Abstract
Background and Objectives
Chronic axonal polyneuropathy is a common disease of the peripheral nervous system with increasing prevalence with age. Typical neurologic signs are present in patients with polyneuropathy but may also occur in individuals without disease. Owing to limited knowledge on normal aging of the peripheral nervous system, it can be difficult to distinguish peripheral nerve dysfunction due to disease from variations in normal aging. Therefore, we described the changes in neurologic examination and nerve conduction studies that accompany aging in the general population.
Methods
In this cross-sectional population-based study, we screened participants for chronic polyneuropathy in a controlled environment using standardized methods including a symptom questionnaire, neurologic examination, and nerve conduction studies (NCS). Inclusion criteria were 40 years or older and living in a suburb of Rotterdam, the Netherlands. Participants not diagnosed with chronic polyneuropathy, based on the discussion of findings in the screening by an expert team, were included to determine the effect of age (range 41–96 years) on features of neurologic examination and NCS using frequency calculations and quantile regression analysis.
Results
In total, 4,179 participants (mean age 64.5 ± 12.7 years, 54.9% female) were included of whom 3,780 (90.5%) did not fulfil the criteria for polyneuropathy. In the population without polyneuropathy, the frequency of normal features at neurologic examination declined with age, most pronounced for vibration sense at the hallux (from 6.6 [SD ± 1.5] in 40–49 years to 3.6 [SD ± 3.1] in 80 years or older) and Achilles tendon reflexes (absent in 9% in 40–49 years up to 33% in 80 years or older). Superficial pain sensation and patellar tendon reflexes remained stable over time. Sural sensory nerve action potential (SNAP) amplitude declined with age from 11.2 μV in 40–49 years to 3.3 μV in 80 years or older. Nonrecordable SNAP amplitudes were found in 25.1% of the participants older than 80 years, more often in men (30.3%) than in women (21.0%).
Discussion
This study showed the effect of age on features of neurologic examination and sural nerve amplitude in the general population. These findings are helpful to distinguish features suggesting polyneuropathy from variations of normal aging of the peripheral nervous system.
Introduction
Chronic axonal polyneuropathy is a chronic disease of the peripheral nervous system, and the diagnosis is based on the presence of symptoms, typical signs at neurologic examination, and findings at nerve conduction studies (NCS). Symptoms, such as tingling, pain, and/or numbness, usually start at the most distal part of the nerve in the extremities because of the length-dependent nature of the disease.
The prevalence of chronic axonal polyneuropathy increases with age1; however, how aging effects different components of the peripheral nervous system has not extensively been studied. Some typical signs of polyneuropathy, such as absent tendon reflexes or lowered vibration sense, are also observed in older individuals without polyneuropathy. Lack of knowledge on aging of the peripheral nervous system can make it difficult to distinguish peripheral nerve dysfunction from variation in normal aging, and this may lead to both under and over diagnosis of chronic axonal polyneuropathy.2 Information on age-related changes in neurologic examination is relatively scarce and is mostly derived from retrospective studies with selected samples of (self-declared) healthy or relatively young participants.2-15 Studies on changes in nerve function while aging primarily focused on nerve conduction slowing rather than changes due to axonal damage; the studies were relatively small, consisted of selected samples and differed regarding technique, temperature control, and use of devices.15-21
It is important to quantify the effects of aging on the peripheral nervous system in the general population as this may help decide whether findings of neurologic examination and NCS are within the normal range for a specific age or aid to diagnose polyneuropathy. Therefore, we prospectively examined aging of peripheral nerves using standardized methods in a controlled environment of a large population-based study.
Methods
Population
This study is embedded in the Rotterdam Study, a population-based study including middle-aged and older persons. The aims of this study were to unravel etiology, natural history, preclinical course, and potential targets for intervention for chronic diseases and include a variety of diseases (e.g., cardiovascular, neurologic, endocrine, respiratory diseases, among others).22 Participants are aged 40 years and older and are living in a district of Rotterdam, the Netherlands. The study is ongoing since 1990, and from 2013 onward, participants are also screened for the presence of chronic axonal polyneuropathy. From June 2013 until January 2020, 4,334 participants were screened. Participants with an insufficient screening were excluded (N = 155) as described in the next paragraph. In total, the results of 4,179 participants were used for this study.
