Abstract
Patients with uncontrolled, allergic severe asthma may be prescribed biologic therapies to reduce exacerbations and improve disease control. Randomized controlled trials (RCTs) of these therapies have differed in design, with varying results overall and by baseline blood eosinophil count (BEC). This study describes published annualized asthma exacerbation rate (AAER) reductions from RCTs in patients with allergic severe asthma, overall and by baseline BEC category. A literature search was performed to identify published phase 3 RCT data of US Food and Drug Administration-approved biologics for severe asthma in patients with severe, uncontrolled asthma and confirmed sensitization to perennial aeroallergens. Analyses focused on AAER reduction versus placebo in the overall population and/or in those with an elevated or low BEC at baseline or screening. Baseline serum total immunoglobulin E levels varied between RCT populations. In patients with allergic severe asthma across all BEC categories, data were available for tezepelumab, dupilumab, benralizumab and omalizumab only; the greatest AAER reduction was observed with tezepelumab. In patients with allergic severe asthma and BECs of ≥ 260 cells/µL or ≥ 300 cells/μL, AAER reductions were observed with all biologics (tezepelumab, dupilumab, mepolizumab, benralizumab and omalizumab); the greatest AAER reduction was observed with tezepelumab and the smallest AAER reduction was observed with omalizumab. In patients with allergic severe asthma and BECs of < 260 cells/µL or < 300 cells/μL (regardless of historical BEC), an AAER reduction was observed with tezepelumab but not with benralizumab or omalizumab. Differential mechanisms of action may explain the differences in results observed between biologics. Among patients with allergic severe asthma, the efficacy of biologics in RCTs varied considerably overall and by BEC. Tezepelumab was the only biologic to demonstrate AAER reductions consistently across all subgroups. These differences can inform provider treatment decisions when selecting biologic treatments for patients with allergic severe asthma.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02647-2.
Keywords: Biologic, Blood eosinophil, Perennial allergy, Efficacy, Exacerbations, Randomized placebo-controlled trial, Severe asthma, Allergic asthma, Literature review
Key Summary Points
| Randomized controlled trials (RCTs) of biologics in patients with severe asthma have demonstrated that efficacy varies in the overall allergic population and according to baseline blood eosinophil count (BEC). |
| In the absence of head-to-head trials evaluating efficacy in patients with allergic severe asthma to date, a literature review was conducted to describe the effects of biologics on the annualized asthma exacerbation rate (AAER) in the overall allergic severe asthma population and by elevated and low baseline BEC in placebo-controlled, phase 3 RCTs. |
| Among patients with allergic severe asthma, all biologics demonstrated efficacy in reducing the AAER in the overall population; greater reductions in the AAER were observed with higher baseline BEC. |
| Efficacy in reducing the AAER varied between individual biologics, likely due to their differing mechanisms of action, and differences in study design, study populations and inclusion/exclusion criteria between trials. |
| The findings in this study can help clinicians to compare efficacy data as well as inform provider treatment decisions when selecting biologic treatments for patients with allergic severe asthma overall and for those with or without coexisting eosinophilic inflammation. |
Introduction
Allergic asthma is a common phenotype of asthma [1]; it is present in up to approximately 60% of adult patients with moderate-to-severe asthma [2], and its prevalence is increasing [3]. The Global Initiative for Asthma (GINA) guidelines recommend biologics as add-on therapies in patients with uncontrolled, allergic severe asthma to improve disease control [4]. Of the six current US Food and Drug Administration (FDA)-approved biologics for moderate or severe asthma, four have indications for patients with an eosinophilic phenotype (benralizumab, mepolizumab, reslizumab and dupilumab [dupilumab is also indicated for oral corticosteroid-dependent asthma]) [5–8], one (omalizumab) is indicated for allergic asthma [9], and one (tezepelumab) has no restriction by asthma phenotype [10]. Benralizumab (Fasenra, AstraZeneca), mepolizumab (Nucala, GSK) and reslizumab (Cinqair, Teva Pharmaceuticals) target the interleukin (IL)-5/eosinophil pathway [5, 6, 8], while dupilumab (Dupixent, Sanofi/Regeneron) inhibits the activity of IL-4 and IL-13 [7]. Omalizumab (Xolair, Genentech/Novartis Pharmaceuticals) targets circulating immunoglobulin E (IgE), preventing IgE from interacting with mast cells and basophils [9]. Tezepelumab (Tezspire, Amgen/AstraZeneca) targets thymic stromal lymphopoietin (TSLP) and therefore inhibits the activity of multiple type 2 inflammatory pathways such as IL-4, IL-5 and IL-13, and tezepelumab has also been shown to reduce airway hyperresponsiveness, likely due to effects on mast cells and smooth muscle [10–13].
Allergic asthma is generally defined by sensitization to a perennial aeroallergen and is associated with elevated serum IgE levels; eosinophilic airway inflammation is also commonly present. As a result, patients with allergic asthma often have elevated blood and sputum eosinophil levels [14]. The allergic and eosinophilic asthma phenotypes are not mutually exclusive, and there is a high degree of overlap between these phenotypes in the population. It can be challenging to treat patients with severe asthma who have complex and overlapping asthma phenotypes, because they are likely to be eligible for multiple biologic treatments. The IDEAL observational study found that, in patients not currently receiving omalizumab, there was a 27–73% overlap in eligibility for mepolizumab (eosinophilic asthma) and omalizumab (allergic asthma) [15]. Similarly, a pooled analysis of clinical trials of patients with moderate-to-severe asthma found that approximately 40% of patients with allergic severe asthma have elevated blood eosinophil counts (BECs; ≥ 300 cells/µL) [16]. An International Severe Asthma Registry study found that a large proportion of adults with severe asthma had both elevated BECs and elevated serum total IgE levels [17]. In general, higher BECs in patients with asthma are associated with a better response to all of the available biologics [4, 18], including omalizumab treatment of allergic asthma [19]. In light of these considerations, providers face a difficult decision when selecting the most appropriate biologic therapy as an additional controller medication for patients with allergic severe asthma.
To date, no head-to-head trials of these biologic therapies in severe asthma have been conducted, meaning that judgments of comparative efficacy must be based on randomized controlled trials (RCTs) that differ in design. The efficacy of biologic therapies by BEC in severe asthma has been previously systematically summarized [20]; however, no such review has been conducted for patients with allergic severe asthma. For several of the available biologics, efficacy in patients with allergic severe asthma has only been described in post hoc subanalyses. Other complexities arise when attempting to compare trial data because of varying definitions of allergic severe asthma. Whereas the most relevant definition would be severe asthma with perennial aeroallergen sensitization for which regular allergen exposure drives the patient’s symptoms, few trials have collected or reported data on whether allergic symptoms were driving patients’ disease. Moreover, many RCT analyses have required specific levels of serum total IgE in addition to perennial aeroallergen sensitization, owing to the range of total IgE levels associated with approved omalizumab dosing regimens.
