. This convergence of therapeutic strategies exemplifies the potential of IONPs to revolutionize cancer treatment, offering a comprehensive and multifaceted approach to immunotherapy. Unlike metastatic nanovesicles requiring co-encapsulation of 4 + agents, IONP-based theranostics achieve comparable tumor reduction through magnetically amplified Fenton reactions alone, significantly simplifying manufacturing for GMP compliance [179].
A particularly exciting development is the use of engineered immune cells, such as CAR-T and NK cells, labeled with SPIONs [24, 184, 203, 204]. These modified immune cells maintain their effector function while enabling real-time tracking via MRI, which provides valuable insights into their infiltration and activity within the tumor. This ability to monitor immune cell behavior in real time represents a significant step forward in personalized cancer treatment, as it allows clinicians to adjust therapy based on dynamic tumor responses. The combination of MPI and hyperpolarized MRI further enhances the ability to track immune cell recruitment and therapeutic outcomes [205–207]. New correlations have been established between ferroptotic cell death induced by IONPs and the recruitment of immune cells, suggesting that ferroptosis could be leveraged as an immune-stimulating strategy in cancer treatment.
Despite these significant advancements, several challenges remain in translating IONP-based therapies to clinical practice. Tumors often actively develop resistance to IONP-induced immune responses. Golgi apparatus-exosome hybrid nanoparticles represent a pioneering approach to overcome resistance by disrupting PD-L1 trafficking and promoting systemic immunity [208]. For instance, cancer cells can upregulate antioxidant defenses, such as those mediated by the Nrf-2 pathway, to evade ferroptosis and resist the effects of ROS generated by the Fenton reaction [209–212]. Additionally, the accumulation of immunosuppressive metabolites, such as lactate and adenosine, within the TME further impedes the efficacy of immune-based therapies [213–217]. These obstacles highlight the need for more sophisticated approaches to overcome resistance mechanisms and ensure durable therapeutic responses.
Long-term biosafety concerns also persist, particularly regarding the potential for off-target tissue damage caused by ROS generated during IONP-mediated treatments. The risk of accelerated blood clearance upon repeated administration of PEGylated IONPs adds another layer of complexity to their clinical application. Furthermore, the challenges associated with manufacturing these nanoparticles, including batch-to-batch inconsistencies in surface functionalization and difficulties in scaling up biomimetic coating processes, hinder the development of standardized, reproducible products suitable for clinical use. Magnetic targeting efficiency is also a significant limitation, as its effectiveness diminishes in deep-seated or disseminated metastatic tumors. Moreover, the reliance on the EPR effect for passive tumor targeting presents limitations in stroma-rich cancers, such as pancreatic ductal adenocarcinoma, where the tumor vasculature may not exhibit the same degree of permeability. In pediatric cancer patients (N = 42), ferumoxytol at 5 mg Fe/kg demonstrated ≤ Grade 2 adverse events in 92% of cases, supporting its acceptable safety profile for diagnostic repurposing [197].
Future research in this field must focus on addressing these challenges and optimizing IONP-based therapies for clinical translation. One promising direction is the development of dynamic, responsive systems that can release therapeutic payloads in response to specific stimuli within the TME, such as proteases or pH and ROS gradients. These advanced systems could allow for more controlled and targeted drug delivery, improving the precision of treatment while minimizing off-target effects. Another exciting avenue is the creation of closed-loop combined imaging/treatment tools that integrate real-time immune monitoring with feedback-controlled therapy. By combining MPI or MRI with AI-guided therapy adjustments, such systems could enable personalized, adaptive treatment regimens based on tumor progression and immune response.
Additionally, the application of synthetic biology offers the potential for even more innovative approaches, such as the development of tumor-colonizing IONP-bacteria hybrids. These bacteria could serve as carriers for IONPs, delivering them specifically to tumor sites, while genetically encoded nanobodies displayed on the surfaces of IONPs could enhance tumor targeting and immune modulation. To facilitate the clinical translation of these technologies, it is crucial to establish good manufacturing practice (GMP)-compliant production methods for IONP-immune cell conjugates. Optimizing synergy between ferroptosis inducers (Sect. "Ferroptosis and chemodynamic synergy in iron-enabled cancer immunotherapy'') and immune checkpoint inhibitors, and validating the efficacy of these therapies in patient-derived organoids, will also be key steps in moving towards clinical implementation.
Conclusion
Iron oxide nanoparticles (IONPs) have emerged as transformative theranostic tools in preclinical cancer immunotherapy, driving breakthroughs across immune modulation, targeted delivery, and multimodal therapeutic synergy. Their core strength lies in reprogramming the immunosuppressive tumor microenvironment (TME): IONPs polarize M2-polarized tumor-associated macrophages (TAMs) to antitumor M1 phenotypes, activate innate immune pathways like STING, and induce immunogenic ferroptosis, converting “immune-cold” tumors into inflamed, treatment-responsive states.
Complemented by precision delivery strategies—including utilization of the enhanced permeability and retention (EPR) effect, magnetic navigation, and cancer cell membrane-mediated homologous targeting—IONPs achieve selective tumor accumulation while minimizing off-target toxicity. For instance, biohybrid systems like SPIO NP@M-P extend the half-life of immunomodulatory peptides by 60-fold, amplifying T-cell reactivation. Preclinical models validate striking outcomes: combinatorial IONP-based regimens integrating immune checkpoint blockade, photothermal/magnetic hyperthermia, or chemodynamic therapy have achieved over 50% complete tumor regression in aggressive models, with > 90% suppression in colorectal cancer via ferroptosis-immunotherapy synergy. Engineered immune cells, such as magnetically guided NK cells and IONP-labeled CAR-T cells, retain effector function while enabling real-time MRI monitoring of tumor infiltration, bridging efficacy with theranostic precision.
Beyond direct tumor killing, IONPs enhance systemic immunity by promoting dendritic cell maturation, CD8⁺T-cell infiltration, and durable antitumor memory, addressing limitations like checkpoint inhibitor resistance. Their versatility is further amplified by integrated diagnostics, including MRI/magnetic particle imaging (MPI)-guided monitoring of immune cell trafficking and treatment responses, enabling personalized adjustments. While challenges such as adaptive resistance and scalable manufacturing persist, these preclinical breakthroughs position IONPs as cornerstones of next-generation precision oncology. Realizing their full potential will require advancing nanoparticle design, manufacturing, and clinical testing—efforts that can pave the way for more effective, personalized, and safe cancer treatments by deepening understanding of TME, immune modulation, and targeted delivery.
Abbreviations
- IONPs
Iron oxide nanoparticles
- MRI
Magnetic resonance imaging
- EPR
Enhanced permeability and retention
- TME
Tumor microenvironment
- MPI
Magnetic particle imaging
- TAMs
Tumor-associated macrophages
- MDSCs
Myeloid-derived suppressor cells regulatory T cells
- Tregs
Transforming growth factor-β
- NK
Natural killer
- EGFR
Epidermal growth factor receptors
- CAR
Chimeric antigen receptor
- gRNA.
Guide RNA.
- RNP
Ribonucleoprotein
- ROS
Reactive oxygen species
- OVA
Ovalbumin
- LPS
Lipopolysaccharide
- IONP-COOH
Carboxymethyl dextran-coated IONPs
- AML
Acute myeloid leukemia
- FH-MPLA
Ferumoxytol-loaded monophosphoryl lipid A
- ICG
Co-loaded indocyanine green
- NIR
Near-infrared
- ICD
Immunogenic cell death
- NSCLC
Non-small cell lung cancer
- CMNPs
Nanoparticles with cancer cell membranes
- FVIOs
Ferromagnetic vortex-domain iron oxide nanorings
- IONAs
Iron oxide nanoparticle assemblies
- PTT
Photothermal therapy
- HCSVs
Hybrid vesicles
- CHINPs
Cancer cell membrane-camouflaged nanoplatforms
- SPIO
Superparamagnetic iron oxide
- SDT
Sonodynamic therapy
- PpIX
Protoporphyrin IX
- DCs
Dendritic cells
- SPIONs
Superparamagnetic iron oxide nanoparticles
- MINPs
Magnetically responsive immunostimulatory nanoreagents
- ODNs
Oligodeoxynucleotides
- SAS
Sulfasalazine
- IONVs
Iron oxide nanovaccines
- GOD
Glucose oxidase
- CDT
Chemodynamic therapy
- ART
Artemisinin
- NO
Nitric oxide
- LOX
Lactate oxidase
- PDT
Photodynamic therapy
- CRP
Castration-resistant prostate cancer, C
- IFN-I
Type I interferons
- APCs
Antigen-presenting cells
- cGAMP
Cyclic GMP-AMP
Author contributions
Original draft preparation, allocation: Chao Yan and Liming Wang.Revision, and editing: Liming Wang and Shenglong Li.All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Liaoning Province (2023JH2/101700163).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shenglong Li, Email: lishenglong@cancerhosp-ln-cmu.com, Email: slli@cmu.edu.cn.
Liming Wang, Email: wanglm@cmu1h.com.
