Objectives:
Pediatric intestinal pseudo-obstruction (PIPO) is a heterogeneous condition characterized by impaired gastrointestinal propulsion, a broad clinical spectrum, and variable severity. Several molecular bases underlying primary PIPO have been identified, of which autosomal dominant ACTG2-related visceral myopathy is the most common in both familial or sporadic primary PIPO cases. We present a family with autosomal recessive ACTG2-related disease in which both parents have mild gastrointestinal symptoms and sons have severe PIPO and bladder dysfunction.
Methods:
Clinical genome sequencing was performed on the patients and the mother. Immunohistochemistry was performed on intestinal tissue from the patients to show expression levels of the ACTG2.
Results:
Genome sequencing identified a 6.8 kb 2p13.1 loss that includes the ACTG2 gene and a maternally inherited missense variant p.Val10Met in the ACTG2 gene.
Discussion:
This case demonstrates that monoallelic hypomorphic ACTG2 variants may underly mild primary gastrointestinal symptoms, while biallelic mild variants can cause severe diseases. The Deletions of the noncoding ACTG2 exon can be an under-recognized cause of mild gastrointestinal symptoms unidentifiable by exome sequencing, explaining some instances of interfamilial variability with an apparent autosomal dominant inheritance. Genome sequencing is recommended as a genetic work-up for primary or idiopathic PIPO because of genetic heterogeneity.
Keywords: ACTG2-related disorder, chronic intestinal pseudo-obstruction, genome sequencing, megacystis microcolon intestinal hypoperistalsis syndrome, visceral myopathy
What Is Known
Causes of pediatric intestinal pseudo-obstruction (PIPO) are genetically heterogeneous.
Autosomal dominant ACTG2-related visceral myopathy due to either dominant-negative or gain-of-function missense variants is the most common genetic cause of PIPO, and severe cases present as megacystis microcolon intestinal hypoperistalsis syndrome (MMIHS).
Three cases of ACTG2-related visceral myopathy suggesting autosomal recessive inheritance have been reported previously.
Variants in other genes associated with the actin-myosin apparatus can cause PIPO and MMIHS.
What Is New
We illustrate sibling cases who have an autosomal recessive form of PIPO/MMIHS due to an exonic deletion and a missense variant in trans, solidifying the autosomal recessive inheritance of ACTG-related visceral myopathy.
Deletion of noncoding exon 1 of the ACTG2 gene can affect the function of the gene.
A mild variant of the ACTG2 gene can be a cause of severe familial constipation.
Genome sequencing is able to detect pathogenic variants missed by exome sequencing.
INTRODUCTION
Pediatric intestinal pseudo-obstruction (PIPO) is a chronic intestinal pseudo-obstruction in children defined by the 2018 European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines (1) as the “chronic inability of the gastrointestinal tract to propel its contents and mimicking mechanical obstruction in the absence of any lesion occluding the gut” and is classified by etiologies—primary, secondary, and idiopathic (1). Primary causes include myopathy, neuropathy, or mesenchymopathy of inherited or inflammatory origin. Recent advances in molecular technology have led to discoveries of molecular etiologies and modes of inheritance for primary PIPO, including autosomal dominant (EDN3, EDNRB, SOX10, ACTG2, RET), autosomal recessive (LMOD1, MYH11, MYLK, MYL9, PDCL3, POLG1, TYMP, RAD21, SGOL1), and X-linked (FLNA, L1CAM). Variants in genes associated with the actin-myosin apparatus (ACTG2, ACTA2, LMOD1, MYL9, MYH11, MYLK) lead to visceral myopathy that can present as PIPO or a more severe prenatal/neonatal form, megacystis microcolon intestinal hypoperistalsis syndrome (MMIHS). This condition may involve extraintestinal organs, including the urinary tract.
