Abstract
Background
Osteosarcoma and Ewing‘s sarcoma in children and adolescents require age-specific interdisciplinary diagnosis and treatment to achieve optimal therapeutic outcomes.
Methods
The diagnosis and treatment of malignant bone tumors in childhood and adolescence are presented in the light of publications retrieved by a selective search, pertinent guidelines, and the authors‘ extensive experience in an interdisciplinary cancer center.
Results
Bone sarcomas make up approximately 5% of all malignancies in children and adolescents; the most common types are Ewing‘s sarcoma and osteosarcoma. Patients are often not referred to a specialized center until long after the onset of symptoms, as they and their physicians rarely consider the possibility of a bone tumor, and the symptoms are often trivialized. Bone pain of unknown origin, swelling, and functional limitations should be investigated with conventional x-rays. Lesions of unclear origin should be biopsied after a meticulous clinical and radiologic evaluation. Multimodal treatment consists of neoadjuvant chemotherapy, limb-preserving resection if possible, and radiotherapy where indicated. In multicenter studies, patients with osteosarcoma achieve event-free survival in 64% of cases if their disease is localized, and 28% if it is metastatic; the corresponding figures for patients with Ewing‘s sarcoma are 80% and 27%, respectively.
Conclusion
With implementation of the current treatment recommendations, most children and adolescents with malignant bone tumors can be treated successfully with curative intent. These patients should be referred to a sarcoma center for diagnosis and treatment.
cme plus
This article has been certified by the North Rhine Academy for Continuing Medical Education. Participation in the CME certification program is possible only over the internet: cme.aerzteblatt.de. The deadline for submission is 15 June 2024..
Primary bone tumors are generally rare and, when they do occur, are mostly of benign etiology. Bone sarcomas make up approximately 5% of all malignancies in children and adolescents (1), but often pose diagnostic and therapeutic challenges. The current WHO classification includes, from a histological perspective, more than 20 different bone sarcomas, whereby only osteosarcoma and Ewing’s sarcoma play a significant role in children and adolescents (2). Osteosarcoma, which is usually highly malignant and whose cells form bone or osteoid, is the most common malignant bone tumor with an incidence of 3.8/1,000,000 children and adolescents (1, 3). It has two age peaks, in the second and the seventh decade of life, and is usually found in the metaphysis of long bones, particularly in the knee region (70%) and more rarely in the trunk region (10%) (3, 4). Ewing’s sarcoma belongs to the group of small round blue cell tumors. Molecular pathological evidence of translocation involving the EWSR1 locus is characteristic (5). With an incidence of 3.5/1,000,000, it is the second most common malignant bone tumor in children and adolescents (1). The predilection age is likewise the second decade of life. Preferred localizations include the pelvis, followed by the diaphysis of long bones of the lower extremities (6). Both tumor entities should be considered as systemic disease, since approximately 80% of patients have occult or manifest metastases, usually to the lung and/or skeletal system, at the time of diagnosis (6, 7).
Methods
A selective search was carried out in PubMed and Google Scholar, and guidelines were also taken into consideration (6– 8). Based on this as well as the extensive experience gained at an interdisciplinary tumor center, the diagnosis and treatment of malignant bone tumors—excluding tumors in the head region—are described.
Symptoms and differential diagnosis
The cardinal symptoms of malignant bone tumors include local pain, functional limitations, and as a late sign, swelling. Benign bone tumors, on the other hand, are usually asymptomatic in children and adolescents, meaning that they are discovered as an incidental finding on radiological examination (9). Patients and their parents often associate what is in fact tumor pain with trauma or a sports injury, which can lead to a delay in diagnosis. Symptom duration is subject to wide variability, ranging from several days to weeks. It is not uncommon for bone tumors to first come to light as a result of exertion-induced pain or only after a pathological fracture has occurred. Depending on localization, specific symptoms may occur in some cases. For example, Ewing’s sarcoma of the chest wall may be primarily associated with breathing difficulties and manifest with the clinical picture of pneumonia. Infiltration of the spinal canal can cause symptoms ranging from neurological deficits to paraplegia.
Since medical history is usually nonspecific, patients often present to a center only after symptoms have persisted for a long time and symptomatic treatment has been given without sufficient diagnostic work-up (10, 11).
Whereas benign tumors may reveal no or minimal findings on clinical examination, malignant tumors are often associated with palpable tissue swelling that may be painful on palpation. Local clinical findings in malignant tumors can sometimes produce the impression of an infectious event or osteomyelitis (10). Pain is usually persistent, undulating in nature, and often perceived as worsening at night. On clinical examination, restricted movement or even loss of function in the neighboring joint is apparent.
From a differential diagnostic perspective, persistent bone pain in childhood and adolescence should prompt consideration of the following clinical pictures:
Benign bone tumors
Osteomyelitis and spondylitis
Aseptic bone necrosis
Perthes’ disease
Femoral head epiphysiolysis and forms of rheumatic disease
Diseases of other organ systems such as leukemia, lymphoma, and neuroblastoma.
Even healthy children may complain of bilateral bone pain that is then often labeled as growing pains (12). Pathological fractures are also seen in benign bone tumors, tumor-like bone lesions, and more rarely in leukemia, lymphoma, or neuroblastoma. In the case of pathological fractures, no osteosynthesis should be carried out until a sarcoma has been excluded by biopsy, but instead, plaster cast immobilization performed where necessary in order to avoid contamination of healthy tissue with malignant cells.
Diagnostic imaging
According to the guideline on musculoskeletal pain in children and adolescents that was updated in 2020, diagnostic imaging should be performed if the physical examination yields abnormal findings, accompanying symptoms are present, or laboratory tests yield pathological values (8).
