Summary:
Vascularized fibular epiphyseal transfer (VFET) offers a functional advantage in pediatric limb salvage due to the preservation of growth potential and an articular surface for remodeling. This review summarizes the available evidence on the clinical characteristics and outcomes of pediatric reconstruction applying VFET at different recipient sites and with varying techniques. VFET was used to reconstruct the proximal humerus, distal radius or ulna, proximal femur, distal fibula, calcaneus, and mandible. Although most often harvested on the anterior tibial artery, VFET has also been performed using the peroneal artery, the inferior lateral genicular artery, and a dual pedicle. Recipient site flap inset most often involved fixation with plates and/or screws as well as soft tissue reconstruction using a retained slip of biceps femoris tendon. Outcomes included limb growth, range of motion, and strength. The most common reported complications were bone flap fracture and peroneal nerve palsy. The anterior tibial artery was the most applied pedicle with reliable limb growth, but with the added risk of postoperative peroneal palsy. Bone flap fracture most often occurred at the proximal humerus and femur recipient sites. Plate fixation and the combined use of allograft had lower instances of bone flap fracture. This review highlights how the anticipated dynamic growth and remodeling this free flap offers in the long term must be weighed against its complexity and potential complications.
Takeaways
Question: What are the applications and associated outcomes of vascularized fibular epiphyseal transfer (VFET) in pediatric reconstruction?
Findings: Of the 40 included studies, VFET was most applied for proximal humerus reconstruction with use of the anterior tibial artery pedicle. Limb function, including growth, range of motion, and strength, was preserved at varying levels depending on pedicle and recipient site. The most common complications were bone flap fracture and peroneal nerve palsy.
Meaning: The vascular pedicle, method of fixation, and recipient site applied during VFET may influence potential pediatric limb function and complications.
INTRODUCTION
Pediatric limb salvage has become a standard surgical treatment method for osteosarcoma and traumatic injury.1 Pediatric limb reconstruction is more than “replacing like with like,” as it must take into consideration the following: growth preservation in a skeletally immature patient, the reconstructed limb’s functional outcome into adulthood, multiple tissue type reconstruction, joint integrity, and need for appropriate immobilization postoperatively. This adds to the concerns that surround