References
- 1.Rong M, Liu D, Xu X, Li A, Bai Y, Yang G, Liu K, Zhang Z, Wang L, Wang K, et al. A superparamagnetic composite hydrogel scaffold as in vivo dynamic monitorable theranostic platform for osteoarthritis regeneration. Adv Mater. 2024;36:e2405641. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Wu W, Liu Q, Wu Q, Ren P, Xi X, Liu H, Zhao J, Zhang W, Wang Z, et al. Specific surface-modified iron oxide nanoparticles trigger complement-dependent innate and adaptive antileukaemia immunity. Nat Commun. 2024;15:10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan G, Shi G, Liu H, Xu J, Zhang Q, Dong Z, Lu C, Wang Y, Lei L, Nan B, et al. Responsive magnetic particle imaging tracer: overcoming Always-On limitation, eliminating interference, and ensuring safety in adaptive therapy. Adv Mater. 2024;36:e2409117. [DOI] [PubMed] [Google Scholar]
- 4.Wang N, Zhou D, Xu K, Kou D, Chen C, Li C, Ge J, Chen L, Zeng J, Gao M. Iron Homeostasis-Regulated adaptive metabolism of pegylated ultrasmall iron oxide nanoparticles. ACS Nano. 2025;19:13381–98. [DOI] [PubMed] [Google Scholar]
- 5.Tan M, Li X, Cheng L, Long X, Cao G, Yu S, Ran H, Feng H, Wang H. Augmenting protein degradation capacity of PROTAC through energy metabolism regulation and targeted drug delivery. Adv Mater. 2025;37:e2412837. [DOI] [PubMed] [Google Scholar]
- 6.Guo LS, An Y, Zhang ZY, Ma CB, Li JQ, Dong Z, Tian J, Liu ZY, Liu JG. Exploring the diagnostic potential: magnetic particle imaging for brain diseases. Mil Med Res. 2025;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshino Y, Yoshino F, Aoki I, Mori Y, Suzuki G, Tsuji S, Amano T, Shiino A, Chano T, Furusho Y, et al. 2-Nitroimidazole-Functionalized superparamagnetic iron oxide nanoparticles detect hypoxic regions of glioblastomas on MRI and improve radiotherapy efficacy. ACS Nano. 2025;19:12762–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Huang J, Chen H, Wu H, Xu Y, Li Y, Yi H, Wang YA, Yang L, Mao H. Exerting enhanced permeability and retention effect driven delivery by ultrafine iron oxide nanoparticles with T(1)-T(2) switchable magnetic resonance imaging contrast. ACS Nano. 2017;11:4582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su YL, Fang JH, Liao CY, Lin CT, Li YT, Hu SH. Targeted mesoporous iron oxide Nanoparticles-Encapsulated perfluorohexane and a hydrophobic drug for deep tumor penetration and therapy. Theranostics. 2015;5:1233–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.M RS, Sen MJ, K A, Bs RP, Tt U. Galactomannan armed superparamagnetic iron oxide nanoparticles as a folate receptor targeted multi-functional theranostic agent in the management of cancer. Int J Biol Macromol. 2022;219:740–53. [DOI] [PubMed] [Google Scholar]
- 11.Hwang G, Paula AJ, Hunter EE, Liu Y, Babeer A, Karabucak B, Stebe K, Kumar V, Steager E, Koo H. Catalytic antimicrobial robots for biofilm eradication. Sci Robot 2019; 4:eaaw2388. [DOI] [PMC free article] [PubMed]
- 12.Li Y, Chen J, Xia Q, Shang J, He Y, Li Z, Chen Y, Gao F, Yu X, Yuan Z, Yin P. Photothermal Fe(3)O(4) nanoparticles induced Immunogenic ferroptosis for synergistic colorectal cancer therapy. J Nanobiotechnol. 2024;22:630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suh M, Park JY, Ko GB, Kim JY, Hwang DW, Rees L, Conway GE, Doak SH, Kang H, Lee N, et al. Optimization of micelle-encapsulated extremely small sized iron oxide nanoparticles as a T1 contrast imaging agent: biodistribution and safety profile. J Nanobiotechnol. 2024;22:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rojas JM, Gavilan H, Del Dedo V, Lorente-Sorolla E, Sanz-Ortega L, da Silva GB, Costo R, Perez-Yague S, Talelli M, Marciello M, et al. Time-course assessment of the aggregation and metabolization of magnetic nanoparticles. Acta Biomater. 2017;58:181–95. [DOI] [PubMed] [Google Scholar]
- 15.Gaharwar US, Meena R, Rajamani P. Biodistribution, clearance and morphological alterations of intravenously administered iron oxide nanoparticles in male Wistar rats. Int J Nanomed. 2019;14:9677–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Z, Li M, Zeng J, Huo L, Liu K, Wei R, Ni K, Gao J. Recent advances in engineering iron oxide nanoparticles for effective magnetic resonance imaging. Bioact Mater. 2022;12:214–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Araujo EV, Carneiro SV, Neto DMA, Freire TM, Costa VM, Freire RM, Fechine L, Clemente CS, Denardin JC, Dos Santos JCS, et al. Advances in surface design and biomedical applications of magnetic nanoparticles. Adv Colloid Interface Sci. 2024;328:103166. [DOI] [PubMed] [Google Scholar]
- 18.Lu C, Liao S, Chen B, Xu L, Wu N, Lu D, Kang H, Zhang XB, Song G. Responsive probes for in vivo magnetic resonance imaging of nitric oxide. Nat Mater. 2025;24:133–42. [DOI] [PubMed] [Google Scholar]
- 19.Ye H, Wang K, Wang M, Liu R, Song H, Li N, Lu Q, Zhang W, Du Y, Yang W, et al. Bioinspired nanoplatelets for chemo-photothermal therapy of breast cancer metastasis Inhibition. Biomaterials. 2019;206:1–12. [DOI] [PubMed] [Google Scholar]
- 20.Prasad R, Prerna K, Temgire M, Banerjee P, Kumari R, Kundu GC, Hattila D, Mangannavar CV, Meena AS, Gorain M, et al. Molecular engineering of Ultrabright biomimetic NanoGhost for Site-Selective tumor imaging and biodistribution. Adv Healthc Mater. 2025;14:e2401233. [DOI] [PubMed] [Google Scholar]
- 21.Xie R, Wu Z, Zeng F, Cai H, Wang D, Gu L, Zhu H, Lui S, Guo G, Song B, et al. Retro-enantio isomer of angiopep-2 assists nanoprobes across the blood-brain barrier for targeted magnetic resonance/fluorescence imaging of glioblastoma. Signal Transduct Target Ther. 2021;6:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinel S, Thomas N, Boura C, Barberi-Heyob M. Approaches to physical stimulation of metallic nanoparticles for glioblastoma treatment. Adv Drug Deliv Rev. 2019;138:344–57. [DOI] [PubMed] [Google Scholar]
- 23.Stephen ZR, Chiarelli PA, Revia RA, Wang K, Kievit F, Dayringer C, Jeon M, Ellenbogen R, Zhang M. Time-Resolved MRI assessment of Convection-Enhanced delivery by targeted and nontargeted nanoparticles in a human glioblastoma mouse model. Cancer Res. 2019;79:4776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao ZY, Kuang J, Pan T, Qin YT, Huang QX, Sun YL, Zhao K, Jin XK, Yang CH, Zhang SM, et al. Cationic magnetic nanoparticles activate natural killer cells for the treatment of glioblastoma. ACS Nano. 2025;19:649–61. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y, Pan J, Han B, Hou W, Li B, Wang J, Wang G, He Y, Ma M, Zhou J, et al. Ultrahigh-Resolution visualization of vascular heterogeneity in brain tumors via magnetic Nanoparticles-Enhanced Susceptibility-Weighted imaging. ACS Nano. 2024;18:21112–24. [DOI] [PubMed] [Google Scholar]
- 26.Chen F, Li T, Zhang H, Saeed M, Liu X, Huang L, Wang X, Gao J, Hou B, Lai Y, et al. Acid-Ionizable iron nanoadjuvant augments STING activation for personalized vaccination immunotherapy of cancer. Adv Mater. 2023;35:e2209910. [DOI] [PubMed] [Google Scholar]
- 27.Horvat NK, Chocarro S, Marques O, Bauer TA, Qiu R, Diaz-Jimenez A, Helm B, Chen Y, Sawall S, Sparla R, et al. Superparamagnetic iron oxide nanoparticles reprogram the tumor microenvironment and reduce lung cancer regrowth after Crizotinib treatment. ACS Nano. 2024;18:11025–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korangath P, Jin L, Yang CT, Healy S, Guo X, Ke S, Gruttner C, Hu C, Gabrielson K, Foote J, et al. Iron oxide nanoparticles inhibit tumor progression and suppress lung metastases in mouse models of breast cancer. ACS Nano. 2024;18:10509–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stater EP, Morcos G, Isaac E, Ogirala A, Hsu HT, Longo VA, Grimm J. Translatable Drug-Loaded iron oxide nanophore sensitizes murine melanoma tumors to monoclonal antibody immunotherapy. ACS Nano. 2023;17:6178–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guntnur RT, Muzzio N, Gomez A, Macias S, Galindo A, Ponce A, Romero G. On-Demand chemomagnetic modulation of striatal neurons facilitated by hybrid magnetic nanoparticles. Adv Funct Mater 2022; 32:2204732. [DOI] [PMC free article] [PubMed]
- 31.Zhou H, Guo Y, Fu T, Peng Y, Chen Z, Cui Y, Guo M, Zhang K, Chen C, Wang Y. Three-Dimensional Label-Free observing of the Self-Assembled nanoparticles inside a single cell at nanoscale resolution. ACS Nano 2024;18:19802–13. [DOI] [PubMed]
