Abstract
Recent evidence suggests changes in circulating microRNA levels may be promising biomarkers for the clinical diagnosis of Alzheimer disease (AD). We hypothesized that whole-blood microRNAs may be useful to identify individuals with established AD. For this purpose, a sample of community-dwelling women (≥55 years old) carrying the ApoE ∊4 allele were clinically evaluated using the American Psychiatric Association/Diagnostic and Statistical Manual of Mental Disorders, Fourth edition and the Alzheimer Disease Assessment Scale–Cognitive Subscale criteria to diagnose probable AD, and the Clinical Dementia Rating scale to stage the dementia. A set of 25 mature microRNAs was rationally selected for evaluation based on experimental evidence of interaction with genes linked to the late-onset AD neuropathology. Whole-blood concentrations were determined by quantitative real-time polymerase chain reaction. Compared to patients without dementia, a median 3-fold decrease in miR-9 levels was found among patients with AD (P = .001). Our findings support blood-borne miR-9 as a candidate biomarker for probable AD, embodied by evidence from the literature of its implication in amyloidogenesis.
Keywords: Alzheimer disease, microRNAs, blood, biomarker, aging
Introduction
Late-onset Alzheimer disease (AD) is the most common dementia disorder worldwide, with an estimated global prevalence of approximately 13.8 million cases. 1 Globally, one new case of AD is estimated to develop every 33 seconds, and nearly 1 million new cases are diagnosed each year. The clinical progression of AD, characterized by progressive loss of recent memory and other cognitive and behavioral impairments, results in permanent inability to perform basic and instrumental activities of daily living. 2,3 Pathologically, AD is often characterized by the presence in the cerebral cortex of senile plaques composed of extracellular amyloid-β protein deposits and intraneuronal neurofibrillary tangles formed by tau hyperphosphorylation. 4 -6
At present, AD can only be diagnosed when a cognitive decline phenotype has been clinically established, and there are no treatments to change the progressive course of this brain disorder. 7,8 There is robust evidence that cellular changes associated with the disease can be identified years before persons show any clinical signs. 9,10 For this reason, researchers are increasingly looking for specific circulating elements in blood or cerebrospinal fluid (CSF), 11,12 aiming to identify physiologic changes to predict the disease development even in symptom-free individuals, in pursuit of strategies to prevent and/or treat AD as early as possible. Several trials and reviews on biomarkers are available or under way worldwide, 9,13 -22 and we draw particular attention to those aimed at circulating microRNAs (miRNAs). 23,24
MicroRNAs are single-stranded, noncoding RNA molecules, with 18 to 25 nucleotides, that have a critical role in regulating target genes usually by binding to the 3′ untranslated region of messenger RNAs, resulting in their translational repression or degradation of target sites. MicroRNAs play a regulatory role in almost all biological processes, including inflammation, proliferation, and apoptosis. 25,26 These molecules are present in all human biofluids. In accessible sources such as CSF and blood, miRNAs have been shown to be particularly stable, probably for being transported in compartmentalized manners as liposomes or lipoproteins, thereby preventing exposure and degradation. Different methods are currently used to measure miRNA levels in biofluids, of which quantitative polymerase chain reaction (PCR) is the most commonly used. 25,27
To date, no biomarker has been informative enough to surpass the accuracy of the clinical diagnosis of AD, despite the high sensitivity and specificity of the combined measurement of amyloid-β peptide and tau protein forms in CSF. 11 In spite of their undoubted informativeness to indicate (and mainly exclude) cases at high risk of AD onset, harvesting CSF by such an invasive procedure as lumbar puncture will hinder these markers from being screened in everyday clinical practice. Therefore, research on blood-borne markers still hold great expectations due to the ease of collection for both routine individual evaluations and population health screening. For this reason, circulating miRNAs are extensively investigated as potential tools for screening, differential diagnosis, and disease progression monitoring. 27,28
About a decade ago, small-scale profiling studies began to be conducted to provide the first clues into changes in miRNA production in the AD brain. 29 Since then, several groups have conducted large-scale genomic studies demonstrating differential miRNA expression patterns not only in brain tissue but also in CSF and blood plasma. 29 -36 The most recent profiling data show that a subset of these small RNAs seems to be specifically altered in the AD brain. This pattern includes miRNAs such as miR-9, 33 miR-15a, 31 miR-29, 30 miR-101, 31 miR-106, 37 miR-107, 36 miR-146, 38 and miR-181c, 39 all of which have been independently demonstrated in 2 or more studies. Interestingly, many of these candidates may play a direct role in modulating the expression of AD-related genes. 40 The present study aimed to compare between individuals with and without probable AD, the whole-blood concentration of a specific subset of miRNAs selected based on their modulatory role in the expression of genes associated with the etiology of AD.