all pediatric reconstruction, including the patient’s emotional and social needs, donor site morbidity, and aesthetic outcome. Although the diaphyseal fibula free flap is a workhorse flap for limb salvage, it is limited in its ability to offer growth potential and articular remodeling. Nonautologous options available for limb salvage include allograft and endoprostheses that can be extendable. In addition to having increased risk of infection, loosening, and implant failure, these methods do not keep up with growth of the pediatric patient.2–6 To meet this challenge, vascularized fibular epiphyseal transfer (VFET) has been used in pediatric reconstruction due to its potential for growth and long-term viability.7–10 In addition to providing an immediate, intact blood supply to allow for primary bone healing, the transfer of the fibular head with its intact physis and a variable length of the diaphysis offers the advantage of limb growth and a remodeling osteoarticular surface at the recipient site. This comprehensive review summarizes evidence of VFET to highlight the methods with the most optimal outcomes. We also present our own experience using this flap to illustrate the perioperative course that motivated us to perform this review.
CASE
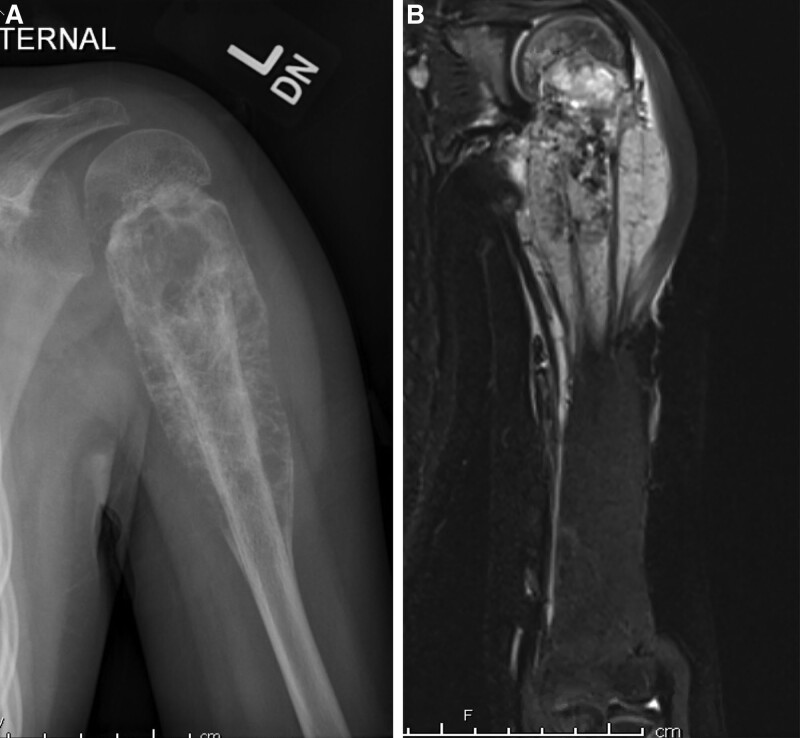
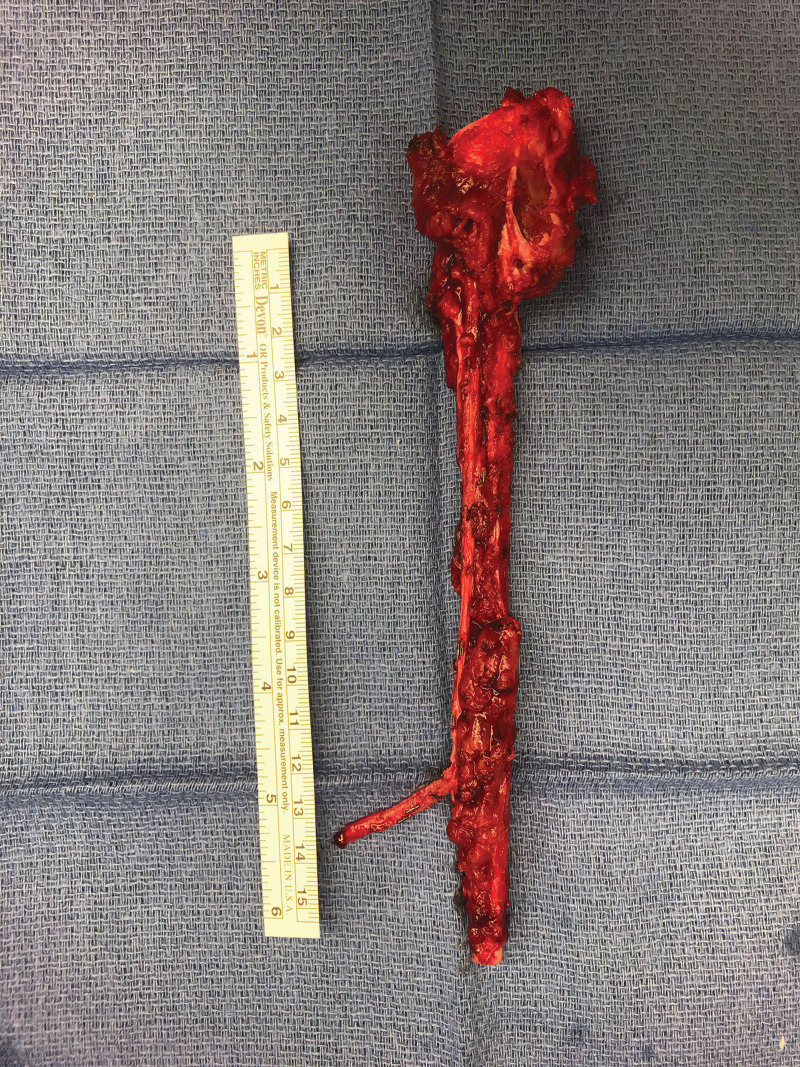
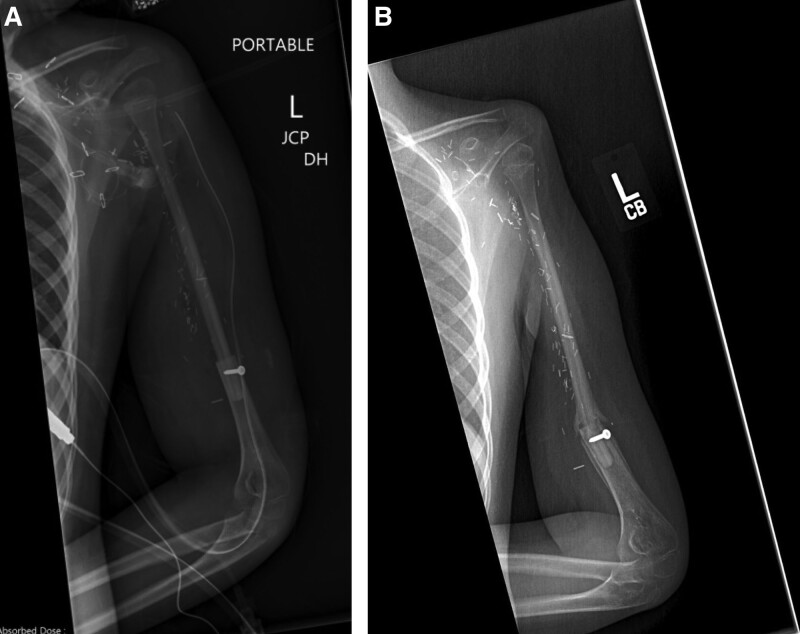
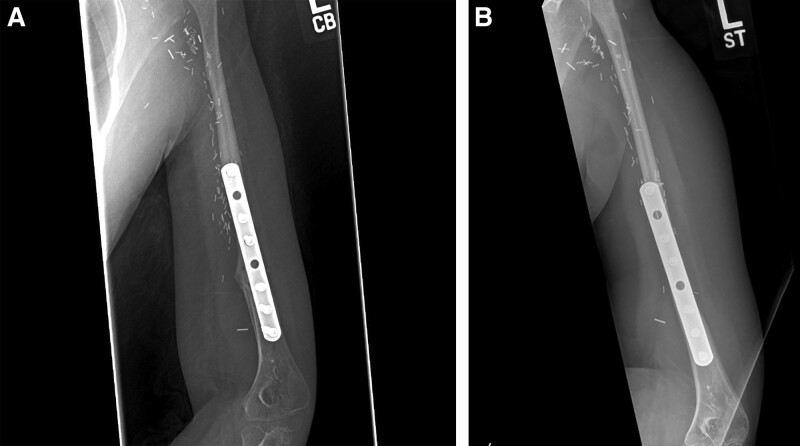
An 8-year-old girl presenting with a 3-month history of left arm pain was diagnosed with a 13-cm osteosarcoma extending through the left humeral head epiphysis (Fig. 1). The VFET was harvested using the method described by Innocenti et al (Fig. 2).11 An end-to-side arterial anastomosis was performed with the brachial artery in a retrograde manner, whereas an antegrade venous anastomosis was performed with a cephalic vein graft due to limited retrograde venous outflow. Osteosynthesis was performed with a bi-cortical screw securing the distal end to minimize disruption of the flap’s blood supply. The flap’s proximal portion was inset within the rotator cuff muscles, recreating the shoulder girdle. At 2 months postoperatively, the patient sustained a fracture at the distal screw site after a fall (Fig. 3), which was repaired with plate fixation. At 6 months follow-up, there was abundant callous formation and osseous healing at the fracture site. Longitudinal flap growth over 21 months was 1.2 cm per year and limb growth was 2.1 cm per year (Fig. 4). Her left upper extremity function was excellent with minimal limitations in shoulder range of motion. She sustained a transient peroneal nerve palsy that resolved after treatment with an ankle orthotic.
