Abstract
Calcineurin inhibitors such as cyclosporine and tacrolimus are immunosuppressant drugs that are known to induce tremors. Non-calcineurin inhibitors such as sirolimus and everolimus have also reportedly been accompanied by tremors, albeit less likely. However, the prevalence rates reported in the literature are notably wide, and the risk profiles for these drug-induced tremors are less understood. We searched PubMed to extract data on the risk of tremors with these drugs when prescribed for various transplant and non-transplant indications. We ascertained whether the risk of drug-induced tremor is influenced by the underlying diagnosis, dosing formulations, drug concentrations, and blood monitoring. We extracted data on treatment strategies and outcomes for tremors. Articles were primarily screened based on English language publications, abstracts, and studies with n ≥ 5, which included case series, retrospective studies, case-controlled studies, and prospective studies. We found 81 eligible studies comprising 33 cyclosporine, 43 tacrolimus, 6 sirolimus, and 1 everolimus that discussed tremor as an adverse event. In the pooled analysis of studies with n > 100, the incidence of tremor was 17% with cyclosporine, 21.5% with tacrolimus, and 7.8% with sirolimus and everolimus together. Regarding the underlying diagnosis, tremor was more frequently reported in kidney transplant (cyclosporine 28%, tacrolimus 30.1%) and bone marrow transplant (cyclosporine 40%, tacrolimus 41.9%) patients compared with liver transplant (cyclosporine 9%, tacrolimus 11.5%) and nontransplant indications (cyclosporine 21.5%, tacrolimus 11.3%). Most studies did not report whether the risk of tremors correlated with drug concentrations in the blood. The prevalence of tremors when using the twice-daily formulation of tacrolimus was nearly the same as the once-daily formulation (17% vs 18%). Data on individual-level risk factors for tremors were lacking. Except for three studies that found some benefit to maintaining magnesium levels, there were minimal data on treatments and outcomes. A large body of data supports a substantive and wide prevalence of tremor resulting from tacrolimus use followed by cyclosporine, especially in patients receiving a kidney transplant. However, there is little reporting on the patient-related risk factors for tremor, risk relationship with drug concentrations, treatment strategies, and outcomes.
Key Points
| A fifth of patients receiving cyclosporine and tacrolimus for treating transplant and nontransplant indications can potentially develop a tremor. |
| Sirolimus and everolimus have a substantially lower risk of neurological toxicity, especially the risk of tremors. |
| Tacrolimus formulations do not seem to impact the risk of tremors. |
Introduction
Drug-induced tremor is a common clinical problem that requires specific criteria for establishing a diagnosis [1]. Tremor is often symmetric and nonprogressive and can reportedly affect any body part. In the history provided by patients, there is an apparent temporal relationship between the initiation of drug therapy and the onset of tremor symptoms. A dose–response relationship is commonly seen with worsening of tremor seen when the dose of the drug dosage is increased and improvement observed when the dosage is reduced. For establishing a diagnosis, it is important to exclude pertinent comorbidities such as hyperthyroidism and hypoglycemia.
Immunosuppressant drugs, including cyclosporine and tacrolimus, commonly lead to tremor as a side effect [2]. These drugs known as calcineurin inhibitors (CNIs) are widely employed to help prevent the rejection of transplanted organs and to treat autoimmune disorders such as inflammatory bowel disease, systemic lupus erythematosus, and rheumatoid arthritis. The neurotoxic potential with the use of these drugs is well known. Tacrolimus, a more potent immunosuppressant drug than cyclosporine, is associated with a greater risk of neurological side effects [3]. The earliest reports of tacrolimus-induced tremor emerged three decades ago when 36% of pediatric patients and 22% of adult patients undergoing orthotopic liver transplantation reported symptoms with drug therapy [4, 5]. Other immunosuppressant drugs such as sirolimus (rapamycin) [6] and everolimus [7] that became subsequently available were also found to potentially induce tremors, albeit at a lower risk. We reviewed the literature to ascertain the risk of tremors with these immunosuppressant drugs when employed for transplant and non-transplant indications. We sought to understand whether the risk of tremors varied with the specific clinical indication, patient characteristics, drug formulations (twice daily vs once daily), drug concentrations, and dosing patterns. We evaluated the strategies considered for mitigating tremors and the eventual outcome with the interventions. We also extracted data on co-occurring neurological side effects that could potentially have common pathophysiological pathways.
Methods
We conducted a scoping review of the literature using the PubMed database. We employed specific search terms comprising both controlled vocabulary words and synonymous free text for extracting data. These included “cyclosporine” [Mesh] OR “cyclosporine” [Title/Abstract] OR “calcineurin inhibitor” [Mesh] OR “calcineurin inhibitor” [Title/Abstract] AND “tremor”. We then applied a similar strategy to search literature for the remaining immunosuppressants of interest such as tacrolimus, sirolimus, and everolimus that had been previously associated with tremor. We included original peer-reviewed literature that was published only in the English language. While our literature search was not limited by study design, we focused on studies with n ≥ 5 thus including case series, retrospective studies, case-controlled studies, and prospective studies. Literature reporting on animal studies was excluded. Studies combining all neurological side effects arising from the use of these drugs such as headaches, seizures, and paresthesia into one category were excluded. The full texts of the abstracts that matched the topic were accessed. We shortlisted studies of interest by examining the abstracts of all included articles. We searched the bibliography of these articles and identified additional relevant articles. Studies with a patient cohort of less than five were excluded from the final analysis. Data from full-text review articles were extracted into a data extraction form. A team of reviewers (authors CL, OM, UK, NJ) extracted the data. Each study was assigned a primary reviewer and a secondary reviewer. We organized data by country, study design, enrollment, underlying medical indication, transplant organ if patient underwent transplant surgery, number of patients developing tremor with immunosuppressant drugs, time frame for tremor development, blood monitoring for drug concentrations, treatment and outcome for tremor, and neurological symptoms that were noted to co-occur as side effects. We defined the timeframe for tremor as early onset if the tremor presented within 6 months of drug administration or otherwise delayed or late onset. While the cut-off value of 6 months was somewhat arbitrary, it was based on a general clinical categorization of the condition as chronic when the duration is more than 6 months. These categorizations were intended to understand whether tremors developed as an acute dose-dependent consequence or resulted from chronic cumulative effects.
Statistical Analyses
We calculated the prevalence rates for tremor induced by each of the drugs as a range and for studies that had a sample size greater than 100 we calculated mean ± standard deviation.
Results
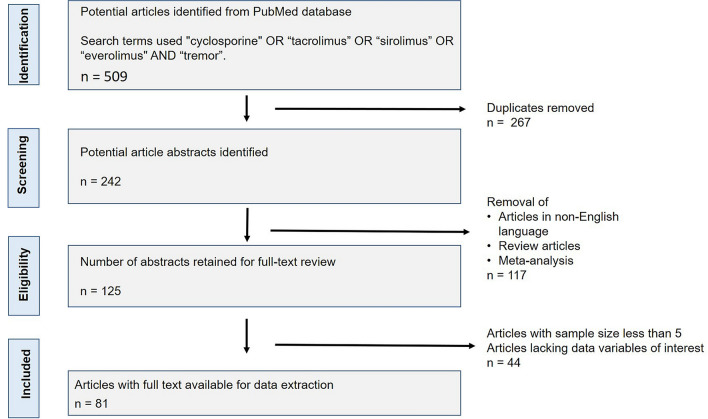
A total of 509 articles published until 2021 were extracted from the PubMed database. Out of these, 267 duplicates were identified and removed. A total of 242 abstracts were screened for the immunosuppressant drug of interest. Upon screening the abstracts, we found 125 articles that could be retained for a full-text review. We then removed the articles published in non-English language, review articles, meta-analyses, and studies with a small sample size (n < 5). Finally, 81 full-text articles were available for data extraction (Fig. 1).
Fig. 1.
Flow diagram following PRISMA (Preferred Reporting Items for Systematic review and Meta-Analyses) guidelines
Cyclosporine and Tremor
We found 33 studies reporting patients developing cyclosporine-induced tremor [8–40] (Table 1). The geographical distribution of data on cyclosporine-induced tremor was as follows: 13 studies from the Americas, 14 from the European Region, two from the Eastern Mediterranean Region, and one from the South-East Asian Region, while one study did not specify the region.
Table 1.
