Abstract
The challenges in controlling the pandemic have been exacerbated by the disease’s morbidity and the emergence of additional COVID-19 variants. The use of emergency vaccines to circumvent these challenges has sparked mixed opinions on their effectiveness. Therefore, we conducted a meta-analysis to assess the efficacy of COVID-19 vaccines on clinical outcomes such as incidence, hospitalization, and ventilation rates in both vaccinated and unvaccinated patients. PubMed, Google Scholar, and Cochrane Central Register of Clinical Trials were searched on April 21, 2022, to extract published articles comparing vaccinated COVID-19 patients versus unvaccinated COVID-19 patients and their clinical outcomes. The clinical outcomes studied were incidence rate, intensive care unit (ICU) admission, mechanical ventilation, and hospitalization rates. The analysis was performed with Review Manager (RevMan) software. Random-effect models were used to calculate pooled odds ratio and corresponding 95% confidence interval (CI). In our meta-analysis, we have identified a total of 250 published findings, encompassing 15 studies that involved a cumulative count of 24,164,227 individuals diagnosed with COVID-19. Being unvaccinated had a significant association with severe clinical outcomes in patients infected with COVID-19. Unvaccinated individuals were 2.36 times more likely to be infected, with a 95% CI ranging from 1.13 to 4.94 (p = 0.02). Unvaccinated subjects with COVID-19 infection were 6.93 times more likely to be admitted to the ICU than their vaccinated counterparts, with a 95% CI ranging from 3.57 to 13.46 (p < 0.0001). The hospitalization rate was 3.37 higher among the unvaccinated compared to those vaccinated, with a 95% CI ranging from 1.92 to 5.93 (p < 0.0001). In addition, patients with COVID-19 infection who are unvaccinated were 6.44 times more likely to be mechanically ventilated than those vaccinated, with a 95% CI ranging from 3.13 to 13.23 (p < 0.0001). Overall, our study revealed that vaccination against COVID-19 disease is beneficial and effective in mitigating the spread of the infection and associated clinical outcomes. However, more awareness and proper education must be made to increase vaccine acceptance. We, therefore, recommend and urge all stakeholders involved in COVID-19 prevention, management, and control to strengthen awareness and educate the people on the effectiveness of COVID-19 vaccination.
Keywords: covid-19 vaccine progress, coronavirus disease (covid-19), covid-19 vaccine, vaccination status, incidence rate, icu admissions, mechanical ventilation, rate of hospitalization
Introduction and background
The Coronaviridae family of viruses includes the recently described severe acute respiratory syndrome coronavirus (SARS-CoV-2), which is the underlying etiology of COVID-19. This mRNA virus causes an inflammatory respiratory infection that mainly affects the lower respiratory tract and is marked by a rise in proinflammatory cytokines like interleukin 6 (IL-6) and interferon-gamma (IFN-γ). The symptoms can range from mild to severe acute respiratory distress syndrome, which can also be accompanied by a fulminant systemic inflammatory response, possibly leading to multiorgan failure and death [1].
As new COVID-19 variations emerge, the Centers for Disease Control and Prevention (CDC) reports an ever-changing virus epidemiology. In North America, there have been 91.8 million cases and 1.4 million deaths since the pandemic began, with 82,217,041 cases and 993,959 deaths in the United States [2]. The case fatality ratio in the United States is 1.2%, while it is 1.1% in Canada and 5.6% in Mexico [2]. In North America, a total of 865,103,192 COVID-19 vaccine doses have been administered, with 575,410,180 doses provided in the United States, comprising single and booster doses [2]. According to the US Coronavirus vaccine tracker, 78% of the population has received at least one dose, and 66% received three doses [3]. Thirty-one percent of the population has received at least one booster dose. On the other hand, in Canada and Mexico, 81.4% and 61.2% of their populations received at least two doses, respectively [4].
As a result of the disease's lethality, researchers are constantly working to combat the virus's devastating effects on both the therapeutic and preventive fronts. Vaccination is considered the safest method of preventing future COVID-19 infections and related clinical outcomes. However, the emergency use authorization of COVID-19 vaccines has sparked skepticism and hampered vaccination acceptance. This could be due to a lack of trust in public health policies, concerns about vaccine safety, or a general aversion to vaccination [5]. In addition, the public appears divided about whether vaccination status affects any component of the COVID-19 disease course. Therefore, we conducted a systematic review and meta-analysis to evaluate the relationship between the clinical outcomes and vaccination status in infected subjects to shed more light on this severe infectious disease.
Review
Materials and methods
Study Design
A meta-analysis of studies was conducted to assess the effectiveness of COVID-19 vaccination. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed to review the studied articles [6]. This review was registered in PROSPERO 2022 (359550).
Eligibility Criteria
The inclusion criteria were studies that reported the effectiveness of COVID-19 vaccination on patient outcomes with COVID-19 infection and studies published after the availability of COVID-19 vaccines. All types of COVID-19 vaccines approved in North America were included in this review.
Population (P): We included cross-sectional, case-control, cohort studies, and randomized controlled trials from North America published in English from March 17, 2020, to July 10, 2022. Case series/reports, conference papers, proceedings, articles available only in abstract form, editorial reviews, letters of communication, commentaries, systematic reviews, and qualitative studies were excluded. Articles in languages other than English or study areas beyond North America were also excluded.
Intervention (I): All types of COVID-19 vaccines approved in North America were included in this review.
Comparison (C): We included studies that compared two groups of patients according to their vaccination status. Individuals who received at least one dose of any COVID-19 vaccine were placed in the "vaccinated group," while individuals who did not receive any vaccine were placed in the "unvaccinated group."
Outcomes (O): Our primary outcome measures were incidence, hospitalization, intensive care unit (ICU) admission rates, and the need for mechanical ventilation.