Here, radiography in two planes forms the basis on which bone tumors, as a result of a possible characteristic internal matrix or skeletal localization, can be classified in 80–90% of cases (13, 14). Furthermore, grading of lytic lesions is possible based on lesion margins and the potential presence of a sclerotic rim. Classification according to Lodwick is carried out based on growth rate (15, 16) (table 1). Here, a higher grade is associated with a higher risk for malignant etiology, meaning that a biopsy should be performed if malignancy is suspected (Lodwick IC, II, III). The radiological examination should include the entire affected bone and neighboring joint regions in order to detect metastases that may be present in the same bone or in a transarticular location in adjacent bone (6, 7).
Table 1. Lodwick classification of bone tumors based on conventional radiography (e15).
| Grade | Morphology |
| IA | Sharply defined geographic lesion with sclerotic rim |
| IB | Well-defined geographic lesion, little or nosclerotic rim, involvement and protrusion of cortical bone possible |
| IC | Geographic lesion, ill-defined margins,little/no sclerotic rim, cortical penetration possible |
| II | Geographic lesion, ill-defined margins,moth-eaten pattern, cortical destruction, usually without a sclerotic rim |
| III | Moth-eaten pattern, tumor permeation, no sclerotic rim |
The assessment of possible soft tissue involvement is the domain of magnetic resonance imaging with dedicated tumor scanning protocols and contrast administration (17). This also enables the important distinction to be made between reactive edematous changes (T1 iso- to hyperintense to muscle, but hypointense to surrounding bone) and bone marrow suppression by tumor cells (T1 hypointense to muscle). To exclude pulmonary metastases, computed tomography (CT) of the lungs is required. CT of the tumor region is generally reserved for cases with an unclear internal matrix on X-ray or for intervention planning. Three-phase bone scintigraphy is used to detect polyostotic lesions or metastases. However, due to the variable tracer uptake here, it is not possible to specifically classify lesions of unclear origin. This investigation is increasingly being replaced by positron emission tomography/CT scan (FDG-PET/CT), which enables distant metastases to be detected, prognostic conclusions to be drawn, as well as an assessment of treatment response over the course of therapy to be made based on metabolic activity (18– 20).
Biopsy
Lesions that are unequivocally identified as benign on the basis of clinical and radiological findings can either be monitored or undergo direct surgical/interventional treatment (21). When malignancy is suspected, all equivocal lesions should always be biopsied for histological confirmation of the diagnosis following thorough clinical and radiological evaluation (6, 7, 21, 22). Patients should be referred for biopsy to a specialist center for bone sarcomas (6, 7). Open biopsy is the method of choice in order to obtain sufficient vital tumor tissue for conventional histology and immunohistochemistry as well as to take fresh material for molecular genetic analysis (6, 7). Obtaining a punch biopsy (preferably ≥ 16 G) with multiple punches can be carried out as an alternative in individual justified cases (6, 7). Here, the access route should be carefully planned, excluding any critical neurovascular structures and joints in view of subsequent surgical excision. No additional compartments should be opened, and precise hemostasis should be achieved, where necessary by introducing a bone cement filling in order to avoid the spread of tumor cells via hematoma (21). Postoperatively, a Redon drain should be placed on the limbs in combination with compression and immobilization for at least 2 days (21).
General treatment strategy
The multimodal treatment of osteosarcoma and Ewing’s sarcoma in childhood and adolescence should be carried out in accordance with pediatric oncology guidelines (23) at an experienced pediatric oncology center, if possible in the setting of clinical trials. Prior to initiation, treatment needs to be defined on a case-by-case basis at an interdisciplinary tumor conference involving pediatric hematology/oncology, tumor orthopedics, pathology, radiology (with a focus on pediatric radiology), and radiation oncology. It is absolutely imperative to avoid complete tumor resection before preoperative chemotherapy has been concluded, even in the case of well-demarcated tumors, in order to be able to make a histological assessment of the primary tumor’s response to chemotherapy. The aim of surgical treatment is complete tumor resection with free margins, including biopsy scar and biopsy channel (6, 7), while at the same time achieving the optimal functional and cosmetic outcome. This is consistent with the “wide resection” called for by Enneking (table 2) (24). As a general rule, limb-preserving resection should be preferred and is performed today in approximately 90% of bone sarcomas. However, the risk of re-operation, functional aspects, and the possibilities offered by modern prosthetic treatment need to be taken into consideration in the decision-making process (25). Patient counseling as well as the surgical procedures themselves require extensive experience and must be carried out by interdisciplinary teams at experienced sarcoma centers.
Table 2. Surgical resection margins according to Enneking (e27).
| Type | Resection plane | Pathologic outcome |
| Intralesional | In the lesion | Positive resection margin (R1, R2) |
| Marginal | Outside the capsule, but in the reactive zone of surrounding tissue | Positive resection margin (R1) |
| Wide | Outside the reactive zone in healthy tissue | Negative resection margin (R0) |
| Radical | Outside the compartment | Negative resection margin (R0) |
The reconstruction of the resected bone segment can be achieved by a variety of reconstructive procedures, whereby endoprosthetic segmental joint replacement with modular tumor endoprostheses has become established for joint reconstruction (26). Following the resection of tumors close to the knee joint—particularly in young children and highly active patients—and as a secondary intervention following failure of endoprosthetic replacement, Borggreve rotation, in which the ankle joint is rotated 180° in a dorsal direction and takes over the function of the knee joint, represents a good alternative (26, 27). In the case of purely diaphyseal resections, biological reconstruction by means of callus distraction as well as autologous and/or allogeneic transplants in combination with well-established osteosynthesis methods can be undertaken (Figure) (26). Pelvic resections, which represent a surgical challenge, have been performed more precisely and sparingly in recent years by using navigation and thanks to the computer-assisted design and manufacture of resection templates (CAD/CAM). The reconstruction of complex defects has been significantly improved by producing patient-specific prostheses and implants using 3D printing methods (26, 28– 30).