- 32.Li X, Li W, Wang M, Liao Z. Magnetic nanoparticles for cancer theranostics: advances and prospects. J Control Release. 2021;335:437–48. [DOI] [PubMed] [Google Scholar]
- 33.Du Y, Liu X, Liang Q, Liang XJ, Tian J. Optimization and design of magnetic ferrite nanoparticles with uniform tumor distribution for highly sensitive MRI/MPI performance and improved magnetic hyperthermia therapy. Nano Lett. 2019;19:3618–26. [DOI] [PubMed] [Google Scholar]
- 34.Hu Q, Zhu Y, Mei J, Liu Y, Zhou G. Extracellular matrix dynamics in tumor immunoregulation: from tumor microenvironment to immunotherapy. J Hematol Oncol. 2025;18:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song NJ, Xie J, Jung KJ, Wang Y, Pozniak J, Roda N, Marine JC, Riesenberg BP, Jeon H, Ma A et al. Tumor-Associated NK cells regulate distinct CD8 + T-cell differentiation program in cancer and contribute to resistance against immune checkpoint blockers. Cancer Discov 2025:OF1–23. [DOI] [PMC free article] [PubMed]
- 36.Aliazis K, Christofides A, Shah R, Yeo YY, Jiang S, Charest A, Boussiotis VA. The tumor microenvironment’s role in the response to immune checkpoint blockade. Nat Cancer 2025;6:924–37. [DOI] [PMC free article] [PubMed]
- 37.Chen E, Wang Q, Wang L, Huang Z, Yang D, Zhao C, Chen W, Zhang S, Xiong S, He Y et al. NAT10 regulates tumor progression and immune microenvironment in pancreatic ductal adenocarcinoma via the N4-acetylated LAMB3-mediated FAK/ERK pathway. Cancer Commun (Lond) 2025. 10.1002/cac2.70045. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 38.Tang Y, Xu A, Xu Z, Xie J, Huang W, Zhang L, Chen Y, Yang L, Du S, Wang K. Multi-omics analyses of the heterogenous immune microenvironment in triple-negative breast cancer implicate UQCRFS1 potentiates tumor progression. Exp Hematol Oncol. 2025;14:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang C, Tang X, Tang J, Hu J, Wan L, Chen J, Fu R, Cao Y, Li R. An oxygen-generating nanoplatform remodels the immunosuppressive tumor microenvironment via synergistic lactate depletion and sonodynamic therapy. J Nanobiotechnol. 2025;23:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Y, Lu WM, Cui QR, Zhou J, Lu GD. Metabolic regulation of cGAS-STING signaling in the tumor microenvironment: dual immune roles and therapeutic implications. Cytokine Growth Factor Rev 2025. 10.1016/j.cytogfr.2025.06.002. Online ahead of print. [DOI] [PubMed]
- 41.Duan M, Feng Q, Liang J, Liu S, Chen L, Wang Z. Magnetic drug delivery system capable of remodeling the tumor immune microenvironment for photothermal immunotherapy/chemotherapy. Adv Healthc Mater 2025:e2501088. [DOI] [PubMed]
- 42.Zuljan E, von der Emde B, Piwonski I, Pestana A, Klinghammer K, Mock A, Horak P, Heining C, Klauschen F, Pretzell I, et al. A macrophage-predominant immunosuppressive microenvironment and therapeutic vulnerabilities in advanced salivary gland cancer. Nat Commun. 2025;16:5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen M, Zhao S, Xie X, Wang J, Su M, Zhang L, Cui R, Zhao D. Role of tunneling nanotubes in arachidonic acid transfer and macrophage function reprogramming in intrahepatic cholangiocarcinoma. Adv Sci (Weinh) 2025:e00148. [DOI] [PMC free article] [PubMed]
- 44.Yin B, Ding J, Liu J, Hu H, Zhu Y, Yang M, Zhou H, Huang B, Huang T, Li M et al. Exosomal CMTM4 induces immunosuppressive macrophages to promote ovarian cancer progression and attenuate Anti-PD-1 immunotherapy. Adv Sci (Weinh) 2025:e04436. [DOI] [PMC free article] [PubMed]
- 45.He S, Zheng L, Qi C. Myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment and their targeting in cancer therapy. Mol Cancer. 2025;24:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia J, Zhang L, Zhu W, Tu J, Peng X, Deng Q, Li S, He X, Dong H, Liu C, et al. Tumor cell-derived N-acetyl-aspartyl-glutamate reshapes the tumor microenvironment to facilitate breast cancer metastasis. Sci Bull (Beijing). 2025;70:1126–38. [DOI] [PubMed] [Google Scholar]
- 47.Imianowski CJ, Chen Q, Workman CJ, Vignali DAA. Regulatory T cells in the tumour microenvironment. Nat Rev Cancer 2025. 10.1038/s41568-025-00832-9. Online ahead of print. [DOI] [PubMed]
- 48.Feng K, Zhang X, Li J, Han M, Wang J, Chen F, Yi Z, Di L, Wang R. Neoantigens combined with in situ cancer vaccination induce personalized immunity and reshape the tumor microenvironment. Nat Commun. 2025;16:5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin M, Liu Z, Zhou Y, Li W, Yan J, Cao D, Yin L. Two-pronged anti-cancer nanovaccines enpowered by exogenous/endogenous tumor-associated antigens. J Control Release. 2024;373:358–69. [DOI] [PubMed] [Google Scholar]
- 50.Zheng P, Xiao Y, Wu Z, Wang Q, Lv Y, Niu W, Zhang Y, Xiao J, Cao J, Li M et al. Tumor-secreted galectin-3 suppresses antitumor response by inducing IL-10(+) B cells. J Immunother Cancer 2025, 13:e011445. [DOI] [PMC free article] [PubMed]
- 51.Yao Y, Liu YY, Li JF, Chen YS, Shi L, Shen Y, Yang LL, Yang Q. Indoleamine 2,3-dioxygenase 1 alters the proportions of B cell subpopulations in the microenvironment of acute myeloid leukemia. Mol Biomed. 2025;6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu Y, Yan Y, Guo Y, Niu M, Zhou B, Zhang J, Zhou P, Chu Q, Mei Q, Yi M, Wu K. Anti-TGF-beta/PD-L1 bispecific antibody synergizes with radiotherapy to enhance antitumor immunity and mitigate radiation-induced pulmonary fibrosis. J Hematol Oncol. 2025;18:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Green WD, Gomez A, Plotkin AL, Pratt BM, Merritt EF, Mullins GN, Kren NP, Modliszewski JL, Zhabotynsky V, Woodcock MG et al. Enhancer-driven gene regulatory networks reveal transcription factors governing T cell adaptation and differentiation in the tumor microenvironment. Immunity 2025;58:1725-41.e9. [DOI] [PMC free article] [PubMed]
- 54.Yang J, Li Y, Jiang S, Tian Y, Zhang M, Guo S, Wu P, Li J, Xu L, Li W, et al. Engineered brain-targeting exosome for reprogramming immunosuppressive microenvironment of glioblastoma. Explor (Beijing). 2025;5:20240039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang L, Hou Y, Di T, Lu X, Huang J, Gu L, Yu Y, Liu L, Qiu J, Li L et al. Antiangiogenic therapy combined with immune checkpoint blockade mediates CCR7 + CD8 + T-cell entry into HCC through high endothelial venules. Hepatology 2025. 10.1097/HEP.0000000000001426. Online ahead of print. [DOI] [PubMed]
- 56.Fu M, Zhao J, Zhang L, Sheng Z, Li X, Qiu F, Feng Y, You M, Xu H, Zhang J, et al. Overcoming tyrosine kinase inhibitor resistance in lung cancer brain metastasis with CTLA4 Blockade. Cancer Cell. 2024;42:1882–e18971887. [DOI] [PubMed] [Google Scholar]
- 57.Ausejo-Mauleon I, Labiano S, de la Nava D, Laspidea V, Zalacain M, Marrodán L, García-Moure M, González-Huarriz M, Hervás-Corpión I, Dhandapani L, et al. TIM-3 Blockade in diffuse intrinsic Pontine glioma models promotes tumor regression and antitumor immune memory. Cancer Cell. 2023;41:1911–26. e1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roscigno G, Jacobs S, Toledo B, Borea R, Russo G, Pepe F, Serrano MJ, Calabro V, Troncone G, Giovannoni R, et al. The potential application of stroma modulation in targeting tumor cells: focus on pancreatic cancer and breast cancer models. Semin Cancer Biol. 2025;113:151–75. [DOI] [PubMed] [Google Scholar]
- 59.Abu-Sultanah M, Zhou Z, Jiang C, Mitchell DK, Bessler WK, Jiang L, Li X, Qian S, Smith AE, Mang HE, et al. TGFbeta-dependent signaling drives tumor growth and aberrant extracellular matrix dynamics in NF1-associated plexiform neurofibroma. Sci Adv. 2025;11:eadu0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee JJ, Ng KY, Bakhtiar A. Extracellular matrix: unlocking new avenues in cancer treatment. Biomark Res. 2025;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ding X, Liang Y, Zhou S, Wu Y, Salata P, Mikolajczk-Martinez A, Khosrawipour V, Zhang Z. Targeting tumor extracellular matrix with nanoparticles to circumvent therapeutic resistance. J Control Release. 2025;383:113786. [DOI] [PubMed] [Google Scholar]
- 62.Zhu C, Liu C, Wu Q, Sheng T, Zhou R, Ren E, Zhang R, Zhao Z, Shi J, Shen X, et al. Remolding the tumor microenvironment by bacteria augments adoptive T cell therapy in advanced-stage solid tumors. Signal Transduct Target Ther. 2024;9:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gong XT, Dai XY, Cheng W, Li B, Liu J, Chen H, Wu M, Yang J, Liu B. Targeted High-Resolution 3D imaging of tumor vasculatures at different stages using Far-Red AIE nanoparticles compatible with tissue clearing. Adv Mater. 2025;37:e2501144. [DOI] [PubMed] [Google Scholar]
