Abstract
Ferroptosis, an iron-dependent form of cell death, holds promise for cancer therapy. However, the intricate link between ferroptosis and oncogenic mutations remains unclear. Here we show that SMARCA4, a well-established tumour suppressor whose deficiency is associated with poor prognosis and resistance to treatments, sensitizes non-small cell lung cancer (NSCLC) cells to ferroptosis. Mechanistically, SMARCA4 promotes chromatin accessibility and expression of ALDH16A1. Surprisingly, ALDH16A1 lacks ALDH enzymatic activity, but binds to the anti-ferroptotic oxidoreductase thioredoxin (TXN), facilitating its translocation to the lysosome and subsequent degradation. Meanwhile, ALDH16A1 directly inhibits TXN’s oxidoreductase function by occluding its active site. We also show that either restoring ALDH16A1 levels or inhibiting TXN significantly enhances the effectiveness of chemo/immunotherapy in a ferroptosis-dependent manner in SMARCA4-deficient NSCLC. Collectively, our findings elucidate an intricate SMARCA4-ALDH16A1-TXN stability/function dual regulatory axis that governs ferroptosis and informs a therapeutic strategy for overcoming resistance to chemotherapy or immunotherapy in SMARCA4-deficient NSCLC.
Subject terms: Cancer therapeutic resistance, Non-small-cell lung cancer, Cell death
SMARCA4 deficiency is associated with poor prognosis in non-small cell lung cancer (NSCLC). Here the authors report that loss of SMARCA4 in NSCLC cells is associated with resistance to ferroptosis, mediated via inhibition of ALDH16A1 expression and thioredoxin degradation.
Introduction
Ferroptosis is a unique form of iron-dependent, regulated cell death characterized by the excessive accumulation of lipid peroxides on cellular membranes. The delicate balance between ferroptosis-inducing activities and intracellular defence systems, primarily consisting of the SLC7A11/GPX4-dependent glutathione (GSH) system, FSP1- and DHODH-dependent ubiquinol system, and GCH1-dependent tetrahydrobiopterin (BH4) system, is crucial for maintaining this equilibrium. These anti-ferroptotic systems detoxify lipid peroxides, preventing membrane rupture and ferroptotic cell death1,2. However, when the pro-ferroptotic forces overwhelm the anti-ferroptotic mechanisms, often due to genetic or pharmacological perturbations in an iron-rich environment, excess hydrogen peroxide reacts with iron in a Fenton reaction, generating highly reactive hydroxyl radicals that attack the bis-allylic moieties of polyunsaturated fatty acid-containing phospholipids located on plasma membrane or membrane organelles, ultimately leading to ferroptosis3–5.
In recent years, there has been a surge in interest in harnessing ferroptosis as a therapeutic strategy against cancer. Studies have shown that various anti-cancer approaches, including chemotherapy, radiotherapy and immunotherapy, can trigger ferroptosis, suggesting its potential to enhance the efficacy of existing cancer therapies and overcome resistance6–9. Notably, specific genetic alterations, such as loss-of-function mutations in TP53 and KEAP1, commonly observed in non-small cell lung cancer (NSCLC), can confer sensitivity or resistance to ferroptosis, with important therapeutic implications5,10,11.
Motivated by these findings, we investigate the role of other tumour suppressors in ferroptosis regulation and the underlying mechanisms. SMARCA4, the ATPase, a key subunit of the SWI/SNF chromatin remodelling complex, is a recognized tumour suppressor due to its roles in cell fate specification and DNA repair12,13. Its deficiency, observed in approximately 8% of NSCLC patients, is associated with poor prognosis and resistance to conventional chemotherapy14–18. Through a genome-wide CRISPR/Cas9 screen in NSCLC cell lines, we discover that SMARCA4 knockout significantly protects tumour cells from ferroptotic cell death.
Our further investigation reveals that SMARCA4 promotes chromatin accessibility and enhances the expression of ALDH16A1, an uncharacterized “cargo adaptor” that transports thioredoxin, an anti-ferroptotic oxidoreductase, to lysosomes for degradation, thereby promoting ferroptosis. We also propose that this mechanism can be leveraged to improve SMARCA4-deficiency NSCLC’s response to immunotherapy.
In this work, we uncover a critical mechanism by which SMARCA4 deficiency enables NSCLC cells to evade ferroptosis, thereby rationalizing the targeting of the SMARCA4/ALDH16A1/TXN/ferroptosis axis as a promising therapeutic approach for patients with SMARCA4-deficient NSCLC.
Results
SMARCA4 promotes ferroptosis in NSCLC cells
Tumour suppressor loss in cancer cells can confer ferroptotic resistance or vulnerability, making them potential therapeutic targets. To identify ferroptosis-related tumour suppressors, we conducted a genome-wide CRISPR/Cas9 screen in human NSCLC cell line PC9. To achieve this, the cells were transduced with a CRISPR library encompassing 19,060 genes, with a total of 114,155 single-guide RNAs (sgRNAs) (six per gene), followed by a two-day selection using the GPX4 inhibitor RSL3 as a ferroptosis inducer (FIN) (Fig. 1a and Supplementary Data 1). sgRNAs targeting known ferroptosis drivers, such as ACSL4 and RETSAT, were enriched in cells surviving RSL3 treatment, whereas anti-ferroptosis genes DHODH, FSP1 and SLC7A11 were depleted (Fig. 1b). These results align with our previous data and those of other research groups.
Fig. 1. CRISPR/Cas9 screening links SMARCA4 to ferroptosis.
a Schematic outline of the CRISPR/Cas9 RSL3 screening workflow in PC9 cell line. Created in BioRender. Bi, G. (2025) https://BioRender.com/fjloxzm. b CRISPR/Cas9 screening results of several genes of interests and well-established ferroptosis regulators. c SMARCA4 protein levels in PC9, H1975, HCC827, A549 and H23 NSCLC cell lines determined by western blotting. d SMARCA4 protein levels in Cas9-NC and SMARCA4-KO PC9, H1975, HCC827 cells determined by western blotting. e Cell viability in Cas-NC and SMARCA4-KO PC9, H1975 and HCC827 cells treated with RSL3 for 6 h. f SMARCA4 protein levels in A549 and H23 cells with indicated genotypes determined by western blotting. g Cell viability in A549 and H23 with indicated genotypes treated with 1000 nM RSL3 for 6 h. h Cell viability in A549 and H23 cells with indicated genotypes treated with 1000 nM RSL3 combined with or without DFO (100 μM), Fer-1 (10 μM), z-VAD-FMK (10 μM), or necrosulfonamide (0.5 μM) for 6 h. i Lipid peroxidation in PC9 and A549 cells with indicated genotypes treated with RSL3 (PC9: 200 nM; A549: 1000 nM) for 3 h. j Transmission electron microscopy images of A549 cells with indicated genotypes treated with 1000 nM RSL3 for 4 h. Scale bars: 4 μm. k Representative brightfield images of patient-derived organoids treated with 10 μM RSL3 for 96 h. Scale bars: 100 μm. Data are presented as the mean ± SD, n = 3 independent experiments. Consistent results were observed across three biological replicates in (c, d, f, j, k). Unpaired two-tailed Student’s t-tests are used. Source data are provided as a Source Data file.
Among the tumour suppressors, KEAP1, a known pro-ferroptosis factor promoting NRF2 proteasomal degradation, emerged as a high-scoring hit in PC9, reinforcing the robustness of our screening model5. Notably, SMARCA4, encoding the ATPase subunit of the SWI/SNF complex and a frequently mutated tumour suppressor in NSCLC, was identified as a potential pro-ferroptosis gene. To systematically investigate SMARCA4 role in ferroptosis regulation, we selected five NSCLC cell lines commonly used in ferroptosis-related studies and confirmed their SMARCA4 status as indicated in the Cancer Cell Line Encyclopedia (CCLE) database19. These included three wild-type (WT) lines (PC9, H1975 and HCC827) and two with SMARCA4 deficiencies (A549 with a frame-shift deletion, H23 with a missense mutation). SMARCA4 expression or deficiency was verified by western blot (Fig. 1c). CRISPR/Cas9-mediated knockout of SMARCA4 in the WT cells significantly inhibited ferroptosis induced by RSL3 and IKE, another key FIN targeting SLC7A11 (Figs. 1d, e and S1a, b). Colony formation assays also demonstrated consistent results, demonstrating a stable phenotype (Fig. S1c). Conversely, ectopic restoration of intact SMARCA4, but not its ATPase-dead mutant (K785R), sensitized A549 and H23 cells to FINs, suggesting ATPase activity is crucial for SMARCA4 regulatory role in ferroptosis (Figs. 1f, g and S1d, e). The increased cell death was abolished by ferroptosis inhibitors ferrostatin-1 (Fer-1) and deferoxamine (DFO), but not by apoptosis (z-VAD(OMe)-FMC) or necroptosis (necrosulfonamide) inhibitors, confirming SMARCA4 specific role in ferroptosis (Fig. 1h). Comparable results were observed with other FINs, including GPX4 inhibitor ML210 and class 3 FIN56 that inhibits both GPX and CoQ pathway (Fig. S1f). Additionally, SMARCA4 depletion in human fibrosarcoma HT1080 and colon cancer HCT116 cells, both of which possess WT SMARCA4, promoted resistance to RSL3-induced cell death, indicating this property is not limited to NSCLC (Fig. S1g, h).
Phospholipid peroxidation, a hallmark of ferroptosis, was assessed using BODIPY-C11 staining. SMARCA4 knockout significantly reduced RSL3-induced lipid peroxidation in PC9, while its restoration had opposite effects in A549 (Fig. 1i). Moreover, transmission electron microscopy revealed that A549 cells treated with RSL3 exhibited reduced numbers of mitochondrial cristae with increased membrane density, another characteristic morphologic ferroptosis feature and this effect was markedly amplified in SMARCA4-restored cells (Fig. 1j).
Organoid refers to the self-organized 3D tissue that is typically derived from stem cells or cancer cells, and which mimics the key functional, structural and biological complexity of the original organs. Compared to 2D cultures and animal models, patient-derived organoid (PDO) cultures offer patient specificity while recapitulating in vivo tissue structures and functions, thus providing an ideal platform for anti-cancer drug discovery and signalling transduction analysis20. As such, we developed PDO models using tumour samples of the NSCLC patients receiving surgical resection in our institute. SMARCA4 status was confirmed by immunohistochemistry (Fig. S1i). We found that RSL3 treatment inhibited SMARCA4-WT PDO’s growth and led to a morphological change, while PDOs from SMARCA4-deficient patients retained normal size and relatively intact structure (Fig. 1k).
SMARCA4 and its paralog SMARCA2, respectively encode one of the two mutually exclusive ATPase of the SWI/SNF complex. The two proteins share 75% identity, and SMARCA4-dificient cells have been shown to depend on SMARCA2, which means simultaneously depleting the two paralogs might be lethal for specific cell lines12,21. Unlike frequently mutated SMARCA4, SMARCA2 is rarely mutated but often epigenetically silenced in tumours22. SMARCA2 knockout in PC9 and H1975 only slightly decreased sensitivity to RSL3, indicating SMARCA4 more dominant role in anti-ferroptosis effects (Fig. S1j, k). Together, our data demonstrate that SMARCA4 is a potent pro-ferroptosis gene.
SMARCA4 enhances ALDH16A1’s chromatin accessibility and expression
To delve deeper into how SMARCA4 modulates ferroptosis, we initially assessed whether alterations in SMARCA4 disrupted canonical ferroptosis-related mechanisms. Our findings indicated that neither SMARCA4-KO in PC9 cells nor its restoration in A549 cells significantly impacted the expression levels of key ferroptosis regulators, such as ACSL4, SLC7A11, FSP1 and GPX4 (Fig. S2a). Additionally, alterations in SMARCA4 did not affect GSH levels, labile iron content, or lipid composition (Fig. S2b–d and Supplementary Data 2). Concordant with Cancer Dependency Map (DepMap) analysis, we also verified that single SMARCA4 knockout or restoration did not notably alter cellular proliferation rates (Fig. S2e, f)23.
