Abstract
Mesothelioma is a type of late‐onset cancer that develops in cells covering the outer surface of organs. Although it can affect the peritoneum, heart, or testicles, it mainly targets the lining of the lungs, making pleural mesothelioma (PMe) the most common and widely studied mesothelioma type. PMe is caused by exposure to fibres of asbestos, which when inhaled leads to inflammation and scarring of the pleura. Despite the ban on asbestos by most Western countries, the incidence of PMe is on the rise, also facilitated by a lack of specific symptomatology and diagnostic methods. Therapeutic options are also limited to mainly palliative care, making this disease untreatable. Here we present an overview of biological aspects underlying PMe by listing genetic and molecular mechanisms behind its onset, aggressive nature, and fast‐paced progression. To this end, we report on the role of deubiquitinase BRCA1‐associated protein‐1 (BAP1), a tumour suppressor gene with a widely acknowledged role in the corrupted signalling and metabolism of PMe. This review aims to enhance our understanding of this devastating malignancy and propel efforts for its investigation.
Keywords: asbestos, BAP1 and therapy, mesothelioma
Pleural mesothelioma (PMe) is a rare but lethal pathology in which the carcinogen asbestos has a proven causative role. Asbestos travels inside the lungs and airways to prime degeneration of mesothelioma cells. Pathogenic molecular mechanisms and pathways are here outlined as well as ways for clinical intervention for this malignancy, which remains untreatable.
Abbreviations
- 5‐hmC
5‐hydroxymethylcytosine
- AE1/3
pan‐cytokeratin antibody cocktail
- AKT
protein kinase B
- AP‐1
activator protein 1
- ASX
additional sex combs
- ASXL1
additional sex combs like 1
- ASXL2
additional sex combs like 2
- BAP1
BRCA1 associated protein 1
- BARD1
BRCA1 associated RING domain 1
- BCL‐2
B‐cell lymphoma 2
- BRCA1
breast cancer associated protein 1
- Ca2+
calcium ions concentration
- CDK4
cyclin‐dependent kinase 4
- CDK6
cyclin‐dependent kinase 6
- CDKN2A
cyclin‐dependent kinase inhibitor 2A
- CR
calretinin
- CSC
cancer stem cell
- DNA
deoxyribonucleic acid
- Dvl
dishevelled
- EGFR
epidermal growth factor receptor
- EMT
epithelial to mesenchymal transition
- EPP
extrapleural pneumonectomy
- ERK
extracellular signal‐regulated kinases
- FOXK1
forkhead box protein K1
- FOXK2
forkhead box protein K2
- GSH
glutathione
- HCF1
host cell factor C1
- HEG1
heart development protein with EGF like domains 1
- ICIs
immune check points inhibitors
- IHC
immunohistochemistry
- IMRT
intensity modulated radiotherapy
- IP3R2
type 3 inositol‐1,4,5‐trisphosphate receptor
- IP3R3
type 3 inositol‐1,4,5‐trisphosphate receptor
- MBD5
methyl‐CpG binding domain protein 5
- MBD6
methyl‐CpG binding domain protein 6
- MCL‐1
induced myeloid leukaemia cell differentiation protein 1
- Merlin
moesin‐ezrin‐radixin‐like protein
- miRNA
micro RNA
- MIS
mesothelioma in situ
- MMe
malignant mesothelioma
- mTOR
mammalian target of rapamycin
- NF2
neurofibromin 2
- OCT4
octamer‐binding transcription factor 4
- OVT
oncolytic viral therapy
- P/D
pleurectomy/decortication
- p14ARF
alternative open reading frame of CDKN2A gene in Homo sapiens
- p16INK4A
cyclin‐dependent kinase inhibitor p16
- p19ARF
alternative open reading frame of CDKN2A gene in Mus musculus
- p53
tumour protein p53
- pAKT
phosphorylated protein kinase B
- PDT
photodynamic treatment
- PMe
pleural mesothelioma
- PRC1
polycomb repressor complex 1
- PR‐DUB
polycomb repressive deubiquitinase
- PTEN
phosphatase and tension homologue
- RNA
ribonucleic acid
- ROS
reactive oxygen species
- RTK
receptor tyrosine kinase
- SLC7A11
solute carrier family 7 member 11
- SOMAscan
slow Off‐Rate modified Aptamer proteomic analysis
- TNF‐α
tumour necrosis factor alpha
- TPDS
tumour predisposition syndrome
- UCH
ubiquitin carboxy‐terminal hydrolase
- ULD
UCH37‐like domain
- WNT
wingless and INT‐1
- WT1
Wilms' tumour 1
- YAP1
yes‐associated protein 1
- YY1
Yin Yang 1
1. Introduction
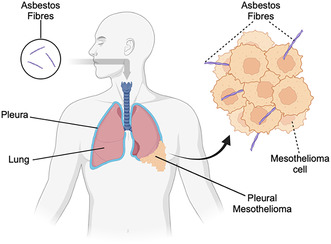
The mesothelium refers to the layer of tissues (epithelium) that surrounds most body cavities and the organs of the chest (pleura and pericardium), abdominal cavity (peritoneum and mesentery), and pelvis (including the tunica vaginalis that surrounds the testes) [1]. It functions to protect internal structures and aid in movement and breathing. Such functions are supported by the cells—called mesothelial cells—which are commonly found in pleural, peritoneal, and pericardial fluids [2]. Mesothelial cells tend to have a large, round, and centrally placed nucleus with a generous amount of basophilic cytoplasm. In response to inflammation or infection, mesothelial cells lose their cytoplasm and converge into clusters. Several medical problems may involve the mesothelium and its resident population such as pleural and pericardial effusions, adhesions, and malignant mesothelioma (MMe) [3]. MMe has an incidence rate ranging between 7 and 40 cases per million [4], killing approximately 40 000 people worldwide per year [5]. Currently, there is a lack of suitable treatments to revert its fatal prognosis as well as of reliable markers allowing for an early diagnosis. MMe develops in lining tissues of the body such as the pericardium, tunica vaginalis, peritoneum, and the pleura [6] (Fig. 1). Pleural mesothelioma (PMe), which includes the parietal layer of the internal chest cavity and the visceral line which covers the lungs [7], accounts for more than 70% of all MMe cases. PMe can develop on either layer of the pleura and spreads to the other [8], resulting in the growth of the tumour around the affected lung, which can lead to breathing difficulties caused by the accumulation of pleural fluids in the thoracic cavity [9]. Importantly, PMe is histologically distinguishable according to the cell type involved in the pathology [10], i.e. (a) epithelioid, (b) sarcomatoid, or (c) biphasic [11] (Fig. 2). Epithelioid mesothelioma is the most common type, tends to be less aggressive, and spreads more slowly compared to sarcomatoid and biphasic ones [12]. Sarcomatoid mesothelioma is associated with the worst prognosis and is the most aggressive and difficult to treat of the three types, accounting for 10–20% of the PMe cases [13]. The biphasic type is a mix of the previous two [14] and its prognosis may depend upon which cell type is most abundant in the tumour. Epithelioid PMe is composed of polygonal, oval, or cuboidal cells, whilst sarcomatoid PMe cells have a spindle shape [15]. The most widely accepted method currently to define PMe pathology progression is the Tumour Node Metastasis (TNM) staging system [16]. See Box 1 on Tumour Node Metastasis (TNM) classification.
Fig. 1.

Tissues affected by mesothelioma. Representation of the most common tissues of origin for the mesothelioma pathology. Source: www.asbestos.com/mesothelioma. Created with Biorender.com.
Fig. 2.

Mesothelioma cell types. Illustration of the three possible cell types involved in mesothelioma. Created with Biorender.com.
Box 1. TNM Classification uses three components to identify cancer stage.
Tumour extent (T): The location and size of the primary tumour site, which can range from T0 if there is no evidence of the tumour, to T4 when the tumour has spread from the pleura to nearby tissues and organs.
Lymph node involvement (N): Whether the cancer has spread to nearby lymph nodes, from N0 to N2 based on how far from the primary tumour the affected lymph nodes are.
Metastasis (M): M0 for no evidence and M1 the for presence of distant metastases, depending on whether the tumour is localised or has spread to distant areas of the body.
The clinician will then score each component and assigns a value. A combination of component scores establishes the mesothelioma stage, between I and IV.
Stage IA: T1, N0, M0
Stage IB: T2 or 3, N0, M0
Stage II: T1 or 2, N1, M0
Stage IIIA: T3, N1, M0
Stage IIIB: T1‐3, N2, M0 or T4, any N, M0
Stage IV: any T, any N, M1
Assessment of cancer progression through the staging system is essential to plan the best possible treatments, but as of today a cure for this pathology has yet to be achieved. For this reason, the average life expectancy for PMe patients is between 8 and 22 months after diagnosis [17], depending on stage and histological subtype. Symptoms include dry cough, shortness of breath, and chest pain [4], which often appear at a late stage of the pathology and since these are common symptoms of other diseases, they may lead to misdiagnosis [18].
This occurs despite an awareness of the main risk factor of the pathology is exposure to asbestos [6]. Even though genetic background [19] and simian virus (SV40) [20, 21] infections have been contemplated as triggers of the condition, accumulation of asbestos in respiratory routes is the prominent one. Asbestos is a term used to describe different mineral species [22], of which the most common is chrysotile. Chrysotile, also known as white asbestos [23], makes up 99% of the total types of asbestos produced worldwide [24]. White asbestos has been widely used in the past century in the textile and building industries, due to its durability and physical properties [22, 25], its usage has been nonetheless banned in more than 65 countries since 1970 for the threat it poses to human health via its fibre release into the air [26, 27]. Asbestos modifies DNA and induces chronic inflammation [28, 29], thereby impairing lung function. However, the damage caused to an organism can take up to 50 years to manifest and PMe is indeed a cancer characterised by a very long latency period [30]. The incidence peak of the pathology is believed to have happened already [31] in the Western countries that have banned the use of the mineral. However, highly populated countries such as China, Russia, and India [32] are continuing the mining of asbestos and its usage in construction indicates that the number of PMe patients is expected to rise globally.