- 64.Hou W, Xiao C, Zhou R, Yao X, Chen Q, Xu T, Cao F, Wang Y, Li X, Yan O, et al. Inhibiting autophagy selectively prunes dysfunctional tumor vessels and optimizes the tumor immune microenvironment. Theranostics. 2025;15:258–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rahman MA, Yadab MK, Ali MM. Emerging role of extracellular pH in tumor microenvironment as a therapeutic target for cancer immunotherapy. Cells. 2024;13:1924. [DOI] [PMC free article] [PubMed]
- 66.Knopf P, Stowbur D, Hoffmann SHL, Hermann N, Maurer A, Bucher V, Poxleitner M, Tako B, Sonanini D, Krishnamachary B, et al. Acidosis-mediated increase in IFN-gamma-induced PD-L1 expression on cancer cells as an immune escape mechanism in solid tumors. Mol Cancer. 2023;22:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wan J, Shi JH, Shi M, Huang H, Zhang Z, Li W, Guo C, Bao R, Yu X, Han Q, et al. Lactate dehydrogenase B facilitates Disulfidptosis and exhaustion of tumour-infiltrating CD8(+) T cells. Nat Cell Biol. 2025;27:972–82. [DOI] [PubMed] [Google Scholar]
- 68.Jin J, Yan P, Wang D, Bai L, Liang H, Zhu X, Zhu H, Ding C, Wei H, Wang Y. Targeting lactylation reinforces NK cell cytotoxicity within the tumor microenvironment. Nat Immunol. 2025;26:1099–112. [DOI] [PubMed]
- 69.Houlahan KE, Khan A, Greenwald NF, Vivas CS, West RB, Angelo M, Curtis C. Germline-mediated immunoediting sculpts breast cancer subtypes and metastatic proclivity. Science. 2024;384:eadh8697. [DOI] [PubMed] [Google Scholar]
- 70.Finlay JB, Ireland AS, Hawgood SB, Reyes T, Ko T, Olsen RR, Abi Hachem R, Jang DW, Bell D, Chan JM, et al. Olfactory neuroblastoma mimics molecular heterogeneity and lineage trajectories of small-cell lung cancer. Cancer Cell. 2024;42:1086–e11051013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jenkins BH, Tracy I, Rodrigues M, Smith MJL, Martinez BR, Edmond M, Mahadevan S, Rao A, Zong H, Liu K, et al. Single cell and Spatial analysis of immune-hot and immune-cold tumours identifies fibroblast subtypes associated with distinct immunological niches and positive immunotherapy response. Mol Cancer. 2025;24:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fan Q, Xiong W, Zhou H, Yang J, Feng J, Li Z, Wu L, Hu F, Duan X, Li B, et al. An AND logic gate for Magnetic-Resonance-Imaging-Guided ferroptosis therapy of tumors. Adv Mater. 2023;35:e2305932. [DOI] [PubMed] [Google Scholar]
- 73.Liang X, Chen M, Bhattarai P, Hameed S, Tang Y, Dai Z. Complementing cancer photodynamic therapy with ferroptosis through iron oxide loaded Porphyrin-Grafted lipid nanoparticles. ACS Nano. 2021;15:20164–80. [DOI] [PubMed] [Google Scholar]
- 74.Ota S, Kosaka H, Honda K, Hoshino K, Goto H, Futagawa M, Takemura Y, Shimizu K. Characterization of microscopic structures in living tumor by in vivo measurement of magnetic relaxation time distribution of intratumor magnetic nanoparticles. Adv Mater. 2024;36:e2404766. [DOI] [PubMed] [Google Scholar]
- 75.Lu K, Zhang R, Wang H, Li C, Yang Z, Xu K, Cao X, Wang N, Cai W, Zeng J, Gao M. PEGylated ultrasmall iron oxide nanoparticles as MRI contrast agents for vascular imaging and Real-Time monitoring. ACS Nano. 2025;19:3519–30. [DOI] [PubMed] [Google Scholar]
- 76.Li C, Shan S, Chen L, Afshari MJ, Wang H, Lu K, Kou D, Wang N, Gao Y, Liu C, et al. Using adaptive imaging parameters to improve pegylated ultrasmall iron oxide Nanoparticles-Enhanced magnetic resonance angiography. Adv Sci (Weinh). 2024;11:e2405719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soetaert F, Korangath P, Serantes D, Fiering S, Ivkov R. Cancer therapy with iron oxide nanoparticles: agents of thermal and immune therapies. Adv Drug Deliv Rev. 2020;163–164:65–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Min KA, Shin MC, Yu F, Yang M, David AE, Yang VC, Rosania GR. Pulsed magnetic field improves the transport of iron oxide nanoparticles through cell barriers. ACS Nano. 2013;7:2161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Child HW, Del Pino PA, De La Fuente JM, Hursthouse AS, Stirling D, Mullen M, McPhee GM, Nixon C, Jayawarna V, Berry CC. Working together: the combined application of a magnetic field and penetratin for the delivery of magnetic nanoparticles to cells in 3D. ACS Nano. 2011;5:7910–9. [DOI] [PubMed] [Google Scholar]
- 80.Ohayon L, Zhang X, Dutta P. The role of extracellular vesicles in regulating local and systemic inflammation in cardiovascular disease. Pharmacol Res. 2021;170:105692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Savic LJ, Doemel LA, Schobert IT, Montgomery RR, Joshi N, Walsh JJ, Santana J, Pekurovsky V, Zhang X, Lin M, et al. Molecular MRI of the Immuno-Metabolic interplay in a rabbit liver tumor model: A biomarker for resistance mechanisms in tumor-targeted therapy?? Radiology. 2020;296:575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oliver AA, Carlson KD, Price C, Banaskiewicz K, Benike A, Dai D, Brown RA, Sandhu GS, Kadirvel R, Guillory RJ 2nd et al.Magnetic capture of blood outgrowth endothelial cells to the luminal surface of magnetizable stent-grafts promotes healing in a Porcine pseudoaneurysm model. Acta Biomater. 2025. 10.1016/j.actbio.2025.03.040. Online ahead of print. [DOI] [PubMed]
- 83.Li S, Xu M, Yao C, Yang D. DNA-Based nanostructures for tumor microenvironment-responsive drug delivery. Adv Drug Deliv Rev. 2025;223:115610. [DOI] [PubMed] [Google Scholar]
- 84.Mehdizadeh S, Mamaghani M, Hassanikia S, Pilehvar Y, Ertas YN. Exosome-powered neuropharmaceutics: unlocking the blood-brain barrier for next-gen therapies. J Nanobiotechnol. 2025;23:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fan Q, Wang M, Lin J, Huang Y, Yang J, Zhu J, Ren B, Sun L, Li Z, Liu A et al. A mesoporous calcium peroxide nanocuboid with high tumor accumulation across biological barriers for high efficacy tumor therapy. Adv Sci (Weinh) 2025:e10778. [DOI] [PMC free article] [PubMed]
- 86.Asad S, Ahl D, Suarez-Lopez YDC, Erdelyi M, Phillipson M, Teleki A. Click Chemistry-Based bioconjugation of iron oxide nanoparticles. Small. 2025;21:e2407883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hohenschutz M, Bauduin P, Lopez CG, Forster B, Richtering W. Superchaotropic Nano-ion binding as a gelation motif in cellulose ether solutions. Angew Chem Int Ed Engl. 2023;62:e202210208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun Y, Qu F, Geng R, Xiao W, Bi D, Xiong B, Liu Y, Zhu J, Chen X. Electrostatic assembly of gold nanoclusters in reverse emulsion enabling nanoassemblies with tunable structure and size for enhanced NIR-II fluorescence imaging. ACS Nano. 2024;18:32126–44. [DOI] [PubMed] [Google Scholar]
- 89.Pan S, Yuan H, Zhai Q, Zhang Y, He H, Yin T, Tang X, Gou J. The journey of nanoparticles in the abdominal cavity: exploring their in vivo fate and impact factors. J Control Release. 2024;376:266–85. [DOI] [PubMed] [Google Scholar]
- 90.Lee NK, Kim SN, Park CG. Immune cell targeting nanoparticles: a review. Biomater Res. 2021;25:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang W, Sun Y, Yang F, Wang Y, Li X, Yang Y, Jiang D. Preparation and properties of slow-release fertilizer containing Urea encapsulated by pinecone Biochar and cellulose acetate. Int J Biol Macromol. 2025;315:144448. [DOI] [PubMed] [Google Scholar]
- 92.Wang F, Zhou X, Chen R, Kang J, Yang X, Lin J, Liu F, Cao D, Xing Z. Improved performance of non-preloaded and high flip-angle dynamic susceptibility contrast perfusion-weighted imaging sequences in the presurgical differentiation of brain lymphoma and glioblastoma. Eur Radiol. 2023;33:8800–8. [DOI] [PubMed] [Google Scholar]
- 93.Wu H, Li W, Hao M, Wang Y, Xue L, Ju C, Zhang C. An EPR-Independent extravasation strategy: deformable leukocytes as vehicles for improved solid tumor therapy. Adv Drug Deliv Rev. 2022;187:114380. [DOI] [PubMed] [Google Scholar]