First described in 2012, ACTG2-related visceral myopathy (MIM 155310) is recognized as the most common molecular cause of familial or sporadic (de novo) PIPO, characterized by variable degrees of smooth muscle dysfunction involving the intestine and bladder (2). ACTG2-related visceral myopathy was initially reported as an autosomal dominant condition with variable severity and frequent de novo occurrences (3,4). More recently, PIPO cases due to a homozygous truncating ACTG2 variant p. Arg168* (5), a homozygous arginine substitution ACTG2 variant p.Arg336Trp (6), and a homozygous ACTG2 exon 6 deletion c.589_613 + 163del188 (7) have been reported, suggesting that some mild variants are associated with an autosomal recessive inheritance. Here, we describe a unique familial quartet illustrating carriers of mild ACTG2 variants with mild gastrointestinal symptoms and their children with severe PIPO.
METHODS
Cases
Brothers with PIPO were referred to our facility at ages 18 and 8 years, respectively. The older brother was a healthy child with normal prenatal history, including prenatal ultrasound and normal cognitive and motor development. He started to have chronic constipation upon introducing solid foods around 2 years, a common practice in his birth country. He preferred milk and resisted oral food. Constipation and food aversion worsened over time, leading to growth failure, although he remained active and participated in swimming. At 14 years, he had acute volvulus of the large intestine requiring a right hemicolectomy. A few days later, he had a small bowel obstruction requiring partial resection and ileostomy. He had been mostly intolerant of oral food intake and dependent on exclusive total parenteral nutrition (TPN) via a central line. He would place a nasogastric tube as needed for decompression. Biopsies performed at an outside hospital showed hypertrophy of the muscularis mucosa and lamina propria, mucosal atrophy, and normal ganglion cells in the small and remnant large intestine. Antroduodenal manometry performed at age 18 revealed a mixed myopathic and neuropathic motility disorder, with low amplitude contractions and a lack of motor migrating complexes. He has been able to tolerate some oral food over time, while still receiving the majority of his caloric intake parenterally.
The younger brother had a similar infantile course as his older brother developing PIPO at a younger age of 6. He developed acute volvulus of the large intestine requiring right hemicolectomy, followed by a small bowel obstruction requiring partial small bowel resection and ileostomy, becoming TPN-dependent around age 14. He has been on anticoagulant therapy due to a history of central-line-related thrombosis. He develops enuresis when he receives too much fluid and suffers from vitamin D deficiency and osteopenia, a known complication of chronic TPN and intestinal failure due to fat malabsorption. Antroduodenal manometry performed at the age of 8 years revealed a motility pattern very similar to that in the older brother. While he is able to consume food, most of his nutrition is provided by TPN. In both brothers, pyridostigmine was started at low doses (10 mg daily) and slowly increased over time up to 80 mg TID (8). Both brothers seemed to benefit from it, at least initially, with less abdominal distension, increased oral intake and higher ostomy output.
The mother, maternal aunts, and maternal grandmother have had severe childhood-onset constipation requiring large amounts of laxatives. The father reportedly had chronic diarrhea and nephrolithiasis and died at age 50 due to lung cancer. Written informed consent was obtained from all patients.
Genome Sequencing
Genome sequencing (GS) was performed on DNA extracted from whole-blood samples collected from the mother and the 2 brothers; the father was not available for testing. Extracted DNA was prepared for next-generation sequencing using the Illumina TruSeq PCR-free kit. Samples were sequenced on the Illumina HiSeq X system with paired-end 150 base pair reads at the Illumina Clinical Services Laboratory in San Diego, CA. The data were aligned according to the Human Reference Genome (GRCh37/hg19) and analyzed using the Strelka caller for single nucleotide variants and Canvas caller for copy number variants. Expansions of short tandem repeats were identified using ExpansionHunter.
Immunohistochemistry
Paraffin-embedded intestinal tissues from the brothers were obtained as part of the clinical work-up. Intestinal tissues used as control were obtained from the Pathology Department repository of the Erasmus University Medical Center. Immunostainings were performed as previously described (Halim et al, 2016), using specific antibodies against ACTG2 (1:100; Novus Biologicals), neurofilament (1:600; Monosan), and smooth muscle actin α2 (ACTA2; ready to use; Dako). All sections were counterstained with hematoxylin. All images were taken with the NanoZoomer 2.0-HT (Hamamatsu Photonics) and analyzed with the NanoZoomer Digital Pathology viewer software (Hamamatsu Photonics).