Methods
Subjects and Study Outline
Participants were recruited from patients receiving care in the Center for Geriatric Medicine at the University Hospital of Brasília, affiliated with Universidade de Brasília, Brazil, which is a local referral center for the medical care of older adults, with emphasis on the treatment of cognitive disorders. Since age, gender, and apolipoprotein E (APOE) genotype are the main nonmodifiable risk factors known for AD onset, 41 we recruited a convenience sample constituted solely by female patients aged ≥55 years carrying the ∊4 allele. Because participants had been recruited for previous clinical and molecular studies, 42 -44 with their APOE genotypes determined following a classic technique 45 and available in database, selective recruitment of ∊4 carriers (homozygotes and heterozygotes) was possible a priori. In addition, early admission to the center (occurring until 2012) allowed patients with dementia to be evaluated for conversion to other dementia diagnoses, while cognitively intact patients at baseline were monitored to exclude the possibility of cognitive decline during the period. Therefore, all dementia cases who showed no diagnostic conversion (and were characterized as sporadic, nonfamilial cases of late onset without signs of an associated vascular component) were considered eligible to constitute the AD group. Likewise, only patients in the control group who showed no clinically important decline during the period were enrolled. For this purpose, clinical reassessments were performed at least once for each patient between 36 and 60 months after the cognitive evaluation on admission to the center.
The study was approved by the research ethics committee of the School of Medicine of the Universidade de Brasília, being the experiments conducted in accord with the Helsinki Declaration. Written informed consent was obtained from all participants and their caregivers for further collection of biological material.
Ambulatory Evaluation
Baseline clinical assessment and clinical follow-up of all patients included the administration of the Mini-Mental State Examination (MMSE) to screen for cognitive impairment. 46 Suspected cases were investigated based on the criteria of the American Psychiatric Association/Diagnostic and Statistical Manual of Mental Disorders, Fourth edition, for confirmation of dementia and of the Alzheimer Disease Assessment Scale–Cognitive subscale for diagnosis of the probable Alzheimer type. 47 The severity of dementia was determined using the Clinical Dementia Rating scale. 48
Other variables investigated in these evaluations included lifestyle habits, the presence of comorbidities (eg, diabetes and hypertension), medical conditions (eg, obesity and previous traumatic brain injury), incidental and previous psychiatric disorders (eg, Parkinson disease, depression), and medication in use.
MicroRNA Selection
To select the set of miRNAs to be investigated, we searched the DIANA Tools (TarBase v7.0) for miRNAs experimentally validated as interacting with target genes linked to the neuropathology of the late-onset form of AD, focusing on genes encoding key proteins as detailed in the review conducted by Moraes et al, 43 as follows: the apolipoprotein E (ApoE), the amyloid precursor protein (APP), presenilins 1 (Psen1) and 2 (Psen2), the tau protein (MAPT), clusterin (Clu), the phosphatidylinositol-binding clathrin assembly protein (Picalm), and the β-site APP cleaving enzyme 1 (Bace1).
A manual search was performed by entering each gene name individually, and human(hsa)-miRNAs were eligible for study if the database reported an association (positive result) and had the source tissue specified. From the list of miRNAs yielded, a subsequent Kyoto Encyclopedia of Genes and Genomes analysis allowed narrowing the selection to 25 miRNAs based on the selection of pathways implicated in neurophysiology (Figure 1).
Figure 1.
Rationale for selection of the microRNAs analyzed. KEGG indicates Kyoto Encyclopedia of Genes and Genomes (www.genome.jp/kegg/).
Blood Samples and Total RNA Extraction
After the minimum clinical follow-up period for each patient, whole-blood samples were collected by venipuncture into vacutainer tubes containing EDTA (Becton Dickinson, Curitiba, Paraná, Brazil). White blood cell counts (total and of common subsets) were determined by an automated procedure, Cell-Dyn 3700 (Abbott, Chicago, Illinois, USA). For RNA protection, samples were fractionated as 0.7-mL aliquots in the presence of an equal volume of RNALater (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Each aliquot was immediately frozen for subsequent extraction of total RNAs, purified using the mirVANA PARIS kit (Life Technologies, Carlsbad, Califórnia, USA), according to the manufacturer’s instructions with the modification of using multiple (2 or 3) consecutive partitions of the lysate with phenol–chloroform (Invitrogen) if necessary. After extraction, quantification and sample analysis were conducted using a NanoDrop Lite spectrophotometer (Thermo Fisher Scientific), with quality control performed by assessing the optimal density ratio of 260:280. Total RNA samples were stored at −20°C for further analysis.
Relative Quantification of MiRNAs by Quantitative Real-Time-PCR
Expression analysis of the miRNAs selected for study was performed using commercially available using stem-loop TaqMan RT-qPCR miRNA assays for quantitative procedures (Applied Biosystems, Foster City, California). The TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems) was used to prepare complementary DNA (cDNA). In this case, each reaction contained 20 ng total RNA, 12.5-nM heptaplex stem-loop miRNA-specific primers, 2-mM dNTPs, 100 U MultiScribe Reverse Transcriptase, and 5 U RNase inhibitor at a final volume of 10 μL. Reaction was incubated for 30 minutes at 16°C, for 30 minutes at 42°C, and 5 minutes at 85°C for enzyme inactivation.
Relative amounts of miRNAs were calculated using the comparative cycle threshold (Ct) method. The total level of each miRNA was evaluated by normalization to levels of a small nucleolar RNA (RNU43), eligible based on prior evidence of its usefulness (and of family members) to normalize miRNA expression in blood samples, 49 with its concentration in our context assessed for stability across samples by means of the comparative cycle threshold (ΔCt) method. With this approach, the RNU43 was rendered suitable as reference (internal control), and relative quantification were run using the 2−ΔΔCt method. 50 For each relative quantification assay of individual miRNAs, we used 0.75 μL TaqMan MicroRNA Primer, 1.2 μL cDNA (in serial dilutions as described later in this section), 7.5 μL 2× TaqMan Universal PCR Master Mix without UNG, and 5.55 μL nuclease-free water, in total 15 μL reactions run using the Eco Real-Time PCR System, version 4.0 (Ilumina, San Diego, California), with cycling at 50°C for 2 minutes, at 95°C for 10 minutes, followed by 45 cycles of 15 seconds at 95°C and 1 minute at 60°C. All reactions were run in duplicate, and the individual mean Ct values were determined for each sample. The cycles of miRNAs and of the endogenous reference from each sample were set up on the same reaction plate to minimize inter-assay variation.
