ABSTRACT
Cisplatin resistance is a major therapeutic challenge in non-small cell lung cancer (NSCLC). Herein, the regulatory role of long non-coding RNA (lncRNA) ITGB2-AS1 in regulating NSCLC cisplatin resistance was investigated. NSCLC cisplatin resistance cells were constructed using A549 and H1975 cells. Cell viability and proliferation were detected by MTT assay and colony formation assay, respectively. Cell apoptosis and cell cycle were examined by flow cytometry. GSH, MDA, ROS, and Fe2+ levels were measured by the corresponding kits. The expressions of ferroptosis-negative regulation genes (GPX4 and SLC7A11) were determined by qRT-PCR and western blot. Molecular interactions were analyzed by RNA pull-down, RIP, ChIP, and dual-luciferase reporter assays. The effects of ITGB2-AS1 silencing on NSCLC cisplatin resistance in vivo were elevated by the tumor xenograft experiment. ITGB2-AS1 expression was increased in NSCLC patients and cisplatin-resistant NSCLC cells, which was positively correlated with ferroptosis-negative regulation genes. ITGB2-AS1 knockdown suppressed resistant cell proliferation and promoted cell apoptosis and ferroptosis. ITGB2-AS1 increased NAMPT expression by binding to FOSL2, thereby repressing p53 expression. The ITGB2-AS1 knockdown also inhibited NSCLC cisplatin resistance in vivo. ITGB2-AS1 promoted NSCLC cisplatin resistance by inhibiting p53-mediated ferroptosis via activating the FOSL2/NAMPT axis.
KEYWORDS: Non-small cell lung cancer, cisplatin resistance, ferroptosis, LncRNA ITGB2-AS1, NAMPT
Introduction
Lung cancer is one of the deadliest cancer types in the world1. NSCLC accounts for ~ 85% of lung cancer cases2. Lung adenocarcinoma (LUAD) is one of the most common subgroups of NSCLC3. Cisplatin-based chemotherapy is the first-line treatment for unresectable NSCLC4. However, most patients with NSCLC will eventually die of recurrent and progressive disease because of resistance to cisplatin5. Cisplatin resistance in NSCLC is considered a multifactorial process, while the specific mechanisms of cisplatin resistance in NSCLC are still poorly understood.
Cell death is critical for preventing hyperproliferative diseases, including cancer6. Ferroptosis is an iron-dependent form of cell death characterized by the consumption of glutathione (GSH) and the accumulation of reactive oxygen species (ROS)7. The selenoenzyme glutathione peroxidase 4 (GPX4) is currently recognized as a central inhibitor of ferroptosis, and its activity depends on glutathione produced by cystine-glutamate anti-transporter solute carrier family seven member 11 (SLC7A11) activation, which are considered anti-ferroptosis molecules8. As widely reported, ferroptosis is closely related to tumor growth and chemoresistance9. As proof, ferroptosis induction could reduce cisplatin resistance in gastric cancer6. It has been suggested that inducing ferroptosis may be an ideal solution for overcoming cisplatin resistance in NSCLC. However, the relationship between ferroptosis and NSCLC cisplatin resistance has yet to be elucidated.
Long non-coding RNAs (LncRNAs) refer to RNA molecules with transcripts longer than 200 nucleotides (nts)10. As well known, lncRNAs are involved in mediating NSCLC progression11. For instance, lncRNA XIST knockdown inhibited NSCLC progression11. LncRNA ITGB2-AS1 is an oncogene in various malignant tumors12. However, the expression and the role of lncRNA ITGB2-AS1 in NSCLC remain unclear. Herein, it was predicted that lncRNA ITGB2-AS1 expression was increased in LUAD using the GEPIA database, which caught our attention. However, the function of lncRNA ITGB2-AS1 in NSCLC cisplatin resistance is largely unknown.
FOS-like antigen 2 (FOSL2), a member of the activator protein 1 transcription factor family, promotes malignant behaviors of cancer cells13. Notably, FOSL2 overexpression promoted NSCLC cell proliferation, invasion, and migration14, suggesting FOSL2 was a risk factor facilitating NSCLC progression. As reported, lncRNAs could regulate the downstream targets by acting on transcription factors15. In the current study, it was predicted that there was the ITGB2-AS1/FOSL2/NAMPT regulatory network in NSCLC by LncMAP, which remained to investigate. NAMPT is a key rate-limiting enzyme in the synthesis of nicotinamide adenine dinucleotide (NAD+), which can determine the level of NAD+ in metabolism to regulate cell metabolism and cell viability16. NAMPT is an important target for tumor therapy as its inhibitors retarded the progression of human malignancies17. More importantly, NAMPT was significantly upregulated in NSCLC tumor tissues, and its expression was inversely correlated with the five-year survival of patients18. Our study found that ITGB2-AS1 could regulate NAMPT expression through interacting with FOSL2 using LncMAP prediction. Nevertheless, the role of ITGB2-AS1/FOSL2/NAMPT in regulating ferroptosis and cisplatin resistance during NSCLC progression remains unclear, which deserves further study.
p53 inhibits cancer development by inducing cell cycle arrest, senescence, and apoptosis19. However, the relationship between p53 and NSCLC cisplatin resistance remains unknown. It has been reported that p53 acts as the downstream target of NAMPT in regulating various biological processes20. Notably, recent studies have identified p53 could regulate ferroptosis. p53 was first reported to sensitize cells to ferroptosis through transcriptionally repressing the expression of SLC7A1119. In addition, p53 was reported to increase ferroptosis in cancer cells to inhibit radio resistance in cancer cells21. Therefore, we speculated that p53 was the downstream molecule of the ITGB2-AS1/FOSL2/NAMPT axis in regulating ferroptosis and cisplatin resistance in NSCLC.
Based on the above evidences, it was speculated that lncRNA ITGB2-AS1 promoted NAMPT expression via binding to transcription factor FOSL2 to inhibit p53-mediated ferroptosis, thereby facilitating cisplatin resistance in NSCLC, which provided novel perspectives for NSCLC treatment in patients with cisplatin resistance.
Materials and methods
Clinical samples collection
There were 25 NSCLC tissues and normal adjacent lung tissues were collected from diagnosed NSCLC patients who received surgical resection at Yuebei People’s Hospital, Shantou University. All samples were stored at −80°C. All patients were clearly diagnosed by pathology and had not receive radiotherapy or chemotherapy before surgery. This study was approved by the Ethics Committee of Yuebei People’s Hospital, Shantou University Hospital, and all participants signed informed consent.