Determining the Population Without Polyneuropathy
All participants were screened for polyneuropathy by repeatedly trained research personnel in a controlled environment. Screening procedure quality checks are regularly conducted.23 The polyneuropathy screening procedure and the categorization into no, possible, probable, and definite polyneuropathy have been published before.23 It consisted of 3 components: a symptom questionnaire, neurologic examination, and NCS. The symptoms questionnaire consisted of 12 questions about common symptoms that had to be present for at least 3 months in both feet and/or legs. The questions could be answered with never, sometimes, or (almost) continuously. The questionnaire was deemed positive when at least 3 of the typical polyneuropathy signs were present, depending whether the symptoms were sometimes present or (almost) continuously, based on the Erasmus-Polyneuropathy Symptom Score.23,24 Neurologic examination of both legs involved sensory testing, tendon reflexes, and dorsal flexion of the feet. Sensory testing included vibration sense and superficial pain sensation. Vibration sense was tested at the hallux of both feet using a Rydel-Seiffer graduated tuning fork ranging from score 0 (lowest) to 8 (highest).25 Superficial pain sensation was tested using a pin from the knees descending to the toes while investigating abnormal pain sensations (both hypoesthesia and hyperesthesia). Patellar and ankle tendon reflexes were tested in sitting position, with the use of the Jendrassik maneuver if needed, and classified as absent, minimal, or normal. Neurologic examination was deemed abnormal when at least 2 features were abnormal of which absent Achilles tendon reflexes, abnormal vibration sense, and abnormal pinprick were deemed most informative.23 NCS was performed with a Nicolet Viking Quest (Natus Medical Incorporated, San Carlos, CA). The sural nerves were stimulated bilaterally along the calf, and the sensory nerve action potentials (SNAPs) were recorded behind the lateral malleolus with surface electrodes (14 cm). SNAP amplitudes were measured from baseline to peak. Sural SNAP amplitude <4.0 μV was considered abnormal.23,26 Limb temperature was measured at the ankle and determined by 2 averaged measurements.
The results of the screening per participant were discussed in an expert panel (P.D., J.D., N.T., and R.H.), and participants were categorized as “no,” “possible,” “probable,” or “definite” polyneuropathy based on the abnormalities in the components of our screening and irrespective of their cause. In addition, the medical records of participants were reviewed for diagnosis of polyneuropathy by a neurologist, as this was considered superior to our screening.23 Participants with “definite” polyneuropathy fulfilled all diagnostic criteria for polyneuropathy, e.g., all components of the screening were deemed abnormal (symptoms, neurologic examination, and NCS). Participants with “no” polyneuropathy had no symptoms, neurologic signs of polyneuropathy, or abnormalities at NCS. Participants with “probable” polyneuropathy had at least 2 abnormal components in the screening, e.g., typical symptoms and multiple abnormalities in neurologic examination, but normal sural SNAP amplitudes. These participants were considered at high risk for developing polyneuropathy as they have symptoms and multiple signs, but not (yet) fulfilled all diagnostic criteria. Participants with possible polyneuropathy had at most one deviant component of the screening, e.g., only symptoms, only abnormal findings at neurologic examination, or only low sural SNAP amplitudes.23 In these cases, the findings in the screening procedure were considered nonspecific for polyneuropathy and rather attributed to aging or other nonpolyneuropathy diseases. To study normal aging of the peripheral nervous system, the results need to be generalizable to the population in which the diagnostic dilemmas arise. Therefore, we decided not to study a highly selected, screened population without any symptoms or signs as this would result in a low generalizability of the results. Therefore, participants with “no” and “possible” polyneuropathy were considered as the population without polyneuropathy. For this study, we excluded participants with clinically relevant symptoms or signs compatible with “definite” and “probable” polyneuropathy. Participants were also excluded if ≥2 of the components of our screening were missing (N = 155).
Data Analysis
Population characteristics were described using means (standard deviation) or median (interquartile range) for continuous measures based on their distribution. Categorical measures were described as number (%). For all further analysis, the population without polyneuropathy was used.
