Abstract
Background
A first febrile urinary tract infection (UTI) is a common condition in children, and pathways of management have evolved over time.
Objective
To determine the extent to which pediatricians and surgeons differ in their investigation and management of a first febrile UTI, and to evaluate the justifications for any divergence of approach.
Materials and methods
A literature search was conducted for papers addressing investigation and/or management following a first febrile UTI in children published between 2011 and 2021. Searches were conducted on Medline, Embase, and the Cochrane Controlled Trials Register. To be eligible for inclusion, a paper was required to provide recommendations on one or more of the following: ultrasound (US) and voiding cystourethrogram (VCUG), the need for continuous antibiotic prophylaxis and surgery when vesicoureteral reflux (VUR) was detected. The authorship required at least one pediatrician or surgeon. Authorship was categorized as medical, surgical, or combined.
Results
Pediatricians advocated less imaging and intervention and were more inclined to adopt a “watchful-waiting” approach, confident that any significant abnormality, grades IV–V VUR in particular, should be detected following a second febrile UTI. In contrast, surgeons were more likely to recommend imaging to detect VUR (p<0.00001), and antibiotic prophylaxis (p<0.001) and/or surgical correction (p=0.004) if it was detected, concerned that any delay in diagnosis and treatment could place the child at risk of kidney damage. Papers with combined authorship displayed intermediate results.
Conclusion
There are two distinct directions in the literature regarding the investigation of an uncomplicated first febrile UTI in a child. In general, when presented with a first febrile UTI in a child, physicians recommend fewer investigations and less treatment, in contrast to surgeons who advocate extensive investigation and aggressive intervention in the event that imaging detects an abnormality. This has the potential to confuse the carers of affected children.
Supplementary Information
Supplementary material is available at 10.1007/s00247-023-05771-x.
Keywords: Antibiotic prophylaxis, Cystography, Pediatric, Review, Urinary tract infections, Vesico-ureteral reflux
Introduction
Febrile urinary tract infections (UTIs) are common in children. In infants presenting with unexplained fever the prevalence of UTI is 7%, reaching 20% in uncircumcised boys by three months of age [1]. These febrile UTIs are said to lead to pyelonephritic scarring in up to 30% of cases, and can be the first sign of a congenital abnormality of the kidney and urinary tract, the most frequent being vesicoureteral reflux (VUR), which occurs in one-third of cases [2]. The observation that VUR is a risk factor for recurrent infection, and the finding of an association between VUR (primarily high-grade) and chronic kidney damage, originally led to a push from the medical and surgical world to try to detect VUR after a febrile UTI, with the implementation of continuous antibiotic prophylaxis or surgical correction in the event of finding it. In recent years, there has been a reassessment of the role of VUR and acquired pyelonephritic scarring as risk factors for progressive chronic kidney disease and other long-term consequences [3, 4], and thus of the need to investigate children following a first febrile UTI.
The role of the pediatric radiologist is to decide whether a voiding cystourethrogram (VCUG), the standard test for VUR, is justified in an individual patient, and if so to perform and interpret it. A first febrile UTI in a young child can present to a pediatrician or a pediatric surgeon.
We are aware of an interesting paper that appeared in the BMJ some years ago entitled “Phenotypic differences between male physicians, surgeons and film stars: comparative study,” where evidence-based medicine was contrasted with confidence-based medicine [5]. A further paper by Stirrat also provided an insight into surgical thinking [6]. In multidisciplinary discussions among radiologists, physicians, and surgeons at our institutions, it has become apparent that stark differences have developed in the medical and surgical approaches to investigation and management of this common condition.
To what extent are differences in approach to febrile UTIs present in the literature, and if so, what are the justifications? To answer these questions, we reviewed the literature, comparing the approaches of physicians and surgeons to investigation and treatment.
Methods
Searches were conducted on Medline, Embase, and the Cochrane Controlled Trials Register for reviews and studies in English, where the authors might make recommendations on the investigation and/or management of UTIs in children published between January 2011, the year of the revised American Academy of Pediatrics (AAP) recommendations [7] and December 2021. The websites of professional societies were also accessed for their guidelines, if available, on the investigation and management of a first febrile UTI in children. The Medline search strategy is included in Supplementary Material 1. The search strategy was adapted to the syntax and subject headings of Embase and the Cochrane Controlled Trials Register. To be eligible for inclusion, a paper was required to provide recommendations on one or more of the following: imaging in the form of ultrasound (US) and voiding cystourethrogram (VCUG), the need for continuous antibiotic prophylaxis, and surgery when VUR was detected. 99mTc-dimercaptosuccinic acid scans are not commonly used after a first febrile UTI and were thus not included. All titles were reviewed, duplicates removed, abstracts read, and the full texts of potential articles obtained. The study selection was performed independently by two of the authors of this manuscript (I.K.H. and G.M., both with over 30 years of experience in pediatric nephrology) based on titles and abstracts; non-English language papers were excluded at this stage. Disagreement in selection and full-text review was resolved by consensus. One hundred and thirty-five papers, addressing investigation and/or management following a first febrile UTI, were selected for inclusion (Fig. 1).
Fig. 1.
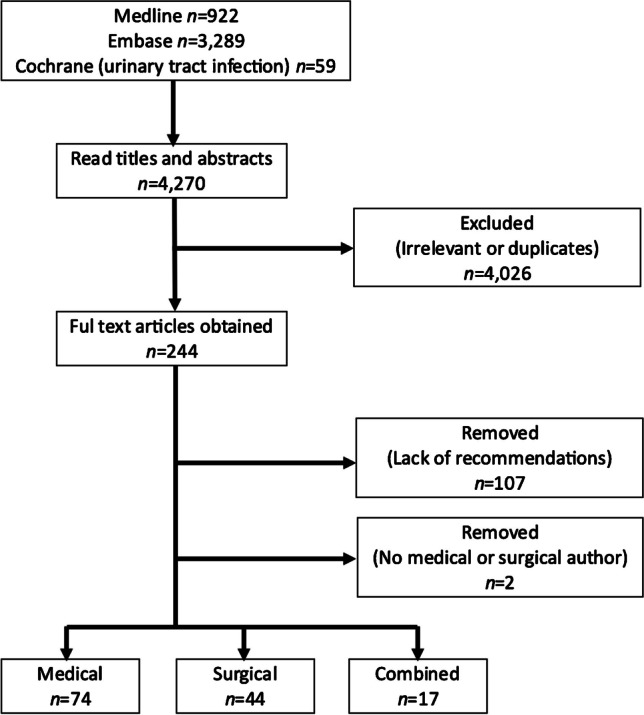
PRISMA flowchart
The papers were divided into reviews and studies, with authorship classified as medical, surgical, or combined, according to author status and affiliations recorded in the articles. Where information was inadequate, authors were searched within their affiliated institutions on the internet. Statistical analysis was performed using the chi-squared test for comparison of proportions, with P-values <0.05 considered significant. The extended Cochran-Armitage test was used to evaluate the association between recommendation and authorship groups (surgeons, physicians, combined). Statistical analysis was performed using the open-source statistical software R [8].
Results
Where recommendations on imaging have been made, ultrasound, being a non-invasive procedure without a radiation burden, is universally recommended following a first febrile UTI by physicians and surgeons alike, apart from the Caring for Australian and New Zealanders with Kidney Impairment (CARI) guidelines that recommend the test only in specific circumstances (Table 1). Most studies and reviews conducted by physicians, in contrast with surgeons, do not support performance of a VCUG following a first febrile UTI, or continuous antibiotic prophylaxis and surgery in the event VUR, particularly of milder grades, is found. The differences are statistically significant in all cases (Table 2). The papers with combined authors demonstrate intermediate results between physicians and surgeons, with the results closer to those of physicians. Including the “both” category in the analysis and considering the variables as ordered (surgeons>both>physicians), the association with all three outcomes is highly significant (extended Cochran-Armitage test): recommending VCUG: P<0.0001, recommending continuous antibiotic prophylaxis: P<0.001, recommending surgery: P=0.003.
Table 1.