Study data on cyclosporine use and tremor
| Author(s) | Country | Study design | Study duration | Grafted organ/etiology | Cohort size (n) | Dosing protocol (mg/kg/day) | Target or mean trough in ng/mL | Tremor prevalence, n (%) | Early vs late | Treatment method/outcome | Other neurologic side effects |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Asćerić et al. [8] | Bosnia | Observational | 18 mo | Kidney | 30 | Oral: 2–5 mg/kg/day | 122.5–280.5 | 4 (7.5) | NS | NR | NR |
| Briggs et al. [9] | UK, Norway, Finland, Sweden, Germany | Prospective, open, multicenter, parallel-group | 3 mo | Kidney | 58 | Changed if blood trough concentrations >300 ng/mL. | 150–300 | 1 (1.7) | NS | NR | NR |
| Caccavo et al. [10] | Italy | Prospective, non-randomized | 24 mo | SLE | 30 | Oral 2.5–5 mg/kg/day reduced by 0.5 mg/kg/day if cr level increased by >30% baseline value | NR | 2 (7) | Late | Therapy withdrawn after 5 mo, ONR | Paresthesia, fatigue |
| Conti et al. [11] | Italy | Retrospective | N/A | SLE | 56 | Initial: oral 5 mg/kg/day maintenance: oral 3 mg/kg/day; dose reduction when tremor present | NR | 6 (5.4) | Early | Discontinuation (n = 3), resolved | Headache, paresthesia, insomnia, dizziness |
| David-Neto et al. [12] | Brazil | Observational | 4 h | Kidney | 46 | Mean dose: oral 6 ± 1.9 mg/kg/day; reduction of dose in 10 children with highest AUC values: 7.5 ± 2.5 to 4.8 ± 2.3 mg/kg per day; Cmax positively correlated with intensity of tremor | 203 ± 75 | 20 (43.5) | NS | Reduction of dosage; intensity of tremor diminished | NR |
| Dehghani et al. [13] | Turkey | Prospective | ~30 mo | Liver | 4 | Convulsions on CSA, converted to tacrolimus | NR | 1 (25) | NS | NR | Convulsions, insomnia, headache, muscle cramps, paresthesia, weakness |
| Erro et al. [14] | Italy | Observational | NR | Kidney | 32 | NR | Therapeutic range | 20 (62) | NS | NR | NR |
| Frank et al. [15] | USA | Prospective | NR | Liver | 29 | Continuous IV: 2–8 mg/mL adjusted to 250–350 mg/mL; changed to FK506 rescue therapy | 250–350 | 1 (3.4) | NS | NR | Headaches, seizures, sleep disorders |
| Hami et al. [16] | Iran | Observational | 6 mo | Kidney | 50 | Day 1 post-transplant: oral ~10 mg/kg subsequently: 5-mg/kg/day dose adjustment according to trough concentration | ~250–350 | 18 (36) | Early | NR | Headache |
| Kahan et al. [17] | USA | Clinical | 72 mo | Kidney | 402 | Living related donor: 5-day loading continuous IV cadaveric renal graft: pre-operation oral 14 mg/kg, IV 2.5 mg/kg | 100–250 | 79 (20) | Early and late | NR | Seizure, paresthesia |
| Mahdi et al. [18] | Canada | Prospective | NR | IBD | 10 | Initial: IV 4 mg/kg in two divided doses maintenance: 250–400 ug/L trough concentrations | 250–400 | 1 (0.1) | NS | Withdrawal resolved | NR |
| Martin et al. [19] | UK | Open-label | 18 mo | HTLV-1 Spastic paraparesis | 7 | Oral 2.5–5 mg/kg/day; dose adjusted trough concentrations | 80–100 | 1 (0.14) | Early | Treatment cessation; resolved | NR |
| Menegaux et al. [20] | USA | Retrospective | NR | Liver | 27 | Induction: 2–3 mg/kg/day, IV continuous infusion, moved to oral administration after digestive reactivation | 300–500 | 2 (7) | Early | Unclear | Encephalopathy seizure, stroke, brachial plexopathy, neuropathy headache, tremor, facial paralysis, spinal cord deficit, Parkinson’s disease |
| Miller et al. [21] | USA | Prospective | 40 mo | Bone marrow | 45 | Induction (day 1–22): IV 2.5 mg/kg/day maintenance: oral 6.0 mg/kg/day; modified to maintain therapeutic concentrations | 325–375 (RIA) | 16 (35) | NS | NR | Paresthesia, seizures, somnolence |
| Minuk et al. [22] | Canada | Prospective | 12 mo | Liver | 12 | Induction: oral 2.5 mg/kg/day maintenance: oral adjusted to fit therapeutic concentrations | Serum: 100–200 | 2 (16.7) | Late | No treatment; ONR | Headaches, paresthesia |
| Munhoz et al. [23] | Brazil | Observational | 7 mo | Bone marrow | 60 | Maintenance oral 5 mg/kg/day | NR | 13 (21.7) | Early | NR | NR |
| Navazo et al. [24] | Spain | Non-randomized | ~12–36 mo | IBD | 11 | Induction: oral 7–7.5 mg/kg/day | Plasma induction: 250–300 ng/mL | 7 (63.6) | Early | Oral magnesium supplement control of tremor | Headache |
| Neuhaus et al. [25] | Germany, UK, France, Sweden | Open, randomized, parallel-group | 22 mo | Liver | 273 | Induction: IV 1–6 mg/kg⋅BW/day Maintenance: oral 8–15 mg/kg⋅BW/day | NR | 27 (9.9) | Early | NR | Coma, somnolence, encephalopathy, convulsion, dysarthria, psychosis, confusion, delirium, paranoid reaction, insomnia, depression, headache, neuropathy |
| Pescovitz et al. [26] | USA | Randomized, prospective, open-label, multicenter | 6 mo | Kidney | 15 | Baseline oral 487.5 mg/month: 3–6 mo ~300 mg | 224–347 ng/mL | 7 (46.7) | Late | NR | Headache, insomnia |
| Pirsch et al. [27] | USA | Randomized, open-label | 21 mo | Kidney | 207 | Induction: oral 5.0 mg/kg BW/day maintenance: dependent on target trough |
Induction blood: 150–400 Maintenance blood: 100–300 |
70 (33.8) | Early | Dose reduction, ONR | Headache, insomnia, paresthesia, dizziness, anxiety |
| Pistoia et al. [28] | Italy | Prospective | NR | Connective tissue, disorder | 9 | 3–10 mg/kg/day maintenance: 3–5 mg/kg/day tapering to maintenance dosage | NR | 1 (11.1) | NS | Temporary tapering; control of tremor | NR |
| Schmidt et al. [29] | NR | Retrospective | NR | Bone marrow | 51 | 2–5 mg/kg/day 30% dose reduction if bilirubin or cr started to rise | Plasma concentration >250 ng/mL to reduce dose severity | 51 (NR) | Early | NR | NR |
| Sheth et al. [30] | Canada | Prospective | 3 mo | Liver, kidney | 10 | NR | NR | 3 (30) | Early | NR | Headache |
| Sood et al. [31] | India | Retrospective, cross-sectional | 12–62 mo | IBD | 24 | Induction: IV 4 mg/kg/day Maintenance: oral, microemulsion 4 mg/kg/day | NR | 9 (37.5) | Early | NR | Paresthesia, headache, peripheral neuropathy |
| Stack et al. [32] | UK | Clinical | ~60 mo | IBD | 22 | Induction: 4 mg/kg/day Maintenance: 6 mg/kg/day | Serum: 100–200 | 4 (18) | Early | NR | Headaches, paresthesia |
| Stange et al. [33] | Germany | Clinical | 10 wk | IBD | 13 | Maintenance: oral 25–600 mg, twice daily | Blood: 200–800 | 4 (30.8) | Early | NR | Hyperesthesia |
| Steinsson et al. [34] | Iceland | Controlled, multicenter | 18 mo | Psoriatic arthritis | 8 | induction: oral 3.5 mg/kg/day | Blood: <500 | 1 (12.5) | Early | Discontinue | NR |
| Thompson et al. [35] | USA | Retrospective | NR | Bone marrow | 12 | IV 3 mg/kg/day or oral 12.5 mg/kg/day; dosage held or reduced with seizure | 200–400 | 2 (18.2) | Mid | Withholding drug and replacing magnesium | Seizures, ataxia, depression, transient aphasia |
| Tolou-Ghamari et al. [36] | Iran | Population-based | NR | Kidney | 75 | Dose unspecified, but oral and twice daily | 16.5–1261 | 35 (46.7) | NS | NR | Headache, anxiety |
| Trocha et al. [37] | Germany | Prospective | NR | Liver | 14 | Group 1: oral 100–325 mg group 2: unspecified | NR | 7 (50) | Early | NR | Anarthria, dysarthria, seizures, confusion, coma, polyneuropathy |
| Wakefield and McCluskey [38] | Australia | Open, uncontrolled | 48 mo | SLE | 22 | Initial: oral 10 mg/kg/day modified to reach 5 mg/kg/day dosage below led to relapse | NR | 6 (27.3) | Early and late | NR | NR |
| Wakefield and McCluskey [39] | Australia | Open, uncontrolled | NR | SLE | 7 | Induction, oral 10 mg/kg/day; maintenance oral 5 mg/kg/day | NR | 5 (71.4) | Late | NR | Facial pain |
| Wijdicks et al. [40] | USA | Retrospective | NR | Liver | 227 |
Induction: 10-mg/kg/day IV emulsion maintenance: oral dose modified to therapeutic goal timeline |
NR | 13 (5.7) | Early and late | Dose reduction, persistence (n = 1) Amelioration (n = 12) | Pain, headaches, paresthesia, seizures, sleep difficulty, leg cramps |
AUC area under the curve, BW body weight, cr creatinine, Cmax maximum concentration, CSA cyclosporine, h hours, IBD inflammatory bowel disease, IV intravenous, mo months, N/A not applicable, NR not reported, NS not specified, ONR outcome not reported, SLE systemic lupus erythematosus, wk weeks
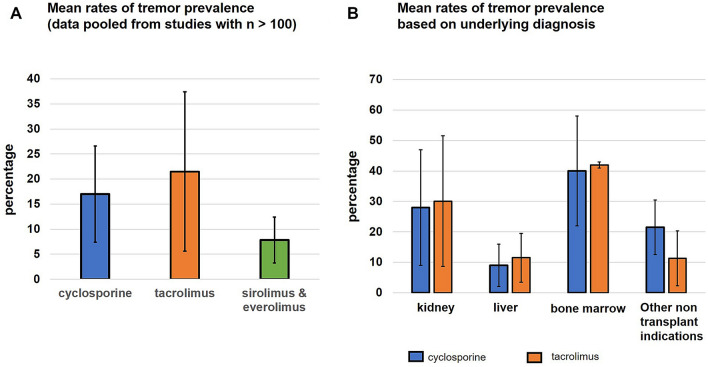
Data were extracted from mostly prospectively conducted studies. Although the prevalence of cyclosporine-induced tremors in all studies taken together appeared to be wide (range 0.1–71.4%), the assessment of prevalence in larger cohorts with n > 100 (Fig. 2A) was determined to be mean ± standard deviation 17.1 ± 6.3% (189/1109 patients; five studies). Most studies did not report the time frame of tremor development (n = 18). A total of 11 studies reported an early-onset tremor, while one study reported late-onset chronic development of tremors. Three studies reported patients developing both early-onset and late-onset tremors. One study with a large number of kidney transplant patients (n = 402) found 20% of participants developed tremor between 1 and 3 months, 12% between 4 and 6 months, and 10% between 7 and 12 months [17].
Fig. 2.
A Bar graph. The colored bars represent the mean prevalence rate (in percentage) for tremors induced by cyclosporine, tacrolimus, and non-calcineurin inhibitor drugs (sirolimus and everolimus). Data were combined for studies that had a large n of > 100. The black whisker lines represent the standard deviations for these mean rates. B Bar graph. The colored bars represent the mean prevalence rate (in percentage) for tremor induced by cyclosporine and tacrolimus for kidney transplant, liver transplant, bone marrow transplant, and non-transplant indications. The black whisker lines represent the standard deviations for these mean rates
Most studies reported the risk of tremor in the context of kidney (n = 9) and liver (n = 7) transplantation, with one study including patients with both kidney and liver transplantation (n = 1). Tremor was also reported in the context of inflammatory bowel disease (n = 5), autoimmune/connective tissue diseases (n = 4), bone marrow transplantation (n = 4), autoimmune disorder of the eye (n = 2), and chronic disease of the spinal cord (n = 1). The risk of cyclosporine-induced tremor was more likely in patients with kidney (28 ± 16.1%) and bone marrow transplants (40 ± 14.7%) compared with liver transplants (9 ± 7.7%). When combining data for nontransplant indications, a prevalence of 21.5 ± 9.7% was noted (Fig. 2B).
Most studies reported their target or mean for monitoring the serum and plasma concentrations of cyclosporine (Table 1). However, data on longitudinal monitoring of drug concentrations and, more importantly, the correlation of blood concentrations with tremor intensity were lacking. One observational study with pediatric renal transplant recipients found that the area under the curve correlated positively with the intensity of tremors [12].
Some studies reported co-occurring neurological side effects such as headaches (n = 14), paresthesia/hypoesthesia/hyperesthesia (n = 14), seizures (n = 8), and insomnia/sleep disorders (n = 5). The more serious neurotoxic adverse effects such as encephalopathy (n = 2) and coma (n = 2) were reported by a small number of studies. Occasionally, studies reported co-occurring ataxia (n = 1) and parkinsonism (n = 1).
Treatments for tremors varied, and ranged from no treatment specified (n = 21), tapering to eventual discontinuation of the drug (n = 6), change to a lower dosage (n = 5), or supplementation with magnesium (n = 2). None of the studies reported data on potential risk factors and patient demographics for developing tremors.
