Abstract
Introduction
This study examined user behavior, e-cigarette dependence, and device characteristics on nicotine intake among users of pod-mod e-cigarettes.
Aims and Methods
In 2019–2020, people who use pod-mods in the San Francisco Bay Area completed questionnaires and provided a urine sample for analysis of total nicotine equivalents (TNE). The relationship between TNE and e-cigarette use, e-cigarette brands, e-liquid nicotine strength, e-cigarette dependence, and urine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), as a measure of combustible cigarette exposure, were examined.
Results
Of 100 participants (64% male, 71% in the 18–34 age group, 45% white), 53 used JUUL primarily, 12 used Puff Bar primarily, and 35 used other brands, including Suorin; 48 participants reported current cigarette smoking. Participants most often reported use of e-liquid with 4.5%–6.0% nicotine (68%), fruit (35%), tobacco (28%), and menthol or mint flavors (26%), used e-cigarettes on 25.5 (SD = 6.3) days a month, 10.2 (SD = 14.2) times a day, and 40% used 1–2 pods/cartridges per week. In bivariate analysis, urinary TNE was higher with greater frequency (days used) and intensity (number of pods used) of e-cigarette use, e-cigarette dependence, and combustible cigarette use. In multivariable analysis, days of e-cigarette use in the last 30 days, number of pods used per week, and NNAL levels were significantly associated with TNE. There was no significant impact of e-liquid nicotine strength on TNE.
Conclusions
Nicotine intake among people who used pod-mod e-cigarettes increased with e-cigarette consumption and e-cigarette dependence, but not with e-liquid nicotine strength. Our findings may inform whether FDA adopts a nicotine standard for e-cigarettes.
Implications
The study examined how device and user characteristics influence nicotine intake among pod-mod e-cigarette users. Nicotine intake increased with frequency (days of e-cigarette use in past 30 days) intensity of use (number of pods used per day) and e-cigarette dependence but not with the flavor or nicotine concentration of the e-liquids. Regulation of nicotine concentration of e-liquids is unlikely to influence nicotine exposure among adult experienced pod-mod users.
Introduction
Electronic cigarettes (e-cigarettes), battery-powered devices used to deliver nicotine in the form of a non-combustion-generated aerosol, have continued to evolve since their introduction on the global market in 2007. Early e-cigarette devices, namely, cig-a-likes, pen-shaped tanks, and variable voltage/power mods, remain on the market, but in recent years pod-mod e-cigarettes have become increasingly popular.1,2 At the time of the study, JUUL was the most popular pod e-cigarette on the US market.3 However, Puff Bar, a disposable pod e-cigarette, has surpassed JUUL as the most popular e-cigarette among middle and high school students who use e-cigarettes.4 While the shapes of pod e-cigarettes vary, most are small rectangular USB stick-shaped devices with removable pods or cartridges or are entirely disposable.5 The pods or cartridges contain a heating element and the e-liquid solution.
Pod-mod e-cigarette liquids usually contain high nicotine concentrations in salt form through formulations with various weak organic acids.6 The addition of acids results in a lower pH at which a greater percentage of nicotine is protonated and has lower volatility in the aerosol than free-base nicotine.7 Because of lower volatility and less interaction with upper airway irritant receptors, despite the high nicotine concentration of the e-liquids, inhalation of nicotine salt formulations is experienced as less harsh than freebase nicotine.8,9 Given the ease of concealment of these small devices, lower power and thus small plumes of aerosol produced, high nicotine salt concentrations, and “sleek” design,10 the rise in popularity of pod-mod e-cigarettes may have contributed to the rapid rise in e-cigarette use among teens after 2018.5 On the other hand, because of lower coil heating temperatures, higher e-liquid nicotine concentrations and lower exposure to aerosol, pod-mod e-cigarette use may have a lower toxicological risk than high-powered e-cigarettes and might be a safer product for use among adult vapers as a step towards harm reduction of tobacco use.
