Abstract
Objectives
A reduced adrenal reserve-associated cortisol production relative to the enhanced needs of chronic inflammation (disproportion principle) has been observed in rheumatoid arthritis (RA). We examined the possible clinical value of diurnal cortisol measurements in active RA on treatment response prediction.
Methods
Diurnal cortisol production (measured at: 08–12:00/18:00–22:00) was assessed by electrochemiluminescence immunoassay in 28 consecutive patients with moderately/highly active RA, as well as 3 and 6 months after treatment initiation or/escalation. Twenty-eight COVID-19 patients and 28 age-matched healthy individuals (HC) served as controls.
Results
Saliva diurnal cortisol production in patients with RA was similar to that of HC, despite 12-fold higher serum C reactive protein (CRP) levels, and lower than COVID-19 patients (area under the curve: RA: 87.0±37.6 vs COVID-19: 146.7±14.3, p<0.001), having similarly high CRP. Moreover, a disturbed circadian cortisol rhythm at baseline was evident in 15 of 28 of patients with RA vs 4 of 28 and 20 of 28 of HC and COVID-19 patients, respectively. Treatment-induced minimal disease activity (MDA) at 6 months was achieved by 16 of 28 patients. Despite comparable demographics and clinical characteristics at baseline, non-MDA patients had lower baseline morning cortisol and higher adrenocorticotropic hormone (ACTH) levels compared with patients on MDA (cortisol: 10.9±4.0 vs 18.4±8.2 nmol/L, respectively, p=0.005 and ACTH: 4.8±3.3 vs 2.4±0.4 pmol/L, respectively, p=0.047). Baseline morning cortisol <13.9 nmol/L predicted non-MDA at 6 months (75% sensitivity, 92% specificity, p=0.006). Prospective measurements revealed that individualised diurnal cortisol production remained largely unchanged from baseline to 3 and 6 months.
Conclusions
An impaired adrenal reserve is present in patients with RA. Further studies to confirm that assessment of diurnal cortisol production may be useful in guiding treatment decisions and/or predicting treatment response in RA are warranted.
Trial registration number
Keywords: Arthritis, Rheumatoid; Glucocorticoids; Arthritis
WHAT IS ALREADY KNOWN ON THIS TOPIC.
An altered circadian rhythm of adrenal cortisol production relative to the enhanced needs of systemic high-grade inflammation has been proposed for patients with rheumatoid arthritis (RA), coined as the ‘disproportion principle’.
WHAT THIS STUDY ADDS
An impaired adrenal reserve is indeed present in patients with active RA, as shown by prospective measurements of diurnal cortisol production. In the current absence of relevant biomarkers, we found that low baseline saliva cortisol morning levels (<13.9 nmol/L) can predict with 75% sensitivity and 92% specificity an inadequate clinical response following 6-month treatment in a real-life setting.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Further research is required to confirm whether detection of an impaired adrenal reserve before initiating or escalating treatment for active RA may assist treatment decisions and/or predict treatment response.
Introduction
Adrenocortical dysfunction, termed also as ‘relative’ or ‘functional’ adrenal insufficiency, has been described in patients with rheumatoid arthritis (RA) without any prior risk of, or evidence of, overt adrenal insufficiency.1–8 On the other hand, low doses of glucocorticoids are often an important component in long-term management of many of these patients, despite the current wide use of biological disease-modifying antirheumatic drugs (bDMARDs) and efforts for glucocorticoid exposure minimisation, as stated by the 2022 updated European Alliance of Associations for Rheumatology (EULAR) recommendations for RA management.9 For example, the SΕΜΙRΑ (Steroid EliMination In Rheumatoid Arthritis) Study showed that patients with RA, who were on remission/low disease activity with a combination treatment of 5 mg prednisone/day and tocilizumab, relapsed more frequently when tapering of low-dose glucocorticoids was attempted, compared with those who continued treatment including low-dose glucocorticoids.10 Moreover, we have examined patients with chronic inflammatory rheumatic diseases, including RA, being in remission under DMARDs (including bDMARDs) and low-dose glucocorticoids who relapsed upon tapering of the latter. In these particular patients, we found that both baseline cortisol levels and time-integrated cortisol responses to tetracosactide stimulation (Synacthen test) were lower compared with healthy controls, despite the absence of established adrenal insufficiency.11 Notably, the reduced adrenal response was independent of age and disease duration or the duration of prior treatment with glucocorticoids, pointing to the presence of a relative adrenal insufficiency in these patients.11 Although current evidence on underlying mechanisms of adrenocortical dysfunction in some patients with RA, coined as the ‘disproportion principle’,12 13 remains inconclusive, contributing factors may include chronic stress and chronic inflammation.13 14
Diurnal cortisol production displays a circadian rhythm in healthy individuals, with the highest levels in the morning and a gradual decline throughout the day, reaching the lowest levels around midnight.15 During acute stress16–18 or acute infection,19 20 the rhythm is disturbed and the afternoon and/or midnight cortisol levels do not drop. Indeed, a disturbed circadian cortisol rhythm with abnormally high afternoon and night cortisol levels was found in patients with even mild or moderate COVID-19 compared with healthy controls.21 Nevertheless, the potential differential effects on adrenal reserve caused by chronic exposure to proinflammatory cytokines versus acute inflammatory stimulus and/or chronic versus acute emotional stress have not been systematically studied. In addition, whether an impaired cortisol responsiveness to chronic stress and inflammation may affect the treatment response in RA remains unknown. Therefore, in patients with active RA in a contemporary real-life setting, (a) we attempted to confirm the presence of an impaired adrenal reserve in comparison with patients with acute infection and healthy individuals, and (b) we prospectively tested the hypothesis that assessment of diurnal cortisol production may assist in the prediction of treatment response in patients who initiate or escalate DMARD treatment. We used saliva instead of serum for the dynamic assessment of cortisol levels during the day as a non-invasive, easily collected (stress-free) method that provides a more accurate representation of the biologically active free cortisol.