Information Sources
On April 21, 2022, a systematic search was performed. We searched three databases: PubMed, Cochrane, and Google Scholar. A snowball search to identify additional studies was carried out by searching the reference lists of publications eligible for full-text review and using Google Scholar to locate and screen studies citing them. Finally, we updated the database search on July 7, 2022, and the snowball and additional searches on July 8, 2022.
Search Strategy
The search was done using the generic free-text search terms developed based on the Patient/Population, Intervention, Comparable group,/Control, and Outcomes (PICO) model to define the clinical question and aid in finding clinically relevant evidence in the literature. P = "COVID-19" AND "NORTH AMERICA, I = "COVID-19 VACCINE, C = "VACCINATION STATUS" OR "VACCINATED" AND "UNVACCINATED," O = "INCIDENCE RATE" OR "NUMBER OF DAYS ILL" OR "NO OF DAYS ON ADMISSION, "OR "NEED FOR ASSISTED VENTILATION" OR "MORTALITY." The search terms were kept broad to encompass all possibilities for applicable studies. All studies published from March 17, 2020, to July 10, 2022, were retrieved to assess their eligibility for inclusion in this study. We limited our search to full-text articles in the English language. To find additional eligible studies, reference lists of included citations were cross-checked.
Selection Process
All records identified by our search strategy were exported to Rayyan Intelligent Systematic Review software (Rayyan System Inc. Cambridge, Massachusetts, USA). Duplicate articles were removed from the list. Three researchers (AO, AI, and OP) independently reviewed the title and abstract of the first 100 records and discussed inconsistencies until consensus was obtained. Then, in pairs, the researchers screened the titles and abstracts of all articles retrieved. In the case of disagreement, a final agreement on which articles to screen full-text was made by discussion. When necessary, the third researcher was consulted to make the final decision.
Additionally, two researchers (referred to as AO and AI) independently evaluated complete articles to determine their suitability for inclusion. In instances where there were disagreements, a consensus on eligibility was reached through discussion, and if necessary, a third researcher (OP) was consulted. The search methodology was documented in the PRISMA flow chart, which depicted the studies that were included as well as those excluded with accompanying justifications. Exclusion reasons included: reason 1: absence of comparable groups (i.e., vaccinated vs. unvaccinated), reason 2: unavailability of the complete text, and reason 3: lack of relevance to the research question, encompassing insufficient data on patient health outcomes, as illustrated in Figure 1.
Figure 1. Flow diagram of the inclusion criteria of studies found eligible in the meta-analysis.
* indicates studies reviewed. ** indicates studies excluded.
Data Collection Process
We designed a data extraction form that two review authors (MFM and KO) used to extract data from eligible studies. Extracted data were compared, with discrepancies resolved through discussion. KO entered data into Review Manager 5 software (Review Manager 2014), double-checking this for accuracy.
Data Outcomes
The data included the first author, year of publication, study location, study design, setting, population characteristics of the COVID-19 patients with various comorbidities, number of doses, sample size, proportion, and data to calculate effect estimates. The outcomes recorded for the meta-analysis were the incidence, hospitalization, ICU admission, and need for assisted ventilation due to COVID-19 infection. The incidence was defined as the number of new COVID-19 infections, considering the population at risk since 2020 [7]. Hospitalization with COVID-19 was defined as admission within 14 days of testing positive for SARS-CoV-2 via PCR test and included those who tested positive within two days of discharge [7]. Assisted ventilation was defined as using devices to support respiration in hospitalized COVID-19 patients [7]. Finally, ICU admission was defined as the number of patients admitted into the ICU after a positive PCR test for COVID-19.
Effect Measures
The effectiveness of COVID-19 vaccination on infected patients was reported in pooled estimate proportion with a 95% confidence interval. In addition, we analyzed dichotomous outcomes by calculating each study's risk ratio (RR) of a patient outcome (i.e., incidence, hospitalization, ICU admission, and need for mechanical ventilation).
Synthesis Methods
The analysis was performed with Review Manager (RevMan, RevMan International Inc., New York, USA) software. First, a generic inverse variance with a random-effects model was applied to pool the proportion of the studies' data. The heterogeneity was assessed by I2 statistic and p-value. If the p-value is < 0.05 or I2 > 50%, the assumption of homogeneity was rejected, and a random-effects model was adopted.
Study Risk of Bias Assessment
Critical appraisal for data quality was assessed using the Joanna Briggs Institute (J.B.I.) meta-analysis for cross-sectional, case-control, cohort studies, and randomized clinical trials. The risk of bias for the observation study (case-control and cohort) was assessed by nine criteria: appropriateness of the sample frame; appropriateness of study participants sampled; adequate sample size, description of study subjects and the setting; sample size justification; power description, or variance and effect estimates; valid methods for the identification of the condition; a standard and reliable requirement measured; appropriateness of statistical analysis; and adequate response rate. The criteria of the risk assessment were represented by "yes," "no," "unclear," or "not available." The score for yes was one (1) and zero (0) for the rest. The risk of bias was considered low when the total score was more than 70%, moderate when 50%-69%, and high when up to 0-49% (ref). Two authors performed bias assessments independently.
Results
We identified 250 published papers in database searching. These articles included a total of 24,164,227 COVID-19 patients. From the initial search, 240 articles in total were identified from PubMed and 10 from the Cochrane database. After duplicate removal, 167 articles were excluded based on the inclusion and exclusion criteria. We finally selected 14 articles for the meta-analysis (Table 1).
Table 1. Sample size of selected studies and their characteristics.
