Summary
Sensitivity to face-to-face stimuli configurations, which likely indicates interaction, seems to appear early in infants’ development, and recently a preference for face-to-face (vs. other spatial configurations) has been shown to occur in macaque monkeys. It is unknown, however, whether such a preference is acquired through experience or as an evolutionary-given biological predisposition. Here, we exploited a precocial social animal, the domestic chick, as a model system to address this question. Visually naive chicks were tested for their spontaneous preferences for face-to-face vs. back-to-back hen dyads of point-light displays depicting biological motion. We found that female chicks have a spontaneous preference for the facing interactive configuration. Males showed no preference, as expected due to the well-known low social motivation of males in this highly polygynous species. These findings support the idea of an innate and sex-dependent predisposition toward social and interacting stimuli in a vertebrate brain such as that of chicks.
Subject areas: Neuroscience, Developmental neuroscience, Social sciences
Graphical abstract

Highlights
-
•
Naive female chicks approach face-to-face rather than back-to-back point-light hens
-
•
It reveals a biological predisposition to recognize socially relevant configurations
-
•
Modulation by sex could be driven by the polygynous mating system of the species
Neuroscience; Developmental neuroscience; Social sciences
Introduction
The brains of young vertebrate animals are equipped with animacy detectors, mechanisms to recognize the presence of other animals in the environment. For example, newborn humans and newly hatched chicks quickly detect face-like stimuli1,2,3 and exhibit preferences for self-propelled4,5,6,7,8,9 and biological motion.10,11,12,13,14,15
This sensitivity to animacy signals not only helps organisms to spot the presence of other living beings in their vicinity but also lays the foundation for the development of social interactions.16,17 Stimuli involving multiple agents that interact with each other may provide information about social events. One example is when two bodies are positioned face-to-face and therefore suggest an interaction compared to a back-to-back positioning.18,19
In humans, visual sensitivity to the difference between face-to-face and back-to-back bodies appears early in development18 and evolves into a preference for face-to-face bodies, which is shared with monkeys.20 In those studies, the looking times of six-month-old infants were computed while presenting simultaneously two dyads showing face-to-face and back-to-back bodies, respectively, finding a significant difference in looking times toward the two types of dyads.18 The same looking-time paradigm was adopted in adult humans and macaques, confirming the different attention driven by the two types of stimuli, and thus, the relevance of such configurations.20 Those results are compatible with the evidence of perceptual adaptation for efficient processing of seemingly interacting (e.g., face-to-face) bodies. In fact, it has been shown that, in conditions of visual noise, facing bodies are more likely to be detected and recognized than the same two bodies facing away from each other.21,22 Other studies showed that the face-to-face positioning of bodies impacts the very early, preattentive stages of visual perception,19,23 up to visual memory.24,25 The behavioral advantage in processing facing people has a counterpart in neuroimaging results showing that, in humans, body-selective regions of the visual cortex respond more strongly to multiple bodies that appear to be interacting (i.e., face-to-face), relative to unrelated bodies.26,27
However, whether the visual preference for face-to-face bodies is acquired through experience or rather represents an example of an evolutionarily given predisposition is unknown, and it is the issue we aim to address here.
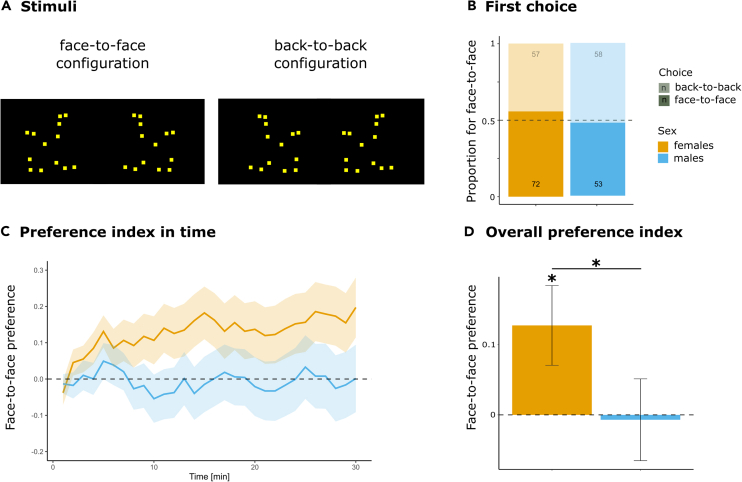
We tested newly hatched visually naive chicks for their spontaneous preferences for a pair of biological motion patterns representing two point-light hen silhouettes walking either face-to-face or back-to-back (Figure 1A). We measured the first approach and time spent near the two stimuli in both male and female newly hatched chicks.
