Abstract
Background
Whether endoscopic submucosal dissection (ESD) applies to undifferentiated-type early gastric cancer (UEGC) remains controversial. We aimed to analyze the risk factors for lymph node metastasis (LNM) in UEGC and evaluate the feasibility of ESD.
Methods
This study included 346 patients with UEGC who underwent curative gastrectomy between January 2014 and December 2021. Univariate and multivariate analyses of the correlation between clinicopathological features and LNM were conducted, and the risk factors for exceeding the expanded ESD indications were evaluated.
Results
The overall LNM rate in UEGC was 19.94%. Among the preoperatively assessable factors, submucosal invasion (odds ratio [OR] = 4.77, 95% confidence interval [CI]: 2.14–10.66) and > 2 cm(OR = 2.49, 95% CI: 1.20–5.15) were independent risk factors for LNM, while postoperative independent risk factors were > 2 cm (OR = 3.35, 95% CI: 1.02–5.40) and lymphovascular invasion(OR = 13.21, 95% CI: 5.18–33.70). Patients who met the expanded indications had a low LNM risk (4.1%). Additionally, tumors located in the cardia (P = 0.03), non-elevated type (P < 0.01) were independent risk factors for exceeding the expanded indications in UEGC.
Conclusions
ESD may be applicable for UEGC meeting the expanded indications, and preoperative evaluation should be cautious when the lesion is non-elevated type or located in the cardia.
Trial registration
Chinese Clinical Trial Registry (12/05/2022 ChiCTR2200059841).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-023-02771-x.
Keywords: Undifferentiated, Gastric cancer, Lymph node metastasis, Endoscopic submucosal dissection, Retrospective analysis
Background
Early gastric cancer (EGC) is defined as tumor tissue confined to the mucosa or submucosa, regardless of lymph node metastasis (LNM) [1]. The presence or absence of LNM affects not only the selection of the treatment modality but also the patient’s prognosis [2]. Endoscopic resection, represented by endoscopic submucosal dissection (ESD), is unanimously recommended by international guidelines because of its low surgical trauma, fewer complications, and complete preservation of tissue and organ function [3]. ESD has gradually become an effective alternative for EGC without LNM. Therefore, the preoperative evaluation of LNM is particularly important.
Evidence is now available that the risk of LNM in EGC can be predicted based on lesion size, depth, histological type, ulceration, and lymphovascular invasion (LVI) [4]. However, the possibility of endoscopic treatment for undifferentiated-type EGC (UEGC) is controversial because of its relatively high risk of LNM compared with differentiated EGC [5]. The previous guidelines listed non-ulcerative undifferentiated intramucosal carcinoma and tumor size of ≤ 2 cm as the expanded indication of ESD [6], and the recent Japanese guidelines included it in the absolute indication [7]; however, ESD for UEGC is still not widespread in China because of the lack of confidence in curative resection by both endoscopists and patients. This study aimed to analyze the correlation between the clinicopathological features and LNM in UEGC and to explore the feasibility of ESD in a Chinese population with UEGC.
Methods
Patients
This study retrospectively included 346 patients with UEGC who underwent curative gastrectomy with intraoperative lymph node dissection at The First Affiliated Hospital of Anhui Medical University between January 2014 and December 2021. All included patients underwent partial gastrectomy with intraoperative lymph node dissection and were initially diagnosed and reviewed by two pathologists as having UEGC. The exclusion criteria were as follows: (1) history of radiochemotherapy; (2) remnant gastric cancer or recurrent cancer of the remnant stomach; (3) other malignant tumors of the stomach, such as gastrointestinal stromal tumors, lymphomas, and neuroendocrine tumors; (4) serious hematologic diseases and other diseases that may affect the study results. This study followed the ethical principles of medical research involving human subjects in the Declaration of Helsinki and was approved by the ethics committee of our hospital(Quick-PJ2022-05-37). We have also registered with the Chinese Clinical Trial Registry (12/05/2022 ChiCTR2200059841).
Data collection
Using the hospital electronic medical record system, clinicopathological data were collected as comprehensively as possible, about the selection of candidate variables, we took into account the actual clinical demands and also referred to other similar studies, including sex, age, body mass index (BMI), smoking history, drinking history, tumor size and location, based on the Japanese classification of gastric carcinoma [8]. The preoperative hematological indexes mainly included the latest preoperative hematological examination of neutrophils, lymphocytes, monocytes, platelets, hemoglobin (Hb), albumin, and tumor markers (carcinoembryonic antigen, carbohydrate antigen CA19-9, and CA125), peripheral blood neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and monocyte-to-lymphocyte ratio (MLR).
