Abstract
We report 3 cases of patients with a history of extra-mammary cancer who presented with breast nodules, leading to diagnostic challenges and occasional misleading imaging findings. These cases highlight the significance of radiologists considering breast metastases as a potential component of the differential diagnosis when assessing patients with a history of cancer who exhibit palpable breast nodules. Furthermore, these cases underscore the importance of integrating various imaging techniques with histological and immunohistochemical analyses of the lesions to achieve precise diagnoses, ultimately ensuring the highest quality of care for these patients.
Keywords: Breast metastases, Extra-mammary cancer, Breast imaging
Introduction
Secondary breast locations for extra-mammary tumors account for approximately 0.5%-6.6% of all breast cancers [1]. These secondary tumors can originate from a wide variety of primary sites, with the most common being melanoma, hematologic malignancies (leukemia and lymphoma), ovarian carcinoma and pulmonary carcinoma [2,3].
Since imaging plays a crucial role in detecting and characterizing these metastases, this article aims to explore the imaging aspects and potential challenges associated with breast metastases originating from extra-mammary cancers.
Cases description
Challenging case 1: Clusters of microcalcifications do not always indicate primary breast neoplasia
A 70-year-old woman underwent a breast imaging assessment after experiencing breast nodules and elevated calcium levels in her blood test. She has a medical history of infiltrating squamous cell carcinoma of the vulva with widespread metastatic involvement in the lymph nodes, pleura, left lung, peritoneum, liver, adrenal glands, gluteal muscles, and skeleton (including the skull, vertebral column, pelvis, femurs, and humeri). She received treatment with radiotherapy and chemotherapy for this condition.
At mammography, multiple subcentimeter round opacities in both breasts were observed, along with 2 clusters of microcalcifications respectively in the equatorial outer region and upper outer quadrant of the right breast, indicating abnormally increased activity of glandular cells (see Fig. 1).
Fig. 1.
Bilateral mammography in mediolateral oblique incidence displaying multiple rounded opacities in all 4 breast quadrants (indicated by dashed arrows) along with 2 clusters of microcalcifications located in the equatorial outer region and upper outer quadrant of the right breast (indicated by solid arrows).
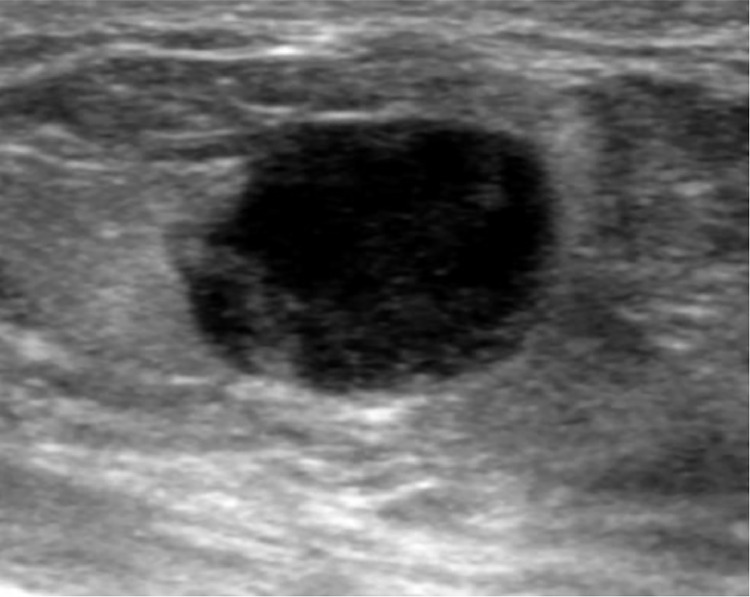
Ultrasound imaging also revealed hyperechoic nodular lesions with slightly microlobulated margins within the breast parenchyma, with the largest measuring 8 mm in diameter and located in the upper outer quadrant of the right breast (see Fig. 2). Subsequent histological and immunohistochemical analyses of this lesion, classified as BIRADS 5 and obtained by microbiopsy under ultrasound guidance (using a 16G needle), confirmed that it was breast metastasis originating from vulvar cancer (see Fig. 3).
