Abstract
Atrial Fibrillation (AF) and gastrointestinal (GI) cancers are age-related diseases with shared environmental risk factors and underlying biological mechanisms. This study aimed to assess the association between AF and GI cancers on a global scale, analyzing incidence data from 204 countries. This ecological study utilized data from the Global Burden of Disease. Spearman's correlation and logistic regression analyses were employed to assess the association between AF and specific GI cancers, including esophagus cancer (EC), colon and rectum cancer (CRC), liver cancer (LC), pancreatic cancer (PC), and stomach cancer (SC). AF, CRC and PC exhibited increasing crude incidence rates from 2000 to 2019, whereas EC and SC demonstrated decreasing trends specifically in females. From 2000 to 2010, there was a noticeable fall in the incidence rate of LC, which was followed by a minor growth through 2019. The age-standardized incidence rate (ASIR) of AF was positively correlated with CRC and PC, but a negative relationship with AF was revealed for EC. Unexpectedly, no significant relationship was discovered for SC and LC associated with AF. Logistic regression analysis revealed a positive correlation between a country's ASIR of AF and its ASIR of CRC, LC and PC. Conversely, these countries demonstrated a decreased ASIR for EC. Our findings showed a significant correlation between national incidence rates of AF with CRC and PC, worldwide. Countries with higher ASIR of AF had higher ASIR of CRC and PC. Additional research is necessary to confirm the association between GI cancers and AF at the individual level.
Keywords: Atrial fibrillation, Gastrointestinal cancers, Incidence, Global burden of disease, Association
1. Introduction
Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia, impacting nearly 60 million individuals globally [1]. The total burden is predicted to increase by approximately 60 % by 2050(2). AF carries an elevated risk of stroke, dementia, heart failure, and mortality [3]. Apart from aging, several cardiovascular conditions, including hypertension, valvular disease, and heart failure, are well-documented risk factors for AF development [2,4]. Similarly, non-cardiovascular factors such as diabetes, obesity, obstructive sleep apnea, surgical history, and alcohol consumption have been established as contributors to AF etiology [2,4]. At the same time, cancer also is a significant health burden globally, with an anticipated 23.6 million new cases and 10.0 million deaths in 2019 [5]. Cardiovascular disease ranks as the second most prevalent cause of morbidity and mortality for cancer survivors [6].
Growing scientific evidence suggests a bidirectional association between AF and cancer [[7], [8], [9], [10]]. Age, physical inactivity, dietary habits, hypertension, diabetes mellitus, and obesity are among the shared risk factors for these two diseases [2,11,12]. Systemic inflammation and autonomic dysfunction seem to contribute to this interrelationship [7]. Gastrointestinal (GI) cancers represent a significant and growing contribution to the global cancer burden, with projections indicating future increases in both new cases and mortality rates [13]. Nonetheless, the relationship between AF and GI cancers remains unexplored in existing research.
The present ecological study aimed to elucidate the relationship between five major GI cancers and AF by analyzing incidence data from 204 countries, sourced from the 2019 Global Burden of Disease (GBD) study.
2. Materials and methods
2.1. Data source
The GBD 2019, initiated by the Institute for Health Metrics and Evaluation, offers a detailed analysis of the epidemiological landscape of 369 diseases and injuries across 204 countries and territories between 1990 and 2019. The general methodology employed in the GBD 2019 study has been previously published [14]. Data about the incidence of AF and the major GI cancers were obtained from the Global Health Data Exchange website (http://ghdx.healthdata.org/gbdresults-tool). Furthermore, to reflect the uncertainty in the incidence estimate, a 95 % uncertainty interval (UI) was derived alongside the point estimate. In the GBD Study, this UI was determined by selecting the 2.5th and 97.5th ordered values from 1000 random draws of the posterior distribution.
