Abstract
Prednisolone, used as a standard initial treatment for immune thrombocytopenia (ITP), is an important risk factor for osteoporosis. Recently, we found that prescription of bisphosphonate during initial loading of prednisolone may prevent reduction in bone mineral density and development of glucocorticoid-induced osteoporosis (GIO) in older patients with ITP receiving prolonged steroid therapy. In this review, I describe the treatment options for older patients with ITP, and present the best practices for screening, evaluating, and diagnosing ITP. I also summarize the literature from 2017 to 2022 on the treatment options for ITP, including discussions on the contraindications and side effects, with an emphasis on GIO, and the relative merits of bisphosphonates as a co-treatment for prevention of GIO. Finally, I present a perspective and an expert recommendation on how older patients with ITP would best be served in the future.
Keywords: Immune thrombocytopenia, Glucocorticoid-induced osteoporosis, Older patients, Bisphosphonate
Background
Glucocorticoid therapy is the standard initial treatment for immune thrombocytopenia (ITP), an autoimmune disease characterized by isolated thrombocytopenia and mucocutaneous bleeding, and has been identified as an important risk factor for osteoporosis [1, 2] (Fig. 1). Glucocorticoids are associated with the risk of bone loss, which is most pronounced in the first few months of use [3]. Recently, strategies that avoid glucocorticoid side effects have been favored, and a strong emphasis has been placed on shared decision making, especially for second-line therapies such as early administration of thrombopoietin receptor agonist (TPO-RA) [4].
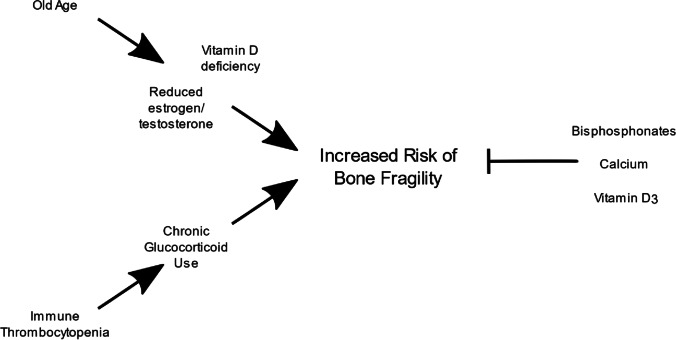
Fig. 1.
Diagram highlighting how risk factors associated with older age may exacerbate the risk of glucocorticoid-induced osteoporosis during treatment of immune thrombocytopenia and how various treatments, including bisphosphonates, are used to mitigate this risk
Glucocorticoids increase the risk of fracture early in the treatment course during the phase of rapid bone loss and at higher levels of bone mineral density (BMD) compared with postmenopausal osteoporosis [5]. An increased risk of fracture was reported for prednisolone doses as low as 2.5–7.5 mg daily [6]. However, the increased risk of fracture in patients taking glucocorticoids declined rapidly during 1 year of therapy [7]. Fracture risk assessment using the 2017 American College of Rheumatology (ACR) guidelines requires the evaluation of clinical risk factors for fracture and BMD [7]. BMD is commonly expressed as the T-score. A T-score ≤ − 2.5 is consistent with a diagnosis of osteoporosis, while a T-score between − 1.0 and − 2.5 is classified as low bone mass (osteopenia) and a T-score ≥ − 1.0 is normal [8]. For patients aged 40–90 years, data for clinical risk factors and BMD can be entered into a validated algorithm (FRAX®; available online at www.sheffield.ac.uk/FRAX/tool.jsp), which calculates the 10-year probabilities of major osteoporotic (clinical vertebral, hip, humerus, wrist) and hip fractures.
Significant negative correlations for BMD with total and mean daily steroid doses were reported for glucocorticoid-induced osteoporosis (GIO) in patients with ITP scheduled to receive long-term steroid treatment, and bisphosphonate was identified as an effective agent for prevention and treatment of GIO [9]. We recently investigated the prevention of GIO-related bone fractures in older patients with ITP receiving prolonged steroid therapy [10]. To fill the evident gap in the management of older patients with ITP, I conducted a summary of the recent literature on GIO occurring secondary to ITP treatment in older adults.
Primary and secondary immune thrombocytopenia
ITP is a diagnosis of exclusion, being defined as isolated thrombocytopenia (peripheral blood platelet count < 100,000/μL) without anemia or leukopenia and without another apparent cause of thrombocytopenia [2, 4]. Primary ITP is ITP acquired through autoimmune mechanisms leading to platelet destruction and platelet underproduction and is not triggered by an apparent associated condition. A presumptive diagnosis of primary ITP is reached when the history, physical examination, and laboratory test findings, including a review of peripheral blood smear findings, do not reveal other potential etiologies for thrombocytopenia.
Secondary ITP, comprising approximately 20% of all ITP cases, is ITP associated with other conditions like autoimmune diseases, such as systemic lupus erythematosus (SLE), antiphospholipid syndrome, and immune thyroid diseases, lymphoproliferative diseases, such as chronic lymphocytic leukemia and autoimmune lymphoproliferative syndrome, and viral and bacterial infections, such as Helicobacter pylori infection, human immunodeficiency virus (HIV) infection, hepatitis C virus (HCV) infection, cytomegalovirus infection, varicella zoster virus infection, and coronavirus disease.
The incidence of primary ITP in adults ranges from 1.6 to 3.9 per 100,000 people/year. After a first peak in childhood, a second peak is observed in adults aged > 65 years, reaching nine per 100,000 people/year in men aged > 75 years [11, 12].
Treatment options for primary and secondary immune thrombocytopenia
First-line therapy
Although not all patients with thrombocytopenia require therapies to increase the platelet count, these treatments are used in patients at increased risk of bleeding based on a number of factors including platelet count, comorbidities, patient values and concerns, and tolerability of therapies [13]. The majority of ITP patients with platelet count < 20,000/μL, especially those with platelet count < 10,000/μL, receive these treatments, even if they have no bleeding symptoms, because of the increased risk of bleeding for platelet count < 20,000/μL and even greater risk for platelet count < 10,000/μL. The majority of ITP patients with minor bleeding, particularly mucosal bleeding including blood blisters in the mouth, and platelet count < 50,000/μL are also treated. Data to support an association between mucosal bleeding and more serious bleeding are limited, and a retrospective study on 112 ITP patients who presented to the emergency department with bleeding and platelet count < 20,000/μL found that only six patients with oral mucosal bleeding subsequently developed more serious bleeding [4].
Glucocorticoids and intravenous immunoglobulin
Glucocorticoids and intravenous immunoglobulin (IVIG) both raise the platelet count, but differ in their mechanisms of action, rapidity of platelet count increase, adverse effects, and costs. For critical bleeding, glucocorticoid and IVIG are given together. In severe bleeding, minor bleeding with surgery, or severe thrombocytopenia without bleeding, glucocorticoid or IVIG alone is typically given. Glucocorticoid therapy is the standard initial treatment for ITP, because glucocorticoids are less expensive and can be easily administered in outpatient settings without infusion. IVIG is generally reserved for settings in which there is a need to raise the platelet count within 12–24 h, or for patients who cannot tolerate glucocorticoids due to diabetes mellitus or significant adverse effects. Differences between glucocorticoids and IVIG include the faster action of IVIG (1–3 days) compared with glucocorticoids (2–14 days), but the efficacies (both short-term and long-term) are similar.