Fig. 2.
Ultrasound image revealing a hyperechoic nodular lesion located in the upper outer quadrant of the right breast (10:00) and characterized by slightly microlobulated margins within the breast parenchyma.
Fig. 3.
(A) Histological appearance of a breast metastasis (H and E), exhibiting fragments of adipo-connective tissue (indicated by asterisks) infiltrated by neoplastic proliferation composed of marrow-sized cells. These cells have pale eosinophilic cytoplasm and round nuclei, occasionally enlarged and nucleolated (indicated by dashed arrows). (B) Immunohistochemical analysis showing positivity for cytokeratins AE1/AE3, indicating a cancerous lesion. (C) Immunohistochemical expression of the P63 marker, suggesting squamous cell carcinoma. (D) Lack of immunohistochemical expression of the P16 marker, which is typically associated with HPV infection. Scale is 100µm.
Challenging case 2: In a patient with an oncological history and the recent appearance of a breast nodule, a mammography can provide falsely reassuring results
A 36-year-old woman sought urgent medical attention due to palpable induration in her right breast and right leg. Her medical history included a prior diagnosis of mastitis and thyroid MALT lymphoma, which had been treated with total thyroidectomy and she was currently undergoing radiotherapy.
Upon examination, the mammography revealed 2 rounded opacities, each measuring 10 mm in diameter, with regular contours, and located in the lower inner quadrant. Notably, there were no clusters of microcalcifications or lymphadenopathy observed (Fig. 4).
Fig. 4.
Right mammography captured in mediolateral oblique (left) and craniocaudal (right) incidence, displaying 2 rounded opacities measuring 10 mm in diameter. These opacities are located in the lower inner quadrant (indicated by the arrows). Notably, there are no microcalcification clusters or signs of adenomegaly.
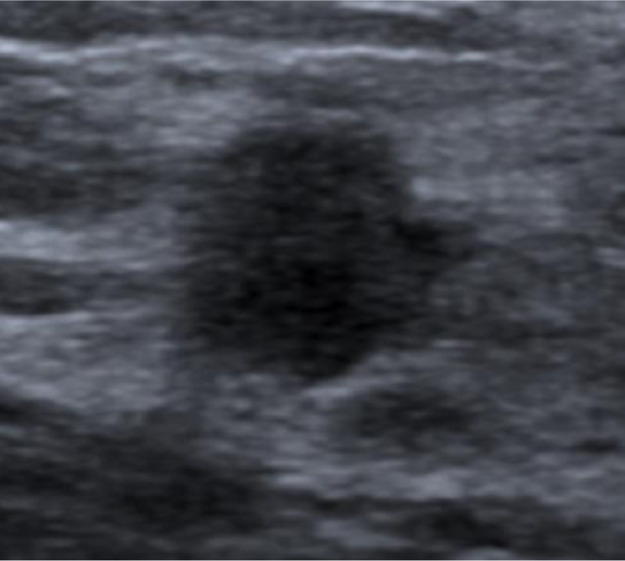
Subsequent ultrasound evaluation revealed hypoechoic masses with relatively indistinct borders, heterogeneous content, and signs of peripheral vascularization (Fig. 5).
Fig. 5.
Ultrasound image revealing the presence of a hypoechoic mass in the lower inner quadrant (4:00) of the right breast with relatively indistinct contours and heterogeneous internal content.
Additionally, an ultrasound examination of the leg uncovered a lesion with a similar architectural pattern.
Histological analysis of the samples, classified as BIRADS 4B and obtained by microbiopsy under ultrasound guidance using a 16G needle, confirmed the presence of the previously diagnosed lymphoma.
Challenging case 3: Be alert to a hypermetabolic area on PET-CT
A 19-year-old woman presented after discovering a mobile nodule measuring 1 cm at the junction of the upper quadrants of her left breast during self-examination.
This patient has been diagnosed with a grade III ovarian neuroendocrine tumor with hepatic and bone metastases (in the spine and left iliac bone) as well as peritoneal carcinomatosis. She has undergone bilateral ovariectomy and received several rounds of chemotherapy as part of her treatment plan.