Summary of published recommendations for the investigation and management of a first febrile urinary tract infection, categorized according to authorship (2011 to 2021)
| Recommending VCUG | Recommending continuous antibiotic prophylaxis | Recommending surgery | ||
|---|---|---|---|---|
| Reviews | Physicians | 5% (2/41) | 10% (4/41) | 8% (1/12) |
| Surgeons | 64% (7/11) | 46% (6/13) | 44% (4/9) | |
| Both | 0% (0/8) | 0% (0/6) | 0% (0/3) | |
| Studies | Physicians | 24% (8/34) | 22% (2/9) | 25% (1/4) |
| Surgeons | 80% (20/25) | 57% (4/7) | 78% (7/9) | |
| Both | 57% (4/7) | 50% (1/2) | 50% (1/2) | |
| Reviews and studies | Physicians | 13% (10/75) | 12% (6/50) | 13% (2/16) |
| Surgeons | 75% (27/36) | 50% (10/20) | 61% (11/18) | |
| Both | 27% (4/15) | 13% (1/8) | 20% (1/5) |
VCUG voiding cystourethrogram
Table 2.
Comparison between recommendations published by physicians and surgeons
| Recommending VCUG | Recommending continuous antibiotic prophylaxis | Recommending surgery | |
|---|---|---|---|
| Reviews | χ2 20.92 df1 P<0.00001 | χ2 12.61 df1 P<0.001 | χ2 3.70 df1 P=0.055 |
| Studies | χ2 18.43 df1 P<0.0001 | χ2 2.05 df1 P=0.15 | χ2 3.26 df1 P<0.07 |
| Reviews and studies | χ2 41.63 df1 P<0.00001 | χ2 11.70 df1 P<0.001 | χ2 8.48 df1 P=0.004 |
VCUG voiding cystourethrogram
Reviews against imaging and interventions
Of the 51 reviews that support a reduction in VCUGs following a first febrile UTI, 39 are medical [4, 9–46], four surgical [47–50], eight combined [2, 51–57], and 45 are reference medical guidelines. The six reviews that do not, note the minimal benefit of interventions in reducing febrile UTI (including no reduction in scarring) and the opportunity to reduce cost, radiation exposure, and stress for the child and family [23, 27, 40, 44, 47, 50].
The 50 reviews against continuous antibiotic prophylaxis in the event of detection of VUR (37 medical [4, 9–11, 13, 15–25, 27–30, 32–38, 40, 41, 45, 46, 58–63], seven surgical [47–50, 64–66], six combined [51, 53, 56, 57, 67, 68]) variously cited National Institute for Health and Care Excellence and American Academy of Pediatrics guidelines, Cochrane, a range of randomized controlled trials (RCTs), and a long-term Swedish follow-up study [69]. Of note, the low incidence of recurrent febrile UTIs and scarring even in the control untreated groups led to questioning the value of any intervention [39].
Those against surgical intervention (11 medical [4, 9, 11, 13, 16, 17, 21, 29, 40, 59, 70], five surgical [47–50, 64], three combined [53, 56, 57]) cited meta-analyses and Cochrane reviews showing minimal benefit or benefit no better than continuous antibiotic prophylaxis.
Reviews for voiding cystourethrograms and interventions
Of the 11 reviews for imaging with VCUGs, eight were surgical, citing the European Association of Urology - European Society for Paediatric Urology (EAU-ESPU) and/or American Urological Association (AUA) guidelines [71, 72], outdated American Academy of Pediatrics 1999 guidelines [73], concerns that delaying a VCUG until after a second febrile UTI placed children at risk of significant scarring [74], the high incidence of VUR associated with a febrile UTI [75], poor compliance with prophylaxis [31], concerns that antibiotic prophylaxis promotes drug resistance [76], and other urologists who support intervention [50]. The three medical reviews for imaging with VCUGs referred to the outdated American Academy of Pediatrics 1999 guidelines [77], Cincinnati guidelines [60], and Indian guidelines [78].
Of the ten reviews for continuous antibiotic prophylaxis, six were surgical, three referenced the RIVUR trial [2] as supportive of continuous antibiotic prophylaxis [79–81], one acknowledged that continuous antibiotic prophylaxis was of questionable value but best “err on the side of caution” [82], one referenced the American Urological Association guideline [71], and one cited increasing antibiotic resistance as a reason to consider surgery as a first line treatment [65]. The four medical reviews for continuous antibiotic prophylaxis cited the outdated American Academy of Pediatrics 1999 guidelines [43, 77, 78] and the RIVUR study demonstrating some benefit [42].
All four reviews for surgical intervention were authored by surgeons. They cited the American Urological Association guidelines, the PRIVENT and Swedish Reflux trials [71, 75, 81], and concerns regarding increased antibiotic resistance with continuous antibiotic prophylaxis [65].
Studies evaluating imaging and interventions
With the National Institute for Health and Care Excellence guidelines restricting ultrasound to infants less than 6 months of age, and both the National Institute for Health and Care Excellence and the American Academy of Pediatrics no longer recommending VCUG following an uncomplicated first febrile UTI, 39 studies applied the newer guidelines to a retrospective fully investigated cohort to determine what would have been missed, at least until a second febrile UTI. As expected, all studies determined that omitting a VCUG would have resulted in fewer cases of VUR being detected. This was deemed acceptable in 20 studies (13 medical [83–95], five surgical [96–100], two combined [101, 102]), with the newer guidelines, relying on US, considered able to detect most significant abnormalities including grade IV–V VUR, while often missing lesser grade II–III VUR with absent or mild dilatation, that would be found in the event of recurrent UTI, with savings in terms of reduced imaging costs and radiation exposure. In contrast, performance of a VCUG was deemed necessary in 19 studies (11 surgical [98, 103–112], six medical [113–118], two combined [119, 120]) with concern expressed that the abnormalities missed, in particular VUR of any grade, would place children at an indeterminate but possibly significant risk of morbidity.
Other studies by pediatricians were six in total and included two papers on compliance with the National Institute for Health and Care Excellence and revised American Academy of Pediatrics guidelines [121–123], two RCTs [2, 124], a meta-analysis of continuous antibiotic prophylaxis for prevention of scarring [125], and a study of scarring and cancer risk following VCUG [126]. Other publications by surgeons were four in total and included a retrospective assessment of UTI post-surgery, citing a febrile UTI incidence of 6.5% [127] whereas the only prospective RCT where children were maintained on continuous antibiotic prophylaxis post-surgery had a febrile UTI incidence of 21% [128], a study of surgical technique with recommendations on investigation and management [129], and a lower urinary tract dysfunction study [130].
Of the 58 studies that reported on surgical technique for correction of VUR, all but one [129] were excluded from this review, as no recommendations were made regarding investigation or management. The excluded papers are listed in Supplementary Material 2. Furthermore, only ten papers gave indications for surgery including breakthrough UTI despite antibiotic prophylaxis, deteriorating kidney function, and parental preference, while 44 were without indications for performing surgery. The 57 excluded papers represented the largest group of studies with an implicit assumption that VUR is a disease to be cured.
Discussion
The literature search performed during the 11-year period from 2011, when the revised American Academy of Pediatrics guidelines on the investigation and management of a first febrile UTI in infancy were published, largely concurring with the earlier National Institute for Health and Care Excellence guidelines, demonstrated significant differences in approach between physicians and surgeons in terms of imaging, antibiotic prophylaxis, and surgery in the event of VUR detection. It should be clarified that this is not a systematic review or meta-analysis, which represent scholarly syntheses of evidence on a subject to inform healthcare decisions. While physicians have largely embraced evidence-based medicine, surgeons in many cases have not; thus, any systematic review or meta-analysis would potentially exclude large portions of the surgical literature and thus make any assessment of conflicting views between the two groups impossible.
An analysis of the papers identifies some indication of the justifications given by physicians and surgeons for the divergence of opinion. Physicians as a group, in line with the newer evidence-based guidelines, advocate less imaging and intervention, and are inclined to adopt a “watchful-waiting” approach, confident that any significant abnormality, grade IV–V VUR in particular, should be picked up following a second febrile UTI. In contrast, surgeons as a group are more likely to recommend imaging to detect VUR, with antibiotic prophylaxis and/or surgical correction if it is detected, concerned that any delay in diagnosis and treatment could place the child at risk of kidney damage. This divergence of approach between physicians and surgeons often confuses the family of the child, regarding the choice of how to best proceed with the diagnostic and therapeutic process.
