Abstract
Purpose
Severe traumatic brain injury (TBI) leads to acute coma and may result in prolonged disorder of consciousness (pDOC). We aimed to determine whether right median nerve electrical stimulation is a safe and effective treatment for accelerating emergence from coma after TBI.
Methods
This randomised controlled trial was performed in 22 centres in China. Participants with acute coma at 7–14 days after TBI were randomly assigned (1:1) to either routine therapy and right median nerve electrical stimulation (RMNS group) or routine treatment (control group). The RMNS group received 20 mA, 300 μs, 40 Hz stimulation pulses, lasting 20 s per minutes, 8 h per day, for 2 weeks. The primary outcome was the proportion of patients who regained consciousness 6 months post-injury. The secondary endpoints were Glasgow Coma Scale (GCS), Full Outline of Unresponsiveness scale (FOUR), Coma Recovery Scale-Revised (CRS-R), Disability Rating Scale (DRS) and Glasgow Outcome Scale Extended (GOSE) scores reported as medians on day 28, 3 months and 6 months after injury, and GCS and FOUR scores on day 1 and day 7 during stimulation. Primary analyses were based on the intention-to-treat set.
Results
Between March 26, 2016, and October 18, 2020, 329 participants were recruited, of whom 167 were randomised to the RMNS group and 162 to the control group. At 6 months post-injury, a higher proportion of patients in the RMNS group regained consciousness compared with the control group (72.5%, n = 121, 95% confidence interval (CI) 65.2–78.7% vs. 56.8%, n = 92, 95% CI 49.1–64.2%, p = 0.004). GOSE at 3 months and 6 months (5 [interquartile range (IQR) 3–7] vs. 4 [IQR 2–6], p = 0.002; 6 [IQR 3–7] vs. 4 [IQR 2–7], p = 0.0005) and FOUR at 28 days (15 [IQR 13–16] vs. 13 [interquartile range (IQR) 11–16], p = 0.002) were significantly increased in the RMNS group compared with the control group. Trajectory analysis showed that significantly more patients in the RMNS group had faster GCS, CRS-R and DRS improvement (p = 0.01, 0.004 and 0.04, respectively). Adverse events were similar in both groups. No serious adverse events were associated with the stimulation device.
Conclusion
Right median nerve electrical stimulation is a possible effective treatment for patients with acute traumatic coma, that will require validation in a confirmatory trial.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-023-07072-1.
Keywords: Coma, Right median nerve, Electrical stimulation, Traumatic brain injury
Take-home message
| The results of this prospective, multi-centre randomised controlled trial showed possible effect of right median nerve electrical stimulation for the treatment of coma at the early stage of 7–14 days post traumatic brain injury. A confirmatory trial is needed prior to implementation of this approach into clinical practice. Further research is required to determine if right median nerve stimulation can be used to improve coma from other etiologies, in a wider time window, and over a longer period of evaluation. |
Background
Traumatic brain injury (TBI) may lead to an impaired level of consciousness depending on injury severity [1]. In the acute phase, coma is defined by impairments in arousal and awareness and is commonly diagnosed when the Glasgow Coma Scale score (GCS) is equal to or less than 8 [2]. Recent large-scale observational studies reported that 42% of patients admitted to the intensive care unit (ICU) with TBI in China and 37% in Europe had a GCS ≤ 8 [1, 3]. An estimated 0.63–7.33% of patients presenting in coma following severe TBI are in a vegetative state 6 months after injury, and the incidence various significantly between centres [4, 5]. Failure to emerge from coma, or emergence into a disorder of consciousness, presents important management and ethical problems, raising questions about the appropriateness of continued clinical care, and sometimes triggering decisions about withdrawal of life supporting treatments (WLST) [6].
Till now, there are few effective treatments to enhance coma recovery at the early stage post-injury. The intracranial instability and the need to monitor and treat patients in the intensive care unit limit the applicability of invasive stimulations, such as deep brain or spinal cord stimulation. Pharmaceuticals show temporary effectiveness at the chronic stage of TBI [7], but no evidence supports benefit in acute coma patients. Till now, we do not have proven interventions that could promote emergence from coma [7, 8].
The potential of non-invasive transcutaneous electrical stimulation to enhance arousal and emergence from coma or a disorder of consciousness has been subject of increasing interest. Right median nerve stimulation (RMNS), which was first used to treat paralysed limbs following trauma, was initially explored in small studies conducted in the United States of America (USA) as approach to improve the conscious level [9].
RMNS may improve unconsciousness through various mechanisms, including (1) enhancing synapses of the spinoreticular component of the median nerve pathway with neurons of the ascending reticular activating system (ARAS); (2) increase of blood perfusion and (3) increased endogenous neurotrophic factors, such as brain-derived neurotrophic fact (BDNF), leading to the survival of more neurons [8, 10]. Understanding the clinical efficacy will, however, require more exploration in the laboratory with translation of the bench findings into the clinical scene towards a more precise model for targeting treatment.
Small pilot studies and single-centre controlled trials described the potential of the technique to accelerate emergence from coma [11, 12]. These results, suggesting a promising novel strategy of coma awakening, motivated a large-scale study to investigate the efficacy of RMNS in comatose patients after severe TBI.
We report on a multicenter, randomised, controlled trial to assess the efficacy of right median nerve electrical stimulation in promoting emergence from coma, when applied early (7–14 days) after TBI and continuing for two weeks[13, 14]. The primary endpoint was the percentage of patients regaining consciousness 6 months after injury. The secondary endpoints were GCS, Full Outline of Unresponsiveness scale (FOUR), Coma Recovery Scale-Revised (CRS-R), Disability Rating Scale (DRS) and Glasgow outcome scale extended (GOSE) scores on day 28, 3 months and 6 months after injury, and GCS and FOUR scores on day 1 and day 7 during stimulation.
Methods
Study design and participants
This study is a prospective, multi-centre, randomised controlled trial, conducted in 22 large, experienced, specialised neurosurgical centres across China (see electronic supplementary material, ESM). The study enrolled patients between 18 and 65 years, who suffered closed TBI 7–14 days before enrolment, with GCS 4–8 and a GCS motor score < 5 assessed on enrolment. Patients with unstable vital signs, history of epilepsy, severe cardiac arrhythmia, or pacemaker implantation were excluded from the study. Full inclusion and exclusion criteria are provided in the appendix (see ESM).