Fig. 1.
Case example of VFET utilizing ATA pedicle for proximal humerus reconstruction. A, Preoperative plain film demonstrating osteosarcoma of left humerus involving the physis. B, Preoperative MRI.
Fig. 2.
Vascularized fibular epiphyseal flap.
Fig. 3.
Case example of VFET utilizing ATA pedicle for proximal humerus reconstruction. A, Plain film immediately postoperatively. B, Plain film at 2 months postoperatively demonstrating fracture at fibula insertion site.
Fig. 4.
Case example of VFET utilizing ATA pedicle for proximal humerus reconstruction. A, Plain film at 6 months postoperatively after plate fixation for bone flap fracture. B, Plain film at 21 months postoperatively.
METHODS
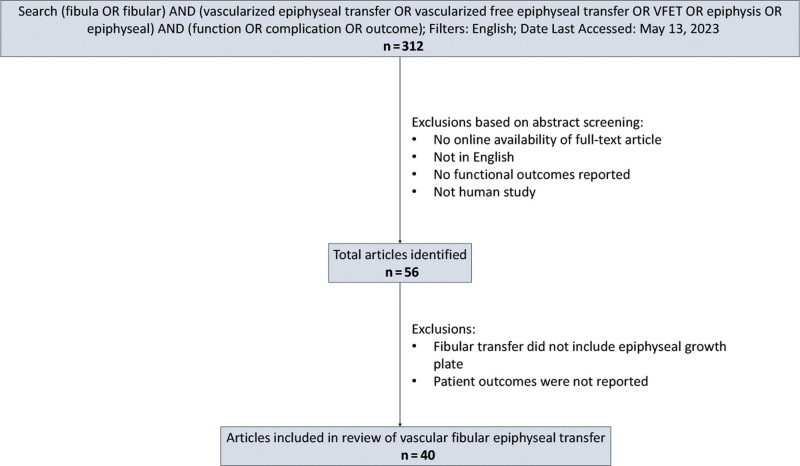
A literature review of VFET pediatric reconstruction was conducted using the PubMed database with the search strategy displayed in Figure 5. Inclusion criteria entailed prospective/retrospective cohort studies and case series. Exclusion criteria included patients receiving fibula transfer without the epiphysis, no online availability, and no outcomes reporting. Information extracted from studies included demographics, indication, clinical characteristics, description of surgical technique, and outcomes.
Fig. 5.
VFET literature review study selection process.
RESULTS
The initial search yielded 312 articles for which the title and abstract were assessed according to the inclusion and exclusion criteria. Of these, 56 articles were read to identify the 40 articles from which information was evaluated. Supplementary Table 1 details the articles’ clinical characteristics. (See table, Supplemental Digital Content 1, http://links.lww.com/PRSGO/C824.) Table 1 details the summary of VFET technique considerations extracted from these articles to help guide surgical planning.2,3,5–42
Table 1.
Summary of VFET Technique Considerations to Guide Surgical Planning
| Summary of Technique Considerations | |
|---|---|
| Harvest | |
| Vascular pedicle | |
| ATA | Reports of limb growth with average range of 0.44–1.72 cm/y Risk of postoperative peroneal palsy due to close proximity of deep peroneal nerve during dissection |
| Peroneal artery | Reports of average limb growth of 0.5 cm/y Associated with premature growth plate closure |
| ILGA | Limited used reported in distal radius/ulna reconstruction No reports of peroneal palsy |
| Dual pedicle | Potential benefit of maintaining adequate perfusion to both fibular head and diaphysis Increased complexity due to multiple anastomoses and increased donor site morbidity due to one-vessel runoff |
| Periosteum | May promote bony union at recipient site |
| Recipient site inset | |
| Fixation method | |
| Plates + screws | Most common fixation method Offers more stability, and may be less prone to postoperative fracture |
| Screws | Less stability, prone to postoperative fracture |
| External fixation | Most commonly used for hip reconstruction |
| Utilization of biceps femoris tendon | Used for ligamentous reconstruction at the recipient site |
| Management of donor site | |
| Knee stability | Suture remaining slip of biceps femoris tendon and lateral collateral ligament to lateral tibia |
| Ankle stability | Leave at least 6 cm fibula distally Consider tibiofibular syndesmotic screw |
| Peroneal nerve | Neurorrhaphy if divided during dissection |
| Modifications | |
| Combined used with allograft | Offers additional structural support while allowing for epiphyseal growth Offers more space for screw purchase and reattachment of surrounding structures |
| Pedicled flap without microsurgery | Ipsilateral pedicled flap based on retrograde anterior tibial artery for reconstruction of lateral malleolus or calcaneus Avoids complexity of microvascular anastomosis |
| Double barrel with vascularized fibula flap | Peroneal-based VFET connected in series with peroneal-based diaphyseal free fibula flap for proximal femur reconstruction |
Indications
VFET has been used for cases in which bony growth is critical, including malignancy, congenital deformity, trauma, and septic arthritis. Oncologic indications most commonly included osteosarcoma and Ewing sarcoma.2,3,5,6,8–10,12–14,16–19,21,23–25,27,31–33,36–38,40 Congenital deformities included radial longitudinal deficiency and ulnar pseudarthrosis.19,22,23,27,30,35 Traumatic injuries involved the lateral malleolus, calcaneus, radius, and ulna.6,15,20,24,27 Across all indications, the most common recipient site was the humerus.