The dynamic range of each assay was determined by amplification reactions with parallel use of serial dilutions of the same sample initially at 3.33 ng/μL, with a minimum set of 3 independent samples, followed by simple linear regression analysis of the values obtained. 50 Thus, comparable proportionality ranges between Ct scores and logarithmic values of template concentrations were established for each miRNA and endogenous control. Based on the dynamic range, the efficiency of amplification (E) of each reaction was calculated using the equation E = 10(−1/slope) − 1.
Statistical Analysis
The normality of data distribution was assessed by exploratory analysis for discrete dependent variables. Comparisons between groups of normally distributed continuous clinical variables were performed by Student t test for independent samples, while the χ2 test was used to compare the frequencies of medical conditions. Comparisons of non-normally distributed variables (including circulating miRNA levels) were performed by the Wilcoxon (Mann-Whitney U) test. Also, binary logistic regressions were used to assess the impact of the variable on the odds ratio (OR) for AD. A P value <.05 was considered significant for these analyses.
Particularly for nonparametric analyses involving the miRNA data, a 2-tailed P value was set as significant following the Bonferroni correction for k independent variables tested across one same trait (k = 25 tests, then α = .002). All analyzes were run using SPSS for Windows, version 17.0.
Results
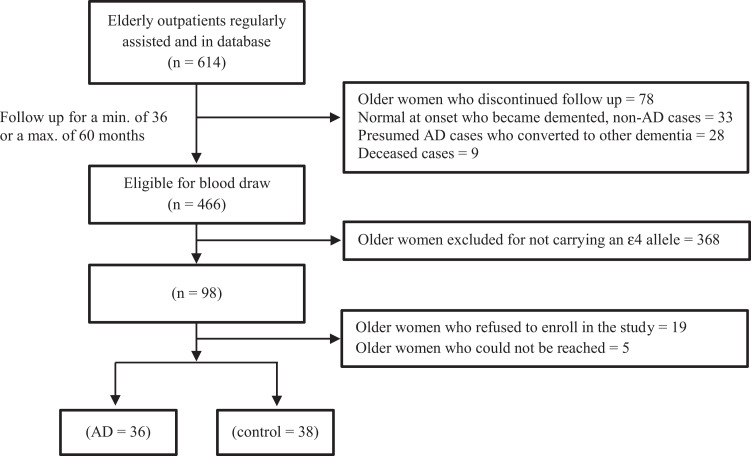
After screening for eligibility using medical as well as research records of 614 patients, whole-blood samples could be obtained from a total of 74 older women (38 controls and 36 patients with probable AD), with recruitment exclusions and failures as depicted in Figure 2. Social and biological data as well as classical clinical features were compared in an initial evaluation between the control group (patients without dementia) and the AD group. The groups did not differ significantly in age or other clinical variables, including counts of total white blood cells (Table 1) and of classic leukocyte subpopulations (data not shown). As expected, MMSE scores differed between the 2 groups. The groups were comparable in terms of years of schooling. On top of all participants being ∊4 carriers, the frequency of the ∊2∊4, ∊3∊4, and ∊4∊4 genotypes was evaluated and found similar across groups, with a predominance the ∊3∊4 genotype, as expected. Groups were also comparable in terms of frequency of users of psychoactive agents (grouped as antipsychotics, antidepressants, and anticonvulsants) as well as in terms of users of the most common metabolic agents (antihypertensive and hypoglycemic drugs).
Figure 2.
Composition of the sample of older women considered for analyses. AD indicates Alzheimer disease.
Table 1.
Comparison of Continuous (Mean and Standard Deviation or Median and Interquartile Range) and Categorical Variables (Proportion [%]) Across Control and AD Older Women.
| Control, n = 38 | AD, n = 36 | P a | |
|---|---|---|---|
| Variable | Mean (or Median) | Mean (or Median) | |
| Age (years) | 76.4 ± 9.2 | 79.5 ± 6.0 | .106 |
| WC (cm) | 93.7 ± 11.6 | 91.4 ± 12.2 | .411 |
| SBP (mm Hg) | 137.9 ± 18.3 | 131.8 ± 18.4 | .159 |
| DBP (mm Hg) | 80.9 ± 17.4 | 76.9 ± 10.1 | .235 |
| MMSE (score) | 24.0 (22.0-26.0) | 12.0 (7.2-19.5) | <.001b |
| Total WBC (109/L) | 6.4 (5.5-7.3) | 6.7 (5.9-7.8) | .435 |
| % | % | ||
| ∊3∊4 genotype | 66.6 | 86.8 | .115c |
| ≤4 years schooling | 42.1 | 61.1 | .216c |
| CDR ≤ 2 | – | 33.3 | – |
| Hachinski ≤4 | – | 86.1 | – |
| Use of | |||
| AChE inhibitors | – | 52.8 | – |
| rNMDA antagonist | – | 19.4 | – |
| Antipsychotics | 2.6 | 0.0 | .883c |
| Antidepressants | 10.5 | 5.6 | .286c |
| Anticonvulsants | 0.0 | 2.6 | .796c |
| Antihypertensives | 65.8 | 50.0 | .162c |
| Antidiabetics | 21.0 | 13.9 | .322c |
Abbreviations: AChE, acetylcholinesterase; AD, Alzheimer disease; CDR, Clinical Dementia Rating; DBP, diastolic blood pressure; MMSE, Mini-Mental State Examination; rNMDA, receptor of N-methyl d-aspartate; SBP, systolic blood pressure; WBC, white blood cells; WC, waist circumference.
