Abstract
Although the pathogenesis of endometriosis is not fully understood, it is often considered to be an inflammatory disease. An increasing number of studies suggest that differential expression of anti-inflammatory cytokines (e.g., interleukin-4 and -10, and transforming growth factor-β1) occurs in women with endometriosis, including in serum, peritoneal fluid and ectopic lesions. These anti-inflammatory cytokines also have indispensable roles in the progression of endometriosis, including by promoting survival, growth, invasion, differentiation, angiogenesis, and immune escape of the endometriotic lesions. In this review, we provide an overview of the expression, origin, function and regulation of anti-inflammatory cytokines in endometriosis, with brief discussion and perspectives on their future clinical implications in the diagnosis and therapy of the disease.
Keywords: Endometriosis, Cytokine, Anti-inflammatory, IL-10, TGF-β, Endometrial stromal cells, Macrophage, T cells, NK cells
Introduction
Endometriosis is one of the most common gynecological diseases. It is a chronic disorder that affects 10% of all women of reproductive age, characterized by the presence of endometrial tissue outside the uterine cavity [1]. This disease can cause long-term chronic pelvic pain, dysmenorrhea, severe dyspareunia, infertility and pelvic-organ dysfunction [2]. Despite improvements in the diagnosis and understanding of endometriosis, many patients with endometriosis suffer due to ineffective non-invasive diagnostic methods and treatments, which are often associated with multiple side effects and high-recurrence rates [3, 4]. Therefore, endometriosis profoundly impairs the quality of life of the patients with a negative impact on social and family life, and high-healthcare costs [5, 6].
Although the pathophysiology of endometriosis is not fully understood, accumulating evidence indicates that combinations of hormonal, immunologic, genetic and environmental factors are involved in the origin and development of endometriosis [1]. Evidence suggests that aberrant immunologic and inflammatory responses, in particular, increased levels of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6 and IL-17A, are present in the serum, peritoneal fluid (PF) and ectopic lesions of patients with endometriosis [7–16]. Due to the crucial role of inflammatory immune responses in the development and progression of endometriosis, this disease is considered to be an immune-related chronic inflammatory disease [17–19]. Subsequently, these pro-inflammatory cytokines have been investigated as possible non-invasive serum biomarkers for diagnosis [13, 14, 20–22] or potential targets for treatment of endometriosis [23–29], although certain findings have been contradictory [30–33].
The human immune response is mediated by a highly complex network of regulatory elements. Among these, anti-inflammatory cytokines are a series of immunoregulatory molecules that control the pro-inflammatory cytokine response [34]. Under physiological conditions, anti-inflammatory cytokines limit the potentially damaging effects of sustained or excessive inflammatory reactions. Under pathological conditions, anti-inflammatory mediators may have insufficient control over pro-inflammatory activities in immune-mediated diseases, or overcompensate and inhibit the immune response, increasing the risk of systemic infection.
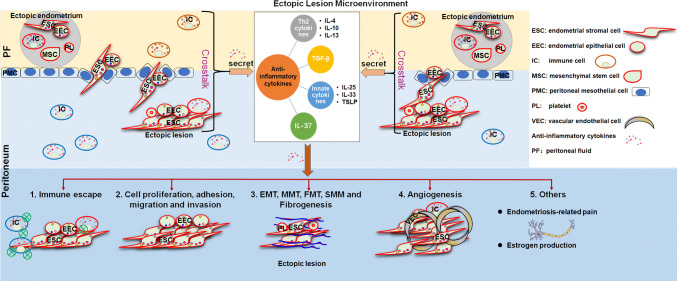
As early as the 1990s, studies reported that anti-inflammatory cytokines (such as IL-10 and IL-4) were also elevated in the peripheral blood and PF of patients with endometriosis [35–37]; however, this did not receive widespread attention. In the past 10 years, the differential expression of these anti-inflammatory cytokines and their role in the pathogenesis of endometriosis has been gradually revealed. From an immunological viewpoint, studies have indicated that endometriosis is not just an inflammatory disorder, but a disease associated with pro-inflammation and anti-inflammation activity. Therefore, the aim of this review is to discuss the changes, cell and tissue origin, regulatory factors and role of anti-inflammatory cytokines associated with endometriosis, and in particular, the communication between endometrial cells and immune cells in the ectopic lesion microenvironment (Fig. 1).
Fig. 1.
Schematic presentation of the anti-inflammatory cytokines that contribute to development of endometriosis. In the endometriotic microenvironment, endometrial cells, peritoneal mesothelial cells and various immune cells (such as macrophages, and T and natural killer cells) produce anti-inflammatory cytokines (e.g., IL-4, IL-10 and TGF-β1), which have a critical role in accelerating the growth, adhesion, invasion, epithelial-mesenchymal transition (EMT), mesothelial-to-mesenchymal transition (MMT), fibroblast-to-myofibroblast transdifferentiation (FMT), smooth muscle metaplasia (SMM), fibrogenesis and angiogenesis of ectopic lesions, and impairment of immune surveillance
Anti-inflammatory cytokines
T helper (TH) 2 cytokines
IL-4 and IL-10 family proteins are the main Th2 anti-inflammatory cytokines. Several lines of evidence indicate that the Th2 immune response is associated with endometriosis [38, 39]. As shown in Table 1, accumulating evidence has demonstrated that anti-inflammatory cytokines IL-4 [38–41] and IL-10 [40, 42–46] are sharply increased in peripheral lymphocytes and the ectopic endometrium of women with endometriosis. Notably, a significant increase in IL-4 [44, 47, 48] and IL-10 levels [39, 46, 48–55] in PF from women with endometriosis was observed, particularly in cases of advanced endometriosis [44, 48, 50, 53, 54]; however, three other research groups reported no change in IL-4 and/or IL-10 in the serum and PF of endometriosis patients [56–58]. Inconsistencies may be due to differences in patient selection, such as whether the patients were infertile or not and whether there was variation endometriosis subtypes (ovarian endometrioma, superficial peritoneal endometriosis, deep infiltrating endometriosis). Additionally, adolescents with endometriosis were reported to have significantly increased IL-4 in serum and PF, suggesting that IL-4 could be a potential biomarker for identifying endometriosis [47]. Furthermore, IL-10 promoter 592A/C and 819T/C polymorphisms have been reported to be associated with endometriosis risk in a Chinese population [51, 59], and considered to increase susceptibility to advanced endometriosis [43]. As a type of Th2 cytokine, IL-13 shares homology with IL-4, interacts with IL-4 receptor and attenuates monocyte/macrophage function (downregulates the production of TNF-α, IL-1 and IL-8 by monocytes) [60]. Chegini et al. have reported that IL-13 is elevated in the endometriotic endometrium and PF in patients with endometriosis [61]; however, no difference in IL-13 in the peripheral blood [14, 62] and PF [49, 63] was observed in endometriosis patients. Differences in the clinical staging of disease and the phases of the menstrual cycle in patients may be factors that cause the inconsistent conclusions among different studies.
Table 1.
The change of anti-inflammatory cytokines in endometriosis
| Classification | Cytokine | Location | Change (EMS vs Control) | References |
|---|---|---|---|---|
| Th2 cytokine | IL-4 | Peripheral blood | ↑ | [38–41, 47] |
| NS | [46] | |||
| Peritoneal fluid | ↑ | [44, 47, 48] | ||
| NS | [46] | |||
| Ectopic lesion | ↑ | [38, 39] | ||
| IL-10 | Peripheral blood | ↑ | [40, 42–44, 46, 55] | |
| NS | [46, 57, 58] | |||
| Peritoneal fluid | ↑ | [39, 46, 48–54] | ||
| NS | [46, 57, 58] | |||
| Ectopic lesion | ↑ | [42, 45] | ||
| IL-13 | Peripheral blood | NS | [14, 40, 62] | |
| Peritoneal fluid | NS | [49, 63] | ||
| ↑ | [61] | |||
| Ectopic lesion | ↑ | [61] | ||
| Growth factor | TGF-β | Peripheral blood | ↓ | [46, 67] |
| Peritoneal fluid | ↑ | [46, 48, 54, 74–77] | ||
| Ectopic lesion | ↑ | [45, 48, 68–73] | ||
| Innate cytokine | IL-25 | Peripheral blood | ↑ | [97] |
| Peritoneal fluid | – | – | ||
| Ectopic lesion | – | – | ||
| IL-33 | Peripheral blood | ↑ | [95] | |
| Peritoneal fluid | ↑ | [95] | ||
| NS | [55] | |||
| Ectopic lesion | ↑ | [95, 96] | ||
| TSLP | Peripheral blood | ↑ | [94] | |
| Peritoneal fluid | ↑ | [94] | ||
| Ectopic lesion | – | – | ||
| Other cytokines | IL-1RA | Peripheral blood | ↑ | [40, 98] |
| NS | [99] | |||
| Peritoneal fluid | ↑ | [98] | ||
| ↓ | [99] | |||
| Ectopic lesion | – | – | ||
| IL-37 | Peripheral blood | ↑ | [44, 58, 104] | |
| Peritoneal fluid | ↑ | [58, 104] | ||
| Ectopic lesion | ↑ | [103] |
NS, no significant difference; –, no data
Transforming growth factor (TGF)-β
TGF-β is a multifunctional growth factor that has important regulatory effects on many developmental and physiological processes. TGF-β was initially identified as a potent chemotactic cytokine that initiates inflammation and its overexpression mediates inflammation in the skin [64]; however, the severe and uncontrolled inflammatory reactions observed in the TGF-β knockout mouse indicated that the physiological role of TGF-β is predominantly as an anti-inflammatory cytokine [65]. Its immunosuppressive functions include the inhibition or reversal of macrophage activation, resulting in downregulated release of pro-inflammatory cytokines, and reactive oxygen and nitrogen species [66]. Numerous studies have confirmed that the levels of TGF-β are significantly higher in the peripheral blood [46, 67], ectopic endometrium (e.g., endometriotic cystic wall) [45, 48, 68–73] and PF [46, 48, 54, 74–77] of patients with endometriosis than in controls.