Due to the relative recency of pod-mod e-cigarettes on the market, data on nicotine delivery, addictiveness, and use patterns are still scarce. A recent cross sectional analysis of participants in a prospective observational study compared users of pod e-cigarettes (n = 37) to those of third-generation mod devices (box mods) (n = 48) and found that despite differences in product and e-liquid characteristics, there was no significant difference in urinary cotinine, a measure of nicotine exposure, between the two groups.11 Other studies have been conducted among pod users12–14 but these studies did not examine how device characteristics, e-cigarette dependence, and e-cigarette consumption influenced nicotine intake with biomarkers. In this study, we examined correlates of nicotine intake among pod-mod users, utilizing total nicotine equivalents, the gold-standard biomarker of nicotine intake. This addition to the literature will inform the FDA’s regulation of pod-mod characteristics, such as nicotine concentration of the e-liquids.
Thus, we conducted a descriptive study to assess correlates of nicotine exposure among adult pod-mod e-cigarette users. Understanding correlates of nicotine intake among adults who use pod-mods will help us understand the effectiveness of these devices as sources of nicotine among this population, especially in the context of the changing tobacco regulatory landscape. We hypothesize that nicotine intake among adult pod-mod users is influenced by device characteristics and user behavior with the primary outcome being total nicotine equivalents as a biomarker of nicotine intake. This study is one of the first studies on pod-mod e-cigarettes to include both biomarker data and e-cigarette dependence measures and will fill important research gaps to inform e-cigarette regulation.
Methods
We enrolled 100 pod-mod e-cigarette users in an observational study conducted in the San Francisco Bay Area between July 2019 and December 2020. Inclusion criteria were participants aged 18 to 70 years old who reported using a pod-mod e-cigarette at least 10 days out of the past 30 days. While participants could be exclusive users of e-cigarettes, no restrictions were placed on participants’ use of other tobacco products or other substances, such as cannabis. Individuals were only excluded from the study if they were concurrent users of nicotine-containing medications. One participant was included in the study who used their e-cigarette on 7 days out of the past 30 days. Results were examined with and without this participant and the findings were the same.
Study Procedures
Participants were recruited via Craigslist, Facebook, and/or flyers and asked to complete an online REDCap eligibility survey. If eligible, participants either attended an in-person study visit at Zuckerberg San Francisco General Hospital (ZSFG) or completed the study via a mobile health visit at their place of residence (during the COVID pandemic). No instructions were given to participants regarding their tobacco product usage on the day of, or days leading up to the study visit. During both study visit formats, participants underwent the same procedures as described below.
Participants completed a battery of questionnaires to assess their demographics, e-cigarette dependence (Penn State Electronic Cigarette Dependence Index [PSECDI]),15 and patterns of e-cigarette, other tobacco products, alcohol, and substance use. Patterns of use questions were asked about participants’ product use over the past 3 days. For each day, participants answered questions on the type of product(s) they used “today,” how much of the product was used (eg number of cartridges), and times of use. For e-cigarette use specifically, some questions included, “On days that you can use your e-cigarette freely, how soon after you wake up do you first use your e-cigarette?,” “Have you used an e-cigarette so far today?,” “How many times did you use an e-cigarette today?,” “At what time did you last use an e-cigarette today?” and “Approximately how many puffs did you take?”
Participants provided a urine sample, photos were taken of their devices, and a sample of their pod or e-liquid were subsequently purchased for pending lab analysis. Participants were compensated $50 in either cash or gift cards upon completion of the study visit.
The study was approved by the Human Research Protection Program at the University of California San Francisco. Written, informed consent was obtained from each participant and all participants were financially compensated.
Analytical Chemistry
Urinary concentrations of nicotine and the nicotine metabolites, cotinine and 3-hydroxycotinine (and their glucuronides), as well as nicotine-N-oxide, cotinine-N-oxide, nornicotine, and norcotinine were measured by liquid chromatography-tandem mass spectrometry.16 The sum of these seven analytes (termed TNE7) accounts for more than 90% of nicotine excretion, is not significantly affected by genetic/metabolic variability among participants, and is considered the gold standard measure of nicotine intake.17 Urine TNE7 was computed as the sum of the molar concentrations of the analytes and was corrected for creatinine concentration.18 We will use “TNE” instead of TNE7 throughout the text.
