Abstract
Background
Intraoperative hypotension (IOH) is a common side effect of non-cardiac surgery that might induce poor postoperative outcomes. The relationship between the IOH and severe postoperative complications is still unclear. Thus, we summarized the existing literature to evaluate whether IOH contributes to developing severe postoperative complications during non-cardiac surgery.
Methods
We conducted a comprehensive search of PubMed, Embase, Cochrane Library, Web of Science, and the CBM from inception to 15 September 2022. The primary outcomes were 30-day mortality, acute kidney injury (AKI), major adverse cardiac events (myocardial injury or myocardial infarction), postoperative cognitive dysfunction (POCD), and postoperative delirium (POD). Secondary outcomes included surgical-site infection (SSI), stroke, and 1-year mortality.
Results
72 studies (3 randomized; 69 non-randomized) were included in this study. Low-quality evidence showed IOH resulted in an increased risk of 30-day mortality (OR, 1.85; 95% CI, 1.30–2.64; P < .001), AKI (OR, 2.69; 95% CI, 2.15–3.37; P < .001), and stroke (OR, 1.33; 95% CI, 1.21–1.46; P < .001) after non-cardiac surgery than non-IOH. Very low-quality evidence showed IOH was associated with a higher risk of myocardial injury (OR, 2.00; 95% CI, 1.17–3.43; P = .01), myocardial infarction (OR, 2.11; 95% CI, 1.41–3.16; P < .001), and POD (OR, 2.27; 95% CI, 1.53–3.38; P < .001). Very low-quality evidence showed IOH have a similar incidence of POCD (OR, 2.82; 95% CI, 0.83–9.50; P = .10) and 1-year-mortality (OR, 1.66; 95% CI, 0.65–4.20; P = .29) compared with non-IOH in non-cardiac surgery.
Conclusion
Our results suggest IOH was associated with an increased risk of severe postoperative complications after non-cardiac surgery than non-IOH. IOH is a potentially avoidable hazard that should be closely monitored during non-cardiac surgery.
Keywords: Intraoperative hypotension, IOH, Non-cardiac surgery, Severe postoperative complications
1. Introduction
Intraoperative hypotension (IOH) is a common side-effect during non-cardiac surgery that has received much attention recently because of its frequent occurrence and presumed adverse consequences [1]. More than 90% of patients receiving anesthesia for the surgery are expected to experience one or more IOH [2]. Factors such as age, surgical type, anesthetic drugs, surgical manipulation, and existing comorbidities can contribute to developing IOH [3,4]. IOH reduces perfusion of organs and results in major kidney damage, neurological or cardiac events, and even death [5]. The rate of acute kidney injury, 30-day mortality, myocardial injury, and postoperative delirium can be up to 23.7%, 8.0%, 2.3%, and 14%, respectively. However, not all studies have reported an association between IOH and postoperative complications [6]. Clinical studies on the association between IOH during non-cardiac surgery and postoperative adverse outcomes remain unclear and controversial [[6], [7], [8]].
Until now, no widely accepted definition of IOH has been available [9]. The incidence of IOH varies in the literature depending on its definition used [9]. Several reviews [10,11] have focused on the association of IOH with postoperative morbidity and mortality. However, the duration of IOH was not reported, and the selected outcomes are not comprehensive in these reviews, meaning this important topic has yet to be fully explored. Furthermore, several large cohort studies [20,24,43,44] focusing on this issue have been published recently.
Therefore, the authors decided to conduct an updated meta-analysis to evaluate the effects of IOH on postoperative organ dysfunction and severe postoperative complications in non-cardiac surgery.
2. Methods
Our study aim to appraise the association of IOH with severe postoperative complications in patients undergoing non-cardiac surgery. We wrote the review based on Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (registration number: CRD42021278672) [12].
2.1. Data sources, search strategy, and eligibility criteria
We conducted a comprehensive literature search of PubMed, Embase, Cochrane Library, Web of Science, and the Chinese Biomedical Literature database (CBM) from inception to 10 July 2022. Weekly e-mail alert was received on the basis of a previously developed search strategy saved in PubMed (i.e., ‘MyNCBI’) and Embase for any new potential studies. The literature search was rerun for all relevant databases on 15 September 2022. No limits or filters on the searches were applied. We also hand-searched the references of selected studies for potentially relevant articles. Combinations of the following Medical Subject Headings (MeSH) terms and keywords were used: hypotension, hypotensive, blood pressure, artery pressure, arterial pressure, systolic pressure, diastolic pressure, vascular hypotension, low blood pressure, intraoperative period, intraoperative periods, low mean arterial pressure, intraoperative, intra-operative, complication, complications, mortality, fatal outcome, organ damage, organ failure, postoperative cognitive dysfunction, kidney injury, AKI, heart failure, myocardial damage, renal failure, cerebrovascular accident, non-cardiac surgery, postoperative. Appendix S1 shows the detailed search strategy.
Studies were eligible if they met the predefined inclusion and exclusion criteria described in Appendix S2.
2.2. Study selection
Two reviewers (JY and HW) independently screened titles and abstracts for potential inclusion. A full-text assessment was made to determine the final inclusion of the potentially relevant articles. Between each assessment, we discussed the results to reach a consensus on interpreting the inclusion criteria. We resolved any disagreements regarding study eligibility by consensus, and a third reviewer (XDD) was consulted if necessary. The relevant study authors were contacted for clarification if the information required to assess eligibility was unavailable or unclear. We used EndNote software version X8 (Clarivate Analytics) to identify duplicate publications. When a hospital or institution published their cases more than once, if the recruitment periods overlapped, we included the paper with a bigger sample to minimize the possibility of double counting [13].
2.3. Data extraction
All data were collected using a predefined standardized form. Two authors (HL and SSX) extracted the data independently and in duplicate. We resolved discrepancies through discussion to achieve a consensus. A pilot test was performed to ensure consistency in the data extraction process before the formal data collection. Severe postoperative complications were defined according to the Clavien-Dindo classification (grade III, IV, or V) [14].
The primary outcomes were 30-day mortality, acute kidney injury (AKI), major adverse cardiac events (myocardial injury or myocardial infarction), postoperative cognitive dysfunction (POCD), and postoperative delirium (POD). Secondary outcomes included surgical-site infection (SSI), stroke, and 1-year mortality. The following data were extracted as follows: general characteristics of included studies (author names, title, publication date), type of the study, sample size, the definition of IOH, study subject characteristics (demographic characteristics, age, sex, comorbidities, type of surgery), outcome measures and analyses (number of events, types of postoperative patient outcomes). Study authors were contacted to obtain missing information or clarify the available information. However, we have yet to receive a response at the time of submission.
2.4. Risk of bias and certainty of evidence
Two reviewers (HL and XY) assessed the risk of bias in included studies using the Cochrane Risk of Bias tool 2.0 (RoB 2) for randomized trials and the Newcastle-Ottawa scale (NOS) for observational cohort and case-control studies [15,16]. There are five domains of bias included in RoB 2: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results. Three levels can be assigned to each domain: low risk of bias, some concerns, or high risk of bias. For cohort and case-control studies, there were three grouping items of the NOS scale as follows: selection (maximum 4 points), comparability (maximum 2 points), and exposure/outcomes (maximum 3 points). An observational study can had a maximum of nine stars. We categorized the observational study as poor (0–3 points), fair (4–6 points), or high (≥7 points) quality. Any disagreements that arose between the reviewers were resolved through discussion. A third reviewer (XDD) was to settle unresolved disputes. We used the GRADEpro guideline development tool (GDT) app to rate evidence and present it in a summary of findings table [17].
2.5. Data analysis
Characteristics of each study and results were described and tabulated. Heterogeneity between studies was calculated using the I2statistic, which describes the percentage of total variation across studies attributable to heterogeneity rather than chance. The degree of heterogeneity was classified into four categories: low (I2 ≤ 25%), moderate (25% < I2 ≤ 50%), large (50%< I2 ≤ 75%), or very large (I2 > 75%). I2 > 75% is considered to indicate substantial heterogeneity. We used the an inverse variance, a fixed-effects method if I2 was less than 50%. For I2 was 50% or greater. a random-effects method was used. Publication bias was assessed by visually inspecting a funnel plot and also evaluated by using the Egger test. A two-sided p-value less than 0.05 will be considered statistically significant. All analyses will be performed using Stata statistical software version 13.0 (StataCorp, College Station, Texas).
We planned a sensitivity analysis, excluding studies at high risk of bias (e.g., NOS score less than four stars) to assess the robustness of our findings. Considering transplant surgery had distinctly different patient populations and surgical manipulation from non-cardiac surgery, we planned subgroup analyses by the type of surgery (transplant surgery vs. non-transplant surgery).
3. Results
3.1. Search results and study selection
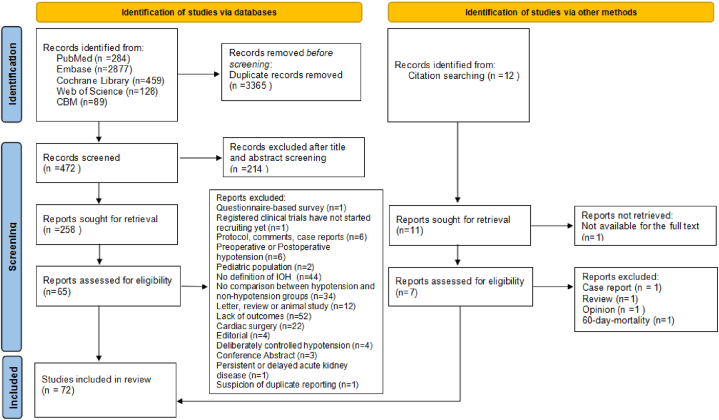
The initial search in PubMed (n = 284), Embase (n = 2877), Cochrane Library (n = 459), Web of Science (n = 128), and the CBM (n = 89) resulted in 3837 articles. Twelve articles were identified through hand-searching. A total of 269 potentially eligible records were identified after screening (n = 214) and removing duplicates (n = 3365). 197 articles were excluded after full-text assessment (reasons for exclusion are described in Appendix S3). Two articles focused on similar outcomes were reported by the same hospital, and the study period overlapped [18,19]. Thus, we only included the study with a bigger sample to minimize the possibility of double counting [19]. The flowchart of the study selection process is presented in Fig. 1.
