Abstract
Background:
We aimed to describe nephrotoxic medication exposure and investigate associations between exposure and acute kidney injury (AKI) in the neonatal intensive care unit during the first postnatal week.
Design/Methods:
Secondary analysis of the AWAKEN cohort. We evaluated nephrotoxic medication exposure during the first postnatal week and associations with AKI using time-varying Cox proportional hazard regressions models.
Results:
Of 2162 neonates, 1616 (74.7%) received ≥1 nephrotoxic medication. Aminoglycoside receipt was most common (72%). AKI developed in 211(9.8%) neonates and was associated with a nephrotoxic medication exposure (p<0.01). Nephrotoxic medication excluding aminoglycoside (aHR 3.14, 95% CI 1.31-7.55) and aminoglycoside with another nephrotoxic medication (aHR 4.79, 95% CI 2.19-10.50) were independently associated with AKI and severe AKI (stage 2/3), respectively.
Conclusions:
Nephrotoxic medication exposure in critically ill infants is common during the first postnatal week. Specific nephrotoxic medication exposure, principally aminoglycosides with another nephrotoxic medication, are independently associated with early AKI.
Keywords: renal disease, renal failure, nephrotoxicity, kidney failure
Introduction
Studies confirm acute kidney injury (AKI) occurs commonly in critically ill neonates and is associated with adverse outcomes irrespective of the specific sub-population being studied.1, 2, 3, 4 Nephrotoxic medications are a potentially preventable cause of AKI, particularly in neonates in the neonatal intensive care unit (NICU), but remain understudied.5, 6, 7, 8, 9 A few single center studies have systematically evaluated nephrotoxic medication exposures in critically ill neonates, but these have primarily been conducted in only the most premature infants.5, 8 These studies suggest nearly all extremely premature neonates are exposed to at least one nephrotoxic medication during their hospital stay, and the neonates with the greatest cumulative exposure are at the highest risk for AKI. A multicenter evaluation of the epidemiology and impact of nephrotoxic medication exposures in a broad neonatal population was recently published but utilized an administrative database with a low rate of reported AKI.7 While this study is helpful, there are significant limitations encountered when using billing codes for AKI identification, in part due to the low rates of AKI recognition among providers. Thus, a multicenter evaluation detailing nephrotoxic medication exposures and associations with AKI using creatinine-based definitions may improve our understanding of the epidemiology of nephrotoxic medication exposures in critically ill neonates and help to delineate opportunities to improve care.
The Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) study conducted by the Neonatal Kidney Collaborative captured data on all high-risk neonates admitted to the NICU across 24 institutions between January 1 and March 31, 2014.10 AWAKEN documented that 30% of critically ill neonates had at least 1 episode of AKI, and AKI was associated with increased length of stay and mortality.
The current study is a secondary analysis of the AWAKEN database that seeks to: describe the epidemiology of nephrotoxic medication exposure in the first postnatal week for neonates in the NICU and investigate associations between nephrotoxic medication exposure and subsequent early AKI (i.e., AKI within first week of life). We hypothesized that nephrotoxic medication exposures would be common in high-risk neonates during the first postnatal week and these exposures would be associated with early AKI.
Methods
Study Population
The methodology and protocol for the AWAKEN study have previously been published; specific inclusion and exclusion criteria were designed to ensure inclusion of only those neonates at highest risk for AKI.10, 11 Each center received approval from their Institutional Review Board or Human Research Ethics Committee.
Data Collection
A detailed description of data collection for AWAKEN has been previously published.10, 11
Nephrotoxic Medications
The primary exposure was a nephrotoxic medication during the first postnatal week. Nephrotoxic medications of interest included acyclovir, amphotericin B, aminoglycosides, piperacillin-tazobactam, vancomycin, and nonsteroidal anti-inflammatory drugs (NSAIDS: including indomethacin and ibuprofen). Given the frequency with which aminoglycosides are prescribed in this population, aminoglycosides were considered separately. Nephrotoxic medication exposure categories were defined as: no nephrotoxic medication, nephrotoxic medications excluding aminoglycosides, aminoglycoside alone, and aminoglycoside and another nephrotoxic medication.
In neonates <29 weeks of gestational age at birth, aminoglycosides are routinely prescribed at intervals of every 36-48 hours. To capture the number of days exposed, we included all days of presumed aminoglycoside coverage in the blood stream. To do this, in the total number of days exposed, we included days when a dose was administered (i.e., “dosed” days) as well as days when an aminoglycosides dose was not given (i.e., “non-dosed” days) if a dose had been given on the days preceding and following the ‘non-dosed’ day.
Acute Kidney Injury Definition
The primary outcome of interest was early neonatal AKI, defined by the modified, neonatal Kidney Disease: Improving Global Outcomes (KDIGO) definition, using the serum creatinine criteria only, and limited to the first postnatal week.12 Severe AKI was defined as KDIGO stage 2 or higher. Urine output was not used to diagnose AKI for this analysis as these data were not reliably collected across all study centers.
Statistical Analysis
Baseline maternal and neonatal characteristics were compared between neonates among the four nephrotoxic medication exposure categories using chi-square tests for categorical variables and Student’s t tests or analysis of variance (or Kruskal-Wallis if appropriate) for continuous variables. Details of nephrotoxic medication exposure including number of patients exposed and cumulative days of exposure during the first postnatal week are detailed for the entire cohort and examined by gestational age categories (22-28, 29-35, and ≥36 weeks of gestational age). Evaluation was performed for each individual nephrotoxic medication (acyclovir, amphotericin B, aminoglycosides, piperacillin-tazobactam, vancomycin), for any NSAID (i.e., including indomethacin and ibuprofen), and for indomethacin and ibuprofen individually. Total potential cumulative days of possible exposure were calculated based on whether the neonate was under follow-up for a given day during the first postnatal week. Comparisons were made between gestational age categories and medication exposure using chi-square tests and between cumulative days and days hospitalized during the first week of life using Poisson means test. Similarly, nephrotoxic medication exposure was detailed by early AKI status. Heat maps were constructed by postnatal day for the study population overall and by AKI stage (i.e., no AKI, stage 1 AKI, and stage 2/3 “severe” AKI) by calculating for each combination of postnatal day and gestational age, the average number of nephrotoxic medication that were administered that day.
