Abstract
Untethered microrobots can be used for cargo delivery (e.g., drug molecules, stem cells, and genes) targeting designated areas. However, it is not enough to just reach the lesion site, as some drugs can only play the best therapeutic effect within the cells. To this end, folic acid (FA) was introduced into microrobots in this work as a key to mediate endocytosis of drugs into cells. The microrobots here were fabricated with biodegradable gelatin methacryloyl (GelMA) and modified with magnetic metal–organic framework (MOF). The porous structure of MOF and the hydrogel network of polymerized GelMA were used for the loading of enough FA and anticancer drug doxorubicin (DOX) respectively. Utilizing the magnetic property of magnetic MOF, these microrobots can gather around the lesion site with the navigation of magnetic fields. The combination effects of FA targeting and magnetic navigation substantially improve the anticancer efficiency of these microrobots. The result shows that the cancer cells inhibition rate of microrobots with FA can be up to 93%, while that of the ones without FA was only 78%. The introduction of FA is a useful method to improve the drug transportation ability of microrobots, providing a meaningful reference for further research.
Introduction
At present, untethered microrobots have achieved remarkable achievements in minimally invasive surgery [1,2], drug delivery [3–5], environmental remediation [6–8], and tissue engineering [9,10]. These microrobots can be actuated wirelessly by external energy such as magnetic field [3,11,12], light [13–15], ultrasound [16,17], chemical reaction [18–20], electric field [21], etc. Among the numerous driving energy sources, the magnetic field has good biosafety, deeper tissue penetration, and a large control range. By controlling the direction and intensity of the magnetic field, the magnitude and direction of the torque applied on the microrobot would be adjusted to guide the microrobot's motion [22]. This drive mode presents a great ability with high temporal and spatial control and fast response.
For the sake of safety for biomedical applications, the development of biodegradable magnetic microrobots can avoid the practical problem that microrobots are difficult to recycle and may cause side effects to the human body. To this end, many biodegradable microrobot systems have been developed [3,23–25]. Whereinto, the newly developed one based on gelatin methacryloyl (GelMA) and iron-doped zeolitic imidazolate framework (Fe@ZIF-8) hold great potential for cargo delivery [26]. Due to the 3-dimensional (3D) network structure of GelMA hydrogel and the high-porosity structure of Fe@ZIF-8, this system has a strong cargo loading capacity [27–30]. Moreover, the good magnetic properties of metal–organic framework (MOF) also provide the prerequisite for the magnetic actuation of microrobots. Magnetic artificial bacterial flagellar (ABF) microrobots, which have the design of microhelical structures, can convert their nonreciprocal rotating motion into a translational motion under a rotating magnetic field, so as to push themselves forward efficiently [31–33]. However, after reaching the designated position, microrobots generally cannot assist drugs to enter cells. With only the manner of free diffusion, the speed and concentration of drugs entering cells are limited, leading to a relatively poor therapeutic efficacy.
To endow the microrobot with specific cancer cell targeting ability and to promote the ingestion of drugs by cells, a commonly used cancer-targeting molecule folic acid (FA) was introduced to microrobots in this work. FA is a small-molecule ligand with high bioaffinity, which could specifically recognize folate receptor (FR) [34–36]. The selective overexpression of the FR on the surface of cancer cells provides FA the ability to promote the uptake of drugs by cancer cells through receptor-ligand-mediated endocytosis [37–39]. By combining FA with the microrobot, the resulting drug delivery system can not only locate the lesion area with the control of magnetic fields but also bring the loaded drug into the cytoplasm through endocytosis.
In this work, a folate-targeting magnetic microrobot system that consists of biodegradable GelMA-based ABF microhelix and FA-loaded Fe@ZIF-8 nanoparticles was developed, for which therapeutic drugs can be loaded into the hydrogel network of the microrobots for cancer therapy (Fig. 1). For simplification, Fe@ZIF-8 is generally marked as MOF in this work, unless otherwise specified. Thus, the developed system can be represented by ABF-MOF(FA), and ABF-MOF(FA)-DOX (doxorubicin) represents this system loaded with therapeutic drugs (DOX). With the directional manipulation of an external magnetic field, the microrobot can be navigated and fixed at the lesion site to ensure therapeutic drugs gather around cells. The full binding of FA on the microrobots and FR on the surface of cancer cells can trigger the occurrence of endocytosis, resulting in the entering of MOF(FA) and DOX into the cells. Accumulation of microrobots near cells is beneficial to the interaction of receptor and ligand, which can further improve the therapeutic efficiency. The results indicated that microrobots with FA showed more obvious cell inhibition compared to those without FA. Therefore, the ABF-MOF(FA) drug delivery system, which combines magnetic manipulation and the active targeting of FA, has a promising application prospect for cancer treatment.
Fig. 1.

The illustration of magnetically controlled ABF microrobots for folate-targeted cancer therapy.