The main outcome variables were measured and classified as follows: the tumor size was calculated based on the longest diameter of the tumor on histology. According to the guidelines of the Japanese Gastric Cancer Association [8], tumor location was classified as follows: cardia, corpus (including fundus), incisura angularis, and antrum. The depth of invasion was divided into two categories: mucosal (M) and submucosal (SM). According to World Health Organization (WHO) criteria, the histological types included in the study were poorly differentiated adenocarcinoma (por), mucinous adenocarcinoma (muc), signet ring cell carcinoma (sig), and their mixed type. According to the Paris classification [9], the macroscopic type was divided into three groups: elevated type (type 0-I, 0-IIa, or a combination of these two types), flat (0-IIb), and depressed type (0-IIc, 0-III, or a combination of these types). The depth of the ulcer was up to or beyond the mucous membrane, and an active ulcer or scar was considered an ulcer on endoscopy. All surgically resected specimens were at 5-mm intervals, and the lymph nodes were sliced into two segments. When the tumor cells occurred in the tubular space arranged by endothelial cells or in the wall of blood vessels, LVI was considered. LNM was analyzed and judged by pathologists based on the results of hematoxylin-eosin staining.
Statistical analysis
All statistical analyses were carried out using SPSS 26.0 software (IBM Inc, New York). Continuous data in accordance with the normal distribution are expressed as the mean ± standard deviation, those that do not conform to the normal distribution are expressed by the median and quartile spacing, and categorical data are expressed as the number of cases and frequency (%). In the univariate analysis, continuous data were compared using the t-test or Mann–Whitney U test, and categorical data were compared using the chi-square test or Fisher’s exact test. Variables with P < 0.05 in the univariate analysis were included in the binary logistic regression for multivariate analysis and were considered statistically significant at P < 0.05 (two-sided).
Results
Clinicopathological characteristics
The study included 346 patients who underwent total or subtotal gastrectomy, all of whom were pathologically diagnosed with UEGC. The study population comprised 187 males and 159 females, with a mean age of 56.3 ± 10.8 years, with individuals aged ≥ 60 years accounting for 139 cases and individuals aged ≤ 59 years accounting for 207 cases. There were 190 cases of lesions measuring ≤ 2 cm and 156 cases with lesions measuring > 2 cm, including 153 cases of por (44.22%), 42 cases of muc (12.14%), 19 cases of sig (5.49%), 85 cases of por mixed with signet ring cells (24.57%), and 47 cases of por with mucinous components (13.58%).
Risk factors for LNM in UEGC
LNM occurred in 69 of 346 patients (19.94%), and there were no statistically significant differences in age, sex, smoking and alcohol history, NLR, MLR, tumor markers, location, and histological type in the LNM (+) group compared with the LNM (-) group (P > 0.05). There were significant differences between the two groups in terms of the BMI, PLR, Hb, and macroscopic type. Their size was larger, and the proportions of ulceration, SM infiltration, and LVI were also significantly higher (P < 0.05) (Table 1).
Table 1.
Incidence of lymph node metastasis and clinicopathological characteristics in undifferentiated-type early gastric cancer
| LNM(-) | LNM(+) | X2 | P value | |
|---|---|---|---|---|
| Gender | 2.88 | 0.089 | ||
| Female | 121 | 38 | ||
| Male | 156 | 31 | ||
| Age(years) | 2.46 | 0.116 | ||
| < 60 | 160 | 47 | ||
| ≥ 60 | 117 | 22 | ||
