Abstract
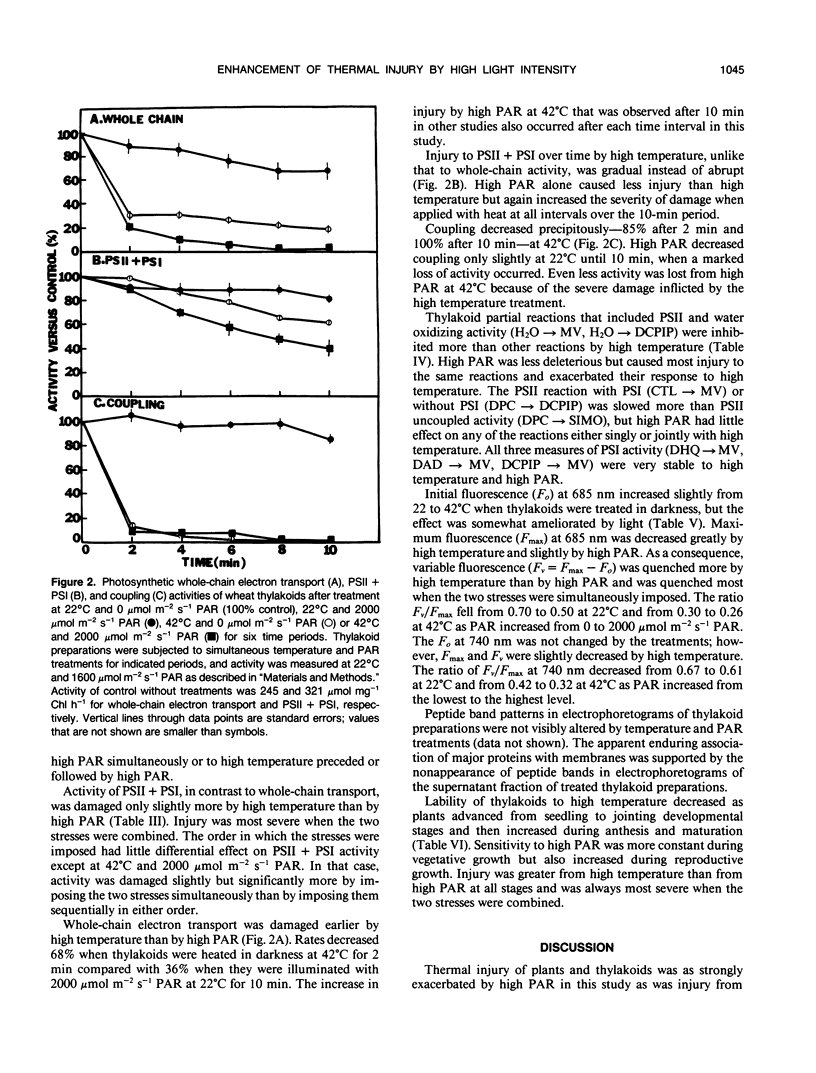
Thermal inhibition and photoinhibition of plants, which may occur simultaneously in nature, were investigated to determine whether the two causal stresses interact and to characterize any interactions that occurred. Photosynthetic rates of wheat (Triticum aestivum L. cv Len) seedlings declined gradually after temperature treatment increased from 22 to 42°C or after photosynthetically active radiation (PAR) treatment increased from 450 to 2000 micromoles per square meter per second and fell rapidly after the stresses were simultaneously imposed. Stomatal conductance and internal CO2 were affected little, indicating the interaction occurred in chloroplasts. Thylakoid whole chain electron transport, quantum yield, and saturating PAR intensity were decreased by high temperature and an additional amount by high PAR treatments. Photosystem reactions involving water oxidation were inhibited more than other reactions, and chlorophyll fluorescence transients indicated most inhibition was on the photooxidizing side of photosystem II. Injury was influenced little by the order in which the stresses were imposed and was always most severe when they were combined. Release of proteins from thylakoid membranes was not detected. Lability to the stresses was lowest in thylakoids from vegetative stage plants and increased as plants matured. We concluded that thermal injury is accentuated by high PAR, the two stresses may act at a common site near the water oxidizing complex, and their interaction may be involved in photosynthetic decline during adverse conditions.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armond P. A., Schreiber U., Björkman O. Photosynthetic Acclimation to Temperature in the Desert Shrub, Larrea divaricata: II. Light-harvesting Efficiency and Electron Transport. Plant Physiol. 1978 Mar;61(3):411–415. doi: 10.1104/pp.61.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U., Tyankova L., Santarius K. A. Effects of freezing on biological membranes in vivo and in vitro. Biochim Biophys Acta. 1973 Jan 2;291(1):23–37. doi: 10.1016/0005-2736(73)90057-6. [DOI] [PubMed] [Google Scholar]
- Kyle D. J., Ohad I., Arntzen C. J. Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4070–4074. doi: 10.1073/pnas.81.13.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Hoffman-Falk H., Marder J. B., Edelman M. Regulation of protein metabolism: Coupling of photosynthetic electron transport to in vivo degradation of the rapidly metabolized 32-kilodalton protein of the chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1380–1384. doi: 10.1073/pnas.81.5.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort D. R., Izawa S. Studies on the Energy-coupling Sites of Photophosphorylation: V. Phosphorylation Efficiencies (P/e(2)) Associated with Aerobic Photooxidation of Artificial Electron Donors. Plant Physiol. 1974 Mar;53(3):370–376. doi: 10.1104/pp.53.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz W., Lam E., Chollar S., Munt D., Malkin R. Topography of the protein complexes of the chloroplast thylakoid membrane : studies of photosystem I using a chemical probe and proteolytic digestion. Plant Physiol. 1985 Feb;77(2):389–397. doi: 10.1104/pp.77.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles S. B., Osmond C. B. Photoinhibition of intact attached leaves of c(3) plants illuminated in the absence of both carbon dioxide and of photorespiration. Plant Physiol. 1979 Dec;64(6):982–988. doi: 10.1104/pp.64.6.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis E. Influence of light on the heat sensitivity of the photosynthetic apparatus in isolated spinach chloroplasts. Plant Physiol. 1982 Nov;70(5):1530–1534. doi: 10.1104/pp.70.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]