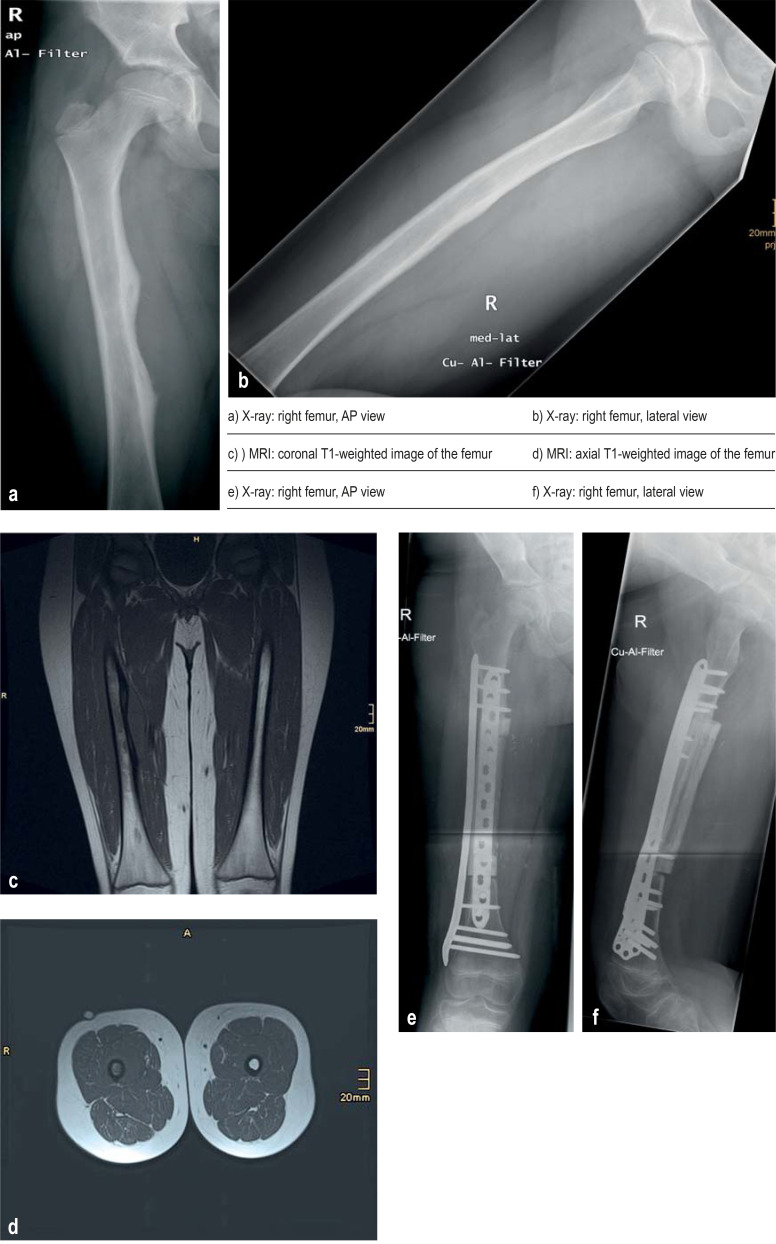
Figure:
Ewing’s sarcoma in an 11-year-old boy with an approximately 2-month history of pain in the right femur
a, b) Initial X-rays of the right femur show a periosteal reaction in the medial region.
c, d) T1-weighted magnetic resonance imaging (MRI) indicates an intraosseous lesion with a periosteal and soft tissue reaction.
e, f) Following neoadjuvant treatment as recommended by the German Society for Pediatric Oncology and Hematology (GPOH) in the Ewing registry, wide resection and biological reconstruction with allogeneic femoral diaphysis, fibula with vascular pedicle, and plate osteosynthesis were performed.
Primary bone tumors of the spine are resected by means of en-bloc spondylectomy, assuming wide resection necessary to meet Enneking criteria is possible. For the assessment of resectability, the Weinstein–Borian–Biagnini (WBB) classification has been widely accepted (31).
In German-speaking countries, the extent of tumor cell necrosis induced by preoperative chemotherapy is based on the Salzer-Kuntschik classification (32). Postoperatively, patients receive further postoperative chemotherapy. In the case of metastatic disease, metastases that have not completely regressed under chemotherapy should also be surgically resected. In Ewing’s sarcoma, radiotherapy plays a valuable role preoperatively, postoperatively, and as radiotherapy alone in inoperable tumors or excessively high surgical morbidity. In osteosarcomas, on the other hand, radiotherapy is intended only for inoperable tumors and recurrences.
Multimodal treatment of osteosarcoma
Only a maximum of 10% of all osteosarcoma patients can be cured with tumor resection alone; without adjuvant chemotherapy, patients almost always develop local recurrences and/or lung metastases within a matter of months (33, 34). The introduction of systemic chemotherapy significantly improved patients’ chances of cure to 64% (localized disease) and 28% (metastatic disease) (table 3) (35– 38). Prognostically significant factors include the presence of distant metastases at the time of diagnosis, tumor localization, tumor size, the degree of radicality achieved, and the histological response to preoperative chemotherapy (4, e1). On the basis of previous studies, patients receive 10-week preoperative chemotherapy with a combination of high-dose MTX, doxorubicin, and cisplatin (MAP), after which definitive tumor resection is performed, followed by postoperative chemotherapy with MAP up to week 29 (e2– e4). For countries with limited resources, alternative MTX-free chemotherapy regimens have been developed (e5). Since additional cycles with ifosfamide and etoposide (MAPIE) in patients showing insufficient histological response failed to improve outcome, no adjustment of chemotherapy is undertaken in such cases (e6). Drugs such as ifosfamide, etoposide, and carboplatin are generally reserved for cases of recurrence. Complementary immunotherapy with interferon also failed to improve cure rates in patients with good histological response (e7).