The detailed trial protocol has been published [15]. The study protocol and consent forms have been approved by the Ethics Committee of Renji Hospital (NO: Renji Lunshen [2016] 001(2)) and the local institutional review boards of each participating site. The trial was registered on ClinicalTrials.gov (ID number NCT02645578), and performed in accordance with the Declaration of Helsinki and the guidelines of Good Clinical Practice. Reporting follows the Consort guidelines.
Randomisation and masking
All eligible participants were randomly assigned (1:1) to the stimulation group (RMNS group) or the non-stimulation group (control group), stratified by study centre using a block randomisation scheme with a block size of 4. The randomisation sequence was generated using SAS PROC PLAN (SAS Institute Software, Cary, NC, USA), and was carried out by an independent statistician. The randomisation allocation was mailed in an opaque envelope to an unmasked study physician in each participating centre, who set the stimulation device with the appropriate stimulation settings according to the group assignment each day. The stimulation device was also applied to the control group but without electric current being administered. Outcome assessors were masked to group assignment.
Procedures
Baseline information collected at presentation to the emergency room included demographics, time of admission, cause of injury, whether accompanied by multiple trauma, injury severity score, initial GCS score, computed tomography (CT) findings, pupillary light reflex, and the need of craniotomy. Baseline assessment was conducted by an independent assessor. Baseline prognostic risk was calculated according to the International Mission for Prognosis and Analysis of Clinical Trials (IMPACT) core model [16].
Patients were enrolled on days 7–14 post-injury and then assigned to trial arms. Trial interventions were initiated on the day of enrolment and continued for two weeks. Patients in the RMNS group received right median nerve stimulation in addition to routine treatment, which included management targeting intracranial hypertension and a variety of supportive treatments [17]. The electrical treatment was delivered via two electrodes that were applied on the volar surface of the right forearm for patients [11]. An electrical neuromuscular stimulator (Nuohe, Shanghai) supplied trains of asymmetric biphasic pulses at an amplitude of 15–20 mA (as tolerated with no obvious change of vital signals including heart rate, respiratory rate and blood pressure) with a pulse width of 300 μs at 40 Hz ON for 20 s and OFF for 40 s. The electrical stimulation treatment lasted for 8 h per day for 2 weeks. Patients assigned to the control group had their devices set to similar settings, but with no electric current applied. Over the period of trial intervention, patients in both arms continued to receive standard intensive care management. All enrolled patients received routine treatments according to Brain Trauma Foundation (BTF) guidelines during hospital stay.
Clinical assessments were performed on day 1 and day 7 of the stimulation protocol, and on day 28, 3 months and 6 months after injury. Assessments included GCS, FOUR, CRS-R, DRS and GOSE scores. Data on all adverse events and serious adverse events were recorded prospectively at each therapy session and at all outcome assessments. Assessments were done by the same assessor at baseline and at follow-up at each site, who was blind to the assignment.
Outcomes
The primary endpoint was the percentage of patients regaining consciousness 6 months after injury. Consciousness was defined as complete wakefulness and awareness of self and environment, and the ability to obey commands and intact light and deep reflexes. Death before primary endpoint assessment was treated as a competing risk, and analysed with competing risks regression.
The secondary endpoints were GCS, FOUR, CRS-R, DRS and GOSE scores on day 28, 3 months and 6 months after injury, and GCS and FOUR scores on day 1 and day 7 during stimulation. In addition, the duration of unconsciousness, duration of mechanical ventilation and length of ICU stay were also recorded as secondary endpoints. The GOSE was additionally classified into favourable (GOSE ≥ 5) and unfavourable outcomes (GOSE ≤ 4).
The safety of RMNS was assessed by the incidence of adverse events within 6 months post-injury, including but not limited to (1) seizures, (2) increased intracranial pressure and (3) intracranial bleeding.
Statistical analysis
On the basis of a previous single-centre randomised controlled trial (RCT) study on RMNS [12], we calculated that enrolment of 334 patients (167 per group) would provide the trial with 80% power to detect a between-group difference of 13.6% in the proportions of patients regaining consciousness at 6 months (primary outcome; 46.2% in the control group vs. 59.8% in RMNS group) at a significance level of 0.05 (one sided test). Assuming 12% withdrawal or loss to follow-up from the trial, we aimed to recruit a total of 380 patients (190 per group). A total of 64 missing data were detected in the trial, of which 17 had missing data at baseline, 6 for the primary endpoint, and 41 for secondary endpoints. A last observation carried forward approach was used for imputation of data in the primary and secondary outcome analysis.
All efficacy and safety analyses were done on the intention-to-treat population, defined as all participants assigned to two groups, regardless of actual treatment received and regardless of the number of stimulation completed. A per-protocol analysis was defined a priori to include participants who completed the stimulation and follow-up without major protocol violations, which could affect or compromise the safety or efficacy of the treatment.
The primary outcome is presented as a total percentage per group, and treated as a categorical variable. Between group differences for primary outcome measures were assessed using chi-squared test. Multivariable competing risks regression model (Fine and Gray model) was also fit, with death before regaining consciousness treated as a competing risk. Besides, to adjust for centre effects, a mixed-effect Cox regression model was established with centres treated as random effect. During secondary outcome analysis, continuous variables were assessed using the Wilcox test, and dichotomous GOSE was assessed using chi-squared test. Post hoc subgroup analyses were also conducted. p values were reported for comparisons between the two groups for the analyses of consciousness rate. Trajectory analysis was performed to estimate trajectories of GCS, FOUR, CRS-R and DRS scores and identify groups using random quadratic effect latent class trajectory modelling, and the optimal number of classes was chosen based on Akaike information criterion (AIC), Bayesian information criteria (BIC) and posterior probabilities and relative entropy. Detailed methods for latent class trajectory modelling were shown in Methods part of appendix (see ESM). Cochran–Armitage test was used to determine statistically significant trends in class variation between groups.
