Abstract
Purpose:
Histone deacetylase (HDAC) inhibition has been shown to induce pharmacologic “BRCAness” in cancer cells with proficient DNA repair activity. This provides a rationale for exploring combination treatments with HDAC and PARP inhibition in cancer types that are insensitive to single-agent PARP inhibitors (PARPi). Here, we report the concept and characterization of a novel bifunctional PARPi (kt-3283) with dual activity toward PARP1/2 and HDAC enzymes in Ewing sarcoma cells.
Experimental Design:
Inhibition of PARP1/2 and HDAC was measured using PARP1/2, HDAC activity, and PAR formation assays. Cytotoxicity was assessed by IncuCyte live cell imaging, CellTiter-Glo, and spheroid assays. Cell-cycle profiles were determined using propidium iodide staining and flow cytometry. DNA damage was examined by γH2AX expression and comet assay. Inhibition of metastatic potential by kt-3283 was evaluated via ex vivo pulmonary metastasis assay (PuMA).
Results:
Compared with FDA-approved PARP (olaparib) and HDAC (vorinostat) inhibitors, kt-3283 displayed enhanced cytotoxicity in Ewing sarcoma models. The kt-3283-induced cytotoxicity was associated with strong S and G2–M cell-cycle arrest in nanomolar concentration range and elevated DNA damage as assessed by γH2AX tracking and comet assays. In three-dimensional spheroid models of Ewing sarcoma, kt-3283 showed efficacy in lower concentrations than olaparib and vorinostat, and kt-3283 inhibited colonization of Ewing sarcoma cells in the ex vivo PuMA model.
Conclusions:
Our data demonstrate the preclinical justification for studying the benefit of dual PARP and HDAC inhibition in the treatment of Ewing sarcoma in a clinical trial and provides proof-of-concept for a bifunctional single-molecule therapeutic strategy.
Translational Relevance.
Clinical use of PARP inhibitors (PARPi) is mostly limited to cancers that have defects in the homologous recombination DNA repair machinery, such as deleterious mutations in BRCA1 or BRCA2. While preclinical data showed encouraging antitumor effects, there was an underwhelming response to first-generation PARPi in an Ewing sarcoma phase II clinical trial. Obtaining durable responses with PARP inhibition in Ewing sarcoma will require deployment of strategies that can increase the efficacy of PARP inhibition. Histone deacetylase (HDAC) inhibition is a known inducer of pharmacologic “BRCAness” and constitutes an attractive approach based on established sensitivity of Ewing sarcoma cells to single-agent HDAC inhibition. Here, we describe and characterize the performance of a dual-specificity single-molecule inhibitor of PARP1/2 and HDAC enzymes in Ewing sarcoma model systems. Our data provide an argument for testing dual PARP-HDAC inhibition strategies in clinical trials.
Introduction
PARP proteins catalyze PARylation of cellular proteins using an ADP-ribose subunit of NAD+ as the donor (1). The human genome encodes 17 PARP enzymes; PARP1–3 has critical functions in DNA repair and PARP1 is the most characterized (2). PARP1 is essential for the repair of single-strand DNA breaks (SSB), which is the most common type of breakpoint lesion in cellular DNA (3). When cells encounter SSB, PARP1 binds the lesion and initiates a PARylation cascade of itself and histones embedded in the chromatin surrounding the SSB lesions. This PARylation event serves as a signal to recruit the SSB repair machinery to patch the lesions before and during DNA replication in the S-phase of the cell cycle (4). Efficient SSB repair is important to prevent replication stress and the more severe double-strand break (DSB) lesions that occur in S-phase when unrepaired SSB lesions collide with the replication forks (3). DSB lesions in S-phase are mainly repaired by homologous recombination (HR) that relies on proteins such as BRCA1 and BRCA2 (5). Deleterious mutations in BRCA1/2 are found in subsets of breast, ovarian, and prostate tumors, and sporadically in other solid tumor indications (6, 7). These HR-deficient tumors are indirectly dependent on proficient PARP enzyme activity to avoid accumulation of catastrophic DSBs in S-phase and initiation of cell death (4). This dependency has paved the way for PARP inhibition as a therapeutic strategy to create synthetic lethality in tumor cells with BRCA1/2-deficiencies (8).
Currently, there are four approved PARP inhibitors (PARPi) in the clinic: olaparib (approved in 2014), rucaparib (approved in 2016), niraparib (approved in 2017), and talazoparib (approved in 2018). These PARPi have been widely deployed in cancers with defects in HR DNA repair activity caused by BRCA1/2 mutations (9). Encouraged by the success of PARPi in BRCA1/2-mutated cancers, research attention has been expanded toward cancer subtypes where HR repair is compromised due to molecular events other than BRCA1/2 mutations. For example, tumors with mutations in RAD51, an enzyme acting downstream of BRCA1/2 in the HR repair pathway, are also sensitive to PARP inhibition (10). This concept is commonly referred to as “BRCAness” and includes all events that mimic BRCA1/2 loss in the context of HR repair (11).
In HR-proficient cancers, the state of “BRCAness” can be mimicked pharmacologically by inhibition of proteins that impact BRCA1/2 expression. This potentially invites opportunities to broaden the use of PARPi beyond current clinical practice. For example, impairing dynamic chromatin events related to DNA replication and repair such as histone acetylation can induce pharmacologic “BRCAness” through indirect regulation of HR components (12). Recent studies in leukemia, breast cancer, liver cancer, glioblastoma, prostate cancer, and anaplastic thyroid cancer models demonstrated suppression of HR activity with histone deacetylase (HDAC) inhibition that further supports the synergistic potential of HDAC and PARP inhibition (13–18).
Ewing sarcoma is a highly metastatic bone and soft tissue tumor affecting mainly children and young adults, with a dismal 5-year survival rate of 15% to 30% for metastatic disease (19). Ewing sarcoma is defined by the presence of specific gene fusion events involving EWSR1 and the erythroblast transformation–specific (ETS) transcription factor FLI1 (85%) or other ETS-family transcription factors (15%), most often ERG (20). These gene fusions encode chimeric oncoproteins (e.g., EWS-FLI1 or EWS-ERG) that drive Ewing sarcoma initiation and progression.
Ewing sarcoma cells are sensitive to PARPi in vitro and this sensitivity depends on EWS-FLI1 (21). Ewing sarcoma cell line–derived xenografts in mice display sensitivity to FDA-approved PARPi similar to the responses seen with the standard-of-care chemotherapy temozolomide (22). Ewing tumors have been shown to display “BRCAness” (23) and a subset (∼10%) of patient samples harbor mutations in DNA repair proteins such as BRCA or RAD51 (24). Despite these deficiencies, patients failed to produce durable responses to single-agent PARP inhibition in a phase II single-agent trial in Ewing sarcoma with olaparib despite encouraging preclinical data (25). By further inducing pharmacologic “BRCAness” through combination therapies, we can potentially increase the efficacy of PARPi against Ewing tumors and resensitize resistant cells, though a small subset of patients may still benefit from PARPi monotherapy. Here, HDAC inhibition seems attractive based on previously established sensitivity of Ewing sarcoma cells to HDAC inhibitors (26, 27). In this study, we have characterized and evaluated kt-3283, a novel dual-specificity single-molecule inhibitor of PARP1/2 and HDAC enzymes in Ewing sarcoma model systems.
Materials and Methods
Cell culture
CHLA10, TC32, and A673 cells were obtained from coauthor Dr. Poul Sorensen. Identities of all human Ewing sarcoma cell lines were confirmed by STR profiling at the Laboratory Corporation of America (Labcorp). All cell lines were confirmed free of Mycoplasma using the MycoAlert mycoplasma detection kit (Lonza) and maintained at 37°C with 5% CO2 and 95% humidity. CHLA10 cells (RRID:CVCL_6583) were maintained in Iscove's modified Dulbecco's medium (Hyclone, catalog No. SH30228.01) containing 1x Insulin-Transferrin-Selenium (Thermo Fisher Scientific, catalog No. 41400045) and 20% FBS (Gibco, catalog No. A3160401). TC32 cells (RRID:CVCL_7151) were maintained in RPMI-1640 (Gibco, catalog No. 11875119) with 10% FBS and 1x GlutaMAX supplement (Thermo Fisher Scientific, catalog No. 35050061). A673 cells (RRID:CVCL_0080) were maintained in DMEM (Gibco, catalog No. 11995065) supplemented with 10% FBS. Cells were used for experiments within 25 passages of thawing.
HDAC activity assay
In vitro HDAC activity was measured using the FLUOR DE LYS HDAC fluorometric activity assay kit (Enzo Life Sciences, catalog No. BML-AK500–0001) following the manufacturer's protocol. IC50 values were calculated using a four-parameter variable slope nonlinear regression in GraphPad Prism 8 (GraphPad Software Inc.; RRID:SCR_002798).