RESULTS
GS Identifies the Presence of Compound Heterozygous Variants in ACTG2 in the 2 Affected Brothers
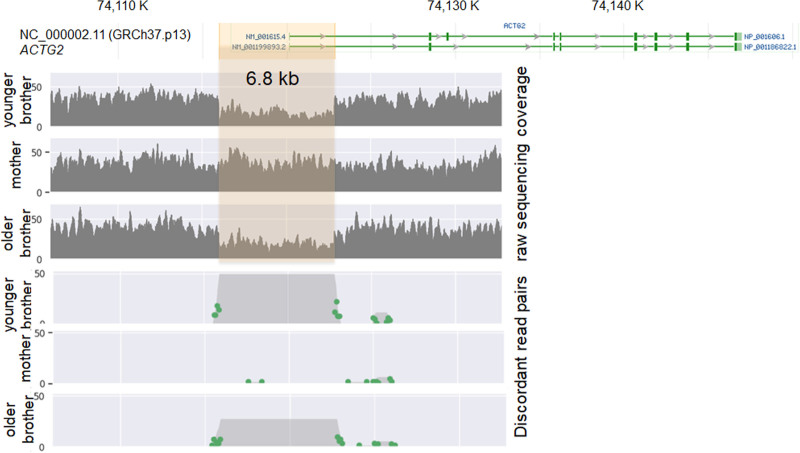
The short-read GS identified a maternally inherited NM_001615.3:c.28G>A (p.Val10Met) variant in the ACTG2 gene and a 6.8 kb 2p13.1 loss [NG_034140.1:g.906_7729del(GRCh37)] in both brothers. The 2p13.1 loss includes the entire noncoding exon 1 and partial intron 1 of the ACTG2 gene (Fig. 1). The presence of the deletion in the brothers was orthogonally confirmed via targeted array comparative genomic hybridization by a clinical laboratory. The p.Val10Met variant was not found in the Genome Aggregation Database in a region of good sequencing coverage. Multiple in silico algorithms predict that the p.Val10Met variant would impact protein function. This variant was also interpreted as likely pathogenic, based on the American College of Medical Genetics guidelines (9). The 6.8 kb deletion at 2p13.1 or deletions of similar regions are not reported in the DatabasE of genomiC varIation and Phenotype in Humans using Ensembl Resources; https://www.deciphergenomics.org or population Database of Genomic Variants (http://dgv.tcag.ca/dgv/app/home). The deletion was interpreted as a variance of unknown significance according to the American College of Medical Genetics (ACMG) guidelines on copy number variant interpretation (10).
FIGURE 1.
2p13.1 deletion [chr2:g.74115998_74122821del(GRCh37)] detected in the family.
Expression Levels of ACTG2 in the Affected Brothers
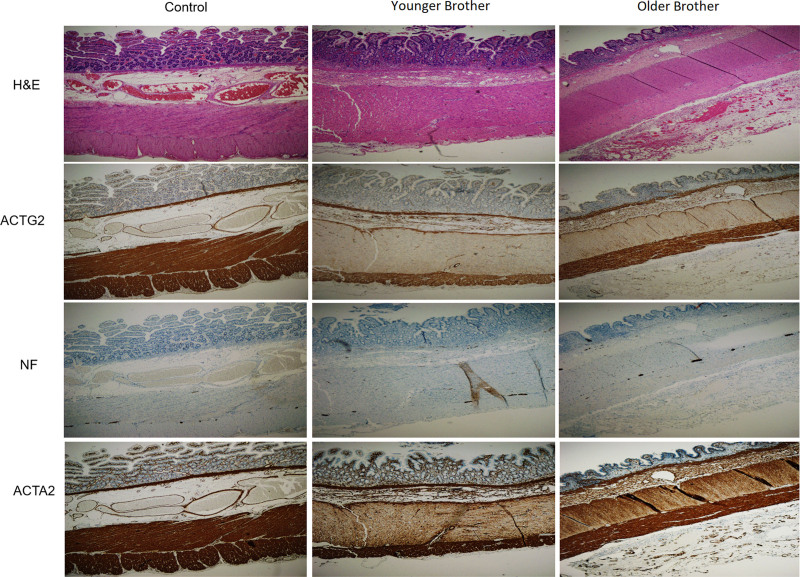
Immunohistochemistry was performed in intestinal material collected during previous surgeries to investigate ACTG2 expression levels in the brothers. Intestinal specimens collected during autopsy from neonatal controls were used for comparison. There seems to be a slight visual decrease in the expression levels of ACTG2 on specimens from the brothers compared with controls (Fig. 2). No difference was detected in the expression of neurofilament and alpha-smooth muscle actin (ACTA2), suggesting no major structural abnormalities of the neurons and smooth muscle, respectively.