IL-25, IL-33, and thymic stromal lymphopoietin (TSLP)
The endogenous cytokines IL-25 (or IL-17E) [78–80], IL-33 [81, 82], and TSLP [83–86] have a role in the maturation of Th2 cells via dendritic cell activation. IL-25 [87–89], IL-33 [88, 89], and TSLP [89, 90] also induce activation of and IL-13 production from innate immune cells, including type 2 innate lymphoid cells. These cytokines have important regulatory roles in allergic diseases, such as asthma [89]. Previous studies have reported a higher prevalence of allergic disease among women with endometriosis [91, 92]. Type 1 allergies develop in a Th2 cytokine environment. Increasing evidence has shown that IL-33 and TSLP are increased in the peripheral blood [93–95], endometriotic endometrium [93–96], and PF [94] of patients with endometriosis. Notably, IL-33 and soluble ST2 (sST2) levels in PF or endometriotic lesions were correlated with endometriosis severity [95, 96]. However, Jaeger-Lansky et al. observed a similar level of IL-33 in the PF between endometriosis and control individuals [55]; however, it should be noted that the PF collected in this study was a mixture of free fluid in the recto-uterine pouch and fluid from genital organs washed with saline solution [55]. One study reported that levels of IL-25 are higher in PF from endometriosis patients; however, IL-25 levels were not correlated with disease stage [97]. Unfortunately, IL-25 levels in peripheral and endometriotic lesions were not analyzed in this study.
IL-1 receptor antagonist (IL-1RA) and IL-37
The IL-1 family is the largest family of interleukins, which comprises 11 members, including pro-inflammatory agonists (IL-1β, IL-18 and IL-36), and defined or putative antagonists (IL-1RA and IL-37) with anti-inflammatory activities. An elevated level of IL-1R was detected in serum [40, 98] and PF [98] from patients with endometriosis. Opposingly, Zhang et al. reported that there was a lower level of IL-1RA in PF from endometriosis patients, particularly those with dysmenorrhea [99]. This conflicting result may be due to the different criteria for patient selection used between the studies; for example, all patients in the former study were infertile [98]. IL-37 inhibits innate and adaptive immune functions by reducing the expression of pro-inflammatory cytokines (TNF-α, IL-6 and IL-17) [100, 101], and its suppressive effect on inflammatory factor may be mediated by inhibition of nuclear factor-kappa B (NF-κB) [102]. IL-37 was reported to be highly expressed in eutopic and ectopic endometrium of women with ovarian endometriosis, particularly ectopic endometrium [103]. In addition, IL-37 was elevated in peripheral blood [44, 58, 104] and PF [58, 104] from patients with endometriosis.
As the disease progresses, the levels of pro-inflammatory cytokines in PF and/or ectopic lesions gradually increase, while anti-inflammatory cytokines tend to increase in the later stage of the disease [39, 48, 50, 95, 98, 104–106]; however, there is still a lack of larger datasets that have measured the concentration of pro-inflammatory and anti-inflammatory cytokines in endometriosis. If large sample studies are conducted, the dynamic changes of these pro-inflammatory and anti-inflammatory cytokines in disease progression may be elucidated. Although pro-inflammatory and anti-inflammatory cytokines are reported to increase in the peripheral blood of endometriosis patients, researchers still question their diagnostic value for endometriosis [32, 33]. Similarly, their use for disease diagnosis requires further assessment in large sample studies.
Origin
Immune cell
Endometriotic lesions have a higher concentration of T lymphocytes than the eutopic endometrium [19, 107]. Foxp3-expressing CD4+ regulatory T cells (Tregs) are an important subset of T lymphocytes with a crucial role in the maintenance of self-tolerance and immune homeostasis, and are involved in various human diseases, such as autoimmune diseases and cancer [108–110]. As the two key functional cytokines, IL-10 and TGF-β are secreted by Tregs in ectopic lesions and PF, and are significantly increased in patients with endometriosis (Fig. 2) [54, 74, 77, 111–113]. As opposed to pro-inflammatory interferon (IFN)-γ+Th17 cells, IL-10 production by Th17 cells is critical for limiting autoimmunity and inflammatory responses. This unique subset of Th17 cells simultaneously secretes IL-10 and IL-17A in the PF of women with endometriosis [48]. Macrophages are the most prevalent type of immune cells in the PF [114], and their number and activation [115], and their production of cytokines (such as IL-6, IL-10, IL-12 and TGF-β1) [77, 116] are increased in endometriosis. Co-culture of macrophages and endometrial stromal cells (ESCs) increases the production of IL-10 and TGF-β [48, 117–119] and further stimulates TGF-β secretion by Tregs [54, 113, 120]. As the classical allergy mediators, mast cells (MCs) are also present in high numbers in endometriotic lesions [121–123]. MCs and Th2 cells may be the most important sources of high IL-25 in PF [97]. Interestingly, a population of aryl hydrocarbon receptor-expressing MCs that express IL-17 and IL-10 has been recently identified in the endometrium [124]. Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immature myeloid cells that have a major role in immunosuppression in cancer, inflammation and other diseases [125, 126]. Increased MDSCs have been confirmed in peripheral blood, PF and endometriotic lesions of endometriosis patients by several groups [127–130]. IL-10, GM-CSF and ARG1 are important effectors secreted by MDSCs; therefore, further studies are required clarify whether MDSC are also associated with the high IL-10 level in endometriosis.
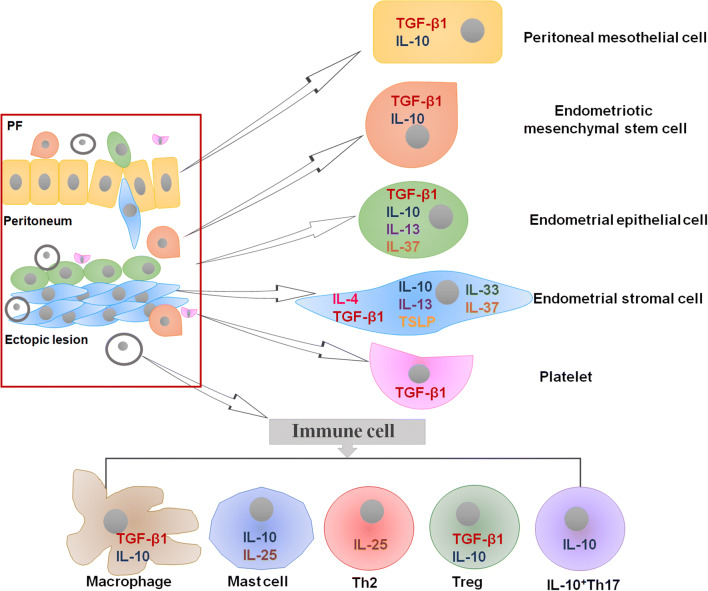
Fig. 2.
Schematic presentation of cell population and the origin of anti-inflammatory cytokines in endometriotic milieu. PF peritoneal fluid
ESCs/endometrial epithelial cells (EECs)
Immunohistochemical analysis has demonstrated that IL-4-positive cells accumulate around blood vessels in the stroma of endometriotic tissue [131]. IL-10 and TGF-β are highly expressed in ESCs [45, 48, 118, 119, 132] and EECs [72] from endometriotic endometrium, and EECs have been found to be the primary source of IL-13 expression [61]. Subsequently, the same research team confirmed that ESCs and EECs isolated from endometrium expressed IL-13 in primary culture [133], and TSLP is also produced and secreted by the isolated ESCs [94, 134, 135]. Our previous study demonstrated that endometriotic ESCs secrete high levels of IL-33 [118], which was further confirmed by a recent report [96]; however, no study has investigated the secretion of IL-25, IL-33 and TSLP by ECCs, which requires further research. Immunohistochemical staining revealed cytoplasmic and nuclear expression of IL-37 in endometrial glandular epithelial cells and stromal cells [103].
Endometriotic mesenchymal stem cells (MSCs)
The presence of regenerating healthy endometrial MSCs [136, 137], menstrual blood stromal stem cells (MenSCs) [138, 139] and endometriotic MSCs [139–142] has recently been reported, with the cell types exhibiting similar phenotypes. These endometrial MSCs are involved in the pathogenesis of endometriosis [139–142]. Reverse transcription-polymerase chain reaction analysis revealed that the anti-inflammatory cytokine TGF-β was significantly downregulated and IL-10 was significantly increased in endometriotic MSCs [143]. Another group recently reported that endometriotic MSCs secrete high levels of TGF-β [144]. Additionally, MenSCs are another source of IL-10 in endometriosis [138].
Peritoneal mesothelial cells (PMCs)
In women with endometriosis, peritoneal areas adjacent to endometriosis lesions express higher TGFB1 mRNA levels than distal sites [76]. Researchers also speculate that cells present in the peritoneum are a major source of IL-10 [50]. PMCs are the largest cell population within the peritoneal cavity. TGF-β1 protein is detectable in conditioned media from primary cultures of human peritoneal mesothelial cells (HPMCs) [76, 145], suggesting that the peritoneum, and in particular, the peritoneal mesothelium, is a source of TGF-β1, which is enhanced around endometriotic lesions [76, 146]. Shed menstrual tissue, ectopic endometrial cells and macrophages are thought to be additional sources of TGF-β1 present in the PF of women with endometriosis [147].