Table 3. Important study data on the treatment of osteosarcoma.
| Study type | Study arm | Patient number | EFS | p | OS | p | Reference |
| MAP vs. MAPIE in the first-line treatment of patients with inadequate histological response | |||||||
| RCT | Total | 618 | 3-Year EFS | 0.86*1 | 3-Year OS | 0.86*1 | Marina NM et al. 2016 (e6) |
| MAP | 310 | 55% | 72% | ||||
| MAPIE | 308 | 53% | 77% | ||||
| Additional interferon alpha-2b in first-line treatment if histological response is good | |||||||
| RCT | Total | 716 | 3-Year EFS | 0.21*1 | 5-Year OS | n.r. | Bielack SS et al. 2015 (e7) |
| MAP | 359 | 74% | 81% | ||||
| MAP + IFN alpha 2a | 357 | 77% | 84% | ||||
| Muramyl tripeptide in the first-line treatment of localized osteosarcoma | |||||||
| 2 × 2 Factorial RCT | Total | 662 | 4-Year EFS | 0.08*2 | 4-Year OS | 0.03*2 | Meyers PA et al. 2008 (e8, e26) |
| MAP | 168 | 66% | 78% | ||||
| MAP MTP | 163 | 65% | 82% | ||||
| MAPI | 163 | 60% | 77% | ||||
| MAPI MTP | 168 | 76% | 86% | ||||
| Sorafenib for recurrent/refractory osteosarcoma | |||||||
| Phase 2 | 35 | Median EFS of 4 months | Median OS of 7 months | Grignani G et al. 2012 (e10) | |||
| Sorafenib and everolimus for recurrent/refractory osteosarcoma | |||||||
| Phase 2 | 38 | Median EFS of 5 months | Median OS of 11 months | Grignani G et al. 2015 (e9) | |||
| Regorafenib for recurrent/refractory osteosarcoma | |||||||
| Phase 2 RCT | Total | 40 | 1-Year EFS | n.r. | Median OS | n.r. | Duffaud F et al. 2019 (e11) |
| Placebo | 14 | 0 | 5.9 Months | ||||
| Regorafenib | 26 | 62% | 11.3 Months | ||||
| Regorafenib for recurrent/refractory osteosarcoma | |||||||
| Phase 2 RCT | Total | 42 | Median PFS | 0.017*1 | Median OS | 0.62*1 | Davis LE et al. 2019 (e12) |
| Placebo | 20 | 1.7 Months | 13.4 Months | ||||
| Regorafenib | 22 | 3.6 Months | 11.1 Months | ||||
| Cabozantinib for recurrent/refractory osteosarcoma | |||||||
| Phase 2 | 45 | 2-Year PFS 9.5% | 2-Year OS 23.3% | Italiano A et al. 2020 (e13) | |||
*1 Cox regression analysis of the intention-to-treat cohort, *2 comparison of chemotherapy without versus with MTP
Drugs: A, doxorubicin; E, etoposide; I, ifosfamide; M, high-dose methotrexate; MTP, muramyl tripeptide; P, cisplatin
EFS, event-free survival; IFN, interferon; n.r., not reported; n.s., not significant; OS, overall survival; PFS, progression-free survival, RCT, randomized controlled trial; vs., versus
The drug muramyl tripeptide (MTP) is approved in Germany for the treatment of non-metastatic osteosarcoma; however, its approval is based on only a single clinical trial (e8). It is not yet possible to assess the value of molecularly targeted treatments for osteosarcoma. Preclinical data point to the off-label use of multikinase inhibitors such as sorafenib (e9, e10), regorafenib (e11, e12), and cabozantinib (e13). Patients without initial metastasis achieve a 5-year EFS (EFS, event-free survival) of 60% and a 5-year OS (OS, overall survival) of 76% with the abovementioned therapies. The prognosis is better still in the case of initially localized disease and complete surgical remission (5-year EFS of 64%, 5-year OS of 79%). For initially metastatic osteosarcoma, on the other hand, the overall prognosis is poor (5-year EFS of 28%, 5-year OS of 45%) (e1).
Multimodal treatment of Ewing’s sarcoma
It was not until intensive chemotherapy was introduced that it was possible to achieve significant cure rates in Ewing’s sarcoma (table 4). Prognostically relevant factors include the presence of metastases, histological response to preoperative chemotherapy, localization, and the extent of tumor resection (e14). Patients with osteomedullary metastasis have a significantly poorer prognosis (3-year EFS of 27%) compared to patients with isolated pulmonary metastasis (3-year EFS of 54%) (e14– e16).
Table 4. Important study data on the first-line treatment of Ewing’s sarcoma.
| Study type | Study arm | Patient number | EFS | p | OS | p | Reference |
| Maintenance treatment in the standard-risk group | |||||||
| RCT | Total | 856 | 3-Year EFS | n.s. | 3-Year OS | n.s. | Le Deley et al. 2014 (e16) |
| VAI | 425 | 78.2% | 85.5% | ||||
| VAC | 431 | 75.4% | 85.9% | ||||
| Maintenance treatment in the standard-risk group | |||||||
| RCT | Total | 155 | 3-Year EFS | 0.72 | 3-Year OS | 0.80 | Paulussen et al. 2008 (e17) |
| VAIA# | 76 | 74% | 86% | ||||
| VACA# | 79 | 73% | 90% | ||||
| Additional etoposide in the high-risk group | |||||||
| RCT | Total | 492 | 3-Year EFS | 0.12 | 3-Year OS | 0.23 | Paulussen et al. 2008 (e17) |
| VAIA# | 240 | 47% | 59% | ||||
| EVAIA# | 252 | 52% | 62% | ||||
| Type of induction chemotherapy | |||||||
| RCT | Total | 640 | Hazard ratio | n.r. | Hazard ratio | n.r. | Brennan et al. 2020 (e27) |
| VIDE | 320 | 1.0 | 1.0 | ||||
| VDC/IE | 320 | 0.70 | 0.64 | ||||
| Interval between VDC/IE induction cycles | |||||||
| RCT | Total | 568 | 5-Year EFS | 0.048 | 5-Year OS | 0.056 | Womer et al. 2012 (e19) |
| 21 Days | 284 | 65% | 77% | ||||
| 14 Days | 284 | 73% | 83% | ||||
| VAI vs. BuMel in the case of inadequate histological response or initial tumor volume >200 ml | |||||||
| RCT | Total | 240 | 3-Year EFS | 0.026* | 3-Year OS | 0.028* | Whelan et al. 2018 (e22) |
| VAI | 118 | 56.7% | 72.2% | ||||
| BuMel | 122 | 69.0% | 78.0% | ||||
| VAI vs. BuMel bei in the case of lung metastasis | |||||||
| RCT | Total | 287 | 3-Year EFS | 0.16* | 3-Year OS | 0.99* | Dirksen et al. 2019 (e14) |
| VAI | 143 | 50.6% | 68.0% | ||||
| BuMel | 144 | 56.6% | 68.2% | ||||
