Abstract
The overconsumption of highly caloric and palatable foods has caused a surge in obesity rates in the past half century, thereby posing a healthcare challenge due to the array of comorbidities linked to heightened body fat accrual. Developing treatments to manage body weight requires a grasp of the neurobiological basis of appetite. In this Review, we discuss advances in neuroscience that have identified brain regions and neural circuits that coordinate distinct phases of eating: food procurement, food consumption, and meal termination. While pioneering work identified several hypothalamic nuclei to be involved in feeding, more recent studies have explored how neuronal populations beyond the hypothalamus, such as the mesolimbic pathway and nodes in the hindbrain, interconnect to modulate appetite. We also examine how long-term exposure to a calorically dense diet rewires feeding circuits and alters the response of motivational systems to food. Understanding how the nervous system regulates eating behaviour will bolster the development of medical strategies that will help individuals to maintain a healthy body weight.
The term obesity is an absurdly monolithic label encompassing the many physiological and psychological consequences of a positive energy balance1. Myriad elements contribute to increased food intake, including genetics, socioeconomic status, food availability, aggressive marketing, engineered palatable products, stress, anxiety, appetitive cues, memories, medications, resistance to internal satiety signals, depression, and misinformation about health and nutrition. Because many of these societal influences cannot be faithfully replicated in a controlled laboratory setting, most work has instead focused on investigating the neurobiological underpinnings of eating to understand how individuals may develop obesity. Perhaps a common myth among the misinformed is that body weight regulation, largely driven by food consumption, is under strict volitional control, whereby one steadfastly determines what, when, and how much to eat. In reality, our neurobiology has evolved to ensure that we ingest the necessary calories to have a surplus, which is far more advantageous than a dearth of energy stores to meet everyday requirements. To efficiently and enduringly combat this evolutionary pressure to overconsume food, anti-obesity therapeutics should be designed to hijack the central mechanisms underlying eating at multiple loci. In this piece, we review the neural circuits underlying the phasic complexity and sequential nature of feeding, which can be summed up in a collection of behaviours and divided into three discrete acts2: (1) an appetitive step comprised of foraging and approaching food, (2) a consummatory stage, whereby a self-propagating feedback loop promotes ingestion, and (3) a termination phase, through which a host of mechanical and chemical signals act in concert to shut off feeding (Fig. 1).
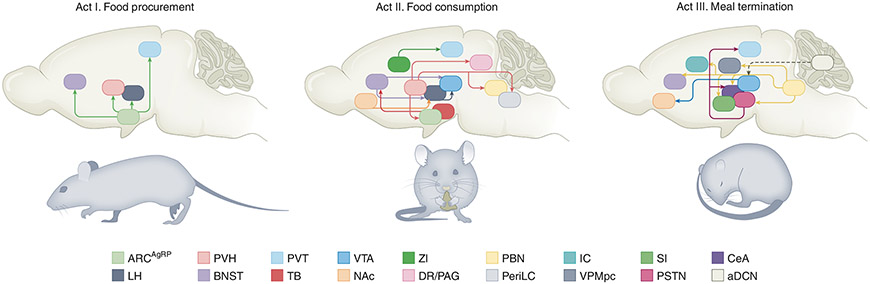
Fig. 1 ∣. Neural circuitry underlying the three acts of appetite.
Left, act I: food procurement. Inhibitory ARCAgRP neuronal projections to the BNST, PVH, LH, and PVT promote food seeking and subsequent food intake. Middle, act II: food consumption. Activation of LHVGAT and inhibition of LHVGLUT2 neurons increases food intake through direct projections to the VTA. Inhibitory circuits from the BNST and NAcD1R neurons feed into this circuit to modulate consumption. GABAergic TBSST neurons, via projections to the BNST and PVH, and ZI neurons, via projections to the PVT, potently drive ingestive behaviours. Excitatory PVH neurons regulate food intake through projections to the ARC, DR/PAG, PBN, and periLC. Right, act III: meal termination. Stimulation of excitatory PBNCGRP suppresses food intake, generates learnt and defensive responses, and induces malaise via projections to the CeA, BNST, SI, PSTN, VPMpc, and IC. Activation of excitatory aDCN neurons reduces meal size by increasing striatal dopamine levels and attenuating the phasic dopamine response to subsequent food consumption. Solid arrows indicate direct connections. Dashed arrows indicate indirect connections. ARC, arcuate nucleus of the hypothalamus; AgRP, Agouti-related peptide; BNST, bed nucleus of the stria terminalis; PVH, paraventricular nucleus of the hypothalamus; LH, lateral hypothalamus; PVT, paraventricular nucleus of the thalamus; VGAT, vesicular gamma-aminobutyric acid transporter; VGLUT2, vesicular glutamate transporter 2; VTA, ventral tegmental area; D1R, dopamine 1 receptor; TB, tuberal nucleus; SST, somatostatin; ZI, zona incerta; DR, dorsal raphe nucleus; PAG, periaqueductal grey; PBN, parabrachial nucleus; periLC, peri-locus coeruleus; CeA, central amygdala; SI, substantia innominata, PSTN, parasubthalamic nucleus; VPMpc, parvicellular portion of the ventroposteromedial nucleus; IC, visceral insular cortex; aDCN, anterior deep cerebellar nuclei.
The brain’s role in shaping motivated behaviours was established over a century ago, with people with tumours, lesions, and additional pathologies exhibiting maladaptive feeding episodes. A high volume of these reports pinpointed the hypothalamus as a common target of these afflictions3-19, as the lateral hypothalamus (LH), paraventricular hypothalamus (PVH), ventral medial hypothalamus (VMH), dorsal medial hypothalamus (DMH), and arcuate nucleus (ARC) were all shown to control eating to various degrees. Layered on top of hypothalamic gating of feeding was the emergence of the dopaminergic mesolimbic system’s role in influencing motivational aspects of food substrates, including its rewarding properties, learnt responses towards acquisition probability, and the predictive and associative memories related to food procurement20-24. Although this groundbreaking work implicated distinct regions of the brain in appetite, or the desire to eat food, the techniques carried significant limitations, including a lack of anatomical precision, cell-type specificity, and targeted site of action. Additionally, many of the methods lacked reversibility, making it difficult to interpret direct versus indirect outcomes of the initial manipulation. These constraints were often a challenge to achieving reproducibility among participants or between laboratories, providing a broad and disjointed understanding of the central mechanisms underlying appetite.
This obstacle heralded a generation of genetically driven cell manipulations to globally knock out, selectively delete, or overexpress genes encoding critical peptides and receptors using transgenic mice. These strategies identified and validated many modulators of energy balance, including the adipocyte-derived hormone leptin and its cognate leptin receptor (LEPR)25-27, the melanocortin system28-32, and the gut-derived incretin glucagon-like peptide 1 (GLP1) and its targeted glucagon-like peptide 1 receptor (GLP1R)33-35. Although they significantly increased our understanding of the precise cell types and signalling schemes underlying feeding behaviours, these manipulations occur throughout development; thus, compensation for genetic perturbations is difficult to detect. Bypassing this drawback, several studies found that acute inactivation of hypothalamic neurons in adulthood using diphtheria-toxin-mediated ablation approaches severely impacted feeding36-40. While this addresses developmental compensatory concerns, it raises additional issues, including the ill-defined temporal kinetics and permanent nature of the manipulation, as well as only testing for cell-type requirement, and not sufficiency, in mediating consummatory behaviours. Given these caveats, alternative techniques need to be employed for molecularly defined neural circuit analysis.
Recent breakthroughs in neuroscience allow for more targeted approaches to tackle these empirical questions. For the past two decades, researchers have used tools that grant both spatial and temporal precision of molecularly or behaviourally defined cell types to investigate their role in conducting food intake. Optogenetic and chemogenetic techniques allow scientists to explain behaviour through necessity and sufficiency claims, and in vivo optical imaging and electrical recordings are used to reinforce these findings by assessing endogenous, real-time neural activity in behaving animals. These approaches have been combined with cell-specific anterograde and retrograde tracing, ex vivo acute brain slice electrophysiology and imaging, single-cell RNA sequencing, upgraded (and often automated) equipment for more precise measurements and less experimenter intervention, innovative tracking solutions, and effective assay design to study the central mechanisms underlying feeding behaviours with an uncanny level of detail.
Prologue: appetite for calories
Appetite describes the desire to eat a specific food item regardless of the body’s energy state, and hunger is a sensation that arises when the body is in need of caloric repletion following food deprivation and/or excess energy expenditure. This perception arises from multiple peripheral signals such as ghrelin, an orexigenic hormone released by the stomach41,42 that increases during periods of fasting and quickly decreases on caloric consumption43,44. Ghrelin is transported from the periphery to the central nervous system, where it acts on multiple brain areas to guide food seeking and feeding behaviours. Furthermore, exogenous administration of ghrelin is sufficient to increase food intake, body weight, and adiposity in a dose-dependent manner45-47.
To maintain proper energy balance and prevent overconsumption, anorexigenic hormones such as leptin help to promote satiety, the sensation of being sated or full in between meals, and satiation, the progressive sensation of fullness during a meal that eventually leads to meal termination. Leptin is produced mainly by adipose tissues; its circulating levels positively correlate with adipose tissue size and thus energy stores48. Similar to ghrelin, leptin encodes the body’s current caloric state, but in the opposite way: leptin levels are low during fasting and increased following food consumption49. Mice lacking leptin show a drastic escalation in food intake and body weight26. Administration of leptin, in contrast, blunts appetite and can be used to reverse obesity in some people and rodent models50,51.
Hunger states can therefore be viewed as the integration of orexigenic and anorexigenic peripheral signals that act on the central nervous system to restore energy homeostasis. Though several neurons expressing receptors for hunger and satiety signals are found throughout the brain, many of them are concentrated in the ARC. Often serving as an entry point for humoral signals, the ARC is optimally situated because it is adjacent to the third ventricle and the median eminence, a structure that has an incomplete blood–brain barrier owing to its fenestrated capillaries. Food deprivation causes expansion of these fenestrated capillaries, allowing for circulating nutritional signals to easily reach and exert their effects on ARC neurons52.
Act I: food procurement
ARCAgRP neurons encode the properties of a starved state.
