Abstract
Purpose
The aim of this systematic review was to investigate the available data on the epidemiology of oculocutaneous albinism (OCA) around the world, and to determine whether a generalizable, worldwide prevalence figure could be proposed.
Methods
Extensive literature search strategies were conducted, interrogating PubMed, Scopus, and Web of Science, to locate relevant literature. Ultimately 34 studies reporting original data were included for analysis.
Results
Findings showed that most data were outdated, and only 6 of 34 articles (18%) were published after 2010. There were few good studies with sound methodology and large, clearly defined population samples. Only a small proportion of countries worldwide (26/193 [13%]) have produced prevalence figures for OCA. By continent, African studies were disproportionately represented (15/34 [44%]). The highest prevalence rates (range, 1 in 22 to 1 in 1300; mean, 1 in 464) were reported in population isolates. The mean prevalence from four African countries was 1 in 4264 (range, 1 in 1755 to 1 in 7900). Prevalence for three countries in Europe (mean, 1 in 12,000; range, 1 in 10,000 to 1 in 15,000) may be underestimated, as the phenotype, in fair-skinned populations, may be missed or misdiagnosed as ocular albinism or isolated visual impairment. Population rates may vary depending on local cultural factors (e.g., consanguineous matings) and may change over time.
Conclusions
The prevalence of OCA varies widely between continents and population groups, and it is often influenced by local factors. It was not possible, therefore, to determine a single, generalizable worldwide prevalence rate for OCA, although continental rates for Africa and Europe are useful.
Keywords: albinism, Africa, epidemiology, frequency, prevalence, worldwide
The use of data, including prevalence data, is an essential component for decision-making within health care systems. Prevalence data, specifically, can be used to assess the burden of a disorder, influence health care policy, encourage dialogue and raise public awareness, in any country, so appropriate research is both necessary and worthwhile.
Oculocutaneous albinism (OCA) is a group of inherited pigment disorders in which melanin biosynthesis is decreased or absent.1 OCA occurs in populations all over the world at varying frequencies,2,3 but there are few good prevalence studies.4 Several types are described (OCA1–7), each being caused by mutations in a different gene.5 Phenotypically, the eyes6 and skin7 are affected, resulting in poor visual acuity8 and a predisposition to skin cancer. Intellectual maturity has been shown to be within the normal range.9 Squamous and basal cell carcinomas occur, mostly on the head and neck areas,10 and are the cause of mortality in some individuals with OCA. In Africa, myths and superstitions surround the condition and this has led to human rights violations and erroneous beliefs regarding the cause of the disorder with, in certain instances, fatal consequences.11
OCA, specifically OCA2, is the most common Mendelian autosomal recessive condition in southern Africa, occurring with a prevalence of approximately 1 in 4000 African individuals.12,13 Prevalence figures for OCA for the rest of sub-Saharan Africa (central, East, and West Africa) show a relatively similar picture (however, in these regions, where malaria is endemic, sickle cell anemia is the most common autosomal recessive condition, owing to positive selection for the HbS beta globin mutation). Only one case of OCA1 has been described in Africa14; however, this type of OCA occurs relatively frequently in Europe (e.g., in Denmark 44% of tested cases had OCA1).15 Two European studies have found that OCA occurs in approximately 1 in 14,000 people in Denmark15 and 1 in 12,000 in the Netherlands.16 Although much of the worldwide prevalence data for OCA were generated some time ago, their publication led to an appreciation of the frequency of albinism in Africa. This in turn led to the establishment of clinics, parent support groups and advocacy groups, and, further, directly focused academic research, over several decades, including research on prevalence rates.
One worldwide figure that could be applied to countries without their own data might be useful. Presently many countries, where no prevalence studies have been performed, sometimes rely on the old estimate of 1 in 17,000.17 This figure was obtained from a study carried out on a mixed ancestry population (mostly of European and African descent) living in North Carolina and may not accurately reflect the prevalence rate of populations from different ancestries. Examining the worldwide prevalence data will help to clarify the epidemiology of OCA, avoid under- or over-estimating its frequency in specific populations, and can lead to the provision of improved and more reliable information to local health departments.
The present study aims to establish the prevalence rates for OCA worldwide. The objectives are to investigate relevant data published in the literature over time, to establish for which countries and populations information exists, to assess and compare findings in and between countries and, finally, to determine whether it is possible to arrive at a useful, broad, worldwide population-based estimate of prevalence.
Methods
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed for this systematic review.18
Protocol and Registration
At the outset, this project was not registered with the international Prospective Register of Systematic Reviews (PROSPERO), and there were no registered reviews on PROSPERO relating to the epidemiology of albinism at the time. The project protocol was developed by the three authors together during the planning phase of the project.