The highest score or best finding within a participant was used for analysis of vibration sense, superficial pain sensation, tendon reflexes, and NCS as chronic axonal polyneuropathy is characterized by symmetrical symptoms and signs because it is usually a symmetric, length-dependent disease. Tendon reflexes were divided into normal, minimal, or absent. Superficial pain sensation was categorized as normal, diminished up to the ankle or midtibia level and the knee or above. Vibration sense at the hallux was scored using a Rydel-Seiffer graduated tuning fork ranging from 0 (lowest) up to 8 (highest). The results for vibration sense were categorized based on previous published reference values as low (0–3), reduced (4–6), or high (7–8).25 Frequencies of features of neurologic examination were calculated per age decade in the population without polyneuropathy.
To assess values for the sural SNAP amplitude per age in the population without polyneuropathy, we used quantile regression analysis, as the assumptions for linear regression, such as linearity and normality, could not be met. Smoothness of the fitted curves of quantile regression is influenced by the degrees of freedom. Degrees of freedom were determined by the introduction of splines for age, and the model with the best performance and best fitted curves was chosen. To adjust for sex, we calculated sex-standardized prevalence. We did not correct for skin temperature as the preferred temperature differs per clinic. The age range for the sural nerve amplitude curves comprised age 40–90 years as this study included very few participants older than 90 years with NCS.
We performed 3 sensitivity analyses. First, to investigate how participants with “possible” polyneuropathy affected the results of neurological examination by excluding them from the population without polyneuropathy. Second, we also investigated the percentages of abnormal neurologic examination in the other polyneuropathy groups (possible, probable, and definite). Third, to verify the effect of temperature on the results of the sural SNAP amplitude, participants with a skin temperature <30°C were excluded.
Standard Protocol Approvals, Registrations, and Patient Consents
The Rotterdam Study has been approved by the Medical Ethics Committee of Erasmus MC (Registration No. MEC 02.1015) and by the Dutch Ministry of Health, Welfare, and Sport (Population Screening Act WBO, License No. 1071272-159521-PG). The Rotterdam Study Personal Registration Data collection is filed with the Erasmus MC Data Protection Officer under Registration No. EMC1712001. The Rotterdam Study has been entered into the Netherlands National Trial Register and into the World Health Organization International Clinical Trials Registry Platform under shared Catalogue No. NTR6831. All participants provided written informed consent to participate in this study and to have their information obtained from treating physicians.
Data Availability
Data can be obtained on request. Requests should be directed toward the management team of the Rotterdam Study (secretariat.epi@erasmusmc.nl), which has a protocol for approving data requests.
Results
In total, 4,179 participants of the Rotterdam Study were included. The mean age of the population was 64.5 (±12.7) years, and 54.9% were female. The population with polyneuropathy comprised the persons with definite (N = 178) and probable polyneuropathy (N = 221), and the population without polyneuropathy comprised persons with possible (N = 798) or no polyneuropathy (N = 2,982) (Table 1).
Table 1.
Population Characteristics
Features at neurologic examination in the population without polyneuropathy differed with age, resulting in lower frequencies of normal or reduced features in older age. This was most pronounced for Achilles tendon reflexes and vibration sense at the hallux (Table 2 and Figure 1). Interestingly, superficial pain sensation and patellar tendon reflexes remained quite stable over time (Figure 1). The largest differences were observed in participants older than 60 years, especially for vibration sense followed by the presence of Achilles tendon reflexes (Table 2 and Figure 2). Vibration sense declined from mean 6.6 (SD ± 1.5) in 40–50-year-old participants to mean 3.6 (±3.1) in older than 80-year-old participants without polyneuropathy (Table 2).
Table 2.
Features of Neurologic Examination in the Population Without Chronic Polyneuropathy
Figure 1. Presence of Features of Neurologic Examination per Age Decade in the Population Without Chronic Polyneuropathy.