PARP1 and PARP2 activity assay
In vitro PARP1 activity was measured using the HT Universal Colorimetric PARP assay kit (R&D Systems, catalog No. 4677–096-K), and PARP2 activity was measured using the PARP2 colorimetric assay kit (BPS Bioscience, catalog No. 80581) following the manufacturer's protocol. IC50 values were calculated using a four-parameter variable slope nonlinear regression in GraphPad Prism 8 (GraphPad Software Inc.).
PAR formation assay
Cellular PAR formation assays were used to measure the ability of a tested compound to inhibit polymerization of PAR. CHLA10 cells were plated on a black, clear-bottom 96-well plate and allowed to attach overnight. Cells were pretreated with increasing concentrations of test inhibitors for 30 minutes at 37°C before H2O2 was added to a final concentration of 25 mmol/L and incubated for 5 minutes at room temperature. After two washes with 0.1% Tween-20 in PBS (PBS-T) and two washes with PBS, cells were fixed with prechilled 70:30 methanol:acetone for 15 minutes at −20°C. Cells were washed with PBS, twice with 3% BSA in PBS (BSA-PBS) and again with PBS and then blocked with 3% BSA-PBS for 30 minutes at room temperature. Following two washes with PBS and one wash with 3% BSA-PBS, cells were incubated for 1 hour at room temperature with anti-PAR/pADPr monoclonal antibody (R&D Systems, catalog No. 4335-MC-100; RRID:AB_2572318) diluted 1:250 in 3% BSA-PBS. Plates were washed twice with 3% BSA-PBS, once with PBS, twice with PBS-T, twice with PBS, and once with 3% BSA-PBS and then incubated with goat anti-mouse IgG-FITC (Thermo Scientific, catalog No. F-2761; RRID:AB_2536524) diluted 1:1,000 in 3% BSA-PBS for 1 hour at room temperature. After washing twice with 3% BSA-PBS, once with PBS, twice with PBS-T and three times with PBS, 100-μL PBS per well was added and plates were imaged on an IncuCyte S3 system (Sartorius). Fluorescence was quantified using the IncuCyte analysis software. Values were normalized to no primary antibody control and % PAR formation was calculated by normalizing to DMSO control. IC50 values were then calculated using a four-parameter variable slope nonlinear regression in GraphPad Prism 8 (GraphPad Software Inc.). The average IC50 value ± SD of three biological replicates was calculated.
Cell viability assay
Cells were plated on a 96-well plate (1,000–5,000 cells per well) in 100-μL appropriate medium and allowed to attach overnight. The 100-μL medium containing DMSO or increasing concentration of test compound was added to each well. Cells were maintained at 37°C with 5% CO2 and 95% humidity for 10 days for CHLA10 and 3 days for TC32 and A673. CellTiter-Glo viability assay was carried out for CHLA10. The wells were equilibrated at room temperature for 30 minutes after removing the media (150 μL per well), followed by the addition of CellTiter-Glo assay reagent, and CellTiter-Glo assay reagent was added to the wells. The plates were gently shaken on an orbital shaker for 2 minutes and incubated at room temperature for 10 minutes in the dark. Luminescence was measured using a Tecan Infinite M200Pro microplate reader. All measurements were carried out in triplicate. For TC32 and A673, the plates were imaged on an Incucyte S3 live cell imaging system after the treatments and % confluency was measured using the Incucyte software (Sartorius; RRID:SCR_023147). Values were normalized to media only and DMSO controls to calculate % cell survival. EC50 values were calculated using a four-parameter variable slope nonlinear regression in GraphPad Prism 8 (GraphPad Software Inc.). The mean EC50 value ± SD was calculated using three biological replicates.
Cell-cycle analysis
Cell-cycle profiles were evaluated via propidium iodide (PI) staining and flow cytometry. CHLA10 and TC32 cells were plated in 10-cm plates with a cell density of 1.5×106 cells/plate and 2.0×106 cells/plate, respectively. The media was replaced the following day with serum-free media for 24 hours. Cells were treated with olaparib, vorinostat, and kt-3283 in a dose-escalating manner for 24 hours for TC32 and 48 hours for CHLA10. Combination treatments of olaparib with vorinostat and olaparib with belinostat were evaluated at equimolar concentration of kt-3283 (0.7 and 1 μmol/L for TC32 and CHLA10 cells, respectively). Cells were harvested, and 1.0×106 cells from each treatment were fixed in 70% ethanol overnight at −30°C. The cell suspension was then washed with cold PBS and stained with a PI solution (50 μg/mL PI, 0.1 mg/mL RNase A, 0.05% Triton X-100 in PBS) and incubated at 37°C for 40 minutes in the dark. Cells were then washed with PBS, filtered through a 40-μm strainer and resuspended with 500-μL PBS. Samples were then examined by flow cytometry and analyzed in FlowJo v10 (RRID:SCR_008520).
Alkaline comet assay
Cells were plated in a six-well plate at a density of 1×106 cells/well and allowed to settle overnight. Cell media was replaced with serum-free media for 24 hours, then treated with DMSO or test compound for 24 hours at 37°C with 5% CO2 and 95% humidity. Cells were harvested as instructed in Trevigen's CometAssay protocol and combined with molten LMAgarose at a ratio of 1:10 and pipetted onto CometSlides. Cells were placed in the dark for 30 minutes and immersed in lysis solution overnight at 4°C. Slides were immersed in Alkaline Unwinding solution for 1 hour at 4°C in the dark, then placed in a gel electrophoresis tray and immersed in Alkaline Electrophoresis solution with an applied voltage of 25V for 30 minutes. Samples were washed with dH2O and 70% ethanol before staining with SYBR Gold. Samples were then viewed using a fluorescence microscope. Acquired images were analyzed using OpenComet on ImageJ (NIH; RRID:SCR_003070).
Immunofluorescence
CHLA10 cells were seeded on glass coverslips in a 24-well plate at a density of 3.5×105 cells/well and allowed to settle overnight. Media was replaced with serum-free media for 24 hours, then cells were treated with DMSO or increasing concentrations of test compound for 24 hours. Cells were fixed with 4% paraformaldehyde for 30 minutes at room temperature and permeabilized with 0.5% Triton-X in PBS before probing with anti-phospho-histone H2AX (Ser 139) rabbit antibody (Cell Signaling Technology, catalog No. 2577; RRID:AB_2118010) overnight at 4°C. Cells on coverslips were then washed with PBS and probed with goat anti-rabbit IgG Alexa Fluor 488 (Abcam, catalog No. ab150077; RRID:AB_2630356) and mounted with VECTASHIELD antifade mounting medium with DAPI solution onto microscope slides. Cells were then visualized on a confocal microscope (Olympus FV3000). Acquired images were analyzed by quantifying the foci using ImageJ (NIH).
Western blot analysis
Cells were plated in a six-well plate to 70% to 80% confluency. After allowing cells to settle overnight, media was replaced with serum-free media for 24 hours. Cells were treated with DMSO or increasing concentrations of test compound for 24 hours. Cells were harvested in RIPA lysis buffer combined with protease and phosphatase inhibitors. Protein yield was assessed using Pierce BCA Protein Assay Kit (Thermofisher, catalog No. 23225) and quantified using a spectrophotometer plate reader (TECAN) at 562 nm. A total of 20-μg protein extracts were loaded per well in 4% to 15% Mini-PROTEAN TGX Precast Protein gels (Bio-Rad, catalog No. 4561084). After electrophoresis, proteins were transferred onto a 0.2 μmol/L nitrocellulose membrane. The membrane was blocked with LICOR Odyssey Blocking Buffer in PBS. After blocking, the membrane was incubated with anti–phospho-histone H2AX (Ser 139) rabbit antibody (Cell Signaling Technology, catalog No. 2577; RRID:AB_2118010) and H2AX rabbit antibody (Abcam, catalog No. ab11175; RRID:AB_297814) at 4°C overnight and incubated with donkey anti-rabbit IRDye800CW secondary antibody (LI-COR, catalog No. 926–32213; RRID:AB_621848) for 1 hour at room temperature. The membrane was washed with 1X TBS with 1% tween-20 (TBS-T) before being scanned with an Odyssey scanner (LI-COR).
Spheroid formation assay
CHLA10-tdTomato or TC32-tdTomato cells (2,500 per well) were added to a 96-well clear round bottom ultralow attachment microplate (Corning, catalog No. 7007) and allowed to form spheroids for 24 hours or until they reached 200 to 300 μm in diameter. Medium containing DMSO or an increasing concentration of test compound was added to each well, and spheroid growth was monitored for 4 days posttreatment using the IncuCyte Spheroid Analysis system (Sartorius). Images from day 0 and day 4 posttreatment were analyzed using the IncuCyte Spheroid Analysis software module. Day 4 values were normalized using a normalization factor from day 0 values, and EC50 values were calculated using a four-parameter variable slope nonlinear regression in GraphPad Prism 8 (GraphPad Software Inc.). The mean EC50 value ± SD was calculated using three biological replicates.