Fig. 1.
PRISMA Flow Diagram.
3.2. Study characteristics
A total of 72 studies published between 1998 and 2022 were included in the systematic review and meta-analysis [7,[19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89]]. These articles were published in English, Portuguese, and Chinese (with sample sizes ranging from 28 to 358,391). Sixty-nine (95.83%) were observational studies, and three (4.17%) were randomized controlled trials (RCTs). The included studies showed heterogeneity in the participant characteristics, the definition of IOH, and the duration of IOH. Twenty-eight studies investigated the effect of IOH on AKI, 4 studied myocardial injury, 10 studied myocardial infarction, 4 reported stroke, 17 reported postoperative delirium (POD), 7 reported postoperative cognitive decline (POCD), 7 studies reported 30-day mortality, 2 reported 1-year mortality, and 3 reported surgical-site infection (SSI). The main characteristics of the included studies are presented in Table 1. Appendix S4 shows the definition of the outcomes used in the included studies.
Table 1.
Characteristics of the included studies.
| First author, yr | Study design | Study period | Country | Setting | Age (yr) | Sex (male, %) | Comorbidity (%) | Type of surgery | Type of anesthesia | Outcomes | sample size | No. of IOH | Definition and duration of IOH | Main findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Park 2020 | Cohort | 2004–2015 | South Korea | Seoul National University Hospital; Seoul National University Bundang Hospital | 58 (44–70) | 47.4 | Not reported | General, Neurosurgery, Orthopedics, Urologic, Thoracic surgery | GA; LA | AKI | 75,224 | 53,180 | MAP<65 mmHg; Not reported |
IOH is associated with higher risks of postoperative AKI after noncardiac surgery |
| Van Waes2016 | Cohort | 2010/01/01–2011/12/31 | Canada | University Health Network Hospital Toronto; University Medical Center Utrecht | 73.7 (7.8) | 69.1 | 61.6 | Vascular surgery | GA; LA | Myocardial injury; myocardial infarction; 30-day mortality | 890 | 450 | MAP<60 mmHg; >1 min |
IOH was associated with postoperative myocardial injury |
| Liao 2020 | Cohort | 2017/11–2019/11 | China | Affiliated Hospital of Qingdao University | 59 (52–65) | 60.8 | 40.0 | Liver resection | GA | AKI | 796 | 164 | MAP<65 mmHg; >10 min |
IOH was associated with AKI in age ≥65 years patients following liver resection |
| Sun 2015 | Cohort | 2009/11–2012/12 | Canada | Toronto General Hospital | 61 (14) | 47.0 | Not available | Noncardiac | GA | AKI | 5127 | 3337 | MAP<65 mmHg; >5 min |
IOH is associated with postoperative AKI |
| Tang 2019 | Cohort | 2011/12/01–2016/07/01 | China | Third Xiangya Hospital | 18–60 | 55.6 | Not available | Noncardiac | GA | AKI | 4952 | 144 | MAP<55 mmHg; >10 min |
There was a considerably increased risk of postoperative AKI when IOH last for more than 10 min |
| Braüner2020 | Cohort | 2018/01/01–2018/12/31 | Denmark | Copenhagen University Hospital | 82.4 (8.8) | 73.2 | 46.2 | Hip fracture | GA | AKI | 299 | 114 | MAP≦55 mmHg; >5 min |
AKI was common following hip fracture surgery and associated with increased mortality |
| Davison 2022 | Cohort | 2015/01–2019/12 | USA | Providence Sacred Heart Medical Center | 62.3 (16.6) | 54.0 | 57.0 | Noncardiac | GA | AKI | 3507 | 1732 | MAP<55 mmHg; >10 min |
IOH was an independent risk factor for AKI |
| Tobar2018 | Cohort | 2010/01–2013/03 | Chile | Hospital Clínico Universidad de Chile | 73 (7) | 39.3 | Not reported | elective open colon surgery | GA | POD | 28 | 17 | MAP↓>20%; Not reported |
Post-operative lactate and rSO2 variables were not associated with delirium |
| Jang 2019 | Cohort | 2011/01–2015/01 | South Korea | Anam Hospital | 77.6 (65–97) | 26.6 | 56.8 | Femoral neck fracture | GA | AKI | 248 | 44 | MAP<55 mmHg; >5 min |
AKI was found to occur frequently after surgery for femur neck fracture |
| Sessler 2018 | RCT | 2011/01–2013/12 | 22 countries | 88 centers in 22 countries | 69 (10) | 53.0 | 86.0 | Noncardiac | GA; LA | Myocardial infarction | 9765 | 3404 | SBP<90 mmHg require treatment; >10 min |
IOH associated with a composite of myocardial infarction and death |
| Hallqvist2017 | Cohort | 2012/10–2013/05; 2015/10–2016/04 |
Sweden | Karolinska University Hospital | 67 (58–74) | 53.0 | 44.0 | Noncardiac | GA; LA | AKI | 470 | 286 | SBP↓>40%; >5 min |
There was a high incidence of perioperative AKI in noncardiac surgery |
| Wang 2019 | Cohort | 2018/04/04–2018/12/28 | China | Shanghai Eye, Ear, Nose, and Throat Hospital | 65.5 (6.4) | 99.1 | 34.6 | Laryngectomy | LA | POD | 323 | 28 | SBP↓>30%; >30 min |
IOH lasting at least 30 min is a risk factor associated with POD |
| Xu2015 | Cohort | 2008/03/01–2010/02/28 | China | Five university hospitals located in different regions of China | 69.7 (6.4) | 52.0 | 56.6 | Noncardiac | GA; LA | Myocardial infarction | 1422 | 455 | SBP↓>30%; >10 min |
IOH is an independent risk factor of major adverse cardiac events in Chinese elderly patients who underwent non-cardiac surgery |
| Alghanem2020 | Cohort | 2010/01–2015/01 | Jordan | The University of Jordan | 71.7 (14.2) | 52.8 | 77.3 | traumatic hip surgery | GA; Spinal anesthesia | AKI; myocardial infarction | 502 | 91 | SBP↓≧30%; >10 min |
There was an association between IOH and post-operative complications in patients undergoing traumatic hip surgery |
| Monk 2015 | Cohort | 2001–2008 | USA | Six Veterans Affairs medical centers | 59.5 (12.8) | 92.8 | Not reported | Noncardiac | GA; LA | 30-day mortality | 18,756 | 3407 | MAP<55 mmHg; >5 min |
IOH is associated with increased 30-day operative mortality |
| Hirsch 2015 | Cohort | 2001/06–2006/04 | USA | University of California | 73.6 (6.2) | 49.4 | Not reported | Noncardiac | GA; LA | POD | 540 | 32 | MAP<50 mmHg; Not reported |
IOH had predictive value in postoperative delirium |
| Robinson 2009 | Cohort | 2006/10–2007/07 | USA | Denver Veterans Affairs Medical Center | 64 (9) | 97.0 | Not reported | Noncardiac | GA; LA | POD | 144 | 78 | SBP<90 mmHg; Not reported |
Delirium is common in elderly patients after a major operation |
| Tognoni2011 | Cohort | Not reported | Italy | University-Hospital San Martino | 74.3 (0.4) | 90.0 | 55.6 | Urological | GA; LA | POD | 90 | 25 | SBP<90 mmHg; Not reported |
Age, cognitive and functional status, previous history of delirium and IOH are the best predictor of POD |
| Langer 2019 | RCT | 2014/11–2016/04 | Italy | Policlinico Ca’ Granda Hospital | 80 (4) | 47.5 | Not reported | Noncardiac | GA | POD; POCD | 101 | 50 | MAP↓>10%; Not reported |
IOH did not correlate with POD or POCD in elderly patients undergoing general anesthesia for non-cardiac surgery |
| Kobayashi 2021 | Cohort | 2009/04/01–2018/03/31 | Japan | Jichi Medical University Saitama Medical Center | ≥50 | 64.3 | 56.8 | Noncardiac | GA | AKI | 6296 | 5800 | SBP<90 mmHg; >5 min |
IOH is associated with AKI |
| Salmasi2017 | Cohort | 2005/01/06–2014/03/01 | USA | Cleveland Clinic | 59 (15) | 44.3 | Not reported | Noncardiac | GA; LA | Myocardial injury; AKI | 57,315 | 41,085 | MAP<65 mmHg; Not reported |
IOH is progressively related to both myocardial injury and AKI |
| Babazade2016 | Cohort | 2009–2013 | USA | Cleveland Clinic | Not reported | 57.0 | 41.0 | Noncardiac | GA | SSI | 2521 | 801 | MAP<55 mmHg; >1 min |
There is no association between IOH and SSI after colorectal surgery |