Tacrolimus and Tremor
Studies reporting tacrolimus-induced tremor listed in Table 2 [4, 5, 9, 13–15, 20, 25, 27, 37, 41–73] mainly comprised kidney (n = 18) and liver transplantation (n = 17), with the rest including bowel-related diseases (inflammatory bowel disease, n = 7), heart transplant (n = 2), rheumatoid arthritis (n = 1), and bone marrow transplant (n = 1). Reports of tremor was geographically distributed as follows: 20 studies from the Americas, 13 from Europe, eight from the Western-Pacific Region, and two from the Eastern-Mediterranean Region.
Table 2.
Study data on tacrolimus use and tremor
| Author(s) | Country/continent | Design | Study duration | Graft organ/etiology | Cohort size (n) | Dosing protocol | Target or mean trough in ng/mL | Tremor (n) | Treatment method and outcome | Other neurological side effects |
|---|---|---|---|---|---|---|---|---|---|---|
| Alissa et al. [41] | Saudi Arabia | Retrospective | NS | Liver | 338 | Day 1: 0.05– 0.1 mg/kg/day every 12 h; maintenance: 7–10 ng/mL within first 3 mo; dose reduction or switch to CSA if intolerance | 10 July | 6 (10%) | Switching to CSA (n = 4); reduction in dose (n = 2) | Seizures, psychosis, agitation, tremors, confusion, twitching, behavioral changes |
| Alloway et al. [42] | USA | Prospective, 3-sequence, open-label, multicenter | 12 mo | Liver | Immediate release: 59; ER-Tac: 59; extension phase ER-Tac: 49 | Tacrolimus: oral, twice daily, fixed-dose at investigator discretion; ER-Tac: oral, once daily, fixed-dose at investigator discretion; extension phase ER-Tac: oral, remained on Tac for an additional 50 wk | Blood: 5–15 ER-Tac and Tac | Tac: 1 (1.7%); ER-Tac: 4 (6.8%); extension phase; ER-Tac: 1 (2%) | NR | Dizziness, fatigue, headache, insomnia, back pain |
| Baumgart et al. [43] | Germany | Retrospective, observational single-center | 12 mo | IBD | 31 | Oral 0.1 mg/kg/day, divided into two doses (n = 37) IV 0.01 mg/kg/day (n = 1) | Serum: 4–6 | 3 (9.7%) | Dose tapering ONR | Paresthesia |
| Baumgart et al. [44] | Germany | Retrospective observational single-center | ~2 y | IBD | 53 | Oral 0.1 mg/kg⋅ BW/day, in 2 divided doses (n = 51); induction IV 0.01 mg/kg BW/day (n = 2) | Serum: 4–8 | 5 (9.4%) | NR | Paresthesia |
| Boschetti et al. [45] | France | Retrospective | 12 mo | IBD | 30 | Induction (12 wk) oral 0.15–0.15 mg/kg BW, 2× day; maintenance: adjusted to achieve target trough concentrations | Induction serum: 10–15; maintenance serum: 5–10 | 6 (20%) | Med stopped (n = 3), ONR | Headache |
| Briggs et al. [9] | Europe | Prospective, open, multicenter, parallel-group | 3 mo | Kidney | 61 | Induction: oral 0.2 mg/kg, divided into two doses; maintenance: oral, adjusted to achieve target trough concentrations | Blood: 10–20 | 10 (16.4%) | NR | NR |
| Bulatova et al. [46] | Jordan | Multicenter, cross-sectional, observational | 16 mo | Kidney | 154 | Oral and personalized doses to achieve therapeutic concentrations | Blood: 4–7 | 61 (40%) | NR | Seizures, headache, paresthesia, sleep disturbance, asthenia, dizziness |
| Burkhalter et al. [47] | USA | Prospective | 5.6 mo | Liver | 100 | Induction IV 0.075–1 mg/kg BW; maintenance: oral 0.3 mg/kg/day; modified to achieve target concentrations or prevent graft rejection and hepatic or renal dysfunction | Serum: 0.5–1.5 | 1 (1%) | Discontinuation but tremor persisted | Central pontine myelinolysis coma, delirium, dysarthria, brain abscess, TIA |
| Chand et al. [48] | USA | Retrospective | 39 mo | Kidney, heart | 22 | Total: oral 0.1–0.3 mg/kg/day, in two doses; maintain therapeutic concentrations or in response to complications such as post-transplant lymphoproliferative disease | Induction: 15–25; maintenance: 5–15 | 1 (9%) | NR, remission of symptoms | Headache |
| Chen et al. [49] | China | Prospective, multicenter clinical | 24 mo | Kidney | 14 | Induction: oral 0.05 mg/kg/day mean dose; (1–6 mo): oral 5.4 ± 1.7 mg/day mean dose; (6–12 mo): oral 3.8 ± 1.3 mg/day | Induction blood: 5–10 maintenance blood: 4–6 | 1 (7.1%) | Reduction in dosage, remission | NR |
| Choi et al. [50] | USA | Retrospective | 10.9 mo | Liver | 25 | IR-Tac: oral 4 mg, daily; LCP-Tac: oral 3 mg, daily; modified according to target level designations | Monotherapy: month 0–1 blood: 8–10; month 1– 6 blood: 5–8; maintenance blood: 3–5 | 6 (32%) | Switch to LCP-Tac formulation, 88% tremor improved | NR |
| De Simone et al. [51] | Italy | Single-center, retrospective | ~45 mo | Liver | 178 | Oral, dose not reported; adjusted for participant needs and to attain desired trough concentrations | Blood: 6–15 | 32 (17.9%) | NS | Headache |
| Dehghani et al. [13] | Turkey | Single-center, observational | ~36 mo | Liver | 44 | NR | Mean trough: 12.4 ± 6.3 | 6 (13.6%) | Dose reduction, ONR | Headache, insomnia, weakness, convulsion paresthesia, muscle cramps, dizziness |
| DuBay et al. [52] | USA | Open-label, multicenter, randomized phase II | 12 mo | Liver | ER-Tac: 29; IR-Tac: 29 | ER-Tac: oral 0.07–0.13 mg/kg/day, once daily; IR-Tac: oral 0.10–0.15, divided 2× day | Induction blood: 5–20; maintenance blood: 5–15 | ER-Tac: 8 (27.6%); IR-Tac: 10 (34.5%) | NS | Headache, back pain, nausea, insomnia |
| Eidelman et al. [53] | USA | Group 1: retrospective chart review; Group 2: prospective; Group 3: surveillance | 2 mo | Liver, lungs, heart, kidney | Group 1: 23; group 2: 294; group 3: 83 | Induction (IV 0.075 mg/kg 4-h infusion per 12 h; modified to IV 0.15-mg/kg continuous infusion; maintenance: oral, 0.15 mg/kg per 12 h | Plasma: 2.5 Plasma mean: 3.4 ± 3 | Group 1: 2; group 2: reported, NS; group 3: NR (overall, 8.7%) | Resolved following treatment discontinuation | Akinetic mutism, aphasia, dysarthria, dysesthesia, encephalopathy, headache, mood disturbances, seizures, sleep and visual disturbances |
| Erro et al. [14] | Italy | Prospective | transplant patients between February 1988 and August 2016; clinical exam day | Kidney | 67 | Oral, dose NR | Within therapeutic range; NR observed those with tremor did show higher plasma concentrations than those without | 55 (82%) | NS | Mild cerebellar and neuropathic symptoms |
| Fan et al. [54] | China | Prospective, multicenter clinical | 24 mo | Kidney | 24 | Induction: oral 0.05 mg/kg/d, 2× day at 12-h intervals | Induction blood: 5–10; maintenance blood: 4–6 | 2 (8.3%) | Reduction in dosage, remission | NR |
| Frank et al. [15] | USA | Prospective | NS | Liver | Group 1 (rescue): 20; group 2 (prophy):7 | Oral, 0.3 mg/kg/day, in 2 divided doses; based on renal function or possible rejection | NR, plasma was utilized | Group 1: 4 (20%) group 2: 2 (28%) | Dose reduction, remission | Headache, seizures, sleep disorders, focal white and gray matter lesions, necrotizing angiopathy |
| January et al. [55] | USA | Single-center, retrospective | ~24 mo | Kidney | LCP-Tac: 84; IR-Tac: 42 | LCP-Tac: oral 0.08 mg/kg, once daily; IR-Tac: oral 0.1 mg/kg once daily; maintain therapeutic concentrations | Induction blood: 7–10; maintenance blood: 3–7 | 9 (7%) | NS | NS |
| Karasawa et al. [56] | Japan | Retrospective | 60 mo | Kidney | 26 | Induction: oral 3 mg/day, evening administration | Blood: <8 ng/mL | 3 (11.5%) | Reduction in dosage, remission | Facial nerve paralysis, trigeminal neuralgia |
| Katari et al. [57] | USA | Observational | 48 mo | Kidney | 22 | Induction: IV 0.075–0.1 mg/kg/day; maintenance: oral 0.30 mg/kg/day | Blood: 5–20; plasma: 0.5–1.5 | 2 (9%) | NS | NS |
| Langone et al. [58] | USA | 2-sequence, open-label, prospective phase IIIb, multicenter, clinical | ~15 mo | Kidney | 38 | Dose personalized, conversion to once-daily ER-Tac from twice-daily capsule therapy, used 0.70 conversion for non-black and 0.85 for black participants | Blood: 3–12 | All had tremor prior to conversion | Formulation adjustment (capsule to ER-Tac), amelioration of tremor severity | NS |
| Menegaux,et al. [20] | USA | Retrospective | Unclear | Liver | 64 adult 10 peds | NS | Whole blood: <20 | 4 adults 1 peds (7%) | Dosage reduction or remission | Encephalopathy, seizure, stroke, peripheral neuropathy headache, facial paralysis, spinal cord deficit, Parkinson's disease |
| Mok et al. [59] | China | Prospective | 12 mo | Kidney | 21 | Oral 4 mg/day, in two divided doses | NR | 1 (3%) | NS | Facial twitching, dyspepsia, cramps |
| Neu et al. [60] | USA | Retrospective | ~27 mo | Kidney | 14 | Initial: oral 0.03 mg/kg/d; modified to achieve target trough concentrations | Blood: 5–12 | 7 (50%) | NS | Seizures myalgias, fatigue, hyperesthesia, headache, insomnia |
| Neuhaus et al. [25] | Europe | Open, randomized, parallel-group | 22 mo | Liver | 267 | Induction: 0.075 mg/kg BW IV infusion over 4 h, repeated every 12 h; maintenance: oral 0.3 mg/kg BW/day | NS | 43 (16.1%) | NR | Coma, somnolence, encephalopathy, convulsion, aphasia, psychosis, confusion, delirium, insomnia, depression, headache, neuropathy |
| Pirsch et al. [27] | USA | Randomized, open-label | 21 mo | Kidney | 205 | Induction: oral 0.1 mg/kg BW/day; maintenance: oral and dependent on individual |
Induction blood: 1 0–25; maintenance blood: 5–15 |
111 (54.1) | Dose reduction, ONR | Headache, insomnia, paresthesia, dizziness, anxiety |
| Reding et al. [61] | Belgium | Prospective, clinical | 23.9 mo | Liver | 23 | Postoperative (n = 3): 0.14–0.16 mg/kg/day for 4–19 days, transitioned to 0.2–0.33 mg/kg/day postoperative maintenance (n = 20): 0.2–0.33 mg/kg/day | Plasma: 0.1–1 | 1 (4.4%) | NR | Myalgia, coma, depression seizures |