To increase our understanding of the efficacy of biologics in patients with allergic severe asthma, this review describes reductions in annualized asthma exacerbation rates (AAERs) in patients with allergic severe asthma analyzed in phase 3 RCTs. The review focuses on each biologic’s data in the broadest population of patients with confirmed sensitization to perennial aeroallergens for whom results have been published. Given that biologic efficacy has been shown to vary with BEC and the clinical challenge of treating patients with allergic and eosinophilic disease, we also summarize the extent to which AAER reduction in patients with allergic severe asthma varies according to baseline BEC levels.
Methods
A literature search of PubMed was performed on May 4, 2023, using the search string in Table S1 (see Supplementary Material) to identify peer-reviewed publications reporting phase 3 RCT data of FDA-approved biologics for severe asthma (omalizumab [9], benralizumab [8], mepolizumab [5], reslizumab [6], dupilumab [7] and tezepelumab [10]) in patients with uncontrolled, allergic severe asthma. The definition of severe, uncontrolled asthma was required to be consistent with the GINA 2023 report [4], and allergic asthma was defined as laboratory-confirmed sensitization to perennial aeroallergens. Early RCTs of omalizumab that enrolled a broad allergic asthma population that did not meet the GINA 2023 definition of severe asthma were excluded from this analysis. The literature search aimed to target studies containing data comparing AAER outcomes between the biologic and placebo in the overall allergic population and/or in those with an elevated or low BEC at baseline or screening; elevated and low BECs were defined as ≥ 300 cells/μL and < 300 cells/μL (or similar), respectively. If data for these specific groups were not available, the closest available subgroup was utilized (i.e., ≥ 260 cells/μL or < 260 cells/μL from the omalizumab EXTRA study) [19, 21]. Additionally, to ensure the standardization of reporting across studies, AAER outcomes were extracted and captured as rate ratios (biologic treatment:placebo). Data on other outcomes (such as lung function and asthma symptom and health-related quality of life scores) were not reported by BEC subgroups with sufficient consistency across studies to enable cross-trial comparisons. As a result, these data have not been included in this review, but they are available in the original publications.
There were no restrictions on how confirmed sensitization to perennial aeroallergens was defined (e.g., through results of a skin prick test or serum testing for allergen-specific IgE). If data for all patients with confirmed sensitization to perennial aeroallergens were not available for a specific biologic, published data for the most inclusive definition of allergic severe asthma were used (i.e., the population with the fewest restrictions to patient inclusion).
Lastly, when conducting the literature search, there were no publication date or language restrictions, nor were there any restrictions on patient age group or other aspects of the RCT design. This review is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Characteristics of the Included Studies and Patient Populations
The literature search returned 45 results, which were reviewed for inclusion suitability. Forty articles did not meet the inclusion requirements, e.g., they did not report phase 3 RCT data or did not contain relevant AAER data. In addition to the five articles that met the inclusion criteria, there were two primary publications not captured in the focused literature search that were identified by the authors to contain relevant analyses. Data from the primary publications of the NAVIGATOR (tezepelumab) and EXTRA (omalizumab) trials were included to enable comparisons. Therefore, overall, a total of seven publications from six different RCTs were identified that contained analyses and/or subanalyses that met the inclusion criteria (Table 1) [18, 19, 21–25]. All biologics approved for severe asthma were represented except for reslizumab. The definitions of an asthma exacerbation used across analyses were similar (Table 1). Only omalizumab had a phase 3 RCT (the EXTRA trial) that exclusively recruited patients with uncontrolled, allergic severe asthma [21]. For the other trials, results in patients with allergic severe asthma were prespecified subanalyses or post hoc analyses.
Table 1.
Characteristics of phase 3 trials and analyses of biologics in patients with allergic severe asthma
| Biologic | Trial/allergic severe asthma analysisa | Intervention | Eligible age range, years/BW, kg | Study duration, weeks | All patients in trial/patients with allergic severe asthma, n | Total IgE level eligibility criterion for all patients in parent trial, IU/mL | Main asthma criteria for all patients in the parent trial | Additional criteria for analysis of patients with allergic severe asthma | Definition of asthma exacerbation as an outcome for patients with allergic severe asthma |
|---|---|---|---|---|---|---|---|---|---|
| Omalizumab [19, 21] | EXTRA/same as main analysis (NCT00314574) |
Omalizumab minimum 0.008 mg/kg/IgE [IU/mL] Q2W SC or 0.016 mg/kg/IgE [IU/mL] Q4W SC |
12–75/30–150 | 48 | 850/850 | 30–700 |
•Uncontrolled, moderate-to- severe asthma with perennial allergy •History of allergic severe asthma for ≥ 1 year before screening •Asthma not well controlled despite treatment with high-dose ICSb and LABA ± other controllers •≥ 1 documented asthma exacerbation requiring SCS rescue during the past 12 months •Percent predicted pre-BD FEV1 of 40–80% |
•Allergy to a relevant perennial aeroallergen •Positive skin test or RAST in the 12 months before screening •Allergens: dog, cat, cockroach, Dermatophagoides farinae and D. pteronyssinus |
•Worsening asthma symptoms requiring SCS treatment for ≥ 3 days or, for patients receiving long-term OCS, ≥ 20 mg increase in mean daily dose of oral prednisone or equivalent |
| Benralizumab [22] | SIROCCO and CALIMA/post hoc analysis of pooled datac (NCT01928771, NCT01914757) | Benralizumab 30 mg Q8W SCd | 12–75/≥ 40 | 48 and 56 | 2295/1375 | No restrictions |
•Severe, uncontrolled asthma requiring: –Treatment with medium- or high-dose ICSe + LABA for ≥ 1 year before enrollment –Treatment with high-dose ICSe + LABA ± OCS and additional asthma controllers for ≥ 3 months before enrollment •≥ 2 asthma exacerbations needing SCS treatment or a temporary increase in mean OCS dose in the 12 months before enrollment •ACQ-6 score of ≥ 1.5 at enrollment •Pre-BD FEV1 of < 80%f •Post-BD FEV1 reversibility of ≥ 12% and ≥ 200 ml in the 12 months before enrollment |