| Sessler 2012 | Cohort | 2005/01–2012/12 | USA | Cleveland Clinic | >40 | Not reported | Not reported | Noncardiac | GA | 30-day mortality | 24,120 | 7695 | MAP<70 mmHg; Not reported |
Hospital stay and mortality are increased in patients having a low blood pressure |
| Hsieh 2016 | Case-control | 2005/01–2011/12 | USA | Cleveland Clinic | 72 (11) | 37 | 80 | Noncardiac | GA | Stroke | 502 | 387 | MAP<75 mmHg; Not reported |
There is no association between IOH and postoperative stroke |
| Yu2018 | Cohort | 2005/01–2015/12 | South Korea | Asan Medical Center | 54.8 (12.5) | 61.8 | 42.0 | Percutaneous nephrolithotomy | LA | AKI | 662 | 176 | MAP<70 mmHg; >1 min |
IOH is an important risk factor for AKI |
| Hallqvist2016 | Cohort | 2012/10–2013/05 | Sweden | Karolinska University Hospital | 67 (57–74) | 47.0 | 43.0 | Noncardiac | GA; LA | Myocardial injury; myocardial infarction | 300 | 34 | SBP↓>50%; >1 min |
There was an association between IOH and myocardial damage |
| Wachtendorf2022 | Cohort | 2005–2017 | USA | Massachusetts General Hospital; Beth Israel Deaconess Medical Center | 52.8 (16) | 48.5 | 38.2 | Noncardiac | GA | POD | 316,717 | 140,047 | MAP<55 mmHg; >15 min |
IOH is associated with POD |
| Wongtangman2021 | Cohort | 2005/11–2017/09 | USA | Massachusetts General Hospital; Beth Israel Deaconess Medical Center | 53.0 (16.1) | 49.0 | 40.4 | Noncardiac | GA | Stroke | 358,391 | 160,109 | MAP<55 mmHg; >15 min |
IOH is not associated with stroke |
| Williams-Russo 1999 | RCT | 1993/03–1995/08 | USA | Hospital for Special Surgery | 55–65 | 55.0 | Not reported | Total hip replacement | Epidural anesthesia | POD, myocardial infarction | 235 | 117 | MAP<55 mmHg; Not reported |
Elderly patients can safely receive controlled hypotensive epidural anesthesia |
| Boos 2005 | Case-control | 2001/09/24–2001/12/07 | Brazil | Hospital Governador Celso Ramos | 53.9 (20.9) | 61.8 | Not reported | Noncardiac | GA; LA | POCD | 55 | 23 | SBP↓>30%; >5 min |
Mini-Mental State Examination is an independent predictor of POCD |
| Roshanov2017 | Cohort | 2007/08–2011/01 | / | 12 centers in eight regions (Brazil, spain, Canada, Australia, USA, Hong Kong, Colombia, Malaysia) | 64.8 (11.8) | 48.5 | Not reported | Noncardiac | GA; LA | Myocardial injury; stroke; 30-d-mortality | 14,687 | 4162 | MAP<90 mmHg; >1 min |
Withholding angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers before major noncardiac surgery was associated with a lower risk of death |
| Sabaté2011 | Cohort | 2007/10–2008/06 | Spain | Twenty-three hospitals in Catalonia | 67 (47–81) | 48.3 | Not reported | Noncardiac | GA; LA | Combined cardiac events, including myocardial infarction, stroke. | 3387 | 313 | MAP↓>20%; Not reported |
IOH is an independent risk factors for adverse cardiac events |
| Patti 2011 | Cohort | 2007/02–2009/02 | Italy | V. Cervello Hospital | 69.0 (2.8) | 40.0 | Not reported | Colorectal surgery | GA; LA | POD | 100 | 18 | MAP<60 mmHg; Not reported |
POD is common after colorectal surgery |
| Vasivej2016 | Case-control | 2009/01–2013/12 | Thailand | Songklanagarind Hospital; hospital of Prince of Songkla University | 58.6 (14.7) | 51.9 | 42.9 | Noncardiac | GA | Stroke | 210 | 22 | MAP<65 mmHg; Not reported |
IOH is a predictors for stroke |
| Thakar2007 | Cohort | 2003/01–2005/01 | USA | University of Cincinnati Medical Center | 43 (10) | 16.5 | 57.0 | Gastric bypass | GA | AKI | 491 | 100 | MAP<60 mmHg; Not reported |
Postoperative AKI is not infrequent after gastric bypass surgery |
| Barone 2002 | Case-control | 1996–1999 | USA | Stamford Hospital | 74 (11) | 55.0 | 40.0 | Noncardiac | GA; LA | Myocardial infarction | 60 | 25 | SBP<100 mmHg; >10 min |
IOH associated with perioperative myocardial infarction |
| Nakamura 2009 | Case-control | 1980–2007 | Japan | University of Miyazaki | 70.8 (9.4) | 70.9 | 87.5 | Thoracic aortic operation | GA; LA | 30-day mortality | 72 | 3 | SBP<70 mmHg; Not reported |
stent graft repair had low mortality |
| Sharma 2006 | Case-control | 1997/07–2003/08 | USA | University of Pittsburgh Medical Center | 42.3 (9.2) | 19.0 | 56.0 | Laparoscopic gastric surgery | GA; LA | AKI | 1800 | 1114 | SBP<100 mmHg; >5 min |
AKI is not common after laparoscopic gastric bypass |
| Kim 2016 | Cohort | 2012/11–2013/12 | South Korea | Severance Hospital | 72 (5) | 37.9 | 63.2 | Lumbar spinal surgery | GA; LA | POCD | 87 | 9 | MAP↓>60%; Not reported |
IOH is associated with POCD |
| Nakatani 2022 | Cohort | 2016/04–2020/12 | Japan | Nara Medical University | 75 (69–80) | 80.6 | 88.6 | Transurethral resection | GA; LA | POD | 324 | 53 | MAP<60 mmHg; Not reported |
Older age is associated with POD |
| Xue 2016 | Cohort | 2010/06–2015/02 | China | First People's Hospital of Lianyungang | 74.8 (6.4) | / | Not reported | Transurethral resection of prostate | GA; Spinal anesthesia | POD | 358 | 78 | MAP<65 mmHg; Not reported |
Older age is associated with POD |
| Ansaloni2010 | Cohort | 2005/05/16–2006/03/25 | Italy | St Orsola-Malpighi University Hospital | 75.8 (7.4) | 40.9 | 51.5 | Noncardiac | GA; LA | POD | 357 | 39 | MAP<65 mmHg; >5 min |
POD is common in elderly patients after elective surgery |
| Shen 2022 | Cohort | 2016/01–2020/12 | China | Xuanwu Hospital | 81.3 (4.4) | 51.8 | 62.7 | Aabdominal surgery | GA | AKI | 573 | 116 | MAP<65 mmHg; Not reported |
IOH is a risk factor of AKI |
| Zhang 2021 | Cohort | 2015/04/01–2018/06/30 | China | Peking University Cancer Hospital | 58.9 (10.7) | 73.4 | 27.4 | Resection for gastric cancer | GA | SSI | 880 | 92 | SBP<90 mmHg; >10 min |
IOH increase the incidence of SSI |
| Kheterpal2009 | Cohort | 2002–2006 | USA | University of Michigan Medical School | >18 | 51.2 | Not reported | Noncardiac | GA; LA | Myocardial infarction | 7740 | 2854 | MAP<60 mmHg; Not reported |
IOH can predict cardiac adverse events |
| White 2016 | Cohort | 2013/05/01–2013/07/31 | UK | The National Hip Fracture Database | >18 | / | Not reported | Hip fracture | GA; Spinal anesthesia | 30-day mortality | 10,302 | 8163 | MAP<75 mmHg; Not reported |
IOH increased mortality |
| Marcantonio1998 | Cohort | Not reported | USA | Brigham and Women's Hospital | 67 (9) | 45.0 | Not reported | Noncardiac | GA; LA | POD | 1341 | 352 | MAP↓>33%; Not reported |
IOH associated with POD |
| Tallgren2007 | Cohort | 2002/04–2003/12 | Finland | Helsinki University Hospital | 66 (58–73) | 86.9 | 66.7 | Infrarenal aortic repair | GA | AKI | 69 | 11 | MAP<60 mmHg; >15 min |
IOH is a risk factor of AKI |
| Zhang 2012 | Cohort | Not reported | China | Beijing Tiantan Hospital | 67.9 (5.6) | 33.8 | Not reported | Arthroplasty surgeries | GA | POCD | 68 | 3 | MAP↓>30%; Not reported |
Arthroplastic surgery under isoflurane inhalation anesthesia causes differential serum protein expression in elderly patients |
| House 2016 | Cohort | 2007/01/01–2012/08/07 | USA | Vanderbilt University Medical Center | 54 (13) | 52.9 | 42.4 | Noncardiac | GA | Myocardial infarction | 46,799 | 38,213 | MAP<60 mmHg; Not reported |
Surgical Apgar score is associated with myocardial injury |
| Yang 2016 | Cohort | 2012/01–2014/03 | China | First Affiliated Hospital of Dalian Medical University | 81 (6) | 48.5 | 43.6 | Noncardiac | GA | POD | 480 | 29 | SBP↓>30%; Not reported |
IOH associated with POD |
| Yue 2013 | Cohort | 2007/04–2012/03 | China | Zhongshan Hospital | Not reported | 78.8 | Not reported | Abdominal aortic aneurysm repair | GA | AKI | 71 | 16 | MAP<65 mmHg; Not reported |