Figure 1.
Stimuli and chicks’ spontaneous choice
(A) Example of a frame for face-to-face (left) and back-to-back (right) configurations.
(B) First choice proportion for face-to-face configuration. Proportion of time spent to face-to-face configuration (preference index) as a function of time (C) and overall for the 30 min of test (D; mean±se, ∗ p < 0.05). GIF files with the moving stimuli are available in the data repository Figshare: https://figshare.com/articles/dataset/Innate_sensitivity_to_face-to-face_biological_motion_dataset/23713299.
Results
To test whether the chicks were more likely to first approach the face-to-face over the back-to-back stimuli, we tracked the animals’ first choice (Figure 1B).
No statistically significant differences were observed in the first approach (between sexes comparison χ2(1) = 1.25, p = 0.26; overall binomial probability for facing configuration 0.52, 95% CI: 0.46–0.59, p = 0.5).
After the first approach, choice proved to stabilize over time. Indeed, we performed a longitudinal analysis with time and sex as factors, to investigate whether response changes across the 30 min of test. This did not reveal any significant main effect (time: 1.5, df = 3.6, p = 0.2; sex 2.5, df = 1, p = 0.1; interaction: 0.8, df = 3.6, p = 0.5). Still, a trend diverging from random choice after the first minutes could be observed in female (Figure 1C).
To test whether the chicks were more likely to spend most of the overall time close to the face-to-face over the back-to-back stimuli, we computed the proportion of time animals spent close to the face-to-face configuration during the overall 30 min of the experiment (Figure 1D). In addition, we considered again sex as a factor, taking into account previous evidence of sex differences in the interaction with social stimuli.28,29,30,31
A permutation test on the proportion of time spent close to the stimuli revealed a sex difference (permutation p = 0.047, effect size Cohen’s d = 0.2). Females spontaneously chose to spend more time near the face-to-face configuration (Z = −2.16, p = 0.03, effect size Cohen’s d = 0.2; post-hoc achieved power 60%), while males did not exhibit any preference (Z = −0.18, p = 0.9, effect size Cohen’s d = 0.01).
Discussion
We found that female chicks spontaneously preferred to remain close to a pair of face-to-face hen silhouettes rather than back-to-back. This finding provides evidence that the recognition of face-to-face configuration, likely indicative of interaction, which has been found in infants, adult humans, and adult macaque monkeys, is observed also in birds and does not derive from experience but it is likely to be biologically predisposed in the vertebrate brain. Moreover, given that previous studies in infants never reported a preference for face-to-face stimuli, but only a capacity for discrimination,18,20 the present study is the first report of such an early preference.
While previous studies have demonstrated discrimination and preference for biological motion versus random motion and inverted biological stimuli (reviewed by Vallortigara17), our study is also the first to reveal a preference for “face-to-face” interactions between agents over “back-to-back” interactions. Interestingly, this finding shows a different relevant level of information than all previously cited works that investigated spontaneous preferences, e.g. the tendency to approach possible instead of impossible figures,32 to prefer consonant music,33 or to complete over fragmented objects.34 Indeed, in all these cases, chicks were proved to show an innate sensitivity to “natural” configurations in the environment that meet the Gestalt rules of continuity, proximity, and order35 as well as the expectation of consonance with the physical rules governing nature. With the present findings, instead, we showed a level of predisposition that reflects expectations about the social world, in opposition to the expectations about the physical world. This could also explain sex differences in this specific predisposition.