Cell culture and induction of cisplatin resistance
Human normal lung epithelial cells (BEAS−2B cells), lung squamous cell carcinoma cells (SK-MES−1, NCI-H520, and Calu−1 cells), lung adenocarcinoma (LUAD) cells (A549, H1975, and PC-9 cells), and 293T cells were purchased from ATCC (VA, USA). All cells were cultured in DMEM (Gibco, MD, USA) containing 10% FBS (Gibco) with 5% CO2 at 37°C. A549/DDP and H1975/DDP cells were constructed as previously described22. Cells were treated with cisplatin for 2 d as a cycle. After completing three cycles of stimulation with the same concentration of cisplatin, the dose was increased from 2 μg/ml to 50 μg/ml.
Cell transfection and treatment
The short hairpin RNA of ITGB2-AS1 (sh-ITGB2-AS1), the overexpression plasmid of FOSL2 (Oe-FOSL2), the short hairpin RNA of FOSL2 (sh-FOSL2), the overexpression plasmid of NAMPT (Oe-NAMPT), and their negative controls were all obtained from GenePharma (Shanghai, China). Cells were transfected with above plasmids using Lipofectamine™ 3000 (Invitrogen, CA, USA) for 48 h. For ferroptosis activation or inhibition, cells were incubated with ferroptosis agonist Erastin (10 μM, Selleck Chemicals, TX, USA) or the antagonist Ferrostatin−1 (10 μM, Selleck Chemicals) for 24 h.
3-(4, 5-Dimethylthiazolyl2)−2, 5-diphenyltetrazolium bromide (MTT) assay
Cells were cultured in 96‐well plates (3 × 103 cells/well) for 24 h. Then, cells were incubated with 20 μl MTT (5 mg/mL, Sangon, Shanghai, China) for 4 h at 37°C. After cells were incubated with 100 μL DMSO for 2 h, the absorbance at 490 nm was detected by a microplate reader (Bioteke, Beijing, China).
Colony formation assay
Cells (1 × 105/well) were seeded in 6-well plates and cultured for 7 d. Cells were subsequently stained with 0.1% crystal violet (Solarbio, Beijing, China) for 10 min, and the colonies formed were counted manually.
Measurement of Fe2+
Cells were harvested and homogenized with PBS. The supernatant was collected for Fe2+ level detection using an iron assay kit (Abcam, Cambridge, UK, ab83366). Briefly, the samples were incubated with an assay buffer for 30 min and subsequently incubated with an iron probe for 60 min. The absorbance was subsequently detected by a microplate reader (Bioteke).
Measurement of GSH, malondialdehyde (MDA) and ROS
The levels of GSH, MDA, and ROS were determined using GSH content detection kit (Solarbio, BC1170), MDA assay kit (Abcam, ab118970), and ROS detection kit (Beyotime, Shanghai, China, S0033S). Cells were collected by centrifugation at 1000 rpm for 10 min. Then, all operation steps were carried out in strict accordance with the instructions.
Cell cycle and apoptosis assay
For the cell cycle assay, cells (1.5 × 106) were collected and fixed using 70% ethanol. Cells were stained with 50 μg/ml PI (Sigma-Aldrich) for 30 min at 37°C. For cell apoptosis, cells (1.5 × 106) were collected and washed with PBS twice. Cells were re-suspended in 500 μl of 1× Annexin-binding buffer (Beyotime) and incubated with 10 μl Annexin V-FITC and 5 μl PI stain (Beyotime) for 10 min at room temperature in the dark. Cells were analyzed in FACScan using Cell Quest software (BD, NJ, USA).
Dual-luciferase reporter gene assay
The FOSL2-binding site in the promoter of NAMPT was predicted using the JASPAR database (http://jaspar.genereg.net/). Wild-type (wt) and mutant-type (mut) reporter plasmids of NAMPT sequences were amplified by PCR and cloned into the PGL3 vector (GenePharma). Site-directed mutagenesis of the FOSL2 binding site in NAMPT promoter was performed using a site-directed mutagenesis kit (Stratagene, CA, USA). Then, cells were co-transfected with NAMPT-WT, NAMPT-MUT1, NAMPT-MUT2, or NAMPT-MUT1/2 plasmids and Oe-FOSL2 or Oe-NC for 48 h. The luciferase activity was assessed by a dual-luciferase reporter assay system (Promega).
Bioinformatics analysis
The Starbase database (https://starbase.sysu.edu.cn/) is a database of gene expression data for 32 cancers derived from 10,882 RNA-seq and 10,546 miRNA-seq data. Starbase is used to predict the expressions of miRNAs, lncRNAs, pseudogenes, and mRNAs in tumors. In our study, the Starbase database was employed to predict ITGB2-AS1 expression in LUAD. The LncMAP database (http://bio-bigdata.hrbmu.edu.cn/LncMAP/index.jsp) is a database designed to predict lncRNA-transcription factor-gene regulatory networks associated with multiple tumors based on transcriptome expression profile data analysis in The Cancer Genome Atlas (TCGA) database. Herein, the regulatory network of ITGB2-AS1-FOSL2-NAMPT in NSCLC was predicted by the LncMAP database.
RNA pull-down assay
LncRNA ITGB2-AS1 or NC was labeled with biotin (Roche, Basel, Switzerland) and transcribed using the Biotin RNA Labeling Mix (Roche) and T7 RNA polymerase (Roche). Then, cells were lysed with the lysis buffer mixed with 80 U/mL RNasin (Promega, WI, USA). Subsequently, cell extract (2 μg) was incubated with biotinylated RNA (100 pmol) for 1 h at 4°C and the RNA – protein complex was isolated using the streptavidin‐coupled agarose beads (Invitrogen). The retrieved protein was analyzed by western blot.
RNA immunoprecipitation (RIP) assay
Cells were lysed with a complete RIP lysis buffer (Millipore, MA, USA). Then, cell extract was then incubated with IgG (Abcam, 1:50, ab172730) and FOSL2 (Cell Signaling Technology, MA, USA, 1:50, #19967) antibodies at 4°C overnight. The RNA samples were purified, and the ITGB2-AS1 transcripts enrichment was determined by qRT-PCR.