Materials and Methods
3D printing of the helix microstructures
GelMA, a derivative of type B gelatin, not only has biodegradability but also can be photopolymerized. Combined with 2-photon lithography technology, submicron GelMA spiral microstructures can be prepared for drug delivery [40,41]. The GelMA-based microstructures in this work were fabricated using direct-laser-writing system Nanoscribe GT2, following the process in previous literatures [40]. Specifically, GelMA (200 mg/ml) and sodium photoinitiator 3,3′-(((1E, 1′E)-(2-oxcyclopentane-1,3-diylidene) bis (methanylylidene)) bis (4,1-phenylene) bis (methylazanediyl)) dipropanoate (P2CK) (1 mg/ml) were dissolved in phosphate-buffered saline (PBS) buffer at 45 °C to prepare the precursor solution (all the PBS mentioned in this work is 1×PBS). A drop of the precursor solution was dropped onto the center of a glass substrate, and then the direct laser writing was performed using a 25× objective lens with a laser power of 50 mW and a scan speed of 30,000 μm/s, setting a piezo settling time of 10 s and a galvo acceleration of 1 v/ms2. During the printing, a near-infrared femtosecond laser was used as the light source to scan in the precursor solution droplet to induce the photopolymerization. The scanning trajectory of the near-infrared laser is determined by the code generated by Describe (a professional software provided by the Nanoscribe company) according to the 3D model of the designed microrobot. The microrobot was designed to have the body length of 140 μm and the screw diameter of 50 μm. After the printing, the sample was placed in PBS at 45 °C for 30 min to remove the unpolymerized precursor solution to obtain the micro helixes.
The synthesis of MOF and MOF(FA)
The Fe@ZIF-8 (MOF) was synthesized according to the protocol reported by Terzopoulou et al. [26]. FeSO4.7H2O (2.5 ml; 0.43 M) and 2.5 ml of Zn(OAc)2.2H2O (0.55 M) were mixed and then added to 5 ml of 2-methylimidazole (5.5 M) aqueous solutions (all solutions were prepared with deionized water). Under sealing conditions, the mixture solution was stirred for 24 h at room temperature. Then, the reaction solution was subsequently washed several times with deionized water and lyophilized to obtain the final product. Fe@ZIF-8 (FA) (MOF(FA)) was prepared with the following protocol: 20 mg of FA was dissolved in 20 ml of dimethyl sulfoxide, followed by adding 20 mg of MOF. The mixture was treated by sonication for 1 h, followed by stirring at 500 rmp for 24 h. Excess-free FA was removed by repeated centrifugation washing steps. Finally, a black solid product was obtained after freeze-drying with a lyophilizer (scientz-12N/A). The FA loading rate was calculated as follows:
Assembly of the ABF microrobot
The microrobots were assembled as follows: the above-printed ABF microstructures were soaked in a MOF(FA) or MOF solution at a concentration of 20 mg/ml for 3 h to obtain microrobots fully decorated with magnetic nanoparticles (ABF-MOF(FA)/ABF-MOF). Excess MOF(FA) or MOF was removed by washing with PBS buffer more than 3 times. The magnetic microrobots were then immersed in 2 mg/ml DOX aqueous solution at 45 °C for 2.5 h to obtain a microrobot (ABF-MOF(FA)-DOX or ABF-MOF-DOX) loaded with the therapeutic drug DOX. Excess DOX was washed away with PBS buffer (4 °C) several times. PBS buffer with low temperature was used here to avoid the release of DOX, which has been loaded in the microrobots, in the washing step.
Characterization
After preparation and assembly steps, optical microscope images of GelMA-based ABF-MOF(FA) and ABF-MOF(FA)-DOX were taken for feature validation. Furthermore, we confirmed the successful preparation of MOF by a scanning electron microscope (SEM). Physical property test was done to characterize the magnetism of MOF and MOF(FA) using a physical property measurement system (PPMS-VSM). Subsequently, to prove the successful loading of FA in MOF, fluorescence luminescences of MOF(FA), MOF, and FA aqueous solutions at 365-nm excitation were measured and compared with each other, with the equipment of a Synergy H1 multimode microplate reader (BioTek Instruments).
Drug loading and releasing properties
The drug release capacity of ABF-DOX was characterized. Initially, we soaked 500 ABFs in 2 mg/ml DOX aqueous solution at 45 °C for 2.5 h and then washed them with PBS buffer solution several times to obtain the preliminary drug-loading system. Then, we immersed them in PBS with pH = 5.3 and conducted a drug release test at 37 °C. At selected time intervals, 500 μl of release solution was collected for testing and supplemented with an equal volume of fresh PBS buffer solution. The amount of the released DOX was evaluated by measuring the absorption of the release solution at 479 nm with ultraviolet (UV)/visible spectrophotometry. All experiments were repeated 3 times independently to minimize bias.