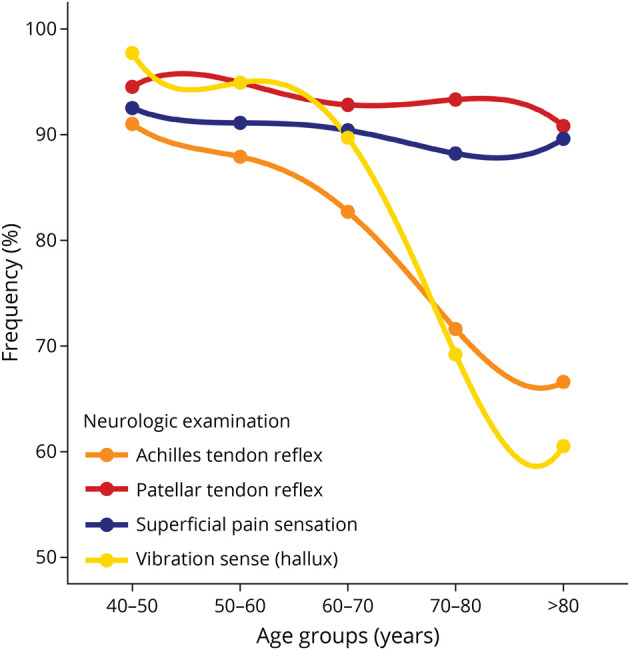
Frequency of presence of Achilles tendon reflex (orange), patellar tendon reflex (red), superficial pain sensation (blue), and vibration sense (yellow). The presence of neurologic features comprised normal or minimal tendon reflexes, normal superficial pain sensation on the feet, and vibration sense ≥4 using the graduated tuning fork (0–8).
Figure 2. Details per Component of Features of Neurologic Examination per Age Decade in the Population Without Chronic Polyneuropathy.
Frequency of signs of neurologic examination of tendon reflexes, superficial pain sensation, and vibration sense, classified as normal (“normal,” “7–8), minimal (“minimal,” “from toes to ankle,” “4–6”), or abnormal (“absent,” “from toes to tibia or higher,” “0–3”).
Figure 2 showed features of neurologic examination subdivided in “normal,” “reduced,” or “absent.” An increase for “reduced” or “absent” patellar and Achilles tendon reflexes was observed with increasing age (Figure 2 and Table 2). Similar patterns were observed for vibration sense and less evident for superficial pain sensation (Figure 2). No relevant sex differences were observed in the features of neurologic examination (eFigure, links.lww.com/WNL/D31).
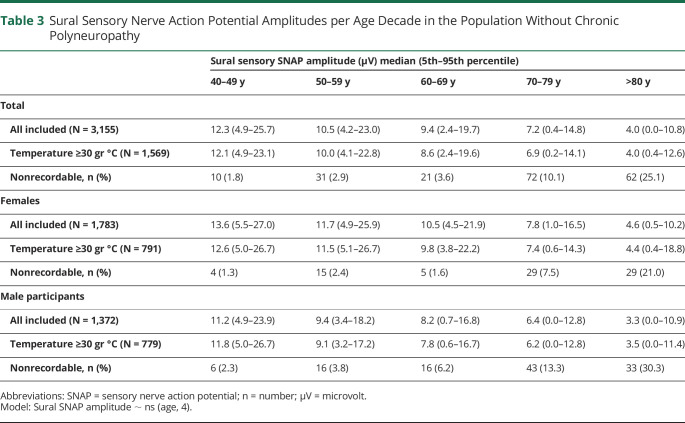
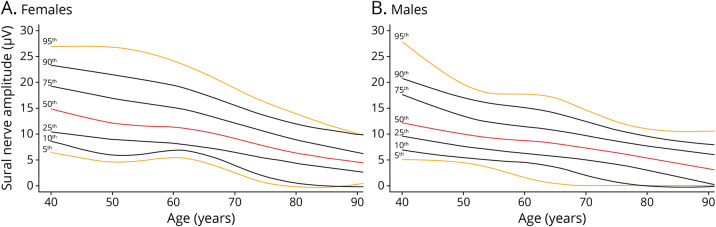
Sural SNAP amplitude was nonlinearly associated with age in the population without polyneuropathy. Sural SNAP amplitude declined with age with a slightly more rapid decay in participants older than 65 years, especially in women. Overall, lower sural SNAP amplitudes were observed in men than women (Figure 3 and Table 3). Per decade difference, nonrecordable SNAP amplitudes increase up to 25.1% of the participants older than 80 years in our population,with higher frequencies in men (30.3%) than in women (21.0%) (Table 3).