Platelets
Considering that ectopic endometrium experiences cyclical and repeated bleeding [148], a basic sign of tissue injury, Guo et al. recently reported that platelets have important roles in the development of endometriosis [149–151]. Activated platelets release TGF-β1 [152], and the activation of platelets in endometriosis is significantly higher than in controls [153]. Tanshinone IIA (Tan IIA) is a major lipophilic component extracted from the root of Salvia miltiorrhiza Bunge that can inhibit the function of platelets and the formation of thrombus [154, 155]. Immunohistochemical analysis has demonstrated that Tan IIA treatment significantly decreased TGF-β1 staining and platelet aggregation in ectopic endometrium [156]. Treatment of platelets with endometriotic ESCs increased the concentration of TGF-β1 in a density-dependent manner [157]. These results suggest that the increased activation of platelets is also an important source of TGF-β1 in endometriosis.
Function
Immune escape
Endometriotic lesions are detectable at a pathological stage, and it is extremely rare to be able to microscopically detect the earlier phases of attachment and proliferation of endometrial tissue in the peritoneum. Numerous studies have attempted to elucidate why ectopic implants develop resistance to scavenging by the immune system due to (at least in part) the altered function of macrophages, natural killer (NK) cells and T cells. It has been demonstrated that T cell reactivity and NK cytotoxicity are decreased in the PF of women with endometriosis, and an increase in the number and activation of peritoneal macrophages with impaired phagocytic activity has been reported [19].
An increase in CD4+CD25+ Tregs can suppress the proliferation of CD4+CD25− effector T cells [54]. As the two key cytokines secreted by Tregs, IL-10 and TGF-β1 are likely to be involved in this process [54]. Anti-inflammatory cytokines, IL-10 and TGF-β1, produced by endometriotic ESCs, macrophages and platelets can weaken the cytotoxic activity of NK cells, which is not due to increased NK cell apoptosis or decreased NK cell proliferation; it is caused by reduced expression of activating receptors, natural killer group 2 member D (NKG2D) and NK receptor (NKp46), reduced perforin production and increased expression of inhibitory receptor killer cell immunoglobulin-like receptor 2DL1 in NK cells [119, 158–160].
Additionally, endometriotic ESCs induce the differentiation of activated macrophages and reduce the phagocytic activity of macrophages in co-culture, which can be reversed by the addition of an IL-33 inhibitor, suggesting that IL-33 produced by endometriotic ESCs contributes to the reduced phagocytic ability of macrophages in endometriosis [118]. It has been reported that a decrease in ST2 suppresses breast cancer progression and metastasis in mice, mainly by via enhanced cytotoxic activity of NK cells [161]. These results suggest that IL-33 may reduce the cytotoxicity of NK cells during endometriosis. Thus, the functional changes induced by anti-inflammatory cytokines contribute to immunosurveillance evasion of ectopic endometrial cells.
Inflammation regulation
Pro-inflammatory and anti-inflammatory factors/responses antagonize each other to maintain physiological homeostasis and pathological immune imbalance. Mancini et al. reported that stimulation with 10 fg/ml IL-10 inhibits NF-κB p65 nuclear localization in endometriotic epithelial (12Z) cells and stromal (22B) cells lines; however, a marked change in IL-1β levels was not observed in vitro [162]. This study did not analyze the effects of IL-10 on the growth of ESCs and EECs further in vitro or in vivo. A recent study reported that IL-37 inhibits the expression of pro-inflammatory cytokines, IL-1β, IL-6 and TNF-α, and anti-inflammatory IL-10 in human ESCs in vitro [163]. Treatment of endometriosis model mice with recombinant human IL-37 reduced the size and weight of endometriotic-like lesions and reduced expression of IL-1β, IL-6, IL-10 and TNF-α in PF [163]; however, a limitation of this study was that human, not mouse, IL-37 was used in the animal experiments, which makes it unclear whether this result can accurately reflect the process in vivo. Currently, there is limited evidence that elevated anti-inflammatory cytokines in the late stage of disease can alleviate disease activity by controlling inflammation.
By contrast, Miller et al. reported that in vitro stimulation with IL-33 increased the production of pro-inflammatory cytokines, IL-1α and TNF-α, by human umbilical venule endothelial cells (HUVECs), and the production of chemokine (C-X-C motif) ligand 1 (CXCL1) and IL-6 by 12Z cells [96]. In a syngeneic mouse model of endometriosis, IL-33 injections caused an increase in pro-inflammatory cytokines within the plasma [96]. Therefore, an increase in anti-inflammatory cytokines in the later stage of the disease potentially has a dual role in the immune regulation of the endometriotic microenvironment. On one hand, the anti-inflammatory cytokines accelerate disease development by promoting immunosurveillance evasion of ectopic endometrial cells and inducing inflammation; and on the other hand, they reduce disease activation by restricting the inflammation. Further research is required.
Cell proliferation, apoptosis, adhesion, migration and invasion
Abnormal cellular characteristics (high proliferative ability, adhesion, migration and invasion, and low levels of apoptosis and autophagy) present in the endometrium of women with endometriosis contribute to ectopic survival and growth of endometrial tissue outside of the uterine cavity [164–171]. Research has revealed that IL-4 induces phosphorylation of p38 mitogen-activated protein kinase (MAPK), stress-activated protein kinase/c-Jun kinase and p42/44 MAPK, and that inhibitors of these kinases suppress IL-4-induced proliferation of human endometriotic ESCs in vitro [172]. Blocking IL-4 significantly reduces the number and volume of total ectopic lesions, downregulates the expression of integrinβ1, matrix metalloproteinase (MMP)-3, MMP-9, E-cadherin and β-catenin in ectopic lesions, and inhibits the development of endometriosis in a syngeneic mouse model [173]. These findings suggest that proliferation, growth adhesion and invasion of endometriotic endometrium induced by locally produced IL-4 are involved in the development of endometriosis.
Suen et al. [42] demonstrated that depletion of IL-10 activity in a mouse model of surgically induced endometriosis significantly decreased the size of endometrial lesions, which supported the findings of an earlier report [174]. Subsequent research revealed that the secretion of IL-10 and TGF-β1 by Tregs increased MMP-2 expression and decreased tissue inhibitor of metalloproteinase 1 (TIMP1) expression. IL-10 and TGF-β1 secretion also stimulated the proliferation and invasion of ESCs in vitro, and the growth of ectopic lesions in vivo [54, 175]. Additionally, IL-10 can cooperate with IL-17A to promote growth, adhesion, invasion and implantation of endometriotic ESCs, and reduce apoptosis in vitro and in vivo [48]. Compared with blocking IL-10 or IL-17A alone, the anti-endometriosis effect is improved by simultaneously blocking IL-10 and IL-17A [48].
In addition to the decrease of immune cell activity, studies in mice and women have indicated that increasing levels of TGF-β1 are associated with an increase in ectopic endometrial cell survival, attachment, invasion, and proliferation, during endometriosis lesion development [146, 176]. Bristol-Gould et al. [177] have developed a mouse model of ovarian endosalpingiosis and found that the onset of this disease is dependent on TGF-β/activin/mothers against decapentaplegic homolog 2 (also known as SMAD family member 2 or Smad2). Treatment with a TGF-β type 1 receptor (TGFβR1) inhibitor can diminish disease progression in mice, especially when used synergistically with a histone acetyltransferase inhibitor [178]. In vitro experiments revealed that TGF-β1 can enhance integrin-mediated cell–cell adhesion by activating the Smad signaling pathway [71, 72, 179] and promoting attachment of cells to the HMrSV5 (human peritoneal mesothelial cell line) cell monolayer, and increase the Matrigel invasion of ESCs, potentially by upregulating the expression of Versican V1 [180]. Most interestingly, treatment of endometriotic EECs and normal EECs with TGF-β1 dramatically increases Smad-dependent and TGFβR1-dependent secretion of plasminogen activator inhibitor-1 (PAI-1) in these cells. Recombinant PAI-1 can increase cellular de-adhesion of endometriotic EECs and normal EECs [132], suggesting that TGF-β1 regulates peritoneal wound repair, and the dynamic imbalance between adhesion formation and de-adhesion of endometriotic cells in endometriosis [176] TGFβR1 expression is significantly higher in the high-migratory ectopic endometriotic tissues, and wound-closure assay, transwell assay and confocal imaging of F-actin cellular distribution have further demonstrated that TGF-β1 significantly increases the migration ability of endometriotic ESCs by upregulating the expression of octamer-binding transcription factor 4 (OCT4) and migration-related genes (SNAIL, SLUG and TWIST) [181]. TGF-β signal blockade using a TGFβRI inhibitor, A83-01, fully attenuates the platelet-induced cell migratory and invasive propensity of endometriotic ESCs in vitro [182]. Moreover, TGF-β1 significantly induces the expression of colony stimulating factor 1 receptor (c-fms) mRNA and c-fms cell-surface expression, and enhances transmesothelial invasion by EM42 cells (an immortalized EEC line) and primary EECs in a three-dimensional in vitro model of the peritoneum [183]. However, cellular proliferation and attachment of EECs to PMCs were not influenced by TGF-β1 [183].