Patients and methods
Study protocol
In this single-centre, 6-month prospective cohort study, we consecutively enrolled 30 patients with RA fulfilling the 2010 American College of Rheumatology (ACR)/EULAR classification criteria22 with moderately or highly active disease (28-joint Disease Activity Score (DAS28) >3.2),23 24 of whom 28 (24 female) completed the study (one patient withdrew informed consent and one patient was lost to follow-up). In total, 13 of 28 patients were newly diagnosed who were going to initiate treatment with DMARD and 15 of 28 required escalation of DMARD treatment due to inadequate response (addition of bDMARDs, conventional synthetic or targeted synthetic DMARDs, or change of a bDMARD with or without addition of glucocorticoids), according to EULAR recommendations (2019 update) for patients with active RA.25 Exclusion criteria were the following: (1) glucocorticoid treatment (at any dose) in the past 6 months, (2) chronic kidney disease stage III or IV, (3) antineoplastic treatment, (4) thyroid-stimulating hormone >5 IU/L, (5) Cushing syndrome, (6) hypo/hyperparathyroidism, (7) use of oestrogen replacement therapy or oral contraceptives, (8) treatment with insulin or haemoglobin A1c >7.5%, (9) body mass index >35, (10) pregnancy.
Blood and saliva samplings, as well as clinical assessments, were performed at baseline, and at 3 and 6 months of follow-up. Patients’ characteristics, medications, comorbidities, family medical history, laboratory and clinical work-up were recorded at the time of blood/saliva sampling, in compliance with the General Data Protection Regulation policy of the hospital. Based on the standard clinical care of our department, treating physicians use EULAR/ACR recommendations (described in 201925 and its later 2022 update9) for assessing disease activity. Therefore, for each patient, each of the four core set variables was registered in the patient’s medical record upon each visit (ie, tender joint count, swollen joint count, patient’s global assessment, erythrocyte sedimentation rate (ESR) and C reactive protein (CRP)). According to these measurements, a DAS28–ESR score was calculated at each visit. DAS28–ESR ≤2.85 was used to report minimal disease activity (MDA) during follow-up.26
Assessment of rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA) was performed at baseline, and patients with positive RF and /or ACPA were considered seropositive. In addition to clinical examination and DAS28 assessments, patients were asked to complete the Perceived Stress Scale Questionnaire-1427 and the Hamilton Rating Scale Questionnaire for depression,28 both validated for the Greek population,29–31 at baseline, 3 and 6 months. Patients were treated as per clinician’s judgement with any combination of DMARDs or biologics, and with or without the addition of glucocorticoids (glucocorticoid regimens were not exceeding 15 mg/day of prednisolone or equivalent), following the EULAR recommendations for RA treatment.25 The treating physicians were blinded to the baseline cortisol values.
Twenty-eight age-matched and apparently healthy volunteers (16 females) were recruited from the hospital personnel during the same time period and served as controls. Blood and saliva measurements in the control group were conducted solely at baseline, with no further assessments throughout the course of the study. Additionally, blood and saliva measurements of 28 age-matched patients with acute COVID-19 (14 females), which were assessed at one time point, were obtained from our previously published cohort,21 and no additional evaluations were conducted thereafter. The exclusion criteria used for patients with RA were also followed in the above groups. All participants signed an informed consent form and the procedures followed were in accordance with the 1975/1983 Declaration of Helsinki. Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research. The study was registered in ClinicalTrials.gov (NCT05671627).
Sampling procedures and measurements
Blood samples were obtained from patients with RA and controls at morning hours (08:00) after an overnight fast, for the measurement of plasma adrenocorticotropic hormone (ACTH) and serum CRP. Plasma ACTH was measured using solid-phase, two-site chemiluminescence immunoassays on an IMMULITE 2000 Immunoassay System (Siemens Healthcare Diagnostics Products, UK) (online supplemental file). Saliva samples were obtained from patients with RA and controls the next day at prescheduled time points, namely at 08:00, 12:00, 18:00 and 22:00 to test saliva cortisol levels, as previously described.21 Patients who were on glucocorticoids at 3 and/or 6 months of follow-up were asked to stop medication at least for 48 hours before blood and saliva sampling. All participants were asked to refrain from eating and brushing their teeth for 1 hour before the collection. The collection of saliva was performed using the Salivette device (Sarstedt, Nümbrecht, Germany) and the samples were stored at 0–4°C. Saliva cortisol was measured by an electrochemiluminescence immunoassay on the automated analyser Cobas e411-Roche Diagnostics (Mannheim). The detection limit was 1.49 nmol/L, and the intra-assay and interassay coefficients of variation were 6.1% and 11.8%, respectively, at the concentration of 3.8 nmol/L (online supplemental file). Morning serum cortisol was also measured using the electrochemiluminescence immunoassay on the automated analyser Cobas e411-Roche Diagnostics (reference range reported by the manufacturer: 172–496.5 nmol/L).
rmdopen-2023-003575supp001.pdf (193.1KB, pdf)
Definition of disturbed circadian cortisol rhythm
In healthy conditions, cortisol levels are expected to gradually decline during the day.15 For the purpose of this study, we defined as ‘disturbed’ cortisol rhythm the lack of this decline in saliva cortisol in at least one of the three following time points; (1) from 08:00 to 12:00, (2) from 12:00 to 18:00, and (3) from 18:00 to 22:00.
Statistical analysis
Continuous variables are presented as mean±SD or mean±SEM, regardless of normality of distribution.32 Differences in continuous variables between two groups were assessed by independent samples t-test or the non-parametric Mann-Whitney U test, when appropriate; categorical variables were assessed by two-tailed Fisher’s exact test. Comparisons among three groups were performed by one-way analysis of variance (ANOVA) followed by Bonferroni multiple comparisons test or the non-parametric Kruskal-Wallis followed by Dunn’s multiple comparisons test. Changes in repeated measurements within the group of patients with RA were assessed by repeated measurements ANOVA or the non-parametric Friedman’s test, as applicable. Univariable and multivariable linear regression models were used to adjust for various confounders (eg, sex, age or disease duration). We employed receiver operating characteristic (ROC) curve analysis to evaluate the predictive ability of baseline morning saliva cortisol levels to distinguish patients with RA who achieved MDA at 6 months after treatment from those who did not. We used the point closest to (0.1) corner in the ROC curve33 to define the optimal cut-off point value. Since morning saliva cortisol values at baseline exhibited a complex and rather non-linear relationship with the final outcome, we dichotomised the variable in order to have a clear threshold that could effectively separate the two groups of patients with RA. Logistic regression was then applied to examine the association between low baseline morning saliva cortisol and the presence of MDA at 6 months while controlling for potential confounding effects of age, sex, CRP levels and disease duration.