A two-tailed p value of 0.05 or less was used to define statistical significance. For secondary outcomes analysis, 19 comparisons were performed, so adjusted alpha was set to 0.003 according to Bonferroni adjustment. Statistical analyses were done using R (version 3.5.0), with R Studio (version 1.1.447) used as the implementation integrated development environment. The proportional hazards assumption was assessed by means of residual diagnostic tests using “cox.zph” function in Package “survival” (version 3.3-1). Plot of Cumulative incidence function was done with the “cuminc” function in “cmprsk” package (version 2.2-11). Multivariable competing risks regression model was done with the “crr” function in the “cmprsk” package (version 2.2-11). Multivariable mixed-effect Cox proportional hazards regression was done with the “coxme” function in the “coxme” package (version 2.2-16). Trajectory analysis was done with the “hlme” function in the “lcmm” package (version 1.9.5). The study was registered with ClinicalTrials.gov, NCT02645578.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
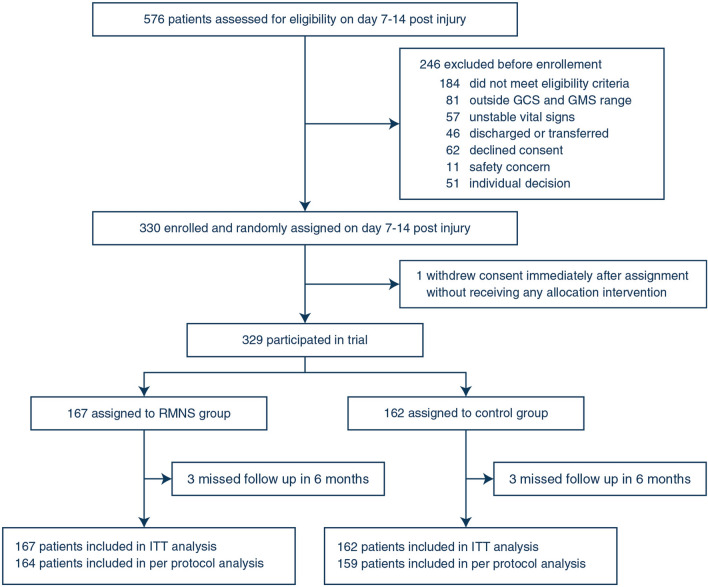
Between Mar 26, 2016, and Oct 18, 2020, 576 participants were screened for eligibility (Fig. 1). A total of 184 patients did not meet eligibility criteria, legal representatives of 62 potential participants declined consent, and 1 withdrew consent immediately after randomisation before initiating study intervention. The study included 329 patients in total, which were randomly assigned (intention-to-treat population) to treatment. 167 were assigned to the RMNS group and 162 to the control group. Six patients were lost to follow-up and a total of 164 in RMNS group and 159 patients in control group were included in the per-protocol analysis. The trial could not be fully blinded to the clinical treating team, as active stimulation could elicit twitching of the stimulated muscles in some patients. Time to study treatment after injury was similar between groups (8 days [interquartile range (IQR) 7–10] vs. 8 days [IQR 7–8], p = 0.27).
Fig. 1.
Trial profile. GCS Glasgow Coma Scale, GMS Glasgow Coma Scale Motor Part, RMNS right median nerve electrical stimulation, ITT intention to treat
There were no significant protocol deviations that affected the rights, safety, or wellbeing of participants or the scientific integrity of the study. Baseline demographics are shown in Table 1. Groups were well matched at baseline. Moreover, we calculated baseline prognostic risk according to the IMPACT core model to further assess comparability between groups. Baseline risks were comparable with death being predicted in 57 patients in the RMNS group and 57 patients in the control group.
Table 1.
Baseline demographics and characteristics of the intention-to-treat population
| Control (N = 162) | RMNS (N = 167) | p value | |
|---|---|---|---|
| Demographic characteristics | |||
| Gender | |||
| Male (%) | 118 (72.8) | 115 (68.9) | 0.502 |
| Female (%) | 44 (27.2) | 52 (31.1) | |
| Age (median [IQR], years old) | 50 [39, 58] | 48 [37, 56] | 0.078 |
| At admission | |||
| Injury causes | |||
| Road traffic incidence | 94 (58) | 101 (60.5) | 0.760 |
| Fallen injury | 39 (24.1) | 36 (21.6) | |
| Violence | 11 (6.8) | 8 (4.8) | |
| Others | 18 (11.1) | 22 (13.2) | |
| Initial GCS (median [IQR]) | 6 [5, 7] | 6 [4, 7] | 0.094 |
| Marshall Score (median [IQR]) | 5 [3, 5] | 5 [3, 5] | 0.185 |
| Subarachnoid haemorrhage (%) | |||
| Yes | 137 (84.6) | 150 (89.8) | 0.207 |
| No | 25 (15.4) | 17 (10.2) | |
| Initial pupillary reflex (%) | |||
| Both present | 81 (50) | 87 (52.1) | 0.639 |
| One absent | 31 (19.1) | 36 (21.6) | |
| Both absent | 50 (30.9) | 44 (26.3) | |
| Operation (%) | |||
| Yes | 131 (80.9) | 129 (77.2) | 0.502 |
| No | 31 (19.1) | 38 (22.8) | |
| At enrolment | |||
| Systolic BP (median [IQR], mmHg) | 130 [119, 138.75] | 125 [116, 137] | 0.062 |
| SpO2 (median [IQR], %) | 99 [98, 100] | 99 [98, 100] | 0.109 |
| GCS (median [IQR]) | 6 [5, 7] | 6 [5, 7] | 0.980 |
| Pupillary reflex (%) | |||
| Both present | 119 (73.5) | 131 (78.4) | 0.567 |
| One absent | 15 (9.3) | 13 (7.8) | |
| Both absent | 28 (17.3) | 23 (13.8) | |
| FOUR (median [IQR]) | 9 [7, 11] | 10 [7, 11] | 0.671 |
| CRS-R (median [IQR]) | 2 [2, 4] | 2 [2, 4] | 0.871 |
| DRS (median [IQR]) | 26 [25, 27] | 26 [25, 27] | 0.956 |
Data are n (%) or median [IQR]. Some percentages can add up to more than 100% due to rounding. RMNS right median nerve electrical stimulation, GCS Glasgow coma scale, FOUR Full Outline of Unresponsiveness scale, CRS-R Coma Recovery Scale-Revised, DRS Disability Rating Scale. Statistical significance assessed using chi-square test for categorical variables, and Wilcoxon rank sum test for continuous variables
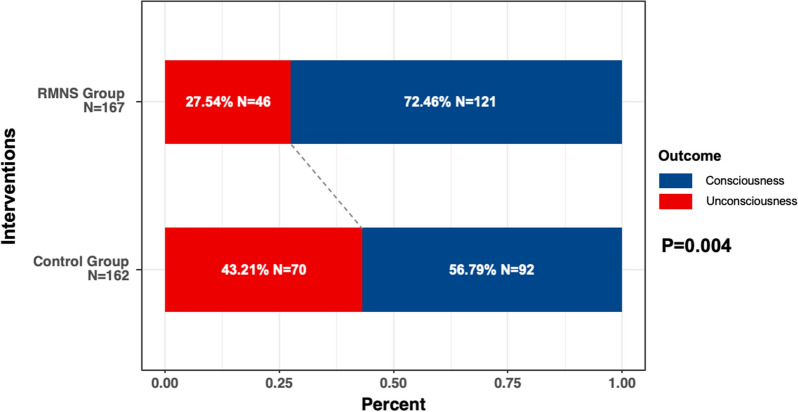
The primary analysis showed that the percentage of patients regaining consciousness 6 months after injury was 15.66% (95% confidence interval (CI) 5.46–25.87%) higher in the RMNS group than in the control group (n = 121, 72.46%, 95% CI 65.23–78.67% vs. n = 92, 56.79%, 95% CI 49.09–64.17%, p = 0.004; Fig. 2).