Agouti-related peptide (AgRP)-expressing neurons in the ARC (ARCAgRP) are stimulated by ghrelin and inhibited by leptin53-55. In line with these hormonal effects, electrophysiology and fibre photometry recordings have revealed that ARCAgRP neurons are most active when animals are fasted, or food-deprived, due in part to an increase in excitatory tone56-61. In rodents with ad libitum access to food, ARCAgRP neuronal activity is intertwined with diurnal rhythm. ARCAgRP neurons are mostly quiescent at the onset of the light cycle, during which rodents reduce energy expenditure after having consumed most of their daily caloric intake in the dark cycle. ARCAgRP neuronal firing rate, however, slowly ramps up as the light cycle progresses, gearing up the animal for food seeking and consumption59.
ARCAgRP neurons release AgRP, neuropeptide Y (NPY), and γ-aminobutyric acid (GABA), all of which have been characterized as involved in food intake and metabolism62-66. Chemogenetic inhibition of ARCAgRP neurons reduces food intake in hungry mice67,68, and ARCAgRP neuronal ablation in adult mice leads to a cessation of feeding37. Additionally, ARCAgRP perturbations govern nutrient partitioning by favouring fat storage on consumption69,70, lower energy expenditure and dissipation through inhibition of intrascapular brown adipose tissue (iBAT) thermogenesis triggered by decreased sympathetic nerve activity and a rapid re-programming of the iBAT gene expression profile67,71,72, and potentiate the rewarding properties of food through the mesolimbic system60,73,74. Optogenetic or chemogenetic activation of ARCAgRP neurons, however, is sufficient to drive voracious food seeking and consumption67,75. More recent work has shown that activation of ARCAgRP neurons is sufficient to dampen other motivational systems—such as thirst, innate fear, nociception, aggression, courtship, parenting, sleep, and self-preservation—often in favour of feeding76-82.
Role of ARCAgRP neurons in food seeking.
While initially thought to drive food consumption specifically, several recent reports instead suggest that ARCAgRP neurons are primarily involved in food seeking, or the appetitive phase, more so than the consummatory phase of feeding (Box 1). Following up on observations that artificial ARCAgRP activation in the absence of food robustly increased locomotor activity often associated with foraging67,81,83, three paradigm-shifting studies revealed the proactive and pre-emptive nature of these neurons. Using distinct neural-recording approaches, it was shown that ARCAgRP neurons robustly exhibit a homogenous inhibitory response within seconds of food presentation, preceding the first bite of food59,84,85 (Fig. 2a). This suppression of activity was specific to the sensory detection of food including cues conditioned to predict food delivery, confirming a degree of learning at the neural level of this population59,84-87. This inhibition is at least in part emanating from an upstream glutamatergic LH–GABAergic DMH circuit with the capacity to shut down feeding behaviour88,89. The sustained silencing of ARCAgRP neurons is dependent on future caloric consumption and scaled to the number of calories detected in the gut84,86,90,91. While the magnitude of ARCAgRP inhibition is higher in hungry versus sated animals, palatable food substrates lead to greater suppression and are even detectable in well-fed animals59,84.
Box 1 ∣. Motivational valence of ARCAgRP neurons.
How does ARCAgRP neuronal firing prompt an animal to search for food? A simple explanation for this inquiry is to determine the valence associated with the activity of these cells. Mice fail to perform operant responses to shut off ARCAgRP activity, which at first pass indicates that these neurons do not motivate behaviour by negative reinforcement. However, given the persistent effects of ARCAgRP stimulation on feeding behaviour that endure even after they are silenced, mice may be unable to associate an action with acute inhibition of these neurons. In fact, sated mice can be trained to display an avoidance of ARCAgRP neuron photostimulation in the absence of food, and fasted mice exhibit a conditioned preference for places paired with ARCAgRP neuron inhibition85. Thus, ARCAgRP neurons transmit a negative valence signal, which may motivate mice to procure food to mitigate or counteract this signal. However, mice engage in lever pressing to optically activate their ARCAgRP neurons if food is present, demonstrating that stimulation can be positively reinforcing. Although not intrinsically rewarding, as is the case with midbrain dopamine neurons203, mice will self-stimulate their ARCAgRP neurons to enhance the incentive value of food when it is directly in front of them and after a learnt positive association has been established93. This supports a model in which ARCAgRP neuron activity results in an enduring potentiation of the rewarding properties of food, likely through enhanced mesolimbic dopamine activity and subsequent dopamine release in the nucleus accumbens of the striatum60,73. Therefore, the exact nature of the valence produced by ARCAgRP neuronal activity is context-dependent. These findings support a model that in the absence of food, ARCAgRP neuronal firing drives an aversive signal that mice attempt to mollify by seeking food, whose properties subsequently become more rewarding once it is found. This sustained positive valence of food during consumption is likely not driven by ARCAgRP neurons themselves because their activity is already greatly reduced at the onset of the meal, but by downstream targets that prompt the consummatory phase of feeding.
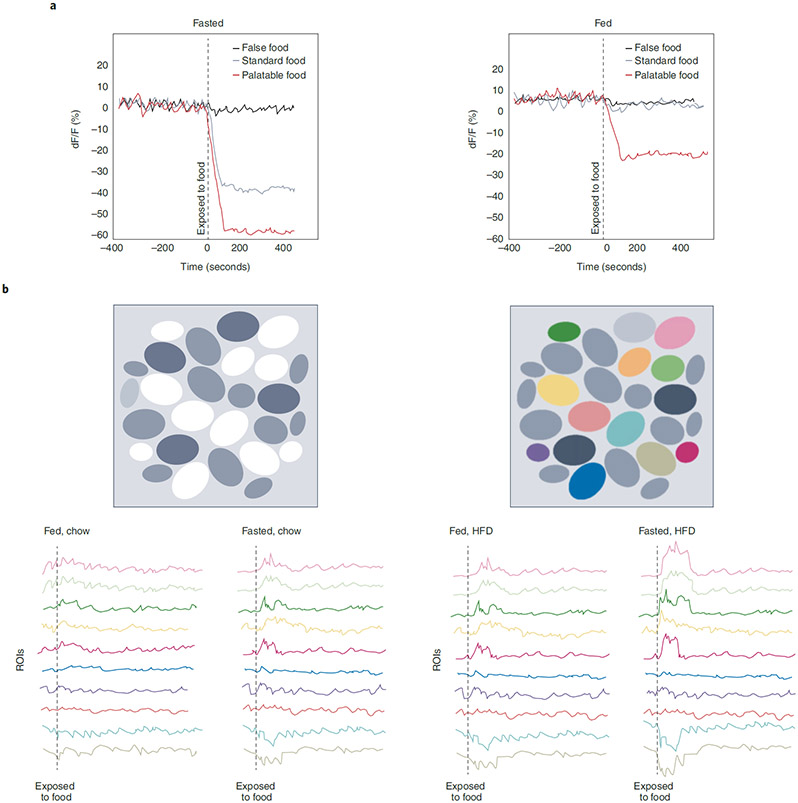
Fig. 2 ∣. Feeding neurons conform to energy status and food palatability.
Real-time recordings of neurons regulating appetite reveal stronger responses under periods of caloric deprivation and towards energy-dense food substrates. These changes can be determined directly by in vivo electrical-wire recordings and/or indirectly via the monitoring of calcium dynamics using genetically encoded calcium sensors (GECIs) that serve as a proxy of neural activity. These excitatory or inhibitory response properties can occur prior to, at the onset of, or after consumption. a, Example of optical-fibre photometry traces in fasted (left) or fed (right) mice expressing the GECI GCaMP (a synthetic fusion of green fluorescent protein, calmodulin and M13, a peptide sequence from myosin light-chain kinase) aligned to exposure of food (dotted vertical line). Although population dynamics are insensitive to false food (black traces), activity is rapidly and robustly suppressed to standard food, but in only energy-deficient mice (silver traces). Palatable-food presentation increases the magnitude of this inhibitory response and is even observed in calorically replete mice (red traces). dF/F in this case refers to the difference between initial fluorescence intensity at the resting state and after food exposure. Adapted from ref. 84 b, Example of single-photon microendoscopic or two-photon individual cellular activity (regions of interest, ROIs) in fasted or fed mice expressing GCaMP, aligned to exposure to food. In vivo miniepifluorescence image of GCaMP expression (top left). Schematized cell map of an example animal’s GCaMP-expressing neurons during a free-access feeding task (top right). The same neurons can be tracked between sessions (coloured cells). Calcium traces of individual neurons tracked in response to chow (left) or HFD (right) in fed versus fasted animals. Individual cells exhibit a variety of responses to food with activation (shifts upwards) or inhibition (shifts downwards) observed before, during, or after consumption. These responses are often exacerbated in hungry animals and towards energy-rich food, such as a HFD. Some neurons are non-responsive to food.
This anticipatory physiological phenomenon was recapitulated by optogenetically stimulating ARCAgRP neurons prior to, but not during, food availability92,93. Under this paradigm, mice still showed a robust increase in food intake, even with as little as 1 minute of pre-stimulation, or even if food presentation was delayed by minutes after priming had ended93. Food consumption that follows ARCAgRP stimulation is likely due to the sustained activity of downstream targets as opposed to activity of ARCAgRP neurons themselves. NPY is uniquely required for this long-lasting hunger signal94,95, providing a molecular correlate linking ARCAgRP neural dynamics with feeding behaviours. Collectively, these findings propose an updated role for ARCAgRP neurons: their main responsibility is to guide the animal in caloric need to a food source. Supporting this notion, ARCAgRP neurons are required for the elevated locomotion and exploration, presumably to discover food, near the onset of scheduled feeding96.
Downstream targets of ARCAgRP neurons.
To further understand how ARCAgRP neurons orchestrate feeding, as well as the sensory detection and value of food, studies have dissected the contributions of multiple ARCAgRP projections within and beyond the hypothalamus80,87,97,98. By expressing channelrhodopsin-2 in axons, it was shown that stimulating ARCAgRP projections to the PVH, anterior bed nucleus of the stria terminalis (aBNST), or LH was sufficient to induce food intake at a comparable level to that following ARCAgRP soma photostimulation (Fig. 1). Although photoactivation of projections to the paraventricular nucleus of the thalamus (PVT) promoted attraction to food odours87 and led to a mild increase in food intake, projections to the central amygdala (CeA), periaqueductal grey (PAG), and parabrachrial nucleus (PBN) revealed no effect. Further studies uncovered that activation of ARCAgRP terminal fields in the medial amygdala (MeA) and medial preoptic area (MPOA) are sufficient to support food intake78,80. Moreover, retrograde tracing established a one-to-one anatomical architecture whereby each ARCAgRP projection field is a separate communication channel without collateralization97. Reinforcing this view, transcriptional profiling unveiled multiple ARCAgRP subtypes and state-dependent changes in gene expression99,100. Thus, despite the observed homogenous activity response to food-related cues and ghrelin97, there is a functional, molecular, and structural heterogeneity to ARCAgRP signalling.