Search Strategy
The search of the literature to retrieve relevant articles was conducted on 26 March 2021 across three databases: PubMed, Scopus, and Web of Science. No filters applied restrictions to language or date of publication. Search Builder version 1.0 was used to set-up the search query.19 This query included the following terms: “albinism,” “oculocutaneous albinism,” “prevalence,” “frequency,” “rate of occurrence,” “epidemiology,” “incidence,” “birth rate,” and “community rate.”
Study Selection
Rayyan software20 was used for article management and study selection. Duplicated articles were scanned for and deleted by KF. Abstracts were reviewed and assessed by KF, RK, and JK, and studies that were not relevant were removed. All remaining articles were assessed through full text by KF, RK, and JK. Articles found to provide evidence relating to the investigation of this study were approved for data extraction. Additional articles referenced in the approved articles, that were not found in the initial data search, and relevant chapters in books, were identified and subsequently included into the study if they contained original data (e.g., Kromberg, 2018,3 Lund and Roberts, 201821). During the data extraction process articles were subjected to the Joanna Briggs Institute (JBI) critical appraisal checklist for studies reporting prevalence data.22
Data Collection Process and Data Items
The following data were extracted (where possible) for all articles included in the study by KF and JK: The first author and year of publication; the country and/or city in which the study was conducted; the sample or population size; the sex ratio of the samples; the infrastructure through which the study was conducted (e.g., hospital, school, survey); any reported mating patterns in the population or community; the setting of the population: rural or urban; any reported statistics or observations regarding the lifespan of the participants; the type of albinism reported; prevalence as reported by the authors; frequency as reported by the authors; any reported cultural incidences of note, such as occupation, age distribution, stigma, and treatment of participants within their communities; and any additional comments. This project followed all the ethical standards for research without direct contact with human or animal subjects.
Results
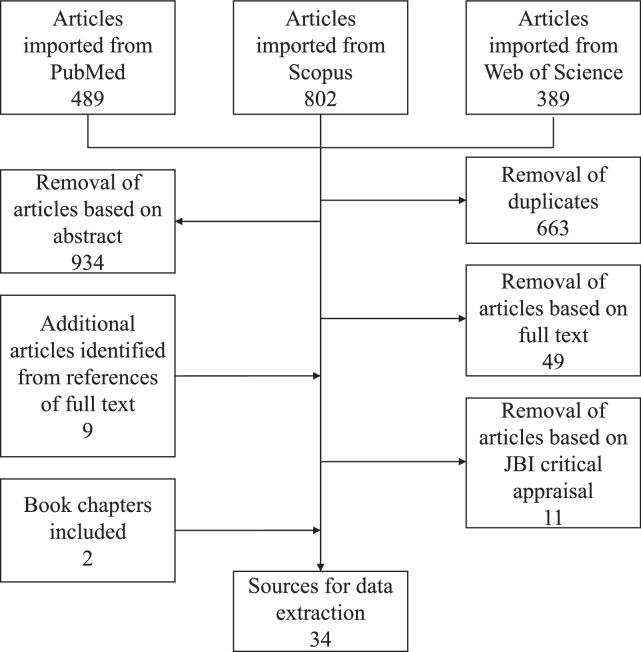
Altogether, 1680 articles were identified during the literature search of the three chosen databases. The article selection steps are illustrated in the Figure. Filtering included the removal of duplicates; removal based on abstracts (i.e., the article abstract indicated the absence of prevalence data); removal based on full text reads (i.e., the article did not contain relevant prevalence data upon reading the full text of the article); and removal based on poor article quality as identified by the JBI critical appraisal checklist.
Figure.
Flow chart illustrating the procedure of source selection.
After selection, 32 articles remained, and these were screened for the required data on prevalence. In addition to these 32 articles, 2 book chapters containing relevant and original prevalence data were identified, and these were included for downstream data extraction. Ultimately, the total number of included sources was 34. Information extracted from the 34 sources provided data across 6 continents with some better represented than others (Table). The sources, dated between 1913 and 2018, were mostly outdated and only 6 of the 34 (18%) were published after 2010.
Table.