In a syngeneic mouse model of endometriosis, injection with IL-33 caused an increase in the proliferation of endometriotic lesions [96]. TSLP stimulates the production of cytokines chemokine (C–C motif) ligand 2 (CCL2) and IL-8 (also known as CXCL8) by ESCs, and promotes the growth of ESCs via c-Jun N-terminal kinase (JNK) and NF-κB signaling pathways in vitro [134]. Notably, endometrial tissue conditioned media enhances the adhesive capability of human endometriotic tissues in mice, and co-treatment with IL-1RA dramatically reduces this effect [184]; however, the role of other anti-inflammatory cytokines, IL-13 and IL-25, in the regulation ESC and EEC biological functions requires further study.
As described above, these anti-inflammatory cytokines can promote the ectopic growth, adhesion, and implantation of ESCs, and even cooperate with pro-inflammatory cytokines to form a local cytokine regulation network and further accelerate the origin and progression of endometriosis.
Epithelial-mesenchymal transition (EMT), mesothelial-to-mesenchymal transition (MMT), fibroblast-to-myofibroblast transdifferentiation (FMT), smooth muscle metaplasia (SMM) and fibrogenesis
EMT is a process by which epithelial cells lose polarized organization of the cytoskeleton and cell-to-cell contacts, acquiring the high motility that is characteristic of MSCs. These changes are thought to be prerequisites for the original establishment of endometriotic lesions [185, 186]. In human endometriotic ESCs, TGF-β1 dose-dependently increases the gene and protein levels of OCT4, SNAIL and N-cadherin, and silencing of endogenous OCT4 significantly suppresses these TGF-β1-induced effects. This demonstrates that TGF-β1, in co-operation with OCT4, contributes to the process of EMT in endometriosis [181]. Matsuzaki et al. [187] demonstrated that stimulation with TGF-β1 initiates a partial EMT-like process in EECs grown on polyacrylamide gel substrates (a soft matrix). Additionally, TGF-β1 has long been regarded as a major regulator of fibrosis [176, 185, 188, 189]; for example, long-term TGF-β1 stimulation induces phosphorylation of nuclear receptor subfamily 4 group A member 1 in a protein kinase B (also known as AKT)-dependent manner, which then promotes the expression of fibrotic markers in vitro and stimulates fibrogenesis in mice with endometriosis [190]. Endometriotic MSCs significantly promote fibrogenesis in ovarian endometrioma through the Wnt/β-catenin pathway via paracrine production of TGF-β1 and Wnt1 [144]. Notably, activated platelets, promote EMT and FMT in endometriosis through the release of TGF-β1, resulting in the increased cell contractility, collagen production, and, ultimately, fibrosis [182]. Exposure of endometriotic ESCs to activated platelets induces the increased expression of alpha smooth muscle actin (α-SMA) and markers of differentiated smooth muscle cells [182]. SMM has been frequently observed in peritoneal, ovarian, extragenital, and pleuropulmonary endometriosis [191]. Another common process in peritoneal fibrotic tissue is MMT, by which mesothelial cells in the peritoneal cavity transform into myofibroblasts under pathological conditions. In a mouse model, blockade of TGF-β resulted in molecular reprogramming of markers associated with the mesenchymal conversion of mesothelial cells and a significant decrease in the severity of peritoneal adhesions [192].
Although there are no reports regarding the role of other anti-inflammatory cytokines (e.g., IL-4 and IL-10) in EMT and FMT during endometriosis, a large number of studies have demonstrated that IL-4 [193], IL-10 [194], IL-13 [195], and IL-33 [196] promote EMT in cancer cells and human kidney cells. Anti-inflammatory cytokine IL-4 and pro-inflammatory cytokine IL-17A provide a Th2/Th17-polarized inflammatory environment, in which TGF-β1 induces bronchial and alveolar EMT processes [193, 197, 198]; however, IL-37 decreases the EMT of lung cancer cells by inhibiting IL-6 expression [199]. Serum IL-33 concentration is positively correlated with leiomyoma features, such as fibroid weight and size, and the number of fibroids [200]. Additionally, IL-13 [201] promotes liver fibrosis, and IL-10 inhibits fibrosis [202]. The reports discussed indicate that the aforementioned anti-inflammatory cytokines, together with TGF-β1, may participate in the regulation of EMT, MMT, FMT and SMM in endometriosis, and pro-inflammatory cytokines may also have a synergistic role in these effects, which is worth further attention and research.
Angiogenesis
Angiogenesis is a key process involved in the successful establishment and growth of endometriotic lesions [203, 204]. Immunohistochemical analysis has shown that eotaxin-positive cells colocalize with IL-4-positive cells and accumulate around the blood vessels in the stroma of endometriotic tissue. IL-4 can increase secretion of eotaxin from ESCs and may further promote angiogenesis, and the subsequent development of endometriosis [131, 205]. In contrast, blocking IL-4 leads to the reduced expression of vascular endothelial growth factor (VEGF) in the endometriotic lesions of mice and may further impair vascularization of the ectopic endometrium [173]. Interestingly, VEGF and IL-10 concentrations are positively correlated in the PF of endometriosis patients, but IL-10 neutralizing antibody did not affect the release of VEGF by neutrophils treated with PF from patients with endometriosis [206].
Yong et al. reported that TGF-β1 can upregulate VEGF-A expression in PMCs by increasing expression of inhibitor of DNA binding (ID) 1 [207]. The luciferase activity of a VEGF promoter construct was increased in the presence of either TGF-β1 or hypoxia, and combined treatment with hypoxia and TGF-β1 resulted in a much higher production of VEGF by ESCs [70]. Furthermore, TGF-β1 and pro-inflammatory cytokines, IL-1β or TNF-α, can synergistically promote IL-8 and VEGF expression in ESCs via the p38/extracellular-signal-regulated kinase (ERK) 1/2 signaling pathways, and stimulate the angiogenesis of HUVECs in vitro [120]. As mentioned above, TGF-β1 has been associated with changes in ectopic endometrial and peritoneal cell metabolism, and the initiation of neoangiogenesis and endometriosis lesion development [146].
In vitro studies have shown that stimulation with IL-33 significantly increases the levels of angiogenic factors, including VEGF and platelet-derived growth factor-AA, by EECs and stimulates tubulogenesis in HUVECs [96]. Endometriotic lesions from IL-33-treated mice were highly vascularized and exhibited increased proliferation of endometriotic lesions, which echoed the results discussed above [96]. IL-8 is considered to be an important angiogenic factor [208]. As previously described, TSLP upregulates the production and secretion of IL-8 from ESCs in vitro [134]. It has also been established that TSLP secreted by cervical cancer cells stimulates proliferation, activation and angiogenesis of HUVECs, but does not alter HUVEC apoptosis in vitro [209]. Therefore, it is likely that TSLP has a role in angiogenesis associated with endometriosis, with further studies required to confirm this function.
Others
It has been reported that elevated serum IL-33 is correlated with the intensity of painful pre-operative symptoms, and with the extent and severity of deeply infiltrating endometriosis [93]. Alterations in Treg and NK cell-related cytokines are associated with deep infiltrating rectosigmoid endometriosis, and TGF-β1, IL-7 and IL-15 expression is linked to dyspareunia, dysmenorrhea and cyclic dyschezia in patients with endometriosis [45]. However, the regulatory roles and molecular mechanisms of IL-33 and TGF-β1 in endometriosis-related pain are almost entirely unknown.
Prostaglandin E2 (PGE2) stimulates P450 aromatase (P450arom) expression in ESCs and increases the production of estrogens [210]. IL-4 was shown to have no effect on the expression of P450arom mRNA, whereas IL-4 and PGE2 in combination significantly augmented the production of estrone from dehydroepiandrosterone (DHEA) in vitro. These findings suggest that the combination of IL-4 and PGE2 may enhance estrogen production in endometriotic tissues, suggesting a complex mechanism exists whereby Th2 immune responses augment inflammation-dependent estrogen production, which promotes disease progression [211].
Regulation
Chemokines
Chemokines produced in the endometriotic microenvironment contribute to a feed-forward cascade of events, which promote the recruitment of leukocytes to the peritoneal cavity and regulate the proliferation and invasion of ESCs in patients with endometriosis. These chemokines include regulated on activation, normal T cell expressed and secreted (RANTES; also known as CCL5), CCL2, CXCL8 and thymus-expressed chemokine (also known as CCL25) [212–215]. As depicted in Table 2, under the regulation of 17β-estradiol (E2) and dioxin 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), RANTES secreted by ESCs can increase macrophage recruitment, and induce tolerant phenotypes by upregulating IL-10 and downregulating IL-23. In turn, these macrophages inhibit apoptosis and enhance the growth of ESCs [117]. Subsequently, our studies show that ESC-derived CCL25 enhances the production and secretion of IL-10 and TGF-β in Tregs by activating the AKT/signal transducer and activator of transcription 3 (STAT3) signaling pathway [54]. Treatment with CCL25 also enhances IL-10 secretion by CD33+CD14+CD11b+HLA-DR− monocytic MDSCs (M-MDSCs) in vitro [129]. Taken together, these findings indicate that chemokines are involved in the regulation of anti-inflammatory cytokines in the endometriotic environment.
Table 2.