In order to estimate the daily production of cortisol, we used serial measurements of saliva cortisol during the day and calculated the area under the curve (AUC) by integrating the values of the saliva cortisol over the prespecified time points (o8:00–12:00/18:00–22:00), using the trapezoidal method, as previously described.34 Due to integration of diurnal cortisol measurements, AUC is expressed as a numerical value without any associated units.
Statistical analysis was conducted with Stata V.13, SPSS V.27 and GraphPad Prism V.7.05.
As this was an explanatory study and due to (1) lack of prior knowledge, (2) the small available sample size and (3) the longitudinal design of the study, power calculation was not conducted in advance. In addition, and in line with good practice, the reported p values in this analysis are used to inform statistical strengths of findings rather than significance.35
Results
Impaired adrenal reserve in patients with active RA
Twenty-eight patients with RA were examined (mean age: 56±10 years, disease duration: 76±103 months, ranging from 2 to 336 months), of whom 13 of 28 were seronegative, in line with recent studies demonstrating an increase in the incidence of seronegative RA over the last decades36 37 (demographics, disease characteristics and management of patients with RA are presented in the online supplemental file 1). Questionnaires for stress and depression revealed that the majority of patients with active RA (19 of 28) were severely stressed, whereas half of them (14 of 28) were moderately or severely depressed at the time of baseline sampling.
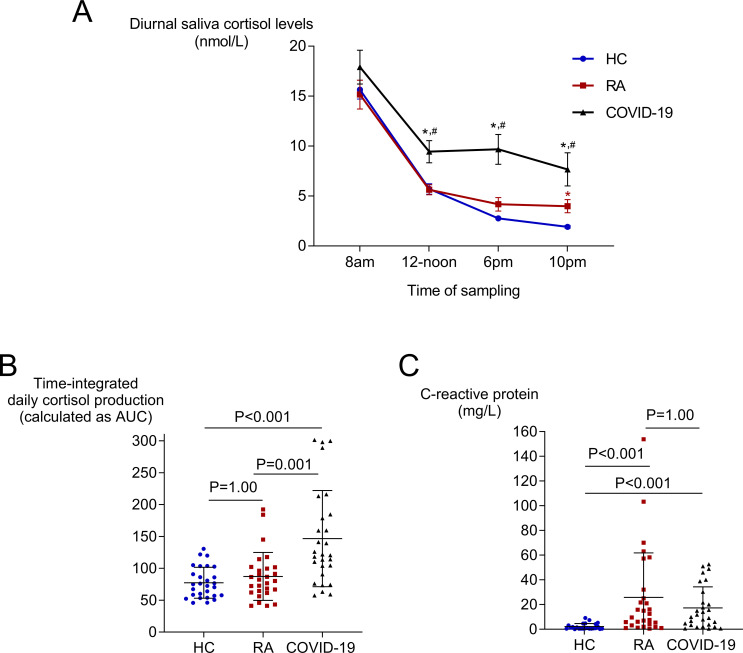
No significant differences were observed in plasma ACTH levels between patients with RA and healthy controls (3.6±2.6 vs 3.8±2.1 pmol/L, respectively). Notably, morning, noon and evening saliva cortisol measurements, as well as the time-integrated daily cortisol production, as assessed by the AUC, were comparable between patients with active RA and age-matched healthy controls (figure 1A,B), despite the presence of 12-fold higher CRP levels in RA (figure 1C). Night cortisol levels, however, were higher in patients with RA than controls (4.0±3.5 vs 1.9±0.8 nmol/L, respectively, p=0.005) (figure 1A). The results remained essentially the same when we performed a linear regression analysis adjusting for sex (online supplemental table 1). On the other hand, patients with active RA had lower time-integrated daily cortisol production compared with age-matched COVID-19 patients (AUC: 87±38 vs 147±75, respectively, p<0.001, figure 1B), despite the presence of comparably high serum CRP levels (figure 1C). This difference was mostly driven by the significantly higher noon, evening and night cortisol levels seen in the context of the acute inflammatory response in COVID-19 patients, as expected (figure 1A).
Figure 1.
Diurnal saliva cortisol levels (A), time-integrated daily saliva cortisol production (B) and serum CRP levels (C) at baseline in patients with RA, COVID-19 patients and healthy controls (HC). Saliva cortisol levels were measured at 08:00, 12:00, 18:00 and 22:00, and the time-integrated daily saliva cortisol production was calculated as the total area under the curve (AUC). Lines in (A) represent mean±SEM, and in (B) and (C) represent mean±SD. P values are derived from one-way ANOVA followed by Bonferroni multiple comparisons test or the non-parametric Kruskal-Wallis followed by Dunn’s multiple comparisons test for measurements that did not follow a normal distribution (saliva cortisol measurements did not follow a normal distribution). *P<0.05 compared with controls; #p<0.05 compared with patients with RA. ANOVA, analysis of variance; CRP, C reactive protein; RA, rheumatoid arthritis.
Further analyses in subgroups of patients with RA (eg, seropositive vs seronegative patients, glucocorticoid-naive vs glucocorticoid-experienced patients) did not reveal striking differences (online supplemental tables 2 and 3). Finally, in a subset of 20 patients with RA, in whom serum samples were available at baseline, morning serum cortisol was not associated with saliva cortisol obtained the next morning, probably reflecting the significant alterations in the cortisol-binding protein cleavage in patients with RA, as previously described by Nenke et al.38
Disturbed circadian cortisol rhythm in active RA
Next, we examined individual diurnal measurements of saliva cortisol to detect possible disturbed circadian rhythms. We found that 15 of 28 of patients with active RA and 20 of 28 of COVID-19 patients demonstrated a disturbed circadian rhythm, in contrast to 4 of 28 healthy controls (both p<0.01). As shown in table 1, when we compared disease characteristics between patients with active RA with disturbed or intact circadian cortisol rhythm, we found that a disturbed rhythm was more frequently observed in females and in patients with shorter disease duration. Regarding age, disease activity, ESR, CRP, stress and depression levels, no significant differences were observed. Notably, time-integrated daily saliva cortisol production was higher in those patients with disturbed compared with patients with intact circadian rhythm (AUC: 96±36 vs 77±39, p=0.04), mostly driven by higher night saliva cortisol levels (5.5±4.0 vs 2.2±1.6 nmol/L, p<0.001, respectively), despite comparable ACTH levels (table 1).