Physicians have for the most part embraced evidence-based medicine following its inception at McMaster University in 1991 [131]. In 2007, the BMJ conducted an international poll to determine the most important medical milestones in healthcare. Evidence-based medicine came seventh, ahead of the computer and medical imaging [132]. Surgeons have been more tempered in accepting the evidence-based concept [133]. It would be easy to be dismissive of surgeons who do not practice evidence-based medicine; however, physicians had similar reservations initially [134]. Stirrat, who has previously published on the challenge of evaluating surgical procedures [135], explored the experiential nature of surgery and reasons for the slow uptake of evidence-based surgery. He acknowledges the benefits of evidence-based medicine but expresses concern regarding over-reliance on RCTs, and a lack of generalizability of evidence to individual patients. These remain valid concerns, particularly when there is absent, incomplete, or conflicting evidence as well as the recognition that RCTs determine net results in the groups studied, while the probabilities are not precisely transferable to all individuals within the groups [136]. These may be factors leading to less enthusiasm for the tenets of evidence-based medicine amongst surgeons. He also noted that surgeons often used historical controls, comparing the results of a new operation with those obtained using another procedure. This introduces serious bias due to the assumption that nothing has changed apart from the new procedure, with incorrect conclusions in 40–60% of such studies. Weil [137], in a commentary on the lack of RCTs in surgery, noted that “The case series remains a favored method of clinical investigation in surgery. Case series are easy to perform, require less resources in terms of personnel and funds, can be performed at a single center, and, for many surgeons, represent a means to illustrate their surgical method and skills.” This concern is particularly relevant to the large number of studies on surgical technique to correct VUR, where success rates in resolving VUR were paramount, with little or no discussion on the indications or outcomes.
Following the recognition by improved antenatal ultrasound of congenital abnormalities of the kidney and urinary tract as a major reason for extensive renal damage, along with RCTs demonstrating medical intervention (continuous antibiotic prophylaxis) to be largely ineffective in preventing febrile UTIs, with no effect on scarring [125], updated evidence-based guidelines in the medical literature have led to a marked reduction in the investigations performed, and treatment prescribed by physicians following a first febrile UTI [138] (Table 3). In contrast, many surgeons remain focused on VUR, as a disease to be investigated for and treated, in the absence of any prospective RCTs demonstrating benefit for their intervention, so much so that the largest group of studies in the surgical literature assess surgical techniques for correcting VUR without alluding to any indications for the procedures.
Table 3.
Published guidelines for imaging following a first febrile urinary tract infection
| Guideline | Ultrasound | VCUG | Late DMSA |
|---|---|---|---|
|
NICE 2007 [139] |
<6/12 Yes >6/12 atypical UTI |
No unless abnormal US or atypical UTI | Atypical UTI |
| AAP 2011 [7] | Yes | No unless abnormal US | No |
| ISPN 2012 [140] | Yes | No unless abnormal US or risk factors | Abnormal US and/or VUR |
| CARI 2014 [141] | No unless absent antenatal US, atypical UTI, mass, poor stream, slow response | No unless recurrent febrile UTIs or US suggestive of posterior urethral valve | No unless reduced kidney function |
| Canadian 2014 [142] | Yes | No unless abnormal US suggestive of obstruction or high grade VUR | |
| EAU-ESPU 2015 [143]a | Yes |
Yes consider after second febrile UTI in boys >1 year with option of a “top down” approach performing DMSA instead and VCUG if positive |
No DMSA if VCUG positive or VCUG if DMSA positive |
| Urology Section AAP 2012 [74]b | Yes | Yes | No |
aThe EUA-ESPU guidelines were updated in 2021 (https://doi.org/10.1016/j.jpurol.2021.01.037), restricting a VCUG to those with an abnormal ultrasound or atypical UTI. The EUA-ESPU guidelines remain referenced in the article as these were the guidelines quoted by authors in the publications reviewed
bThe Urology Section of the AAP published a dissenting view on the updated AAP guidelines regarding the investigation and management of a first febrile UTI, concerned that lack of a VCUG placed children with unrecognized VUR at risk of pyelonephritis and scarring
AAP American Academy of Pediatrics, CARI Caring for Australian and New Zealanders with Kidney Impairment, EAU-ESPU European Association of Urology – European Society of Pediatric Urology, DMSA 99mTc-dimercaptosuccinic acid scan, ISPN Italian Society of Pediatric Nephrology, NICE National Institute for Health and Care Excellence, US ultrasound, UTI urinary tract infection, VCUG voiding cystourethrogram
This is not universally the case, however, with other surgeons stating that “vesicoureteral reflux is a phenotype not a disease” and is thus inconsequential [47]. Furthermore, RCTs in children with VUR that compared continuous antibiotic prophylaxis with surgical correction, demonstrated a low incidence of subsequent scarring with no significant difference between treatment arms, although no study has had a no-treatment control group [144–146].
An additional factor often raised is the mode of practice. Physicians and surgeons graduate from the same medical schools, after which their training diverges. Physicians focus on lifestyle counselling and medication where appropriate, often involving a long-term palliative rather than a curative approach, with the ability to re-assess management as newer information becomes available. Surgeons perform definitive procedures that historically were emergency interventions, such as appendectomy, with a predominance of elective procedures only developing in recent times. Regardless, given the invasive nature and irreversibility of operations, surgeons must have a strong belief in the curative nature of their interventions to convince patients, or the parents of patients, to consent. Are the differences phenotypic with different inherent traits determining the training path undertaken, as suggested in the BMJ paper [5], or are they the consequence of different approaches to training, as proposed by Stirrat [6]?
Comments such as “the literature obfuscates more than it clarifies” [82], “these guidelines do not reflect the real world” [129], and assertions that some guidelines (National Institute for Health and Care Excellence, American Academy of Pediatrics and Italian included) “are driven by economic and health care issues” [143], pervade the surgical literature. The admonition “You can’t handle the truth!” has even been applied to the pediatric community when they debate the need for continuous antibiotic prophylaxis in children following a UTI [82].
The 2008 National Institute for Health and Care Excellence evidence-based guidelines on UTI in infants and young children were the first to advocate reduced imaging and intervention [139]. Thereafter, published physician-instigated guidelines largely concurred with the National Institute for Health and Care Excellence, recommending additional imaging only when ultrasound was abnormal or in the few cases where recurrent febrile UTIs occurred [7, 140–142]. Otherwise, they promoted a “watchful waiting” approach, with less emphasis on VUR given the lack of evidence that intervention is of any clinical benefit, except possibly for high grade IV–V VUR, which accounts for <5% of cases and is likely to be detected in the event of a second febrile UTI.
The European Association of Urology - European Society for Paediatric Urology 2015 was the only guideline formulated by surgeons that addresses investigation and management of a first febrile UTI in children. It advocated VCUG to detect VUR [143] during the study period of this paper. The guideline has been recently updated, recommending VCUG following a first febrile UTI where an ultrasound was abnormal or the infection was due to an atypical organism [147]. The authors of the guideline did not reference any RCT and lament the low quality of evidence cited, acknowledging most of the recommendations are based on “panel and expert opinion” [148]. The relative lack of prospective well-designed studies involving elective surgical procedures (such as correction of VUR) is highlighted by data from major publicly-funded research bodies such as the UK’s National Institute for Health Research and Medical Research Council. Their combined spend on surgical research has been reported as <2% of the total research budget of £1.53 billion, even though 30% of National Health Service patients receive surgical care [149]. Similar funding levels are reported in other countries [137].
There appear to be two distinct directions in the literature on the investigation and management of febrile UTIs in children, one published predominantly by physicians in medical journals, and the other predominantly by surgeons in surgical journals. In undertaking this comparative study, we came to realize that, as physicians, almost all our reading has been in the medical literature, with the likelihood that surgeons may restrict their reading to the surgical literature. If we are to achieve consensus on the optimal management of conditions that cross boundaries between the physician and surgeon, as in the case of childhood febrile UTI, then improved collaboration in research as well as publications highlighting differences in points of view in both literatures is essential. Pediatric radiologists participate in multidisciplinary meetings and work closely with both pediatric nephrologists and surgeons. They may be in a good position to drive both research and consensus in the future.