Pulmonary metastasis assay
Procedures involving mice were approved by a local animal care committee, University of British Columbia. tdTomato-expressing TC32 and A673 cells (1 × 106 cells/100 μL saline) were injected into the tail vein of 6- to 8-week-old immune-compromised NSG female mice (Jax Laboratories). After injection, mice were euthanized via isoflurane and CO2 asphyxiation according to local animal care standard operating procedures. Lungs were insufflated via gravity perfusion with a prewarmed (37°C) 1:1 mixture of fully supplemented PneumaCult-ALI media (STEMCELL Technologies, catalog No. 05001) and 1.2% low-melting point agarose (Lonza) as previously described (28). The pluck (heart and lungs) was carefully removed and placed in ice-cold PBS (supplemented with 1X penicillin/streptomycin) for 20 minutes to allow for agarose solidification. Small lung slices (∼2 mm x 4 mm) were obtained by manual cutting with sterile surgical scissors, and five to 12 slices per condition were chosen for serial imaging at 0 and 14 days postinjection/-treatment. Lung slices were maintained in vitro on gelatine sponges partially submerged in 2-mL PneumaCult media ± compound in a six-well plate; media ± compound was refreshed every 3 days. On the day of imaging, lung slices from each group were transferred to a small 35-mm Petri dish with a glass coverslip bottom (IBIDI) to permit aseptic widefield fluorescence imaging. The lung slices were imaged on an inverted Zeiss Observer.Z1 Colibri microscope using 2.5X objective. Lung tumor burden (% tumor burden) for a lung slice is calculated as the quotient of the summed area of tdTomato lesions and total area of each lung slice, multiplied by 100, as previously described (29). ImageJ software was used for image processing. This calculation was performed for all lung slices (n = 5–12 lung slices) per experimental group. Average values of percent lung tumor burden per group were compared and analyzed in Graph Prism 8 (GraphPad Software Inc.).
IHC and histopathology
Formalin-fixed, paraffin-embedded pulmonary metastasis assay (PuMA) lung tissue sections were freshly cut and analyzed for CD99 immunoexpression using Ventana Discovery Ultra autostainer (Ventana Medical Systems, Tucson, Arizona). In brief, baked and deparaffinized tissue sections were incubated in Tris-based buffer (CC1, Ventana) at 95°C for 64 minutes to retrieve antigenicity, followed by incubation with anti-CD99 rabbit polyclonal antibody (Abcam, catalog No. ab27271; RRID:AB_470945) at room temperature for 1 hour. Bound primary antibody was visualized with the UltraMap DAB anti-Rb Detection Kit (Ventana). All stained slides were digitized with Leica scanner (Aperio AT2, Leica Microsystems; Concord, Ontario, Canada) at magnification equivalent to 40X. The images were subsequently stored in the Aperio eSlide Manager (Leica Microsystems) at the Vancouver Prostate Centre. The IHC positive areas were reviewed by a research pathologist (H.Z. Oo), along with their corresponding hematoxylin and eosin (H&E) sections to confirm the presence of Ewing sarcoma cells.
Statistical analysis
Data are presented as mean ± SD. Statistical analysis for cell-cycle profiling, comet assay, spheroid assay, and PuMA assay were performed using GraphPad Prism 8.0 (GraphPad Software, Inc.). Statistical analysis of the cell-cycle profiles was done using a multiple t test and was only performed on the profiles that displayed a change in cell-cycle profile greater than 5%, as these were considered to be biologically relevant changes. Statistics for the comet assay were determined using a Mann–Whitney nonparametric test. Statistical analysis of the spheroid assay was analyzed using unpaired t test, and PuMA assay was done using Kruskal–Wallis nonparametric test (n.s. = not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
Data availability statement
The data generated in this study are available upon request from the corresponding author.
Results
A bispecific small molecule with dual activity against PARP1/2 and HDAC enzymes
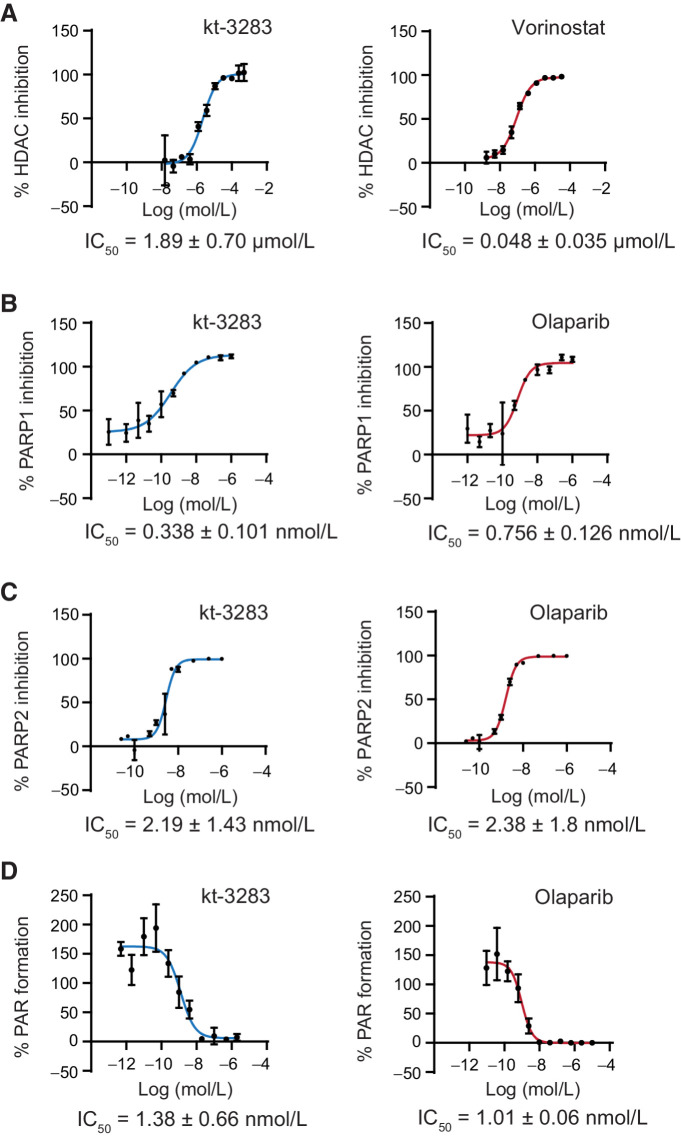
Through medicinal chemistry cycles, we developed a small-molecule inhibitor prototype (kt-3283) with dual activity against PARP1/2 and HDACs. In vitro activity assay kits were used to determine inhibition of PARP1, PARP2, and HDAC by kt-3283 compared with the FDA-approved PARPi olaparib and the HDAC inhibitor vorinostat. A wide concentration range of each compound was used to determine IC50 values. kt-3283 had an IC50 value of 1.89 μmol/L for inhibition of HDACs, while vorinostat was about 40-fold lower at 0.05 μmol/L (Fig. 1A; Supplementary Fig. S1A). The PARP1 and PARP2 inhibitory activities of kt-3283 were comparable with olaparib, with IC50 values for kt-3283 at 0.338 nmol/L and 2.19 nmol/L, respectively (Fig. 1B and C; Supplementary Fig. S1B and S1C). To further validate the ability of kt-3283 to inhibit PARP1/2 activity, we used a cellular PAR synthesis assay to determine the level of PAR formation. Comparable with olaparib, we determined an IC50 of 1.38 nmol/L for the inhibition of PAR formation in cells treated with kt-3283 (Fig. 1D; Supplementary Fig. S1D). These data indicate that kt-3283 is able to inhibit both PARP1/2 and HDAC enzymes.
Figure 1.
Characterization of a bispecific small molecule with dual activity against PARP1/2 and HDAC enzymes. A, In vitro HDAC activity in HeLa nuclear extracts treated with kt-3283 or vorinostat. B, Recombinant PARP1 activity in vitro after treatment with kt-3283 and olaparib. C, Recombinant PARP2 activity in vitro after treatment with kt-3283 and olaparib. D, PAR formation in CHLA10 cells treated with kt-3283 and olaparib. Values were normalized to control, and IC50 was calculated as the concentration required to produce 50% inhibition of activity from nonlinear regression plots using GraphPad Prism8 software. Data shown are the mean values of n = 3 replicates with representative graphs.
