Abstract
Background
Rare diseases (RDs) affect approximately 8% of all people or > 400 million people globally. The Australian Government’s National Strategic Action Plan for Rare Diseases has identified the need for a national, coordinated, and systematic approach to the collection and use of RD data, including registries. Rare disease registries (RDRs) are established for epidemiological, quality improvement and research purposes, and they are critical infrastructure for clinical trials. The aim of this scoping review was to review literature on the current state of RDRs in Australia; to describe how they are funded; what data they collect; and their impact on patient outcomes.
Methods
We conducted a literature search on MEDLINE, EMBASE, CINAHL and PsychINFO databases, in addition to Google Scholar and grey literature. Dissertations, government reports, randomised control trials, conference proceedings, conference posters and meeting abstracts were also included. Articles were excluded if they did not discuss RDs or if they were written in a language other than English. Studies were assessed on demographic and clinical patient characteristics, procedure or treatment type and health-related quality of life captured by RDRs or databases that have been established to date.
Results
Seventy-four RDRs were identified; 19 were global registries in which Australians participated, 24 were Australian-only registries, 10 were Australia and New Zealand based, and five were Australian jurisdiction-based registries. Sixteen “umbrella” registries collected data on several different conditions, which included some RDs, and thirteen RDRs stored rare cancer-specific information. Most RDRs and databases captured similar types of information related to patient characteristics, comorbidities and other clinical features, procedure or treatment type and health-related quality of life measures. We found considerable heterogeneity among existing RDRs in Australia, especially with regards to data collection, scope and quality of registries, suggesting a national coordinated approach to RDRs is required.
Conclusion
This scoping review highlights the current state of Australian RDRs, identifying several important gaps and opportunities for improvement through national coordination and increased investment.
Keywords: Rare disease, Minimum data set, Registry, Patient outcomes, National approach
Background
Rare diseases (RDs), by definition, affect fewer than five in 10,000 people [1, 2]. Estimates of the total number of RDs vary between countries and studies, due to differing definitions and challenges with data collection. However, it is cited that there are more than 7000 different RDs [3]. Approximately 80% of RDs are of genetic origin. As genomic technology evolves, new RDs are discovered regularly [1]. Although individual RDs are rare, the total number of Australians living with a RD is sizeable. Approximately 8% of Australians live with a RD, equating to around two million people [4].
RDs often manifest in childhood and become chronic; some are life threatening and others lead to significant disability [5, 6]. Diagnosis of RDs is often delayed, due to limited knowledge, lack of exposure to and awareness of healthcare professionals to RDs and uncertainty about referral pathways. Inherent features of RDs, including heterogeneity, complexity and low patient numbers result in a lack of data, evidence and knowledge [1], making data collection and registries critical for RDs.
Rare disease registries (RDR) are established to collect RD data and in some cases, monitor clinical outcomes [1, 7]. Registries, if populated with high-quality clinical data over extended periods of time, can assist with health service planning, epidemiological research, clinical trial recruitment and post-marketing drug surveillance [1, 8]. RDRs can play a vital role in understanding disease trajectories and help developing clinical trials for RD that meet safety and efficacy criteria despite low patient numbers; with many recruiting fewer than 100 patients worldwide [9].
The critical role of RDRs is globally recognised by the RD community and policy makers [1, 6, 10]. International experts jointly identified ten key principles of RDRs, including: the need for RDRs to be a global priority; the importance of scope and focus; interoperability and harmonisation; consistency through minimum core data elements; links with biobank data; inclusion of patient reported data; sustainability; and governance and building knowledge [11].
Nonetheless, in Australia, data for most RDs are not being captured through routine health information systems or registries, and there is no coordinated strategy to collect, measure, build and translate already existing information on RDs [1].
On behalf of the Australian Government, Rare Voices Australia (RVA), the national peak body for Australians living with a RD [12], led the collaborative development of the National Strategic Action Plan for Rare Diseases (the Action Plan) [13], which was informed by extensive multi-stakeholder consultation. The Action Plan was launched in February 2020 with bipartisan support and RVA is now leading its collaborative implementation. The Action Plan called for the development of a national, person-centred approach to RDRs to support national standards, best practice and minimum data sets. It further highlighted the need for investment into RDRs, which aligns with worldwide agreement on the value and importance of RDRs [13].
Under Pillar 3, Research and Data, of the Action Plan, Priority 3.1 calls on the sector to ‘Enable coordinated and collaborative data collection to facilitate the monitoring and cumulative knowledge of rare diseases, informing care management, research and health system planning’ [13]. The first step to this end is outlined in Implementation step 3.1.4.1, ‘Develop a summary report of all existing Australian and relevant international rare disease registries’ [13], which provided the impetus for this scoping review.
This scoping review investigates RDRs collecting Australian data in the literature, including an audit of what RDRs and databases exist in Australia, how they are funded, what data they collect and their impact on patient outcomes. It is the first step to understanding existing datasets and informing national coordination of RDRs that aligns with international best practice for RD data collection, including establishment of minimum data sets. A national approach to RDR’s in Australia would enable improved monitoring and the accumulation of knowledge about RDs to inform clinical practice, research, government investment in RD and health service planning [14]. Data collected should also inform planning and investment decisions for other government services and departments playing an essential role in the holistic care of patients, carers and families living with rare disease including, the National Disability Insurance Agency, Department of Communities and Housing, Department of Education, and other financial support services.
Methods
Protocol
A protocol for this scoping review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews (PRISMA-ScR) format [15].
Information sources
To identify relevant studies, we searched four databases: MEDLINE, EMBASE, CINAHL and PsychINFO, and the Google Scholar from inception through November 2022. Grey literature was also included. For each article selected for inclusion, abstracts and full articles were obtained. Reference lists of the included studies and systematic reviews were examined during the review. To capture as many relevant studies as possible, we did not establish a time frame for when articles were published. Dissertations, government reports, randomised control trials, conference proceedings, conference posters and meeting abstracts were included.
Search strategy
The search strategy was developed by three researchers (RR, MC and CM). We used Medical Subject Heading (MeSH) keywords and free text search terms. The most up to date version of Chrome Web browser was used in our searches. The database records and details of how the literature search was undertaken were maintained at each stage of the review process. Also, a manual search using Google and Google Scholar was performed. The key search terms were ([“rare” or “disease” or “rare diseases” or rare adj disease* or condition* or disorder* or illness* or infection* or diagnosis*] and [“genetic” or “genetic, population” or “genetics, medical” or “genetics, microbial” or “human genetics”] and [“registry” or “registries” or “database” or “dataset” or “library” or “record” or “archive”] and [“Australia” or “Victoria” or “New South Wales” or “South Australia” or “Western Australia” or “Tasmania” or “Queensland” or “Northern Territory” or “Melbourne” or “Sydney” or “Adelaide” or “Canberra” or “Hobart” or “Brisbane” or “Darwin” or “Perth”]). We adapted the search strategy to the search requirements of the remaining databases mentioned above. The terms were combined by means of Boolean operators.
Eligibility criteria
Quantitative and qualitative studies describing existing RDs and RDRs were included. International studies without Australian data, were excluded from this review, as they do not provide information on Australia’s RDR landscape. Furthermore, studies of diseases that are not rare were also excluded. Articles were excluded if they did not discuss a RD or if they were written in a language other than English.
Study selection
The study selection process was made up of two phases. In phase one, two researchers conducted the initial search of the literature (RR and CM). The second phase consisted of screening the literature, where three researchers (RR, MC and CM) independently screened the titles and abstracts of all articles identified through the search strategy to determine eligibility and classify studies as relevant, possibly relevant and irrelevant. Results were discussed by all researchers to resolve any inconsistencies in study selection and a final list of relevant studies were made.
Data management and analysis
The results from the database searches were extracted into EndNote™ X9 software, a management software for references that allows the identified references to be organized into different electronic databases. The results were combined into a single EndNote folder. Duplicated studies were identified and removed.
Data were extracted from relevant articles and internet material using a standardised data extraction form in Microsoft Excel. The main data points extracted include: objectives of the registry, details of registry management, population size, data captured by the registry, demographic or clinical or patient-reported outcome measures (PROMs) and quality of life (QoL) data, funding information, ethics and consent models of the registry.
Results
General description of the literature
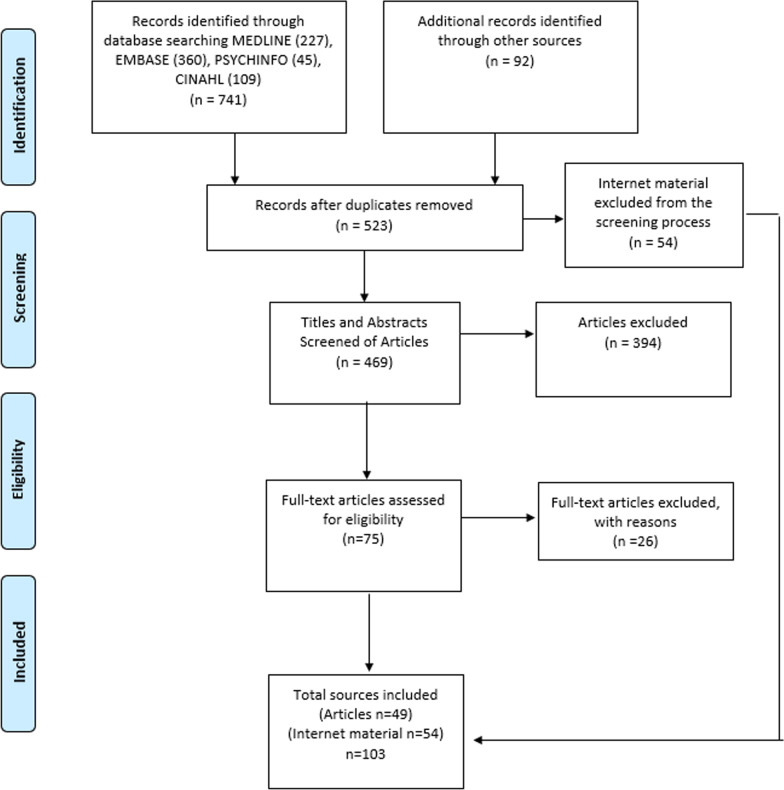
The search of MEDLINE, EMBASE, CINAHL and PsycINFO databases yielded in 741 documents (Fig. 1). A further 92 documents of grey literature were identified. After removing duplicates, 523 documents were screened for titles and abstracts. Of those, full copies of 75 relevant articles were retrieved. Fifty-four online publications (e.g. webpages and reports) of Australian RDRs and online databases were included. The screening of full-texts resulted in 49 publications. A total of 103 publications and online data sources were used in the data extraction and data analysis process.
Fig. 1.
Study flow. Details the flow of information through the different phases of the review; maps out the number of records identified, included and excluded
Publications included in the final review were published between 2002 and 2021. Five (10.2%) articles were published in 2017 and six (12.2%) were published in 2019, 2020 and 2021. The remaining articles were published between the 2002 and 2016.