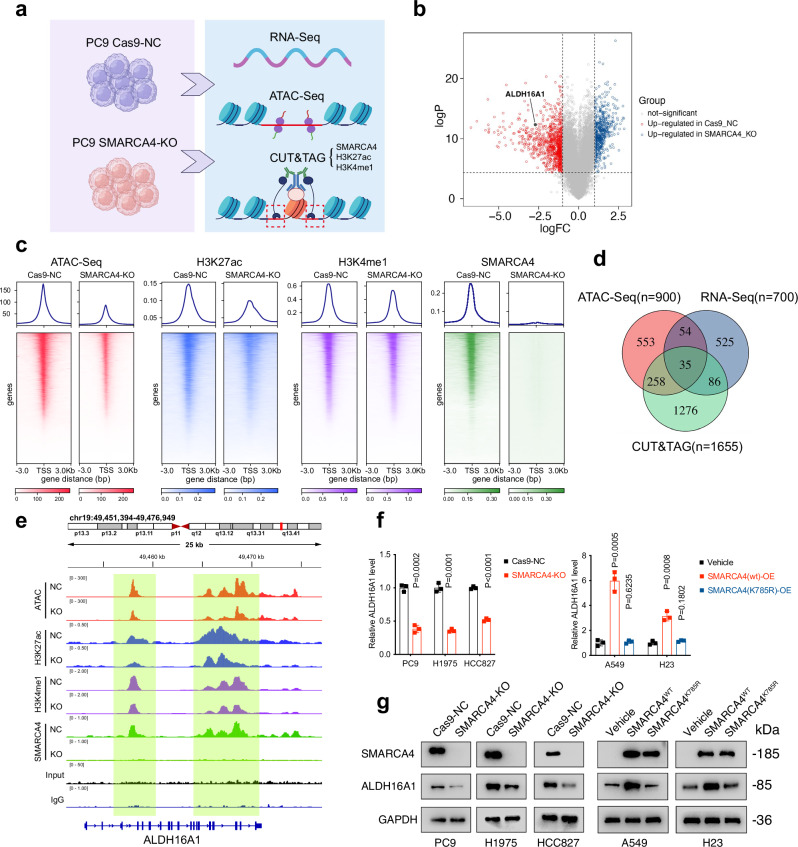
Next, we profiled the genome-wide occupancy of SWI/SNF complexes in SMARCA4-WT and -KO PC9 cells using CUT&TAG. Guided by their localization at distal enhancer sites24,25, we identified genes with active enhancer regions (marked by H3K27ac and H3K4me1) enriched with SMARCA4 peaks as potential SMARCA4 targets. We complemented this with RNA-Seq and ATAC-Seq for insights into RNA expression and chromatin accessibility (Fig. 2a–c and Supplementary Data 3–5). By integrating these data, we pinpointed hub genes that exhibited both reduced chromatin accessibility and expression upon SMARCA4 depletion.
Fig. 2. SMARCA4 regulates ALDH16A1’s chromatin accessibility and expression.
a Schematic outline of the identification of SMARCA4’s downstream targets using ATAC-Seq, CUT&TAG and RNA-Seq in Cas9-NC and SMARCA4-KO PC9 cells. Created in BioRender. Bi, G. (2025) https://BioRender.com/fjloxzm. b Volcano plots showing the differentially expressed genes between Cas9-NC and SMARCA4-KO PC9 cells. c Heatmap for SMARCA4, H3K27ac, H3K4me1 (CUT&TAG) levels and ATAC-Seq chromatin accessibility in Cas9-NC and SMARCA4-KO PC9 cells across merged SMARCA4 sites. d Venn diagram showing the intersection of potential SMARCA4 targets identified by ATAC-Seq, CUT&TAG and RNA-Seq. e Representative browser track of ATAC-Seq and SMARCA4 (CUT&TAG) on the ALDH16A1 locus in indicated PC9 cells. f, g mRNA (f) and protein (g) levels of ALDH16A1 in PC9, H1975, HCC827, A549 and H23 cells with indicated genotypes, determined by qPCR and western blotting. Data are presented as the mean ± SD, n = 3 independent experiments. Consistent results were observed across three biological replicates in (g). Unpaired two-tailed Student’s t tests are used. Source data are provided as a Source Data file.
Among the 35 genes identified, several known SMARCA4 targets like FOSB and JUN were present, validating the robustness of our multi-omics approach (Fig. 2d and Supplementary Data 6)13. Among these potential targets, we specifically choose ALDH16A1 for further investigation because our research team has concentrated on the role ALDHs plays in the regulation of ferroptosis in lung adenocarcinoma for years26–28. Supportively, the above-mentioned CRISPR/Cas9 data also indicated that deleting ALDH16A1 conferred robust ferroptosis resistance in PC9, explaining SMARCA4 pro-ferroptotic role (Figs. 1b and 2d, e). The CUT&TAG-Seq results were further confirmed by qPCR (Fig. S2g). Our bioinformatic analysis also unveiled a mild positive correlation between SMARCA4 and ALDH16A1 expression in RNA-Seq data from both NSCLC patients and cell lines harbouring WT SMARCA4, sourced from TCGA and CCLE databases, respectively (Fig. S2h). Furthermore, SMARCA4-KO in PC9, H1975 and HCC827 cells significantly decreased ALDH16A1 mRNA and protein levels, whereas restoring functional SMARCA4, not its ATPase-dead mutant K785R, upregulated ALDH16A1 expression (Fig. 2f, g). In contrast, SMARCA2 KO failed to mimic SMARCA4 effects on ALDH16A1 (Fig. S2i). Collectively, these findings underscore that ALDH16A1 is a direct target of SMARCA4 chromatin remodelling and transcription-activating properties.
The “unorthodox ALDH family member” ALDH16A1 mediates SMARCA4’s ferroptosis-promoting effect in an ALDH-independent manner
Having elucidated the regulatory interplay between SMARCA4 and ALDH16A1, we subsequently investigated the potential involvement of ALDH16A1 in ferroptosis across several NSCLC cell lines. In line with our CRISPR/Cas9 findings, employing sgRNA to delete ALDH16A1 significantly attenuated, whereas its reinstatement augmented, RSL3/IKE-induced ferroptotic cell death and lipid peroxidation. Consistent with the differential baseline expression levels of ALDH16A1 in these cell lines, the magnitude of ferroptosis resistance induced by ALDH16A1 knockout in A549 and H23 cells (SMARCA4def) is less pronounced compared to PC9 and H1975 (SMARCA4wt) (Figs. 3a–c and S3a, b). Further, the cell death inhibitor assay conclusively demonstrated that the ferroptosis-promoting effect of ALDH16A1 was exclusively mitigated by DFO and Fer-1 (Fig. 3d). Notably, concurrent ALDH16A1 knockout almost entirely abrogated the impact of SMARCA4 depletion or restoration on ferroptosis (Fig. 3e, f). These cumulative findings suggest that ALDH16A1 sensitizes NSCLC cells to ferroptotic cell death, thereby contributing to SMARCA4 ferroptosis-promoting property.
Fig. 3. ALDH16A1 mediates SMARCA4’s pro-ferroptosis property.
a Protein levels of ALDH16A1 in PC9, H1975, A549 and H23 cells with indicated genotypes. b Cell viability in PC9, H1975, A549 and H23 cells with indicated genotypes treated with RSL3 for 6 h. c Lipid peroxidation in PC9 and A549 cells with indicated genotypes treated with RSL3 (PC9: 200 nM; A549: 1000 nM) for 3 h. d Cell viability in Vehicle and ALDH16A1-OE PC9 and A549 cells treated with RSL3 (PC9: 200 nM; A549: 1000 nM) combined with or without DFO (100 μM), Fer-1 (10 μM), z-VAD-FMK (10 μM), or necrosulfonamide (0.5 μM) for 6 h. e Protein levels of SMARCA4 and ALDH16A1 in PC9, H1975, A549 and H23 cell lines with indicated genotypes determined by western blotting. f Cell viability in PC9, H1975, A549 and H23 cells with indicated genotypes treated with RSL3 (PC9: 200 nM; H1975, A549 and H23: 1000 nM) for 6 h. Data are presented as the mean ± SD, n = 3 independent experiments. Consistent results were observed across three biological replicates in (a, e). Unpaired two-tailed Student’s t tests are used. Source data are provided as a Source Data file.
Aldehydes constitute a class of reactive molecules generated during intracellular metabolism, particularly under external stimuli like radiotherapy or chemotherapy29–31s. Accumulated aldehydes frequently form adducts with DNA, RNA and proteins, triggering oxidative damage and, in turn, further aldehyde generation. Aldehyde dehydrogenases (ALDHs), a superfamily of NAD(P)+-dependent enzymes, catalyse the oxidation of diverse aldehydes into less toxic and more soluble carboxylic acids, thus affording cells a survival advantage32. Specifically, in the context of ferroptosis, ALDHs are crucial for detoxifying lipid peroxidation products such as malondialdehyde and 4-hydroxynonenal (4-HNE), thereby safeguarding cells from exaggerated ferroptotic cascades. Conventionally, ALDHs are viewed as ferroptosis inhibitors, operating in parallel to canonical GPX4/GSH and FSP1/CoQ10 pathways27,28,33,34. However, our revelation of ALDH16A1 ferroptosis-promoting role presents an unexpected contrast.
The distinctive nature of ALDH16A1 may stem from its unique architecture. While most ALDH family members span 400–570 amino acids (aa) and harbour a single ALDH domain, ALDH16A1 is longer (802 aa) and comprises two ALDH domains: 1–494 aa and 525–802 aa, linked by a linker sequence. Notably, mammalian ALDH16A1 lacks Cys-302, a conserved residue crucial for the catalytic function of all ALDH family members (Fig. S3c). Moreover, ALDH16A1 lacks discernible binding pockets for substrates (aldehydes) or cofactors (NAD+). Consequently, ALDH16A1 is regarded as a “dead” or “pseudo” enzyme within the ALDH family35. In support, we confirmed that manipulating SMARCA4 or ALDH16A1 levels did not alter intracellular ALDH activity (Fig. S3d). Thus, the underlying mechanism governing ALDH16A1 moonlight function in ferroptosis regulation necessitates further exploration.
ALDH16A1 interacts with thioredoxin and promotes its lysosomal degradation
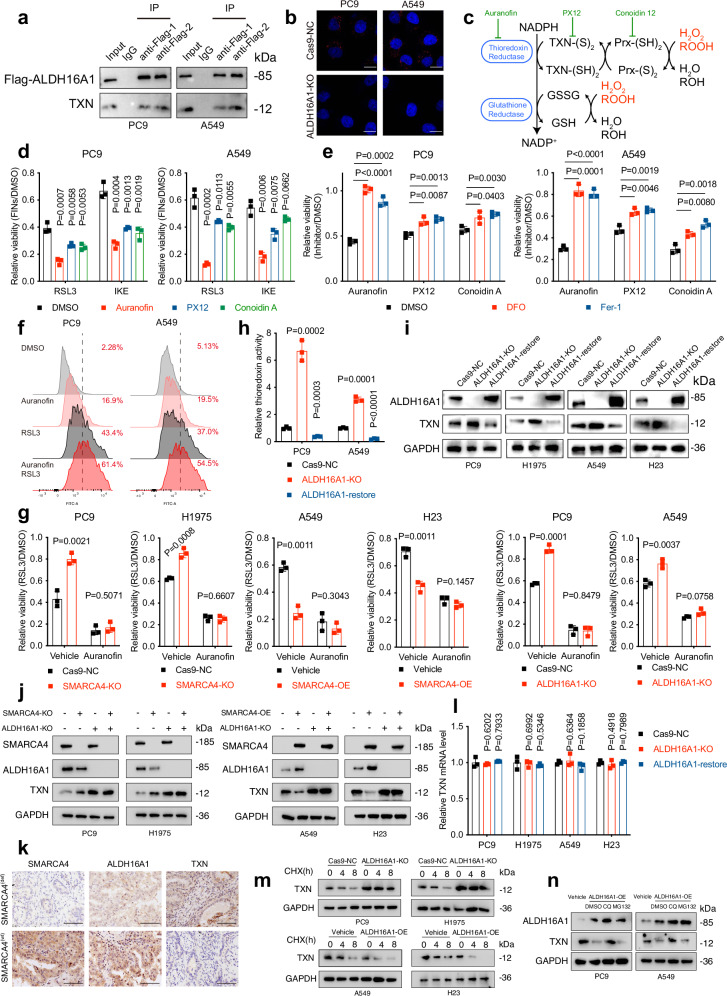
Similar to its upstream factor SMARCA4, ALDH16A1 did not affect canonical ferroptosis-related pathways, including GSH levels, labile iron concentrations, lipid metabolism and key ferroptosis-regulating proteins (Fig. S3e–h and Supplementary Data 2). Given that ALDH16A1 lacks enzymatic activity, we conducted mass spectrometry analyses of ALDH16A1 immunoprecipitants and observed a potential interaction between ALDH16A1 and thioredoxin (TXN) (Fig. 4a and Supplementary Data 7). TXN is a ubiquitous 12 kDa oxidoreductase that exhibits antioxidative properties through a GPX4/FSP1-independent mechanism36. We validated this association using co-immunoprecipitation and Duolink proximity ligation assay (PLA) (Fig. 4b).