*Cox regression analysis of the intention-to-treat cohort
Drugs: A, actinomycin; A#, doxorubici; BuMel, high-dose chemotherapy with busulfan and melphalan; C, cyclophosphamide; D; doxorubicin; E, etoposide
EFS, event-free survival; I, ifosfamide; n.r., not reported; n.s., not significant; OS, overall survival; RCT, randomized controlled trial; V, vincristine, vs., versus
Treatment is broken down into induction chemotherapy, tumor resection, and postoperative consolidation. Induction chemotherapy generally includes the drugs vincristine, ifosfamide, cyclophosphamide, doxorubicin, and/or etoposide in various combinations (e14, e17, e18). In this context, cyclophosphamide appears to be noninferior to ifosamide (e16, e17). Interval-compressed chemotherapy is able to improve outcomes (table 3) (e19). Since the combination of the four drugs, vincristine, ifosfamide, doxorubicin, and etoposide (VIDE), is inferior to alternating VDC cycles (VDC: vincristine, doxorubicin, cyclophosphamide) and IE cycles (IE: ifosfamide, etoposide), VDC/IE is currently recommended as the standard regimen for induction chemotherapy (e20, e21). Postoperative consolidation generally comprises further cycles with vincristine, actinomycin D, and ifosfamide (VAI) or vincristine, actinomycin D, and cyclophosphamide (VAC). High-dose chemotherapy with busulfan/melphalan and autologous stem cell transplantation (HDCT) improves prognosis in localized Ewing’s sarcoma with insufficient histological response or initially large tumors > 200 mL (e22). On the other hand, in patients with lung metastasis only, busulfan/melphalan HDCT was not superior to maintenance treatment with VAI cycles and lung irradiation (e14).
Consolidation radiotherapy of the primary tumor and selected metastases plays a significant role in the treatment of Ewing’s sarcoma. In patients with lung metastases detectable on initial diagnosis, whole-lung radiotherapy is also necessary. It is not uncontroversial whether radiotherapy can be omitted following confirmed R0 resection and complete histological response (e23). Radiotherapy in young patients should preferably be provided in the form of proton therapy, which lowers the burden on surrounding tissue (e23– e25). Patients with localized Ewing’s sarcoma achieve a 3-year EFS of 75–80% and a 3-year OS of 85%, patients with lung metastasis a 3-year EFS of 54 %, and Ewing’s sarcoma patients with osteomedullary metastasis a 3-year EFS of 27% and a 3-year OS of 34% (e14– e16).
Summary
For children with osteosarcoma and Ewing’s sarcoma, interdisciplinary diagnosis and treatment in centers specialized in these diseases is recommended for treatment success. Multimodal treatment comprising neoadjuvant chemotherapy, limb-preserving tumor resection if possible, and radiotherapy where indicated significantly improved the prognosis of these patients. In multicenter studies, osteosarcoma patients achieve an event-free survival of between 64% (localized) and 28% (metastatic), and Ewing’s sarcoma patients of between 80% (localized) and 27% (metastatic). Disease recurrence is usually seen within the first year, predominantly in the form of lung or local recurrence, meaning that consistent follow-up care is urgently required. This also ensures the timely detection of complications related to reconstruction and should be carried out lifelong in the case of endoprostheses.
Questions on the article in issue 24/2023: The Diagnosis and Treatment of Osteosarcoma and Ewing’s Sarcoma in Children and Adolescents.
The submission deadline is 15 June 2024. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
Bone sarcomas account for approximately what percentage of all malignancies in children and adolescents?
5%
10%
25%
40%
60%
Question 2
What is the most common location of osteosarcoma?
Diaphysis of long bones
Metaphysis of long bones
Proximal epiphysis of the femur
Metaphysis of short bones
In the fontanelle region of the skull
Question 3
What characterizes Ewing’s sarcoma?
Large oval blue cell tumor
Small flat red cell tumor
Small oval red cell tumor
Large round green cell tumor
Small round blue cell tumor
Question 4
Which statement applies?
Malignant bone tumors are usually discovered as incidental findings.
Ewing’s sarcoma is usually accompanied by paralysis.
Benign bone tumors are usually asymptomatic.
Most metastases of malignant bone tumors occur in the brain.
Bone fractures promote the development of malignant bone tumors.
Question 5
Which of the following symptoms is suggestive of Ewing’s sarcoma?
Numbness in the region of the tumor
Pale skin color in the region of the tumor
Pain is usually only present in the morning.
Palpable tissue swelling in the region of the tumor
No restriction of movement occurs.
Question 6
For which grade of the Lodwick classification of bone tumors is the morphology described as a moth-eaten pattern with tumor permeation and no marginal sclerosis?
Grade II
Grade IA
Grade IB
Grade IC
Grade III
Question 7
In what percentage of bone sarcomas is it possible today to perform limb-preserving resection?
Approximately 10%
Approximately 25%
Approximately 50%
Approximately 75%
Approximately 90%
Question 8
Which joint is replaced in Borggreve rotation?
Wrist
Metacarpophalangeal joint
Knee joint
Hip joint
Elbow joint
Question 9
What is the maximum percentage of osteosarcoma patients that can be cured by tumor resection alone?
Approximately 5%
Approximately 10%
Approximately 20%
Approximately 50%
Approximately 80%
Question 10
As a general rule, with what does osteosarcoma treatment begin?