IVIG may be used in patients who cannot tolerate glucocorticoids or wish to avoid glucocorticoid toxicities, or added to glucocorticoid treatment in patients who require a rapid platelet count increase before an invasive procedure, because it raises the platelet count more rapidly than glucocorticoids. The efficacy of glucocorticoids compared with IVIG was demonstrated in a trial that randomly assigned 122 patients with previously untreated acute primary ITP (platelet count ≤ 20,000/μL) to intravenous high-dose methylprednisolone (HDMP; 15 mg/kg/day) or IVIG (0.7 g/kg/day) on days 1–3, followed by a second randomization to placebo or oral prednisone (1 mg/kg/day) on days 4–21 [14]. After monitoring the patients for 1 year, the major results included similar 1-year response rates in patients receiving HDMP or IVIG, and better responses in patients who followed initial HDMP or IVIG with 3 weeks of oral prednisone rather than placebo in the second randomization [14]. For patients who received initial therapy followed by prednisone, the 1-year response rates were 47% (HDMP) and 46% (IVIG). Meanwhile, in patients who received initial therapy followed by placebo, the 1-year response rates were 32% (HDMP) and 29% (IVIG). The increase in platelet count was faster with IVIG than with HDMP (platelet count > 50,000/μL at day 5 observed in 79% of patients receiving IVIG and 60% of patients receiving HDMP). Both treatments were generally well tolerated. There were no deaths or life-threatening hemorrhages. Thus, it may be reasonable to use IVIG in patients for whom glucocorticoids are ineffective and vice versa. Glucocorticoids raise the platelet count in approximately two-thirds of patients with ITP. Most responses occur within 2–5 days, but a period of up to 2 weeks may be required. The mechanism is uncertain but may involve increased apoptotic death of autoantibody-producing lymphocytes and downregulation of macrophage activity responsible for platelet phagocytosis [15].
Prednisolone is typically administered at 1 mg/kg orally once per day (range, 0.5–2 mg/kg daily) for 1–2 weeks, followed by a gradual taper, typically completed within 6 weeks [2, 4]. Administration of glucocorticoids for longer than 6 weeks should be avoided to minimize side effects [4]. If there is no platelet count response after 2 weeks, a faster taper over 1 week can be used. The relative efficacies of shorter or longer tapers of oral prednisone have not been evaluated in randomized trials. Clinical experience suggests that shorter tapers (≤ 6 weeks) are associated with similar efficacy and reduced toxicity compared with longer tapers. For patients who experience a decrease in platelet count during the prednisone taper, an additional course or an alternative therapy can be given. The choice among glucocorticoids is individualized [2, 4].
Dexamethasone
The most common dexamethasone treatment regimens are typically administered at 40 mg orally or intravenously once per day for 4 days with no taper [4]. The intravenous route is generally used for critical or severe bleeding. For minor bleeding or thrombocytopenia without bleeding, dexamethasone can be given orally or intravenously. This can be repeated for additional cycles, up to three times in total [2]. Reasons to prefer dexamethasone over prednisone include faster responses, reduced risk of dose confusion, no need for dose tapering, completion of therapy in 4 days, and fewer bleeding events. Reasons to prefer prednisone over dexamethasone include greater ability to titrate the therapy to match the patient’s individual response. Complete long-term remission with glucocorticoids has been reported in approximately 20% of patients, based on uncontrolled studies. These long-term remission rates may simply reflect the natural history of ITP, with spontaneous resolution independent of glucocorticoid use. Outcomes with different glucocorticoid regimens were compared in a 2016 meta-analysis of nine randomized trials that included 1138 patients with previously untreated ITP who received high-dose dexamethasone for three cycles or oral prednisone 1 mg/kg for 2–4 weeks [16]. Dexamethasone 40 mg daily is approximately equivalent to prednisone 4 mg/kg daily, based on the per-milligram potency for dexamethasone being approximately 7.5 times greater than that for prednisone.
Glucocorticoids for older patients with immune thrombocytopenia
Younger patients may have greater platelet count improvement following therapy than older patients. This was illustrated in a cohort of 117 patients with ITP who were observed for approximately 3 years on various therapies [17]. The proportions of patients with platelet count > 30,000/μL at 6 months after completing therapy were similar in patients aged ≤ 60 or > 60 years (91% versus 87%). However, the number of patients with platelet count > 100,000/μL was much higher in patients aged ≤ 60 years (64% versus 17%). Patients without a sufficient platelet count increase on glucocorticoids should have their diagnosis re-evaluated. Furthermore, patients without a sufficient platelet count increase on glucocorticoids and patients with rapid recurrence of thrombocytopenia following glucocorticoid tapering or discontinuation should be observed or transitioned to other therapies rather than continuing glucocorticoids. According to a consensus guideline in 2019, excessive use of glucocorticoids is a common error in ITP management [2].
Although short-term glucocorticoid administration for older patients with ITP is generally safe and well tolerated, glucocorticoids are associated with both short-term and long-term adverse effects. Short-term effects include mood alterations or emotional lability, insomnia, hyperglycemia, and dyspepsia. These adverse effects may be seen with higher or more prolonged dosing, but can also occur with standard dosing and short courses of therapy. Routine prophylaxis for gastrointestinal toxicity, such as use of proton pump inhibitors, is not generally advised in asymptomatic patients, but can be appropriate for older patients with ITP. Long-term effects include infections, cataracts, and osteoporosis. Attention to bone health is necessary, especially in older patients at increased risk, as outlined in a 2019 guideline from the British Society for Haematology [1]. Considerations about osteoporosis generally apply after approximately 3 months of therapy, but may be relevant at an earlier point in older patients with ITP at high risk for low BMD. Some glucocorticoid toxicities may be intolerable to patients, and in these cases, it is appropriate to use IVIG or second-line therapies [18, 19], including TPO-RAs [10], instead. Although prolonged glucocorticoid use should not be routinely advocated, rare patients may receive long-term treatment with low-dose glucocorticoids (e.g., prednisone ≤ 5 mg/day). For such patients, calcium and vitamin D supplementation is appropriate to reduce the risk of osteoporosis, along with monitoring of BMD. Evidence to support this practice for older patients with ITP was presented in our recent study [10].
Second-line therapy
Second-line therapy options include splenectomy, rituximab, TPO-RAs, other immunosuppressive agents, and combination regimens. Some trials have evaluated intensification of therapy, such as use of multiple agents including glucocorticoid plus rituximab, glucocorticoid plus TPO-RA, and glucocorticoid plus mycophenolate mofetil (MMF). Some of these trials demonstrated improved response rates with multiagent therapy at 6 or 12 months, often at the cost of greater toxicity, but the follow-up was insufficient to determine whether intensification of upfront therapy led to greater cure rates [20]. A randomized trial on glucocorticoid plus MMF versus glucocorticoid alone showed a greater likelihood of achieving platelet count > 30,000/μL in the MMF group [21]. However, there was no difference in the bleeding rates, and the patients who received MMF had decreased quality of life, including more fatigue and worse physical health. Although further studies are warranted, the absence of evidence for increased cure rates renders these mixed results insufficiently compelling to recommend multiagent therapy in the initial treatment setting [22].
Splenectomy, rituximab, and TPO-RAs are the three principal choices for second-line therapy, but differ in their mechanisms of action [4]. While all three therapies are effective for raising the platelet count in the majority of patients, they show marked differences in their application, duration, cost, burden, and adverse effect profiles (Table 1) [23].
Table 1.
Immune thrombocytopenia therapies and comparisons of their advantages and disadvantages
| Therapies | Advantages | Disadvantages |
|---|---|---|
| First-line therapy | ||
| Glucocorticoids |
Effective Inexpensive Oral administration Tolerable short-term toxicities |
Uncommon durable response after discontinuation Serious long-term toxicities |
| Rescue therapy | ||
| IVIG | Rapid response |
Cost Frequent side effects |
| Second-line therapy | ||
| Splenectomy |
Effective Long duration of response |
Surgical risks Infectious risks from asplenia Thrombosis risk |
| Rituximab | Non-surgical |
Less effective than splenectomy Shorter remission Risks of viral reactivation (e.g., HBV) Risks of other toxicities (e.g., anaphylaxis, pulmonary toxicity) |
| TPO-RAs |
Effective Non-surgical Non-immunosuppressive Potential for self-administration |
Cost Requirement for continuous administration |
IVIG intravenous immunoglobulin, HBV hepatitis B virus, TPO-RAs thrombopoietin receptor agonists
Splenectomy is a one-time permanent surgical procedure that is the most likely of the three therapies to result in a durable response [24]. Because some patients can experience spontaneous remission within the first year, splenectomy is generally deferred until at least 1 year has elapsed since diagnosis, if feasible. Splenectomy may be a good choice for patients who wish to undergo a single potentially curative surgical procedure and who are willing to accept the increased risks of infection and venous thromboembolism. Previous studies indicated that younger patients had a higher response rate than older patients, but a specific age cutoff for effective splenectomy could not be determined [25, 26]. One study reported that the duration of response following splenectomy and the likelihood of a response may be reduced in patients aged > 65 years [24].