| Riva et al. [62] | Argentina | Retrospective, single-center cohort | 30 mo | Liver | 72 | Oral 0.1 mg/kg/day, adjusted for trough concentrations | Month (1–6), blood: 7–8; month (6–12), blood: 5–7; maintenance blood: 5 | 1 (1.4%) | NR | NR |
| Rostaing et al. [63] | USA | Randomized, double-blind, double-dummy, multicenter phase III | 24 mo | Kidney | ER-Tac: 268; IR-Tac: 275 | ER-Tac: oral 0.17 mg/kg/day, once daily; IR-Tac: oral 0.1 mg/kg/day, 2× day; modified to maintain target trough concentrations |
Induction blood: 6–11; maintenance blood: 4–11 |
ER-Tac: 59 (22%); IR-Tac: 51 (18.5%) | Discontinuation and ONS | NR |
| Sánchez Fructuoso et al. [64] | Spain | Multicenter, retrospective, single-cohort conversion | 5 mo | Kidney | 365 | NS | Blood: 7–8.4 | Pre-conversion: 76 (23%); post-conversion: 43 (11.8%) | Switch to ER formulation, improvement in symptoms | Headache, concentration issues, insomnia |
| Tamaki et al. [65] | Japan | Clinical | 3.9 mo | IBD | 14 | 0.01–0.02 mg/kg/day (n = 3), days 1–14: IV, oral for remaining; oral 0.1–0.2 mg/kg/day (n = 11) | Induction serum: 10–15; maintenance serum: 5–10 | 1 (7%) | Dose reduction, remission | Paresthesia |
| Thorp et al. [66] | USA | Prospective clinical | 12 mo | Kidney | 16 | Oral 0.1 mg/kg, 2× day adjusted based on blood trough concentrations taken during first week; considered stable following 3 measurements in target range | Blood: 5–20 | 1 (6.25%) | No treatment, tremor reported as well tolerated | Headaches |
| Trocha et al. [37] | Germany | Prospective cohort | ~30 mo | Liver | 23 | Group 1 + 2 induction: 0.05–0.30 mg/kg BW 2× day; group 1 maintenance: 6–9 mg/day; group 2 maintenance: altered to maintain target plasma concentrations at investigator discretion | Plasma 0.3–3.0 | 14 (77.8%) | Dose reduction, amelioration of tremor | Anarthria, dysarthria, confusion apathy, coma, polyneuropathy |
| Truffinet et al. [67] | France | Retrospective | NS | IBD | 8 | Induction: oral 0.05–0.2 mg/kg, 12 h; maintenance: oral, NR | Induction blood: 8–15; maintenance blood: 5–10 | 1 (12.5%) | Discontinuation of treatment, ONR | Paresthesia |
| Turunc et al. [68] | Turkey | Single-center, retrospective | 6 mo | Kidney | ER-Tac: 52; IR-Tac 63 | Day 2 (pre-transplantation) ER-Tac: oral 0.2 mg/kg/day; day 2 (pre-transplantation) IR-Tac: oral 0.15 mg/kg/day; lowered over the first month following achievement of target concentrations | Blood (day 1–30): 8–12; blood (day 31–180): 4–11; blood (day 180–end): 6–8 | ER-Tac: 4 (7.6%); IR-Tac: 9 (14.3%) | NS | NR |
| Uemoto et al. [5] | Japan | Prospective, clinical | ~33 mo | Liver | 61 | Postoperative: IV 0.06-mg/kg infusion, postoperative: concurrent oral 0.15 mg/kg per 12 h and above infusion | Blood: 10–20 | 12 (23%) | NR | Convulsions |
| Uemoto et al. [69] | Japan | Prospective, clinical | ~30 mo | Liver | 22 |
Postoperative (when using a rescue immunosuppressant drug, n = 14): IV 0.075-mg/kg infusion per 12 h, overlapping oral 0.15 mg/kg during transition from IV to oral, postoperative (n = 8): 0.03 mg/kg per 12 h |
Plasma: 0.15–0.50, blood: 10–30 | 8 (36%) | Considered manageable; no treatment required, ONR | Insomnia |
| Watson et al. [70] | USA | Retrospective chart review | NS | IBD | 46 | Oral 0.1 mg twice/day; adjusted to yield target trough concentrations | Induction blood: 10–15; maintenance blood: 5–10 | 21 (46%) | Weaning/cessation of treatment, spontaneously resolved/resolved with other treatments | Headache, seizure |
| Wijdicks et al. [4] | USA | Group 1: comparative; Group 2: open-label, multicenter, randomized parallel comparison | 44 mo | Liver | Group 1: 21; group 2: 23 | Postoperative (group 1): 0.075 mg/kg/6 h continuous IV infusion; maintenance (group 1): oral 0.15 mg/kg/12 h; maintenance (group 2, high dose): 0.03 mg/kg/12 h IV; continuous infusion or oral 0.075 mg/kg/12 h | Blood: <60, Plasma: <5 | 10 (4.4%) | Dose reduction, remission (n = 7), persistence (n = 3) | Psychosis temporary apraxia, pseudobulbar affect, suicidal behavior, tonic-clonic generalized seizures |
| Yamamoto et al. [71] | Japan | Retrospective, observational, single-center | Unclear | IBD | 27 | Induction (n = 4): 0.01 mg/kg BW IV infusion then moved to oral formulation, maintenance (n = 23): oral 0.1 mg/kg BW |
Induction blood: 10–15; maintenance blood: 5–10 |
7 (25.9%) | NR | Headache |
| Yanik et al. [72] | USA | Retrospective chart review | ~6 mo | BM | Related: 11; unrelated: 20 | Inpatient: 0.03 mg/kg/day continuous IV infusion; outpatient: oral, quadruple IV-calculated dose until no evidence of GVHD, tapered by 25% per month until discontinued | Serum: 5–15 | 13 (32%) | Magnesium supplementation, remission attributed to hypomagnesemia development (n = 7), tremor persistence (n = 6) | Focal complex seizure |
| Yocum et al. [73] | USA | Open-label, long-term safety | ~12 mo | RA | 896 | Oral, 3 mg/day | Blood: 2–3 | 81 (9%) | Withdrawal from study (n = 14), ONS | Asthenia, back pain, dizziness, headache |
BM bone marrow, cr creatinine, CSA cyclosporine, ER extended release, GVHD graft-vs-host disease, h hours, IBD inflammatory bowel disease, IV intravenous, med medication, mo months, N/A not applicable, NR not reported, NS not specified, ONR outcome not reported, peds pediatric age group, RA rheumatoid arthritis, SLE systemic lupus erythematosus, TIA transient ischemic attack, wk weeks
Although many studies involved a prospective design, a wide range (0.1–71.4%) for tremor was reported. Data extracted from larger cohorts (n > 100) revealed a frequency of 21.5 ± 8.4% (490/2276; 12 studies) for tremor (Fig. 2A). In one of the largest studies by Yocum et al. (n > 800), the prevalence of tremor was observed to be lower (9.1%) [73]. The risk of tremor in nontransplant indications was about 11.3 ± 9.7% (Fig. 2B). While most studies did not report the time frame, some specified the tremor as early onset (n = 10) and some reported as late onset (n = 4). Three studies reported patients with tremors in both settings. Most studies reported the risk of tremor in the context of kidney (n = 18) and liver (n = 17) transplantation, with one study including patients with both kidney and liver transplantation (n = 1). Tremor was also reported in the context of inflammatory bowel disease (n = 7) and autoimmune/connective tissue diseases (n = 1). The risk of tacrolimus-induced tremor was higher in patients with kidney transplants (30.1 ± 19.7%) and bone marrow transplants (41.9 ± 1.7%) compared with patients with liver transplants (11.5 ± 9.8%).
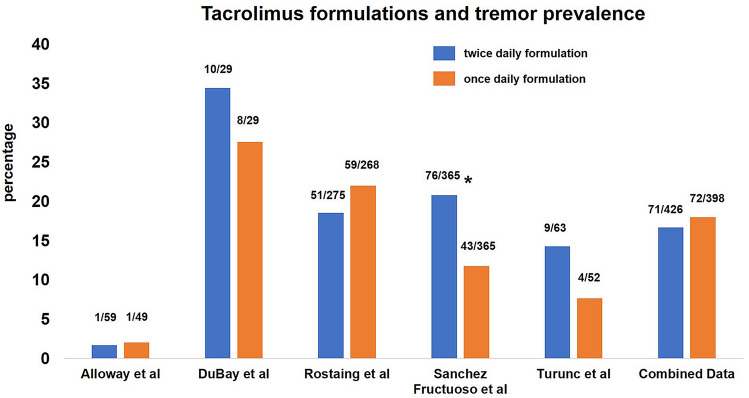
Similar to cyclosporine, the majority of the studies employed plasma monitoring of drug concentrations for therapeutic dosing (Table 2); however, longitudinal monitoring and, more importantly, data on whether blood concentrations correlated with tremor intensity were lacking. A few studies compared the risk of tremor with twice-daily/immediate-release versus once-daily/prolonged or extended-release tacrolimus [42, 52, 63, 64, 68]. The incidence of tremor with the twice-daily formulation had a range of 1.7–34.5% compared with the once-daily formulation range of 2–27.5% (Fig. 3). In one study, tremor was less likely to develop when converted to a once-daily extended-release formulation that was prepared with Meltdose technology (improved tacrolimus bioavailability) [64]. Similarly, in another study, conversion to extended-release tacrolimus led to a reduction in tremor intensity [58]. However, several other studies did not observe a statistically significant difference in the incidence of tremors between twice-daily immediate-release and once-daily extended-release formulations [42, 52, 63]. Some studies reported co-occurring neurological side effects such as headaches (n = 16), seizures (n = 12), paresthesia/hypoesthesia/hyperesthesia (n = 9), and insomnia/sleep disorders (n = 8). A small number of studies reported more serious neurotoxic effects, such as encephalopathy (n = 3), visual disturbances (n = 2), and coma (n = 5). Occasionally studies reported co-occurring ataxia (n = 1) and parkinsonism (n = 1).
Fig. 3.
Bar graph. The colored bars represent the mean prevalence rate (in percentage) for tremors induced by twice-daily and once-daily formulations of tacrolimus. The x-axis includes the various studies that report tremors occurring using these two formulations. The numbers above the bar represent the number of patients out of the total cohort size in individual studies that developed a tremor. Sanchez Fructuoso et al. included and compared data for immediate and prolonged release in one group versus extended release (Meltdose technology) in the other group. The asterisk indicates that the blue bar for the twice-daily formulation also includes data for patients receiving the prolonged-release formulation
None of the studies presented data on patient demographics and potential risk factors for tremors. Many studies also did not specify whether a treatment for tremor was employed (n = 20). Of the studies that described the various strategies for mitigating tremor, we found some changed to a lower dosage (employed by n = 11 studies), one study switched patients to another immunosuppressant drug such as cyclosporine, some studies used longer acting tacrolimus (n = 5), tapered tacrolimus to eventual discontinuation (n = 3), and supplemented with magnesium (n = 1).