10-Week preoperative chemotherapy (MAP)
Definitive tumor resection
Short chemotherapy (2 weeks) with etoposide
10-Week tumor-specific antibody therapy
Tumor irradiation and whole-lung irradiation
Acknowledgments
Translated from the original German by Christine Rye.
Footnotes
Conflict of interest statement
The authors declares that no conflicts of interest exists.
References
- 1.Erdmann FKP, Grabow D, Spix C. German Childhood Cancer Registry—Annual Report 2019 (1980-2018) Institute of Medical Biostatistics, Epidemiology and Informatics (IMBEI) at the University Medical Center of the Johannes Gutenberg University. 2020 [Google Scholar]
- 2.(WHO) WHOCoTEB. WHO. Lyon, France: 2020. WHO classification of tumours. Soft tissue and bone tumors. 5th edition Volume 3. International Agency for Research on Cancer. [Google Scholar]
- 3.Esiashvili N, Goodman M, Marcus RB Jr. Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: surveillance epidemiology and end results data. J Pediatr Hematol Oncol. 2008;30:425–430. doi: 10.1097/MPH.0b013e31816e22f3. [DOI] [PubMed] [Google Scholar]
- 4.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 5.Le Deley MC, Delattre O, Schaefer KL, et al. Impact of EWS-ETS fusion type on disease progression in Ewing’s sarcoma/peripheral primitive neuroectodermal tumor: prospective results from the cooperative Euro-E.W.I.N.G. 99 trial. J Clin Oncol. 2010;28:1982–1988. doi: 10.1200/JCO.2009.23.3585. [DOI] [PubMed] [Google Scholar]
- 6.(GPOH) GfPOuH. S1-Leitlinie Ewing-Sarkom des Kinder- und Jugendalters. https://register.awmf.org/de/leitlinien/detail/025-006“. (last accessed on 15 May 2023) [Google Scholar]
- 7.(GPOH) GfPOuH. S1-Leitlinie Osteosarkome. www.awmf.org/leitlinien/detail/ll/025-005.html2021 (last accesed on 15 May 2023) [Google Scholar]
- 8.(DGKJ) DGfK-uJeV. S2k-Leitlinie: Muskuloskelettale Schmerzen bei Kindern und Jugendlichen—Ein Algorithmus zur differenzialdiagnostischen Abklärung eines häufigen Leitsymptoms in der Kinder- und Jugendmedizin. www.awmf.org/uploads/tx_szleitlinien/027-073m_S2k_Muskuloskelettale-Schmerzen-Kinder-Jugendliche%E2%80%93Algorithmus__2020-12.pdf (last accessed on 1 March 2022) [Google Scholar]
- 9.Luedtke LM, Flynn JM, Ganley TJ, Hosalkar HS, Pill SG, Dormans JP. The orthopedists’ perspective: bone tumors, scoliosis, and trauma. Radiol Clin North Am. 2001;39:803–821. doi: 10.1016/s0033-8389(05)70312-2. [DOI] [PubMed] [Google Scholar]
- 10.Rechl H, Kirchhoff C, Wortler K, Lenze U, Topfer A, von Eisenhart-Rothe R. [Diagnosis of malignant bone and soft tissue tumors] Orthopade. 2011;40:931–941. doi: 10.1007/s00132-011-1821-7. quiz 42-3. [DOI] [PubMed] [Google Scholar]
- 11.Grimer RJ, Briggs TW. Earlier diagnosis of bone and soft-tissue tumours. J Bone Joint Surg Br. 2010;92:1489–1492. doi: 10.1302/0301-620X.92B11.24326. [DOI] [PubMed] [Google Scholar]
- 12.Lehmann HS, Blache D, Drynan E, Tshewang P, Blignaut DJC, Musk GC. Optimum drug combinations for the sedation of growing boars prior to castration. Animals (Basel) 2017;7 doi: 10.3390/ani7080061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Temple HT, Scully SP, Aboulafia AJ. Benign bone tumors. Instr Course Lect. 2002;51:429–439. [PubMed] [Google Scholar]
- 14.Yildiz C, Erler K, Atesalp AS, Basbozkurt M. Benign bone tumors in children. Curr Opin Pediatr. 2003;15:58–67. doi: 10.1097/00008480-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Lodwick GS, Wilson AJ, Farrell C, Virtama P, Dittrich F. Determining growth rates of focal lesions of bone from radiographs. Radiology. 1980;134:577–583. doi: 10.1148/radiology.134.3.6928321. [DOI] [PubMed] [Google Scholar]
- 16.Caracciolo JT, Temple HT, Letson GD, Kransdorf MJ. A modified lodwick-madewell grading system for the evaluation of lytic bone lesions. AJR Am J Roentgenol. 2016;207:150–156. doi: 10.2214/AJR.15.14368. [DOI] [PubMed] [Google Scholar]
- 17.Erlemann R. [MRI morphology of bone tumors and tumor-like lesions] Radiologe. 2010;50:61–80. doi: 10.1007/s00117-009-1845-8. quiz 1. [DOI] [PubMed] [Google Scholar]
- 18.Davis JC, Daw NC, Navid F, et al. 18F-FDG uptake during early adjuvant chemotherapy predicts histologic response in pediatric and young adult patients with osteosarcoma. J Nucl Med. 2018;59:25–30. doi: 10.2967/jnumed.117.190595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costelloe CM, Chuang HH, Daw NC. PET/CT of osteosarcoma and Ewing sarcoma. Semin Roentgenol. 2017;52:255–268. doi: 10.1053/j.ro.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Annovazzi A, Ferraresi V, Anelli V, et al. [18F]FDG PET/CT quantitative parameters for the prediction of histological response to induction chemotherapy and clinical outcome in patients with localised bone and soft-tissue Ewing sarcoma. Eur Radiol. 2021;31:7012–7021. doi: 10.1007/s00330-021-07841-w. [DOI] [PubMed] [Google Scholar]
- 21.Leithner A, Windhager R. [Guidelines for the biopsy of bone and soft tissue tumours] Orthopade. 2007;36:167–174. doi: 10.1007/s00132-006-1039-2. quiz 75. [DOI] [PubMed] [Google Scholar]
- 22.Bruns J, Delling G, Henne-Bruns D, Hossfeld DK. Biopsy of tumors of the musculoskeletal system. Dtsch Arztebl Int. 2008;105:492–497. doi: 10.3238/arztebl.2008.0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gemeinsamer Bundesausschuss. Richtlinie über Maßnahmen zur Qualitätssicherung für die stationäre Versorgung von Kindern und Jugendlichen mit hämato-onkologischen Krankheiten gemäß § 136 Abs. 1 Satz 1 Nr. 2 SGB V für nach § 108 SGB V zugelassene Krankenhäuser. www.g-ba.de/richtlinien/47/ (last accessed on 16 October 2022) [Google Scholar]