Of the 49 publications used in the scoping review, 20 (40.8%) were published in Australia, seven (14.3%) were published in the United States (US), six (12.2%) in the Netherlands, two (4.1%) in the United Kingdom (UK) and one each (2.04%) was published in Japan and Canada.
Rare disease registries
Seventy-four different RDRs and databases were described in these studies (Table 1). Nineteen (25.7%) of those were global registries that included Australian data, 24 (32.4%) were Australian national registries and covered all jurisdictions, 10 (13.5%) were Australian and New Zealand (ANZ) and five (6.8%) were Australian state and jurisdiction-based registries. Sixteen (21.6%) “umbrella” registries, which represent numerous registries that have agreed to work under a unified registry, collected data from several RDs or conditions, which included some RDs. Thirteen (17.6%) registries stored data that were specific to rare forms of cancer.
Table 1.
General description and characteristics of rare disease registries, databases and organisations identified in the scoping review
| Name | Size | Year established | Type | Patient/clinician; single/multicentre | Data entry method | Reporting | Funding source | Consent model |
|---|---|---|---|---|---|---|---|---|
| International Collaborative Gaucher Group Gaucher Registry | 12,000 participants | 1994 | Global |
Clinician; Multicentre |
Web-based data entry | NS | Genzyme | Opt in |
| Global Retrospective Registry for Danon Disease | 82 patients (2021) | 2006 | Global |
Clinician; Multicentre |
E-mail & telephone | NS | NS | NS |
| Global Registry of Acute Coronary Events | 10,2000 patients (2021) | 1999–2009 | Global |
Clinician; Multicentre |
NS | NS | NS | Opt in |
| International & SIOP-E Diffuse Intrinsic Pontine Glioma (DIPG) Registries | Estimated > 1000 patients (2020) | 2011 | Global |
Clinician; Multicentre |
Online web application & database | Annual report | The DIPG Collaborative | Consent required |
| International Dysferlinopathy Registry | NS | 2012 | Global |
Clinician; Multicentre |
Online | NS | The Jain Foundation | Consent required |
| International Schwannomatosis Database | 389 patients (2017) | 2011 | Global |
Clinician; Multicentre |
Online | NS | Industry | Consent required |
| International Pachyonychia Congenita Research Registry | 2000 patients (2018) | 2004 | Global |
Patient; Multicentre |
Web-based data entry | NS | Pachyonychia Congenita Project Non-profit Organization | Consent required |
| Wolfram Syndrome International Registry | 21 patients (2009) | 2009 | Global |
Patient; Multicentre |
NS | NS | NS | Consent required |
| The Global Fukutin Related Protein Registry | 622 patients (2018) | 2011 | Global |
Patient; Multicentre |
Web-based data entry | Newsletters | LGMD2iFund & CureLGMD2i | Voluntary opt in/opt out |
| Cantú Syndrome Registry | 74 (2019) | 2012 | Global |
Patient; Multicentre |
Paper form, electronic form through REDCap | Newsletters | NS | Consent & recruitment procedures vary |
| GNE Myopathy Registry | 432 patients (2021) | 2014 | Global |
Patient; Multicentre |
Online | Newsletters | Translational Research in Europe-Assessment & Treatment of Neuromuscular Diseases network | Electronic consent |
| Global Prader-Willi Syndrome Registry | 1696 participants (2019) | 2015 | Global |
Patient; Multicentre |
Online | NS | Foundation for Prader-Willi Research | Opt in |
| Global Angelman Syndrome Registry | > 1300 families | 2016 | Global |
Patient; Multicentre |
Online database | NS | Foundation for Angelman Syndrome Therapeutics AUS | Consent required |
| Idiopathic Thrombocytopenic Purpura Natural History Study Registry | 1448 patients (2021) | 2017 | Global |
Patient; Multicentre |
Web-based data entry | Annual reports & fundraisers | Platelet Disorder Support Association | Voluntary participation |
| Friedreich’s Ataxia Global Patient Registry | 1000+ patients (2021) | 2019 | Global |
Patient; Multicentre |
Online | Australian annual reports; newsletters | Friedreich’s Ataxia Research Alliance | Consent required |
| The Myotubular & Centronuclear Myopathy Patient Registry | 228 patients (2018) | 2013 | Global |
Patient; Multicentre |
Online | Newsletters | Myotubular Trust, Muscular Dystrophy UK & Audentes Therapeutics | Opt in |
| International Niemann-Pick Disease Registry | 1000 patients | 2013 | Global |
Patient; Multicentre |
Web-based data entry | Annual reports; newsletters | Consumers, Health, Agriculture & Food Executive Agency grant | Consent required |
| Limb-Girdle Muscular Dystrophy Type 2A Patient Registry | NS | NS | Global |
Patient; Multicentre |
Online | NS | Fundraisers | NS |
| International Fragile X Premutation Registry | > 2300 patients (2014) | 2009 | Global |
Patient; Multicentre |
Online | NS | CDC | Consent required |
| Australian & New Zealand Fontan Registry | 1682 patients | 2009 | ANZ |
Clinician; Multicentre |
Web-based data entry | Annual report, registry publications | Heart foundation, MCRI, ANZ trustees, Heart Kids, Australian Government | Opt out |
| Thrombotic Microangiopathies Registry | 340 patients (2020) | 2009 | ANZ |
Clinician; Multicentre |
Online REDCap database | Newsletter | Monash University (In kind) | NS |
| Australasian Epidermolysis Bullosa Registry | 417 patients (2012) | 2006 | ANZ |
Clinician; Multicentre |
Online database | Research publications | The Dystrophic Epidermolysis Bullosa Research Association of Australia | Opt in |
| Lymphoma & Related Diseases Registry | 4500 patients (2021) | 2016 | ANZ |
Clinician; Multicentre |
Web-based data entry | NS | Industry partners | Opt out |
| Myeloma & Related Diseases Registry | 4747 patients (2021) | 2012 | ANZ |
Clinician; Multicentre |
Web-based data entry, Paper QoL | Research publications, annual report, 6-monthly reports to sites, newsletter | Industry partners, Myeloma Australia | Opt out |
| Australia & New Zealand Transplant Registry* | 29,997 patients (2021) | 1977 | ANZ |
Clinician; Multicentre |
Online or postal | Annual report | Government, Industry | Opt out |
| Australian & New Zealand Registry of Advanced Glaucoma* | > 2000 | 2007 | ANZ |
Clinician; Multicentre |
Paper & online | Newsletter | Government, Industry | NS |
| Australasian Interstitial Lung Disease Registry | > 2000 | 2016 (Australia) | ANZ |
Clinician; Multicentre |
Retrospective data entered at 1st clinic visit with prospective data—after each subsequent visit | NS | Lung Foundation | Consent required |
| The Australasian Registry Network of Orphan Lung Diseases | 186 patients | Jul 2009 & Jun2014 | ANZ |
Clinician; Multicentre |
NS | Publication | Lung Foundation & Mr Ivan Cash | Opt in |
| Australia & New Zealand Familial Hypercholesterolaemia Registry | 1500 patients (2021) | 2015 | ANZ |
Clinician; Multicentre |
EMR | Annual report | NS | Opt in |
| Australian National Creutzfeldt-Jakob Disease Registry | 1321 cases (2019) | 1993 | AUS |
Clinician; Multicentre |
Forms completed by clinicians, families | Annual Reports | Government | Opt in |
| The Australian Rett Syndrome Database | 311 patients (2007) | 1993 | AUS |
Clinician; Multicentre |
Family & clinical questionnaire | Newsletter | New South Wales Rett Syndrome Association & Rett Syndrome Australian Research Fund | Opt in |
| Lymphangioleiomyomatosis Registry | 100 patients (2014) | 2006 | AUS |
Clinician; Multicentre |
NS | Newsletter | Not-for-profit charity | NS |
| Australian Genetic Heart Disease Registry | 1600 patients (2013) | 2007 | AUS |
Patient, Multicentre |
Online database | Bi annual newsletter | Agnes & Berel Gings, Pfizer, Boston Scientific, Cardiomyopathy Association of Australia | Opt in |
| Neonatal Alloimmune Thrombocytopenia Registry | 76 patients (2015) | 2009 | AUS |
Clinician; Multicentre |
Online database | Periodic newsletters | Monash University's Department of Epidemiology & Preventative Medicine | Opt out |
| Australian Idiopathic Pulmonary Fibrosis Registry | 647 patients (2017) | 2012 | AUS |
Clinician; Multicentre |
Web-based data entry | Newsletters | Educational grants & government research funding | Opt in |
| The Aplastic Anaemia & Other Bone Marrow Failure Syndromes Registry | NS | 2013 | AUS |
Clinician; Multicentre |
NS | NS | Maddie Riewoldt's vision | Opt out |
| Haemoglobinopathy Registry | 378 patients | 2013 | AUS |
Clinician; Multicentre |
Online | On funders request | Industry partners, Thalassaemia & Sickle Cell Australia, Thalassaemia Society of NSW | Opt out |
| Australian Bronchiectasis Registry | 1053 patients (2018) | 2015 | AUS |
Clinician; Multicentre |
Web-based data entry | NS | National Health & Medical Research Council practitioner fellowship | Opt in |
| Monash Public Health Genomics* | NS | 2015 | AUS |
Clinician; Multicentre |
NS | NA | NA | NA |
| PLANET | NS | 2019 | AUS |
Clinician; Multicentre |
Online database /Mobile App | NS | Australian Government, Ipsen, Melbourne University | Opt in |
| Australian Cystic Fibrosis Data Registry | 3538 patients | 1996 (data collection in 1998) | AUS |
Clinician; Multicentre |
Online database | Annual reports & quarterly Newsletters | Cystic Fibrosis Australia, Vertex Pharmaceuticals | Opt in & Opt out |
| Australian Inherited Retinal Disease Registry | 9298 patients (2020) | 2009 | AUS |
Clinician; Multicentre |
NS | Annual Reports | Retina Australia (WA) | Opt in |
| Australian Calciphylaxis Registry | 47 patients (2014–2019) | 2014 | AUS |
Clinician; Multicentre |
Online database | Nil | Amgen Australia | Opt in |
| Bosentan Patient Registry | 528 patients | 2004–2007 | AUS |
Patient; Multicentre |
NS | Nil | Actelion Pharmaceuticals | Opt in |
| The Australian Neuromuscular Disease Registry (covers Spinal muscular atrophy | 448 patients (December, 2021) | NS | AUS |
Patient; Multicentre |
Online | Monthly & on demand |
Biogen & Novartis, Philanthropic support Save our Sons & Muscular Dystrophy NSW |
Opt in |
| Hypersomnolence Australia, Idiopathic Hypersomnia Patient Registry | NS | NS | AUS |
Patient; Multicentre |
Web-based data entry | Newsletter | Non-profit charitable organisation | Opt in |
| HHT Alliance (Hereditary Haemorrhagic Telangiectasia) | 108 patients | 2014 | AUS |
Clinician; Multicentre |
Online database | NS | The Royal Melbourne Hospital Foundation | Opt in |
| Australian Mitochondrial Disease Foundation Patient Registry | 486 patients (2020) | 2014 | AUS |
Patient; Multicentre |
Online form | Nil | The Mito Foundation, charity | Opt in |
| Leukodystrophy Australia | > 300 patients and families | NS | AUS |
Patient; Multicentre |
NS | Annual report | Australian Government’s Medical Research Fund | Opt in |
| Australian Autoinflammatory Diseases Registry | ~ 200 people | 2016 | AUS |
Clinician; Multicentre |
REDCap database | On demand | Research grant | Opt in |
| Australian Bleeding Disorders Registry | 3127 patients (2021) | 1988 | AUS |
Industry; Multicentre |
Paper form, mobile app, webpage | Annual report | National Blood Authority (NBA) | Opt in |
| Australian Motor Neurone Disease Registry | 827 (in 2009) | 2004 | AUS |
Clinician; Multicentre |
NS | Nil | Charity—run by volunteers | Opt in & Opt out |
| The Rare Genetic Lipid Disorders Registry | 16 patients (2019) | 2019 | AUS |
Patient; Multicentre |
Online database | NS | NS | Opt in |