Ultrasound examination revealed an oval-shaped hypoechoic lesion measuring 10 x 8 mm, with slightly indistinct borders. No signs of hypervascularization or axillary lymphadenopathy were observed (see Fig. 6).
Fig. 6.
PET-CT scan revealing moderate hypermetabolic activity (maximal Standardized Uptake Value - SUV of 2,8) of a nodule located at the junction of the upper quadrants of the left breast (indicated by the arrow).
A follow-up PET-CT indicated moderate hypermetabolic activity (maximal Standardized Uptake Value - SUV of 2,8) in the nodule. Subsequently, a microbiopsy of the lesion, classified as BIRADS 4C, was performed under ultrasound guidance using a 16G needle. The histological analysis strongly supported a diagnosis of neuroendocrine carcinoma (see Fig. 7).
Fig. 7.
Ultrasound image displaying an oval-shaped hypoechoic lesion at the junction of the upper quadrants of the left breast, measuring 10 mm x 8 mm, characterized by slightly blurred contours and without any signs of hypervascularization.
Discussion
Breast metastases are rare, often occurring in the absence of breast cancer risk factors, typically in the context of polymetastatic tumors. However, in some cases, they may indicate an underlying primary cancer [3].
About half of the extramammary metastases to the breast are situated in the upper outer quadrant, a pattern attributed to the abundance of vascularity, lymphatic drainage, and glandular tissue [4]. The left breast may be equally as affected as the right and approximately 25% of cases exhibit bilateral involvement [5].
The diagnostic challenge lies in distinguishing between benign and malignant tumors, as well as primary breast tumors and breast metastases, to ensure appropriate management, given their significant differences [6].
Examination often reveals superficial, mobile, and well-defined nodules without skin or nipple retraction, owing to the absence of desmoplastic reaction [7]. These nodules can be easily mistaken for benign breast tumors. Nevertheless, the sudden appearance of a rapidly growing breast lesion should raise the suspicion of malignancy. Palpable axillary lymph nodes are present in approximately half of the cases [8].
Imaging presentations of these metastases are also nonspecific, although the presence of multiple lesions can aid in diagnosis [1].
On mammography, these nodules are typically well-circumscribed without architectural distortion [9]. Microcalcifications, as seen in case 1, are rare and more commonly associated with psammomas in ovarian cancers [10]. Often, the appearance is similar to that of benign tumors, such as fibroadenomas, as in case 2.
Ultrasound appearances vary but often include rounded, hypoechoic nodules with circumscribed, indistinct, or microlobulated margins [11]. They may sometimes show vascularity on color Doppler imaging and posterior enhancement [12].
MRI becomes particularly valuable when there is diagnostic uncertainty or inconclusive results from other imaging modalities. It provides excellent soft tissue contrast and can aid in distinguishing breast metastases from other benign or malignant breast lesions. Metastatic lesions typically exhibit intermediate signal intensity on T2-weighted images and early enhancement with washout kinetics during contrast-enhanced dynamic sequences [13].
The increasing use of PET-CT in current practice for assessing the extension and follow-up of extra-mammary cancers can occasionally lead to the incidental detection of breast metastases, as seen in case 3 [14]. PET-CT may also help differentiate primary breast cancer from metastatic disease [15].
From a practical standpoint, it is advisable to initially perform a breast microbiopsy under ultrasound guidance, emphasizing the importance of comparing the results with the histological and cytological data of the primary tumor and the patient's clinical history [16].
In general, histological features combined with immunohistochemical analysis facilitate the differentiation of breast lesions as primary or metastatic in origin [17]. In most cases, tissue abnormalities on histological study do not resemble primary breast carcinoma, often providing valuable clues about the nature and location of the primary tumor. Negative cytokeratin 7 markers and hormone receptor negativity in immunohistochemistry suggest breast lesions of metastatic origin [18].