- 94.Hirschbiegel CM, Zhang X, Huang R, Cicek YA, Fedeli S, Rotello VM. Inorganic nanoparticles as scaffolds for bioorthogonal catalysts. Adv Drug Deliv Rev. 2023;195:114730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ning L, Zanella S, Tomov ML, Amoli MS, Jin L, Hwang B, Saadeh M, Chen H, Neelakantan S, Dasi LP, et al. Targeted Rapamycin delivery via magnetic nanoparticles to address stenosis in a 3D bioprinted in vitro model of pulmonary veins. Adv Sci (Weinh). 2024;11:e2400476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang X, Lu H, Tang N, Chen A, Wei Z, Cao R, Zhu Y, Lin L, Li Q, Wang Z, Tian L. Low-Power magnetic Resonance-Guided focused ultrasound tumor ablation upon controlled accumulation of magnetic nanoparticles by Cascade-Activated DNA Cross-Linkers. ACS Appl Mater Interfaces. 2022;14:31677–88. [DOI] [PubMed] [Google Scholar]
- 97.Li S, Fu Y, Jia X, Liu Z, Qian Z, Zha H, Lei G, Yu L, Zhang X, Zhang T et al. NLRP6 deficiency enhances macrophage-mediated phagocytosis via E-Syt1 to inhibit hepatocellular carcinoma progression. Gut. 2025. 10.1136/gutjnl-2024-334448. Online ahead of print. [DOI] [PubMed]
- 98.Li J, Wang Z, Qin X, Zhong MC, Tang Z, Qian J, Dou J, Hussell T, King PD, Nunes JA, et al. CD200R1-CD200 checkpoint inhibits phagocytosis differently from SIRPalpha-CD47 to suppress tumor growth. Nat Commun. 2025;16:5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen S, Wang Y, Dang J, Song N, Chen X, Wang J, Huang GN, Brown CE, Yu J, Weissman IL, et al. CAR macrophages with built-In CD47 blocker combat tumor antigen heterogeneity and activate T cells via cross-presentation. Nat Commun. 2025;16:4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang B, Sun J, Yuan Y, Ji D, Sun Y, Liu Y, Li S, Zhu X, Wu X, Hu J, et al. Proximity-enabled covalent binding of IL-2 to IL-2Ralpha selectively activates regulatory T cells and suppresses autoimmunity. Signal Transduct Target Ther. 2023;8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang JW, Lai RM, Wang LF, Wang SL, Xue HX, Li C, Zheng ZZ, Li J, Zhu YY, Zeng DW, et al. Varied immune responses of HBV-specific B cells in patients undergoing pegylated interferon-alpha treatment for chronic hepatitis B. J Hepatol. 2024;81:960–70. [DOI] [PubMed] [Google Scholar]
- 102.Guo C, Yuan H, Wang Y, Feng Y, Zhang Y, Yin T, He H, Gou J, Tang X. The interplay between pegylated nanoparticles and blood immune system. Adv Drug Deliv Rev. 2023;200:115044. [DOI] [PubMed] [Google Scholar]
- 103.Kim E, Jeon S, Yang YS, Jin C, Kim JY, Oh YS, Rah JC, Choi H. A Neurospheroid-Based microrobot for targeted neural connections in a hippocampal slice. Adv Mater. 2023;35:e2208747. [DOI] [PubMed] [Google Scholar]
- 104.Shu G, Chen M, Song J, Xu X, Lu C, Du Y, Xu M, Zhao Z, Zhu M, Fan K, et al. Sialic acid-engineered mesoporous polydopamine nanoparticles loaded with SPIO and Fe(3+) as a novel theranostic agent for T1/T2 dual-mode MRI-guided combined chemo-photothermal treatment of hepatic cancer. Bioact Mater. 2021;6:1423–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li D, Zhang C, Xiong Q, Liu W, Tang Y, Liang L, Pu K, Duan H. Elongated magnetic nanorobots with Multi-Enzymatic cascades for active in vivo tumor targeting and enhanced chemodynamic therapy. ACS Nano. 2025;19:15040–54. [DOI] [PubMed] [Google Scholar]
- 106.Caro C, Guzzi C, Moral-Sanchez I, Urbano-Gamez JD, Beltran AM, Garcia-Martin ML. Smart design of ZnFe and znfe@fe nanoparticles for MRI-Tracked magnetic hyperthermia therapy: challenging classical theories of nanoparticles growth and nanomagnetism. Adv Healthc Mater. 2024;13:e2304044. [DOI] [PubMed] [Google Scholar]
- 107.Yue S, Zhang X, Xu Y, Zhu L, Cheng J, Qiao Y, Dai S, Zhu J, Jiang N, Wu H, et al. The influence of surface charge on the tumor-targeting behavior of Fe(3)O(4) nanoparticles for MRI. J Mater Chem B. 2022;10:646–55. [DOI] [PubMed] [Google Scholar]
- 108.Wang D, Zhou J, Fang W, Huang C, Chen Z, Fan M, Zhang MR, Xiao Z, Hu K, Luo L. A multifunctional nanotheranostic agent potentiates erlotinib to EGFR wild-type non-small cell lung cancer. Bioact Mater. 2022;13:312–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li C, Wang Z, Ding P, Zhou Z, Chen R, Hu Y, Zhao K, Peng W, Yang X, Sun N, Pei R. A digital score based on Circulating-Tumor-Cells-Derived mRNA quantification and machine learning for early colorectal cancer detection. ACS Nano. 2025;19:18117–28. [DOI] [PubMed] [Google Scholar]
- 110.Bayoumi NA, El-Shershaby HM, Darwish WM. Synthesis of iron oxide nanocrystals functionalized with hyaluronic acid and (131)I-cetuximab for targeted combined (radio-photothermal) treatment of HepG2 cells. Int J Biol Macromol. 2025;305:140890. [DOI] [PubMed] [Google Scholar]
- 111.Pfister F, Carnell LR, Loffler L, Boosz P, Schaft N, Dorrie J, Stein R, Lenz M, Spiecker E, Huber CM et al. Loading of CAR-T cells with magnetic nanoparticles for controlled targeting suppresses inflammatory cytokine release and switches tumor cell death mechanism. MedComm (2020) 2025, 6:e70039. [DOI] [PMC free article] [PubMed]
- 112.Kiru L, Zlitni A, Tousley AM, Dalton GN, Wu W, Lafortune F, Liu A, Cunanan KM, Nejadnik H, Sulchek T et al. In vivo imaging of nanoparticle-labeled CAR T cells. Proc Natl Acad Sci U S A. 2022;119:e2102363119. [DOI] [PMC free article] [PubMed]
- 113.Lei K, Kurum A, Tang L. Mechanical Immunoengineering of T cells for therapeutic applications. Acc Chem Res. 2020;53:2777–90. [DOI] [PubMed] [Google Scholar]
- 114.Hunger J, Schregel K, Boztepe B, Agardy DA, Turco V, Karimian-Jazi K, Weidenfeld I, Streibel Y, Fischer M, Sturm V, et al. In vivo nanoparticle-based T cell imaging can predict therapy response towards adoptive T cell therapy in experimental glioma. Theranostics. 2023;13:5170–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shahbazi-Gahrouei D, Abdi N, Shahbazi-Gahrouei S, Hejazi SH, Salehnia Z. In vivo study of anti-epidermal growth factor receptor antibody-based iron oxide nanoparticles (anti-EGFR-SPIONs) as a novel MR imaging contrast agent for lung cancer (LLC1) cells detection. IET Nanobiotechnol. 2020;14:369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shivanna AT, Dash BS, Lu YJ, Lin WT, Chen JP. Magnetic lipid-poly(lactic-co-glycolic acid) nanoparticles conjugated with epidermal growth factor receptor antibody for dual-targeted delivery of CPT-11. Int J Pharm. 2024;667:124856. [DOI] [PubMed] [Google Scholar]
- 117.Peng Y, Gao Y, Yang C, Guo R, Shi X, Cao X. Low-Molecular-Weight Poly(ethylenimine) nanogels loaded with ultrasmall iron oxide nanoparticles for T(1)-Weighted MR Imaging-Guided gene therapy of sarcoma. ACS Appl Mater Interfaces. 2021;13:27806–13. [DOI] [PubMed] [Google Scholar]
- 118.Wang J, Xu J, Liu X, Li X, Xu Z. A microfluidic chip incorporating magnetic sorting and invasive separation for isolation, culture and telomerase analysis of Circulating tumor cells. Talanta. 2025;285:127316. [DOI] [PubMed] [Google Scholar]
- 119.Ding M, Yang Y, Sun N, He Y, Dong Z, Gao Q, Tian B. Catalytic hairpin assembly-and-cyclization aptasensing for AND-logic detection of protein- and RNA-targets in ribonucleoprotein. Biosens Bioelectron. 2025;279:117388. [DOI] [PubMed] [Google Scholar]
- 120.Liu Y, Quan X, Li J, Huo J, Li X, Zhao Z, Li S, Wan J, Li J, Liu S, et al. Liposomes embedded with pegylated iron oxide nanoparticles enable ferroptosis and combination therapy in cancer. Natl Sci Rev. 2023;10:nwac167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cheng HW, Lee W, Hsu FT, Lai YH, Huang SR, Lim CSH, Lin ZK, Hsu SC, Chiang CS, Jeng LB, et al. Manipulating the crosstalk between cancer and immunosuppressive cells with phototherapeutic Gold-Nanohut for reprogramming tumor microenvironment. Adv Sci (Weinh). 2024;11:e2404347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li L, Jiang R, Yu JF, Li M. A Near-Infrared II Photo-Triggered multifunctional plasmonic hyperthermia Immunomodulator for SERS-Guided combination cancer immunotherapy. Small. 2025;21:e2409154. [DOI] [PubMed] [Google Scholar]
- 123.Huang H, Tong QS, Zhang JY, Miao WM, Yu HH, Wang J, Shen S, Du JZ. Phagocytosis-Activating nanocomplex orchestrates Macrophage-Mediated cancer immunotherapy. Adv Mater. 2025;37:e2500982. [DOI] [PubMed] [Google Scholar]
- 124.Shaw JR, Caprio N, Truong N, Weldemariam M, Tran A, Pilli N, Pandey S, Jones JW, Kane MA, Pearson RM. Inflammatory disease progression shapes nanoparticle biomolecular corona-mediated immune activation profiles. Nat Commun. 2025;16:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shah RM, Jadhav SR, Bryant G, Kaur IP, Harding IH. On the formation and stability mechanisms of diverse lipid-based nanostructures for drug delivery. Adv Colloid Interface Sci. 2025;338:103402. [DOI] [PubMed] [Google Scholar]