- 24.Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res. 1986:9–24. [PubMed] [Google Scholar]
- 25.Kaneuchi Y, Yoshida S, Fujiwara T, Evans S, Abudu A. Limb salvage surgery has a higher complication rate than amputation but is still beneficial for patients younger than 10 years old with osteosarcoma of an extremity. J Pediatr Surg. 2022:;57:702–709. doi: 10.1016/j.jpedsurg.2022.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Windhager R, Funovics P, Panotopoulos J, Hobusch G, Schinhan M. [Growing prostheses after sarcoma resection in children and adolescents] Orthopade. 2019;48:563–571. doi: 10.1007/s00132-019-03753-2. [DOI] [PubMed] [Google Scholar]
- 27.Gotta J, Bielack S, Hecker-Nolting S, et al. When your ankle becomes a knee—long-term functional outcome and quality of life with a rotationplasty after resection of malignant limb tumors. Klin Padiatr. 2022;234:154–162. doi: 10.1055/a-1681-1916. [DOI] [PubMed] [Google Scholar]
- 28.Benady A, Gortzak Y, Sofer S, et al. Internal hemipelvectomy for primary bone sarcomas using intraoperative patient specific instruments—the next step in limb salvage concept. BMC Musculoskelet Disord. 2022;23 doi: 10.1186/s12891-022-05918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dominkus M, Darwish E, Funovics P. Reconstruction of the pelvis after resection of malignant bone tumours in children and adolescents. Recent Results Cancer Res. 2009;179:85–111. doi: 10.1007/978-3-540-77960-5_8. [DOI] [PubMed] [Google Scholar]
- 30.Fujiwara T, Medellin Rincon MR, Sambri A, et al. Limb-salvage reconstruction following resection of pelvic bone sarcomas involving the acetabulum. Bone Joint J. 2021;103-B:795–803. doi: 10.1302/0301-620X.103B4.BJJ-2020-0665.R1. [DOI] [PubMed] [Google Scholar]
- 31.Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine. Terminology and surgical staging. Spine (Phila Pa 1976) 1997;22:1036–1044. doi: 10.1097/00007632-199705010-00020. [DOI] [PubMed] [Google Scholar]
- 32.Salzer-Kuntschik M, Delling G, Beron G, Sigmund R. Morphological grades of regression in osteosarcoma after polychemotherapy—study COSS 80. J Cancer Res Clin Oncol. 1983;106(Suppl 21-4.) doi: 10.1007/BF00625047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campanacci M, Bacci G, Bertoni F, Picci P, Minutillo A, Franceschi C. The treatment of osteosarcoma of the extremities: twenty year’s experience at the Istituto Ortopedico Rizzoli. Cancer. 1981;48:1569–1581. doi: 10.1002/1097-0142(19811001)48:7<1569::aid-cncr2820480717>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 34.Campanacci M, Bacci G, Pagani P, Giunti A. Multiple-drug chemotherapy for the primary treatment of osteosarcoma of the extremities. J Bone Joint Surg Br. 1980;62-B:93–101. doi: 10.1302/0301-620X.62B1.6927980. [DOI] [PubMed] [Google Scholar]
- 35.Bielack S, Beck J, Delling G, et al. [Neoadjuvant chemotherapy of osteosarcoma Results of the cooperative studies COSS-80 and COSS-82 after 7 and 5 years] Klin Padiatr. 1989;201:275–284. doi: 10.1055/s-2008-1026715. [DOI] [PubMed] [Google Scholar]
- 36.Goorin AM, Perez-Atayde A, Gebhardt M, et al. Weekly high-dose methotrexate and doxorubicin for osteosarcoma: the Dana-Farber Cancer Institute/the Children’s Hospital–study III. J Clin Oncol. 1987;5:1178–1184. doi: 10.1200/JCO.1987.5.8.1178. [DOI] [PubMed] [Google Scholar]
- 37.Purfürst C, Beron G, Torggler S, Kotz R, Salzer-Kuntschik M, Winkler K. [Results of the COSS-77 and COSS-80 studies on adjuvant chemotherapy in osteosarcoma of the extremities] Klin Padiatr. 1985;197:233–238. doi: 10.1055/s-2008-1033974. [DOI] [PubMed] [Google Scholar]
- 38.Winkler K, Beron G, Delling G, et al. Neoadjuvant chemotherapy of osteosarcoma: results of a randomized cooperative trial (COSS-82) with salvage chemotherapy based on histological tumor response. J Clin Oncol. 1988;6:329–337. doi: 10.1200/JCO.1988.6.2.329. [DOI] [PubMed] [Google Scholar]
- E1.Smeland S, Bielack SS, Whelan J, et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer. 2019;109:36–50. doi: 10.1016/j.ejca.2018.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Whelan JS, Bielack SS, Marina N, et al. EURAMOS-1, an international randomised study for osteosarcoma: results from pre-randomisation treatment. Ann Oncol. 2015;26:407–414. doi: 10.1093/annonc/mdu526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.(GPOH) GfPOuH. S1-Leitlinie Osteosarkome. www.awmf.org/leitlinien/detail/ll/025-005.html2021“ (last accesed on 15 May 2023 [Google Scholar]
- E4.Casali PG, Bielack S, Abecassis N, et al. Bone sarcomas: ESMO-PaedCan-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv79–iv95. doi: 10.1093/annonc/mdy310. [DOI] [PubMed] [Google Scholar]