Rituximab typically requires four weekly intravenous administrations [27] and may need to be re-administered. Rituximab may be a good choice for patients who wish to avoid surgery and prefer not to take long-term medications. However, its effect is often short-lived, necessitating redosing or use of another second-line agent [28–34]. Rituximab is an immunosuppressive agent, and its use has been avoided during the coronavirus disease-2019 (COVID-19) pandemic because it blunts the immune response to vaccinations, including COVID-19 vaccination, probably for at least 6 months. New-onset ITP or exacerbation of existing ITP has been reported following COVID-19 vaccination [35]. However, the risk is low, and vaccination remains the best way to reduce serious disease, hospitalization, and death from COVID-19 [36]. Adverse effects of rituximab include infusion reactions and prolonged immunosuppression, which can result in reactivation of HBV infection [37]. Progressive multifocal leukoencephalopathy (PML) has been reported following rituximab therapy for ITP, and many of the affected patients had been pretreated with other immunosuppressive agents in addition to rituximab [37]. Boxed warnings for infusion reactions, HBV reactivation, mucocutaneous reactions, and PML are included in the prescribing information.
TPO-RAs typically require administration for an extended period of time, although their responses are sometimes maintained after cessation of treatment. A TPO-RA may be a good choice for patients who wish to avoid surgery and the immunosuppressive effects of splenectomy or rituximab and who are less concerned about the need to take medications for an extended period of time, including the associated costs and burdens. Temporary use of a TPO-RA may be appropriate during the COVID-19 pandemic as a means of avoiding immunosuppressive therapy, especially for older patients. If splenectomy is chosen, it is generally preferable to wait at least 1 year from the time of diagnosis in case spontaneous remission occurs. During this time, a TPO-RA may be used temporarily while surgery is being scheduled and preoperative vaccines are being administered. A TPO-RA may also be useful for patients who require a temporary increase in platelet count in preparation for splenectomy and do not have an adequate platelet count response to glucocorticoids [38]. The 2019 ASH guideline on ITP makes weak recommendations for TPO-RA over rituximab, and for rituximab over splenectomy, but it emphasizes the potential usefulness of all three treatments and the importance of patient characteristics, such as age, ITP history, and comorbidities, values, and preferences in the final decision [4]. Approximately 80% of patients with ITP have a significant platelet count increase in response to TPO-RA therapy [39]. Several reports have documented sustained responses in some patients with ITP after TPO-RA discontinuation, with response rates of 30% to 50%, although the observation periods were relatively short (< 1 year) [40]. In most patients, these agents do not induce remission, and prolonged maintenance therapy is usually required. If treatment is discontinued, the platelet count generally returns to the baseline level or lower. Available TPO-RAs for ITP include romiplostim, eltrombopag, and avatrombopag. These three agents are all effective, but their relative efficacies for ITP have not been evaluated in randomized trials. The 2019 ASH guideline does not express a preference for a particular TPO-RA, and suggests a choice between romiplostim or eltrombopag based on patient preference for route of administration [4]. The choice is based on availability, cost, comorbidities, and patient preference and can be individualized. Some patients have a strong preference for one administration route (oral or subcutaneous) over the other.
Comparisons among second-line therapies
Splenectomy, rituximab, and TPO-RAs have not been directly compared in randomized trials [26]. However, the reported initial response rates are higher for splenectomy than for rituximab (60%–80% versus 55%–65%), and the responses with splenectomy are more durable, often lasting for many years or even indefinitely for splenectomy versus 1–2 years for rituximab [28, 41]. There are also significant differences in the short- and long-term risks of splenectomy versus rituximab [41–43]. Rituximab-related morbidities include infections, such as pneumonia and sepsis, and thrombotic complications, such as venous thromboembolism and myocardial infarction. There are significant differences in the short- and long-term risks and burdens of rituximab versus TPO-RAs. While rituximab causes immunosuppression and may only work transiently, TPO-RAs are highly effective but require extended administration in many cases. Other therapies may be appropriate if splenectomy, rituximab, and/or TPO-RAs are ineffective or cannot be used. As a result, the choice of therapy is highly dependent on patient values and preferences. All three options require patient assistance to balance the risks and benefits of the approach.
Bisphosphonates and alternative treatment options as prophylaxis against glucocorticoid-induced osteoporosis in older patients with immune thrombocytopenia
Use of bisphosphonates in older patients with ITP who are to receive long-term steroid therapy should be followed up in all patients receiving any dose of glucocorticoids for ≥ 3 months to minimize bone loss [7]. The dose and duration of glucocorticoid therapy should be as low as possible, because even doses considered replacement doses or chronic inhaled glucocorticoids can cause bone loss [44]. Patients should complete weightbearing exercises to prevent both bone loss and muscle atrophy. Patients should also avoid smoking and excess alcohol, and take measures to prevent falls. Evidence for the use of vitamin D3 to supplement bisphosphonate treatment in patients at risk of GIO was reported [7]. The ACR Task Force osteoporosis guidelines suggest that all patients taking glucocorticoids at any dose with an anticipated duration of ≥ 3 months should maintain a total calcium intake of 1000–1200 mg/day and vitamin D intake of 600–800 international units/day through diet and/or supplements [7]. Glucocorticoids induce a negative calcium balance by decreasing intestinal calcium absorption and increasing urinary calcium excretion [45]. Therefore, calcium supplementation may attenuate bone loss in patients taking glucocorticoids. In a meta-analysis of five randomized trials comparing calcium and vitamin D (cholecalciferol or active vitamin D metabolite) with calcium alone or placebo in patients taking glucocorticoids, significant improvements in the lumbar spine and radial BMD were noted in the calcium and vitamin D group (weighted mean differences between the treatment group and control group of 2.6% and 2.5%, respectively) [3]. The incidence of new nontraumatic fractures did not differ significantly in two trials (odds ratio [OR] 0.6, 95% CI 0.1–2.4). In one of the larger studies included in the meta-analysis, 96 patients with rheumatoid arthritis receiving low-dose glucocorticoid therapy (mean prednisone dose, 5.6 mg daily) were randomly assigned to calcium carbonate (1000 mg of elemental calcium daily) plus vitamin D3 (500 international units/day) or placebo [46]. The between-group differences in the annual rate of change were 2.65% (95% CI 0.73–4.57) and 2.08% (95% CI 0.43–3.73) for the spine and trochanter BMD, respectively, favoring calcium and vitamin D supplementation. Although calcium and vitamin D supplementation is necessary, it is generally not sufficient to prevent bone loss and fracture in patients taking high-dose glucocorticoids [47–49]. Vitamin D metabolites with greater activity than vitamin D itself, such as calcitriol and alfacalcidol, have been evaluated for the prevention and treatment of glucocorticoid-induced bone loss [50].
Calcitriol (1,25-dihydroxyvitamin D; the most active metabolite of vitamin D) plus calcium protected against spine bone loss more effectively than calcium alone in patients taking glucocorticoids [48, 51]. A meta-analysis of five trials on active vitamin D metabolites in patients exposed to corticosteroids reported a beneficial effect of vitamin D metabolites on lumbar spine BMD [52]. However, there were insufficient data to address fracture prevention. Calcitonin is not used for the treatment or prevention of GIO, because more effective drugs (e.g., bisphosphonates, teriparatide) are available for prevention of bone loss and reduction of fracture risk. Another concern is that long-term use of calcitonin for osteoporosis has been associated with an increase in cancer rates [53].
Active vitamin D metabolites are not commonly used because of the risks of hypercalcemia and hypercalciuria in patients whose urinary calcium excretion is already increased and because more effective therapies are available [54]. The superior efficacy of bisphosphonates compared with an active vitamin D metabolite for preventing glucocorticoid-induced bone loss has been demonstrated in several randomized trials [55, 56]. Although not statistically significant, one trial showed that fewer patients randomly assigned to alendronate had new vertebral fractures compared with patients assigned to alfacalcidol (three versus eight patients) [57].