MMWR: Morbidity and Mortality Weekly Report, CDC: Centers for Disease Control and Prevention, MR: mortality rate, ICU: intensive care unit, HR: hospitalization, MV: need for mechanical ventilation, CC: COVID cases, VT: tidal volume.
| Author (Year) | Study Area | Study Type Design | Type of Journal | Total Number of Patients | Outcomes Analyzed | Vaccine Type |
| Corchado-Garcia et al. (2021) [8] | USA | Retrospective cohort | JAMA Network | 97,787 | CC | Janssen (J&J) |
| Naleway et al. (2021) [9] | USA | Retrospective cohort | MMWR (CDC) | 482,464 | HR, MV, CC, ICU, MR | Pfizer, Moderna, Janssen (J&J) |
| Johnson et al. (2022) [10] | USA | Retrospective cohort | MMWR (CDC) | 9,678,557 | CC, MR | Unspecified |
| Danza et al. (2022) [11] | USA | Cross-sectional | MMWR (CDC) | 422,966 | HR, MV, ICU, MR, | Pfizer, Moderna, Janssen (J&J) |
| White et al. (2021) [12] | USA | Retrospective cohort | The New England Journal of Medicine | 22,232 | CC | Pfizer, Moderna |
| Dunkle et al. (2022) [13] | USA, Mexico | Randomized control trial | The New England Journal of Medicine | 25,452 | CC | Novavax |
| Olson et al. (2022) [14] | USA | Case-control | The New England Journal of Medicine | 1,222 | HR, VT, ICU, MR | Pfizer |
| Polinski et al. (2022) [15] | USA | Retrospective cohort | JAMA Network | 2,067,431 | HR, CC | Janssen (J&J) |
| Griffin et al. (2021) [16] | USA | Cross-sectional | MMWR (CDC) | 43,127 | HR, MV, CC, ICU, MR | Pfizer, Moderna, Janssen (J&J) |
| Tenforde et al. (2021) [17] | USA | Case-control | MMWR (CDC) | 1,440 | Unspecified | |
| Taylor et al. (2021) [18] | USA | Cross-sectional | MMWR (CDC) | 7,615 | Unspecified | |
| Chung et al. (2021) [19] | Canada | Cross-sectional | The BMJ | 324,033 | CC | Unspecified |
| Tenforde et al. (2022) [20] | USA | Case-control | JAMA Network | 1,983 | HR, MR | Pfizer, Moderna |
| Xu et al. (2022) [21] | USA | Retrospective cohort | MMWR (CDC) | 10,987,919 | MR | Pfizer, Moderna, Janssen (J&J) |
In a review examining the effectiveness of COVID-19 vaccination on patient outcomes, the authors designed tables presenting each included study, the citations, study design, country, sample size, median age, gender, and ethnic distribution of vaccinated and unvaccinated patients (Tables 2-4). Patient comorbidities and types of COVID-19 vaccine used for various studies have also been elaborated in Tables 5, 6. In this analysis, the incidence of COVID-19 infection, hospitalization, ICU admission, and mechanical ventilation in various studies are considered clinical outcomes in patients with COVID-19 infection.
Table 2. Summary of data on demographics.
| Author (Year) | Total Number of Patients | N (%) Vaccinated | N (%) Unvaccinated | N (%) Female | N (%) Male | Age Range of Patients |
| Corchado-Garcia et al. (2021) [8] | 97,787 | 8,889 (9.1) | 88,898 (90.9) | 48,537 (49.6) | 49,239 (50.4) | 18-75 |
| Naleway et al. (2021) [9] | 482,464 | 344,848 (71.5) | 137,616 (28.5) | 251,552 (52.1) | 230,552 (47.8) | 18-75 |
| Johnson et al. (2022) [10] | 9,678,557 | 2,866,517 (29.6) | 6,812,040 (70.4) | - | - | 18-65+ |
| Danza et al. (2022) [11] | 422,966 | 281,038 (66.4) | 141,928 (33.6) | 224,173 (53) | 184,134 (43.5) | 18-80+ |
| White et al. (2021) [12] | 22,232 | 18,242 (82.1) | 3,990 (17.9) | - | - | |
| Dunkle et al. (2022) [13] | 25,452 | 17,312 (68) | 8,140 (32) | 12,271 (48.2) | 13,181 (51.8) | 18-95 |
| Olson et al. (2022) [14] | 1,222 | 345 (28.2) | 868 (71.8) | - | - | 12-18 |
| Polinski et al. (2022) [15] | 2,067,431 | 422,034 (20.4) | 1,645,397 (79.6) | 1,159,374 (56) | 908,057 (43.9) | 18+ |
| Griffin et al. (2021) [16] | 43,127 | 12,326 (28.6) | 30,801 (71.4) | 21,743 (50.4) | 20,425 (47.4) | 16-80+ |
| Tenforde et al. (2021) [17] | 1,440 | 307 (21.3) | 1,133 (78.7) | 598 (41.5) | 842 (58.5) | 18+ |
| Taylor et al. (2021) [18] | 7,615 | 782 (10.3) | 6,061 (89.7) | 3,255 (42.7) | 3,568 (46.9) | 18-65+ |
| Chung et al. (2021) [19] | 324,033 | 21,272 (6.6) | 302,761 (93.4) | 185,539 (57.3) | 138,494 (42.7) | 16-80+ |
| Tenforde et al. (2022) [20] | 1,983 | 314 (15.8) | 1,669 (84.2) | 969 (48.9) | 1,014 (51.1) | 18-65+ |
| Xu et al. (2022) [21] | 10,987,919 | 6,398,361 (58.2) | 4,589,557 (41.8) | 5,946,533 (54.1) | 5,041,385 (45.9) | 12-85+ |
Table 4. Summary of data on demographics of unvaccinated patients.