Given the growing global attention to this disease, we review what is known about PMe by detailing (a) mechanisms of carcinogenesis, (b) signalling pathways, (c) proven genetic determinants besides diagnostics, and (d) treatments. This overview represents a core‐based knowledge of the disease, which is aimed at assisting both researchers and clinicians.
2. Asbestos‐induced PMe pathogenesis
The mesothelium is an important structure that serves not only to protect and lubricate movements of organs, but also facilitates fluid transport, blood clotting, and helps resistance against infections and cancers. While it can aid in controlling tumours, it is also particularly sensitive to asbestos [3]. The first line of evidence showing that asbestos exerts regulatory effects in pleural mesothelial cells came from a study in which asbestos fibres caused the induction of c‐fos and c‐jun proto‐oncogenes [33] in human cells. It is yet ill‐defined how asbestos influences the biology of mesothelial cells, but the currently accepted view is that the induced cytotoxicity leads to DNA damage and/or frustrates phagocytosis, triggering chronic inflammation [34, 35]. During the latency period, from exposure to the carcinogen and pathology outbreak, several core aspects of mesothelial cells change, spanning chromosomal integrity and epigenetic modifications (e.g. promoter hypermethylation at tumour suppressor loci) [36, 37]. Currently, there are four main hypotheses regarding the pathogenesis of asbestos‐induced mesothelioma (Fig. 3A–D).
The first one pertains to the production of a large amount of reactive oxygen species (ROS). ROS are dangerous oxygen forms, and are highly reactive due to their unstable nature [38]. These molecules can be produced directly from the exposed surface of the fibre, or by phagocytic cells such as macrophages, which engulf asbestos fibres but are not able to digest them (Fig. 3A). ROS can then interact with DNA, leading to DNA damage lesions and mutations. It is nonetheless important to consider that it might be difficult for ROS to reach the DNA in their active state, as they would probably react with membranes and molecules on their way to the nucleus. Therefore, one of the other three mechanisms below, or a combination of them, could better explain the specific action of these dangerous fibres on the cells.
The second hypothesis indicates that asbestos fibres are engulfed directly by mesothelial cells and can physically interfere with the mitotic process of the cell cycle (Fig. 3D). Tangling of asbestos fibres with chromosomes or mitotic spindles may result in chromosomal structural abnormalities during cell division [39] (Fig. 3B).
The third one proposes that asbestos traps other molecules on its surface [40], such as proteins and other chemical compounds, resulting in the accumulation of hazardous molecules, including carcinogens (Fig. 3C).
Finally, the fourth hypothesis suggests that mesothelial cells and macrophages exposed to asbestos fibres release a variety of cytokines and growth factors, such as tumour necrosis factor‐α, interleukin‐1β, transforming growth factor‐β, and platelet‐derived growth factor, which induce inflammation and tumour promotion [41]. This is probably due to the ability of asbestos to directly interact and activate surface receptors (Fig. 3D). Indeed, it has been demonstrated that asbestos fibres activate the epidermal growth factor receptor (EGFR) in mesothelial cells and in turn the downstream extracellular signal‐regulated kinases (ERK) [42] cascade. Moreover, the activation of the pathway is coupled by an upregulation of EGFR mRNA and protein levels [43], leading to an amplification of the signal. One of the principal effects following the upregulation of the ERK cascade in PMe is the activation of the transcription factor activator protein 1 (AP‐1) [44], which controls several cellular processes, such as cellular proliferation, differentiation, and apoptosis [45].
Fig. 3.

Possible mechanisms of carcinogenesis caused by asbestos. Representation of the proposed mechanisms of action of asbestos fibres in mesothelioma progression. (A) DNA damage caused by ROS produced by macrophages after asbestos phagocytosis. (B) Abnormal cell cycle due to asbestos fibres entangling chromosomes during mitosis. (C) Activation of receptors localised on the cell surface by asbestos fibres. (D) Transport of cancerous chemical compounds inside the cell carried on the surface of the fibres. Created with Biorender.com.
Therefore, asbestos exhibits pleiotropic effects, linked to aberrant transcriptional responses, cell proliferation, and transformation. Either through direct interactions or via the generation of ROS, asbestos activates cell signalling pathways that regulate gene expression and cell fate.
3. Pathogenesis beyond asbestos
Even though approximately 80% of individuals developing PMe have been previously exposed to asbestos, fewer than 5% of asbestos workers develop PMe [46]. This suggests that genetic traits may be relevant for the development of the pathology. For instance, a higher incidence of PMe has been observed in certain families among residents exposed to asbestos [47]. Cytogenetic studies have shown that mesothelioma cells carry highly complex and variable chromosomal aberration. However, other pathogenic mechanisms have been unveiled and are reported below.
3.1. Chromosomal and genetic changes
Loss‐of‐heterozygosity analyses have demonstrated frequent deletions of specific sites within chromosome arms 1p, 3p, 6q, 9p, 13q, 15q, and 22q [48]. Three of these regions are most frequently altered: the tumour suppressors cyclin‐dependent kinase inhibitor 2A (CDKN2A) at 9p21, neurofibromin 2 (NF2) at 22q12, and the BRCA1‐associated protein 1 (BAP1) at 3p21.1. Homozygous deletion appears to be the major mechanism affecting CDKN2A, whereas inactivating mutations coupled with allelic loss occur at the NF2 locus [48]. More recently, BAP1 was determined to be the gene involved in the mutations or deletions at 3p21.1 [49]. BAP1 is a tumour suppressor protein [50], mainly by acting on DNA repair, cell death, and gene transcription. The CDKN2A locus on chromosome 9p21 encodes two tumour suppressor proteins, namely p16INK4A and p14ARF [51]. Mesothelioma, as opposed to many other cancers, rarely has mutated TP53 [52]. However, loss of p14ARF indirectly inactivates p53, since the degradation of p53 is blocked by p14ARF activity [53]. P53 is an important tumour suppressor, essential for the regulation of cell division, senescence, and apoptosis [54]. The protein p16INK4A, instead, controls cell cycle and division by suppressing CDK4 and CDK6 activity [55]. Moreover, in murine models it has been shown that hypermethylation of p16 INK4A or p19 ARF (orthologous of the human p14 ARF ) precedes mesothelioma accompanied by silencing of CDKN2A and loss of p16INK4A and p19ARF proteins, suggesting that epigenetic alterations may play an important role in gene regulation leading to PMe [37].
3.2. Merlin signalling
Moesin‐ezrin‐radixin‐like protein (Merlin) is the protein encoded by the NF2 gene that affects multiple signalling cascades, including cell adhesion, small GTPases, receptor tyrosine kinase (RTK), Hippo, and the mammalian target of rapamycin (mTOR) pathways [56]. Given its plethora of functions, the loss of Merlin in PMe has been linked not only to increased proliferation, but also increased invasiveness, spreading, and migration [56]. In patients with no detectable NF2 mutations, Merlin was found to be phosphorylated at Ser518, and thus inactivated [57], confirming the role of Merlin in PMe development.
More specifically, its role in PMe seems to be tightly linked to Hippo and mTOR signalling cascades. Besides the recurring mutations of the LST1 and LST2 kinases, arising from loss of NF2 gene function [56], Merlin interplays with the Hippo pathway by influencing the transcriptional co‐activator Yes‐associated protein 1 (YAP1). The latter is known to be involved in mesothelial cell growth through the upregulation of cell cycle‐promoting genes [58]. YAP1 activity is normally inhibited by Merlin, through phosphorylation and cytoplasmic retention [59].
3.3. Noncoding miRNAs
Not only conventional coordinators of gene transcription such as transcription tactors have been linked to PMe onset, but also microRNAs (miRNAs). miRNAs are small, single‐stranded, noncoding RNA molecules containing 21–23 nucleotides that regulate gene expression by blocking the translation of target messenger RNA (mRNA) [60]. It has been demonstrated that several miRNAs are differentially expressed in mesothelioma cells compared to normal or immortalised mesothelial cells [61, 62]. Most of the differences involve the downregulation of miRNA directed at silencing proto‐oncogenes such as BCL‐2, OCT4, MCL‐1, and others [63]. This leads to a deregulation of essential cellular processes, such as differentiation, proliferation, apoptosis, and metabolism [64]; hence, promoting cancer progression. The downregulation of miRNA expression in PMe is usually due to chromosomal aberrations [63] (e.g. miR‐31 [65]). However, not all differences in miRNA expression are related to a downregulation, given that some miRNAs are abundantly overexpressed in PMe cells [61], such as those targeting CDKN2A and NF2 [61]. Deregulation of the miRNA processing could therefore hold pathogenic relevance in PMe patients.
3.4. mTOR and AKT signalling
There are several signalling pathways exploited by PMe during onset and rapid progression. The mammalian target of rapamycin (mTOR) is an important serine/threonine protein kinase, involved in fundamental processes such as autophagy, mitochondrial biogenesis, protein and lipid biosynthesis, and growth. Its defective function in PMe is linked with the fast progression of the disease as well as with its late onset [66]. Recently, it was demonstrated how pharmacologically targeting the mTOR complex [67] it is possible to inhibit malignant cell growth in vitro and in vivo tumour tissues. mTOR‐regulating signals are predominately linked to a lack of nutrients (specifically amino acids) [68] and are negatively regulated by Merlin, which is repressed by phosphorylated AKT (pAKT) [69, 70]. The constant activation of AKT in PMe could be due to the absence of phosphatase and tension homologue (PTEN), which is either mutated or completely absent in some cases [71, 72]. PTEN acts as a tumour suppressor by negatively regulating intracellular levels of phosphatidylinositol‐3,4,5‐trisphosphate, and phosphorylation levels of AKT [73]. Notably, the undetectable level of this protein in PMe might not only arise from mutations but also from hyperactivation of the Notch‐1 signalling pathway, which controls the transcriptional regulation of PTEN [74]. Moreover, a further mechanism of inactivation of this protein could be related to the effect of asbestos fibres on ROS production since PTEN is also frequently inactivated by H2O2 [75].