Indeed, our experiment revealed a divergence between females and males, with the latter showing no preference. This finding is interesting in itself and aligns with the species’ natural history and the existing ethological literature28,29,30,31,36 (see also for non-human primates Brown et al. study37), which indicates that males typically display lower levels of interest in social stimuli and interactions with them compared to females, as part of being a strict polygynous species. Indeed, within adult fowls, there is usually a dominant rooster and many hierarchically organized hens,38 favoring the evolution of solitary territorial males and more socially prone females.39,40
Moreover, the two kinds of stimuli used here clearly provided perceptual cues associated with affiliative responses, which are known to be stronger in females. There is evidence that chicks tend to align to the apparent direction of movement of point-light displays.14 Thus, facing dyads would elicit approach responses, irrespective of which of the two point-light hens the chick aligns to; instead, non-facing dyads would elicit withdrawal responses.
Additionally, an approach is needed to perform social pecking, which is the main tool for social recognition in young chicks.41 Again, social pecking, which is more pronounced in females,30 requires proper orienting toward conspecifics, as in facing dyads.
Of course, social interaction is a very general term and may comprise different aspects. For instance, point-light displays facing in the same direction may signal the increased likelihood of a resource between the hens (e.g., corn on the ground between them) that the chicks may be attracted to. This seems unlikely, however, because newly hatched chicks have reserves in the yolk sac for the first two days and are primarily interested in imprinting on a social partner rather than on food at this age.41 Besides, there are no obvious reasons why females should be more interested in food than males. It is more likely that chicks may prefer to congregate with groups of hens, which should motivate attraction to two facing hens rather than two hens walking in opposite directions. Whatever the underlying motivation, it is clear that the face-to-face signal to which chicks respond reflects a predisposition in their brain.
Limitations of the study
The present paper only deals with the issue of the origin (innate or learned) of the face-to-face preference and not with the particular visual cues that generate it (see for specific investigations on this topic Goupil and Papeo works18,19,21). However, the fact that only females exhibit the preference supports the idea that it is the biological meaning of the face-to-face interaction that elicits interest and not some low-level aspect of the visual stimulation.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| Innate sensitivity to face-to-face biological motion dataset | This paper | Figshare: https://figshare.com/articles/dataset/Innate_sensitivity_to_face-to-face_biological_motion_dataset/23713299 |
| Experimental models: Organisms/strains | ||
| White Leghorn chicks (Gallus gallus) | Azienda Agricola Crescenti, Brescia, IT | Strain: Ross 308 |
| Software and algorithms | ||
| DeepLabCut | Mathis et al.42 | http://www.mackenziemathislab.org/deeplabcut |
| R studio | Posit | https://posit.co/download/rstudio-desktop/ |
| G-Power 3.1 | Faul et al.43 | https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower |
| ImprintSchedule | Zanon et al.44 | https://github.com/MirkoZanon/ImprintSchedule |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Giorgio Vallortigara (giorgio.vallortigara@unitn.it).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
Behavioral data have been deposited at Figshare and are publicly available as of the date of publication. DOIs are listed in the key resources table.
-
•
All original code has been deposited at Figshare and is publicly available as of the date of publication. DOIs are listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
We studied 240 White Leghorn chicks of the Ross 308 strain (129 females) obtained from a local hatchery (Azienda Agricola Crescenti, Brescia). The estimation was based on an effect of 0.28 (from studies on chicks’ spontaneous preference for motion cues, with similar setup and paradigm; see e.g. Rosa Salva’s work45) with the alpha level at 5% and 80% power. The chicks were incubated at the University of Trento under controlled conditions (37.7°C, and 60% humidity). In our pursuit of investigating innate predispositions, we took precautionary measures to ensure that the experimental subjects remained free from external stimuli immediately preceding the experiment. To achieve this, chicks were incubated in the dark in individual small boxes.
A few hours just after hatching (variable between 2 to 10, during which chicks were always kept in the dark), animals were singularly placed in rectangular arenas (60 cm x 90 cm x 90 cm) with high-frequency screens (120 Hz) at opposite ends44 to undergo the dual-choice spontaneous task lasting 30 minutes.
The experimental procedures received ethical approval from the University of Trento's Ethical Committee and the Italian Ministry of Health (permit number 324/2022-PR).
Method details
After hatching, chicks were placed in a rectangular arena (60 cm x 90 cm x 90 cm) with high-frequency screens (120 Hz) at opposite ends.44 One screen displayed face-to-face walking point-light silhouette hens, while the other showed a back-to-back configuration (Figure 1A). The stimuli, displayed as GIFs at 13.7 frames per second, were presented during the whole test duration while recording the chicks' behaviors. Previous studies with analogous paradigms focused on the first 6-minute choice; to better evaluate possible adjustments and time variations in the chicks’ spontaneous preference we decided here to record the behaviour for 30 minutes.