Ewing sarcoma cells are highly sensitive to dual PARP1/2 and HDAC inhibition
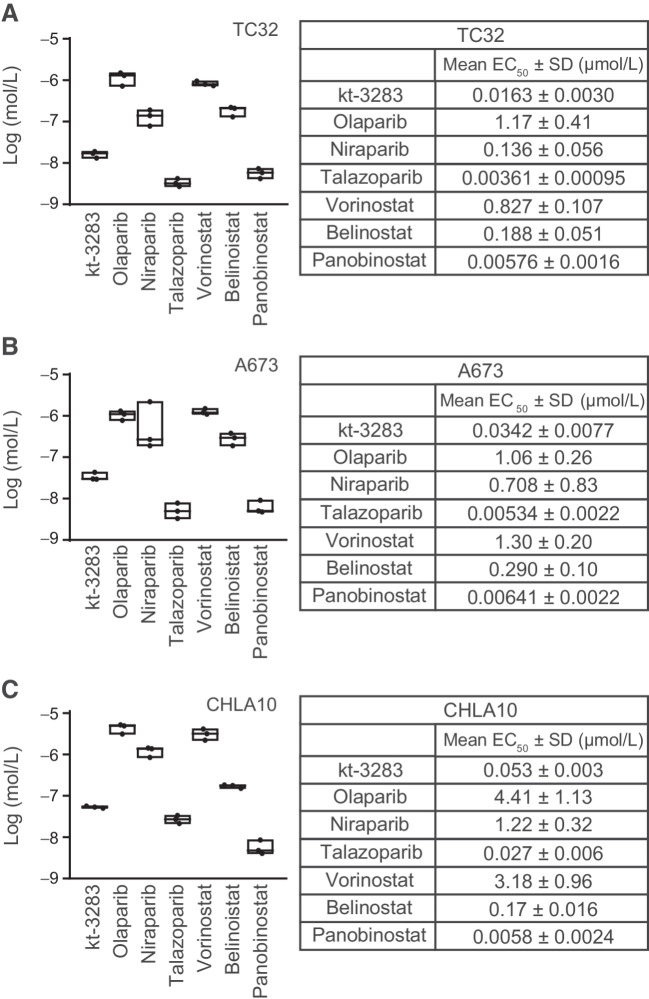
To investigate the effect of kt-3283 on cell growth, we performed cell viability assays in three Ewing sarcoma cell lines, TC32, A673, and CHLA10. Using an IncuCyte S3 live cell imaging system to evaluate cell viability, we determined the EC50 values after 3-day treatment with increasing concentrations of kt-3283 or clinically relevant PARP or HDAC inhibitors in TC32 and A673 cells. These include three FDA-approved PARPi, olaparib, niraparib, and talazoparib, and three HDAC inhibitors, vorinostat, belinostat and panobinostat, two of which are currently FDA approved. kt-3283 demonstrated higher efficacy in suppression of cell viability than olaparib, niraparib, vorinostat, and belinostat with an EC50 of 0.0163 μmol/L in TC32 cells (Fig. 2A; Supplementary Fig. S2A). We also detected a similar effect of kt-3283 in A673 cells with a much lower EC50 value of 0.0342 μmol/L compared with olaparib, niraparib, vorinostat, or belinostat treatment alone (Fig. 2B; Supplementary Fig. S2B). However, treatment with talazoparib or panobinostat showed a more potent inhibitory effect in both cell lines compared with kt-3283, although all values are in the lower nmol/L range (Fig. 2A and B; Supplementary Fig. S2A and S2B). To further validate our findings, we also performed CellTiter-Glo viability assay to determine the EC50 values of these tested compounds after 10 days of treatment in CHLA10 cells. Consistent with the IncuCyte assay, the EC50 value (0.053 μmol/L) of kt-3283 treatment in CHLA10 cells was also much lower than olaparib, niraparib, vorinostat, and belinostat (Fig. 2C; Supplementary Fig. S2C). Cell viability assays of CHLA10 cells by Incucyte S3 (3-day treatment) and CellTiter-Glo (10-day treatment) methods were found to be comparable (Supplementary Fig. S2D). Taken together, our data demonstrate a potent inhibitory effect of kt-3283 in Ewing sarcoma cells compared with most FDA-approved PARP or HDAC inhibitors.
Figure 2.
Ewing sarcoma cells are highly sensitive to dual PARP1/2 and HDAC inhibition. A, In TC32 cells, cell viability was examined by IncuCyte S3 live cell imaging system following 3-day treatments with increasing concentrations of indicated compounds. EC50 values were calculated as the concentration required for 50% cell viability. n = 3. B, EC50 values of tested compounds were also determined in A673 cells using the same experimental condition as in (A). C, EC50 values of indicated inhibitors were determined using CellTiter-Glo cell viability assays in CHLA10 cells. Cells were exposed to 10-day treatments of increasing concentrations of the inhibitors, and the EC50 values were calculated as the concentration required for 50% cell viability. n = 3.
kt-3283 induces S and G2/M cell-cycle arrest in Ewing sarcoma cells
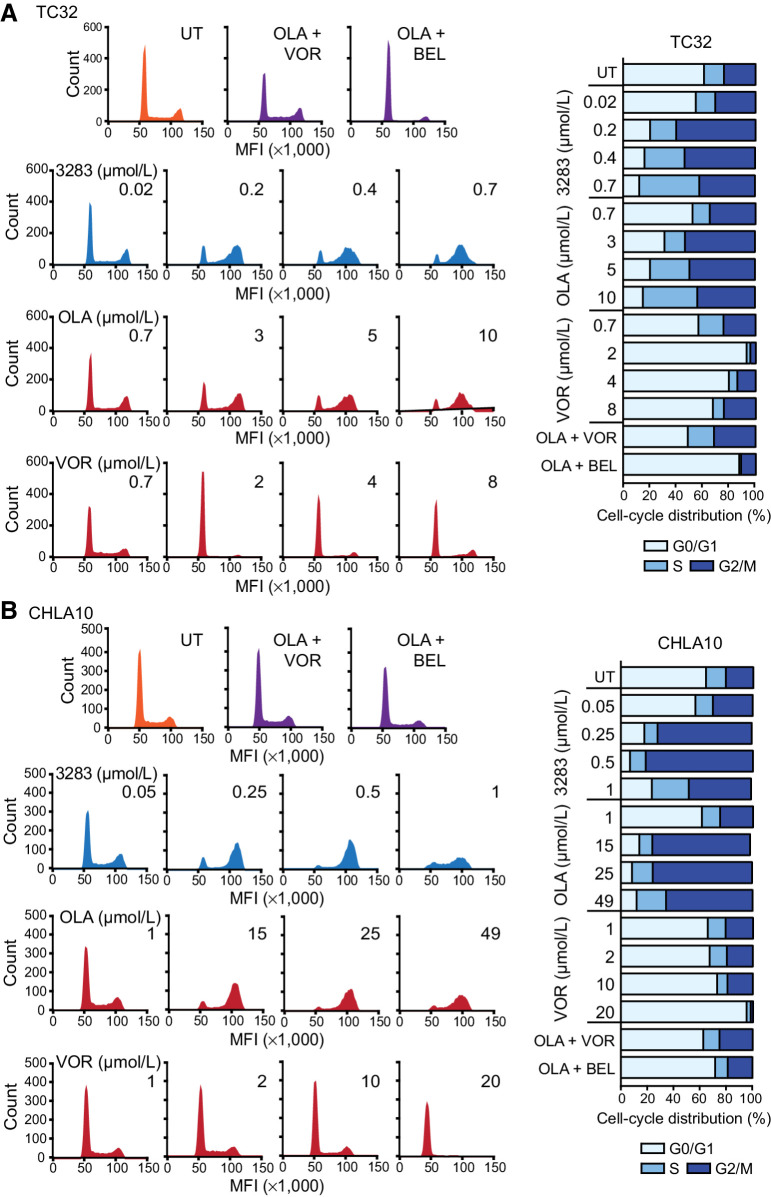
PARPi and HDAC inhibitors constantly induce S-G2/M and G0/G1 cell-cycle arrest, respectively as PARP regulates replication fork progression and HDAC play a major role in regulating the expression of cell-cycle checkpoint proteins including cyclin-dependent kinases, cyclin D1 and p21 (30). Here, we examined the cell-cycle profiles of CHLA10 and TC32 cells upon treatment with kt-3283 (3283), olaparib (OLA), and vorinostat (VOR) in both single and combination regimens. Serum-starved cells were treated with increasing concentrations of kt-3283 in complete medium for 24 or 48 hours, and displayed strong S and G2/M arrest at and above 0.2 μmol/L in TC32 and 0.25 μmol/L in CHLA10 cells. Similar cell-cycle arrest was only observed with olaparib treatment at concentrations higher than 3 μmol/L in TC32 and 15 μmol/L in CHLA10, respectively (Fig. 3A and B; Supplementary Fig. S3A–S3C). Combination treatment with olaparib and vorinostat/belinostat (BEL) at equimolar concentrations of kt-3283 (0.7 μmol/L and 1 μmol/L for TC32 and CHLA10 cells, respectively) had almost no influence on cell-cycle phases compared with control (Fig. 3A and B; Supplementary Fig. S3A–S3C). These data demonstrate kt-3283 has stronger potency to induce S and G2/M cell-cycle arrest than olaparib alone or in combination with vorinostat or belinostat in Ewing sarcoma cells.