•Positive Phadiatop test reaction (multiallergen FEIA) •Allergens: house dust mites; cat and dog dander; mold spores; and tree, grass and weed pollen •High-dose ICS and LABA useg •Exclusions: combined serum IgE concentration range and baseline weight > 600 kU/L to •700 kU/L and > 60 kg, •> 500 kU/L to 600 kU/L and > 70 kg, or •> 300 kU/L to 500 kU/L and > 90 kg |
•Worsening of asthma that led to use of SCS treatment (or temporary increase in stable OCS for ≥ 3 days or a single depo-injectable dose of corticosteroid), or an asthma-related ER or urgent care visit that required SCS treatment, or asthma-related hospitalization •Worsening of asthma was defined as any new or increased symptoms or signs that were concerning to the patient or related to an asthma daily diary alert |
| Mepolizumab [24] | MENSA/post hoc analysis (NCT01691521) | Mepolizumab 75 mg Q4W IV/100 mg Q4W SC (pooled doses) | ≥ 12/no BW restriction | 32 | 576/181 | No restrictions |
•Severe eosinophilic asthma •BEC of ≥ 300 cells/μL related to asthma in the previous year; or ≥ 150 cells/μL related to asthma at screening •≥ 2 exacerbations needing SCS treatment in the year before baseline despite regular treatment with high-dose ICSh in the 12 months before screening, plus additional controller(s) ± OCS for ≥ 3 months •Percent predicted pre-BD FEV1 of < 80% at screeningi •≥ 1 of the following: FEV1 reversibility ≥ 12%, positive results on methacholine or mannitol challenge, FEV1 variability (≥ 20%) between two visits in the past 12 months |
•Sensitivity to allergens (atopy) based on IgE titers of IgE ≥ 0.35 kU/L to 17.5 kU/L at baseline •Allergens: house dust mite (D. farinae and D. pteronyssinus), dog dander, cat dander, Alternaria alternata and cockroach |
•Clinically significant exacerbations defined as worsening of asthma requiring SCS treatment for ≥ 3 days and/or an ER visit and/or hospitalization |
| Dupilumab [23] | LIBERTY ASTHMA QUEST/post hoc analysis (NCT02414854) | Dupilumab 200 mg Q2W SC or dupilumab 300 mg Q2W SC | ≥ 12/ < 30 | 52 | 1902/1083 | ≥ 30 |
•Moderate-to- severe, uncontrolled asthma •Asthma worsening in the previous year leading to hospitalization, emergency medical care or SCS treatment for ≥ 3 days •Treatment with medium- or high-dose ICSg for ≥ 12 months before screening plus up to two additional controllers •ACQ-5 score of ≥ 1.5 •Percent predicted pre-BD FEV1 of ≤ 80%f •FEV1 reversibility of ≥ 12% and 200 mL |
•≥ 1 aeroallergen-specific IgE of ≥ 0.35 kU/L •Allergens: D. farinae, D. pteronyssinus, A. alternata, Cladosporium herbarum, cat dander, dog dander, German cockroach, oriental cockroach and Aspergillus fumigatus •No skin prick allergy testing was performed •Total IgE ≥ 30 IU/mL •Criteria were based on eligibility for omalizumab in the USA, with the additional stipulation of a total IgE of > 700 IU/mL (which omalizumab therapy is not indicated for in the USA) |
•Severe exacerbation defined as deterioration of asthma leading to SCS treatment for ≥ 3 days, or hospitalization or an ER visit leading to treatment with SCS |
| Tezepelumab [18, 25] | NAVIGATOR/prespecified analysis (NCT03347279) | Tezepelumab 210 mg Q4W SC | 12–80/no BW restriction | 52 | 1059/680 | No restrictions |
•Severe, uncontrolled asthma •Medium- or high-dose ICSg for ≥ 12 months before screening and ≥ 1 additional controller medication ± OCS •Morning percent predicted pre-BD FEV1 of < 80%f •Post-BD FEV1 reversibility of ≥ 12% and ≥ 200 mL during the 12 months before screening •≥ 2 asthma exacerbations in the 12 months before informed consent |
•Positive FEIA test was defined as ≥ 1 aeroallergen-specific IgE of ≥ 0.35 kU/L •Allergens: cat dander, dog dander, cockroach, dust mite (D. farinae and D. pteronyssinus) and mold mix |
•Worsening of asthma symptoms that led to hospitalization, an ED visit that resulted in the use of SCS treatment for ≥ 3 consecutive days, or the use of SCS treatment for ≥ 3 days |
ACQ Asthma Control Questionnaire, BD bronchodilator, BEC blood eosinophil count, BW bodyweight, ED emergency department, ER emergency room, FEIA fluorescence enzyme immunoassay, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, ICS inhaled corticosteroid, IgE immunoglobulin E, IV intravenous, LABA long-acting β2 agonist, OCS oral corticosteroid, Q2W every 2 weeks, Q4W every 4 weeks, Q8W every 8 weeks, RAST radioallergosorbent test, SC subcutaneous, SCS systemic corticosteroid
aAdditional study design details in this table were extracted from the primary publications for each study [26–29]
bHigh-dose ICS was a minimum dose of 500 μg of fluticasone dry-powder inhaler twice daily (or equivalent) for at least 8 weeks before screening
cIn the benralizumab SIROCCO and CALIMA study populations, patients with baseline BECs of ≥ 300 cells/μL and < 300 cells/μL were recruited at a ratio of approximately 2:1, respectively; the authors stated that results were reweighted to adjust for this differential recruitment
dThe SIROCCO and CALIMA trials also produced data for benralizumab 30 mg Q4W SC; this regimen is recommended for the first two doses of treatment according to the US prescribing information, after which benralizumab should be administered Q8W
e250–500 μg (medium) or > 500 μg (high) per day of fluticasone propionate administered by means of a dry-powder inhaler or equivalent
fPre-BD FEV1 < 90% for patients 12–17 years of age
gDaily dose of ≥ 500 µg of fluticasone propionate or equivalent
hHigh-dose ICS was defined as a dose of ≥ 880 μg of fluticasone propionate or the equivalent by inhalation per day
iFEV1 of < 90% of the predicted normal for patients younger than 18 years of age or an FEV1/FVC ratio of < 0.8
Differences in the main eligibility criteria for patients enrolled in the phase 3 trials are summarized in Table 1. Patient populations in the reviewed trials of tezepelumab (NAVIGATOR) [18], dupilumab (LIBERTY ASTHMA QUEST) [26], omalizumab (EXTRA) [21] and benralizumab (SIROCCO/CALIMA) [27, 28] included patients with perennial allergy across all BEC thresholds (i.e., without any BEC exclusion criteria at enrollment). To the best of our knowledge, mepolizumab data for this overall allergic severe asthma patient population have not been reported because mepolizumab RCTs required an elevated historical BEC for enrollment (e.g., the mepolizumab MENSA trial required that all patients had a BEC of ≥ 150 cells/µL at screening or ≥ 300 cells/µL at some time during the previous year) [29].