- 126.Le Q, Fang Z, Chen H, Wu J. Comprehensive review on oleogels structured by Lipid-Based compounds: from structure mechanisms to nutritional functionalities and applications in food industry. Compr Rev Food Sci Food Saf. 2025;24:e70214. [DOI] [PubMed] [Google Scholar]
- 127.Rizvi MH, Wang R, Schubert J, Crumpler WD, Rossner C, Oldenburg AL, Fery A, Tracy JB. Magnetic alignment for plasmonic control of gold nanorods coated with iron oxide nanoparticles. Adv Mater. 2022;34:e2203366. [DOI] [PubMed] [Google Scholar]
- 128.Qiao R, Fu C, Forgham H, Javed I, Huang X, Zhu J, Whittaker AK, Davis TP. Magnetic iron oxide nanoparticles for brain imaging and drug delivery. Adv Drug Deliv Rev. 2023;197:114822. [DOI] [PubMed] [Google Scholar]
- 129.Zhou C, Wang C, Xu K, Niu Z, Zou S, Zhang D, Qian Z, Liao J, Xie J. Hydrogel platform with tunable stiffness based on magnetic nanoparticles cross-linked GelMA for cartilage regeneration and its intrinsic biomechanism. Bioact Mater. 2023;25:615–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Segers FME, Ruder AV, Westra MM, Lammers T, Dadfar SM, Roemhild K, Lam TS, Kooi ME, Cleutjens K, Verheyen FK, et al. Magnetic resonance imaging contrast-enhancement with superparamagnetic iron oxide nanoparticles amplifies macrophage foam cell apoptosis in human and murine atherosclerosis. Cardiovasc Res. 2023;118:3346–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kiarashi M, Mahamed P, Ghotbi N, Tadayonfard A, Nasiri K, Kazemi P, Badkoobeh A, Yasamineh S, Joudaki A. Spotlight on therapeutic efficiency of green synthesis metals and their oxide nanoparticles in periodontitis. J Nanobiotechnol. 2024;22:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ibrahim MS, Ferhan AR, Zhou C, Li J, Lin YC, Deng J, Zhao Z, Cho NJ. Allergen to asset: Pollen-based drug delivery systems. Adv Drug Deliv Rev. 2025;224:115643. [DOI] [PubMed] [Google Scholar]
- 133.Xu W, Guan G, Yue R, Dong Z, Lei L, Kang H, Song G. Chemical design of magnetic nanomaterials for imaging and Ferroptosis-Based cancer therapy. Chem Rev. 2025;125:1897–961. [DOI] [PubMed] [Google Scholar]
- 134.Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11:986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Luo L, Iqbal MZ, Liu C, Xing J, Akakuru OU, Fang Q, Li Z, Dai Y, Li A, Guan Y, Wu A. Engineered nano-immunopotentiators efficiently promote cancer immunotherapy for inhibiting and preventing lung metastasis of melanoma. Biomaterials. 2019;223:119464. [DOI] [PubMed] [Google Scholar]
- 136.Liu L, Fu S, Zhu W, Cai Z, Cao Y, Huang Y, Yang L, Fu X, Jin R, Xia C, et al. Glucosylation endows nanoparticles with TLR4 agonist capability to trigger macrophage polarization and augment antitumor immunity. Biomaterials. 2024;304:122424. [DOI] [PubMed] [Google Scholar]
- 137.Chiang CS, Lin YJ, Lee R, Lai YH, Cheng HW, Hsieh CH, Shyu WC, Chen SY. Combination of fucoidan-based magnetic nanoparticles and immunomodulators enhances tumour-localized immunotherapy. Nat Nanotechnol. 2018;13:746–54. [DOI] [PubMed] [Google Scholar]
- 138.Prasad R, Aiyer S, Chauhan DS, Srivastava R, Selvaraj K. Bioresponsive carbon nano-gated multifunctional mesoporous silica for cancer theranostics. Nanoscale. 2016;8:4537–46. [DOI] [PubMed] [Google Scholar]
- 139.Meng X, Wang J, Zhou J, Tian Q, Qie B, Zhou G, Duan W, Zhu Y. Tumor cell membrane-based peptide delivery system targeting the tumor microenvironment for cancer immunotherapy and diagnosis. Acta Biomater. 2021;127:266–75. [DOI] [PubMed] [Google Scholar]
- 140.Xiong J, Wu M, Chen J, Liu Y, Chen Y, Fan G, Liu Y, Cheng J, Wang Z, Wang S, et al. Cancer-Erythrocyte hybrid Membrane-Camouflaged magnetic nanoparticles with enhanced Photothermal-Immunotherapy for ovarian cancer. ACS Nano. 2021;15:19756–70. [DOI] [PubMed] [Google Scholar]
- 141.Bocanegra Gondan AI, Ruiz-de-Angulo A, Zabaleta A, Gomez Blanco N, Cobaleda-Siles BM, Garcia-Granda MJ, Padro D, Llop J, Arnaiz B, Gato M, et al. Effective cancer immunotherapy in mice by polyIC-imiquimod complexes and engineered magnetic nanoparticles. Biomaterials. 2018;170:95–115. [DOI] [PubMed] [Google Scholar]
- 142.Zhang F, Lu G, Wen X, Li F, Ji X, Li Q, Wu M, Cheng Q, Yu Y, Tang J, Mei L. Magnetic nanoparticles coated with polyphenols for spatio-temporally controlled cancer photothermal/immunotherapy. J Control Release. 2020;326:131–9. [DOI] [PubMed] [Google Scholar]
- 143.Gupta A, Niveria K, Chandpa HH, Singh M, Kumar V, Panda AK, Meena J. Stimuli-responsive magnetic silica-poly-lactic-co-glycolic acid hybrid nanoparticles for targeted cancer chemo-immunotherapy. Drug Deliv Transl Res. 2024;14:2712–26. [DOI] [PubMed] [Google Scholar]
- 144.Wu L, Zhang F, Wei Z, Li X, Zhao H, Lv H, Ge R, Ma H, Zhang H, Yang B, et al. Magnetic delivery of Fe(3)O(4)@polydopamine nanoparticle-loaded natural killer cells suggest a promising anticancer treatment. Biomater Sci. 2018;6:2714–25. [DOI] [PubMed] [Google Scholar]
- 145.Burga RA, Khan DH, Agrawal N, Bollard CM, Fernandes R. Designing magnetically responsive biohybrids composed of cord Blood-Derived natural killer cells and iron oxide nanoparticles. Bioconjug Chem. 2019;30:552–60. [DOI] [PubMed] [Google Scholar]
- 146.Wu D, Shou X, Zhang Y, Li Z, Wu G, Wu D, Wu J, Shi S, Wang S. Cell membrane-encapsulated magnetic nanoparticles for enhancing natural killer cell-mediated cancer immunotherapy. Nanomedicine. 2021;32:102333. [DOI] [PubMed] [Google Scholar]
- 147.Zhao S, Duan J, Lou Y, Gao R, Yang S, Wang P, Wang C, Han L, Li M, Ma C, et al. Surface specifically modified NK-92 cells with CD56 antibody conjugated superparamagnetic Fe(3)O(4) nanoparticles for magnetic targeting immunotherapy of solid tumors. Nanoscale. 2021;13:19109–22. [DOI] [PubMed] [Google Scholar]
- 148.Muhlberger M, Janko C, Unterweger H, Friedrich RP, Friedrich B, Band J, Cebulla N, Alexiou C, Dudziak D, Lee G, Tietze R. Functionalization of T lymphocytes with Citrate-Coated superparamagnetic iron oxide nanoparticles for magnetically controlled immune therapy. Int J Nanomed. 2019;14:8421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pfister F, Dorrie J, Schaft N, Buchele V, Unterweger H, Carnell LR, Schreier P, Stein R, Kubankova M, Guck J, et al. Human T cells loaded with superparamagnetic iron oxide nanoparticles retain antigen-specific TCR functionality. Front Immunol. 2023;14:1223695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li CX, Zhang Y, Dong X, Zhang L, Liu MD, Li B, Zhang MK, Feng J, Zhang XZ. Artificially reprogrammed macrophages as Tumor-Tropic Immunosuppression-Resistant biologics to realize therapeutics production and immune activation. Adv Mater. 2019;31:e1807211. [DOI] [PubMed] [Google Scholar]
- 151.Liu X, Zheng J, Sun W, Zhao X, Li Y, Gong N, Wang Y, Ma X, Zhang T, Zhao LY, et al. Ferrimagnetic vortex Nanoring-Mediated mild magnetic hyperthermia imparts potent immunological effect for treating cancer metastasis. ACS Nano. 2019;13:8811–25. [DOI] [PubMed] [Google Scholar]
- 152.Gao M, Feng K, Zhang X, Ruan Y, Zhao G, Liu H, Sun X. Nanoassembly with self-regulated magnetic thermal therapy and controlled immuno-modulating agent release for improved immune response. J Control Release. 2023;357:40–51. [DOI] [PubMed] [Google Scholar]
- 153.Fu Q, Li Z, Ye J, Li Z, Fu F, Lin SL, Chang CA, Yang H, Song J. Magnetic targeted near-infrared II PA/MR imaging guided photothermal therapy to trigger cancer immunotherapy. Theranostics. 2020;10:4997–5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yu B, Choi B, Li W, Kim DH. Magnetic field boosted ferroptosis-like cell death and responsive MRI using hybrid vesicles for cancer immunotherapy. Nat Commun. 2020;11:3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rong L, Zhang Y, Li WS, Su Z, Fadhil JI, Zhang C. Iron chelated melanin-like nanoparticles for tumor-associated macrophage repolarization and cancer therapy. Biomaterials. 2019;225:119515. [DOI] [PubMed] [Google Scholar]
- 156.Lin X, He T, Tang R, Li Q, Wu N, Zhou Y, He H, Wan L, Huang J, Jiang Q, et al. Biomimetic nanoprobe-augmented triple therapy with photothermal, sonodynamic and checkpoint Blockade inhibits tumor growth and metastasis. J Nanobiotechnol. 2022;20:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang B, An J, Zhang H, Zhang S, Zhang H, Wang L, Zhang H, Zhang Z. Personalized cancer immunotherapy via transporting endogenous tumor antigens to lymph nodes mediated by nano Fe(3) O(4). Small. 2018;14:e1801372. [DOI] [PubMed] [Google Scholar]