In vitro cytotoxicity (MTT)
To evaluate the targeting effect of FA in the anticancer process, the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) method was used to detect the cytotoxicity of therapeutic drugs with human breast cancer (MCF-7) cells. MCF-7 cells were seeded into a 48-well plate (15,000 cells per well) and cultured in a cell culture medium (90% Dulbecco's modified eagle medium, 10% fetal bovine serum, and 0.5% cyanine streptomycin antibacterial) under physiological conditions (37 °C and 5% CO2) for 24 h. Then, a mixture of MOF(FA) (0.05 mg/ml) and DOX (10 μg/ml or 5 μg/ml) was added to the cell cultures to be the experimental groups. The control group was distinguished by replacing the MOF(FA) with the MOF. The cell viability percentage was calculated by MTT viability assay at 24 and 48 h after the treatment. For the MTT assay, the experiment samples were treated with 5 mg/ml MTT solution prepared with PBS buffer solution and incubated at 37 °C for 4 h. Then, dimethyl sulfoxide (100 μl per well) was added to dissolve the formazan deposited in the living cells. The light absorption values of the samples were measured at 490 nm with a full-wavelength multifunctional microplate detector (Synergy H1) to indirectly reflect the number of living cells.
Anticancer efficiency of microrobots
We evaluated the inhibitory effect of the microrobot drug delivery system on MCF-7 cells through live/dead cell staining experiments, in which the living cells appeared in green and the dead cells appeared in red in the fluorescence images. The MCF-7 cells and microrobots were seeded into a 6-well plate. After incubation of 24 and 48 h, the culture medium was replaced by 2 μM calcein-AM and 4 μM propidium iodide. Then, the samples were incubated for 30 min, followed by being washed with PBS buffer solution. Finally, the fluorescence images were recorded using an inverted fluorescence microscope (Olympus x75).
Magnetic actuation and control of the microrobots
The magnetic field generator (MFG-100, Magnebotix, Zurich, Switzerland) was used to generate a rotating magnetic field for the movability test of the microrobots. A microprobe (T-4-22 model) was used to release and place microrobots on a clean petri dish subtract. Within a rotating magnetic field of 20 mT, the moving performance of the microrobots was observed and evaluated. The movability test of microrobots was also demonstrated in both a cross-shaped microchannel and a petri dish with cells landed inside.
Fabrication of microchannel
As shown in Fig. 2, a Y-shaped microchannel was fabricated to be used for an application demonstration, within which drug-loaded microrobots were navigated by a magnetic field to a target area to exert the anticancer effect. First, a layer of SU-8 2150 photoresist (500 μm) was coated on the glass substrate with a spin coater (Fig. 2A). Then, the photoresist was polymerized by UV irradiation for 30 s with a photomask that has a Y-shape pattern (Fig. 2B). Next, after removing the photomask, the sample was developed within a solution of propylene glycol methyl ether acetate (Fig. 2C). As the result, a clear Y-shape SU-8 pattern attached to the glass substrate was obtained (Fig. 2D). Afterward, the glass substrate was covered by a layer of polydimethylsiloxane (PDMS) solution and heated at 110 °C for 1 h (Fig. 2E). In the end, a PDMS Y-shaped microchannel was obtained by removing the glass substrate with the SU-8 pattern. The areas at both ends of the microchannel are marked as hole 1 and hole 2, respectively (Fig. 2F).
Fig. 2.
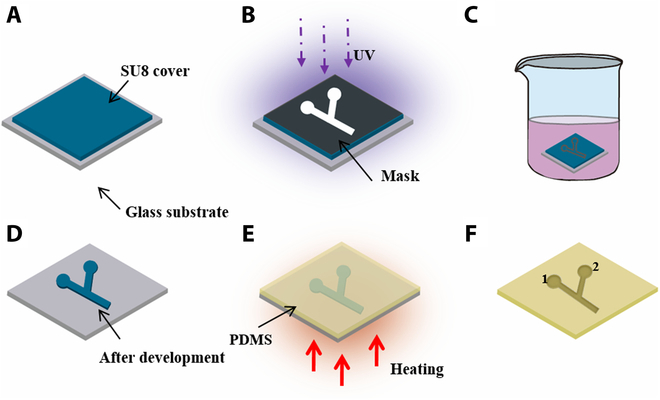
Schematic illustration of the Y-shape microchannel fabrication process. (A) A layer of SU-8 coated on a glass substrate. (B) UV polymerization of SU-8 with a Y-shape photomask. (C) Development of photopolymerized SU-8. (D) The obtained SU-8 Y-shape pattern. (E) Heating of PDMS to fabricate the microchannel. (F) The obtained Y-shape PDMS microchannel.
Application demonstration experiment
Within the Y-shaped microchannel, 1,000 MCF-7 cells were seeded in hole 1 and hole 2. After 24 h, the cells adhered to the wall and grew normally. Then, 10 microrobots were navigated toward hole 1 with a rotating magnetic field (20 mT, 6 Hz), while no microrobots were guided toward hole 2. After the microrobots reached hole 1, the cells in the microchannel were continuously incubated for another 24 h. After the entire 48-h incubation, dead cells were stained with propidium iodide, and the survival states of the cancer cells were observed with a fluorescence microscope.