Older patients with the highest risk for fracture are the most likely to benefit from drug therapy. Thus, selection of patients based on fracture risk, as determined by a combination of BMD and clinical risk factors, is desirable. Patients with established osteoporosis (history of fragility fracture or BMD T-score ≤ − 2.5) are at the highest risk for fracture. For patients without established osteoporosis, fracture risk can be assessed using a fracture risk calculator, such as FRAX. FRAX estimates the 10-year probability of fracture in untreated patients aged 40–90 years using femoral neck BMD and clinical risk factors, including glucocorticoid exposure. FRAX does not account for glucocorticoid dose or duration, and therefore FRAX risk estimates must be corrected by the glucocorticoid dose [58]. For patients taking prednisolone > 7.5 mg/day or equivalent, the risk estimate should be increased by 15% for major osteoporotic fracture and 20% for hip fracture [58].
In North America, reasonable glucocorticoid-corrected thresholds to indicate high, moderate, and low risks of fracture are as follows [3]: high risk, 10-year probability of hip fracture or combined major osteoporotic fracture of ≥ 3% or 20%, respectively; moderate risk, 10-year probability of hip fracture or combined major osteoporotic fracture of 1%–3% or 10%–19%, respectively; and low risk, 10-year probability of hip fracture or combined major osteoporotic fracture of ≤ 1% or < 10%, respectively. Some patients receiving glucocorticoids are at high risk, even if they fail to meet the FRAX criteria for high risk. For patients with clinical risk factors for fracture and low lumbar spine BMD, but normal femoral neck BMD, FRAX is likely to underestimate the fracture risk. This situation is particularly likely in patients taking glucocorticoids, which are more prone to cause osteoporosis in the spine than in the hip. Thus, intervention guidelines with or without the use of FRAX provide only general clinical guidance. Treatment should remain individualized through shared decision making between patients and clinicians.
For men aged ≥ 50 years and postmenopausal women who are initiating or receiving chronic treatment with any dose of glucocorticoids for any duration and have osteoporosis (previous fragility fracture and/or BMD T-score ≤ − 2.5) at initial assessment, pharmacological therapy is recommended. For high-risk men aged ≥ 50 years and postmenopausal women who are initiating or receiving chronic treatment with any dose of glucocorticoids for any duration and have T-scores between − 1.0 and − 2.5, pharmacological therapy is suggested. A reasonable threshold to indicate high risk in some settings is a glucocorticoid-corrected, FRAX-calculated, 10-year probability of hip or combined major osteoporotic fracture of ≥ 3 or 20%, respectively. For men aged > 50 years and postmenopausal women with T-scores between − 1.0 and − 2.5 who have a glucocorticoid-corrected, FRAX-calculated absolute risk below these thresholds, pharmacological therapy is suggested if they are taking ≥ 7.5 mg/day of prednisone or its equivalent for an anticipated duration of ≥ 3 months. These recommendations are based on randomized trial data showing that pharmacological therapy improves BMD in patients taking glucocorticoids [59, 60] and additional randomized trial data showing that pharmacological therapy reduces fracture in men and postmenopausal women with established osteoporosis [61].
Bisphosphonates are the first-line therapy for prevention and treatment of GIO in men because they are known to reduce fracture risk. Men who develop symptomatic hypogonadism should also be treated with testosterone for its benefits on muscle, energy, and libido, as well as on bone [62, 63]. A number of testosterone preparations are available for the treatment of testosterone deficiency. Alendronate or risedronate is preferred because of clinical trial data demonstrating efficacy in men and women with GIO. For patients who cannot tolerate oral bisphosphonates or who have difficulty with the dosing requirements or adherence, intravenous zoledronic acid is an acceptable alternative.
Parathyroid hormone (PTH; teriparatide) is typically reserved for patients with severe osteoporosis (T-score ≤ − 3.5 in the absence of fracture or T-score ≤ − 2.5 plus fragility fracture). Teriparatide is also an option for patients who cannot tolerate any of the available bisphosphonates or who continue to suffer fracture after 1 year of bisphosphonate therapy. Denosumab is another alternative therapeutic option for patients at high risk for fracture. However, due to the increased risk of vertebral fracture after discontinuation of denosumab, the need for a careful exit strategy should be discussed with patients prior to initiation of its cessation.
There are substantial data supporting the use of antiresorptive agents, such as bisphosphonates, for the prevention and treatment of glucocorticoid-induced bone loss. In a meta-analysis of 27 randomized trials evaluating bisphosphonates (alone or in combination with calcium and vitamin D) versus calcium and vitamin D (alone or with placebo) for the prevention and treatment of GIO, significant improvements in the lumbar spine (absolute difference 3.5%) and femoral neck (absolute difference 2.1%) BMD were noted in the bisphosphonate group [64]. There was also a reduction in the risk of new vertebral fracture with bisphosphonate treatment (44 versus 77 per 1000 persons in the bisphosphonate and no treatment groups, respectively; risk ratio [RR] 0.57, 95% CI 0.35–0.91). The reduction in the risk of nonvertebral fracture did not reach statistical significance (42 versus 55 per 1000 persons; RR 0.79, 95% CI 0.47–1.33). The therapeutic efficacy of bisphosphonates in patients with GIO has been thought to be related to their ability to promote osteoclast apoptosis [65]. However, glucocorticoids may negate the pro-apoptotic effect of bisphosphonates, suggesting that bisphosphonates may prevent glucocorticoid-induced bone loss by prolonging the lifespan of osteoblasts [65, 66].
The efficacy of alendronate in patients receiving glucocorticoid therapy was demonstrated in a study involving 477 patients aged 17–83 years who were randomly assigned to receive one of two doses of alendronate or placebo [51]. The mean BMD of the lumbar spine increased by 2.1% and 2.9% over 48 weeks in the patients receiving 5 and 10 mg of alendronate daily, respectively, but decreased by 0.4% in the patients receiving placebo. The femoral neck, trochanter, and total body BMD also increased significantly in the alendronate groups. Patients receiving alendronate had fewer new vertebral fractures compared with those receiving placebo (2.3% versus 3.7%), although fracture was not a primary outcome. These benefits were maintained for 2 years, representing a 12-month extension of a previously completed 1-year trial of daily alendronate [67]. Once-weekly alendronate (70 mg) showed similar improvement of BMD [68], and a retrospective cohort study found that alendronate was associated with a significantly lower risk of hip fracture in older patients taking oral prednisolone [69].
Risedronate is also effective for the prevention and treatment of osteoporosis, including GIO [70, 71]. In a 1-year study of risedronate versus placebo in 290 patients receiving glucocorticoid therapy (prednisone ≥ 7.5 mg/day for ≥ 6 months), the lumbar spine and femoral neck BMD increased by 2.7% and 1.8%, respectively, in the risedronate group, compared with no change in the placebo group [70]. The relative risk of vertebral fracture (a secondary outcome) was reduced by 70%.
The efficacy of zoledronic acid for the prevention and treatment of GIO was demonstrated in a 1-year randomized trial of intravenous zoledronic acid (5 mg once) or daily oral risedronate (5 mg) in 288 patients who recently started glucocorticoids (prevention group) and 545 patients who had been taking glucocorticoids for > 3 months (treatment group) [71]. In an analysis of patients who received the study drugs and had baseline and follow-up BMD measurements, zoledronic acid and risedronate increased the mean BMD of the lumbar spine in both the prevention (2.6% and 0.6%, respectively) and treatment (4.1% and 2.7%, respectively) groups. The study was not designed to evaluate fractures, which occurred in three and five patients in the risedronate and zoledronic acid groups, respectively. During the first 3 days, the occurrence of adverse events (predominantly arthralgias, fever, and flu-like symptoms) was greater in the zoledronic acid group.