Fig. 4. ALDH16A1 interacts with ferroptosis suppressor TXN.
a Flag and TXN proteins immunoprecipitated with Flag-ALDH16A1 and total lysates used for immunoprecipitation (IP). b Representative images of PLA signals between endogenous ALDH16A1 and TXN in PC9 and A549 cells, as detected by Duolink PLA assay. Scale bars: 5 μm. c Schematic depiction of TXN-based antioxidative system. d Cell viability in PC9 and A549 cells treated with RSL3 for 6 h or IKE (PC9: 2 μM; A549: 40 μM) for 24 h following pre-treatment with 1 μM auranofin, PX-12, or conoidin A for 6 h. e Cell viability in PC9 and A549 cells treated with 10 μM auranofin, PX-12, or conoidin A combined with or without DFO (100 μM) or Fer-1 (10 μM) for 24 h. f Lipid peroxidation in PC9 and A549 cells treated with RSL3 for 3 h following pre-treatment with 1 μM auranofin for 6 h. g Cell viability in PC9, H1975, A549 and H23 cells with indicated genotypes treated with RSL3 for 6 h following pre-treatment with auranofin for 6 h. h Relative TXN activity in PC9 and A549 cells with indicated genotypes determined by fluorometric assay. i ALDH16A1 and TXN protein levels in PC9, H1975, A549 and H23 cells with indicated genotypes. j SMARCA4, ALDH16A1 and TXN protein levels in PC9, H1975, A549 and H23 cells with indicated genotypes. k SMARCA4, ALDH16A1 and TXN protein levels in SMARCA4 (wt) and deficient NSCLC tumour tissues determined by immunohistochemistry (Scale bars: 100 μm, n = 37). l mRNA levels of TXN in PC9, H1975, A549 and H23 cells with indicated genotypes. m TXN protein levels in PC9, H1975, A549 and H23 cells with indicated genotypes treated with 100 μg/mL CHX for 0, 4 or 8 h. n TXN protein levels in PC9, H1975, A549 and H23 cells with indicated genotypes treated with 100 μg/mL CHX combined with or without 10 μM CQ or MG132 for 8 h. CQ, chloroquine. Data are presented as the mean ± SD, n = 3 independent experiments. Consistent results were observed across three biological replicates in (a, b, i, j, k, m, n). Unpaired two-tailed Student’s t tests are used. Source data are provided as a Source Data file.
Mechanistically, TXN transfers electrons to thioredoxin peroxidases (Prxs) using a pair of SH groups as reducing equivalents to reduce peroxides such as H2O2 and ROOH. This process generates the oxidized form of TXN (TXN-S2), which can be recycled back to its reduced form (TXN-SH2) by thioredoxin reductase (TXNR) at the expense of NADPH (Fig. 4c)36,37. Although TXN often goes unnoticed compared to its “colleagues” GPX4 and FSP1, the TXN system still represents an indispensable component of the intracellular antioxidant system. Previous studies have shown that inhibiting TXN renders cells vulnerable to oxidative stress, leading to lipid peroxidation and subsequent ferroptotic cell death38,39. We tested the ferroptosis-inducing effects of three types of TXN system antagonists acting on different targets at varying doses. When treating cells with relatively subtoxic doses of TXN antagonists that did not compromise cell viability, cells became significantly more sensitive to subsequent RSL3 or IKE treatment, whereas higher doses of TXN antagonists directly induced cell death, which could be partially reversed by ferroptosis inhibitors DFO and Fer-1, suggesting ferroptosis involvement (Fig. 4d, e). Among the three antagonists, we chose auranofin for subsequent TXN-related experiments due to its optimal ferroptosis-inducing and sensitizing effects. Combining auranofin with RSL3 produced a significant additive effect in inducing lipid peroxidation (Figs. 4f and S4a). Colony formation assays also demonstrated consistent results (Fig. S4b). Furthermore, in the presence of auranofin, knocking out or overexpressing SMARCA4 and ALDH16A1 failed to desensitize or sensitize cells to ferroptosis any more (Fig. 4g). Therefore, we conclude that TXN mediates the pro-ferroptotic properties of SMARCA4/ALDH16A1.
Next, we explored whether the interaction with ALDH16A1 regulates TXN function or abundance. Using a thioredoxin fluorometric assay and western blot, we found that ALDH16A1 knockout significantly increased TXN enzymatic activity and protein levels in NSCLC cell lines, whereas its restoration had the opposite effect (Fig. 4h, i). Consistently, upstream SMARCA4 depletion had a similar impact on TXN expression, but ALDH16A1 knockout abrogated this effect (Fig. 4j). Besides, in the cell lines used in the present study, the protein levels of ALDH16A1 were relatively lower in SMARCA4def A549 and H23 in comparison with SMARCA4wt PC9 and H1975, while TXN exhibited opposite results (Fig. S4c). We also correlated the abundance of these proteins in a cohort of 37 human NSCLC surgical specimens and observed that SMARCA4-positive NSCLC correlated with significantly higher ALDH16A1 and lower TXN protein levels (Figs. 4k and S4d).
However, alterations in ALDH16A1 did not affect TXN mRNA levels, suggesting that ALDH16A1 regulates TXN at the post-translational level (Fig. 4i, l). To verify this, we assessed whether ALDH16A1 influenced TXN protein stability using a cycloheximide (CHX) assay, which determines protein degradation rates when protein synthesis is inhibited. As shown in Figs. 4m and S4d, ALDH16A1 depletion markedly extended TXN’s protein half-life, while ALDH16A1 overexpression accelerated TXN degradation. Since autophagy-lysosome and ubiquitin-proteasome systems are the two primary pathways for protein degradation, we treated cells with either the lysosome inhibitor chloroquine (CQ) or the proteasome inhibitor MG132. The enhanced TXN degradation caused by ALDH16A1 was blocked by CQ but not MG132, indicating the involvement of the autophagy-lysosome pathway (Fig. 4n). In summary, these data demonstrate that ALDH16A1 interacts with TXN and triggers its degradation via the autophagy-lysosome pathway.
ALDH16A1 promotes TXN transport to lysosome
We subsequently conducted immunofluorescence staining of endogenously expressed TXN in PC9 and A549 cells, both with and without ALDH16A1 knockout, and observed reduced lysosomal localization of TXN in the ALDH16A1-KO cells (Figs. 5a and S5a). We dismissed the notion that ALDH16A1-induced lysosomal degradation of TXN could be attributed solely to effects on lysosomes themselves, as alterations in ALDH16A1 did not impact the number of lysosomes (Fig. S5b).
Fig. 5. ALDH16A1 simultaneously impairs TXN’s protein stability and function.
a Immunofluorescence showing the colocalization of TXN (green) with lysosome (marked by LAMP1, red) in PC9 and A549 cells with ALDH16A1-KO or restoration. Scale bars: 10 μm. b Immunofluorescence showing the distribution of TXN and ALDH16A1 (green) in lysosome (marked by LAMP1, red). Scale bars: 10 μm. c TXN protein levels in PC9 and A549 cells. d Immunofluorescence showing the colocalization of TXN (green) with lysosome (red) in PC9 and A549 cells with indicated genotypes. Scale bars: 10 μm. e Cell viability in PC9 and A549 cells with indicated genotypes treated with RSL3 for 6 h. f The structure of ALDH16A1-TXN complex predicted by AlphaFold2. ALDH16A1 N-terminal (1–494aa): cyan; ALDH16A1 C-terminal (525–802aa): blue; TXN: orange. The active site Cys32-Cys35 in TXN sequence and predicted interacting sites are labelled. g Flag and HA-TXN proteins immunoprecipitated with Flag-ALDH16A1 (full-length, 1–494aa, or 525–802aa) or HA-TXN and total lysates used for immunoprecipitation (IP) in HEK-293T cells. h Cell viability in PC9 and A549 cells with indicated genotypes treated with RSL3 for 6 h. i Immunofluorescence showing the colocalization of TXN (green) with lysosome (red) in PC9 and A549 cells with indicated genotypes. Scale bars: 10 μm. j Cell viability in PC9 and A549 cells with indicated genotypes treated with RSL3 for 6 h. k Representative images of PLA signals between edited ALDH16A1 and endogenous TXN in PC9 and A549 cells, as detected by Duolink PLA assay. Scale bars: 5 μm. l Immunofluorescence showing the colocalization of TXN (green) with lysosome (red) in PC9 and A549 cells with indicated genotypes. Scale bars: 10 μm. m Cell viability in PC9 and A549 cells with indicated genotypes treated with RSL3 for 6 h following pre-treatment with 10 μM Chloroquine for 8 h. n Relative TXN activity in PC9 and A549 cells with indicated genotypes treated with 10 μM CQ for 8 h, determined by fluorometric assay. Data are presented as the mean ± SD, n = 3 independent experiments. Consistent results were observed across three biological replicates in (a–d, g, i, k, l). Unpaired two-tailed Student’s t tests are used. Source data are provided as a Source Data file.
To further explore the underlying mechanisms of this observation, we questioned whether ALDH16A1 might function as a cargo adaptor, specifically mediating the transport of TXN to lysosomes for degradation. Analysis of the ALDH16A1 amino acid sequence uncovered the presence of a canonical KFERQ-like motif (609–613aa, RLERQ), suggesting the potential involvement of endosomal microautophagy or chaperone-mediated autophagy process40–42. Reinforcing this, immunofluorescence staining also demonstrated lysosomal localization of ALDH16A1 (Figs. 5b and S5a). To ascertain whether ALDH16A1 utilizes the KFERQ motif to shuttle TXN to lysosomes, we restored the ALDH16A1 variant lacking the 609–613 region (ALDH16A1Δ609–613) in ALDH16A1-KO cells. We found that this KFERQ-deficient ALDH16A1 failed to destabilize TXN protein or enhance the colocalization of TXN with the lysosomal marker LAMP1 (Figs. 5c, d and S5c). Furthermore, ALDH16A1Δ609–613 did not exhibit the pro-ferroptotic properties observed with full-length ALDH16A1 (Figs. 5e and S5d). Collectively, these experiments indicate that ALDH16A1 acts as a cargo adaptor, promoting the transport of TXN to lysosomes for degradation, thereby contributing to its ferroptosis-sensitizing effect.
The two ALDH domains are both required to destabilize TXN and impair its function
To investigate the pivotal functional domains of ALDH16A1 in its interaction and regulation of TXN, we utilized AlphaFold2 (https://alphafold.ebi.ac.uk) to predict the structure of the ALDH16A1-TXN heterodimer43. The resulting model revealed that TXN is embedded between the two ALDH domains of ALDH16A1, suggesting that both the N- and C-terminal domains, despite lacking canonical ALDH activity, play a role in the interaction with TXN (Fig. 5f). To substantiate this prediction, we cloned the two domains (N-terminal, 1–494 aa; C-terminal, 525–802 aa) into plasmids and transduced them into HEK-293T cells expressing HA-TXN. Additionally, a plasmid containing full-length ALDH16A1 served as a positive control. As anticipated, Co-IP experiments demonstrated that neither of the isolated ALDH16A1 domains could bind to TXN alone (Fig. 5g). Consistent with these structural findings, we observed that restoring either of the two domains in ALDH16A1-KO PC9 and A549 cells failed to mimic the full-length ALDH16A1 ability to promote ferroptosis or TXN’s lysosomal transport and degradation (Figs. 5h, i and S5e–g).
Based on the AlphaFold2-predicted ALDH16A1-TXN structure, we further identified three potential interaction sites between the two proteins (Fig. 5f). Two of these sites are located on the C-terminal region (ALDH16A1PRO567-TXNASP61 and ALDH16A1GLN737-TXNTRP31), while the third is on the N-terminal region (ALDH16A1PRO389-TXNGLY91/SER90). Separately deleting one of the three binding sites in ALDH16A1 sequence to different extents, while simultaneous depletion of all of them completely, impaired ALDH16A1’s pro-ferroptotic property and interaction with TXN (Figs. 5j, k and S5h, i). Moreover, these ALDH16A1 mutants failed to promote TXN’s lysosomal translocation and suppress its protein levels as full-length ALDH16A1 did (Figs. 5l and S5j, k). These results indicate that the three binding sites, distributed across the two ALDH domains of ALDH16A1, mediate its regulatory effects on TXN.
It is noteworthy that when lysosomal function was inhibited with CQ, although ALDH16A1 impact on TXN protein levels was abrogated, the cells’ sensitivity to FINs remained altered by ALDH16A1 modulation (Figs. 5m and S5l). This suggests the existence of mechanisms beyond the ALDH16A1/TXN-lysosomal degradation pathway. Notably, we found that TXN activity was still modulated by ALDH16A1 depletion or restoration, even in the presence of lysosomal inhibitors, indicating that ALDH16A1 may directly block TXN’s function as an oxidoreductase (Fig. 5n). Previous studies have identified the evolutionary conserved C-G-P-C motif between Cys32 and Cys35 as the catalytic centre of TXN37. According to the ALDH16A1-TXN binding model predicted by AlphaFold2, when ALDH16A1 and TXN interact, the active site Cys32-Cys35 is spatially positioned within the binding interface of the two proteins (Fig. 5f). This suggests that ALDH16A1 may block TXN’s active site, preventing it from interacting with other TXN system components like TXNR, peroxidases and NADPH, thereby impairing TXN’s normal function. In summary, ALDH16A1 sensitizes cells to ferroptosis through its interaction with TXN, not only by promoting TXN’s lysosomal degradation but also by directly impairing its oxidoreductase activity.