Fig. 2.
Percentage of patients who regained consciousness in both groups at 6 months after injury
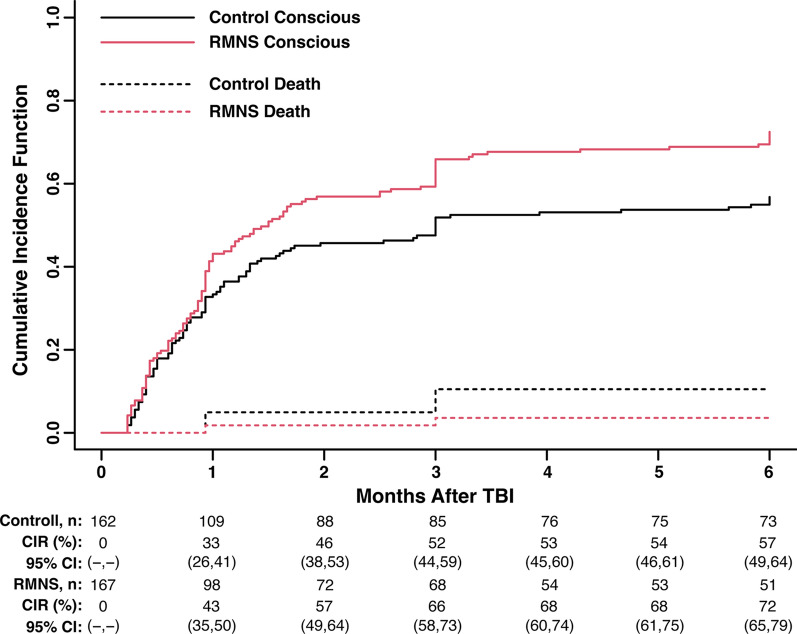
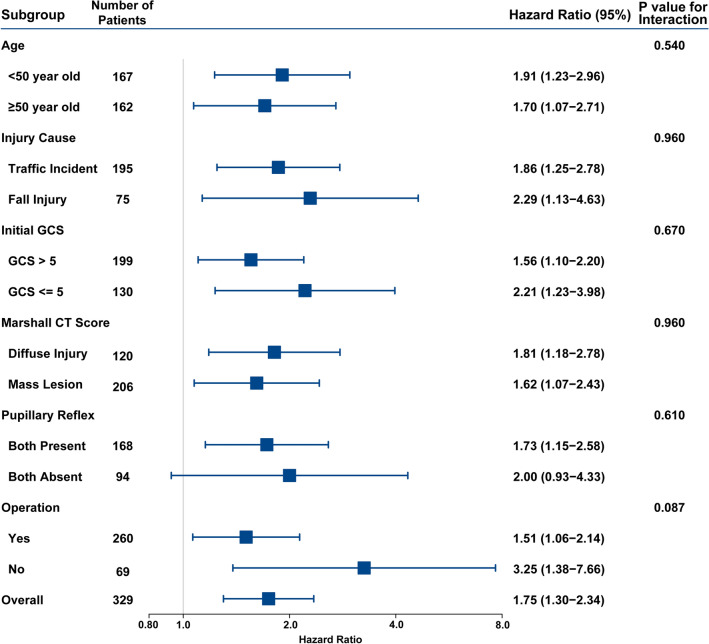
Exploratory analysis for 6 months consciousness was also performed. Cumulative incidence for consciousness showed that significantly more patients regained consciousness over time in the RMNS group (Fig. 3). A total of 24 patients (7.29%, 95% CI 4.95–10.62%) died during the study, among whom 17 patients belong to the control group, and 7 patients belong to the RMNS group. Multivariable competing risks regression model revealed that RMNS was significantly associated with a higher conscious rate at 6 months after injury, adjusting for baseline variables including age, gender, GCS and CT findings (hazard ratio 1.75 [95% CI 1.30–2.34], p = 0.0002, Fig. 4). Besides, in multivariable mixed-effect Cox regression model, adjusting for centre effects showed similar results (hazard ratio 1.73 [95% CI 1.27–2.36], p = 0.0005, Table S1). For those who regained consciousness during study, the median time to emergence from coma in the RMNS group was 28 (IQR 15–51) days post-injury, which was similar to control group (27 days, IQR 14–48, p = 0.47).
Fig. 3.
Cumulative incidence for consciousness and death in both groups
Fig. 4.
Subgroup analysis of consciousness rate. Shown are the results of subgroup analyses of the RMNS effect on consciousness rate. Ratios of consciousness in the RMNS group, as compared with the control group, are shown along with 95% confidence intervals. The results were adjusted for baseline variables including age, gender, GCS and CT findings
In secondary outcome comparisons, a threshold of alpha less than 0.003 was set after Bonferroni adjustment. GOSE was significantly increased in the RMNS group compared with the control group at 3 months (5 [IQR 3–7] vs. 4 [IQR 2–6], p = 0.002) and 6 months (6 [IQR 3–7] vs. 4 [IQR 2–7], p = 0.0005). Significantly more patients had favourable outcome in RMNS group at 3 months (106, 63.47% vs. 66, 40.74%, p < 0.0001) and 6 months (115, 68.86% vs. 65, 40.12%, p < 0.0001). The FOUR score was significantly higher in RMNS group compared with the control group at day 28 (15 [13–16] vs. 13 [11–16], p = 0.002). Detailed results are shown in table S2. Results for all outcomes were similar on the per-protocol analysis (table S3).
No difference was found in length of ICU stay after admission between groups (median 17 days [IQR 12–22] in the control group vs. 18 days [IQR 13–25] in the RMNS group, p = 0.50). No difference was found in length of ventilation after admission between groups (median 0 days [IQR 0–10] in control group vs. 0 days [IQR 0–10] in RMNS group, p = 0.59).