Figure 3.
kt-3283 induces S and G2/M cell-cycle arrest in Ewing sarcoma cells. A, TC32 cells were synchronized at G0/G1 phase by serum starvation for 24 hours before treatments with kt-3283, olaparib, or vorinostat as indicated in complete medium for 48 hours. Then, cell-cycle profiles were examined by PI staining followed by flow cytometric analysis as described in Materials and Methods. B, Cell-cycle analysis was also performed in CHLA10 cells treated with kt-3283, olaparib, or vorinostat as indicated for 24 hours using the same experimental procedures as in (A).
kt-3283 treatment induces DNA damage in Ewing sarcoma cells
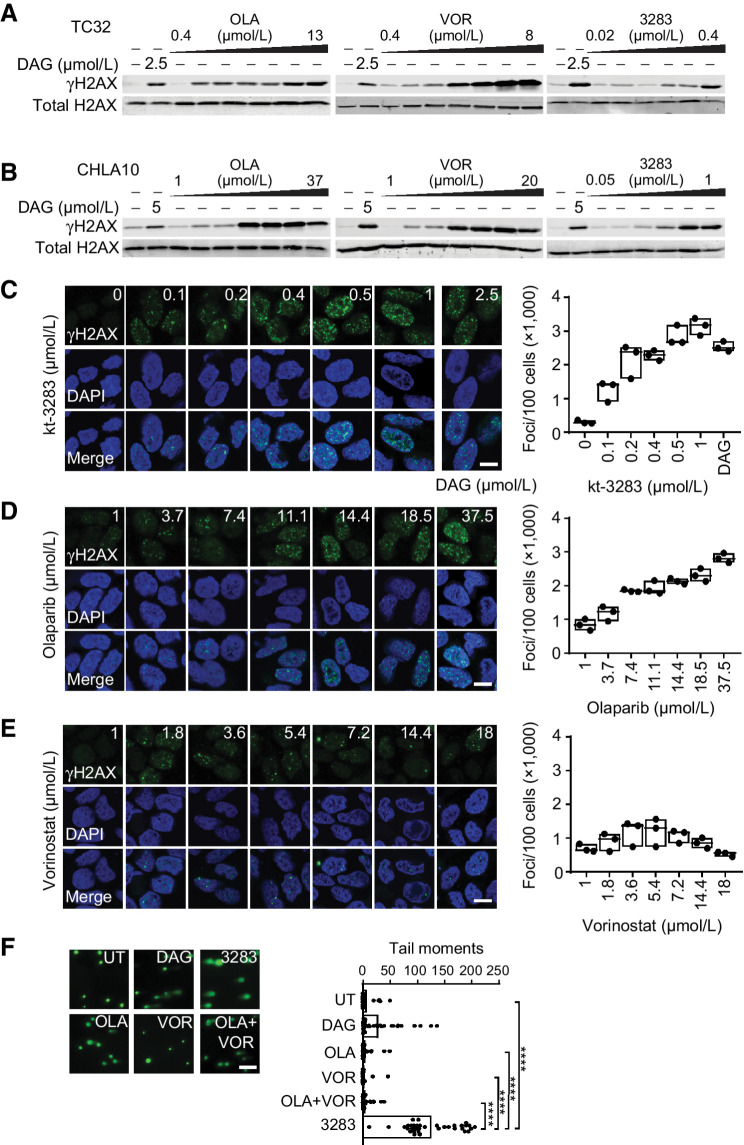
PARPi and HDAC inhibitors have been reported to induce DNA damage in cells (31). We investigated the effect of kt-3283 on DNA damage compared with olaparib and vorinostat treatment in Ewing sarcoma cells using Western blotting, immunofluorescence, and comet assay. Phosphorylated histone variant H2AX (γH2AX) is a surrogate marker for DSB in DNA (32). Dianhydrogalactitol (DAG) was included as a positive control because previous studies in our group showed that DAG induces replication-dependent DNA damage in a variety of cancer cell lines (33). Treatment with kt-3283, olaparib, or vorinostat induced γH2AX expression in a dose-dependent manner in both TC32 and CHLA10 cells. In comparison to olaparib and vorinostat, kt-3283 was able to induce γH2AX expression at a much lower concentration range (Fig. 4A and B; Supplementary Fig. S4A and S4B). Moreover, CHLA10 cells treated with kt-3283 or olaparib also showed dose-dependent γH2AX foci formation in immunofluorescence followed by confocal microscopy imaging with kt-3283 at a much lower concentration range (Fig. 4C and D; Supplementary Fig. S4C and S4D). However, vorinostat that induced G0/G1 cell-cycle arrest (Fig. 3A and B; Supplementary Fig. S3A and S3B) demonstrated milder DNA damage foci formation in CHLA10 cells (Fig. 4E; Supplementary Fig. S4E). In addition, TC32 cells only showed increased γH2AX expression by Western blotting upon treatment with 0.35 μmol/L of kt-3283 but not with 0.35 μmol/L of olaparib, vorinostat, or olaparib + vorinostat (Supplementary Fig. S4G). This observation was also confirmed using equimolar concentrations of these compounds in CHLA10 cells by Western blotting and immunofluorescence (Supplementary Fig. S4H and S4I). To further consolidate our data, we also employed alkaline comet assay, as it can detect both SSB and DSB in cells. There was significant DNA damage in CHLA10 cells treated with 1 μmol/L kt-3283 but not with 1 μmol/L olaparib or vorinostat or 1 μmol/L olaparib + 1 μmol/L vorinostat (Fig. 4F; Supplementary Fig. S4F). In summary, our data show kt-3283 is able to induce DNA damage in Ewing sarcoma cells at a much lower concentration range than olaparib or vorinostat.
Figure 4.
kt-3283 treatment induces DNA damage in Ewing sarcoma cells. A, TC32 cells treated with 2.5 μmol/L DAG or increasing doses of olaparib (0.4–13 μmol/L), vorinostat (0.4–8 μmol/L), or kt-3283 (0.02–0.4 μmol/L) for 24 hours and analyzed for γH2AX expression by Western blotting. B, CHLA10 cells treated with 5 μmol/L DAG or increasing doses of olaparib (1–37 μmol/L), vorinostat (1–20 μmol/L), or kt-3283 (0.05–1 μmol/L) for 24 hours and analyzed as in A. C–E, CHLA10 cells treated with DAG (2.5 μmol/L) or increasing doses of kt-3283 (0–1 μmol/L) (C), olaparib (1–37.5 μmol/L) (D), or vorinostat (1–18 μmol/L) (E) for 24 hours were analyzed for γH2AX foci by immunofluorescence and confocal microscopy imaging. Scale bar represents 10 μm. F, CHLA10 cells treated with 1 μmol/L kt-3283, olaparib, vorinostat, or olaparib + vorinostat followed by comet assay as described in “Materials and Methods.” 5 μmol/L DAG was included as positive control. Scale bar represents 200 μm. ****, P < 0.0001.