This study encompassed all forms of atrial flutter and AF, including paroxysmal, persistent, and permanent/chronic subtypes. The GI cancers investigated were colon and rectum cancer (CRC), esophagus cancer (EC), liver cancer (LC), pancreatic cancer (PC), and stomach cancer (SC). Furthermore, we analyzed four distinct subgroups of LC: LC due to hepatitis B (LC_B), hepatitis C (LC_C), alcohol use (LC_alcohol), and non-alcoholic steatohepatitis (LC_NASH), to explore their individual relationships with AF. The analysis variables comprised the year of estimation (2000, 2010, and 2019) and gender (male and female).
2.2. Statistical analysis
The following incidence indicators were employed in the current study: absolute incidence number, incidence rate, and age-standardized incidence rate (ASIR). The incidence rate refers to the count of new cases within a specific population, typically expressed as the number per 100,000 people. To account for variations in the age structure of the population over time and to facilitate comparisons, the age-standardized incidence rate (ASIR) per 100,000 people was used to assess differences in the burden of disease across historical periods, genders, and geographic locations. ASIR was calculated by a direct standardization method, with basing on the global population of GBD(14).
A Spearman correlation test assessed the relationship between AF and GI cancers, expressed as a correlation coefficient (r) with corresponding 95 % confidence intervals (CIs). Countries and territories were then dichotomized into two groups based on the median value of the ASIR of AF and GI cancers: low- and high-ASIR countries. Logistic regression analysis evaluated the influence of GI cancers on elevated ASIR of AF at the national level, presenting results as odds ratios (ORs) with 95 % CIs. Statistical analyses were performed using R, version 4.0.3, with statistical significance set at a p-value <0.05.
3. Results
Globally, 4,720,324 (95 % UI 3,644,331 to 5,961,597) new cases for AF, 534,563 (95 % UI 466,513 to 595,342) new cases for EC, 1,269,806 (95 % UI 1,150,487 to 1,399,817) new cases for SC, 2,166,168 (95 % UI 1,996,298 to 2,342,842) new cases for CRC, 530,297 (95 % UI 486,175 to 573,635) new cases for PC and 534,364 (95 % UI 486,550 to 588,639) new cases for LC were reported in 2019 (Fig. 1). Males consistently presented a higher likelihood of being affected compared to females (Fig. 1). Among new cases of LC, 40.96 % were secondary to hepatitis B, 28.49 % to hepatitis C, 18.43 % to alcohol use, and 6.80 % to NASH (Fig. 1B and Fig. 1C).
Fig. 1.
New cases of AF and gastrointestinal cancers in 2019 are presented. The number of new cases of AF and GI cancers for both genders combined. in 2019 (A). The proportion of new cases of different subgroups of LC, for males (B) and females (C). Abbreviation: AF: atrial fibrillation; CRC: colon and rectum cancer; EC: esophagus cancer; LC: liver cancer; PC: pancreatic cancer; SC: stomach cancer; LC_alcohol: LC due to alcohol use; LC_B: LC due to hepatitis B; LC_C: LC due to hepatitis c; LC_NASH: LC due to non-alcoholic steatohepatitis; LC_ other: LC due to other.
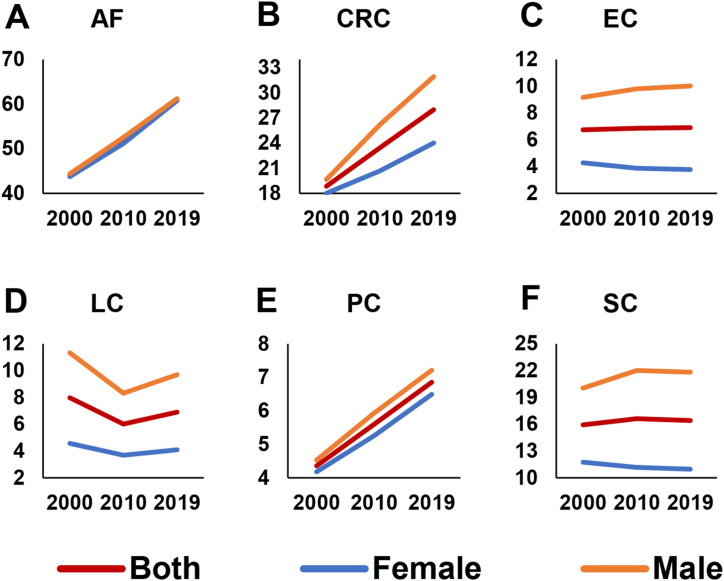
Global crude incidence rates of AF exhibited an upward trend for both sexes in the years 2000, 2010, and 2019 (Fig. 2). Similarly, CRC and PC incidence rates followed an increasing trend (Fig. 2). In contrast, incidence rates of EC and SC demonstrated a decreasing trend for females (Fig. 2). From 2000 to 2010, there was a noticeable fall in the incidence rate of LC, which was followed by a minor growth through 2019. Meanwhile, similar trends were seen in the four subgroups of LC (Fig. 3).