Monitoring for glucocorticoid-induced osteoporosis
There are several published guidelines for monitoring the response to osteoporosis therapy. Although all recommend follow-up BMD testing, there is no consensus on the optimal frequency of monitoring and the preferred site to monitor. We typically use dual-energy X-ray absorptiometry to measure the BMD of the lumbar spine and hip at the initiation of glucocorticoid therapy and after 1 year of treatment. If the BMD is stable or improved, we perform the measurements less frequently (every 2–3 years) thereafter. If glucocorticoids are discontinued and the BMD remains stable, measurements at 5-year intervals may be sufficient. The finding of a BMD decrease greater than the least significant change or a new fracture in a treated patient should trigger additional evaluation for contributing factors, which may include poor adherence to therapy, inadequate gastrointestinal absorption, inadequate intake of calcium and vitamin D, or development of a disease or disorder with adverse skeletal effects. Switching from oral bisphosphonates to intravenous zoledronic acid may be effective in patients with poor absorption or poor compliance with the oral regimen. Alternative options for patients who fail oral bisphosphonate therapy are similar to those for patients with osteoporosis in general. There may be a significant increase in BMD after discontinuation of exogenous glucocorticoid therapy or reversal of endogenous Cushing’s syndrome [72]. As an example, one study examined spine BMD after successful treatment for Cushing’s disease in 20 patients, all of whom had marked osteoporosis of the lumbar spine and femoral neck [72]. There was no change in BMD for 6 months, the time required for gradual reversal of increased osteoclastic activity, after which the BMD increased. However, patients who have experienced a fracture may have a permanent deformity.
Perspectives and recommendations
The purpose of this section is to summarize the provided literature and place it into the context of current recommendations for prevention of GIO in older ITP patients. Given that the present review focuses on the literature generated since 2017, it may also be pertinent to summarize some of the key findings in the report by Hill et al. [1], which covers 1960–2017.
Regarding the GIO treatment recommendations by various clinical bodies worldwide [7, 73], the guidelines do not address older patients as a specific cohort. The recently published literature provides additional insights into this cohort. Recent papers on ITP and/or GIO in older patients support the continued recommendation of bisphosphonates to mitigate the development of GIO in older ITP patients, as indicated in our previous report [10] (Fig. 2). Bisphosphonates are currently the recommended first-line treatment for GIO, according to the guideline of the Japanese Society for Bone and Mineral Research [74]. This recommendation is almost 8 years old and is limited to drugs currently approved for osteoporosis treatment in Japan. A new guideline will be published in 2023. However, this new guideline will not specifically address older ITP patients. The 2022 ACR guideline for the prevention and treatment of GIO describes sequential treatments recommended when the initial osteoporosis therapy and glucocorticoid therapy are discontinued depending on the fracture risk [75]. Further studies are needed to evaluate the utility of new diagnostic tools [76, 77] and to conduct direct comparisons between the efficacies of GIO therapies [78].
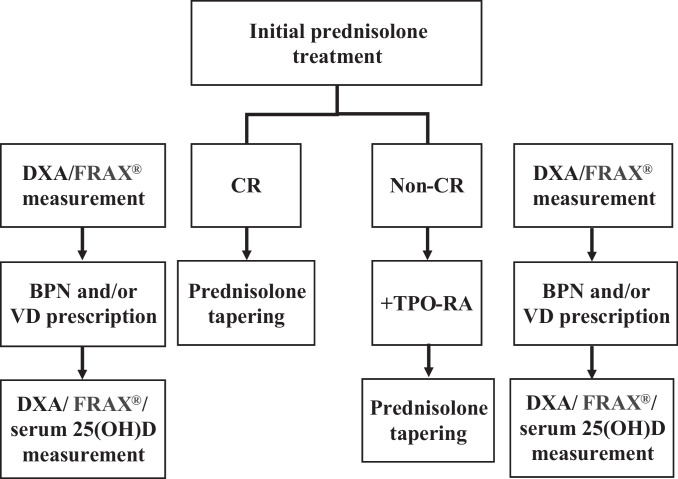
Fig. 2.
Flow chart highlighting the decision-making process for treatment of glucocorticoid-induced osteoporosis in older patients with immune thrombocytopenia. If a complete response (CR) is achieved on prednisolone therapy, a gradual taper of prednisolone is initiated and typically completed within 6 weeks. If no platelet count response is observed after 2 weeks of prednisolone therapy, a thrombopoietin receptor agonist (TPO-RA) can be added and a faster taper of prednisolone over 1 week can be used. Data for dual-energy X-ray absorptiometry (DXA) and femoral neck bone mineral density measurement are typically entered into FRAX® (a validated algorithm; available online at www.sheffield.ac.uk/FRAX/tool.jsp) to define the risk-adapted approach to bisphosphonate (BPN) treatment during the initial loading and tapering phases of prednisolone therapy. Active vitamin D (VD) is usually prescribed for women. Serum 25-hydroxyvitamin D (25(OH)D) levels are measured during the tapering phase of prednisolone therapy to ensure adequate VD levels for continuous VD therapy in women and additional VD therapy in men
Author contribution
SY designed, prepared, and reviewed the manuscript. SY met the International Committee of Medical Journal Editors criteria for authorship of this article as a whole, and provided approval for this version to be published.
Funding
The article processing charges were funded by the author. This work was supported by JSPS KAKENHI Grant Number 22K12887. The author thanks Alison Sherwin, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Declarations
Ethics approval
This was a retrospective review study with no experimental interventions.
Informed consent
This was a retrospective review study with requirement for informed consent.
Conflict of interest
The author declares no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hill QA, Grainger JD, Thachil J, Provan D, Evans G, Garg M, Bradbury C, Bagot C, Kanis JA, Compston JE, British Society of Haematology in conjunction with the UK ITP forum The prevention of glucocorticoid- induced osteoporosis in patients with immune thrombocytopenia receiving steroids: a British Society for Haematology Good Practice Paper. Br J Haematol. 2019;185:410–417. doi: 10.1111/bjh.15735. [DOI] [PubMed] [Google Scholar]
- 2.Provan D, Arnold DM, Bussel JB, Chong BH, Cooper N, Gernsheimer T, Ghanima W, Godeau B, González-López TJ, Grainger J, Hou M, Kruse C, McDonald V, Michel M, Newland AC, Pavord S, Rodeghiero F, Scully M, Tomiyama Y, Wong RS, Zaja F, Kuter DJ. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3:3780–3817. doi: 10.1182/bloodadvances.2019000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319–1328. doi: 10.1007/s00198-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 4.Neunert C, Terrell DR, Arnold DM, Buchanan G, Cines DB, Cooper N, Cuker A, Despotovic JM, George JN, Grace RF, Kühne T, Kuter DJ, Lim W, McCrae KR, Pruitt B, Shimanek H, Vesely SK. American society of hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3:3829–3866. doi: 10.1182/bloodadvances.2019000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossini M, Viapiana O, Vitiello M, Malavolta N, La Montagna G, Maddali Bongi S, Di Munno O, Nuti R, Manzini CU, Ferri C, Bogliolo L, Mathieu A, Cantatore F, Del Puente A, Muratore M, Grassi W, Frediani B, Saviola G, Delvino P, Mirone L, Ferraccioli G, Tripi G, Piazza I, Gatti D. Prevalence and incidence of osteoporotic fractures in patients on long-term glucocorticoid treatment for rheumatic diseases: the Glucocorticoid Induced OsTeoporosis TOol (GIOTTO) study. Reumatismo. 2017;69:30–39. doi: 10.4081/reumatismo.2017.922. [DOI] [PubMed] [Google Scholar]