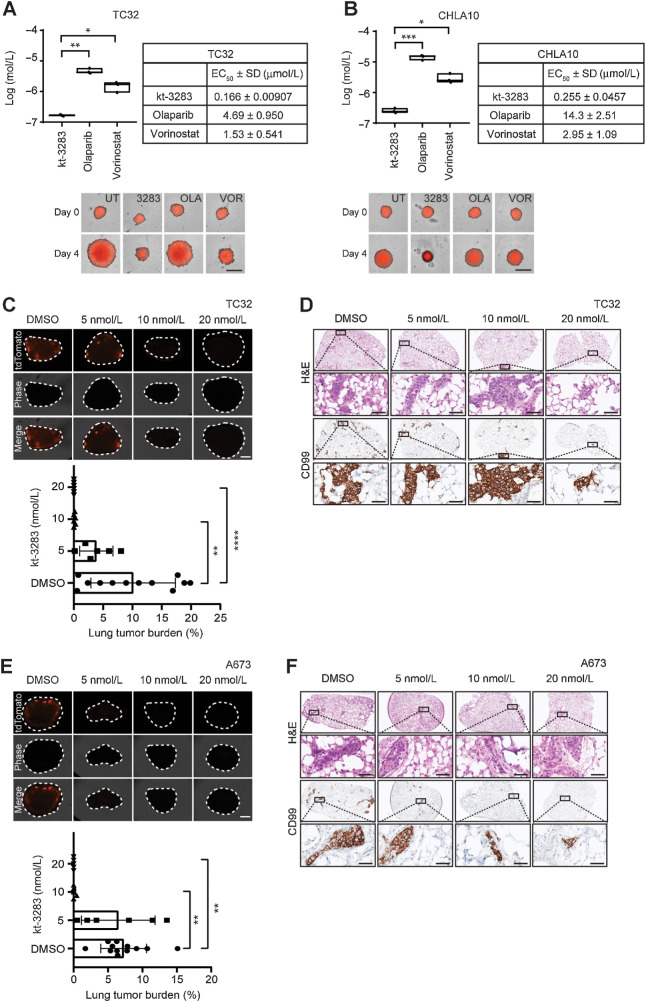
kt-3283 inhibits three-dimensional spheroid growth and metastasis of Ewing sarcoma cells
Spheroids are three-dimensional (3D) cell aggregates that can mimic tumor behavior more accurately compared with two-dimensional cell cultures (34). To further validate our data, we investigated the effect of kt-3283 on the 3D spheroid model with Ewing sarcoma cells. TC32 and CHLA10 spheroids were established to 200 to 300 μm followed by treatment with increasing concentrations of kt-3283, olaparib, or vorinostat. The growth of the spheroids was monitored and quantified for 4 days using the IncuCyte S3 imaging system. The EC50 values of kt-3283 in suppression of spheroid growth were much lower than olaparib and vorinostat in both TC32 and CHLA10 models (Fig. 5A and B; Supplementary Fig. S5A and S5B). Spheroid assays with both TC32 and CHLA10 cells demonstrate equivalent activity of kt-3283 and talazoparib but 10 times lower efficacy of panobinostat compared with kt-3283 (Supplementary Fig. S5C and S5D). We also examined the effect of kt-3283 on metastatic growth of Ewing sarcoma cells in an ex vivo PuMA. Here, colonization of td-Tomato expressing TC32 in mouse lungs was inhibited by kt-3283 at concentrations as low as 10 nmol/L (Fig. 5C). Parallel H&E and CD99 IHC staining (Fig. 5D) confirmed that the tdTomato fluorescent signals in the lung tissues were Ewing sarcoma cells. Similarly, kt-3283 inhibited colonization of td-Tomato expressing A673 cells at concentrations as low as 10 nmol/L (Fig. 5E and F). Fluorescence per cell of both td-Tomato expressing TC32 and A673 cells were confirmed not to be affected by treatment with kt-3283 by fluorescence microscopy (Supplementary Fig. S5E and S5F). A pilot study in nude mice showed no sign of toxicity as indicated by weight and blood cell counts after intraperitoneal treatment with 30 mg/kg kt-3283 (twice a day) for 4 days (Supplementary Table S1). These data suggest that kt-3283 is a potent inhibitor of Ewing sarcoma lung metastasis and that it is more effective than olaparib or vorinostat alone in inhibiting 3D growth in Ewing sarcoma spheroids.
Figure 5.
kt-3283 inhibits 3D spheroid growth and metastasis of Ewing sarcoma cells. A, TC32 spheroid growth following 4 days of treatment with increasing concentrations of kt-3283, olaparib, or vorinostat was monitored using the IncuCyte Spheroid Analysis system. The EC50 values were calculated as the concentration required for 50% inhibition of growth from nonlinear regression plots using GraphPad Prism8 software. Representative images of TC32 spheroids at day 0 and day 4 with DMSO, 1 μmol/L kt-3283, 1 μmol/L olaparib, or 1 μmol/L vorinostat are shown with scale bars representing 400 μm. *, P < 0.05; **, P < 0.01. B, CHLA10 spheroid growth following 4-day treatment with increasing concentrations of kt-3283, olaparib, or vorinostat was also examined using the same experimental procedures as mentioned in A. Representative images of CHLA10 3D spheroids at day 0 and day 4 with DMSO, 1 μmol/L kt-3283, 1 μmol/L olaparib, or 1 μmol/L vorinostat are shown with scale bars representing 400 μm. *, P < 0.05; ***, P < 0.001. C, Lung tumor burden following 14 days of treatment with vehicle, 5,10, or 20 nmol/L of kt-3283. n = 5–12. Representative fluorescence images of tdTomato TC32 cells in lung slices following 14 days of treatment with 5, 10, or 20 nmol/L of kt-3283. Scale bar represents 1 mm. **, P < 0.01; ****, P < 0.0001. D, Representative H&E and CD99 staining images of TC32 Ewing sarcoma cells in PuMA lung sections following 14 days of treatment with 5, 10, or 20 nmol/L of kt-3283. The respective zoomed images from the insets (top) are shown below each image. Scale bar represents 50 μm. E, Lung tumor burden following 14 days of treatment with vehicle, 5,10 or 20 nmol/L of kt-3283. n = 5–12. Representative fluorescence images of tdTomato A673 cells in lung slices following 14 days of treatment with 5, 10, or 20 nmol/L of kt-3283. Scale bar represents 1 mm. **, P < 0.01. F, Representative H&E and CD99 staining images of A673 Ewing sarcoma cells in PuMA lung sections following 14 days of treatment with 5, 10 or 20 nmol/L of kt-3283. The respective zoomed images from the insets (top) are shown below each image. Scale bar represents 50 μm.
Discussion
Pharmacologic “BRCAness” can potentially offer a path to take PARP inhibition beyond the BRCA1/2 mutation space and counter potential resistance to PARPi therapy. Epigenetic modifiers such as HDAC as well as DNA and histone methyltransferases are attractive targets for induced “BRCAness” in BRCA1/2 proficient cancer scenarios (14, 16, 35–39). At present, four clinical trials using a PARPi in combination with the HDACi vorinostat (NCT03259503 and NCT03742245), the DNA methyltransferase inhibitor decitabine (NCT02878785), and the EZH2 histone methyltransferase inhibitor SHR2554 (NCT04355858), are currently ongoing.
Previously, the FDA had approved three pan-HDACi drugs (vorinostat, belinostat, and panobinostat) and one HDAC1/2-selective HDACi (romidepsin) for treatment of hematologic cancers (40). Panobinostat, however, had its FDA approval revoked in 2022 for the treatment of multiple myeloma (Docket No. FDA-2022-N-0352). Histone acetylation attenuates chromatin structure and plays a critical role in recognition and repair of DNA lesions (41). HDACi-induced downregulation of key HR proteins including BRCA1, BRCA2, and RAD51 has been established in a variety of cancer types, and HDACi treatment sensitizes cancer cells to PARPi (14–17, 39, 42). This corroborative activity of HDAC and PARP inhibition is particularly interesting in the context of HR-proficient cancer types where PARPi therapy has limited effect on its own, such as Ewing sarcoma. However, dose-limiting toxicity with HDACi therapy is not uncommon in solid tumors (e.g., breast cancer and sarcomas) and has been preventing some therapeutic effects as stand-alone and in treatment combinations (43, 44). The HDACi ingredient must be carefully adjusted to prevent over-lapping toxicity events arising from the combination with other therapeutic moieties, which can be challenging when working with different pharmacokinetics profiles.
We have characterized a novel bifunctional PARP-HDAC single-molecule inhibitor, kt-3283, in Ewing sarcoma models to evaluate the potential benefit of combined PARP-HDAC inhibition over stand-alone PARPi or HDACi treatments. TC32, A673, and CHLA10 cell lines were chosen as they are commonly used and well-validated Ewing sarcoma cell models. TC32 cells express mutated STAG2 (45), TC32, and A673 are p16/p14 null, and CHLA10 and A673 cells both express nonfunctional p53 protein, all of which may contribute to their responsiveness to PARPi (46). The design of kt-3283 was made with chemical inspiration from olaparib and vorinostat as the most well-studied PARP and HDAC inhibitors currently FDA approved. Olaparib and vorinostat were used as the main comparators against kt-3283 for this reason. kt-3283 has similar PARPi activity as olaparib and slightly lower HDACi activity than vorinostat. However, the dual activity of kt-3283 was 30 to 80 times more cytotoxic to Ewing sarcoma cells than olaparib, and 30 to 60 times more cytotoxic than vorinostat alone. While panobinostat appeared to have higher efficacy in Ewing sarcoma cell lines, this can be attributed to the toxicity of panobinostat, as seen in clinical trials when dose-limiting toxicity has restricted effective use in solid tumors (47). This is likely also the case with talazoparib. While talazoparib is the most potent FDA-approved PARPi to date, it also showed clinical toxicities more similar to other chemotherapeutic agents than the other approved PARPi, including anemia, thrombocytopenia, and neutropenia (48).
kt-3283 also induces cell-cycle arrest and DNA damage in Ewing sarcoma cells in much lower concentrations than olaparib and vorinostat. The cell-cycle arrest pattern of kt-3283 was more similar to that of olaparib than the combination treatments, as the PARPi activity of kt-3283 was stronger than the HDAC inhibitor portion of the drug. In TC32 cells, the combination of 0.7 μmol/L olaparib and 0.7 μmol/L belinostat showed some arrest in G0/G1, consistent with the lower EC50 value for TC32 cells treated with belinostat. When compared in 3D spheroid models, kt-3283 showed efficacy at 30 to 40 times lower concentrations than olaparib and at five to 10 times lower concentrations than vorinostat. Spheroid models treated with talazoparib indicated equivalent efficacy to kt-3283 but 10 times lower efficacy by panobinostat compared with kt-3283. This may be attributed to the overall toxicity of panobinostat as previously mentioned. kt-3283 also hinders metastatic growth of Ewing sarcoma cells in an ex vivo PuMA model, with a strong inhibitory effect using as little as 10 nmol/L of inhibitor. While the PuMA model does not fully capture the entire metastatic process, it measures both seeding and growth of cancer cells in the lung. As hematologic toxicity of PARP and HDAC inhibitors is of concern, a pilot study of kt-3283 performed in mice showed no evidence of toxicity based on weight loss and blood cell counts.