AKI is common after abdominal aortic aneurysm repair |
| Ellis 2018 | Cohort | 2013/06–2017/04 | Australia | University of Queensland | 18–70 | 61.4 | 54.4 | Nephrectomy | GA | AKI | 184 | 44 | MAP<60 mmHg; ≥5 min |
Preoperative dehydration may be associated with postoperative acute kidney injury |
| Deiner2015 | Cohort | Not reported | USA | Mount Sinai Hospital | 74 .0 (5.4) | 51.9 | 23.4 | Noncardiac | GA | POCD | 77 | 21 | MAP<55 mmHg; >5 min |
Burst suppression may be protective for POCD |
| Brinkman 2015 | Cohort | 2011/09–2013/01 | Canada | University of Manitoba | 67.9 (9.1) | 65.0 | 67.5 | Open repair of abdominal aortic aneurysms | GA | AKI | 40 | 35 | MAP<65 mmHg; Not reported |
AKI is common after abdominal aortic aneurysm repair |
| Ishikawa 2014 | Cohort | 2009/01/01–2009/12/31 | Japan | Keiyukai Sapporo Hospital | 67 (60–75) | 55.0 | 16.7 | Elective open surgery for colorectal cancer | GA | SSI | 224 | 33 | SBP<80 mmHg; >5 min |
SSI is common for elective open surgery |
| Yeheyis2021 | Cohort | 2017/08/01–2020/03/30 | Ethiopia | School of Medicine, Addis Ababa University | 54 (12.08) | 39.0 | 11.0 | Esophagectomy | GA | 30-day mortality | 54 | 28 | SBP<90 mmHg; >5 min |
IOH is not associate with mortality |
| Jing 2021 | Cohort | 2017/03–2019/12 | China | China-Japan Friendship Hospital | 59 (51–62) | 83.0 | 73.0 | Lung transplantation | GA | AKI | 191 | 41 | MAP<65 mmHg; Not reported |
AKI is common after lung transplantation |
| Knight 2022 | Cohort | 2013/06–2017/06 | USA | University of Pittsburgh Medical Center | 51 (14) | 57.0 | 59.0 | Lung transplantation | GA | AKI | 245 | 244 | MAP≦65 mmHg; >15 min |
IOH is associate with AKI after lung transplant |
| Balci 2017 | Cohort | 2014/03–2015/08 | Turkey | Kartal Kosuyolu Training Hospital | 46.1 (15.4) | 63.3 | 42.9 | Lung transplantation | GA | AKI | 30 | 15 | MAP<70 mmHg; Not reported |
AKI is common after lung transplantation |
| Joosten2021 | Cohort | 2014–2019 | Belgium | Erasme Hospital | 57 (48–64) | 70.0 | 62.0 | Liver transplantation | GA | AKI | 205 | 203 | MAP<65 mmHg; Not reported |
AKI is common after liver transplant |
| Xu2010 | Cohort | 2004/01–2005/09 | China | First Affiliated Hospital, Zhejiang University School of Medicine | 45 (9) | 86.3 | Not reported | Liver transplantation | GA | AKI | 102 | 28 | SBP<90 mmHg; >15 min |
IOH is associate with AKI after lung transplant |
| Mizota2017 | Cohort | 2008/03–2015/04 | Japan | Kyoto University Hospital | 55 (44–61) | 48.9 | Not reported | Liver transplantation | GA | AKI | 231 | 198 | MAP<50 mmHg; >1 min |
AKI is common after liver transplant |
| Chen 2017 | Cohort | 2003/01–2011/02 | China | Zhongshan Hospital | 50.4 (9.6) | 89.2 | 92.9 | Liver transplantation | GA | AKI | 566 | 54 | SBP<90 mmHg; >15 min |
Post-liver transplantation AKI is common |
| Wyssusek2015 | Cohort | 2009/01–2012/08 | Queensland | Princess Alexandra hospital | 49 (12) | 69.1 | 48.5 | Liver transplantation | GA | AKI | 97 | 33 | SBP<90 mmHg; >15 min |
AKI is common after liver transplant |
| Cai 2006 | Cohort | 2004/08–2004/11 | China | Zhongshan Hospital | 69.7 (5.23) | 82.3 | 46.7 | Noncardiac | GA | POCD | 79 | 31 | MAP<60 mmHg; >5 min |
IOH is a risk factor of POCD |
| Wang 2015 | Cohort | 2011/01–2012/12 | China | Yongchuan hospital | >18 | 49.5 | 24.4 | Lumbar spinal | GA; Spinal anesthesia | POD | 200 | 95 | MAP<65 mmHg; Not reported |
IOH is a risk factor of POD after spinal operation |
| Wang 2013 | Cohort | 2010/05–2012/08 | China | Qingdao municipal hospital | 74.0 (8) | 53.5 | 62.5 | Hip replacement | Epidural anesthesia | POD; POCD | 200 | 33 | MAP<65 mmHg; Not reported |
IOH is a risk factor of POD after hip joint replacement |
| Ji2016 | Cohort | 2012/06–2015/08 | China | Yangpu hospital | 73 (6) | Not reported | Not reported | Laparoseopic | GA | POD | 213 | 108 | MAP↓>20%; MAP<65 mmHg |
IOH associate with POD |
| Xie 2021 | Cohort | 2019/11–2020/11 | China | Beijing cancer hospital | 63 (13) | 63.7 | Not reported | Radical resection for colorectal cancer | GA | AKI | 543 | 359 | SBP<60 mmHg; Not reported |
IOH associate with AKI after radical resection of malignant colorectal cancer |
| Monk 2005 | Cohort | Not reported | USA | Shands Hospital | 51 (37–65) | 36.5 | 33.1 | Noncardiac | GA | 1-year-mortality | 1064 | 203 | SBP<80 mmHg; Not reported |
Death during the first year after surgery is primarily associated with the natural history of preexisting conditions |
| Bijker2009 | Cohort | 2002/02–2003/08 | Netherlands | University Medical Center Utrecht | 52 (15.8) | 51.6 | 22.3 | Noncardiac | GA; LA | 1-year-mortality | 1705 | 88 | SBP<80 mmHg; >1 min |
IOH is not associate with 1-year mortality |
IOH = intraoperative hypotension; GA = general anesthesia; LA = local anesthesia; POCD = postoperative cognitive dysfunction; POD = post operative delirium; SSI = surgical-site infection; AKI = acute kidney injury; MAP = mean arterial pressure; SBP = systolic blood pressure.
3.3. Assessments of risk of bias for each included study
The risk of bias was mostly low-to-moderate for observational studies, and only three cohort [36,83,89] studies were graded as poor quality, indicating that most of the studies were of fair-to-high quality (Appendix S5). Two randomized studies [29,45] were at low risk of bias, and one [38] was at medium risk (Appendix S6).
3.4. Primary outcomes
3.4.1. 30-Day mortality
Seven studies [7,21,34,47,53,72,89] reported the association between IOH and risk of 30-day mortality and included 68,881 participants and 1377 deaths (2.00%). Low-quality evidence showed that, compared with non-IOH, IOH was associated with increased 30-day mortality (OR, 1.85; 95% CI, 1.30–2.64; P < .001; I2 = 83%) after non-cardiac surgery (Fig. 2).
Fig. 2.
Forest Plot of odds ratio (OR) for 30-day mortality for IOH VS Non-IOH during non-cardiac surgery.
3.4.2. AKI
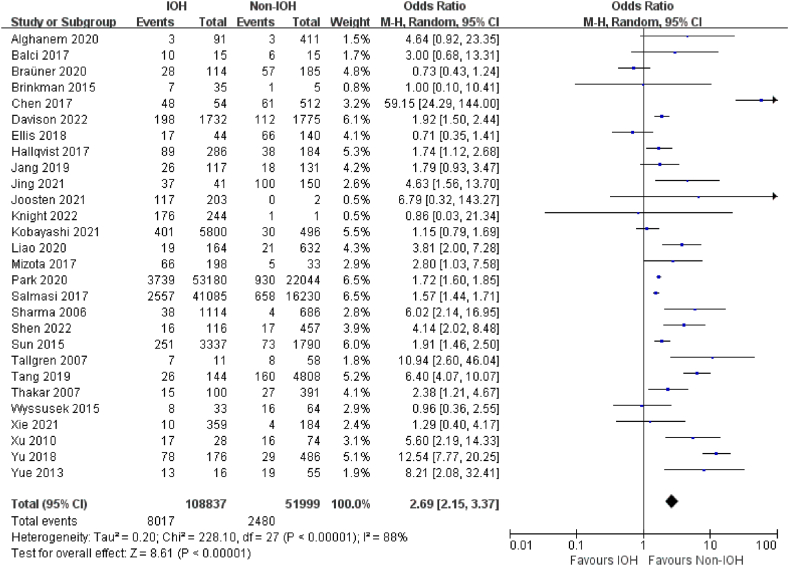
Twenty-eight studies [19,20,[22], [23], [24], [25], [26],28,30,33,41,51,54,59,63,67,68,70,[73], [74], [75], [76], [77], [78], [79], [80],85,88] investigated the effect of IOH on AKI and included 160,836 participants and 10,497 AKI (6.52%). Low-quality evidence showed that IOH was associated with a higher risk of postoperative AKI (OR, 2.69; 95% CI, 2.15–3.37; P < .001; I2 = 88%) within 7 days after non-cardiac surgery than non-IOH (Fig. 3).
Fig. 3.
Forest Plot of AKI for IOH VS Non-IOH during non-cardiac surgery.
Major adverse cardiac events (myocardial injury or myocardial infarction)
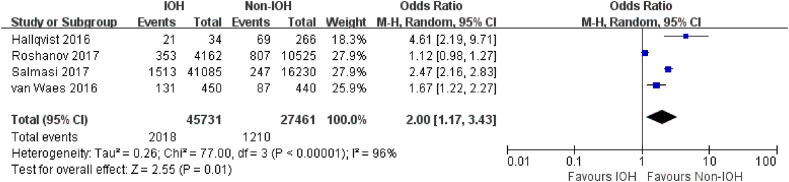
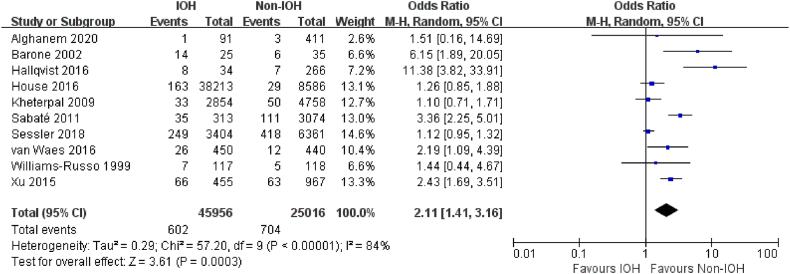
Four studies [19,21,42,47] reported myocardial injury, and 10 studies [21,29,32,33,42,45,48,52,61,65] addressed myocardial infarction. Very low-quality evidence showed that adult patients with hypotension during non-cardiac surgery were more likely to have a postoperative myocardial injury (OR, 2.00; 95% CI, 1.17–3.43; P = .01; I2 = 96%) (Fig. 4). The association between IOH and myocardial infarction was similar, with IOH associated with an increased risk of myocardial infarction (OR, 2.11; 95% CI, 1.41–3.16; P < .001; I2 = 84%; very low certainly) after non-cardiac surgery (Fig. 5).
Fig. 4.
Forest Plot of myocardial injury for IOH VS Non-IOH during non-cardiac surgery.
Fig. 5.
Forest Plot of myocardial infarction for IOH VS Non-IOH during non-cardiac surgery.