Surgical Techniques
Flap Harvest and Donor Site Management
The anterior tibial artery (ATA), which supplies the proximal two-thirds of the fibula as detailed by Taylor et al,43 was the predominant vascular pedicle harvested.2,3,7,10,12–17,19–21,24,26,31,35,36,40,42 Dissection of the ATA was challenging due to its close relation with the deep peroneal nerve and the fragility of its short recurrent epiphyseal branch that supplies the fibular head. The ATA was often applied in a reverse flow manner to provide a longer pedicle during flap inset without necessitating a vein graft.3,29 Harvesting the flap on the peroneal artery (PA), which provides the primary endosteal and periosteal blood supply to the fibular shaft, offered an easier dissection but less direct blood supply to the fibular head.6,16,18,27,33 Several case series used a bi-pedicled approach using both the ATA and PA to optimize blood supply to the growth plate and fibular shaft.5,30,32,37,38 Several authors have raised concerns about using a dual pedicle due to the increased complexity and potential morbidity at both the recipient and donor sites, such as anastomotic thrombosis and future complications associated with one-vessel runoff of the lower extremity.21,38,44 After harvesting VFET with a dual pedicle, Lovic et al restored blood flow through the distal ATA to the foot with a great saphenous vein bypass connected from the PA proximally.37 Yang et al successfully used the inferior lateral genicular artery (ILGA), a shorter pedicle than the ATA that nourishes the fibular head, in distal radial and ulnar reconstruction in six patients.22,23 Morsy et al performed an anatomical study in 28 cadavers that detailed the characteristics of the vessels supplying the fibula and concluded that both the reverse flow ATA and ILGA can be used successfully in VFET.45
If a major motor branch of the peroneal nerve was divided while dissecting the pedicle during flap harvest, Innocenti et al recommended performing neurorrhaphy to prevent permanent peroneal palsy.21 Several articles also advocated harvesting vascularized fibular periosteum along with the flap to promote bony union at the recipient site.19,21,25,29,36 During flap dissection, a strip of the biceps femoral tendon was often harvested to provide ligamentous reconstruction during flap inset at the recipient site.3,8,17,20 To prevent secondary knee instability after fibula harvest, the lateral collateral ligament and remaining biceps femoris tendon were reinserted to the proximal lateral tibia.10,13,15–17 To maintain ankle stability after VFET harvest, a general guideline is to preserve the distal 6 cm of fibula.46 In addition, a syndesmotic screw to secure the remaining fibula to the tibia distally can be used to prevent and/or delay valgus deformity of the ankle.32,46
Recipient Site Flap Inset
The type of fixation used to secure the VFET in the recipient site should offer stability while limiting how much the nearby soft tissue and vessels are disturbed. The predominant method of fixation was the combined use of plates and screws.9,12–14,17,20,23,29,30,32,33,36,37,40 Although a plate bridging the flap may provide stable fixation to maintain postoperative alignment, there has been concern that it can interrupt the blood supply and prevent flap thickening over time.29 Several cases therefore involved fixation with screws alone.7,31,36,40 External fixation and intramedullary nailing were primarily used in hip reconstruction.5,25,26
Following inset of VFET at the recipient site, muscle reattachment and soft tissue reconstruction were performed to support the reconstructed joint.13,18,37 Examples of ligamentous reconstruction using the biceps femoral tendon included wrapping it around the coracoid process of the scapula or anchoring it to the glenoid capsule for shoulder stabilization,3,10,14 weaving it into the residual capsule to stabilize the radiocarpal joint in distal radius and ulna reconstruction,8,22,23 helping reconstruct calcaneal ligaments in ankle reconstruction,15,20,31 and suturing it into the remaining capsular ligament of the TMJ in mandible reconstruction.17
Postoperative protocols entailed immobilization followed by rehabilitation with progressive range of motion and weight-bearing of the salvaged extremity at variable timepoints, as displayed in Supplemental Table 1. (See table, Supplemental Digital Content 1, http://links.lww.com/PRSGO/C824.)