Regarding the specific analyses or subanalyses for patients with allergic severe asthma, there were some differences in the definition of allergic sensitization (Table 1). The omalizumab analysis was the only one to allow perennial allergy to be assessed with a skin prick or radioallergosorbent test for aeroallergens [19]; all other analyses used a laboratory test such as a fluorescence enzyme immunoassay for serum allergen-specific IgE. The aeroallergen panels for omalizumab and mepolizumab did not include molds [21, 24]. The Phadiatop assay for benralizumab was a multiallergen test that combined perennial and seasonal allergens [30, 31]. Results for this mixed perennial and seasonal allergy population were included because they were the only data available for benralizumab in patients with allergic severe asthma.
Importantly, because of limitations in the panels of perennial aeroallergens used, none of the analyses would necessarily have captured all patients with perennial aeroallergen sensitization. Moreover, no analyses reported confirmation that such sensitization was a cause of asthma symptoms, except for one published estimate for tezepelumab [25]. There were no restrictions on patients’ baseline serum total IgE concentrations in the benralizumab, mepolizumab or tezepelumab analyses. For omalizumab, total IgE concentrations had to be 30–700 IU/mL, based on the dosing guidance in the US prescribing information. For the dupilumab analyses, total IgE concentrations had to be ≥ 30 IU/mL.
The published baseline characteristics of patients in the analyses reviewed are summarized in Table 2. Of note, there was a broad distribution of serum total IgE concentrations within each trial, as well as differences between trial populations, consistent with the total IgE ranges required for enrollment. Patients participating in trials of benralizumab and mepolizumab generally had a higher mean number of exacerbations in the 12 months before study commencement (in both the treatment and placebo arms [27–29]; placebo data not shown in Table 2) than those participating in trials of omalizumab and dupilumab, which is also consistent with differences in study inclusion criteria. For tezepelumab, like benralizumab and mepolizumab, patients were required to have ≥ two exacerbations in the 12 months before study commencement [18]. The median baseline BEC of the analyzed study populations was highest for benralizumab (384 cells/µL for all patients with atopy), followed by tezepelumab and dupilumab (group medians ranged from 240 cells/µL to 290 cells/µL); this measure was not reported for the overall study population in the omalizumab EXTRA study. The mean baseline BEC for the mepolizumab study population was 310 cells/µL (geometric mean).
Table 2.
Baseline characteristics of patients with allergic severe asthma in phase 3 trials of biologics
| Omalizumab based on BW and serum total IgE from EXTRA Q2W or Q4W SC [9, 19, 21] | Benralizumab pooled data from SIROCCO and CALIMA Q4W or Q8W SC [22] | Mepolizumab MENSA [24] 75 mg Q4W IV or 100 mg Q4W SC | Dupilumab QUEST [23] | Tezepelumab NAVIGATOR [25] 210 mg Q4W SC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 848 | All patients with atopy N = 1375a | All patients with atopy N = 181 | N = 1083 | All patients with perennial allergy sensitization n = 680 | |||||||
| Placebo n = 421 | Omalizumab n = 427 | Low BEC subgroup < 260 cells/µL n = 383 | Elevated BEC subgroup ≥ 260 cells/µL n = 414 | Placebo 1.14 ml SC n = 183 | Dupilumab 200 mg Q2W SC n = 360 | Placebo 2 ml SC n = 179 | Dupilumab 300 mg Q2W SC n = 361 | ||||
| Age, years, mean (SD) | 45.3 (13.9) | 43.7 (14.3) | 45 (14) | 44 (15) | 46.8 (14.6) | 50 (14) | 44.0 (16.8) | 45.5 (16.0) | 44.1 (14.9) | 43.9 (15.8) | 45.8 (16.6) |
| Female, n (%) | 295 (70.1) | 262 (61.4) | 242 (63) | 279 (67) | 824 (60) | 99 (55) | 101 (55.2) | 196 (54.4) | 114 (63.7) | 216 (59.8) | 412 (60.6) |
| BMI, kg/m2, mean (SD) | 31.5 (7.3) | 32 (7.8) | NR | NR | 29.0 (7.0) | 28.3 (6.2) | 29.3 (7.35) | 28.47 (6.35) | 28.78 (6.88) | 28.91 (6.91) | 28.2 (7.0) |
| Duration of asthma, years, mean (SD) | 24.7 (15.8) | 22.8 (15.4) | NR | NR | NR | 21 (14) | NR | NR | NR | NR | NR |
| Age at asthma onset, years, mean (SD) | NR | NR | NR | NR | NR | NR | 20.9 (17.9) | 23.0 (19.5) | 20.9 (16.9) | 20.8 (17.8) | NR |
| Pre-BD FEV1, l, mean (SD) | NR | NR | NR | NR | 1.775 (0.615) | NR | 1.84 (0.64) | 1.85 (0.64) | 1.84 (0.61) | 1.88 (0.58) | 1.93 (0.74) |
| Percent-predicted FEV1, mean (SD) | 64.4 (13.9) | 65.4 (15.2) | 67 (15) | 64 (15) | 58.0 (14.4) | 62 (19) | NR | NR | NR | NR | 63.6 (18.2) |
| OCS use, n (%) | 71 (16.9)b | 73 (17.1)b | 41 (11) | 91 (22) | 155 (11)c | 44 (24) | NR | NR | NR | NR | 51 (7.5)c |
| Serum total IgE, IU/mL, median (min, max) or [IQR] |
175.1 (133.7) Mean (SD) |
178.7 (134.5) Mean (SD) |
156 (123) Mean (SD) |