| Smoking | 0.767 | 0.381 | ||
| Absence | 222 | 52 | ||
| Presence | 55 | 17 | ||
| Drinking | 0.042 | 0.838 | ||
| Absence | 230 | 58 | ||
| Presence | 47 | 11 | ||
| BMI(kg/m2) | 22.44 ± 3.68 | 21.01 ± 4.47 | 0.031 | |
| NLR | 1.89(1.41–2.53) | 1.92(1.56–3.15) | 0.156 | |
| PLR | 123.19(91.61-162.08) | 143.54(113.56-183.87) | 0.014 | |
| MLR | 0.19(0.15–0.26) | 0.20(0.157–0.29) | 0.192 | |
| Hb(g/L) | 132(119–144) | 123(106-138.5) | 0.005 | |
| CEA(ng/ml) | 1.86(1.2–3.1) | 1.66(1.12–2.16) | 0.163 | |
| CA125(U/ml) | 9.43(7.05–13.7) | 9.25(6.16–15.97) | 0.673 | |
| CA199(U/ml) | 8.98(5.31–13.55) | 8.41(6.05–13.76) | 0.127 | |
| Alb(g/L) | 42.91 ± 4.31 | 42.20 ± 4.21 | 0.431 | |
| Size(cm) | 10.34 | 0.001 | ||
| ≤ 2 | 164 | 26 | ||
| > 2 | 113 | 43 | ||
| Ulcer | 11.32 | 0.001 | ||
| Absence | 147 | 21 | ||
| Presence | 130 | 48 | ||
| Location | 4.25 | 0.23 | ||
| cardia | 21 | 1 | ||
| corpus | 67 | 16 | ||
| Incisura angularis | 87 | 21 | ||
| antrum | 102 | 31 | ||
| Macroscopic type | 6.39 | 0.04 | ||
| elevated | 18 | 3 | ||
| flat | 63 | 7 | ||
| depressed | 196 | 59 | ||
| Pathological type | 4.01 | 0.405 | ||
| sig | 17 | 2 | ||
| por | 117 | 36 | ||
| muc | 37 | 5 | ||
| por + muc | 38 | 9 | ||
| por + sig | 68 | 17 | ||
| Depth | 18.95 | < 0.001 | ||
| M | 169 | 22 | ||
| SM | 108 | 47 | ||
| LVI | 83.29 | < 0.001 | ||
| Absence | 259 | 34 | ||
| Presence | 18 | 35 | ||
BMI: body mass index;NLR: neutrophil-to-lymphocyte ratio;PLR: platelet-to-lymphocyte ratio;MLR: monocyte-to-lymphocyte ratio;Hb: hemoglobin;Alb:albumin;por:poorly differentiated adenocarcinoma,muc:mucinous adenocarcinoma, sig: signet ring cell carcinoma;M: mucosal;SM:submucosal; LVI: lymphovascular invasion;LNM: lymph node metastasis;
Multivariate analysis of risk factors for LNM in UEGC
With LNM as the outcome variable, as shown in Table 2, in which factors that could be assessed by laboratory tests and endoscopy before surgery were included in the preoperative group (except LVI), and postoperative assessable factors including LVI, the results of the preoperative analysis showed that lesions measuring > 2 cm (odds ratio [OR] = 2.49, 95% confidence interval [CI]: 1.20–5.15) and SM infiltration (OR = 4.77, 95% CI: 2.14–10.66) were independent risk factors for LNM (P < 0.05); among the postoperative factors, size (OR = 3.35, 95% CI: 1.02–5.40) and LVI (OR = 13.21, 95% CI: 5.18–36.70) were independent risk factors, especially LVI, presenting the highest OR. Postoperative risk factors(size + LVI) were better than preoperative factors(size + depth) in predicting LNM based on multivariate analysis(AUROC 0.76 vs. 0.70), but the difference was not significant(Z = 1.89, P = 0.59)(Fig. 1).
Table 2.
Multivariate logistic regression analysis for lymph node metastasis in undifferentiated-type early gastric cancer
| Factor | Preoperative | Postoperative | ||
|---|---|---|---|---|
| OR(95%CI) | P | OR(95%CI) | P | |
| BMI | 0.94(0.85–1.04) | 0.229 | 0.94(0.84–1.06) | 0.329 |
| PLR | 1.00(0.99–1.01) | 0.240 | 1.00(0.99–1.01) | 0.537 |
| Hb(g/L) | 0.99(0.98–1.01) | 0.178 | 1.00(0.98–1.01) | 0.808 |
| Macroscopic type | ||||
| flat | 1(Ref.) | 1(Ref.) | ||
| elevated | 0.33(0.03–3.70) | 0.370 | 0.15(0.01–2.06) | 0.154 |
| depressed | 0.77(0.20–2.94) | 0.705 | 1.14(0.26–4.93) | 0.861 |
| Size(>2 cm) | 2.49(1.20–5.15) | 0.014 | 3.35(1.02–5.40) | 0.044 |
| UL(+) | 1.87(0.67–5.21) | 0.233 | 1.46(0.48–4.42) | 0.506 |
| Depth(SM) | 4.77(2.14–10.66) | < 0.001 | 2.46(0.99–6.10) | 0.052 |
| LVI(+) | 13.21(5.18–33.70) | < 0.001 | ||
BMI: body mass index;PLR: platelet-to-lymphocyte ratio;Hb: hemoglobin;UL: ulcer;SM:submucosal; LVI: lymphovascular invasion; OR: odds ratio;CI:Confidence intervals
Fig. 1.