| Myeloma Database in the Royal Adelaide Hospital, South Australia | 743 patients | 2009–2019 | State |
Clinician; Multicentre |
NS | NS | NS | NS |
| South Australian Scleroderma Register | 859 patients (2021) | 1993 | State |
Industry; Multicentre |
Online | Reported in other public reports | Australian Rheumatology Association | Informed consent |
| The Glomerular Disease Registry | 120 patients (2020) | 2018 | State |
Clinician; Multicentre |
NS | Annual report | The George Institute for Global Health | Informed consent |
| Huntington's Disease Research Participant Registry | NS | 2019 | State |
Patient; Multicentre |
Online | NS | NS | Voluntary |
| Perth Demyelinating Diseases Database | 983 patients (2009) | NS | State |
Clinician; Single |
Online | NS | NS | Informed consent |
| The South Australia Pathology iSYS database# | 20 patients (2017) | NS | Umbrella |
Industry; Single |
Online | NS | NS | NS |
| Medical Genome Reference Bank* | 4000 participants | 2016 | Umbrella |
Industry; Multicentre |
Web-based | NS | NS | NS |
| Australian Cancer Database# | 353 cases with mucosal melanomas | 1982 | Umbrella |
Industry; Multicentre |
Online | NS | NS | NS |
| Australian Childhood Cancer Registry# | 1269 patients (2020) | 1983 | Umbrella |
Industry; Multicentre |
NS | NS | NS | NS |
| Australian Paediatric Surveillance Unit Database# | 1429 | 1993 | Umbrella |
Industry; Multicentre |
APSU report card | NS | NS | NS |
| Queensland Oncology Online Database# | 18 patients (2015–2017) | 2005–2017 | Umbrella |
Industry; Single |
Retrospective | NS | NS | NS |
| State-wide database based at Royal Perth Hospital, Western Australia# | 526 patients (Feb 2015-Sept2017) | NS | Umbrella |
Clinician; Single |
Online database | NS | NS | NS |
| Gynaecological tumour registry of King George V Hospital | 20 patients | 1987–2000 | Umbrella |
Clinician; Single |
Online database | NS | NS | NS |
| South Australian Clinical Cancer Registry# | 140 patients | 1980's | Umbrella |
Industry; Multicentre |
Online database | NS | NS | NS |
| South Australian Pathology Database# | NS | 1960s | Umbrella | Industry | Online database | NS | NS | NS |
| The Victorian Cancer Council Registry# | 251 patients (Jan 1998 to Dec2013) | NS | Umbrella | Industry | Online database | NS | NS | NS |
| Western Australia Register of Developmental Anomalies# | 30,000 births annually | 2011 | Umbrella | Clinician | Online database | NS | NS | NS |
| Western Australia Cancer Registry# | 95 patients (2021) | 1981 | Umbrella | Clinician | Electronic & paper records | NS | NS | NS |
| PMCC Prospective Database on Cervix Cancer Patients | NS | 1998 | Umbrella | Clinician | Online database | NS | NS | NS |
| University of Sydney Endocrine Surgical Unit Database | 21 patients (Jul 1958 to Jun 2010) | 1957 | Umbrella | Clinician | Online database | NS | NS | NS |
ANZ Australia & New Zealand; APSU Australian Paediatric Surveillance Unit; AUS Australia; DIPG diffuse intrinsic pontine glioma; CDC Centre for Disease Control and Prevention; LGMD limb girdle muscular dystrophy; MCRI Murdoch Children’s Research Institute; NBA National Blood Authority; NHMRC National Health and Medical Research Council; NS not stated; NSW New South Wales; PMCC Peter MacCallum Cancer Centre; SIOPE; European Society for Paediatric Oncology; UK United Kingdom; WA Western Australia
*Includes information of registries, databases and institutions collecting/researching people with rare disease conditions
#Information on State and Umbrella rare disease registries and databases listed in the table is relevant to specific disease or condition
Most of the registries, 47 (63.5%), mentioned in these studies, were established in the 2000s. The Neuroendocrine Tumour Registry (PLANET) [16, 17], Friedreich’s Ataxia Global Patient Registry [18] and Huntington’s Disease Research Participant Registry [19, 20] were established most recently, in 2019.
Forty-two (56.8%) registries used solely an online web-based data entry method. While only five (6.8%) registries use either, both paper and online web-based data entry or paper-based data entry only.
Population size
The population size captured in each registry varied. At the time of this review, only 16 (21.6%) of the RDRs captured population of > 1000 patients. Five (6.8%) registries captured data of > 4000 patients. One registry, International Collaborative Gaucher Group (ICGG) Gaucher Disease Registry [21, 22], captured data of > 120,000 participants since it was established in 1994.
Data management
Data management varied among the RDRs identified in this scoping review. Within the global registries, most of the data were entered by patients, their caregivers or other family members. Six global registries were clinician-initiated with the data entered by clinicians (ICGG Gaucher Disease Registry [22], the Global Retrospective Registry for Danon Disease [23, 24], the International and SIOP-E Diffuse Intrinsic Pontine Glioma (DIPG) Registries [25, 26], the International Dysferlinopathy Registry [27] and the International Schwannomatosis Database [28]).
In ANZ and Australian national registries, most registries collect their data online, with data entry completed by clinicians and registry staff. However, two of the Australian patient registries, Australian Bleeding Disorders Registry (ABDR) [29] and the PLANET registry [17], direct patients to enter their data via a mobile application.
Limited information was available on data management of the jurisdiction-based or “umbrella” registries identified in this scoping review.
Data collected by the registries and databases
Most of the RDRs collected similar types of data, including patient demographics, clinical and diagnostic variables, procedure and treatment information, and PROMs.
Table 2 summarises data captured by RDRs and databases, described in this scoping literature review.
Table 2.
Data captured by rare disease registries and databases
| Registry name | Demographic variables | Diagnostic/clinical variables | Treatment/procedure details | QoL data | Timing of data collection |
|---|---|---|---|---|---|
| International Collaborative Gaucher Group Gaucher Registry | Gender, age | Diagnosis details, clinical, biochemical, & therapeutic characteristics regardless disease severity | Treatment status & choice, splenectomy status & date, treatment with alglucerase/imiglucerase | QoL questions—NS | (1) Baseline window: 12 months before to 1 month following date of imiglucerase initiation, (2) 10-year window: 8.5 to 11.5 years, (3) 20-year window: 17 to 23 years |
| Global Retrospective Registry for Danon Disease | Gender, age | Diagnostic details; genetic details; Baseline echocardiographic data | NS | NS | NS |
| Global Registry of Acute Coronary Events | Initials, DOB, address, telephone, physician, cardiologist details | Medical history, presentation symptoms, hospital dates, ECG findings, laboratory pathology results | Procedures, cardiac interventions, thrombolytics, medications, in-hospital events, discharge status | NS | 6 months after discharge |
| International & SIOP-E Diffuse Intrinsic Pontine Glioma (DIPG) Registries | Country, gender, age | Diagnosis, date of diagnosis, imaging, signs & symptoms & physical exam at diagnosis, response evaluations, central pathology review characteristics, central imaging review characteristics, & molecular profile | Treatment data- chemotherapy, biopsies, pathology data | NS | Ongoing after registration |
| International Dysferlinopathy Registry | Basic demographics | Diagnosis details; symptoms &family history | NS | NS | NS |
| International Schwannomatosis Database | Contact details, DOB gender | Diagnosis details; symptoms & family history | NS | NS | NS |
| International Pachyonychia Congenita Research Registry | Name, surname, gender, DOB, address, doctor details | Age of diagnosis, disease characteristics, family characteristics | Images of disease areas, genetic testing details, medications | QOL symptom & pain questions | Once a year, target follow-up for 1 year |
| Wolfram Syndrome International Registry | Age, gender | Diagnosis details, family history | Genetic testing, DNA samples, & biological samples (blood & urine) | NS | NS |
| The Global Fukutin Related Protein Registry | Name, age, address, email, phone number | Diagnosis, motor function, pain, ventilation & family history, symptoms, lung, heart & cognitive function, contractures, six-minute walk distance & muscle strength | Medications, non-invasive/invasive ventilation use, brain/muscle MRI | NS | Annually |
| Cantú Syndrome Registry | Name, age, address, email, phone number | Medical history & health information, specific ABCC9 or KCNJ8 variant in the family (if any) | NS | NS | Annually |
| GNE Myopathy Registry | Age, 18+ | Muscle Biopsy or genetic testing. General medical history; Level of physical activity | Medications | QoL questions | 6 months, 12 months & Annually |
| Global Prader-Willi Syndrome Registry | Socioeconomic details, biological family history | Diagnostic details & medical history | Medications, supplements | Behaviour & mental health | As often as required |
| Global Angelman Syndrome Registry | Name, DOB, DOD, gender | Newborn & infancy history; history of diagnosis & results; Illnesses or medical problems; Behaviour & Development; Epilepsy history; Sleep Disturbance Scale for Children Pathology & Diagnostics | Medications & interventions | NS | Periodically |
| Idiopathic Thrombocytopenic Purpura Natural History Study Registry | Name, surname, age, gender, country, race, insurance, education, employment | Diagnostics tests, clinical visits, family history, & pregnancy/childbirth | Medications & frequencies/dosage, diet, surgeries | Enjoyment of life, fatigue, sleep, pain, social activities, mental health, physical health, PTSD symptoms, & financial impact | Within patient's own timeframe |
| Friedreich’s Ataxia Global Patient Registry | Name, DOB, gender | Diagnosis, medical history, functional mobility | NS | QoL—NS | 1 month before anniversary, then annually |
| The Myotubular & Centronuclear Myopathy Patient Registry | Contact details & demographics; family history | Clinical diagnosis; genetic mutation; Genetic report & muscle biopsy report; Details of clinician & genetic testing/muscle biopsy centres | Best & current motor function & wheelchair use; Respiratory function, ventilation type & chest infections, Feeding & heart function; Neuromuscular examinations & scoliosis surgery | NS | NS |
| International Niemann-Pick Disease Registry | Individually identifying data: name, address | Diagnosis, health status, disease characteristics | Treatment, lab results, imaging tests | QoL questions—NS | Every six months |
| Limb-Girdle Muscular Dystrophy Type 2A Patient Registry | Name, DOB, gender, email, phone number, state, country, clinic name | Genetic diagnosis, genetic mutation, current ambulatory (walking) status, other family members with muscular dystrophy | Medications | NS | NS |
| International Fragile X Premutation Registry | Name, DOB, gender, contact details | Limited medical history | NS | NS | NS |