Results and Disscussion
Fabrication and characterization
Figure 3A to C shows the optical images of a 3D printed microhelix, an assembled magnetic microrobot, and a drug (DOX)-loaded microrobot, respectively. The main body of the microrobot is transparent (Fig. 3A). With the uniform distribution of MOF(FA) on the surface of the microrobot, its optical image becomes darker (Fig. 3B). After DOX loading, the color of the microrobot framework turns to be red, indicating that DOX was loaded successfully into the hydrogel network of the microrobot (Fig. 3C), and the inset in Fig. 3C is a SEM image of ABF-MOF(FA)-DOX. It can also be noted that the micro helix becomes slightly smaller after DOX loading. This might mainly be because of the shrinkage of GelMA caused by dehydration during multiple cleaning steps with PBS (4 °C). Figure 3D shows the SEM image of MOF, which has a typical ZIF-8 dodecahedron shape with particle size values of 300 ± 50 nm. After FA loading, the structure of MOF(FA) becomes irregular (Fig. 3E), and the crystallinity is reduced, as shown in the x-ray diffraction (XRD) results (Fig. 3F). However, the VSM result (Fig. 3G) indicates that the ferrimagnetism of these nanoparticles does not change obviously because of folate loading. Using the calculation method mentioned above, the loading rate of FA in MOF is about 10.2%. When dispersed in water, MOF and MOF(FA) can both be quickly collected with magnets, which ensures the effective separation and collection of them (Fig. 3H). In addition, to verify that FA has been successfully loaded in MOF, the emission spectra of MOF-FA, MOF, and FA were investigated. As shown in Fig. 3I, being consistent with pure FA, MOF(FA) has the strongest emission peak at 450 nm under the excitation wavelength of 365 nm, while MOF without FA has no such fluorescence characteristics. Based on the above results, the microrobot has 2 substantial advantages: (a) the whole system is very suitable as a cargo carrier and its assembly process is easy; and (b) compared with other metal coating used solely to provide magnetism for microrobots, magnetic MOF can provide both magnetism and cargo loading ability for microrobots.
Fig. 3.
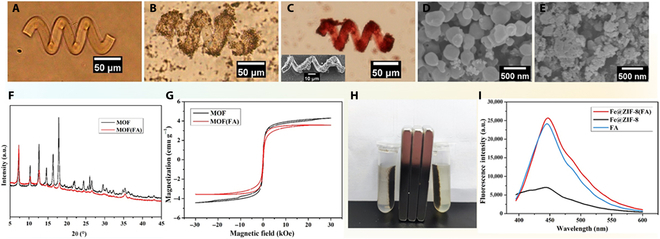
(A and B) The optical images of ABF and ABF-MOF(FA), respectively. (C) The optical image and SEM image of ABF-MOF(FA)-DOX. (D and E) The SEM image of MOF and MOF(FA). (F) XRD analysis of MOF and MOF(FA), respectively. (G) Magnetization curves of soft magnetic MOF and MOF(FA). (H) Collection of MOF (left) and MOF(FA) (right) with a magnet. (I) Fluorescence emission spectra of MOF(FA), MOF, and pure FA.
Drug loading and release properties
As the surrounding environment of cancer cells is weakly acidic, the drug release ability of GelMA-based microstructures was tested at acidic environment here. Figure 4 illustrates the curve of DOX release from GelMA-based microstructures in PBS buffer with pH = 5.3. At a constant temperature of 37 °C, the release amount of DOX gradually increased in the first 96 h and then began to grow slowly. This result indicates that the microrobot based on GelMA has a certain drug encapsulation ability and drug release ability.
Fig. 4.
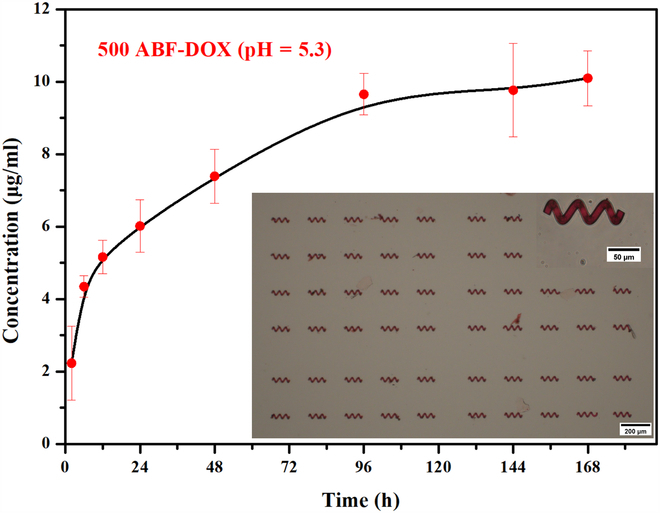
Drug release curve of ABF-DOX at 37 °C and pH 5.3.