- 6.van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13:777–787. doi: 10.1007/s001980200108. [DOI] [PubMed] [Google Scholar]
- 7.Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE, Humphrey MB, Lane NE, Magrey M, Miller M, Morrison L, Rao M, Robinson AB, Saha S, Wolver S, Bannuru RR, Vaysbrot E, Osani M, Turgunbaev M, Miller AS, McAlindon T. 2017 American college of rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2017;69:1521–1537. doi: 10.1002/art.40137. [DOI] [PubMed] [Google Scholar]
- 8.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group Osteoporos Int. 1994;4:368–381. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 9.Nomura S, Kurata Y, Tomiyama Y, Takubo T, Hasegawa M, Saigo K, Nishikawa M, Higasa S, Maeda Y, Hayashi K. Effects of bisphosphonate administration on the bone mass in immune thrombocytopenic purpura patients under treatment with steroids. Clin Appl Thromb Hemost. 2010;16:622–627. doi: 10.1177/1076029609350889. [DOI] [PubMed] [Google Scholar]
- 10.Yamasaki S, Kamezaki K, Ito Y, Horiuchi T. Bisphosphonate use for glucocorticoid-induced osteoporosis in elderly patients with immune thrombocytopenia receiving prolonged steroid therapy: a single institute retrospective Study. Hematol Rep. 2022;14:276–285. doi: 10.1016/j.schres.2022.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frederiksen H, Schmidt K. The incidence of idiopathic thrombocytopenic purpura in adults increases with age. Blood. 1999;94:909–913. doi: 10.1182/blood.V94.3.909.415k02_909_913. [DOI] [PubMed] [Google Scholar]
- 12.Kohli R, Chaturvedi S. Epidemiology and clinical manifestations of immune thrombocytopenia. Hamostaseologie. 2019;39:238–249. doi: 10.1055/s-0039-1683416. [DOI] [PubMed] [Google Scholar]
- 13.Neunert C, Noroozi N, Norman G, Buchanan GR, Goy J, Nazi I, Kelton JG, Arnold DM. Severe bleeding events in adults and children with primary immune thrombocytopenia: a systematic review. J Thromb Haemost. 2015;13:457–467. doi: 10.1111/jth.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godeau B, Chevret S, Varet B, Lefrère F, Zini JM, Bassompierre F, Chèze S, Legouffe E, Hulin C, Grange MJ, Fain O, Bierling P, French ATIP Study Group Intravenous immunoglobulin or high-dose methylprednisolone, with or without oral prednisone, for adults with untreated severe autoimmune thrombocytopenic purpura: a randomised, multicentre trial. Lancet. 2002;359:23–29. doi: 10.1016/S0140-6736(02)07275-6. [DOI] [PubMed] [Google Scholar]
- 15.Mizutani H, Furubayashi T, Imai Y, Kashiwagi H, Honda S, Take H, Kurata Y, Yonezawa T, Tarui S, Ikehara S. Mechanisms of corticosteroid action in immune thrombocytopenic purpura (ITP): experimental studies using ITP-prone mice, (NZW x BXSB) F1. Blood. 1992;79:942–947. doi: 10.1182/blood.V79.4.942.942. [DOI] [PubMed] [Google Scholar]
- 16.Mithoowani S, Gregory-Miller K, Goy J, Miller MC, Wang G, Noroozi N, Kelton JG, Arnold DM. High-dose dexamethasone compared with prednisone for previously untreated primary immune thrombocytopenia: a systematic review and meta-analysis. Lancet Haematol. 2016;3:e489–e496. doi: 10.1016/S2352-3026(16)30109-0. [DOI] [PubMed] [Google Scholar]
- 17.Cortelazzo S, Finazzi G, Buelli M, Molteni A, Viero P, Barbui T. High risk of severe bleeding in aged patients with chronic idiopathic thrombocytopenic purpura. Blood. 1991;77(1):31–33. doi: 10.1182/blood.V77.1.31.31. [DOI] [PubMed] [Google Scholar]
- 18.Guidry JA, George JN, Vesely SK, Kennison SM, Terrell DR. Corticosteroid side-effects and risk for bleeding in immune thrombocytopenic purpura: patient and hematologist perspectives. Eur J Haematol. 2009;83:175–182. doi: 10.1111/j.1600-0609.2009.01265.x. [DOI] [PubMed] [Google Scholar]
- 19.Guidry JA, Watson S, George JN, Vesely SK, Terrell DR. Addendum to corticosteroid side effects and risk for bleeding in immune thrombocytopenic purpura: patient perspectives. Eur J Haematol. 2009;83:497–498. doi: 10.1111/j.1600-0609.2009.01322.x. [DOI] [PubMed] [Google Scholar]
- 20.Cuker A, Prak ET, Cines DB. Can immune thrombocytopenia be cured with medical therapy? Semin Thromb Hemost. 2015;41:395–404. doi: 10.1055/s-0034-1544001. [DOI] [PubMed] [Google Scholar]
- 21.Bradbury CA, Pell J, Hill Q, Bagot C, Cooper N, Ingram J, Breheny K, Kandiyali R, Rayment R, Evans G, Talks K, Thomas I, Greenwood R. Mycophenolate mofetil for first-line treatment of immune thrombocytopenia. N Engl J Med. 2021;385:885–895. doi: 10.1056/NEJMoa2100596. [DOI] [PubMed] [Google Scholar]
- 22.Bolton-Maggs PHB, George JN. Immune thrombocytopenia treatment. N Engl J Med. 2021;385:948–950. doi: 10.1056/NEJMe2110953. [DOI] [PubMed] [Google Scholar]
- 23.Bylsma LC, Fryzek JP, Cetin K, Callaghan F, Bezold C, Mehta B, Wasser JS. Systematic literature review of treatments used for adult immune thrombocytopenia in the second-line setting. Am J Hematol. 2019;94:118–132. doi: 10.1002/ajh.25301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Porras JR, Escalante F, Pardal E, Sierra M, Garcia-Frade LJ, Redondo S, Arefi M, Aguilar C, Ortega F, de Cabo E, Fisac RM, Sanz O, Esteban C, Alberca I, Sanchez-Barba M, Santos MT, Fernandez A, Gonzalez-Lopez TJ, representing the Grupo de Trombosis y Hemostasia de Castilla y León Safety and efficacy of splenectomy in over 65-yrs-old patients with immune thrombocytopenia. Eur J Haematol. 2013;91:236–241. doi: 10.1111/ejh.12146. [DOI] [PubMed] [Google Scholar]
- 25.Kojouri K, Vesely SK, Terrell DR, George JN. Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood. 2004;104:2623–2634. doi: 10.1182/blood-2004-03-1168. [DOI] [PubMed] [Google Scholar]
- 26.Fabris F, Tassan T, Ramon R, Carraro G, Randi ML, Luzzatto G, Moschino P, Girolami A. Age as the major predictive factor of long-term response to splenectomy in immune thrombocytopenic purpura. Br J Haematol. 2001;112:637–640. doi: 10.1046/j.1365-2141.2001.02615.x. [DOI] [PubMed] [Google Scholar]
- 27.Cragg MS, Walshe CA, Ivanov AO, Glennie MJ. The biology of CD20 and its potential as a target for mAb therapy. Curr Dir Autoimmun. 2005;8:140–174. doi: 10.1159/000082102. [DOI] [PubMed] [Google Scholar]
- 28.Chugh S, Darvish-Kazem S, Lim W, Crowther MA, Ghanima W, Wang G, Heddle NM, Kelton JG, Arnold DM. Rituximab plus standard of care for treatment of primary immune thrombocytopenia: a systematic review and meta-analysis. Lancet Haematol. 2015;2:e75–81. doi: 10.1016/S2352-3026(15)00003-4. [DOI] [PubMed] [Google Scholar]
- 29.Ghanima W, Khelif A, Waage A, Michel M, Tjønnfjord GE, Romdhan NB, Kahrs J, Darne B, Holme PA, RITP study group Rituximab as second-line treatment for adult immune thrombocytopenia (the RITP trial): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:16531661. doi: 10.1016/S0140-6736(14)61495-1. [DOI] [PubMed] [Google Scholar]
- 30.Auger S, Duny Y, Rossi JF, Quittet P. Rituximab before splenectomy in adults with primary idiopathic thrombocytopenic purpura: a meta-analysis. Br J Haematol. 2012;158:386–398. doi: 10.1111/j.1365-2141.2012.09169.x. [DOI] [PubMed] [Google Scholar]
- 31.Arnold DM, Dentali F, Crowther MA, Meyer RM, Cook RJ, Sigouin C, Fraser GA, Lim W, Kelton JG. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med. 2007;146:25–33. doi: 10.7326/0003-4819-146-1-200701020-00006. [DOI] [PubMed] [Google Scholar]