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was conducted by using the ChIP kit (Beyotime). Briefly, cells were transfected with sh-NC, sh-FOSL2, or sh-ITGB2-AS1, fixed and quenched. DNA was fragmented by sonication. Then, the cell lysate was incubated with anti-FOSL2 (Cell Signaling Technology, 1:50, #19967) or anti-IgG (Abcam, 1:100, ab172730) at 4°C overnight. Then, DNA enrichment was collected after the samples were incubated with dynabeads protein G (Invitrogen) for 2 h. Immunoprecipitated DNAs were analyzed by qRT-PCR.
Tumor xenograft in vivo
A total of 24 female BALB/c nude mice (6-week-old) were purchased from SLACOM (Shanghai, China). Mice were housed in individual cages and provided with free food and water. A549 (200 μl, 2 × 104) cells transfected with sh-NC or sh-ITGB2-AS1 were injected into the back of mice. Nude mice were randomly assigned to three groups (8 mice/group): Control group, sh-NC group, and sh-ITGB2-AS1 group. After 7 d, mice were injected intraperitoneally with 15 mg/kg cisplatin every 3 d. The tumor volumes were calculated as follows: V = lw2/2 (l: length, w: width). The mice were euthanized on the 28th day. Tumor tissues were collected and weighed. The level of Ki67 (cell proliferation-associated antigen) was detected by immunohistochemistry (IHC). The animal experiments were approved by the Ethics Committee of Yuebei People’s Hospital, Shantou University Hospital.
Quantitative real-time polymerase chain reaction (Qrt-PCR)
Total RNA was extracted with TRIzol (Thermo Fisher Scientific, MA, USA). RNA was reverse transcribed to cDNA was acquired by the Reverse Transcription Kit (Toyobo, Tokyo, China). Then, RNA quantification was assessed by SYBR (Thermo Fisher Scientific). GAPDH was used as the reference gene. The data were analyzed with the 2−ΔΔCT method. The primers were listed as follows (5’−3’):
LncRNAITGB2-AS1(F): AAGGCAGGTGAGTGTAGGAAGGAG;
LncRNAITGB2-AS1(R): GGAAGGCAGAGGAGGGAGGAAC.
SLC7A11 (F): GGTCCATTACCAGCTTTTGTACG;
SLC7A11 (R): - AATGTAGCGTCCAAATGCCAG.
GPX4 (F): AGTGGATGAAGATCCAACCCAAGG;
GPX4 (R): GGGCCACACACTTGTGGAGCTAGA.
FOSL2 (F): CAGAAATTCCGGGTAGATATGCC;
FOSL2 (R): GGTATGGGTTGGACATGGAGG.
NAMPT (F): CGGCAGAAGCCGAGTTCAA;
NAMPT (R): GCTTGTGTTGGGTGGATATTGTT.
GAPDH (F): GGAGCGAGATCCCTCCAAAAT;
GAPDH (R): GGCTGTTGTCATACTTCTCATGG.
Western blot
Total proteins were extracted using RIPA (Thermo Fisher Scientific), and the protein concentration was estimated through the BCA kit (Beyotime). Proteins were separated using the 10% SDS-page gel and further transferred into a polyvinylidene fluoride (PVDF) membrane (Millipore). The membranes were blocked and subsequently incubated overnight with antibodies against SLC7A11 (Abcam, 1:1000, ab216876), GPX4 (Abcam, 1:1000, ab125066), FOSL2 (Cell Signaling Technology, 1:1000, #19967), NAMPT (Cell Signaling Technology, 1:1000, #86634), p53 (Abcam, 2 μg/ml, ab26), p-p53 (Abcam, 1:1000, ab33889), and GAPDH (Abcam, 1:2500, ab9485). The membranes were then incubated with the corresponding secondary antibody (Abcam, 1:5000, ab7090) for 60 min following washed with PBST. The bands were visualized using an ECL detection kit (Beyotime).
Statistical analysis
All our data were obtained from three independent experiments. Statistical data were analyzed using SPSS 19.0 (IBM, Armonk, NY) and expressed as means ±SD. The differences between two groups and multiple groups were analyzed by Student’s t-tests and one-way analysis of variance (ANOVA), respectively. The p values less than 0.05 were considered significant.
Results
LncRNA ITGB2-AS1 was upregulated in NSCLC tissues, and its expression was negatively correlated with ferroptosis
It was reported that ITGB2-AS1 is an oncogene in various human malignant tumors23,24, while the expression and the role of ITGB2-AS1 in NSCLC remained unknown. Herein, it was predicted that lncRNA ITGB2-AS1 was significantly upregulated in LUAD using Starbase (Figure 1a). Subsequently, qRT-PCR results showed that ITGB2-AS1 was significantly upregulated in NSCLC tissues (Figure 1b). Meanwhile, we found that the expressions of ferroptosis markers (SLC7A11 and GPX4) were also increased in NSCLC tissues (Figure 1b-c). Additionally, ITGB2-AS1 expression was positively correlated with GPX4 expression and SLC7A11 expression (Figure 1d). Finally, the relationship between ITGB2-AS1 expression and the clinical characteristics of NSCLC patients was analyzed. Clinical samples were divided into high ITGB2-AS1 expression and low ITGB2-AS1 expression groups based on the median ITGB2-AS1 expression, and it was observed that ITGB2-AS1 high expression was positively correlated with histological cell type, TNM stage, and lymph node metastasis, while it was not correlated with other parameters, such as gender, age, smoking and differentiation (Table 1). Collectively, lncRNA ITGB2-AS1 was a risk factor affecting NSCLC progression, and its expression was negatively correlated with ferroptosis.
Figure 1.

LncRNA ITGB2-AS1 was upregulated in NSCLC tissues, and its expression was negatively correlated with ferroptosis.
(a) ITGB2-AS1 expression in LUAD was predicted by Starbase. (b) ITGB2-AS1, SLC7A11, and GPX4 expressions in NSCLC (n = 25) were assessed by qRT-PCR. (c) SLC7A11 and GPX4 in NSCLC protein levels were assessed by western blot (n = 25). (d) The correlation analysis of ITGB2-AS1 expression, SLC7A11 expression, and GPX4 expression were performed using Pearson correlation analysis. *p < 0.05, **p < 0.01, ***p < 0.001.
Table 1.