How should the pediatric radiologist respond when asked to perform a VCUG in an otherwise normal child with a first febrile UTI? Firstly, the obvious disadvantages of the VCUG must be considered. Although a diagnostic study can be achieved with a relatively low radiation dose by using careful technique and modern equipment [150], in practice the range of doses is extremely wide [151]. It is worth noting that the European diagnostic reference level for dose-area product of 70 µGy·m2 for a child aged between one month and four years [150] corresponds to an effective dose of about 700 µSv, although this is at the upper end of a very wide range [151]. The risk of induced cancer from this exposure depends on a controversial conversion factor, but for the purposes of informed consent could reasonably be estimated at one in 5,000. Most VCUG procedures are uneventful, but severe complications have been reported, including fatal sepsis [152]. Finally, the psychological trauma of the procedure is difficult to quantify, but is probably significant, both for the patient and their carers [153, 154].
The risk of a child without other congenital abnormalities of the kidney and urinary tract developing chronic kidney disease as a result of repeated febrile UTIs associated with VUR is very low [3, 39, 155]. The shorter-term benefit of the possible prevention of further febrile UTIs by continuous antibiotic prophylaxis or a surgical intervention must be small, given that the probability of recurrence after a first febrile UTI is also known to be low [39]. One small non-randomized study showed no advantage in terms of quality of life in those children who underwent surgery over those managed non-surgically [156]. Studies with no control group are unlikely to provide useful data because the natural history of VUR is improvement through childhood. In the absence of a RCT of surgical versus non-surgical management of unselected patients found to have VUR after a first febrile UTI, there is no evidence that performing VCUG is of benefit in this context. Given that there are well-established risks of VCUG, it must be regarded as unwarranted. McAlister [152], writing nearly 50 years ago, gave sound and succinct advice: “Cystography is not a benign procedure. It can result in death and a host of complications. It should be undertaken only when its findings have a reasonable chance of altering patient management.”
Potential limitations of the approach used here include exclusion of papers published in languages other than English, and coverage of a limited, although recent, time period.
Conclusion
There are two distinct directions in the literature regarding the investigation of an uncomplicated first febrile UTI in a child. In general, when presented with a first febrile UTI in a child, physicians recommended fewer investigations and less treatment, in contrast to surgeons who advocated extensive investigation and aggressive intervention in the event that imaging detects an abnormality. This has the potential to confuse the carers of affected children.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
I.K.H. and G.M. performed the literature search. All authors contributed to writing the manuscript and approved the final version.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions Funded in part by the Simon Lee Foundation, simonleefoundation.com.au.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflicts of interest
None
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008;27:302–308. doi: 10.1097/INF.0b013e31815e4122. [DOI] [PubMed] [Google Scholar]
- 2.RIVUR Trial Investigators Antimicrobial prophylaxis for children with vesicoureteral reflux. N Engl J Med. 2014;370:2367–2376. doi: 10.1056/NEJMoa1401811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toffolo A, Ammenti A, Montini G. Long-term clinical consequences of urinary tract infections during childhood: a review. Acta Paediatr. 2012;101:1018–1031. doi: 10.1111/j.1651-2227.2012.02785.x. [DOI] [PubMed] [Google Scholar]
- 4.Montini G, Tullus K, Hewitt I. Febrile urinary tract infections in children. N Engl J Med. 2011;365:239–250. doi: 10.1056/NEJMra1007755. [DOI] [PubMed] [Google Scholar]
- 5.Trilla A, Aymerich M, Lacy AM, Bertran MJ. Phenotypic differences between male physicians, surgeons, and film stars: comparative study. BMJ. 2006;333:1291–1293. doi: 10.1136/bmj.39015.672373.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stirrat GM. Ethics and evidence based surgery. J Med Ethics. 2004;30:160–165. doi: 10.1136/jme.2003.007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management, Roberts KB Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128:595–610. doi: 10.1542/peds.2011-1330. [DOI] [PubMed] [Google Scholar]
- 8.R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/. Accessed 18 May 2023
- 9.Agostiniani R, Mariotti P. The natural history of vesicoureteral reflux. J Matern Fetal Neonatal Med. 2011;24:2–3. doi: 10.3109/14767058.2011.607557. [DOI] [PubMed] [Google Scholar]
- 10.Bhat RG, Katy TA, Place FC. Pediatric urinary tract infections. Emerg Med Clin North Am. 2011;29:637–653. doi: 10.1016/j.emc.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Finnell SME, Carroll AE, Downs SM, Subcommittee on Urinary Tract Infection Technical report-Diagnosis and management of an initial UTI in febrile infants and young children. Pediatrics. 2011;128:e749–770. doi: 10.1542/peds.2011-1332. [DOI] [PubMed] [Google Scholar]
- 12.Tullus K. Pediatrics: AAP recommends reduced imaging after first febrile UTI. Nat Rev Urol. 2011;9:11–12. doi: 10.1038/nrurol.2011.174. [DOI] [PubMed] [Google Scholar]
- 13.Keren R. Management of primary vesicoureteral reflux in children: editorial commentary. Pediatr Clin North Am. 2012;59:835–838. doi: 10.1016/j.pcl.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Burke M. Is it time to change how I diagnose, treat, and manage young children with urinary tract infections? Hosp Pediatr. 2012;2:45–46. doi: 10.1542/hpeds.2011-0017-2. [DOI] [PubMed] [Google Scholar]
- 15.Roberts KB. Revised AAP guideline on UTI in febrile infants and young children. Am Fam Physician. 2012;86:940–946. [PubMed] [Google Scholar]
- 16.Tullus K. What do the latest guidelines tell us about UTIs in children under 2 years of age. Pediatr Nephrol. 2012;27:509–511. doi: 10.1007/s00467-011-2077-5. [DOI] [PubMed] [Google Scholar]
- 17.Williams GJ, Hodson EH, Isaacs D, Craig JC. Diagnosis and management of urinary tract infection in children. J Paediatr Child Health. 2012;48:296–301. doi: 10.1111/j.1440-1754.2010.01925.x. [DOI] [PubMed] [Google Scholar]
- 18.Bitsori M, Galanakis E. Pediatric urinary tract infections: diagnosis and treatment. Expert Rev Anti Infect Ther. 2012;10:1153–1164. doi: 10.1586/eri.12.99. [DOI] [PubMed] [Google Scholar]
- 19.Davis A, Obi B, Ingram M. Investigating urinary tract infections in children. BMJ. 2013;346:e8654. doi: 10.1136/bmj.e8654. [DOI] [PubMed] [Google Scholar]
- 20.Kari JA, Tullus K. Controversy in urinary tract infection management in children: a review of new data and subsequent changes in guidelines. J Trop Pediatr. 2013;59:465–469. doi: 10.1093/tropej/fmt054. [DOI] [PubMed] [Google Scholar]
- 21.Paintsil E. Update on recent guidelines for the management of urinary tract infections in children: the shifting paradigm. Curr Opin Pediatr. 2013;25:88–94. doi: 10.1097/MOP.0b013e32835c14cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tullus K. A review of guidelines for urinary tract infections in children younger than 2 years. Pediatr Ann. 2013;42:52–56. doi: 10.3928/00904481-20130222-10. [DOI] [PubMed] [Google Scholar]