Albinism Prevalence Data Categorized by Continent
| Location | Population Description | Prevalence | Reference (Authors, Year) |
|---|---|---|---|
| Australia: Oceania | |||
| Polynesia | Polynesians in Tuvalu | 1 in 669 | Johanson et al. 201024 |
| Africa | |||
| Botswana | Tswana village (isolate) | 1 in 1307 | Kromberg 20183 |
| Cameroon | Bamileke group | 1 in 11,900 | Aquaron 198025 |
| Bamileke group | 1 in 7900 | Aquaron 199026,* | |
| Cameroon | 1 in 28,000 | Aquaron 199026 | |
| Egypt | Pediatric Hospital patients | 1 in 5843 | Mohamed et al. 201027 |
| Namibia | National Census | 1 in 1755 | Lund & Roberts 201821,* |
| Nigeria | Lagos city | 1 in 2858 | Barnicot 195228 |
| Benin city | 1 in 7000 | Barnicot 195228 | |
| East Central State | 1 in 15,000 | Okoro 197529 | |
| Ibo (population isolate) | 1 in 1100 | Okoro 197529 | |
| South Africa | Eastern Cape province | 1 in 3759 | Oettle 196330 |
| Eastern Cape province | 1 in 3000 | Rose 197431 | |
| Soweto, Johannesburg | 1 in 3900 | Kromberg & Jenkins 198212 | |
| Vhavhenda population | 1 in 2239 | Lund et al. 200732 | |
| Swaziland | Hhohho district | 1 in 1900 | Kromberg 20183 |
| Tanzania | Hospital survey | 1 in 1400 | Luande 198533 |
| National Census | 1 in 2673 | Lund & Roberts 201821,* | |
| Zimbabwe | School children in Harare | 1 in 2883 | Kagore & Lund 199534 |
| School children | 1 in 4728 | Lund 199613,* | |
| Tonga (population isolate) | 1 in 1000 | Lund et al. 199723 | |
| Asia | |||
| Borneo | Iban population | 1 in 5−10,000 | Abrahams 197235 |
| Japan | Old estimate | 1 in 47,000 | Neel et al. 194936 |
| Europe | |||
| Denmark | National register | 1 in 14,000 | Gronskov et al. 200915,* |
| France | Old estimate (1910) | 1 in 10−20,000 | Pearson et al. 19132 |
| Germany | Old estimate (1910) | 1 in 20,000 | Pearson et al. 19132 |
| Italy | Old estimate | 1 in 23−26,000 | Pearson et al. 19132 |
| The Netherlands | General population | 1 in 15,000 | Van Dorp 198737,* |
| Medical Centre patients | 1 in 12,000 | Kruijt et al. 201816 | |
| Northern Ireland | Whole population | 1 in 10,000 | Froggatt 196038,* |
| Norway | Old estimate (1898) | 1 in 25,000 | Pearson et al. 19132 |
| Russia | Old estimate (1909) | 1 in 100,000 | Pearson et al. 19132 |
| Scotland | Old estimate | 1 in 15−25,000 | Pearson et al. 19132 |
| Spain | Spanish gypsies/Gutanos, | 1 in 1200 | Gamella & Nunez- Negrillo 201339 |
| SE Spain (isolate) | |||
| Spanish gypsies (isolate) | 1 in 3300 | Gamella & Nunez-Negrillo 201339 | |
| Non-gypsy population | 1 in 50,000 | Martinez-Frias & Bermejo 199240 | |
| North America | |||
| Canada | British Columbia | 1 in 20,600 | McLeod & Lowry 197541 |
| United States of America | |||
| Arizona | Hopi Indians (isolate) | 1 in 227 | Woolf 196542 |
| Hopi Indians (isolate) | 1 in 231 | Woolf & Dukepoo 196943 | |
| Navajo | 1 in 3750 | Woolf 196542 | |
| 1 in 1500−2000 | Yi et al. 200344 | ||
| New Mexico | Jemez (isolate) | 1 in 140 | Woolf 196542 |
| Laguna (isolate) | 1 in 2000 | Woolf 196542 | |
| San Juan (isolate) | 1 in 500 | Woolf 196542 | |
| Zuni (isolate) | 1 in 247 | Woolf 196542 | |
| North Carolina | State population | 1 in 17,000 | Witkop et al. 197017 |
| Central America | |||
| Panama | San Blas, Cuna (isolate) | 1 in 210 | Stout 194645 |
| San Blas, Cuna (isolate) | 1 in 167 | Keeler 196446 | |
| South America | |||
| Brazil | Caingang Indians (isolate) | 1 in 27 | Salzano 196147 |
| North Brazilian Island (isolate) | 1 in 22 | Freire-Maia et al. 197848 | |
African and European studies with baseline population samples >1,000,000.
Altogether 15 of the 34 sources (44%), including the 2 book chapters,3,21 were from the African continent. These 15 sources provided prevalence statistics across 9 African countries (6 were in southern Africa). The second highest output was from Europe with seven articles reporting rates across 10 countries, followed by North America with 5 articles, South and Central America with 4 articles, Asia with 2 articles, and Australia-Oceania with only 1 article. Lack of any original published prevalence data from India and China was noted.