Association between ITGB2-AS1 expression and clinical features of NSCLC patients.
| Clinical features | ITGB2-AS1 |
P value | |
|---|---|---|---|
| High (n = 13) | Low (n = 12) | ||
| Gender | 0.6882 | ||
| Male | 7 | 8 | |
| Female | 6 | 4 | |
| Age (years) | 0.4283 | ||
| <65 | 4 | 6 | |
| ≥65 | 9 | 6 | |
| Histological cell type | 0.0414* | ||
| Adenocarcinoma | 8 | 2 | |
| Squamous cell carcinoma | 5 | 10 | |
| TNM stage | 0.0414* | ||
| I+II | 2 | 7 | |
| III+IV | 11 | 5 | |
| Lymph node metastasis | 0.0472* | ||
| Negative | 4 | 9 | |
| Positive | 9 | 3 | |
| Smoking | 0.4338 | ||
| Yes | 8 | 5 | |
| No | 5 | 7 | |
| Differentiation | 0.3783 | ||
| Well/moderate | 5 | 2 | |
| Poor | 8 | 10 | |
NSCLC, non‐small‐cell lung cancer; TNM, tumor node metastasis. *p < .05.
LncRNA ITGB2-AS1 knockdown suppressed cisplatin resistance in NSCLC cells
As widely described, cisplatin resistance is a primary factor in NSCLC relapse25. The role of ITGB2-AS1 in NSCLC cisplatin resistance was investigated. First, we found that ITGB2-AS1 expression was increased in lung squamous cell carcinoma cells (SK-MES−1, NCI-H520, and Calu−1 cells) and LUAD cells (A549, H1975, and PC−9 cells) compared with that in BEAS−2B cells (Figure 2a). Considering that ITGB2-AS1 expression was highest in A549 and H1975 cells among all these NSCLC cells, A549 and H1975 cells were selected for subsequent experiments. A549 and H1975 cells were exposed to increasing concentrations of cisplatin to construct cisplatin-resistant cells as previously described22. Results revealed that the IC50 values of A549/DDP and H1975/DDP cells were greater than those of the corresponding cisplatin-sensitive cells (Figure 2b). ITGB2-AS1 expression was significantly upregulated in resistant cells (Figure 2c). To further probe the relationship between ITGB2-AS1 expression and NSCLC cisplatin resistance, ITGB2-AS1 knockdown was induced in resistant cells (Figure 2d). ITGB2-AS1 knockdown inhibited the vitality of resistant cells (Figure 2e). Moreover, ITGB2-AS1 knockdown reduced the number of colonies (Figure 2f and Fig. S1a), increased the number of G0/G1 phase cells, decreased the number of S phase cells (Figure 2g and Fig. S1b), and promoted cell apoptosis (Figure 2h and Fig. S1c) in resistant cells. All these results suggested that ITGB2-AS1 silencing inhibited cisplatin resistance in NSCLC cells.
Figure 2.
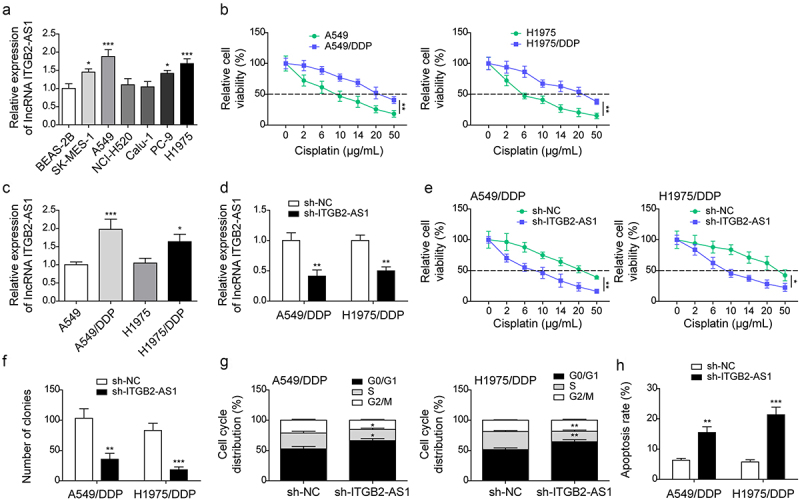
LncRNA ITGB2-AS1 knockdown suppressed cisplatin resistance in NSCLC cells.
(a) LncRNA ITGB2-AS1 expression in cells was examined by qRT-PCR. Subsequently, cisplatin-resistant cells were established. (b) MTT assay was carried out to analyze IC50. (c) ITGB2-AS1 expression in NSCLC cells and cisplatin-resistant cells was detected by qRT-PCR. ITGB2-AS1 knockdown was induced in A549/DDP and H1975/DDP cells: (d) ITGB2-AS1 expression was detected using qRT-PCR. (f) Cell viability was evaluated by MTT assay. (f) Cell proliferation was analyzed using colony formation assay. Flow cytometry was carried out to analyze cell cycle (g) and the apoptosis (h). *p < 0.05, **p < 0.01, ***p < 0.001.
ITGB2-AS1 knockdown repressed cisplatin resistance in NSCLC by promoting ferroptosis
Ferroptosis is a potential way to kill chemotherapy resistance cancer cells26. Herein, we found that ROS, MDA, and Fe2+ levels were significantly reduced in resistant cells compared to the parental cells, while GSH level was increased (Figure 3a-b), suggesting that ferroptosis was reduced in resistant cells. To further investigate the correlation between ITGB2-AS1 and ferroptosis in resistant NSCLC cells, ITGB2-AS1 knockdown was induced in resistant cells with the supplement of the ferroptosis agonist Erastin and the antagonist Ferrostatin−1. ITGB2-AS1 knockdown or Erastin treatment significantly reduced the resistant cell vitality, which was abolished by Ferrostatin−1 treatment (Figure 3c). In addition, ITGB2-AS1 knockdown or Erastin treatment resulted in increased ROS and MDA levels as well as reduced GSH level, whereas these effects were neutralized by Ferrostatin−1 treatment (Figure 3d). Meanwhile, ITGB2-AS1 knockdown or Erastin treatment increased Fe2+ level and reduced SLC7A11 and GPX4 protein levels in resistant cells, whereas these effects were abolished by Ferrostatin−1 treatment (Figure 3e-f). Moreover, ITGB2-AS1 knockdown or Erastin treatment inhibited cell proliferation, increased G0/G1 phase cell numbers, decreased S phase cell numbers, and increased cell apoptosis of resistance cells, which was reversed by Ferrostatin−1 treatment (Figure 4a-c and Fig. S2a-c). Collectively, the above results suggested that ITGB2-AS1 knockdown promoted ferroptosis to suppress cisplatin resistance in NSCLC cells.