| Australian & New Zealand Fontan Registry | Height, Weight, BMI | Birth defects, congenital abnormalities, obstetric complications/interventions. cardiovascular health; condition specific information, general health, imaging, medication & supplements, reproductive health, linkage to other health databases | Re-intervention event(s), pacemaker status, cardiac transplant status, site of cardiac transplant procedure, Fontan take-down status, site of Fontan take-down procedure | PedsQL | NS |
| Thrombotic Microangiopathies Registry | Name, sex, DOB, doctor’s name | The circumstances and symptoms leading up to the diagnosis of TMA, other illnesses, medications, or history which could influence the onset & treatment of TMA | The type of treatment, the response of the illness to those treatments & any complications of the illness or treatment; relevant laboratory test & scan results | NS | NS |
| Australasian Epidermolysis Bullosa Registry | Age, sex, ethnicity, geographical & disease subtype distribution, past & current medical history, family history | Diagnostic laboratory studies, areas of blistering or erosion, EB type/subtype, electron microscopy, immunofluorescence, genetic mutation, complications (tracheolaryngeal, gastrointestinal, weight & growth development, ocular, genitourinary, skin cancers, renal disease, anaemia, swallowing problems) & clinical outcomes | Electron microscopy, immunofluorescence | NS | NS |
| Lymphoma & Related Diseases Registry | Demographics outcomes- overall & progression-free survival, duration of response & time to next treatment. Long-term outcomes- through linkage with cancer & death registries | Diagnoses, health status at diagnosis. Laboratory and imaging results at diagnosis, therapy, chemotherapy, autologous & allogeneic stem cell transplantation, & maintenance and supportive therapies: outcomes, long-term outcomes | Therapy | QoL | NS |
| Myeloma & Related Diseases Registry | General health diagnosis, gender, age | Diagnoses, clinical & laboratory results, therapy, decisions & complications, clinical outcomes | Autologous &allogeneic stem cell transplantation, therapy complications, laboratory results | EQ-5D-5L | NS |
| Australia & New Zealand Transplant Registry* | Name, gender, DOB, ethnic background, details of transfers between treating units | Baseline comorbidities, paediatric data; parenthood outcomes | Transplant Event, graft failure event | NS | Real time data collected on all new incident patients commencing RRT |
| Australian & New Zealand Registry of Advanced Glaucoma* | Fist name, surname, DOB, gender, address, phone number, email, eye clinician | Patient clinical details, diagnosis, BCVA, highest recorded IOP, refraction, central corneal thickness, cup disc ratio | Glaucoma surgery, laser trabeculoplasty | NS | NS |
| Australasian Interstitial Lung Disease Registry | Sex, age, ethnicity, mortality | Symptoms, clinical findings, occupational & environmental exposures, family history & co-morbid disease | Current & past medication lists (including oxygen use), results of other investigations including: serum blood markers, high resolution CT chest findings, blood gases, bronchoscopy +/− biopsy, active & past treatments specific to any form of ILD, adverse effects or incidents | Shortness of Breath Questionnaire, St George Respiratory Questionnaire & HADS | Baseline, Mortality data is reviewed every 6 months |
| The Australasian Registry Network of Orphan Lung Diseases | Reported disease, patient initials, DOB, postcode, medical record number, new case or follow up, physician details | NS | NS | NS | NS |
| Australia & New Zealand Familial Hypercholesterolaemia Registry | Personal & contact details, doctor’s details, relatives’ details for index patients, family pedigree, details on founder effect origin, family linkage details, clinical data at follow up assessments: hypertension, diabetes, antithrombotic, biochemistry profiles, & death | Family history, clinical history, physical examination, plasma LDL-cholesterol, biochemistry profile, risk factors and clinical trials, ‘Dutch Lipid Clinic Network Score’, ‘FH Diagnostic category’, ‘LDL-cholesterol adjusted for treatment’, ‘BMI’, genetic data (genotype & gene variants), laboratory reports | Medication details, drug intolerances, imaging tests: carotid ultrasonography, echocardiograms, coronary artery calcium scores, angiograms, & nuclear perfusion scans, e type, frequency, & complications | NS | NS |
| Australian National Creutzfeldt-Jakob Disease Registry | Full name, MRN, DOB, Address, doctor name, Hospital; Survival/mortality | Relevant medical conditions, including investigations, FNI outcome classification | Required to list any surgeries, transplants, grafts, blood donations/products or organs, dental/surgical care, renal dialysis, tattoos or piercings, or acupuncture | NS | From diagnosis—death |
| The Australian Rett Syndrome Database | Full name DOB, address, age at diagnosis, mothers age at subjects’ birth, mothers & fathers’ occupation, education, birth order | Genetic: MECP2 test, result & mutation identified, clinical severity | Treatment type dependant on symptoms | NS | Every 2 to 3 years |
| Lymphangioleiomyomatosis Registry | Full name, DOB, address, phone, email, gender, NOK | Date of diagnosis, spirometry and, when available, lung volumes and diffusing capacity), arterial blood gases or oximetry, walking and resting oxygen titration, cardiopulmonary stress testing, cause-specific mortality, functional status, & clinical events associated with lung transplantation | NS | NS | NS |
| Australian Genetic Heart Disease Registry | DOB, gender, ethnicity, COB, height & weight, family history | Clinical diagnosis, age at diagnosis, sudden cardiac death event, other serious medical conditions, symptoms, medications, genetic testing results, echo, ECG, cardiac MRI, exercise stress tests | Details of interventions, AICD indications, device details, adverse events & appropriate shocks. Use of services, cardiac investigations & clinical genetics consults | NS | Annually |
| Neonatal Alloimmune Thrombocytopenia Registry | Maternal demographics, neonatal demographics, postnatal features | Clinical case description, laboratory investigations (antibody investigations- immunofluorescence tests, glycoprotein binding ELISA assays & HLA antibody testing) | Transfusions, antenatal therapy, therapy & outcome, clinical outcomes | NS | Antenatal & Postnatal |
| Australian Idiopathic Pulmonary Fibrosis Registry | Demographics, smoking history, family history, medication history | Symptoms, objective investigational data relevant comorbidities (gastroesophageal reflux disease) | High-resolution computed tomography scans & any surgical lung biopsy specimens collected | QoL | Every 6 months |
| The Aplastic Anaemia & Other Bone Marrow Failure Syndromes Registry | Demographic details Clinical context, including possible precipitants, family history, including IBMFS, IPF & liver disease | Clinical presentation, laboratory test results at initial presentation & follow-up reviews | Immunosuppressive therapy, bone marrow or haematopoietic stem cell transplant, & supportive therapy; Clinical outcomes, including details of any relapse, complications, performance status indicators & disease progression | Nil | Data is collected through routine clinical visits |
| Haemoglobinopathy Registry | Full name, DOB, Medicare no, MRN, doctors’ details, ethnicity, gender | Height, weight, symptoms, diagnostic laboratory results, clinical &imaging results, complications of disease, family history | Therapy, clinical outcomes, transfusions, | NS | Multiple occasions (not specified) |
| Australian Bronchiectasis Registry | Full name, DOB, gender, Postal address, email address, COB | Age at diagnosis, diagnosis tests, clinical visits, comorbidities, genetic variations | Spirometry results, CT chest, radiology reports, airway cultures, medications | QoL-B | Baseline data, spirometry & CT details measured at enrolment, airway cultures obtained for 2 years prior to & 3mths after enrolment |
| Monash Public Health Genomics* | NA | The program conducts research centred around the genetic analyses of large cohorts, clinical trials and patient registries | Maintenance & supportive therapies; | NA | NA |
| PLANET | Full name, gender, DOB, clinician details | Bristol Stool Scale, Vital measurement (height & weight) ECOG scale—level of function | Compare types of treatments including; SSA PRRT, Chemo (Carbo/Etop), CAPTEM, Everolimus, Sunitinib, Telotristat & Immunolocy | QLQ-C30 & QLQGINET21 | Periodically dependant on various treatments |
| Australian Cystic Fibrosis Data Registry | Name, Gender, DOB, COB, DOD | Diagnostic data; genetic mutation, initial diagnosis testing, clinical measures; lung function & BMI status, pulmonary infections & complications | Transplant history, hospital admissions, IVAb | Nil | Quarterly |
| Australian Inherited Retinal Disease Registry | Gender, DOB & contact details, ophthalmologist /referring doctor, family history & pedigree structure | Clinical data, results of electrophysiology tests, psychophysical measurement, ophthalmic examinations & genetic variations | Topical therapies, gene therapies, clinical trials | NS | NS |
| Australian Calciphylaxis Registry | Full name, DOB | Laboratory data, clinical background & presentation as well as therapeutic strategies & outcomes. Laboratory results | Treatment dialysis prescription, medications & treatment outcomes. Photos of skin lesions | Nil | Initial 5-year period of data collection, all treating units were contacted for patient follow-up data ongoing periodically, including calciphylaxis resolution & mortality |
| Bosentan Patient Registry | Name, gender, DOB | Health & vital statistics, clinical characteristics | Concomitant medications, length of treatment | Nil | At enrolment & at each 6-monthly visit |
| The Australian Neuromuscular Disease Registry | Full name, DOB, COB, gender, postal & email address | Genetic information, year of diagnosis, diagnostic tests, clinical visits, comorbidities | Clinical management, therapies, laboratory results, medications, frequency, dosages, surgical procedures | NS | 6 & 12 months |
| Hypersomnolence Australia, Idiopathic Hypersomnia Patient Registry | Full name, DOB, email, postal address | Diagnosis, Multiple Sleep Latency Test results | Dose/type of medications, other medications, sleep apnoea status, treatment for sleep apnoea, other concerns | Nil | Once off |
| HHT Alliance (Hereditary Haemorrhagic Telangiectasia) | Full name, DOB address & Country of residence | Age & details of first diagnosis, physician details, family history of HHT | Surgical therapies, medical therapies, treatments manage complications | NS | Initial entry & subsequent update of available information as required |
| Australian Mitochondrial Disease Foundation Patient Registry | Full name DOB, DOD, gender, email & postal address | Clinical diagnosis, biochemical diagnosis, genetic diagnosis, type of mitochondrial disease | NS | Nil | 6 & 12 months |
| Leukodystrophy Australia | Full name DOB, gender, email & postal address, COB | Year of diagnosis, diagnosis test, genetic results, family history of disease | Nil | Survival/mortality | Initial diagnosis, 6mths, 12 months & 24 months |
| Australian Autoinflammatory Diseases Registry | Full name, DOB, gender, email & postal address | Age at diagnosis, diagnostic testing, family history & genetic results | Clinical management, therapy, laboratory results | Nil | Once off—initial recruitment |