FIGURE 2.
Immunohistochemistry performed in intestinal material obtained from the 2 PIPO patients and a neonatal control, shows distribution and expression of ACTG2 in the cytoplasm of smooth muscle cells of the muscularis mucosa, blood vessels and, the circular and longitudinal muscle of muscularis propria. No significant changes were detected in the composition of the intestine of patients and control, shown by HE staining and immunohistochemistry for NF and ACTA2. Scale bars: 800 μm. HE = hematoxylin and eosin; NF = neurofilament; PIPO = pediatric intestinal pseudo-obstruction.
DISCUSSION
The brothers with PIPO were found with a missense variant involving a nonarginine residue (Val10Met) and a deletion involving the noncoding 5’ untranslated region (UTR) of the ACTG2 gene. The missense variant was inherited from the mother with severe chronic constipation, which can be a mild manifestation of ACTG2-related disease. The deletion was presumably inherited from the father, who suffered from chronic diarrhea.
The spectrum of the ACTG2-related disease typically includes MMIHS with urinary tract involvement such as megacystis and megaureter, recurrent urinary tract infections, and bladder dysfunction. Intestinal involvement includes malrotation, microcolon, abdominal pain, constipation, diarrhea, and functional obstruction. Some patients respond to oral anti-cholinesterase drug pyridostigmine (11).
ACTG2 (ENSG00000163017) is predominantly expressed in the smooth muscle of the intestine, bladder, and uterus with some expression in the aorta (12). Structurally, it consists of 9 exons (ENST00000345517; NM_001615.3) and encodes smooth muscle alpha-actin, a protein of 376 amino acids (ENP00000295137). Exon 1 and the initial part of exon 2 form the 5’UTR, with translation starting in the middle of exon 2. Smooth muscle gamma-actin (ACTG2) is one of the 6 actin isoforms sharing 93% amino acid identity; skeletal muscle alpha-actin (ACTA1), smooth muscle aortic alpha-actin (ACTA2), cytoskeletal gamma-actin (ACTG1), smooth muscle alpha-actin (ACTA2), cardiac muscle alpha-actin (ACTC1), and cytoplasmic beta-actin (ACTB) (13). These isoforms can partly compensate for each other. Variants of each actin gene are associated with human diseases, inherited mostly as autosomal dominant traits. A heterozygous pathogenic variant in ACTA1 is associated with nemaline myopathy, ACTA2 with familial thoracic aortic aneurysms, ACTG1 with nemaline myopathy, ACTC1 with hypertrophic cardiomyopathy, and ACTB with Baraitser-Winter syndrome. While the majority of pathogenic variants in the actin genes are missense, likely loss-of-function variants (small deletion, indel, frameshift, splice-site) are registered in the ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) and LOVD v3.0 (https://www.lovd.nl/3.0/home) databases. Loss-of-function variants in Acta1, Acta2, and Actc1 have been shown to be pathogenic in mouse models (14).