Table 1.
Baseline characteristics and diurnal saliva cortisol levels in patients with RA with disturbed and intact cortisol rhythm
| Disturbed cortisol rhythm (n=15) |
Intact cortisol rhythm (n=13) |
P value | |
| Female sex, n | 15/15 | 9/13 | 0.035 |
| Age (years) | 58±10 | 55±11 | 0.210 |
| Disease duration (months) | 49±81 | 108±118 | 0.046 |
| Seropositive RA, n | 7/15 | 8/13 | 0.476 |
| ESR, mm/1st hour | 42±33 | 31±20 | 0.427 |
| CRP levels (mg/L) (RV: <0.5 mg/L) |
35±45 | 16±18 | 0.525 |
| DAS28–ESR | 5.5±1.2 | 5.3±0.8 | 0.315 |
| DAS28–CRP | 5.2±1.4 | 4.9±0.9 | 0.308 |
| Plasma ACTH levels (pmol/L) RV of the assay: morning measurements: 1.9–11.4 pmol/L |
4.0±3.4 | 3.2±1.3 | 0.771 |
| Saliva cortisol, 08:00 (nmol/L) | 15.9±7.6 | 14.3±7.8 | 0.525 |
| Saliva cortisol, 12:00 (nmol/L) | 5.5±2.4 | 5.8±3.0 | 0.892 |
| Saliva cortisol, 18:00 (nmol/L) | 5.2±4.1 | 3.0±2.4 | 0.072 |
| Saliva cortisol, 22:00 (nmol/L) | 5.5±4.0 | 2.2±1.6 | <0.001 |
| Time-integrated daily saliva cortisol production—AUC | 96±36 | 77±39 | 0.041 |
| Moderately and severely stressed patients, n | 15/15 | 12/13 | 0.464 |
| Moderately and severely depressed patients, n | 9/15 | 5/13 | 0.449 |
Values are shown as mean±SD. Categorical data are shown as absolute number.
Independent samples Student’s t-test or Mann-Whitney U test was performed for comparisons between groups, as applicable. Two-tailed Fisher’s exact test was used to compare categorical data.
ACTH, adrenocorticotropic hormone; AUC, area under the curve; CRP, C reactive protein; DAS28, 28-joint Disease Activity Score; ESR, erythrocyte sedimentation rate; RA, rheumatoid arthritis; RV, reference values.
Diurnal production of cortisol in patients with active RA at baseline predicts treatment response at 6 months
At 6 months, 16 of 28 patients with RA had achieved MDA (DAS28–ESR ≤2.85). Despite the fact that no differences in age, disease duration, ESR, CRP, DAS28, and levels of stress and depression were evident at baseline between these two subgroups (table 2), morning baseline saliva cortisol was lower in those in non-MDA after 6 months of treatment compared with patients with MDA (10.9±4.0 vs 18.4±8.2 nmol/L, respectively, p=0.005), as well as compared with healthy controls (p=0.007). Interestingly, despite lower cortisol levels in these patients, their ACTH levels were higher than in patients achieving MDA (4.8±3.3 vs 2.4±0.4 pmol/L, respectively, p=0.047), probably reflecting a feedback regulatory mechanism towards the lower morning cortisol levels (table 2). In addition, disease duration was numerically higher in patients who did not achieve MDA after 6 months, compared with the rest of the patients, although not reaching statistical significance (p=0.133) (table 2).
Table 2.
Baseline characteristics and saliva cortisol levels in patients with active RA stratified according to treatment outcome at 6 months
| Baseline characteristics | Patients not achieving MDA at 6 months (n=12) |
Patients on MDA at 6 months (n=16) |
P value |
| Female sex, n | 11/12 | 13/16 | 0.613 |
| Age (years) | 56.7±9.4 | 56.8±11.9 | 0.984 |
| Disease duration (months) | 105±113 | 55±92 | 0.133 |
| Seropositive RA, n | 6/12 | 9/16 | 1.000 |
| ESR, mm/1st hour | 36±27 | 38±30 | 0.829 |
| Serum CRP levels (mg/L) (RV: <0.5 mg/L) |
30±48 | 23±25 | 0.873 |
| DAS28–ESR | 5.5±0.9 | 5.3±1.1 | 0.569 |
| DAS28–CRP | 5.2±1.2 | 4.9±1.2 | 0.611 |
| Plasma ACTH levels (pmol/L) RV of the assay: morning measurements: 1.9–11.4 pmol/L |
4.8±3.3 | 2.4±0.4 | 0.047 |
| Saliva cortisol, 08:00 (nmol/L) | 10.9±4.0 | 18.4±8.2 | 0.005 |
| Saliva cortisol, 12:00 (nmol/L) | 6.0±2.9 | 5.4±2.5 | 0.302 |
| Saliva cortisol, 18:00 (nmol/L) | 2.7±2.0 | 5.3±4.2 | 0.042 |
| Saliva cortisol, 22:00 (nmol/L) | 4.4±4.5 | 3.7±2.7 | 0.698 |
| Time-integrated daily saliva cortisol production (AUC) | 74±29 | 97±41 | 0.053 |
| Disturbed cortisol rhythm, n | 7/12 | 8/16 | 0.718 |
| Moderately and severely stressed patients, n | 12/12 | 15/16 | 1.000 |
| Moderately and severely depressed patients, n | 8/12 | 6/12 | 0.252 |
Values are shown as mean±SD. Categorical data are shown as absolute number.
Independent samples Student’s t-test or Mann-Whitney U test was performed for comparisons between groups, as applicable. Two-tailed Fisher’s exact test was used to compare categorical data.
ACTH, adrenocorticotropic hormone; AUC, area under the curve; CRP, C reactive protein; DAS28, 28-joint Disease Activity Score; ESR, erythrocyte sedimentation rate; MDA, minimal disease activity; RA, rheumatoid arthritis; RV, reference values.