a Significance verified by Student t test.
b Significance verified by the Wilcoxon test.
c Significance verified by the χ2 test.
We then evaluated the whole-blood concentration of the human miRNAs subjected to relative quantification (by qRT-PCR) in both control and patients with AD. All 25 target miRs and the internal control could be detected in the samples. Correlational analyses revealed no influence of classic AD risk factors in titers of any of the miRNAs evaluated across age (continuous trait) or APOE (∊2∊4/∊3∊4 vs ∊4∊4), regardless of performing a whole-sample analyses or taking each group (AD or control) at a time. Of all miRNAs evaluated, only the concentration of miR-9-5p differed between the groups (P = .001), with a median 3-fold decrease in circulating levels in patients with AD compared with controls (Table 2). A tendency for a relevant difference (if considered the conventional P < .05 threshold), although not achieving significance according to our standards, was observed for miR-21-5p and miR-29b-3p levels. Subsequent analyses using binary regression reassured miR-9-5p levels as importantly accounting for the phenotype of AD, confirming cognitively preserved older women to be more likely to express higher total circulating levels of the miRNA (OR = 1.041; 95% confidence interval, 1.003-1.080; P = .035) in the sample, with no such contribution occurring from levels of miR-21-5p (P = .520) or miR-29b-3p (P = .057).
Table 2.
Description of the hsa-MicroRNA Identification and Relative Levels Across Control and Older Women With AD.a
| miR Target Strand | Order of Magnitude | Control, n = 38 | AD, n = 36 | P b |
|---|---|---|---|---|
| miR-1-3p | 100 | 2.90 (1.02-14.50) | 3.03 (1.33-8.09) | .875 |
| miR-1-2-5p | 10−1 | 2.15 (0.89-8.75) | 1.16 (0.36-3.61) | .134 |
| miR-9-5p | 10−1 | 3.27 (1.84-6.42) | 1.02 (0.66-2.76) | .001 |
| miR-16-2-3p | 10−1 | 7.70 (0.78-70.08) | 8.59 (1.66-26.11) | .990 |
| miR-21-5p | 10−4 | 2.81 (1.02-15.18) | 1.47 (0.35-3.48) | .038 |
| miR-27b-3p | 10−1 | 0.89 (0.46-1.89) | 0.66 (0.29-1.35) | .171 |
| miR-29b-3p | 100 | 0.31 (0.10-0.63) | 0.14 (0.06-0.25) | .014 |
| miR-30a-3p | 100 | 2.50 (0.52-14.64) | 2.32 (0.69-4.44) | .525 |
| miR-34a-5p | 100 | 1.80 (0.65-4.66) | 2.79 (0.69-5.10) | .650 |
| miR-34c-5p | 10−1 | 0.66 (0.32-1.37) | 0.90 (0.36-1.96) | .320 |
| miR-92a-3p | 10−5 | 0.86 (0.42-1.58) | 0.74 (0.34-2.22) | .791 |
| miR-100-5p | 10−1 | 2.00 (0.21-5.38) | 1.00 (0.26-6.20) | .830 |
| miR-126-3p | 10−4 | 1.32 (0.55-2.60) | 1.20 (0.45-2.40) | .873 |
| miR-130a-3p | 10−3 | 2.84 (0.74-7.62) | 3.98 (0.76-6.74) | .697 |
| miR-141-3p | 100 | 1.67 (0.42-4.46) | 1.21 (0.29-4.00) | .673 |
| miR-145-5p | 10−1 | 1.05 (0.39-3.08) | 0.72 (0.44-1.89) | .402 |
| miR-146a-5p | 10−3 | 2.01 (0.67-11.16) | 1.62 (0.65-2.72) | .329 |
| miR-155-5p | 10−2 | 7.53 (2.94-22.36) | 6.68 (3.37-9.37) | .530 |
| miR-181a-5p | 10−3 | 1.88 (0.53-4.07) | 0.98 (0.49-4.72) | .424 |
| miR-181c-5p | 100 | 0.84 (0.34-1.99) | 0.80 (0.23-1.54) | .270 |
| miR-183-5p | 10−3 | 1.05 (0.60-5.12) | 1.02 (0.42-2.98) | .364 |
| miR-200a-3p | 100 | 2.16 (0.82-6.90) | 1.75 (0.95-3.49) | .456 |
| miR-221-3p | 10−2 | 1.12 (0.52-2.90) | 1.18 (0.52-1.96) | .615 |
| miR-371-3p | 100 | 0.98 (0.36-2.20) | 0.92 (0.38-3.40) | .858 |
| miR-373-5p | 10−2 | 0.22 (0.14-0.34) | 0.20 (0.11-0.27) | .496 |
Abbreviations: AD, Alzheimer disease; hsa, Human sapiens.
a Data are expressed as median (and interquartile range) for continuous traits with non-Gaussian distribution.
b P values are results from the Wilcoxon test.