The SMARCA4/ALDH16A1/TXN pathway enhances the efficacy of chemotherapy and immunotherapy
Our team has revealed an important role of ferroptosis in combination chemotherapy with cisplatin (CDDP)/pemetrexed (PEM) induced cell death, that CDDP/PEM triggers ferroptosis in lung adenocarcinoma cells and altering cells’ response to ferroptosis would be a promising strategy to overcome chemoresistance7. These evidences prompt us to examine whether SMARCA4/ALDH16A1/TXN pathway’s pro-ferroptotic effect is also involved in this process. As expected, depletion of either SMARCA4 or ALDH16A1 significantly desensitized cells to CDDP/PEM treatment, while TXN inhibitor generated opposite effect (Fig. S6a). Besides, recent studies have unveiled the crucial role of ferroptosis in cancer immunology and immunotherapy. In essence, CD8+ cytotoxic T cells secrete interferon gamma (IFNγ), which subsequently downregulates SLC7A11 expression, thereby sensitizing cancer cells to ferroptosis and bolstering the anti-tumour efficacy of immune checkpoint blockades (ICBs)8. Given the aforementioned ferroptosis-sensitizing function of the SMARCA4/ALDH16A1/TXN pathway, we postulated that tumour cells with SMARCA4 deficiency or ALDH16A1 inhibition would exhibit greater resistance to CD8+ T cell-mediated killing. To validate this hypothesis, we accessed publicly available data from 56 melanoma patients who underwent tumour biopsy and RNA-sequencing during anti-CTLA4/anti-PD1-based therapy (GSE91061)44. The data revealed that sensitivity to immunotherapy is associated with higher ALDH16A1 expression, which is consistent with our in vitro an in vivo result (Fig. S6b). We further categorized these patients into high/low ALDH16A1 expression groups and observed that 7 out of 14 (50%) patients with high ALDH16A1 expression were responsive to ICBs, compared to only 6 out of 42 (14.3%) in the low-ALDH16A1 group (Fig. 6a). This observation primarily implies that relatively higher ALDH16A1 expression confers greater benefits to cancer patients undergoing immunotherapy. Considering that SMARCA4 is rarely (<2%) mutated/deleted in melanoma45, although SMARCA4’s mutation information is absence in this database, we also investigated the correlation between SMARCA4’s expression and patients’ response to immunotherapy. However, we failed to obtain a significant result. This discrepancy might be due to the limited sample volume and the distinct biological nature of NSCLC and melanoma. Further large-volume pan-cancer clinical research is warranted to identify potential indicators of immunotherapeutic effect.
Fig. 6. SMARCA4-ALDH16A1-TXN axis promotes ferroptosis in vivo and enhanced immunotherapeutic efficacy.
a Correlation between expression levels of ALDH16A1 and immunotherapeutic response in melanoma (GSE91061). Chi-square test is performed. b, c Experimental design for co-culture of OVA+ and Luc+ expressing LLC and activated OT-1 cells (b). Created in BioRender. Bi, G. (2025) https://BioRender.com/fjloxzm. The viability of treated LLC is measured using CCK8 after 24 h co-culture with OT-1 cells (c). d–f Experimental design of the A549 mice xenograft assay. Groups of mice were treated as indicated (n = 5 per group) (d). The image (e) and the final weight (f) of resected tumours from A549 mice xenografts, and the growth of tumour volumes were also shown (g). h Representative immunohistochemical images of the resected tumours in each group. Scale bars: 100 μm). Consistent results were observed across three biological replicates. i–l Experimental design of the LLC mice xenograft assay. Groups of mice were treated as indicated (n = 5 per group) (i). The image (j) and the final weight (k) of resected tumours from A549 mice xenografts, and the growth of tumour volumes were also shown (l). m Dot plots of the tumour-infiltrating lymphocytes from a representative mouse in each group. The percentages of CD45+CD8+ T cells and CD45+CD4+ T cells were measured by flow cytometry. Data are presented as the mean ± SD, n = 3 independent experiments unless otherwise stated. Unpaired two-tailed Student’s t tests are used. Source data are provided as a Source Data file.
Furthermore, we established ovalbumin (OVA) and luciferase (Luc) co-expressing Lewis lung carcinoma (LLC) cells and cocultured them with OVA-specific CD8+ T cells (Fig. 6b). LLC cell viability was assessed by quantifying luciferase activity. As depicted in Figs. 6c and S7a, Smarca4 knockout in LLC cells significantly downregulated Aldh16a1 and upregulated Txn proteins, and protected the cells from OT-1 cell-mediated tumour cell death. Additionally, ectopic overexpression of ALDH16A1 in LLC cells or the administration of a subtoxic dose of auranofin markedly enhanced OT-I-mediated cell death in both Cas9-NC and Smarca4-KO cells to a comparable extent (Fig. 6c). In summary, these findings indicate that SMARCA4 deficiency attenuates the cytotoxicity of CD8+ T cells via the ALDH16A1/TXN pathway.
The SMARCA4/ALDH16A1/TXN axis sensitizes NSCLC against ferroptosis and immune checkpoint blockers in vivo
We subsequently examined the role of the SMARCA4-ALDH16A1-TXN pathway in NSCLC ferroptosis in vivo. Human SMARCA4-OE or control A549 cells were subcutaneously implanted into the left flank of immunodeficient nude mice. IKE was selected as the FIN due to its remarkable stability in animals46. Consistent with our cellular assay findings, IKE treatment substantially inhibited tumour growth, and SMARCA4 overexpression further augmented this inhibitory effect. Moreover, the injection of adeno-associated virus (AAV) carrying the ALDH16A1 coding sequence or auranofin treatment enhanced IKE’s toxicity in both control and SMARCA4-overexpressing tumours. This indicates that, in the presence of ectopically expressed ALDH16A1 or a TXN inhibitor, SMARCA4 overexpression does not further sensitize tumours to ferroptosis (Figs. 6d–g and S7b). In line with this, staining for 4-hydroxynonenal (4-HNE), a marker of ferroptosis, in these xenograft tumour samples further validated the hypothesis that the SMARCA4/ALDH16A1/TXN axis elevates ferroptotic lipid peroxidation induced by IKE (Fig. 6h).
Given that CD4+ and CD8+ T cells are activated during cancer immunotherapy, we hypothesized that the SMARCA4/ALDH16A1/TXN axis might synergize with PD-1 blockers to exert enhanced anti-tumour activity in vivo. To test this, LLC-bearing C57BL/6 mice were treated with systemic PD-1 antibody. Anti-PD-1 treatment significantly impeded tumour growth and elevated the infiltration of cytotoxic CD4+ and CD8+ T cells, particularly the activated CD8+ T cell subset (IFNγ+ CD8+ T cells) (Figs. 6i–m and S8a, b). Notably, the therapeutic efficacy was notably diminished by liproxstatin-1, implying the occurrence of ferroptosis in this setting. Furthermore, the anti-tumour and immune-activating effects of anti-PD1 were substantially diminished in SMARCA4-knockout (KO) tumours, whereas this protective effect was largely reversed by ALDH16A1-OE or auranofin administration (Figs. 6j–m and S7c). In all animal studies, drug treatments did not significantly alter animal weights, suggesting their good tolerability in vivo (Fig. S8c). Therefore, these findings suggest that the SMARCA4-ALDH16A1-TXN axis promotes tumour cell ferroptosis, thereby enhancing anti-tumour immunity both in vitro and in vivo.
Discussion
The growing interest in ferroptosis within oncology stems from its recognition as a natural tumour-suppressing mechanism and its potential to enhance immunotherapy responses and surmount existing cancer therapies3. Intriguingly, abnormal activation of oncogenes or silencing of tumour suppressors remodels cancer cells’ metabolic networks to fulfil their heightened demands for nutrients and energy, essential for uncontrolled proliferation. This reprogramming can reveal novel metabolic vulnerabilities to ferroptosis. For example, RB1 inactivation in prostate cancer, associated with resistance to standard anti-tumour therapy and poor prognosis, paradoxically sensitizes cancer cells to ferroptosis by upregulating ACSL4 and enriching arachidonic acid-containing phospholipids47. Conversely, specific oncogenic mutations may confer an advantage to cancer cells in evading ferroptosis. TP53, the most frequently mutated tumour suppressor gene, promotes ferroptosis by suppressing genes like SLC7A11, SAT1 and VKORC1L1, whereas p53 loss confers ferroptosis resistance10,48,49. Similarly, mutant KRAS in lung cancers upregulates FASN, fostering fatty acid synthesis and phospholipid reprofiling to combat oxidative stress and evade ferroptosis50. In sum, identifying and destroying the key “arm” that the oncogenic mutations utilize to defend against ferroptosis represent a promising strategy to overcome the resistance to cancer therapy caused by these mutations in certain contents.
In our current study, leveraging CRISPR/Cas9 screening, we identified SMARCA4 as a ferroptosis-promoting tumour suppressor gene. Integrating multiple high-throughput datasets, we demonstrated that ALDH16A1 is a direct transcriptional target of SMARCA4, mediating its ferroptosis-inducing effects independently of ALDH activity. Furthermore, ALDH16A1 interacts with the anti-ferroptotic protein TXN, promoting its lysosomal degradation and potentially inhibiting its oxidoreductase function by blocking its active site (Cys32-Cys35), as implied by the binding model predicted by AlphaFold2. Thus, this dual mechanism simultaneously destabilizes and disables TXN. Our findings reveal that disruption of the SMARCA4/ALDH16A1/TXN axis not only protects NSCLC from ferroptosis-induced cell death but also impairs ferroptosis-associated immunotherapy efficacy in vitro and in vivo (Fig. 7).
Fig. 7. A schematic model depicting the SMARCA4-ALDH16A1-TXN stability/function-ferroptosis axis.
In this work, we identify SMARCA4 as a ferroptosis-promoting tumour suppressor gene. Its direct transcriptional target, ALDH16A1, interacts with TXN, promoting its lysosomal degradation and inhibiting its oxidoreductase function, thereby mediating SMARCA4’s pro-ferroptosis property. Created in BioRender. Bi, G. (2025) https://BioRender.com/fjloxzm.
The chromatin remodeller SWI/SNF complex is commonly considered as a tumour suppressor for their roles in the regulation of specific transcriptional programs such as cell differentiation and lineage specifications. Approximately 25% of cancers harbour SWI/SNF gene aberrations12. In contrast to classical oncogenes and tumour suppressors like RAS and TP53, which have been studied for more than 40 years, the oncogenic role of SWI/SNF mutations has only recently gained recognition. As revealed in multiple clinical research, nearly 10% NSCLC patients were identified to possess SMARCA4 mutation. In agreement with its tumour-suppressing property, SMARCA4 mutations, especially those “class I” mutations, which are characterized by SMARCA4 protein loss, are associated with poorer survival. Co-mutations with KRAS, TP53 and STK11 make the prognosis even worse15–17. Currently, a consensus on therapeutic approaches for SMARCA4-deficient NSCLC is lacking. Researchers have proposed various survival dependencies in SMARCA4-deficient NSCLC. For instance, Zhu et al. uncovered that SMARCA4/2 loss represses expression of the glucose transporter GLUT1, causing reduced glycolysis and increased dependency on glutamine-fuelled OXPHOS, suggesting the SMARCA4/2 deficient cancers might be sensitive to the inhibitors targeting OXPHOS or glutamine uptake51. Meanwhile, obvious progress has been made in SWI/SNF inhibitors, as SMARCA4 deficiency might result in synthetic lethal dependencies on other SWI/SNF components such as SMARCA2. Nonetheless, this phenomenon is context dependent, and its therapeutic translation is still underway. Notably, SMARCA4-deficient NSCLC typically responds poorly to conventional chemotherapies18, possibly due to SMARCA4 loss inhibiting chemotherapy-induced apoptosis by disrupting intracellular Ca2+ release52. Our prior work established ferroptosis’s role in chemotherapy-induced cell death7. The present study uncovered the ferroptosis resistance in SMARCA4-deficient NSCLC, offering an alternative explanation for its chemoresistance. Supportively, the CRISPR/Cas9 screening data using ML210 as FIN performed by Zou et al. also included SMARCA4 as a potential ferroptosis promoter53. However, a contradictory result was reported in pancreatic cancer by Bhat et al. The authors carried out an excellent study and proposed a model that SMARCA4/2 colocalize with NRF2 on genome to enhance the transcription of its target genes, most of which are related to anti-oxidative response, thus leading to a ferroptosis-sensitization54, highlighting SMARCA4 context-dependent impact on ferroptosis. Their conclusion was supported by efficient experimental data and bioinformatic analysis based on Depmap’s high-throughput data. Considering the complicated intracellular ferroptosis regulating network and the substantial difference in mutational background among various cell lines, cells’ response to ferroptosis might also be regulated by other factors beyond SMARCA4, thus leading up to the opposing results in different context. Given that the cell lines enrolled in this study all possess EGFR (PC9 and H1975) or KRAS (A549 and H23), we questioned whether SMARCA4’s impact on ferroptosis depends on these two commonly mutated gene? We then further validated the key findings in our study in H1299 cell line, a commonly used NSCLC cell line possessing WT EGFR and KRAS but deficient SMARCA4, and obtained consistent results (Fig. S9). Therefore, as the core subunit of SWI/SNF, SMARCA4 overall impact on ferroptosis hinges on its specific “dominant” targets, which may vary across cell lines and warrant further investigations.