ROC curve analysis performed to evaluate the predictive ability of baseline morning saliva cortisol levels to distinguish patients with RA who achieved MDA at 6 months after treatment from those who did not revealed that a value <13.9 nmol/L could predict failure to achieve MDA with 75% sensitivity and 92% specificity (p=0.006) (figure 2). The predictive value of low saliva morning cortisol was independent of age, sex, CRP levels and disease duration (overall adjusted OR: 53.7, 95% CI: 3.9 to 744). Further analysis demonstrated that of those seven patients who displayed both morning saliva cortisol <13.9 nmol/L and disturbed diurnal cortisol rhythm at baseline, none achieved MDA after 6 months of treatment.
Figure 2.
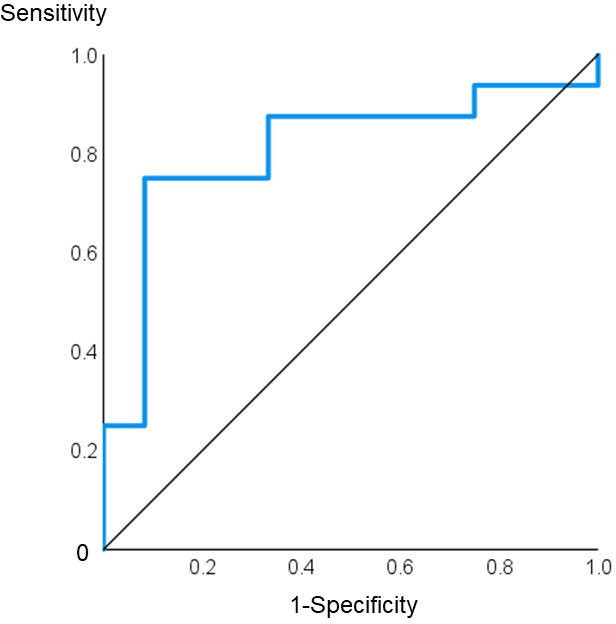
Receiver operating characteristic curve for baseline morning saliva cortisol levels predicting no minimal disease activity (DAS >2.85) at 6 months in patients with RA. AUC: 0.807, 95% CI: 0.634 to 0.981, p=0.006. AUC, area under the curve; DAS, Disease Activity Score; RA, rheumatoid arthritis.
In the subset of patients with RA (n=20), in whom both serum and plasma samples were available at baseline, we also calculated the cortisol/ACTH ratio, to elaborate upon the functionality of the hypothalamic–pituitary–adrenal (HPA) axis. At 6 months, the cortisol/ACTH ratio was significantly lower in those patients who did not achieve MDA (10 of 20) compared with patients who achieved MDA (10 of 20) at 6 months (online supplemental table 4), mostly driven by the significantly higher plasma ACTH levels in the non-MDA group.
Individualised diurnal production of cortisol is comparable overtime and not associated with treatment response
Finally, we compared diurnal cortisol production over time in the whole cohort, as well as between patients who achieved MDA at 6 months versus the remaining patients. We found that individualised time-integrated daily cortisol production from baseline to 3 and 6 months was comparable between the two subgroups, despite significant treatment-induced changes in the disease activity and the degree of severe stress (online supplemental table 5). Similarly, individualised serial measurements of saliva cortisol during the day did not change significantly from baseline to 3 and 6 months.
Discussion
By assessing diurnal saliva cortisol production in real-life patients with active RA, we found an impaired adrenal reserve as shown by (a) the lack of differences compared with healthy controls in both time-integrated daily cortisol production and individual serial cortisol measurements during the day, despite the presence of systemic inflammation, and (b) the lower diurnal saliva cortisol production compared with COVID-19 patients despite the comparable inflammatory status. In addition, we found an increased proportion of patients with disturbed cortisol rhythm in the RA cohort compared with healthy controls and comparable with the proportion seen in COVID-19 patients. These findings further confirm the role of adrenocortical dysfunction in the pathophysiology and progression of RA.39
Along this line, we also found that at the baseline of the present prospective study, lower morning saliva cortisol with corresponding higher plasma ACTH levels was present in those patients not achieving MDA after 6 months of treatment. Moreover, we report that low morning saliva cortisol levels (<13.9 nmol/L) in patients with active RA before initiation or escalation of drug treatment may serve as a prognostic biomarker in identifying those patients who would be unlikely to achieve MDA. Interestingly, the serum cortisol/ACTH ratio was also significantly lower in those patients who did not achieve MDA compared with patients who did at 6 months. This finding supports the notion that the problem lies in the adrenal glands, which, despite the increased stimulation of ACTH secretion from the pituitary gland, are not able to follow, suggesting that the adrenal cortices are either less sensitive and/or hypotrophic/hypoplastic in patients with RA. Certainly, our data cannot preclude adrenocortical atrophy/hypoplasia because of pre-existing hypofunction of the hypothalamic–pituitary unit of the HPA axis, as occurs in the arthritis-susceptible Lewis rat strain, a model of many autoimmune/inflammatory disorders.40 41
Despite several attempts, including machine learning utilisation,42–44 no single established biomarker has been reported in a real-life setting.45 Finally, the lack of noteworthy changes of diurnal production of saliva cortisol over time, in spite of the significant treatment-induced changes in disease activity and degree of stress, provides additional evidence that the impaired adrenal reserve in RA is probably an intrinsic condition associated with the underlying pathophysiological molecular mechanisms and thus may not be readily reversible.
In patients with RA, symptoms follow circadian rhythms with impaired function due to pain and joint stiffness being most severe in the early morning46 47 associated with increased proinflammatory cytokines, such as tumour necrosis factor and interleukin 6, occurring during late night hours.48 49 Increased endogenous nocturnal production of cortisol could alleviate these morning symptoms,13 but as we have shown here, the adrenal response to stress and inflammation is impaired in these patients. Several studies have reported that the optimal time for delivery of glucocorticoid treatment would be during the night, in order to target the effects of nocturnal proinflammatory stimuli50–53 and probably compensate for the presence of an impaired adrenal reserve.