| Author (Year) | N (%) Unvaccinated | N (%) Female | N (%) Male | Mean/Median Age | N (%) White | N (%) Asian | N (%) Black | N (%) Hispanic | N (%) Native American | N (%) Native Hawaiian/Pacific Islander | N (%) Multiple Races/Others/Unknown |
| Corchado-Garcia et al. (2021) [8] | 88,898 | 44,140 (49.7) | 44,748 (50.3) | 51.7±16.7 | 79,692 (89.6) | 1,605 (1.8) | 2 281 (2.6) | - | 267 (0.3) | 100 (0.1) | 3,953 (4.4) |
| Naleway et al. (2021) [9] | 137,616 | 63,841 (46.4) | 73,592 (53.5) | 37 | 83,474 (60.7) | 3,930 (2.9) | 4 851 (3.5) | - | 588 (0.4) | 1,021 (0.7) | 43,752 (31.8) |
| Johnson et al. (2022) [10] | 6,812,040 | - | - | - | - | - | - | - | - | - | - |
| Danza et al. (2022) [11] | 141,928 | 69,382 (48.9) | 66,163 (46.6) | 35 | 20,529 (14.5) | 7,451 (5.2) | 12,319 (8.7) | - | 342 (0.2) | 1,429 (1) | 19,214 (1305) |
| White et al. (2021) [12] | 3,990 | - | - | - | - | - | - | - | - | - | - |
| Dunkle et al. (2022) [13] | 8,140 | 4,009 (49.3) | 4,131 (50.7) | 47 | 6,184 (76) | 366 (4.5) | 900 (11) | - | 498 (6.1) | 10 (0.1) | 177 (2.2) |
| Olson et al. (2022) [14] | 868 | - | - | 15 | 358 (41.2) | - | 197 (22.7) | 191 (22) | - | - | 122(14) |
| Polinski et al. (2022) [15] | 1,645,397 | 922,937 (56.1) | 722,460 (43.9) | 54.5±17.5 | - | - | - | - | - | - | - |
| Griffin et al. (2021) [16] | 30,801 | 15,472 (50.2) | 14,517 (47.1) | 32 | 5,620 (18.2) | 961 (3.1) | 4,755 (15.4) | 10,183 (33.1) | 51 (0.2) | 161 (0.5) | 8,551 (27.8) |
| Tenforde et al. (2021) [17] | 1,133 | 463 (40.9) | 670 (59.1) | 55 | 638 (56.3) | - | 200 (17.7) | 200 (17.7) | - | - | 95 (8.4) |
| Taylor et al. (2021) [18] | 6,061 | 2,897 (47.8) | 3,144 (51.9) | - | 2,894 (47.7) | 405 (6.7) | 1 839 (30.3) | 841 (13.9) | 63 (1) | - | - |
| Chung et al. (2021) [19] | 302,761 | 170,280 (56.2) | 132,481 (43.8) | - | - | - | - | - | - | - | |
| Tenforde et al. (2022) [20] | 1,669 | 831 (49.8) | 838 (50.2) | 53 | 717 (43) | - | 453 (27.1) | 381 (22.8) | - | - | 118 (7.1) |
| Xu et al. (2022) [21] | 4,589,557 | 2,498,171 (54.4) | 2,091,386 (45.6) | - | 1,982,834 (43.2) | 633,212 (13.8) | 262,766 (5.7) | 1,201,784 (26.2) | - | - | 508,961 (11.1) |
Table 5. Summary of patient comorbidities for vaccinated patients.
| Author (Year) | Total number vaccinated | N (%) chronic kidney disease | N (%) Diabetes | N (%) Chronic lung disease | N (%) cardiovascular disease | N (%) Immunodeficiency disorder | N (%) Neuromuscular/Neurological disorder |
| Corchado-Garcia et al. (2021) [8] | 8,889 | - | - | - | - | - | - |
| Naleway et al. (2021) [9] | 344,848 | 32 (0.009) | 24 (0.007) | 24 (0.007) | - | - | - |
| Johnson et al. (2022) [10] | 2,866,517 | - | - | - | - | - | 10 (0.0003) |
| Danza et al. (2022) [11] | 281,038 | - | - | - | - | - | - |
| White et al. (2021) [12] | 18,242 | - | - | - | - | - | - |
| Dunkle et al. (2022) [13] | 17,312 | - | - | - | - | - | - |
| Olson et al. (2022) [14] | 345 | - | 28 (8.1) | 81 (23.5) | 27 (7.8) | - | - |
| Polinski et al. (2022) [15] | 422,034 | 21,904 (5.2) | 69,272 (16.5) | 50,486 (12) | 191,134 (45.3) | 1,563 (0.4) | 121,537 (28.8) |
| Griffin et al. (2021) [16] | 12,326 | - | - | - | - | - | - |
| Tenforde et al. (2021) [17] | 307 | - | 140 (45.6) | 91 (29.6) | 252 (82) | 123 (40) | - |
| Taylor et al. (2021) [18] | 782 | - | - | - | - | - | - |
| Chung et al. (2021) [19] | 21,272 | - | - | - | - | - | - |
| Tenforde et al. (2022) [20] | 314 | - | 112 (35.7) | 100 (31.8) | 236 (75.2) | 128 (40.8) | - |
| Xu et al. (2022) [21] | 6,398,361 | - | - | - | - | - | - |
Table 6. Summary of patient comorbidities for unvaccinated patients.