In vitro cytotoxicity (MTT)
According to the drug release data in Fig. 4, a relatively large concentration (10 μg/ml) and a relatively intermediate concentration (5 μg/ml) of DOX that can be released by the microhelix were selected in the test groups here and the concentrations of MOF and MOF(FA) were selected as 0.05 mg/ml. As depicted in Fig. 5, high cell viability (close to 100%) of MOF and MOF(FA) groups was observed, indicating the biocompatibility of MOF and FA. In contrast, relatively low cell viability of MOF-DOX groups and MOF(FA)-DOX groups indicates their substantial growth inhibition to cancer cells, and high DOX concentration also results in better inhibition. Under the same experimental conditions and culture time, the survival rate of cancer cells in the group with FA was distinctly lower than that without FA, which was attributed to endocytosis of DOX mediated by the binding of FA to FR overexpressed on the surface of cancer cells. With more anticancer drugs entering the cell and acting on the nucleus, groups with FA have better cancer cell inhibitory effect. Taking the cases with 10 μg/ml DOX and 48-h treatment, the MOF(FA)-DOX group shows a marked cell growth inhibition with 7% cell survival rate, while the cell survival rate of the MOF-DOX group is 22%. The MTT results verify that FA in our microrobot system plays an important role in enhancing the targeting therapeutic effect.
Fig. 5.

Viability analysis of MCF-7 cells treated with different samples for 24 and 48 h.
Anticancer efficiency of microrobots
To demonstrate the inhibitory effect of drug-loaded microrobots on breast cancer cells, live/dead cell viability assays have been used for the evaluation. As illustrated in Fig. 6, MCF-7 cells incubated with ABF-MOF(FA) (the control group) grew normally, and notable proliferation was observed within 48 h. This suggested that the microrobots based on GelMA, MOF, and FA had good biocompatibility for MCF-7 cells and barely affect cell growth. Contrasting to the continuously increasing live cell density of the control group, the live cell densities of the ABF-MOF(FA)-DOX and ABF-MOF-DOX groups were both decreasing over time. This indicated that the therapeutic drug DOX can be effectively released from the microrobot and plays a role in inhibiting MCF-7 cells. Furthermore, the live cell density in the ABF-MOF(FA)-DOX group is less than that in the ABF-MOF-DOX group, while the dead cell densities in these 2 groups show an opposite relationship, especially at 48 h. This result is consistent with the previous assumption that the introducing of FA improves the tumor targeting ability of the microrobot system, enhancing the therapeutic effect.
Fig. 6.
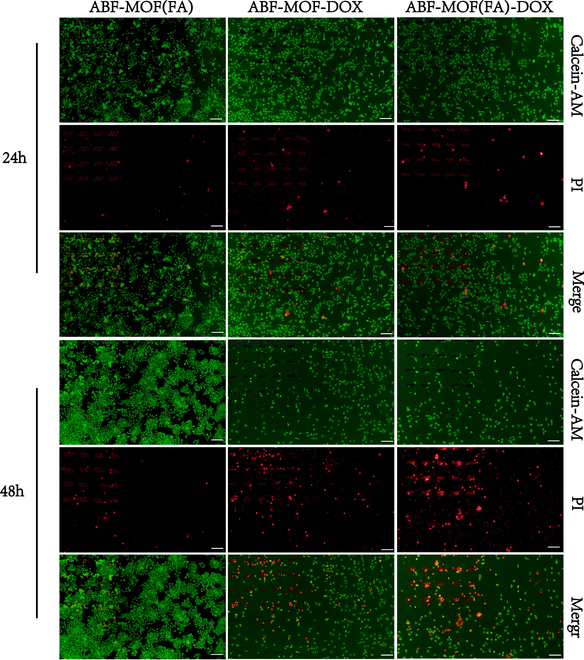
Live/dead staining images of MCF-7 cells treated with ABF-MOF(FA), ABF-MOF-DOX, and ABF-MOF(FA)-DOX for 24 and 48 h. Scale bar, 200 μm. PI, propidium iodide.
Magnetic actuation and control of the microrobots
As an important property of microrobots for drug delivery, the movability of the microrobots has also been demonstrated in this work. We first tested the swimming performance of the microrobots within a rotating magnetic field of 20 mT. By gradually increasing the frequency of the applied rotating magnetic field from 1 to 10 Hz, it was demonstrated that the movement speed of the microrobot increased first and then decreased, and the speed reached the maximum at the frequency of 6 Hz. Furthermore, the targeting capability of the microrobots was also demonstrated within a microchannel and cell environment. Figure 7A shows the motion of an ABF-MOF(FA)-DOX microrobot in a cross-microchannel. Under a rotating magnetic field with the intensity of 15 mT and the rotation frequency of 3 Hz, the microrobot can smoothly swim in the microchannel, and the moving direction can be changed by adjusting the direction of the magnetic field rotation axis, so as to realize the timely lane-changing swimming in the microchannel. Moreover, the microrobot has also been manipulated in a cancer cells environment. As depicted in Fig. 7B, in a petri dish filled with MCF-7 cells, the motion of a microrobot was demonstrated within a smooth, narrow path that was reserved in advance. With the control of a rotating magnetic field (20 mT, 2 Hz), the microrobot can move flexibly toward a corner that is surrounded by cells and difficult to reach. The corresponding movies are provided in the Supplementary Materials (Movies S1 and S2). These results indicate that our microrobots can be accurately manipulated by a magnetic field and perform specific tasks.
Fig. 7.