Effects of a high-fat diet on ARCAgRP neurons.
Diet-induced obesity (DIO) in mice has been shown to cause a decrease in fasting-induced refeeding with standard chow or a high-fat diet (HFD)101,102. This reduction in food intake can be elevated by photostimulating ARCAgRP neurons, indicating that fasting does not fully activate ARCAgRP neurons in DIO mice103. Longitudinal in vivo recordings reveal that long-term exposure to a HFD reduces the basal activity of ARCAgRP neurons60, although slice recordings show elevated intrinsic excitability of ARCAgRP neurons following HFD exposure104,105, demonstrating some discrepancy between in vivo and ex vivo studies.
ARCAgRP neurons in fasted, HFD-challenged mice exhibit a diminished inhibitory response to standard chow, suggesting that less palatable food is not sufficient to completely attenuate the drive to seek food (Box 2). However, further suppression of ARCAgRP activity can be achieved by HFD presentation60,103. Extended HFD exposure also disrupts the response of the mesolimbic reward system to food. Dopaminergic neurons in the ventral tegmental area (VTA) of HFD-challenged mice show a diminished response to standard chow while maintaining an increase in activity on HFD consumption60. Taken together, these studies demonstrate that exposure to a HFD rewires homeostatic and hedonic feeding systems to drive an animal to prefer and seek out more caloric foods, thereby promoting overeating and obesity.
Box 2 ∣. Feeding neurons conform to energy status and food palatability.
A shared feature among neurons responding to food stimuli is the modulatory influence of both the internal energy state of the animal and hedonic palatability, often scaling with the length of caloric deprivation and the number of calories, respectively (Fig. 2). Cells react more strongly to food substrates when animals are in a caloric deficit, and this response is intensified with high energy composition, often of food with elevated fat content. For example, ARCAgRP neurons exhibit little to no inhibitory response to chow when animals are sated but are rapidly and robustly reduced in the hungry state59,84,85, when food holds incentive worth. Another explanation for this enhanced state-dependent response to food may lie in the diametric baseline activity under opposing energy conditions. ARCAgRP neurons are active under caloric deprivation but are relatively quiescent following satiation56,58-60; thus, the inhibitory response to food is more pronounced in the fasted state when baseline activity is high. Comparable observations have been made in many feeding nodes where activity is contingent on energetic status84,88,108,112,113,117,162,163,180,195.
Palatability is associated with food pleasantness, ranging from its enhanced sensory properties to post-digestive gut-to-brain feedback that prolongs bouts of consumption204. Palatable foods are often energy dense, engineered to heighten sensory perception and attraction, and from an evolutionary perspective, highly preferred. Just as hunger in humans increases the subjective reports of palatability205, energy-rich food presentation to fasted animals augments neural activity responses compared to normal chow60,84,88,103,108,112,113,117,162,163,195, and owing to the strong preference for these palatable foods, neural activity changes are evident even under conditions of energy surfeit. Although acute inhibition to these palatable foods is primarily driven by their enhanced sensory properties, the durable suppression often calibrates with the number of calories consumed either via active eating or through direct infusion into the gastrointestinal tract84,86,90,91. Moreover, recent studies have pinpointed neurons that are preferentially activated by palatable food109,165. Thus, palatability is a critical factor in controlling appetite206.
ARCAgRP neurons thus play a critical role in food procurement, the first phase of feeding that begins with hunger. ARCAgRP projections demonstrate heterogeneity in their capacity to drive voracious food seeking. These findings suggest that ARCAgRP neurons could be divided in multiple subpopulations, not just in terms of their projections but also their transcriptomic profiles. Though past studies typically looked at only total food intake when investigating the role of cell types and/or projections in feeding, further studies are needed to elucidate how different subpopulations of ARCAgRP neurons orchestrate multiple aspects of feeding behaviour that may not necessarily affect total food intake, such as meal count, meal size, or motivation to obtain food. The functional heterogeneity of ARCAgRP neurons could arise from other peptides, neurotransmitters, or receptors that they co-express. Future experiments can take advantage of newer genetic tools to manipulate ARCAgRP neurons that overlap with the expression of other genes and dissect their role in feeding behaviour.
Interestingly, additional populations of inhibitory ARC neurons have been identified that have the capacity to drive food intake and body weight gain106, including those marked by tyrosine hydroxylase107, prepronociceptin108 or somatostatin (SST)100. Although the orexigenic circuitry and neural dynamics of these subtypes remain unresolved, these populations have been shown to synapse on downstream targets similar to those of ARCAgRP neurons100,107,109.
Although ARCAgRP neurons exhibit some functional and transcriptomic heterogeneity, they show a homogenous, rapid decrease in activity once hungry animals find food, suggesting that they are mainly necessary for food seeking and not consumption per se. The residual, low-rate spiking of ARCAgRP neurons at the beginning of the meal, along with the sustained activity of downstream ARCAgRP targets, may encode a persistent drive to eat and thus underlie the next phase of feeding: the consumption and ingestion of food.
Act II: food consumption
Control of feeding by the LH.
While the rhythmic actions of ingestion are moderated by hindbrain motor circuits110, the command of consummatory properties, including meal size, duration, frequency, and rate, occurs in multiple brain nodes (Fig. 1). Cell types within these anatomical structures manifest divergent responses to food intake and have the capacity to bidirectionally modulate feeding behaviours (Fig. 2b). The LH and PVH sit directly downstream from ARCAgRP neurons and thus are ideally situated to receive the metaphorical hand-off from food seeking to ingestion. In particular, the LH is a richly heterogeneous structure on the basis of gene expression, function, and structural organization111-113. In the LH of fasted rats, extracellular glutamate increases and returns to baseline on meal termination114. Optogenetic activation of LH vesicular glutamate transporter 2 (SLC17A6 or VGLUT2) neurons in mice evokes a decrease in food intake and is aversive115. Additionally, during an operant-conditioning paradigm in which mice were trained to discriminate fasted and sated periods, LHVGLUT2 activation attenuated the sensation of hunger in fasted mice116. Furthermore, LHVGLUT2 neurons receive inhibitory inputs from the BNST, and activation of this pathway triggers consumption in sated mice, whereas inhibition suppresses food intake in food-deprived mice115.
Optogenetic or chemogenetic activation of vesicular GABA transporter (SLC32A1 or VGAT) LH neurons promotes food intake and positive valence in sated mice, whereas acute and/or chronic inhibition of these neurons decreases food intake and attenuates weight gain117. In vivo calcium imaging revealed that distinct, predominantly non-overlapping populations of LHVGAT neurons become active during appetitive and consummatory feeding behaviours, although unlike ARCAgRP neurons, these responses were heterogeneous117. Interestingly, when mice were trained to discriminate between fasted and sated states, activation of LHVGAT neurons did not evoke a hunger sensation, suggesting that these neurons help sustain, rather than induce, hunger states116. Further partitioning of LHVGAT cells found that a subset of these cells, namely the leptin-receptor-expressing neurons, project to the VTA118, and activation of this pathway promotes motivation to obtain food119 and mediates appetitive learning120.
Non-GABAergic clusters121 of LH neurons expressing pro-melanin concentrating hormone (PMCH) and hypocretin (HCRT) have long been implicated in appetite regulation122-124. Intracerebroventricular administration of either PMCH122,125-129 or HCRT123,130 increases food intake. Ablation of LHPMCH neurons leads to a lean phenotype and obesity resistance131, and ablation of LHHCRT neurons decreases food consumption132,133. Paradoxically, ablation of LHHCRT neurons induces an increase in body weight and obesity132,133, possibly by regulation of energy expenditure. Chemogenetic activation of LHHCRT neurons increases food intake132,133, while inactivation leads to overeating and obesity134. However, optogenetic and chemogenetic activation of LHPMCH neurons did not evoke food intake unless stimulation was temporarily paired with consumption. Moreover, activation of LHPMCH neurons alone is rewarding135. A recent study suggested that the effect of LHPMCH neurons on overeating is related to increased impulsivity rather than to an increased motivation for food, and that the connections between these neurons with the ventral hippocampus (vHP) seem to play a role in driving increased impulsive responding128.
Although it has been observed that some animals receiving electrical stimulation in the LH selectively consume food136, others display selection of alternative actions depending on the presence of external variables to interact with, including drinking137, predatory attack138, gnawing139, or sexual activity140. Artificial manipulation of LHVGAT neurons not only regulates consumption of caloric foods but also of non-nutritional foods and inedible objects116,141. Moreover, specific photostimulation of the projection from the LHVGAT to the VTA resulted in erratic licking and gnawing independent of caloric presentation117,142. Although LH-evoked goal-directed behaviour appears to lack specificity to food, newer techniques have reinforced previous observations that animals find activation of these consummatory neurons positively rewarding117,142,143.
LH-VTA-NAc feeding loop.
The LH sends inhibitory and excitatory projection to the VTA, and LHVGAT neurons drive feeding by inhibiting VTA neurons142 (Fig. 1). Photoactivation of the LHVGAT–VTA pathway increases food intake in sated mice and incites food seeking in aversive conditions. In a conditioned feeding task, electrical recordings in the LH uncovered activity of a subpopulation of neurons during the conditioned response of entering a food delivery area, but not during the consumption of food; another subpopulation reacted to food predictive cues via the VTA, suggesting a preparatory contribution to feeding in addition to a consummatory one142. The increase in LHVGAT activity inhibits VTA GABAergic neurons, promoting dopamine (DA) release into the nucleus accumbens (NAc), whereas the activity of LHVGLUT2 neurons decreases DA release into the NAc144. The NAc comprises two major neuronal populations: medium spiny neurons (MSN) expressing dopamine D1 or D2 receptors. NAcD1 projections are an important source of inhibition of LH GABAergic neurons. NAcD1 MSN activity is lower during feeding, and activation of NAcD1 MSNs suppresses LH GABAergic neurons to stop feeding, whereas inhibition of the NAcD1–LH pathway promotes food consumption145.