Table 3.
Sural Sensory Nerve Action Potential Amplitudes per Age Decade in the Population Without Chronic Polyneuropathy
Figure 3. Percentile Curves of Sural Sensory Nerve Action Potential Amplitude in the General Population Without Chronic Polyneuropathy, Stratified for Sex.
Percentile curves for the sural sensory nerve action potential amplitude for female (panel A, N = 1,783) and male participants (panel B, N = 1,372). Lines correspond to the 5th (orange), 10th (black), 25th (black), 50th (red), 75th (black), 90th (black), and 95th (orange) percentile.
The first sensitivity analysis, in which participants with possible polyneuropathy were excluded from the population without diagnosis polyneuropathy (N = 2,982), minimally changed the results as expected based on the current diagnostic criteria for polyneuropathy and our categorization of polyneuropathy based on the results of screening. In general, absent Achilles tendon reflexes were slightly less common as the percentage of the participants with normal reflexes was slightly higher. A similar pattern was observed for vibration sense, especially in older age. As these changes were only marginal, we concluded that dividing the groups, no/possible polyneuropathy (the “population without polyneuropathy”) and probable/definite polyneuropathy (the “population with polyneuropathy”) were reliable for the purpose of this study (eTable 1, links.lww.com/WNL/D30). The second sensitivity analysis showed a similar pattern regarding the presence of neurologic features while aging in possible, probable, and definite polyneuropathy (eTables 2–4, links.lww.com/WNL/D30).
The third sensitivity analysis comprised the effect of temperature on the outcome sural SNAP amplitude. After excluding participants with skin temperature <30°C from the population without polyneuropathy (N = 1,569), the results remained quite similar compared with all participants, as presented in Table 3.
Discussion
This study showed that age-related changes of features of peripheral nerve function frequently occur, and this must be considered by physicians when interpreting neurologic examination and NCS. The frequency of normal features at neurologic examination in the general population without polyneuropathy, especially vibration sense at the hallux and Achilles tendon reflexes, declined with increasing age. These age-related changes in neurologic examination may be interpreted as abnormal and may lead to the erroneous consideration or diagnosis of polyneuropathy in older age groups. Interestingly, superficial pain sensation and patellar tendon reflexes remained quite stable over time, implying these features may be more informative in older age. Sural SNAP amplitudes decline with increasing age, and a faster decline is observed in older age. Men often have lower sural amplitudes than women. Nonrecordable sural amplitudes are mostly noticed from the sixth decade of life.
Age-related changes in neurologic examination have been noticed in the past, but most studies collected the data retrospectively.2-13,15 The definition of healthy individuals differed per study ranging from self-declared healthy individuals to individuals without any diseases or even without risk factors that may influence the risk for polyneuropathy, resulting in a highly selective population. In those studies, testing for chronic axonal polyneuropathy included neurologic examination frequently without NCS that may have resulted in an underestimation or overestimation of abnormalities at neurologic examination. By determining the effects of aging on the peripheral nervous system in our screened population, we provided estimates of normal features and abnormalities in neurologic examination per age decade. Estimates by age decades are important, as we found a relatively high number of participants with abnormal features at neurologic examination that were largely explained by the higher estimates in older age. Similarly, the decline observed in sural SNAP amplitude with advancing age has been described before, based on studies that primarily focused on nerve conduction velocities, including a relatively young, selective population of middle-aged persons in relatively small numbers.17-21,26,27 This may have affected the generalizability to the older population of interest in whom the diagnostic doubts of interpretation of the results of NCS prevail.
We observed sex differences in the results of sural SNAP amplitude, but not in neurologic examination. In previous studies, the influence of sex on NCS has been described and most often been attributed to differences in height or leg length between men and women.16,17 To adjust for sex, we made percentile curves for men and women separately. Interestingly, the influence of sex was not observed in features of neurologic examination. Possibly, this may demonstrate that neurologic examination is a coarser, composite method of testing in which subtle differences may not be detected.