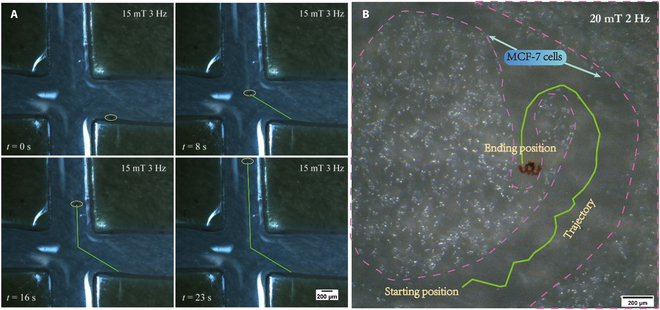
(A and B) The swimming trajectory images of a ABF-MOF(FA)-DOX microrobot was driven by a rotating magnetic field in the microchannel and the cell environment. Scale bar, 200 μm.
Application demonstration
To demonstrate the anticancer effect of the folate-modified microrobots right after they have been navigated to a target location with a magnetic field, an application demonstration experiment was conducted in a Y-shaped PDMS microchannel (Fig. 8A). As shown in Fig. 8B and Movie S3, the magnetic navigation of a group of microrobots toward hole 1 within the microchannel was demonstrated. The survival states of the cancer cells after the entire 48-h incubation are indicated by both bright-light and fluorescence images (Fig. 8C to F). As cells would shrink and become round after death, many dead cells around the microrobots in hole 1 were observed (Fig. 8C), while the cells grew normally in hole 2, where no microrobots reached (Fig. 8D). Furtherly, the dead cell staining results also show that the density of dead cells around microrobots in hole 1 (Fig. 8E) is remarkably higher than the density of dead cells in hole 2 (Fig. 8F), where no microrobot exists. The results proved that, after being navigated to the designated location by a magnetic field, DOX-loaded folate targeting magnetic microrobots (ABF-MOF(FA)-DOX) can produce an obvious anticancer effect within 24 h.
Fig. 8.

(A) A photo of the Y-shape microchannel. (B) A snapshot of microrobots being navigated in the Y-shape microchannel. (C and D) Bright-light images of hole 1 and hole 2 after the entire 48-h incubation, respectively. (E and F) Fluorescence images of dead cells in hole 1 and hole 2 after the entire 48-h incubation, respectively.
Conclusion
In conclusion, we proposed a magnetic microrobot platform with FA targeting function for enhanced cancer cell inhibition. These microrobots can transport drugs to cancer cell regions and increase the ingestion of drugs by cancer cells through receptor-ligand-mediated endocytosis. Due to the high drug loading capacity of GelMA hydrogel and MOF porous structures, drug and FA were successfully loaded in the hydrogel network and MOF nanoparticles of the microrobots, respectively. The successful loadings have been confirmed by UV/visible spectroscopy and microscopic images. The MTT assay and the live/dead staining experiments demonstrated that the existence of FA can substantially enhance the inhibition ability of therapeutic agents to cancer cells activity. Besides, the live/dead staining experiments also confirmed the good biocompatibility of the folate-modified microrobots when no DOX was loaded inside. The motion experiment proved that the microrobots can achieve directional movement in a cross-microchannel and cell environment under the actuation of a rotating magnetic field. In addition, an application experiment in the Y-shaped channel successfully demonstrated both the magnetic actuation and the enhanced targeted anticancer effect of folate-modified magnetic microrobots entirely. With the advantages of high loading capacity, controllable navigation, and enhancing cancer cell targeting and inhibition, the proposed folate-targeting magnetic microrobot system hold great potential in clinical cancer treatment.
Acknowledgments
The authors would like to thank M. Yang, a doctoral student at the Southern University of Science and Technology, for helping with the SEM and XRD characterizations. Funding: This work is funded by the Shenzhen Institute of Artificial Intelligence and Robotics for Society (AC01202101106). Competing interests: The authors declare that there are no competing interests.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Supplementary Materials
Data file: Supporting Information.
Audio files: Movies S1 to S3.