196 (140) Mean (SD) |
305.1 (3.7, 46,983.8) |
201 (1.30) Geometric mean (SD logs) |
337.0 [147.0–629.0] |
304.0 [137.0–835.5] |
315.0 [142.0–763.0] |
326.0 [152.0–762.0] |
367.2 (2.6, 12,823.2) |
| BEC, cells/µL, median (min, max) or [IQR] | NR | NR |
152 (58) Mean (SD) |
535 (336) Mean (SD) |
384 (0, 3640) |
310 (0.97) Geometric mean (SD logs) |
290.0 [150.0–490.0] |
240.0 [120.0–470.0] |
260.0 [160.0–440.0] |
240.0 [140.0–430.0] |
260 (0, 8170) |
| FeNO, ppb, median (min, max) |
29.2 (29.7) Mean (SD) |
28.5 (26.9) Mean (SD) |
20 (14) Mean (SD) |
37 (33) Mean (SD) |
NR | NR | 27.0 (15.0, 50.0) | 25.0 (16.0, 45.0) | 30.0 (17.5, 53.0) | 24.0 (14.0, 42.0) | 32.0 (5.0, 258.0) |
| Severe exacerbations in the past 12 months, mean (SD) | 1.9 (1.5) | 2.0 (2.2) | 2 (1) | 2 (2) | 2.8 (1.6) | 3.5 (2.5)d | 1.89 (1.48) | 1.98 (2.99) | 2.22 (1.99) | 1.79 (1.33) | NRe |
BD bronchodilator, BEC blood eosinophil count, BMI body mass index, BW bodyweight, FeNO fractional exhaled nitric oxide, FEV1 forced expiratory volume in 1 s, IgE immunoglobulin E, IQR interquartile range, IV intravenous, NR not reported, OCS oral corticosteroid, Q2W every 2 weeks, Q4W every 4 weeks, Q8W every 8 weeks, SC subcutaneous, SD standard deviation
aPatient characteristics for the atopic population in SIROCCO and CALIMA do not correspond exactly to the analysis presented here because they include both dosages of benralizumab (i.e., 30 mg Q4W continuously and 30 mg Q4W for the first three doses then 30 mg Q8W); we present outcome data only for the latter regimen, which is specified in the US label [8]
bEither regular OCS use at baseline or at least four exacerbations in the previous year requiring OCS use
cMaintenance OCS
dNumber of exacerbations in the 12 months before screening
eIn NAVIGATOR, there were 414 (60.9%) and 266 (39.1%) patients with two and more than two exacerbations in the 12 months before the study, respectively
AAER Outcomes in Patients with Allergic Severe Asthma (All Baseline BECs)
In patients with perennial allergy and any BEC, omalizumab, benralizumab, dupilumab and tezepelumab were all associated with a significantly lower AAER after treatment for approximately 1 year compared with placebo (data for mepolizumab were not available; Fig. 1). Although direct comparisons between studies should be made with caution owing to differing study designs, the benefit of tezepelumab over 1 year was greater than that of the other biologics. While 95% confidence intervals (CIs) overlapped for the AAER ratios versus placebo between tezepelumab and dupilumab 300 mg and 200 mg doses, respectively, as shown in Fig. 1, there was no overlap in the 95% CIs for the AAER rate ratio versus placebo between tezepelumab (rate ratio: 0.42 [95% CI 0.33–0.53], corresponding to a 58% [47–67%] reduction in AAER) and benralizumab (rate ratio: 0.66 [95% CI 0.54–0.81]) or omalizumab (rate ratio: 0.75 [95% CI 0.61–0.92]). Indeed, the rate ratio of 0.75 for omalizumab versus placebo is on the borderline of being clinically meaningful [32]. An exploratory analysis of the tezepelumab data demonstrated that AAER reductions versus placebo were very similar in patients in whom perennial aeroallergen sensitization had a confirmed relationship with asthma symptoms (rate ratio: 0.40 [95% CI 29–57]) [25].
Fig. 1.
Reduction in AAER by biologic therapy in patients with allergic severe asthma (all baseline BEC levels). aRegardless of serum total IgE level; brequired a serum total IgE level of ≥ 30 IU/mL; cpooled trials; drequired a serum total IgE level of 30–700 IU/mL. AAER annualized asthma exacerbation rate, BEC blood eosinophil count, CI confidence interval, IgE immunoglobulin E, Q2W every 2 weeks, Q4W every 4 weeks, Q8W every 8 weeks, SC subcutaneous
AAER Outcomes in Patients with Allergic Severe Asthma and Elevated Baseline BECs
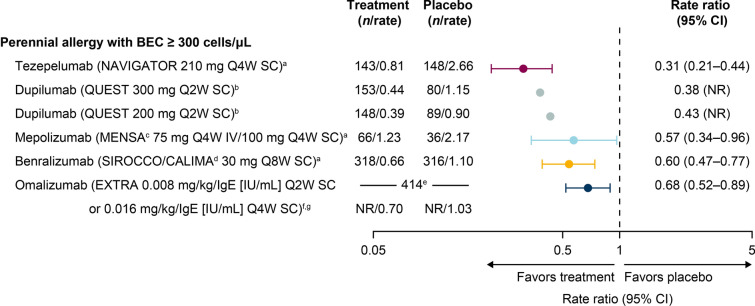
All biologics demonstrated efficacy in reducing the AAER in patients with allergic severe asthma and elevated baseline BECs (≥ 300 cells/µL or ≥ 260 cells/µL) (Fig. 2). The high efficacy in this population is consistent with the positive association between BECs and treatment efficacy rates observed in patients with severe asthma in general [20]. The rank order trend of these biologics remained the same as demonstrated for all baseline BECs: tezepelumab had the lowest rate ratio (0.31 [95% CI 0.21–0.44]), corresponding to the greatest efficacy, and omalizumab had the highest rate ratio (0.68 [95% CI 0.52–0.89]), corresponding to the lowest efficacy, with the two separated by non-overlapping CIs. The rate ratios for dupilumab were 0.38 and 0.43 for the 300 mg and 200 mg doses, respectively (95% CIs were not reported). Mepolizumab and benralizumab reduced the AAER by a similar magnitude (rate ratios: 0.57 [95% CI 0.34–0.96] and 0.60 [95% CI 0.47–0.77], respectively).
Fig. 2.