- 23.Williams G, Craig J. RIVUR trial offers confirmatory evidence for a small but real benefit of antibiotics for UTI prevention in children. Evid Based Med. 2014;19:229–230. doi: 10.1136/ebmed-2014-110070. [DOI] [PubMed] [Google Scholar]
- 24.Cara-Fuentes G, Gupta N, Garin EH. The RIVUR study: a review of its findings. Pediatr Nephrol. 2015;30:703–706. doi: 10.1007/s00467-014-3021-2. [DOI] [PubMed] [Google Scholar]
- 25.Simoes e Silva AC, Oliveira EA. Update on the approach of urinary tract infection in childhood. J Pediatr (Rio J) 2015;91:S2–10. doi: 10.1016/j.jped.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Stephens GM, Akers S, Nguyen H, Woxland H. Evaluation and management of urinary tract infections in the school-aged child. Prim Care. 2015;42:33–41. doi: 10.1016/j.pop.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Tasian G, Furth S. Narrowing the focus: what we now know (and still don't know) about antibiotic prophylaxis for children with vesicoureteral reflux. Am J Kidney Dis. 2015;65:214–216. doi: 10.1053/j.ajkd.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Tullus K. Vesicoureteric reflux in children. Lancet. 2015;385:371–379. doi: 10.1016/S0140-6736(14)60383-4. [DOI] [PubMed] [Google Scholar]
- 29.Hewitt IK, Montini G. Pediatric febrile urinary tract infections: the current state of play. Ital J Pediatr. 2011;37:57. doi: 10.1186/1824-7288-37-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korbel L, Howell M, Spencer JD. The clinical diagnosis and management of urinary tract infections in children and adolescents. Paediatr Int Child Health. 2017;37:273–279. doi: 10.1080/20469047.2017.1382046. [DOI] [PubMed] [Google Scholar]
- 31.Yeung CK, Chowdhary SK, Sreedhar B. Minimally invasive management for vesicoureteral reflux in infants and young children. Clin Perinatol. 2017;44:835–849. doi: 10.1016/j.clp.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Robinson JL, Le Saux N. Management of urinary tract infections in children in an era of increasing antimicrobial resistance. Expert Rev Anti Infect Ther. 2016;14:809–816. doi: 10.1080/14787210.2016.1206816. [DOI] [PubMed] [Google Scholar]
- 33.Traisman ES. Clinical management of urinary tract infections. Pediatr Ann. 2016;45:e108–111. doi: 10.3928/00904481-20160316-01. [DOI] [PubMed] [Google Scholar]
- 34.Morello W, La Scola C, Alberici I, Montini G. Acute pyelonephritis in children. Pediatr Nephrol. 2016;31:1253–1265. doi: 10.1007/s00467-015-3168-5. [DOI] [PubMed] [Google Scholar]
- 35.Delbet JD, Lorrot M, Ulinski T. An update on new antibiotic prophylaxis and treatment for urinary tract infections in children. Expert Opin Pharmacother. 2017;18:1619–1625. doi: 10.1080/14656566.2017.1383383. [DOI] [PubMed] [Google Scholar]
- 36.Sutton AG, Chandler N, Roberts KB. Recent studies on the care of first febrile urinary tract infection in infants and children for the pediatric hospitalist. Rev Recent Clin Trials. 2017;12:269–276. doi: 10.2174/1574887112666170816143639. [DOI] [PubMed] [Google Scholar]
- 37.Balighian E, Burke M. Urinary tract infections in children. Pediatr Rev. 2018;39:3–12. doi: 10.1542/pir.2017-0007. [DOI] [PubMed] [Google Scholar]
- 38.Finnell SME. Urinary tract infection in children: an update. Open J Urol Nephrol. 2015;295, 8:92–95. [Google Scholar]
- 39.Hewitt I, Montini G. Vesicoureteral reflux is it important to find? Pediatr Nephrol. 2021;36:1011–1017. doi: 10.1007/s00467-020-04573-9. [DOI] [PubMed] [Google Scholar]
- 40.Hewitt IK, Montini G. Kidney damage associated with vesicoureteric reflux. Curr Opin Pediatr. 2021;33:247–251. doi: 10.1097/MOP.0000000000000996. [DOI] [PubMed] [Google Scholar]
- 41.Veauthier B, Miller MV. Urinary tract infections in young children and infants: common questions and answers. Am Fam Physician. 2020;102:278–285. [PubMed] [Google Scholar]
- 42.Inouye A. Urologic conditions in infants and children: urinary tract infection and vesicoureteral reflux. FP Essent. 2020;488:25–34. [PubMed] [Google Scholar]
- 43.Thergaonkar RW, Hari P. Current management of urinary tractinfection and vesicoureteral reflux. Indian J Pediatr. 2020;87:625–632. doi: 10.1007/s12098-019-03099-9. [DOI] [PubMed] [Google Scholar]
- 44.Williams G, Craig JC. Long-term antibiotics for preventing recurrent urinary tract infection in children. Cochrane Database Syst Rev. 2019;4:CD001534. doi: 10.1002/14651858.CD001534.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garin EH. Primary vesicoureteral reflux; what have we learnt from the recently published randomized, controlled trials? Pediatr Nephrol. 2019;34:1513–1519. doi: 10.1007/s00467-018-4045-9. [DOI] [PubMed] [Google Scholar]
- 46.Simoes ESAC, Oliveira EA, Mak RH. Urinary tract infection in pediatrics: an overview. J Pediatr (Rio J) 2020;96:65–79. doi: 10.1016/j.jped.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bandari J, Docimo SG. Vesicoureteral reflux is a phenotype, not a disease: a population-centered approach to pediatric urinary tract infection. J Pediatr Urol. 2017;13:378–382. doi: 10.1016/j.jpurol.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 48.Desai DJ, Gilbert B, McBride CA. Paediatric urinary tract infections: diagnosis and treatment. Aust Fam Physician. 2016;45:558–563. [PubMed] [Google Scholar]
- 49.Lopez PJ, Celis S, Reed F, Zubieta R. Vesicoureteral reflux: current management in children. Curr Urol Rep. 2014;15:447. doi: 10.1007/s11934-014-0447-9. [DOI] [PubMed] [Google Scholar]
- 50.Petcu CT, Stehr E, Isaac JP, Desai D. Management of paediatric recurrent urinary tract infections and challenges in special patient populations. Aust J Gen Pract. 2021;50:458–464. doi: 10.31128/AJGP-03-21-5922. [DOI] [PubMed] [Google Scholar]
- 51.Becknell B, Schober M, Korbel L, Spencer JD. The diagnosis, evaluation and treatment of acute and recurrent pediatric urinary tract infections. Expert Rev Anti Infect Ther. 2015;13:81–90. doi: 10.1586/14787210.2015.986097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hudson A, Romao RLP, MacLellan D. Urinary tract infection in children. CMAJ. 2017;189:E608. doi: 10.1503/cmaj.160656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koyle MA, Shifrin D. Issues in febrile urinary tract infection management. Pediatr Clin North Am. 2012;59:909–922. doi: 10.1016/j.pcl.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 54.Roupakias S, Sinopidis X, Karatza A, Varvarigou A. Predictive risk factors in childhood urinary tract infection, vesicoureteral reflux, and renal scarring management. Clin Pediatr (Phila) 2014;53:1119–1133. doi: 10.1177/0009922813515744. [DOI] [PubMed] [Google Scholar]
- 55.Subcommittee on Urinary Tract Infection Reaffirmation of AAP Clinical Practice Guideline: the diagnosis and management of the initial urinary tract infection in febrile infants and young children 2–24 months of age. Pediatrics. 2016;138(6):e20163026. doi: 10.1542/peds.2016-3026. [DOI] [PubMed] [Google Scholar]
- 56.Caillaud C, Lacreuse I, Fothergill H, et al. Observational, medical or surgical management of vesicoureteric reflux. Acta Paediatr. 2013;102:222–225. doi: 10.1111/apa.12118. [DOI] [PubMed] [Google Scholar]
- 57.Mattoo TK, Shaikh N, Nelson CP. Contemporary management of urinary tract infection in children. Pediatrics. 2021;147(2):e2020012138. doi: 10.1542/peds.2020-012138. [DOI] [PubMed] [Google Scholar]
- 58.Lo V, Wah Y, Maggio L. FPIN's clinical inquiries: antibiotic prophylaxis to prevent recurrent UTI in children. Am Fam Physician. 2011;84:3–4. [PubMed] [Google Scholar]
- 59.Nagler EV, Williams G, Hodson EM, Craig JC (2011) Interventions for primary vesicoureteric reflux. Cochrane Database Syst Rev:CD001532 [DOI] [PubMed]