To examine the association between nephrotoxic medication exposure and AKI, a time-varying Cox proportional hazard regressions model was used to estimate hazard ratios (HRs) and associated 95% confidence intervals (CIs). The inclusion of nephrotoxic medication exposure category as a time-varying covariate allows subjects to contribute follow-up time to multiple exposure categories (e.g. a neonate receiving aminoglycoside and another nephrotoxic medication could contribute time as no nephrotoxic medication exposure, aminoglycoside alone, and aminoglycoside plus another nephrotoxic medication). Findings were reported as crude and adjusted HRs with associated 95% CIs. Potential confounders were entered into the model as a covariate if they were statistically significant in the baseline maternal and neonatal characteristic analysis (p<0.05). Checks for proportionality suggested that gestational age was non-proportional; as a result, models were stratified by gestational age category (i.e., 22-28, 29-35, and ≥36 weeks of gestational age) to account for differences in the baseline risk and non-proportional changes in hazard of AKI by gestational age. Adjusted models included 5-minute Apgar score, gestational age captured as a continuous value, birthweight, maternal hypertension, maternal bacterial infection, out-born delivery, mode of delivery, and neonatal sex, race, and ethnicity. A secondary analysis including only those neonates with follow-up and data available beyond three days of life was also conducted.
All analyses were performed using SAS (version 9.4; SAS Institute, Inc. Cary, NC, USA), and statistical significance was determined a priori to be at the level α=0.05 level. All methods and results are reported in accordance with the STROBE guidelines.13
Results
Nephrotoxic Medication Epidemiology
Of the 2,162 enrolled neonates, nearly 75% (1,616/2,162) were exposed to at least 1 nephrotoxic medication within the first postnatal week. When evaluating the exposures using the pre-determined exposure categories, 546 (25.3%) received no nephrotoxic medication, 62 (2.9%) received a nephrotoxic medication excluding aminoglycoside, 1,315 (60.8%) received an aminoglycoside alone, and 239 (11.1%) received an aminoglycoside with another nephrotoxic medication (Table 1). In our cohort, 276 (12.8%) were born at 22-28 weeks of gestational age, 958 (44.3%) were born at 29-35 weeks of gestational age, and 928 (42.9%) were born at ≥36 weeks of gestational age.
Table 1.
Comparison of Abridged Maternal and Neonatal Characteristics by Administration of Nephrotoxic Medications Category
| No nephrotoxic medication (n=546) |
Nephrotoxic medication, excluding aminoglycosides (n=62) |
Aminoglycoside alone (n=1315) |
Aminoglycoside and another nephrotoxic medication (n=239) |
p value* | |
|---|---|---|---|---|---|
| MATERNAL CHARACTERISTICS | |||||
| Hypertensive diseases during pregnancy (%)** | 166 (30.4) | 9 (14.5) | 239 (18.2) | 38 (15.9) | <0.0001 |
| Maternal infection (%) | |||||
| Bacterial | 30 (5.5) | 6 (9.7) | 140 (10.6) | 25 (10.5) | 0.0056 |
| Viral | 11 (2.0) | 0 (0.0) | 39 (3.0) | 11 (4.6) | 0.1135 |
| Mode of delivery (%) | |||||
| Vaginal | 188 (34.5) | 41 (66.1) | 563 (42.8) | 93 (38.9) | <0.0001 |
| Scheduled C-section | 102 (18.7) | 6 (9.7) | 157 (11.9) | 18 (7.5) | |
| Unscheduled C-section | 209 (38.3) | 12 (19.4) | 535 (40.7) | 120 (50.2) | |
| Unknown | 46 (8.4) | 3 (4.8) | 60 (4.6) | 8 (3.3) | |
| NEONATAL CHARACTERISTICS | |||||
| Mean gestational age±SD (weeks) | 35.0±3.6 | 36.0±4.5 | 34.1±4.3 | 31.4±6.1 | <0.0001 |
| <29 weeks of gestation (%) | 23 (4.2) | 6 (9.7) | 142 (10.8) | 105 (43.9) | <0.0001 |
| 29-35 weeks of gestation (%) | 253 (46.3) | 14 (22.6) | 647 (49.2) | 44 (18.4) | |
| ≥36 weeks of gestation (%) | 270 (49.5) | 42 (67.7) | 526 (40.0) | 90 (37.7) | |
| Mean birth weight±SD (g) | 2400±933 | 2547±960 | 2313±957 | 1886±1208 | <0.0001 |
| Male sex (%) | 280 (51.3) | 34 (54.8) | 787 (59.8) | 132 (55.2) | 0.0072 |
| Race (%) | |||||
| White | 308 (56.4) | 31 (50.0) | 739 (56.2) | 134 (56.1) | 0.0096 |
| Black | 115 (21.1) | 4 (6.5) | 251 (19.1) | 43 (18.0) | |
| Other | 123 (22.5) | 27 (43.5) | 325 (24.7) | 62 (25.9) | |
| Ethnicity (%) | |||||
| Hispanic/Latino | 78 (14.3) | 2 (3.2) | 193 (14.7) | 20 (8.4) | 0.0002 |
| Non-Hispanic/Latino | 367 (67.2) | 53 (85.5) | 910 (69.2) | 195 (81.6) | |
| Unknown | 101 (18.5) | 7 (11.3) | 212 (16.1) | 24 (10.0) | |
| Outborn (%) | 228 (41.8) | 47 (75.8) | 511 (38.9) | 112 (46.9) | <0.0001 |
| Caffeine exposure (%) | 67 (12.3) | 9 (14.5) | 359 (27.3) | 102 (42.7) | <0.0001 |
| Median 1-minute Apgar [IQR] | 8 [5-8] | 8 [5-9] | 7 [4-8] | 6 [3-8] | <0.0001 |
| Median 5-minute Apgar [IQR] | 9 [7-9] | 9 [8-9] | 8 [7-9] | 8 [6-9] | <0.0001 |
Estimated from a chi-square for categorical and t-test or Kruskal-Wallis test for continuous variables
Includes pre-eclampsia, eclampsia, chronic hypertension
Nearly all examined maternal and neonatal characteristics varied by exposure group (Table 1 includes abridged variables pertinent to analysis; supplemental Table 1 includes all maternal and neonatal variables).