Statistical Methods
Participant demographic information and self-reported tobacco and e-cigarette use are presented as counts and/or proportions; continuous variables are presented as mean and standard deviation. Analysis of variance was used to test differences in mean TNE (log-transformed due to log-normal distribution) across predictor variables. We used a urinary creatinine-adjusted NNAL cutoff point of 10 pg/mg creatinine to biochemically discriminate between cigarette smokers and nonsmokers with or without secondhand smoke exposure.19 NNAL, which has a terminal half-life of 10–18 days,20 indicates cigarette smoking within the past 6 to 12 weeks even if users are infrequent/nondaily smokers.
Three multivariable models were used to further assess the relationship between urine TNE and various predictor variables. NNAL and/or self-report smoking were used to indicate combustible cigarette use among e-cigarette users (ie dual use). The first model included all participants. The second model examined e-cigarette only users by excluding biochemically verified participants who smoked cigarettes (n = 61 participants were included). The third model examined e-cigarette-only users by excluding biochemically-verified smokers and self-reported smokers (n = 44 participants were included). Covariates were chosen a priori and included demographic variables, measures of frequency and intensity of e-cigarette use, e-cigarette dependence, and e-cigarette characteristics, such as e-liquid nicotine concentration. We included these variables because both user characteristics and device characteristics have been shown to predict nicotine intake among e-cigarette users.21
Results
The demographic characteristics of the participants are presented in Table 1. Of the 100 participants enrolled, 64% were male, 71% were between 18 and 34 years old, 45% were white, and 94% had some college education or more.
Table 1.
Demographic information of pod-mod e-cigarette users from the San Francisco Bay Area enrolled in a cross-sectional study in 2019–2020
| Demographic variable | Number of participants (N = 100) |
|---|---|
| Sex | |
| Male | 64 |
| Female | 36 |
| Age | |
| 18–34 | 71 |
| 35–49 | 22 |
| 50–64 | 7 |
| Race | |
| Asian | 21 |
| Black | 8 |
| Latino | 10 |
| White | 45 |
| Mixed/other | 16 |
| Education | |
| Some high school/high school | 6 |
| Some college/two-year degree | 49 |
| Four-year degree | 41 |
| Advanced degree | 4 |
| Household income | |
| Less than 25k | 33 |
| 25 000–49 999 50 000–74 999 |
23 |
| 75 000 or more | 21 |
Pod-mod E-cigarette Use
Pod-mod e-cigarettes and use patterns are presented in Table 2. Most participants (53%) used JUUL while 12% used Puff Bar and 35% used other pod-mod e-cigarette brands, including Smok, Blu, and Suorin. Most participants used their e-cigarettes on 21–30 days out of the past 30 days (72%), 40% used their e-cigarettes at least 10 times on days they vaped, where one “time” is defined as 15 puffs or a session which lasts around 10 min, as has been used in the Penn State E-cigarette Dependence Index.15 Regarding intensity of consumption, 77% of participants used one or more pods/cartridges per week. Most participants (77%) used high nicotine concentration e-liquids (4.5% to 6.0% nicotine concentration by weight). The most often used flavors were fruit (35%), tobacco (28%), and menthol/mint (26%).
Table 2.