Fig. 2.
Crude incidence rate of AF and gastrointestinal cancers for both genders in the years 2000, 2010 and 2019. Abbreviation: AF: atrial fibrillation; CRC: colon and rectum cancer; EC: esophagus cancer; LC: liver cancer; PC: pancreatic cancer; SC: stomach cancer.
Fig. 3.
Crude incidence rate of different subgroups of LC for both genders in the years 2000, 2010 and 2019. Abbreviation: LC: liver cancer; LC_alcohol: LC due to alcohol use; LC_B: LC due to hepatitis B; LC_C: LC due to hepatitis c; LC_NASH: LC due to non-alcoholic steatohepatitis.
Associating national ASIR for AF and GI cancers in 204 countries in 2019, AF was positively correlated with CRC 0.619 (r = 0.619, 95 % CI: 0.455, 0.784; P < 0.001) and PC (r = 2.529, 95 % CI: 1.768, 3.290; P < 0.001), but a negative relationship with AF was revealed for EC (r = −1.106, 95 % CI: −1.761, −0.451; P = 0.001) (Fig. 4). Unexpectedly, no significant correlation was observed between SC and AF (r = −0.147, 95 % CI: −0.583, 0.289; p = 0.506) or between LC and AF (r = 0.133, 95 % CI: −0.172, 0.439; p = 0.392). As the development of LC is primarily attributed to four distinct causes, we proceeded to investigate the relationship between AF and the various pathogenetic subtypes of LC. A positive correlation was observed between AF and LC_alcohol(r = 1.169, 95 % CI: 0.077, 2.262; P = 0.036). However, no significant correlation was found with LC_B (r = −0.011, 95 % CI: −0.848, 0.826; p = 0.979), LC_C (r = 0.370, 95 % CI: −0.571, 1.312; p = 0.439), or LC_NASH (r = 0.879, 95 % CI: −3.029, 4.787; p = 0.658) (Fig. 5).
Fig. 4.
Association between AF and gastrointestinal cancers in 2019. Abbreviation: AF: atrial fibrillation; CRC: colon and rectum cancer; EC: esophagus cancer; LC: liver cancer; PC: pancreatic cancer; SC: stomach cancer.
Fig. 5.
Association between AF and different subgroups of LC in the year 2019. Abbreviation: AF: atrial fibrillation; LC: liver cancer; LC_alcohol: LC due to alcohol use; LC_B: LC due to hepatitis B; LC_C: LC due to hepatitis c; LC_NASH: LC due to non-alcoholic steatohepatitis.
Further analysis using logistic regression revealed that countries with high ASIR of AF exhibited significantly higher ASIR of CRC (OR = 4.000, 95 % CI: 2.235, 7.160; p < 0.001), LC (OR = 2.609, 95 % CI: 1.483, 4.590; p = 0.001), and PC (OR = 2.609, 95 % CI: 1.483, 4.590; p = 0.001). Conversely, these countries demonstrated a decreased ASIR of EC (OR = 0.510, 95 % CI: 0.292, 0.890; p = 0.018) (Fig. 6). In addition, a positive correlation was observed between high ASIR of AF and elevated ASIR of LC_alcohol (OR = 4.000, 95 % CI: 2.235, 7.160; p < 0.001) as well as LC_C (OR = 3.361, 95 % CI: 1.893, 5.969; p < 0.001) (Fig. 6).