Table 2 shows the percent of patients exposed and the number of cumulative patient-days exposed for each of the nephrotoxic medications. Aminoglycosides were the most commonly administered nephrotoxic medication with nearly 72% of neonates (1,554/2,162) receiving an aminoglycoside (alone or in combination with another nephrotoxic medication), with a cumulative exposure of almost 6,000 days during the first postnatal week (41.5% of all possible patient-days). Aminoglycoside receipt varied by gestational age. NSAIDs were the second most commonly administered nephrotoxic medication, specifically indomethacin. When examining each specific nephrotoxic medication among the gestational age categories, the highest proportion of neonates exposed to each specific medication were infants born between 22-28 weeks of gestation for all nephrotoxic medications except acyclovir and piperacillin-tazobactam which were both most commonly administered to neonates ≥36 weeks of gestational age. Specific nephrotoxic medication receipt and exposure duration during the first week of life varied significantly between gestational age categories for all medications except amphotericin B and piperacillin-tazobactam.
Table 2.
Exposure to Nephrotoxic Medications by Medication and Weeks Gestational Age Category
| Specific Nephrotoxic Medication/Group |
Whole population (n=2,162) |
WGA: 22-28 weeks (n=276) |
WGA: 29-35 weeks (n=958) |
WGA: ≥36 weeks (n=928) |
p value** |
|---|---|---|---|---|---|
| Total days hospitalized (N) | 14,355 | 1,908 | 6,522 | 5,925 | <0.0001 |
| Acyclovir | |||||
| N (%) of patients exposed | 83 (3.8) | 2 (0.7) | 12 (1.3) | 69 (7.5) | <0.0001 |
| Cumulative days* (% total) | 201 (1.4) | 3 (0.2) | 33 (0.5) | 165 (2.8) | <0.0001 |
| Amphotericin B | |||||
| N (%) of patients exposed | 9 (0.4) | 2 (0.7) | 3 (0.3) | 4 (0.4) | 0.6428 |
| Cumulative days* (% total) | 17 (0.1) | 6 (0.3) | 3 (0.0) | 8 (0.1) | 0.3031 |
| Aminoglycoside | |||||
| N (%) of patients exposed | 1,554 (71.8) | 247 (89.5) | 691 (72.1) | 616 (66.4) | <0.0001 |
| Cumulative days* (% total) | 5,964 (41.5) | 1,136 (59.5) | 2,684 (41.2) | 2,144 (36.2) | <0.0001 |
| Piperacillin-tazobactam | |||||
| N (%) of patients exposed | 66 (3.1) | 6 (2.2) | 28 (2.9) | 32 (3.4) | 0.5311 |
| Cumulative days* (% total) | 229 (1.6) | 10 (0.5) | 110 (1.7) | 109 (1.8) | <0.0001 |
| Vancomycin | |||||
| N (%) of patients exposed | 71 (3.3) | 18 (6.5) | 17 (1.8) | 36 (3.9) | 0.0002 |
| Cumulative days* (% total) | 189 (1.3) | 35 (1.8) | 46 (0.7) | 108 (1.8) | <0.0001 |
| Any NSAID | |||||
| N (%) of patients exposed | 106 (4.9) | 95 (34.4) | 9 (0.9) | 2 (0.2) | <0.0001 |
| Cumulative days* (% total) | 283 (2.0) | 254 (13.3) | 25 (0.4) | 4 (0.1) | <0.0001 |
| Indomethacin | |||||
| N (%) of patients exposed | 83 (3.8) | 78 (28.3) | 4 (0.4) | 1 (0.1) | <0.0001 |