Reduction in AAER by biologic therapy in patients with allergic severe asthma and BECs of ≥ 300 cells/μL. aRegardless of serum total IgE level; brequired a serum total IgE level of ≥ 30 IU/mL; cpooled doses; dpooled trials; epatient n numbers are reported for the overall population (breakdown by treatment group was not given); frequired a serum total IgE level of 30–700 IU/mL; gthe EXTRA trial of omalizumab reported patients with BECs of ≥ 260 cells/µL. AAER annualized asthma exacerbation rate, BEC blood eosinophil count, CI confidence interval, IgE immunoglobulin E, IV intravenous, NR not reported, Q2W every 2 weeks, Q4W every 4 weeks, Q8W every 8 weeks, SC subcutaneous
AAER Outcomes in Patients with Allergic Severe Asthma and Low Baseline BECs
In patients with allergic severe asthma and low baseline BECs (< 300 cells/µL or < 260 cells/µL), tezepelumab was effective in reducing the AAER relative to placebo to a clinically meaningful extent (rate ratio: 0.55 [95% CI 0.40–0.75]). Benralizumab demonstrated a numerical trend of reducing the AAER, but the upper limit of the 95% CI exceeded one (rate ratio: 0.74 [95% CI 0.54–1.02]). Omalizumab did not demonstrate a meaningful AAER reduction relative to placebo (rate ratio: 0.91 [95% CI 0.66–1.24]). There were no data matching our inclusion criteria for this patient population in dupilumab or mepolizumab analyses (Fig. 3). However, in a post hoc analysis of the MENSA trial (a mepolizumab study that required enrolled patients to have a BEC of ≥ 150 cells/µL at screening or ≥ 300 cells/µL during the previous year) [24], the exacerbation rate ratios for patients with atopic asthma with baseline BECs of ≥ 150–300 cells/µL and under 150 cells/µL were 0.58 (95% CI 0.22–1.58) and 0.22 (95% CI 0.07–0.65), respectively. Of note, the patient numbers in these subgroups were small (BEC ≥ 150–300 cells/µL, n = 39; BEC < 150 cells/µL, n = 38), and all patients with BECs < 150 cells/µL would have had a recent history of BECs of ≥ 300 cells/µL per the study enrollment criteria for the MENSA trial.
Fig. 3.
Reduction in AAER by biologic therapy in patients with allergic severe asthma and BECs of < 300 cells/μL. Data that matched our inclusion criteria for this patient population were unavailable in the dupilumab or mepolizumab analyses. aRegardless of serum total IgE level; bpooled trials; cpatient n numbers are reported for the overall population (breakdown by treatment group was not given); drequired a serum total IgE level of 30–700 IU/mL; ethe EXTRA trial of omalizumab reported patients with BECs of < 260 cells/µL. AAER annualized asthma exacerbation rate, BEC blood eosinophil count, CI confidence interval, IgE immunoglobulin E, NR not reported, Q2W every 2 weeks, Q4W every 4 weeks, Q8W every 8 weeks, SC subcutaneous
Discussion
The varying magnitudes of the AAER reductions observed across biologics can be explained in part by their different mechanisms of action [33, 34]. Specifically, benralizumab (anti-IL-5 receptor) and mepolizumab (anti-IL-5) directly affect eosinophilic inflammation [5, 8] but there is no robust evidence to suggest they affect other pathways or biomarkers such as fractional exhaled nitric oxide (FeNO) [35, 36] or serum total IgE levels. Omalizumab binds free IgE and consequently blocks the interaction of IgE with high-affinity IgE receptors on mast cells and basophils; however, omalizumab has limited effects on other pathways, as evidenced by small reductions in BECs and FeNO levels [9, 19]. Dupilumab (anti-IL-4 receptor) blocks the activity of IL-4 and IL-13 [7] but has no direct effect on IL-5-driven inflammation, as evidenced by transient elevations in BEC rather than reductions [37], and no demonstrable impact on airway eosinophil levels [38]. Interestingly, an analysis of the dupilumab QUEST RCT data demonstrated a consistent trend of lower efficacy with dupilumab treatment among patients with evidence of allergic asthma than in patients without evidence of allergic asthma: among patients with a baseline BEC of ≥ 150 cells/μL, AAER reductions versus placebo for those receiving dupilumab 200 mg and 300 mg were 42% and 55% in those with allergic asthma and 71% and 63% in those not meeting the definition of allergic asthma, respectively [23]. Finally, tezepelumab (anti-TSLP) has broad suppressive effects on type 2 inflammatory pathways, reducing the activity of IL-4, IL-5 and IL-13, as evidenced by reductions in blood and airway eosinophil counts, and FeNO and serum total IgE levels [10, 11]. Tezepelumab has also been shown to reduce airway hyperresponsiveness [12, 13], likely through type 2-dependent and -independent effects on airway smooth muscle and mast cell activation [39–41]. In addition to varying mechanisms of action of the biologics discussed, differences in study design, study populations and inclusion/exclusion criteria between trials could also contribute to the observed differences in AAER reductions.
Whereas all biologics demonstrated efficacy in patients with allergy and elevated baseline BECs, the magnitude of the efficacy was proportional to the breadth of the biologic’s mechanism of action, with tezepelumab and dupilumab (300 mg dose) demonstrating the highest exacerbation reductions and omalizumab demonstrating the lowest. These findings are supported by a recent Bayesian meta-analysis of biologic efficacy in eosinophilic severe asthma, which concluded that tezepelumab and dupilumab had the greatest efficacy of the biologics evaluated (tezepelumab, dupilumab, benralizumab and mepolizumab); additionally, a recent real-world comparative study demonstrated lower efficacy with omalizumab compared with anti-IL-5/5R biologics (benralizumab, mepolizumab and reslizumab) among patients with allergic and eosinophilic disease [42, 43]. Meanwhile, in patients with allergic severe asthma and a low baseline BEC, only tezepelumab was effective in reducing the AAER relative to placebo. This difference is likely derived from the distinct mechanism of action of tezepelumab compared with other current asthma biologics. Tezepelumab blocks the activity of TSLP, and the broad effects of TSLP blockade, including effects on mast cells, may be particularly relevant among patients with allergic severe asthma in whom mast cell activation may play a central role in disease.
To the best of our knowledge, this is the first literature review to summarize biologic efficacy data for patients with allergic severe asthma in the overall patient population (all BECs) and by baseline BEC. Additional data for patients with different BEC categories would be valuable because some baseline BEC subgroups were not available for some FDA-approved biologics. As noted previously, the multiple differences between trials are an important limitation of this study; these differences included the specific definitions used to identify patients with allergic severe asthma. While comparisons of available published data provide useful mechanistic and clinical insights, the results presented in this review are not equivalent to the findings of head-to-head, randomized studies using the same patient population. It would be beneficial if future trials describe the efficacy of biologics in patients with severe asthma whose symptoms are clinically confirmed to be driven by perennial aeroallergen sensitization and exposures; these patients comprise the allergic severe asthma population in its truest sense. To date, such data are only available for tezepelumab [25].