Population isolates accounted for a large proportion (15/34 [44%]) of the extracted sources. These isolates were defined by the authors as populations that were geographically and culturally isolated from neighboring populations, within or between countries, and were therefore highly unlikely to interact and/or reproduce with individuals from neighboring areas. Data from such isolates showed that they had higher rates of albinism than any of the countries reporting prevalence rates. Where both a large general population sample and a population isolate was studied in the same country, exemplified by two Zimbabwe studies,13,23 the isolate showed a significantly higher prevalence than the general population, as expected.
In the four African countries with studies that had adequate methodology and large population samples (>1,000,000), the mean prevalence rate was 1 in 4264, with a range of 1 in 1755 to 1 in 7900. In the three European countries with studies with adequate methodology and similar large samples (>1,000,000), the mean rate was 1 in 13,000 (range, 1 in 10,000 to 1 in 15,000).
The Table shows that prevalence data are only available for a few countries (26/193 [13%]) and many areas around the world have no published prevalence data at all (with the limitation that filtering removed non-English articles). The findings also show that the reported prevalence rates of OCA vary widely in different populations, for example, 1 in 14,000 in Denmark,15 versus 1 in 1755 in Namibia,21 and even in the same population over time, for example, over 31 years in the Netherlands.16,37
Discussion
Countries With Data
The data obtained in this review from the different countries were based on research of varying quality, reliability, and validity. In some cases, information was obtained from general observations in hospital clinics,27 whereas in others, data were based on clinical research and counting of health center cases with an educated guess as to the value of the denominator,33 and only a few were based on sound research principles and large community-based samples.12,21 Nevertheless, what can be deduced is that albinism (usually OCA2)49 has a higher reported prevalence rate in individuals of African ancestry than in those of European origin. Further, albinism is more common in isolates than in large population groups, as might be expected for a recessive condition in small and inbred populations.
There is some debate that the low rates reported in European countries might be inaccurate50,51 because the phenotype is not as easily distinguishable in the general population and therefore not as well-recognized. Affected children might be diagnosed as having ocular albinism (OA) or low vision rather than OCA. For example, the findings in two Irish studies, completed 50 years apart, when compared, show the prevalence in Northern Ireland to be higher (1 in 10,000 reported in 1960 vs. 1 in 6600 reported in 2014) once the children with albinism in eye clinics and schools for the partially sighted are diagnosed and considered.38,50
The outdated statistics presented in 1913, in a monograph by Pearson et al.,2 for countries such as Russia and Norway, are educated guesses at best. They are based on estimates made by doctors working in various countries who were contacted by Pearson and his colleagues and asked to estimate the prevalence of albinism. These estimates are presented here because no research has been undertaken, and published, to produce better prevalence figures in those countries.
The highest rates of albinism in the world are found in population isolates. Two Brazilian isolates showed particularly high rates (1 in 22 on a small coastal island,48 and 1 in 27 among the Caingang Indians living in reservations in the South-East of Brazil),47 followed by the isolates in New Mexico (1 in 140)42 and Arizona (1 in 227 in the Hopi Indians).42 These isolated populations exist within geographical barriers and have been living and reproducing (with endogamous mating patterns) within these confines for several generations. For example, the 5000 Hopi Indians in Arizona live on the flat tops of three mesas, where they settled several centuries ago. There are data from two isolated populations in Africa: one group live in a secluded Zambezi River valley in Northern Zimbabwe (Tonga population of 11,000 people, rate 1 in 1000)23 and the other in a large remote village in Botswana (Tswana population of 18,000, rate 1 in 1300).3 The population size of these African isolates is larger than those found in the Americas, and consequently the OCA rates are lower.
There is more prevalence information from countries in Africa than from countries on other continents. This is partly because albinism is a more obvious disorder in darker skinned populations, than in lighter skinned peoples living elsewhere in the world. Second, albinism is surrounded by myths and superstitions in Africa, which made it of interest, not only to the early explorers, medical doctors, geneticists, scientists, and epidemiologists, but also to social scientists such as psychologists and anthropologists. Third, a large-scale, long-term, wide-ranging research project on albinism was carried out over four decades in southern Africa, resulting in many novel findings and publications on the condition.52
The data presented in most studies in this review were collected from urban based samples. However, rates derived from urban and rural studies often differ. For example, the Namibia census data showed a rural rate of 1 in 1459, whereas the urban rate was 1 in 2409.21 Factors such as cultural habits, mating patterns, migration, founder effect, and gene frequencies, as well as community attitudes, can affect prevalence rates, so that country-wide data cannot be extrapolated from data collected at a specific local site.