| Cumulative days* (% total) | 225 (1.6) | 212 (11.) | 10 (0.2) | 3 (0.1) | <0.0001 |
| Ibuprofen | |||||
| N (%) of patients exposed | 23 (1) | 17 (6) | 5 (0.5) | 1 (0.1) | <0.0001 |
| Cumulative days* (% total) | 58 (0.4) | 42 (2.2) | 15 (0.2) | 1 (0.0) | <0.0001 |
Cumulative days in first week of life. NSAID, nonsteroidal anti-inflammatory drug
p-value comparing gestational age categories and medication exposure estimate from chi-square test and for cumulative days and days hospitalized using a Poisson means test.
Primary outcome: AKI in the First Postnatal Week
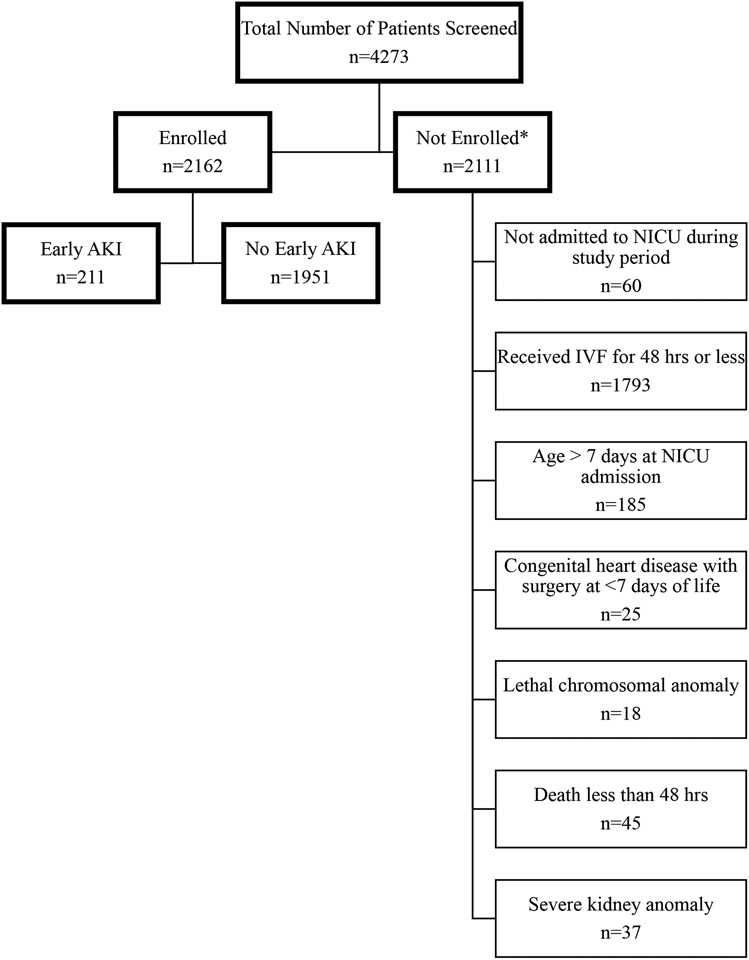
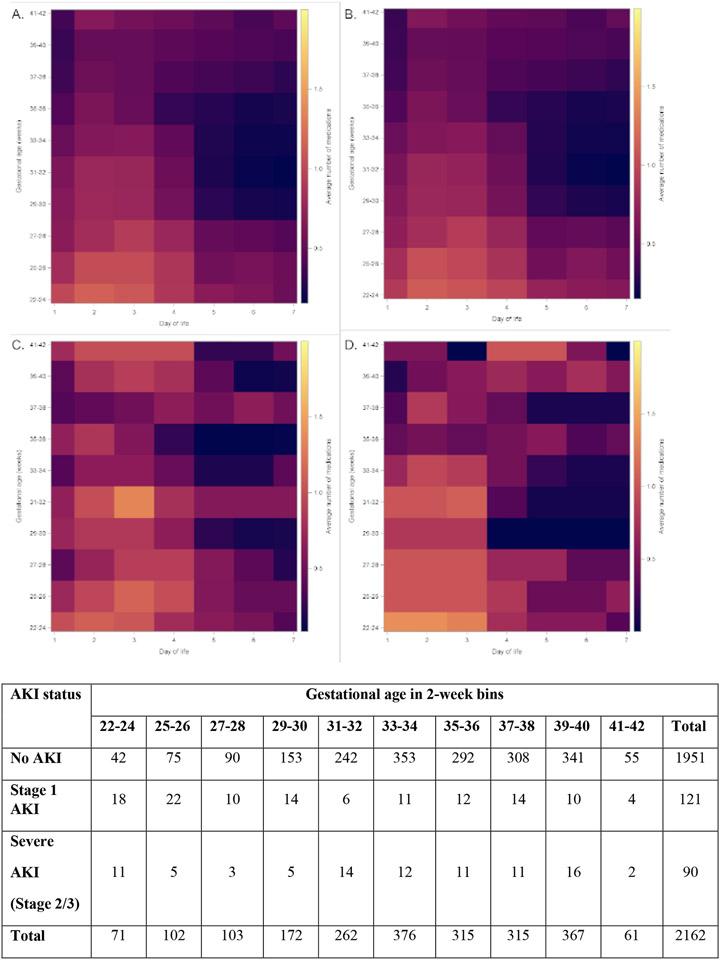
Early AKI of any stage occurred in 211 (9.8%), and 90 neonates experienced severe early AKI (4.2% of total cohort, 19.7% of patients with AKI; Figure 1). Supplemental Table 2 details the specific nephrotoxic medication exposures by AKI stage. For each specific nephrotoxic medication, the highest proportion of exposed patients experienced stage 1 AKI. Figure 2 includes heat maps depicting average number of nephrotoxic medications by both weeks of gestational age and day of life. In most cases, regardless of specific cohort, the most frequent nephrotoxic medication exposures occur in the first days after birth.
Figure 1. Study Flow Diagram.
AKI, acute kidney injury; NICU, neonatal intensive care unit; IVF, intravenous fluids; hrs, hours. *some patients were excluded for more than one reason.
Figure 2. Heat Map of Nephrotoxic Medication Exposure by Gestational Age and Postnatal Day.
Heat maps demonstrating average number of nephrotoxic medications (y-axis on right) by gestational age groups (2-week bins, y-axis on left) and day of life (x-axis). Panel A. All neonates in cohort (n=2,162). Panel B. All neonates without early AKI (n=1,951). Panel C. All neonates with early, stage 1 AKI (n=121). Panel D. All neonates with early, severe AKI (n=90)
Overall, nephrotoxic medication exposure was associated with AKI (Table 3). Specifically, compared to those who were not exposed to a nephrotoxic medication, those exposed to a nephrotoxic medication excluding an aminoglycoside had an increased hazard of any stage early AKI (HR 2.65, 95% CI 1.13-6.23), and nephrotoxic medication receipt excluding an aminoglycoside remained independently associated with any stage early AKI (aHR 3.14, 95% CI 1.31-7.55) after adjusting for potential confounders. Nephrotoxic medications excluding aminoglycoside receipt was not associated with severe AKI. Among those neonates with ≥3 days of follow-up data available (n=2,060), nephrotoxic medication excluding aminoglycoside receipt was associated with increased hazard for any stage early AKI (HR 3.00, 95% CI 1.03-8.78), and this increased hazard for any stage early AKI persisted after adjustment for confounders (aHR 3.54, 95% CI 1.18-10.64).
Table 3.