| Australian Bleeding Disorders Registry | Title, Full name, alias, DOB, address, phone email, doctor details | Date diagnosed, bleeding disorder, severity, baseline factor date/level, weight, height | Treatment regimen, product name, total dose, frequency | Nil | NS |
| Australian Motor Neurone Disease Registry | Patient demographics, site of onset of disease | Changes in disease, complications related to disease progression, diagnosis date | Treatment type & impact of new treatments & interventions | Nil | First presentation 3 months 6 months, approx. 12 months after your first visit, then ongoing every 6 months |
| The Rare Genetic Lipid Disorders Registry | Personal & contact details | Clinical history, family history, first physical examination. Genetic data & imaging, laboratory data | Management & treatment plans | Nil | Longitudinal follow up forms (not specified) |
| Myeloma Database in the Royal Adelaide Hospital, South Australia | Personal & contact details | Paraprotein subtype, cytogenic abnormalities at diagnosis, sites of involvement; biopsy-proven extramedullary myelomas involving skin, organ or CNS | Treatment details: autologous & allogenic stem cell transplants | NS | NS |
| South Australian Scleroderma Register | Demographics, sex, age at disease onset, residency & occupation at disease onset & 10 years before first symptoms, siblings, familial history of scleroderma, disease subtype | Incidence, clinical phenotype, possible precipitating factors, seasonality at presentation, presence of autoantibodies, survival; organ involvement, available serology | Immunofluorescence, counter immune-electrophoresis, enzyme-linked immunosorbent assay, line immunoblot | HAQ, SF-36, PROMIS-29 Profile 2.0, UCLA SCTC GIT 2.0, FACIT | Annual |
| The Glomerular Disease Registry | Basic demographics details | Medical history& details of disease diagnosis | DNA & blood samples | NS | Every 6 months through medical records & physician reviews |
| Huntington's Disease Research Participant Registry | Name, contact details, DOB, age, gender | Handedness (left or right h& dominant), description of health in terms of Huntington’s disease (diagnosis/genetics), general health-related questions | NS | NS | 4 times a year |
| Perth Demyelinating Diseases Database | Gender, family history of MS, age at onset of initial symptoms, clinical course | EDSS score, MSSS score, VEP, disease duration | HLA-DRB1 genotyping, blood samples, MRI findings, cerebrospinal fluid studies | NS | NS |
AICD automatic implantable cardioverter-defibrillator; BCVA best corrected visual acuity; BMI Body Mass Index; CAPTEM capecitabine and temozolomide; CNS Central Nervous System; COB Country of Birth; CT computed tomography; DIPG Diffuse Intrinsic Pontine Glioma; DNA deoxyribonucleic acid; DOB Date of Birth; DOD date of death; EB Epidermolysis Bullosa; ECG electrocardiogram; ECOG Eastern Cooperative Oncology Group; ELISA Enzyme linked Immunosorbent Assay; EQ-5D European Quality of Life Five Dimension; FACIT Functional Assessment of Chronic Illness Therapy; FH Familial Hypercholesterolaemia; HADS Hospital Anxiety & Depression Scale; HAQ Health Assessment Questionnaire; HLA Human Leukocyte Antigen; HLA-DRB1 Major Histocompatibility Complex, Class II, DR Beta; IBMFS Inherited Bone Marrow Failure Syndromes, ILD Interstitial Lung Disease;IOP Intraocular pressure; IVAb Intravenous Antibiotics;IPF Idiopathic Lung Fibrosis; LDL; Low-density Lipoprotein; MECP2 Methylcytosine-binding Protein 2; MRI Magnetic Resonance Imaging; MRN Medical Records Number; MS Multiple Sclerosis; MSSS Multiple Sclerosis Severity Scale; NA not applicable; NS Not Stated; NOK Next of Kin; PedsQL; Paediatric Quality of; PROMIS - 29 Profile 2.0: Patient-Reported Outcomes Measurement Information System - 29 Profile version 2.0; PRRT Peptide Receptor Radionuclide Therapy; PTSD Posttraumatic Stress Disorder; QoL quality of life; QoL-B quality of life questionnaire; QLQ-C30 European Organisation for Research and Treatment of Cancer. Core Quality of Life Questionnaire; QLQGINET21 Questionnaire for Patients with Gastrointestinal Neuroendocrine Tumours; RRT renal replacement Therapy; SIOPE; European Society for Paediatric Oncology; SSA Somatostatin Analogues; SF 36 Short Form 36; TMA Thrombotic Microangiopathies; UCLA SCTC GIT University of California Los Angeles Scleroderma Clinical Trial Consortium Gastrointestinal Tract; VEP visual evoked potential
Demographic data
Demographic variables included given names, surnames, age, gender, country, address, email, phone number, death date, race/ethnicity, insurance, education, employment status, family history, next of kin, clinic and doctor’s details. The information was anonymised for most RDRs and databases. Individually identifying data was only collected in the International Niemann-Pick Disease Registry [30, 31]. As the development of the International Niemann-Pick Disease Registry is ongoing, the use of identified data will aid in the registries planning for linkages to clinical and patient datasets.
Diagnostic/clinical data variables
Diagnostic/clinical data variables generally included some or all of the following: (1) the presence of disease symptoms and clinical examination findings; (2) investigation findings such as pathology investigation and biopsy/cytology results, medical imaging investigations, genetic profile, and resulting date of diagnosis; (3) functional and behavioural status and support needs related to the disease; and (4) the presence of disease sequelae, including morbidity and death.
For paediatric RDs, a broader paediatric history was also often included (Table 2). Some clinical and diagnostic information was unique to each RDR. For example, the ICGG Gaucher Disease Registry [22, 32] collected additional details on biochemical and therapeutic characteristics, while the DIPG registry [25] captured symptom duration, cranial neve palsy, pyramidal signs, cerebellar signs, tumour material, imaging details, signs and symptoms and physical exam at diagnosis, response evaluations, central pathology review characteristics, and molecular profile. The Australian Bronchiectasis Registry records spirometry results, computerized tomography (CT) chest scans, radiology reports, airway cultures and pulmonary exacerbations as their baseline data [33, 34], while the Neonatal Alloimmune Thrombocytopenia (NAIT) records maternal and paternal testing result details [35, 36].
Treatment/procedure details
Disease management information included: (1) medical management, including medications; (2) surgical and procedural information; (3) exacerbation/critical care episodes; and (4) supportive care, such as nutritional and allied health interventions. This information was similar across all the registries; however, additional information was available for individual RDRs. Only three ANZ RDRs collected surgical procedures and treatment information.
PROMs and QoL data
PROMs and QoL data were captured by five global registries (ICGG [22], International Pachyonychia Congenita Research Registry [37, 38], GNE Myopathy Registry [39], Friedreich’s Ataxia Global Patient Registry [18], Immune Thrombocytopenia (ITP) Natural History Study Registry [40]), which captured physical and mental health, post-traumatic stress disorder symptoms, social activities and financial impact.
Only four ANZ RDRs captured PROMs and QoL information. These include the ANZ Fontan Registry [41], Myeloma and Related Diseases Registry (MRDR) [42], Lymphoma and Related Diseases Registry (LARDR) [43] and the Australasian Interstitial Lung Disease Registry (AILDR) [44]. Amongst the Australian RDRs, 24 registries collected PROMs. Similar to most RDRs discussed in this review, the specific PROMs collected varied depending on the registry.
Timing of data collection
Most global RDRs and databases captured data annually; however, some had specific data entry requirements. For example, the ICGG Gaucher Disease Registry collected data at multiple time points: (1) baseline (2 months before to 1 month following the date of imiglucerase initiation), (2) 10-year window (8.5–11.5 years), and 3) 20-year window (17–23 years) [22]. In the Global Angelman Syndrome registry participants may receive a request to update data periodically [45, 46]. The GNE Myopathy Registry [39] contacted their patients at 6 months and yearly. Friedreich’s Ataxia Global Patient Registry [18], Cantú Syndrome Registry [47, 48] and the International Pachyonychia Congenita Research Registry [37] collected data annually.
While there was limited data available on the timing of data collection throughout the ANZ RDRs, it was noted that the ANZDATA registry distributes an annual survey for all dialysis and transplant units in ANZ on the 31st of December [49]. The AILDR data are collected at baseline, and mortality data are reviewed every 6 months with dates of death and lung transplantation recorded as determined by clinical records and/or death certificates [44]. Families and carers in the Australian Rett Syndrome Database are invited to contribute information every 2–3 years about changes in the health or function of the individual with Rett syndrome for whom they care [50]. To assess patient outcomes in the Australian Calciphylaxis Registry (ACR) [51], following the initial 5-year period of data collection, all treating units are contacted for patient follow-up data, including calciphylaxis resolution and mortality. Other registries and databases capture data annually.
Registry reporting and funding
Annual reports, newsletters and research publications were the main reporting outputs for 32 (43%) RDRs identified in this review (Table 2). Funding the operations of the registries and databases varied from industry partners, non-profit charity organisations and fundraisers and government grants.
Discussion
Registries are often established to describe patterns of care, and to understand variation in treatments and outcomes and predictors of prognosis and QoL. Establishing and maintaining a registry requires substantial resources, infrastructure and sustained funding [52].
The present scoping review examined publicly-available data on existing RDRs and databases that included Australian participants. The findings of this review are an important first step towards informing the development of a national framework for the RDRs in Australia.
Seventy-four different registries and databases of RDs collecting Australian data were identified; 19 of them were global registries, 24 were Australian-only registries, 10 were ANZ based, and five Australian jurisdiction-based registries. Sixteen “umbrella” registries collected data on several rare conditions, which included some RDs, and thirteen registries stored rare cancer-specific information.
The population size captured in each registry varied, with many being relatively small. This highlights the challenges associated with diagnosis and reporting, as few clinicians have experience in managing individuals with RDs. Lack of clinician awareness can lead to significant delays in diagnosis or individuals remaining undiagnosed, creating future RD data deficits [53].