Figure 3.

ITGB2-AS1 knockdown repressed cisplatin resistance in NSCLC by promoting ferroptosis.
(a-b) The levels of ROS, MDA, GSH, and Fe2+ levels in cells were examined by kits. A549/DDP and H1975/DDP cells were transfected with sh-NC, sh-ITGB2-AS1, treated with Erastin and Ferrostatin-1. (c) Cell viability was assessed by MTT assay. (d-e) ROS, MDA, GSH, and Fe2+ levels were analyzed by kits. (f) SLC7A11 and GPX4 levels were examined by western blot. The data were expressed as mean ± SD. All data were obtained from at least three replicate experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 4.

ITGB2-AS1 knockdown repressed cisplatin resistance in NSCLC by promoting ferroptosis.
(a) Cell proliferation was assessed by colony formation assay. Cell cycle (b) and the apoptosis (c) were analyzed using flow cytometry. *p < 0.05, **p < 0.01, ***p < 0.001.
ITGB2-AS1 upregulated NAMPT by binding to FOSL2, thereby repressing p53 expression
It was predicted that there was the ITGB2-AS1-NAMPT-FOSL2 regulatory network in NSCLC using LncMAP (Figure 5a). In our study, it was observed that FOSL2 and NAMPT were upregulated in NSCLC tissues (Figure 5b-c). Moreover, the ITGB2-AS1 expression was positively correlated with FOSL2 expression and the NAMPT expression, and FOSL2 expression was positively correlated with NAMPT expression (Figure 5d). Meanwhile, FOSL2 and NAMPT were highly expressed in resistant cells compared to those in cisplatin-sensitive cells (Figure 5e-f). Subsequently, RNA pull-down and RIP assays further confirmed that ITGB2-AS1 bound with FOSL2 (Figure 6a-b). It was predicted that there were two potential binding sites between FOSL2 and NAMPT promoter using JASPAR (Figure 6c). The dual-luciferase reporter gene assay results revealed that FOSL2 overexpression enhanced the luciferase activity of NAMPT‐WT vector as well as NAMPT‐MUT2 but had no significant effect on that of NAMPT‐MUT1 and NAMPT‐MUT 1/2 (Figure 6d), suggesting that FOSL2 could directly bind to the site 1 of NAMPT promoter. Subsequently, the ChIP assay showed that the NAMPT enrichment was reduced after FOSL2 knockdown and ITGB2-AS1 knockdown (Figure 6e). Moreover, ITGB2-AS1 silencing reduced the levels of FOSL2 and NAMPT in resistant cells, which was abolished by FOSL2 overexpression (Figure 6f). p53 activated ferroptosis during tumor suppression19. ITGB2-AS1 knockdown reduced the nuclear p53 level and increased the cytoplasmic p53 level in resistant cells, while this effect was reversed by FOSL2 overexpression (Figure 6g). In conclusion, ITGB2-AS1 promoted NAMPT expression by interacting with FOSL2 to reduce p53 expression.
Figure 5.

ITGB2-AS1 upregulated NAMPT expression by binding to FOSL2.
(a) The regulatory network of ITGB2-AS1, FOSL2, and NAMPT in LUAD was predicted using LncMAP. (b-c) FOSL2 and NAMPT expressions in tissues were determined by qRT-PCR and western blot, respectively (n = 25). (d) The correlation analysis among ITGB2-AS1, FOSL2, and NAMPT expressions was performed using Pearson correlation analysis. (e-f) FOSL2 and NAMPT expression levels in cells were determined by qRT-PCR and western blot. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 6.

ITGB2-AS1 upregulated NAMPT by binding to FOSL2, thereby repressing p53 expression.
(a-b) The interaction between ITGB2-AS1 and FOSL2 was analyzed using RNA pull-down and RIP assays. (c) The binding sites between FOSL2 and NAMPT promoter were predicted by using JASPAR. (d-e) Dual-luciferase reporter gene and ChIP assays were carried out to analyze the interaction between FOSL2 and NAMPT. (f) Resistant cells were co-transfected with sh-ITGB2-AS1 and Oe-FOSL2, and FOSL2 and NAMPT levels in cells were analyzed by western blot. (g) Resistant cells were co-transfected with sh-ITGB2-AS1 and Oe-FOSL2, and nuclear p53 and cytoplasmic p53 levels were analyzed by western blot. *p < 0.05, **p < 0.01, ***p < 0.001.
ITGB2-AS1 regulated ferroptosis in cisplatin-resistant NSCLC cells via targeting NAMPT
To explore the function of NAMPT in regulating ITGB2-AS1-mediated ferroptosis inhibition in NSCLC, both ITGB2-AS1 knockdown and NAMPT overexpression were induced in resistant cells. As demonstrated in Figure 7a, ITGB2-AS1 knockdown reduced NAMPT expression in cells, which was abrogated by NAMPT overexpression. In addition, NAMPT overexpression reduced ROS, MDA, and Fe2+ levels and increased SLC7A11 and GPX4 protein levels and GSH level in resistant cells, which reversed the effect of ITGB2-AS1 knockdown (Figure 7b-d). It was also turned out that NAMPT overexpression promoted resistance cell proliferation, reduced the number of G0/G1 phase cells, increased the number of S phase cells, and inhibited cell apoptosis, which eliminated ITGB2-AS1 knockdown’s effects (Figure 7e-h and Fig. S3a-c). In summary, NAMPT overexpression eliminated the promoting effect of ITGB2-AS1 knockdown on ferroptosis and the inhibitory effect on cisplatin resistance in NSCLC cells.
Figure 7.

ITGB2-AS1 regulated ferroptosis in cisplatin-resistant NSCLC cells via targeting NAMPT.
A549/DDP and H1975/DDP cells were classified into sh-NC group, sh-ITGB2-AS1 group, sh-ITGB2-AS1 + Oe-NC and sh-ITGB2-AS1 + Oe-NAMPT group. (A) NAMPT protein level in cells was assessed using western blot. (B-C) ROS, MDA, GSH, and Fe2+ levels in cells were analyzed by kits. (D) SLC7A11 and GPX4 levels in cells were examined by western blot. (E) MTT assay was employed to evaluate cell viability. (F) Cell proliferation was analyzed using colony formation assay. Flow cytometry was carried out to analyze cell cycle (G) and the apoptosis (H). *p < 0.05, **p < 0.01, ***p < 0.001.