Fig. 6.
Association between AF and gastrointestinal cancers at national level by the logistic regression analyses. Abbreviation: AF: atrial fibrillation; CRC: colon and rectum cancer; EC: esophagus cancer; LC: liver cancer; PC: pancreatic cancer; SC: stomach cancer; LC_alcohol: LC due to alcohol use; LC_B: LC due to hepatitis B; LC_C: LC due to hepatitis c; LC_NASH: LC due to non-alcoholic steatohepatitis.
It was worth noting that the intensities of the association between the ASIR of AF and GI cancer consistently diminished between 2000 and 2019 (Fig. 7). The correlation coefficient between AF and CRC decreased from 0.738 (95 % CI: 0.581, 0.895; p < 0.001) in 2000 to 0.619 (95 % CI: 0.455, 0.784; p < 0.001) in 2019. Similarly, the correlation coefficient between AF and PC decreased from 3.032 (95 % CI: 2.273, 3.791; p < 0.001) to 2.529 (95 % CI: 1.768, 3.290; p < 0.001) over the same period. In contrast, the correlation coefficient between AF and EC increased from −0.815 (95 % CI: −1.342, −0.288; p = 0.003) to −1.106 (95 % CI: −1.761, −0.451; p = 0.001) between 2000 and 2019.
Fig. 7.
Comparison of correlation coefficients (r) of AF and gastrointestinal cancers between 2000 and 2019. Abbreviation: AF: atrial fibrillation; CRC: colon and rectum cancer; EC: esophagus cancer; LC: liver cancer; PC: pancreatic cancer; SC: stomach cancer; LC_alcohol: LC due to alcohol use; LC_B: LC due to hepatitis B; LC_C: LC due to hepatitis c; LC_NASH: LC due to non-alcoholic steatohepatitis.
4. Discussion
This study investigated the relationship between the incidence of AF and GI cancers based on GBD study 2019. Previous research has established an association between AF and GI cancers [7,9,10]. To the best of our knowledge, our findings provided novel evidence suggesting a positive correlation between the ASIR of AF and the ASIR of CRC and PC. Specifically, countries with higher ASIR of AF consistently presented higher ASIR of CRC and PC.
Some theories may explain the positive correlation between AF and CRC and PC. Firstly, this relationship could be mediated by systemic inflammation because inflammatory markers have been shown to be elevated in both AF and cancer [8,15]. Secondly, autonomic dysfunction is the common pathophysiological background of AF and cancer. A disorder of sympathetic and parasympathetic system activities is associated with AF [16], and cancer patients also exhibit signs of altered autonomic activity or dysfunction [17,18]. Thirdly, another mechanism causing the development of AF is pulmonary microembolism due to hypercoagulability in the neoplastic state. Lastly, AF and cancer share several risk factors, including age, physical inactivity, dietary habit, hypertension, diabetes mellitus, and obesity [2,11,12]. Therefore, the statistical association is biologically reasonable, and further investigation into its mechanisms is required.
The strength of the association between the ASIR of AF and CRC and PC consistently decreased from 2000 to 2019. This decreasing trend may be attributed to improvements in the therapeutic management of AF, particularly in developed nations, coupled with a significant rise in the incidence of GI cancers in developing countries [11,19].
Different from previous studies [9,10], results from this study demonstrated that the ASIR of AF was negatively correlated with the ASIR of EC. In addition, no significant correlation between AF and SC and LC was found in this study. It is likely that methodological differences between studies contribute to the disparate findings. Therefore, this result should be interpreted with caution, and more studies in this field are warranted.
LC_alcohol exhibited a positive association with AF. Alcohol consumption is a well-established co-risk factor for the development of AF [20, 21]. Long-term alcohol intake induces progressive atrial structural remodeling [22], and acute alcohol consumption exerts direct cellular effects on atrial myocytes and influences autonomic nervous function, creating an electrophysiological environment conducive to AF initiation and maintenance [22]. Habitual alcohol consumption increases the likelihood of developing AF through its direct impact on the left atrial substrate and its interaction with other risk factors, such as hypertension, left ventricular dysfunction and obstructive sleep apnea [22].