E-cigarette use and device characteristics of pod-mod e-cigarette users from the San Francisco Bay Area (2019–2020). Frequencies are given for all participants and by the primary pod-mod e-cigarette brand used (JUUL, Puff Bar, or other brands)
| E-cigarette use/device characteristic | All participants (N = 100) |
Primary brand use | ||
|---|---|---|---|---|
| JUUL (n = 53) |
Puff Bar (n = 12) |
Other (n = 35) |
||
| Number of days of e-cigarette use in last 30 days, n (%) | ||||
| 7–10* | 6 | 2 (3.8) | 2 (17) | 2 (5.7) |
| 11–20 | 22 | 14 (26) | 4 (33) | 4 (11) |
| 21–30 | 72 | 37 (70) | 6 (50) | 29 (83) |
| Number of times e-cigarette used per day, n (%) | ||||
| 1–4 | 33 | 18 (34) | 5 (42) | 10 (29) |
| 5–9 | 27 | 21 (40) | 1 (8.3) | 5 (14) |
| ≥10 | 40 | 14 (26) | 6 (50) | 20 (57) |
| Number of pods/cartridges used per week, n (%) | ||||
| <1 | 20 | 8 (15) | 3 (25) | 9 (26) |
| 1–2 | 40 | 17 (32) | 5 (42) | 18 (51) |
| 3–4 | 19 | 14 (26) | 2 (17) | 3 (8.6) |
| ≥5 | 18 | 14 (26) | 1 (8.3) | 3 (8.6) |
| N/A | 3 | 0 (0) | 1 (8.3) | 2 (5.7) |
| E-liquid nicotine strength, n (%) | ||||
| 0.3%–0.6% | 1 | 0 (0) | 0 (0) | 1 (2.9) |
| 1.2%–2.5% | 9 | 1 (1.9) | 0 (0) | 8 (23) |
| 2.7%–3.0% | 10 | 10 (19) | 0 (0) | 0 (0) |
| 4.5%–6.0% | 77 | 42 (79) | 12 (100) | 23 (66) |
| Unknown | 3 | 0 (0) | 0 (0) | 3 (8.6) |
| Most frequently used flavor, n (%) | ||||
| Tobacco | 29 | 27 (51) | 0 (0) | 2 (5.7) |
| Menthol/mint | 26 | 20 (38) | 0 (0) | 6 (17) |
| Fruit | 35 | 6 (11) | 9 (75) | 20 (57) |
| Dessert/candy | 2 | 0 (0) | 0 (0) | 2 (5.7) |
| Other | 8 | 0 (0) | 3 (25) | 5 (14) |
*One participant used the e-cigarette on 7 of the last 30 days while 5 used their e-cigarette on 10 of the last 30 days.
Tobacco Cigarette Smoking
Of all participants, 48% reported using tobacco cigarettes in the past 30 days, including 18% who smoked daily, 16% who smoked at least weekly, and 14% who smoked at least monthly. Furthermore, 39% reported being former smokers, and 13% were self-reported never-smokers. To the question of whether they had smoked a cigarette on the day of the study visit “today,” 78% of participants self-reported “No” while 18% reported smoking at least one tobacco cigarette (4% did not respond to the question). Among the 18 who had smoked “today,” 17 had smoked 1–10 cigarettes and one had smoked 11–20 cigarettes. Using the urinary NNAL cutoff point of 10 pg/mg creatinine to discriminate between smokers and nonsmokers, 61% of the participants were classified as nonsmokers, and 39% were classified as smokers.
Nicotine intake
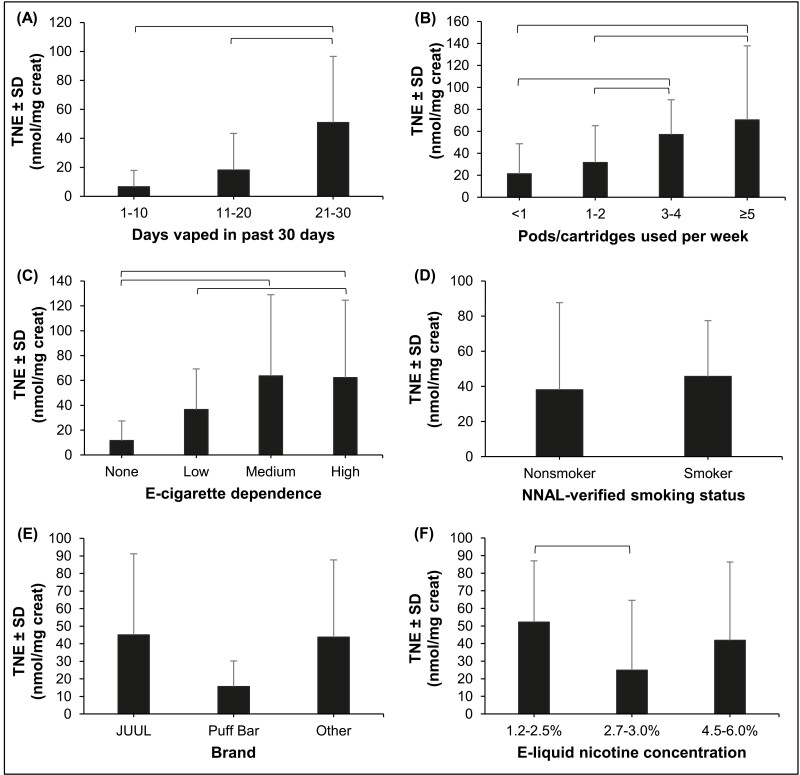
Supplementary Table S1 presents bivariate analyses of TNE, as a measure of nicotine intake, across demographic and product use characteristics. Significant differences in TNE levels were observed across a number of pods/cartridges used per week, days vaped in the last 30 days, a number of times e-cigarettes are used per day, and NNAL-verified smoking status (see Figure 1). TNE did not vary across sex, age, and race in the univariate analysis. Also, no differences were observed in TNE levels across self-reported smoking status, e-cigarette brand (JUUL vs. non-JUUL brands), and e-liquid flavor. TNE differed significantly across e-liquid nicotine concentration in the univariate analysis, but no clear pattern was found. Within the category of nonsmokers (validated by NNAL), there was no significant difference in mean TNE for those who self-identified as “Never Smokers” when compared to those identifying as “Former Smokers.”