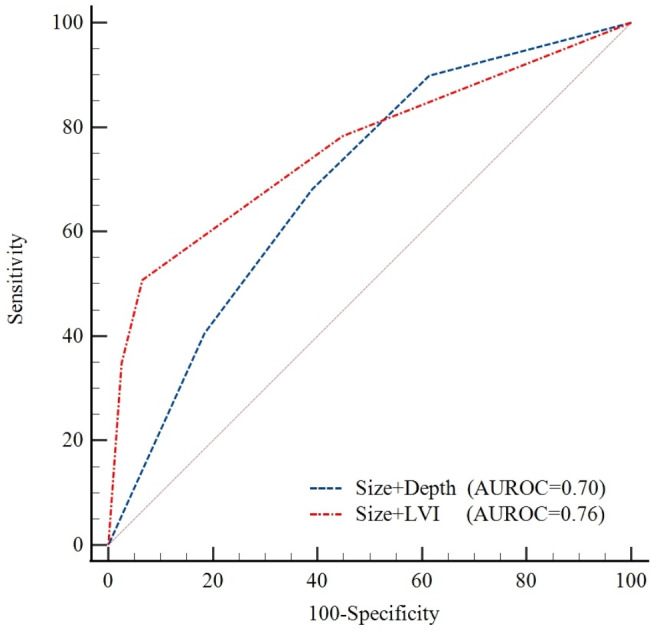
ROC curve of the risk prediction for lymph node metastasis of undifferentiated-type early gastric cancer
LVI: lymphovascular invasion; AUROC:Area under the receiver operating characteristic curve
Subgroup analysis of LNM in UEGC
LNM in UEGC was stratified according to three risk factors, including size, depth, and ulceration, in which size and depth were independent risk factors in the multivariate analysis, and ulceration was included in the guidelines, as detailed in Table 3. The results showed that for lesions measuring ≤ 1 cm, regardless of ulceration, no postoperative LNM in the included participants (0/39), undifferentiated intramucosal cancer without ulceration, and lesions measuring ≤ 2 cm, which met the expanded indications for ESD, had a comparatively low rate of LNM (4.10%). When the lesion was ≤ 3 cm, the risk of LNM was higher than before, even for ulcer-negative intramucosal carcinoma (5.05%). When the lesion infiltrated the SM, the risk of LNM was as high as 20% (1/5), even for ulcer-negative intramucosal carcinoma measuring ≤ 1 cm, which was not suitable for endoscopic treatment.
Table 3.
Rate of lymph node metastasis according to three risk factors [% (n)]
| Size (cm) |
M | SM | ||||||
|---|---|---|---|---|---|---|---|---|
| UL- | 95%CI(%) | UL+ | 95%CI(%) | UL- | 95%CI(%) | UL+ | 95%CI(%) | |
| ≤ 1 | 0(0/24) | 0 | 0(0/15) | 0 | 20.0(1/5) | 35.5–75.5 | 10.0(1/10) | -12.6-32.6 |
| > 1,≤2 | 6.1(3/49) | -0.8-13.1 | 15.4(4/26) | 0.5–30.2 | 15.0(3/20) | 2.1–32.1 | 34.1(14/41) | 19-0.49.3 |
| > 2,≤3 | 7.7(2/26) | -3.3-18.7 | 27.3(6/22) | 7.1–47.5 | 7.7(1/13) | -9.1-24.5 | 30.6(11/36) | 14.7–46.4 |
| >3 | 31.2(5/16) | 5.7–58.8 | 15.4(2/13) | -7.3-38.1 | 40.0(6/15) | 11.9–68.1 | 66.7(10/15) | 39.6–93.7 |
M: mucosal;SM:submucosal;UL:ulcer
Risk of exceeding the ESD expanded indications in UEGC
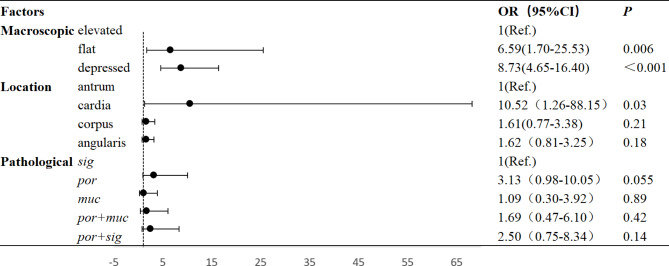
According to the guidelines, among the 346 cases included in this study, a total of 73 cases met the expanded indication, and 273 cases exceeded the indications. The univariate analysis showed that the macroscopic type, location, and pathological type were associated with exceeding the expanded indications (P < 0.05), Their multivariate analysis are presented in Fig. 2, the multivariate regression analysis showed that depressed or flat lesions(non-elevated type) and tumor located in the cardia were independent risk factors for exceeding the expanded indications (P < 0.05) (Supplementary 1).
Fig. 2.
Multivariate logistic regression analysis of undifferentiated-type early gastric cancer according to endoscopic submucosal dissection indications. por:poorly differentiated adenocarcinoma,muc:mucinous adenocarcinoma, sig: signet ring cell carcinoma;LNM: lymph node metastasis; OR: odds ratio;CI:Confidence intervals
Characteristics of patients with LNM in expanded indications
Further analysis showed that three cases (4.10%) in the ESD indications group still had LNM, including two cases invading the M but not the SM; the clinicopathological features are listed in Table 4. All lesions were flat, two were cases of por mixed with mucinous components, and the other was a case of pure muc. Notably, the largest diameter in all three lesions was 2.0 cm, which may be a specific feature to differentiate from lymph node-negative patients.