Given the positive association between the ASIR of AF and CRC and PC, clinicians are likely to conduct more intensive screening for these subgroups. However, the impact of regular screening on clinical outcomes remains uncertain. Future research should aim to address this knowledge gap and explore the relationship between AF and the risk of adverse cardiovascular events, as well as optimal management strategies.
Given the positive association between the ASIR of AF and CRC and PC, clinicians are likely to conduct more intensive screening for these subgroups. However, it is still not clear whether regular screening could improve clinical outcomes. Future research must address this dilemma. Additionally, current knowledge gaps exist regarding the relationship between AF and the risk of subsequent adverse cardiovascular events. The optimal management strategies for these patients also require further elucidation.
Several limitations should be properly acknowledged. Firstly, the study may suffer from what is known as the ecological fallacy, which does not allow conclusions regarding individual risk to be drawn from aggregate data [23]. The unit of analysis for this study was the country as a collective, rather than the individual. Therefore, the findings may lack generalizability to the entire population of AF patients with GI cancers. Another limitation of the current study pertains to variations in the recording of disease burden across countries and regions, which may differ in terms of both quality and quantity. The study utilized GBD cross-sectional data and did not have the ability to evaluate the causal relationship between GI cancers and AF. Moreover, this study does not include an estimation of potential confounding variables that may affect the interaction between GI cancers and AF. Lastly, due to the database's nature, it does not offer detailed information on cancer types beyond broader classifications. Consequently, our study was unable to differentiate between specific types of GI cancers, such as adenocarcinoma or squamous cell carcinoma. This limitation restricted our ability to conduct subgroup analyses or discuss potential differences in associations based on histological variations. Future research should utilize more extensive pathological datasets to gain a nuanced understanding of the complex relationship between AF and various GI cancers, as this is a critical aspect that requires further exploration. Despite these limitations, this study is the first to demonstrate the correlation between the global incidence of GI cancers and AF at the country level.
Our findings showed a significant correlation between national incidence rates of AF with CRC and PC, worldwide. Countries with higher ASIR of AF had higher ASIR of CRC and PC. Although epidemiological data help to highlight unmet needs for disease management, additional research is necessary to confirm the association of GI cancers and AF at an individual level.
Funding
No funding was received.
Data availability statement
The data underpinning this article originate from publicly available sources: http://ghdx.healthdata.org/gbd-results-tool.
CRediT authorship contribution statement
Weipeng Huang: Software, Methodology, Conceptualization. Shangbo Xu: Writing – original draft, Data curation. Haoyue Zhou: Visualization, Investigation. Weibiao Ji: Validation, Software. Yangbo Chen: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We extend our gratitude to the Institute for Health Metrics and Evaluation staff and their collaborators for making these data publicly available.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e29929.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Roth G.A., Mensah G.A., Johnson C.O., et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akboga M.K., Inanc I.H., Keskin M., Sabanoglu C., Gorenek B. Current evidence on prevention of atrial fibrillation: modifiable risk factors and the effects of risk factor intervention. Cardiol. Rev. 2023;31:70–79. doi: 10.1097/CRD.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 3.Weng L.C., Choi S.H., Klarin D., et al. Heritability of atrial fibrillation. Circ. Cardiovasc. Genet. 2017;10 doi: 10.1161/CIRCGENETICS.117.001838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen J.H., Andreasen L., Olesen M.S. Atrial fibrillation-a complex polygenetic disease. Eur. J. Hum. Genet. : EJHG (Eur. J. Hum. Genet.) 