Figure 1.
Differences in average urinary total nicotine equivalents (TNE) across (A) number of days vaped in the last 30 days; (B) number of pods/cartridges used per week; (C) e-cigarette dependence, as measured by the Penn State Electronic Cigarette Dependence Index (PSECDI); (D) NNAL-verified smoking status (cigarette nonsmoker vs. cigarette smoker); (E) brand of pod-mod; and (F) e-liquid nicotine concentration. Bars indicate significant differences in urinary TNE across categories of each variable (α < 0.05). The TNE measured was TNE7, the molar sum of nicotine and 6 of its metabolites. SD = standard deviation.
Multivariable analysis was performed including all participants (Table 3), with the covariates age, race, e-liquid nicotine strength, pods/cartridges used per week, days of e-cigarette use in the last 30 days and urine NNAL as a measure of combustible cigarette use. The most significant predictors of TNE were days of e-cigarette use in the last 30 days (p < .001), pods/cartridges used per week (p < .001), and urinary NNAL (p < .001). TNE was also positively related to age. Race and e-liquid nicotine strength were not significant predictors of TNE. When only e-cigarette users were considered (models 2 and 3), the most significant predictors of TNE were days of e-cigarette use in the last 30 days (p < .001) and pods/cartridges used per week (p < .001). As before, age but not race and e-liquid nicotine strength was significant predictor of TNE.
Table 3.
Predictors of nicotine intake among pod-mod e-cigarette users, as measured by urinary total nicotine equivalents (TNE)
| Variable | df | F | P |
|---|---|---|---|
| Model 1: All participants | |||
| Age | 1 | 6.36 | .014 |
| Race | 4 | 1.56 | .19 |
| E-liquid nicotine strength | 1 | 0.67 | .42 |
| Pods per week | 3 | 6.81 | <.001 |
| EC use in last 30 days | 1 | 21.1 | <.001 |
| NNAL (pg/mg creatinine) | 1 | 11.8 | <.001 |
| Model 2: E-cigarette only users (NNAL-verified nonsmokers) | |||
| Age | 1 | 5.63 | .02 |
| Race | 4 | 1.38 | .25 |
| E-liquid nicotine strength | 1 | 0.59 | .44 |
| Pods per week | 3 | 6.02 | <.001 |
| EC use in last 30 days | 1 | 18.7 | <.001 |
| Model 3: E-cigarette only users (self-report and NNAL-verified) | |||
| Age | 1 | 13.64 | <.001 |
| Race | 4 | 1.34 | .28 |
| E-liquid nicotine strength | 1 | 3.74 | .06 |
| Pods per week | 3 | 9.74 | <.001 |
| EC use in last 30 days | 1 | 10.34 | <.001 |
The TNE measured was TNE7, the molar sum of nicotine and 6 of its metabolites. In Model 1, all participants were included, and independent variables included demographics, e-cigarette use behavior and device characteristics, and urinary NNAL. In Model 2, biochemically verified cigarette smokers were excluded (n = 39), and the model included demographics and e-cigarette use and device characteristics. In Model 3, self-reported smokers and biochemically verified cigarette smokers (n = 56) were excluded and the model included demographics and e-cigarette use and device characteristics.