Effects of diet-induced obesity on LH plasticity.
Studies using in vivo functional imaging have demonstrated that LHVGLUT2 neurons encode satiety states, and that their reward-encoding properties are modified during obesity112 (Fig. 2b). In particular, LHVGLUT2 neurons from DIO mice become progressively less responsive to sucrose consumption and less active at rest112, suggesting that these neurons lose their ability to limit feeding when mice consume a HFD. In a binge-eating model, only 3 days of exposure to a highly palatable food is capable of inducing long-term potentiation on inhibitory inputs from the NAc to both LHVGLUT2 and LHVGAT neurons. Moreover, such potentiation is absent in mice fed with regular chow. Thus, increased inhibition onto LHVGLUT2 neurons triggered by exposure of highly palatable foods can induce overeating146.
Control of feeding by the PVH.
Several lesion, pharmacological, and genetic knock-out studies have demonstrated the crucial role of the PVH in appetite control4,7,30,147-149. The majority of PVH neurons are marked by the transcription factor single-minded 1 (SIM1), which is required for proper development150, and acute ablation of these cells or a subset of cells characterized by expression of melanocortin 4 receptor (MC4R) leads to obesity151-153. Further supporting the role of PVH neuronal subtypes in dictating consummatory behaviour, real-time silencing of numerous PVH neuronal subtypes increased food intake, while activation decreased food intake68,152,154-157. Activation of SIM1-, MC4R-, or oxytocin-expressing PVH neurons occluded photoactivated ARCAgRP–PVH-evoked feeding68,154. Interestingly, while recordings of GLP1R or corticotropin-releasing hormone (CRH) population dynamics demonstrate a sharp and sustained increase or decrease in activity in response to food presentation, respectively152,158, those marked by MC4R exhibited only a transient change in response to food, likely owing to their heterogeneity. Single-cell endomicro-scopic resolution of individual PVHMC4R cells confirmed this neural diversity, as cells show varying responses to food introduction, subsequent consumption, and energy state159. Unbiased two-photon imaging from the PVH uncovered neurons enriched in Crh that co-express Vglut2 and Npy1r that respond to caloric ingestion160.
Control of feeding by the periLC.
Satiety-promoting PVH neurons send dense efferents to the brainstem, particularly the pons, where glutamatergic communication acts to signal fullness. Synaptic silencing of PVHSIM1 axons to the midbrain, near the ventral–lateral portion of the PAG and dorsal raphe, promptly and robustly stimulated feeding in sated animals161. Additional studies employing both gain- and loss-of-function experiments pinpointed a critical appetite-regulating projection from the PVH to the parabrachial complex, made up of the peri-locus coeruleus (periLC) and central lateral parabrachial nucleus (PBN)152,154. Although the molecular identity of these downstream neurons remains to be elucidated, a seminal study using hindbrain calcium imaging in freely moving mice found that periLCVGLUT2 neurons are selectively tuned to ingestive behaviours and are scalably inhibited by palatability and the internal milieu162. Reducing periLCVGLUT2 neural activity is rewarding and escalates consumption by augmenting palatability and prolonging ingestion duration162. A fundamental difference between PVH and periLC versus LH neural activation is that the former does not elicit fictive ingestive behaviours or alter lick frequency, but the latter often does. This finding suggests that, although LH circuits can control the motor actions of consumption through such pathways as the substantia nigra–superior colliculus–medullary reticular formation–motor nuclei circuits, PVH and periLC neurons have a more modulatory role during eating, whereby inhibition prolongs consumption152,154,162-164.
Control of feeding by other brain sites.
A number of studies have pinpointed additional neuronal subtypes in discrete regions that regulate food intake. These include inhibitory SST neurons of the tuberal nucleus (TN), which are preferentially activated by palatable food and drive overconsumption through signalling to the PVH and BNST108,165. Another population of GABAergic cells in the zona incerta (ZI) robustly stimulates food intake via projections to the PVT166. Additionally, impairment of cholinergic signalling in the basal forebrain promotes food intake through hypothalamic communication167. Temperature-sensitive neurons of the MPOA can also regulate food intake through synapses to ARCAgRP or PVH neurons166,168.
Overall, several lines of research suggest that ARCAgRP downstream nodes act as a neural hub modulating the transition from food seeking to consumption. Among those targets, the activation of GABAergic neurons of the LH promotes food consumption, in part through communication with the midbrain dopamine system, and this is opposed by the actions of glutamatergic neurons, which act as a break on feeding. Despite the reliability of this system in modulating feeding, it is malleable and susceptible to maladaptation by exposure to less nutritional, highly caloric diets, which can contribute to the development of obesity. Another downstream target of ARCAgRP neurons that regulate consumption is the PVH, a region that is marked by heterogeneity, particulary in PVHMC4R neurons that display varying responses to food presentation and ingestion. The PVH and LH are intertwined, with a plethora of brain nuclei that act in concert to mediate consumption (Fig. 2). Neuronal activities that encode levels of hunger and satiation are then conveyed from hypothalamic nuclei to regions in the hindbrain to eventually signal the end of the meal.
Act III: meal termination
Multimodal signalling of satiation.
A merger of interoceptive signals arising from the periphery and exteroceptive processes from the environment leads to the end of a feeding bout. Meal termination is characterized by the cessation of interactions aimed at consumption, including reaching, holding, biting, chewing, and swallowing food. Ending a meal is influenced by several distinct but overlapping processes. First, gastrointestinal, blood-borne, and descending hypothalamic signals converge on the hindbrain and medulla when caloric need has been appropriately met. Satiation is associated with a range of peripherally derived hormone signals—such as cholecystokinin (CCK), GLP1, amylin, leptin, neurotensin, serotonin, insulin, and peptide YY—that communicate with neurons to wind down eating169. Second, mechanoreceptors in the digestive tract distend to accommodate food during a meal. As the volume of stomach content is increased, food intake decreases and information related to mechanical stretch and nutritional content is conveyed mainly via vagal afferents170 to the nucleus of the solitary tract (NTS)171-173, located in the caudal brainstem172-175. In the NTS, sensory peripheral information is integrated and processed to be relayed to the dorsal motor nucleus of the vagus, which provides parasympathetic motor input to the gastrointestinal tract to modulate gastric and intestinal motility, tone, secretion, and emptying to regulate meal termination and nutrient absorption176. Third, competing processes involved with the expression of rival motivated behaviours may shift an animal away from eating and towards a new goal, such as mating or territorial defence77,78,177. Last, aversive and nociceptive signals, such as anxiety, illness, malaise, pain, itch, and inflammation, are potent satiety stimuli capable of suppressing feeding despite caloric need178-181.
Role of the PBN in halting food consumption.
Several lines of evidence have positioned the PBN, a part of the central gustatory and the visceral sensory system, as a neural hub for feeding suppression and taste memories178,179,181-183 (Fig. 1). The majority of work has centred on a subpopulation of excitatory neurons in the outer external lateral PBN marked by the expression of calcitonin gene-related protein (CALCA or CGRP). These cells respond broadly to visceral stimuli, including nausea, itch, and pain, and are tuned to increasing intensities of aversive stimuli178,180,184 (Fig. 3 and Box 3). Activation of PBNCGRP neurons, including a subset projecting to the CeA, potently suppresses food intake regardless of internal need for calories while silencing mitigates the inhibition of feeding induced by pain, sickness, and exogenous administration of anorectic hormones178,184. These effects are probably mediated in part by inputs from ARCAgRP (refs. 90,92), area postrema185, and NTS neurons186-189 and outputs to the CeA178,190 and parasubthalamic nucleus (PSTN)191. A subpopulation of GABAergic neurons marked by protein kinase C-δ (PKCδ) are responsive to and required for the influence of anorexigenic agents on food intake192. CeAPKCδ neuron stimulation abrogates food intake independent of anxiolytic effects192. It should be noted that an alternative projection from NTS neurons expressing calcitonin receptors (CALCR) to the PBN supports meal termination without producing aversion independent of PBNCGRP signalling189,193. Further parsing of NTSCALCR neurons uncovered a neuronal subset that expresses prolactin releasing hormone, which has the capacity to suppress food intake and reduce body weight without inducing conditioned taste aversion194.
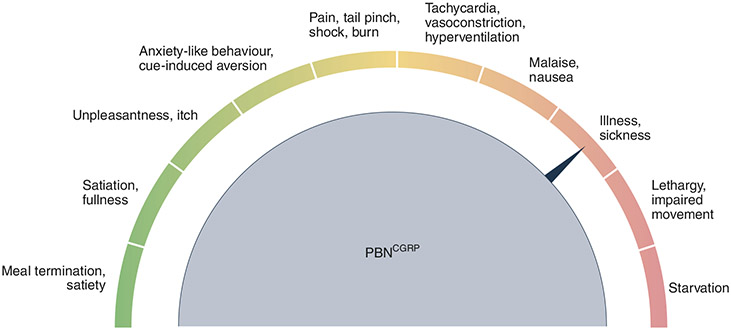
Fig. 3 ∣. Fine tuning of satiation circuits is required to avoid aversive outcomes.
The most common side effect of effective anti-obesity drugs is visceral malaise, including nausea, vomiting, and gastrointestinal issues. This likely stems from the overlapping features of cell types, such as PBNCGRP neurons. Modest activation may signal meal termination or satiety, ultimately leading to the feeling of a full stomach. However, further stimulation could result in unpleasantness, including anxiety-like behaviour, pain, and malaise brought on by physiological changes, including tachycardia, vasoconstriction, and hyperventilation. Even stronger rousing of these circuits could ultimately culminate in severe sickness, impaired movement, and starvation. Therefore, understanding how this information is encoded at distinct brain nodes will aid in the careful crafting of therapeutics that curb overeating without negative consequences.
Box 3 ∣. Feeling full, but not too full.