The management of breast metastases is complex and requires a multidisciplinary approach. In the majority of cases, palliative chemotherapy is the recommended treatment, either alone or in conjunction with local radiotherapy, depending on the primary tumor type [19]. Unlike primary breast cancer, mastectomy and axillary lymph node dissection offer no benefit in treating metastatic breast cancer [17]. In the absence of other metastases, lumpectomy with excision of metastatic adenopathy can be considered [2].
The prognosis for breast metastases is generally poor, with a median expected survival of approximately 1 year [20].
Conclusion
Breast metastases originating from extra-mammary tumors represent a rare occurrence that demands a multidisciplinary approach. Imaging assumes a pivotal role in both the detection and occasionally challenging characterization of these lesions [21].
In cases where patients have a history of tumors and present with breast lumps, metastasis to the breast should always be considered as part of the differential diagnosis. The early detection and precise diagnosis of breast metastases are paramount, as they can guide appropriate treatment strategies and ultimately enhance patient outcomes [22],[23].
Patient consent
All patient data was anonymized and consent was obtained for scientific work and publication.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Jian-Yong Z, Guang-Ping Z, Xue W, Shi-Min Z, Zhen-Guo Z. Breast metastasis of cervical cancer: a case report and systematic literature review. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.974592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koch A., Richter-Marot A., Wissler M.P., Baratte A., Mathelin C. Métastases mammaires de cancers d'origine extra-mammaire : état des lieux et difficultés diagnostiques. Gynécol Obstét Fertil. 2013;41(11):653–659. doi: 10.1016/j.gyobfe.2013.09.013. ISSN 1297-9589. [DOI] [PubMed] [Google Scholar]
- 3.Khouchani M, Benchakroun N, Tahri A, Tawfiq N, Jouhadi H, Acharki A., et al. Métastase intramammaire d’un cancer vulvaire : à propos d’un cas avec revue de la littérature. Cancer/Radiothérapie. 2008;12(Issue 2):120–125. doi: 10.1016/j.canrad.2007.11.001. ISSN 1278-3218. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Wahab R, Brown AL, Guarnieri B, Lewis K, Mahoney MC, et al. Extramammary metastases to the breast. Radiographics. 2023;43:10. doi: 10.1148/rg.230036. [DOI] [PubMed] [Google Scholar]
- 5.Moreno-Astudillo L, Villaseñor-Navarro Y, Sánchez-Goytia V, Porras-Reyes F, Lara-Mercado A, Sollozo-Dupont I. A case series of breast metastases from different extramammary malignancies and their literature review. Case Rep Radiol. 2019;2019 doi: 10.1155/2019/9454201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semba R, Horimoto Y, Arakawa A, Saito M. Metastatic breast tumors from extramammary malignancies: a case series. Surg Case Rep. 2021;7(1):154. doi: 10.1186/s40792-021-01235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreno M, Matschinski GN, Czarnobai I, de Oliveira A, Boff TC. An uncommon case of vulvar cancer metastatic to breast. Exp Oncol. 2021;43(1):92–95. doi: 10.32471/exp-oncology.2312-8852.vol-43-no-1.15843. [DOI] [PubMed] [Google Scholar]
- 8.DeLair DF, Corben AD, Catalano JP, Vallejo CE, Brogi E, Tan LK. Non-mammary metastases to the breast and axilla: a study of 85 cases. Mod Pathol. 2013;26(3):343–349. doi: 10.1038/modpathol.2012.191. [DOI] [PubMed] [Google Scholar]