Conclusion
An effective treatment is needed for patients with allergic severe asthma and elevated or low baseline BECs. Many patients with allergic asthma have concurrent eosinophilia, but there is also a sizable number of patients with allergic asthma and low baseline BECs [16]. The efficacy of biologics in reducing the AAER in RCTs varied considerably overall and by baseline BEC. All biologics with available data were effective in patients with perennial allergy and baseline BECs of ≥ 300 cells/μL, and greater efficacy was seen with biologics with broader mechanisms of action. Only tezepelumab demonstrated a meaningful AAER reduction in patients with allergic severe asthma regardless of baseline BEC. The differences between biologics observed in this study can help to inform provider treatment decisions when selecting biologic treatments for patients with allergic severe asthma, overall and for those with or without coexisting eosinophilic inflammation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Medical Writing and Editorial Assistance
Medical writing support was provided by Lisa Simpson, PhD, of PharmaGenesis London, London, UK, with funding from AstraZeneca and Amgen Inc.
Author Contributions
Christopher S. Ambrose contributed to the design, literature search string and data acquisition. A medical writer performed the literature search, article screening and data extraction. Christopher S. Ambrose and Jean-Pierre Llanos contributed to the data analysis. All authors significantly contributed to the data interpretation and development of the manuscript content, and critically reviewed each draft of the manuscript. All authors approved the final version of the submitted manuscript and were responsible for the decision to publish the manuscript. All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Funding
This study and the journal’s Rapid Service and Open Access Fees were funded by AstraZeneca, Wilmington, DE, USA, and Amgen Inc., Thousand Oaks, CA, USA.
Data Availability
All data and materials used in this research are freely available. References have been provided.
Declarations
Conflict of Interest
Jonathan A. Bernstein has served as a consultant for Amgen, AstraZeneca, Genentech, Merck, Novartis and Sanofi Regeneron; has participated in research with Amgen, AstraZeneca, Genentech, Merck, Novartis and Sanofi Regeneron; and has received speaker fees from AstraZeneca, Genentech, GSK, Novartis, Optinose and Sanofi Regeneron. Jean-Pierre Llanos is an employee of Amgen and owns stock in Amgen. Gillian Hunter, Neil Martin and Christopher S. Ambrose are employees of AstraZeneca and may own stock or stock options in AstraZeneca.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Footnotes
Prior Presentation: Findings from this literature review were previously presented in an oral presentation at the American College of Allergy, Asthma and Immunology Annual Meeting, November 10–14, 2022, Louisville, KY, USA; however, the literature search dates for this manuscript differ from the published abstract (Bernstein JA et al. Ann Allergy Asthma Immunol 2022;129:S3) and oral presentation.
References
- 1.Schatz M, Rosenwasser L. The allergic asthma phenotype. J Allergy Clin Immunol Pract. 2014;2(6):645–648. doi: 10.1016/j.jaip.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Tran TN, Zeiger RS, Peters SP, Colice G, Newbold P, Goldman M, et al. Overlap of atopic, eosinophilic, and TH2-high asthma phenotypes in a general population with current asthma. Ann Allergy Asthma Immunol. 2016;116(1):37–42. doi: 10.1016/j.anai.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 3.Backman H, Räisänen P, Hedman L, Stridsman C, Andersson M, Lindberg A, et al. Increased prevalence of allergic asthma from 1996 to 2006 and further to 2016-results from three population surveys. Clin Exp Allergy. 2017;47(11):1426–1435. doi: 10.1111/cea.12963. [DOI] [PubMed] [Google Scholar]
- 4.Global Initiative for Asthma. Global strategy for asthma management and prevention. 2023. Available from: https://ginasthma.org/wp-content/uploads/2023/05/GINA-2023-Full-Report-2023-WMS.pdf. Accessed 3 May 2023.
- 5.FDA. NUCALA® (mepolizumab) Prescribing Information. 2017. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125526s004lbl.pdf (Accessed April 14, 2023).
- 6.FDA. CINQAIR® (reslizumab) Prescribing Information. 2016. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761033lbl.pdf (Accessed April 14, 2023).
- 7.FDA. DUPIXENT® (dupilumab) Prescribing Information. 2017. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf (Accessed April 14, 2023).
- 8.FDA. FASENRA™ (benralizumab) Prescribing Information. 2017. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761070s000lbl.pdf (Accessed April 14, 2023).
- 9.FDA. XOLAIR® (omalizumab) Prescribing Information. 2016. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/103976s5225lbl.pdf (Accessed April 14, 2023).
- 10.FDA. TEZSPIRE™ (tezepelumab) Prescribing Information. 2021. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761224s000lbl.pdf (Accessed April 14, 2023).
- 11.Corren J, Pham TH, Garcia Gil E, Salapa K, Ren P, Parnes JR, et al. Baseline type 2 biomarker levels and response to tezepelumab in severe asthma. Allergy. 2022;77(6):1786–1796. doi: 10.1111/all.15197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sverrild A, Hansen S, Hvidtfeldt M, Clausson CM, Cozzolino O, Cerps S, et al. The effect of tezepelumab on airway hyperresponsiveness to mannitol in asthma (UPSTREAM) Eur Respir J. 2022;59(1):2101296. doi: 10.1183/13993003.01296-2021. [DOI] [PubMed] [Google Scholar]
- 13.Diver S, Khalfaoui L, Emson C, Wenzel SE, Menzies-Gow A, Wechsler ME, et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2021;9(11):1299–1312. doi: 10.1016/S2213-2600(21)00226-5. [DOI] [PubMed] [Google Scholar]
- 14.Oppenheimer J, Hoyte FCL, Phipatanakul W, Silver J, Howarth P, Lugogo NL. Allergic and eosinophilic asthma in the era of biomarkers and biologics: similarities, differences and misconceptions. Ann Allergy Asthma Immunol. 2022;129(2):169–180. doi: 10.1016/j.anai.2022.02.021. [DOI] [PubMed] [Google Scholar]
- 15.Albers FC, Mullerova H, Gunsoy NB, Shin JY, Nelsen LM, Bradford ES, et al. Biologic treatment eligibility for real-world patients with severe asthma: The IDEAL study. J Asthma. 2018;55(2):152–160. doi: 10.1080/02770903.2017.1322611. [DOI] [PubMed] [Google Scholar]