References
- 1.York PA, Peña R, Kent D, Wood RJ. Microrobotic laser steering for minimally invasive surgery. Sci Robot. 2021;6(50):Article eabd5476. [DOI] [PubMed] [Google Scholar]
- 2.Xie L, Pang X, Yan X, Dai Q, Lin H, Ye J, Cheng Y, Zhao Q, Ma X, Zhang X, et al. Photoacoustic imaging-trackable magnetic microswimmers for pathogenic bacterial infection treatment. ACS Nano. 2020;14(3):2880–2893. [DOI] [PubMed] [Google Scholar]
- 3.Lee H, Kim D-i, Kwon S-h, Park S. Magnetically actuated drug delivery helical microrobot with magnetic nanoparticle retrieval ability. ACS Appl Mater Interfaces. 2021;13(17):19633–19647. [DOI] [PubMed] [Google Scholar]
- 4.Gong D, Celi N, Zhang D, Cai J. Magnetic biohybrid microrobot multimers based on chlorella cells for enhanced targeted drug delivery. ACS Appl Mater Interfaces. 2022;14(5):6320–6330. [DOI] [PubMed] [Google Scholar]
- 5.Zhang F, Zhuang J, Li Z, Gong H, Avila BE, Duan Y, Zhang Q, Zhou J, Yin L, Karshalev E, et al. Nanoparticle-modified microrobots for in vivo antibiotic delivery to treat acute bacterial pneumonia. Nat Mater. 2022;21(11):1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Zhang M-J, Zhang S-Y, Shi L, Yang Y-M, Liu Z, Ju X-J, Xie R, Wang W, Chu L-Y. Simple and continuous fabrication of self-propelled micromotors with photocatalytic metal–organic frameworks for enhanced synergistic environmental remediation. ACS Appl Mater Interfaces. 2020;12(31):35120–35131. [DOI] [PubMed] [Google Scholar]
- 7.Zarei M, Zarei M. Self-propelled micro/nanomotors for sensing and environmental remediation. Small. 2018;14(30):Article 1800912. [DOI] [PubMed] [Google Scholar]
- 8.Vilela D, Parmar J, Zeng Y, Zhao Y, Sánchez S. Graphene-based microbots for toxic heavy metal removal and recovery from water. Nano Lett. 2016;16(4):2860–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu D, Zhang T, Guo Y, Liao Y, Wu Z, Jiang H, Lu Y.. Biohybrid magnetic microrobots for tumor assassination and active tissue regeneration. ACS Appl Bio Mater. 2022;5(12):5933–5942. [DOI] [PubMed] [Google Scholar]
- 10.Rahman MA, Wang Z, Ohta AT. Collaborative micromanipulation using multiple bubble microrobots in an open reservoir. Micro Nano Lett. 2017;12(11):891–896. [Google Scholar]
- 11.Fu Q, Guo S, Yamauchi Y, Hirata H, Ishihara H. A novel hybrid microrobot using rotational magnetic field for medical applications. Biomed Microdevices. 2015;17(2):31. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Hu C, Schurz L, De Marco C, Chen X, Pané S, Nelson BJ. Surface-chemistry-mediated control of individual magnetic helical microswimmers in a swarm. ACS Nano. 2018;12(6):6210–6217. [DOI] [PubMed] [Google Scholar]
- 13.Zeng H, Wasylczyk P, Wiersma DS, Priimagi A. Light robots: Bridging the gap between microrobotics and Photomechanics in soft materials. Adv Mater. 2018;30(24):Article e1703554. [DOI] [PubMed] [Google Scholar]
- 14.He Q, Wang Z, Wang Y, Wang Z, Li C, Annapooranan R, Zeng J, Chen R, Cai S. Electrospun liquid crystal elastomer microfiber actuator. Sci Robot. 2021;6(57):Article eabi9704. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Liang Y, Gao W, Dong R, Wang C. Emulsion hydrogel soft motor actuated by thermal stimulation. 2017;9(49):43211–43219. [DOI] [PubMed] [Google Scholar]
- 16.Kaynak M, Ozcelik A, Nourhani A, Lammert PE, Crespi VH, Huang TJ. Acoustic actuation of bioinspired microswimmers. 2017;17(3):395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao Y, Zhang J, Fang B, Zhao X, Hao N. Acoustics-actuated microrobots. 2022;13(3):481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Chen C, Wu J, Ju H. Bubble-propelled jellyfish-like micromotors for DNA sensing. ACS Appl Mater Interfaces. 2019;11(14):13581–13588. [DOI] [PubMed] [Google Scholar]
- 19.Panda A, Reddy AS, Venkateswarlu S, Yoon MJN. Bio-inspired self-propelled diatom micromotor by catalytic decomposition of H2O2 under low fuel concentration. Nanoscale. 2018;10(34):16268–16277. [DOI] [PubMed] [Google Scholar]
- 20.Umebara M, Sugai N, Murayama K, Sugawara T, Akashi Y, Morita Y, Kato R, Komatsu T. Catalase-driven protein microtube motors with different exterior surfaces as ultrasmall biotools. Mater Adv. 2021;2(19):6428–6438. [Google Scholar]
- 21.Kosa G, Shoham M, Zaaroor M. Propulsion method for swimming microrobots. IEEE Trans Robot. 2007;23(1):137–150. [Google Scholar]
- 22.Abbott JJ, Peyer KE, Lagomarsino MC, Zhang L, Dong L, Kaliakatsos IK, Nelson BJ. How should microrobots swim? Int J Robot Res. 2009;28(11–12):1434–1447. [Google Scholar]