Technique Modifications
The combined use of VFET with allograft has been demonstrated in humeral reconstruction to provide additional structural support while allowing for epiphyseal growth.13,14 Lovic et al combined the use of a dual pedicle VFET with radius allograft for proximal femur reconstruction.37 In these cases, the allograft’s articular surface was removed, and the diaphysis was reamed to allow telescoping of the VFET.13,14,37 The allograft also provided more space for screw purchase for fixation and reattachment of surrounding muscles. VFET has also been used as an ipsilateral pedicled flap for reconstruction of the lateral malleolus and calcaneus without the necessity of microvascular anastomosis.6,12,15,31 Santanelli di Pompeo et al performed a double barrel vascularized dual fibula transfer in which a VFET was connected in series with a contralateral free fibula flap for femur reconstruction.18
Outcomes
Limb Growth
Reports of longitudinal bone growth varied within the range of 0.44–1.72 cm per year.2,3,9,10,13–15,17,24,34–36,38 The variation in longitudinal bone growth may be partly attributed to the vascular pedicle utilized and the recipient site. The PA as the pedicle was associated with an average limb growth of 0.5 cm per year and premature epiphyseal closure,27,34 while the use of the ATA had a range of 0.44–1.72 cm per year.2,3,9,13–15,17,24,35,36 Mughal et al reported a limb growth range of 0.8–1.21 cm per year with a dual pedicle.38 While overall limb growth was not reported with use of the ILGA in radial longitudinal deficiency reconstruction, the mean variance between the distal ends of the radius and ulna improved to 7 mm from 23 mm preoperatively over an average of 3.5 years.22 Limb growth with the femur as a recipient site was reported at 0.5 cm per year compared with 0.44–1.72 cm per year with the humerus, 0.77 cm per year with the mandible, and 0.5 to 1.1 cm per year with the radius.3,9,10,13,14,17,24,34–36 By transplanting the fibula to a new site, subsequent bone growth may vary in accordance with Wolff law to meet new biomechanical stresses.2,9,47,48 Because the fibula is similar in size to the radius, ulna, and mandible, the flap must undergo less hypertrophy and remodeling upon transfer to handle these recipient sites’ biomechanical stresses compared with the humerus and femur. Another important consideration is the necessity to match the growth of adjacent bone to prevent any resultant deformity. In a two-bone region like the forearm, symmetric growth must be maintained between the radius and ulna to prevent deviation of the upper extremity.21 Innocenti et al reported that six of eight patients who underwent distal radius reconstruction with VFET using the ATA had symmetric growth with the ipsilateral ulna and, therefore, maintained wrist alignment.21 In a case series where VFET used the PA for distal radius reconstruction, premature epiphyseal closure of the radius caused radial deviation due to asymmetric growth of the ipsilateral ulna.27
Range of Motion/Strength
Decreases in limb range of motion and strength are to be expected after reconstruction due to the initial defect at the recipient site that may include resection of bone and surrounding soft tissues including muscle and nerve. For instance, osteosarcoma resection of the proximal humerus sometimes involved resection of the axillary nerve in addition to muscles such as the deltoid, infraspinatus, teres minor, and subscapularis.13,14 Data on limb range of motion and strength following VFET, therefore, highlight how this reconstructive procedure may contribute to preserved limb function. For instance, proximal femur reconstruction with VFET was associated with retained hip flexion of 100–103 degrees, hip extension of 3–10 degrees, and 30 degrees abduction.37,42 After humeral reconstruction with VFET, arm forward flexion ranged from 10 degrees to 80 degrees, and arm abduction ranged from 10 degrees to 113 degrees.7,10,13,14,24,33,44 In two patients who underwent radius reconstruction with VFET, palmar flexion to dorsal extension was 45-0-10 degrees in one patient at 2-year follow-up, and 15-0-10 degrees in the second patient at 15-year follow-up.27 Pronation/supination was 10-0-70 degrees and 10-0-30 degrees, respectively.
Objective measures of limb strength after VFET were rarely described. In three patients who underwent wrist reconstruction, grip strength was reported as ranging from 20% to 28.7% of the contralateral hand.27 In a case of humeral reconstruction of an 8-year-old patient, postoperative motor strength at 6 months was rated as 4 of 5.33 Several case series, however, documented functional scores using the Musculoskeletal Tumor Society (MSTS) scoring system that consists of six domains translated to a total score of 0% to 100%, with a higher score indicating better function.49 Debarge et al reported an MSTS range of 66% to 87% in three patients who underwent VFET hip reconstruction for management of septic arthritis.26 Average MSTS scores for humeral reconstruction with VFET ranged from 60%–100% in several case series.10,13,14,33,40 Gebert et al reported an MSTS score of 80% and 93% in two patients who underwent ulna reconstruction, and 93% in two patients who underwent radius reconstruction.40
Complications
Complications after VFET included poor wound healing,20 hardware malfunction,20,26,30 growth arrest,27,32 nonunion,24 joint dislocation,5,26 anastomotic failure,22 infection,16,36 and avascular necrosis.10 Management of symptomatic hardware, bony nonunion, joint deformity/deviation, or arterial failure often entailed return to the operating room.2,5,10,16,22,26,30,34,36 The most common complications after VFET pediatric reconstruction, however, were bone flap fracture and peroneal nerve palsy.