Table 4.
Characteristics of undifferentiated-type early gastric cancer with endoscopic submucosal dissection extended indication but with lymph node metastasis
| Gender | Age(years) | Size(cm) | Location | Depth | Form | Pathological type | LVI | |
|---|---|---|---|---|---|---|---|---|
| 1 | male | 82 | 2.0*1.5 | Incisura angularis | mm | flat | muc | positivity |
| 2 | female | 46 | 2.0*1.5 | antrum | lp | flat | por + muc | negative |
| 3 | male | 61 | 2.0*1.0 | corpus | mm | flat | por + muc | negative |
por:poorly differentiated adenocarcinoma,muc:mucinous adenocarcinoma, mm:muscularis mucosa;lp:lamina propria; LVI: lymphovascular invasion
Discussion
As one of the top five malignant tumors worldwide in terms of morbidity and mortality, gastric cancer has adversely affected human health [10]. Owing to the widespread eradication of Helicobacter pylori, the incidence of intestinal gastric cancer has shown a decreasing trend, and UEGC, represented by por and sig, has gained attention [11]. As UEGC has a higher risk of LNM and a relatively poor prognosis [12], preoperative assessment of the risk of LNM should be particularly cautious. Our study showed that lesions measuring > 2 cm and SM infiltration were preoperatively independent risk factors for LNM, patients who met the expanded indications had a relatively low risk of LNM, and endoscopic resection was still possible for intramucosal carcinomas of < 1 cm, even with ulceration.
In agreement with previous studies [13, 14], our study showed that UEGC occurred mostly in the lower and middle part of the stomach, with a flat and depressed form, and the LNM rate was 19.94%, slightly higher than the 11.3% reported by Lee IS [15]. In agreement with other studies [16], LVI is the most prominent independent risk factor of LNM. As for preoperatively assessable factors, an increased risk of LNM was observed when the size of the lesion was > 2 cm. We cannot ignore lesions that are near the cut-off value in size,in our study, three patients who were eligible for ESD based on the expanded indications but presented with LNM had lesions measuring 2.0 cm, due to postoperative specimen shrinkage, the actual size may exceed 2 cm.
In addition, the LNM rates of UEGC in the M and SM were 11.5% and 30.3%, respectively, which is comparable to those reported in previous studies (11.2% and 33.0%, respectively) [17]. There is also a difference in the risk of LNM between the SM1 and SM2 or deeper, but since this study mainly included surgical specimens retrospectively, it is regrettable that no further submucosa subdivision could be performed. Our study also demonstrated that the macroscopic type and ulceration were associated with LNM; however, they were not independent risk factors. No relevance was found between the history of smoking, alcohol consumption, chronic diseases, tumor markers, and LNM, and this is consistent with the findings of Minghan Ren [18]. Similar to another study [19], PLR showed statistical differences in the univariate analysis but not in the multivariate analysis. In addition, BMI and Hb in the LNM group were lower than those of the control group. A study [20] on biopsy specimens of EGC with undifferentiated components also confirmed that BMI is a protective factor for LNM, which may be related to energy consumption along with tumor expansion.
Regarding the pathological type, a previous study [17] suggested that the rate of LNM of sig was similar to that of differentiated EGC and significantly lower than that of UEGC. Our study found no LNM in sig smaller than 2 cm regardless of the ulceration and pure por smaller than 2 cm without ulceration. This suggests that the risk of LNM in pure sig and por may be low, which has been confirmed in several studies [15, 21, 22]. This difference does not preclude an association with promoter CpG island hypermethylation [23]; however, due to the small sample size, no statistical difference was observed in the present study. In additional to the risk of LNM in sig, tumors larger than 2 cm have an increased risk of incomplete resection due to underestimation of their size, and endoscopic resection should be performed with caution [24]. Moreover, patients that meet the expanded indications for ESD but present with LNM comprise a mucinous component. A Korean study [25] also concluded that the risk of LNM is higher in muc than in other UEGC because of extracellular mucins which can act as a medium to promote tumor cell infiltration [26].
For intramucosal carcinoma measuring < 1 cm, regardless of whether there is an ulcer or not, no LNM was observed in our data, and endoscopic treatment was relatively suitable. We confirmed that the rate of LNM meeting the expanded indications for ESD was 4.1%, higher than the Korean analogous study (1.56%) [27]. this may be related to the difference in the pathological diagnostic system after surgery between China and Korea, and most Korean pathologists refer to Japanese standards to diagnose cancer based on nuclear and architectural heterogeneity, which is more aggressive compared with the WHO standards; thus, have a lower risk of LNM.