It is well appreciated that sickness brought on by fever or infection suppresses appetite. This is presumably due to shared central sites that encode the sensation of unpleasantness with appetite control (Fig. 3). Nodes like PBNCGRP neurons can be thought of as knobs for the satiety system, whereby a slight adjustment results in satiety but excessive turning leads to profound visceral malaise. While these cells respond to refeeding and satiation92,180, they are also robustly activated by threats of diverse origin178,180,181,184,207. Moreover, activation of PBNCGRP neurons soundly reduces food intake via meal termination but can also produce freezing and anxiety-like behaviour in conjunction with tachycardia or parasympathetic responses, depending on the stimulation frequency or how far the knob is twisted92,178,184,208. The aversive affective state transmitted by these neurons was reaffirmed using a real-time place preference assay, which demonstrated that mice choose to avoid a space paired with PBNCGRP neuron activation. Thus, the spectrum between satiety and sickness is tunable to the length and intensity of stimulation, as acute activation of PBNCGRP neurons reduces meal size but chronic activation leads to severe anorexia and starvation92,178. PBNCGRP neurons are likely just one of many systems in the brain that impact both appetite and illness as these states are intrinsically intertwined. In fact, the most common side effect of efficacious anti-obesity medications is nausea and other associated gastrointestinal discomforts, including diarrhea, vomiting, and constipation209-212. Balancing this tightrope between feeling full but not too full to the point of sickness is a major obstacle in the development of modern-day therapeutics to blunt appetite.
Although food deprivation lowered PBNCGRP activity relative to the sated state, likely owing to a combination of increased inhibitory ARCAgRP neuronal68,92 and BNST input195 and decreased excitatory input from anorexigenic neurons in the NTS92,188,195, these neurons became more active as feeding progressed180. Further supporting their contribution to meal termination, silencing PBNCGRP neurons increases meal duration without affecting the total amount of food consumed92. Reinforcing this notion, some PBNCCK neurons respond to leptin, and leptin injections into the PBN dose-dependently reduce cumulative food intake and habitual meal size, but not meal number186,196. Interestingly, gastric bypass activates PBNCGRP neurons197, suggesting that these neurons may play a role in appetite loss and weight loss resulting from this surgery198. However, it should be noted that PBNCGRP activation is aversive, emulating conditioned taste aversion, and results in avoidance of learnt cues paired with their elevated activity179. The strength of the conditioned taste aversion is modulated by the frequency and duration of PBNCGRP stimulation181. Furthermore, PBNCGRP neurons are required for the acquisition and expression of aversive taste memories181.
Cerebellar control of meal termination.
In addition to the PBN, a recent report found that cerebellar activity acts as a ‘brake’ to reduce meal size and duration, but not frequency or rate199 (Fig. 1). Neurons in the anterior deep cerebellar nuclei (aDCN) are engaged by feeding or direct nutrient infusion in the gut. Selective activation of aDCN neurons substantially decreased food intake, independent of caloric need. The underlying mechanism for this aDCN-induced meal termination is enacted via increasing striatal dopamine levels and attenuating the phasic dopamine response to subsequent food consumption. These changes may diminish meal size by reducing the reward value of additional consumption similar to hindbrain satiation networks that modulate food motivation based on interoceptive state through dopamine signalling23,200. Supporting the translational relevance of these findings, individuals with the genetic disorder Prader–Willi syndrome (PWS), who are characterized by obesity and a lack of satiation201, demonstrate dysregulated neural activity to food cues while fasted or after eating compared with healthy controls. This lack of cerebellar engagement in response to food cues may result in the extreme hyperphagia reported in individuals with PWS.
Therefore, several mechanisms interact to orchestrate the termination of a meal. Satiation hormones together with gastrointestinal mechanoreceptors signal for the fulfillment of caloric demand to the brainstem, enabling the suppression of food consumption. In addition, aversive and nociceptive stimuli are powerful feeding suppressors, inhibiting intake even in conditions of caloric deficiency. The PBN has a predominant role in mediating suppression of food consumption induced by fullness, aversion, illness, and pain.
A growing body of evidence is advancing towards the understanding of extrahypothalamic brain loci that modulate feeding behaviours. Dysregulation of these brain regions can contribute to eating disorders; however, the mechanisms by which diet and feeding habits could impact their function and the roles of phenotypically identifiable subpopulations of neurons are far from understood. The need for translational research is compelling and could shed light into how different neural feeding circuits modulate food consumption and become susceptible to maladaptive changes. The impact of diet nutrient composition and specific nutrients in neuronal activity is also something to be considered. Although past studies have characterized how ARCAgRP neurons sense different macronutrients86,90,202, this level of scrutiny has not yet been applied to other cell types or brain nodes.
Epiologue: managing obesity into the future
Recognizing obesity as a chronic disease helps to destigmatize the prevalent assumption that obesity is a product of inadequate self-discipline. Despite lifestyle and behavioural interventions, losing body weight is a daunting task in a world designed to make it so. Our understanding of the altered biological mechanisms and pathophysiology associated with excess fat accumulation helps us to understand why our best efforts often fall short of our goals for long-term weight loss. This growing barrier to improving general health has led to alternative obesity treatment strategies, including pharmacological mediation. While the quest for highly efficient anti-obesity medications has been challenging from both a technical and societal perspective, the work described above, outlining the molecular mechanisms and neural circuits overseeing appetite regulation, has served to navigate drug discovery and therapeutic interventions in attempts to abate overconsumption. Integrating the latest advances in our understanding of appetite homeostasis along with the continued identification of novel drug targets leads to an optimistic view of plausible treatments that could reduce body weight by more than 25%. To realize this objective, anti-obesity medications should be designed in a way to collectively impact the separate acts of feeding discussed in this Review. Having the means to simultaneously curb craving, hunger, and food-seeking behaviours, along with the rate and duration of food consumption, would greatly benefit us in our struggle with obesity. This multi-branched targeting of these distinct processes could combat the profound amount of redundancy and regulation built into our systems designed to sustain our current body weight. Although monotherapy would be preferred for simplicity, a combination of pharmacological agents will have a higher probability of meeting the demands required to hit each of these phases and ultimately pare down caloric intake. Optimizing approaches on the basis of these and future findings to enhance and prolong efficacy of sustained weight loss is a unified goal of scientists and clinicians that is becoming more and more realistic with each passing day and discovery.
Acknowledgements
The authors acknowledge with gratitude S. Sarsfield for comments on the manuscript. Y. A. is supported by the National Institute on Drug Abuse Intramural Research Program (NIDA IRP), US National Institutes of Health (NIH). M. K. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases Intramural Research Program (NIDDK IRP), US National Institutes of Health (NIH).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Collaborators, G. B. D. O. et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med 377, 13–27 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sternson SM & Eiselt AK Three pillars for the neural control of appetite. Annu Rev. Physiol 79, 401–423 (2017). This review served as inspiration for dividing up the discrete phases of feeding into three acts.
- 3.Anand BK, Dua S & Shoenberg K Hypothalamic control of food intake in cats and monkeys. J. Physiol 127, 143–152 (1955). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aravich PF & Sclafani A Paraventricular hypothalamic lesions and medial hypothalamic knife cuts produce similar hyperphagia syndromes. Behav. Neurosci 97, 970–983 (1983). [DOI] [PubMed] [Google Scholar]
- 5.Bergen HT, Mizuno TM, Taylor J & Mobbs CV Hyperphagia and weight gain after gold-thioglucose: relation to hypothalamic neuropeptide Y and proopiomelanocortin. Endocrinology 139, 4483–4488 (1998). [DOI] [PubMed] [Google Scholar]
- 6.Gold RM, Quackenbush PM & Kapatos G Obesity following combination of rostrolateral to VMH cut and contralateral mammillary area lesion. J. Comp. Physiol. Psychol 79, 210–218 (1972). [DOI] [PubMed] [Google Scholar]
- 7.Gold RM, Jones AP & Sawchenko PE Paraventricular area: critical focus of a longitudinal neurocircuitry mediating food intake. Physiol. Behav 18, 1111–1119 (1977). [DOI] [PubMed] [Google Scholar]
- 8.Holzwarth-McBride MA, Hurst EM & Knigge KM Monosodium glutamate induced lesions of the arcuate nucleus. I. Endocrine deficiency and ultrastructure of the median eminence. Anat. Rec 186, 185–205 (1976). [DOI] [PubMed] [Google Scholar]
- 9.Holzwarth-McBride MA, Sladek JR Jr. & Knigge KM Monosodium glutamate induced lesions of the arcurate nucleus. II. Fluorescence histochemistry of catecholamines. Anat. Rec 186, 197–205 (1976). [DOI] [PubMed] [Google Scholar]
- 10.Leibowitz SF Reciprocal hunger-regulating circuits involving alpha- and beta-adrenergic receptors located, respectively, in the ventromedial and lateral hypothalamus. Proc. Natl Acad. Sci. USA 67, 1063–1070 (1970). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leibowitz SF, Hammer NJ & Chang K Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol. Behav 27, 1031–1040 (1981). [DOI] [PubMed] [Google Scholar]
- 12. Teitelbaum P & Epstein AN The lateral hypothalamic syndrome: recovery of feeding and drinking after lateral hypothalamic lesions. Psychol. Rev 69, 74–90 (1962). This study was part of a revoultion of lesion experiments demonstrating the necessity of hypothalamic structures in the regulation of body weight homeostasis.
- 13.Boghossian S, Park M & York DA Melanocortin activity in the amygdala controls appetite for dietary fat. Am. J. Physiol. Regul. Integr. Comp. Physiol 298, R385–R393 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Booth DA Mechanism of action of norepinephrine in eliciting an eating response on injection into the rat hypothalamus. J. Pharmacol. Exp. Ther 160, 336–348 (1968). [PubMed] [Google Scholar]
- 15.Grill HJ, Ginsberg AB, Seeley RJ & Kaplan JM Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J. Neurosci 18, 10128–10135 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grossman SP Eating or drinking elicited by direct adrenergic or cholinergic stimulation of hypothalamus. Science 132, 301–302 (1960). [DOI] [PubMed] [Google Scholar]
- 17.Williams DL, Kaplan JM & Grill HJ The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation. Endocrinology 141, 1332–1337 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Hoebel BG & Teitelbaum P Hypothalamic control of feeding and self-stimulation. Science 135, 375–377 (1962). [DOI] [PubMed] [Google Scholar]
- 19.Tenen SS & Miller NE Strength of electrical stimulation of lateral hypothalamus, food deprivation, and tolerance for quinine in food. J. Comp. Physiol. Psychol 58, 55–62 (1964). [DOI] [PubMed] [Google Scholar]
- 20.Schultz W. Dopamine neurons and their role in reward mechanisms. Curr. Opin. Neurobiol 7, 191–197 (1997). [DOI] [PubMed] [Google Scholar]
- 21.Salamone JD, Correa M, Mingote S & Weber SM Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J. Pharmacol. Exp. Ther 305, 1–8 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Wise RA The parsing of food reward. Am. J. Physiol. Regul. Integr. Comp. Physiol 291, R1234–R1235 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Palmiter RD Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 30, 375–381 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Zhou QY & Palmiter RD Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 83, 1197–1209 (1995). [DOI] [PubMed] [Google Scholar]
- 25.Cohen P. et al. Selective deletion of leptin receptor in neurons leads to obesity. J. Clin. Invest 108, 1113–1121 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halaas JL et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–546 (1995). [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994). This study identified that mutations in the ob gene encoding leptin resulted in profound obsesity and type II diabetes.