Sirolimus and Everolimus, and Tremor
While multi-drug therapy for immunosuppression means that these drugs were frequently co-administered with CNIs, a few studies have reported the occurrence of tremor in the context of sirolimus alone, without the presence of a CNI (n = 5) [14, 26, 74–76] (Table 3). The geographical distribution of reports on sirolimus-induced tremor was as follows: three studies from the Americas, one from Europe, and one from the Western Pacific regions. These studies primarily included patients with a kidney transplant; one study included patients with heart and liver transplants. When combining data from all studies, tremors were seen in 22 out of 402 patients, with an average of 12.9% (range 0.48–44%). In the post-marketing surveillance from Korea (n = 209, largest), the prevalence of tremor was 0.48%. While there was no comment on the timeframe of tremor development (n = 3), tremor was reported as early onset by one study and late onset by one study. Two studies reported specific trough concentrations, and one described trough concentrations as within the therapeutic range. No treatment was provided for tremors (n = 3), and the tremors were described as self-limiting (n = 2). One study reported headaches, insomnia, and fatigue and another study reported depression, polyneuropathy, transient ischemic attacks, seizures, and stroke to co-occur with tremor.
Table 3.
Study data on sirolimus or everolimus use and tremor
| Author(s) | Country | Study design | Study duration | Grafted organ/etiology | Cohort size (n) | Dosing protocol | Target or mean trough in ng/mL | Tremor prevalence n (%) | Treatment method/outcome | Other neurologic side effects |
|---|---|---|---|---|---|---|---|---|---|---|
| Jeon et al. [74] | Korea | Prospective, open-label, non-comparative, observational post-marketing surveillance | 6 mo | Kidney | 209 | 1.8 ± 0.7 | Immunoassay: 5.5 ± 2.6, HPLC: 7.8 ± 4.2 | 1 (0.48%) | No treatment, defined as self-limited | NR |
| Chinnock et al. [75] | USA | Retrospective | NS | Kidney | 20 | NR | 8 May | 1 (5%) | No treatment, self-limited, symptoms resolved at conclusion of study | NR |
| Erro et al. [14] | Italy | Prospective | NS | Kidney | 27 | NR | Assumed to be within therapeutic range | 12 (44%) | NR | NR |
| Pescovitz et al. [26] | USA | Prospective, randomized, open-label, multicenter | 0.5 mo | Kidney | 30 | Induction (days 1–3): 15 mg/day, oral solution; maintenance (day 4–end): oral 10-mg/day tablets; to reduce drug-related toxicity while maintaining immunosuppression, at investigator discretion | Induction blood: 10–25; maintenance blood: 8–15 | 3 (10%) | NR | Headache, insomnia, fatigue |
| Van de Beek et al. [76] | USA | Retrospective | NS follow-up was ~6 y | Heart, liver, kidney | 313 | NR | NR | 5 (5%) | No treatment, ONR | Depression, polyneuropathy TIAs, seizures, stroke |
| Tedesco-Silva et al. [77] | 42 countries | Randomized open-label, 2-arm | 1 y | Kidney | 1014 | Adjusted for target trough concentrations | Blood: 3–8 | 98 (9.7%) | Dose adjustment, ONR | Insomnia |
HPLC high-performance liquid chromatography, mo months, NR not reported, NS not specified, ONR outcome not reported, TIA transient ischemic attack, y years
Tremor related to the use of everolimus was reported in a recent study in the setting of a kidney transplant. Unlike sirolimus, everolimus was administered in conjunction with low-dose CNI therapy. The tremor prevalence was noted to be about 9.7% among 1014 patients across 42 countries [77]. Dose adjustment with everolimus was observed to reduce the intensity of tremor.
Discussion
In the current review of the literature, we found that the prevalence of tremor with the use of tacrolimus was only modestly higher than with cyclosporine. A few studies found tremors also developed using sirolimus and everolimus. Tremors appeared more frequently when immunosuppressant drugs were prescribed for transplant versus nontransplant indications. Tremors more likely developed in patients with kidney and bone marrow transplants than in patients with liver transplants.
Calcineurin Inhibitors (Cyclosporine and Tacrolimus)
In the early 1960s, azathioprine and prednisolone were the main immunosuppressive agents used after solid organ transplant surgery [3]. However, these drugs were associated with a 50% rate of acute rejection leading to reduced allograft survival [78]. Cyclosporine was introduced in 1982 and the drug with potent immunosuppression properties worked as a CNI. With availability of this drug, the incidence of acute rejection after solid organ transplant surgery significantly dropped to 25% [79]. Furthermore, there were substantial improvements in the rates of allograft survival [80] but 20–39% of patients were observed to develop tremor [81]. The neurotoxicity risk with cyclosporine in bone marrow transplant recipients was found to be as low (4.2–28.8%) [82, 83]. A few years later in 1987, tacrolimus, another CNI agent, was introduced for immunosuppression purposes [84]. Compared with cyclosporine, tacrolimus was 100 times more potent, thus leading to further lowering of the incidence of acute rejection and improvement of graft survival rates. Tacrolimus became an integral part of induction and maintenance immunosuppression therapy. Currently, more than 90% of solid organ transplant recipients are discharged with a CNI‐based immunosuppressive regimen and the majority of them receive tacrolimus instead of cyclosporine [3, 85, 86]. Some studies compared the safety and efficacy profiles of the two drugs. In a phase III US multicenter trial, at the 1-year follow-up after transplant surgery, there was a significantly low incidence of acute rejection in the tacrolimus group (30.7%) versus the cyclosporine group (46.4%). At 5 years, the rate of patients with serum creatinine levels >150 μg/L was lower in the tacrolimus group (40.4%) versus the cyclosporine group (62%), but tremor and paresthesia were more common in the tacrolimus group (54.1%) versus the cyclosporine group (33.8%) [27]. In one study, over 70% of patients had resolution of neurological side effects arising from tacrolimus therapy when switched to cyclosporine [87]. In another study with prospectively collected single-center data, the prevalence rates of tremor were even higher with cyclosporine (62%) and tacrolimus (82%) [14]. Webster and associates, in a meta-analysis of 4102 patients, found significant reductions in graft loss in tacrolimus-treated recipients compared with cyclosporine, but more tremor, headache, diarrhea, dyspepsia, and vomiting [88]. In contrast to these data, our literature review found that tremor prevalence rates with cyclosporine (17%) and tacrolimus (21.5%) were lower overall and shared a similar likelihood of developing tremors.
Non-CNI Inhibitors (Sirolimus and Everolimus)
Cyclosporine (rapamycin) forms a complex with cyclophilin, whereas sirolimus/rapamycin bind to a family of immunophilins called FK506 (same as tacrolimus). While acting on the FK binding protein-12, sirolimus, which interferes with the cellular signal transduction pathways, does not inhibit calcineurin [89]. Sirolimus has gained attention as an immunosuppressive therapy in organ transplantation mainly owing to its unique mechanism of action, reduced risk of organ toxicity, and its ability to synergize with other immunosuppressant drugs without overlapping toxicity [6]. One study directly compared sirolimus with cyclosporine to find a lower risk of tremor (10% vs 46%) [26].
Everolimus is a second-generation rapamycin derivative with functions of mammalian target of rapamycin inhibition similar to sirolimus but with a higher potency [7, 90]. The two drugs exhibit significant differences in their pharmacokinetic, pharmacodynamic, and toxicodynamic properties, resulting in distinct clinical profiles. In one large study involving kidney transplant patients who were randomized to receive either everolimus with lower than typical doses of CNI or mycophenolic acid with standard doses of CNI, the investigators found that nearly 9.7% of patients developed tremor when exposed to everolimus compared with 13.5% with standard exposure CNIs [77]; these findings suggest that the dose is an important contributor. When combining data from all sources, sirolimus and everolimus indeed appear to have a lower propensity to induce tremors than cyclosporine and tacrolimus agents (Fig. 2).
Risk Factors for Tremor Development
As the therapeutic window is narrow, CNI agents require individual dose titration and mandate serum concentration strict monitoring to achieve a correct balance between maximizing efficacy and minimizing dose-related toxicity. Transplant surgeries in the past found low tacrolimus concentrations to correlate with rejections, whereas high concentrations were associated with nephrotoxicity [91, 92]. Some studies have suggested the following risk factors including elevated blood concentrations of cyclosporine or tacrolimus [93], administration of drugs through the intravenous route, co-administration of drugs that inhibit cyclosporine and tacrolimus metabolism (e.g., high-dose methylprednisolone), and the presence of hypomagnesemia leading to the development of tremor [53, 94]. In our data analysis, tremors seemed to be more frequent in kidney transplant patients compared with liver transplant patients. Unfortunately, the prevalence of data on drug-induced tremor in bone marrow transplants was inadequate, and the heart transplant data were not individually presented (combined with kidney transplant data). In our analysis, most studies did not specifically find a correlation between tremor intensity and drug concentrations. While most studies tended to monitor the trough concentrations, one study in the literature showed that the incidence of adverse reactions with cyclosporine was not different whether the area under the curve or trough concentration was monitored [95]. Few studies mentioned whether the tremor was early onset during the induction phase (drug concentrations relatively high) or the chronic maintenance phase when lower doses were employed. Finally, the studies included in our review did not ascertain whether a higher propensity of drug-induced tremors accompanied older age, male sex, and polypharmacy [1, 80].
Does Switching Twice-Daily Tacrolimus to a Once-Daily Formulation Lower the Risk?
Several clinical and nonclinical studies have shown that the pharmacokinetics of twice-daily and once-daily tacrolimus are significantly different [42]. Once-daily extended-release formulation has about a 30% greater relative bioavailability, about a 30% lower peak-to-trough fluctuation, and a consistently lower daily dose than prolonged-release tacrolimus [63, 96–98]. In one pooled analysis of over 800 kidney transplant recipients, a longer-acting once-daily formulation was found to be as effective as twice-daily tacrolimus [99]. The STRATO clinical trial found a lower risk of tremor with the once-daily extended-release formulation compared with twice-daily tacrolimus formulations [58]. In one study examining the real-world experience with conversion, a pre-conversion incidence of tremor (20.8%) was found to be substantially lower post-conversion (11.8%, p < 0.0001) [64]. In another study, among patients who were switched from twice-daily to once-daily formulations because of tremors, 88% reported a significant amelioration in symptoms [50]. In contrast to these data, some studies did not find a significant difference between the two formulations in the prevalence of tremors [42, 63]. In our assessment of combined data, we found that the tremor developing with a twice-daily tacrolimus formulation (17%) was comparable to a once-daily formulation (18%). Thus, further research could potentially resolve the current inconsistencies in findings.
Potential Mechanisms for Tremor
While the exact mechanism underlying the immunosuppressant-induced tremor is unknown, we speculate that several factors contribute to the pathophysiology. The intracellular binding proteins for both cyclosporine (cyclophilin) and tacrolimus (FK binding protein-12 or FKBP-12) [100, 101] are enriched in the central nervous system [102]. Cyclosporine and tacrolimus via calcineurin inhibition likely modulates the activity of both excitatory NMDA and inhibitory GABA receptors [80]. The pathophysiological mechanism for tremor oscillations is robustly linked to a dysfunction of these receptors [103]. Given the symptoms of ataxia co-occurring in some patients [14, 104], there is no doubt that the cerebellum is involved; a critical substrate also for the pathogenesis of tremor. It could be speculated that the use of cyclosporine and tacrolimus lowers the threshold for tremor generation for patients predisposed to developing these symptoms (a two-hit hypothesis). Many clinicians find that the tremors improve with a lowering of the dosage or discontinuation of the CNI drugs; however, some patients continue to exhibit bothersome tremors despite these measures, suggesting that the drugs could induce permanent damage in the tremor circuitry. The lower prevalence or risk of tremors with non-CNI drugs is intriguing, either related to the paucity of data or mechanistically, the drugs do not affect the tremor circuitries, but this will need further work.