The regulatory factors of anti-inflammatory cytokines in endometriosis
| Classification | Regulatory factor | Function | References |
|---|---|---|---|
| Chemokine | RANTES | Recruits more macrophages and induces them into tolerant phenotype by upregulating IL-10 and downregulating IL-23 | [117] |
| CCL25 | Enhances the production and secretion of IL-10 and TGF-β1 in Tregs and M-MDSCs | [54, 129, 212] | |
| Proinflammatory cytokine | IL-1β | Increases the secretion of IL-4 | [216] |
| Contributes to the Th2 immune response by improving the secretion of TSLP | [94] | ||
| TNF-α | Increases the secretion of IL-13 | [133] | |
| IFN-γ | Inhibits the IL-1β-induced TSLP secretion from ESCs | [94] | |
| IL-33 | Upregulates the levels of IL-10 and TGF-β1 in macrophages in co-culture unit | [118] | |
| IL-27 | Induces IL-10 production of Th17 cells | [48] | |
| IL-8 and IL-23 | Induces the differentiation of COX-2highCD16−NK cells with high levels of IL-10 and TGF-β1 | [217] | |
| Hormone/hormonal drugs | 17β estradiol | Regulates GATA3 and promotes Th2 cytokines expression of EECs | [221, 222] |
| Stimulates the secretion of TSLP by ESCs | [134, 135, 223] | ||
| Induces IL-10 production of Th17 cells by promoting IL-27 from macrophages and ESCs | [48] | ||
| Results in a rapid induction of IL-13 and IL-15 expression in ESCs and EECs but not a sustained induction | [133] | ||
| Urocortin | Urocortin 2 increases TNF-α and IL-4 while urocortin 3 induces an increase of IL-4 secretion | [228] | |
| GnRH analogs | Inhibits IL-1, IL-2, IL-8, and IL-13 levels in the serum with recombinant IL-2 | [233] | |
| Danazol | Suppresses IL-2 and interferon-γ were but upregulates IL-4 and IL-10 and increases Tregs population among splenocytes | [235, 236] | |
| Non-steroidal drugs | Macrolide | Increases IL-10 expression in the rat endometriosis model | [237] |
| PLGA/anti-CTLA-4 | Downregulates IL-10 and TGF-β secreted by Tregs | [175] | |
| Sodium TAN IIA sulfonate | Reduces the expression of CD41, TGF-β1, p-Smad3, α-SMA and collagen I in ectopic lesions | [156] | |
| G-Rg3 | Decreases mRNA levels of Ki-67, fibronectin, TGF-β1, MMP2 and MMP9 significantly in human ESCs | [245] | |
| PPD | Activates the cytotoxicity and decreases IL-10 production of NK cells in endometriotic milieu | [246] | |
| EGCG | Decreases the TGF-β1-dependent increase in the mRNA expression of fibrotic markers | [188] | |
| Metabolite/metabolic enzyme | SNAP | Decreases plasma IL-2, IFN-γ, and IL-4 and increases IL-10, and enables the growth of implant in a murine model of endometriosis | [256] |
| IDO1 | Triggers macrophages to display the tolerant cytokine profile (the markedly raised level of IL-10 and TGF-β1 and depressed level of IL-12p70) by upregulating IL-33 in ectopic ESCs | [118] | |
| 1-MT | Decreases differentiation of Treg cells in an ESC-Tregs co-culture unit, especially IL-10+ Treg cells | [113] | |
| Glucose | Triggers production of TGF-β1 which plays a pivotal role in the progression of peritoneal fibrosis by inducing numerous pro-fibrotic events | [259] | |
| Induces TGF-β1 production in HPMCs in vitro | [145] | ||
| LXA4 | Downregulates the pro-inflammatory cytokines IL-1β and IL-6, as well as modulating TGF-β isoform expression within endometriotic lesions and in peritoneal fluid cells | [264] | |
| Platelet | Increases concentration of TXB2, thrombin, and TGF-β1 | [157, 182] | |
| Others | MRC2 | Negatively regulates Treg differentiation in the ectopic lesion, especially that for TGF-β+ Treg | [113] |
| Hypoxia | Leads to high level of TGF-β1 by ESCs | [179] | |
| TNF-binding protein-1 | Negatively modulates the expression of TGF-β in endometriotic lesions from baboons | [265] | |
| KLF11 | Binds to specific elements located in the promoter regions of key fibrosis-related genes from the Collagen, MMP and TGF-β1 families in ESCs, and results in transcriptional repression of these genes | [266] |
Pro-inflammatory cytokines
It has been reported that the pro-inflammatory cytokine IL-1β significantly increases the secretion of IL-4 [216]. In addition, IL-1β contributes to the Th2 immune response by promoting the secretion of TSLP, and this effect is dependent on p42/44 MAPK, p38 MAPK and stress-activated protein kinase (SAPK)/JNK signaling pathways [94]. IL-4 enhances the IL-1β-induced TSLP secretion from ESCs, while IFN-γ inhibits it [94]. Roberts et al. [133] confirmed that primary ESCs and EECs isolated and cultured from endometrium express IL-13. Pro-inflammatory cytokine TNF-α and anti-inflammatory cytokine TGF-β1 both significantly increase the secretion of IL-13 from EECs and ESCs in vitro. Our previous study demonstrated that IL-33 derived from ESCs can upregulate the levels of IL-10 and TGF-β1 in macrophages in a co-culture [118], and IL-27 secreted by ESCs and macrophages induces IL-10 production by Th17 cells in the endometriotic environment [48]. IL-8 and IL-23 produced from ectopic lesions induce the differentiation of cyclooxygenase (COX)-2highCD16−NK cells, which express high levels of IL-10 and TGF-β1 [217]. As stated previously, there are interactions between pro-inflammatory (e.g., IL-1β, TNF-α, and IFN-γ) and anti-inflammatory cytokines (e.g., IL-4, IL-10, IL-13, IL-33, TGF-β, and TSLP) in the microenvironment of ectopic lesions. The co-existence of high levels of pro-inflammatory and anti-inflammatory cytokines is involved in immune escape by endometriotic lesions and inflammation regulation.
Hormones and hormonal drugs
It is generally established that endometriosis is an estrogen-dependent disease [218]. Endometriotic lesions are associated with hormonal imbalance, including an increase in estrogen synthesis, metabolism and progesterone resistance. As a Th2 cell-specific transcription factor, the binding sites for GATA binding protein-3 (GATA-3) are present in the promoter regions of all Th2 cytokines [219]. This can promote differentiation of Th2 cells and induce the expression of Th2 cytokines, such as IL-4 and IL-10 [220]. GATA-3 is time- and dose-dependently regulated by E2, and promotes Th2 cytokine expression (e.g., IL-4 and IL-10) by EECs [221], which is consistent with previous evidence of the stimulatory regulation role of E2 in rat IL-10 expression [222]. In addition, E2 stimulates the secretion of TSLP by ESCs in a dose-dependent manner [134, 135, 223]. Under external (TCDD) and local (high levels of estrogen, IL-6 and TGF-β) environmental regulation, IL-27 secreted by macrophages and ESCs induces IL-10 production by Th17 cells in endometriosis in vitro and in vivo [48]. Treatment of EECs and ESCs with E2 and medroxyprogesterone acetate (MPA) results in a rapid induction of IL-13 and IL-15 expression [133]. Interestingly, prolonged MPA exposure, especially > 10 days, does not lead to a sustained induction of IL-13 and IL-15 [133]. These studies indicate that estrogen and MPA may indirectly contribute to the regulation of immune and endometriotic lesion implantation and growth by modulating these anti-inflammatory cytokines, and the potential mechanisms involved require further research.
The presence of neuroendocrine cells in the eutopic endometrium of women with endometriosis has been reported [224]. There is evidence that corticotrophin-releasing hormone [225] and urocortin [226] are present in endometriotic tissue, and that the levels of these neuropeptides are dysregulated in endometriosis [227]. Stimulation of cultured ESCs with urocortin 2 significantly increases TNF-α and IL-4, while urocortin 3 induces an increase in IL-4 secretion in vitro [228]. These results indicate that these neuropeptides may also be involved in the pathogenesis of endometriosis via the regulation of pro-inflammatory and anti-inflammatory cytokines.
Therapy with gonadotropin-releasing hormone (GnRH) analogs is effective for pain control in women with symptomatic endometriosis and has been considered the gold standard treatment for two decades. Currently, GnRH analogs are second-line therapy, when primary treatments fail, are contraindicated or are not tolerated [229–231]. Post-surgical treatment with GnRH analogs may be useful in reducing pain and in delaying recurrence of symptoms in patients with incomplete treatment [232]. Velasco et al. [233] found that patients receiving GnRH analogs alone had high IL-1, IL-2, IL-8 and IL-13 levels in their serum; however, administration of recombinant IL-2 plus GnRH analogs had a tendency to reduce cytokine production. Whether the anti-endometriosis effect of GnRH analogs depends on the regulation of these cytokines is unknown.
Danazol (the synthetic androgen 2,3-isoxazol), a derivative of 17α-ethynyl testosterone, has mild androgenic and strong anti-estrogenic activity. It can inhibit gonadotropin release, determine the competitive inhibition of steroidogenic enzymes, suppress cell proliferation, modulate immunologic function and reduce endometriosis-associated pain [231, 234]. In a murine model, danazol treatment significantly prolonged the survival of fully mismatched cardiac allografts. Furthermore, in danazol-treated mice, IL-2 and IFN-γ were suppressed, whereas IL-4 and IL-10 were upregulated in splenocytes, and the splenocyte Treg population was increased [235, 236]. However, whether danazol regulates the level of pro-inflammatory and anti-inflammatory cytokines in the endometriotic environment requires further investigation.
Non-steroidal drugs
As the pathogenesis of endometriosis involves immune responses, the immunomodulatory effect of macrolides has been a research focus. Clarithromycin and telithromycin increase IL-10 expression in rat endometriosis lesions induced by autotransplantation of endometrium, and also inhibit the growth of endometriotic lesions [237]; however, the mechanism needs further study.
Cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) is essential for the suppressive function of Tregs. Poly lactic-co-glycolic acid (PLGA) encapsulation of CTLA-4 antibody (PLGA/anti-CTLA-4) as a protein delivery vehicle exhibited superior potential as an endometriosis treatment in a mouse model compared to CTLA-4 antibody used alone. Most strikingly, in an in vitro experiment, PLGA/anti-CTLA-4 inhibited the proliferation and invasion of ectopic ESCs in a co-culture system, and downregulated IL-10 and TGF-β1 secretion by Tregs [175]. However, there is no clear evidence that CTLA-4 antibody regulates cytokine levels and has anti-endometriosis activity in vivo.
Tanshinone (TAN) is a pharmacologically active diterpenoid extracted from the roots of S. miltiorrhiza Bunge (a plant used in traditional Chinese medicine as a remedy for ‘blood stasis’, which is a term used in traditional Chinese medicine to describe hypercoagulability). TAN can inhibit platelet aggregation [238], and suppress TGF-β1/Smad3 and NF-κB signaling pathways, thus reducing fibrogenesis [239, 240]. Recent research has reported that sodium TAN IIA sulfonate injection significantly reduces the levels of CD41, TGF-β1, p-Smad3, α-SMA and collagen I in ectopic lesions, inhibits EMT, FMT, SMM and fibrogenesis, and reduces lesion weight in mouse endometriosis models [156]. However, large-scale clinical studies are required to assess the clinical value of TAN as an anti-endometriosis therapy.
As a traditional medicinal herb, ginseng is widely used in Asian countries and North America. Ginsenosides, such as ginsenoside-Rg3 (G-Rg3), G-Rf and G-Rh2, are the main components extracted from ginseng and have various pharmaceutical activities, including anti-oxidant, immunomodulatory, anti-inflammatory and anti-tumor effects [241, 242]. As two metabolites of ginsenoside, protopanaxadiol (PPD), and protopanaxatriol also exhibit similar activities [242–244]. Recent studies have shown that G-Rg3, G-Rf and PPD have powerful anti-endometriosis activities [245–248]. G-Rg3 reduces the proliferation, invasion and fibrosis of endometriosis. In vitro, the mRNA levels of Ki-67, fibronectin, TGF-β1, MMP-2 and MMP-9 were significantly decreased by G-Rg3 treatment in human ESCs [245]. In vivo, G-Rg3A reduced the size and fibrosis of mouse endometrial lesions [227], and inhibited angiogenesis in rat endometrial lesions [247]. G-Rf decreases the volume of the endometriotic implants, and writhing responses, endometriosis-associated dysmenorrhea and inflammation in a surgically induced rat endometriosis model [248]. PPD has anti-estrogen receptor α (ERα) effects, and induces the autophagy of ESCs, activates cytotoxicity and decreases IL-10 production by NK cells in a human ESC-NK co-culture model [246]. However, the clinical evidence of the anti-endometriosis effect of ginsenosides is still limited.
Epigallocatechin-3-gallate (EGCG) is one of the most abundant polyphenols present in green tea. It has been reported that EGCG reduces the size of endometriotic implants by inhibition of cell proliferation, angiogenesis, and induction of apoptosis in mouse endometriotic implants [249–252]. Furthermore, EGCG treatment significantly decreased the TGF-β1-induced in the mRNA expression of fibrotic markers, and the TGF-β1-stimulated activation of MAPK and Smad signaling pathways in endometriotic ESCs, suggesting that anti-fibrosis activity of EGCG may be dependent on the suppression of TGF-β1 in endometriosis [188]. However, there is no epidemiological evidence of a link between green tea consumption and the occurrence and treatment of endometriosis.
Metabolite and metabolic enzyme
Oxidative stress has been identified in the peritoneal cavity of women with endometriosis [253]. Nitric oxide (NO) is associated with chronic pelvic pain experienced by patients with endometriosis [254]. NO also has an important role in the regulation of local immune responses [255]. Intraperitoneal injections of S-nitroso-N-acetyl-penicillamine (SNAP), an NO donor, decreased plasma IL-2, IFN-γ and IL-4, increased IL-10 and enabled the growth of implants in a murine model of endometriosis [256].
Indoleamine 2,3-dioxygenase-1 (IDO1) is highly expressed in ectopic endometrium, and it can enhance the proliferation and invasion of ESCs in via an indirect immune regulation-independent mechanism [257] and directly [113, 118, 258]. Additionally, IL-33 upregulated by IDO1 in ectopic ESCs can trigger macrophages to have a tolerant cytokine profile (with markedly increased IL-10 and TGF-β1 levels, and a reduced level of IL-12p70) [118]. In turn, these macrophages promote the proliferation and invasion of ESCs [258]. Interestingly, ESC-derived TGF-β1 also upregulates IDO levels in NK cells in co-culture [160]. A specific inhibitor of IDO1, 1-methyl-tryptophan (1-MT), decreases differentiation of Treg cells in an ESC-Treg co-culture, particularly IL-10+ Treg cells [113]. Additionally, the total number and weight of mouse endometriotic lesions was notably decreased in a group of mice that received 1-MT [113].
Glucose is used as an osmotic agent in peritoneal dialysis solution, but glucose-based solutions are associated with increases in cytokines. For example, glucose-mediated production of TGF-β1 has a pivotal role in the progression of peritoneal fibrosis by inducing numerous pro-fibrotic events, such as EMT, fibroblast proliferation and extracellular matrix protein deposition [259]. Furthermore, high glucose can also induce TGF-β1 production in HPMCs in vitro, and silencing of FBJ murine osteosarcoma viral oncogene homolog (FOS) expression can significantly weaken this effect [145]. These studies discussed above suggest that high glucose may affect the immune status of the ectopic lesion microenvironment. It has been found that the prevalence of diabetes in women with endometriosis is similar to that of the general population [260]. However, Wang et al. reported that long-term hyperestrogenemia and type II diabetes increased the risk of malignant transformation of endometriosis in rats [261]; however, whether this is associated with changes in immune regulation caused by high glucose is unknown.
Lipoxins are endogenous eicosanoids predominantly produced via a transcellular biosynthetic pathway that have both anti-inflammatory and pro-resolving properties [262]. Lipoxins are estrogenic in vitro and in vivo as they can bind to ERα in EECs and counteract E2-mediated responses [263]. Lipoxin 4 (LXA4) treatment significantly reduces endometriotic lesion size, downregulates pro-inflammatory cytokines, IL-1β and IL-6, and modulates TGF-β isoform expression within endometriotic lesions and in PF cells [264]. However, it is unclear whether the regulation of these cytokines by LXA4 is involved in the inhibition of endometriotic lesion growth.
Secretion of thrombin and thromboxane A2 (TXA2) by ESCs can induce platelet activation and aggregation. Meanwhile, treatment of platelets further increased the concentration of TXB2, thrombin and TGF-β1 [157, 182]. These findings establish that there is a crosstalk between endometriotic lesions and platelets via thrombin and TXA-dependent mechanisms, which potentially contributes to the upregulation of TGF-β1 in endometriosis.
Others
Mannose receptor C, type 2 (MRC2) expression was notably decreased in ectopic ESCs, which negatively regulates Treg differentiation in ectopic lesions, particularly that of TGF-β1+ Tregs [113]. These results indicate that an estrogen-IDO1-MRC2 axis is involved in the differentiation and TGF-β1 production of Tregs, and is associated with the development of endometriosis by promoting immune escape of ectopic lesions. Immunohistochemistry analysis of human tissues has shown that TGF-β1 exhibits the same expression trend as hypoxia-inducible factor (HIF)-1α in ESCs and EECs, with the highest staining observed in ectopic lesions. Additionally, hypoxia increases TGF-β1 expression by ESCs in endometriosis [179]. Recombinant human TNF-binding protein-1 negatively modulates the expression of TGF-β in endometriotic lesions from baboons [265]. Furthermore, the Sp/Kruppel-like factor (KLF) family transcription factor, KLF11, can bind to specific elements located in the promoter regions of key fibrosis-related genes of the collagen, MMP and TGF-β1 families in ESCs, resulting in transcriptional repression of these genes [266]. Further research is required to analyze the underlying mechanism.
Conclusions and future perspectives
Chronic inflammation has an important role in the onset and development of endometriosis, particularly at the early stages. Therefore, it is often stated that endometriosis is an inflammatory disease from the immunological point of view; however, studies have also shown that anti-inflammatory cytokines produce by various cell types (e.g., immune cells, ESCs, EECs, MSCs, peritoneal mesothelial cells, and platelets) accumulate in the microenvironment of ectopic lesions. A lack of knowledge regarding the cytokine regulatory changes from the perspective of disease progression and role of anti-inflammatory factors in the local environment of endometriosis has led to less awareness and acceptance of the importance of anti-inflammatory factors in the pathogenesis of endometriosis. With the continuous research advances and in-depth studies, it can be concluded that anti-inflammatory cytokines also have a critical role in accelerating the growth, adhesion, invasion, EMT, MMT, FMT, SMM, fibrogenesis, angiogenesis, and impairment of immune surveillance in endometriosis; thus, endometriosis is not simply an inflammatory disease, and it should be redefined as a disease with a combined inflammatory and anti-inflammatory status.
However, there is a lack of large-scale studies using cytokine arrays to systematically analyze the dynamic changes in pro-inflammatory and anti-inflammatory cytokines during endometriosis disease progression. There is a complex and interactive regulatory imbalance between pro-inflammatory cytokines/anti-inflammatory cytokines that is regulated by sex hormones, particularly estrogen, local metabolism, and the pro-inflammatory cytokines and anti-inflammatory cytokines themselves. This leads to the co-existence of high concentrations of pro-inflammatory and anti-inflammatory cytokines in endometriosis. Similar to tumors, anti-inflammatory factors may contribute to the new adaptive growth pattern of the ectopic endometrium by creating a tolerant endometriotic environment and have an important role in immune escape by ectopic lesions. Whether this process depends on inflammation regulation of the microenvironment of ectopic lesions by anti-inflammatory cytokines remains unclear. In addition, how the co-existence of pro-inflammatory and anti-inflammatory cytokines is generated and the regulatory mechanisms involved in endometriosis is not fully understood, which warrants further investigated.