3.5. Calretinin and C‐met signalling
The sustained activation of AKT leading to Merlin repression and mTOR exploitation in PMe has been linked to calretinin (CR) [76, 77], which is overexpressed in certain types of tumours and pathologies, including colon carcinoma and PMe [78]. Mostly characterised as an intracellular Ca2+ effector and buffer [79], its expression promotes cellular growth, survival, and invasiveness inducing the epithelial to mesenchymal transition (EMT) [73]. In murine cortical neurons, it has been demonstrated that CR expression is regulated by an AP2‐like element present in the promoter region of the gene [80]. This element does not influence CR transcription in PMe [81], indicating different regulation between neuronal and non‐neuronal cells. A further activator of the AKT/mTOR pathway in PMe is speculated to be the hepatocyte growth factor receptor (c‐Met), the dysregulation of which contributes to the regulation of cell growth, motility, and invasion, as well as confers tumours the ability to metastasize [82]. Expressed in most PMe patients but not in healthy mesothelial cells [83], its inhibition by a specific drug (PHA‐665752) resulted in the arrest of the cell cycle and reduction of the activity of AKT and ERK signalling pathways [84].
3.6. NF‐kB and WNT signalling
In PMe, the proteasome is also overexpressed, which in combination with the activation of AKT causes activation of the nuclear translocator factor k‐light chain enhancer of activated B‐cells (NF‐kB) [44, 85]. In human mesothelial cells exposed to asbestos fibres [86], NF‐kB is translocated to the nucleus, resulting in the expression of prosurvival genes such as BCL‐2 [87]. A further stimulus for NF‐kB activation in PMe is tumour necrosis factor‐α (TNF‐α), which is abundant in PMe [86]. The Wingless and INT‐1 (WNT) pathway is also deregulated in PMe. The WNT signalling pathway regulates developmental processes, cell proliferation and polarity, and its upregulation is caused by overexpression of the activating protein Dishevelled (Dvl) [88]. Among the proteins capable of redesigning cancer cell signalling, greater consideration for its prominence in PMe was gained by the BRCA1‐associated protein 1 (BAP1). BAP1 is one of the most mutated genes in PMe. Loss of BAP1 nuclear staining is considered a reliable indicator of malignancy, particularly for epithelioid histology, and therefore part of the panel for the differential diagnosis.
All this highlights that PMe pathogenesis is multifactorial, mirrored by the complex genetic which characterises the transformed cells causing the disease (Fig. 4).
Fig. 4.

Schematic representation of the genes and protein most commonly deregulated in PMe. Representation of a PMe cell with dysregulated pathways and molecular mediator differently expressed or regulated during the onset of the pathology indicated. Created with Biorender.com.
4. Role of the BRCA1‐associated protein 1 (BAP1) in malignant mesothelioma
BAP1 is a ubiquitin carboxy‐terminal hydrolase of 729 aa, encoded in humans by the BAP1 gene [89]. In its native form it assumes a molecular mass of 80.4 kDa and its structure contains three domains: (a) a catalytic carboxy‐terminal hydrolase (UCH) domain, localised at the first 240 aa of the N‐terminus, which removes ubiquitin from ubiquitylated substrates; (b) a linker region, which includes a host cell factor C1 (HCF1) binding motif; and (c) the C‐terminal region, which comprises a UCH37‐like domain (ULD) and two nuclear localization sequences [90, 91] (Fig. 5). BAP1 was discovered in 1998, following a two‐hybrid screening and named after its interaction with breast cancer‐associated protein 1 (BRCA1) [92]. BRCA1, by interacting with BRCA1‐associated RING domain 1 (BARD1) forms a tumour suppressor complex that regulates the DNA damage response via its E3 ubiquitin ligase activity [93].
Fig. 5.

Schematic representation of BAP1 sequence, domains, and binding sites. Representation of BAP1 illustrating the functional domains and known binding sites to other proteins and complexes. UCH, ubiquitin carboxyl‐terminal hydrolase domain; ULD, UCH37‐like domain; NLS, nuclear localization sequence.
BAP1 associates with the complex by binding and deubiquitylating BARD1 [94], thereby modulating the DNA damage response of BRCA1–BARD1. BAP1 interacts with several other proteins in the cell [95, 96, 97] and its DNA repair activity could also be linked to its interaction with the proteins MBD5 and MBD6 [98]. Contrary to what was originally thought, BAP1 localization and activity are not exclusively nuclear, and can also be found in the cytosol [99] (Fig. 6). Given its protective role, BAP1 loss is established as “a foe” when it comes to tumour susceptibility and development. However, patients carrying germline BAP1 mutations in PMe [100, 101, 102] and cutaneous melanoma [103] show an improved survival and a better prognosis, suggesting that the presence of a mutated BAP1 might mitigate tumour aggressiveness (a “friendly” aspect). Moreover, in uveal melanoma, somatic BAP1 mutations cause metastasis with a probability of 74%, while the same has been observed in only 36% of the patients carrying a germline mutation [104]. A possible explanation for the differences reported is that in patients carrying a germline mutation, often a somatic mutation is also observed, thus probably inducing a complete inactivation of the protein [105]. What is clear is that PMe in carriers of BAP1 mutations are almost exclusively of the epithelioid subtype [101, 106], they are well‐differentiated, and have an overall nonaggressive morphology, consistent with prolonged survival. In addition, even though wildtype BAP1 affects sensitivity to gemcitabine [107], very recent work has shown that mutation of the gene as well as its ablation improves the response to platin/pemetrexed [108].
Fig. 6.

Scheme of the different BAP1 functions in the cell. BAP1 is a tumour‐suppressor protein that exerts its role via the coordination of several different cellular mechanisms. In the nucleus, it is involved in gene expression regulation, DNA damage repair, and chromatin remodelling, while in the cytosol it can stabilise the calcium channel IP3R3. The downstream effects on a cell include the regulation of cell death via apoptosis and ferroptosis. Created with Biorender.com.
The question of how the lack of a tumour suppressor protein like BAP1 can lead to an improvement in patient's outcome remains open. This positive effect may be due to the involvement of Ca2+ homeostasis in tumour progression. It has been reported that a few Ca2+ signalling effectors promote the cancer stem cell (CSC) state and are associated with cell resistance to cancer treatments [109]. In liver cancer, for example, Ca2+ oscillation mediated by IP3R2 plays a central role in CSC self‐renewal [110]. The recent findings on the role of BAP1 in the stabilisation of the IP3R3 receptor may support this theory. It is therefore possible indeed that malfunctions in BAP1, even though leading to cancer progression, may prevent the formation of CSCs, thereby reducing the aggressiveness of the cancerous cells.
5. Targets for molecular diagnosis
5.1. Diagnostic protein markers
Histological analysis is usually not sufficient for PMe diagnosis. It may indeed be hard to distinguish sarcomatoid mesothelioma from sarcomatoid carcinoma of the lung [111] and epithelioid mesothelioma from adenocarcinoma or reactive mesothelial cells [15]. Therefore, an immunohistochemistry (IHC) approach is required using antibodies specific for PMe biomarkers. Even though biomarkers with 100% sensitivity and specificity [112] for MMe are still missing, a few promising candidates have been discovered. Namely, by using a combination of Wilms' tumour 1 (WT1) protein, CAM5.2, and AE1/3 cytokeratins, it is possible to distinguish between sarcomatoid carcinoma and sarcomatoid mesothelioma with a specificity of 97.7% [113]. CR is also a useful diagnostic protein to distinguish mesothelioma from adenocarcinoma, with a sensitivity of 95% and specificity of 87% [114]. Another promising readout is the heart development protein with EGF‐like domains 1 (HEG1), which for the epithelioid subtype offers a sensibility comparable to CR, but with a higher specificity [115]. The discrimination between benign mesothelial proliferation and epithelial mesothelioma is rather difficult. To this end, the evaluation of the nuclear absence of BRCA1‐associated‐protein 1 (BAP1) and 5‐hydroxymethylcytosine (5‐hmC) emerges as a valid strategy [116]. Furthermore, a loss of BAP1 has been associated with mesothelioma in situ (MIS) [117]: a condition currently acknowledged as a precursor to PMe with an epithelioid component [118].
It is worth mentioning that there are blood‐based biomarkers that can help inform diagnosis, thus aiding the development of a screening methodology beyond classical histology, perhaps allowing for an early detection of PMe. To this goal, fibulin‐3 has been proposed as a possible detectable protein in the serum, even though there is still controversy that would explain a lack of attention towards this blood‐based marker [119]. Mesothelin is present in the blood too, and a study conducted on more than 4000 [120] patients has shown that it could be used as a serum marker to confirm PMe due to its high specificity (95%), although a sensitivity score of 32% does not advocate for this protein as a suitable diagnostic tool. However, encouraging recent studies have demonstrated that when mesothelin is scored in combination with CR, it is possible to reach a sensitivity of 75% [121] without losing any specificity.