During explorative latent class trajectory analysis, random quadratic models were chosen based their better fitting performance (supplementary Table S4). Trajectory analysis for GCS score classified patients with TBI into four classes (supplementary Fig. S1 and table S5, S6), i.e. class with very slow GCS improvement, slow improvement, moderate improvement and rapid improvement. The Cochran–Armitage test showed that, compared with control group, significantly more patients in the RMNS group were present in classes with faster GCS improvement (p = 0.01). Further analysis showed that compared with the control group, significantly more patients who received the active treatment were allocated to classes with faster CRS-R and DRS improvement (p = 0.004 and 0.04, respectively, supplementary Figs. S2, S3 and table S7, S8). Difference in FOUR score trajectory was not significant between groups (p = 0.32, supplementary Fig. S4, Table S9).
Additional post hoc subgroup analyses were performed to evaluate any effects on primary outcome (Fig. 4). The result of subgroup analysis showed similar results with the overall primary outcome analysis that consciousness rate was improved in RMNS group. None of the P values for interaction were significant in any subgroup analyses.
Complications were similar in both groups (Table 2). Eighty-nine (53.29%) of 167 patients in the RMNS group and 92 (56.79%) of 162 patients in the control group had at least one complication (p = 0.60, total of 126 events vs 134 events). Most complications were common complications of severe TBI, and included pulmonary infection, hydrocephalus, deep vein thrombosis, urinary tract infection, and central nervous system infection. Three patients in the RMNS group and one patient in the control group had seizures, which was controlled with diazepam and sodium valproate. One patient in the control group developed paroxysmal sympathetic hyperactivity. No patients experienced intracranial bleeding in RMNS group, and one patient was reported to have delayed epidural haemorrhage in the control group. No patients showed increases in intracranial pressure above pre-treatment levels during the period of treatment in either group. There were no unexpected adverse events, or serious adverse events reported associated with the stimulation device.
Table 2.
Adverse events in two groups
| Control group (N = 162) | RMNS group (N = 167) | p value | |
|---|---|---|---|
| Any adverse events | 92 (56.79%) | 89 (53.29%) | 0.60 |
| Central nervous system | |||
| Seizure | 1 (0.62%) | 3 (1.8%) | 0.62 |
| PSH | 1 (0.62%) | 0 | 0.49 |
| Hydrocephalus | 7 (4.32%) | 8 (4.79%) | 1 |
| Encephalitis infection | 5 (3.09%) | 3 (1.8%) | 0.50 |
| Subdural hygroma | 1 (0.62%) | 0 | 0.49 |
| Epidural haemorrhage | 1 (0.62%) | 0 | 0.49 |
| Ischaemia cerebrovascular | 1 (0.62%) | 1 (0.6%) | 1 |
| Diabetes insipidus | 1 (0.62%) | 1 (0.6%) | 1 |
| Respiratory system | |||
| Pneumonitis | 89 (54.94%) | 82 (49.1%) | 0.34 |
| Pleural effusion | 5 (3.09%) | 3 (1.8%) | 0.50 |
| Pneumothorax | 1 (0.62%) | 1 (0.6%) | 1 |
| ARDS | 1 (0.62%) | 0 | 0.49 |
| Urinary system | |||
| Bladder infection | 4 (2.47%) | 4 (2.4%) | 1 |
| Acute kidney injury | 1 (0.62%) | 1 (0.6%) | 1 |
| Gastrointestinal system | |||
| Gastric haemorrhage | 5 (3.09%) | 3 (1.8%) | 0.50 |
| Alanine aminotransferase increased | 4 (2.47%) | 1 (0.6%) | 0.21 |
| Cardiovascular system | |||
| Deep vein thrombosis | 3 (1.85%) | 3 (1.8%) | 1 |
| Pulmonary embolism | 0 | 1 (0.6%) | 1 |
| Hypotension | 0 | 1 (0.6%) | 1 |
| Asystole | 0 | 1 (0.6%) | 1 |
| Blood system | |||
| Anaemia | 3 (1.85%) | 1 (0.6%) | 0.37 |
| INR increased | 1 (0.62%) | 1 (0.6%) | 1 |
| Bloodstream infection | 0 | 2 (1.2%) | 0.50 |
| Others | |||
| Skin ulceration | 1 (0.62%) | 2(1.2%) | 1 |
| Electrolyte disturbance | 1 (0.62%) | 1 (0.6%) | 1 |
| Ascites | 2 (1.23%) | 0 | 0.24 |
| Wound infection | 2 (1.23%) | 0 | 0.24 |
| Hypoalbuminemia | 0 | 1 (0.6%) | 1 |
| Drug allergy | 0 | 1 (0.6%) | 1 |
RMNS right median nerve electrical stimulation, PSH paroxysmal sympathetic hyperactivity, ARDS acute respiratory distress syndrome. Statistical significance assessed using chi-square test or Fisher’s exact test
Discussion
In this trial involving participants with a traumatic coma 7–14 days after brain injury, right median nerve electrical stimulation 8 h per day for two weeks showed a clinically meaningful effect on emergence from coma, as also on improvement of conscious level and functional outcome (e.g. GCS, CRS-R and DRS). Substantial limitations in trial design and conduct, including underpowering, incomplete blinding and risk of subjectivity in outcome assessments, however, warrant caution in drawing too strong conclusions. We do not consider the results of sufficient strength to motivate implementation into clinical practice, and suggest the need for a confirmatory study, in which some of the weakness of the current trial can be addressed.
In our study, multiple secondary outcomes were evaluated at various time points after injury. These included the FOUR score and CRS-R score at 6 months (to describe the level of consciousness), and the GOSE and DRS (to characterise functional outcome). GOSE at 6 months were significantly better in the treatment group. These results suggest that transcutaneous electrical stimulation has potential for improving functional recovery. Further, the trajectory analyses on different secondary outcome variables, which describe the course of recovery over 6 months post-injury, showed that patients receiving RMNS had more rapid improvement of GCS, CRS-R and DRS. Together, these data support the concept that the intervention may be effective in improving conscious level and the overall functional outcome in patients of traumatic coma.