High-quality trials since late 50s demonstrated the clinical efficacy of low-dose glucocorticoids in the management of RA54 55 and the beneficial effect on structural damage.56 Contemporary multicentre randomised clinical trials have proved efficacy of low-dose glucocorticoids in RA and favourable effects versus placebo or tapering, validating and replicating the earlier findings. In the GLORIA trial (a pragmatic Glucocorticoid Low-dose Outcome in Rheumatoid Arthritis trial), researchers compared the results of 2 years of prednisone (5 mg/day, equivalent to a dosage of 20 mg hydrocortisone in replacement therapy) or placebo added to optimised standard care in patients with established RA, showing that prednisone, even in this low dose, significantly reduced disease activity and joint damage progression.57 In the SEMIRA Study,10 continuation of low-dose prednisone (5 mg/day) in patients who achieved low disease activity with tocilizumab was safe and demonstrated a better disease control than tapering.
Taken together, the documented efficacy of complementary low-dose prednisone may be related to our findings of an impaired adrenal reserve in patients with RA described here and supports the addition of glucocorticoids in RA treatment regimens, as a potential ‘replacement’ therapy.11 58
The main limitations of our pilot study include the following: first, the sample size was relatively small and patients with both early and established RA were included. Second, as an explanatory analysis, it lacked power size calculation. Third, a unified treatment protocol was not used, and clinical parameters recorded did not include all the core set measures as per the EULAR/ACR collaborative recommendations for reporting disease activity in clinical trials of patients with RA.59 Our study design, however, reflects the routine clinical practice in RA management in a real-life clinical setting. Fourth, stress and depression were not systematically assessed in the control groups; therefore, their relative contribution on the degree of adrenal reserve exhaustion in RA cannot be assessed. Finally, at this point, we do not have data from a control group of patients with RA with inactive disease at baseline. However, the lack of differences in the diurnal cortisol production from baseline to 3 and 6 months in our cohort, despite significant treatment-induced changes in the disease activity, offers an indirect insight on the potential differences of the adrenal response between patients with active and inactive RA.
To conclude, our prospective data in patients with active RA suggest that, at least in some patients, the cortisol production is impaired, and this may be due to the fact that the adrenal functional reserve has been exhausted. In turn, an impaired adrenal reserve may contribute to the perpetuation of inflammation. Whether this condition could be partly or fully reversible after long-term disease remission remains to be elucidated. Identifying early in the course of the disease those patients with impaired adrenal reserve is challenging. A simple and easy-to-use method, such as measuring morning saliva cortisol levels, could be a valuable prognostic biomarker in clinical practice.
Footnotes
Contributors: Conceptualisation—MPY and PPS. Data curation—all authors. Data analysis—MPY, MGF, NIV and PPS. Funding acquisition—PPS. Investigation—all authors. Methodology—MPY and NIV. Writing (original draft)—MPY. Writing (review and editing)—all authors. Guarantor—PPS.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data of major outcomes are available on request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and was approved by the Scientific Committee of Laikon University Hospital (38/21-01-2021). Participants gave informed consent to participate in the study before taking part.
References
- 1.Chikanza IC, Petrou P, Kingsley G, et al. Defective hypothalamic response to immune and inflammatory stimuli in patients with rheumatoid arthritis. Arthritis Rheum 1992;35:1281–8. 10.1002/art.1780351107 [DOI] [PubMed] [Google Scholar]
- 2.Gudbjörnsson B, Skogseid B, Oberg K, et al. Intact Adrenocorticotropic hormone secretion but impaired Cortisol response in patients with active rheumatoid arthritis. J Rheumatol 1996;23:596–602. [PubMed] [Google Scholar]
- 3.Crofford LJ, Kalogeras KT, Mastorakos G, et al. Circadian relationships between interleukin (IL)-6 and hypothalamic-pituitary-adrenal axis hormones: failure of IL-6 to cause sustained Hypercortisolism in patients with early untreated rheumatoid arthritis. J Clin Endocrinol Metab 1997;82:1279–83. 10.1210/jcem.82.4.3852 [DOI] [PubMed] [Google Scholar]
- 4.Gutiérrez MA, García ME, Rodriguez JA, et al. Hypothalamic-pituitary-adrenal axis function in patients with active rheumatoid arthritis: a controlled study using insulin Hypoglycemia stress test and prolactin stimulation. J Rheumatol 1999;26:277–81. [PubMed] [Google Scholar]
- 5.Cutolo M, Foppiani L, Prete C, et al. Hypothalamic-pituitary-Adrenocortical axis function in premenopausal women with rheumatoid arthritis not treated with glucocorticoids. J Rheumatol 1999;26:282–8. [PubMed] [Google Scholar]
- 6.Cutolo M, Foppiani L, Minuto F. Hypothalamic-pituitary-adrenal axis impairment in the pathogenesis of rheumatoid arthritis and Polymyalgia Rheumatica. J Endocrinol Invest 2002;25(10 Suppl):19–23. [PubMed] [Google Scholar]
- 7.Straub RH, Paimela L, Peltomaa R, et al. Inadequately low serum levels of steroid hormones in relation to Interleukin-6 and tumor necrosis factor in untreated patients with early rheumatoid arthritis and reactive arthritis. Arthritis Rheum 2002;46:654–62. 10.1002/art.10177 [DOI] [PubMed] [Google Scholar]
- 8.Imrich R, Vigas M, Rovensky J, et al. Adrenal plasma steroid relations in glucocorticoid-naive premenopausal rheumatoid arthritis patients during insulin-induced Hypoglycemia test compared to matched normal control females. Endocr Regul 2009;43:65–73. [PubMed] [Google Scholar]