Quantification and statistical analysis
We used DeeplabCut42 to track animals’ coordinates, from which we determined the first choice and the time spent near both stimuli. The evaluation was solely based on the animal’s position, considering a stimulus as approached whenever the animal was 30 cm or less from the screen displaying it.
We calculated a preference index for the face-to-face configuration dividing the total time spent close to this stimulus by the total time in proximity of both stimuli.
We used a χ2 test to examine sex differences in the first choice and a binomial test to assess the preference for the face-to-face configuration. For the analysis in time, a longitudinal non-parametric test was conducted with minutes and sex as factors (using the nparLD R package). This is a rank-based test on the marginal distribution functions: using minutes as an ordered factor we can investigate differences in time (null hypothesis: equal marginal cumulative distributions at the different time points). Briefly, the preference index was converted to ranks, data were stratified in independent (i.e., sex) and repeated (i.e., minutes) factors, while mean rank differences between these groups and their interactions were tested using non-parametric ANOVA-type statistics (ATS).
The overall preference (during the cumulative 30 minutes) was evaluated through a permutation ANOVA, and non-parametric Wilcoxon Rank Sum tests were conducted to assess deviations from chance for each sex.
Power analysis and calculation of post-hoc achieved power were performed with G-Power 3.1 software.43
Acknowledgments
This work was supported by the European Research Council Grant ERC-2011-ADG_20110406, Project 461295517, Predisposed mechanisms for social orienting (PREMESOR) (to G.V.) and European Research Council Starting Grant Project: THEMPO, grant agreement 758473 (to L.P.) We are thankful to Isaia Meneghelli for his help in collecting data.
Author contributions
G.V., L.P., M.Z., and B.S.L. conceived the experiment. M.Z. collected the data. M.Z. and B.S.L. performed the data analysis. M.Z. and B.S.L. wrote the manuscript. G.V. and L.P. revised the manuscript. All authors contributed to the final version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: January 4, 2024
References
- 1.Buiatti M., Di Giorgio E., Piazza M., Polloni C., Menna G., Taddei F., Baldo E., Vallortigara G. Cortical route for facelike pattern processing in human newborns. Proc. Natl. Acad. Sci. USA. 2019;116:4625–4630. doi: 10.1073/pnas.1812419116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosa Salva O., Regolin L., Vallortigara G. Faces are special for newly hatched chicks: Evidence for inborn domain-specific mechanisms underlying spontaneous preferences for face-like stimuli. Dev. Sci. 2010;13:565–577. doi: 10.1111/j.1467-7687.2009.00914.x. [DOI] [PubMed] [Google Scholar]
- 3.Simion F., Giorgio E.D. Face perception and processing in early infancy: Inborn predispositions and developmental changes. Front. Psychol. 2015;6:969. doi: 10.3389/fpsyg.2015.00969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Giorgio E., Loveland J.L., Mayer U., Rosa Salva O., Versace E., Vallortigara G. Filial responses as predisposed and learned preferences: Early attachment in chicks and babies. Behav. Brain Res. 2017;325:90–104. doi: 10.1016/j.bbr.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Di Giorgio E., Lunghi M., Simion F., Vallortigara G. Visual cues of motion that trigger animacy perception at birth: the case of self-propulsion. Dev. Sci. 2017;20 doi: 10.1111/desc.12394. [DOI] [PubMed] [Google Scholar]