Crude and Adjusted Hazards Ratios* (HRs) and Associated 95% Confidence Intervals (CIs) for the Association between Nephrotoxic Medication and Aminoglycoside Administration and Acute Kidney Injury
| No nephrotoxic medication (n=546) |
Nephrotoxic medication, excluding aminoglycoside (n=62) |
Aminoglycoside alone (n=1315) |
Aminoglycoside and another nephrotoxic medication (n=239) |
|
|---|---|---|---|---|
| OVERALL | ||||
| Any Stage AKI | ||||
| N, % | 28 (5.1) | 9 (14.5) | 126 (9.6) | 48 (20.1) |
| Crude HR (95% CI) | Referent | 2.65 (1.13-6.23) | 1.26 (0.88-1.80) | 1.80 (1.03-3.13) |
| Adjusted HR (95% CI) | Referent | 3.14 (1.31-7.55) | 1.25 (0.86-1.82) | 1.68 (0.95-2.97) |
| Stage 2 or 3 AKI | ||||
| N, % | 15 (14.3) | 4 (18.2) | 52 (20.3) | 19 (26.0) |
| Crude HR (95% CI) | Referent | 2.28 (0.52-9.97) | 1.57 (0.88-2.79) | 4.56 (2.14-9.72) |
| Adjusted HR (95% CI) | Referent | 2.51 (0.56-11.36) | 1.74 (0.94-3.21) | 4.79 (2.19-10.50) |
| NEONATES WITH ≥3 DAYS FOLLOW-UP | ||||
| Any Stage AKI | ||||
| N, % | 14 (2.7) | 6 (10.2) | 74 (5.9) | 24 (11.2) |
| Crude HR (95% CI) | Referent | 3.00 (1.03-8.78) | 1.85 (1.14-3.01) | 2.68 (1.37-5.24) |
| Adjusted HR (95% CI) | Referent | 3.54 (1.18-10.64) | 1.92 (1.16-3.19) | 2.68 (1.35-5.33) |
| Stage 2 or 3 AKI | ||||
| N, % | 5 (5.3) | 3 (14.3) | 36 (15.1) | 12 (18.2) |
| Crude HR (95% CI) | Referent | 1.58 (0.20-12.51) | 2.00 (0.96-4.18) | 4.18 (1.66-10.53) |
| Adjusted HR (95% CI) | Referent | 2.12 (0.25-17.74) | 2.65 (1.16-6.06) | 5.38 (1.99-14.56) |
Estimated from a Cox proportional hazards regression stratified by gestational age category (22-28, 29-35, and ≥36 weeks) with nephrotoxic medication exposure entered as a time-varying covariate
Adjusted for 5-minutd APGAR, continuous gestational age, birthweight, maternal hypertension, bacterial infection, outborn delivery, mode of delivery, and neonatal sex, race, and ethnicity
In the entire cohort, aminoglycoside alone receipt was not associated with increased hazard for any stage AKI or severe AKI. However, among those neonates with ≥3 days of follow-up data available, aminoglycoside alone was associated with increased hazard for any stage early AKI (HR 1.85, 95% CI 1.14-3.01) and remained independently associated after adjustment for confounders (aHR 1.92, 95% CI 1.16-3.19). An aminoglycoside alone was also independently associated with increased hazard for severe AKI (aHR 2.65, 95% CI 1.16-6.06).
When examining aminoglycoside receipt with another nephrotoxic medication in the entire cohort compared to no nephrotoxic medication receipt, aminoglycoside and another nephrotoxic medication was associated with increased hazard for any stage early AKI (HR 1.80, 95% CI 1.03-3.13), however this association was no longer significant after adjustment for confounders. An aminoglycoside and another nephrotoxic medication was also associated with increased hazard for severe AKI (HR 4.56, 95% CI 2.14-9.72), and this association remained after adjusting for confounders (aHR 4.79, 95% CI 2.19-10.50). Similarly, when examining nephrotoxic medication receipt among neonates with ≥3 days of follow-up data available, an aminoglycoside and another nephrotoxic medication was associated with increased hazard for any stage early AKI (HR 2.68, 95% 1.37-5.24), and this association remained after adjustment for confounders (aHR 2.68, 95% CI 1.35-5.33). An aminoglycoside and another nephrotoxic medication was also associated with severe AKI (HR 4.18, 95% CI 1.66-10.53) which again remained after adjustment for confounders (aHR 5.38, 95% CI 1.99-14.56).
Discussion
This secondary analysis of the AWAKEN study demonstrates that exposure to nephrotoxic medications is frequent with nearly 75% of neonates receiving at least one nephrotoxic medication during the first postnatal week in the NICU. Aminoglycosides account for the most frequent nephrotoxic medication exposure with nearly 72% of our cohort receiving an aminoglycoside, either alone or in combination with another nephrotoxic medication, for a cumulative exposure of almost 6,000 days (41.5% of all possible patient-days) during the first postnatal week. For most nephrotoxic medications, the highest proportion of patient’s exposed was among those born neonates born at 22-28 weeks of gestational age. In addition to describing the epidemiology of early nephrotoxic medication exposure, we demonstrated that specific nephrotoxic medication regimens were associated with AKI; aminoglycosides during the first week of life was independently associated with any stage AKI, and exposure to an aminoglycosides with another nephrotoxic medication was independently associated with severe neonatal AKI. When examining those infants with data available at ≥ 3 days of life, we detected a similar pattern; both nephrotoxic medication excluding aminoglycoside and aminoglycoside with another nephrotoxic medication were independently associated with any stage early AKI, and aminoglycoside and another nephrotoxic medication was again also independently associated with severe AKI. Additionally, aminoglycoside alone was independently associated with severe AKI.
Investigations of nephrotoxic medication utilization in the NICU to date have largely been conducted in very low birthweight (VLBW, i.e., BW <1,500 grams) and extremely low birthweight (ELBW, i.e., BW <1,000 grams) infants though a recent multi-center database analysis of a broad NICU cohort has also recently been published. In VLBW/EBLW neonates, our findings align with those of previous studies; we detected high rates of nephrotoxic medication exposure (as high as 87%), with the most common exposures being antibiotics, and we showed strong associations between nephrotoxic medication exposure and AKI.1, 5, 8 In the recent multicenter, retrospective analysis of the Children’s Hospital Association Pediatric Health Information System (PHIS) database, again, similar to our findings, these authors reported high nephrotoxic medication exposure rates (74%), with the highest exposures occurring in neonates born at <28 weeks of gestational age. Aminoglycosides were the most frequently administered nephrotoxic medication, and there was a strong association between nephrotoxic medication and AKI (aRR 3.68, 95% CI 2.85-4.75).7 While this publication has greatly enhanced our understanding of the epidemiology of nephrotoxic medication in the NICU, the authors were limited to defining AKI by the International Classification of Disease (ICD)-9 and 10 diagnosis codes. Failure to recognize and code AKI by the clinician will result in underreporting of AKI prevalence.14 Furthermore, the timing of nephrotoxic medication exposures and AKI in this cohort is undefined. In contrast, we focused on nephrotoxic medication exposures and AKI within the first 7 days of life, and the use of a time-varying Cox model allows for nephrotoxic medication exposure status for each neonate to change relative to AKI across the first 7 days of life. The first postnatal week is of particular importance as nephrotoxic medication exposure during nephrogenesis, which continues after birth in prematurely born infants, could interfere with nephron endowment, exacerbating risk for AKI, specifically tubular dysfunction.15
Successful reduction in nephrotoxic medication-associated AKI can be achieved through careful medication stewardship suggesting nephrotoxic medication exposure often remains a modifiable risk factor for neonatal AKI. In neonates with a hemodynamically significant patent ductus arteriosus for example, acetaminophen is a less nephrotoxic alternative to NSAIDs; acetaminophen may actually be associated with lower rates of AKI in some populations.16, 17 Goldstein et al. developed the Nephrotoxic Injury Negated by Just-in-time Action (NINJA) program in pediatric patients to identify those at high risk for AKI due to nephrotoxic medication exposures and accordingly increase serum creatinine monitoring for these patients. Their efforts resulted in sustained, decreased nephrotoxic medication-associated AKI rates.18, 19, 20 Stoops et al. introduced NINJA into the NICU and found that ‘Baby NINJA’ similarly reduced nephrotoxic medication exposures and AKI rates in the NICU.21 Dissemination of such a program across all NICUs has the potential to reduce nephrotoxic medication exposures and thus AKI rates.