Recently, the exploration of the “moonlighting” functions of known proteins has garnered significant attention among researchers55,56. Typically, ALDHs mitigate oxidative stress by metabolizing a broad spectrum of aldehydes57. However, with the assistance of AlphaFold2, we have proposed an intriguing hypothesis wherein ALDH16A1 functions as a “cargo adaptor,” facilitating the transport of TXN to lysosomes. This ALDH-independent moonlight function redefines ALDH16A1, transcending its previous status as a mere “dead enzyme.” Given the overly expansive target range of SMARCA4, directly restoring its function in NSCLC patients poses considerable risks, as SMARCA4 may even exhibit oncogenic properties in certain contexts13,58,59. Furthermore, the clinical application of chemosynthetic TXN inhibitors is hindered by their nonspecific toxicity. In our current study, we validated the feasibility of restoring ALDH16A1 in mice to potentiate ferroptosis induced by IKE or immunotherapy. We employed AAVs as a delivery vector due to their minimal pathogenicity and ability to establish long-term gene expression in vivo60. Consequently, restoring ALDH16A1 appears to be a relatively safe approach to reverse the ferroptosis-resistant phenotype in SMARCA4-deficient NSCLC. Further clinical trials are essential to substantiate its efficacy and safety.
Considering TXN’s pivotal role in the intracellular antioxidative system, modulating its levels renders cells vulnerable to oxidative stress, leading to lipid peroxidation and ultimately ferroptotic cell death. Prior research has demonstrated that pharmacological and genetic inhibition of the TXN system sensitizes cancer cells to ferroptosis or directly induces cell death, which can be mitigated by specific ferroptosis inhibitors37,38,61. Our study corroborates the anti-ferroptotic properties of the TXN system. Theoretically, the TXN system operates in parallel with canonical anti-ferroptotic mechanisms, including GPX4/GSH, FSP1/CoQ, GCH/BH4 and DHODH/CoQ2,62. These pathways exhibit functional overlaps and complement each other when one or more are compromised, particularly within specific organelles, as many of these proteins are distributed across both the cytosol and mitochondria.
Remarkably, our study found that ALDH16A1 transports TXN to lysosomes for degradation. By degrading iron-storage protein ferritin and selenocysteine-rich protein SEPP1, lysosomes maintain iron and selenium pools, both of which are crucial determinants of cellular ferroptosis sensitivity63–65. Moreover, lysosomal degradation of ferroptosis-related proteins has been proposed66,67. It has been reported that TXN is degraded in lysosome by cathepsin D, but how TXN is transported to lysosome still remains unclear68,69. Our findings offer a preliminary answer to this question.
Intriguingly, previous researchers have reported that among patients with SMARCA4-deficient NSCLC who received first-line platinum doublet chemotherapy, the median progression-free survival was only 38 days18. Therefore, insight mechanistic study is warranted to improve these tumours’ response to chemotherapy. Xue et al. proposed a model that SMARCA4/2 loss inhibits chemotherapy-induced apoptosis through disrupting intracellular organelle calcium ion (Ca2+) release in these cancers52. Based on our previous research focusing on the role of ferroptosis in chemoresistance, we confirmed that interfering SMARCA4/ALDH16A1/TXN axis could be a promising strategy to reverse the chemoresistance in a ferroptosis-dependent manner. Meanwhile, clinical studies have shown that SMARCA4-deficient NSCLC patients benefit from ICB treatment, indicating a potential vulnerability in this NSCLC subtype15,16. Supportively, a large sample size clinical research has suggested that NSCLC patients with homozygous truncating SMARCA4 mutations had significantly worse OS on checkpoint immunotherapy compared to WT patients70. In recent years, the intricate interplay between ferroptosis and tumour immunity has garnered significant research interest. On the one hand, ferroptosis contributes to CD8+ T cells induced cell death during cancer immunotherapy. On the other hand, ferroptotic cancer cells can release immunostimulatory damage-associated molecular patterns, which assists the recruitment and activation of immune system71. Our study, through bioinformatic analysis and in vitro/in vivo experiments, confirmed that combining PD-1 antibody treatment with ALDH16A1 overexpression or TXN inhibitors synergistically enhances anti-tumour immunity in SMARCA4-deficient NSCLC. Thus, targeting the SMARCA4/ALDH16A1/TXN/ferroptosis pathway represents a promising strategy to magnify the efficacy of immunotherapy in this challenging NSCLC subtype.
In conclusion, by identifying SMARCA4 as a pro-ferroptotic tumour suppressor, our study illuminates the role of the SMARCA4/ALDH16A1/TXN axis in ferroptosis regulation and its underlying mechanisms. Given the high mutation rate of SMARCA4 in NSCLC and its refractory clinical characteristic, our findings uncover a targetable vulnerability in SMARCA4-deficient NSCLC and provide a rationale for clinical studies to explore whether targeting this pathway can enhance the efficacy of chemotherapy and immunotherapy by promoting ferroptosis in this difficult-to-treat cancer subtype.
Methods
The use of patient samples and animal models in this research complies with all relevant ethical regulations of Zhongshan Hospital Fudan University, including the Institutional Review Board and Institutional Animal Care and Use Committee.
Cell lines and culture condition
Cells were maintained at 37 °C under a 5% CO₂ humidified atmosphere. The human NSCLC lines PC9 (SCSP-5085), H1975 (SCSP-597), HCC827 (SCSP-538), A549 (TCHu150), H23 (SCSP-581), H1299 (SCSP-589), fibrosarcoma cell line HT1080 (TCHu170), colorectal cancer cell line HCT116 (TCHu 99), human embryonic kidney cell line HEK-293T (GNHu17), and mouse Lewis lung cancer cell line LLC (SCSP-5252), were obtained from the Chinese Academy of Science Cell Bank and maintained in DMEM (Hyclone, U.S.A) containing 10% fetal bovine serum (ScienCell, U.S.A) along with 100 U/mL penicillin/streptomycin/amphotericin B. All experiments were conducted using early-passage cells (under 30 passages). Prior to use, the cell lines underwent short tandem repeat analysis for authentication in 2024 and were passaged every 3–5 days based on their growth kinetics.
To monitor potential Mycoplasma contamination, cells were routinely screened every two months through PCR detection. For this purpose, antibiotic-free culture supernatant was harvested following 7 days of cell growth. DNA was then isolated and cleaned up using silica-gel columns (TIANGEN, China). PCR amplification was carried out with hot-start Taq DNA polymerase following manufacturer’s instructions. The amplified products were electrophoresed on 1.3% agarose-TAE gels stained with ethidium bromide and analysed under UV light. A distinct band ranging from 515 to 525 bp would indicate Mycoplasma contamination. Only cell cultures confirmed to be Mycoplasma-free were utilized for further experimental procedures.
Compounds
The following compounds were purchased from TargetMol (U.S.A): RSL3 (T3646), IKE (T5523), ML210 (T8375), FIN56 (T4066), ferrostatin-1 (T6500), deferoxamine mesylate (T1637), Z-VAD(OMe)-FMK (T6013), necrosulfonamide (T7129), cycloheximide (T1225), chloroquine (T8689), MG132 (T2154), liproxstatin-1 (T2376). The following compounds were purchased from MedChemExpress (U.S.A): auranofin (HY-B1123), PX-12 (HY-13734), Conoidin A (HY-116090).
Human LUAD samples
Tumour specimens and matched adjacent normal tissues were collected from patients undergoing surgical resection for NSCLC in the Department of Thoracic Surgery at Zhongshan Hospital, Fudan University, between May 2021 and August 2024 (n = 43; 25 females, 18 males). The study protocol was approved by the Institutional Review Board of Zhongshan Hospital (Approval No: Y2024-727), and written informed consent was obtained from all participants. All procedures adhered to the principles of the Declaration of Helsinki. The expressing status of SMARCA4 was confirmed by IHC staining for all tissues. 6 of 43 pieces of fresh tissues were used for organoid construction (female 4, male 2).
CRISPR/Cas9 screening
The genome-wide CRISPR-Cas9 knockout screening system was implemented using an RNA-guided approach based on the architecture described by Zhang et al. This platform employs a pooled single-guide RNA (sgRNA) library comprising 114,155 unique sgRNAs targeting 19,060 human protein-coding genes (accessible via Addgene: https://www.addgene.org/pooled-library/zhang-human-gecko-v2/). The sgRNA library was sourced from Genechem Co., Ltd (Shanghai, China) and utilised for subsequent genome-wide functional screening. Briefly, PC9 cells were seeded and lentivirus sgRNA library were transfected at an MOI of 0.8, followed by positive selection with 1.2 μg/mL puromycin. After transfection, cells were treated with 0.1 μM RSL3 or DMSO for 48 h, an optimal time window for cell’s stable resistance to ferroptosis to emerge without causing complete cell death. Genomic DNA from each group were extracted and subjected to paired-end sequencing on the Illumina HiSeq platform (Illumina, San Diego, CA, USA). If a gene essential for tumour cell survival is knocked out by an sgRNA, those sgRNAs specific to that gene would be scarcely present in the surviving population. Therefore, the average Log2(Foldchange) of the abundance of all sgRNAs targeting the gene, before and after RSL3 treatment, were calculated. ΔLog2(FC) indicates [Log2(FCRSL3)–Log2(FCDMSO)]7,72,73. Gene-level significance was determined by one-sided MAGeCK-RRA algorithm (v0.5.9) to identify enriched or depleted genes, with false discovery rate controlled by Benjamini–Hochberg procedure. Genes with |ΔLog2(FC) > 1| were considered as potential candidates (581 enriched and 1217 depleted).
Immunoblotting
For protein extraction, cells were lysed in RIPA buffer (Beyotime) supplemented with protease and phosphatase inhibitors (Beyotime). The extracted proteins were then denatured by heating at 100 °C for 10 min in 5× SDS-PAGE loading buffer (EpiZyme Biotech, China). Approximately 15–20 μg of protein per sample was resolved by SDS-PAGE (EpiZyme Biotech) and subsequently transferred to polyvinylidene fluoride membranes (Merck-Millipore, U.S.A). The membranes were blocked with 5% nonfat milk and probed overnight at 4 °C with the following antibodies: SMARCA4 (1:1000, A2117, Abclonal, U.S.A), SMARCA2 (1:1000, A23291, Abclonal), ALDH16A1 (1:500, sc-398693, Santa Cruz, U.S.A), TXN (1:1000, A7638, Abclonal), ACSL4 (1:1000, abs106075, Absin, China), FSP1 (1:2000, A22278, Abclonal), Flag (1:1000, #14793, Cell Signalling Technology, U.S.A), HA (1:1000, #3724, Cell Signalling Technology), GPX4 (1:1000, CY6959, Abways, China), SLC7A11 (1:1000, DF12509, Affinity), GAPDH (1:2000, AF0006, Beyotime). Following extensive washing with Tris-buffered saline containing Tween-20, the membranes were incubated with appropriate secondary antibodies at room temperature. Protein bands were detected using the NcmECL Ultra chemiluminescence kit (New Cell & Molecular Biotech Co., Ltd). Uncropped and unprocessed scans of key immunoblot are included in the Source Data file.
CRISPR/Cas9-mediated gene knockout
CRISPR/Cas9-mediated gene knockout was performed in the cell lines using sequence-specific sgRNAs. The sgRNA sequences were cloned into GV392 lentiviral vectors, which co-express hSpCas9 and carry either puromycin or blasticidin resistance markers. The following sgRNA sequences were utilised: sgRNA-Control: 5′-CGCTTCCGCGGCCCGTTCAA-3′; sgSMARCA4: 5′- GGCCGAGGAGTTCCGCCCAG-3′; sgSMARCA2: 5′- CTGCAAGCTGCAGCGTTTCG-3′; sgALDH16A1: 5′- TGTTCGAGAGGTTCGAGACG-3′, sgSmarca4: 5′-GCATGTTCAGAGCCGCCGAG-3′. Lentiviral particles were produced by packaging the constructed vectors in HEK-293T cells. All vector design and lentivirus construction were carried out by Genechem Technology (Shanghai, China).
Overexpression plasmid constructs
Lentiviral particles carrying the full-length or mutant SMARCA4/ALDH16A1 genes were produced in HEK-293T cells using GV341 plasmid vectors. To prevent cleavage in cell lines stably expressing Cas9-sgRNA, silent mutations were introduced within the sgRNA-targeted sequences. All plasmid design and lentiviral packaging were conducted by Genechem Technology.
Cell viability assay
Cell viability was assessed with the Cell Counting Kit-8 (CCK8, Topscience). For cytotoxicity evaluation, 5000–10,000 cells per well were plated in 96-well plates, while proliferation assays used 1000 cells per well. After 24 h of incubation, cells were subjected to the specified treatments. Next, 10 μL of CCK8 reagent was added to each well (containing 100 μL medium) and incubated at 37 °C with 5% CO₂ for 1 h. Absorbance readings at 450 nm were obtained using a microplate reader (Biotek, U.S.A).
Colony formation assay
Cells were plated in six-well plates at a density of 500 cells per well (A549 and PC9) during their logarithmic growth phase. Following a 14-day incubation in complete medium supplemented with or without treatments (DMSO, RSL3, or auranofin), the cells were fixed using 4% methanol for 30 min and stained with 1% crystal violet.