- 9.Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying Antirheumatic drugs: 2022 update. Ann Rheum Dis 2023;82:3–18. 10.1136/ard-2022-223356 [DOI] [PubMed] [Google Scholar]
- 10.Burmester GR, Buttgereit F, Bernasconi C, et al. Continuing versus tapering glucocorticoids after achievement of low disease activity or remission in rheumatoid arthritis (SEMIRA): a double-blind, Multicentre, randomised controlled trial. Lancet 2020;396:267–76. 10.1016/S0140-6736(20)30636-X [DOI] [PubMed] [Google Scholar]
- 11.Yavropoulou MP, Filippa MG, Panopoulos S, et al. Impaired adrenal cortex Reserve in patients with rheumatic and musculoskeletal diseases who relapse upon tapering of low glucocorticoid dose. Clin Exp Rheumatol 2022;40:1789–92. 10.55563/clinexprheumatol/x78tko [DOI] [PubMed] [Google Scholar]
- 12.Imrich R, Vlcek M, Aldag JC, et al. An Endocrinologist’s view on relative Adrenocortical insufficiency in rheumatoid arthritis. Ann N Y Acad Sci 2010;1193:134–8. 10.1111/j.1749-6632.2009.05362.x [DOI] [PubMed] [Google Scholar]
- 13.Straub RH, Cutolo M. Glucocorticoids and chronic inflammation. Rheumatology (Oxford) 2016;55(suppl 2):ii6–14. 10.1093/rheumatology/kew348 [DOI] [PubMed] [Google Scholar]
- 14.Filippa MG, Tektonidou MG, Mantzou A, et al. Adrenocortical dysfunction in rheumatoid arthritis: alpha narrative review and future directions. Eur J Clin Invest 2022;52:e13635. 10.1111/eci.13635 [DOI] [PubMed] [Google Scholar]
- 15.Kirschbaum C, Hellhammer DH. Salivary Cortisol in Psychobiological research: an overview. Neuropsychobiology 1989;22:150–69. 10.1159/000118611 [DOI] [PubMed] [Google Scholar]
- 16.Sephton SE, Sapolsky RM, Kraemer HC, et al. Diurnal Cortisol rhythm as a Predictor of breast cancer survival. J Natl Cancer Inst 2000;92:994–1000. 10.1093/jnci/92.12.994 [DOI] [PubMed] [Google Scholar]
- 17.Pervanidou P, Kolaitis G, Charitaki S, et al. Elevated morning serum interleukin (IL)-6 or evening salivary Cortisol concentrations predict Posttraumatic stress disorder in children and adolescents six months after a motor vehicle accident. Psychoneuroendocrinology 2007;32:991–9. 10.1016/j.psyneuen.2007.07.001 [DOI] [PubMed] [Google Scholar]
- 18.Bougea AM, Spandideas N, Alexopoulos EC, et al. Effect of the emotional freedom technique on perceived stress, quality of life, and Cortisol salivary levels in tension-type headache sufferers: a randomized controlled trial. Explore (NY) 2013;9:91–9. 10.1016/j.explore.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 19.Chrousos GP, Kaltsas G. Post-SARS sickness syndrome manifestations and Endocrinopathy: how, Why, and so what? Clin Endocrinol (Oxf). Clin Endocrinol (Oxf) 2005;63:363–5. 10.1111/j.1365-2265.2005.02361.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Acevo CA, Arrendondo-Loza E, Salinas-Carmona MC, et al. Cortisol and perceived stress are associated with Cytokines levels in patients infected with influenza B virus. Cytokine 2021;138. 10.1016/j.cyto.2020.155400 [DOI] [PubMed] [Google Scholar]
- 21.Yavropoulou MP, Filippa MG, Mantzou A, et al. Alterations in Cortisol and Interleukin-6 secretion in patients with COVID-19 suggestive of Neuroendocrine-immune adaptations. Endocrine 2022;75:317–27. 10.1007/s12020-021-02968-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aletaha D, Neogi T, Silman AJ, et al. Rheumatoid arthritis classification criteria: an American college of rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 23.Aletaha D, Ward MM, Machold KP, et al. Remission and active disease in rheumatoid arthritis: defining criteria for disease activity States. Arthritis Rheum 2005;52:2625–36. 10.1002/art.21235 [DOI] [PubMed] [Google Scholar]
- 24.Wells G, Becker J-C, Teng J, et al. Validation of the 28-joint disease activity score (Das28) and European League against rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the Das28 based on Erythrocyte sedimentation rate. Ann Rheum Dis 2009;68:954–60. 10.1136/ard.2007.084459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying Antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. 10.1136/annrheumdis-2019-216655 [DOI] [PubMed] [Google Scholar]
- 26.Wells GA, Boers M, Shea B, et al. Minimal disease activity for rheumatoid arthritis: a preliminary definition. J Rheumatol 2005;32:2016–24. [PubMed] [Google Scholar]
- 27.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–96. [PubMed] [Google Scholar]
- 28.HAMILTON M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andreou E, Alexopoulos EC, Lionis C, et al. Perceived stress scale: Reliability and validity study in Greece. Int J Environ Res Public Health 2011;8:3287–98. 10.3390/ijerph8083287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katsarou A, Panagiotakos D, Zafeiropoulou A, et al. Validation of a Greek version of PSS-14; a global measure of perceived stress. Cent Eur J Public Health 2012;20:104–9. 10.21101/cejph.a3698 [DOI] [PubMed] [Google Scholar]
- 31.Mitsikostas DD, Thomas AM. Comorbidity of headache and depressive disorders. Cephalalgia 1999;19:211–7. 10.1046/j.1468-2982.1999.019004211.x [DOI] [PubMed] [Google Scholar]
- 32.Lydersen S. Mean and standard deviation or median and Quartiles Tidsskr Nor Laegeforen 2020;140. 10.4045/tidsskr.20.0032 [DOI] [PubMed] [Google Scholar]
- 33.Perkins NJ, Schisterman EF. “The inconsistency of "optimal" Cutpoints obtained using two criteria based on the receiver operating characteristic curve”. Am J Epidemiol 2006;163:670–5. 10.1093/aje/kwj063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pruessner JC, Kirschbaum C, Meinlschmid G, et al. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003;28:916–31. 10.1016/s0306-4530(02)00108-7 [DOI] [PubMed] [Google Scholar]
- 35.Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307–12. 10.1111/j.2002.384.doc.x [DOI] [PubMed] [Google Scholar]