Bone Flap Fracture
Bone flap fracture was most commonly reported in patients who underwent proximal humerus and femur VFET reconstruction.5,8,10,16,19,25,33,36,40 In a multi-institutional retrospective review, 40.7% of patients who underwent proximal humerus VFET reconstruction sustained postoperative fractures.36 In a meta-analysis of 62 patients who underwent VFET at different recipient sites, postoperative fracture was the most common complication at a rate of 35%.44 Fracture was not isolated to a fixation method, as it occurred with external fixation, intramedullary nailing, plates with screws, or screws alone.5,7,9,16,36 All patients who underwent humeral reconstruction with the combined use of VFET with allograft did not sustain fractures.13,14,37 Most fractures were treated nonoperatively with immobilization,5,9,10,16,36 whereas others were managed with plate fixation.5,21,36
Peroneal Nerve Palsy
Peroneal nerve palsy occurred exclusively in cases using the ATA as the vascular pedicle. Review of multiple case series using the ATA demonstrated transient and permanent peroneal nerve palsy at rates of 30%–59.3% and 15%–20%, respectively.2,8–10,12,13,24,25,36,38,40 A meta-analysis showed that the majority of VFET cases using the ATA experience a transient foot drop, and approximately 8% of patients can expect permanent foot drop.44 While the peroneal nerve can be avoided during dissection of the PA and ILGA, branches of the peroneal nerve are frequently encountered and require division during dissection of the ATA. Patients with peroneal nerve palsy showed gradual recovery with an ankle orthotic and physical therapy.13,26 Tendon transfer may be considered for patients with persistent peroneal nerve palsy.21
DISCUSSION
This comprehensive review demonstrates reliable growth outcomes in VFET pediatric reconstruction. Included studies ranging over 20 years in over 10 countries detailed variations in indications, surgical techniques, and outcomes. These articles illustrated how VFET continues to be optimized over time, as certain methods become less in use, such as the PA as the sole pedicle due to decreased limb growth. Patients and their families should be informed about potential limb growth, bone flap fracture, and peroneal nerve palsy considering the recipient site and vascular pedicle to be utilized in VFET.
The most validated surgical technique and reliable limb growth involved use of the ATA as the pedicle. Although the ILGA and a dual pedicle were feasible pedicle options in VFET, the smaller size of the ILGA and the added difficulty/morbidity potentially associated with a dual pedicle may pose limitations. The ATA is not without its own complexity, however, due to its close association with the deep peroneal nerve and subsequent risk of peroneal nerve palsy. The pedicle choice should take into consideration the ideal size of the pedicle, diaphysis necessary for recipient site reconstruction, and the technical abilities of the surgeon.
The most common recipient site reconstructed with VFET was the proximal humerus after osteosarcoma resection. This recipient site, in addition to the proximal femur, was more prone to bone flap fracture compared with the distal radius, ulna, or mandible. Bone flap fracture after VFET may be attributed to poor immobilization in the pediatric population, ability of the fibula’s small caliber to withstand biomechanical stresses at the recipient site, and the type of fixation method used.14 The new biomechanical stresses and initial size mismatch in bone caliber are greater at the humerus and femur. Screw fixation alone has been previously advocated to minimize disruption of the fibular endosteal and periosteal blood supply, but this method may provide minimal fixation.7 Plate fixation and the combined use of allograft may offer more stability of the VFET as it undergoes growth and remodeling to adapt to its recipient site and may therefore potentially decrease the postoperative bone flap fracture rate in pediatric patients who may have difficulty maintaining strict adherence to activity restrictions.
The impaired function of the reconstructed limb following VFET is expected, given the necessary resection of bone and surrounding soft tissues to extirpate osteosarcoma, the initial defect from a severe traumatic injury, or a congenital deformity. It is also important to consider other potential factors that may affect postoperative functional outcomes, such as the patient’s age, nutrition status, access to rehabilitation resources such as physical therapy, and chemotherapy/radiation therapy.36 VFET not only offers limb preservation and some function, it offers a psychological benefit compared with limb amputation.1 While other methods such as diaphyseal free flaps, allografts, and protheses also allow for limb preservation, they do not offer the growth potential associated with VFET. Further investigation is warranted to assess how functional outcomes of strength, range of motion, joint stability, and overall patient satisfaction compare between VFET and the other reconstructive options for limb salvage.
CONCLUSIONS
This comprehensive review demonstrates reliable growth outcomes in VFET pediatric reconstruction at various recipient sites and with different techniques, most commonly involving the ATA as the pedicle and the proximal humerus as the recipient site. The method of flap fixation and associated soft tissue reconstruction should take into consideration the biomechanical forces the VFET must accommodate at the recipient site. Patients and their families should be counseled about potential limb growth, bone flap fracture, and peroneal nerve palsy based on the recipient site and vascular pedicle to be utilized in the VFET procedure.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Supplementary Material