- 6.Di Giorgio E., Lunghi M., Vallortigara G., Simion F. Newborns’ sensitivity to speed changes as a building block for animacy perception. Sci. Rep. 2021;11:542. doi: 10.1038/s41598-020-79451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mascalzoni E., Regolin L., Vallortigara G. Innate sensitivity for self-propelled causal agency in newly hatched chicks. Proc. Natl. Acad. Sci. USA. 2010;107:4483–4485. doi: 10.1073/pnas.0908792107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorenzi E., Mayer U., Rosa-Salva O., Vallortigara G. Dynamic features of animate motion activate septal and preoptic areas in visually naïve chicks (Gallus gallus) Neuroscience. 2017;354:54–68. doi: 10.1016/j.neuroscience.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Rosa Salva O., Grassi M., Lorenzi E., Regolin L., Vallortigara G. Spontaneous preference for visual cues of animacy in naïve domestic chicks: The case of speed changes. Cognition. 2016;157:49–60. doi: 10.1016/j.cognition.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Lemaire B.S., Vallortigara G. Life is in motion (through a chick’s eye) Anim. Cogn. 2023;26:129–140. doi: 10.1007/s10071-022-01703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemaire B.S., Rosa Salva O., Fraja M., Lorenzi E., Vallortigara G. Spontaneous preference for unpredictability in the temporal contingencies between agents’ motion in naive domestic chicks. Proc. Biol. Sci. 2022;289:20221622. doi: 10.1098/rspb.2022.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simion F., Regolin L., Bulf H. A predisposition for biological motion in the newborn baby. Proc. Natl. Acad. Sci. USA. 2008;105:809–813. doi: 10.1073/pnas.0707021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallortigara G., Regolin L., Marconato F. Visually inexperienced chicks exhibit spontaneous preference for biological motion patterns. PLoS Biol. 2005;3:e208–e1316. doi: 10.1371/journal.pbio.0030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vallortigara G., Regolin L. Gravity bias in the interpretation of biological motion by inexperienced chicks. Curr. Biol. 2006;16:279–280. doi: 10.1016/j.cub.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 15.Matsushima T., Miura M., Patzke N., Toji N., Wada K., Ogura Y., Homma K.J., Sgadò P., Vallortigara G. Fetal blockade of nicotinic acetylcholine transmission causes autism-like impairment of biological motion preference in the neonatal chick. Cereb. Cortex Commun. 2022;3 doi: 10.1093/texcom/tgac041. tgac041–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorenzi E., Vallortigara G. The Cambridge Handbook of Animal Cognition. Cambridge University Press; 2021. Evolutionary and Neural Bases of the Sense of Animacy; pp. 295–321. [Google Scholar]
- 17.Vallortigara G. MIT press Cambridge; 2021. Born Knowing. [Google Scholar]
- 18.Goupil N., Papeo L., Hochmann J.R. Visual perception grounding of social cognition in preverbal infants. Infancy. 2022;27:210–231. doi: 10.1111/infa.12453. [DOI] [PubMed] [Google Scholar]
- 19.Papeo L. Twos in human visual perception. Cortex. 2020;132:473–478. doi: 10.1016/j.cortex.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Goupil N., Rayson H., Serraille E., Massera A., Ferrari P.F., Hochmann J.-R., Papeo L. Visual preference for socially relevant spatial relations in humans and monkeys. OSF Preprints. 2023 doi: 10.31219/osf.io/8zsc9. Preprint at. [DOI] [PubMed] [Google Scholar]
- 21.Papeo L., Stein T., Soto-Faraco S. The Two-Body Inversion Effect. Psychol. Sci. 2017;28:369–379. doi: 10.1177/0956797616685769. [DOI] [PubMed] [Google Scholar]
- 22.Papeo L., Abassi E. Seeing social events: The visual specialization for dyadic human-human interactions. J. Exp. Psychol. Hum. Percept. Perform. 2019;45:877–888. doi: 10.1037/xhp0000646. [DOI] [PubMed] [Google Scholar]
- 23.Xu Z., Chen H., Wang Y. Invisible social grouping facilitates the recognition of individual faces. Conscious. Cognit. 2023;113 doi: 10.1016/j.concog.2023.103556. [DOI] [PubMed] [Google Scholar]
- 24.Ding X., Gao Z., Shen M. Two Equals One: Two Human Actions During Social Interaction Are Grouped as One Unit in Working Memory. Psychol. Sci. 2017;28:1311–1320. doi: 10.1177/0956797617707318. [DOI] [PubMed] [Google Scholar]
- 25.Paparella I., Papeo L. Chunking by Social Relationship in Working Memory. Vis. cogn. 2022;30:354–370. [Google Scholar]