Measurement of lipid peroxidation by BODIPY-C11
Briefly, cells (10⁵ per well) were plated in 12-well plates and allowed to adhere overnight. Following the indicated treatments, cells were detached using trypsin, rinsed with phosphate-buffered saline (PBS, Beyotime, China), and resuspended in fresh medium containing 4 μM BODIPY 581/591 C11 dye (Thermo Fisher, U.S.A). Incubation proceeded at 37 °C under 5% CO₂ for 30 min. After washing, samples were analysed for lipid peroxidation using a BD FACS Aria III flow cytometer (BD Bioscience, U.S.A) equipped with a 488-nm laser. Data processing was conducted using FlowJo software (TreeStar, Woodburn, OR, U.S.A). A Figure exemplifying the gating strategy is provided in Fig. S8d.
Transmission electron microscopy
The samples for transmission electron microscopy were prepared as previously described74. Treated cells grown in 6 cm dishes were first fixed with 2.5% glutaraldehyde. Following three washes with 0.1 M phosphate buffer (pH 7.4), the cells underwent post-fixation using 1% osmic acid in phosphate buffer. After additional buffer washes, the samples were dehydrated, embedded, and cured at 60 °C for 48 h. Ultrathin sections were then prepared, sequentially stained with uranyl acetate and lead citrate, and allowed to dry overnight. Finally, the sections were imaged using a Hitachi transmission electron microscope (Japan).
Patient-derived organoid (PDO)
Freshly obtained LUAD (lung adenocarcinoma) tumour tissues underwent a series of processing steps. Initially, they were fragmented into pieces, thoroughly rinsed with an ice-cooled culture buffer, and subsequently subjected to digestion procedures as outlined in the instructions provided by the human lung cancer PDO culture medium kit (D1 Medical Technology, Shanghai, China). Following centrifugation, the resulting precipitate was resuspended in a solution of matrigel (YEASEN) at a 25-fold volume ratio and distributed into a 24-well plate. After gelation at 37 °C for 10 min, 500 μL culture medium were introduced into each well to facilitate the establishment of PDOs. The images of PDOs were acquired using an Olympus IX71 microscope (Olympus, Japan).
In the context of drug sensitivity assessments, PDOs were plated onto 96-well plates. Following exposure to specific agents, cell viability was quantitatively determined utilizing the CellTiter-Lumi Luminescent 3D Cell Viability Assay Kit (Beyotime). Considering that tumour cells are often replaced over time by normal lung cells in the initial biopsy, we made sure that the organoids-related assays in this study were performed within passage 2–3, where tumour purity is most reliably maintained. The combination of morphological and functional data in our previous studies provides compelling evidence for maintained tumour characteristics27,28.
Labile iron pool measurement
Cells (1 × 105 per well) were plated in 24-well plates and treated after 24 h of culture. Briefly, protein-bound iron was released by incubating cell lysates with acidic KMnO4 (Solarbio, China). The liberated iron was then detected by reaction with an iron-sensing cocktail, with absorbance measured at 550 nm using a microplate reader. Data were normalized to total protein content.
Reduced GSH measurement
Following treatment, cells were detached with trypsin, collected by centrifugation, and washed with PBS. The pellets were then suspended in 60 μL protein removal reagent and subjected to two freeze-thaw cycles alternating between liquid nitrogen and 37 °C water baths. After additional incubation at 4 °C for 5 min, samples were centrifuged at 10,000 × g for 10 min. The resulting supernatant was collected for reduced GSH quantification using a commercial assay kit (Beyotime).
Pseudotargeted lipidomic analysis
Sample preparation
A549 (SMARCA4def and SMARCA4restore) and PC9 (SMARCA4wt and SMARCA4ko) cells were plated separately in 10-cm dishes at a density of 1 × 10⁶ cells per dish. Following attachment, cells were washed with PBS, harvested using a cell scraper, and pelleted by centrifugation. Cell pellets were flash-frozen in liquid nitrogen and processed using an adapted Folch extraction method75. Briefly, pellets were resuspended in 600 µL of methanol/water (1:1, v/v) spiked with isotopically labelled internal standard mixtures sourced from Avanti Polar Lipids (USA) and Sigma-Aldrich (USA). After adding 600 µL chloroform, samples were sonicated for 3 min and extracted ultrasonically in an ice-water bath for 10 min, then incubated at 4 °C for 30 min. The chloroform phase was collected and dried under vacuum centrifugation. The residue was re-extracted under identical conditions. Combined extracts were reconstituted in isopropanol/methanol (1:1, v/v), vortexed for 30 s, sonicated for 3 min, and centrifuged at 13,000 rpm and 4 °C for 10 min. Approximately 200 µL of supernatant per sample was collected for further analysis. A quality-control sample was generated by pooling equal volumes of supernatant from all individual samples26.
Liquid chromatography with tandem mass spectrometry (LC-MS) analysis
The liquid chromatography system comprised an ExionLC™ platform equipped with a binary high-pressure mixing pump (integrated degasser), a thermostatically controlled autosampler, and a column oven. The chromatographic separation was carried out using an UPLC HSS T3 column (1.7 μm, 2.1 × 100 mm) under the following conditions: autosampler temperature, 55 °C; injection volume, 5 μL; mobile phase A, acetonitrile/water (60:40, v/v) with 0.1% formic acid and 10 mM ammonium formate; mobile phase B, acetonitrile/methanol (10:90, v/v) containing 0.1% formic acid and 10 mM ammonium formate; flow rate, 0.35 mL/min. A 20-min gradient programme was applied: 0% B held for 1.5 min, increased linearly to 55% B at 5 min, 60% at 10 min, 70% at 13 min and 90% at 15 min, followed by a rise to 100% B over 1 min and held for 2 min. The system was then returned to initial conditions and re-equilibrated for 2 min. Mass spectrometric detection was performed on a QTRAP® 6500+ instrument (Sciex, USA) fitted with an IonDrive™ Turbo V ion source, operating in scheduled multiple reaction monitoring (MRM) mode with polarity switching. Source parameters were set as: curtain gas, 35 psi; collision gas, medium; ion spray voltage, −4.5 kV/+5.5 kV; ion source gas 1, 40 psi; ion source gas 2, 45 psi.
Data processing and analysis
The raw LC–MS data were processed and annotated with the MRMPROBS software package. Subsequent principal component analysis and differential lipid analysis were conducted using R (R Foundation for Statistical Computing, Vienna, Austria).
ATAC-Seq
The ATAC-Seq was performed according to the Omni-ATAC protocol76. Briefly, 100,000 cells seeded in 6-cm dishes per condition were used and rinsed with PBS followed by lysis in 50 mL of cold ATAC-seq resuspension buffer (10 mM Tris-HCl pH 7.4, 10 mM NaCl, 3 mM MgCl2, 0.1% Tween-20, 0.1% NP-40 and 0.1% digitonin) for 5 min. Next, cells were washed with 1 ml of resuspension buffer supplemented with 0.1% Tween-20 and centrifuged at 500 × g for 10 min. Cell pellets were resuspended in 50 μL transposition reaction mix containing 25 μL 2× TD buffer, 2.5 μL transposase, 16.5 μL PBS, 0.5 μL 1% digitonin, 0.5 μL 10% Tween-20 and 5 μL nuclease-free water. The system was shaked at 37 °C for 30 min on a thermomixer. The TIANquick Mini Purification Kit was used for DNA purification (DP203, TIANGEN, China). A standard ATAC-seq amplification protocol with 7 cycles of amplification was used to amplify the library77. ATAC-seq libraries were sequenced on NovaSeq 6000 (Illumina) using 37-bp paired-end sequencing.
The ATAC-Seq data was analysed using deeptools package78. The ATAC-Seq data were first aligned to hg38 human reference genome. Then, comparative analysis was performed by a standard normalization method, and peaks were determined using the MACS 3.0.0 algorithm. False peaks were removed according to the ENCODE blacklist.
Quantitative real-time PCR (qRT-PCR)
RNA was isolated from cells with TRIzol reagent (TIANGEN), followed by cDNA synthesis using the Hifair® II 1st Strand cDNA Synthesis Kit (Yeasen Biotechnology, China) with integrated gDNA removal. Quantitative PCR analysis was conducted in triplicate with the Hifair® III SYBR Green RT-qPCR Kit (Yeasen Biotechnology) on an ABI QuantStudio 5 instrument (Thermo Fisher, U.S.A). Gene expression levels were calculated by the 2−ΔΔCt method after normalizing Ct values to GAPDH. All primers were commercially synthesized (Sangon Biotech). GAPDH: F: AGAAGGCTGGGGCTCATTTG, R: AGGGGCCATCCACAGTCTTC; SMARCA4: F: GACCAGCACTCCCAAGGTTAC, R: CTGGCCCGGAAGACATCTG; ALDH16A1: F: CACCTCGCTGGAGTACGGA; R: CCATTCACATAGTGGCCCAAG; TXN: F: GTGAAGCAGATCGAGAGCAAG, R: CGTGGCTGAGAAGTCAACTACTA.
CUT&TAG
CUT&TAG library construction
The CUT&Tag assay was carried out with the Hyperactive™ In-Situ ChIP Library Prep Kit for Illumina (Vazyme Biotech, China; cat. no. TD901-TD902)79. Briefly, concanavalin A-coated magnetic beads (ConA beads) were combined with resuspended cells and incubated at room temperature. Permeabilisation was achieved using the non-ionic detergent digitonin. Cells bound to ConA beads were then sequentially incubated with primary antibodies—SMARCA4 (Abclonal, A2117; 1:100), H3K27ac (Cell Signalling Technology, #8173; 1:50), and H3K4me1 (Cell Signalling Technology, #5326; 1:50)—followed by secondary antibody and Hyperactive pA-Tn5 Transposase. This enabled targeted fragmentation of DNA bound by the proteins of interest. The cleaved fragments were subsequently ligated to P5 and P7 adapters via Tn5 transposase activity, and adapter-ligated libraries were amplified by PCR using P5 and P7 primers. PCR products were purified and quality-assessed on an Agilent 2100 Bioanalyzer (Agilent Technologies, USA). Final libraries were sequenced on an Illumina NovaSeq 6000 instrument, generating 150 bp paired-end reads for downstream analysis. Besides, qPCR was also performed using the purified PCR products to confirm the sequencing results. The following primers were used: Primer 1: F: GTTCAAGACCAGCCTGACCAACA, R: CGCTATTCTCCCGCCTCAATCTC; primer 2: F: GGCTGTTAGGCGGTTCTCACATT, R: TGGCACGATCTCGGCTCACT; primer 3: F: TGAGGCAGGAGAATGGCGTGAA, R: GGTTCGGGCGGTGAGAAACATC.
Data analysis
The raw sequence data were firstly quality trimmed by fastp software to obtain the clean reads. Then the clean reads were aligned to the Human genome GRCh38.p12 using Bowtie2 and subsequently analysed by the SEACR software to detect genomic regions enriched for multiple overlapping DNA fragments (peaks) that we considered to be putative binding sites. Peaks were called using Macs2 (v2.12) for each antibody relative to IgG as control. Peaks were then annotated using ChipSeeker software80. Visualization of peak distribution along genomic regions of interested genes was performed with IGV. The heatmaps were generated with Deeptools using computeMatrix reference-point and plotHeatmap order with either 6 kb windows centred on each site (p 10 \–binSize 50 \–referencePoint TSS \-a 3000 -b 3000 \-R hg38.tss.bed \). Read density matrices were further normalized to Z-scores to enable comparison of signal patterns across peaks. Rows in heatmaps show the normalized read density at different peaks and are rank ordered by SMARCA4 occupancy, which was calculated based on the peak intensity.
RNA sequencing (RNA-seq) and bioinformatic analysis
RNA-seq and corresponding bioinformatic analyses were carried out using the Illumina HiSeq platform (Illumina, USA) by Shanghai OE Biotech Co., Ltd, as previously described7,81. Differentially expressed genes (DEGs) were identified with the limma package (3.62.2), which implements an empirical Bayesian approach to estimate gene expression changes by using the moderated t-test. |log FC| > 0.5 and a p-value < 0.05 were considered as a cut-off criterion to screen for DEGs.
Determination of ALDH activity
The ALDH activity was measured using the ALDH Activity Assay Kit (Colorimetric) (Abcam, ab155893, U.S.A.) according to the protocol provided by the manufacturer. Briefly, 1 × 106 cells were homogenized with 200 μL ice-cold ALDH Assay Buffer for 10 min on ice, followed by spinning down at 12,000 rpm for 5 min to remove nuclei and insoluble material. 40 μL supernatant and 10 μL buffer was added into the 96-well plate. Then, 50 μL reaction mix containing ALDH assay buffer, ALDH substrate mix, and acetaldehyde, was added to each well containing the control or tested samples. The mixed samples were incubated at room temperature for 30 min and the OD values were tested at a wavelength of 450 nm using a Molecular Devices microplate reader (Biotek).
Co-immunoprecipitation (Co-IP) and proteome profiling
Protein A/G magnetic beads (ThermoFisher Scientific, U.S.A.) were incubated with the antibodies of interest overnight at 4 °C on rotator and crosslinked with DSS for 30 min, followed by washing with NP-40 Lysis Buffer for two times. Then, cell lysates were incubated with pre-treated beads overnight at 4 °C on rotator. Immunoprecipitated complexes were collected with magnetic stand, rinsed, and subjected to proteome profiling or boiling with SDS loading buffer for western blotting.