Combination therapies can work in a synergistic or additive manner by simultaneously targeting different pathways in cells. Unfortunately, combination therapies that include chemotherapeutic agents can be toxic to patients and often have to be administered sequentially in clinical settings, sometimes with reduced biological efficacy. Given the possibility for toxicities with PARPi and HDACi monotherapies and the likelihood of additive toxicities from sequential administration of combination therapies, it is important to develop drugs with better tumor targeting capabilities to hopefully mitigate adverse effects, such as single-molecule dual function agents. This offers a powerful rationale for the development of a dual-activity small molecule such as kt-3283. Importantly, the clinical combination of a dual-activity small molecule with other therapeutic modalities is also an intriguing option that could be further explored in the future.
Combining PARP and HDAC inhibition into one single molecule may also offer a convenient way to prevent resistance to PARPi therapy. For example, Ewing sarcoma and many other solid tumor indications epigenetically suppress expression of the tumor suppressor gene Schlafen 11 (SLFN11), which leads to resistance to DNA damage-inducing agents, including PARPi therapy (49). It is important to note that HDACi treatment prompts reexpression of SLFN11 and resensitization to PARPi (50). In summary, our work provides preclinical justification for studying a novel single-molecule PARP-HDAC inhibitor in Ewing sarcoma with improved cytotoxicity and DNA damage activity as compared with PARPi and HDACi alone. This concept will likely be relevant in cancer indications beyond Ewing sarcoma and potentially offers an opportunity to suppress therapeutic resistance.
Supplementary Material
Replicate graphs of HDAC, PARP1, PARP2 and PARylation assays
Replicate graphs of cell viability assays and comparison of cell viability assay methods
Replicates of cell cycle assays and statistics
Replicate data of DNA damage assays
Replicate graphs of spheroid assays and cell fluorescence data
Blood cell panel counts and weight loss percentage of in vivo toxicity study
Materials and methods for in vivo toxicity and cellular fluorescence assays
Acknowledgments
This work was supported by a Robert J. Arceci Innovation Award from St. Baldrick's Foundation, a St. Baldrick's Foundation/Martha's Better Ewing Sarcoma Treatment (BEST) grant (No. 663113), and an Accelerate grant awarded to L. Ramos by the Mathematics of Information Technology and Complex Systems (Mitacs) and Rakovina Therapeutics Inc. This project was also supported in part by advisory services and research and development funding from the National Research Council of Canada Industrial Research Assistance Program (NRC IRAP).
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
L. Ramos reports other support from Rakovina Therapeutics during the conduct of the study and other support from Rakovina Therapeutics outside the submitted work. S. Truong reports other support from Rakovina Therapeutics during the conduct of the study and other support from Rakovina Therapeutics outside the submitted work. B. Zhai reports grants from St. Baldrick's Foundation, Mathematics of Information Technology and Complex Systems (Mitacs), Rakovina Therapeutics Inc., and the National Research Council of Canada Industrial Research Assistance Program (NRC IRAP) during the conduct of the study. J. Joshi reports other support from Rakovina Therapeutics during the conduct of the study and other support from Rakovina Therapeutics outside the submitted work. F. Ghaidi reports other support from Rakovina Therapeutics during the conduct of the study and other support from Rakovina Therapeutics outside the submitted work. H. Adomat reports other support from Vancouver Prostate Centre during the conduct of the study. J. Bacha reports personal fees from Rakovina Therapeutics Inc. during the conduct of the study, personal fees from Rakovina Therapeutics Inc. outside the submitted work, and has a pending U.S. provisional patent assigned to and owned by Rakovina Therapeutics Inc. J. Langlands reports personal fees from Rakovina Therapeutics outside the submitted work and is a consultant to Rakovina Therapeutics Inc. M. Daugaard reports grants and other support from Rakovina Therapeutics during the conduct of the study, other support from Rakovina Therapeutics outside the submitted work, and has a patent for Next-Generation PARP Inhibitors pending. No disclosures were reported by the other authors.
Authors' Contributions
L. Ramos: Data curation, formal analysis, validation, investigation, visualization, methodology, writing–original draft, writing–review and editing. S. Truong: Conceptualization, data curation, formal analysis, supervision, validation, investigation, visualization, methodology, writing–original draft, project administration, writing–review and editing. B. Zhai: Conceptualization, supervision, validation, methodology, writing–review and editing. J. Joshi: Data curation, formal analysis, validation, investigation, visualization, methodology, writing–review and editing. F. Ghaidi: Data curation, formal analysis, validation, investigation, visualization, methodology, writing–review and editing. M.M. Lizardo: Data curation, formal analysis, supervision, validation, investigation, visualization, methodology, writing–original draft, project administration, writing–review and editing. T. Shyp: Data curation, formal analysis, validation, investigation, visualization, methodology, writing–review and editing. S.H.Y. Kung: Investigation, methodology. A.M. Rezakhanlou: Investigation, methodology. H.Z. Oo: Formal analysis, supervision, visualization, methodology, project administration. H. Adomat: Data curation, formal analysis, validation, investigation, visualization, writing–review and editing. S. Le Bihan: Resources, supervision, writing–review and editing. C. Collins: Resources, supervision. J. Bacha: Conceptualization, resources, supervision, funding acquisition. D. Brown: Conceptualization, resources, supervision, funding acquisition. J. Langlands: Conceptualization, resources, supervision, funding acquisition. W. Shen: Conceptualization, resources, funding acquisition. N. Lallous: Resources, supervision, methodology. P.H. Sorensen: Resources, supervision, funding acquisition, methodology. M. Daugaard: Conceptualization, resources, supervision, funding acquisition, methodology, writing–original draft, writing–review and editing.
References
- 1. Bock FJ, Chang P. New directions in poly(ADP-ribose) polymerase biology. FEBS J 2016;283:4017–31. [DOI] [PubMed] [Google Scholar]
- 2. Curtin NJ, Szabo C. Poly(ADP-ribose) polymerase inhibition: past, present, and future. Nat Rev Drug Discov 2020;19:711–36. [DOI] [PubMed] [Google Scholar]
- 3. Caldecott KW. Single-strand break repair and genetic disease. Nat Rev Genet 2008;9:619–31. [DOI] [PubMed] [Google Scholar]
- 4. Caldecott KW. Protein ADP-ribosylation and the cellular response to DNA strand breaks. DNA Repair 2014;19:108–13. [DOI] [PubMed] [Google Scholar]
- 5. McKinnon PJ, Caldecott KW. DNA strand break repair and human genetic disease. Annu Rev Genomics Hum Genet 2007;8:37–55. [DOI] [PubMed] [Google Scholar]
- 6. Maxwell KN, Domchek SM. Cancer treatment according to BRCA1 and BRCA2 mutations. Nat Rev Clin Oncol 2012;9:520–8. [DOI] [PubMed] [Google Scholar]
- 7. de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med 2020;382:2091–102. [DOI] [PubMed] [Google Scholar]
- 8. Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005;434:917–21. [DOI] [PubMed] [Google Scholar]
- 9. Cai Z, Liu C, Chang C, Shen C, Yin Y, Yin X, et al. Comparative safety and tolerability of approved PARP inhibitors in cancer: a systematic review and network meta-analysis. Pharmacol Res 2021;172:105808. [DOI] [PubMed] [Google Scholar]
- 10. Yang H, Wei Y, Zhang Q, Yang Y, Bi X, Yang L, et al. CRISPR/Cas9-induced saturated mutagenesis identifies Rad51 haplotype as a marker of PARP inhibitor sensitivity in breast cancer. Mol Med Rep 2022;26:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abbotts R, Dellomo AJ, Rassool FV. Pharmacologic induction of BRCAness in BRCA-proficient cancers: expanding PARP inhibitor use. Cancers 2022;14:2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lord CJ, Ashworth A. BRCAness revisited. Nat Rev Cancer 2016;16:110–20. [DOI] [PubMed] [Google Scholar]