Treatment and Outcomes
There are no standard guidelines for treating tremor resulting from the use of cyclosporine, tacrolimus, sirolimus, and everolimus. In our review of the data, most studies that reported the prevalence of tremor did not address the potential treatment modalities. Some studies implemented the discontinuation or reduction of the dosage to achieve remission or amelioration. Some studies reported that control of magnesium levels could be a potential strategy to lower the possibility of developing tremor [24, 35]. Some patients with immunosuppressant drug-induced tremor may respond to treatments proven to be of benefit for other tremor disorders such as propranolol and primidone [105, 106]. These potential modalities will need systematic studies in large well-characterized cohort of immunosuppressant drug-induced tremors.
We acknowledge that there are pitfalls in interpreting the data extracted from the literature. Pooled analyses using statistical tests could not be conducted as the included studies did not use the same study design, populations were not homogeneous, and the reporting methods used by most studies were quite heterogeneous. Many studies did not report the onset of tremors and whether there was a definitive temporal relationship between drug initiation and tremor. Most studies did not list the specific method for clinical or electrophysiological characterization. Although kidney and bone marrow transplant studies reportedly had a higher prevalence rate of tremor, we acknowledge that there could be a publication bias. Moreover, some data could not be analyzed as diverse etiologies were combined in a single dataset, such as kidney and heart transplant data. Finally, specific risk factors applicable to the emergence of tremors were not addressed.
Conclusions
In summary, a number of studies have revealed a substantive prevalence of tremors with the use of tacrolimus followed by cyclosporine. The propensity for drug-induced tremor appears to be higher in kidney transplant patients compared with bone marrow transplants, liver transplants, and other non-transplant indications. A few studies have found tremors to develop but at a lower risk with sirolimus and everolimus. A once-daily formulation has not consistently revealed a lower prevalence of tremors compared to the twice-daily formulation. No large-scale consistent data have supported a clear relationship between higher blood concentrations of drugs and a greater risk of tremors. Some patients have persistent tremors despite lowering the dose or discontinuation, indicating a mechanism beyond a simple dose-dependent relationship. There is little reporting on the patient-related risk factors for tremor, risk relationships with drug concentrations, treatment strategies, and outcomes. Future studies should address these critical knowledge gaps to improve the quality of life for patients receiving immunosuppressant therapies.
Declarations
Funding
No funding was received for the preparation of this article.
Conflicts of Interest/Competing Interests
Aparna Wagle Shukla, Caroline Lunny, Omar Mahboob, Uzair Khalid, Malea Joyce, Nivedita Jha, Nandakumar Nagaraja and Ashutosh M. Shukla have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Original data were extracted from PubMed articles. Upon request, the authors can provide the extracted data for individual studies.
Code Availability
Not applicable.
Authors’ Contributions
AWS: research project: conception, organization, execution; Manuscript preparation: writing of the first draft, writing of the final manuscript. CL: research project: organization, execution; OM: manuscript preparation: review and critique, writing of the final manuscript; UK: research project: execution; MJ: research project: execution; NJ: research project: execution; NN: Manuscript preparation: review and critique, writing of the final manuscript; AS: research project: conception; Manuscript preparation: writing of the first draft, review and critique, writing of the final manuscript.
References
- 1.Baizabal-Carvallo JF, Morgan JC. Drug-induced tremor, clinical features, diagnostic approach and management. J Neurol Sci. 2022;435:120192. doi: 10.1016/j.jns.2022.120192. [DOI] [PubMed] [Google Scholar]
- 2.Morgan JC, Kurek JA, Davis JL, Sethi KD. Insights into pathophysiology from medication-induced tremor. Tremor Hyperkinet Move (N Y). 2017;7:442. doi: 10.5334/tohm.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shrestha BM. Two decades of tacrolimus in renal transplant: basic science and clinical evidences. Exp Clin Transplant. 2017;15:1–9. doi: 10.6002/ect.2016.0157. [DOI] [PubMed] [Google Scholar]
- 4.Wijdicks EF, Wiesner RH, Dahlke LJ, Krom RA. FK506-induced neurotoxicity in liver transplantation. Ann Neurol. 1994;35:498–501. doi: 10.1002/ana.410350422. [DOI] [PubMed] [Google Scholar]
- 5.Uemoto S, Tanaka K, Honda K, et al. Experience with FK506 in living-related liver transplantation. Transplantation. 1993;55:288–292. doi: 10.1097/00007890-199302000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31:335–340. doi: 10.1016/S0009-9120(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 7.Yang MT, Lin YC, Ho WH, Liu CL, Lee WT. Everolimus is better than rapamycin in attenuating neuroinflammation in kainic acid-induced seizures. J Neuroinflamm. 2017;14:15. doi: 10.1186/s12974-017-0797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asćerić M, Avdić S, Nukić S, Vrabac-Mujcinagić M. Intensive observation of toxic side effects after several-year of cyclosporin treatment in kidney transplant patient. Bosnian J Basic Med Sci. 2007;7:132–135. doi: 10.17305/bjbms.2007.3067. [DOI] [PubMed] [Google Scholar]
- 9.Briggs D, Dudley C, Pattison J, et al. Effects of immediate switch from cyclosporine microemulsion to tacrolimus at first acute rejection in renal allograft recipients. Transplantation. 2003;75:2058–2063. doi: 10.1097/01.TP.0000069041.48226.DD. [DOI] [PubMed] [Google Scholar]
- 10.Caccavo D, Laganà B, Mitterhofer AP, et al. Long-term treatment of systemic lupus erythematosus with cyclosporin A. Arthritis Rheum. 1997;40:27–35. doi: 10.1002/art.1780400106. [DOI] [PubMed] [Google Scholar]
- 11.Conti F, Priori R, Alessandri C, Spinelli FR, Medda E, Valesini G. Safety profile and causes of withdrawal due to adverse events in systemic lupus erythematosus patients treated long-term with cyclosporine A. Lupus. 2000;9:676–680. doi: 10.1191/096120300676096627. [DOI] [PubMed] [Google Scholar]
- 12.David-Neto E, Lemos FBC, Arai Furusawa E, et al. Impact of cyclosporin A pharmacokinetics on the presence of side effects in pediatric renal transplantation. J Am Soc Nephrol. 2000;11:343–349. doi: 10.1681/ASN.V112343. [DOI] [PubMed] [Google Scholar]
- 13.Dehghani SM, Honar N, Inaloo S, et al. Neuromuscular complication after liver transplant in children: a single-center experience. Exp Clin Transplant. 2010;8:9–13. [PubMed] [Google Scholar]
- 14.Erro R, Bacchin R, Magrinelli F, et al. Tremor induced by calcineurin inhibitor immunosuppression: a single-centre observational study in kidney transplanted patients. J Neurol. 2018;265:1676–1683. doi: 10.1007/s00415-018-8904-x. [DOI] [PubMed] [Google Scholar]
- 15.Frank B, Perdrizet GA, White HM, Marsh JW, Lemann W, Woodle ES. Neurotoxicity of FK 506 in liver transplant recipients. Transplant Proc. 1993;25:1887–1888. [PubMed] [Google Scholar]
- 16.Hami M. Cyclosporine effects on pediatric kidney recipients. Nephro Urol Mon. 2012;4:491–492. doi: 10.5812/numonthly.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahan BD, Flechner SM, Lorber MI, Golden D, Conley S, Van Buren CT. Complications of cyclosporine-prednisone immunosuppression in 402 renal allograft recipients exclusively followed at a single center for from one to five years. Transplantation. 1987;43:197–204. doi: 10.1097/00007890-198702000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Mahdi G, Israel DM, Hassall E. Cyclosporine and 6-mercaptopurine for active, refractory Crohn's colitis in children. Am J Gastroenterol. 1996;91:1355–1359. [PubMed] [Google Scholar]
- 19.Martin F, Castro H, Gabriel C, et al. Ciclosporin A proof of concept study in patients with active, progressive HTLV-1 associated myelopathy/tropical spastic paraparesis. PLoS Negl Trop Dis. 2012;6:e1675. doi: 10.1371/journal.pntd.0001675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menegaux F, Keeffe EB, Andrews BT, et al. Neurological complications of liver transplantation in adult versus pediatric patients. Transplantation. 1994;58:447–450. doi: 10.1097/00007890-199408270-00010. [DOI] [PubMed] [Google Scholar]
- 21.Miller KB, Schenkein DP, Comenzo R, et al. Adjusted-dose continuous-infusion cyclosporin A to prevent graft-versus-host disease following allogeneic bone marrow transplantation. Ann Hematol. 1994;68:15–20. doi: 10.1007/BF01695914. [DOI] [PubMed] [Google Scholar]
- 22.Minuk GY, Bohme CE, Burgess E, et al. Pilot study of cyclosporin A in patients with symptomatic primary biliary cirrhosis. Gastroenterology. 1988;95:1356–1363. doi: 10.1016/0016-5085(88)90373-3. [DOI] [PubMed] [Google Scholar]
- 23.Munhoz RP, Teive HA, Germiniani FM, et al. Movement disorders secondary to long-term treatment with cyclosporine A. Arq Neuropsiquiatr. 2005;63:592–596. doi: 10.1590/S0004-282X2005000400007. [DOI] [PubMed] [Google Scholar]
- 24.Navazo L, Salata H, Morales S, et al. Oral microemulsion cyclosporine in the treatment of steroid-refractory attacks of ulcerative and indeterminate colitis. Scand J Gastroenterol. 2001;36:610–614. doi: 10.1080/003655201750163051. [DOI] [PubMed] [Google Scholar]
- 25.Neuhaus P, McMaster P, Calne R, et al. Neurological complications in the European multicentre study of FK 506 and cyclosporin in primary liver transplantation. Transplant Int. 1994;7(Suppl. 1):S27–31. doi: 10.1111/j.1432-2277.1994.tb01305.x. [DOI] [PubMed] [Google Scholar]