Data management and entry varied among the RDRs, with data either being entered by patients/caregivers/family members, clinicians or by the registry staff. Most of the Australian-only RDRs identified in this review were clinician-led. For ultra RDs where patients are few, building a patient registry is an intuitive first step to determining the number of people affected, their geographical distribution and basic demographic and clinical characteristics of the disease. The scope of these registries may evolve over time, maturing from a community effort as a means for a basic understanding of patient and disease characteristics, to a supportive mechanism for research funding and attracting input from health service providers to registry data collection [54].
Identifying a common minimum data set for RDRs is a challenge due to the heterogeneous nature of RDs. The RDR data sets identified in our review generally comprised a variety of data elements within the domains of demographic; diagnostic/clinical; treatment/procedure and PROMs and QoL data, with individual variables being unique to RDs. In 2011, the European Commission funded the EPIRARE project (‘Building Consensus and Synergies for the European Union (EU) Registration of Rare Disease Patients’), prior to the establishment of a European Platform for RDRs, which aims to support a harmonized approach to European RDRs [55–57]. A ‘set of common data elements for Rare Diseases Registration’ was produced by a Working Group coordinated by the Joint Research Centre and composed of experts from EU projects: EUCERD Joint Action, EPIRARE and RD-Connect.
The European Platform on RD Registration (EU RD Platform; European Commission) identified 16 common data elements for the initial registration of people with RDs onto a RDR. These are grouped in the following categories:
Pseudonym.
Personal information (date-of-birth, sex).
Patient status (alive, dead, long-term follow-up, opted out).
Care pathway (date of initial specialist contact).
Disease history (Age at symptom onset; age at diagnosis).
Diagnosis (Disease diagnosis, Genetic diagnosis, undiagnosed).
Research (contact for research, data for secondary use, biobank).
Disability (functioning, disability).
While a detailed mapping of each Australian registry’s dataset against elements was beyond the remit of this scoping review, a majority of identified RDRs collected items that aligned with the broad categories recommended by EPIRARE [58]. In addition, a number of RDRs in Australia collected follow-up clinical and patient-reported information.
PROMs and QoL data were captured only by some of the RDRs identified in our review. PROMs are increasingly being introduced into clinical registries in Australia, providing a person-centred perspective on the expectations and impact of treatment [59]. Including PROMs in clinical registries offers numerous advantages [60]. First, incorporation of the patient voice helps keep outcome measurements of care person-centred. Further, symptom burden, QoL and satisfaction with care are dynamic variables that cannot be recreated accurately through retrospection; they are essentially lost if not captured “in the moment”. PROMs data collection has also been supported by the EPIRARE [58].
Frequency of data collection was not consistent throughout the RDRs captured in this review. Determining regular intervals and time points for data capture in RDRs can be challenging due to low patient numbers and frequent loss to follow up. Overtime, retention of patients and providers can also be difficult, so registry developers should build in mechanisms for monitoring and follow-up [61].
RDR reporting information was not available for more than half of the RDRs identified in this review. This could be because these RDRs were not established with a purpose for quality improvement as an outcome [62]. This is a noteworthy limitation of many RDRs in Australia, that should be addressed by a more coordinated national approach, including alignment with the recently published National Strategy for Clinical Quality Registries and Virtual Registries [63].
Our review identified opportunities to learn from successful RDRs, as well as from those that have not been sustained. Established in 2009, the Australasian Registry Network of Orphan Lung Diseases (ARNOLD) is one example of an unsustainable registry [64]. ARNOLD aimed to provide prevalence data for multiple orphan lung diseases in both the paediatric and adult populations [64]. During its operation ARNOLD obtained national data relating to 30 rare and extremely rare lung diseases. However, the registry faced several barriers. Data were limited by the under-reporting of patient identifiers and other details. Only 35% of notifications to the registry included postcode, birthdate or patient initials, so duplicate notifications could not be identified, which may have led to over-reporting of some rare conditions. Another limitation of this registry was specific diseases were not well defined, so reporting relied on individual physician diagnoses, which may have led to inconsistencies. Nonetheless, the registry operators noted that many physicians recognised the importance of contributing to a web-based RDR to monitor quality of care [64].
An exemplar RDR is the Australian Cystic Fibrosis Data Registry (ACFDR) [65, 66]. The ACFDR has been funded, from its commencement, by Cystic Fibrosis Australia through a combination of fundraising, philanthropy and industry support. The ACFDR collects information from people with cystic fibrosis from the time of diagnosis to the time of death or lung transplantation. Data is entered into the registry by clinicians and data managers from 23 public centres. Data completeness has been enhanced over the last few years through an industry-sponsored data quality program, leading to increased acceptability of the data and increasing use for research and quality improvement. The success of the ACFDR is attributed to sustained funding over a prolonged period, broad clinical and community support, and experienced registry management.
The concept of a single national RDR or database in Australia is worth consideration and has been suggested previously [1]. National RDRs have been also established in other countries, including China [67], Italy [68] and other European countries [69]. In 2019, researchers in Slovenia developed a national RDR pilot informed by focus groups with experts from leading institutions in the field of RD [70]. The results indicated that effective development of a national RDR requires a series of systemic changes and many considerations [70].
The European Society for Immunodeficiencies (ESID) Registry was utilised as a platform for the German Network on Primary Immunodeficiency Diseases (PID-NET) to perform queries on RDRs and extract the data in the context of PID-NET [71, 72]. To interconnect RDRs the Open Source Registry System was implemented, based on a federated search functionality, ultimately making data from the ESID registry available in an interoperable manner and without losing sovereignty [73].
For several years, there have been activities ongoing in Germany under the Medical Informatics Initiative to digitally bring the hospitals together, combining the development of IT infrastructure, scientific research projects, as well as the promotion of junior researchers and education in medical informatics [74]. This initiative has been realized within five consortia of which two are mainly using Open-Source software [75], namely the consortia MIRACUM [76] and DiFUTURE [77]. They work on distributed data analysis using Data Shield [78] and the Personal Health Train [79], the latter designed for distributed machine learning. A combination of the aforementioned methods could potentially lead to a robust solution allowing RD data to remain within the registries without creating a new umbrella RDR, to use attachable structure leveraged by established tools, in order to interconnect RDRs nationally and internationally.
To achieve this in Australia, well-coordinated and well-funded efforts should involve all stakeholders, as well as alignment of all medical, organisational and technological aspects in accordance with the long-term public healthcare objectives and infrastructure for clinical trials. With differing perspectives on key issues related to RDRs, such as their sustainability, data custodianship, data entry requirements, and other challenges including limited patient numbers, a lack of infrastructure and workforce, funding sources and governance structures, the development of formal policies to support coordination and sustainability of Australian RDRs has been complex and difficult.
Such initiatives require behavioural, regulatory, financial, organizational, process, policy, clinical, and many other changes at all levels of the healthcare system. Dedicated funding should be allocated for eligible RDRs to support their reach to recruit every possible consenting patient across Australia and ensure full coverage of the eligible clinical population. Incentives for hospital and health services should encourage staff to continue dedicating their time to RD data collection. Investing in the infrastructure and staffing would assist in streamlining and simplifying the maintenance and data entry demands of new and existing RDRs across Australia. Finally, RDRs should be promoted in hospitals and relevant clinics, and collaborations with relevant international registries should be sought [13]. As we take steps towards a nationally coordinated approach we should leverage data and learnings from existing RDR captured in this review. Information regarding RDRs operating in Australia could be consolidated into a repository to support the development of new RDRs. Aligning with the previous work of EPIRARE and the EU RD Platform in defining a minimum data set, a national set of best practice principles for RDRs in Australia could be developed, using the ACFDR as an exemplar. This could include principles of success derived from this review, as well as individual RDR experiences, including:
Clinician-led, person-centred governance.
Low burden approvals.
Ongoing sustainable funding.
Low burden data collection & linkage with existing data sources.
Mechanisms for consumer contribution.
Prospective, incidence-focused registries that address knowledge gaps.
Enablement of multiple data uses, with consumer opt-out provisions.
Broad data uses, including for reporting, epidemiology, secondary research, access to clinical trials.
Support consumer connection and mechanisms to report back to registry participants.
Data interoperability/harmonization—data sharing/linkage between datasets/registries.
Research and data are one of the three key pillars in the Action Plan [13]. Limited data is a common feature of RDs, often resulting in high uncertainty, which impacts every part of people’s lives. People are faced with impossible choices based on incomplete knowledge and unclear pathways. In development of the Action Plan the sector highlighted comprehensive, high quality collection, and effective use of RD data as one of four critical enablers of effective RD policy [13]. RDRs have unique advantages that can be leveraged for success. These advantages include engaged communities who value data and knowledge and are highly attuned to the benefits of RDRs in facilitating access to participation in research and clinical trials. In addition, the benefits of novel therapies and precision medicine for RDs are likely to encourage the establishment of future RDRs for the support of post-marketing surveillance.
Strengths and limitations
This study has systematically and comprehensively reviewed publicly-reported data collected by RDRs with Australian participants, as reported in the literature. To appreciate the findings in this review, the following limitations should be considered. First, we have likely missed several RDRs and databases that collect RD information because a lot of data is kept in secure electronic health records despite our comprehensive search strategy, including an internet search in addition to a literature search of four large electronic databases. Second, most publications or grey literature sources did not provide detailed information on the data items captured by RDRs and databases. For state-wide and “umbrella” registries, the information and numbers reported are accurate at the date of publication of the source and for the particular condition, as these registries may also capture other RDs and conditions. We have identified a few state/jurisdiction and “umbrella” registries that capture data from various conditions, including RDs. Registry information is relevant to the conditions only listed in the tables. This has been noted in the legends and in the text. Since we focussed on the registries collecting data from people with RDs, we are aware that not all RDs captured by the state and “umbrella” registries may be described in this scoping review. Third, RDR literature did not usually report on the impact of registries on patient outcomes, nor did they detail enablers and barriers to meeting registry objectives. This requires further investigation and follow-up with registry managers.
Conclusion
The findings of this scoping review highlight the heterogeneity of RDRs collecting Australian data, with variations noted in the mechanisms for data entry, data items captured, scope, outputs, and sources of funding. This heterogeneity calls attention to the challenges of establishing and maintaining RDRs in Australia and the interest in a nationally consistent approach, as highlighted in the Action Plan [13]. Nevertheless, the different characteristics and needs of individual RDs in Australia make interoperability difficult, especially given the absence of a nationally consistent minimum dataset for RDs.
An initial step would be to consolidate information regarding existing RDRs to support the development of new registries. This should be followed by development of Australian RDRs best practice consensus guidelines, aligned with existing international standards, with goals to improve clinical practice and outcomes, access to research and clinical trials, and support the RD community. Investment in a nationally coordinated and consistent approach to data collection for RD in Australia will improve our understanding and quantification of the national burden of RDs, and their impact on patients, carers and families. A national strategy for RD data collection will also require cross-jurisdictional government engagement and agreement. This requires systemic policy reform, but is a critical way for governments to keep pace with international direction and respond to the Action Plan for the best possible health and wellbeing outcomes for Australians living with a RD.