E-cigarette Dependence Measures
A higher level of e-cigarette dependence, as indicated by a shorter time after awakening to first e-cigarette use and higher Penn State Electronic Cigarette Dependence Index score, was significantly associated with TNE (Supplementary Table S1). There was a significant inverse relationship between time to first e-cigarette use and the Penn State E-Cigarette Dependence Index score (p < .001).
Discussion
We present novel data on predictors of nicotine intake in pod-mod users, including e-liquid nicotine concentration and flavor, and how the level of e-cigarette use and dependence are associated with nicotine exposure. As one of the few studies with biomarkers of nicotine exposure in pod-mod users, this study fills in some important knowledge gaps about the use of these popular e-cigarette devices.
The strongest predictors of nicotine intake among adult pod-mod users were consumption related variables (days of e-cigarette use in the last 30 days, number of pods/cartridges used per week), tobacco cigarette smoking or smoke exposure and e-cigarette dependence measures. When exclusive e-cigarette users were analyzed separately, only consumption related variables were significant predictors of nicotine intake. Nicotine intake did not differ by brand (JUUL vs. non-JUUL e-cigarette brands), sex, race, and device characteristic, such as e-liquid nicotine concentration and flavors. The amount of nicotine consumed in this group is largely driven by frequency and intensity of e-cigarette use, which are related to the users’ physical dependence on nicotine and/or e-cigarettes as indicated by the PSECDI.
Pod-mod e-cigarettes can contain a wide range of nicotine concentrations and flavors, including some of the highest nicotine concentrations among all e-cigarette products on the U.S. market. There is a concern that high nicotine concentrations may expose users to higher amounts of nicotine than those with lower nicotine concentration, and as such may be more addictive or harmful. However, our findings show that users’ nicotine intake is not related to the e-liquid nicotine concentration, similar to the findings of a recent comparison between pod users and third-generation mod e-cigarettes.11 This is likely because e-cigarette users engage in compensatory puffing, such as more intense puffing when using devices with lower nicotine concentration, as has been shown elsewhere.22,23 While concerns about the addictiveness of high nicotine content pod-mod devices like JUUL remain, especially concerns about teen use,24 the increased frequency of use of devices with lower nicotine concentrations leads to increased exposure to e-cigarette-related toxicants23 and likely increased health risk. Although several countries, including those in the European Union, have restricted e-cigarette nicotine concentration to 20 mg/mL, our findings suggest that restrictions on e-cigarette nicotine content alone may have limited impact on the health consequences of e-cigarette use among experienced adult e-cigarette users.
Age was a significant predictor of TNE in the multivariate models. While age may not necessarily be a predictor of nicotine intake on its own, age may be a predictor of years of e-cigarette use and thus e-cigarette dependence and e-cigarette consumption and nicotine intake. This likely explains why TNE increased with age.
Our data support the relationship between two commonly used dependence measures of nicotine self-administration. As e-cigarette dependence, measured by the PSECDI, increased, TNE levels increase significantly. Likewise, we found a significant relationship between the time to first reported use of their e-cigarette after waking and the PSECDI score with TNE levels. Participants who used their devices first thing in the morning had higher TNE levels compared to those who had their first use later in the day.
Limitations of our study include a small convenience sample of predominantly white males from one region of the United States. The size limits our ability to assess subgroup differences and to make broad generalizations about pod e-cigarettes. Further, our study findings may not be generalizable to other parts of the US since smoking and vaping behaviors and demographics of vapers may differ in the San Francisco Bay Area compared to vapers in other parts of the country. However, our finding that e-liquid nicotine concentration did not predict nicotine intake is similar to that of a recent study of pod and third-generation mod users from New York.11
Conclusion
In a convenience sample of 100 pod e-cigarette users in the San Francisco Bay Area, we found the most significant predictors of nicotine intake to be consumption related variables, tobacco cigarette use, and level of e-cigarette dependence. E-liquid nicotine strength and e-liquid flavors were not found to be significantly associated with TNE levels. Finally, we found that TNE levels were associated with the level of e-cigarette dependence measured by the PSECDI.