Although pathological criteria were not fully harmonized, patients meeting the expanded indications for ESD have a lower risk than the serious postoperative complications [28, 29] in our study. Long-term survival of patients with ESD and surgical operation also did not show clear differences (propensity score matching, P = 0.33) [30]. Takizawa’s multicenter study [31] further confirms the safety and efficacy of ESD in patients with UEGC based on the expanded indications. In our center, we performed ESD in 13 patients with UEGC (until Dec. 2021), two patients were lost to follow-up. Additional surgery were performed in 7 cases, one of them had positive LNM(1/7). The other 4 cases were followed up until now, only 1 case had distant LNM. There were no LNM(+) in patients with expanded indications for ESD (Supplementary 2). With the gradual convergence of pathological diagnostic criteria in Japan, endoscopic resection of UEGC that meets the expanded indications has become possible in China.
Owing to the unique biological behavior of UEGC, it is still challenging to determine its size and depth using electronic staining endoscopy and endoscopic ultrasonography [32–34]. This study suggests that tumors located in the cardia (P = 0.03) and depressed-type or flat-type tumors (P < 0.001) were independent risk factors for exceeding the expanded indications. Ohara [35] also showed that depressed lesions are independently associated with LVI and SM infiltration. Owing to the greater constriction at the cardia, even with adequate gas injection, the lesions are difficult to observe with non-extended signs under endoscopy, and the depth is easily underestimated. When the lesion has the above characteristics, caution should be exercised when assessing its indications for ESD preoperatively.
At present, there are few large sample studies on the risk of LNM in UEGC in China, and our study, being a single-center retrospective study, does not exclude the problems of selection bias. Inadequate postoperative specimen retrieval and shallow depth judgment are other limitations of this study. Besides, this is a retrospective study in which patients did not undergo preoperative endoscopic ultrasonography, which has been shown to be valuable for the assessment before ESD [36]. Also limited by the retrospective study design, the depth of infiltration and ulceration involved were derived from postoperative pathological findings, which is slightly different from the preoperative endoscopic evaluation.
Conclusion
We believe that ESD can be considered for undifferentiated intramucosal carcinoma measuring < 2 cm without ulceration, and the risk of exceeding the expanded indication is high when the macroscopic type is non-elevated type or the lesion is located in the cardia. ESD should be evaluated carefully, and the treatment strategy should be further confirmed by high-quality survival and prognostic studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Author Contribution
Pengyue Zhang,Tingting Xu, Hui Feng, Zhen Zhu and Jingjing Wang performed most of the data analysis, manuscript writing and statistical analyses; Yalei Wang supervised the entire study and participated in study design.All authors reviewed the manuscript.
Funding
None.
Data Availability
The datasets generated or analyzed during this study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study followed the ethical principles of medical research involving human subjects in the Declaration of Helsinki. This study was approved by the ethics committee of the First Affiliated Hospital of Anhui Medical University(Quick-PJ2022-05-37). Since this study was conducted using medical records/biospecimens obtained from previous clinical practice, the informed consent is waived off by the ethics committee of the First Affiliated Hospital of Anhui Medical University.
Consent for publication
All authors have approved to this submission to your journal. Its publication is also approved tacitly by the responsible authorities where the work was carried out.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20(1):1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kunisaki C, Akiyama H, Nomura M, Matsuda G, Otsuka Y, Ono H, Nagahori Y, Hosoi H, Takahashi M, Kito F, et al. Significance of long-term follow-up of early gastric cancer. ANN SURG ONCOL. 2006;13(3):363–9. doi: 10.1245/ASO.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 3.Song W, Qiao X, Gao X. A comparison of endoscopic submucosal dissection (ESD) and radical surgery for early gastric cancer: a retrospective study. World J Surg Oncol 2015;13(1). [DOI] [PMC free article] [PubMed]