- E5.Daw NC, Neel MD, Rao BN, et al. Frontline treatment of localized osteosarcoma without methotrexate: results of the St Jude Children‘s Research Hospital OS99 trial. Cancer. 2011;117:2770–2778. doi: 10.1002/cncr.25715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Marina NM, Smeland S, Bielack SS, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol. 2016;17:1396–1408. doi: 10.1016/S1470-2045(16)30214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E7.Bielack SS, Smeland S, Whelan JS, et al. Methotrexate, Doxorubicin, and Cisplatin (MAP) plus maintenance pegylated Interferon Alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: first results of the EURAMOS-1 good response randomized controlled trial. J Clin Oncol. 2015;33:2279–2287. doi: 10.1200/JCO.2014.60.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E8.Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival—a report from the Children‘s Oncology Group. J Clin Oncol. 2008;26:633–638. doi: 10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- E9.Grignani G, Palmerini E, Ferraresi V, et al. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: a non-randomised phase 2 clinical trial. Lancet Oncol. 2015;16:98–107. doi: 10.1016/S1470-2045(14)71136-2. [DOI] [PubMed] [Google Scholar]
- E10.Grignani G, Palmerini E, Dileo P, et al. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group study. Ann Oncol. 2012;23:508–516. doi: 10.1093/annonc/mdr151. [DOI] [PubMed] [Google Scholar]
- E11.Duffaud F, Mir O, Boudou-Rouquette P, et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2019;20:120–133. doi: 10.1016/S1470-2045(18)30742-3. [DOI] [PubMed] [Google Scholar]
- E12.Davis LE, Bolejack V, Ryan CW, et al. Randomized double-blind phase II study of regorafenib in patients with metastatic osteosarcoma. J Clin Oncol. 2019;37:1424–1431. doi: 10.1200/JCO.18.02374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E13.Italiano A, Mir O, Mathoulin-Pelissier S, et al. Cabozantinib in patients with advanced Ewing sarcoma or osteosarcoma (CABONE): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:446–455. doi: 10.1016/S1470-2045(19)30825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E14.Dirksen U, Brennan B, Le Deley MC, et al. High-dose chemotherapy compared with standard chemotherapy and lung radiation in Ewing sarcoma with pulmonary metastases: results of the European Ewing Tumour Working Initiative of National Groups, 99 Trial and EWING 2008. J Clin Oncol. 2019;37:3192–3202. doi: 10.1200/JCO.19.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E15.Ladenstein R, Pötschger U, Le Deley MC, et al. Primary disseminated multifocal Ewing sarcoma: results of the Euro-EWING 99 trial. J Clin Oncol. 2010;28:3284–3291. doi: 10.1200/JCO.2009.22.9864. [DOI] [PubMed] [Google Scholar]
- E16.Le Deley MC, Paulussen M, Lewis I, et al. Cyclophosphamide compared with ifosfamide in consolidation treatment of standard-risk Ewing sarcoma: results of the randomized noninferiority Euro-EWING99-R1 trial. J Clin Oncol. 2014;32:2440–2448. doi: 10.1200/JCO.2013.54.4833. [DOI] [PubMed] [Google Scholar]
- E17.Paulussen M, Craft AW, Lewis I, et al. Results of the EICESS-92 study: two randomized trials of Ewing‘s sarcoma treatment-cyclophosphamide compared with ifosfamide in standard-risk patients and assessment of benefit of etoposide added to standard treatment in high-risk patients. J Clin Oncol. 2008;26:4385–4393. doi: 10.1200/JCO.2008.16.5720. [DOI] [PubMed] [Google Scholar]
- E18.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing‘s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- E19.Womer RB, West DC, Krailo MD, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children‘s Oncology Group. J Clin Oncol. 2012;30:4148–4154. doi: 10.1200/JCO.2011.41.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E20.Anderton J, Moroz V, Marec-Berard P, et al. International randomised controlled trial for the treatment of newly diagnosed EWING sarcoma family of tumoursEURO EWING 2012 Protocol. Trials. 2020;21 doi: 10.1186/s13063-019-4026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E21.(GPOH) GfPOuH. S1-Leitlinie Ewing-Sarkom des Kinder- und Jugendalters. https://register.awmf.org/de/leitlinien/detail/025-006“. (last accessed on 15 May 2023) [Google Scholar]
- E22.Whelan J, Le Deley MC, Dirksen U, et al. High-dose chemotherapy and blood autologous stem-cell rescue compared with standard chemotherapy in localized high-risk Ewing sarcoma: results of Euro-E.W.I.N.G.99 and Ewing-2008. J Clin Oncol. 2018;36 doi: 10.1200/JCO.2018.78.2516. JCO2018782516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E23.Foulon S, Brennan B, Gaspar N, et al. Can postoperative radiotherapy be omitted in localised standard-risk Ewing sarcoma? An observational study of the Euro-E.W.I.N.G group. Eur J Cancer. 2016;61:128–136. doi: 10.1016/j.ejca.2016.03.075. [DOI] [PubMed] [Google Scholar]
- E24.Worawongsakul R, Steinmeier T, Lin YL, et al. Proton therapy for primary bone malignancy of the pelvic and lumbar region—data from the prospective registries ProReg and KiProReg. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.805051. 805051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E25.Kharod SM, Indelicato DJ, Rotondo RL, et al. Outcomes following proton therapy for Ewing sarcoma of the cranium and skull base. Pediatr Blood Cancer. 2020;67 doi: 10.1002/pbc.28080. e28080. [DOI] [PubMed] [Google Scholar]
- E26.Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23:2004–2011. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- E27.Brennan B, Kirton L, Marec-Berard P, et al. Comparison of two chemotherapy regimens in Ewing sarcoma (ES): Overall and subgroup results of the Euro Ewing 2012 randomized trial (EE2012) J Clin Oncol. 2020;38 [Google Scholar]