Collectively, the studies mentioned above and our own highlight that aminoglycoside antibiotic use, in particular, is incredibly frequent and strongly associated with AKI. In our cohort, especially when examining neonates with data available at ≥3 days of life, exposure to aminoglycosides alone or an aminoglycoside with another nephrotoxic medication were independently associated with any stage early AKI and severe AKI, respectively. Notably, the hazard ratios were significantly larger for neonates exposed to aminoglycoside with an additional nephrotoxic medication suggesting that a combination of nephrotoxic medications including an aminoglycoside is most harmful. Gentamicin, the most frequently used aminoglycoside in neonates, remains an important standard of care for empiric antibiotic coverage in neonates at risk for early-onset sepsis and is among the most frequently prescribed medications in the NICU.9, 22 Unfortunately, studies of empiric coverage with alternative, less nephrotoxic antibiotics are associated with increased mortality.23 While utilization of gentamicin in the NICU in the first week of life may be non-modifiable standard of care, alternative, less-nephrotoxic antibiotics may be appropriate later in the NICU course.6, 22 Additionally, though not specifically addressed in this study, duration of therapy with any nephrotoxic medication should be carefully discussed by multi-disciplinary teams and utilization dictated by culture results (in the case of antibiotics) and clinical course. A recent quality improvement study investigating the safety and efficacy of reducing empiric antibiotic duration, including gentamicin, from at least 48 hours to 24 hours reduced antibiotic exposure in 77% of preterm infants in whom early onset sepsis was ruled out.24 In the case that prolonged gentamicin use is required and suitable alternative is inappropriate, careful monitoring of gentamicin levels is likely necessary though elevated gentamicin troughs do not always result in AKI.25
The strengths of our study include the following: a large, multicenter representative cohort, daily documentation of medications received, the presence or absence of early AKI was determined via chart review, and the time-varying Cox proportional hazard regression modeling allows for precise determination of the association between nephrotoxic medication exposure and AKI. Our study is limited by the retrospective nature, the inclusion of many but not all potential nephrotoxic medications, the high number of out-born infants raises the concern for missed exposures prior to transfer and missed serum creatinine measurements, and the lack of indications for nephrotoxic medication use. Additionally, significant variability in the frequency with which serum creatinine measurements were obtained at each center likely influenced AKI rates in the AWAKEN cohort.26 Finally, neonates, particularly VLBW and ELBW neonates, have a great number of possible risk factors for AKI during the first postnatal week such patent ductus arteriosus, sepsis, fluid imbalance, hypoxic-ischemic insults, and hypotension. It is impossible to definitively determine the exact cause of AKI or to affirm that all AKI in the first postnatal week is attributable to nephrotoxic medication-associated insult as early AKI is likely multifactorial.
In summary, we identified that during the first postnatal week, nephrotoxic medication exposure, particularly aminoglycosides, is frequent in the NICU and associated with AKI. Combination therapy including an aminoglycoside with another nephrotoxic medication is particularly concerning and most strongly associated with severe AKI. Improving our understanding of the epidemiology of nephrotoxic medication exposure in the NICU and these associations with AKI may lead to improvements in care through potential modification of neonatal AKI risk factors.
Supplementary Material
Acknowledgements:
We acknowledge the outstanding work of the following clinical research personnel and colleagues for their involvement in the AWAKEN study:
Ariana Aimani, Samantha Kronish, Ana Palijan, MD, and Michael Pizzi (Montreal Children’s Hospital, McGill University Health Centre, Montreal, QC, Canada)
Laila Ajour, BS, and Julia Wrona, BS (University of Colorado, Children’s Hospital Colorado, Aurora, CO, USA)
Melissa Bowman, RN (Golisano Children’s Hospital, University of Rochester, Rochester, NY, USA)
Teresa Cano, RN, Marta G. Galarza, MD, Wendy Glaberson, MD, Aura Arenas Morales, MD, and Denisse Cristina Pareja Valarezo, MD (Holtz Children’s Hospital, University of Miami, Miami, FL, USA)
Sarah Cashman, BS, and Madeleine Stead, BS (University of Iowa Children’s Hospital, Iowa City, IA, USA)
Jonathan Davis, MD, and Julie Nicoletta, MD (Floating Hospital for Children at Tufts Medical Center, Tufts University School of Medicine, Boston, Massachusetts);
Alanna DeMello (British Columbia Children’s Hospital, Vancouver, Canada);
Lynn Dill, RN, and Emma Perez-Costas, PhD (The University of Alabama at Birmingham);
Ellen Guthrie, RN (MetroHealth Medical Center, CaseWestern Reserve University, Cleveland, Ohio);
Nicholas L. Harris, BS, and Susan M. Hieber, MSQM (C.S. Mott Children’s Hospital, University of Michigan, Ann Arbor);
Katherine Huang and Rosa Waters (University of Virginia Children’s Hospital, Charlottesville);
Judd Jacobs, Ryan Knox, BS, Hilary Pitner,MS, and Tara Terrell (Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio);
Nilima Jawale,MD (Maimonides Medical Center, Brooklyn, New York);
Emily Kane (Australian National University, Canberra);
Vijay Kher, DM, and Puneet Sodhi, MBBS (Medanta Kidney Institute, Medanta the Medicity, Gurgaon, Haryana, India);
Grace Mele (New York College of Osteopathic Medicine, Westbury);
Patricia Mele, DNP (Stony Brook Children’s Hospital, Stony Brook, New York);
Charity Njoku, Tennille Paulsen, and Sadia Zubair (Texas Children’s Hospital, Baylor College of Medicine, Houston);
Emily Pao (University of Washington, Seattle Children’s Hospital, Seattle);
Becky Selman, RN, and Michele Spear, CCRC (University of New Mexico Health Sciences Center, Albuquerque);
Melissa Vega, PA-C (The Children’s Hospital at Montefiore, Bronx, New York)
Leslie Walther, RN (Washington University in St Louis, Missouri).