Previous studies showed that temperature may influence the results of NCS. However, how to correct the skin temperature remains argued as the effect is likely different for healthy and damaged nerves, especially for conduction velocities.28-31 Furthermore, skin temperature and near-nerve temperature may differ depending on skin and tissue volume characteristics such as nerve depth, vascularization, and fat layer.31 Methodologically adjusting for temperature was not rated accurately enough in previous studies,28,29 and therefore, we included all participants irrespective of skin temperature. We conducted a sensitivity analysis on skin temperature and only found limited effects on sural SNAP amplitude results, even in the extreme percentiles.
The strengths of this study are the population-based setting, resembling the general population. All participants included were screened for chronic axonal polyneuropathy that included also NCS, minimizing the risk for misclassification of participants that would result in an overestimation of the results. The screening was performed by a trained personnel in a controlled environment using standardized methods reducing the risk that methodological differences may explain the results. Furthermore, this prospective study includes large numbers of participants over the full age range in which chronic axonal polyneuropathy usually occurs.
Limitations of this study are that “normal population” was defined as free of polyneuropathy but not of other chronic diseases. As our results are of most interest for the interpretation and diagnosis of chronic axonal polyneuropathy, we presume it is unlikely this may have influenced the results. Within the framework of the Rotterdam Study, participants were categorized in 4 categories of polyneuropathy to increase the accuracy of the data. However, when studying clinical outcomes, this categorization needs to be translated to the clinical setting resembling the general population. Hence, we deemed participants with possible polyneuropathy (nonspecific symptoms and signs) as part of the population without polyneuropathy and performed a sensitivity analysis to check this assumption. On the one hand, we cannot rule out that some participants (especially the persons with low sural nerve amplitudes) may develop polyneuropathy in the future, as longitudinal data are not yet available. On the other hand, we can also not rule out that some participants with normal aging were excluded from the analyses as they were categorized as probable polyneuropathy (especially the persons without any symptoms). Furthermore, owing to logistic reasons in this population-based study, we were not able to control skin temperature before conducting NCS. To check the consequences of this limitation, we performed a sensitivity analysis that showed only minimal changes of the results after excluding participants with lower skin temperature.
In conclusion, this study provides readily, clinically relevant values that may be used when interpreting the findings of neurologic examination and NCS in the general population as changes emerge while aging, especially in participants older than 60 years. This study may help to interpret features of neurologic examination and NCS in persons with older age.
Glossary
- NCS
nerve conduction studies
- SNAP
sensory nerve action potentials
Appendix. Authors

Footnotes
CME Course: NPub.org/cmelist
Study Funding
Prinses Beatrix Spierfonds (W.OR17-10).
Disclosure
P.A. van Doorn and M.A. Ikram received a grant from Prinses Beatrix Spierfonds for neuromuscular diseases (Grant No. W.OR17-10) to conduct this study and report no other disclosures relevant to this manuscript. N.E. Taams, J. Drenthen, and R. Hanewinckel report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Taams NE, Drenthen J, Hanewinckel R, Ikram MA, van Doorn PA. Prevalence and risk factor profiles for chronic axonal polyneuropathy in the general population. Neurology. August 25, 2022. doi. 10.1212/WNL.0000000000201168 [DOI] [PubMed] [Google Scholar]