- 4.Sekiguchi M, Oda I, Taniguchi H, Suzuki H, Morita S, Fukagawa T, Sekine S, Kushima R, Katai H. Risk stratification and predictive risk-scoring model for lymph node metastasis in early gastric cancer. J GASTROENTEROL. 2016;51(10):961–70. doi: 10.1007/s00535-016-1180-6. [DOI] [PubMed] [Google Scholar]
- 5.Feng H, Wang Y, Cao L, Zhang C, Sun B, Zhao Y, Xu J. Lymph node metastasis in differentiated-type early gastric cancer: a single-center retrospective analysis of surgically resected cases. SCAND J GASTROENTERO. 2015;51(1):48–54. doi: 10.3109/00365521.2015.1054425. [DOI] [PubMed] [Google Scholar]
- 6.Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24(1):1–21. [DOI] [PMC free article] [PubMed]
- 7.Ono H, Yao K, Fujishiro M, Oda I, Uedo N, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition) Dig Endosc. 2021;33(1):4–20. doi: 10.1111/den.13883. [DOI] [PubMed] [Google Scholar]
- 8.Japanese classification of gastric carcinoma: 3rd English edition, Gastric Cancer. 2011;14(2):101–112. [DOI] [PubMed]
- 9.The Paris endoscopic Classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. GASTROINTEST ENDOSC. 2003;58(6 Suppl):3–S43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 10.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 11.Choi Y, Gwack J, Kim Y, Bae J, Jun JK, Ko KP, Yoo KY. Long term trends and the future gastric cancer mortality in Korea: 1983 ~ 2013. CANCER RES TREAT. 2006;38(1):7–12. doi: 10.4143/crt.2006.38.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Cui JG, Huang F, Zhang A, Li C, Zhao ZC, Li WD, Fu WH. Prognostic factors for Survival in Node-Negative gastric Cancer patients who underwent curative resection. SCAND J SURG. 2017;106(3):235–40. doi: 10.1177/1457496916677878. [DOI] [PubMed] [Google Scholar]
- 13.Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12(3):148–52. doi: 10.1007/s10120-009-0515-x. [DOI] [PubMed] [Google Scholar]
- 14.Horiuchi Y, Ida S, Yamamoto N, Nunobe S, Ishizuka N, Yoshimizu S, Ishiyama A, Yoshio T, Hirasawa T, Tsuchida T, et al. Feasibility of further expansion of the indications for endoscopic submucosal dissection in undifferentiated-type early gastric cancer. Gastric Cancer. 2020;23(2):285–92. doi: 10.1007/s10120-019-01003-0. [DOI] [PubMed] [Google Scholar]
- 15.Lee IS, Lee S, Park YS, Gong CS, Yook JH, Kim BS. Applicability of endoscopic submucosal dissection for undifferentiated early gastric cancer: mixed histology of poorly differentiated adenocarcinoma and signet ring cell carcinoma is a worse predictive factor of nodal metastasis. Surg Oncol. 2017;26(1):8–12. doi: 10.1016/j.suronc.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Lu P, Lu Y, Liu C, Xu H, Wang S, Chen J. Predictive factors of lymph node metastasis in undifferentiated early gastric cancers and application of endoscopic mucosal resection. SURG ONCOL. 2010;19(4):221–6. doi: 10.1016/j.suronc.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Jin X, Wu W, Zhao J, Song S, Zhang C, Sun W, Lv B. Clinical Features and Risk Factors for Lymph Node Metastasis in Early Signet Ring Cell Gastric Cancer. Front Oncol 2021;11. [DOI] [PMC free article] [PubMed]
- 18.Ren M, Qi X, Chu Y, Yu Y, Chen Y, Zhang P, Mao T, Tian Z. Risk of Lymph Node Metastasis and Feasibility of Endoscopic Treatment in Ulcerative Early gastric Cancer. ANN SURG ONCOL. 2021;28(4):2407–17. doi: 10.1245/s10434-020-09153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu GS, Tian SB, Wang H, Ma MG, Liu Y, Du HS, Long YP. Preoperative neutrophil lymphocyte ratio and platelet lymphocyte ratio cannot predict Lymph Node Metastasis and Prognosis in patients with early gastric Cancer: a single Institution Investigation in China. Curr Med Sci. 2018;38(1):78–84. doi: 10.1007/s11596-018-1849-6. [DOI] [PubMed] [Google Scholar]
- 20.Zou Y, Wu L, Yang Y, Shen X, Zhu C. Risk factors of tumor invasion and node metastasis in early gastric cancer with undifferentiated component: a multicenter retrospective study on biopsy specimens and clinical data. Annals of Translational Medicine. 2020;8(6):360. doi: 10.21037/atm.2020.02.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asakawa Y. Stratifying the risk of lymph node metastasis in undifferentiated-type early gastric cancer. WORLD J GASTROENTERO. 2015;21(9):2683. doi: 10.3748/wjg.v21.i9.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huh CW, Jung DH, Kim J, Lee YC, Kim H, Kim H, Yoon SO, Youn YH, Park H, Lee SI, et al. Signet ring cell mixed histology may show more aggressive behavior than other histologies in early gastric cancer. J SURG ONCOL. 2013;107(2):124–9. doi: 10.1002/jso.23261. [DOI] [PubMed] [Google Scholar]