3.4.3. POCD and POD
Only six non-randomized studies [46,55,64,69,81,83] and one randomized trial [38] with a sample size of 643 participants reported the outcomes of POCD (49 of 150 vs. 78 of 493[19.75%]). Very low-quality evidence showed that adult patients with or without IOH during non-cardiac surgery had a similar likelihood of POCD (OR, 2.82; 95% CI, 0.83–9.50; P = .10; I2 = 77%) (Appendix S7). In the analysis of POD [27,31,[35], [36], [37], [38],43,45,49,[56], [57], [58],62,66,[82], [83], [84]], results with very low-quality evidence showed that a higher risk of POD was associated with IOH (OR, 2.27; 95% CI, 1.53–3.38; P < .001; I2 = 81%) compared with non-IOH (Appendix S8).
3.5. Secondary outcomes
3.5.1. SSI
Three studies reported SSI (178 of 1031 vs. 354 of 2594[14.68%]). One [39] of these studies reported no difference between the IOH and non-IOH groups regarding the development of SSI (OR, 1.10; 95% CI, 0.87–1.38). However, the other two studies [60,71] provided evidence that IOH was associated with a higher risk of SSI than non-IOH. Overall, low-quality evidence showed no significant difference (OR, 1.83; 95% CI, 0.93–3.60; P = .08; I2 = 80%) was found between IOH and non-IOH group (Appendix S9).
3.5.2. Stroke
Four studies [40,44,47,50] reported stroke (925 of 164,680 vs. 864 of 209,110[0.48%]). A slight difference was observed between the IOH and non-IOH groups regarding the development of postoperative stroke (OR, 1.33; 95% CI, 1.21–1.46; P < .001; I2 = 26%). Low-quality evidence showed that adult patients with hypotension during non-cardiac surgery had higher odds of developing stroke than non-IOH (Appendix S10).
3.5.3. 1-Year mortality
Only two studies [86,87] reported very low rates of 1-year mortality after non-cardiac surgery (64 of 855 vs. 82 of 1914[5.27%]). Very low-quality evidence showed that compared with non-IOH, patients with IOH had a similar likelihood of 1-year mortality (OR, 1.66; 95% CI, 0.65–4.20; P = .29; I2 = 81%) (Appendix S11).
3.5.4. Sensitivity and subgroup analysis
We conducted several sensitivity analyses to confirm the robustness of our findings. For sensitivity analysis, removing studies with a high risk of bias did not change the significance of the results. Moreover, the sensitivity analysis showed that studies with a high risk of bias contributed greatly to the overall heterogeneity. All three sensitivity analyses indicated that the results of the meta-analysis were robust (Appendix S12–S14). However, the sensitivity analyses showed patients who had hypotension during non-cardiac surgery had higher odds of postoperative SSI compared with non-IOH (OR, 2.50; 95% CI, 1.57–3.97; P < .001; I2 = 0%) (Appendix S15). The test for subgroup differences (I2 = 22.9%) indicates that surgery types (transplant surgery vs. non-transplant surgery) may have contributed to the heterogeneity of AKI (Appendix S16). We found differences between transplant surgery (OR, 4.46; 95% CI, 1.48–13.42; P = .008) and non-transplant surgery (OR, 2.32; 95% CI, 1.86–2.89; P < .001). The transplant surgery group had a broader confidence interval (95% CI, 1.48–13.42) than the non-transplant surgery (95% CI, 1.86–2.89).
3.5.5. Publication bias
There was no evidence of publication bias for outcomes with ten or more studies (Appendix S17–S19). The Egger test demonstrates no significant publication bias for AKI (P = .056), POD (P = .769), and myocardial infarction (P = .085).
3.5.6. Certainty of evidence
Six outcomes had a large effect (strong association, upgrade one level, OR > 2). Nevertheless, the certainty of evidence was mostly low-to-very low as most of the included studies were observational designs in nature (Appendix S20).
4. Discussion
In this systematic review and meta-analysis from 72 studies (69 cohort studies and 3 RCTs), IOH was independently associated with a higher risk of 30-day mortality, AKI, major adverse cardiac events (myocardial injury or myocardial infarction), POD, and stroke after non-cardiac surgery than non-IOH. Our review found very low-quality to low-quality evidence that IOH has a similar likelihood of POCD and 1-year-mortality compared with non-IOH in non-cardiac surgery. Available evidence suggests that IOH has a higher risk of severe postoperative complications after non-cardiac surgery than non-IOH.
The primary aim of this meta-analysis was to investigate the association of IOH with severe postoperative complications in patients undergoing non-cardiac surgery. Overall, our primary findings are supported by similar results from previous systematic reviews [10,90] while adding to the evidence on the outcomes of SSI, stroke, and 1-year-mortality in adult patients who underwent non-cardiac surgery. However, previous systematic reviews only included studies published to June 2019, excluding recently published articles [26,43,56,74]. Furthermore, none of these studies rated the certainty of the evidence or included articles published in other languages.
More than 300 million non-cardiac surgeries are performed worldwide annually [91]. IOH is common during non-cardiac surgery and may be associated with organ ischemia and mortality [87]. Reducing postoperative mortality is a top priority for surgeons worldwide. However, evidence regarding the association between IOH and postoperative mortality conveyed inconsistent and conflicting results, with some studies finding an association between IOH and mortality and some not [92,93]. A multiple-center cohort study used three methods to define the IOH (population thresholds, absolute thresholds, and percent change from baseline blood pressure) [34]. They found IOH was associated with 30-day mortality with any of these definitions. Their results stress the importance of the prevention of hypotension during surgery. Wickham et al. also urged improved management of IOH may be a simple intervention with real potential to reduce morbidity in elderly patients undergoing surgery [94]. Our study showed that IOH significantly increases the risk of 30-day mortality, which is in line with the results of the previous meta-analyses [10,11,95]. However, it is worth noting that the endpoints (30-d mortality, 60-d mortality, 90-day mortality, and 180-d mortality) selected by each study are different, although there have been an increasing number of studies on the correlation between IOH and postoperative mortality in non-cardiac surgery. What's more, it is difficult to weigh the role of IOH in these postoperative mortality rates considering most of it is all all-cause mortality.
Our study found that IOH was also associated with AKI, major adverse cardiac events (myocardial injury or myocardial infarction), and POD. Major adverse cardiovascular events and AKI are leading causes of morbidity and mortality following non-cardiac surgery, with up to one-third of 30-day mortality potentially attributable to myocardial injury [96]. A multicenter retrospective cohort [97] study including 368,222 non-cardiac surgical procedures revealed that IOH increased the occurrence of AKI, myocardial infarction, and stroke. Similarly, a retrospective cohort study of 33,330 non-cardiac surgeries showed mean arterial pressure (MAP) less than 55 mmHg is associated with AKI and myocardial injury even with a short duration of IOH [98]. A recent study by Wesselink and colleagues also confirmed IOH has a graded association with postoperative myocardial injury [99]. IOH was not associated with POCD in our study, consistent with two previous studies containing cardiac and non-cardiac surgeries [90,100]. Meanwhile, IOH was also associated with POD and stroke in this meta-analysis, which is inconsistent with previous systematic reviews or meta-analyses [10,100]. Of note, these authors either included cardiac surgery [100], or only included articles published in English [10]. One explanation is that we included some articles [[81], [82], [83], [84]] published in other languages to minimize the publication bias.
A secondary aim of our review was to determine the relationship between IOH and surgical-site infection, stroke, and 1-year mortality in non-cardiac surgery. The SSI and 1-year mortality were uncommon or rare after non-cardiac surgery. Our results showed that IOH has a similar likelihood of SSI and 1-year-mortality compared with non-IOH in non-cardiac surgery. Only two observational studies [60,71] with small sample sizes reported IOH was associated with a higher risk of SSI. But the largest retrospective cohort study [39] showed no association between IOH and SSI, probably because the outcomes are overwhelmingly determined by other baseline and surgical factors. All these three studies were abdominal surgery. Only two studies [86,87] contributed data for the analysis of 1-year mortality; No association was observed between IOH and 1-year mortality.
The heterogeneity of the included articles in this paper is high, which is affected by the variability of definitions used for IOH and the different thresholds examined. The lack of uniform criteria was a reason for the discrepancy among researchers. The baseline comparability of inclusion was inconsistent, with many studies targeted at high-risk groups and broad groups suffering from varying degrees of severe disease. The results of pre-specified sensitivity and subgroup analysis indicate that the surgical types may partly contribute to the heterogeneity. Various definitions of outcomes may also explain the high heterogeneity and make it difficult to appraise the occurrence of postoperative complications. The Perioperative Quality Initiative-3 workgroup has stated in its consensus statement that mean arterial pressure (MAP) should be maintained >60–70 mmHg during non-cardiac surgery to reduce postoperative complications [101]. A universally accepted standard definition of hypotension would facilitate further studies. Notably, most of the included studies were observational, resulting in the low certainty of evidence. Thus, further high-quality, including well-designed randomized trials of different surgery types in the general population, is urgently needed.
Collectively, our findings provide low to very low-quality evidence to support that IOH is a modifiable risk factor associated with severe postoperative complications. Thus, monitoring blood pressure during non-cardiac surgery could confer additional benefits.
4.1. Strengths and limitations
Our study had some strengths: First, we included several important clinical outcomes directly relevant to postoperative complications management after non-cardiac surgery. Second, articles with different languages were included to minimize publication bias. Third, this meta-analysis has provided an overview of the effect of IOH on multiple postoperative outcomes. We included various outcomes to address the review question comprehensively. Fourth, we rated the certainty of evidence using the GRADEpro approach.
A key limitation is that a high level of heterogeneity among studies was observed in most outcomes concerning the surgical types, definition and duration of IOH. Thus, the results of this review pertaining to outcomes with substantial heterogeneity should be interpreted accordingly. Furthermore, most of the included studies were observational, resulting in the low certainty of evidence. Only three randomized trials were identified for the relationship between IOH and postoperative complications. Thus, further high-quality research is urgently needed.
5. Conclusions
Our results suggest IOH was associated with an increased risk of severe postoperative complications after non-cardiac surgery than non-IOH. For patients undergoing non-cardiac surgery, IOH is a potentially avoidable hazard that should be closely monitored, and corresponding measures should be taken to reduce severe postoperative complications. There is also an urgent need for a universally accepted standard definition of IOH and further high-quality research.
Funding
This manuscript did not receive any funding.