- 2.Dyck PJ, Overland CJ, Low PA, et al. Signs and symptoms versus nerve conduction studies to diagnose diabetic sensorimotor polyneuropathy: Cl vs. NPhys trial. Muscle Nerve. 2010;42(2):157-164. doi. 10.1002/mus.21661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowditch MG, Sanderson P, Livesey JP. The significance of an absent ankle reflex. J Bone Joint Surg Br. 1996;78(2):276-279. [PubMed] [Google Scholar]
- 4.Bryndum B, Marquardsen J. The tendon reflexes in old age. Gerontol Clin (Basel). 1964;6:257-265. doi. 10.1159/000244838 [DOI] [PubMed] [Google Scholar]
- 5.Clarke CE, Davies P, Wilson T, Nutbeam T. Comparison of the tendon and plantar strike methods of eliciting the ankle reflex. J Neurol Neurosurg Psychiatry. 2003;74(9):1351-1352. doi. 10.1136/jnnp.74.9.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Michele G, Filla A, Coppola N, et al. Influence of age, gender, height and education on vibration sense. A study by tuning fork in 192 normal subjects. J Neurol Sci. 1991;105(2):155-158. doi. 10.1016/0022-510x(91)90139-x [DOI] [PubMed] [Google Scholar]
- 7.Dyck PJ, Litchy WJ, Lehman KA, Hokanson JL, Low PA, O'Brien PC. Variables influencing neuropathic endpoints: the rochester diabetic neuropathy study of healthy subjects. Neurology. 1995;45(6):1115-1121. doi. 10.1212/wnl.45.6.1115 [DOI] [PubMed] [Google Scholar]
- 8.Ellenberg M. The deep reflexes in old age. JAMA. 1960;174:468-469. doi. 10.1001/jama.1960.03030050010003 [DOI] [PubMed] [Google Scholar]
- 9.Hilz MJ, Axelrod FB, Hermann K, Haertl U, Duetsch M, Neundorfer B. Normative values of vibratory perception in 530 children, juveniles and adults aged 3-79 years. J Neurol Sci. 1998;159(2):219-225. doi. 10.1016/s0022-510x(98)00177-4 [DOI] [PubMed] [Google Scholar]
- 10.Impallomeni M, Kenny RA, Flynn MD, Kraenzlin M, Pallis CA. The elderly and their ankle jerks. Lancet. 1984;1(8378):670-672. doi. 10.1016/s0140-6736(84)92181-0 [DOI] [PubMed] [Google Scholar]
- 11.Martina IS, van Koningsveld R, Schmitz PI, van der Meche FG, van Doorn PA. Measuring vibration threshold with a graduated tuning fork in normal aging and in patients with polyneuropathy. European Inflammatory Neuropathy Cause and Treatment (INCAT) group. J Neurol Neurosurg Psychiatry. 1998;65(5):743-747. doi. 10.1136/jnnp.65.5.743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odenheimer G, Funkenstein HH, Beckett L, et al. Comparison of neurologic changes in “successfully aging” persons vs the total aging population. Arch Neurol. 1994;51(6):573-580. doi. 10.1001/archneur.1994.00540180051013 [DOI] [PubMed] [Google Scholar]
- 13.Vrancken AF, Kalmijn S, Brugman F, Rinkel GJ, Notermans NC. The meaning of distal sensory loss and absent ankle reflexes in relation to age: a meta-analysis. J Neurol. 2006;253(5):578-589. doi. 10.1007/s00415-005-0064-0 [DOI] [PubMed] [Google Scholar]
- 14.Chronic symmetric symptomatic polyneuropathy in the elderly: a field screening investigation in two Italian regions. I. Prevalence and general characteristics of the sample. Italian General Practitioner Study Group (IGPSG). Neurology. 1995;45(10):1832-1836. doi: 10.1212/wnl.45.10.1832 [DOI] [PubMed] [Google Scholar]
- 15.Vrancken AF, Franssen H, Wokke JH, Teunissen LL, Notermans NC. Chronic idiopathic axonal polyneuropathy and successful aging of the peripheral nervous system in elderly people. Arch Neurol. 2002;59(4):533-540. doi. 10.1001/archneur.59.4.533 [DOI] [PubMed] [Google Scholar]