Most known ACTG2 pathogenic variants found in autosomal dominant ACTG2-related visceral myopathy are missense. Arginine substitutions are common pathogenic variants often associated with severe phenotypes and poor outcomes (2). The Arg257Cyn variant impairs the formation and function of actin filaments composed of γ-actin (13). The gain-of-function or dominant-negative effect of missense variants at the Arg38, Arg148, Arg178, and Arg257 residues have been suggested as the autosomal dominant pathogenic mechanism (3,4). The case with a homozygous p.Arg336Trp variant reported by Matera et al (6) suggests that the missense p.Arg336Trp variant leads to loss-of-function. The p.Val10Met variant seen in our family likely leads to mild disease in a heterozygous state, as evidenced in the mother, aunt, and grandmother with severe chronic constipation.
Exonic ACTG2 deletions and truncating variants have been reported in cases with visceral myopathy, suggesting loss-of-function as an alternative etiology (15,16). Specifically, Monies et al (5) reported a homozygous truncating ACTG2 variant c.631C>T (p.Arg211*; reported as c.631C>T using the transcript NM_ 001199893.1) in a 16-year-old girl with visceral myopathy, with affected family members heterozygous for the variant presenting with chronic constipation. Moreover, Lee et al (16) described a deletion of ACTG2 exons 8–9 resulting in decreased protein expression in proximal and distal intestinal histology in an 11-year-old girl with PIPO, although the zygosity, parental origin of the variant, or parental phenotypes were not described. If the deletion was heterozygous with possibly de novo inheritance, it would indicate haploinsufficiency due to loss-of-function. If the deletion was homozygous, each possibly inherited from a carrier parent, it would suggest the second report of recessive inheritance possibly due to loss-of-function. Additionally, Kraatari et al (15) identified heterozygous microdeletion 2p13.1 of 84 kb, including exons 2–9 of the ACTG2 gene and the entire DGUOK gene in a 10-year-old boy with PIPO. This patient was dependent on parenteral nutrition, presenting with infantile periodic abdominal pain, vomiting and growth deficiency. Most recently, James et al (7) reported a homozygous exon 6 deletion c.589_613 + 163del188 in a 13-month-old boy with severe visceral myopathy. We speculate that the exon 1 deletion of ACTG2 leads to loss-of-function, supported by their father’s chronic diarrhea and a small decrease of intestinal ACTG2 expression in the brothers, although the immunohistochemistry results need to be interpreted with care. Diarrhea has been reported in some reported cases of ACTG2-disease. The father possibly could have had other gastrointestinal complaints though we were not able to substantiate his phenotype. One could argue that the severe presentation in the brothers and milder presentation in the mother and other maternal family members could be explained by interfamilial variability of the ACTG2 disease. Examples of 5’UTR variants causing human diseases by disturbing upstream open-reading frames have been reported (17). 5’UTR and 3’UTR variants in a gene may affect transcript stability and translation through RNA structural changes (18). In addition, 5’UTR variants that create an upstream start codon or disrupt upstream open-reading frames can cause human diseases (19–22) and represent an under-recognized pathogenic variant class (17) not necessarily detectable by exome sequencing (ES). Deletion of noncoding exons at other genetic loci, ATP7A exon 1, has been reported in patients with Menkes disease (23).
The family reported by Monies et al (5) was the first description suggesting autosomal recessive inheritance in ACTG2-related visceral myopathy with the same variant leading to severe diseases in a homozygous state and mild disease in a heterozygous state. Interestingly, another sister with homozygous p.Arg168* variant only had chronic constipation similar to their parents, suggesting incomplete penetrance (5). Further, Matera et al (6) reported a homozygous arginine substitution c.1006C>T (p.Arg336Trp) in siblings with an MMIHS phenotype, which were inherited from asymptomatic parents. The 13-year-old boy with severe visceral myopathy with a homozygous exon 6 deletion reported by James et al (7) was the third report of autosomal recessive inheritance with variable expressivity and incomplete penetrance. Neither parent had any gastrointestinal symptoms, while a maternal half-sister had intermittent constipation. The mother was heterozygous for the deletion suggesting autosomal recessive inheritance; the father and the half-sister were not available for testing. Our case solidifies the principle that some ACTG2 variants lead to mild gastrointestinal phenotypes in the monoallelic state and severe disease in a biallelic state. Variable expressivity and incomplete penetrance have been shown in both monoallelic and biallelic states. Loss-of-function is speculated to be the mechanism for these mild variants.