Author contributions
Dr. Duan had full access to all of the data in the study and takes responsibility for the integrity of the data. JHC, MT, JY, and HW drafted the manuscript. JC contributed to the design of the search strategy. JY and HW did the study selection. HL and SSX collected the data. HL and XY assessed the risk of bias. XW did the statistical analysis. All authors read, provided feedback and approved the final version.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e15997.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wesselink E.M., Kappen T.H., Torn H.M., Slooter A.J.C., van Klei W.A. Intraoperative hypotension and the risk of postoperative adverse outcomes: a systematic review. Br. J. Anaesth. 2018;121:706–721. doi: 10.1016/j.bja.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 2.García P.S., Brown C.H. Phenylephrine or ephedrine for intraoperative hypotension? Consider the cerebral microcirculation. Anesthesiology. 2021;135:775–777. doi: 10.1097/ALN.0000000000003975. [DOI] [PubMed] [Google Scholar]
- 3.Wijnberge M., Geerts B.F., Hol L., Lemmers N., Mulder M.P., Berge P., et al. Effect of a machine learning-derived early warning system for intraoperative hypotension vs standard care on depth and duration of intraoperative hypotension during elective noncardiac surgery: the HYPE randomized clinical trial. JAMA. 2020;323:1052–1060. doi: 10.1001/jama.2020.0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saengrung S., Kaewborisutsakul A., Tunthanathip T., Phuenpathom N., Taweesomboonyat C. Risk factors for intraoperative hypotension during decompressive craniectomy in traumatic brain injury patients. World Neurosurg. 2022;162:e652–e658. doi: 10.1016/j.wneu.2022.03.102. [DOI] [PubMed] [Google Scholar]
- 5.Yu Q., Qi J., Wang Y. Intraoperative hypotension and neurological outcomes. Curr. Opin. Anaesthesiol. 2020;33:646–650. doi: 10.1097/ACO.0000000000000904. [DOI] [PubMed] [Google Scholar]
- 6.Kertai M.D., White W.D., Gan T.J. Cumulative duration of "triple low" state of low blood pressure, low bispectral index, and low minimum alveolar concentration of volatile anesthesia is not associated with increased mortality. Anesthesiology. 2014;121:18–28. doi: 10.1097/ALN.0000000000000281. [DOI] [PubMed] [Google Scholar]
- 7.Sessler D.I., Sigl J.C., Kelley S.D., Chamoun N.G., Manberg P.J., Saager L., et al. Hospital stay and mortality are increased in patients having a "triple low" of low blood pressure, low bispectral index, and low minimum alveolar concentration of volatile anesthesia. Anesthesiology. 2012;116:1195–1203. doi: 10.1097/ALN.0b013e31825683dc. [DOI] [PubMed] [Google Scholar]
- 8.Willingham M.D., Karren E., Shanks A.M., O'Connor M.F., Jacobsohn E., Kheterpal S., et al. Concurrence of intraoperative hypotension, low minimum alveolar concentration, and low bispectral index is associated with postoperative death. Anesthesiology. 2015;123:775–785. doi: 10.1097/ALN.0000000000000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinberg L., Li S.Y., Louis M., Karp J., Poci N., Carp B.S., et al. Reported definitions of intraoperative hypotension in adults undergoing non-cardiac surgery under general anaesthesia: a review. BMC Anesthesiol. 2022;22:69. doi: 10.1186/s12871-022-01605-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wijnberge M., Schenk J., Bulle E., Vlaar A.P., Maheshwari K., Hollmann M.W., et al. Association of intraoperative hypotension with postoperative morbidity and mortality: systematic review and meta-analysis. BJS open. 2021;5 doi: 10.1093/bjsopen/zraa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu W.J., Hou B.L., Kwong J.S.W., Tian X., Qian Y., Cui Y., et al. Association between intraoperative hypotension and 30-day mortality, major adverse cardiac events, and acute kidney injury after non-cardiac surgery: a meta-analysis of cohort studies. Int. J. Cardiol. 2018;258:68–73. doi: 10.1016/j.ijcard.2018.01.137. [DOI] [PubMed] [Google Scholar]
- 12.Page M.J., Moher D., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai J., Tang M., Gao Y., Zhang H., Yang Y., Zhang D., et al. Cesarean section or vaginal delivery to prevent possible vertical transmission from a pregnant mother confirmed with COVID-19 to a neonate: a systematic review. Front. Med. (Lausanne) 2021;8 doi: 10.3389/fmed.2021.634949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clavien P.A., Barkun J., de Oliveira M.L., Vauthey J.N., Dindo D., Schulick R.D., et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann. Surg. 2009;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 15.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clin. Res. Ed.) 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 16.Wells G., Shea B., O'Connell D. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. 2010. http://www3.med.unipmn.it/dispense_ebm/2009-2010/Corso%20Perfezionamento%20EBM_Faggiano/NOS_oxford.pdf Available from:
- 17.Guyatt G., Oxman A.D., Akl E.A., Kunz R., Vist G., Brozek J., et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 18.Ahuja S., Mascha E.J., Yang D., Maheshwari K., Cohen B., Khanna A.K., et al. Associations of intraoperative radial arterial systolic, diastolic, mean, and pulse pressures with myocardial and acute kidney injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2020;132:291–306. doi: 10.1097/ALN.0000000000003048. [DOI] [PubMed] [Google Scholar]
- 19.Salmasi V., Maheshwari K., Yang D., Mascha E.J., Singh A., Sessler D.I., et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126:47–65. doi: 10.1097/ALN.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 20.Park S., Lee H.C., Jung C.W., Choi Y., Yoon H.J., Kim S., et al. Intraoperative arterial pressure variability and postoperative acute kidney injury. Clin. J. Am. Soc. Nephrol. : CJASN. 2020;15:35–46. doi: 10.2215/CJN.06620619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Waes J.A., van Klei W.A., Wijeysundera D.N., van Wolfswinkel L., Lindsay T.F., Beattie W.S. Association between intraoperative hypotension and myocardial injury after vascular surgery. Anesthesiology. 2016;124:35–44. doi: 10.1097/ALN.0000000000000922. [DOI] [PubMed] [Google Scholar]
- 22.Liao P., Zhao S., Lyu L., Yi X., Ji X., Sun J., et al. Association of intraoperative hypotension with acute kidney injury after liver resection surgery: an observational cohort study. BMC Nephrol. 2020;21:456. doi: 10.1186/s12882-020-02109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun L.Y., Wijeysundera D.N., Tait G.A., Beattie W.S. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123:515–523. doi: 10.1097/ALN.0000000000000765. [DOI] [PubMed] [Google Scholar]
- 24.Tang Y., Zhu C., Liu J., Wang A., Duan K., Li B., et al. Association of intraoperative hypotension with acute kidney injury after noncardiac surgery in patients younger than 60 Years Old. Kidney Blood Pres. Res. 2019;44:211–221. doi: 10.1159/000498990. [DOI] [PubMed] [Google Scholar]
- 25.Braüner Christensen J., Aasbrenn M., Sandoval Castillo L., Ekmann A., Giver Jensen T., Pressel E., et al. Predictors of acute kidney injury after hip fracture in older adults. Geriatr. Orthop. Surg. Rehabil. 2020;11 doi: 10.1177/2151459320920088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davison E., Affleck A., Daratha K.B. Intraoperative hypotension and acute kidney injury in non-cardiac surgery at a large tertiary care medical center. AANA J. 2022;90:58–63. [PubMed] [Google Scholar]
- 27.Tobar E., Abedrapo M.A., Godoy J.A., Llanos J.L., Díaz M.J., Azolas R., et al. Impact of hypotension and global hypoperfusion in postoperative delirium: a pilot study in older adults undergoing open colon surgery. Braz. J. Anesthesiol. (Elsevier) 2018;68:135–141. doi: 10.1016/j.bjane.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jang W.Y., Jung J.K., Lee D.K., Han S.B. Intraoperative hypotension is a risk factor for postoperative acute kidney injury after femoral neck fracture surgery: a retrospective study. BMC Muscoskel. Disord. 2019;20:131. doi: 10.1186/s12891-019-2496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sessler D.I., Meyhoff C.S., Zimmerman N.M., Mao G., Leslie K., Vásquez S.M., et al. Period-dependent associations between hypotension during and for four days after noncardiac surgery and a composite of myocardial infarction and death: a substudy of the POISE-2 trial. Anesthesiology. 2018;128:317–327. doi: 10.1097/ALN.0000000000001985. [DOI] [PubMed] [Google Scholar]
- 30.Hallqvist L., Granath F., Huldt E., Bell M. Intraoperative hypotension is associated with acute kidney injury in noncardiac surgery: an observational study. Eur. J. Anaesthesiol. 2018;35:273–279. doi: 10.1097/EJA.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y., Yu H., Qiao H., Li C., Chen K., Shen X. Risk factors and incidence of postoperative delirium in patients undergoing laryngectomy. Otolaryngology-Head and Neck Surgery. 2019;161:807–813. doi: 10.1177/0194599819864304. [DOI] [PubMed] [Google Scholar]
- 32.Xu L., Yu C., Jiang J., Zheng H., Yao S., Pei L., et al. Major adverse cardiac events in elderly patients with coronary artery disease undergoing noncardiac surgery: a multicenter prospective study in China. Arch. Gerontol. Geriatr. 2015;61:503–509. doi: 10.1016/j.archger.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Alghanem S.M., Massad I.M., Almustafa M.M., Al-Shwiat L.H., El-Masri M.K., Samarah O.Q., et al. Relationship between intra-operative hypotension and post-operative complications in traumatic hip surgery. Indian J. Anaesth. 2020;64:18–23. doi: 10.4103/ija.IJA_397_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monk T.G., Bronsert M.R., Henderson W.G., Mangione M.P., Sum-Ping S.T., Bentt D.R., et al. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. 2015;123:307–319. doi: 10.1097/ALN.0000000000000756. [DOI] [PubMed] [Google Scholar]