| Author (Year) | N (%) Unvaccinated | N (%) Chronic Kidney Disease | N (%) Diabetes | N (%) Chronic Lung Disease | N (%) Cardiovascular Disease | N (%) Immunodeficiency Disorder | N (%) Neuromuscular/Neurological Disorder |
| Corchado-Garcia et al (2021) [8] | 88,898 | - | - | - | - | - | - |
| Naleway et al (2021) [9] | 137,616 | 37 (0.03) | 98 (0.07) | 22 (0.02) | - | - | - |
| Johnson et al. (2022) [10] | 6,812,040 | - | - | - | - | - | 15 (0.0002) |
| Danza et al. (2022) [11] | 141,928 | - | - | - | - | - | - |
| White et al. (2021) [12] | 3,990 | - | - | - | - | - | - |
| Dunkle et al. (2022) [13] | 8,140 | - | - | - | - | - | - |
| Olson et al. (2022) [14] | 868 | - | 72 (8.3) | 241 (27.8) | 69 (8) | - | - |
| Polinski et al. (2022) [15] | 1,645,397 | 83,640 (5.08) | 267,659 (16.3) | 197,398 (12) | 741,703 (45.1) | 6,255 (0.38) | 472,062 (28.7) |
| Griffin et al. (2021) [16] | 30,801 | - | - | - | - | - | - |
| Tenforde et al. (2021) [17] | 1,133 | - | 323 (28.5) | 213 (18.8) | 571 (50.4) | 109 (9.6) | - |
| Taylor et al. (2021) [18] | 6,061 | - | - | - | - | - | - |
| Chung et al. (2021) [19] | 302,761 | - | - | - | - | - | - |
| Tenforde et al. (2022) [20] | 1,669 | - | 425 (25.5) | 327 (19.6) | 814 (48.8) | 191 (11.4) | - |
| Xu et al. (2022) [21] | 4,589,557 | - | - | - | - | - | - |
Table 3. Summary of data on demographics of vaccinated patients.
| Author (Year) | N (%) Vaccinated | N (%) Female | N (%) Male | Mean/Median Age | N (%) White | N (%) Asian | N (%) Black | N (%) Hispanic | N (%) Native American | N (%) Native Hawaiian/Pacific Islander | N (%) Multiple Races/Others/Unknown |
| Corchado-Garcia et al. (2021) [8] | 8,889 | 4,397 (49.5) | 4,491 (50.5) | 52.4±16.9 | 7,945 (89.4) | 191 (2.1) | 274 (3.1) | - | 31 (0.3) | 12 (0.1) | 436 (4.9) |
| Naleway et al. (2021) [9] | 344,848 | 187,711 (54.5) | 156,960 (45.5) | 50 | 242,110 (70.2) | 22,828 (6.6) | 8,224 (2.4) | - | 12,880 (0.4) | 1,931 (0.6) | 68,475 (19.9) |
| Johnson et al. (2022) [10] | 2,866,517 | - | - | - | - | - | - | - | - | - | |
| Danza et al. (2022) [11] | 281,038 | 154,791 (55.1) | 117,971 (42) | 36 | 46,612 (16.6) | 26 384 (9.4) | 15,991 (5.7) | - | 530 (0.2) | 2,348 (0.8) | 40,538 (14.4) |
| White et al. (2021) [12] | 18,242 | - | - | - | - | - | - | - | - | - | - |
| Dunkle et al. (2022) [13] | 17,312 | 8,262 (47.7) | 9,050 (52.3) | 47 | 13,140 (75.9) | 761 (4.4) | 1,893 (10.9) | - | 1,074 (6.2) | 47 (0.3) | 397 (2.3) |
| Olson et al. (2022) [14] | 345 | - | - | 16 | 143 (41.4) | - | 68 (19.7) | 94 (27.2) | - | - | 49 (14.2) |
| Polinski et al. (2022) [15] | 422,034 | 236,437 (56) | 185,597 (44) | 54.7±17.4 | - | - | - | - | - | - | - |
| Griffin et al. (2021) [16] | 12,326 | 6,271 (50.9) | 5,908 (47.9) | 36 | 3,718 (30.2) | 1,009 (8.2) | 819 (6.6) | 3 961 (32.1) | 19 (0.2) | 49 (0.4) | 2,447 (19.9) |
| Tenforde et al. (2021) [17] | 307 | 135 (44) | 172 (56) | 69 | 191 (62.2) | - | 49 (16) | 47 (15.4) | - | - | 20 (6.5) |
| Taylor et al. (2021) [18] | 782 | 358 (45.8) | 424 (54.2) | - | 480 (61.4) | 48 (6.1) | 157 (20.1) | 85 (10.9) | 11 (1.4) | - | - |
| Chung et al. (2021) [19] | 21,272 | 15,259 (71.7) | 6,013 (28.3) | 51.8 | - | - | - | - | - | - | - |
| Tenforde et al. (2022) [20] | 314 | 138 (44) | 176 (56) | 67 | 201 (64) | - | 55 (17.5) | 44 (14) | - | - | 14 (4.5) |
| Xu et al. (2022) [21] | 6,398,361 | 3,448,362 (53.9) | 2,949,999 (46.1) | - | 2,778,730 (43.4) | 633,212 (10) | 341,189 (5.3) | 1.409,187 (22) | - | - | 880,523 (13.8) |
Figure 2 displays for each study included in the meta-analysis the summary statistics (number of events and sample size) for the unvaccinated and vaccinated groups, the odds ratio and its 95% confidence interval, heterogeneity, and test for overall effect for the dichotomous outcome, and the incidence of COVID-19 infection. COVID-19 patients were compared between those who received the COVID-19 vaccine and those who did not. Seven studies, including 1,871,595 patients, reported having COVID-19 infection. The odds ratio of COVID-19 infection between patients with COVID-19 vaccination and patients without was 2.36, with a 95% CI ranging from 1.13 to 4.94. The result was statistically significant, indicating that unvaccinated patients with COVID-19 infection are 2.36 times more likely to have COVID-19 infection if exposed to the virus than vaccinated patients (p = 0.02). In addition, we did a heterogeneity test with results of I2 = 100%, p ≤ 0.00001.