- 23.Park S, Kook MC, Kim YW, Cho N, Kim T, Kang GH. Mixed-type gastric cancer and its association with high-frequency CpG island hypermethylation. VIRCHOWS ARCH. 2010;456(6):625–33. doi: 10.1007/s00428-010-0916-6. [DOI] [PubMed] [Google Scholar]
- 24.Kim MN, Kim HK, Shim CN, Lee HJ, Lee H, Park JC, Shin SK, Lee SK, Lee YC. Tumour size is related to the curability of signet ring cell early gastric cancer with endoscopic submucosal dissection: a retrospective single centre study. DIGEST LIVER DIS. 2014;46(10):898–902. doi: 10.1016/j.dld.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Ahn H, Chung WC, Kim Y, Ryu S, Lim E. Clinical outcomes of mucinous gastric carcinomas compared with non-mucinous and Signet Ring Cell Carcinomas. Korean J Gastroenterol. 2020;76(6):297–303. doi: 10.4166/kjg.2020.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi JS, Kim MA, Lee HE, Lee HS, Kim WH. Mucinous gastric carcinomas. CANCER-AM CANCER SOC. 2009;115(15):3581–90. doi: 10.1002/cncr.24422. [DOI] [PubMed] [Google Scholar]
- 27.Ahn JY, Kim YI, Shin WG, Yang HJ, Nam SY, Min BH, Jang JY, Lim JH, Kim J, Lee WS, et al. Comparison between endoscopic submucosal resection and surgery for the curative resection of undifferentiated-type early gastric cancer within expanded indications: a nationwide multi-center study. Gastric Cancer. 2021;24(3):731–43. doi: 10.1007/s10120-020-01140-x. [DOI] [PubMed] [Google Scholar]
- 28.Tokunaga M, Kondo J, Tanizawa Y, Bando E, Kawamura T, Terashima M. Postoperative intra-abdominal complications assessed by the Clavien-Dindo classification following open and laparoscopy-assisted distal gastrectomy for early gastric cancer. J GASTROINTEST SURG. 2012;16(10):1854–9. doi: 10.1007/s11605-012-1981-8. [DOI] [PubMed] [Google Scholar]
- 29.Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, Ryu SW, Lee HJ, Song KY. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report–a phase III multicenter, prospective, randomized Trial (KLASS Trial) ANN SURG. 2010;251(3):417–20. doi: 10.1097/SLA.0b013e3181cc8f6b. [DOI] [PubMed] [Google Scholar]
- 30.Lim JH, Kim J, Kim SG, Chung H. Long-term clinical outcomes of endoscopic vs. surgical resection for early gastric cancer with undifferentiated histology. SURG ENDOSC. 2019;33(11):3589–99. doi: 10.1007/s00464-018-06641-6. [DOI] [PubMed] [Google Scholar]
- 31.Takizawa K, Ono H, Hasuike N, Takashima A, Minashi K, Boku N, Kushima R, Katayama H, Ogawa G, Fukuda H, et al. A nonrandomized, single-arm confirmatory trial of expanded endoscopic submucosal dissection indication for undifferentiated early gastric cancer: Japan Clinical Oncology Group study (JCOG1009/1010) Gastric Cancer. 2021;24(2):479–91. doi: 10.1007/s10120-020-01134-9. [DOI] [PubMed] [Google Scholar]
- 32.Kobara H, Mori H, Fujihara S, Kobayashi M, Nishiyama N, Nomura T, Kato K, Ishihara S, Morito T, Mizobuchi K, et al. Prediction of invasion depth for submucosal differentiated gastric cancer by magnifying endoscopy with narrow-band imaging. ONCOL REP. 2012;28(3):841–7. doi: 10.3892/or.2012.1889. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto S, Nishida T, Kato M, Inoue T, Hayashi Y, Kondo J, Akasaka T, Yamada T, Shinzaki S, Iijima H, et al. Evaluation of endoscopic ultrasound image quality is necessary in endosonographic assessment of early gastric cancer invasion depth. Gastroenterol Res Pract. 2012;2012:194530. doi: 10.1155/2012/194530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ninomiya Y, Yanagisawa A, Kato Y, Tomimatsu H. Unrecognizable intramucosal spread of diffuse-type mucosal gastric carcinomas of less than 20 mm in size. ENDOSCOPY. 2000;32(8):604–8. doi: 10.1055/s-2000-16506. [DOI] [PubMed] [Google Scholar]
- 35.Ohara Y, Toshikuni N, Matsueda K, Mouri H, Yamamoto H. The superficial elevated and depressed lesion type is an independent factor associated with non-curative endoscopic submucosal dissection for early gastric cancer. SURG ENDOSC. 2016;30(11):4880–8. doi: 10.1007/s00464-016-4825-x. [DOI] [PubMed] [Google Scholar]
- 36.Kim J, Kim SG, Chung H, Lim JH, Choi JM, Park JY, Yang HJ, Han SJ, Oh S, Kim MS, et al. Clinical efficacy of endoscopic ultrasonography for decision of treatment strategy of gastric cancer. SURG ENDOSC. 2018;32(9):3789–97. doi: 10.1007/s00464-018-6104-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated or analyzed during this study are available from the corresponding author on reasonable request.