Currently, the value of pro-inflammatory factors in the diagnosis of endometriosis is controversial [32, 33]. For diagnostic or evaluation purposes, reliable pro-inflammatory and anti-inflammatory cytokine arrays with high sensitivity and specificity must be identified. More realistic diagnostic tools will consist of a panel of biomarkers, including pro-inflammatory and anti-inflammatory cytokines, and other serum markers (e.g., CA-125), but the establishment of cytokine-based approaches for the management of endometriosis still represents a major challenge. These techniques, together with other non-invasive tools, such as ultrasound and magnetic resonance imaging (MRI), may markedly improve the diagnosis of endometriosis and the life quality of patients.
A single anti-inflammatory treatment (e.g., anti-TNF-α or anti-IFN-γ) is effective in mouse [29], rat [26, 27], baboon [23, 267] or human [24] models; however, they are less effective or ineffective [30, 31] in clinical trials, particularly in patients with severe and deep infiltrating endometriosis. As discussed earlier, many anti-endometriosis drugs or potential therapies, including hormonal drugs and immunoregulators, simultaneously affect the level and profile of cytokines in endometriosis. Therefore, compared with suppressing pro-inflammatory cytokines alone, targeting both pro-inflammatory and anti-inflammatory cytokines and/or enhancing immune surveillance by innate immune cells (macrophages and NK cells) may be more effective for the treatment of endometriosis. For therapeutic purposes, novel measures combining targeting of pro-inflammatory and anti-inflammatory factors present in the endometriotic lesion microenvironment, without inducing severe side effects, are required.
Furthermore, individualized therapies may be necessary for immune-associated interventions to treat endometriosis, and systematic immunization assessment is required before treatment. Therefore, the co-existence of pro-inflammatory and anti-inflammatory cytokines in endometriosis offers many possibilities for the establishment of novel diagnostics, models and therapeutic approaches, which requires further research.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (nos. 91542108, 81471513, 31671200, 81601354, 31600735 and 81571509), the Shanghai Rising-Star Program (no. 16QA1400800), the Innovation-oriented Science and Technology Grant from NPFPC Key Laboratory of Reproduction Regulation (CX2017-2), the Program for Zhuoxue of Fudan University, the National Science Foundation of Jiangsu Province (no. BK20160128), the Fundamental Research Funds for the Central Universities (no. 021414380180), the Open Project Program of Shanghai Key Laboratory of Female Reproductive Endocrine-Related Diseases (CN) (no. 14DZ2271700).
Author contributions
MQL designed and wrote the review, and supervised and critically reviewed the complete manuscript. WJZ and HLY performed the literature search, drafted the manuscript and prepared the figures. JS, KKC and JM helped to perform revisions and critically discussed the completed manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be considered as potential conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wen-Jie Zhou and Hui-Li Yang co-first author.
References
- 1.Giudice L, Kao L. Endometriosis. Lancet. 2004;364(9447):1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 2.Culley L, Law C, Hudson N, et al. The social and psychological impact of endometriosis on women’s lives: a critical narrative review. Hum Reprod Update. 2013;19(6):625–639. doi: 10.1093/humupd/dmt027. [DOI] [PubMed] [Google Scholar]
- 3.Guo SW. Recurrence of endometriosis and its control. Hum Reprod Update. 2009;15(4):441–461. doi: 10.1093/humupd/dmp007. [DOI] [PubMed] [Google Scholar]
- 4.Simpson PD, McLaren JS, Rymer J, et al. Minimising menopausal side effects whilst treating endometriosis and fibroids. Post Reprod Health. 2015;21(1):16–23. doi: 10.1177/2053369114568440. [DOI] [PubMed] [Google Scholar]
- 5.Simoens S, Hummelshoj L, D’Hooghe T. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update. 2007;13(4):395–404. doi: 10.1093/humupd/dmm010. [DOI] [PubMed] [Google Scholar]
- 6.De Graaff AA, D’Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international crosssectional survey. Hum Reprod. 2013;28(10):2677–2685. doi: 10.1093/humrep/det284. [DOI] [PubMed] [Google Scholar]
- 7.Mori H, Sawairi M, Nakagawa M, et al. Peritoneal fluid interleukin-1 beta and tumor necrosis factor in patients with benign gynecologic disease. Am J Reprod Immunol. 1991;26(2):62–67. doi: 10.1111/j.1600-0897.1991.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 8.Rier SE, Zarmakoupis PN, Hu X, et al. Dysregulation of interleukin-6 responses in ectopic endometrial stromal cells: correlation with decreased soluble receptor levels in peritoneal fluid of women with endometriosis. J Clin Endocrinol Metab. 1995;80(4):1431–1437. doi: 10.1210/jcem.80.4.7714120. [DOI] [PubMed] [Google Scholar]
- 9.Hirata T, Osuga Y, Hamasaki K, et al. Interleukin (IL)-17A stimulates IL-8 secretion, cyclooxygensase-2 expression, and cell proliferation of endometriotic stromal cells. Endocrinology. 2008;149(3):1260–1267. doi: 10.1210/en.2007-0749. [DOI] [PubMed] [Google Scholar]
- 10.Milewski Ł, Barcz E, Dziunycz P, et al. Association of leptin with inflammatory cytokines and lymphocyte subpopulations in peritoneal fluid of patients with endometriosis. J Reprod Immunol. 2008;79(1):111–117. doi: 10.1016/j.jri.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Carmona F, Chapron C, Martinez-Zamora MA, et al. Ovarian endometrioma but not deep infiltrating endometriosis is associated with increased serum levels of interleukin-8 and interleukin-6. J Reprod Immunol. 2012;95(1–2):80–86. doi: 10.1016/j.jri.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Ahn SH, Edwards AK, Singh SS, et al. IL-17A contributes to the pathogenesis of endometriosis by triggering proinflammatory cytokines and angiogenic growth factors. J Immunol. 2015;195(6):2591–2600. doi: 10.4049/jimmunol.1501138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luisi S, Pinzauti S, Regini C, et al. Serum markers for the noninvasive diagnosis of endometriosis. Womens Health (Lond) 2015;11(5):603–610. doi: 10.2217/whe.15.46. [DOI] [PubMed] [Google Scholar]
- 14.Malutan AM, Drugan T, Costin N, et al. Pro-inflammatory cytokines for evaluation of inflammatory status in endometriosis. Cent Eur J Immunol. 2015;40(1):96–102. doi: 10.5114/ceji.2015.50840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sikora J, Mielczarek-Palacz A, Kondera-Anasz Z, et al. Peripheral blood proinflammatory response in women during menstrual cycle and endometriosis. Cytokine. 2015;76(2):117–122. doi: 10.1016/j.cyto.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Gogacz M, Winkler I, Bojarska-Junak A, et al. Increased percentage of Th17 cells in peritoneal fluid is associated with severity of endometriosis. J Reprod Immunol. 2016;117:39–44. doi: 10.1016/j.jri.2016.04.289. [DOI] [PubMed] [Google Scholar]
- 17.Christodoulakos G, Augoulea A, Lambrinoudaki I, et al. Pathogenesis of endometriosis: the role of defective ‘immunosurveillance’. Eur J Contracept Reprod Health Care. 2007;12(3):194–202. doi: 10.1080/13625180701387266. [DOI] [PubMed] [Google Scholar]
- 18.Jiang L, Yan Y, Liu Z, et al. Inflammation and endometriosis. Front Biosci (Landmark Ed) 2016;21:941–948. doi: 10.2741/4431. [DOI] [PubMed] [Google Scholar]
- 19.Riccio LDGC, Santulli P, Marcellin L, et al. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2018;50:39–49. doi: 10.1016/j.bpobgyn.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Martínez S, Garrido N, Coperias JL, et al. Serum interleukin-6 levels are elevated in women with minimal-mild endometriosis. Hum Reprod. 2007;22(3):836–842. doi: 10.1093/humrep/del419. [DOI] [PubMed] [Google Scholar]
- 21.May KE, Conduit-Hulbert SA, Villar J, et al. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update. 2010;16(6):651–674. doi: 10.1093/humupd/dmq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mihalyi A, Gevaert O, Kyama CM, et al. Non-invasive diagnosis of endometriosis based on a combined analysis of six plasma biomarkers. Hum Reprod. 2010;25(3):654–664. doi: 10.1093/humrep/dep425. [DOI] [PubMed] [Google Scholar]
- 23.Falconer H, Mwenda JM, Chai DC, et al. Treatment with anti-TNF monoclonal antibody (c5N) reduces the extent of induced endometriosis in the baboon. Hum Reprod. 2006;21(7):1856–1862. doi: 10.1093/humrep/del044. [DOI] [PubMed] [Google Scholar]