5.2. The role of microRNAs as diagnostic markers
The screening for sarcomatoid mesothelioma via a blood test remains challenging, since it appears to be quite difficult to find specific protein markers linked to a histological subtype. Hope in this direction is brought by the detection of miRNAs. Previous analysis of tissue samples showed that for the correct diagnosis of PMe, the detection of differentially expressed miRNA [62] stands as a valid option. The deregulation of several miRNAs is a characteristic of PMe, regardless of histologic subtypes [122], and an assay validated on 68 samples based on the detection of three miRNAs reached a sensitivity of 100% and a specificity of 94% against adenocarcinoma [123]. In addition, it has been shown that miRNA and DNA molecules can be released from the cells into the body fluids [124, 125], in which they are remarkably stable, being protected from endogenous RNAse activity [126]. In keeping with this, the circulating upregulated microRNAs miR‐197‐3p, miR‐1281, and miR‐32‐3p have been proposed as potential new biomarkers [127] in PMe, but further studies will be needed to prove the efficacy of such a screening methodology for the detection of both epithelioid and sarcomatoid cell types. Lastly, for the same purpose, a novel interesting screening technique termed SOMAscan proteomics has been proposed, in which the presence of thousands of proteins in the serum is scored simultaneously. Recently, it has been shown that it is possible to detect PMe with a 75% sensitivity and 88.2% specificity using this method [128], which is an encouraging result but still not enough by itself. Perhaps soon, it will be possible to increase the sensitivity and specificity of this assay by producing a better screening array for this pathology.
6. Therapeutic tools
There is no cure for PMe and most of the current strategies of intervention are palliative, aimed at extending the lifespan of the patients. The multimodality treatment that sees surgery followed by radiotherapy and chemotherapy is the adopted standard of care, even though high incidences of failure and recurrence are still registered [129].
A recent cross‐sectional study has found that the incidence of PMe's death toll is constantly increasing worldwide, and particularly so in regions with limited resources [130]. Furthermore, the survival rate 5 years postdiagnosis is around 10% [131], corroborating that currently available treatments for PMe have little impact. Patient candidates recommended for radical surgery represent a lower percentage (around 20%) [132] and those are younger individuals. The surgical procedures consist of: (a) extrapleural pneumonectomy (EPP), en‐bloc resection of the lung, pleura, pericardium, and diaphragm, or (b) the pleurectomy/decortication (P/D), a lung‐sparing surgery that involves removing the tumours and the affected pleura [133]. A cure is not generally achieved following EPP [134], so the liberation of the tumour mass with P/D in a multimodal treatment scenario has been mostly adopted in recent years [135], mainly as a palliative [136].
The utility of P/D is still under debate and a recent study in the UK was conducted to assess whether the adoption of this procedure is effectively advantageous in terms of survivability and quality of life [137], but the results have yet to be published. It is important to note that although the multimodality treatment including surgery, chemotherapy and radiotherapy has been proposed for many years, it is not indicated as a standard of care [136].
Radiotherapy alone does not appear to be effective in resolving the pathology for PMe [138], although intensity‐modulated radiotherapy (IMRT) following surgery [139, 140] is mainly beneficial in easing the pain and symptoms.
Chemotherapy too shows marginal efficacy in PMe [136], considering the high resistance phenomena with efficacy just under 30% in the patients [55]. The standard chemotherapy protocol, used just in patients with unresectable disease, is a combination of a platinum drug (cisplatin or carboplatin) and pemetrexed [132, 141], which is a coadjuvant to increase cytotoxicity.
The addition of antiangiogenic drugs such as bevacizumab [142] to this combination can increase the therapy's efficacy [136]. More recently, attention has shifted to immune checkpoint inhibitors (ICI), to activate the immune system to fight the disease. ICI uses monoclonal antibodies directed to specific receptors present on cytotoxic T‐cells [143], which inhibits their function against the cells of our body. By blocking these receptors (or their ligands) the T‐cells can exert their function when they recognise specific epitopes on the cancerous cells. In a randomised phase III study conducted on 605 patients, Baas et al. [144] showed that the administration of ipilimumab/nivolumab significantly increases the overall survival of the patients to 18 months, compared to 14 with chemotherapy alone. This is now a standard treatment for PMe in the USA, Australia, and the UK. The approval has nonetheless raised criticisms by several experts who have questioned the clinical and pharmaeconomic benefit of this approach compared to standard chemotherapy, following a thorough analysis [145, 146, 147, 148, 149]. There are also a few other experimental approaches that are currently being explored for the treatment of this pathology. For example, photodynamic treatment (PDT) is based on a chemical accumulated in tumour cells activated by a specific wavelength of light to produce reactive singlet oxygen (1O2). This technique showed potential as therapy after surgical resection [150].
Another option is the use of miRNA [150]: the overexpression of miR‐31 miR‐29c, or miR‐145172 in PMe cell lines has been shown to induce a decrease in proliferation, migration, invasion, and colony formation. Despite the promising findings in vitro, the only clinical study [151] so far completed failed to deliver conclusive positive results.
Finally, oncolytic viral therapy (OVT) involves the use of engineered viruses to kill the tumorigenic cells [152] via lysis and parallel stimulation of the immune system. In PMe, various viruses have been manipulated such as adenovirus, HSV Type 1, vaccinia virus, and measles virus. Even though clinical studies are still ongoing, OVT did not prove to be effective alone [153], but useful in combination with chemotherapy and surgery [154], thus indicating a possible adjuvant rather than curative for this approach.
The current lack of a standard of care for PMe highlights that a deeper knowledge of biology and its pathogenesis is indispensable to designing effective approaches, as well as designating standards for clinical trials to measure success.
7. Conclusion
The progress made in the comprehension of genetic and molecular mechanisms involved in the aetiopathogenesis of PMe has not yet evolved into therapeutic protocols or predictive biomarking strategies. This means that more needs to be done to explore the cell biology of the PMe's onset and progression. The right focus on securing translational impact has left several of the molecular hits, which recently emerged, not fully characterised. In the same way, those that have been used instead far more robustly linked with the disease are still in need of further clarification. This is epitomised by the evidence of the dual role acknowledged for BAP1, considered as a “friend” in healthy subjects, but as a “foe” in PMe patients. This is indicative that the full picture of the underlying dysregulated biology in PMe is not yet fully elucidated. However, the literature reviewed here highlights that in PMe physiopathology, two promising aspects of this disease remain understudied: signalling and metabolism. Despite the contributions on Ca2+ signalling, the role of other intracellular second messengers or respiration by‐products holding a similar role (e.g. ROS) remains unclear. Equally, a deeper knowledge of metabolic pathways and metabolites would better inform hidden oncological aspects of PMe, thus offering new avenues for personalised therapies. Formation and maintenance of a CSC population, tumour micromovements and T‐cells function are all strictly dependent on modified metabolism. In keeping with this, the dynamics of interorganelle contacts are also unexplored, which determine foci of metabolic and signalling events. Their full understanding will empower the ambition for tailored therapeutic strategies and improved diagnosis in this aggressive malignancy.
Conflict of interest
The authors declare no conflicts of interest.
Author contributions
Conceptualization, MR and MC; Writing: Original Draft, MR and MC; Writing: Review & Editing, MR, LM, and MC.
Acknowledgements
The research activities led by MC are supported by the following funders: The ERC Consolidator Grant COG2018‐819600_FIRM; BBSRC [BB/M010384/1; BB/N007042/1]; The Petplan Charitable Trust; LAM‐Bighi Grant; FIRB [RBFR13P392], Italian Health Ministry [IFO14/01/R/52], the Italian Association for Cancer Research (AIRC) MFAG21903, Fondation ARC pour la Recherche sur le Cancer ARCLEADER2022020004901, Rotary Foundation, and the Gruppo Italiano Mesotelioma G.I.Me Charity.
References
- 1. Mutsaers SE, Herrick SE. Mesothelial cells. In: Geoffrey JL, Steven DS, editors. Encyclopedia of Respiratory Medicine, Four‐Volume Set. Oxford: Academic Press; 2006. p. 47–52. [Google Scholar]
- 2. Valenciano AC, Rizzi TE. Abdominal, thoracic, and pericardial effusions. In: Valenciano AC, Cowell RL, editors. Cowell and Tyler's Diagnostic Cytology and Hematology of the Dog and Cat. St. Louis, MO: Elsevier Mosby; 2020. p. 229–246. [Google Scholar]
- 3. Mutsaers SE. The mesothelial cell. Int J Biochem Cell Biol. 2004;36(1):9–16. 10.1016/S1357-2725(03)00242-5 [DOI] [PubMed] [Google Scholar]
- 4. Robinson BWS, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005;353:1591–1603. [DOI] [PubMed] [Google Scholar]
- 5. Odgerel CO, Takahashi K, Sorahan T, Driscoll T, Fitzmaurice C, Yoko‐o M, et al. Estimation of the global burden of mesothelioma deaths from incomplete national mortality data. Occup Environ Med. 2017;74(12):851–858. 10.1136/oemed-2017-104298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tischoff I, Tannapfel A. Mesotheliom. Pathologe. 2017;38(6):547–560. 10.1007/s00292-017-0364-z [DOI] [PubMed] [Google Scholar]
- 7. D'Agostino HP, Edens MA. Physiology, Pleural Fluid. Treasure Island, FL: StatPearls Publishing; 2019. [PubMed] [Google Scholar]
- 8. Boutin C, Schlesser M, Frenay C, Astoul P. Malignant pleural mesothelioma. Eur Respir J. 1998;12:4–981. [DOI] [PubMed] [Google Scholar]
- 9. What is pleural mesothelioma? Full overview & what to expect. Accessed 30 Jun 2020. https://www.asbestos.com/mesothelioma/pleural/
- 10. Bueno R, Reblando J, Glickman J, Jaklitsch MT, Lukanich JM, Sugarbaker DJ. Pleural biopsy: a reliable method for determining the diagnosis but not subtype in mesothelioma. Ann Thorac Surg. 2004;78(5):1774–1776. 10.1016/j.athoracsur.2004.05.007 [DOI] [PubMed] [Google Scholar]
- 11. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–1260. [DOI] [PubMed] [Google Scholar]
- 12. Van Schil PE, Opitz I, Weder W, De Laet C, Domen A, Lauwers P, et al. Multimodal management of malignant pleural mesothelioma: where are we today? Eur Respir J. 2014;44(3):754–764. 10.1183/09031936.00207213 [DOI] [PubMed] [Google Scholar]
- 13. Kim KC, Vo HP. Localized malignant pleural sarcomatoid mesothelioma misdiagnosed as benign localized fibrous tumor. J Thorac Dis. 2016;8(6):E379–E384. 10.21037/jtd.2016.03.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Borczuk AC, Chan JKC, Cooper WA, Dacic S, Kerr KM, Lantuejoul S, et al. Thoracic Tumours . Vol. 2 (Dilani L, Valerie AW, Beiko W, lan AC, editors). International Agency for Pesearch on Cancer (lABC); 2021. https://publications.iarc.fr/581. Accessed 25 Sep 2023.