- 16.Horowitz SH, Krarup C. Conduction studies of the normal sural nerve. Muscle Nerve. 1992;15(3):374-383. doi. 10.1002/mus.880150318 [DOI] [PubMed] [Google Scholar]
- 17.Trojaborg WT, Moon A, Andersen BB, Trojaborg NS. Sural nerve conduction parameters in normal subjects related to age, gender, temperature, and height: a reappraisal. Muscle Nerve. 1992;15(6):666-671. doi. 10.1002/mus.880150606 [DOI] [PubMed] [Google Scholar]
- 18.Falco FJ, Hennessey WJ, Goldberg G, Braddom RL. Standardized nerve conduction studies in the lower limb of the healthy elderly. Am J Phys Med Rehabil. 1994;73(3):168-174. doi. 10.1097/00002060-199406000-00005 [DOI] [PubMed] [Google Scholar]
- 19.Stetson DS, Albers JW, Silverstein BA, Wolfe RA. Effects of age, sex, and anthropometric factors on nerve conduction measures. Muscle Nerve. 1992;15(10):1095-1104. doi. 10.1002/mus.880151007 [DOI] [PubMed] [Google Scholar]
- 20.Bouche P, Cattelin F, Saint-Jean O, et al. Clinical and electrophysiological study of the peripheral nervous system in the elderly. J Neurol. 1993;240(5):263-268. doi. 10.1007/BF00838158 [DOI] [PubMed] [Google Scholar]
- 21.Hanewinckel R, Ikram MA, Franco OH, Hofman A, Drenthen J, van Doorn PA. High body mass and kidney dysfunction relate to worse nerve function, even in adults without neuropathy. J Peripher Nerv Syst. 2017;22(2):112-120. doi. 10.1111/jns.12211 [DOI] [PubMed] [Google Scholar]
- 22.Ikram MA, Brusselle G, Ghanbari M, et al. Objectives, design and main findings until 2020 from the Rotterdam Study. Eur J Epidemiol. 2020;35(5):483-517. doi. 10.1007/s10654-020-00640-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanewinckel R, Drenthen J, van Oijen M, Hofman A, van Doorn PA, Ikram MA. Prevalence of polyneuropathy in the general middle-aged and elderly population. Neurology. 2016;87(18):1892-1898. doi. 10.1212/WNL.0000000000003293 [DOI] [PubMed] [Google Scholar]
- 24.Hanewinckel R, van Oijen M, Taams NE, et al. Diagnostic value of symptoms in chronic polyneuropathy: the Erasmus polyneuropathy symptom score. J Peripher Nerv Syst. 2019;24(3):235-241. doi. 10.1111/jns.12328 [DOI] [PubMed] [Google Scholar]
- 25.Merkies IS, Schmitz PI, van der Meche FG, van Doorn PA. Reliability and responsiveness of a graduated tuning fork in immune mediated polyneuropathies. The Inflammatory Neuropathy Cause and Treatment (INCAT) Group. J Neurol Neurosurg Psychiatry. 2000;68(5):669-671. doi. 10.1136/jnnp.68.5.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buschbacher RM. Sural and saphenous 14-cm antidromic sensory nerve conduction studies. Am J Phys Med Rehabil. 2003;82(6):421-426. [PubMed] [Google Scholar]
- 27.Rivner MH, Swift TR, Malik K. Influence of age and height on nerve conduction. Muscle Nerve. 2001;24(9):1134-1141. doi. 10.1002/mus.1124 [DOI] [PubMed] [Google Scholar]
- 28.Franssen H, Wieneke GH, Wokke JH. The influence of temperature on conduction block. Muscle Nerve. 1999;22(2):166-173. doi. [DOI] [PubMed] [Google Scholar]
- 29.Notermans NC, Franssen H, Wieneke GH, Wokke JH. Temperature dependence of nerve conduction and EMG in neuropathy associated with gammopathy. Muscle Nerve. 1994;17(5):516-522. doi. 10.1002/mus.880170508 [DOI] [PubMed] [Google Scholar]
- 30.Rutkove SB. Effects of temperature on neuromuscular electrophysiology. Muscle Nerve. 2001;24(7):867-882. doi. 10.1002/mus.1084 [DOI] [PubMed] [Google Scholar]
- 31.Drenthen J, Blok JH, van Heel EB, Visser GH. Limb temperature and nerve conduction velocity during warming with hot water blankets. J Clin Neurophysiol. 2008;25(2):104-110. doi. 10.1097/WNP.0b013e31816a3b28 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be obtained on request. Requests should be directed toward the management team of the Rotterdam Study (secretariat.epi@erasmusmc.nl), which has a protocol for approving data requests.