- 28.Butler AA et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 141, 3518–3521 (2000). [DOI] [PubMed] [Google Scholar]
- 29. Fan W, Boston BA, Kesterson RA, Hruby VJ & Cone RD Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385, 165–168 (1997). This is one of three studies that demonstrated the essential role of the melanocortin system on the control of energy balance.
- 30. Huszar D. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141 (1997). This is one of three studies that demonstrated the essential role of the melanocortin system on the control of energy balance.
- 31. Ollmann MM et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278, 135–138 (1997). This is one of three studies that demonstrated the essential role of the melanocortin system on the control of energy balance.
- 32.Yaswen L, Diehl N, Brennan MB & Hochgeschwender U Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat. Med 5, 1066–1070 (1999). [DOI] [PubMed] [Google Scholar]
- 33.Scrocchi LA et al. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat. Med 2, 1254–1258 (1996). [DOI] [PubMed] [Google Scholar]
- 34.Scrocchi LA, Marshall BA, Cook SM, Brubaker PL & Drucker DJ Identification of glucagon-like peptide 1 (GLP-1) actions essential for glucose homeostasis in mice with disruption of GLP-1 receptor signaling. Diabetes 47, 632–639 (1998). [DOI] [PubMed] [Google Scholar]
- 35.Hansotia T. et al. Double incretin receptor knockout (DIRKO) mice reveal an essential role for the enteroinsular axis in transducing the glucoregulatory actions of DPP-IV inhibitors. Diabetes 53, 1326–1335 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Gropp E. et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat. Neurosci 8, 1289–1291 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Luquet S, Perez FA, Hnasko TS & Palmiter RD NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310, 683–685 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Wu Q, Whiddon BB & Palmiter RD Ablation of neurons expressing agouti-related protein, but not melanin concentrating hormone, in leptin-deficient mice restores metabolic functions and fertility. Proc. Natl Acad. Sci. USA 109, 3155–3160 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xi D, Gandhi N, Lai M & Kublaoui BM Ablation of Sim1 neurons causes obesity through hyperphagia and reduced energy expenditure. PLoS ONE 7, e36453 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhan C. et al. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J. Neurosci 33, 3624–3632 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kojima M. et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660 (1999). [DOI] [PubMed] [Google Scholar]
- 42.Muller TD et al. Ghrelin. Mol. Metab 4, 437–460 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tschop M, Smiley DL & Heiman ML Ghrelin induces adiposity in rodents. Nature 407, 908–913 (2000). [DOI] [PubMed] [Google Scholar]
- 44.Drazen DL, Vahl TP, D’Alessio DA, Seeley RJ & Woods SC Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology 147, 23–30 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Wren AM et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 141, 4325–4328 (2000). [DOI] [PubMed] [Google Scholar]
- 46.Wren AM et al. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab 86, 5992 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Nakazato M. et al. A role for ghrelin in the central regulation of feeding. Nature 409, 194–198 (2001). [DOI] [PubMed] [Google Scholar]
- 48.Frederich RC et al. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat. Med 1, 1311–1314 (1995). [DOI] [PubMed] [Google Scholar]
- 49.Ahima RS et al. Role of leptin in the neuroendocrine response to fasting. Nature 382, 250–252 (1996). [DOI] [PubMed] [Google Scholar]
- 50. Farooqi IS et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med 341, 879–884 (1999). This study successfully translated rodent findings to a patient with congenital leptin deficiency.
- 51.Farr OM, Gavrieli A & Mantzoros CS Leptin applications in 2015: what have we learned about leptin and obesity? Curr. Opin. Endocrinol. Diabetes Obes 22, 353–359 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schaeffer M. et al. Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons. Proc. Natl Acad. Sci. USA 110, 1512–1517 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baskin DG et al. Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res. 848, 114–123 (1999). [DOI] [PubMed] [Google Scholar]
- 54.Willesen MG, Kristensen P & Romer J Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology 70, 306–316 (1999). [DOI] [PubMed] [Google Scholar]
- 55.Wilson BD et al. Physiological and anatomical circuitry between Agouti-related protein and leptin signaling. Endocrinology 140, 2387–2397 (1999). [DOI] [PubMed] [Google Scholar]
- 56. Takahashi KA & Cone RD Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology 146, 1043–1047 (2005). This study demonstrated that internal state of an animal drives activity of appetite-regulating neurons using acute brain slice electrophysiology.
- 57.Yang R. et al. Restoring leptin signaling reduces hyperlipidemia and improves vascular stiffness induced by chronic intermittent hypoxia. Am. J. Physiol. Heart Circ. Physiol 300, H1467–H1476 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu T. et al. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron 73, 511–522 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mandelblat-Cerf Y. et al. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. eLife 4, e07122 (2015). This is one of three studies that demonstrated that activity of hypothalamic neurons are rapidly and robustly altered via anticipation of food consumption using in vivo electrophysiology.
- 60. Mazzone CM et al. High-fat food biases hypothalamic and mesolimbic expression of consummatory drives. Nat. Neurosci 23, 1253–1266 (2020). This study used longitudinal recordings of hypothalamic neurons and mesolimbic dopamine to demonstrate that palatable food exposure diminishes the capacity of chow diets to alleviate the negative valence associated with hunger and the rewarding properties of food discovery.
- 61.Krashes MJ et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507, 238–242 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clark JT, Kalra PS, Crowley WR & Kalra SP Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology 115, 427–429 (1984). [DOI] [PubMed] [Google Scholar]
- 63.Rossi M. et al. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology 139, 4428–4431 (1998). [DOI] [PubMed] [Google Scholar]
- 64.Semjonous NM et al. Coordinated changes in energy intake and expenditure following hypothalamic administration of neuropeptides involved in energy balance. Int. J. Obes 33, 775–785 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stratford TR & Kelley AE GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J. Neurosci 17, 4434–4440 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krashes MJ, Shah BP, Koda S & Lowell BB Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab. 18, 588–595 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krashes MJ et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest 121, 1424–1428 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Atasoy D, Betley JN, Su HH & Sternson SM Deconstruction of a neural circuit for hunger. Nature 488, 172–177 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cavalcanti-de-Albuquerque JP, Bober J, Zimmer MR & Dietrich MO Regulation of substrate utilization and adiposity by Agrp neurons. Nat. Commun 10, 311 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Joly-Amado A. et al. Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning. EMBO J. 31, 4276–4288 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burke LK et al. mTORC1 in AGRP neurons integrates exteroceptive and interoceptive food-related cues in the modulation of adaptive energy expenditure in mice. eLife 6, e22848 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steculorum SM et al. AgRP neurons control systemic insulin sensitivity via myostatin expression in brown adipose tissue. Cell 165, 125–138 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alhadeff AL et al. Central leptin signaling transmits positive valence. Brain Res. 1724, 146441 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reichenbach A. et al. Metabolic sensing in AgRP neurons integrates homeostatic state with dopamine signalling in the striatum. eLife 11, e72668 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Aponte Y, Atasoy D & Sternson SM AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci 14, 351–355 (2011). This study was the first to show that acute optogenetic activation of hypothalamic AgRP neurons emulated a fasted state driving food intake.
- 76.Burnett CJ et al. Hunger-driven motivational state competition. Neuron 92, 187–201 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burnett CJ et al. Need-based prioritization of behavior. eLife 8, e44527 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Padilla SL et al. Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nat. Neurosci 19, 734–741 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alhadeff AL et al. A neural circuit for the suppression of pain by a competing need state. Cell 173, 140–152 e115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li XY et al. AGRP neurons project to the medial preoptic area and modulate maternal nest-building. J. Neurosci 39, 456–471 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dietrich MO, Zimmer MR, Bober J & Horvath TL Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell 160, 1222–1232 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Goldstein N. et al. Hypothalamic neurons that regulate feeding can influence sleep/wake states based on homeostatic need. Curr. Biol 28, 3736–3747 (2018). This study investigated the intersection between feeding circuits and sleep architecture finding reciprocal influences of these two essential behaviors.
- 83.Jikomes N, Ramesh RN, Mandelblat-Cerf Y & Andermann ML Preemptive stimulation of AgRP neurons in fed mice enables conditioned food seeking under threat. Curr. Biol 26, 2500–2507 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chen Y, Lin YC, Kuo TW & Knight ZA Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 160, 829–841 (2015). This is one of three studies that demonstrated that activity of hypothalamic neurons are rapidly and robustly altered via anticipation of food consumption using in vivo optical-fibre photometry.
- 85. Betley JN et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521, 180–185 (2015). This is one of three studies that demonstrated that activity of hypothalamic neurons are rapidly and robustly altered via anticipation of food consumption using in vivo single-photon microendoscopy.