ITGB2-AS1 silencing repressed NSCLC tumor growth and cisplatin resistance in vivo
To probe the effect of ITGB2-AS1 on NSCLC cisplatin resistance in vivo, mice were injected with A549 cells stably transfected with sh-NC or sh-ITGB2-AS1 and intraperitoneally injected with cisplatin. As displayed in Figure 8a-c, ITGB2-AS1 silencing suppressed NSCLC tumor growth. ITGB2-AS1 expression significantly downregulated in the sh-ITGB2-AS1 group (Figure 8d). Additionally, IHC showed that ITGB2-AS1 silencing reduced Ki67 (proliferation marker) level in tumor tissues (Figure 8e). Moreover, after ITGB2-AS1 knockdown, FOSL2, NAMPT, SLC7A11, and GPX4 expressions in tumor tissues were significantly reduced (Figure 8f). In total, ITGB2-AS1 silencing suppressed NSCLC cisplatin resistance in vivo.
Figure 8.

ITGB2-AS1 silencing repressed NSCLC tumor growth and cisplatin resistance in vivo.
A549 cells transfected with sh-ITGB2-AS1 and sh-NC were inoculated into nude mice, and then all mice were received cisplatin treatment. (a-c) The size and weight of tumors were measured. (d) qRT-PCR was employed to analyze the ITGB2-AS1 expression. (e) Ki67 level in tumor tissues was evaluated using IHC. (f) Western blot was employed to examine FOSL2, NAMPT, SLC7A11, and GPX4 levels. n = 8. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
Although many molecular medical studies have been carried out to explain the mechanism of chemotherapy resistance in NSCLC, it is difficult to distinguish the real effective targets for regulating chemotherapy resistance. Ferroptosis is a newly discovered form of cell death27. As widely illustrated, ferroptosis induction contributes to the discovery of new therapeutic strategies for cancer28. Herein, it was found that lncRNA ITGB2-AS1 suppressed p53-mediated ferroptosis to promote cisplatin resistance in NSCLC by targeting the FOSL2/NAMPT axis.
LncRNAs play important roles in cisplatin resistance in NSCLC. As proof, lncRNA SNHG1 was markedly upregulated in NSCLC cisplatin-resistant tissues and cells, and its silencing promoted NSCLC cell cisplatin sensitivity29. LncRNA ITGB2-AS1 is identified as a classic oncogene in various malignancies30,31. However, the expression level and role of ITGB2-AS1 in NSCLC remain unknown. Herein, we first found that ITGB2-AS1 expression was increased in NSCLC tissues and NSCLC cisplatin-resistant cells, and its expression was positively correlated with the clinical characteristics of NSCLC patients. Notably, ITGB2-AS1 silencing inhibited resistant cell growth and promoted the apoptosis. As well known, some lncRNAs act as an extensive role in modulating ferroptosis in cancer32. Our results demonstrated that ITGB2-AS1 expression was positively correlated with the expressions of anti-ferroptosis molecules (GPX4 and SLC7A11). Subsequently, ITGB2-AS1 knockdown suppresses cisplatin resistance in NSCLC cells by promoting ferroptosis. All our results suggested that ITGB2-AS1 overexpression promoted cisplatin resistance in NSCLC by inhibiting ferroptosis.
It has been widely illustrated that transcription factors and lncRNAs are key regulators in cancer development33. It was predicted that ITGB2-AS1 could regulate NAMPT expression by interacting with transcription factor FOSL2 using LncMAP. The high expression of FOSL2 in NSCLC is related to high invasiveness34 and high proliferation35 during cancer progression. Notably, FOSL2 upregulation was related to cisplatin resistance in human malignant tumors36. However, the function of FOSL2 in regulating NSCLC cisplatin resistance is unknown. In the current research, FOSL2 was highly expressed in NSCLC tissues and cisplatin-resistant NSCLC cells. Additionally, this current report was the first one to indicate that ITGB2-AS1 increased FOSL2 expression by directly binding to FLSO2. Subsequently, we further explored the regulatory effect of ITGB2-AS1/FOSL2 on NSCLC cisplatin resistance. NAMPT is upregulated in a variety of tumors, including NSCLC37. Notably, NAMPT upregulation contributed to chemotherapy resistance in cancer cells by activating oxidative phosphorylation38. Herein, NAMPT was highly expressed in NSCLC tissues and cisplatin-resistant NSCLC cells. The binding relationship between FOSL2 and NAMPT promoter was ultimately confirmed with ChIP and luciferase reporter assays. Moreover, the NAMPT overexpression abrogated ITGB2-AS1 knockdown’s facilitation on ferroptosis and ITGB2-AS1 knockdown’s repression on cisplatin resistance in NSCLC cells. Our study illustrated for the first time that ITGB2-AS1/FOSL2/NAMPT axis activation promoted cisplatin resistance in NSCLC by repressing ferroptosis.
p53 achieved its tumor suppressive effect by inducing ferroptosis39. p53 was the downstream target of NAMPT in regulating some biological processes20. In this study, the ITGB2-AS1 knockdown reduced nuclear p53 level and increased cytoplasmic p53 level in resistant cells, which was reversed by FOSL2 overexpression. Our results revealed that p53 served as the target of the ITGB2-AS1/FOSL2/NAMPT axis in regulating ferroptosis and cisplatin resistance during NSCLC progression.
Collectively, our study displayed that ITGB2-AS1 repressed p53-mediated ferroptosis to promote NSCLC cisplatin resistance by activating the FOSL2/NAMPT axis, which illustrated that ITGB2-AS1 had a potential therapeutic value for cisplatin resistance improvement in NSCLC.
Supplementary Material
Biographies
Huiyong Chen, received M.D. degree from the Southern Medical University in 2022. Now, she is a docimaster in the Yuebei People's Hospital. Her research focus on the molecular mechanism of lung cancer.
Linhui Wang, received M.M. degree from the Chongqing Medical University in 2012. Now, he is an attending physician in the Yuebei People's Hospital. His research focus on the pathological diagnosis of lung and mediastinal tumors.
Lin Zhou, received M.M. degree from the Fourth Military Medical University in 2011. Now, she works in the thoracic Surgery department of Yuebei People's Hospital. Her research focus on the treatment of lung cancer and esophageal cancer.