- 60.White B. Diagnosis and treatment of urinary tract infections in children. Am Fam Physician. 2011;83:409–415. [PubMed] [Google Scholar]
- 61.Brandstrom P, Hansson S. Long-term, low-dose prophylaxis against urinary tract infections in young children. Pediatr Nephrol. 2015;30:425–432. doi: 10.1007/s00467-014-2854-z. [DOI] [PubMed] [Google Scholar]
- 62.Mattoo TK, Thomas R. Routine prophylaxis is not necessary to prevent renal scarring in children with urinary tract infection. Evid Based Med. 2017;22:208. doi: 10.1136/ebmed-2017-110784. [DOI] [PubMed] [Google Scholar]
- 63.Larcombe J. Urinary tract infections in children: recurrent infections. Clin Evid. 2015;06:306. [PMC free article] [PubMed] [Google Scholar]
- 64.Coleman R. Early management and long-term outcomes in primary vesico-ureteric reflux. BJU Int. 2011;108:3–8. doi: 10.1111/j.1464-410X.2011.10698.x. [DOI] [PubMed] [Google Scholar]
- 65.Kutasy B, Coyle D, Fossum M. Urinary tract infection in children: management in the era of antibiotic resistance - a pediatric urologist's view. Eur Urol Focus. 2017;3:207–211. doi: 10.1016/j.euf.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 66.Storm DW, Braga LH, Cooper CS. Continuous antibiotic prophylaxis in pediatric urology. Urol Clin North Am. 2018;45:525–538. doi: 10.1016/j.ucl.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 67.Awais M, Rehman A, Baloch NU, et al. Evaluation and management of recurrent urinary tract infections in children: state of the art. Expert Rev Anti Infect Ther. 2015;13:209–231. doi: 10.1586/14787210.2015.991717. [DOI] [PubMed] [Google Scholar]
- 68.Koyle MA, Elder JS, Skoog SJ, et al. Febrile urinary tract infection, vesicoureteral reflux, and renal scarring: current controversies in approach to evaluation. Pediatr Surg Int. 2011;27:337–346. doi: 10.1007/s00383-011-2863-y. [DOI] [PubMed] [Google Scholar]
- 69.Wennerstrom M, Hansson S, Jodal U, et al. Renal function 16 to 26 years after the first urinary tract infection in childhood. Arch Pediatr Adolesc Med. 2000;154:339–345. doi: 10.1001/archpedi.154.4.339. [DOI] [PubMed] [Google Scholar]
- 70.Williams GJ, Craig JC, Carapetis JR. Preventing urinary tract infections in early childhood. Adv Exp Med Biol. 2013;764:211–218. doi: 10.1007/978-1-4614-4726-9_18. [DOI] [PubMed] [Google Scholar]
- 71.Sung J, Skoog S. Surgical management of vesicoureteral reflux in children. Pediatr Nephrol. 2012;27:551–561. doi: 10.1007/s00467-011-1933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arlen AM, Cooper CS. New trends in voiding cystourethrography and vesicoureteral reflux: Who, when and how? Int J Urol. 2019;26:440–445. doi: 10.1111/iju.13915. [DOI] [PubMed] [Google Scholar]
- 73.Tanaka ST, Brock JW., 3rd Pediatric urologic conditions, including urinary infections. Med Clin North Am. 2011;95:1–13. doi: 10.1016/j.mcna.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 74.Wan J, Skoog SJ, Hulbert WC, et al. Section on Urology response to new guidelines for the diagnosis and management of UTI. Pediatrics. 2012;129:e1051–1053. doi: 10.1542/peds.2011-3615. [DOI] [PubMed] [Google Scholar]
- 75.Rensing A, Austin P. The diagnosis and treatment of vesicoureteral reflux: an update. Open J Urol Nephrol. 2015;8:96–103. doi: 10.2174/1874303X01508010096. [DOI] [Google Scholar]
- 76.Roy P, Ramesh S, MittalMittal R, Mehta S. Emerging landscape of antibiotic resistance and use of endoscopic injection in vesicoureteral reflux. J Assoc Physicians India. 2018;66:68–72. [PubMed] [Google Scholar]
- 77.Schlager TA (2016) Urinary tract infections in infants and children. Microbiol Spectr 4(5) 10.1128/microbiolspec.UTI-0022-2016 [DOI] [PubMed]
- 78.Mishra OP, Abhinay A, Prasad R. Urinary infections in children. Indian J Pediatr. 2013;80:838–843. doi: 10.1007/s12098-013-1118-4. [DOI] [PubMed] [Google Scholar]
- 79.Afshar K. Randomized intervention for children with VesicoureReflux (RIVUR) Study: a new look at an old question? Commentary on antimicrobial prophylaxis for children with vesicoureteral reflux. Urology. 2014;84:991–992. doi: 10.1016/j.urology.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 80.Lee T, Park JM. Vesicoureteral reflux and continuous prophylactic antibiotics. Investig Clin Urol. 2017;58:S32–S37. doi: 10.4111/icu.2017.58.S1.S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hajiyev P, Burgu B. Contemporary management of vesicoureteral reflux. Eur Urol Focus. 2017;3:181–188. doi: 10.1016/j.euf.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 82.Greenfield SP. Antibiotic prophylaxis in pediatric urology: an update. Curr Urol Rep. 2011;12:126–131. doi: 10.1007/s11934-010-0164-y. [DOI] [PubMed] [Google Scholar]
- 83.Adibi A, Gheysari A, Azhir A, et al. Value of sonography in the diagnosis of mild, moderate and severe vesicoureteral reflux in children. Saudi J Kidney Dis Transpl. 2013;24:297–302. doi: 10.4103/1319-2442.109582. [DOI] [PubMed] [Google Scholar]
- 84.Bayram MT, Kavukcu S, Alaygut D, et al. Place of ultrasonography in predicting vesicoureteral reflux in patients with mild renal scarring. Urology. 2014;83:904–908. doi: 10.1016/j.urology.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 85.Garout WA, Kurdi HS, Shilli AH, Kari JA. Urinary tract infection in children younger than 5 years. Etiology and associated urological anomalies. Saudi Med J. 2015;36:497–501. doi: 10.15537/smj.2015.4.10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hannula A, Venhola M, Perhomaa M, et al. Imaging the urinary tract in children with urinary tract infection. Acta Paediatr. 2011;100:e253–259. doi: 10.1111/j.1651-2227.2011.02391.x. [DOI] [PubMed] [Google Scholar]
- 87.Hung TW, Tsai JD, Liao PF, Sheu JN. Role of renal ultrasonography in predicting vesicoureteral reflux and renal scarring in children hospitalized with a first febrile urinary tract infection. Pediatr Neonatol. 2016;57:113–119. doi: 10.1016/j.pedneo.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 88.Lee JH, Kim MK, Park SE. Is a routine voiding cystourethrogram necessary in children after the first febrile urinary tract infection? Acta Paediatr. 2012;101:e105–109. doi: 10.1111/j.1651-2227.2011.02507.x. [DOI] [PubMed] [Google Scholar]
- 89.Pauchard JY, Chehade H, Kies CZ, et al. Avoidance of voiding cystourethrography in infants younger than 3 months with Escherichia coli urinary tract infection and normal renal ultrasound. Arch Dis Child. 2017;102:804–808. doi: 10.1136/archdischild-2016-311587. [DOI] [PubMed] [Google Scholar]
- 90.Pennesi M, L'Erario I, Travan L, Ventura A. Managing children under 36 months of age with febrile urinary tract infection: a new approach. Pediatr Nephrol. 2012;27:611–615. doi: 10.1007/s00467-011-2087-3. [DOI] [PubMed] [Google Scholar]
- 91.Abdulnour HA, Williams JL, Kairalla JA, Garin EH. Does hydronephrosis predict the presence of severe vesicoureteral reflux? Eur J Pediatr. 2012;171:1605–1610. doi: 10.1007/s00431-012-1775-8. [DOI] [PubMed] [Google Scholar]
- 92.Pennesi M, Amoroso S, Pennesi G, et al. Is ultrasonography mandatory in all children at their first febrile urinary tract infection? Pediatr Nephrol. 2021;36:1809–1816. doi: 10.1007/s00467-020-04909-5. [DOI] [PubMed] [Google Scholar]
- 93.Kobayashi Y, Mishina H, Michihata N, et al. Indication for voiding cystourethrography during first urinary tract infection. Pediatr Int. 2019;61:595–600. doi: 10.1111/ped.13835. [DOI] [PubMed] [Google Scholar]
- 94.Alberici I, La Manna A, Pennesi M, et al. First urinary tract infections in children: the role of the risk factors proposed by the Italian recommendations. Acta Paediatr. 2019;108:544–550. doi: 10.1111/apa.14506. [DOI] [PubMed] [Google Scholar]