Footnotes
Published online 18 October 2023.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Wang J, Tang J, Tan X, et al. Amputation predisposes to higher cancer-specific mortality than limb salvage surgery in pediatric patients with osteosarcoma of the limbs: a propensity matching analysis. Front Surg. 2022;9:817051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zelenski N, Brigman BE, Levin LS, et al. The vascularized fibular graft in the pediatric upper extremity: a durable, biological solution to large oncologic defects. Sarcoma. 2013;2013:321201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishiura R, Sawaizumi M. Long-term results of vascularized proximal fibula epiphyseal transfer based on the anterior tibial artery in retrograde fashion. Clin Case Rep. 2020;8:1069–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsuda Y, Tsoi K, Stevenson JD, et al. Extendable endoprostheses in skeletally immature patients: a study of 124 children surviving more than 10 years after resection of bone sarcomas. J Bone Joint Surg Am. 2020;102:151–162. [DOI] [PubMed] [Google Scholar]
- 5.Petersen MM, Hovgaard D, Elberg JJ, et al. Vascularized fibula grafts for reconstruction of bone defects after resection of bone sarcomas. Sarcoma. 2010;2010:524721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajacic N, Dashti H. Reconstruction of the lateral malleolus using a reverse-flow vascularized fibular head: a case report. Microsurgery. 1996;17:158–161. [DOI] [PubMed] [Google Scholar]
- 7.Erdmann D, Garcia RM, Blueschke G, et al. Vascularized fibula-based physis transfer for pediatric proximal humerus reconstruction. Plast Reconstr Surg. 2013;132:281e–287e. [DOI] [PubMed] [Google Scholar]
- 8.Innocenti M, Delcroix L, Manfrini M, et al. Vascularized proximal fibular epiphyseal transfer for distal radial reconstruction. J Bone Joint Surg Am. 2004;86:1504–1511. [DOI] [PubMed] [Google Scholar]
- 9.Shammas RL, Avashia YJ, Farjat AE, et al. Vascularized fibula-based physis transfer: a follow-up study of longitudinal bone growth and complications. Plast Reconstr Surg Glob Open. 2017;5:e1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevenson JD, Doxey R, Abudu A, et al. Vascularized fibular epiphyseal transfer for proximal humeral reconstruction in children with a primary sarcoma of bone. Bone Jt J. 2018;100-B:535–541. [DOI] [PubMed] [Google Scholar]
- 11.Innocenti M, Delcroix L, Romano GF. Epiphyseal transplant: harvesting technique of the proximal fibula based on the anterior tibial artery. Microsurgery. 2005;25:284–292. [DOI] [PubMed] [Google Scholar]
- 12.Long Z, Lu Y, Chen G, et al. Lateral malleolus reconstruction after tumor resection in children: a case report and literature review. Orthop Surg. 2022;14:782–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houdek MT, Wellings EP, Saifuddin H, et al. Composite-free vascularized fibular epiphyseal flap and proximal humeral allograft for proximal humerus reconstruction in a pediatric patient. J Am Acad Orthop Surg Glob Res Rev. 2021;5:e21.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Y, Xiao X, Li M, et al. Use of vascularized fibular epiphyseal transfer with massive bone allograft for proximal humeral reconstruction in children with bone sarcoma. Ann Surg Oncol. 2021;28:7834–7841. [DOI] [PubMed] [Google Scholar]
- 15.Ozols D, Blums K, Krumins M, et al. Entire calcaneus reconstruction with pedicled composite fibular growth plate flap in a pediatric patient. Microsurgery. 2021;41:280–285. [DOI] [PubMed] [Google Scholar]
- 16.Bachy M, Mascard E, Dana C, et al. Clinical and radiological results of vascularized fibular epiphyseal transfer after bone tumor resection in children. Orthop Traumatol Surg Res OTSR. 2020;106:1319–1324. [DOI] [PubMed] [Google Scholar]
- 17.Innocenti M, Mori F, Raffaini M, et al. Mandibular ramus and condyle reconstruction with vascularized proximal fibular epiphyseal transfer in the pediatric patient: a case report. Microsurgery. 2020;40:818–822. [DOI] [PubMed] [Google Scholar]
- 18.Santanelli di Pompeo F, Selvaggi G, Longo B, et al. Double-barrel vascularized dual fibula transfer with epiphyseal growth plate for hip reconstruction: a case report. Microsurgery. 2018;38:572–575. [DOI] [PubMed] [Google Scholar]
- 19.Soldado F, Diaz-Gallardo P, Sena-Cabo L, et al. Vascularized fibular grafts extended with vascularized periosteum in children. Microsurgery. 2017;37:410–415. [DOI] [PubMed] [Google Scholar]
- 20.Bibbo C, Ehrlich DA, Kovach SJ. Reconstruction of the pediatric lateral malleolus and physis by free microvascular transfer of the proximal fibular physis. J Foot Ankle Surg Off Publ Am Coll Foot Ankle Surg. 2015;54:994–1000. [DOI] [PubMed] [Google Scholar]
- 21.Innocenti M, Baldrighi C, Menichini G. Long term results of epiphyseal transplant in distal radius reconstruction in children. Handchir Mikrochir Plast Chir. 2015;47:83–89. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Qin B, Li P, et al. Vascularized proximal fibular epiphyseal transfer for Bayne and Klug type III radial longitudinal deficiency in children. Plast Reconstr Surg. 2015;135:157e–166e. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Zhang G, Huo Z, et al. Reconstruction of the distal ulnar epiphysis with vascularized proximal fibula including epiphysis in children after osteochondroma resection: report of two cases. Plast Reconstr Surg. 2013;132:784e–789e. [DOI] [PubMed] [Google Scholar]