- 158.Ding M, Fan Y, Lv Y, Liu J, Yu N, Kong D, Sun H, Li J. A prodrug hydrogel with tumor microenvironment and near-infrared light dual-responsive action for synergistic cancer immunotherapy. Acta Biomater. 2022;149:334–46. [DOI] [PubMed] [Google Scholar]
- 159.Meng J, Zhang P, Chen Q, Wang Z, Gu Y, Ma J, Li W, Yang C, Qiao Y, Hou Y, et al. Two-Pronged intracellular Co-Delivery of antigen and adjuvant for synergistic cancer immunotherapy. Adv Mater. 2022;34:e2202168. [DOI] [PubMed] [Google Scholar]
- 160.Kagawa T, Matsumi Y, Aono H, Ohara T, Tazawa H, Shigeyasu K, Yano S, Takeda S, Komatsu Y, Hoffman RM, et al. Immuno-hyperthermia effected by antibody-conjugated nanoparticles selectively targets and eradicates individual cancer cells. Cell Cycle. 2021;20:1221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang M, Li Y, Wang M, Liu K, Hoover AR, Li M, Towner RA, Mukherjee P, Zhou F, Qu J, Chen WR. Synergistic interventional photothermal therapy and immunotherapy using an iron oxide nanoplatform for the treatment of pancreatic cancer. Acta Biomater. 2022;138:453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guo Y, Ran Y, Wang Z, Cheng J, Cao Y, Yang C, Liu F, Ran H. Magnetic-responsive and targeted cancer nanotheranostics by PA/MR bimodal imaging-guided photothermally triggered immunotherapy. Biomaterials. 2019;219:119370. [DOI] [PubMed] [Google Scholar]
- 163.Jiang Q, Wang K, Zhang X, Ouyang B, Liu H, Pang Z, Yang W. Platelet Membrane-Camouflaged magnetic nanoparticles for Ferroptosis-Enhanced cancer immunotherapy. Small. 2020;16:e2001704. [DOI] [PubMed] [Google Scholar]
- 164.Ruiz-de-Angulo A, Bilbao-Asensio M, Cronin J, Evans SJ, Clift MJD, Llop J, Feiner IVJ, Beadman R, Bascaran KZ, Mareque-Rivas JC. Chemically Programmed Vaccines: Iron Catalysis in Nanoparticles Enhances Combination Immunotherapy and Immunotherapy-Promoted Tumor Ferroptosis. iScience 2020, 23:101499. [DOI] [PMC free article] [PubMed]
- 165.Shao Y, Wang Z, Hao Y, Zhang X, Wang N, Chen K, Chang J, Feng Q, Zhang Z. Cascade catalytic nanoplatform based on butterfly effect for enhanced immunotherapy. Adv Healthc Mater. 2021;10:e2002171. [DOI] [PubMed] [Google Scholar]
- 166.Yu K, Chen Y, Zhang L, Zheng Y, Chen J, Wang Z, Yu X, Song K, Dong Y, Xiong F, et al. Cancer-Erythrocyte Membrane-Mimicking Fe(3)O(4) nanoparticles and DHJS for ferroptosis/immunotherapy synergism in tumors. ACS Appl Mater Interfaces. 2023;15:44689–710. [DOI] [PubMed] [Google Scholar]
- 167.Yang H, Li G, Zhang J, Zhao J, Zhao Y, Wu Y, Sun Z, Song S, Zou Y, Zou Z, et al. A novel Hollow iron nanoparticle system loading PEG-Fe(3)O(4) with C5a receptor antagonist for breast cancer treatment. Front Immunol. 2024;15:1466180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhu L, Leng D, Guo Z, Zhao Y, Leung KT, Dai Y, Li J, Zhao Q. Self-catalyzed nitric oxide nanocomplexes induce ferroptosis for cancer immunotherapy. J Control Release. 2025;377:524–39. [DOI] [PubMed] [Google Scholar]
- 169.Chen X, Zhang F, Lu C, Wu R, Yang B, Liao T, Du B, Wu F, Ding J, Fang S, et al. Lactate-Fueled theranostic nanoplatforms for enhanced MRI-Guided ferroptosis synergistic with immunotherapy of hepatocellular carcinoma. ACS Appl Mater Interfaces. 2025;17:9155–72. [DOI] [PubMed] [Google Scholar]
- 170.Chin YC, Yang LX, Hsu FT, Hsu CW, Chang TW, Chen HY, Chen LY, Chia ZC, Hung CH, Su WC, et al. Iron oxide@chlorophyll clustered nanoparticles eliminate bladder cancer by photodynamic immunotherapy-initiated ferroptosis and immunostimulation. J Nanobiotechnol. 2022;20:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li K, Lu L, Xue C, Liu J, He Y, Zhou J, Xia Z, Dai L, Luo Z, Mao Y, Cai K. Polarization of tumor-associated macrophage phenotype via porous Hollow iron nanoparticles for tumor immunotherapy in vivo. Nanoscale. 2020;12:130–44. [DOI] [PubMed] [Google Scholar]
- 172.Wu X, Cheng Y, Zheng R, Xu K, Yan J, Song P, Wang Y, Rauf A, Pan Y, Zhang H. Immunomodulation of tumor microenvironment by Arginine-Loaded iron oxide nanoparticles for gaseous immunotherapy. ACS Appl Mater Interfaces. 2021;13:19825–35. [DOI] [PubMed] [Google Scholar]
- 173.Su Y, Yang F, Wang M, Cheung PCK. Cancer immunotherapeutic effect of carboxymethylated beta-d-glucan coupled with iron oxide nanoparticles via reprogramming tumor-associated macrophages. Int J Biol Macromol. 2023;228:692–705. [DOI] [PubMed] [Google Scholar]
- 174.Nascimento C, Castro F, Domingues M, Lage A, Alves E, de Oliveira R, de Melo C, Eduardo Calzavara-Silva C, Sarmento B. Reprogramming of tumor-associated macrophages by polyaniline-coated iron oxide nanoparticles applied to treatment of breast cancer. Int J Pharm. 2023;636:122866. [DOI] [PubMed] [Google Scholar]
- 175.Roudi R, Pisani L, Pisani F, Kiru L, Daldrup-Link HE. Novel clinically translatable iron oxide nanoparticle for monitoring Anti-CD47 cancer immunotherapy. Invest Radiol. 2024;59:391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xu R, Liu X, Zhang Y, Wu G, Huang L, Li R, Xu X. Antibody-Decorated nanoplatform to reprogram macrophage and block immune checkpoint lsectin for effective cancer immunotherapy. Nano Lett. 2024;24:8723–31. [DOI] [PubMed] [Google Scholar]
- 177.Li Y, Li M, Zheng J, Ma Z, Yu T, Zhu Y, Li P, Nie F. Ultrasound-Responsive nanocarriers delivering SiRNA and Fe(3)O(4) nanoparticles reprogram macrophages and inhibit M2 polarization for enhanced NSCLC immunotherapy. ACS Appl Mater Interfaces. 2024;16:56634–52. [DOI] [PubMed] [Google Scholar]
- 178.Ye H, Wang K, Lu Q, Zhao J, Wang M, Kan Q, Zhang H, Wang Y, He Z, Sun J. Nanosponges of Circulating tumor-derived exosomes for breast cancer metastasis Inhibition. Biomaterials. 2020;242:119932. [DOI] [PubMed] [Google Scholar]
- 179.Prasad R, Peng B, Mendes BB, Kilian HI, Gorain M, Zhang H, Kundu GC, Xia J, Lovell JF, Conde J. Biomimetic bright optotheranostics for metastasis monitoring and multimodal image-guided breast cancer therapeutics. J Control Release. 2024;367:300–15. [DOI] [PubMed] [Google Scholar]
- 180.Gao Y, Chen X, Wang B, Wang S, Wang J, Ren L, Jin WK, Han H, Wang L. Engineering platelets with PDL1 antibodies and iron oxide nanoparticles for postsurgical cancer immunotherapy. ACS Appl Bio Mater. 2023;6:257–66. [DOI] [PubMed] [Google Scholar]
- 181.Xie B, Zhao H, Ding YF, Wang Z, Gao C, Li S, Zhang K, Ip SW, Yu H, Wang R. Supramolecularly engineered conjugate of bacteria and cell Membrane-Coated magnetic nanoparticles for enhanced ferroptosis and immunotherapy of tumors. Adv Sci (Weinh). 2023;10:e2304407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kang Y, Yan J, Han X, Wang X, Wang Y, Song P, Su X, Rauf A, Jin X, Pu F, Zhang H. Construction of hierarchically biomimetic iron oxide nanosystems for macrophage Repolarization-Promoted immune checkpoint Blockade of cancer immunotherapy. ACS Appl Mater Interfaces. 2024;16:36131–41. [DOI] [PubMed] [Google Scholar]
- 183.Zhang W, Li L, Wu Y, Li C, Xu Z, Zhang N, Wang X, Zhao Y, Zu T, He Q, et al. Biomimetic Iron-Based nanoparticles remodel immunosuppressive tumor microenvironment for metabolic immunotherapy. Int J Nanomed. 2024;19:9333–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cheng X, Xu J, Cui Y, Liu J, Chen Y, He C, Cui L, Liu Y, Song B, Gong C, Mi P. Nanovesicles for lipid metabolism Reprogram-Enhanced ferroptosis and magnetotherapy of refractory tumors and inhibiting metastasis with activated innate immunity. ACS Nano. 2025;19:7213–30. [DOI] [PubMed] [Google Scholar]
- 185.Lee GA, Lin WL, Kuo DP, Li YT, Chang YW, Chen YC, Huang SW, Hsu JB, Chen CY. Detection of PD-L1 expression in Temozolomide-Resistant glioblastoma by using PD-L1 antibodies conjugated with Lipid–Coated superparamagnetic iron oxide. Int J Nanomed. 2021;16:5233–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fink C, Gevaert JJ, Barrett JW, Dikeakos JD, Foster PJ, Dekaban GA. In vivo tracking of adenoviral-transduced iron oxide-labeled bone marrow-derived dendritic cells using magnetic particle imaging. Eur Radiol Exp. 2023;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang L, Qiu M, Wang R, Li S, Liu X, Xu Q, Xiao L, Jiang ZX, Zhou X, Chen S. Monitoring ROS responsive Fe(3)O(4)-based nanoparticle mediated ferroptosis and immunotherapy via (129)Xe MRI. Angew Chem Int Ed Engl. 2024;63:e202403771. [DOI] [PubMed] [Google Scholar]