- 36.Thomas K, Lazarini A, Kaltsonoudis E, et al. Multicenter cross-sectional study of patients with rheumatoid arthritis in Greece: results from a cohort of 2.491 patients. Mediterr J Rheumatol 2018;29:27–37. 10.31138/mjr.29.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myasoedova E, Davis J, Matteson EL, et al. Is the epidemiology of rheumatoid arthritis changing? results from a population-based incidence study, 1985-2014. Ann Rheum Dis 2020;79:440–4. 10.1136/annrheumdis-2019-216694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nenke MA, Lewis JG, Rankin W, et al. Reduced corticosteroid-binding globulin cleavage in active rheumatoid arthritis. Clin Endocrinol (Oxf) 2016;85:369–77. 10.1111/cen.13081 [DOI] [PubMed] [Google Scholar]
- 39.Straub RH, Cutolo M. Involvement of the hypothalamic--pituitary--adrenal/Gonadal axis and the peripheral nervous system in rheumatoid arthritis: viewpoint based on a systemic Pathogenetic role. Arthritis Rheum 2001;44:493–507. [DOI] [PubMed] [Google Scholar]
- 40.Sternberg EM, Young WS, Bernardini R, et al. A central nervous system defect in biosynthesis of corticotropin-releasing hormone is associated with susceptibility to Streptococcal cell wall-induced arthritis in Lewis rats. Proc Natl Acad Sci USA 1989;86:4771–5. 10.1073/pnas.86.12.4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sternberg EM, Hill JM, Chrousos GP, et al. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in Streptococcal cell wall arthritis-susceptible Lewis rats. Proc Natl Acad Sci U S A 1989;86:2374–8. 10.1073/pnas.86.7.2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guan Y, Zhang H, Quang D, et al. Machine learning to predict anti-tumor necrosis factor drug responses of rheumatoid arthritis patients by integrating clinical and genetic markers. Arthritis Rheumatol 2019;71:1987–96. 10.1002/art.41056 [DOI] [PubMed] [Google Scholar]
- 43.Yoosuf N, Maciejewski M, Ziemek D, et al. Early prediction of clinical response to anti-TNF treatment using multi-Omics and machine learning in rheumatoid arthritis. Rheumatology (Oxford) 2022;61:1680–9. 10.1093/rheumatology/keab521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bouget V, Duquesne J, Hassler S, et al. Machine learning predicts response to TNF inhibitors in rheumatoid arthritis: results on the ESPOIR and ABIRISK cohorts. RMD Open 2022;8:e002442. 10.1136/rmdopen-2022-002442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wientjes MHM, den Broeder AA, Welsing PMJ, et al. Prediction of response to anti-TNF treatment using laboratory biomarkers in patients with rheumatoid arthritis: a systematic review. RMD Open 2022;8:e002570. 10.1136/rmdopen-2022-002570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cutolo M, Villaggio B, Otsa K, et al. Altered circadian rhythms in rheumatoid arthritis patients play a role in the disease’s symptoms. Autoimmun Rev 2005;4:497–502. 10.1016/j.autrev.2005.04.019 [DOI] [PubMed] [Google Scholar]
- 47.Straub RH, Cutolo M. Circadian rhythms in rheumatoid arthritis: implications for pathophysiology and therapeutic management. Arthritis Rheum 2007;56:399–408. 10.1002/art.22368 [DOI] [PubMed] [Google Scholar]
- 48.Cutolo M, Masi AT. Circadian rhythms and arthritis. Rheum Dis Clin North Am 2005;31:115–29, 10.1016/j.rdc.2004.09.005 [DOI] [PubMed] [Google Scholar]
- 49.Cutolo M. Circadian rhythms and rheumatoid arthritis. Joint Bone Spine 2019;86:327–33. 10.1016/j.jbspin.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 50.De Silva M, Binder A, Hazleman BL. The timing of prednisolone dosage and its effect on morning stiffness in rheumatoid arthritis. Ann Rheum Dis 1984;43:790–3. 10.1136/ard.43.6.790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buttgereit F, Doering G, Schaeffler A, et al. Efficacy of modified-release versus standard prednisone to reduce duration of morning stiffness of the joints in rheumatoid arthritis (CAPRA-1): a double-blind, randomised controlled trial. Lancet 2008;371:205–14. 10.1016/S0140-6736(08)60132-4 [DOI] [PubMed] [Google Scholar]
- 52.Buttgereit F, Doering G, Schaeffler A, et al. Targeting pathophysiological rhythms: prednisone Chronotherapy shows sustained efficacy in rheumatoid arthritis. Ann Rheum Dis 2010;69:1275–80. 10.1136/ard.2009.126888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buttgereit F, Mehta D, Kirwan J, et al. Low-dose prednisone Chronotherapy for rheumatoid arthritis: a randomised clinical trial (CAPRA-2). Ann Rheum Dis 2013;72:204–10. 10.1136/annrheumdis-2011-201067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.COPEMAN WSC, SAVAGE O, BISHOP PMF, et al. A study of Cortisone and other steroids in rheumatoid arthritis. Br Med J 1950;2:849–55. 10.1136/bmj.2.4684.849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.A comparison of prednisolone with aspirin or other Analgesics in the treatment of rheumatoid arthritis. A second report by the joint committee of the medical research Council and Nuffield foundation on clinical trials of Cortisone, ACTH, and other therapeutic measures in chronic rheumatic diseases. Ann Rheum Dis 1960;19:331–7. 10.1136/ard.19.4.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kirwan JR. The effect of glucocorticoids on joint destruction in rheumatoid arthritis. The arthritis and rheumatism Council low-dose glucocorticoid study group. N Engl J Med 1995;333:142–6. 10.1056/NEJM199507203330302 [DOI] [PubMed] [Google Scholar]
- 57.Boers M, Hartman L, Opris-Belinski D, et al. Low dose, add-on prednisolone in patients with rheumatoid arthritis aged 65+: the pragmatic randomised, double-blind placebo-controlled GLORIA trial. Ann Rheum Dis 2022;81:925–36. 10.1136/annrheumdis-2021-221957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cutolo M, Paolino S, Gotelli E. Glucocorticoids in rheumatoid arthritis still on first line: the reasons. Expert Rev Clin Immunol 2021;17:417–20. 10.1080/1744666X.2021.1903319 [DOI] [PubMed] [Google Scholar]
- 59.Aletaha D, Landewe R, Karonitsch T, et al. Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Arthritis Rheum 2008;59:1371–7. 10.1002/art.24123 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003575supp001.pdf (193.1KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data of major outcomes are available on request.