Figure 2. COVID-19 infection by vaccination status.
M-H: Mantel-Haenszel statistics.
Figure 3 displays for each study included in the meta-analysis the summary statistics (number of events and sample size) for the unvaccinated and vaccinated groups, the odds ratio and its 95% confidence interval, heterogeneity, and test for overall effect for the dichotomous outcome, and ICU admission from COVID-19 infection. Clinical outcomes in COVID-19 patients were compared between the vaccinated and unvaccinated subjects. Four studies, including 1,789 patients, reported being admitted to the ICU. The odds ratio of ICU admission between patients with COVID-19 vaccination and patients without COVID-19 vaccination was 6.93, with a 95% CI ranging from 3.57 to 13.46. The result was statistically significant, indicating that unvaccinated patients are six times more likely to be admitted to the ICU than their vaccinated counterparts (p < 0.0001). In addition, we did a heterogeneity test with results of I2 = 94%, p ≤ 0.00001.
Figure 3. Intensive care unit (ICU) admission from COVID-19 infection by vaccination status.
M-H: Mantel-Haenszel statistics.
Figure 4 displays for each study included in the meta-analysis the summary statistics (number of events and sample size) for the unvaccinated and vaccinated groups, the odds ratio and its 95% confidence interval, heterogeneity, and test for overall effect for the dichotomous outcome, and hospitalization from COVID-19 infection. Patient outcomes of COVID-19 infection were compared between vaccinated and unvaccinated individuals. Seven studies, including 55,258 patients, reported hospitalization for COVID-19 infection. The odds ratio of hospitalization from COVID-19 infection between patients with COVID-19 vaccination and those without was 3.37, with a 95% CI ranging from 1.92 to 5.93. The result was statistically significant, which indicates that patients with COVID-19 infection who are unvaccinated are 3.37 times more likely to be hospitalized from COVID-19 infection compared to those with COVID-19 infection who are vaccinated (p < 0.0001). In addition, we did a heterogeneity test with results of I2 = 100%, p ≤ 0.00001.
Figure 4. Hospitalization from COVID-19 infection by vaccination status.
M-H: Mantel-Haenszel statistics.
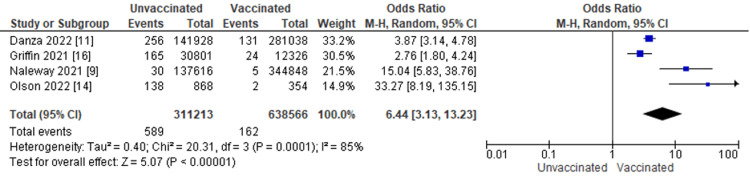
Figure 5 displays for each study included in the meta-analysis the summary statistics (number of events and sample size) for the unvaccinated and vaccinated groups, the odds ratio and its 95% confidence interval, heterogeneity, and test for overall effect for the dichotomous outcome, and need for mechanical ventilation from COVID-19 infection. Clinical outcomes in COVID-19 patients were compared between vaccinated and unvaccinated individuals. Four studies, including 751 patients, reported the need for mechanical ventilation. The odds ratio of mechanical ventilation between patients with COVID-19 vaccination and patients without COVID-19 vaccination was 6.44, with a 95% CI ranging from 3.13 to 13.23. The result was statistically significant, which indicates that patients with COVID-19 infection who are unvaccinated are 6.44 times more likely to be mechanically ventilated than those with COVID-19 infection who are vaccinated (p < 0.0001). In addition, we did a heterogeneity test with results of I2 = 85%, p = 0.0001.
Figure 5. Need for mechanical ventilation from COVID-19 infection by vaccination status.
M-H: Mantel-Haenszel statistics.
Discussion
This study evaluated the effectiveness of COVID-19 vaccination on patients with COVID-19 infection in North America. In this evaluation, several outcomes were analyzed among individuals aged 12-95 years using the following study designs: retrospective cohort, cross-sectional, case-control studies, and randomized control trials. These studies reveal that COVID-19 vaccination confers a certain level of protection against poor outcomes in COVID-19-infected individuals.
Incidence (Infection Rates)
Among patients infected with the COVID-19 disease, being unvaccinated had a higher likelihood of having poorer health outcomes. In addition, unvaccinated individuals had a higher risk of contracting COVID-19 infection, suggesting that vaccines play an important role in boosting the body's immune system and decreasing the severity of the infection in affected patients [8,9].
Comparing the incidence rate of COVID-19 infection among vaccinated and unvaccinated patients in North America, our analysis demonstrated a strong association between unvaccinated patients and COVID-19 infection. Specifically, our study showed that unvaccinated patients are 2.36 times more likely to have COVID-19 infection if exposed to the virus than vaccinated patients. This finding is consistent with the results of studies that highlighted that the risk of COVID-19 infection was highest for unvaccinated persons, and COVID-19 vaccines were protective against COVID-19 infection [10-13]. Also, a similar study demonstrated that the incidence rate among fully vaccinated persons was three times lower than in unvaccinated persons across all sex, race, ethnicity, and age groups evaluated in the study. In addition, another similar retrospective cohort study showed that a single dose of the COVID-19 vaccine effectively protected against recorded COVID-19 infections [14,15]. However, COVID-19 infections were observed in vaccinated individuals but were rare (0.7% of vaccinated individuals) [8]. Most studies that analyzed the incidence rate of COVID-19 infection suggested similar outcomes; however, a retrospective cohort study showed a substantial case rate increase in COVID-19 infection recorded among both vaccinated and unvaccinated persons [10,15]. However, the findings in this study are subject to at least two limitations; first, variable data linkage completeness might have resulted in misclassification, and second, their data represented 62% of the overall US population and, therefore, might not be generalizable.