Changes in Prevalence Over Time
Research suggests that reported prevalence figures can change over time. Two studies from the Netherlands, undertaken 31 years apart, support this finding.16,37 Similarly, Grønskov et al.15,53 suggest that there has been an increased prevalence of albinism (their samples include both OCA and some OA cases) in Denmark over time. It is suggested that changes in prevalence rates are actually due to better and more stringent diagnostic criteria, improved case finding and medical care, and (most likely) better methods of selecting cases (Kruijt, personal communication, 2022), determining prevalence and data collection. Undertaking molecular studies on suspected cases, that might have been misdiagnosed or missed on clinical examination, shows that more individuals are affected with albinism than initially thought.51
Determining a Worldwide Prevalence Rate
It is seemingly not possible to determine a worldwide prevalence rate that could be applied in those countries where rates are not available. Rates seem to be markedly different between populations of African versus non-African descent. Further, local psychosocial and cultural factors differ and can affect prevalence rates. Such factors include mating patterns, negative attitudes to albinism (leading to infanticide, rejection, stigmatization, and neglect),54 positive attitudes (leading to preferential treatment; e.g., not being obliged to work),42 and geographical factors, such as numbers of sunny days, limited cloud cover and high UV risks. For example, in Israel, the OCA prevalence rate of 1 in 7000 (higher than in many other European populations) has been associated with local cultural practices that favor consanguineous marriages (Blumenfeld, personal communication, 2022). Furthermore, the prevalence could be affected by local health management infrastructure and the availability of services. Where these services are adequate and efficient, as in first-world countries, the complications of the condition can be managed effectively, and it becomes less disabling.
Continental prevalence differences are apparent in the findings from this systematic review. Africa has the highest prevalence, with an average of approximately 1 in 4000 for OCA in the scattered countries in which studies have been performed. From the few available European studies, it can be estimated that the rate is about three times lower, averaging at approximately 1 in 13,000. It has been proposed that this rate is an underestimate and molecular studies are supporting this suggestion.51 Further, OA may be diagnosed in some European children rather than OCA, unless molecular studies are performed to confirm the actual diagnosis.
The data from the Far East are limited, but the estimates from China and Japan36,55 suggest that the rates there are lower than those in Europe. In the abstract (in English) of Gong et al.55 (the paper was excluded from the present review owing to language of publication), the authors suggested that the prevalence rate in the Han population in the Shandong province of China was 1 in 18,000. Neel et al.36 studied consanguineous mating in Japan and from the data, on “induced recessive mutations” in their sample, they estimated the rate of albinism in that population to be 1 in 47,000.
Findings Highlight Limitations of the Data
The main limitation on the data used in this review is the lack of good, reliable, published studies on the prevalence of albinism in various countries around the world. Most of the articles identified for attention were outdated (28/34 or 82%, being published before 2010) and very few recent studies have been undertaken. Prevalence data that are ≥10 years old may no longer be valid (prevalence rates were found to be higher by approximately 25% over 31 years in the Netherlands).16 Also, case finding in some European studies included both individuals with OCA and OA (no cases of OA have been reported in African populations, as far as the authors are aware), so that rates in different countries may not be strictly comparable.
A further limitation is associated with the fact that only English language publications were searched. Reports in other languages may have been missed (e.g., Gong et al.55 in Chinese), as may those published as a letter to a journal editor (e.g., Healey et al.50), those not indexed in PubMed, Scopus, or Web of Science, and those where the key words used in the present study were not represented (e.g., Rajab et al.56).
Implications of Findings
The findings from this review draw attention to the fact that individuals with OCA occur in all populations. However, if countries in Africa, with no prevalence data, wish to use an estimated prevalence figure they should use the range of 1 in 4000 to 7000, rather than the previously used rate of 1 in 17,000.17 Also, in European countries for which there are no data, using a range of 1 in 12,000 to 15,000 would be more appropriate than using the prevalence rate of 1 in 17,000.
The data sourced for this project were mostly published many years ago. New and better research studies on prevalence rates, with stringent methodology, improved diagnostic criteria, and large samples, are essential if the epidemiology of OCA in countries around the world is to be better understood.
Further, health care professionals worldwide need to acknowledge that OCA occurs and that individuals with OCA have special needs that must be met. Offering specific and appropriate health services, such as skin and eye care, as well as genetic counseling, would improve the quality of life for people with OCA, allowing them to manage their condition and reach their potential. Further, community education on prevalence rates is required to increase awareness and acceptance of the condition. Providing good diagnostic services, early screening and treatment, and regular monitoring for visual and skin problems, would decrease the burden on health departments of costly ophthalmology and, particularly, oncology treatment, which could otherwise be required later. Such services, particularly in developing countries, would also prevent persons with OCA becoming disabled (and unemployable) and a burden on their families and communities.