It is increasingly recognized that GS and its bioinformatic pipelines have improved the detection of exonic deletion variants compared with ES. Had the ES technology been applied to our family, the exon 1 deletion would not have been detected and the heterozygous p.Val10Met variant could have been filtered out as a benign variant depending on the variant analysis strategy because it was inherited from the mother who does not have the typical PIPO phenotype. In addition, the 6.8 kb 2p13.1 deletion identified by GS would not have been detected by the standard whole-genome SNP microarray technology due to its small size. The noncoding ACTG2 exon 1 is typically not captured in standard ES. This is also illustrated by James et al (7), in which GS detected a homozygous exonic variant that was missed by 2 copy number and indel variant callers.
It is possible that the deletion or other variants in UTR of the ACTG2 are under-recognized as a cause of ACTG2-related visceral myopathy that shows interfamilial variable expressivity or reduced penetrance. Future transcriptional studies of these brothers may clarify the effect of the 5’UTR deletion of the ACTG2 gene in PIPO pathogenesis.
Our case adds to the growing body of evidence that mild ACTG2 variants can cause mild gastrointestinal symptoms in a monoallelic state, which may not be necessarily recognized as a disease, and severe PIPO in the biallelic state. It also illustrates the ability of GS to identify under-recognized UTR pathogenic variants, explaining intrafamilial phenotypic variability in ACTG2-related diseases.
ACKNOWLEDGMENTS
We thank the patients and their parents for their informed consent/assent to publish the details of this case and Andrew Gross for bioinformatics support.
M.M. designed the work and the main conceptual ideas and wrote the article. M.M.A. and J.W. performed immunohistochemical analysis. R.T.H. and A.R.C. performed molecular analysis. R.T.H. and V.P. critically reviewed the study proposal. M.M., K.T., K.M., and C.D.L. provided and cared for study patients. S.G.K., A.R.C., and M.M.A. served as scientific advisors. All authors contributed to the critical editing of the article. C.D.L. and M.M.A. are joint senior authors. C.D.L. is a member of North American Society For Pediatric Gastroenterology, Hepatology & Nutrition.
Footnotes
Drs Alves and Di Lorenzo are joint senior authors.
Dr Di Lorenzo receives consultant fees from Sucampo, Allergan, QOL Inc, Mahana, Innovative Health Solutions, and Mallinckrodt. Dr Kaler receives Consultant fees from Vivet Therapeutics, Inc. (Paris, France) as chair of a clinical trial Data Monitoring Committee. AC is an employee and shareholder of Illumina, Inc. The remaining authors report no conflicts of interest.
Genome Sequencing studies were funded by the Illumina iHope program.
Consents were obtained from the patients and a parent. The study met the criteria for an exemption determination by the Institutional Review Board.