Few of the seventy-four RDRs identified in this review were sustainable funded or reported regular research and quality improvement outputs. This suggests an under-investment in RDRs in Australia compared to more common conditions—diabetes and obesity, heart disease and cancer, which have been prioritised as areas for CQRs by the Australian Commission for Safety and Quality in Health Care [80]. Not many RDRs identified in this review outline a clear purpose or scope, and the data they collect is often incomplete and varied. These factors are contributing to poor data quality and poor levels of clinical awareness and knowledge. To achieve equity in data and knowledge of RD that is aligned with more common conditions, investment in RDRs is vital. Without such investment rare diseases cannot be counted and evidence-based improvements in care and outcomes will not keep pace with rapid advances in medical technologies, particularly in the areas of genomics and precision medicine.
Acknowledgements
We thank to the Scientific and Medical Advisory Committee of Rare Voices Australia. Susannah Ahern: In consultation with the Scientific and Medical Advisory Committee of Rare Voices Australia.
Abbreviations
- ACFDR
Australian Cystic Fibrosis Data Registry
- ABDR
Australian Bleeding Disorders Registry
- ACR
Australian Calciphylaxis Registry
- AILDR
Australasian Interstitial Lung Disease Registry
- ANZ
Australian and New Zealand
- ARNOLD
Australasian Registry Network of Orphan Lung Diseases
- CT
Computerized tomography
- DIPG
Diffuse Intrinsic Pontine Glioma
- ESID
European Society for Immunodeficiencies Registry
- EU
European Union
- ICGG
International Collaborative Gaucher Group
- ITP
Immune Thrombocytopenia
- LARDR
Lymphoma and Related Diseases Registry
- MeSH
Medical Subject Heading
- MRDR
Myeloma and Related Diseases Registry
- NAIT
Neonatal Alloimmune Thrombocytopenia
- PID-NET
German Network on Primary Immunodeficiency Diseases
- PRISMA-ScR
Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews
- PROMs
Patient-Reported Outcome Measures
- QoL
Quality of life
- RD
Rare disease
- RDR
Rare disease registry
- RVA
Rare Voices Australia
- UK
United Kingdom
- US
United States
Author contributions
RR, FH, NM, SA conceptualised the study and supported the study methodology. RR, MC, CM conducted the data analysis and drafted the original manuscript. FH, NM, PL and SA reviewed and edited the manuscript. All authors have read and approved the manuscript.
Funding
This research was funded by Rare Voices Australia.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
As a scoping review, no human was involved or participated in the study, with no necessity for ethical approval and consent to participate.
Consent for publication
As a scoping review, no human was involved or participated in the study, with no necessity for consent for publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lacaze P, Millis N, Fookes M, Zurynski Y, Jaffe A, Bellgard M, et al. Rare disease registries: a call to action. J Intern Med. 2017;47(9):1075–1079. doi: 10.1111/imj.13528. [DOI] [PubMed] [Google Scholar]
- 2.European Commission. Rare diseases. https://ec.europa.eu/health/non_communicable_diseases/rare_diseases_en. Accessed July 6, 2022.
- 3.United States Department of Health & Human Services 2019. FAQs about rare diseases. Available from: https://rarediseases.info.nih.gov/diseases/pages/31/faqs-about-rare-diseases
- 4.Australian Government Department of Health. What we’re doing about rare diseases: Australian Government; 2022. Available from: https://www.health.gov.au/health-topics/chronic-conditions/what-were-doing-about-chronic-conditions/what-were-doing-about-rare-diseases#:~:text=Around%208%25%20of%20Australians%20(2,of%20rare%20diseases%20are%20genetic.
- 5.Bhattacharya K, Millis N, Jaffe A, Zurynski Y. Rare diseases research and policy in Australia: on the journey to equitable care. J Paediatr Child Health. 2021;57(6):778–781. doi: 10.1111/jpc.15507. [DOI] [PubMed] [Google Scholar]
- 6.Jaffe A, Zurynski Y, Beville L, Elliott E. Call for a national plan for rare diseases. J Paediatr Child Health. 2010;46(1–2):2–4. doi: 10.1111/j.1440-1754.2009.01608.x. [DOI] [PubMed] [Google Scholar]
- 7.Lacaze P, Pinese M, Kaplan W, Stone A, Brion M-J, Woods RL, et al. The Medical Genome Reference Bank: a whole-genome data resource of 4000 healthy elderly individuals. Rationale and cohort design. Eur J Hum Genetics EJHG. 2019;27(2):308–316. doi: 10.1038/s41431-018-0279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kölker S, Gleich F, Mütze U, Opladen T. Rare disease registries are key to evidence-based personalized medicine: highlighting the European experience. Front Endocrinol. 2022;13:832063. doi: 10.3389/fendo.2022.832063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckland D. The pros and cons of a rare diseases registry. https://www.raconteur.net/healthcare/the-pros-and-cons-of-a-rare-diseases-registry/. Accessed 15 Nov 2022. 2021.
- 10.Coi A, Santoro M, Villaverde-Hueso A, Di Paola ML, Gainotti S, Taruscio D, et al. The quality of rare disease registries: evaluation and characterization. Public Health Genomics. 2016;19(2):108–115. doi: 10.1159/000444476. [DOI] [PubMed] [Google Scholar]
- 11.EURORDIS-NORD-CORD Joint Declaration of 10 Key Principles for Rare Disease Patient Registries. http://download2.eurordis.org/documents/pdf/EURORDIS_NORD_CORD_JointDec_Registries_FINAL.pdf. Accessed 6 July 2023.
- 12.Rare Voices Australia. https://rarevoices.org.au/. Accessed 6 July 2023.
- 13.The National Strategic Action Plan for Rare Diseases. https://rarevoices.org.au/action-plan/. Accessed 6 July 2023.
- 14.Zurynski Y, Frith K, Leonard H, Elliott E. Rare childhood diseases: how should we respond? Arch Dis Child. 2008;93(12):1071–1074. doi: 10.1136/adc.2007.134940. [DOI] [PubMed] [Google Scholar]
- 15.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 16.Michael M, Chantrill L, Price T, Chan DL, Wakelin K, Cummins M. Real-world management and patient perspectives on QOL with neuroendocrine tumors: an ANZ perspective. Asia Pac J Clin Oncol. 2021;17:3–10. doi: 10.1111/ajco.13587. [DOI] [PubMed] [Google Scholar]
- 17.PLANET Registry. https://neuroendocrine.org.au/planetregistry/. Accessed 14 Nov 2022.
- 18.Friedreich's Ataxia Global Patient Registry. https://www.curefa.org/patient-registry. Accessed 14 Nov 2022.
- 19.Mestre TA, Fitzer-Attas C, Giuliano J, Landwehrmeyer B, Sampaio C. Enroll-HD: a global clinical research platform for Huntington's disease. Mov Disord. 2016;31(Supplement 2):S366. doi: 10.1002/mdc3.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huntington's disease Research Participant Registry (University of Melbourne). http://hrgv.org.au/Research/Current%20Research/ParticipantRegistry.html. Accessed 14 Nov 2022.
- 21.Weinreb NJ, Camelo JS, Jr, Charrow J, McClain MR, Mistry P, Belmatoug N. Gaucher disease type 1 patients from the ICGG Gaucher Registry sustain initial clinical improvements during twenty years of imiglucerase treatment. Mol Genet Metab. 2021;132(2):100–111. doi: 10.1016/j.ymgme.2020.12.295. [DOI] [PubMed] [Google Scholar]
- 22.International Collaborative Gaucher Group (ICGG) Gaucher Registry. https://www.gaucherdisease.org/research/registry/. Accessed 10 Nov 2022.
- 23.Escobedo VS, Nguyen N, Teng D, Bui QM, Ma GS, Brambatti M, et al. Clinical features and outcomes for Danon disease: data from global registry. J Heart Lung Transplant. 2019;38(4 Supplement):S463. doi: 10.1016/j.healun.2019.01.1180. [DOI] [Google Scholar]
- 24.Global Retrospective Registry for Danon Disease. http://www.danondisease.org/. Accessed November 10, 2022.
- 25.van Zanten SEMV, Baugh J, Chaney B, De Jongh D, Aliaga ES, Barkhof F, et al. Development of the SIOPE DIPG network, registry and imaging repository: a collaborative effort to optimize research into a rare and lethal disease. J Neurooncol. 2017;132(2):255–266. doi: 10.1007/s11060-016-2363-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.International DIPG Registry (IDIPGR). https://dipgregistry.org/about/about-the-dipg-dmg-registry/. Accessed 15 Nov 2022.
- 27.International Dysferlinopathy Registry. https://dysferlinregistry.jain-foundation.org/. Accessed 15 Nov 2022.
- 28.International Schwannomatosis Database. https://sid2011.squarespace.com/. Accessed 15 Nov 2022. [Internet].
- 29.Australian Bleeding Disorders Registry. https://www.haemophilia.org.au/about-bleeding-disorders/abdr. Accessed 10 Nov 2022.
- 30.International Niemann-Pick Disease Registry. https://inpdr.org/. Accessed 15 Nov 2022.
- 31.Patterson MC, Mengel E, Wijburg FA, Muller A, Schwierin B, Drevon H, et al. Disease and patient characteristics in NP-C patients: findings from an international disease registry. Orphanet J Rare Dis. 2013;8:12. doi: 10.1186/1750-1172-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellgard MI, Napier KR, Bittles AH, Szer J, Fletcher S, Zeps N, et al. Design of a framework for the deployment of collaborative independent rare disease-centric registries: Gaucher disease registry model. Blood Cells Mol Dis. 2018;68:232–238. doi: 10.1016/j.bcmd.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Australian Bronchiectasis Registry. https://lungfoundation.com.au/research/our-research/bronchiectasis/. Accessed 15 Nov 2022.
- 34.Visser SK, Bye PTP, Fox GJ, Burr LD, Chang AB, Holmes-Liew CL, et al. Australian adults with bronchiectasis: the first report from the Australian Bronchiectasis Registry. Respir Med. 2019;155:97–103. doi: 10.1016/j.rmed.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 35.Neonatal Alloimmune Thrombocytopenia (NAIT) Registry. https://www.monash.edu/medicine/sphpm/units/transfusionresearch. Accessed 15 Nov 2022.
- 36.Scarborough R, Wood E, McQuilten Z, Holdsworth R, Crighton G, Savoia H, et al. Neonatal alloimmune thrombocytopenia (NAIT): initial data from the Australian registry. J Paediatr Child Health. 2015;51(SUPPL. 1):92. [Google Scholar]
- 37.International Pachyonychia Congenita Research Registry. https://www.pachyonychia.org/patient-registry/. Accessed 15 Nov 2022.