- 95.Shaki D, Hodik G, Elamour S, et al. Urinary tract infections in children < 2 years of age hospitalized in a tertiary medical center in Southern Israel: epidemiologic, imaging, and microbiologic characteristics of first episode in life. Eur J Clin Microbiol Infect Dis. 2020;39:955–963. doi: 10.1007/s10096-019-03810-w. [DOI] [PubMed] [Google Scholar]
- 96.Berry CS, Vander Brink BA, Koff SA, et al. Is VCUG still indicated following the first episode of urinary tract infection in boys? Urology. 2012;80:1351–1355. doi: 10.1016/j.urology.2012.03.073. [DOI] [PubMed] [Google Scholar]
- 97.Deader R, Tiboni SG, Malone PS, Fairhurst J. Will the implementation of the 2007 National Institute for Health and Clinical Excellence (NICE) guidelines on childhood urinary tract infection (UTI) in the UK miss significant urinary tract pathology? BJU Int. 2012;110:454–458. doi: 10.1111/j.1464-410X.2011.10801.x. [DOI] [PubMed] [Google Scholar]
- 98.Ristola MT, Hurme T. Consequences of following the new American Academy of Pediatrics guidelines for imaging children with urinary tract infection. Scand J Urol. 2015;49:419–423. doi: 10.3109/21681805.2015.1009485. [DOI] [PubMed] [Google Scholar]
- 99.Ristola MT, Loyttyniemi E, Hurme T. Factors associated with abnormal imaging and infection recurrence after a first febrile urinary tract infection in children. Eur J Pediatr Surg. 2017;27:142–149. doi: 10.1055/s-0036-1572418. [DOI] [PubMed] [Google Scholar]
- 100.Park YW, Kim MJ, Han SW, et al. Meaning of ureter dilatation during ultrasonography in infants for evaluating vesicoureteral reflux. Eur J Radiol. 2015;84:307–311. doi: 10.1016/j.ejrad.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 101.Kovanlikaya A, Kazam J, Dunning A, et al. The role of ultrasonography in predicting vesicoureteral reflux. Urology. 2014;84:1205–1210. doi: 10.1016/j.urology.2014.06.057. [DOI] [PubMed] [Google Scholar]
- 102.Schroeder AR, Abidari JM, Kirpekar R, et al. Impact of a more restrictive approach to urinary tract imaging after febrile urinary tract infection. Arch Pediatr Adolesc Med. 2011;165:1027–1032. doi: 10.1001/archpediatrics.2011.178. [DOI] [PubMed] [Google Scholar]
- 103.Friedman AA, Wolfe-Christensen C, Toffoli A, et al. History of recurrent urinary tract infection is not predictive of abnormality on voiding cystourethrogram. Pediatr Surg Int. 2013;29:639–643. doi: 10.1007/s00383-013-3301-0. [DOI] [PubMed] [Google Scholar]
- 104.Hua L, Linke RJ, Boucaut HA, Khurana S. Micturating cystourethrogram as a tool for investigating UTI in children - an institutional audit. J Pediatr Urol. 2016;12:292 e1–292.e5. doi: 10.1016/j.jpurol.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 105.Juliano TM, Stephany HA, Clayton DB, et al. Incidence of abnormal imaging and recurrent pyelonephritis after first febrile urinary tract infection in children 2 to 24 months old. J Urol. 2013;190:1505–1510. doi: 10.1016/j.juro.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kurtz MP, Chow JS, Johnson EK, et al. Imaging after urinary tract infection in older children and adolescents. J Urol. 2015;193:1778–1782. doi: 10.1016/j.juro.2014.10.119. [DOI] [PubMed] [Google Scholar]
- 107.Logvinenko T, Chow JS, Nelson CP. Predictive value of specific ultrasound findings when used as a screening test for abnormalities on VCUG. J Pediatr Urol. 2015;11:176 e171–177. doi: 10.1016/j.jpurol.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nelson CP, Johnson EK, Logvinenko T, Chow JS. Ultrasound as a screening test for genitourinary anomalies in children with UTI. Pediatrics. 2014;133:e394–403. doi: 10.1542/peds.2013-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ristola MT, Hurme T. NICE guidelines for imaging studies in children with UTI adequate only in boys under the age of 6 months. Pediatr Surg Int. 2013;29:215–222. doi: 10.1007/s00383-012-3257-5. [DOI] [PubMed] [Google Scholar]
- 110.Suson KD, Mathews R. Evaluation of children with urinary tract infection–impact of the 2011 AAP guidelines on the diagnosis of vesicoureteral reflux using a historical series. J Pediatr Urol. 2014;10:182–185. doi: 10.1016/j.jpurol.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 111.Nakamura M, Moriya K, Kon M, et al. Girls and renal scarring as risk factors for febrile urinary tract infection after stopping antibiotic prophylaxis in children with vesicoureteral reflux. World J Urol. 2021;39:2587–2595. doi: 10.1007/s00345-020-03524-1. [DOI] [PubMed] [Google Scholar]
- 112.Lee T, Varda BK, Venna A, et al. Changes in clinical presentation and renal outcomes among children with febrile urinary tract infection: 2005 vs 2015. J Urol. 2021;205:1764–1769. doi: 10.1097/JU.0000000000001597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Coulthard MG, Lambert HJ, Vernon SJ, et al. Guidelines to identify abnormalities after childhood urinary tract infections: a prospective audit. Arch Dis Child. 2014;99:448–451. doi: 10.1136/archdischild-2013-304429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sasaki J, Parajuli N, Sharma P, et al. Utility of post-urinary tract infection imaging in patients with normal prenatal renal ultrasound. Clin Pediatr (Phila) 2012;51:244–246. doi: 10.1177/0009922811420712. [DOI] [PubMed] [Google Scholar]
- 115.Ghobrial EE, Abdelaziz DM, Sheba MF, Abdel-Azeem YS. Value of ultrasound in detecting urinary tract anomalies after first febrile urinary tract infection in children. Clin Pediatr (Phila) 2016;55:415–420. doi: 10.1177/0009922815590224. [DOI] [PubMed] [Google Scholar]
- 116.Capone MA, Balestracci A, Toledo I, Martin SM. Diagnosis of vesicoureteral reflux according to the 1999 and 2011 guidelines of the Subcommittee on Urinary Tract Infection of the American Academy of Pediatrics. Arch Argent Pediatr. 2016;114:129–134. doi: 10.5546/aap.2016.eng.129. [DOI] [PubMed] [Google Scholar]
- 117.Kimata T, Kitao T, Yamanouchi S, et al. Voiding cystourethrography is mandatory in infants with febrile urinary tract infection. Tohoku J Exp Med. 2013;231:251–255. doi: 10.1620/tjem.231.251. [DOI] [PubMed] [Google Scholar]
- 118.Pokrajac D, Sefic-Pasic I, Begic A. Vesicoureteral reflux and renal scarring in infants after the first febrile urinary tract infection. Med Arch. 2018;72:272–275. doi: 10.5455/medarh.2018.72.272-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Massanyi EZ, Preece J, Gupta A, et al. Utility of screening ultrasound after first febrile UTI among patients with clinically significant vesicoureteral reflux. Urology. 2013;82:905–909. doi: 10.1016/j.urology.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 120.Sinha R, Mukherjee D, Sengupta J, et al. Yield of imaging performed as per Indian Society of Pediatric Nephrology guidelines in children with urinary tract infection. Indian Pediatr. 2017;54:749–751. doi: 10.1007/s13312-017-1168-1. [DOI] [PubMed] [Google Scholar]
- 121.Hadjipanayis A, Grossman Z, Del Torso S, et al. Current primary care management of children aged 1–36 months with urinary tract infections in Europe: large scale survey of paediatric practice. Arch Dis Child. 2015;100:341–347. doi: 10.1136/archdischild-2014-306119. [DOI] [PubMed] [Google Scholar]
- 122.Judkins A, Pascoe E, Payne D. Management of urinary tract infection in a tertiary children's hospital before and after publication of the NICE guidelines. Arch Dis Child. 2013;98:521–525. doi: 10.1136/archdischild-2012-303032. [DOI] [PubMed] [Google Scholar]
- 123.Platt C, Larcombe J, Dudley J, et al. Implementation of NICE guidance on urinary tract infections in children in primary and secondary care. Acta Paediatr. 2015;104:630–637. doi: 10.1111/apa.12979. [DOI] [PubMed] [Google Scholar]