- 15. Husain AN, Colby TV, Ordóñez NG, Allen TC, Attanoos RL, Beasley MB, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2017 update of the consensus statement from the international mesothelioma interest group. Arch Pathol Lab Med. 2018;142(1):89–108. 10.5858/arpa.2017-0124-RA [DOI] [PubMed] [Google Scholar]
- 16. Berzenji L, Van Schil PE, Carp L. The eighth TNM classification for malignant pleural mesothelioma. Transl Lung Cancer Res. 2018;7(5):543–549. 10.21037/tlcr.2018.07.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakano T. Systemic treatment of malignant pleural mesothelioma. Gan To Kagaku Ryoho. 2017;44(13):2041–2047. 10.1097/cco.0000000000000053 [DOI] [PubMed] [Google Scholar]
- 18. Zhang W, Wu X, Wu L, Zhang W, Zhao X. Advances in the diagnosis, treatment and prognosis of malignant pleural mesothelioma. Ann Transl Med. 2015;3(13):182. 10.3978/j.issn.2305-5839.2015.07.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kharazmi E, Chen T, Fallah M, Sundquist K, Sundquist J, Albin M, et al. Familial risk of pleural mesothelioma increased drastically in certain occupations: a nationwide prospective cohort study. Eur J Cancer. 2018;103:1–6. 10.1016/j.ejca.2018.07.139 [DOI] [PubMed] [Google Scholar]
- 20. Robinson BWS, Musk AW, Lake RA. Malignant mesothelioma. Lancet. 2005;366:397–408. 10.1016/S0140-6736(05)67025-0 [DOI] [PubMed] [Google Scholar]
- 21. Kroczynska B, Cutrone R, Bocchetta M, Yang H, Elmishad AG, Vacek P, et al. Crocidolite asbestos and SV40 are cocarcinogens in human mesothelial cells and in causing mesothelioma in hamsters. Proc Natl Acad Sci USA. 2006;103(38):14128–14133. 10.1073/pnas.0604544103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wagner GR, Lemen R. Asbestos. In: Heggenhougen HK, editor. International Encyclopedia of Public Health. Boston, MA: Elsevier Inc.; 2008. p. 238–245. [Google Scholar]
- 23. Rudd R. Asbestos and the lung. Medicine. 2008;36(5):261–264. 10.1016/j.mpmed.2008.02.001 [DOI] [Google Scholar]
- 24. Landrigan PJ, Nicholson WJ, Suzuki Y, Ladou J. The hazards of chrysotile asbestos: a critical review. Ind Health. 1999;37(3):271–280. 10.2486/indhealth.37.271 [DOI] [PubMed] [Google Scholar]
- 25. Habashi F. Asbestos. In: Jürgen Buschow KH, Cahn RW, Flemings MC, Ilschner B, Kramer EJ, Mahajan S, et al., editors. Encyclopedia of Materials: Science and Technology. 2nd ed. Amsterdam: Elsevier; 2002. p. 1–5. [Google Scholar]
- 26. Wagner GR, Lemen RA. Asbestos. In: Quah SR, editor. International Encyclopedia of Public Health. Amsterdam: Elsevier Inc.; 2016. p. 176–182. [Google Scholar]
- 27. Chen M, Wang H, Zhang J, Yu C, Liu W, Xu Y. Distribution of asbestos enterprises and asbestosis cases — China, 1997–2019. China CDC Wkly. 2020;2(18):305–309. 10.46234/ccdcw2020.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Korda RJ, Clements MS, Armstrong BK, Law HD, Guiver T, Anderson PR, et al. Risk of cancer associated with residential exposure to asbestos insulation: a whole‐population cohort study. Lancet Public Health. 2017;2(11):e522–e528. 10.1016/S2468-2667(17)30192-5 [DOI] [PubMed] [Google Scholar]
- 29. Furuya S, Chimed‐Ochir O, Takahashi K, David A, Takala J. Global asbestos disaster. Int J Environ Res Public Health. 2018;15(5):1000. 10.3390/ijerph15051000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bianchi C, Giarelli L, Grandi G, Brollo A, Ramani L, Zuch C. Latency periods in asbestos‐related mesothelioma of the pleura. Eur J Cancer Prev. 1997;6:162–166. [PubMed] [Google Scholar]
- 31. Hodgson JT, McElvenny DM, Darnton AJ, Price MJ, Peto J. The expected burden of mesothelioma mortality in Great Britain from 2002 to 2050. Br J Cancer. 2005;92(3):587–593. 10.1038/sj.bjc.6602307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marsili D, Terracini B, Santana VS, Ramos‐Bonilla J, Pasetto R, Mazzeo A, et al. Prevention of asbestos‐related disease in countries currently using asbestos. Int J Environ Res Public Health. 2016;13(5):494. 10.3390/ijerph13050494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heintz NH, Janssen YM, Mossman BT. Persistent induction of c‐fos and c‐jun expression by asbestos. Proc Natl Acad Sci USA. 1993;90(8):3299–3303. 10.1073/pnas.90.8.3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jaurand MC, Fleury‐Feith J. Pathogenesis of malignant pleural mesothelioma. Respirology. 2005;10(1):2–8. 10.1111/j.1440-1843.2005.00694.x [DOI] [PubMed] [Google Scholar]
- 35. Jean D, Daubriac J, Le Pimpec‐Barthes F, Galateau‐Salle F, Jaurand MC. Molecular changes in mesothelioma with an impact on prognosis and treatment. Arch Pathol Lab Med. 2012;136(3):277–293. 10.5858/arpa.2011-0215-RA [DOI] [PubMed] [Google Scholar]
- 36. Røe OD, Anderssen E, Sandeck H, Christensen T, Larsson E, Lundgren S. Malignant pleural mesothelioma: genome‐wide expression patterns reflecting general resistance mechanisms and a proposal of novel targets. Lung Cancer. 2010;67(1):57–68. 10.1016/j.lungcan.2009.03.016 [DOI] [PubMed] [Google Scholar]
- 37. Chernova T, Murphy FA, Galavotti S, Sun XM, Powley IR, Grosso S, et al. Long‐fiber carbon nanotubes replicate asbestos‐induced mesothelioma with disruption of the tumor suppressor gene Cdkn2a (Ink4a/Arf). Curr Biol. 2017;27(21):3302–3314.e6. 10.1016/j.cub.2017.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact. 2014;224:164–175. 10.1016/j.cbi.2014.10.016 [DOI] [PubMed] [Google Scholar]
- 39. Toyokuni S. Mechanisms of asbestos‐induced carcinogenesis. Nagoya J Med Sci. 2009;71:1–10. [PMC free article] [PubMed] [Google Scholar]
- 40. Ottaviani MF, Venturi F, Pokhrel MR, Schmutz T, Bossmann SH. Physicochemical studies on the adsorption properties of asbestos: 2. An EPR and fluorescence study on the adsorption of pyrene. J Colloid Interface Sci. 2001;238(2):371–380. 10.1006/jcis.2001.7529 [DOI] [PubMed] [Google Scholar]
- 41. Sekido Y. Molecular pathogenesis of malignant mesothelioma. Carcinogenesis. 2013;34(7):1413–1419. 10.1093/carcin/bgt166 [DOI] [PubMed] [Google Scholar]
- 42. Zanella CL, Posada J, Tritton TR, Mossman BT. Asbestos causes stimulation of the extracellular signal‐regulated kinase 1 mitogen‐activated protein kinase Cascade after phosphorylation of the epidermal growth factor receptor. Cancer Res. 1996;56:5334–5338. [PubMed] [Google Scholar]
- 43. Zanella CL, Timblin CR, Cummins A, Jung M, Goldberg J, Raabe R, et al. Asbestos‐induced phosphorylation of epidermal growth factor receptor is linked to c‐Fos and apoptosis. Am J Physiol. 1999;277:L684–L693. [DOI] [PubMed] [Google Scholar]
- 44. Heintz NH, Janssen‐Heininger YMW, Mossman BT. Asbestos, lung cancers, and mesotheliomas: from molecular approaches to targeting tumor survival pathways. Am J Respir Cell Mol Biol. 2010;42(2):133–139. 10.1165/rcmb.2009-0206TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ameyar M, Wisniewska M, Weitzman JB. A role for AP‐1 in apoptosis: the case for and against. Biochimie. 2003;85(8):747–752. 10.1016/j.biochi.2003.09.006 [DOI] [PubMed] [Google Scholar]
- 46. Below JE, Cox NJ, Fukagawa NK, Hirvonen A, Testa JR. Factors that impact susceptibility to fiber‐induced health effects. J Toxicol Environ Health B Crit Rev. 2011;14(1–4):246–266. 10.1080/10937404.2011.556052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carbone M, Emri S, Dogan AU, Steele I, Tuncer M, Pass HI, et al. A mesothelioma epidemic in Cappadocia: scientific developments and unexpected social outcomes. Nat Rev Cancer. 2007;7(2):147–154. 10.1038/nrc2068 [DOI] [PubMed] [Google Scholar]
- 48. Murthy SS, Testa JR. Asbestos, chromosomal deletions, and tumor suppressor gene alterations in human malignant mesothelioma. J Cell Physiol. 1999;180(2):150–157. [DOI] [PubMed] [Google Scholar]
- 49. Bott M, Brevet M, Taylor BS, Shimizu S, Ito T, Wang L, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet. 2011;43:668–672. 10.1038/ng.855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cheung M, Testa JR. BAP1, a tumor suppressor gene driving malignant mesothelioma. Transl Lung Cancer Res. 2017;6(3):270–278. 10.21037/tlcr.2017.05.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ferru A, Fromont G, Gibelin H, Guilhot J, Savagner F, Tourani JM, et al. The status of CDKN2A alpha (p16INK4A) and beta (p14 ARF) transcripts in thyroid tumour progression. Br J Cancer. 2006;95(12):1670–1677. 10.1038/sj.bjc.6603479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Esposito V, Baldi A, De Luca A, Claudio PP, Signoriello G, Bolognese A, et al. p53 immunostaining in differential diagnosis of pleural mesothelial proliferations. Anticancer Res. 1997;17(1B):733–736. [PubMed] [Google Scholar]
- 53. Pomerantz J, Schreiber‐Agus N, Liégeois NJ, Silverman A, Alland L, Chin L, et al. The Ink4a tumor suppressor gene product, p19(Arf), interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell. 1998;92(6):713–723. 10.1016/S0092-8674(00)81400-2 [DOI] [PubMed] [Google Scholar]