Funding:
Cincinnati Children’s Hospital Center for Acute Care Nephrology provided funding to create and maintain the Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) study Medidata Rave electronic database. The Pediatric and Infant Center for Acute Nephrology (PICAN) at the University of Alabama at Birmingham (UAB) provided support for web meetings and for the NKC steering committee annual meeting at UAB as well as support for some of the AWAKEN study investigators at UAB (DA, LBJRG) through support from the UAB Department of Pediatrics at Children’s of Alabama, UAB School of Medicine, and UAB’s Center for Clinical and Translational Sciences (National Institutes of Health grant UL1TR001417). The AWAKEN study at The University of New Mexico was supported by the Clinical and Translational Science Center at The University of New Mexico (National Institutes of Health grant UL1TR001449) and by The University of Iowa Institute for Clinical and Translational Science (grant U54TR001356). The AWAKEN study investigators at the Canberra Hospital at the Australian National University Medical School were supported by the Canberra Hospital Private Practice Fund, and investigators at University of Virginia Children’s Hospital were supported by a 100 Women Who Care Grant from the 100 Women Charitable Foundation. The funding sources for this study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Neonatal Kidney Collaborative*
Subrata Sarkar8; Alison Kent9,10; Jeffery Fletcher9; Carolyn L. Abitbol11; Marissa DeFreitas11; Shahnaz Duara11; Jonathan R. Swanson2; Ronnie Guillet10; Carl D’Angio10; Ayesa Mian10; Erin Rademacher10; Maroun J. Mhanna12,13; Rupesh Raina12; Deepak Kumar12; Namasivayam Ambalavanan6; Ayse Akcan Arikan14; Christopher J. Rhee14; Stuart L. Goldstein4; Amy T. Nathan4; Juan C Kupferman15; Alok Bhutada15; Shantanu Rastogi15; Elizabeth Bonachea16; Susan Ingraham16,17; John Mahan16; Arwa Nada16,18; Jennifer Jetton19; Patrick D. Brophy19,10; Tarah T. Colaizy19; Jonathan M. Klein19; F. Sessions Cole20; T. Keefe Davis20,21; Joshua Dower22; Lawrence Milner22; Alexandra Smith22; Mamta Fuloria23; Kimberly Reidy23; Frederick J. Kaskel23; Danielle E. Soranno24,25; Jason Gien24; Aftab S. Chishti5; Sangeeta Hingorani26; Sandra Juul26; Michelle Starr25,26,; Craig S. Wong27; Catherine Joseph27; Tara DuPont27; Robin Ohls27,28; Amy Staples27; Smriti Rohatgi29; Sidharth K. Sethi29; Sanjay Wazir30; Surender Khokhar31; Sofia Perazzo32; Patricio E. Ray2,32; Mary Revenis32; Cherry Mammen33; Anne Synnes33; Pia Wintermark34; Michael Zappitelli34,35; Robert Woroniecki36; Shanthy Sridhar36
8CS Mott Children’s Hospital, University of Michigan, Ann Arbor, MI, USA
9Centenary Hospital for Women and Children, Canberra Hospital, Australian National University Medical School, Canberra, ACT, Australia
10Golisano Children’s Hospital, University of Rochester, Rochester, NY, USA
11Holtz Children’s Hospital, University of Miami, Miami, FL, USA
12MetroHealth Medical Center, Case Western Reserve University, Cleveland, OH, USA
13Ochsner/Louisiana State University Health, Shreveport, LA, USA
14Texas Children’s Hospital, Baylor College of Medicine, Houston, TX, USA
15Maimonides Medical Center, Brooklyn, NY, USA
16Nationwide Children’s Hospital, Columbus, OH, USA
17Kapi'olani Medical Center for Women & Children, Honolulu, HI, USA
18LeBonheur’s Children’s Hospital/University of Tennessee, Memphis, TN, USA
19University of Iowa Children’s Hospital, Iowa City, IA, USA
20Washington University, St Louis, MO, USA
21University of Saskatchewan, Saskatoon, SK, Canada
22Tufts University School of Medicine, Boston, MA, USA
23The Children’s Hospital at Montefiore, Bronx, NY, USA
24University of Colorado, Children’s Hospital Colorado, Aurora, CO, USA
25University of Indiana, Indianapolis, IN, USA
26University of Washington, Seattle Children’s Hospital, Seattle, WA, USA
27University of New Mexico Health Sciences Center, Albuquerque, NM, USA
28University of Utah, Salt Lake City, UT, USA
29Kidney and Urology Institute Medanta—The Medicity, Gurgaon, India
30Cloudnine Hospital, Gurgaon, Haryana, India
31Apollo Cradle, Gurgaon, Haryana, India
32Children’s National Medical Center, George Washington University School of Medicine and the Health Sciences, Washington, DC, USA
33British Columbia Children’s Hospital, Vancouver, BC, Canada
34Montreal Children’s Hospital, McGill University Health Centre, Montreal, QC, Canada
35The Hospital for Sick Children, Toronto, ON, Canada
36Stony Brook School of Medicine, Stony Brook, NY, USA
Footnotes
Conflict of Interest: All authors report no real or perceived conflicts of interest that could affect the study design, collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the manuscript for publication. For full disclosure, we provide the additional list of authors’ other funding sources that are not directly related to this study.
DJA is a consultant for Baxter, Nuwellis, Bioporto, and Seastar. His institution receives grant funding for education and research that is not related to this project from NIH, Baxter, Nuwellis, Medtronic, Bioporto, Portero, and Seastar. He has patents pending on inventions to improve the kidney care of neonates. He is the Founder and Chief Scientific Officer for Zorro-Flow Inc.
HJS received research grants with Baxter. No other disclosures were reported.
KG is a consultant for Bioporto and Potrero Medical.
JRC is on the Executive Board for the Neonatal Kidney Collaborative, a consultant for Medtronics, and an investor in Zorro-Flow.