- 35.Hirsch J., DePalma G., Tsai T.T., Sands L.P., Leung J.M. Impact of intraoperative hypotension and blood pressure fluctuations on early postoperative delirium after non-cardiac surgery. Br. J. Anaesth. 2015;115:418–426. doi: 10.1093/bja/aeu458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson T.N., Raeburn C.D., Tran Z.V., Angles E.M., Brenner L.A., Moss M. Postoperative delirium in the elderly: risk factors and outcomes. Ann. Surg. 2009;249:173–178. doi: 10.1097/SLA.0b013e31818e4776. [DOI] [PubMed] [Google Scholar]
- 37.Tognoni P., Simonato A., Robutti N., Pisani M., Cataldi A., Monacelli F., et al. Preoperative risk factors for postoperative delirium (POD) after urological surgery in the elderly. Arch. Gerontol. Geriatr. 2011;52:e166–e169. doi: 10.1016/j.archger.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 38.Langer T., Santini A., Zadek F., Chiodi M., Pugni P., Cordolcini V., et al. Intraoperative hypotension is not associated with postoperative cognitive dysfunction in elderly patients undergoing general anesthesia for surgery: results of a randomized controlled pilot trial. J. Clin. Anesth. 2019;52:111–118. doi: 10.1016/j.jclinane.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 39.Babazade R., Yilmaz H.O., Zimmerman N.M., Stocchi L., Gorgun E., Kessler H., et al. Association between intraoperative low blood pressure and development of surgical site infection after colorectal surgery: a retrospective cohort study. Ann. Surg. 2016;264:1058–1064. doi: 10.1097/SLA.0000000000001607. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh J.K., Dalton J.E., Yang D., Farag E.S., Sessler D.I., Kurz A.M. The association between mild intraoperative hypotension and stroke in general surgery patients. Anesth. Analg. 2016;123:933–939. doi: 10.1213/ANE.0000000000001526. [DOI] [PubMed] [Google Scholar]
- 41.Yu J., Park H.K., Kwon H.J., Lee J., Hwang J.H., Kim H.Y., et al. Risk factors for acute kidney injury after percutaneous nephrolithotomy: implications of intraoperative hypotension. Medicine. 2018;97 doi: 10.1097/MD.0000000000011580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hallqvist L., Mårtensson J., Granath F., Sahlén A., Bell M. Intraoperative hypotension is associated with myocardial damage in noncardiac surgery: an observational study. Eur. J. Anaesthesiol. 2016;33:450–456. doi: 10.1097/EJA.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 43.Wachtendorf L.J., Azimaraghi O., Santer P., Linhardt F.C., Blank M., Suleiman A., et al. Association between intraoperative arterial hypotension and postoperative delirium after noncardiac surgery: a retrospective multicenter cohort study. Anesth. Analg. 2022;134:822–833. doi: 10.1213/ANE.0000000000005739. [DOI] [PubMed] [Google Scholar]
- 44.Wongtangman K., Wachtendorf L.J., Blank M., Grabitz S.D., Linhardt F.C., Azimaraghi O., et al. Effect of intraoperative arterial hypotension on the risk of perioperative stroke after noncardiac surgery: a retrospective multicenter cohort study. Anesth. Analg. 2021;133:1000–1008. doi: 10.1213/ANE.0000000000005604. [DOI] [PubMed] [Google Scholar]
- 45.Williams-Russo P., Sharrock N.E., Mattis S., Liguori G.A., Mancuso C., Peterson M.G., et al. Randomized trial of hypotensive epidural anesthesia in older adults. Anesthesiology. 1999;91:926–935. doi: 10.1097/00000542-199910000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Boos G.L., Soares L.F., Oliveira Filho G.R. [Postoperative cognitive dysfunction: prevalence and associated factors. Rev. Bras. Anestesiol. 2005;55:517–524. doi: 10.1590/s0034-70942005000500006. [DOI] [PubMed] [Google Scholar]
- 47.Roshanov P.S., Rochwerg B., Patel A., Salehian O., Duceppe E., Belley-Côté E.P., et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: an analysis of the vascular events in noncardiac surgery patIents cOhort evaluatioN prospective cohort. Anesthesiology. 2017;126:16–27. doi: 10.1097/ALN.0000000000001404. [DOI] [PubMed] [Google Scholar]
- 48.Sabaté S., Mases A., Guilera N., Canet J., Castillo J., Orrego C., et al. Incidence and predictors of major perioperative adverse cardiac and cerebrovascular events in non-cardiac surgery. Br. J. Anaesth. 2011;107:879–890. doi: 10.1093/bja/aer268. [DOI] [PubMed] [Google Scholar]
- 49.Patti R., Saitta M., Cusumano G., Termine G., Di Vita G. Risk factors for postoperative delirium after colorectal surgery for carcinoma. Eur. J. Oncol. Nurs. 2011;15:519–523. doi: 10.1016/j.ejon.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 50.Vasivej T., Sathirapanya P., Kongkamol C. Incidence and risk factors of perioperative stroke in noncardiac, and nonaortic and its major branches surgery. J. Stroke Cerebrovasc. Dis. 2016;25:1172–1176. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.051. [DOI] [PubMed] [Google Scholar]
- 51.Thakar C.V., Kharat V., Blanck S., Leonard A.C. Acute kidney injury after gastric bypass surgery. Clin. J. Am. Soc. Nephrol.: CJASN. 2007;2:426–430. doi: 10.2215/CJN.03961106. [DOI] [PubMed] [Google Scholar]
- 52.Barone J.E., Bull M.B., Cussatti E.H., Miller K.D., Tucker J.B. Review of a large clinical series: perioperative myocardial infarction in low-risk patients undergoing noncardiac surgery is associated with intraoperative hypotension. J. Intensive Care Med. 2002;17:250–255. [Google Scholar]
- 53.Nakamura K., Matsuyama M., Yano M., Yano Y., Nagahama H., Nakamura E., et al. Open surgery or stent repair for descending aortic diseases: results and risk factor analysis. Scand. Cardiovasc. J.: SCJ. 2009;43:201–207. doi: 10.1080/14017430802422379. [DOI] [PubMed] [Google Scholar]
- 54.Sharma S.K., McCauley J., Cottam D., Mattar S.G., Holover S., Dallal R., et al. Acute changes in renal function after laparoscopic gastric surgery for morbid obesity. Surg. Obes. Relat. Dis. 2006;2:389–392. doi: 10.1016/j.soard.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 55.Kim J., Shim J.K., Song J.W., Kim E.K., Kwak Y.L. Postoperative cognitive dysfunction and the change of regional cerebral Oxygen saturation in elderly patients undergoing spinal surgery. Anesth. Analg. 2016;123:436–444. doi: 10.1213/ANE.0000000000001352. [DOI] [PubMed] [Google Scholar]
- 56.Nakatani S., Ida M., Wang X., Naito Y., Kawaguchi M. Incidence and factors associated with postoperative delirium in patients undergoing transurethral resection of bladder tumor. JA Clin. Rep. 2022;8:6. doi: 10.1186/s40981-022-00497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xue P., Wu Z., Wang K., Tu C., Wang X. Incidence and risk factors of postoperative delirium in elderly patients undergoing transurethral resection of prostate: a prospective cohort study. Neuropsychiatric Dis. Treat. 2016;12:137–142. doi: 10.2147/NDT.S97249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ansaloni L., Catena F., Chattat R., Fortuna D., Franceschi C., Mascitti P., et al. Risk factors and incidence of postoperative delirium in elderly patients after elective and emergency surgery. Br. J. Surg. 2010;97:273–280. doi: 10.1002/bjs.6843. [DOI] [PubMed] [Google Scholar]
- 59.Shen J., Chu Y., Wang C., Yan S. Risk factors for acute kidney injury after major abdominal surgery in the elderly aged 75 years and above. BMC Nephrol. 2022;23:224. doi: 10.1186/s12882-022-02822-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y., Li S., Yan C., Chen J., Shan F. Perioperative use of glucocorticoids and intraoperative hypotension may affect the incidence of postoperative infection in patients with gastric cancer: a retrospective cohort study. Cancer Manag. Res. 2021;13:7723–7734. doi: 10.2147/CMAR.S333414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kheterpal S., O'Reilly M., Englesbe M.J., Rosenberg A.L., Shanks A.M., Zhang L., et al. Preoperative and intraoperative predictors of cardiac adverse events after general, vascular, and urological surgery. Anesthesiology. 2009;110:58–66. doi: 10.1097/ALN.0b013e318190b6dc. [DOI] [PubMed] [Google Scholar]
- 62.Marcantonio E.R., Goldman L., Orav E.J., Cook E.F., Lee T.H. The association of intraoperative factors with the development of postoperative delirium. Am. J. Med. 1998;105:380–384. doi: 10.1016/s0002-9343(98)00292-7. [DOI] [PubMed] [Google Scholar]
- 63.Tallgren M., Niemi T., Pöyhiä R., Raininko E., Railo M., Salmenperä M., et al. Acute renal injury and dysfunction following elective abdominal aortic surgery. Eur. J. Vasc. Endovasc. Surg. 2007;33:550–555. doi: 10.1016/j.ejvs.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Q., Li S.Z., Feng C.S., Qu X.D., Wang H., Zhang X.N., et al. Serum proteomics of early postoperative cognitive dysfunction in elderly patients. Chin. Med. J. 2012;125:2455–2461. [PubMed] [Google Scholar]
- 65.House L.M., Marolen K.N., St Jacques P.J., McEvoy M.D., Ehrenfeld J.M. Surgical Apgar score is associated with myocardial injury after noncardiac surgery. J. Clin. Anesth. 2016;34:395–402. doi: 10.1016/j.jclinane.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 66.Yang L., Sun D.F., Han J., Liu R., Wang L.J., Zhang Z.Z. Effects of intraoperative hemodynamics on incidence of postoperative delirium in elderly patients: a retrospective study. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2016;22:1093–1100. doi: 10.12659/MSM.895520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yue J.N., Luo Z., Guo D.Q., Xu X., Chen B., Jiang J.H., et al. Evaluation of acute kidney injury as defined by the risk, injury, failure, loss, and end-stage criteria in critically ill patients undergoing abdominal aortic aneurysm repair. Chin. Med. J. 2013;126:431–436. [PubMed] [Google Scholar]