- 16.Chen M, Shepard K, 2nd, Yang M, Raut P, Pazwash H, Holweg CTJ, et al. Overlap of allergic, eosinophilic and type 2 inflammatory subtypes in moderate-to-severe asthma. Clin Exp Allergy. 2021;51(4):546–555. doi: 10.1111/cea.13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denton E, Price DB, Tran TN, Canonica GW, Menzies-Gow A, FitzGerald JM, et al. Cluster analysis of inflammatory biomarker expression in the International Severe Asthma Registry. J Allergy Clin Immunol Pract. 2021;9(7):2680–2688. doi: 10.1016/j.jaip.2021.02.059. [DOI] [PubMed] [Google Scholar]
- 18.Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;384(19):1800–1809. doi: 10.1056/NEJMoa2034975. [DOI] [PubMed] [Google Scholar]
- 19.Hanania NA, Wenzel S, Rosén K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187(8):804–811. doi: 10.1164/rccm.201208-1414OC. [DOI] [PubMed] [Google Scholar]
- 20.Korn S, Cook B, Simpson LJ, Llanos JP, Ambrose CS. Efficacy of biologics in severe, uncontrolled asthma stratified by blood eosinophil count: a systematic review. Adv Ther. 2023;2:2. doi: 10.1007/s12325-023-02514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes-Rivera I, Zhu J, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154(9):573–582. doi: 10.7326/0003-4819-154-9-201105030-00002. [DOI] [PubMed] [Google Scholar]
- 22.Chipps BE, Newbold P, Hirsch I, Trudo F, Goldman M. Benralizumab efficacy by atopy status and serum immunoglobulin E for patients with severe, uncontrolled asthma. Ann Allergy Asthma Immunol. 2018;120(5):504–511. doi: 10.1016/j.anai.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 23.Corren J, Castro M, O'Riordan T, Hanania NA, Pavord ID, Quirce S, et al. Dupilumab efficacy in patients with uncontrolled, moderate-to-severe allergic asthma. J Allergy Clin Immunol Pract. 2020;8(2):516–526. doi: 10.1016/j.jaip.2019.08.050. [DOI] [PubMed] [Google Scholar]
- 24.Prazma CM, Idzko M, Douglass JA, Bourdin A, Mallett S, Albers FC, et al. Response to mepolizumab treatment in patients with severe eosinophilic asthma and atopic phenotypes. J Asthma Allergy. 2021;14:675–683. doi: 10.2147/JAA.S298559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corren J, Ambrose CS, Griffiths JM, Hellqvist A, Lindsley AW, Llanos JP, et al. Efficacy of tezepelumab in patients with evidence of severe allergic asthma: results from the phase 3 NAVIGATOR study. Clin Exp Allergy. 2022;2:2. doi: 10.1111/cea.14256. [DOI] [PubMed] [Google Scholar]
- 26.Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496. doi: 10.1056/NEJMoa1804092. [DOI] [PubMed] [Google Scholar]
- 27.Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–2127. doi: 10.1016/S0140-6736(16)31324-1. [DOI] [PubMed] [Google Scholar]
- 28.FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141. doi: 10.1016/S0140-6736(16)31322-8. [DOI] [PubMed] [Google Scholar]
- 29.Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 30.Vidal C, Gude F, Boquete O, Fernández-Merino MC, Meijide LM, Rey J, et al. Evaluation of the phadiatop test in the diagnosis of allergic sensitization in a general adult population. J Investig Allergol Clin Immunol. 2005;15(2):124–130. [PubMed] [Google Scholar]
- 31.Chupp GL, Bradford ES, Albers FC, Bratton DJ, Wang-Jairaj J, Nelsen LM, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390–400. doi: 10.1016/S2213-2600(17)30125-X. [DOI] [PubMed] [Google Scholar]
- 32.Bonini M, Di Paolo M, Bagnasco D, Baiardini I, Braido F, Caminati M, et al. Minimal clinically important difference for asthma endpoints: an expert consensus report. Eur Respir Rev. 2020;29(156):190137. doi: 10.1183/16000617.0137-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matucci A, Vultaggio A, Maggi E, Kasujee I. Is IgE or eosinophils the key player in allergic asthma pathogenesis? Are we asking the right question? Respir Res. 2018;19(1):113. doi: 10.1186/s12931-018-0813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papapostolou N, Makris M. Allergic asthma in the era of personalized medicine. J Pers Med. 2022;12(7):1162. doi: 10.3390/jpm12071162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hearn AP, Kavanagh J, d'Ancona G, Roxas C, Green L, Thomson L, et al. The relationship between Feno and effectiveness of mepolizumab and benralizumab in severe eosinophilic asthma. J Allergy Clin Immunol Pract. 2021;9(5):2093–2096. doi: 10.1016/j.jaip.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Upham JW, Jurak LM. How do biologicals and other novel therapies effect clinically used biomarkers in severe asthma? Clin Exp Allergy. 2020;50(9):994–1006. doi: 10.1111/cea.13694. [DOI] [PubMed] [Google Scholar]
- 37.Wechsler ME, Klion AD, Paggiaro P, Nair P, Staumont-Salle D, Radwan A, et al. Effect of dupilumab on blood eosinophil counts in patients with asthma, chronic rhinosinusitis with nasal polyps, atopic dermatitis, or eosinophilic esophagitis. J Allergy Clin Immunol Pract. 2022;10(10):2695–2709. doi: 10.1016/j.jaip.2022.05.019. [DOI] [PubMed] [Google Scholar]
- 38.ClinicalTrials.gov. ClinicalTrials.gov. Evaluation of dupilumab's effects on airway inflammation in patients with asthma: EXPEDITION (Study results data). Available from: https://clinicaltrials.gov/study/NCT02573233. Accessed 6 Apr 2023.
- 39.Gauvreau GM, Sehmi R, Ambrose CS, Griffiths JM. Thymic stromal lymphopoietin: its role and potential as a therapeutic target in asthma. Expert Opin Ther Targets. 2020;24(8):777–792. doi: 10.1080/14728222.2020.1783242. [DOI] [PubMed] [Google Scholar]
- 40.Banafea GH, Bakhashab S, Alshaibi HF, Natesan Pushparaj P, Rasool M. The role of human mast cells in allergy and asthma. Bioengineered. 2022;13(3):7049–7064. doi: 10.1080/21655979.2022.2044278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spahn JD, Brightling CE, O'Byrne PM, Simpson LJ, Molfino NA, Ambrose CS, et al. Effect of biologic therapies on airway hyperresponsiveness and allergic response: a systematic literature review. J Asthma Allergy. 2023;16:755–774. doi: 10.2147/JAA.S410592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nopsopon T, Lassiter G, Chen ML, Alexander GC, Keet C, Hong H, et al. Comparative efficacy of tezepelumab to mepolizumab, benralizumab, and dupilumab in eosinophilic asthma: a Bayesian network meta-analysis. J Allergy Clin Immunol. 2023;151(3):747–755. doi: 10.1016/j.jaci.2022.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfeffer PE, Ali N, Murray R, Ulrik C, Tran TN, Maspero J, et al. Comparative effectiveness of Anti-IL5 and Anti-IgE biologic classes in patients with severe asthma eligible for both. Allergy. 2023 doi: 10.1111/all.15711. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and materials used in this research are freely available. References have been provided.