- 38.Forrest CE, Casey G, Mordaunt DA, Thompson EM, Gordon L. Pachyonychia Congenita: a spectrum of KRT6a mutations in Australian patients. Pediatr Dermatol. 2016;33(3):337–342. doi: 10.1111/pde.12841. [DOI] [PubMed] [Google Scholar]
- 39.GNE Myopathy Registry. https://www.gne-registry.org/. Accessed 3 Nov 2022.
- 40.ITP Natural History Study Registry. https://pdsa.org/covid-19. Accessed 15 Nov 2022.
- 41.The Australian & New Zealand Fontan Registry. https://www.fontanregistry.com/. 2009.
- 42.Bergin K, Moore E, McQuilten Z, Wood E, Augustson B, Blacklock H, et al. Design and development of the Australian and New Zealand (ANZ) myeloma and related diseases registry. BMC Med Res Methodol. 2016;16(1):151. doi: 10.1186/s12874-016-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.2020 Annual Report. Lymphoma and Related Diseases Registry (LaRDR). https://lardr.org/wp-content/uploads/2021/05/20210429_LaRDR_AnnualReport.pdf. 2020.
- 44.Moore I, Wrobel J, Rhodes J, Lin Q, Webster S, Jo H, et al. Australasian interstitial lung disease registry (AILDR): objectives, design and rationale of a bi-national prospective database. BMC Pulm Med. 2020;20(1):257. doi: 10.1186/s12890-020-01297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Global Angelman Syndrome Registry. https://angelmanregistry.info/. Accessed 6 July 2023.
- 46.Tones M, Cross M, Simons C, Napier KR, Hunter A, Bellgard MI, et al. Research protocol: the initiation, design and establishment of the Global Angelman Syndrome Registry. J Intellect Disabil Res. 2018;62(5):431–443. doi: 10.1111/jir.12482. [DOI] [PubMed] [Google Scholar]
- 47.Cantu Syndrome Registry. https://cantu.wustl.edu/registry/. Accessed November 15, 2022.
- 48.Grange DK, Roessler HI, McClenaghan C, Duran K, Shields K, Remedi MS, et al. Cantú syndrome: findings from 74 patients in the International Cantú Syndrome Registry. Am J Med Genet C Semin Med Genet. 2019;181(4):658–681. doi: 10.1002/ajmg.c.31753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Souzeau E, Goldberg I, Healey PR, Mills RAD, Landers J, Graham SL, et al. Australian and New Zealand Registry of Advanced Glaucoma: methodology and recruitment. Clin Exp Ophthalmol. 2012;40(6):569–575. doi: 10.1111/j.1442-9071.2011.02742.x. [DOI] [PubMed] [Google Scholar]
- 50.Freilinger M, Bebbington A, Lanator I, De Klerk N, Dunkler D, Seidl R, et al. Survival with Rett syndrome: comparing Rett's original sample with data from the Australian Rett syndrome database. Dev Med Child Neurol. 2010;52(10):962–965. doi: 10.1111/j.1469-8749.2010.03716.x. [DOI] [PubMed] [Google Scholar]
- 51.Ruderman I, Toussaint ND, Hawley CM, Krishnasamy R, Pedagogos E, Lioufas N, et al. The Australian Calciphylaxis Registry: reporting clinical features and outcomes of patients with calciphylaxis. Nephrol Dial Transplant Off Publ Eur Dial Transplant Assoc Eur Renal Assoc. 2021;36(4):649–656. doi: 10.1093/ndt/gfz256. [DOI] [PubMed] [Google Scholar]
- 52.Hickey GL, Grant SW, Cosgriff R, Dimarakis I, Pagano D, Kappetein AP, et al. Clinical registries: governance, management, analysis and applications. Eur J Cardiothorac Surg. 2013;44(4):605–614. doi: 10.1093/ejcts/ezt018. [DOI] [PubMed] [Google Scholar]
- 53.Yashodhara B, Gayatri I, Priya KA, Subhadra P, Konda JK, Qurratulain H. Rare disease advocacy groups and their significance in diagnosis, management, treatment, and prevention of rare diseases. In: He WZ, editor. Rare diseases. Rijeka: IntechOpen; 2019. p. 11. [Google Scholar]
- 54.AHRQ Methods for Effective Health Care. In: Gliklich RE, Dreyer NA, Leavy MB, editors. Registries for evaluating patient outcomes: a user’s guide. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014. [PubMed]
- 55.Taruscio D, Mollo E, Gainotti S, De La Paz MP, Bianchi F, Vittozzi L. The EPIRARE proposal of a set of indicators and common data elements for the European platform for rare disease registration. Archives of Public Health. 2014;72(1):35. doi: 10.1186/2049-3258-72-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taruscio D, Vittozzi L, Choquet R, Heimdal K, Iskrov G, Kodra Y, et al. National registries of rare diseases in Europe: an overview of the current situation and experiences. Public Health Genomics. 2015;18(1):20–25. doi: 10.1159/000365897. [DOI] [PubMed] [Google Scholar]
- 57.Vittozzi L, Gainotti S, Mollo E, Donati C, Taruscio D. A model for the European platform for rare disease registries. Public Health Genomics. 2013;16(6):299–304. doi: 10.1159/000355935. [DOI] [PubMed] [Google Scholar]
- 58.Taruscio D, Mollo E, Gainotti S, de la Paz MP, Bianchi F, Vittozzi L. The EPIRARE proposal of a set of indicators and common data elements for the European platform for rare disease registration. Arch Public Health. 2014;72(1):35. doi: 10.1186/2049-3258-72-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruseckaite R, Maharaj AD, Dean J, Krysinska K, Ackerman IN, Brennan AL, et al. Preliminary development of recommendations for the inclusion of patient-reported outcome measures in clinical quality registries. BMC Health Serv Res. 2022;22(1):276. doi: 10.1186/s12913-022-07657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahern S, Ruseckaite R, Ackerman IN. Collecting patient-reported outcome measures. Intern Med J. 2017;47(12):1454–1457. doi: 10.1111/imj.13633. [DOI] [PubMed] [Google Scholar]
- 61.AHRQ Methods for Effective Health Care. In: Gliklich RE, Dreyer NA, Leavy MB, Christian JB, editors. 21st Century Patient Registries: registries for evaluating patient outcomes: a user’s guide: (3rd Edition), Addendum. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018. [PubMed]
- 62.Hoque DME, Kumari V, Hoque M, Ruseckaite R, Romero L, Evans SM. Impact of clinical registries on quality of patient care and clinical outcomes: a systematic review. PLoS ONE. 2017;12(9):e0183667. doi: 10.1371/journal.pone.0183667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.National Strategy for Clinical Quality Registries and Virtual Registries.https://www.health.gov.au/sites/default/files/documents/2021/02/national-clinical-quality-registry-and-virtual-registry-strategy-2020-2030.pdf. Accessed 6 July 2023.
- 64.Casamento K, Laverty A, Wilsher M, Twiss J, Gabbay E, Glaspole I, et al. Assessing the feasibility of a web-based registry for multiple orphan lung diseases: the Australasian Registry Network for Orphan Lung Disease (ARNOLD) experience. Orphanet J Rare Dis. 2016;11:42. doi: 10.1186/s13023-016-0389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahern S, Salimi F, Caruso M, Ruseckaite R, Bell S, Burke N. The ACFDR Registry Annual Report, 2020. Monash University, Department of Epidemiology and Preventive Medicine, July 2021, Report No. 22. 2021.
- 66.Ahern S, Sims G, Earnest A, Bell SC. Optimism, opportunities, outcomes: the Australian Cystic Fibrosis Data Registry. Intern Med J. 2018;48(6):721–723. doi: 10.1111/imj.13807. [DOI] [PubMed] [Google Scholar]
- 67.Guo J, Liu P, Chen L, Lv H, Li J, Yu W, et al. National rare diseases registry system (NRDRS): China’s first nation-wide rare diseases demographic analyses. Orphanet J Rare Dis. 2021;16(1):515. doi: 10.1186/s13023-021-02130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taruscio D, Kodra Y, Ferrari G, Vittozzi L. The Italian national rare diseases registry. Blood Transfus. 2014;12(Suppl 3):s606–s613. doi: 10.2450/2014.0064-14s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Orphanet Report Series—Rare Disease Registries in Europe. 2021. https://www.orpha.net/orphacom/cahiers/docs/GB/Registries.pdf. Accessed 6 July 2023.
- 70.Stanimirovic D, Murko E, Battelino T, Groselj U. Development of a pilot rare disease registry: a focus group study of initial steps towards the establishment of a rare disease ecosystem in Slovenia. Orphanet J Rare Dis. 2019;14(1):172. doi: 10.1186/s13023-019-1146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scheible R, Kadioglu D, Ehl S, Blum M, Boeker M, Folz M, et al. Enabling external inquiries to an existing patient registry by using the open source registry system for rare diseases: demonstration of the system using the European Society for Immunodeficiencies Registry. JMIR Med Inform. 2020;8(10):e17420. doi: 10.2196/17420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scheible R, Rusch S, Guzman D, Mahlaoui N, Ehl S, Kindle G. The NEW ESID online database network. Bioinformatics. 2019;35(24):5367–5369. doi: 10.1093/bioinformatics/btz525. [DOI] [PubMed] [Google Scholar]
- 73.Scott CM, Wong EM, Joo JE, Dugue P-A, Jung C-H, O'Callaghan N, et al. Genome-wide DNA methylation assessment of 'BRCA1-like' early-onset breast cancer: data from the Australian breast cancer family registry. Exp Mol Pathol. 2018;105(3):404–410. doi: 10.1016/j.yexmp.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gehring S, Eulenfeld R. German medical informatics initiative: unlocking data for research and health care. Methods Inf Med. 2018;57(S01):e46–e49. doi: 10.3414/ME18-13-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Open Source Initiative. About the open source initiative. https://opensource.org/about. Accessed 6 July 2023.
- 76.Prokosch HU, Acker T, Bernarding J, Binder H, Boeker M, Boerries M, et al. MIRACUM: medical informatics in research and care in university medicine. Methods Inf Med. 2018;57(S01):e82–e91. doi: 10.3414/ME17-02-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prasser F, Kohlbacher O, Mansmann U, Bauer B, Kuhn KA. Data integration for future medicine (DIFUTURE) Methods Inf Med. 2018;57(S01):e57–e65. doi: 10.3414/ME17-02-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DataSHIELD. A software solution for secure bioscience collaboration. https://www.datashield.org/. Accessed 6 July 2023.
- 79.de Arruda Botelho Herr M, Graf M, Placzek P, König F, Bötte F, Stickel T, et al. Bringing the algorithms to the data—secure distributed medical analytics using the personal health train (PHT-meDIC). https://arxiv.org/pdf/2212.03481.pdf. 2022.
- 80.Australian Commission for Safety and Quality in Health Care. https://www.safetyandquality.gov.au/. Accessed 6 July 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.