Availability of Data and Materials: Unfortunately, there are currently no agreements for data-sharing in place though we are currently working to make the dataset available.
References
- 1.Arcinue R, Kantak A, Elkhwad M. Acute kidney injury in ELBW infants (< 750 grams) and its associated risk factors. J Neonatal Perinatal Med 2015, 8(4): 349–357. [DOI] [PubMed] [Google Scholar]
- 2.Beken S, Akbulut BB, Albayrak E, Güner B, Ünlü Y, Temur B, et al. Evaluation of neonatal acute kidney injury after critical congenital heart disease surgery. Pediatr Nephrol 2021, 36(7): 1923–1929. [DOI] [PubMed] [Google Scholar]
- 3.Hingorani S, Schmicker RH, Brophy PD, Heagerty PJ, Juul SE, Goldstein SL, et al. Severe Acute Kidney Injury and Mortality in Extremely Low Gestational Age Neonates. Clin J Am Soc Nephrol 2021, 16(6): 862–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Criss CN, Selewski DT, Sunkara B, Gish JS, Hsieh L, McLeod JS, et al. Acute kidney injury in necrotizing enterocolitis predicts mortality. Pediatr Nephrol 2018, 33(3): 503–510. [DOI] [PubMed] [Google Scholar]
- 5.Barhight M, Altaye M, Gist KM, Isemann B, Goldstein SL, Akinbi H. Nephrotoxic Medications and Associated Acute Kidney Injury in Very Low Birth Weight Infants. J Clin Nephrol Res 2017, 4(4). [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy HJ, Thomas B, Van Wyk B, Tierney SB, Selewski DT, Jetton JG. Nephrotoxic medications and acute kidney injury risk factors in the neonatal intensive care unit: clinical challenges for neonatologists and nephrologists. Pediatr Nephrol 2020, 35(11): 2077–2088. [DOI] [PubMed] [Google Scholar]
- 7.Mohamed TH, Abdi HH, Magers J, Prusakov P, Slaughter JL. Nephrotoxic medications and associated acute kidney injury in hospitalized neonates. J Nephrol 2022, 35(6): 1679–1687. [DOI] [PubMed] [Google Scholar]
- 8.Rhone ET, Carmody JB, Swanson JR, Charlton JR. Nephrotoxic medication exposure in very low birth weight infants. J Matern Fetal Neonatal Med 2014, 27(14): 1485–1490. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh EM, Hornik CP, Clark RH, Laughon MM, Benjamin DK Jr., Smith PB. Medication use in the neonatal intensive care unit. Am J Perinatol 2014, 31(9): 811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 2017, 1(3): 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jetton JG, Guillet R, Askenazi DJ, Dill L, Jacobs J, Kent AL, et al. Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates: Design of a Retrospective Cohort Study. Front Pediatr 2016, 4: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Group. KDIGOKAKIW. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney International Supplement 2012, 2: 1–138. [Google Scholar]
- 13.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007, 147(8): 573–577. [DOI] [PubMed] [Google Scholar]
- 14.Schaffzin JK, Dodd CN, Nguyen H, Schondelmeyer A, Campanella S, Goldstein SL. Administrative data misclassifies and fails to identify nephrotoxin-associated acute kidney injury in hospitalized children. Hosp Pediatr 2014, 4(3): 159–166. [DOI] [PubMed] [Google Scholar]
- 15.Gubhaju L, Sutherland MR, Black MJ. Preterm birth and the kidney: implications for long-term renal health. Reprod Sci 2011, 18(4): 322–333. [DOI] [PubMed] [Google Scholar]
- 16.Jasani B, Mitra S, Shah PS. Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Driest SL, Jooste EH, Shi Y, Choi L, Darghosian L, Hill KD, et al. Association Between Early Postoperative Acetaminophen Exposure and Acute Kidney Injury in Pediatric Patients Undergoing Cardiac Surgery. JAMA Pediatr 2018, 172(7): 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein SL, Dahale D, Kirkendall ES, Mottes T, Kaplan H, Muething S, et al. A prospective multi-center quality improvement initiative (NINJA) indicates a reduction in nephrotoxic acute kidney injury in hospitalized children. Kidney Int 2020, 97(3): 580–588. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein SL, Kirkendall E, Nguyen H, Schaffzin JK, Bucuvalas J, Bracke T, et al. Electronic health record identification of nephrotoxin exposure and associated acute kidney injury. Pediatrics 2013, 132(3): e756–767. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein SL, Mottes T, Simpson K, Barclay C, Muething S, Haslam DB, et al. A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int 2016, 90(1): 212–221. [DOI] [PubMed] [Google Scholar]
- 21.Stoops C, Stone S, Evans E, Dill L, Henderson T, Griffin R, et al. Baby NINJA (Nephrotoxic Injury Negated by Just-in-Time Action): Reduction of Nephrotoxic Medication-Associated Acute Kidney Injury in the Neonatal Intensive Care Unit. J Pediatr 2019, 215: 223–228.e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker CJ, Byington CL, Polin RA. Policy statement—Recommendations for the prevention of perinatal group B streptococcal (GBS) disease. Pediatrics 2011, 128(3): 611–616. [DOI] [PubMed] [Google Scholar]
- 23.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics 2006, 117(1): 67–74. [DOI] [PubMed] [Google Scholar]
- 24.Kumar R, Setiady I, Bultmann CR, Kaufman DA, Swanson JR, Sullivan BA. Implementation of a 24-hour empiric antibiotic duration for negative early-onset sepsis evaluations to reduce early antibiotic exposure in premature infants. Infect Control Hosp Epidemiol 2022: 1–6. [DOI] [PubMed] [Google Scholar]
- 25.Landers S, Berry PL, Kearns GL, Kaplan SL, Rudolph AJ. Gentamicin disposition and effect on development of renal function in the very low birth weight infant. Dev Pharmacol Ther 1984, 7(5): 285–302. [DOI] [PubMed] [Google Scholar]
- 26.Charlton JR, Boohaker L, Askenazi D, Brophy PD, D'Angio C, Fuloria M, et al. Incidence and Risk Factors of Early Onset Neonatal AKI. Clin J Am Soc Nephrol 2019, 14(2): 184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.