- 9.Kalli S, Lanfranchi M, Alexander A, Makim S, Freer PE. Spectrum of extramammary malignant neoplasms in the breast with radiologic-pathologic correlation. Curr Probl Diagn Radiol. 2016;45(6):392–401. doi: 10.1067/j.cpradiol.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Susini T, Olivieri S, Molino C, Castiglione F, Tavella K, Viligiardi R. Ovarian cancer initially presenting as intramammary metastases and mimicking a primary breast carcinoma: a case report and literature review. J Womens Health (Larchmt) 2010;19(1):169–174. doi: 10.1089/jwh.2009.1465. [DOI] [PubMed] [Google Scholar]
- 11.Taha A, Khalil A, Khan M, Metafa A. Breast metastasis from thyroid follicular carcinoma in a 90-year-old patient 12 years after thyroidectomy and radiotherapy. Radiol Case Rep. 2022;17(10):3911–3914. doi: 10.1016/j.radcr.2022.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sippo DA, Kulkarni K, Carlo PD, Lee B, Eisner D, Cimino-Mathews A, et al. Metastatic disease to the breast from extramammary malignancies: a multimodality pictorial review. Curr Probl Diagn Radiol. 2016;45(3):225–232. doi: 10.1067/j.cpradiol.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Bitencourt AGV, Gama RRM, Graziano L, Negrão EMS, Sabino SMPS, Watanabe AHU, et al. Breast metastases from extramammary malignancies: multimodality imaging aspects. Br J Radiol. 2017;90(1077):20170197. doi: 10.1259/bjr.20170197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Litmanovich D, Gourevich K, Israel O, Gallimidi Z. Unexpected foci of 18F-FDG uptake in the breast detected by PET/CT: incidence and clinical significance. Eur J Nucl Med Mol Imaging. 2009;36(10):1558–1564. doi: 10.1007/s00259-009-1147-4. [DOI] [PubMed] [Google Scholar]
- 15.Benveniste AP, Marom EM, Benveniste MF, Mawlawi OR, Miranda RN, Yang W. Metastases to the breast from extramammary malignancies - PET/CT findings. Eur J Radiol. Jul 2014;83(7):1106–1112. doi: 10.1016/j.ejrad.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Wan X, Zhang H, Zhang Y, Peng Y. Metastases to the breast from extramammary nonhematological malignancies: case series. Int J Gen Med. 2020;13:1105–1114. doi: 10.2147/IJGM.S276602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charfi S, Krichen Makni S, Khanfir A, Abbes K, Gouiaa N, Fakhfakh I, et al. Les métastases mammaires : étude anatomoclinique de six cas. J Gynécol Obstét Biol Reprod. 2008;37(4):346–352. doi: 10.1016/j.jgyn.2008.02.002. ISSN 0368-2315. [DOI] [PubMed] [Google Scholar]
- 18.Lee AHS, Hodi Z, Soomro I, Sovani V, Abbas A, Rakha E, et al. Histological clues to the diagnosis of metastasis to the breast from extramammary malignancies. Histopathology. 2020;77(2):303–313. doi: 10.1111/his.14141. [DOI] [PubMed] [Google Scholar]
- 19.Williams SA, Ehlers RA, 2nd, Hunt KK, Yi M, Kuerer HM, Singletary SE, et al. Metastases to the breast from nonbreast solid neoplasms: presentation and determinants of survival. Cancer. 2007;110(4):731–737. doi: 10.1002/cncr.22835. [DOI] [PubMed] [Google Scholar]
- 20.Lee SK, Kim WW, Kim SH, Hur SM, Kim S, Choi JH, et al. Characteristics of metastasis in the breast from extramammary malignancies. J Surg Oncol. 2010;101(2):137–140. doi: 10.1002/jso.21453. [DOI] [PubMed] [Google Scholar]
- 21.Choo ALE, Sim LS, Sittampalam K, Tan WC, Tay AZE, Nadarajah R, et al. Breast metastasis from endometrial clear cell carcinoma: A case report and review of the literature. Front Oncol. 2023;12:1070744. doi: 10.3389/fonc.2022.1070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santana Valenciano Á, Juez Sáez LD, Pérez Mies B, Moreno SC, Fidalgo SR, Montero JC. Breast metastases from non-primary breast malignancies: what should we know? Breast Dis. 2023;42(1):223–228. doi: 10.3233/BD-220056. [DOI] [PubMed] [Google Scholar]
- 23.Qi Y, Kong X, Wang X, Zhai J, Fang Y, Wang J. Metastasis to breast from extramammary solid tumors and lymphomas: a 20-year population-based study. Cancer Invest. 2022;40(4):325–336. doi: 10.1080/07357907.2021.2019264. [DOI] [PubMed] [Google Scholar]