- 54. Zilfou JT, Lowe SW. Tumor suppressive functions of p53. Cold Spring Harb Perspect Biol. 2009;1(5):a001883. 10.1101/cshperspect.a001883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li P, Zhang X, Gu L, Zhou J, Deng D. P16 methylation increases the sensitivity of cancer cells to the CDK4/6 inhibitor palbociclib. PLoS One. 2019;14(10):e0223084. 10.1371/journal.pone.0223084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stamenkovic I, Yu Q. Merlin, a “magic” linker between the extracellular cues and intracellular signaling pathways that regulate cell motility, proliferation, and survival. Curr Protein Pept Sci. 2010;11(6):471–484. 10.2174/138920310791824011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Thurneysen C, Opitz I, Kurtz S, Weder W, Stahel RA, Felley‐Bosco E. Functional inactivation of NF2/merlin in human mesothelioma. Lung Cancer. 2009;64(2):140–147. 10.1016/j.lungcan.2008.08.014 [DOI] [PubMed] [Google Scholar]
- 58. Mizuno T, Murakami H, Fujii M, Ishiguro F, Tanaka I, Kondo Y, et al. YAP induces malignant mesothelioma cell proliferation by upregulating transcription of cell cycle‐promoting genes. Oncogene. 2012;31(49):5117–5122. 10.1038/onc.2012.5 [DOI] [PubMed] [Google Scholar]
- 59. Yokoyama T. YAP1 is involved in mesothelioma development and negatively regulated by Merlin through phosphorylation. Carcinogenesis. 2008;29:2139–2146. [DOI] [PubMed] [Google Scholar]
- 60. Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136(4):642–655. 10.1016/j.cell.2009.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Guled M, Lahti L, Lindholm PM, Salmenkivi K, Bagwan I, Nicholson AG, et al. CDKN2A, NF2, and JUN are dysregulated among other genes by miRNAs in malignant mesothelioma‐a miRNA microarray analysis. Genes Chromosomes Cancer. 2009;48(7):615–623. 10.1002/gcc.20669 [DOI] [PubMed] [Google Scholar]
- 62. Busacca S, Germano S, De Cecco L, Rinaldi M, Comoglio F, Favero F, et al. MicroRNA signature of malignant mesothelioma with potential diagnostic and prognostic implications. Am J Respir Cell Mol Biol. 2010;42(3):312–319. 10.1165/rcmb.2009-0060OC [DOI] [PubMed] [Google Scholar]
- 63. Birnie KA, Prêle CM, Thompson PJ, Badrian B, Mutsaers SE. Targeting microRNA to improve diagnostic and therapeutic approaches for malignant mesothelioma. Oncotarget. 2017;8(44):78193–78207. 10.18632/oncotarget.20409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. De Santi C, Melaiu O, Bonotti A, Cascione L, Di Leva G, Foddis R, et al. Deregulation of miRNAs in malignant pleural mesothelioma is associated with prognosis and suggests an alteration of cell metabolism. Sci Rep. 2017;7:3140. 10.1038/s41598-017-02694-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ivanov SV, Goparaju CMV, Lopez P, Zavadil J, Toren‐Haritan G, Rosenwald S, et al. Pro‐tumorigenic effects of miR‐31 loss in mesothelioma. J Biol Chem. 2010;285(30):22809–22817. 10.1074/jbc.M110.100354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122(20):3589–3594. 10.1242/jcs.051011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Grosso S, Marini A, Gyuraszova K, Voorde JV, Sfakianos A, Garland GD, et al. The pathogenesis of mesothelioma is driven by a dysregulated translatome. Nat Commun. 2021;12(1):1–17. 10.1038/s41467-021-25173-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960–976. 10.1016/j.cell.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. James MF, Han S, Polizzano C, Plotkin SR, Manning BD, Stemmer‐Rachamimov AO, et al. NF2/Merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol. 2009;29(15):4250–4261. 10.1128/mcb.01581-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Altomare DA, You H, Xiao GH, Ramos‐Nino ME, Skele KL, de Rienzo A, et al. Human and mouse mesotheliomas exhibit elevated AKT/PKB activity, which can be targeted pharmacologically to inhibit tumor cell growth. Oncogene. 2005;24(40):6080–6089. 10.1038/sj.onc.1208744 [DOI] [PubMed] [Google Scholar]
- 71. Opitz I, Soltermann A, Abaecherli M, Hinterberger M, Probst‐Hensch N, Stahel R, et al. PTEN expression is a strong predictor of survival in mesothelioma patients. Eur J Cardiothorac Surg. 2008;33(3):502–506. 10.1016/j.ejcts.2007.09.045 [DOI] [PubMed] [Google Scholar]
- 72. Nastase A, Mandal A, Lu SK, Anbunathan H, Morris‐Rosendahl D, Zhang YZ, et al. Integrated genomics point to immune vulnerabilities in pleural mesothelioma. Sci Rep. 2021;11(1):1–15. 10.1038/s41598-021-98414-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chalhoub N, Baker SJ. PTEN and the PI3‐kinase pathway in cancer. Annu Rev Pathol. 2009;4:127–150. 10.1146/annurev.pathol.4.110807.092311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Graziani I, Eliasz S, De Marco MA, Chen Y, Pass HI, De May RM, et al. Opposite effects of Notch‐1 and Notch‐2 on mesothelioma cell survival under hypoxia are exerted through the Akt pathway. Cancer Res. 2008;68(23):9678–9685. 10.1158/0008-5472.CAN-08-0969 [DOI] [PubMed] [Google Scholar]
- 75. Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2 . J Biol Chem. 2002;277(23):20336–20342. 10.1074/jbc.M111899200 [DOI] [PubMed] [Google Scholar]
- 76. Blum W, Schwaller B. Calretinin is essential for mesothelioma cell growth/survival in vitro: a potential new target for malignant mesothelioma therapy? Int J Cancer. 2013;133(9):2077–2088. 10.1002/ijc.28218 [DOI] [PubMed] [Google Scholar]
- 77. Wörthmüller J, Blum W, Pecze L, Salicio V, Schwaller B. Calretinin promotes invasiveness and EMT in malignant mesothelioma cells involving the activation of the FAK signaling pathway. Oncotarget. 2018;9(91):36256–36272. 10.18632/oncotarget.26332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Saydan N, Salicio V, Cappelli‐Gotzos B, Gotzos V. Expression of calretinin in human mesothelioma cell lines and cell cycle analysis by flow cytometry. Anticancer Res. 2001;21(1A):181–188. [PubMed] [Google Scholar]
- 79. CALB2 calbindin 2 [Homo sapiens (human)] – Gene – NCBI. https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=ShowDetailView&TermToSearch=794. Accessed 5 Sep 2020.
- 80. Billing‐Marczak K, Buzanska L, Winsky L, Nowotny M, Rudka T, Isaacs K, et al. AP2‐like cis element is required for calretinin gene promoter activity in cells of neuronal phenotype differentiated from multipotent human cell line DEV. Biochim Biophys Acta Gene Struct Expr. 2002;1577(3):412–420. 10.1016/S0167-4781(02)00443-8 [DOI] [PubMed] [Google Scholar]
- 81. Billing‐Marczak K, Ziemińska E, Leśniak W, Łazarewicz JW, Kuźnicki J. Calretinin gene promoter activity is differently regulated in neurons and cancer cells. Role of AP2‐like cis element and zinc ions. Biochim Biophys Acta Gene Struct Expr. 2004;1678(1):14–21. 10.1016/j.bbaexp.2004.01.004 [DOI] [PubMed] [Google Scholar]
- 82. Zhang Y, Xia M, Jin K, Wang S, Wei H, Fan C, et al. Function of the c‐met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol Cancer. 2018;17(1):1–14. 10.1186/s12943-018-0796-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tolnay E, Kuhnen C, Wiethege T, König JE, Voss B, Müller KM. Hepatocyte growth factor/scatter factor and its receptor c‐met are overexpressed and associated with an increased microvessel density in malignant pleural mesothelioma. J Cancer Res Clin Oncol. 1998;124(6):291–296. 10.1007/s004320050171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mukohara T, Civiello G, Davis IJ, Taffaro ML, Christensen J, Fisher DE, et al. Inhibition of the met receptor in mesothelioma. Clin Cancer Res. 2005;11(22):8122–8130. 10.1158/1078-0432.CCR-05-1191 [DOI] [PubMed] [Google Scholar]
- 85. Sartore‐Bianchi A, Gasparri F, Galvani A, Nici L, Darnowski JW, Barbone D, et al. Bortezomib inhibits nuclear factor‐κB‐dependent survival and has potent in vivo activity in mesothelioma. Clin Cancer Res. 2007;13(19):5942–5951. 10.1158/1078-0432.CCR-07-0536 [DOI] [PubMed] [Google Scholar]
- 86. Yang H, Bocchetta M, Kroczynska B, Elmishad AG, Chen Y, Liu Z, et al. TNF‐α inhibits asbestos‐induced cytotoxicity via a NF‐κB‐dependent pathway, a possible mechanism for asbestos‐induced oncogenesis. Proc Natl Acad Sci USA. 2006;103(27):10397–10402. 10.1073/pnas.0604008103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Heckman CA, Mehew JW, Boxer LM. NF‐kB activates Bcl‐2 expression in t(14;18) lymphoma cells. Oncogene. 2002;21:3898–3908. 10.1038/sj/onc/1205483 [DOI] [PubMed] [Google Scholar]
- 88. Uematsu K, Kanazawa S, You L, He B, Xu Z, Li K, et al. Wnt pathway activation in mesothelioma: evidence of dishevelled overexpression and transcriptional activity of β‐catenin. Cancer Res. 2003;63(15):4547–4551. [PubMed] [Google Scholar]
- 89. BAP1 BRCA1 associated protein 1 [Homo sapiens (human)] – Gene – NCBI. https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=8314. Accessed 12 Jun 2023.