In this study, all participants had a TBI event 7–14 days before recruitment and were clinically diagnosed as being in coma. The present data indicate that RMNS treatment initiated in the acute coma stage may achieve meaningful improvement on regaining consciousness, beyond spontaneous recovery. Notably, the treatment was initiated in the early post-injury period, and lasted for 2 weeks, meaning that the intervention was completed within 28 days post-injury. This protocol was in accordance with the original methodology of treating coma patients at early stage [9] and provides an appropriate context for treatment, since the diagnosis of a prolonged disorder of consciousness (pDOC), is defined by the failure to regain consciousness at or beyond 28 days after disease onset [18, 19].
The technique used in this trial provides a non-invasive treatment for traumatic coma patients who are being cared for in intensive care or high dependency units. Compared with other invasive therapeutic choices, which are logistically difficult and have recognised hazards in the acute phase, RMNS involves no intracranial mechanical insults, does not interrupt routine bedside activities, and appears not to increase complications. RMNS warrants future exploration, both for replication and to explore applicability in a wider therapeutic window and a broader etiological spectrum of disease.
Other options for improving conscious level in patients of TBI include pharmacologic, electromagnetic, mechanical, sensory, and regenerative therapies [20–24]. Early intensive neurorehabilitation bundles, combining physical, occupational, speech/language, and neuropsychological therapy, appear to improve long-term functional recovery [25, 26]. However, much of the evidence regarding treatments for pDOC involve small single-centre studies, and few interventions have been tested in appropriately powered multicenter studies [8]. Initially based on the indication or improving muscular power in paralysed limbs, right median nerve stimulation was first explored as an intervention aimed at coma arousal in small sample pilot studies [9], which were subsequently replicated in many countries [10, 11]. However, many of these studies had suboptimal design, and varied enormously in terms of confounders, recruitment criteria, intervention window, and treatment duration. Following the promising findings in a previous phase IIa study [12], we sought to address these deficiencies by employing early right median nerve stimulation, randomised design, and multicenter involvement. The results of the current study achieved similar treatment effects as in our previous study, and the levels of conscious patients were higher, implying an improvement of TBI management among centres. Thus, our results, combined with the previous evidence, provides useful evidence for benefit from an intervention likely to address the problem of coma after traumatic brain injury.
The role of neuromodulation through peripheral nerve stimulation is supported by reports of therapeutic potential in other recent publications [12, 27]. Right median nerve stimulation has also been employed in different diseases, including spinocerebellar ataxia [28], essential tremor [29], and autonomic nervous system dysfunction [30]. The cumulative incidence curve of our study showed long-term effects of RMNS treatment, and this trend was also observed in other studies [11, 12], with a similar electrical stimulation protocol as applied in this study. The mechanism behind it still requires further investigation, but may be due to that RMNS may raise the concentration of neurotrophins, such as brain-derived neurotrophic fact (BDNF), enhance synapses with ARAS, increase blood perfusion, and maintain stabilisation of neurotransmitters, leading to the survival of more neurons and hastening the recovery of coma patients [31]. Besides, the curve also implied that the optimal time window of coma stimulation for comatose TBI patients should be within 3 months. Our current results provide a basis for cautious optimism that the option of RMNS may provide benefit in terms of coma emergence, beyond pharmaceutical or surgical choices available at the early stage.
The limitations of this trial include failure to reach the original target population due to the coronavirus disease 2019 (COVID-19) pandemic, which caused this trial to be underpowered. Although we trained centres for determining emergence from coma, including the date of obeying commands, we acknowledge that the pragmatic clinical bedside approach we adopted towards determining emergence from coma may have introduced an element of subjectivity. Although the trial was intended to be blinded, this did not fully work out in practice since there was partial unblinding due to the muscle contractions in some patients of the intervention group. This experience is relevant to the design of future clinical trials on median nerve stimulation, and we suggest that, for example, covering the right forearm might be considered during stimulation. The subjectivity of primary outcome in an unblinded, and underpowered trial setting warrants a cautious interpretation of conclusions. We acknowledge that the primary outcome measure was not validated. Sedation may influence the primary endpoint assessment, which was minimised by halting sedative agents before evaluation. Aiming to mitigate possible elements of subjectivity in determining emergence from coma, we used multiple endpoints, including CRS-R as semi-quantitative method. The consistency in results across different endpoints lends support to the efficacy signal. We also have no data on consecutive steps from comatose to full conscious status, no data on the level of cognitive function, psychological health measures, and no data in withdrawal of life-sustaining therapy. Withdrawal of life-sustaining therapy is, however, seldom practiced in China. The rehabilitation and surgical treatments of cranial defect and hydrocephalus in both groups are not recorded. Our findings cannot be generalised to coma resulting from other etiologies, or DOC in TBI treated at later time points. Future clinical trials are important to validate these findings and its generalizability to different health care systems with various ethnicities. Additional data are needed to demonstrate the benefits as well as safety/harm of the intervention over one year or even longer term of follow-up. Our data do not provide any information on other protocols for RMNS stimulation.
We conclude that right median nerve stimulation, using the protocol that we adopted, applied within two weeks after TBI, and continued for 2 weeks, results in greater and more rapid emergence from coma at 6 months, when compared with control subjects. Right median nerve stimulation is a possible effective treatment to help achieve improvement in an acute coma following severe traumatic brain injury. If the benefit of the technique is confirmed in subsequent studies, it could provide a valuable addition to the management of patients with DOC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are immensely grateful to Edwin Cooper, who has supported the series of coma awakening study and practice in China over 17 years. We thank our patients and their families for helping us in our efforts to improve care and outcome for TBI.