- 68.Ellis R.J., Del Vecchio S.J., Kalma B., Ng K.L., Morais C., Francis R.S., et al. Association between preoperative hydration status and acute kidney injury in patients managed surgically for kidney tumours. Int. Urol. Nephrol. 2018;50:1211–1217. doi: 10.1007/s11255-018-1901-2. [DOI] [PubMed] [Google Scholar]
- 69.Deiner S., Luo X., Silverstein J.H., Sano M. Can intraoperative processed EEG predict postoperative cognitive dysfunction in the elderly? Clin. Therapeut. 2015;37:2700–2705. doi: 10.1016/j.clinthera.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brinkman R., HayGlass K.T., Mutch W.A., Funk D.J. Acute kidney injury in patients undergoing open abdominal aortic aneurysm repair: a pilot observational trial. J. Cardiothorac. Vasc. Anesth. 2015;29:1212–1219. doi: 10.1053/j.jvca.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 71.Ishikawa K., Kusumi T., Hosokawa M., Nishida Y., Sumikawa S., Furukawa H. Incisional surgical site infection after elective open surgery for colorectal cancer. Int. J. Surg. Oncol. 2014;2014 doi: 10.1155/2014/419712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yeheyis E.T., Kassa S., Yeshitela H., Bekele A. Intraoperative hypotension is not associated with adverse short-term postoperative outcomes after esophagectomy in esophageal cancer patients. BMC Surg. 2021;21:1. doi: 10.1186/s12893-020-01015-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jing L., Chen W., Zhao L., Guo L., Liang C., Chen J., et al. Acute kidney injury following adult lung transplantation. Chin. Med. J. 2021;135:172–180. doi: 10.1097/CM9.0000000000001636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Knight J., Hill A., Melnyk V., Doney L., D'Cunha J., Kenkre T., et al. Intraoperative hypoxia independently associated with the development of acute kidney injury following bilateral orthotopic lung transplantation. Transplantation. 2022;106:879–886. doi: 10.1097/TP.0000000000003814. [DOI] [PubMed] [Google Scholar]
- 75.Balci M.K., Vayvada M., Salturk C., Kutlu C.A., Ari E. Incidence of early acute kidney injury in lung transplant patients: a single-center experience. Transplant. Proc. 2017;49:593–598. doi: 10.1016/j.transproceed.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 76.Joosten A., Lucidi V., Ickx B., Van Obbergh L., Germanova D., Berna A., et al. Intraoperative hypotension during liver transplant surgery is associated with postoperative acute kidney injury: a historical cohort study. BMC Anesthesiol. 2021;21:12. doi: 10.1186/s12871-020-01228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu X., Ling Q., Wei Q., Wu J., Gao F., He Z.L., et al. An effective model for predicting acute kidney injury after liver transplantation. Hepatobiliary Pancreat. Dis. Int.: HBPD INT. 2010;9:259–263. [PubMed] [Google Scholar]
- 78.Mizota T., Hamada M., Matsukawa S., Seo H., Tanaka T., Segawa H. Relationship between intraoperative hypotension and acute kidney injury after living donor liver transplantation: a retrospective analysis. J. Cardiothorac. Vasc. Anesth. 2017;31:582–589. doi: 10.1053/j.jvca.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 79.Chen X., Ding X., Shen B., Teng J., Zou J., Wang T., et al. Incidence and outcomes of acute kidney injury in patients with hepatocellular carcinoma after liver transplantation. J. Cancer Res. Clin. Oncol. 2017;143:1337–1346. doi: 10.1007/s00432-017-2376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wyssusek K.H., Keys A.L., Yung J., Moloney E.T., Sivalingam P., Paul S.K. Evaluation of perioperative predictors of acute kidney injury post orthotopic liver transplantation. Anaesth. Intensive Care. 2015;43:757–763. doi: 10.1177/0310057X1504300614. [DOI] [PubMed] [Google Scholar]
- 81.Cai Y., Xue Z., Zhu B. Risk factors contributing to postoperative cognitive dysfunction in elderly patients. J. Clin. Anesth. 2006;22:608–610. [Google Scholar]
- 82.Wang J., Li Z., Yu Y., Shao G., Wang Q. Risk factors of delirium in elderly patients after spinal operation. J. Chongqing Med. Univ. 2015;40:721–724. [Google Scholar]
- 83.Wang B., Zhang Q., Li J., Liu S., Bi Y. Risk factors of post-operative delirium and cognitive dysfunction in elderly patients undergoing hip joint replacement surgery. J. Clin. Anesth. 2013;29:785–788. [Google Scholar]
- 84.Ji J., Fu L., Guo X. Impact of intraoperative hypotension and blood pressure fluctuations on early postoperative delirium after laparoscopic surgery. J. Chin. Physician. 2016;18:1017–1020. [Google Scholar]
- 85.Xie Y., Yu L., Tan H. Risk factors for postoperative acute kidney injury in patients undergoing radical resection of malignant colorectal cancer. Chin. J. Anesthesiol. 2021;41:430–433. [Google Scholar]
- 86.Monk T.G., Saini V., Weldon B.C., Sigl J.C. Anesthetic management and one-year mortality after noncardiac surgery. Anesth. Analg. 2005;100:4–10. doi: 10.1213/01.ANE.0000147519.82841.5E. [DOI] [PubMed] [Google Scholar]
- 87.Bijker J.B., van Klei W.A., Vergouwe Y., Eleveld D.J., van Wolfswinkel L., Moons K.G., et al. Intraoperative hypotension and 1-year mortality after noncardiac surgery. Anesthesiology. 2009;111:1217–1226. doi: 10.1097/ALN.0b013e3181c14930. [DOI] [PubMed] [Google Scholar]
- 88.Kobayashi Y., Yamaoka K. Analysis of intraoperative modifiable factors to prevent acute kidney injury after elective noncardiac surgery: intraoperative hypotension and crystalloid administration related to acute kidney injury. JA Clin. Rep. 2021;7:27. doi: 10.1186/s40981-021-00429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.White S.M., Moppett I.K., Griffiths R., Johansen A., Wakeman R., Boulton C., et al. Secondary analysis of outcomes after 11,085 hip fracture operations from the prospective UK Anaesthesia Sprint Audit of Practice (ASAP-2) Anaesthesia. 2016;71:506–514. doi: 10.1111/anae.13415. [DOI] [PubMed] [Google Scholar]
- 90.van Zuylen M.L., Gribnau A., Admiraal M., Ten Hoope W., Veelo D.P., Hollmann M.W., et al. The role of intraoperative hypotension on the development of postoperative cognitive dysfunction: a systematic review. J. Clin. Anesth. 2021;72 doi: 10.1016/j.jclinane.2021.110310. [DOI] [PubMed] [Google Scholar]
- 91.Smilowitz N.R., Gupta N., Ramakrishna H., Guo Y., Berger J.S., Bangalore S. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol. 2017;2:181–187. doi: 10.1001/jamacardio.2016.4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kluger M.T., Collier J.M.K., Borotkanics R., van Schalkwyk J.M., Rice D.A. The effect of intra-operative hypotension on acute kidney injury, postoperative mortality and length of stay following emergency hip fracture surgery. Anaesthesia. 2022;77:164–174. doi: 10.1111/anae.15555. [DOI] [PubMed] [Google Scholar]
- 93.Khanna A.K., Maheshwari K., Mao G., Liu L., Perez-Protto S.E., Chodavarapu P., et al. Association between mean arterial pressure and acute kidney injury and a composite of myocardial injury and mortality in postoperative critically ill patients: a retrospective cohort analysis. Crit. Care Med. 2019;47:910–917. doi: 10.1097/CCM.0000000000003763. [DOI] [PubMed] [Google Scholar]
- 94.Wickham A., Highton D., Martin D. Care of elderly patients: a prospective audit of the prevalence of hypotension and the use of BIS intraoperatively in 25 hospitals in London. Perioperat. Med. (London, England) 2016;5:12. doi: 10.1186/s13741-016-0036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.An R., Pang Q.Y., Liu H.L. Association of intra-operative hypotension with acute kidney injury, myocardial injury and mortality in non-cardiac surgery: a meta-analysis. Int. J. Clin. Pract. 2019;73 doi: 10.1111/ijcp.13394. [DOI] [PubMed] [Google Scholar]
- 96.Devereaux P.J., Mrkobrada M., Sessler D.I., Leslie K., Alonso-Coello P., Kurz A., et al. Aspirin in patients undergoing noncardiac surgery. N. Engl. J. Med. 2014;370:1494–1503. doi: 10.1056/NEJMoa1401105. [DOI] [PubMed] [Google Scholar]
- 97.Gregory A., Stapelfeldt W.H., Khanna A.K., Smischney N.J., Boero I.J., Chen Q., et al. Intraoperative hypotension is associated with adverse clinical outcomes after noncardiac surgery. Anesth. Analg. 2021;132:1654–1665. doi: 10.1213/ANE.0000000000005250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Walsh M., Devereaux P.J., Garg A.X., Kurz A., Turan A., Rodseth R.N., et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119:507–515. doi: 10.1097/ALN.0b013e3182a10e26. [DOI] [PubMed] [Google Scholar]
- 99.Wesselink E.M., Wagemakers S.H., van Waes J.A.R., Wanderer J.P., van Klei W.A., Kappen T.H. Associations between intraoperative hypotension, duration of surgery and postoperative myocardial injury after noncardiac surgery: a retrospective single-centre cohort study. Br. J. Anaesth. 2022;129:487–496. doi: 10.1016/j.bja.2022.06.034. [DOI] [PubMed] [Google Scholar]
- 100.Feng X., Hu J., Hua F., Zhang J., Zhang L., Xu G. The correlation of intraoperative hypotension and postoperative cognitive impairment: a meta-analysis of randomized controlled trials. BMC Anesthesiol. 2020;20:193. doi: 10.1186/s12871-020-01097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sessler D.I., Bloomstone J.A., Aronson S., Berry C., Gan T.J., Kellum J.A., et al. Perioperative Quality Initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br. J. Anaesth. 2019;122:563–574. doi: 10.1016/j.bja.2019.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.