Discussion
MiR-9 is a small, noncoding RNA involved in the regulation of the expression of multiple genes. 51 Despite having a highly conserved primary structure, miR-9 expression patterns are not equal among different species. In Drosophila sp, at early embryonic stages, miR-9 is expressed in most epithelial cells, except for the ventral ectoderm. 52 In vertebrates, miR-9 expression is confined to the nervous system, suggesting highly specialized neurogenic and neurophysiological functions. 53 In humans, mature miR-9 consists of 21 nucleotides that is processed by Dicer at the 5′ end of the pre-miR-9. 51 A key function of miR-9 is to regulate the morphological differentiation of postmitotic neurons from neural progenitor cells. 54 In this respect, it is not surprising that studies suggest that miR-9 dysregulation may play a role in neurodegenerative diseases. 29
In AD, it is known that the amyloid-β peptide in its 42 amino acids (Aβ42) insoluble form is produced by sequential enzymatic cleavage of APP by BACE1 and γ-secretase, 55 thus generating an amyloidogenic pathway. Evidence suggests that miR-9 may play a role in negative BACE1 regulation. 56 Lowered miR-9 levels may increase BACE1 expression, thereby increasing Aβ42 production. 31 Therefore, downregulation of miR-9 expression in the central nervous system (CNS) may have pathological implications by promotion of amyloidogenic processing, leading to pronounced Aβ42 aggregation and deposition into senile plaques. 57
In the same vein, a study using the luciferase assay in HEK293 cells showed that miR-9 suppression increases phosphorylated tau levels and amyloidogenesis by overexpression of calcium/calmodulin-dependent protein kinase 2, a target of miR-9. 58,59 Another target of miR-9 is the gene encoding sirtuin 1 (SIRT1), a deacetylase enzyme that interacts with tau and regulates its phosphorylation. 27,60 Studies suggest that SIRT1 plays a role in neuronal protection in patients with AD 61 since regular SIRT1 expression prevents tau hyperphosphorylation, maintaining the axonal transport of neurotransmitters by neurons.
In addition to evidence that miR-9 plays a role in different stages of neurogenesis and neurophysiology, its participation in processes such as apoptosis, inflammation, and oxidative stress seems consistent. 31,54 In view of the involvement of these factors in the development of AD, the authors do not rule out the participation of miR-9 in other pathways contributing to this form of neurodegeneration.
Several studies have demonstrated that miR-9 expression is altered in the AD brain, 40 being either downregulated or upregulated. In patients with AD, there is evidence of lowered miR-9 levels in the anterior temporal cortex along with overexpression in the hippocampus. 29,31 A study comparing transgenic APP23 to nontransgenic mice reported reduced miR-9 levels in the hippocampus of the former animals. 62 In autopsy-derived brain samples, 4 miRNAs (including miR-9) were negatively regulated in patients with AD. 39 Levels of these same miRNAs were compared in the serum of patients with AD, patients with mild cognitive impairment and control patients, with reduced circulating levels of the miR-9 being found among patients with AD. 60 Another independent study determined the concentration of 6 miRNAs (including miR-9) in blood plasma and CSF of patients with and without AD, and once again miR-9 was found in lower levels among patients with AD. 63
All in all, and despite how elusive that a finding from the circulation is to be extrapolated to a specialized milieu as the CNS, the body of evidence reviewed above points out to an overall reduction in miR-9 concentration in human biological samples as diverse as brain tissue, CSF, plasma, and serum in the context of AD. In our case, results suggest that miR-9 concentration is also altered in the whole-blood milieu, supporting the hypothesis that miR-9 may constitute an accessible biomarker for AD. The results of our report are corroborated by another whole-blood-based miRNA expression assay conducted elsewhere, also showing decrease in peripheral concentration of the has-miR-9-5p in AD cases. 64 By demonstrating that such an association persists even when sample subsets are at stake (as the on average >75 years older female ∊4 carriers considered herein), our work tends to pose a contribution to the translational research and literature on biomarkers by expanding the repertoire of possible AD diagnostic elements, tools, and biological sources by providing evidence possibly reduced in biases owing to age, gender, and APOE genotype, as main nonmodifiable risk factors for AD. Likewise, groups were fairly balanced in counts of individuals using metabolic-acting (antidiabetic and antihypertensive) medications and under psychoactive (antipsychotics, antidepressants, or anticonvulsants) pharmacotherapy, rendering results unlikely to be highly influenced by unleveled effects of common drugs across groups. On the consumption of acetylcholinesterase inhibitors and of antagonists of NMDA receptor exclusively by patients with AD (as expected), the authors state to the best of their knowledge not knowing of any effect of those classes on transcription or processing of miR-9.
Despite standardizing participants in terms of relevant nonmodifiable risk factors for AD, our study has limitations. First, no power calculation was performed to estimate an optimum small sample size, what could be rendered as a shortcoming. Second, neither the remarkable genetic admixture of the Brazilian population nor the usual dietary habit and deep hematological features of these patients were considered or controlled for in our analyses. At least, total and differential white blood cell counts were determined and proved unchanged across participants, what may avert suspicion on miR levels being influenced by fluctuation in blood-borne nucleated elements.