- 26.Pescovitz MD, Vincenti F, Hart M, et al. Pharmacokinetics, safety, and efficacy of mycophenolate mofetil in combination with sirolimus or ciclosporin in renal transplant patients. Br J Clin Pharmacol. 2007;64:758–771. doi: 10.1111/j.1365-2125.2007.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pirsch JD, Miller J, Deierhoi MH, Vincenti F, Filo RS. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. FK506 Kidney Transplant Study Group. Transplantation. 1997;63:977–983. doi: 10.1097/00007890-199704150-00013. [DOI] [PubMed] [Google Scholar]
- 28.Pistoia V, Buoncompagni A, Scribanis R, et al. Cyclosporin A in the treatment of juvenile chronic arthritis and childhood polymyositis-dermatomyositis: results of a preliminary study. Clin Exp Rheumatol. 1993;11:203–208. [PubMed] [Google Scholar]
- 29.Schmidt H, Ehninger G, Dopfer R, et al. Correlation between low CSA plasma concentration and severity of acute GvHD in bone marrow transplantation. Blut. 1988;57:139–142. doi: 10.1007/BF00320154. [DOI] [PubMed] [Google Scholar]
- 30.Sheth TN, Ichise M, Kucharczyk W. Brain perfusion imaging in asymptomatic patients receiving cyclosporin. Am J Neuroradiol. 1999;20:853–856. [PMC free article] [PubMed] [Google Scholar]
- 31.Sood A, Midha V, Sood N, et al. Cyclosporine in the treatment of severe steroid refractory ulcerative colitis: a retrospective analysis of 24 cases. Indian J Gastroenterol. 2008;27:232–235. [PubMed] [Google Scholar]
- 32.Stack WA, Long RG, Hawkey CJ. Short- and long-term outcome of patients treated with cyclosporin for severe acute ulcerative colitis. Aliment Pharmacol Ther. 1998;12:973–978. doi: 10.1046/j.1365-2036.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- 33.Stange EF, Fleig WE, Rehklau E, Ditschuneit H. Cyclosporin A treatment in inflammatory bowel disease. Digest Dis Sci. 1989;34:1387–1392. doi: 10.1007/BF01538074. [DOI] [PubMed] [Google Scholar]
- 34.Steinsson K, Jónsdóttir I, Valdimarsson H. Cyclosporin A in psoriatic arthritis: an open study. Ann Rheum Dis. 1990;49:603–606. doi: 10.1136/ard.49.8.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson CB, June CH, Sullivan KM, Thomas ED. Association between cyclosporin neurotoxicity and hypomagnesaemia. Lancet. 1984;2:1116–1120. doi: 10.1016/S0140-6736(84)91556-3. [DOI] [PubMed] [Google Scholar]
- 36.Tolou-Ghamari Z, Mortazavi M, Palizban AA, Najafi MR. The investigation of correlation between Iminoral concentration and neurotoxic levels after kidney transplantation. Adv Biomed Res. 2015;4:59. doi: 10.4103/2277-9175.151876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trocha K, Winkler M, Haas J, Ringe B, Wurster U, Ehrenheim C. Neurological examinations after liver transplantation concerning patients under corticosteroid immunosuppression and either FK 506 or cyclosporin. Transplant Int. 1994;7(Suppl. 1):S43–S49. doi: 10.1111/j.1432-2277.1994.tb01308.x. [DOI] [PubMed] [Google Scholar]
- 38.Wakefield D, McCluskey P. Cyclosporin therapy for severe scleritis. Br J Ophthalmol. 1989;73:743–746. doi: 10.1136/bjo.73.9.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wakefield D, McCluskey P. Cyclosporine: a therapy in inflammatory eye disease. J Ocular Pharmacol. 1991;7:221–226. doi: 10.1089/jop.1991.7.221. [DOI] [PubMed] [Google Scholar]
- 40.Wijdicks EF, Dahlke LJ, Wiesner RH. Oral cyclosporine decreases severity of neurotoxicity in liver transplant recipients. Neurology. 1999;52:1708–1710. doi: 10.1212/WNL.52.8.1708. [DOI] [PubMed] [Google Scholar]
- 41.Alissa DA, Alkortas D, Alsebayel M, et al. Tacrolimus-induced neurotoxicity in early post-liver transplant Saudi patients: incidence and risk factors. Ann Transplant. 2022;27:e935938. doi: 10.12659/AOT.935938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alloway RR, Eckhoff DE, Washburn WK, Teperman LW. Conversion from twice daily tacrolimus capsules to once daily extended-release tacrolimus (LCP-Tacro): phase 2 trial of stable liver transplant recipients. Liver Transplant. 2014;20:564–575. doi: 10.1002/lt.23844. [DOI] [PubMed] [Google Scholar]
- 43.Baumgart DC, Pintoffl JP, Sturm A, Wiedenmann B, Dignass AU. Tacrolimus is safe and effective in patients with severe steroid-refractory or steroid-dependent inflammatory bowel disease: a long-term follow-up. Am J Gastroenterol. 2006;101:1048–1056. doi: 10.1111/j.1572-0241.2006.00524.x. [DOI] [PubMed] [Google Scholar]
- 44.Baumgart DC, Wiedenmann B, Dignass AU. Rescue therapy with tacrolimus is effective in patients with severe and refractory inflammatory bowel disease. Aliment Pharmacol Ther. 2003;17:1273–1281. doi: 10.1046/j.1365-2036.2003.01534.x. [DOI] [PubMed] [Google Scholar]
- 45.Boschetti G, Nancey S, Moussata D, et al. Tacrolimus induction followed by maintenance monotherapy is useful in selected patients with moderate-to-severe ulcerative colitis refractory to prior treatment. Dig Liver Dis. 2014;46:875–880. doi: 10.1016/j.dld.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Bulatova N, Yousef AM, Al-Khayyat G, Qosa H. Adverse effects of tacrolimus in renal transplant patients from living donors. Curr Drug Saf. 2011;6:3–11. doi: 10.2174/157488611794480043. [DOI] [PubMed] [Google Scholar]
- 47.Burkhalter EL, Starzl TE, Van Thiel DH. Severe neurological complications following orthotopic liver transplantation in patients receiving FK 506 and prednisone. J Hepatol. 1994;21:572–577. doi: 10.1016/S0168-8278(94)80103-7. [DOI] [PubMed] [Google Scholar]
- 48.Chand DH, Southerland SM, Cunningham RJ., 3rd Tacrolimus: the good, the bad, and the ugly. Pediatr Transplant. 2001;5:32–36. doi: 10.1034/j.1399-3046.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- 49.Chen W, Liu Q, Liao Y, et al. Outcomes of tacrolimus therapy in adults with refractory membranous nephrotic syndrome: a prospective, multicenter clinical trial. Am J Med Sci. 2013;345:81–87. doi: 10.1097/MAJ.0b013e31824ce676. [DOI] [PubMed] [Google Scholar]
- 50.Choi D, Thaker S, West-Thielke P, Elmasri A, Chan C. Evaluating the conversion to extended-release tacrolimus from immediate-release tacrolimus in liver transplant recipients. Eur J Gastroenterol Hepatol. 2021;33:1124–1128. doi: 10.1097/MEG.0000000000002172. [DOI] [PubMed] [Google Scholar]
- 51.De Simone P, Carrai P, Coletti L, et al. Everolimus vs mycophenolate mofetil in combination with tacrolimus: a propensity score-matched analysis in liver transplantation. Transplant Proc. 2018;50:3615–3620. doi: 10.1016/j.transproceed.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 52.DuBay DA, Teperman L, Ueda K, et al. Pharmacokinetics of once-daily extended-release tacrolimus tablets versus twice-daily capsules in de novo liver transplant. Clin Pharmacol Drug Dev. 2019;8:995–1008. doi: 10.1002/cpdd.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eidelman BH, Abu-Elmagd K, Wilson J, et al. Neurologic complications of FK 506. Transplant Proc. 1991;23:3175–3178. [PMC free article] [PubMed] [Google Scholar]
- 54.Fan L, Liu Q, Liao Y, et al. Tacrolimus is an alternative therapy option for the treatment of adult steroid-resistant nephrotic syndrome: a prospective, multicenter clinical trial. Int Urol Nephrol. 2013;45:459–468. doi: 10.1007/s11255-012-0205-1. [DOI] [PubMed] [Google Scholar]
- 55.January SE, Hagopian JC, Nesselhauf NM, Progar K, Horwedel TA, Santos RD. Clinical experience with extended-release tacrolimus in older adult kidney transplant recipients: a retrospective cohort study. Drugs Aging. 2021;38:397–406. doi: 10.1007/s40266-021-00842-w. [DOI] [PubMed] [Google Scholar]
- 56.Karasawa K, Uchida K, Kodama M, Moriyama T, Nitta K. Long-term effects of tacrolimus for maintenance therapy of lupus nephritis: a 5-year retrospective study at a single center. Rheumatol Int. 2018;38:2271–2277. doi: 10.1007/s00296-018-4154-6. [DOI] [PubMed] [Google Scholar]
- 57.Katari SR, Magnone M, Shapiro R, et al. Clinical features of acute reversible tacrolimus (FK 506) nephrotoxicity in kidney transplant recipients. Clin Transplant. 1997;11:237–242. [PMC free article] [PubMed] [Google Scholar]
- 58.Langone A, Steinberg SM, Gedaly R, et al. Switching STudy of Kidney TRansplant PAtients with Tremor to LCP-TacrO (STRATO): an open-label, multicenter, prospective phase 3b study. Clin Transplant. 2015;29:796–805. doi: 10.1111/ctr.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mok CC, To CH, Yu KL, Ho LY. Combined low-dose mycophenolate mofetil and tacrolimus for lupus nephritis with suboptimal response to standard therapy: a 12-month prospective study. Lupus. 2013;22:1135–1141. doi: 10.1177/0961203313502864. [DOI] [PubMed] [Google Scholar]
- 60.Neu AM, Furth SL, Case BW, Wise B, Colombani PM, Fivush BA. Evaluation of neurotoxicity in pediatric renal transplant recipients treated with tacrolimus (FK506) Clin Transplant. 1997;11:412–414. [PubMed] [Google Scholar]
- 61.Reding R, Wallemacq PE, Lamy ME, et al. Conversion from cyclosporine to FK506 for salvage of immunocompromised pediatric liver allografts: efficacy, toxicity, and dose regimen in 23 children. Transplantation. 1994;57:93–100. doi: 10.1097/00007890-199401000-00017. [DOI] [PubMed] [Google Scholar]
- 62.Riva N, Dip M, Halac E, et al. Survival time to biopsy-proven acute rejection and tacrolimus adverse drug reactions in pediatric liver transplantation. Ther Drug Monitor. 2018;40:401–410. doi: 10.1097/FTD.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 63.Rostaing L, Bunnapradist S, Grinyó JM, et al. Novel once-daily extended-release tacrolimus versus twice-daily tacrolimus in de novo kidney transplant recipients: two-year results of phase 3, double-blind, randomized trial. Am J Kidney Dis. 2016;67:648–659. doi: 10.1053/j.ajkd.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 64.Sánchez Fructuoso A, Ruiz JC, Franco A, et al. Effectiveness and safety of the conversion to MeltDose(®) extended-release tacrolimus from other formulations of tacrolimus in stable kidney transplant patients: a retrospective study. Clin Transplant. 2020;34:e13767. doi: 10.1111/ctr.13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tamaki H, Nakase H, Matsuura M, et al. The effect of tacrolimus (FK-506) on Japanese patients with refractory Crohn's disease. J Gastroenterol. 2008;43:774–779. doi: 10.1007/s00535-008-2229-y. [DOI] [PubMed] [Google Scholar]