- 86.Su Z, Alhadeff AL & Betley JN Nutritive, post-ingestive signals are the primary regulators of AgRP neuron activity. Cell Rep. 21, 2724–2736 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Horio N & Liberles SD Hunger enhances food-odour attraction through a neuropeptide Y spotlight. Nature 592, 262–266 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Garfield AS et al. Dynamic GABAergic afferent modulation of AgRP neurons. Nat. Neurosci 19, 1628–1635 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Berrios J. et al. Food cue regulation of AGRP hunger neurons guides learning. Nature 595, 695–700 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beutler LR et al. Dynamics of gut–brain communication underlying hunger. Neuron 96, 461–475 e465 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bai L. et al. Genetic identification of vagal sensory neurons that control feeding. Cell 179, 1129–1143 e1123 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Campos CA, Bowen AJ, Schwartz MW & Palmiter RD Parabrachial CGRP neurons control meal termination. Cell Metab. 23, 811–820 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen Y, Lin YC, Zimmerman CA, Essner RA & Knight ZA Hunger neurons drive feeding through a sustained, positive reinforcement signal. eLife 5, e18640 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen Y. et al. Sustained NPY signaling enables AgRP neurons to drive feeding. eLife 8, e46348 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Engstrom Ruud L, Pereira MMA, de Solis AJ, Fenselau H & Bruning JC NPY mediates the rapid feeding and glucose metabolism regulatory functions of AgRP neurons. Nat. Commun 11, 442 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tan K, Knight ZA & Friedman JM Ablation of AgRP neurons impairs adaption to restricted feeding. Mol. Metab 3, 694–704 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Betley JN, Cao ZF, Ritola KD & Sternson SM Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 155, 1337–1350 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fu O. et al. Hypothalamic neuronal circuits regulating hunger-induced taste modification. Nat. Commun 10, 4560 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Henry FE, Sugino K, Tozer A, Branco T & Sternson SM Cell type-specific transcriptomics of hypothalamic energy-sensing neuron responses to weight-loss. eLife 4, e09800 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Campbell JN et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat. Neurosci 20, 484–496 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Briggs DI, Lemus MB, Kua E & Andrews ZB Diet-induced obesity attenuates fasting-induced hyperphagia. J. Neuroendocrinol 23, 620–626 (2011). [DOI] [PubMed] [Google Scholar]
- 102.Ueno N, Asakawa A & Inui A Blunted metabolic response to fasting in obese mice. Endocrine 32, 192–196 (2007). [DOI] [PubMed] [Google Scholar]
- 103.Beutler LR et al. Obesity causes selective and long-lasting desensitization of AgRP neurons to dietary fat. eLife 9, e55909 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baver SB et al. Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus. J. Neurosci 34, 5486–5496 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wei W. et al. Diet composition, not calorie intake, rapidly alters intrinsic excitability of hypothalamic AgRP/NPY neurons in mice. Sci. Rep 5, 16810 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhu C. et al. Profound and redundant functions of arcuate neurons in obesity development. Nat. Metab 2, 763–774 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang X & van den Pol AN Hypothalamic arcuate nucleus tyrosine hydroxylase neurons play orexigenic role in energy homeostasis. Nat. Neurosci 19, 1341–1347 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Luo SX et al. Regulation of feeding by somatostatin neurons in the tuberal nucleus. Science 361, 76–81 (2018). [DOI] [PubMed] [Google Scholar]
- 109. Jais A. et al. PNOC(ARC) neurons promote hyperphagia and obesity upon high-fat-diet feeding. Neuron 106, 1009–1025 (2020). This study characterized a novel population of hypothalamic neurons activated upon palatable food consumption with the capacity to promote hyperphagia.
- 110.Wiesenfeld Z, Halpern BP & Tapper DN Licking behavior: evidence of hypoglossal oscillator. Science 196, 1122–1124 (1977). [DOI] [PubMed] [Google Scholar]
- 111.Mickelsen LE et al. Single-cell transcriptomic analysis of the lateral hypothalamic area reveals molecularly distinct populations of inhibitory and excitatory neurons. Nat. Neurosci 22, 642–656 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Rossi MA et al. Obesity remodels activity and transcriptional state of a lateral hypothalamic brake on feeding. Science 364, 1271–1274 (2019). This study performed longitudinal two-photon neural recordings to demonstrate how diet disrupts the function of an endogenous feeding suppression system to promote overeating and obesity.
- 113.Rossi MA et al. Transcriptional and functional divergence in lateral hypothalamic glutamate neurons projecting to the lateral habenula and ventral tegmental area. Neuron 109, 3823–3837 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rada P, Tucci S, Murzi E & Hernández L Extracellular glutamate increases in the lateral hypothalamus and decreases in the nucleus accumbens during feeding. Brain Res. 768, 338–340 (1997). [DOI] [PubMed] [Google Scholar]
- 115.Jennings JH, Rizzi G, Stamatakis AM, Ung RL & Stuber GD The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science 341, 1517–1521 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Siemian JN, Arenivar MA, Sarsfield S & Aponte Y Hypothalamic control of interoceptive hunger. Curr. Biol 31, 3797–3809 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jennings JoshuaH. et al. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 160, 516–527 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leinninger GM et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 10, 89–98 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schiffino FL et al. Activation of a lateral hypothalamic-ventral tegmental circuit gates motivation. PLoS ONE 14, e0219522 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Siemian JN et al. Lateral hypothalamic LEPR neurons drive appetitive but not consummatory behaviors. Cell Rep. 36, 109615 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mickelsen LE et al. Cellular taxonomy and spatial organization of the murine ventral posterior hypothalamus. eLife 9, e58901 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Qu D. et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature 380, 243–247 (1996). [DOI] [PubMed] [Google Scholar]
- 123.Sakurai T. et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585 (1998). [DOI] [PubMed] [Google Scholar]
- 124.de Lecea L. et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl Acad. Sci. USA 95, 322–327 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rossi M. et al. Investigation of the feeding effects of melanin concentrating hormone on food intake — action independent of galanin and the melanocortin receptors. Brain Res. 846, 164–170 (1999). [DOI] [PubMed] [Google Scholar]
- 126.Della-Zuana O. et al. Acute and chronic administration of melanin-concentrating hormone enhances food intake and body weight in Wistar and Sprague–Dawley rats. Int. J. Obes 26, 1289–1295 (2002). [DOI] [PubMed] [Google Scholar]
- 127.Gomori A. et al. Chronic intracerebroventricular infusion of MCH causes obesity in mice. Am. J. Physiol. Endocrinol. Metab 284, E583–E588 (2003). [DOI] [PubMed] [Google Scholar]
- 128.Noble EE et al. Hypothalamus–hippocampus circuitry regulates impulsivity via melanin-concentrating hormone. Nat. Commun 10, 4923 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ludwig DS et al. Melanin-concentrating hormone: a functional melanocortin antagonist in the hypothalamus. Am. J. Physiol. Endocrinol. Metab 274, E627–E633 (1998). [DOI] [PubMed] [Google Scholar]
- 130.Edwards CM et al. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J. Endocrinol 160, R7–R12 (1999). [DOI] [PubMed] [Google Scholar]
- 131.Jeon JY et al. MCH−/− mice are resistant to aging-associated increases in body weight and insulin resistance. Diabetes 55, 428–434 (2006). [DOI] [PubMed] [Google Scholar]
- 132.Inutsuka A. et al. Concurrent and robust regulation of feeding behaviors and metabolism by orexin neurons. Neuropharmacology 85, 451–460 (2014). [DOI] [PubMed] [Google Scholar]
- 133.Hara J. et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 30, 345–354 (2001). [DOI] [PubMed] [Google Scholar]
- 134.González JA et al. Inhibitory interplay between orexin neurons and eating. Curr. Biol 26, 2486–2491 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dilsiz P. et al. MCH neuron activity is sufficient for reward and reinforces feeding. Neuroendocrinology 110, 258–270 (2020). [DOI] [PubMed] [Google Scholar]
- 136. Delgado JM & Anand BK Increase of food intake induced by electrical stimulation of the lateral hypothalamus. Am. J. Physiol 172, 162–168, (1953). This study was the first to show that excitation of the lateral hypothalamus via electrical stimulation augmented food intake.
- 137.Mogenson GJ & Stevenson JA Drinking induced by electrical stimulation of the lateral hypothalamus. Exp. Neurol 17, 119–127 (1967). [DOI] [PubMed] [Google Scholar]
- 138.Woodworth CH Attack elicited in rats by electrical stimulation of the lateral hypothalamus. Physiol. Behav 6, 345–353 (1971). [DOI] [PubMed] [Google Scholar]
- 139.Valenstein ES, Cox VC & Kakolewski JW Modification of motivated behavior elicited by electrical stimulation of the hypothalamus. Science 159, 1119–1121 (1968). [DOI] [PubMed] [Google Scholar]
- 140.Vaughan E & Fisher AE Male sexual behavior induced by intracranial electrical stimulation. Science 137, 758–760 (1962). [DOI] [PubMed] [Google Scholar]
- 141. Navarro M. et al. Lateral hypothalamus GABAergic neurons modulate consummatory behaviors regardless of the caloric content or biological relevance of the consumed stimuli. Neuropsychopharmacology 41, 1505–1512 (2016). This study demonstrated that artificial activation of feeding centers can elicit consummatory responses to inedible, non-caloric objects.
- 142.Nieh EH et al. Decoding neural circuits that control compulsive sucrose seeking. Cell 160, 528–541 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Margules DL & Olds J Identical ‘feeding’ and ‘rewarding’ systems in the lateral hypothalamus of rats. Science 135, 374–375 (1962). [DOI] [PubMed] [Google Scholar]
- 144.Nieh EdwardH. et al. Inhibitory input from the lateral hypothalamus to the ventral tegmental area disinhibits dopamine neurons and promotes behavioral activation. Neuron 90, 1286–1298 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.O’Connor EoinC. et al. Accumbal D1R neurons projecting to lateral hypothalamus authorize feeding. Neuron 88, 553–564 (2015). [DOI] [PubMed] [Google Scholar]
- 146.Thoeni S, Loureiro M, O’Connor EC & Lüscher C Depression of accumbal to lateral hypothalamic synapses gates overeating. Neuron 107, 158–172.e154 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Broberger C, Johansen J, Johansson C, Schalling M & Hokfelt T The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc. Natl Acad. Sci. USA 95, 15043–15048 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tolson KP et al. Postnatal Sim1 deficiency causes hyperphagic obesity and reduced Mc4r and oxytocin expression. J. Neurosci 30, 3803–3812 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Baltasar A. et al. Laparoscopic sleeve gastrectomy: a multi-purpose bariatric operation. Obes. Surg 15, 1124–1128 (2005). [DOI] [PubMed] [Google Scholar]
- 150.Michaud JL, Rosenquist T, May NR & Fan CM Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev. 12, 3264–3275 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xi B, Chandak GR, Shen Y, Wang Q & Zhou D Association between common polymorphism near the MC4R gene and obesity risk: a systematic review and meta-analysis. PLoS ONE 7, e45731 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li MM et al. The paraventricular hypothalamus regulates satiety and prevents obesity via two genetically distinct circuits. Neuron 102, 653–667 e656 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shah BP et al. MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proc. Natl Acad. Sci. USA 111, 13193–13198 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Garfield AS et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat. Neurosci 18, 863–871 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sutton AK et al. Control of food intake and energy expenditure by Nos1 neurons of the paraventricular hypothalamus. J. Neurosci 34, 15306–15318 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sutton AK et al. Paraventricular, subparaventricular and periventricular hypothalamic IRS4-expressing neurons are required for normal energy balance. Sci. Rep 10, 5546 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.An JJ, Liao GY, Kinney CE, Sahibzada N & Xu B Discrete BDNF neurons in the paraventricular hypothalamus control feeding and energy expenditure. Cell Metab. 22, 175–188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim J. et al. Rapid, biphasic CRF neuronal responses encode positive and negative valence. Nat. Neurosci 22, 576–585 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sweeney P, Chen C, Rajapakse I & Cone RD Network dynamics of hypothalamic feeding neurons. Proc. Natl Acad. Sci. USA 118, e2011140118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xu S. et al. Behavioral state coding by molecularly defined paraventricular hypothalamic cell type ensembles. Science 370, eabb2494 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stachniak TJ, Ghosh A & Sternson SM Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus→midbrain pathway for feeding behavior. Neuron 82, 797–808 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Gong R, Xu S, Hermundstad A, Yu Y & Sternson SM Hindbrain double-negative feedback mediates palatability-guided food and water consumption. Cell 182, 1589–1605 (2020). This study highlights a population of hindbrain neurons that increase consumption by enhancing palatbility and prolonging ingestion duration.