- 124.Hari P, Hari S, Sinha A, et al. Antibiotic prophylaxis in the management of vesicoureteric reflux: a randomized double-blind placebo-controlled trial. Pediatr Nephrol. 2015;30:479–486. doi: 10.1007/s00467-014-2943-z. [DOI] [PubMed] [Google Scholar]
- 125.Hewitt IK, Pennesi M, Morello W, et al. Antibiotic prophylaxis for urinary tract infection-related renal scarring: a systematic review. Pediatrics. 2017;139:e20163145. doi: 10.1542/peds.2016-3145. [DOI] [PubMed] [Google Scholar]
- 126.Liao PF, Ku MS, Tsai JD, et al. Comparison of procalcitonin and different guidelines for first febrile urinary tract infection in children by imaging. Pediatr Nephrol. 2014;29:1567–1574. doi: 10.1007/s00467-014-2801-z. [DOI] [PubMed] [Google Scholar]
- 127.Nelson CP, Hubert KC, Kokorowski PJ, et al. Long-term incidence of urinary tract infection after ureteral reimplantation for primary vesicoureteral reflux. J Pediatr Urol. 2013;9:92–98. doi: 10.1016/j.jpurol.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 128.Brandstrom P, Jodal U, Sillen U, Hansson S. The Swedish reflux trial: review of a randomized, controlled trial in children with dilating vesicoureteral reflux. J Pediatr Urol. 2011;7:594–600. doi: 10.1016/j.jpurol.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 129.Chertin B, Arafeh WA, Zeldin A, et al. Endoscopic correction of VUR using Vantris as a new non-biodegradable tissue augmenting substance: three years of prospective follow-up. Urology. 2013;82:201–204. doi: 10.1016/j.urology.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 130.Van Batavia JP, Ahn JJ, Fast AM, et al. Prevalence of urinary tract infection and vesicoureteral reflux in children with lower urinary tract dysfunction. J Urol. 2013;190:1495–1499. doi: 10.1016/j.juro.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 131.Guyatt GH. ACP Journal Club A16. Ann Intern Med. 1991;114(Suppl):2. [Google Scholar]
- 132.Kamerow D. Milestones, tombstones, and sex education. BMJ. 2007;334:0–a. [Google Scholar]
- 133.McGee RG, Craig JC, Rogerson TE, Webster AC. Systematic reviews of surgical procedures in children: quantity, coverage and quality. J Paediatr Child Health. 2013;49:319–324. doi: 10.1111/jpc.12156. [DOI] [PubMed] [Google Scholar]
- 134.Coulthard MG. Vesicoureteric reflux is not a benign condition. Pediatr Nephrol. 2009;24:227–232. doi: 10.1007/s00467-008-0911-1. [DOI] [PubMed] [Google Scholar]
- 135.Stirrat GM, Farrow SC, Farndon J, Dwyer N. The challenge of evaluating surgical procedures. Ann R Coll Surg Engl. 1992;74:80–84. [PMC free article] [PubMed] [Google Scholar]
- 136.Sniderman AD, LaChapelle KJ, Rachon NA, Furberg CD. The necessity for clinical reasoning in the era of evidence-based medicine. Mayo Clin Proc. 2013;88:1108–1114. doi: 10.1016/j.mayocp.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 137.Weil RJ. The future of surgical research. PLoS Med. 2004;1:e13. doi: 10.1371/journal.pmed.0010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hewitt I, Montini G. Vesicoureteral reflux is it important to find? Pediatr Nephrol. 2021;36:1011–1017. doi: 10.1007/s00467-020-04573-9. [DOI] [PubMed] [Google Scholar]
- 139.National Institute of Health and Clinical Excellence (2007) Urinary tract infection in children: diagnosis, treatment and longterm management. https://pubmed.ncbi.nlm.nih.gov/21290637/ [DOI] [PubMed]
- 140.Ammenti A, Cataldi L, Chimenz R, et al. Febrile urinary tract infections in young children: recommendations for the diagnosis, treatment and follow-up. Acta Paediatr. 2012;101:451–457. doi: 10.1111/j.1651-2227.2011.02549.x. [DOI] [PubMed] [Google Scholar]
- 141.McTaggart S, Danchin M, Ditchfield M, et al. KHA-CARI guideline: diagnosis and treatment of urinary tract infection in children. Nephrology (Carlton) 2015;20:55–60. doi: 10.1111/nep.12349. [DOI] [PubMed] [Google Scholar]
- 142.Robinson JL, Finlay JC, Lang ME, et al. Urinary tract infections in infants and children: diagnosis and management. Paediatr Child Health. 2014;19:315–325. doi: 10.1093/pch/19.6.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stein R, Dogan HS, Hoebeke P, et al. Urinary tract infections in children: EAU/ESPU guidelines. Eur Urol. 2015;67:546–558. doi: 10.1016/j.eururo.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 144.International Reflux Study Committee Medical versus surgical treatment of primary vesicoureteral reflux: report of the International Reflux Study Committee. Pediatrics. 1981;67:392–400. doi: 10.1542/peds.67.3.392. [DOI] [PubMed] [Google Scholar]
- 145.Birmingham Reflux Study Group (1983) Prospective trial of operative versus non-operative treatment of severe vesicoureteric reflux: two years' observation in 96 children. Br Med J (Clin Res Ed) 287:171–174 [DOI] [PMC free article] [PubMed]
- 146.Smellie JM, Barratt TM, Chantler C, et al. Medical versus surgical treatment in children with severe bilateral vesicoureteric reflux and bilateral nephropathy: a randomised trial. Lancet. 2001;357:1329–1333. doi: 10.1016/S0140-6736(00)04520-7. [DOI] [PubMed] [Google Scholar]
- 147.t Hoen LA, Bogaert G, Radmayr C, et al. Update of the EAU/ESPU guidelines on urinary tract infections in children. J Pediatr Urol. 2021;17:200–207. doi: 10.1016/j.jpurol.2021.01.037. [DOI] [PubMed] [Google Scholar]
- 148.Radmayr C, Tekgul S. Paediatric urology and the dilemma of low-quality evidence for the management of common urological conditions (vesicoureteral reflux, lower urinary tract dysfunction, undescended testis) in children. Eur Urol Focus. 2017;3:308–309. doi: 10.1016/j.euf.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 149.Chan JK, Shalhoub J, Gardiner MD, et al. Strategies to secure surgical research funding: fellowships and grants. JRSM Open. 2014;5:2042533313505512. doi: 10.1177/2042533313505512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ashworth E, Woods L, Cook JV. Diagnostic reference levels in paediatric fluoroscopy: how does a secondary referral centre compare with 2018 European guidelines? Br J Radiol. 2021;94:20201269. doi: 10.1259/bjr.20201269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tzanis E, Raissaki M, Konstantinos A, et al. Radiation exposure to infants undergoing voiding cystourethrography: the importance of the digital imaging technology. Phys Med. 2021;85:123–128. doi: 10.1016/j.ejmp.2021.05.006. [DOI] [PubMed] [Google Scholar]
- 152.McAlister WH, Cacciarelli A, Shackelford GD. Complications associated with cystography in children. Radiology. 1974;111:167–172. doi: 10.1148/111.1.167. [DOI] [PubMed] [Google Scholar]
- 153.Volkl-Kernstock S, Felber M, Schabmann A, et al. Comparing stress levels in children aged 2–8 years and in their accompanying parents during first-time versus repeated voiding cystourethrograms. Wien Klin Wochenschr. 2008;120:414–421. doi: 10.1007/s00508-008-1001-x. [DOI] [PubMed] [Google Scholar]
- 154.Quas JA, Goodman GS, Bidrose S, et al. Emotion and memory: children's long-term remembering, forgetting, and suggestibility. J Exp Child Psychol. 1999;72:235–270. doi: 10.1006/jecp.1999.2491. [DOI] [PubMed] [Google Scholar]
- 155.Pepper RJ, Trompeter RS. The causes and consequences of paediatric kidney disease on adult nephrology care. Pediatr Nephrol. 2022;37:1245–1261. doi: 10.1007/s00467-021-05182-w. [DOI] [PubMed] [Google Scholar]
- 156.Yao DF, Weinberg AC, Penna FJ, et al. Quality of life in children with vesicoureteral reflux as perceived by children and parents. J Pediatr Urol. 2011;7:261–265. doi: 10.1016/j.jpurol.2011.02.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.