2021;29:1051–1060. doi: 10.1038/s41431-020-00784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kocarnik J.M., Compton K., Dean F.E., et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the global burden of disease study 2019. JAMA Oncol. 2022;8:420–444. doi: 10.1001/jamaoncol.2021.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mertens A.C., Liu Q., Neglia J.P., et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zubair Khan M., Gupta A., Patel K., et al. Association of atrial fibrillation and various cancer subtypes. J. Arrhythmia. 2021;37:1205–1214. doi: 10.1002/joa3.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan M., Zhang Z., Tse G., et al. Association of cancer and the risk of developing atrial fibrillation: a systematic review and meta-analysis. Cardiol. Res. Pract. 2019;2019 doi: 10.1155/2019/8985273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jakobsen C.B., Lamberts M., Carlson N., et al. Incidence of atrial fibrillation in different major cancer subtypes: a Nationwide population-based 12 year follow up study. BMC Cancer. 2019;19:1105. doi: 10.1186/s12885-019-6314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yun J.P., Choi E.K., Han K.D., et al. Risk of atrial fibrillation according to cancer type: a nationwide population-based study. JACC CardioOncol. 2021;3:221–232. doi: 10.1016/j.jaccao.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu L., Mullins C.S., Schafmayer C., Zeißig S., Linnebacher M. A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun. 2021;41:1137–1151. doi: 10.1002/cac2.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mysuru Shivanna L., Urooj A. A review on dietary and non-dietary risk factors associated with gastrointestinal cancer. J. Gastrointest. Cancer. 2016;47:247–254. doi: 10.1007/s12029-016-9845-1. [DOI] [PubMed] [Google Scholar]
- 13.Arnold M., Abnet C.C., Neale R.E., et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159:335–349.e315. doi: 10.1053/j.gastro.2020.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diseases GBD and Injuries C: Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Y., Lip G.Y., Apostolakis S. Inflammation in atrial fibrillation. J. Am. Coll. Cardiol. 2012;60:2263–2270. doi: 10.1016/j.jacc.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 16.Xi Y., Cheng J. Dysfunction of the autonomic nervous system in atrial fibrillation. J. Thorac. Dis. 2015;7:193–198. doi: 10.3978/j.issn.2072-1439.2015.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamiya A., Hiyama T., Fujimura A., Yoshikawa S. Sympathetic and parasympathetic innervation in cancer: therapeutic implications. Clin. Auton. Res. : official J. Clin. Autonomic Res. Soc. 2021;31:165–178. doi: 10.1007/s10286-020-00724-y. [DOI] [PubMed] [Google Scholar]
- 18.Martin R., Delgado J.M., Molto J.M., et al. Cardiovascular reflexes in patients with malignant disease. Ital. J. Neurol. Sci. 1992;13:125–129. doi: 10.1007/BF02226960. [DOI] [PubMed] [Google Scholar]
- 19.Karamitanha F., Ahmadi F., Fallahabadi H. Difference between various countries in mortality and incidence rate of the atrial fibrillation based on human development index in worldwide: data from global burden of disease 2010-2019. Curr. Probl. Cardiol. 2023;48 doi: 10.1016/j.cpcardiol.2022.101438. [DOI] [PubMed] [Google Scholar]
- 20.Csengeri D., Sprünker N.A., Di Castelnuovo A., et al. Alcohol consumption, cardiac biomarkers, and risk of atrial fibrillation and adverse outcomes. Eur. Heart J. 2021;42:1170–1177. doi: 10.1093/eurheartj/ehaa953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang H., Mei X., Jiang Y., et al. Alcohol consumption and atrial fibrillation risk: an updated dose-response meta-analysis of over 10 million participants. Front. Cardiovasc. Med. 2022;9 doi: 10.3389/fcvm.2022.979982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voskoboinik A., Prabhu S., Ling L.H., Kalman J.M., Kistler P.M. Alcohol and atrial fibrillation: a Sobering review. J. Am. Coll. Cardiol. 2016;68:2567–2576. doi: 10.1016/j.jacc.2016.08.074. [DOI] [PubMed] [Google Scholar]
- 23.Sedgwick P. Ecological studies: advantages and disadvantages. BMJ (Clinical research ed) 2014;348:g2979. doi: 10.1136/bmj.g2979. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underpinning this article originate from publicly available sources: http://ghdx.healthdata.org/gbd-results-tool.