- 90. BAP1 – Ubiquitin carboxyl‐terminal hydrolase BAP1 – Homo sapiens (Human) – BAP1 gene & protein. https://www.uniprot.org/uniprot/Q92560. Accessed 13 Jun 2023.
- 91. BAP1 (BRCA1 associated protein‐1 (ubiquitin carboxy‐terminal hydrolase)). http://atlasgeneticsoncology.org/Genes/BAP1ID755ch3p21.html. Accessed 16 Jun 2023.
- 92. Jensen DE, Proctor M, Marquis ST, Gardner HP, Ha SI, Chodosh LA, et al. BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1‐mediated cell growth suppression. Oncogene. 1998;16(9):1097–1112. 10.1038/sj.onc.1201861 [DOI] [PubMed] [Google Scholar]
- 93. Greenberg RA, Sobhian B, Pathania S, Cantor SB, Nakatani Y, Livingston DM. Multifactorial contributions to an acute DNA damage response by BRCA1/BARD1‐containing complexes. Genes Dev. 2006;20(1):34–46. 10.1101/gad.1381306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nishikawa H, Wu W, Koike A, Kojima R, Gomi H, Fukuda M, et al. BRCA1‐associated protein 1 interferes with BRCA1/BARD1 RING heterodimer activity. Cancer Res. 2009;69(1):111–119. 10.1158/0008-5472.CAN-08-3355 [DOI] [PubMed] [Google Scholar]
- 95. Yu H, Mashtalir N, Daou S, Hammond‐Martel I, Ross J, Sui G, et al. The ubiquitin carboxyl hydrolase BAP1 forms a ternary complex with YY1 and HCF‐1 and is a critical regulator of gene expression. Mol Cell Biol. 2010;30(21):5071–5085. 10.1128/mcb.00396-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Campagne A, Lee MK, Zielinski D, Michaud A, le Corre S, Dingli F, et al. BAP1 complex promotes transcription by opposing PRC1‐mediated H2A ubiquitylation. Nat Commun. 2019;10(1):1–15. 10.1038/s41467-018-08255-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lee HS, Lee SA, Hur SK, Seo JW, Kwon J. Stabilization and targeting of INO80 to replication forks by BAP1 during normal DNA synthesis. Nat Commun. 2014;5(1):1–14. 10.1038/ncomms6128 [DOI] [PubMed] [Google Scholar]
- 98. Baymaz HI, Fournier A, Laget S, Ji Z, Jansen PWTC, Smits AH, et al. MBD5 and MBD6 interact with the human PR‐DUB complex through their methyl‐CpG‐binding domain. Proteomics. 2014;14(19):2179–2189. 10.1002/pmic.201400013 [DOI] [PubMed] [Google Scholar]
- 99. Farquhar N, Thornton S, Coupland SE, Coulson JM, Sacco JJ, Krishna Y, et al. Patterns of BAP1 protein expression provide insights into prognostic significance and the biology of uveal melanoma. J Pathol Clin Res. 2018;4(1):26–38. 10.1002/cjp2.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Carbone M, Adusumilli PS, Alexander HR, Baas P, Bardelli F, Bononi A, et al. Mesothelioma: scientific clues for prevention, diagnosis, and therapy. CA Cancer J Clin. 2019;69(5):402–429. 10.3322/caac.21572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pastorino Yyhpmemnip S. A subset of mesotheliomas with improved survival occurring in carriers of BAP1 and other germline mutations. J Clin Oncol. 2018;36:3485–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Baumann F, Flores E, Napolitano A, Kanodia S, Taioli E, Pass H, et al. Mesothelioma patients with germline BAP1 mutations have 7‐fold improved long‐term survival. Carcinogenesis. 2015;36(1):76–81. 10.1093/carcin/bgu227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Liu‐Smith F, Lu Y. Opposite roles of BAP1 in overall survival of uveal melanoma and cutaneous melanoma. J Clin Med. 2020;9(2):411. 10.3390/jcm9020411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ewens KG, Lalonde E, Richards‐Yutz J, Shields CL, Ganguly A. Comparison of germline versus somatic BAP1 mutations for risk of metastasis in uveal melanoma 11 medical and health sciences 1112 oncology and carcinogenesis. BMC Cancer. 2018;18(1):1172. 10.1186/s12885-018-5079-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hassan R, Morrow B, Thomas A, Walsh T, Lee MK, Gulsuner S, et al. Inherited predisposition to malignant mesothelioma and overall survival following platinum chemotherapy. Proc Natl Acad Sci USA. 2019;116:9008–9013. 10.1073/pnas.1821510116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yoshikawa Y, Sato A, Tsujimura T, Emi M, Morinaga T, Fukuoka K, et al. Frequent inactivation of the BAP1 gene in epithelioid‐type malignant mesothelioma. Cancer Sci. 2012;103(5):868–874. 10.1111/j.1349-7006.2012.02223.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Guazzelli A, Meysami P, Bakker E, Demonacos C, Giordano A, Krstic‐Demonacos M, et al. BAP1 status determines the sensitivity of malignant mesothelioma cells to gemcitabine treatment. Int J Mol Sci. 2019;20(2). 10.3390/IJMS20020429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Louw A, Panou V, Szejniuk WM, Meristoudis C, Chai SM, van Vliet C, et al. BAP1 loss by immunohistochemistry predicts improved survival to first‐line platinum and Pemetrexed chemotherapy for patients with pleural mesothelioma: a validation study. J Thorac Oncol. 2022;17(7):921–930. 10.1016/J.JTHO.2022.04.008 [DOI] [PubMed] [Google Scholar]
- 109. Terrié E, Coronas V, Constantin B. Role of the calcium toolkit in cancer stem cells. Cell Calcium. 2019;80:141–151. 10.1016/j.ceca.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 110. Sun C, Shui B, Zhao W, Liu H, Li W, Lee JC, et al. Central role of IP3R2‐mediated Ca2+ oscillation in self‐renewal of liver cancer stem cells elucidated by high‐signal ER sensor. Cell Death Dis. 2019;10(6):1–13. 10.1038/s41419-019-1613-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Inai K. Pathology of mesothelioma. Environ Health Prev Med. 2008;13:60–64. 10.1007/s12199-007-0017-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Porpodis K, Zarogoulidis P, Boutsikou E, Papaioannou A, Machairiotis N, Tsakiridis K, et al. Malignant pleural mesothelioma: current and future perspectives. J Thorac Dis. 2013;5(SUPPL.4):S397–S406. 10.3978/j.issn.2072-1439.2013.08.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kushitani K, Takeshima Y, Amatya VJ, Furonaka O, Sakatani A, Inai K. Differential diagnosis of sarcomatoid mesothelioma from true sarcoma and sarcomatoid carcinoma using immunohistochemistry. Pathol Int. 2008;58(2):75–83. 10.1111/j.1440-1827.2007.02193.x [DOI] [PubMed] [Google Scholar]
- 114. Yaziji H, Battifora H, Barry TS, Hwang HC, Bacchi CE, McIntosh MW, et al. Evaluation of 12 antibodies for distinguishing epithelioid mesothelioma from adenocarcinoma: identification of a three‐antibody immunohistochemical panel with maximal sensitivity and specificity. Mod Pathol. 2006;19(4):514–523. 10.1038/modpathol.3800534 [DOI] [PubMed] [Google Scholar]
- 115. Naso JR, Tsuji S, Churg A. HEG1 is a highly specific and sensitive marker of epithelioid malignant mesothelioma. Am J Surg Pathol. 2020;44:1143–1148. 10.1097/PAS.0000000000001469 [DOI] [PubMed] [Google Scholar]
- 116. Chapel DB, Husain AN, Krausz T. Immunohistochemical evaluation of nuclear 5‐hydroxymethylcytosine (5‐hmC) accurately distinguishes malignant pleural mesothelioma from benign mesothelial proliferations. Mod Pathol. 2019;32:376–386. 10.1038/s41379-018-0159-7 [DOI] [PubMed] [Google Scholar]
- 117. Churg A, Hwang H, Tan L, Qing G, Taher A, Tong A, et al. Malignant mesothelioma in situ. Histopathology. 2018;72(6):1033–1038. 10.1111/HIS.13468 [DOI] [PubMed] [Google Scholar]
- 118. Klebe S, Nakatani Y, Dobra K, Butnor KJ, Roden AC, Nicholson AG, et al. The concept of mesothelioma in situ, with consideration of its potential impact on cytology diagnosis. Pathology. 2021;53(4):446–453. 10.1016/J.PATHOL.2020.12.005 [DOI] [PubMed] [Google Scholar]