Our findings may have regulatory implications. As discussed before, our findings indicate that e-liquid nicotine content is not associated with greater nicotine exposure. An advantage of higher nicotine levels in e-liquids is that the smaller volumes of aerosol generated at lower coil temperatures can deliver desired levels of nicotine with exposure to fewer thermal degradation produced toxicants. Thus, for adults who use e-cigarettes, high nicotine liquid vaping may be safer than lower nicotine liquid vaping. This benefit needs to be balanced against the potential that high nicotine liquids might be more likely to produce e-cigarette or nicotine dependence in youth.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Acknowledgments
We thank Kristina Bello, Trisha Mao, Polly Cheung, Lawrence Chin, and Lisa Yu for performing analytical chemistry. We also thank Drs. Buchbinder, Hilton, Newman, and Neuhaus and the students in the UCSF Training in Clinical Research Program master’s seminar for reviewing drafts of the manuscript.
Contributor Information
Jeremy Giberson, Research Program in Clinical Pharmacology, Division of Cardiology, Department of Medicine, University of California, San Francisco, CA, USA.
Natalie Nardone, Research Program in Clinical Pharmacology, Division of Cardiology, Department of Medicine, University of California, San Francisco, CA, USA.
Newton Addo, Research Program in Clinical Pharmacology, Division of Cardiology, Department of Medicine, University of California, San Francisco, CA, USA.
Sameera Khan, Univiersity of California, Berkeley, CA, USA.
Peyton Jacob, III, Research Program in Clinical Pharmacology, Division of Cardiology, Department of Medicine, University of California, San Francisco, CA, USA; Center for Tobacco Control Research and Education, University of California, San Francisco, CA, USA; Tobacco Center of Regulatory Science, University of California, San Francisco, CA, USA.
Neal Benowitz, Research Program in Clinical Pharmacology, Division of Cardiology, Department of Medicine, University of California, San Francisco, CA, USA; Center for Tobacco Control Research and Education, University of California, San Francisco, CA, USA; Tobacco Center of Regulatory Science, University of California, San Francisco, CA, USA.
Gideon St.Helen, Research Program in Clinical Pharmacology, Division of Cardiology, Department of Medicine, University of California, San Francisco, CA, USA; Center for Tobacco Control Research and Education, University of California, San Francisco, CA, USA; Tobacco Center of Regulatory Science, University of California, San Francisco, CA, USA.
Funding
This study was supported by grants 1 U54HL147127 from the National Heart, Lung, and Blood Institute (NHLBI) and the U.S. Food and Drug Administration (FDA) and P30DA012393 from the National Institute on Drug Abuse (NIDA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH) or the Food and Drug Administration (FDA).
Declaration of Interests
Benowitz is a consultant to Pfizer and Achieve Life Sciences, companies that market or are developing smoking cessation medications, and has served as a paid expert witness in litigation against tobacco companies. The other authors declare no conflict of interest.
Data Availability
Data will be available upon reasonable request.