Conclusions
OCA needs to be recognized, wherever it occurs, and not left untreated. This is especially so in Africa with its high prevalence rates, high risks of skin cancer, and significant human rights violations in certain countries. Skin damage and compromised vision have significant health, social, educational, and financial consequences for the person with OCA, as well as for the family and community. As discussed in the United Nations by Ero in 2019,57 it is important, in Africa, that updated prevalence data be collected and used to justify the need for appropriate health services and community education around the condition.57
In this systematic review, the prevalence of OCA worldwide has been investigated. The comprehensive literature search shows that very few reliable and valid studies have been carried out, rates of albinism in many populations are only estimates, or not available at all, and further research is necessary. The findings reinforce the previously suspected fact that albinism is more prevalent in Africa than elsewhere, and health departments need to be aware of prevalence rates and the need to provide for appropriate health services, all over the continent. Further, albinism is probably underdiagnosed in European communities and more accurate diagnostics are required if people with OCA are to be identified and benefit from the appropriate health and educational services. Appreciating that prevalence figures can be used to leverage health policy, create public awareness, and generally stimulate dialogue, the value of generating good and current data cannot be overemphasized.
Acknowledgments
The assistance of the National Health Laboratory Service (NHLS) and the University of the Witwatersrand in providing the first author with an Honorary Associate Professorship and an office in the Division of Human Genetics, and the meticulous work of research assistant, Dylan J. McCarthy, are acknowledged.
Funding Sources: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Disclosure: J.G.R. Kromberg, None; K.A. Flynn, None; R.A. Kerr, None
References
- 1. Summers CG. Albinism: classification, clinical characteristics, and recent findings. Optom Vis Sci. 2009; 86(6): 659–662. [DOI] [PubMed] [Google Scholar]
- 2. Pearson K, Nettleship E, Usher CH.. A monograph on albinism in man. In: Research Memoirs Biometric Series. Vol VIII. London: Drapers Co; 1913. [Google Scholar]
- 3. Kromberg J. Epidemiology of albinism. In: Kromberg J, Manga P, eds. Albinism in Africa: historical, geographical, medical, genetic, and psychosocial aspects. San Diego: Elsevier, Academic Press; 2018: 57–79. [Google Scholar]
- 4. Hong ES, Zeeb H, Repacholi MH.. Albinism in Africa as a public health issue. BMC Public Health. 2006; 6(1): 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aamir A, Kuht HJ, Grønskov K, Brooks BP, Thomas MG.. Clinical utility gene card for oculocutaneous (OCA) and ocular albinism (OA)—an update. Eur J Hum Genet. 2021; 29(10): 1577–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williams SEI. Albinism and the eye. In: Kromberg J, Manga P, eds. Albinism in Africa: historical, geographical, medical, genetic, and psychosocial aspects. San Diego: Elsevier Academic Press; 2018: 135–149. [Google Scholar]
- 7. Hartshorne S, Manga P.. Dermatological aspects of albinism. In: Kromberg J, Manga P, eds. Albinism in Africa: historical, geographical, medical, genetic, and psychosocial aspects. San Diego: Elsevier Academic Press; 2018: 121–134. [Google Scholar]
- 8. Kammer R. Visual rehabilitation and albinism. In: Kromberg J, Manga P, eds. Albinism in Africa: historical, geographical, medical, genetic and psychosocial aspects. San Diego: Elsevier, Academic Press; 2018: 151–170. [Google Scholar]
- 9. Manganyi NC, Kromberg JGR, Jenkins T.. Studies on albinism in the South African Negro I. Intellectual maturity and body image differentiation. J Biosoc Sci. 1974; 6(1): 107–112. [DOI] [PubMed] [Google Scholar]
- 10. Kromberg JGR, Castle D, Zwane EM, Jenkins T.. Albinism and skin cancer in Southern Africa. Clin Genet. 1989; 36(1): 43–52. [DOI] [PubMed] [Google Scholar]