- 13. Valdez BC, Nieto Y, Yuan B, Murray D, Andersson BS. HDAC inhibitors suppress protein poly(ADP-ribosyl)ation and DNA repair protein levels and phosphorylation status in hematologic cancer cells: implications for their use in combination with PARP inhibitors and chemotherapeutic drugs. Oncotarget 2022;13:1122–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wiegmans AP, Yap PY, Ward A, Lim YC, Khanna KK. Differences in expression of key DNA damage repair genes after epigenetic-induced BRCAness dictate synthetic lethality with PARP1 Inhibition. Mol Cancer Ther 2015;14:2321–31. [DOI] [PubMed] [Google Scholar]
- 15. Liang BY, Xiong M, Ji GB, Zhang EL, Zhang ZY, Dong KS, et al. Synergistic suppressive effect of PARP-1 inhibitor PJ34 and HDAC inhibitor SAHA on proliferation of liver cancer cells. J Huazhong Univ Sci Technolog Med Sci 2015;35:535–40. [DOI] [PubMed] [Google Scholar]
- 16. Rasmussen RD, Gajjar MK, Jensen KE, Hamerlik P. Enhanced efficacy of combined HDAC and PARP targeting in glioblastoma. Mol Oncol 2016;10:751–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yin L, Liu Y, Peng Y, Yu X, Gao Y, Yuan B, et al. PARP inhibitor veliparib and HDAC inhibitor SAHA synergistically cotarget the UHRF1/BRCA1 DNA damage repair complex in prostate cancer cells. J Exp Clin Cancer Res 2018;37:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baldan F, Mio C, Allegri L, Puppin C, Russo D, Filetti S, et al. Synergy between HDAC and PARP inhibitors on proliferation of a human anaplastic thyroid cancer-derived cell line. Int J Endocrinol 2015;2015:978371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zöllner SK, Amatruda JF, Bauer S, Collaud S, de Álava E, DuBois SG, et al. Ewing sarcoma-diagnosis, treatment, clinical challenges, and future perspectives. J Clin Med 2021;10:1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Balamuth NJ, Womer RB. Ewing's sarcoma. Lancet Oncol 2010;11:184–92. [DOI] [PubMed] [Google Scholar]
- 21. Garnett MJ, Edelman EJ, Heidorn SJ, Greenman CD, Dastur A, Lau KW, et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 2012;483:570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brenner JC, Ateeq B, Li Y, Yocum AK, Cao Q, Asangani IA, et al. Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer. Cancer Cell 2011;19:664–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gorthi A, Romero JC, Loranc E, Cao L, Lawrence LA, Goodale E, et al. EWS-FLI1 increases transcription to cause R-loops and block BRCA1 repair in Ewing sarcoma. Nature 2018;555:387–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brohl AS, Patidar R, Turner CE, Wen X, Song YK, Wei JS, et al. Frequent inactivating germline mutations in DNA repair genes in patients with Ewing sarcoma. Genet Med 2017;19:955–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Choy E, Butrynski JE, Harmon DC, Morgan JA, George S, Wagner AJ, et al. Phase II study of olaparib in patients with refractory Ewing sarcoma following failure of standard chemotherapy. BMC Cancer 2014;14:813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sakimura R, Tanaka K, Nakatani F, Matsunobu T, Li X, Hanada M, et al. Antitumor effects of histone deacetylase inhibitor on Ewing's family tumors. Int J Cancer 2005;116:784–92. [DOI] [PubMed] [Google Scholar]
- 27. Schmidt O, Nehls N, Prexler C, von Heyking K, Groll T, Pardon K, et al. Class I histone deacetylases (HDAC) critically contribute to Ewing sarcoma pathogenesis. J Exp Clin Cancer Res 2021;40:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scopim-Ribeiro R, Lizardo MM, Zhang HF, Dhez AC, Hughes CS, Sorensen PH. NSG mice facilitate ex vivo characterization of Ewing sarcoma lung metastasis using the PuMA model. Front Oncol 2021;11:645757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lizardo MM, Sorensen PH. Practical considerations in studying metastatic lung colonization in osteosarcoma using the pulmonary metastasis assay. J Vis Exp 2018:56332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ramaiah MJ, Tangutur AD, Manyam RR. Epigenetic modulation and understanding of HDAC inhibitors in cancer therapy. Life Sci 2021;277:119504. [DOI] [PubMed] [Google Scholar]
- 31. Robert C, Rassool FV. HDAC inhibitors: roles of DNA damage and repair. Adv Cancer Res 2012;116:87–129. [DOI] [PubMed] [Google Scholar]
- 32. Rahmanian N, Shokrzadeh M, Eskandani M. Recent advances in γH2AX biomarker-based genotoxicity assays: a marker of DNA damage and repair. DNA Repair 2021;108:103243. [DOI] [PubMed] [Google Scholar]
- 33. Zhai B, Li Y, Kotapalli SS, Bacha J, Brown D, Steinø A, et al. Dianhydrogalactitol synergizes with topoisomerase poisons to overcome DNA repair activity in tumor cells. Cell Death Dis 2020;11:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jubelin C, Muñoz-Garcia J, Griscom L, Cochonneau D, Ollivier E, Heymann MF, et al. Three-dimensional in vitro culture models in oncology research. Cell Biosci 2022;12:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jones PA, Issa JP, Baylin S. Targeting the cancer epigenome for therapy. Nat Rev Genet 2016;17:630–41. [DOI] [PubMed] [Google Scholar]
- 36. Yamaguchi H, Du Y, Nakai K, Ding M, Chang SS, Hsu JL, et al. EZH2 contributes to the response to PARP inhibitors through its PARP-mediated poly-ADP ribosylation in breast cancer. Oncogene 2018;37:208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abbotts R, Topper MJ, Biondi C, Fontaine D, Goswami R, Stojanovic L, et al. DNA methyltransferase inhibitors induce a BRCAness phenotype that sensitizes NSCLC to PARP inhibitor and ionizing radiation. Proc Natl Acad Sci USA 2019;116:22609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Robert C, Nagaria PK, Pawar N, Adewuyi A, Gojo I, Meyers DJ, et al. Histone deacetylase inhibitors decrease NHEJ both by acetylation of repair factors and trapping of PARP1 at DNA double-strand breaks in chromatin. Leuk Res 2016;45:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ladd B, Ackroyd JJ, Hicks JK, Canman CE, Flanagan SA, Shewach DS. Inhibition of homologous recombination with vorinostat synergistically enhances ganciclovir cytotoxicity. DNA Repair 2013;12:1114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ho TCS, Chan AHY, Ganesan A. Thirty years of HDAC Inhibitors: 2020 insight and hindsight. J Med Chem 2020;63:12460–84. [DOI] [PubMed] [Google Scholar]
- 41. Misteli T, Soutoglou E. The emerging role of nuclear architecture in DNA repair and genome maintenance. Nat Rev Mol Cell Biol 2009;10:243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xiao W, Graham PH, Hao J, Chang L, Ni J, Power CA, et al. Combination therapy with the histone deacetylase inhibitor LBH589 and radiation is an effective regimen for prostate cancer cells. PLoS One 2013;8:e74253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bondarev AD, Attwood MM, Jonsson J, Chubarev VN, Tarasov VV, Schiöth HB. Recent developments of HDAC inhibitors: emerging indications and novel molecules. Br J Clin Pharmacol 2021;87:4577–97. [DOI] [PubMed] [Google Scholar]
- 44. Lanzi C, Cassinelli G. Combinatorial strategies to potentiate the efficacy of HDAC inhibitors in fusion-positive sarcomas. Biochem Pharmacol 2022;198:114944. [DOI] [PubMed] [Google Scholar]
- 45. Brohl AS, Solomon DA, Chang W, Wang J, Song Y, Sindiri S, et al. The genomic landscape of the Ewing sarcoma family of tumors reveals recurrent STAG2 mutation. PLoS Genet 2014;10:e1004475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. May WA, Grigoryan RS, Keshelava N, Cabral DJ, Christensen LL, Jenabi J, et al. Characterization and drug resistance patterns of Ewing's sarcoma family tumor cell lines. PLoS One 2013;8:e80060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shah RR. Safety and tolerability of histone deacetylase (HDAC) inhibitors in oncology. Drug Saf 2019;42:235–45. [DOI] [PubMed] [Google Scholar]
- 48. Boussios S, Abson C, Moschetta M, Rassy E, Karathanasi A, Bhat T, et al. Poly (ADP-ribose) polymerase inhibitors: talazoparib in ovarian cancer and beyond. Drugs R D 2020;20:55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Knelson EH, Patel SA, Sands JM. PARP inhibitors in small cell lung cancer: rational combinations to improve responses. Cancers 2021;13:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yin YP, Ma LY, Cao GZ, Hua JH, Lv XT, Lin WC. FK228 potentiates topotecan activity against small cell lung cancer cells via induction of SLFN11. Acta Pharmacol Sin 2022;43:2119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Replicate graphs of HDAC, PARP1, PARP2 and PARylation assays
Replicate graphs of cell viability assays and comparison of cell viability assay methods
Replicates of cell cycle assays and statistics
Replicate data of DNA damage assays
Replicate graphs of spheroid assays and cell fluorescence data
Blood cell panel counts and weight loss percentage of in vivo toxicity study
Materials and methods for in vivo toxicity and cellular fluorescence assays
Data Availability Statement
The data generated in this study are available upon request from the corresponding author.