Three samples were analysed using proteomics, including two anti-flag and one IgG as control. Proteome profiling was carried out by Shanghai OE Biotech Co., Ltd. Briefly, immunoprecipitated proteins were digested and peptides were separated with liquid chromatography (LC) system (UltiMateTM 3000 RSLCnano System, ThermoFisher Scientific), and detected by Q Exactive Plus mass spectrometer (ThermoFisher Scientific). Data analysis was performed using Proteome Discover software (ver. 2.1). The mass spectrometer (MS) spectra were searched against the Swiss-Prot human sequence database (released at 202003).
Immunofluorescence and Duolink proximity ligation assay (PLA)
PLA was conducted using a PLA kit (Duolink® In Situ, Sigma). Briefly, Cells were seeded in 24-well plates with coverslips and cultured for 24 h. After treating as indicated, the cells were then washed with PBS, fixed with paraformaldehyde, permeabilized with Triton, and blocked with 3% BSA sequentially (Beyotime). Then, prepared cells were incubated with primary antibodies at 4 °C overnight: anti-Flag (1:1000, #8146, CST) and anti-TXN (1:100, A7638, Abclonal). Nuclei were counterstained with DAPI. For immunofluorescence, cells were further incubated with an Alexa Fluor 488-labeled goat anti-rabbit and Alexa Fluor 555-labeled donkey anti-mouse IgG antibody (Beyotime) for 1 h at room temperature. For PLA assay, corresponding in situ PLA probes (mouse for Flag and rabbit for TXN) were then applied for 60 min at 37 °C. The cells were then washed with buffer and the fluorescence images were captured by the Laser Scanning Confocal Microscope FV3000 (Olympus, Japan). Hybridization between the two PLA plus and minus probes leading to fluorescent signal occurs when the distance between the two proteins of interests is less than 40 nm, suggesting potential interaction. The immunofluorescence colocalization were quantified with ImageJ (U.S.A.). Briefly, the grayscale images were opened, and background subtraction was applied. Regions of interest were selected, and colocalization analysis was performed using the “Coloc 2” plugin. Thresholds were set using Costes’ automated method, and the Manders’ overlap coefficients, which was defined as the proportion of one protein co-localized with the other, were calculated.
Determination of TXN activity
The TXN activity was measured using the Thioredoxin Fluorometric Activity Assay Kit (Cayman Chemical, U.S.A.) according to the protocol provided by manufacturer. Briefly, 107 cells were collected with scrapers and homogenized with cold buffer containing 100 mM Tris-HCl, 1.5 mM EDTA, and protease inhibitor. The cell lysates were mixed with assay buffer, diluted TXN reductase, and NADPH assay reagent, in 96-well plate, and incubated at 37 °C for 30 min. Then eosin-labelled insulin was added to the mix, and the fluorescence was measured at excitation and emission wavelengths of 520 and 560 nm, respectively. The results were standardized with the protein concentrations in each sample.
Immunohistochemistry
Tissue samples were collected from tumour xenograft models and surgical resections of LUAD patients. Following paraffin embedding, sections were dewaxed, rehydrated and immunohistochemically stained according to the manufacturer’s instructions (SP-9002, ZSGB-BIO, China). Imaging was performed using an Olympus IX71 microscope (Japan). The primary antibodies applied included: anti-4HNE (1:200, Abcam, ab46545), SMARCA4 (1:100, A2117, Abclonal, U.S.A), ALDH16A1 (1:200, sc-398693, Santa Cruz, U.S.A), TXN (1:200, A7638, Abclonal).
In silico modelling and structural analysis
The predicted structure of human ALDH16A1-TXN complex was obtained from the AlphaFold2 database (https://alphafold.ebi.ac.uk)43,82. The UCSF ChimeraX (U.S.A.) was used to visualize the predicted 3-dimentional structure and potential interacting sites83.
Re-analysis of previously published data
Publicly available data from 56 patients with melanoma who underwent tumour biopsies during immunotherapy were obtained from the Gene Expression Omnibus (GSE91061)44. Patients enrolled in this study received nivolumab (3 mg kg−1 every 2 weeks) until progression or for a maximum of two years. “Sensitive tumours” (n = 13) were defined as those who had complete or partial responses (PR/CR) to anti-PD1 therapy, while “resistant tumours” (n = 43) were those who had progressive or stable disease (PD/SD). These response patterns were based on RECIST v1.1. After interrogating their RNA-Seq data, 14 patients with high ALDH16A1 expression were classified as “High” and 42 patients were categorized as “Low” according to the iterative thresholding method. Chi-square test is performed to examine the relationship between ALDH16A1 levels and patients’ response to immunotherapy.
Co-culture OVA+ Luc+ tumour cells with OT-I cells
Mouse LLC cells with or without Smarca4 knockout and/or Aldh16a1 overexpression were infected with lentivirus-expressing ovalbumin (OVA+) and nano-luciferase (Luc+), which are co-translated and separated by a self-cleaving 2 A peptide. The activity of luciferase was quantified to correct the result if ovalbumin was differentially expressed across different groups of cells.
The OT-I mice (C57BL/6-Tg(TcraTcrb)1100Mjb/Crl) were purchased from the GemPharmatech (Nanjing, China) and housed under specific-pathogen-free conditions with a 12 h light–12 h dark cycle. The mice were sacrificed between 6 and 8 weeks old and the spleens were collected and minced using the flat end of the plunger. The released splenocytes were filtered, suspended in red blood cell lysis buffer (Beyotime) for 2 min, and cultured in RPMI 1640 medium (KeyGEN Biotech, China) containing 2.5 μg/mL OVA257–264 peptide (MedChemExpress), 10 ng/mL of mouse recombinant IL-2 (MedChemExpress), and 40 μM 2-mercaptoethanol (Aladdin, China) at 37 °C for 4 days. The amplified OT-1 cells were then purified using MojoSort™ Mouse CD8 T Cell Isolation Kit (BioLegend, U.S.A.)
The edited OVA+ Luc+ LLC were seeded into 96-well plates overnight and pre-treated with auranofin or DMSO for 6 h. Then, activated OT-I cells were added for co-culture (LLC: OT-1 ratio of 1:1). 24 h later, the supernatant and OT-1 CD8 + T cells were removed, and the viability of residual LLC were measured with CCK8 as mentioned above.
Tumour xenograft experiment
All animal studies were conducted in compliance with the policies of the Institutional Animal Care and Use Committee of Zhongshan Hospital, Fudan University (Approval No: B2022-180R). In compliance with the Chinese national guideline RB/T 173-2018 (Guideline for the Assessment of Humane Endpoints in Animal Experiments), the maximum permitted tumour diameter was set at 15 mm. Six-week-old male homozygous Foxn1nu BALB/c nude mice and C57BL/6J mice were acquired from GemPharmatech (Nanjing, China) and maintained under specific-pathogen-free (SPF) conditions with a 12-h light/dark cycle. Ambient temperature was controlled at 21–23 °C with 45% humidity; food (LabDiet, #5053) and water were provided ad libitum. For xenograft studies, 2 × 10⁶ Vehicle or SMARCA4-overexpressing A549 cells, suspended in 100 μL of cold PBS, were subcutaneously injected into the right flank of each nude mouse. Once tumours became palpable, animals were randomly allocated into 12 treatment groups (initial n = 5 per group). IKE (30 mg/kg) and auranofin (2 mg/kg) dissolved in DMSO/corn oil (Beyotime) and were intraperitoneally injected every two days, as indicated in the corresponding Figures. The AAV-NC and AAV-ALDH16A1 were injected at 2 × 1012 genome copies (GC) in PBS/0.001% via tail vein at D0. As for assays in C57/BL6, 2 × 106 Cas9-NC or Smarca4-KO LLC cells were resuspended in 100 μL cold PBS and subcutaneously injected into the right flank of each mouse. IKE (30 mg/kg), auranofin (2 mg/kg), liproxstatin-1 (10 mg/kg) and 100 μg anti-PD-1 per mice, were intraperitoneally injected at indicated days. The tumour volume was measured using a caliper every four days until the endpoint and calculated according to the equation: v = length × width2 × 1/2. Body weights of mice were measured. The maximal tumour burden permitted by the ethics committee is a length of 1.5 cm, and the maximal tumour burden did not exceed the limit.
Flow cytometry analysis
To quantify the infiltrating levels of CD8 + T cells and CD4 + T cells in tumour xenografts, fresh tumour tissues were harvested, excised, ground and digested with the Mouse Tumour Dissociation Kit (Miltenyi, U.S.A.). Dissociated tumour samples were filtered through the 70 μm strainer, and the suspended in red blood cell lysis buffer (Beyotime) for 2 min. Then the dead cells were removed using the Dead Cell Removal Kit (Miltenyi) according to the protocol provided by manufacturer. The screened alive cells were blocked with 2% TruStain FcX™ (anti-mouse CD16/32) Antibody (BioLegend) on ice for 10 min. The samples were then stained with the following antibodies in dark conditions: Zombie Aqua Fixable Viability Kit (BioLegend, #423101), FITC anti-mouse CD45 (BioLegend, #103107), APC anti-mouse CD4 (BioLegend #100515), PE anti-mouse CD8a (BioLegend #100707), Brilliant Violet 421 anti-mouse IFN-γ (BioLegend, #505829). The cells were then washed with PBS and assessed read on FACS Aria III (BD Bioscience) with 405-nm, 488-nm and 640-nm lasers. The results were analysed in FlowJo software. A Figure exemplifying the gating strategy is provided in Fig. S8e.
Statistical analysis & reproducibility
All experiments were independently performed in at least three times. Unpaired two-tailed Student t-tests was utilized to analyse the statistical significance of differences between two groups using GraphPad Prism software (7.0) and Excel. Two-way analysis of variance was used for grouped analysis. The results are presented as means, and the error bars represent the standard deviation. Bioinformatical analyses were conducted using R software74. P-values < 0.05 were considered statistically significant. All cells and the animals were randomly allocated to experimental groups. The investigators were blinded to allocation during experiments and outcome assessment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
This research was supported by the Natural Science Foundation of Shanghai (No. 24ZR1409900, to G.B.), the Fellowship from the China Postdoctoral Science Foundation (No. 2024M760555, to G.B.), the National Natural Science Foundation of China (No. 82403100, to G.B.; and No. 82473184, to C.Z.), the 18th Special Fund (In-Station) from China Postdoctoral Science Foundation (No. 2025T180549, to G.B.) and the Outstanding Resident Clinical Postdoctoral Program of Zhongshan Hospital Affiliated to Fudan University (to G.B.). The authors thank OE Biotech Co., Ltd. (Shanghai, China) for their assistance in this study.
Author contributions
G.B. and C.Z. designed the research. G.B., J.L. and Y.B. carried out the experiments and analysed the data. G.S., S.R., H.S., X.H. and J.Z. helped with the discussion and interpretation of results. G.B., J.L. and Y.B. wrote the manuscript under the supervision of Q.W., W.J., B.G. and C.Z. All authors have read and approved the manuscript.
Peer review
Peer review information
Nature Communications thanks Ultan McDermott, Liming Wang and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The Cancer Genome Atlas (TCGA) and Broad Institute Cancer Cell Line Encyclopedia (CCLE) data analyzed in this study were obtained at https://portal.gdc.cancer.gov/ and https://sites.broadinstitute.org/ccle/datasets. The raw data files of RNA-Seq generated in this study have been deposited in the SRA database under accession code PRJNA1289880. The raw data files of CRISPR/Cas9, ATAC and CUT&TAG, have been deposited in the SRA database under accession code PRJNA1309296 (https://www.ncbi.nlm.nih.gov/bioproject/ PRJNA1309296). The Co-IP proteomics data generated in this study have been deposited in the ProteomeXchange database under accession code PXD066126 (https://www.iprox.cn/page/ProjectFileList.html?projectId=IPX0012588000). The lipidomics data analysed in this study are available at Figshare (10.6084/m9.figshare.28465319) and Supplementary Data. Previously published data from patients with melanoma who underwent tumour biopsies during anti-CTLA4/anti-PD1-based therapy that were re-analysed here are available at the Gene Expression Omnibus under the accession code GSE91061. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Guoshu Bi, Jiaqi Liang, Yunyi Bian.
Contributor Information
Wei Jiang, Email: jiang.wei1@zs-hospital.sh.cn.
Boyi Gan, Email: BGan@mdanderson.org.
Cheng Zhan, Email: czhan10@fudan.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-63687-6.