- 11. Kajiru I, Mubangizi JC.. Human rights violations of persons with albinism in Tanzania: the case of children in temporary holding shelters. African Human Rights Law Journal;. 2019; 19(1): 246–266. [Google Scholar]
- 12. Kromberg JG, Jenkins T.. Prevalence of albinism in the South African negro. S Afr Med J. 1982; 61(11): 383–386. [PubMed] [Google Scholar]
- 13. Lund PM. Distribution of oculocutaneous albinism in Zimbabwe. J Med Genet. 1996; 33(8): 641–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Badens C, Courrier S, Aquaron R.. A novel mutation (delAACT) in the tyrosinase gene in a Cameroonian black with type 1A oculocutaneous albinism. J Dermatol Sci. 2006; 42(2): 121–124. [DOI] [PubMed] [Google Scholar]
- 15. Grønskov K, Ek J, Sand A, et al.. Birth prevalence and mutation spectrum in Danish patients with autosomal recessive albinism. Invest Ophthalmol Vis Sci. 2009; 50(3): 1058. [DOI] [PubMed] [Google Scholar]
- 16. Kruijt CC, de Wit GC, Bergen AA, Florijn RJ, Schalij-Delfos NE, van Genderen MM.. The phenotypic spectrum of albinism. Ophthalmology. 2018; 125(12): 1953–1960. [DOI] [PubMed] [Google Scholar]
- 17. Witkop CJ, Nance WE, Rawls RF, White JG.. Autosomal recessive oculocutaneous albinism in man. Evidence for genetic heterogeneity. Am J Hum Genet. 1970; 22(1): 55–74. [PMC free article] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, Altman DG.. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009; 6(7): e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kamdar BB, Shah PA, Sakamuri S, Kamdar BS, Oh J.. A novel search builder to expedite search strategies for systematic reviews. Int J Technol Assess Health Care. 2015; 31(1-2): 51–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A.. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016; 5(1): 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lund PM, Roberts M.. Prevalence and population genetics of albinism. In: Kromberg J, Manga P, eds. Albinism in Africa: historical, geographical, medical, genetic, and psychosocial aspects. San Diego: Elsevier, Academic Press; 2018: 81–98. [Google Scholar]
- 22. Moola S, Munn Z, Tufanaru C, et al.. Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, eds. JBI manual for evidence synthesis. Nashville, TN: JBI; 2020. [Google Scholar]
- 23. Lund PM, Puri N, Durham-Pierre D, King RA, Brilliant MH.. Oculocutaneous albinism in an isolated Tonga community in Zimbabwe. J Med Genet. 1997; 34(9): 733–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johanson HC, Chen W, Wicking C, Sturm RA.. Inheritance of a novel mutated allele of the OCA2 gene associated with high incidence of oculocutaneous albinism in a Polynesian community. J Hum Genet. 2010; 55(2): 103–111. [DOI] [PubMed] [Google Scholar]
- 25. Aquaron R. L'albinisme oculo-cutane au Cameroun, A propos de 216 observations. Rev Epidemiol Sante Publique. 1980; 28(1): 81–88. [PubMed] [Google Scholar]
- 26. Aquaron R. Oculocutaneous albinism in Cameroon. A 15-year follow-up study. Ophthalmic Paediatr Genet. 1990; 11(4): 255–263. [DOI] [PubMed] [Google Scholar]
- 27. Mohamed AF, El-Sayed NS, Seifeldin NS.. Clinico-epidemiologic features of oculocutaneous albinism in northeast section of Cairo – Egypt. EJMHG. 2010; 11(2): 167–172. [Google Scholar]
- 28. Barnicot CA. Albinism in South-Western Nigeria. Ann Eugen. 1953; 18(1): 38–73. [DOI] [PubMed] [Google Scholar]
- 29. Okoro AN. Albinism in Nigeria. A clinical and social study. Br J Dermatol. 1975; 92(5): 485–492. [PubMed] [Google Scholar]
- 30. Oettle AG. Skin cancer in Africa. National Cancer Institute Monograph. 1963; 10: 197–214. [Google Scholar]
- 31. Rose EF. Pigment anomalies encountered in the Transkei. S Afr Med J. 1974; 48: 2345–2347. [PubMed] [Google Scholar]
- 32. Lund PM, Maluleke TG, Gaigher I, Gaigher MJ.. Oculocutaneous albinism in a rural community of South Africa: a population genetic study. Ann Hum Biol. 2007; 34(4): 493–497. [DOI] [PubMed] [Google Scholar]
- 33. Luande J, Henschke CI, Mohammed N.. The Tanzanian human albino skin. Natural history. Cancer. 1985; 55(8): 1823–1828. [DOI] [PubMed] [Google Scholar]