Another limitation inherent to this type of study is the difficulty in relating the findings in peripheral blood samples to pathophysiologic processes in specific organs/tissues or body compartments, as is the case of the CNS. In this context, the blood–brain barrier (BBB) is a structure formed by endothelial cells aligned with capillaries that allow the selective passage of substances, particularly controlling the entry of toxic substances. 65 However, microvesicles containing miRNAs (exosomes) are known to cross the BBB from the brain to the blood and vice versa by still unknown mechanisms. 66 -68 Therefore, it is plausible that bioavailable levels of miR-9 in the blood reflect proportions of the same mediator at the level of the CNS.
Conclusion
It is evident that much work is still needed to elucidate and determine the various roles that a given miRNA can play in pathological pathways and in identification of medical conditions. However, we conclude that whole-blood levels of the miR-9-5p are decreased in patients with AD compared with controls. Subsequent clinical studies may confirm the predictive value of this candidate biomarker and whether strategies for the maintenance of miR-9 expression levels can delay aspects related to the neurophysiology and even the symptomatology of AD.
Acknowledgments
The authors thank the participants for their commitment to the protocol. The authors are also grateful to the health-care team of the Center for Geriatric Medicine at the University Hospital of Brasília (HUB), affiliated to the Universidade de Brasília (UnB), for providing due clinical settings for the study.
Authors’ Note: V.C. Souza and G.S. Morais Jr were responsible for RNA processing and relative quantification. A.D. Henriques and W. Machado-Silva obtained and stored blood samples. C.J. Brito and D.I.V. Perez conducted the statistical analyses. E.F. Camargos and C.F. Moraes conducted the medical evaluations. O.T. Nóbrega coordinated the study and, along with V.C. Souza, wrote the original manuscript, which was reviewed by all authors. Data can be made available upon reasonable request. The authors declare minor self-plagiarism by reusing elements from published work of our own to help describing the sample and methods, being provided appropriate reference.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Brazilian National Council for Scientific and Technological Development (CNPq, grant no. 445692/2014-6) and the Research Support Foundation of the Federal District (FAPDF, grant nos. 193.000.967/2015 and 193.000.651/2015). O.T.N. received a CNPq research productivity grant, while V.C.S., G.S.M.J., and A.D.H. received stipends from the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES, FinanceCode 001).
ORCID iD: Otávio Toledo Nóbrega  https://orcid.org/0000-0003-1775-7176
https://orcid.org/0000-0003-1775-7176
References
- 1. Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11(3):332–384. [DOI] [PubMed] [Google Scholar]
- 2. Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88(9):1337–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kumar P, Dezso Z, MacKenzie C, et al. Circulating miRNA biomarkers for Alzheimer’s disease. PLoS One. 2013;8(7):e69807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonda DJ, Lee HG, Camins A, et al. The sirtuin pathway in ageing and Alzheimer disease: mechanistic and therapeutic considerations. Lancet Neurol. 2011;10(3):275–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Femminella GD, Rengo G, Komici K, et al. Autonomic dysfunction in Alzheimer’s disease: tools for assessment and review of the literature. J Alzheimers Dis. 2014;42(2):369–377. [DOI] [PubMed] [Google Scholar]
- 6. Femminella GD, Rengo G, Pagano G, et al. Beta-adrenergic receptors and G protein-coupled receptor kinase-2 in Alzheimer’s disease: a new paradigm for prognosis and therapy? J Alzheimers Dis. 2013;34(2):341–347. [DOI] [PubMed] [Google Scholar]
- 7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). BMC Med. 2013;17:133–137. [Google Scholar]
- 8. DeCarli C. Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurol. 2003;2(1):15–21. [DOI] [PubMed] [Google Scholar]
- 9. Bateman RJ, Xiong C, Benzinger TLS, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jack CR, Jr, Holtzman DM. Biomarker modeling of Alzheimer’s disease. Neuron. 2013;80(6):1347–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Henriques AD, Benedet AL, Camargos EF, Neto PR, Nobrega OT. Fluid and imaging biomarkers for Alzheimer’s disease: where we stand and where to head to. Exp Gerontol. 2018;107:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Langbaum JB, Fleisher AS, Chen K, et al. Ushering in the study and treatment of preclinical Alzheimer disease. Nat Rev Neurol. 2013;9(7):371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Douaud G, Menke RAL, Gass A, et al. Brain microstructure reveals early abnormalities more than two years prior to clinical progression from mild cognitive impairment to Alzheimer’s disease. J Neurosci. 2013;33(5):2147–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Furney SJ, Kronenberg D, Simmons A, et al. Combinatorial markers of mild cognitive impairment conversion to Alzheimer’s disease – cytokines and MRI measures together predict disease progression. J Alzheimers Dis. 2011;26(suppl 3):395–405. [DOI] [PubMed] [Google Scholar]