- 119. Kirschner MB, Pulford E, Hoda MA, Rozsas A, Griggs K, Cheng YY, et al. Fibulin‐3 levels in malignant pleural mesothelioma are associated with prognosis but not diagnosis. Br J Cancer. 2015;113(6):963–969. 10.1038/bjc.2015.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hollevoet K, Reitsma JB, Creaney J, Grigoriu BD, Robinson BW, Scherpereel A, et al. Serum mesothelin for diagnosing malignant pleural mesothelioma: an individual patient data meta‐analysis. J Clin Oncol. 2012;30(13):1541–1549. 10.1200/JCO.2011.39.6671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Johnen G, Gawrych K, Raiko I, Casjens S, Pesch B, Weber DG, et al. Calretinin as a blood‐based biomarker for mesothelioma. BMC Cancer. 2017;17(1):386. 10.1186/s12885-017-3375-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gee GV, Koestler DC, Christensen BC, Sugarbaker DJ, Ugolini D, Ivaldi GP, et al. Downregulated microRNAs in the differential diagnosis of malignant pleural mesothelioma. Int J Cancer. 2010;127(12):2859–2869. 10.1002/ijc.25285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Benjamin H, Lebanony D, Rosenwald S, Cohen L, Gibori H, Barabash N, et al. A diagnostic assay based on MicroRNA expression accurately identifies malignant pleural mesothelioma. J Mol Diagn. 2010;12(6):771–779. 10.2353/jmoldx.2010.090169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ortiz‐Quintero B. Cell‐free microRNAs in blood and other body fluids, as cancer biomarkers. Cell Prolif. 2016;49(3):281–303. 10.1111/cpr.12262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Moretti G, Aretini P, Lessi F, Mazzanti CM, Ak G, Metintaş M, et al. Liquid biopsies from pleural effusions and plasma from patients with malignant pleural mesothelioma: a feasibility study. Cancers (Basel). 2021;13(10):2445. 10.3390/CANCERS13102445/S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova‐Agadjanyan EL, et al. Circulating microRNAs as stable blood‐based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105(30):10513–10518. 10.1073/pnas.0804549105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bononi I, Comar M, Puozzo A, Stendardo M, Boschetto P, Orecchia S, et al. Circulating microRNAs found dysregulated in ex‐exposed asbestos workers and pleural mesothelioma patients as potential new biomarkers. Oncotarget. 2016;7(50):82700–82711. 10.18632/oncotarget.12408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Tsim S, Alexander L, Kelly C, Shaw A, Hinsley S, Clark S, et al. Serum proteomics and plasma Fibulin‐3 in differentiation of mesothelioma from asbestos‐exposed controls and patients with other pleural diseases. J Thorac Oncol. 2021;16(10):1705–1717. 10.1016/j.jtho.2021.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Baldini EH, Richards WG, Gill RR, Goodman BM, Winfrey OK, Eisen HM, et al. Updated patterns of failure after multimodality therapy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2015;149(5):1374–1381. 10.1016/j.jtcvs.2014.10.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Zhai Z, Ruan J, Zheng Y, Xiang D, Li N, Hu J, et al. Assessment of global trends in the diagnosis of mesothelioma from 1990 to 2017. JAMA Netw Open. 2021;4(8):e2120360. 10.1001/JAMANETWORKOPEN.2021.20360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Cancer statistics review, 1975–2017 – SEER statistics. https://seer.cancer.gov/archive/csr/1975_2017/. Accessed 23 Nov 2022.
- 132. Cinausero M, Rihawi K, Sperandi F, Melotti B, Ardizzoni A. Chemotherapy treatment in malignant pleural mesothelioma: a difficult history. J Thorac Dis. 2018;10(Suppl 2):S304–S310. 10.21037/jtd.2017.10.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ricciardi S, Cardillo G, Zirafa CC, Carleo F, Facciolo F, Fontanini G, et al. Surgery for malignant pleural mesothelioma: an international guidelines review. J Thorac Dis. 2018;10(Suppl 2):S285–S292. 10.21037/jtd.2017.10.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Treasure T, Lang‐Lazdunski L, Waller D, Bliss JM, Tan C, Entwisle J, et al. Extra‐pleural pneumonectomy versus no extra‐pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the mesothelioma and radical surgery (MARS) randomised feasibility study. Lancet Oncol. 2011;12(8):763–772. 10.1016/S1470-2045(11)70149-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Rodríguez Panadero F. Diagnóstico y tratamiento del mesotelioma pleural maligno. Arch Bronconeumol. 2015;51(4):177–184. 10.1016/j.arbres.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 136. Scherpereel A, Scherpereel A, Opitz I, Psallidas I, Glatzer M, Rigau D, et al. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur Respir J. 2020;55(6):1900953. 10.1183/13993003.00953-2019 [DOI] [PubMed] [Google Scholar]
- 137. Lim E, Darlison L, Edwards J, Elliott D, Fennell DA, Popat S, et al. Mesothelioma and Radical Surgery 2 (MARS 2): protocol for a multicentre randomised trial comparing (extended) pleurectomy decortication versus no (extended) pleurectomy decortication for patients with malignant pleural mesothelioma On behalf of MARS 2 Trialists. BMJ Open. 2020;10:e038892. 10.1136/bmjopen-2020-038892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ung YC, Yu E, Falkson C, Haynes AE, Stys‐Norman D, Evans WK. The role of radiation therapy in malignant pleural mesothelioma: a systematic review. Radiother Oncol. 2006;80(1):13–18. 10.1016/j.radonc.2006.06.002 [DOI] [PubMed] [Google Scholar]
- 139. Rosenzweig KE, Giraud P. Radiothérapie des mésothéliomes malins pleuraux. Cancer/Radiotherapie. 2017;21(1):73–76. 10.1016/j.canrad.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 140. Chi A, Liao Z, Nguyen NP, Howe C, Gomez D, Jang SY, et al. Intensity‐modulated radiotherapy after extrapleural pneumonectomy in the combined‐modality treatment of malignant pleural mesothelioma. J Thorac Oncol. 2011;6(6):1132–1141. 10.1097/JTO.0b013e3182199819 [DOI] [PubMed] [Google Scholar]
- 141. Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21(14):2636–2644. 10.1200/JCO.2003.11.136 [DOI] [PubMed] [Google Scholar]
- 142. Zalcman G, Mazieres J, Margery J, Greillier L, Audigier‐Valette C, Moro‐Sibilot D, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the mesothelioma avastin cisplatin pemetrexed study (MAPS): a randomised, controlled, open‐label, phase 3 trial. Lancet. 2016;387:1405–1414. [DOI] [PubMed] [Google Scholar]
- 143. Rotte A. Combination of CTLA‐4 and PD‐1 blockers for treatment of cancer. J Exp Clin Cancer Res. 2019;38(1):1–12. 10.1186/S13046-019-1259-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, et al. First‐line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open‐label, phase 3 trial. Lancet. 2021;397(10272):375–386. 10.1016/S0140-6736(20)32714-8 [DOI] [PubMed] [Google Scholar]
- 145. Meirson T, Pentimalli F, Cerza F, Baglio G, Gray SG, Correale P, et al. Comparison of 3 randomized clinical trials of frontline therapies for malignant pleural mesothelioma. JAMA Netw Open. 2022;5(3):e221490. 10.1001/jamanetworkopen.2022.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Messori A, Trippoli S. Current treatments for inoperable mesothelioma: indirect comparisons based on individual patient data reconstructed retrospectively from 4 trials. J Chemother. 2023;35:158–162. 10.1080/1120009X.2022.2061183 [DOI] [PubMed] [Google Scholar]
- 147. Kerrigan K, Jo Y, Chipman J, Haaland B, Puri S, Akerley W, et al. A real‐world analysis of the use of systemic therapy in malignant pleural mesothelioma and the differential impacts on overall survival by practice pattern. JTO Clin Res Rep. 2022;3(3):100280. 10.1016/J.JTOCRR.2022.100280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Yang L, Cao X, Li N, Zheng B, Liu M, Cai H. Cost‐effectiveness analysis of nivolumab plus ipilimumab versus chemotherapy as the first‐line treatment for unresectable malignant pleural mesothelioma. Ther Adv Med Oncol. 2022;14:17588359221116604. 10.1177/17588359221116604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ye ZM, Tang ZQ, Xu Z, Zhou Q, Li H. Cost‐effectiveness of nivolumab plus ipilimumab as first‐line treatment for American patients with unresectable malignant pleural mesothelioma. Front Public Health. 2022;10:947375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Munck C, Mordon SR, Scherpereel A, Porte H, Dhalluin X, Betrouni N. Intrapleural photodynamic therapy for mesothelioma: what place and which future? Ann Thorac Surg. 2015;99(6):2237–2245. 10.1016/j.athoracsur.2014.12.077 [DOI] [PubMed] [Google Scholar]
- 151. van Zandwijk N, Pavlakis N, Kao SC, Linton A, Boyer MJ, Clarke S, et al. Safety and activity of microRNA‐loaded minicells in patients with recurrent malignant pleural mesothelioma: a first‐in‐man, phase 1, open‐label, dose‐escalation study. Lancet Oncol. 2017;18(10):1386–1396. 10.1016/S1470-2045(17)30621-6 [DOI] [PubMed] [Google Scholar]
- 152. Ur Rehman SI, Haaris SM. Oncolytic viral therapy. J Pak Med Assoc. 2017;67(4):658. 10.1097/01.cot.0000669692.29645.ac [DOI] [PubMed] [Google Scholar]
- 153. Pease DF, Kratzke RA. Oncolytic viral therapy for mesothelioma. Front Oncol. 2017;7:179. 10.3389/fonc.2017.00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Acuna SA, Ottolino‐Perry K, Çako B, Tang N, Angarita FA, McCart JA. Oncolytic vaccinia virus as an adjuvant treatment to cytoreductive surgery for malignant peritoneal mesothelioma. Ann Surg Oncol. 2014;21(7):2259–2266. 10.1245/s10434-014-3651-4 [DOI] [PubMed] [Google Scholar]