The Asia Coma Electrical Stimulation (ACES) Participants: Jiemin Yao, Department of Neurosurgery, The Second People’s Hospital of Nanning, Nanning, China. Kai Luo, Department of Neurosurgery, The Second People’s Hospital of Nanning, Nanning, China. Jiarong Li, Department of Neurosurgery, Guangdong Provincial Hospital of Integrated Traditional Chinese and Western Medicine, Foshan, China. Deliang Liu, Department of Neurosurgery, Guangdong Provincial Hospital of Integrated Traditional Chinese and Western Medicine, Foshan, China. Xueming Ou, Department of Neurosurgery, Guangdong Provincial Hospital of Integrated Traditional Chinese and Western Medicine, Foshan, China. Lixin Ruan, Department of Neurosurgery, The Peopleʹs hospital of PingYang, Pingyang, China. Lie Chen, Department of Neurosurgery, The Peopleʹs hospital of PingYang, Pingyang, China. Jun Hong, Department of Neurosurgery, Tangshan Gongren Hospital, Tangshan, China. Shuwei Wang, Department of Neurosurgery, Tangshan Gongren Hospital, Tangshan, China. Haibo Wang, Department of Neurosurgery, Tangshan Gongren Hospital, Tangshan, China. Guodong Zheng, Department of Neurosurgery, Tangshan Gongren Hospital, Tangshan, China. Xide Zhu, Department of Neurosurgery, Linyi People’s Hospital, Linyi, China. Yangyu Cheng, Department of Neurosurgery, Changzhi Second People’s Hospital, Changzhi, China. Liansheng Long, Department of Neurosurgery, South Taihu Hospital, Huzhou, China. Wei Wang, Department of Neurosurgery, South Taihu Hospital, Huzhou, China. Zhonghua Wu, Department of Neurosurgery, South Taihu Hospital, Huzhou, China. Jiancun Wang, Department of Neurosurgery, Zhangjiajie City People’s Hospital, Zhangjiajie, China. Chuanping Huang, Department of Neurosurgery, The 421st Hospital of Chinese People’s Liberation Army, Guangzhou, China. Jin Lei, Brain Injury Centre, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China. Qiuyou Xie, Department of Neurosurgery, Guangzhou General Hospital of Guangzhou Military Region, Guangzhou, China. Xuelei Zhang, Department of Neurosurgery, Lishui City People’s Hospital, Lishui, China. Qinghua Du, Department of Neurosurgery, Lishui City People's Hospital, Lishui, China. Chao Yan, Department of Neurosurgery, Lishui City People’s Hospital, Lishui, China. Jianghong He, 14 Department of Neurosurgery, General Hospital of Beijing Military Region, Beijing, China. Xuebing Yu, Department of Neurosurgery, Shaoxing People's hospital, Shaoxing, China. Shouhua Lv, Department of Neurosurgery, Tengzhou City Center People’s Hospital, Tengzhou, China. Zhaosheng Sun, Department of Neurosurgery, Harrison International Peace Hospital, Hengshui, China. Dai Liu, Department of Neurosurgery, The Affiliated Huaian No. 1 People’s Hospital of Nanjing Medical University, Huaian, China. Xin Li, Department of Neurosurgery, The Brain Hospital of Hunan Province, Changsha, China. Qingping Tang, Department of Neurosurgery, The Brain Hospital of Hunan Province, Changsha, China. Junquan Wang, Department of Neurosurgery, The Brain Hospital of Hunan Province, Changsha, China. Jianxin Zhu, Department of Neurosurgery, Liaocheng Brain Hospital, Liaocheng, China. Xueguang Zhang, Department of Neurosurgery, Liaocheng Brain Hospital, Liaocheng, China. Hanyu Sun, Department of Neurosurgery, Liaocheng Brain Hospital, Liaocheng, China. Xiaoliang Yang, Department of Neurosurgery, Baoji 3rd Hospital of Chinese People’s Liberation Army, Baoji, China. Dongdong Wang, Department of Neurosurgery, Yanjiao People’s Hospital, Sanhe, China. Yijun Bao, Department of Neurosurgery, the Fourth Affiliated Hospital of China Medical University, Shenyang, China.
Author contributions
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated in the concept, design, analysis, writing, or revision of the manuscript. All authors participated in the reported analyses or interpretation of results relevant to their domain of interest. XW, LX, AIRM, DM and GG prepared the draft manuscript and coordinated its finalisation. XW and LX performed statistical analyses and drafting of tables and figures. AIRM, DM, YX, JJ, JF and GG revised the manuscript and provided input regarding the presentation of results and drafting of figures. All authors approved the final manuscript.
Funding
National natural science foundation of China Grant 81671198, 81971699; Clinical Research Plan of Shanghai Hospital Development Center Grant 16CR3011A; Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant 20152212.
Data sharing statement
Researchers who submit a methodologically sound study proposal that is approved by the management committee can have access to the study protocol, individual participant data, data dictionary, analytic code and analysis scripts. A Data Access Agreement is required, and all access must comply with regulatory restrictions imposed on the original study.
Declarations
Conflicts of interest
All authors declare no competing interests.
Ethics statement
The ACES trial has been conducted in accordance with all relevant laws of the People’s Republic of China, including but not limited to, the relevant privacy and data protection laws and regulations (the “Privacy Law”), the relevant laws and regulations on the use of human materials, and all relevant guidance relating to clinical studies from time to time in force including, but not limited to, the ICH Harmonised Tripartite Guideline for Good Clinical Practice (CPMP/ICH/135/95) (“ICH GCP”) and the World Medical Association Declaration of Helsinki entitled “Ethical Principles for Medical Research Involving Human Subjects”. Ethical approval was obtained for all recruiting sites.
Footnotes
The ACES Participants are listed in the Acknowledgement section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiang Wu, Li Xie and Jin Lei are joint first authors.
Yajun Xue, Junfeng Feng and Guoyi Gao have contributed equally.
Contributor Information
Guoyi Gao, Email: gao3@sina.com.