- 66.Thorp M, DeMattos A, Bennett W, Barry J, Norman D. The effect of conversion from cyclosporine to tacrolimus on gingival hyperplasia, hirsutism and cholesterol. Transplantation. 2000;69:1218–1220. doi: 10.1097/00007890-200003270-00029. [DOI] [PubMed] [Google Scholar]
- 67.Truffinet O, Martinez-Vinson C, Guerriero E, Hugot JP, Viala J. Tacrolimus exerts only a transient effectiveness in refractory pediatric Crohn disease: a case series. J Pediatr Gastroenterol Nutr. 2017;64:721–725. doi: 10.1097/MPG.0000000000001338. [DOI] [PubMed] [Google Scholar]
- 68.Turunc V, Ari E, Guven B, Tabendeh B, Yildiz A. Once- vs twice-daily tacrolimus: survival rates and side effects: single-center experience. Transplant Proc. 2019;51:2308–2311. doi: 10.1016/j.transproceed.2019.01.149. [DOI] [PubMed] [Google Scholar]
- 69.Uemoto S, Tanaka K, Tokunaga Y, et al. Long-term use of FK 506 in living related liver transplantation. Transplant Int. 1994;7(Suppl. 1):S81–S84. doi: 10.1111/j.1432-2277.1994.tb01315.x. [DOI] [PubMed] [Google Scholar]
- 70.Watson S, Pensabene L, Mitchell P, Bousvaros A. Outcomes and adverse events in children and young adults undergoing tacrolimus therapy for steroid-refractory colitis. Inflamm Bowel Dis. 2011;17:22–29. doi: 10.1002/ibd.21418. [DOI] [PubMed] [Google Scholar]
- 71.Yamamoto S, Nakase H, Mikami S, et al. Long-term effect of tacrolimus therapy in patients with refractory ulcerative colitis. Aliment Pharmacol Ther. 2008;28:589–597. doi: 10.1111/j.1365-2036.2008.03764.x. [DOI] [PubMed] [Google Scholar]
- 72.Yanik G, Levine JE, Ratanatharathorn V, Dunn R, Ferrara J, Hutchinson RJ. Tacrolimus (FK506) and methotrexate as prophylaxis for acute graft-versus-host disease in pediatric allogeneic stem cell transplantation. Bone Marrow Transplant. 2000;26:161–167. doi: 10.1038/sj.bmt.1702472. [DOI] [PubMed] [Google Scholar]
- 73.Yocum DE, Furst DE, Bensen WG, et al. Safety of tacrolimus in patients with rheumatoid arthritis: long-term experience. Rheumatology. 2004;43:992–999. doi: 10.1093/rheumatology/keh155. [DOI] [PubMed] [Google Scholar]
- 74.Jeon HJ, Lee HE, Yang J. Safety and efficacy of Rapamune® (Sirolimus) in kidney transplant recipients: results of a prospective post-marketing surveillance study in Korea. BMC Nephrol. 2018;19:201. doi: 10.1186/s12882-018-1002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chinnock TJ, Shankel T, Deming D, et al. Calcineurin inhibitor minimization using sirolimus leads to improved renal function in pediatric heart transplant recipients. Pediatr Transplant. 2011;15:746–749. doi: 10.1111/j.1399-3046.2011.01566.x. [DOI] [PubMed] [Google Scholar]
- 76.van de Beek D, Kremers WK, Kushwaha SS, McGregor CG, Wijdicks EF. No major neurologic complications with sirolimus use in heart transplant recipients. Mayo Clin Proc. 2009;84:330–332. doi: 10.1016/S0025-6196(11)60541-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tedesco-Silva H, Pascual J, Viklicky O, et al. Safety of everolimus with reduced calcineurin inhibitor exposure in de novo kidney transplants: an analysis from the randomized TRANSFORM Study. Transplantation. 2019;103:1953–1963. doi: 10.1097/TP.0000000000002626. [DOI] [PubMed] [Google Scholar]
- 78.Pilch NA, Bowman LJ, Taber DJ. Immunosuppression trends in solid organ transplantation: the future of individualization, monitoring, and management. Pharmacotherapy. 2021;41:119–131. doi: 10.1002/phar.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Calne RY. The initial study of the immunosuppressive effects of 6-mercaptopurine and azathioprine in organ transplantation and a few words on cyclosporin A. World J Surg. 1982;6:637–640. doi: 10.1007/BF01657885. [DOI] [PubMed] [Google Scholar]
- 80.Bechstein WO. Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transplant Int. 2000;13:313–326. doi: 10.1111/j.1432-2277.2000.tb01004.x. [DOI] [PubMed] [Google Scholar]
- 81.Walker RW, Brochstein JA. Neurologic complications of immunosuppressive agents. Neurol Clin. 1988;6:261–278. doi: 10.1016/S0733-8619(18)30869-7. [DOI] [PubMed] [Google Scholar]
- 82.Erer B, Polchi P, Lucarelli G, et al. CsA-associated neurotoxicity and ineffective prophylaxis with clonazepam in patients transplanted for thalassemia major: analysis of risk factors. Bone Marrow Transplant. 1996;18:157–162. [PubMed] [Google Scholar]
- 83.Reece DE, Frei-Lahr DA, Shepherd JD, et al. Neurologic complications in allogeneic bone marrow transplant patients receiving cyclosporin. Bone Marrow Transplant. 1991;8:393–401. [PubMed] [Google Scholar]
- 84.Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramman R, Jain A. FK 506 for liver, kidney, and pancreas transplantation. Lancet. 1989;2:1000–1004. doi: 10.1016/S0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 86.Augusto JF, Subra JF, Onno C, et al. Long-term maintenance immunosuppressive regimen with tacrolimus monotherapy. Ann Transplant. 2013;18:368–377. doi: 10.12659/AOT.883979. [DOI] [PubMed] [Google Scholar]
- 87.Abouljoud MS, Kumar MS, Brayman KL, Emre S, Bynon JS. Neoral rescue therapy in transplant patients with intolerance to tacrolimus. Clin Transplant. 2002;16:168–172. doi: 10.1034/j.1399-0012.2002.01054.x. [DOI] [PubMed] [Google Scholar]
- 88.Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. BMJ. 2005;331:810. doi: 10.1136/bmj.38569.471007.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cardenas ME, Zhu D, Heitman J. Molecular mechanisms of immunosuppression by cyclosporine, FK506, and rapamycin. Curr Opin Nephrol Hypertens. 1995;4:472–477. doi: 10.1097/00041552-199511000-00002. [DOI] [PubMed] [Google Scholar]
- 90.Klawitter J, Nashan B, Christians U. Everolimus and sirolimus in transplantation-related but different. Expert Opin Drug Saf. 2015;14:1055–1070. doi: 10.1517/14740338.2015.1040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hedayat S, Kershner RP, Su G. Relationship of whole-blood FK506 concentrations to rejection and toxicity in liver and kidney transplants. J Biopharm Stat. 1996;6:411–424. doi: 10.1080/10543409608835153. [DOI] [PubMed] [Google Scholar]
- 92.Kahan BD, Keown P, Levy GA, Johnston A. Therapeutic drug monitoring of immunosuppressant drugs in clinical practice. Clin Ther. 2002;24:330–350. doi: 10.1016/S0149-2918(02)85038-X. [DOI] [PubMed] [Google Scholar]
- 93.Berden JH, Hoitsma AJ, Merx JL, Keyser A. Severe central-nervous-system toxicity associated with cyclosporin. Lancet. 1985;1:219–220. doi: 10.1016/S0140-6736(85)92053-7. [DOI] [PubMed] [Google Scholar]
- 94.Lucey MR, Kolars JC, Merion RM, Campbell DA, Aldrich M, Watkins PB. Cyclosporin toxicity at therapeutic blood levels and cytochrome P-450 IIIA. Lancet. 1990;335:11–15. doi: 10.1016/0140-6736(90)90137-T. [DOI] [PubMed] [Google Scholar]
- 95.Kuijvenhoven MA, Wilhelm AJ, Meijer E, Janssen J, Swart EL. TRough versus AUC Monitoring of cyclosporine: a randomized comparison of adverse drug reactions in adult allogeneic stem cell recipients (TRAM study) Eur J Haematol. 2021;107:364–369. doi: 10.1111/ejh.13674. [DOI] [PubMed] [Google Scholar]
- 96.Sańko-Resmer J, Boillot O, Wolf P, Thorburn D. Renal function, efficacy and safety postconversion from twice- to once-daily tacrolimus in stable liver recipients: an open-label multicenter study. Transplant Int. 2012;25:283–293. doi: 10.1111/j.1432-2277.2011.01412.x. [DOI] [PubMed] [Google Scholar]
- 97.Kamar N, Cassuto E, Piotti G, et al. Pharmacokinetics of prolonged-release once-daily formulations of tacrolimus in de novo kidney transplant recipients: a randomized, parallel-group, open-label, multicenter study. Adv Ther. 2019;36:462–477. doi: 10.1007/s12325-018-0855-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gaber AO, Alloway RR, Bodziak K, Kaplan B, Bunnapradist S. Conversion from twice-daily tacrolimus capsules to once-daily extended-release tacrolimus (LCPT): a phase 2 trial of stable renal transplant recipients. Transplantation. 2013;96:191–197. doi: 10.1097/TP.0b013e3182962cc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bunnapradist S, Rostaing L, Alloway RR, et al. LCPT once-daily extended-release tacrolimus tablets versus twice-daily capsules: a pooled analysis of two phase 3 trials in important de novo and stable kidney transplant recipient subgroups. Transplant Int. 2016;29:603–611. doi: 10.1111/tri.12770. [DOI] [PubMed] [Google Scholar]
- 100.Parlakpinar H, Gunata M. Transplantation and immunosuppression: a review of novel transplant-related immunosuppressant drugs. Immunopharmacol Immunotoxicol. 2021;43:651–665. doi: 10.1080/08923973.2021.1966033. [DOI] [PubMed] [Google Scholar]
- 101.Bentata Y. Tacrolimus: 20 years of use in adult kidney transplantation. What we should know about its nephrotoxicity. Artific Organs. 2020;44:140–152. doi: 10.1111/aor.13551. [DOI] [PubMed] [Google Scholar]
- 102.Azzi JR, Sayegh MH, Mallat SG. Calcineurin inhibitors: 40 years later, can't live without. J Immunol. 2013;191:5785–5791. doi: 10.4049/jimmunol.1390055. [DOI] [PubMed] [Google Scholar]
- 103.Wagle SA. Reduction of neuronal hyperexcitability with modulation of T-type calcium channel or SK channel in essential tremor. Int Rev Neurobiol. 2022;163:335–355. doi: 10.1016/bs.irn.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 104.Atkinson K, Biggs J, Darveniza P, Boland J, Concannon A, Dodds A. Spinal cord and cerebellar-like syndromes associated with the use of cyclosporine in human recipients of allogeneic marrow transplants. Transplant Proc. 1985;17:1673–1675. [PubMed] [Google Scholar]
- 105.Wagle Shukla A, Lunny C, Hisham I, Cagle J, Malea J, Santos A, Shukla AM. Phenomenology and physiology of tacrolimus induced tremor. Tremor Other Hyperkinet Mov (N Y). 2023;13:2. [DOI] [PMC free article] [PubMed]
- 106.Wagle Shukla A. Diagnosis and treatment of essential tremor. Continuum (Minneap Minn). 2022;28(5):1333–49. [DOI] [PMC free article] [PubMed]