Hospitalization Rates
COVID-19 vaccination plays a significant role in reducing hospital admission. Numerous other literature reviews and meta-analytical studies across the globe echo the same outcomes. All six studies that analyzed hospitalization rates by vaccination status consistently showed statistically significantly higher odds of hospitalization in the unvaccinated than the vaccinated, individually and collectively [14-20]. In addition, the mean age of individuals hospitalized with COVID-19 infection was generally higher among vaccinated people than among unvaccinated. While this systematic review did not stratify the efficacy of the COVID-19 vaccine on hospitalization rate according to the number of vaccine doses received or vaccine type, the literature indicates that mRNA vaccines have an efficacy rate of 85% on hospitalizations with COVID-19 [17]. COVID-19 vaccine has recounted a variability of protection after a single dose of vaccine with an average duration of about 180 days, accounting for the reduced hospitalization in participants with at least a single dose [22]. Another study revealed significantly lower hospitalization rates after at least two doses of the COVID-19 vaccine [22].
Further evidence shows that booster shots provide even better protection against hospitalization from COVID-19 infection as they prolong the duration of protection [11]. Booster shots also offer added protection against certain COVID-19 variants [11]. The hospitalization rate was also generally higher among seniors (65+ years) and immunocompromised individuals due to a higher prevalence of comorbidities in the respective subgroups. Comorbidities were associated with a higher hospitalization rate, even in younger subjects. However, COVID-19 vaccination significantly reduced hospital admission rates and conferred better outcomes for this subpopulation [11,17].
ICU Admissions
After examining the effectiveness of vaccination on the rate of ICU admission for COVID-19-infected patients, our analysis showed that patients who had at least one dose of the vaccine types approved in North America are less likely to develop symptoms that warrant ICU admission and possible supportive care. Specifically, unvaccinated patients are six times more likely to require ICU admission than vaccinated patients across the age group of 18-80. These outcomes were less common in fully vaccinated persons with a booster (0.08%, and 0.03%, respectively) and fully vaccinated persons without a booster (0.12% and 0.05%, respectively) [11]. The findings strengthen and augment evidence from previous meta-analyses, which confirmed that single and double doses of COVID-19 vaccines prevented ICU admission rates at 45% and 96%, respectively [20]. Although the World Health Organization (WHO) and other health bodies have advised at least two doses of the vaccine to offer protection to new and emerging variants, this was not very practical as several countries had shortages of vaccine supply at the peak of the pandemic and could only offer the second dose several weeks after the first dose, for example, Canada had to wait 16 weeks after the first doses were administered.
However, recent literature has shown that the T cell and antibody responses induced by a single dose of the SARS-CoV-2 vaccine were comparable to those occurring in subjects naturally infected with SARS-CoV-2 within weeks or months after infection, re-iterating the protection acquired from even single doses of the vaccine on ICU admission [20]. However, multiple doses of the vaccine have been shown to offer protection against ICU admission and mechanical ventilation, as described by a study that showed that unvaccinated persons were more likely to be admitted to an ICU (0.5%) and require intubation for mechanical ventilation (0.2%).
Mechanical Ventilation
In this study, unvaccinated patients had a higher risk of getting intubated for mechanical ventilation than vaccinated patients, confirming several research studies that reported not getting vaccinated as a risk factor for poor outcomes in COVID-19-infected patients [23]. One possible explanation is that the vaccine produces antibodies that tag the virus and neutralize it, preventing the respiratory system from being damaged. These findings align with recent studies [11], highlighting the importance of the COVID-19 vaccine in protecting against severe COVID-19 infection and alleviating the symptoms that could occur. Efforts channeled into promoting the COVID-19 vaccine and boosters, in coordination with other prevention strategies, would go a long way in preventing the need for mechanical ventilation.
Limitations
The risks of COVID-19 infection are not equal for everyone, as the likelihood of exposure might influence the likelihood of COVID-19 vaccine acceptance and coverage. These discrepancies were not accounted for in our study. Independent analyses of the preventive effect of single doses compared to double and booster doses were not performed. Also, the protective development of the vaccines accepted in North America against different virus variants could not be ascertained. Possible reasons could have been the lack of uniformity in vaccine scheduling and availability in North America.
Conclusions
There has been a lot of skepticism about the efficacy of the COVID-19 vaccines in the population due to the speed of the vaccine rollout. This has affected vaccination acceptance, and this study was done to add to the literature on the effectiveness of COVID-19 vaccination, thereby hoping to increase confidence in acceptance. This meta-analysis showed that COVID-19 vaccination is highly effective in reducing clinical outcomes such as incidence of infection, hospitalization rate, ICU admission, and mechanical ventilation rates from the COVID-19 infection. Overall, our study has shown that receiving the COVID-19 vaccine is beneficial and effective in mitigating the spread of infection and providing better clinical outcomes. We recommend and urge all stakeholders involved in COVID-19 infection prevention, management, and control to strengthen the advocacy and education of the people. All hands should be on deck to ensure that the misconceptions and erroneous beliefs about the effectiveness of the COVID-19 vaccine be corrected based on the scientific evidence from our work and other ongoing research. This will go a long way in reducing the morbidities from COVID-19 and other associated impacts of the COVID-19 pandemic.
The authors have declared that no competing interests exist.