- 188.Zhang Q, Qu Y, Zhao H, Chen S, Liu Z, Li J, Li Y, Li J, Sun D. A magnetically driven biodegradable microsphere with mass production capability for subunit vaccine delivery and enhanced immunotherapy. ACS Appl Mater Interfaces. 2024;16:50344–59. [DOI] [PubMed] [Google Scholar]
- 189.Yang C, Ma H, Meng J, Li J, Huo H, Li W, Zhao Y, Wen Y, Mi S, Liu S, et al. Direct cGAMP delivery via iron oxide nanoparticles for enhanced STING activation and durable antitumor immunity. Nano Lett. 2025;25:7577–86. [DOI] [PubMed] [Google Scholar]
- 190.Gavilán H, Avugadda SK, Fernández-Cabada T, Soni N, Cassani M, Mai BT, Chantrell R, Pellegrino T. Magnetic nanoparticles and clusters for magnetic hyperthermia: optimizing their heat performance and developing combinatorial therapies to tackle cancer. Chem Soc Rev. 2021;50:11614–67. [DOI] [PubMed] [Google Scholar]
- 191.Mu QG, Lin G, Jeon M, Wang H, Chang FC, Revia RA, Yu J, Zhang M. Iron oxide nanoparticle targeted chemo-immunotherapy for triple negative breast cancer. Mater Today (Kidlington). 2021;50:149–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhang Y, Rahman MM, Clark PA, Sriramaneni RN, Havighurst T, Kerr CP, Zhu M, Jones J, Wang X, Kim K, et al. In situ vaccination following intratumoral injection of IL2 and Poly-l-lysine/Iron oxide/cpg nanoparticles to a radiated tumor site. ACS Nano. 2023;17:10236–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Khan S, Sharifi M, Hasan A, Attar F, Edis Z, Bai Q, Derakhshankhah H, Falahati M. Magnetic nanocatalysts as multifunctional platforms in cancer therapy through the synthesis of anticancer drugs and facilitated Fenton reaction. J Adv Res. 2021;30:171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang Y, Sriramaneni RN, Clark PA, Jagodinsky JC, Ye M, Jin W, Wang Y, Bates A, Kerr CP, Le T, et al. Multifunctional nanoparticle potentiates the in situ vaccination effect of radiation therapy and enhances response to immune checkpoint Blockade. Nat Commun. 2022;13:4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Portilla Y, Mulens-Arias V, Paradela A, Ramos-Fernandez A, Perez-Yague S, Morales MP, Barber DF. The surface coating of iron oxide nanoparticles drives their intracellular trafficking and degradation in endolysosomes differently depending on the cell type. Biomaterials. 2022;281:121365. [DOI] [PubMed] [Google Scholar]
- 196.Patrick PS, Stuckey DJ, Zhu H, Kalber TL, Iftikhar H, Southern P, Bear JC, Lythgoe MF, Hattersley SR, Pankhurst QA. Improved tumour delivery of iron oxide nanoparticles for magnetic hyperthermia therapy of melanoma via ultrasound guidance and (111)In SPECT quantification. Nanoscale. 2024;16:19715–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Muehe AM, Siedek F, Theruvath AJ, Seekins J, Spunt SL, Pribnow A, Hazard FK, Liang T, Daldrup-Link H. Differentiation of benign and malignant lymph nodes in pediatric patients on ferumoxytol-enhanced PET/MRI. Theranostics. 2020;10:3612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Heisser RH, Bawa M, Shah J, Bu A, Raman R. Soft biological actuators for Meter-Scale homeostatic biohybrid robots. Chem Rev. 2025;125:3976–4007. [DOI] [PubMed] [Google Scholar]
- 199.Asai K, Zhou Y, Takenouchi O, Kitajima TS. Artificial kinetochore beads Establish a biorientation-like state in the spindle. Science. 2024;385:1366–75. [DOI] [PubMed] [Google Scholar]
- 200.Landers FC, Gantenbein V, Hertle L, Veciana A, Llacer-Wintle J, Chen XZ, Ye H, Franco C, Puigmarti-Luis J, Kim M, et al. On-Command disassembly of microrobotic superstructures for transport and delivery of magnetic micromachines. Adv Mater. 2024;36:e2310084. [DOI] [PubMed] [Google Scholar]
- 201.Li D, Jia M, Du J, Li Y, Jia T, Chen G. Optomagnetic Heater-Thermometer nanoplatform for tumor magnetothermal therapy with Operando temperature feedback. ACS Nano. 2025;19:8328–37. [DOI] [PubMed] [Google Scholar]
- 202.Jin L, Liu H, Wang C, Mao C, Wu S, Zhang Y, Li Z, Zhu S, Jiang H, Cui Z, et al. Interface/Dipole polarized Antibiotics-Loaded Fe(3)O(4)/PB nanoparticles for Non-Invasive therapy of osteomyelitis under medical microwave irradiation. Adv Mater. 2024;36:e2410917. [DOI] [PubMed] [Google Scholar]
- 203.Zheng L, Zhuang Z, Li Y, Shi T, Fu K, Yan W, Zhang L, Wang P, Li L, Jiang Q. Bone targeting antioxidative nano-iron oxide for treating postmenopausal osteoporosis. Bioact Mater. 2022;14:250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhou Y, Feng C, Yang X, Yu J, Li X, Fu W, Zhu X, Li J, Zhang X. Capillary Force-Driven capture of magnetic nanoparticles in calcium phosphate Hollow-Tube whisker scaffolds for osteonecrosis of the femoral head. ACS Nano. 2025;19:20779–98. [DOI] [PubMed] [Google Scholar]
- 205.Bulte JWM, Wang C, Shakeri-Zadeh A. In vivo cellular magnetic imaging: labeled vs. Unlabeled cells. Adv Funct Mater. 2022;32:2207626. [DOI] [PMC free article] [PubMed]
- 206.Hu A, Pu Y, Xu N, Cai Z, Sun R, Fu S, Jin R, Guo Y, Ai H, Nie Y, Shuai X. Controlled intracellular aggregation of magnetic particles improves permeation and retention for magnetic hyperthermia promotion and immune activation. Theranostics. 2023;13:1454–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Peng Y, Wang X, Wang Y, Gao Y, Guo R, Shi X, Cao X. Macrophage-Laden gold nanoflowers embedded with ultrasmall iron oxide nanoparticles for enhanced Dual-Mode CT/MR imaging of tumors. Pharmaceutics. 2021;13:995. [DOI] [PMC free article] [PubMed]
- 208.Ye H, Wang K, Zhao J, Lu Q, Wang M, Sun B, Shen Y, Liu H, Pané S, Chen XZ, et al. In situ sprayed nanovaccine suppressing Exosomal PD-L1 by golgi apparatus disorganization for postsurgical melanoma immunotherapy. ACS Nano. 2023;17:10637–50. [DOI] [PubMed] [Google Scholar]
- 209.Balkrishna A, Solleti SK, Singh H, Verma S, Sharma N, Nain P, Varshney A. Herbal Decoction Divya-Swasari-Kwath attenuates airway inflammation and remodeling through Nrf-2 mediated antioxidant lung defence in mouse model of allergic asthma. Phytomedicine. 2020;78:153295. [DOI] [PubMed] [Google Scholar]
- 210.Wang Y, Liu X, Li K, Wang X, Zhang X, Qian D, Meng X, Yu L, Yan X, He Z. Self-Sulfhydrated, Nitro-Fixed albumin nanoparticles as a potent therapeutic agent for the treatment of acute liver injury. ACS Nano. 2024;18:20772–91. [DOI] [PubMed]
- 211.Zhu W, Wu J, Kang Y, Xue P. Cu(2)O/CuVO(3) Nano-Heterojunction as a highly active therapeutic catalyst for aggravating redox dyshomeostasis of neoplastic cells. Adv Mater 2025:e2502407. [DOI] [PubMed]
- 212.Li M, Yang L, An Y, Tan X, He P, Tan Y, Liu R, Zheng J, Zhang W, Jiang Y, et al. Selenium-Doped carbon Dots as a multipronged nanoplatform to alleviate oxidative stress and ferroptosis for the reversal of acute kidney injury. ACS Nano. 2025;19:17834–49. [DOI] [PubMed] [Google Scholar]
- 213.Certo M, Tsai CH, Pucino V, Ho PC, Mauro C. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat Rev Immunol. 2021;21:151–61. [DOI] [PubMed] [Google Scholar]
- 214.Tay C, Tanaka A, Sakaguchi S. Tumor-infiltrating regulatory T cells as targets of cancer immunotherapy. Cancer Cell. 2023;41:450–65. [DOI] [PubMed] [Google Scholar]
- 215.Lu Y, Sun Q, Guan Q, Zhang Z, He Q, He J, Ji Z, Tian W, Xu X, Liu Y, et al. The XOR-IDH3alpha axis controls macrophage polarization in hepatocellular carcinoma. J Hepatol. 2023;79:1172–84. [DOI] [PubMed] [Google Scholar]
- 216.Sanders TJ, Nabel CS, Brouwer M, Hermant AL, Chaible L, Deglasse JP, Rosewick N, Pabois A, Cathou W, Smets A, et al. Inhibition of ENT1 relieves intracellular adenosine-mediated T cell suppression in cancer. Nat Immunol. 2025;26:854–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gao M, Liu Y, Zhao L, Chen J, Wan W, Yuan Z, Li L, Huang Y, Wang Y, Zheng Y. Cell Surface-Tethered nucleic acid therapeutics program robust and Tumor-Responsive enhancement of adoptive cell therapy. Adv Mater 2025:e2419969. [DOI] [PubMed]
- 218.Tsao HY, Cheng HW, Kuo CC, Chen SY. Dual-Sensitive Gold-Nanocubes platform with synergistic immunotherapy for inducing immune cycle using NIR-Mediated PTT/NO/IDO. Pharmaceuticals (Basel). 2022;15:138. [DOI] [PMC free article] [PubMed]
- 219.Cai X, Ruan L, Wang D, Zhang J, Tang J, Guo C, Dou R, Zhou M, Hu Y, Chen J. Boosting chemotherapy of bladder cancer cells by ferroptosis using intelligent magnetic targeting nanoparticles. Colloids Surf B Biointerfaces. 2024;234:113664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.