References
- 1.Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell149, 1060–1072 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lei, G., Zhuang, L. & Gan, B. The roles of ferroptosis in cancer: tumor suppression, tumor microenvironment, and therapeutic interventions. Cancer Cell.42, 513–534 (2024). [DOI] [PubMed] [Google Scholar]
- 3.Lei, G., Zhuang, L. & Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer. 22, 381–396 (2022). [DOI] [PMC free article] [PubMed]
- 4.Stockwell, B. R. et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell171, 273–285 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun, X. et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology63, 173–184 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lei, G. et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res.30, 146–162 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bi, G. et al. miR-6077 promotes cisplatin/pemetrexed resistance in lung adenocarcinoma via CDKN1A/cell cycle arrest and KEAP1/ferroptosis pathways. Mol. Ther. Nucleic Acids28, 366–386 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang, W. et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature569, 270–274 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim, R. et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature612, 338–346 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang, L. et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature520, 57–62 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koppula, P. et al. A targetable CoQ-FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1 inactive lung cancers. Nat. Commun.13, 2206 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mittal, P. & Roberts, C. W. M. The SWI/SNF complex in cancer - biology, biomarkers and therapy. Nat. Rev. Clin. Oncol.17, 435–448 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Miguel, F. J. et al. Mammalian SWI/SNF chromatin remodeling complexes promote tyrosine kinase inhibitor resistance in EGFR-mutant lung cancer. Cancer Cell41, 1516–1534.e9 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian, Y., Xu, L., Li, X., Li, H. & Zhao, M. SMARCA4: current status and future perspectives in non-small-cell lung cancer. Cancer Lett.554, 216022 (2023). [DOI] [PubMed] [Google Scholar]
- 15.Schoenfeld, A. J. et al. The genomic landscape of SMARCA4 alterations and associations with outcomes in patients with lung cancer. Clin. Cancer Res.26, 5701–5708 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alessi, J. V. et al. SMARCA4 and other SWItch/sucrose nonfermentable family genomic alterations in NSCLC: clinicopathologic characteristics and outcomes to immune checkpoint inhibition. J. Thorac. Oncol.16, 1176–1187 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Cheung, A. H.-K. et al. SMARCA4 deficiency and mutations are frequent in large cell lung carcinoma and are prognostically significant. Pathology56, 504–515 (2024). [DOI] [PubMed] [Google Scholar]
- 18.Dagogo-Jack, I. et al. Clinicopathologic characteristics of BRG1-deficient NSCLC. J. Thorac. Oncol.15, 766–776 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Barretina, J. et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature483, 603–607 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, M. et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun.10, 3991 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffman, G. R. et al. Functional epigenetics approach identifies BRM/SMARCA2 as a critical synthetic lethal target in BRG1-deficient cancers. Proc. Natl. Acad. Sci. USA111, 3128–3133 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadoch, C. et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat. Genet.45, 592–601 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dempster, J. M. et al. Extracting biological insights from the Project Achilles genome-scale CRISPR screens in cancer cell lines. bioRxiv. 2019:720243.
- 24.Mashtalir, N. et al. Modular organization and assembly of SWI/SNF family chromatin remodeling complexes. Cell175, 1272–1288.e2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alver, B. H. et al. The SWI/SNF chromatin remodelling complex is required for maintenance of lineage specific enhancers. Nat. Commun.8, 14648 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bi, G. et al. Retinol saturase mediates retinoid metabolism to impair a ferroptosis defense system in cancer cells. Cancer Res.83, 2387–2404 (2023). [DOI] [PubMed] [Google Scholar]
- 27.Shan, G. et al. Targeting ALDH2 to augment platinum-based chemosensitivity through ferroptosis in lung adenocarcinoma. Free Radic. Biol. Med.224, 310–324 (2024). [DOI] [PubMed] [Google Scholar]
- 28.Bian, Y. et al. Targeting ALDH1A1 to enhance the efficacy of KRAS-targeted therapy through ferroptosis. Redox Biol.77, 103361 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fritz, K. S. & Petersen, D. R. An overview of the chemistry and biology of reactive aldehydes. Free Radic. Biol. Med.59, 85–91 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saiki, J. P. et al. Aldehyde dehydrogenase 3A1 activation prevents radiation-induced xerostomia by protecting salivary stem cells from toxic aldehydes. Proc. Natl. Acad. Sci. USA115, 6279–6284 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Shamma, S. A., Zaher, D. M., Hersi, F., Abu Jayab, N. N. & Omar, H. A. Targeting aldehyde dehydrogenase enzymes in combination with chemotherapy and immunotherapy: an approach to tackle resistance in cancer cells. Life Sci.320, 121541 (2023). [DOI] [PubMed] [Google Scholar]
- 32.Shortall, K., Djeghader, A., Magner, E. & Soulimane, T. Insights into Aldehyde dehydrogenase enzymes: a structural perspective. Front. Mol. Biosci.8, 659550 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yusuf, R. Z. et al. Aldehyde dehydrogenase 3a2 protects AML cells from oxidative death and the synthetic lethality of ferroptosis inducers. Blood136, 1303–1316 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen, X. et al. A noncanonical function of EIF4E limits ALDH1B1 activity and increases susceptibility to ferroptosis. Nat. Commun.13, 6318 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasiliou, V. et al. ALDH16A1 is a novel non-catalytic enzyme that may be involved in the etiology of gout via protein-protein interactions with HPRT1. Chem. Biol. Interact.202, 22–31 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu, X., Zhang, Y., Zhuang, L., Olszewski, K. & Gan, B. NADPH debt drives redox bankruptcy: SLC7A11/xCT-mediated cystine uptake as a double-edged sword in cellular redox regulation. Genes Dis.8, 731–745 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu, J. & Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med.66, 75–87 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Llabani, E. et al. Diverse compounds from pleuromutilin lead to a thioredoxin inhibitor and inducer of ferroptosis. Nat. Chem.11, 521–532 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang, L. et al. Auranofin mitigates systemic iron overload and induces ferroptosis via distinct mechanisms. Signal Transduct. Target. Ther.5, 138 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu, X. et al. Hsc70 promotes anti-tumor immunity by targeting PD-L1 for lysosomal degradation. Nat. Commun.15, 4237 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tekirdag, K. & Cuervo, A. M. Chaperone-mediated autophagy and endosomal microautophagy: joint by a chaperone. J. Biol. Chem.293, 5414–5424 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaushik, S. & Cuervo, A. M. The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol.19, 365–381 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature596, 583–589 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riaz, N. et al. Tumor and Microenvironment evolution during Immunotherapy with Nivolumab. Cell171, 934–949.e16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shain, A. H. et al. The genetic evolution of melanoma from precursor lesions. N. Engl. J. Med.373, 1926–1936 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Zhang, Y. et al. Imidazole ketone erastin induces ferroptosis and slows tumor growth in a mouse lymphoma model. Cell Chem. Biol. 26, 623–633.e9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, M.-E. et al. RB1-deficient prostate tumor growth and metastasis are vulnerable to ferroptosis induction via the E2F/ACSL4 axis. J. Clin. Invest. 133, e166647 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ou, Y., Wang, S.-J., Li, D., Chu, B. & Gu, W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc. Natl. Acad. Sci. USA113, E6806–E6812 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang, X. et al. Regulation of VKORC1L1 is critical for p53-mediated tumor suppression through vitamin K metabolism. Cell Metab. 35, 1474–1490.e8 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bartolacci, C. et al. Targeting de novo lipogenesis and the Lands cycle induces ferroptosis in KRAS-mutant lung cancer. Nat. Commun.13, 4327 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu, X. et al. Alanine supplementation exploits glutamine dependency induced by SMARCA4/2-loss. Nat. Commun.14, 2894 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xue, Y. et al. SMARCA4/2 loss inhibits chemotherapy-induced apoptosis by restricting IP3R3-mediated Ca2+ flux to mitochondria. Nat. Commun.12, 5404 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zou, Y. et al. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat. Chem. Biol.16, 302–309 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhat, K. P. et al. CRISPR activation screens identify the SWI/SNF ATPases as suppressors of ferroptosis. Cell Rep.43, 114345 (2024). [DOI] [PubMed] [Google Scholar]
- 55.Zhang, T. et al. ENO1 suppresses cancer cell ferroptosis by degrading the mRNA of iron regulatory protein 1. Nat. Cancer3, 75–89 (2022). [DOI] [PubMed] [Google Scholar]
- 56.Pan, C., Li, B. & Simon, M. C. Moonlighting functions of metabolic enzymes and metabolites in cancer. Mol. Cell.81, 3760–3774 (2021). [DOI] [PubMed] [Google Scholar]
- 57.Singh, S. et al. Aldehyde dehydrogenases in cellular responses to oxidative/electrophilic stres. Free Radic. Biol. Med. 56, 89–101 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He, T. et al. Targeting the mSWI/SNF complex in POU2F-POU2AF transcription factor-driven malignancies. Cancer Cell42, 1336–1351.e9 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mota, M. et al. Targeting SWI/SNF ATPases in H3.3K27M diffuse intrinsic pontine gliomas. Proc. Natl. Acad. Sci. USA120, e2221175120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang, J.-H., Gessler, D. J., Zhan, W., Gallagher, T. L. & Gao, G. Adeno-associated virus as a delivery vector for gene therapy of human diseases. Signal Transduct. Target. Ther.9, 78 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bai, L. et al. Thioredoxin-1 rescues MPP+/MPTP-induced ferroptosis by increasing glutathione peroxidase 4. Mol. Neurobiol.58, 3187–3197 (2021). [DOI] [PubMed] [Google Scholar]
- 62.Harris, I. S. et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell.27, 211–222 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Hou, W. et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy12, 1425–1428 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao, M. et al. Ferroptosis is an autophagic cell death process. Cell Res.26, 1021–1032 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li, Z. et al. Ribosome stalling during selenoprotein translation exposes a ferroptosis vulnerability. Nat. Chem. Biol.18, 751–761 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu, K. et al. Creatine kinase B suppresses ferroptosis by phosphorylating GPX4 through a moonlighting function. Nat. Cell Biol.25, 714–725 (2023). [DOI] [PubMed] [Google Scholar]
- 67.Zhang, Y. et al. mTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation. Nat. Commun.12, 1589 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Persson, T. et al. Apolipoprotein E4 elicits lysosomal cathepsin D release, decreased thioredoxin-1 levels, and apoptosis. J. Alzheimers Dis.56, 601–617 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haendeler, J. et al. Cathepsin D and H2O2 stimulate degradation of thioredoxin-1: implication for endothelial cell apoptosis. J. Biol. Chem.280, 42945–42951 (2005). [DOI] [PubMed] [Google Scholar]
- 70.Fernando, T. M. et al. Functional characterization of SMARCA4 variants identified by targeted exome-sequencing of 131,668 cancer patients. Nat. Commun.11, 5551 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Friedmann Angeli, J. P., Krysko, D. V. & Conrad, M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer19, 405–414 (2019). [DOI] [PubMed] [Google Scholar]
- 72.Liang, J. et al. MAFF confers vulnerability to cisplatin-based and ionizing radiation treatments by modulating ferroptosis and cell cycle progression in lung adenocarcinoma. Drug Resist. Updat.73, 101057 (2024). [DOI] [PubMed] [Google Scholar]
- 73.Rossiter, N. J. et al. CRISPR screens in physiologic medium reveal conditionally essential genes in human cells. Cell Metab. 33, 1248–1263.e9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bi, G. et al. Polyamine-mediated ferroptosis amplification acts as a targetable vulnerability in cancer. Nat. Commun.15, 2461 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Folch, J., Lees, M. & Sloane Stanley, G. H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem.226, 497–509 (1957). [PubMed] [Google Scholar]
- 76.Corces, M. R. et al. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat. Methods14, 959–962 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buenrostro, J. D., Wu, B., Chang, H. Y. & Greenleaf, W. J. ATAC-seq: a method for assaying chromatin accessibility genome-wide. Curr. Protoc. Mol. Biol. 109, 21.29.1–21.29.9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramírez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res.44, W160–W165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaya-Okur, H. S. et al. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat. Commun.10, 1930 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu, G., Wang, L.-G. & He, Q.-Y. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics31, 2382–2383 (2015). [DOI] [PubMed] [Google Scholar]
- 81.Bi, G. et al. Pan-cancer characterization of metabolism-related biomarkers identifies potential therapeutic targets. J. Transl. Med.19, 219 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Evans, R. et al. Protein complex prediction with AlphaFold-Multimer. bioRxiv. 2021.10.04.463034.
- 83.Pettersen, E. F. et al. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem.25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The Cancer Genome Atlas (TCGA) and Broad Institute Cancer Cell Line Encyclopedia (CCLE) data analyzed in this study were obtained at https://portal.gdc.cancer.gov/ and https://sites.broadinstitute.org/ccle/datasets. The raw data files of RNA-Seq generated in this study have been deposited in the SRA database under accession code PRJNA1289880. The raw data files of CRISPR/Cas9, ATAC and CUT&TAG, have been deposited in the SRA database under accession code PRJNA1309296 (https://www.ncbi.nlm.nih.gov/bioproject/ PRJNA1309296). The Co-IP proteomics data generated in this study have been deposited in the ProteomeXchange database under accession code PXD066126 (https://www.iprox.cn/page/ProjectFileList.html?projectId=IPX0012588000). The lipidomics data analysed in this study are available at Figshare (10.6084/m9.figshare.28465319) and Supplementary Data. Previously published data from patients with melanoma who underwent tumour biopsies during anti-CTLA4/anti-PD1-based therapy that were re-analysed here are available at the Gene Expression Omnibus under the accession code GSE91061. Source data are provided with this paper.