Jingting Liu, received M.M. degree from the Guangzhou Medical University in 2019. Now, she works in the thoracic Surgery department of Yuebei People's Hospital. Her research focus on the thoracic cancer.
Hongliang Liao, received M.M. degree from the Sun Yat-sen University in 2010. Now, he works in the Yuebei People's Hospital. His research focus on the lung cancer and esophageal cancer.
Renping Wan, received bachelor's degree from Jiangxi Medical College in 1994. Now, he works in the Yuebei People's Hospital. His research focus on the thoracic cancer.
Zihao Wan, received bachelor's degree from Guangzhou University of Chinese Medicine in 2023. His research focus on the sports and health.
Funding Statement
Shaoguan Health research project (Y23004)
Disclosure statement
All authors agree with the presented findings, have contributed to the work, and declare no conflict of interest.
Authors’ contributions
Huiyong Chen: Conceptualization; Methodology; Validation;
Linhui Wang: Formal analysis; Investigation; Resources;
Jingting Liu: Data Curation;
Zihao Wan: Writing – Original Draft;
Lin Zhou: Visualization;
Hongliang Liao: Writing – Review & Editing;
Renping Wan: Supervision; Project administration; Funding acquisition
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Yuebei People’s Hospital, Shantou University Hospital, and all participants signed informed consent. The animal experiments were approved by the Ethics Committee of Yuebei People’s Hospital, Shantou University Hospital.
Consent for publication
The informed consent was obtained from study participants.
Abbreviations
- NSCLC
Non-small cell lung cancer
- GSH
Glutathione
- ROS
Reactive oxygen species
- lncRNA
Long non-coding RNA
- FOSL2
FOS-like antigen 2
- NAMPT
Ncotinamide phosphoribosyltransferase
- MTT
3-(4, 5-Dimethylthiazolyl2)−2, 5-diphenyltetrazolium bromide
- MDA
Malondialdehyde
- GPX4
Glutathione peroxidase 4
- SLC7A11
Solute carrier family 7 member 11
- RIP
RNA immunoprecipitation
- ChIP
Chromatin immunoprecipitation
- IHC
Immunohistochemistry
- qRT-PCR
Quantitative real-time polymerase chain reaction
- SD
Standard deviation
- ANOVA
Analysis of variance
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15384047.2023.2223377
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A.. Cancer statistics. CA Cancer J Clin. 2021;71(1):7–13. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Yu H, Pang Z, Li G, Gu T. Bioinformatics analysis of differentially expressed miRnas in non-small cell lung cancer. J Clin Lab Anal. 2021;35(2):e23588. doi: 10.1002/jcla.23588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao L, Lan X, Shi X, Zhao K, Wang D, Wang X, Li F, Huang H, Liu J. Cytoplasmic RAP1 mediates cisplatin resistance of non-small cell lung cancer. Cell Death Disease. 2017;8(5):e2803. doi: 10.1038/cddis.2017.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu D, Wang C, Yu L, Yu R. Induction of ferroptosis by ATF3 elevation alleviates cisplatin resistance in gastric cancer by restraining Nrf2/Keap1/xCT signaling. Cell Mol Biol Lett. 2021;26(1):26. doi: 10.1186/s11658-021-00271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev E, Gleason C, Patel D, Bauer A, Cantley A, Yang W, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2021;17(9):2054–2081. doi: 10.1080/15548627.2020.1810918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mou Y, Wang J, Wu J, He D, Zhang C, Duan C, Li B. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 2019;12(1):34. doi: 10.1186/s13045-019-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47(3):199–208. doi: 10.1038/ng.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Yao L, Zhang M, Jiang J, Yang M, Wang Y. Downregulation of LncRNA-XIST inhibited development of non-small cell lung cancer by activating miR-335/SOD2/ROS signal pathway mediated pyroptotic cell death. Aging (Albany NY. Aging. 2019;11(18):7830–7846. doi: 10.18632/aging.102291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou YL, Xu ZJ, Zhou JD, Zhang T-J, Yao D-M, Ma J-C, Lin J, Qian J. [Overexpression of LncRNA ITGB2-AS1 predicts adverse prognosis in acute myeloid Leukemia]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2021;29(5):1436–1449. doi: 10.19746/j.cnki.issn.1009-2137.2021.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Wan X, Guan S, Hou Y, Qin Y, Zeng H, Yang L, Qiao Y, Liu S, Li Q, Jin T, et al. FOSL2 promotes VEGF-independent angiogenesis by transcriptionnally activating Wnt5a in breast cancer-associated fibroblasts. Theranostics. 2021;11(10):4975–4991. doi: 10.7150/thno.55074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu P, Wang L, Xie X, Hu F, Yang Q, Hu R, Jiang L, Ding F, Mei J, Liu J, et al. Hsa_circ_0001869 promotes NSCLC progression via sponging miR-638 and enhancing FOSL2 expression. Aging (Albany NY. Aging. 2020;12(23):23836–23848. doi: 10.18632/aging.104037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He H, Wu S, Ai K, Xu R, Zhong Z, Wang Y, Zhang L, Zhao X, Zhu X. LncRNA ZNF503-AS1 acts as a tumor suppressor in bladder cancer by up-regulating Ca(2+) concentration via transcription factor GATA6. Cell Oncol (Dordr). 2021;44(1):219–233. doi: 10.1007/s13402-020-00563-z. [DOI] [PubMed] [Google Scholar]