- 163.Li C. et al. Defined paraventricular hypothalamic populations exhibit differential responses to food contingent on caloric state. Cell Metab. 29, 681–694 e685 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Travers JB, Dinardo LA & Karimnamazi H Motor and premotor mechanisms of licking. Neurosci. Biobehav. Rev 21, 631–647 (1997). [DOI] [PubMed] [Google Scholar]
- 165.Mohammad H. et al. A neural circuit for excessive feeding driven by environmental context in mice. Nat. Neurosci 24, 1132–1141 (2021). [DOI] [PubMed] [Google Scholar]
- 166.Zhang X & van den Pol AN Rapid binge-like eating and body weight gain driven by zona incerta GABA neuron activation. Science 356, 853–859 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Herman AM et al. A cholinergic basal forebrain feeding circuit modulates appetite suppression. Nature 538, 253–256 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pinol RA et al. Brs3 neurons in the mouse dorsomedial hypothalamus regulate body temperature, energy expenditure, and heart rate, but not food intake. Nat. Neurosci 21, 1530–1540 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Miller GD Appetite regulation: hormones, peptides, and neurotransmitters and their role in obesity. Am. J. Lifestyle Med 13, 586–601 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schwartz GJ, Salorio CF, Skoglund C & Moran TH Gut vagal afferent lesions increase meal size but do not block gastric preload-induced feeding suppression. Am. J. Physiol 276, R1623–R1629 (1999). [DOI] [PubMed] [Google Scholar]
- 171.Ritter S, Dinh TT & Friedman MI Induction of Fos-like immunoreactivity (Fos-li) and stimulation of feeding by 2,5-anhydro-D-mannitol (2,5-AM) require the vagus nerve. Brain Res. 646, 53–64 (1994). [DOI] [PubMed] [Google Scholar]
- 172.Fan W. et al. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat. Neurosci 7, 335–336 (2004). [DOI] [PubMed] [Google Scholar]
- 173.Kreisler AD, Davis EA & Rinaman L Differential activation of chemically identified neurons in the caudal nucleus of the solitary tract in non-entrained rats after intake of satiating vs. non-satiating meals. Physiol. Behav 136, 47–54 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Blouet C & Schwartz GaryJ. Brainstem nutrient sensing in the nucleus of the solitary tract inhibits feeding. Cell Metab. 16, 579–587 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen J. et al. A vagal–NTS neural pathway that stimulates feeding. Curr. Biol 30, 3986–3998.e3985 (2020). [DOI] [PubMed] [Google Scholar]
- 176.Browning KN & Travagli RA Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr. Physiol 4, 1339–1368 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sutton AK & Krashes MJ Integrating hunger with rival motivations. Trends Endocrinol. Metab 31, 495–507 (2020). [DOI] [PubMed] [Google Scholar]
- 178. Carter ME, Soden ME, Zweifel LS & Palmiter RD Genetic identification of a neural circuit that suppresses appetite. Nature 503, 111–114 (2013). This study identified a neural circuit from the parabrachial nucleus to the amygdala that mediates appetite suppression in conditions when it is unfavorable to eat.
- 179.Carter ME, Han S & Palmiter RD Parabrachial calcitonin gene-related peptide neurons mediate conditioned taste aversion. J. Neurosci 35, 4582–4586 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Campos CA, Bowen AJ, Roman CW & Palmiter RD Encoding of danger by parabrachial CGRP neurons. Nature 555, 617–622 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chen JY, Campos CA, Jarvie BC & Palmiter RD Parabrachial CGRP neurons establish and sustain aversive taste memories. Neuron 100, 891–899 e895 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Reilly S. The parabrachial nucleus and conditioned taste aversion. Brain Res. Bull 48, 239–254 (1999). [DOI] [PubMed] [Google Scholar]
- 183.Phua SC et al. A distinct parabrachial-to-lateral hypothalamus circuit for motivational suppression of feeding by nociception. Sci. Adv 7, eabe4323 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Han S, Soleiman MT, Soden ME, Zweifel LS & Palmiter RD Elucidating an affective pain circuit that creates a threat memory. Cell 162, 363–374 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sabatini PV et al. GFRAL-expressing neurons suppress food intake via aversive pathways. Proc. Natl Acad. Sci. USA 118, e2021357118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Alhadeff AL & Grill HJ Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. Am. J. Physiol. Regul. Integr. Comp. Physiol 307, R465–R470 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Richard JE et al. GLP-1 receptor stimulation of the lateral parabrachial nucleus reduces food intake: neuroanatomical, electrophysiological, and behavioral evidence. Endocrinology 155, 4356–4367 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Roman CW, Derkach VA & Palmiter RD Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat. Commun 7, 11905 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Roman CW, Sloat SR & Palmiter RD A tale of two circuits: CCK(NTS) neuron stimulation controls appetite and induces opposing motivational states by projections to distinct brain regions. Neuroscience 358, 316–324 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Huang D, Grady FS, Peltekian L, Laing JJ & Geerling JC Efferent projections of CGRP/Calca-expressing parabrachial neurons in mice. J. Comp. Neurol 529, 2911–2957 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kim JH et al. A discrete parasubthalamic nucleus subpopulation plays a critical role in appetite suppression. eLife 11, e75470 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cai H, Haubensak W, Anthony TE & Anderson DJ Central amygdala PKC-delta+ neurons mediate the influence of multiple anorexigenic signals. Nat. Neurosci 17, 1240–1248 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cheng W. et al. Calcitonin receptor neurons in the mouse nucleus tractus solitarius control energy balance via the non-aversive suppression of feeding. Cell Metab. 31, 301–312 e305 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cheng W. et al. NTS Prlh overcomes orexigenic stimuli and ameliorates dietary and genetic forms of obesity. Nat. Commun 12, 5175 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Luskin AT et al. Extended amygdala-parabrachial circuits alter threat assessment and regulate feeding. Sci. Adv 7, eabd3666 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Flak JN et al. Leptin-inhibited PBN neurons enhance responses to hypoglycemia in negative energy balance. Nat. Neurosci 17, 1744–1750 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mumphrey MB et al. Eating in mice with gastric bypass surgery causes exaggerated activation of brainstem anorexia circuit. Int J. Obes 40, 921–928 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Evers SS, Sandoval DA & Seeley RJ The physiology and molecular underpinnings of the effects of bariatric surgery on obesity and diabetes. Annu Rev. Physiol 79, 313–334 (2017). [DOI] [PubMed] [Google Scholar]
- 199.Low AYT et al. Reverse-translational identification of a cerebellar satiation network. Nature 600, 269–273 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Alhadeff AL, Rupprecht LE & Hayes MR GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 153, 647–658 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Angulo MA, Butler MG & Cataletto ME Prader–Willi syndrome: a review of clinical, genetic, and endocrine findings. J. Endocrinol. Invest 38, 1249–1263 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Goldstein N. et al. Hypothalamic detection of macronutrients via multiple gut-brain pathways. Cell Metab. 33, 676–687 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Corbett D & Wise RA Intracranial self-stimulation in relation to the ascending dopaminergic systems of the midbrain: a moveable electrode mapping study. Brain Res. 185, 1–15 (1980). [DOI] [PubMed] [Google Scholar]
- 204.Yeomans MR Taste, palatability and the control of appetite. Proc. Nutr. Soc 57, 609–615 (1998). [DOI] [PubMed] [Google Scholar]
- 205.Cabanac M. Physiological role of pleasure. Science 173, 1103–1107 (1971). [DOI] [PubMed] [Google Scholar]
- 206.Sorensen LB, Moller P, Flint A, Martens M & Raben A Effect of sensory perception of foods on appetite and food intake: a review of studies on humans. Int. J. Obes. Relat. Metab. Disord 27, 1152–1166 (2003). [DOI] [PubMed] [Google Scholar]
- 207.Campos CA et al. Cancer-induced anorexia and malaise are mediated by CGRP neurons in the parabrachial nucleus. Nat. Neurosci 20, 934–942 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bowen AJ et al. Dissociable control of unconditioned responses and associative fear learning by parabrachial CGRP neurons. eLife 9, e59799 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Astrup A. et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J. Obes 36, 843–854 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Muller TD, Clemmensen C, Finan B, DiMarchi RD & Tschop MH Anti-obesity therapy: from rainbow pills to polyagonists. Pharm. Rev 70, 712–746 (2018). [DOI] [PubMed] [Google Scholar]
- 211.Pi-Sunyer X. et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N. Engl. J. Med 373, 11–22 (2015). [DOI] [PubMed] [Google Scholar]
- 212.Wilding JPH et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med 384, 989 (2021). [DOI] [PubMed] [Google Scholar]