on behalf of the ACES Participants:
Jiemin Yao, Kai Luo, Jiarong Li, Deliang Liu, Ou Xueming, Lixin Ruan, Lie Chen, Jun Hong, Shuwei Wang, Haibo Wang, Guodong Zheng, Xide Zhu, Yangyu Cheng, Liansheng Long, Wei Wang, Wu Zhonghua, Jiancun Wang, Chuanping Huang, Jin Lei, Qiuyou Xie, Xuelei Zhang, Qinghua Du, Chao Yan, Jianghong He, Xuebing Yu, Shouhua Lv, Zhaosheng Sun, Dai Liu, Xin Li, Qingping Tang, Junquan Wang, Jianxin Zhu, Xueguang Zhang, Hanyu Sun, Xiaoliang Yang, Dongdong Wang, and Yijun Bao
References
- 1.Steyerberg EW, et al. Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: a European prospective, multicentre, longitudinal, cohort study. Lancet Neurol. 2019;18(10):923–934. doi: 10.1016/S1474-4422(19)30232-7. [DOI] [PubMed] [Google Scholar]
- 2.Teasdale GM, Murray L. Revisiting the Glasgow Coma Scale and Coma Score. Intensive Care Med. 2000;26(2):153–154. doi: 10.1007/s001340050037. [DOI] [PubMed] [Google Scholar]
- 3.Gao G, et al. Clinical characteristics and outcomes in patients with traumatic brain injury in China: a prospective, multicentre, longitudinal, observational study. Lancet Neurol. 2020;19(8):670–677. doi: 10.1016/S1474-4422(20)30182-4. [DOI] [PubMed] [Google Scholar]
- 4.McCrea MA, et al. Functional outcomes over the first year after moderate to severe traumatic brain injury in the prospective, Longitudinal TRACK-TBI study. JAMA Neurol. 2021;78(8):982–992. doi: 10.1001/jamaneurol.2021.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang Q, et al. Prevalence of persistent vegetative state in patients with severe traumatic brain injury and its trend during the past four decades: a meta-analysis. NeuroRehabilitation. 2017;40(1):23–31. doi: 10.3233/NRE-161387. [DOI] [PubMed] [Google Scholar]
- 6.van Veen E, et al. Occurrence and timing of withdrawal of life-sustaining measures in traumatic brain injury patients: a CENTER-TBI study. Intensive Care Med. 2021;47(10):1115–1129. doi: 10.1007/s00134-021-06484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giacino JT, et al. Placebo-controlled trial of amantadine for severe traumatic brain injury. N Engl J Med. 2012;366(9):819–826. doi: 10.1056/NEJMoa1102609. [DOI] [PubMed] [Google Scholar]
- 8.Edlow BL, et al. Therapies to restore consciousness in patients with severe brain injuries: a gap analysis and future directions. Neurocrit Care. 2021;35(Suppl 1):68–85. doi: 10.1007/s12028-021-01227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper JB, et al. Right median nerve electrical stimulation to hasten awakening from coma. Brain Inj. 1999;13(4):261–267. doi: 10.1080/026990599121638. [DOI] [PubMed] [Google Scholar]
- 10.Liu JT, et al. Regaining consciousness for prolonged comatose patients with right median nerve stimulation. Acta Neurochir Suppl. 2003;87:11–14. doi: 10.1007/978-3-7091-6081-7_3. [DOI] [PubMed] [Google Scholar]
- 11.Peri CV, et al. Pilot study of electrical stimulation on median nerve in comatose severe brain injured patients: 3-month outcome. Brain Inj. 2001;15(10):903–910. doi: 10.1080/02699050110065709. [DOI] [PubMed] [Google Scholar]
- 12.Lei J, et al. Right median nerve electrical stimulation for acute traumatic coma patients. J Neurotrauma. 2015;32(20):1584–1589. doi: 10.1089/neu.2014.3768. [DOI] [PubMed] [Google Scholar]
- 13.Guoyi Gao EC, Jiang J (2011) Right median nerve stimulation in posttraumatic coma. In: Abstracts from the 10th international neurotrauma symposium (INTS) April 27–30, 2011. 2011, Journal of Neurotrauma: Shanghai, China
- 14.Gao G (2022) Scans: a story of Shanghai coma afferent nerve stimulation on traumatic coma. In: Abstracts from The 15th International Neurotrauma Symposium. 2022, Journal of Neurotrauma: Berlin, Germany
- 15.Wu X, et al. Right median nerve electrical stimulation for acute traumatic coma (the Asia Coma Electrical Stimulation trial): study protocol for a randomised controlled trial. Trials. 2017;18(1):311. doi: 10.1186/s13063-017-2045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steyerberg EW, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5(8):e165. doi: 10.1371/journal.pmed.0050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carney N, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;80(1):6–15. doi: 10.1227/NEU.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 18.Giacino JT, et al. Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology. 2018;91(10):450–460. doi: 10.1212/WNL.0000000000005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondziella D, et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur J Neurol. 2020;27(5):741–756. doi: 10.1111/ene.14151. [DOI] [PubMed] [Google Scholar]
- 20.Thibaut A, et al. Therapeutic interventions in patients with prolonged disorders of consciousness. Lancet Neurol. 2019;18(6):600–614. doi: 10.1016/S1474-4422(19)30031-6. [DOI] [PubMed] [Google Scholar]
- 21.Gosseries O, et al. Amantadine, apomorphine and zolpidem in the treatment of disorders of consciousness. Curr Pharm Des. 2014;20(26):4167–4184. [PubMed] [Google Scholar]
- 22.Raguz M, et al. Structural changes in brains of patients with disorders of consciousness treated with deep brain stimulation. Sci Rep. 2021;11(1):4401. doi: 10.1038/s41598-021-83873-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cain JA, et al. Ultrasonic deep brain neuromodulation in acute disorders of consciousness: a proof-of-concept. Brain Sci. 2022;12(4):428. doi: 10.3390/brainsci12040428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox CS, Jr, et al. Treatment of severe adult traumatic brain injury using bone marrow mononuclear cells. Stem Cells. 2017;35(4):1065–1079. doi: 10.1002/stem.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giacino JT, Katz DI, Whyte J. Neurorehabilitation in disorders of consciousness. Semin Neurol. 2013;33(2):142–156. doi: 10.1055/s-0033-1348960. [DOI] [PubMed] [Google Scholar]
- 26.Seel RT, et al. Specialized early treatment for persons with disorders of consciousness: program components and outcomes. Arch Phys Med Rehabil. 2013;94(10):1908–1923. doi: 10.1016/j.apmr.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 27.Yu YT, et al. Transcutaneous auricular vagus nerve stimulation in disorders of consciousness monitored by fMRI: the first case report. Brain Stimul. 2017;10(2):328–330. doi: 10.1016/j.brs.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Chen CC, et al. Neuromuscular electrical stimulation of the median nerve facilitates low motor cortex excitability in patients with spinocerebellar ataxia. J Electromyogr Kinesiol. 2015;25(1):143–150. doi: 10.1016/j.jelekin.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Reis C, et al. Essential tremor amplitude modulation by median nerve stimulation. Sci Rep. 2021;11(1):17720. doi: 10.1038/s41598-021-96660-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maharjan A, et al. Investigation of the optimal parameters of median nerve stimulation, using a variety of stimulation methods, and its effects on heart rate variability: a systematic review. Neuromodulation. 2022;25:1268–1279. doi: 10.1016/j.neurom.2021.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Cooper EB, Scherder EJ, Cooper JB. Electrical treatment of reduced consciousness: experience with coma and Alzheimer’s disease. Neuropsychol Rehabil. 2005;15(3–4):389–405. doi: 10.1080/09602010443000317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Researchers who submit a methodologically sound study proposal that is approved by the management committee can have access to the study protocol, individual participant data, data dictionary, analytic code and analysis scripts. A Data Access Agreement is required, and all access must comply with regulatory restrictions imposed on the original study.