- 32.Patel VL, Mahévas M, Lee SY, Stasi R, Cunningham-Rundles S, Godeau B, Kanter J, Neufeld E, Taube T, Ramenghi U, Shenoy S, Ward MJ, Mihatov N, Patel VL, Bierling P, Lesser M, Cooper N, Bussel JB. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood. 2012;119:5989–5995. doi: 10.1182/blood-2011-11-393975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nazi I, Kelton JG, Larché M, Snider DP, Heddle NM, Crowther MA, Cook RJ, Tinmouth AT, Mangel J, Arnold DM. The effect of rituximab on vaccine responses in patients with immune thrombocytopenia. Blood. 2013;122:1946–1953. doi: 10.1182/blood-2013-04-494096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khellaf M, Charles-Nelson A, Fain O, Terriou L, Viallard JF, Cheze S, Graveleau J, Slama B, Audia S, Ebbo M, Le Guenno G, Cliquennois M, Salles G, Bonmati C, Teillet F, Galicier L, Hot A, Lambotte O, Lefrère F, Sacko S, Kengue DK, Bierling P, Roudot-Thoraval F, Michel M, Godeau B. Safety and efficacy of rituximab in adult immune thrombocytopenia: results from a prospective registry including 248 patients. Blood. 2014;124:3228–3236. doi: 10.1182/blood-2014-06-582346. [DOI] [PubMed] [Google Scholar]
- 35.Crickx E, Moulis G, Ebbo M, Terriou L, Briantais A, Languille L, Limal N, Guillet S, Michel M, Mahevas M, Godeau B. Safety of anti-SARS-CoV-2 vaccination for patients with immune thrombocytopenia. Br J Haematol. 2021;195:703–705. doi: 10.1111/bjh.17813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Visser C, Swinkels M, van Werkhoven ED, Croles FN, Noordzij-Nooteboom HS, Eefting M, Last-Koopmans SM, Idink C, Westerweel PE, Santbergen B, Jobse PA, Baboe F, RECOVAC-IR Consortium. Te Boekhorst PAW, Leebeek FWG, Levin MD, Kruip MJHA, Jansen AJG. COVID-19 vaccination in patients with immune thrombocytopenia. Blood Adv. 2022;6:1637–1644. doi: 10.1182/bloodadvances.2021006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carson KR, Evens AM, Richey EA, Habermann TM, Focosi D, Seymour JF, Laubach J, Bawn SD, Gordon LI, Winter JN, Furman RR, Vose JM, Zelenetz AD, Mamtani R, Raisch DW, Dorshimer GW, Rosen ST, Muro K, Gottardi-Littell NR, Talley RL, Sartor O, Green D, Major EO, Bennett CL. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the research on adverse drug events and reports project. Blood. 2009;113:4834–4840. doi: 10.1182/blood-2008-10-186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng Y, Duan X, Xu J, Ni X (2011) TPO receptor agonist for chronic idiopathic thrombocytopenic purpura. Cochrane Database Syst Rev CD008235. 10.1002/14651858.CD008235 [DOI] [PMC free article] [PubMed]
- 39.González-López TJ, Pascual C, Álvarez-Román MT, Fernández-Fuertes F, Sánchez-González B, Caparrós I, Jarque I, Mingot-Castellano ME, Hernández-Rivas JA, Martín-Salces M, Solán L, Beneit P, Jiménez R, Bernat S, Andrade MM, Cortés M, Cortti MJ, Pérez-Crespo S, Gómez-Núñez M, Olivera PE, Pérez-Rus G, Martínez-Robles V, Alonso R, Fernández-Rodríguez A, Arratibel MC, Perera M, Fernández-Miñano C, Fuertes-Palacio MA, Vázquez-Paganini JA, Gutierrez-Jomarrón I, Valcarce I, de Cabo E, Sainz A, Fisac R, Aguilar C, Paz Martínez-Badas M, Peñarrubia MJ, Calbacho M, de Cos C, González-Silva M, Coria E, Alonso A, Casaus A, Luaña A, Galán P, Fernández-Canal C, Garcia-Frade J, González-Porras JR. Successful discontinuation of eltrombopag after complete remission in patients with primary immune thrombocytopenia. Am J Hematol. 2015;90:E40–E43. doi: 10.1002/ajh.23900. [DOI] [PubMed] [Google Scholar]
- 40.Bussel JB, Kuter DJ, Pullarkat V, Lyons RM, Guo M, Nichol JL. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood. 2009;113:2161–2171. doi: 10.1182/blood-2008-04-150078. [DOI] [PubMed] [Google Scholar]
- 41.Lal LS, Said Q, Andrade K, Cuker A. Second-line treatments and outcomes for immune thrombocytopenia: A retrospective study with electronic health records. Res Pract Thromb Haemost. 2020;4:1131–1140. doi: 10.1002/rth2.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodeghiero F, Ruggeri M. Is splenectomy still the gold standard for the treatment of chronic ITP? Am J Hematol. 2008;83:91. doi: 10.1002/ajh.21109. [DOI] [PubMed] [Google Scholar]
- 43.Cooper N, Evangelista ML, Amadori S, Stasi R. Should rituximab be used before or after splenectomy in patients with immune thrombocytopenic purpura? Curr Opin Hematol. 2007;14:642–646. doi: 10.1097/MOH.0b013e3282c8ca50. [DOI] [PubMed] [Google Scholar]
- 44.Brynes RK, Orazi A, Theodore D, Burgess P, Bailey CK, Thein MM, Bakshi KK. Evaluation of bone marrow reticulin in patients with chronic immune thrombocytopenia treated with eltrombopag: Data from the EXTEND study. Am J Hematol. 2015;90:598–601. doi: 10.1002/ajh.24011. [DOI] [PubMed] [Google Scholar]
- 45.Zelissen PM, Croughs RJ, van Rijk PP, Raymakers JA. Effect of glucocorticoid replacement therapy on bone mineral density in patients with Addison disease. Ann Intern Med. 1994;120:207–210. doi: 10.7326/0003-4819-120-3-199402010-00005. [DOI] [PubMed] [Google Scholar]
- 46.Homik J, Suarez-Almazor ME, Shea B, Cranney A, Wells G, Tugwell P. Calcium and vitamin D for corticosteroid-induced osteoporosis. Cochrane Database Syst Rev. 2000;1998:CD000952. doi: 10.1002/14651858.CD000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buckley LM, Leib ES, Cartularo KS, Vacek PM, Cooper SM. Calcium and vitamin D3 supplementation prevents bone loss in the spine secondary to low-dose corticosteroids in patients with rheumatoid arthritis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1996;125:961–968. doi: 10.7326/0003-4819-125-12-199612150-00004. [DOI] [PubMed] [Google Scholar]
- 48.Boutsen Y, Jamart J, Esselinckx W, Devogelaer JP. Primary prevention of glucocorticoid-induced osteoporosis with intravenous pamidronate and calcium: a prospective controlled 1-year study comparing a single infusion, an infusion given once every 3 months, and calcium alone. J Bone Miner Res. 2001;16:104–112. doi: 10.1359/jbmr.2001.16.1.104. [DOI] [PubMed] [Google Scholar]
- 49.Sambrook P, Birmingham J, Kelly P, Kempler S, Nguyen T, Pocock N, Eisman J. Prevention of corticosteroid osteoporosis. A comparison of calcium, calcitriol, and calcitonin. N Engl J Med. 1993;328:1747–1752. doi: 10.1056/NEJM199306173282404. [DOI] [PubMed] [Google Scholar]
- 50.Cohen S, Levy RM, Keller M, Boling E, Emkey RD, Greenwald M, Zizic TM, Wallach S, Sewell KL, Lukert BP, Axelrod DW, Chines AA. Risedronate therapy prevents corticosteroid-induced bone loss: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 1999;42:2309–2318. doi: 10.1002/1529-0131(199911)42:11<2309::AID-ANR8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 51.Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, Thamsborg G, Liberman UA, Delmas PD, Malice MP, Czachur M, Daifotis AG. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. N Engl J Med. 1998;339:292–299. doi: 10.1056/NEJM199807303390502. [DOI] [PubMed] [Google Scholar]
- 52.Richy F, Ethgen O, Bruyere O, Reginster JY. Efficacy of alphacalcidol and calcitriol in primary and corticosteroid-induced osteoporosis: a meta-analysis of their effects on bone mineral density and fracture rate. Osteoporos Int. 2004;15:301–310. doi: 10.1007/s00198-003-1570-5. [DOI] [PubMed] [Google Scholar]
- 53.Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis: pathogenesis and management. Ann Intern Med. 1990;112:352–364. doi: 10.7326/0003-4819-112-5-352. [DOI] [PubMed] [Google Scholar]