- 34. Kagore F, Lund PM.. Oculocutaneous albinism among schoolchildren in Harare, Zimbabwe. J Med Genet. 1995; 32(11): 859–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abrahams PH. Albinos in Borneo. Lancet. 1972; 1: 101–102. [Google Scholar]
- 36. Neel J V, Kodani M, Brewer R, Anderson RC.. The incidence of consanguineous matings in Japan, with remarks on the estimation of comparative gene frequencies and the expected rate of appearance of induced recessive mutations. Am J Hum Genet. 1949; 1(2): 156–178. [PMC free article] [PubMed] [Google Scholar]
- 37. Van Dorp DB. Albinism, or the NOACH syndrome. Clin Genet. 1987; 31(4): 228–242. [DOI] [PubMed] [Google Scholar]
- 38. Froggatt P. Albinism in Northern Ireland. Ann Hum Genet. 1960; 24(3): 213–238. [DOI] [PubMed] [Google Scholar]
- 39. Gamella JF, Carrasco-Muñoz EM, Núñez Negrillo AM. Oculocutaneous albinism and consanguineous marriage among Spanish Gitanos or Calé–a study of 83 cases. Coll Antropol. 2013; 37(3): 723–734. [PubMed] [Google Scholar]
- 40. Martínez-Frías ML, Bermejo E.. Prevalence of congenital anomaly syndromes in a Spanish gypsy population. J Med Genet. 1992; 29(7): 483–486. [PMC free article] [PubMed] [Google Scholar]
- 41. McLeod R, Lowry RB.. Incidence of albinism in British Columbia (B.C.). Separation by hairbulb test. Clin Genet. 1976; 9(1): 77–80. [DOI] [PubMed] [Google Scholar]
- 42. Woolf CM. Albinism among Indians in Arizona and New Mexico . Am J Hum Genet. 1965; 17(1): 23–35. [PMC free article] [PubMed] [Google Scholar]
- 43. Woolf CM, Dukepoo FC.. Hopi Indians, inbreeding, and albinism. Science (1979). 1969; 164(3875): 30–37. [DOI] [PubMed] [Google Scholar]
- 44. Yi Z, Garrison N, Cohen-Barak O, et al.. A 122.5-kilobase deletion of the P gene underlies the high prevalence of oculocutaneous albinism type 2 in the Navajo population. Am J Hum Genet. 2003; 72(1): 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stout DB. Further notes on albinism among the San Blas Cuna, Panama. Am J Phys Anthropol. 1946; 4(4): 483–490. [DOI] [PubMed] [Google Scholar]
- 46. Keeler C. The incidence of the Cuna Moon-Child Albinos. J. Hered. 1964; 55(3): 115–120. [DOI] [PubMed] [Google Scholar]
- 47. Salzano FM. Rare genetic conditions among the Gaingang Indians. Ann Hum Genet. 1961; 25(2): 123–130. [DOI] [PubMed] [Google Scholar]
- 48. Freire-Maia N, de Andrade FL, de Athayde-Neto A, et al.. Genetic investigations in a Northern Brazilian island. II. Random drift. Hum Hered. 1978; 28(6): 401–410. [DOI] [PubMed] [Google Scholar]
- 49. Stevens G, van Beukering J, Jenkins T, Ramsay M.. An intragenic deletion of the P gene is the common mutation causing tyrosinase-positive oculocutaneous albinism in southern African Negroids. Am J Hum Genet. 1995; 56(3): 586–591. [PMC free article] [PubMed] [Google Scholar]
- 50. Healey N, McLoone E, Saunders KJ, Jackson AJ, McClelland JF.. Are worldwide albinism prevalence figures an accurate reflection? An incidental finding from a Northern Ireland study. BJO. 2014; 98(7): 990.1–990. [DOI] [PubMed] [Google Scholar]
- 51. Arveiler B, Michaud V, Lasseaux E.. Albinism: an underdiagnosed condition. J Invest Dermatol. 2020; 140(7): 1449–1451. [DOI] [PubMed] [Google Scholar]
- 52. Kromberg J, Manga P, (eds). Albinism in Africa. historical, geographical, medical, genetic, and psychosocial aspects. San Diego: Elsevier Academic Press; 2018. [Google Scholar]
- 53. Grønskov K, Ek J, Brondum-Nielsen K. Oculocutaneous albinism. Orphanet J Rare Dis. 2007; 2(1): 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kromberg JGR, Kerr R.. Oculocutaneous albinism in southern Africa: Historical background, genetic, clinical and psychosocial issues. Afr J Disabil. 2022; 11: 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gong Y, Shao C, Zheng H, Chen B, Guo Y.. Study on genetic epidemiology of albinism. Yi Chuan Xue Bao. 1994; 21(3): 169–172. [PubMed] [Google Scholar]
- 56. Rajab A, Al Rashdi I, Al Salmi Q. Genetic services and testing in the Sultanate of Oman. Sultanate of Oman steps into modern genetics. J Community Genet. 2013; 4(3): 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ero IK. Report of the Independent Expert on the Enjoyment of Human Rights by Persons with Albinism. Geneva: United Nations; 2019. [Google Scholar]