References
- 1.COVID-19: a review on the novel coronavirus disease evolution, transmission, detection, control and prevention. Sharma A, Ahmad Farouk I, Lal SK. Viruses. 2021;13:202. doi: 10.3390/v13020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weekly COVID-19 Epidemiological Update: Issue 33 (20 September 2022) IRIS PAHO: Weekly COVID-19 epidemiological update: Issue 33 (20 September 2022) IRIS PAHO. [ Sep; 2022 ]. 2022. https://iris.paho.org/handle/10665.2/56466 https://iris.paho.org/handle/10665.2/56466
- 3.USAFacts: US coronavirus vaccine tracker. [ Oct; 2022 ]. 2021. https://usafacts.org/visualizations/covid-vaccine-tracker-states https://usafacts.org/visualizations/covid-vaccine-tracker-states
- 4.PAHO: Weekly COVID-19 epidemiological update: region of the Americas, Issue 9. [ Oct; 2022 ]. 2022. https://www.paho.org/en/file/107020/download?token=GmGwUr6b https://www.paho.org/en/file/107020/download?token=GmGwUr6b
- 5.Vaccine hesitancy in the era of COVID-19. Troiano G, Nardi A. Public Health. 2021;194:245–251. doi: 10.1016/j.puhe.2021.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Page MJ, McKenzie JE, Bossuyt PM, et al. BMJ. 2021;372:0. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO: WHO COVID-19 Case definition. [ Oct; 2022 ]. 2022. https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2022.1 https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2022.1
- 8.Analysis of the effectiveness of the Ad26.COV2.S adenoviral vector vaccine for preventing COVID-19. Corchado-Garcia J, Zemmour D, Hughes T, et al. JAMA Netw Open. 2021;4:0. doi: 10.1001/jamanetworkopen.2021.32540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Incidence of SARS-CoV-2 infection, emergency department visits, and hospitalizations because of COVID-19 among persons aged ≥12 years, by COVID-19 vaccination status: Oregon and Washington, July 4-September 25, 2021. Naleway AL, Groom HC, Crawford PM, et al. MMWR Morb Mortal Wkly Rep. 2021;70:1608–1612. doi: 10.15585/mmwr.mm7046a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence: 25 U.S. Jurisdictions, April 4-December 25, 2021. Johnson AG, Amin AB, Ali AR, et al. MMWR Morb Mortal Wkly Rep. 2022;71:132–138. doi: 10.15585/mmwr.mm7104e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SARS-CoV-2 infection and hospitalization among adults aged ≥18 years, by vaccination status, before and during SARS-CoV-2 B.1.1.529 (Omicron) variant predominance: Los Angeles County, California, November 7, 2021-January 8, 2022. Danza P, Koo TH, Haddix M, Fisher R, Traub E, OYong K, Balter S. MMWR Morb Mortal Wkly Rep. 2022;71:177–181. doi: 10.15585/mmwr.mm7105e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Incident SARS-CoV-2 infection among mRNA-vaccinated and unvaccinated nursing home residents. White EM, Yang X, Blackman C, Feifer RA, Gravenstein S, Mor V. N Engl J Med. 2021;385:474–476. doi: 10.1056/NEJMc2104849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. Dunkle LM, Kotloff KL, Gay CL, et al. N Engl J Med. 2022;386:531–543. doi: 10.1056/NEJMoa2116185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Effectiveness of BNT162b2 vaccine against critical COVID-19 in adolescents. Olson SM, Newhams MM, Halasa NB, et al. N Engl J Med. 2022;386:713–723. doi: 10.1056/NEJMoa2117995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durability of the single-dose Ad26.COV2.S vaccine in the prevention of COVID-19 infections and hospitalizations in the US before and during the Delta variant surge. Polinski JM, Weckstein AR, Batech M, et al. JAMA Netw Open. 2022;5:0. doi: 10.1001/jamanetworkopen.2022.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.SARS-CoV-2 infections and hospitalizations among persons aged ≥16 years, by vaccination status: Los Angeles County, California, May 1-July 25, 2021. Griffin JB, Haddix M, Danza P, et al. MMWR Morb Mortal Wkly Rep. 2021;70:1170–1176. doi: 10.15585/mmwr.mm7034e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Association between mRNA vaccination and COVID-19 hospitalization and disease severity. Tenforde MW, Self WH, Adams K, et al. JAMA. 2021;326:2043–2054. doi: 10.1001/jama.2021.19499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Severity of disease among adults hospitalized with laboratory-confirmed COVID-19 before and during the period of SARS-CoV-2 B.1.617.2 (Delta) predominance: COVID-NET, 14 States, January-August 2021. Taylor CA, Patel K, Pham H, et al. MMWR Morb Mortal Wkly Rep. 2021;70:1513–1519. doi: 10.15585/mmwr.mm7043e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe COVID-19 outcomes in Ontario, Canada: test negative design study. Chung H, He S, Nasreen S, et al. BMJ. 2021;374:0. doi: 10.1136/bmj.n1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Effectiveness of severe acute respiratory syndrome coronavirus 2 messenger RNA vaccines for preventing coronavirus disease 2019 hospitalizations in the United States. Tenforde MW, Patel MM, Ginde AA, et al. Clin Infect Dis. 2022;74:1515–1524. doi: 10.1093/cid/ciab687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.COVID-19 vaccination and non-COVID-19 mortality risk: seven integrated health care organizations, United States, December 14, 2020-July 31, 2021. Xu S, Huang R, Sy LS, et al. MMWR Morb Mortal Wkly Rep. 2021;70:1520–1524. doi: 10.15585/mmwr.mm7043e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Liu Q, Qin C, Liu M, Liu J. Infect Dis Poverty. 2021;10:132. doi: 10.1186/s40249-021-00915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Safety and effectiveness of SARS-CoV-2 vaccines: a systematic review and meta-analysis. Ling Y, Zhong J, Luo J. J Med Virol. 2021;93:6486–6495. doi: 10.1002/jmv.27203. [DOI] [PMC free article] [PubMed] [Google Scholar]