- 26.Abassi E., Papeo L. The representation of two-body shapes in the human visual cortex. J. Neurosci. 2020;40:852–863. doi: 10.1523/JNEUROSCI.1378-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abassi E., Papeo L. Category-selective representation of relationships in visual cortex. J. Neurosci. 2023 doi: 10.1523/JNEUROSCI.0250-23.2023. JN-RM-0250-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cailotto M., Vallortigara G., Zanforlin M. Sex differences in the response to social stimuli in young chicks. Ethol. Ecol. Evol. 1989;1:323–327. [Google Scholar]
- 29.Lemaire B.S., Rugani R., Regolin L., Vallortigara G. Response of male and female domestic chicks to change in the number (quantity) of imprinting objects. Learn. Behav. 2021;49:54–66. doi: 10.3758/s13420-020-00446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallortigara G. Affiliation and aggression as related to gender in domestic chicks (Gallus gallus) J. Comp. Psychol. 1992;106:53–57. doi: 10.1037/0735-7036.106.1.53. [DOI] [PubMed] [Google Scholar]
- 31.Vallortigara G., Cailotto M., Zanforlin M. Sex differences in social reinstatement motivation of the domestic chick (Gallus gallus) revealed by runway tests with social and nonsocial reinforcement. J. Comp. Psychol. 1990;104:361–367. doi: 10.1037/0735-7036.104.4.361. [DOI] [PubMed] [Google Scholar]
- 32.Regolin L., Rugani R., Stancher G., Vallortigara G. Spontaneous discrimination of possible and impossible objects by newly hatched chicks. Biol. Lett. 2011;7:654–657. doi: 10.1098/rsbl.2011.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiandetti C., Vallortigara G. Chicks like consonant music. Psychol. Sci. 2011;22:1270–1273. doi: 10.1177/0956797611418244. [DOI] [PubMed] [Google Scholar]
- 34.Regolin L., Vallortigara G., Zanforlin M. Object and spatial representations in detour problems by chicks. Anim. Behav. 1995;49:195–199. [Google Scholar]
- 35.Koffka K. Vol. 44. Routledge; 2013. Principles of Gestalt Psychology. [Google Scholar]
- 36.Workman L., Andrew R.J. Simultaneous changes in behaviour and in lateralization during the development of male and female domestic chicks. Anim. Behav. 1989;38:596–605. [Google Scholar]
- 37.Brown J., Kaplan G., Rogers L.J., Vallortigara G. Perception of biological motion in common marmosets (Callithrix jacchus): By females only. Anim. Cogn. 2010;13:555–564. doi: 10.1007/s10071-009-0306-0. [DOI] [PubMed] [Google Scholar]
- 38.Queiroz S.A., Cromberg V.U. Aggressive behavior in the genus Gallus sp. Rev. Bras. Ciência Avícola. 2006;8:1–14. [Google Scholar]
- 39.Gottier R.F. The dominance-submission hierarchy in the social behavior of the domestic chicken. J. Genet. Psychol. 1968;112:205–226. doi: 10.1080/00221325.1968.10533796. [DOI] [PubMed] [Google Scholar]
- 40.McBride G., Foenander F. Territorial behaviour in flocks of domestic fowls. Nature. 1962;194:102. [Google Scholar]
- 41.Rogers L.J. CABI; 1995. Development of Brain and Behaviour in the Chicken. [Google Scholar]
- 42.Mathis A., Mamidanna P., Cury K.M., Abe T., Murthy V.N., Mathis M.W., Bethge M. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 2018;21:1281–1289. doi: 10.1038/s41593-018-0209-y. [DOI] [PubMed] [Google Scholar]
- 43.Faul F., Erdfelder E., Buchner A., Lang A.G. Statistical power analyses using G∗Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 44.Zanon M., Lemaire B.S., Vallortigara G. Steps towards a computational ethology: an automatized, interactive setup to investigate filial imprinting and biological predispositions. Biol. Cybern. 2021;115:575–584. doi: 10.1007/s00422-021-00886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosa-Salva O., Hernik M., Broseghini A., Vallortigara G. Visually-naïve chicks prefer agents that move as if constrained by a bilateral body-plan. Cognition. 2018;173:106–114. doi: 10.1016/j.cognition.2018.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
-
•
Behavioral data have been deposited at Figshare and are publicly available as of the date of publication. DOIs are listed in the key resources table.
-
•
All original code has been deposited at Figshare and is publicly available as of the date of publication. DOIs are listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.