- 15. Galluzzi S, Geroldi C, Amicucci G, et al. Supporting evidence for using biomarkers in the diagnosis of MCI due to AD. J Neurol. 2013;260(2):640–650. [DOI] [PubMed] [Google Scholar]
- 16. Honea RA, Vidoni ED, Swerdlow RH, Burns JM; Alzheimer’s Disease Neuroimaging Initiatice. Maternal family history is associated with Alzheimer’s disease biomarkers. J Alzheimers Dis. 2012;31(3):659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnson KA, Minoshima S, Bohnen NI, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement. 2013;54(3):476–490. [DOI] [PubMed] [Google Scholar]
- 18. Roe CM, Fagan AM, Grant EA, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurol. 2013;80(19):1784–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shaffer JL, Petrella JR, Sheldon FC, et al. Predicting cognitive decline in subjects at risk for Alzheimer disease by using combined cerebrospinal fluid, MR imaging, and PET biomarkers. Radiology. 2013;266(2):583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stricker NH, Dodge HH, Dowling NM, Han SD, Erosheva EA, Jagust WJ. CSF biomarker associations with change in hippocampal volume and precuneus thickness: implications for the Alzheimer’s pathological cascade. Brain Imag Behav. 2012;6(4):599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vos S, Rossum IV, Burns L, et al. Test sequence of CSF and MRI biomarkers for prediction of AD in subjects with MCI. Neurobiol Aging. 2012;33(10):2272–2281. [DOI] [PubMed] [Google Scholar]
- 22. Yang X, Tan MZ, Qiu A. CSF and brain structural imaging markers of the Alzheimer’s pathological cascade. PLoS One. 2012;7(12): e47406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grasso M, Piscopo P, Confaloni A, Denti MA. Circulating miRNAs as biomarkers for neurodegenerative disorders. Molecules. 2014;19(5):6891–6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keller A, Leidinger P, Bauer A, et al. Toward the blood-borne miRNome of human diseases. Nat Methods. 2011;8(10):841–843. [DOI] [PubMed] [Google Scholar]
- 25. Dorval V, Nelson PT, Hebert SS. Circulating microRNAs in Alzheimer’s disease: the search for novel biomarkers. Front Mol Neurosci. 2013;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goodall EF, Heath PR, Bandmann O, Kirby J, Shaw PJ. Neuronal dark matter: the emerging role of microRNAs in neurodegeneration. Front Cell Neurosci. 2013;7:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Femminella GD, Ferrara N, Rengo G. The emerging role of microRNAs in Alzheimer’s disease. Front Physiol. 2015;6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fransquet PD, Ryan J. Micro RNA as a potential blood-based epigenetic biomarker for Alzheimer’s disease. Clin Biochem. 2018;58:5–14. [DOI] [PubMed] [Google Scholar]
- 29. Lukiw WJ. Micro-RNA speciation in fetal, adult and Alzheimer’s disease hippocampus. Neuroreport. 2007;18(3):297–300. [DOI] [PubMed] [Google Scholar]
- 30. Cogswell JP, Ward J, Taylor IA, et al. Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. J Alzheimer’s Dis. 2008;14(1):27–41. [DOI] [PubMed] [Google Scholar]
- 31. Hebert SS, Horre K, Nicolai L, et al. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proc Natl Acad Sci U S A. 2008;105(17):6415–6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nelson PT, Wang WX. MiR-107 is reduced in Alzheimer’s disease brain neocortex: validation study. J Alzheimers Dis. 2010;21(1):75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iglesias JN, Liu CC, Morgan TE, Finch CE, Zhou XJ. Joint genome-wide profiling of miRNA and mRNA expression in Alzheimer’s disease cortex reveals altered miRNA regulation. PLoS One. 2010;5(2):e8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schipper HM, Maes OC, Chertkow HM, Wang E. MicroRNA expression in Alzheimer blood mononuclear cells. Gene Regul Syst Biol. 2007;1:263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shioya M, Obayashi S, Tabunoki H, et al. Aberrant microRNA expression in the brains of neurodegenerative diseases: miR-29a decreased in Alzheimer disease brains targets neurone navigator 3. Neuropathol Appl Neurobiol. 2010;36(4):320–330. [DOI] [PubMed] [Google Scholar]
- 36. Wang WX, Rajeev BW, Stromberg AJ, et al. The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J Neurosci. 2008;28(5):1213–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hebert SS, Horre K, Nicolai L, et al. MicroRNA regulation of Alzheimer’s amyloid precursor protein expression. Neurobiol dis. 2009;33(3):422–428. [DOI] [PubMed] [Google Scholar]
- 38. Sethi P, Lukiw WJ. Micro-RNA abundance and stability in human brain: specific alterations in Alzheimer’s disease temporal lobe neocortex. Neurosci Lett. 2009;459(2):100–104. [DOI] [PubMed] [Google Scholar]
- 39. Geekiyanage H, Chan C. MicroRNA-137/181c regulates serine palmitoyltransferase and in turn amyloid beta, novel targets in sporadic Alzheimer’s disease. J Neurosci. 2011;31(41):14820–14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Delay C, Mandemakers W, Hebert SS. MicroRNAs in Alzheimer’s disease. Neurobiol Dis. 2012;46(2):285–290. [DOI] [PubMed] [Google Scholar]
- 41. Riedel BC, Thompson PM, Brinton RD. Age, APOE and sex: triad of risk of Alzheimer’s disease. J Steroid Biochem Mol Biol. 2016;160:134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Benedet AL, Moraes CF, Camargos EF, et al. Amerindian genetic ancestry protects against Alzheimer’s disease. Dement Geriatr Cogn Disord. 2012;33(5):311–317. [DOI] [PubMed] [Google Scholar]