- 16.Sun BL, Sun X, Casanova N, Garcia AN, Oita R, Algotar AM, Camp SM, Hernon VR, Gregory T, Cress AE, et al. Role of secreted extracellular nicotinamide phosphoribosyltransferase (eNAMPT) in prostate cancer progression: novel biomarker and therapeutic target. EBioMedicine. 2020;61:103059. doi: 10.1016/j.ebiom.2020.103059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang K, Ni Y, Chen J, Tu Z, Wu X, Chen D, Yao H, Jiang S. Discovery of trans-3-(pyridin-3-yl)acrylamide-derived sulfamides as potent nicotinamide phosphoribosyltransferase (NAMPT) inhibitors for the potential treatment of cancer. Bioorg Med Chem Lett. 2019;29(12):1502–1506. doi: 10.1016/j.bmcl.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Li W, Liu Y, Li ZJ, Shi Y, Deng J, Bai J, Ma L, Zeng XX, Feng SS, Ren JL, et al. Unravelling the role of LncRNA WT1-AS/miR-206/NAMPT axis as prognostic biomarkers in lung adenocarcinoma. Biomolecules. 2021;11(2):203. doi: 10.3390/biom11020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang L, Kon N, Li T, Wang S-J, Su T, Hibshoosh H, Baer R, Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520(7545):57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y, Nasri M, Dannenmann B, Mir P, Zahabi A, Welte K, Morishima T, Skokowa J. NAMPT/SIRT2-mediated inhibition of the p53-p21 signaling pathway is indispensable for maintenance and hematopoietic differentiation of human iPS cells. Stem Cell Res Ther. 2021;12(1):112. doi: 10.1186/s13287-021-02144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei G, Zhang Y, Hong T, Zhang X, Liu X, Mao C, Yan Y, Koppula P, Cheng W, Sood AK, et al. Ferroptosis as a mechanism to mediate p53 function in tumor radiosensitivity. Oncogene. 2021;40(20):3533–3547. doi: 10.1038/s41388-021-01790-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng Z, Zhao G, Zhu H, Nie L, He L, Liu J, Li R, Xiao S, Hua G. LncRNA FOXD3-AS1 promoted chemo-resistance of NSCLC cells via directly acting on miR-127-3p/MDM2 axis. Cancer Cell Int. 2020;20(1):350. doi: 10.1186/s12935-020-01402-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu M, Gou L, Xia J, Wan Q, Jiang Y, Sun S, Tang M, He T, Zhang Y. LncRNA ITGB2-AS1 could promote the migration and invasion of breast cancer cells through up-regulating ITGB2. Int J Mol Sci. 2018;19(7):19(7. doi: 10.3390/ijms19071866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, Lu Y, Shi H, Li X, Zhang Z, Deng X, Yang Y, Wan B. LncRNA ITGB2-AS1 promotes the progression of clear cell renal cell carcinoma by modulating miR-328-5p/HMGA1 axis. Hum Cell. 2021;34(5):1545–1557. doi: 10.1007/s13577-021-00563-7. [DOI] [PubMed] [Google Scholar]
- 25.Siddik ZH. Biochemical and molecular mechanisms of cisplatin resistance. Cancer Treat Res. 2002;112:263–284. [DOI] [PubMed] [Google Scholar]
- 26.Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell. 2019;35(6):830–849. doi: 10.1016/j.ccell.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang C, Zhang X, Yang M, Dong X. Recent progress in ferroptosis inducers for cancer therapy. Adv Mater. 2019;31(51):e1904197. doi: 10.1002/adma.201904197. [DOI] [PubMed] [Google Scholar]
- 29.Ge P, Cao L, Zheng M, Yao Y, Wang W, Chen X. LncRNA SNHG1 contributes to the cisplatin resistance and progression of NSCLC via miR-330-5p/DCLK1 axis. Exp Mol Pathol. 2021;120:104633. doi: 10.1016/j.yexmp.2021.104633. [DOI] [PubMed] [Google Scholar]
- 30.Dai J, Xu LJ, Han GD, Jiang H-T, Sun H-L, Zhu G-T, Tang X-M. Down-regulation of long non-coding RNA ITGB2-AS1 inhibits osteosarcoma proliferation and metastasis by repressing Wnt/β-catenin signalling and predicts favourable prognosis. Artif Cells, Nanomed Biotechnol. 2018;46(sup3):S783–s790. doi: 10.1080/21691401.2018.1511576. [DOI] [PubMed] [Google Scholar]
- 31.Giulietti M, Righetti A, Principato G, Piva F. LncRNA co-expression network analysis reveals novel biomarkers for pancreatic cancer. Carcinogenesis. 2018;39(8):1016–1025. doi: 10.1093/carcin/bgy069. [DOI] [PubMed] [Google Scholar]
- 32.Guo Y, Qu Z, Li D, Bai F, Xing J, Ding Q, Zhou J, Yao L, Xu Q. Identification of a prognostic ferroptosis-related lncRNA signature in the tumor microenvironment of lung adenocarcinoma. Cell Death Discov. 2021;7(1):190. doi: 10.1038/s41420-021-00576-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li D, Yang W, Zhang J, Yang J, Guan R, Yang M. Transcription factor and lncRNA regulatory networks identify key elements in lung adenocarcinoma. Genes (Basel). 2018;9(1):9(1. doi: 10.3390/genes9010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Sun D, Wang Y, Ren F, Pang S, Wang D, Xu S. FOSL2 positively regulates TGF-β1 signalling in non-small cell lung cancer. PLos One. 2014;9(11):e112150. doi: 10.1371/journal.pone.0112150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding R, Jiao L, Sang S, Yin Y, Wang Y, Gong Y, Xu L, Bi L. Anticancer action of xiaoxianxiong tang in non-small cell lung cancer by pharmacological analysis and experimental validation. Evid Based Complement Alternat Med. 2021;2021:1–20. doi: 10.1155/2021/9930082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L, Wang S. Long Non-Coding RNA OIP5-AS1 knockdown enhances CDDP sensitivity in osteosarcoma via miR-377-3p/FOSL2 axis. Onco Targets Ther. 2020;13:3853–3866. doi: 10.2147/OTT.S232918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu HY, Li QR, Cheng XF, WANG G-J, HAO H-P. NAMPT inhibition synergizes with NQO1-targeting agents in inducing apoptotic cell death in non-small cell lung cancer cells. Chin J Nat Med. 2016;14(8):582–589. doi: 10.1016/S1875-5364(16)30068-1. [DOI] [PubMed] [Google Scholar]
- 38.Jones CL, Stevens BM, Pollyea DA, Culp-Hill R, Reisz JA, Nemkov T, Gehrke S, Gamboni F, Krug A, Winters A, et al. Nicotinamide metabolism mediates resistance to venetoclax in relapsed acute myeloid leukemia stem cells. Cell Stem Cell. 2020;27(5):748–764.e4. doi: 10.1016/j.stem.2020.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tarangelo A, Magtanong L, Bieging-Rolett KT, Li Y, Ye J, Attardi LD, Dixon SJ. P53 suppresses metabolic stress-induced ferroptosis in cancer cells. Cell Rep. 2018;22(3):569–575. doi: 10.1016/j.celrep.2017.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