References
- 1. Lee SJ, Rees VW, Yossefy N, Emmons KM, Tan AS.. Youth and young adult use of pod-based electronic cigarettes from 2015 to 2019: a systematic review. JAMA Pediatr. 2020;174(7):714–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barrington-Trimis JL, Leventhal AM.. Adolescents’ use of “pod mod” e-cigarettes—urgent concerns. N Engl J Med. 2018;379(12):1099–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang J, Duan Z, Kwok J, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control. 2019;28(2):146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park-Lee E, Ren C, Sawdey MD, et al. Notes from the field: e-cigarette use among middle and high school students—National Youth Tobacco Survey, United States, 2021. MMWR. 2021;70(39):1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fadus MC, Smith TT, Squeglia LM.. The rise of e-cigarettes, pod mod devices, and JUUL among youth: factors influencing use, health implications, and downstream effects. Drug Alcohol Depend. 2019;201:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harvanko AM, Havel CM, Jacob P, Benowitz NL.. Characterization of nicotine salts in 23 electronic cigarette refill liquids. Nicotine Tob Res. 2020;22(7):1239–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henningfield JE, Pankow JF, Garrett BE.. Ammonia and other chemical base tobacco additives and cigarette nicotine delivery: issues and research needs. Nicotine Tob Res. 2004;6(2):199–205. [DOI] [PubMed] [Google Scholar]
- 8. Hajek P, Pittaccio K, Pesola F, et al. Nicotine delivery and users’ reactions to JUUL compared with cigarettes and other e‐cigarette products. Addiction. 2020;115(6):1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pankow JF. A consideration of the role of gas/particle partitioning in the deposition of nicotine and other tobacco smoke compounds in the respiratory tract. Chem Res Toxicol. 2001;14(11):1465–1481. [DOI] [PubMed] [Google Scholar]
- 10. Kava CM, Soule EK, Seegmiller L, et al. “Taking up a new problem”: context and determinants of pod-mod electronic cigarette use among college students. Qual Health Res. 2021;31(4):703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Felicione NJ, Kaiser L, Leigh NJ, et al. Comparing POD and MOD ENDS users’ product characteristics, use behaviors, and nicotine exposure. Nicotine Tob Res. 2022;25.(3).):498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leavens EL, Nollen NL, Ahluwalia JS, et al. Changes in dependence, withdrawal, and craving among adult smokers who switch to nicotine salt pod-based e-cigarettes. Addiction. 2022;117(1):207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soule EK, Mayne S, Snipes W, et al. Electronic cigarette nicotine flux, nicotine yield, and particulate matter emissions: impact of device and liquid heterogeneity. Nicotine Tob Res. 2022;25(3):412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Do EK, O’Connor K, Perks SN, et al. E-cigarette device and liquid characteristics and E-cigarette dependence: a pilot study of pod-based and disposable E-cigarette users. Addict Behav. 2022;124:107117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Foulds J, Veldheer S, Yingst J, et al. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking e-cigarette users. Nicotine Tob Res. 2015;17(2):186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jacob P, Yu L, Benowitz N.. Determination of nicotine, its major metabolites, and deuterium-labeled isotopomers in human urine using LC-MS/MS. Drug Metab Rev. 2002;34:160. [Google Scholar]
- 17. Feng S, Kapur S, Sarkar M, et al. Respiratory retention of nicotine and urinary excretion of nicotine and its five major metabolites in adult male smokers. Toxicol Lett. 2007;173(2):101–106. [DOI] [PubMed] [Google Scholar]
- 18. Benowitz NL, St Helen G, Nardone N, Cox LS, Jacob P.. Urine metabolites for estimating daily intake of nicotine from cigarette smoking. Nicotine Tob Res. 2019;22(2):288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benowitz NL, Nardone N, Jain S, et al. Comparison of urine 4-(methylnitrosamino)-1-(3)pyridyl-1-butanol and cotinine for assessment of active and passive smoke exposure in urban adolescents. Cancer Epidemiol Biomarkers Prev. 2018;27(3):254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goniewicz ML, Havel CM, Peng MW, et al. Elimination kinetics of the tobacco-specific biomarker and lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Cancer Epidemiol Biomarkers Prev. 2009;18(12):34213421.–342133425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Academies of Sciences Engineering and Medicine. Public Health Consequences of E-Cigarettes. Washington, DC: The National Academies Press. 2018. [PubMed] [Google Scholar]
- 22. Kośmider L, Kimber CF, Kurek J, Corcoran O, Dawkins LE.. Compensatory puffing with lower nicotine concentration e-liquids increases carbonyl exposure in e-cigarette aerosols. Nicotine Tob Res. 2018;20(8):998–1003. [DOI] [PubMed] [Google Scholar]
- 23. Dawkins L, Cox S, Goniewicz M, et al. ‘Real‐world’compensatory behaviour with low nicotine concentration e‐liquid: subjective effects and nicotine, acrolein and formaldehyde exposure. Addiction. 2018;113(10):1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. King BA, Jones CM, Baldwin GT, Briss PA.. The EVALI and youth vaping epidemics—implications for public health. N Engl J Med. 2020;382(8):689–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be available upon reasonable request.