- 43. Moraes CF, Lins TC, Carmargos EF, Naves JO, Pereira RW, Nobrega OT. Lessons from genome-wide association studies findings in Alzheimer’s disease. Psychogeriatrics. 2012;12(1):62–73. [DOI] [PubMed] [Google Scholar]
- 44. Quintas JL, Souza VC, Henriques AD, et al. Lack of association between apolipoprotein E genotypes and cognitive performance in the non-demented elderly. Psychogeriatrics. 2014;14(1):11–16. [DOI] [PubMed] [Google Scholar]
- 45. Donohoe GG, Salomaki A, Lehtimaki T, Pulkki K, Kairisto V. Rapid identification of apolipoprotein E genotypes by multiplex amplification refractory mutation system PCR and capillary gel electrophoresis. Clin Chem. 1999;45(1):143–146. [PubMed] [Google Scholar]
- 46. Nitrini R, Caramelli P, Bottino CMC, Damasceno BP, Brucki SMD, Anghinah R. Diagnóstico de doença de alzheimer no brasil: avaliação cognitiva e funcional. Arq Neuropsiquiatr. 2005;63(3-A):720–727. [DOI] [PubMed] [Google Scholar]
- 47. Nitrini R, Caramelli P, Bottino CMC, Damasceno BP, Brucki SMD, Anghinah R. Diagnóstico de doença de alzheimer no brasil: critérios diagnósticos e exames complementares. Arq Neuropsiquiatr. 2005;63(3-A):713–719. [DOI] [PubMed] [Google Scholar]
- 48. Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurol. 1993;43(11):2412–2414. [DOI] [PubMed] [Google Scholar]
- 49. Hunter MP, Ismail N, Zhang X, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008;3(11):e3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. L Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 51. Minor J, Wang X, Zhang F, et al. Methylation of microRNA-9 is a specific and sensitive biomarker for oral and oropharyngeal squamous cell carcinomas. Oral Oncol. 2012;48(1):73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li Y, Wang F, Lee JA, Gao FB. MicroRNA-9a ensures the precise specification of sensory organ precursors in drosophila. Genes Dev. 2006;20(20):2793–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stark A, Brennecke J, Bushati N, Russell RB, Cohen SM. Animal microRNAS confer robustness to gene expression and have a significant impact on 3′UTR evolution. Cell. 2005;123(6):1133–1146. [DOI] [PubMed] [Google Scholar]
- 54. Aydemir Y, Simkin A, Gascon E, Gao FB. MicroRNA-9: functional evolution of a conserved small regulatory RNA. RNA Biol. 2011;8(4):557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gupta P, Bhattacharjee S, Sharma AR, Sharma G, Lee S, Chakraborty C. miRNAs in Alzheimer disease – a therapeutic perspective. Current Alzheimer Res. 2017;14(11):1198–1206. [DOI] [PubMed] [Google Scholar]
- 56. Holohan KN, Lahiri DK, Schneider BP, Foroud T, Saykin AJ. Functional microRNAs in Alzheimer’s disease and cancer: differential regulation of common mechanisms and pathways. Front Genet. 2012;3:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shaik MM, Tamargo IA, Abubakar MB, Kamal MA, Greig NH, Gan SH. The role of microRNAs in Alzheimer’s disease and their therapeutic potentials. Genes (Basel). 2018;9(4):pii:E174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Coello GM, Courchet J, Pieraut S, Courchet V, Maximov A, Polleux F. The CAMKK2-AMPK kinase pathway mediates the synaptotoxic effects of Aβ oligomers through tau phosphorylation. Neuron. 2013;78(1):94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Salminen A, Kaarniranta K, Haapasalo A, Soininen H, Hiltunen M. AMP-activated protein kinase: a potential player in Alzheimer’s disease. J Neurochem. 2011;118(4):460–474. [DOI] [PubMed] [Google Scholar]
- 60. Geekiyanage H, Jicha GA, Nelson PT, Chan C. Blood serum miRNA: non-invasive biomarkers for Alzheimer’s disease. Exp Neurol. 2012;235(2):491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Julien C, Tremblay C, Emond V, et al. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp Neurol. 2009;68(1):48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schonrock N, Ke YD, Humphreys D, et al. Neuronal microRNA deregulation in response to Alzheimer’s disease amyloid-beta. PLoS One. 2010;5(6):e11070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kiko T, Nakagawa K, Tsuduki T, Furukawa K, Arai H, Miyazawa T. MicroRNAs in plasma and cerebrospinal fluid as potential markers for Alzheimer’s disease. J Alzheimers Dis. 2014;39(2):253–259. [DOI] [PubMed] [Google Scholar]
- 64. Yilmaz SG, Erdal ME, Ozge AA, Sungur MA. Can peripheral microRNA expression data serve as epigenomic (upstream) biomarkers of Alzheimer’s disease? OMICS. 2016;20(8):456–461. [DOI] [PubMed] [Google Scholar]
- 65. Banks WA, Erickson MA. The blood–brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37(1):26–32. [DOI] [PubMed] [Google Scholar]
- 66. Pusic AD, Pusic KM, Kraig RP. What are exosomes and how can they be used in multiple sclerosis therapy? Expert Rev Neurother. 2014;14(4):353–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang Q, Li P, Li A, et al. Plasma specific miRNAs as predictive biomarkers for diagnosis and prognosis of glioma. J Expl Clin Cancer Res. 2012;31(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhuang X, Xiang X, Grizzle W, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19(10):1769–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]