- 54.Okamoto H, Shibazaki N, Yoshimura T, Uzawa T, Sugimoto T. Association between elcatonin use and cancer risk in Japan: A follow-up study after a randomized, double-blind, placebo-controlled study of once-weekly elcatonin in primary postmenopausal osteoporosis. Osteoporos Sarcopenia. 2020;6:15–19. doi: 10.1016/j.afos.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki Y, Ichikawa Y, Saito E, Homma M. Importance of increased urinary calcium excretion in the development of secondary hyperparathyroidism of patients under glucocorticoid therapy. Metabolism. 1983;32:151–156. doi: 10.1016/0026-0495(83)90221-4. [DOI] [PubMed] [Google Scholar]
- 56.Sambrook PN, Kotowicz M, Nash P, Styles CB, Naganathan V, Henderson-Briffa KN, Eisman JA, Nicholson GC. Prevention and treatment of glucocorticoid-induced osteoporosis: a comparison of calcitriol, vitamin D plus calcium, and alendronate plus calcium. J Bone Miner Re. 2003;18:919–924. doi: 10.1359/jbmr.2003.18.5.919. [DOI] [PubMed] [Google Scholar]
- 57.de Nijs RN, Jacobs JW, Lems WF, Laan RF, Algra A, Huisman AM, Buskens E, de Laet CE, Oostveen AC, Geusens PP, Bruyn GA, Dijkmans BA, Bijlsma JW, Investigators STOP. Alendronate or alfacalcidol in glucocorticoid-induced osteoporosis. N Engl J Med. 2006;355:675–684. doi: 10.1056/NEJMoa053569. [DOI] [PubMed] [Google Scholar]
- 58.Kanis JA, Johansson H, Oden A, McCloskey EV. Guidance for the adjustment of FRAX according to the dose of glucocorticoids. Osteoporos Int. 2011;22:809–816. doi: 10.1007/s00198-010-1524-7. [DOI] [PubMed] [Google Scholar]
- 59.Saag KG, Shane E, Boonen S, Marín F, Donley DW, Taylor KA, Dalsky GP, Marcus R. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357:2028–2039. doi: 10.1056/NEJMoa071408. [DOI] [PubMed] [Google Scholar]
- 60.Allen CS, Yeung JH, Vandermeer B, Homik J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev. 2016;10:CD001347. doi: 10.1002/14651858.CD001347.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.MacLean C, Newberry S, Maglione M, McMahon M, Ranganath V, Suttorp M, Mojica W, Timmer M, Alexander A, McNamara M, Desai SB, Zhou A, Chen S, Carter J, Tringale C, Valentine D, Johnsen B, Grossman J. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2008;148:197–213. doi: 10.7326/0003-4819-148-3-200802050-00198. [DOI] [PubMed] [Google Scholar]
- 62.Reid IR, Wattie DJ, Evans MC, Stapleton JP. Testosterone therapy in glucocorticoid-treated men. Arch Intern Med. 1996;156:1173–1177. doi: 10.1001/archinte.1996.00440100065008. [DOI] [PubMed] [Google Scholar]
- 63.Crawford BA, Liu PY, Kean MT, Bleasel JF, Handelsman DJ. Randomized placebo-controlled trial of androgen effects on muscle and bone in men requiring long-term systemic glucocorticoid treatment. J Clin Endocrinol Metab. 2003;88:3167–3176. doi: 10.1210/jc.2002-021827. [DOI] [PubMed] [Google Scholar]
- 64.Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104:1363–1374. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weinstein RS, Chen JR, Powers CC, Stewart SA, Landes RD, Bellido T, Jilka RL, Parfitt AM, Manolagas SC. Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. J Clin Invest. 2002;109:1041–1048. doi: 10.1172/JCI14538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adachi JD, Saag KG, Delmas PD, Liberman UA, Emkey RD, Seeman E, Lane NE, Kaufman JM, Poubelle PE, Hawkins F, Correa-Rotter R, Menkes CJ, Rodriguez-Portales JA, Schnitzer TJ, Block JA, Wing J, McIlwain HH, Westhovens R, Brown J, Melo-Gomes JA, Gruber BL, Yanover MJ, Leite MO, Siminoski KG, Nevitt MC, Sharp JT, Malice MP, Dumortier T, Czachur M, Carofano W, Daifotis A. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001;44:202–211. doi: 10.1002/1529-0131(200101)44:1<202::AID-ANR27>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 67.Stoch SA, Saag KG, Greenwald M, Sebba AI, Cohen S, Verbruggen N, Giezek H, West J, Schnitzer TJ. Once-weekly oral alendronate 70 mg in patients with glucocorticoid-induced bone loss: a 12-month randomized, placebo-controlled clinical trial. J Rheumatol. 2009;36:1705–1714. doi: 10.3899/jrheum.081207. [DOI] [PubMed] [Google Scholar]
- 68.Axelsson KF, Nilsson AG, Wedel H, Lundh D, Lorentzon M. Association between alendronate use and hip fracture risk in older patients using oral prednisolone. JAMA. 2017;318:146–155. doi: 10.1001/jama.2017.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reid DM, Hughes RA, Laan RF, Sacco-Gibson NA, Wenderoth DH, Adami S, Eusebio RA, Devogelaer JP. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European Corticosteroid-Induced Osteoporosis Treatment Study. J Bone Miner Res. 2000;15:1006–1013. doi: 10.1359/jbmr.2000.15.6.1006. [DOI] [PubMed] [Google Scholar]
- 70.Eastell R, Devogelaer JP, Peel NF, Chines AA, Bax DE, Sacco-Gibson N, Nagant de Deuxchaisnes C, Russell RG. Prevention of bone loss with risedronate in glucocorticoid-treated rheumatoid arthritis patients. Osteoporos Int. 2000;11:331–337. doi: 10.1007/s001980070122. [DOI] [PubMed] [Google Scholar]
- 71.Reid DM, Devogelaer JP, Saag K, Roux C, Lau CS, Reginster JY, Papanastasiou P, Ferreira A, Hartl F, Fashola T, Mesenbrink P, Sambrook PN, HORIZON investigators, Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2009;373:1253–1263. doi: 10.1016/S0140-6736(09)60250-6. [DOI] [PubMed] [Google Scholar]
- 72.Hermus AR, Smals AG, Swinkels LM, Huysmans DA, Pieters GF, Sweep CF, Corstens FH, Kloppenborg PW. Bone mineral density and bone turnover before and after surgical cure of Cushing’s syndrome. J Clin Endocrinol Metab. 1995;80:2859–2565. doi: 10.1210/jcem.80.10.7559865. [DOI] [PubMed] [Google Scholar]
- 73.Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, Hope S, Kanis JA, McCloskey EV, Poole KES, Reid DM, Selby P, Thompson F, Thurston A, Vine N, National Osteoporosis Guideline Group (NOGG) UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017;12:43. doi: 10.1007/s11657-017-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suzuki Y. Glucocorticoid and Bone. Updated Japanese guidelines for the management of glucocorticoid-induced osteoporosis. Clin Calcium. 2014;24:1309–1318. [PubMed] [Google Scholar]
- 75.2022 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Published online September 14, 2022. https://www.rheumatology.org/Portals/0/Files/Prevention-Treatment-GIOP-Guideline-Summary.pdf. Accessed 21 Sept 2022
- 76.Jiang B, Feng C, Li C, Tu C. A bibliometric and visualization analysis of glucocorticoid-induced osteoporosis research from 2012 to 2021. Front Endocrinol (Lausanne) 2022;13:961471. doi: 10.3389/fendo.2022.961471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morikawa T, Sakuma M, Nakamura T, Sonoyama T, Matsumoto C, Takeuchi J, Ohta Y, Kosaka S, Morimoto T. Effectiveness of a computerized clinical decision support system for prevention of glucocorticoid-induced osteoporosis. Sci Rep. 2022;12:14967. doi: 10.1038/s41598-022-19079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takahata M, Shimizu T, Yamada S, Yamamoto T, Hasegawa T, Fujita R, Kobayashi H, Endo T, Koike Y, Amizuka N, Todoh M, Okumura J, Kajino T, Iwasaki N. Bone biopsy findings in patients receiving long-term bisphosphonate therapy for glucocorticoid-induced osteoporosis. J Bone Miner Metab. 2022;40:613–622. doi: 10.1007/s00774-022-01323-9. [DOI] [PubMed] [Google Scholar]