REFERENCES
- 1.Thapar N, Saliakellis E, Benninga MA, et al. Paediatric intestinal pseudo-obstruction: evidence and consensus-based recommendations from an ESPGHAN-led expert group. J Pediatr Gastroenterol Nutr. 2018;66:991–1019. [DOI] [PubMed] [Google Scholar]
- 2.Batzir NA, Bhagwat PK, Larson A, et al. Recurrent arginine substitutions in the ACTG2 gene are the primary driver of disease burden and severity in visceral myopathy. Hum Mutat. 2020;41:641–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matera I, Rusmini M, Guo Y, et al. Variants of the ACTG2 gene correlate with degree of severity and presence of megacystis in chronic intestinal pseudo-obstruction. Eur J Hum Genet. 2016;24:1211–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehtonen HJ, Sipponen T, Tojkander S, et al. Segregation of a missense variant in enteric smooth muscle actin γ-2 with autosomal dominant familial visceral myopathy. Gastroenterology. 2012;143:1482–1491.e3. [DOI] [PubMed] [Google Scholar]
- 5.Monies D, Maddirevula S, Kurdi W, et al. Autozygosity reveals recessive mutations and novel mechanisms in dominant genes: implications in variant interpretation. Genet Med. 2017;19:1144–1150. [DOI] [PubMed] [Google Scholar]
- 6.Matera I, Bordo D, Di Duca M, et al. Novel ACTG2 variants disclose allelic heterogeneity and bi-allelic inheritance in pediatric chronic intestinal pseudo-obstruction. Clin Genet. 2021;99:430–436. [DOI] [PubMed] [Google Scholar]
- 7.James KN, Lau M, Shayan K, et al. Expanding the genotypic spectrum of ACTG2-related visceral myopathy. Cold Spring Harb Mol Case Stud. 2021;7:a006085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manini ML, Camilleri M, Grothe R, et al. Application of pyridostigmine in pediatric gastrointestinal motility disorders: a case series. Paediatr Drugs. 2018;20:173–180. [DOI] [PubMed] [Google Scholar]
- 9.Richards S, Aziz N, Bale S, et al. ; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riggs ER, Andersen EF, Cherry AM, et al. Technical standards for the interpretation and reporting of constitutional copy-number variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen). Genet Med. 2020;22:245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Nardo G, Viscogliosi F, Esposito F, et al. Pyridostigmine in pediatric intestinal pseudo-obstruction: case report of a 2-year old girl and literature review. J Neurogastroenterol Motil. 2019;25:508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian J, Kumar A, Szucsik JC, Lessard JL. Tissue and developmental specific expression of murine smooth muscle γ-actin fusion genes in transgenic mice. Dev Dyn. 1996;207:135–144. [DOI] [PubMed] [Google Scholar]
- 13.Hashmi SK, Barka V, Yang C, et al. Pseudo-obstruction-inducing ACTG2R257C alters actin organization and function. JCI Insight. 2020;5:140604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perrin BJ, Ervasti JM. The actin gene family: function follows isoform. Cytoskeleton (Hoboken). 2010;67:630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraatari M, Kokkonen H, Makinen M, Turunen S, Moilanen J, Kuismin O. Visceral myopathy due to a novel deletion of the ACTG2 gene: a case report. In: European Journal of Human Genetics. London, United Kingdom: Nature Publishing Group; 2019:1244–1244. [Google Scholar]
- 16.Lee H, Park S, Oh JT, et al. Oral pyridostigmine-responsive visceral myopathy with ACTG2 mutations: a case series. J Pediatr Gastroenterol Nutr. 2019;68:e16–e17. [DOI] [PubMed] [Google Scholar]
- 17.Whiffin N, Karczewski KJ, Zhang X, et al. ; Genome Aggregation Database Production Team; Genome Aggregation Database Consortium. Characterising the loss-of-function impact of 5’ untranslated region variants in 15,708 individuals. Nat Commun. 2020;11:2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeraati M, Moye AL, Wong JWH, et al. Cancer-associated noncoding mutations affect RNA G-quadruplex-mediated regulation of gene expression. Sci Rep. 2017;7:708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferdinandusse S, Te Brinke H, Ruiter JPN, et al. A mutation creating an upstream translation initiation codon in SLC22A5 5’UTR is a frequent cause of primary carnitine deficiency. Hum Mutat. 2019;40:1899–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bohlen AE von, Böhm J, Pop R, et al. A mutation creating an upstream initiation codon in the SOX9 5’ UTR causes acampomelic campomelic dysplasia. Mol Genet Genomic Med. 2017;5:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Occhi G, Regazzo D, Trivellin G, et al. A novel mutation in the upstream open reading frame of the CDKN1B gene causes a MEN4 phenotype. PLoS Genet. 2013;9:e1003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen Y, Liu Y, Xu Y, et al. Loss-of-function mutations of an inhibitory upstream ORF in the human hairless transcript cause Marie Unna hereditary hypotrichosis. Nat Genet. 2009;41:228–233. [DOI] [PubMed] [Google Scholar]
- 23.Kaler SG, Holmes CS, Goldstein DS, et al. Neonatal diagnosis and treatment of Menkes disease. N Engl J Med. 2008;358:605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]