- 24.Medrykowski F, Barbary S, Gibert N, et al. Vascularized proximal fibular epiphyseal transfer: two cases. Orthop Traumatol Surg Res OTSR. 2012;98:728–732. [DOI] [PubMed] [Google Scholar]
- 25.Soldado F, Fontecha CG, Haddad S, et al. Composite vascularized fibular epiphyseo-osteo-periosteal transfer for hip reconstruction after proximal femoral tumoral resection in a 4-year-old child. Microsurgery. 2012;32:489–492. [DOI] [PubMed] [Google Scholar]
- 26.Debarge R, Chotel F, Gazarian A, et al. Failed vascularized proximal fibular epiphyseal transfer for hip reconstruction following infection in children. J Child Orthop. 2009;3:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papadopulos NA, Weigand C, Kovacs L, et al. The free vascularized fibular epiphyseal transfer: long-term results of wrist reconstruction in young patients. J Reconstr Microsurg. 2009;25:3–13. [DOI] [PubMed] [Google Scholar]
- 28.Innocenti M, Delcroix L, Balatri A. Vascularized growth plate transfer for distal radius reconstruction. Semin Plast Surg. 2008;22:186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Innocenti M, Delcroix L, Romano GF, et al. Vascularized epiphyseal transplant. Orthop Clin North Am. 2007;38:95–101, vii. [DOI] [PubMed] [Google Scholar]
- 30.Bae DS, Waters PM, Sampson CE. Use of free vascularized fibular graft for congenital ulnar pseudarthrosis: surgical decision making in the growing child. J Pediatr Orthop. 2005;25:755–762. [DOI] [PubMed] [Google Scholar]
- 31.de Gauzy JS, Kany J, Cahuzac JP. Distal fibular reconstruction with pedicled vascularized fibular head graft: a case report. J Pediatr Orthop Part B. 2002;11:176–180. [DOI] [PubMed] [Google Scholar]
- 32.Ad-El DD, Paizer A, Pidhortz C. Bipedicled vascularized fibula flap for proximal humerus defect in a child. Plast Reconstr Surg. 2001;107:155–157. [DOI] [PubMed] [Google Scholar]
- 33.Shea KG, Coleman SS, Coleman DA. Growth of the proximal fibular physis and remodeling of the epiphysis after microvascular transfer. A case report. J Bone Joint Surg Am. 1997;79:583–586. [PubMed] [Google Scholar]
- 34.Barrera-Ochoa S, Soldado F, Knörr J. Eight-year follow-up after vascularized fibular epiphyseal transfer for hip reconstruction. Microsurgery. 2017;37:743–744. [DOI] [PubMed] [Google Scholar]
- 35.Sawaizumi M, Maruyama Y, Okajima K, et al. Free vascularised epiphyseal transfer designed on the reverse anterior tibial artery. Br J Plast Surg. 1991;44:57–59. [DOI] [PubMed] [Google Scholar]
- 36.Azoury SC, Shammas RL, Othman S, et al. Outcomes following free fibula physeal transfer for pediatric proximal humerus reconstruction: an international multi-institutional study. Plast Reconstr Surg. 2023;151:805–813. [DOI] [PubMed] [Google Scholar]
- 37.Lovic A, Ortiz-Cruz EJ, Pérez-Rodríguez J, et al. Total hip reconstruction after sarcoma resection in children with a free vascularized fibula without osteotomy of the bone flap: Technique description and case series. J Plast Reconstr Aesthetic Surg JPRAS. 2022;75:3140–3148. [DOI] [PubMed] [Google Scholar]
- 38.Mughal M, Rose V, Sindali K, et al. Dual pedicle epiphyseal transfer for paediatric bony sarcoma reconstruction: technique and review of outcomes. J Plast Reconstr Aesthetic Surg JPRAS. 2022;75:2466–2473. [DOI] [PubMed] [Google Scholar]
- 39.Ozols D, Butnere MM, Kalnina L, et al. Double vascularized fibula proximal growth plate transplantation: novel technique for the radial longitudinal deficiency (RLD) grade IV reconstruction. Tech Hand Up Extrem Surg. 2022;26:98–102. [DOI] [PubMed] [Google Scholar]
- 40.Gebert C, Hillmann A, Schwappach A, et al. Free vascularized fibular grafting for reconstruction after tumor resection in the upper extremity. J Surg Oncol. 2006;94:114–127. [DOI] [PubMed] [Google Scholar]
- 41.Sadek AA, Halim A, Ismail S, et al. Does distal radius reconstruction with free epiphyseal transfer lead to inferior ra- dioulnar dissociation. Ann Clin Case Rep. 2016:1075.
- 42.Manfrini M, Innocenti M, Ceruso M, et al. Original biological reconstruction of the hip in a 4-year-old girl. Lancet Lond Engl. 2003;361:140–142. [DOI] [PubMed] [Google Scholar]
- 43.Taylor GI, Wilson KR, Rees MD, et al. The anterior tibial vessels and their role in epiphyseal and diaphyseal transfer of the fibula: experimental study and clinical applications. Br J Plast Surg. 1988;41:451–469. [DOI] [PubMed] [Google Scholar]
- 44.Kurlander DE, Shue S, Schwarz GS, et al. Vascularized fibula epiphysis transfer for pediatric extremity reconstruction: a systematic review and meta-analysis. Ann Plast Surg. 2019;82:344–351. [DOI] [PubMed] [Google Scholar]
- 45.Morsy M, Sur YJ, Akdag O, et al. Vascularity of the proximal fibula and its implications in vascularized epiphyseal transfer: an anatomical and high-resolution computed tomographic angiography study. Plast Reconstr Surg. 2019;143:172e–183e. [DOI] [PubMed] [Google Scholar]
- 46.Kanaya K, Wada T, Kura H, et al. Valgus deformity of the ankle following harvesting of a vascularized fibular graft in children. J Reconstr Microsurg. 2002;18:91–96. [DOI] [PubMed] [Google Scholar]
- 47.Pho RW, Patterson MH, Kour AK, et al. Free vascularised epiphyseal transplantation in upper extremity reconstruction. J Hand Surg Edinb Scotl. 1988;13:440–447. [DOI] [PubMed] [Google Scholar]
- 48.Wolff J. The classic: on the inner architecture of bones and its importance for bone growth. 1870. Clin Orthop. 2010;468:1056–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Enneking WF, Dunham W, Gebhardt MC, et al. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop. 1993:241–246. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.