- 23.Ceylan H, Yasa IC, Yasa O, Tabak AF, Giltinan J, Sitti M. 3D-printed biodegradable microswimmer for Theranostic cargo delivery and release. ACS Nano. 2019;13(3):3353–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noh S, Jeon S, Kim E, Oh U, Park D, Park SH, Kim SW, Pane S, Nelson BJ, Kim JY, et al. A biodegradable magnetic microrobot based on gelatin methacrylate for precise delivery of stem cells with mass production capability. Small. 2022;18(25):Article 2107888. [DOI] [PubMed] [Google Scholar]
- 25.Llacer-Wintle J, Rivas-Dapena A, Chen XZ, Pellicer E, Nelson BJ, Puigmarti-Luis J, Pane S. Biodegradable small-scale swimmers for biomedical applications. Adv Mater. 2021;33(42):Article 2102049. [DOI] [PubMed] [Google Scholar]
- 26.Terzopoulou A, Wang X, Chen XZ, Palacios-Corella M, Pujante C, Herrero-Martín J, Qin XH, Sort J, DeMello AJ, Nelson B, et al. Biodegradable metal–organic framework-based microrobots (MOFBOTs). 2020;9(20):Article 2001031. [DOI] [PubMed] [Google Scholar]
- 27.Luo Z, Sun W, Fang J, Lee K, Li S, Gu Z, Dokmeci MR, Khademhosseini A. Biodegradable gelatin methacryloyl microneedles for transdermal drug delivery. Adv Healthc Mater. 2019;8(3):Article e1801054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bordini EAF, Ferreira JA, Dubey N, Ribeiro JS, Souza Costa CA, Soares DG, Bottino MC. Injectable multifunctional drug delivery system for hard tissue regeneration under inflammatory microenvironments. ACS Appl Bio Mater. 2021;4(9):6993–7006. [DOI] [PubMed] [Google Scholar]
- 29.Zhao H, Zhao Y, Liu D. pH and H(2)S dual-responsive magnetic metal-organic frameworks for controlling the release of 5-fluorouracil. ACS Appl Bio Mater. 2021;4(9):7103–7110. [DOI] [PubMed] [Google Scholar]
- 30.Baikeli Y, Mamat X, He F, Xin X, Li Y, Aisa HA, Hu G. Electrochemical determination of chloramphenicol and metronidazole by using a glassy carbon electrode modified with iron, nitrogen co-doped nanoporous carbon derived from a metal-organic framework (type Fe/ZIF-8). Ecotoxicol Environ Saf. 2020;204:111066. [DOI] [PubMed] [Google Scholar]
- 31.Xin C, Yang L, Li J, Hu Y, Qian D, Fan S, Hu K, Cai Z, Wu H, Wang D, et al. Conical hollow microhelices with superior swimming capabilities for targeted cargo delivery. Adv Mater. 2019;31(25). [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Hu C, Pane S, Nelson BJ. Dynamic modeling of magnetic helical microrobots. IEEE Robot Autom Lett. 2022;7(2):1682–1688. [Google Scholar]
- 33.Wang W, He XH, Zhang MJ, Tang MJ, Xie R, Ju XJ, Liu Z, Chu LY. Controllable microfluidic fabrication of microstructured materials from nonspherical particles to helices. Macromol Rapid Commun. 2017;38(23):Article 1700429. [DOI] [PubMed] [Google Scholar]
- 34.Bansal D, Gulbake A, Tiwari J, Jain SK. Development of liposomes entrapped in alginate beads for the treatment of colorectal cancer. Int J Biol Macromol. 2016;82:687–695. [DOI] [PubMed] [Google Scholar]
- 35.Xu W, Lou Y, Chen W, Kang Y. Folic acid decorated metal-organic frameworks loaded with doxorubicin for tumor-targeted chemotherapy of osteosarcoma. Biomed Tech. 2020;65(2):229–236. [DOI] [PubMed] [Google Scholar]
- 36.Yang H, Li Y, Li T, Xu M, Chen Y, Wu C, Dang X, Liu Y. Multifunctional core/shell nanoparticles cross-linked polyetherimide-folic acid as efficient Notch-1 siRNA carrier for targeted killing of breast cancer. Sci Rep. 2014;4:7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao X, Li H, Lee RJ. Targeted drug delivery via folate receptors. Expert Opin Drug Deliv. 2008;5(3):309–319. [DOI] [PubMed] [Google Scholar]
- 38.Narmani A, Rezvani M, Farhood B, Darkhor P, Mohammadnejad J, Amini B, Refahi S, Abdi Goushbolagh N. Folic acid functionalized nanoparticles as pharmaceutical carriers in drug delivery systems. Drug Dev Res. 2019;80(4):404–424. [DOI] [PubMed] [Google Scholar]
- 39.Arabian T, Amjad-Iranagh S, Halladj R. Molecular dynamics simulation study of doxorubicin adsorption on functionalized carbon nanotubes with folic acid and tryptophan. Sci Rep. 2021;11(1):24210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Qin X-H, Hu C, Terzopoulou A, Chen X-Z, Huang T-Y, Maniura-Weber K, Pané S, Nelson BJ. 3D printed enzymatically biodegradable soft helical microswimmers. Adv Funct Mater. 2018;28(45):Article 1804107. [Google Scholar]
- 41.Lee BH, Shirahama H, Cho N-J, Tan LP. Efficient and controllable synthesis of highly substituted gelatin methacrylamide for mechanically stiff hydrogels. RSC Adv. 2015;5(128):106094–106097. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data file: Supporting Information.
Audio files: Movies S1 to S3.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.