- 24.Altintas D, Kokcu A, Tosun M, et al. Efficacy of recombinant human interferon alpha-2b on experimental endometriosis. Eur J Obstet Gynecol Reprod Biol. 2008;139(1):95–99. doi: 10.1016/j.ejogrb.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Jerzak M, Niemiec T, Nowakowska A, et al. First successful pregnancy after addition of enoxaparin to sildenafil and etanercept immunotherapy in woman with fifteen failed IVF cycles—case report. Am J Reprod Immunol. 2010;64(2):93–96. doi: 10.1111/j.1600-0897.2010.00826.x. [DOI] [PubMed] [Google Scholar]
- 26.Islimye M, Kilic S, Zulfikaroglu E, et al. Regression of endometrial autografts in a rat model of endometriosis treated with etanercept. Eur J Obstet Gynecol Reprod Biol. 2011;159(1):184–189. doi: 10.1016/j.ejogrb.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 27.Zulfikaroglu E, Kılıc S, Islimye M, et al. Efficacy of anti-tumor necrosis factor therapy on endometriosis in an experimental rat model. Arch Gynecol Obstet. 2011;283(4):799–804. doi: 10.1007/s00404-010-1434-0. [DOI] [PubMed] [Google Scholar]
- 28.Soares SR, Martínez-Varea A, Hidalgo-Mora JJ, et al. Pharmacologic therapies in endometriosis: a systematic review. Fertil Steril. 2012;98(3):529–555. doi: 10.1016/j.fertnstert.2012.07.1120. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Sun L, Hou Z, et al. rhTNFR: Fc suppresses the development of endometriosis in a mouse model by downregulating cell proliferation and invasiveness. Reprod Sci. 2016;23(7):847–857. doi: 10.1177/1933719115620495. [DOI] [PubMed] [Google Scholar]
- 30.Koninckx PR, Craessaerts M, Timmerman D, et al. Anti-TNF-alpha treatment for deep endometriosis-associated pain: a randomized placebo-controlled trial. Hum Reprod. 2008;23(9):2017–2023. doi: 10.1093/humrep/den177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu D, Song H, Shi G. Anti-TNF-α treatment for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2013;28(3):CD008088. doi: 10.1002/14651858.CD008088.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahn SH, Singh V, Tayade C. Biomarkers in endometriosis: challenges and opportunities. Fertil Steril. 2017;107(3):523–532. doi: 10.1016/j.fertnstert.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Rocha AL, Vieira EL, Maia LM, et al. Prospective evaluation of a panel of plasma cytokines and chemokines as potential markers of pelvic endometriosis in symptomatic women. Gynecol Obstet Investig. 2016;81(6):512–517. doi: 10.1159/000443956. [DOI] [PubMed] [Google Scholar]
- 34.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117(4):1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 35.Punnonen J, Teisala K, Ranta H, et al. Increased levels of interleukin-6 and interleukin-10 in the peritoneal fluid of patients with endometriosis. Am J Obstet Gynecol. 1996;174(5):1522–1526. doi: 10.1016/S0002-9378(96)70600-2. [DOI] [PubMed] [Google Scholar]
- 36.Ho HN, Wu MY, Chao KH, et al. Peritoneal interleukin-10 increases with decrease in activated CD4+ T lymphocytes in women with endometriosis. Hum Reprod. 1997;12(11):2528–2533. doi: 10.1093/humrep/12.11.2528. [DOI] [PubMed] [Google Scholar]
- 37.Hsu CC, Yang BC, Wu MH, et al. Enhanced interleukin-4 expression in patients with endometriosis. Fertil Steril. 1997;67(6):1059–1064. doi: 10.1016/S0015-0282(97)81439-2. [DOI] [PubMed] [Google Scholar]
- 38.Antsiferova YS, Sotnikova NY, Posiseeva LV, et al. Changes in the T-helper cytokine profile and in lymphocyte activation at the systemic and local levels in women with endometriosis. Fertil Steril. 2005;84(6):1705–1711. doi: 10.1016/j.fertnstert.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 39.Podgaec S, Abrao MS, Dias JA, Jr, et al. Endometriosis: an inflammatory disease with a Th2 immune response component. Hum Reprod. 2007;22(5):1373–1379. doi: 10.1093/humrep/del516. [DOI] [PubMed] [Google Scholar]
- 40.Maluţan AM, Drugan T, Ciortea R, et al. Serum anti-inflammatory cytokines for the evaluation of inflammatory status in endometriosis. J Res Med Sci. 2015;20(7):668–674. doi: 10.4103/1735-1995.166215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malutan AM, Drugan C, Drugan T, et al. The association between interleukin-4 -590C/T genetic polymorphism, IL-4 serum level, and advanced endometriosis. Cent Eur J Immunol. 2016;41(2):176–181. doi: 10.5114/ceji.2016.60992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suen JL, Chang Y, Chiu PR, et al. Serum level of IL-10 is increased in patients with endometriosis, and IL-10 promotes the growth of lesions in a murine model. Am J Pathol. 2014;184(2):464–471. doi: 10.1016/j.ajpath.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 43.Malutan AM, Drugan C, Walch K, et al. The association between interleukin-10 (IL-10) -592C/A, -819T/C, -1082G/A promoter polymorphisms and endometriosis. Arch Gynecol Obstet. 2017;295(2):503–510. doi: 10.1007/s00404-016-4269-5. [DOI] [PubMed] [Google Scholar]
- 44.Fan YY, Chen HY, Chen W, et al. Expression of inflammatory cytokines in serum and peritoneal fluid from patients with different stages of endometriosis. Gynecol Endocrinol. 2018;34(6):507–512. doi: 10.1080/09513590.2017.1409717. [DOI] [PubMed] [Google Scholar]
- 45.Gueuvoghlanian-Silva BY, Bellelis P, Barbeiro DF, et al. Treg and NK cells related cytokines are associated with deep rectosigmoid endometriosis and clinical symptoms related to the disease. J Reprod Immunol. 2018;126:32–38. doi: 10.1016/j.jri.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Sikora J, Smycz-Kubańska M, Mielczarek-Palacz A, et al. The involvement of multifunctional TGF-β and related cytokines in pathogenesis of endometriosis. Immunol Lett. 2018;201:31–37. doi: 10.1016/j.imlet.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Drosdzol-Cop A, Skrzypulec-Plinta V, Stojko R. Serum and peritoneal fluid immunological markers in adolescent girls with chronic pelvic pain. Obstet Gynecol Surv. 2012;67(6):374–381. doi: 10.1097/OGX.0b013e31825cb12b. [DOI] [PubMed] [Google Scholar]
- 48.Chang KK, Liu LB, Jin LP, et al. IL-27 triggers IL-10 production in Th17 cells via a c-Maf/RORγt/Blimp-1 signal to promote the progression of endometriosis. Cell Death Dis. 2017;8(3):e2666. doi: 10.1038/cddis.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mier-Cabrera J, Jiménez-Zamudio L, García-Latorre E, et al. Quantitative and qualitative peritoneal immune profiles, T-cell apoptosis and oxidative stress-associated characteristics in women with minimal and mild endometriosis. BJOG. 2011;118(1):6–16. doi: 10.1111/j.1471-0528.2010.02777.x. [DOI] [PubMed] [Google Scholar]
- 50.Tabibzadeh S, Becker JL, Parsons AK. Endometriosis is associated with alterations in the relative abundance of proteins and IL-10 in the peritoneal fluid. Front Biosci. 2003;8:a70–a78. doi: 10.2741/1045. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X, Hei P, Deng L, et al. Interleukin-10 gene promoter polymorphisms and their protein production in peritoneal fluid in patients with endometriosis. Mol Hum Reprod. 2007;13(2):135–140. doi: 10.1093/molehr/gal106. [DOI] [PubMed] [Google Scholar]
- 52.Podgaec S, Dias Junior JA, Chapron C, et al. Th1 and Th2 ummune responses related to pelvic endometriosis. Rev Assoc Med Bras (1992) 2010;56(1):92–98. doi: 10.1590/S0104-42302010000100022. [DOI] [PubMed] [Google Scholar]
- 53.Wickiewicz D, Chrobak A, Gmyrek GB, et al. Diagnostic accuracy of interleukin-6 levels in peritoneal fluid for detection of endometriosis. Arch Gynecol Obstet. 2013;288(4):805–814. doi: 10.1007/s00404-013-2828-6. [DOI] [PubMed] [Google Scholar]
- 54.Li MQ, Wang Y, Chang KK, et al. CD4+Foxp3+ regulatory T cell differentiation mediated by endometrial stromal cell-derived TECK promotes the growth and invasion of endometriotic lesions. Cell Death Dis. 2014;5:e1436. doi: 10.1038/cddis.2014.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jaeger-Lansky A, Schmidthaler K, Kuessel L, et al. Local and systemic levels of cytokines and danger signals in endometriosis-affected women. J Reprod Immunol. 2018;130:7–10. doi: 10.1016/j.jri.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 56.Hassa H, Tanir HM, Tekin B, et al. Cytokine and immune cell levels in peritoneal fluid and peripheral blood of women with early- and late-staged endometriosis. Arch Gynecol Obstet. 2009;279(6):891–895. doi: 10.1007/s00404-008-0844-8. [DOI] [PubMed] [Google Scholar]
- 57.Andreoli CG, Genro VK, Souza CA, et al. T helper (Th)1, Th2, and Th17 interleukin pathways in infertile patients with minimal/mild endometriosis. Fertil Steril. 2011;95(8):2477–2480. doi: 10.1016/j.fertnstert.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 58.Jiang J, Jiang Z, Xue M. Serum and peritoneal fluid levels of interleukin-6 and interleukin-37 as biomarkers for endometriosis. Gynecol Endocrinol. 2019;11:1–5. doi: 10.1080/09513590.2018.1554034. [DOI] [PubMed] [Google Scholar]
- 59.Xie J, Wang S, He B, et al. Association of estrogen receptor alpha and interleukin-10 gene polymorphisms with endometriosis in a Chinese population. Fertil Steril. 2009;92(1):54–60. doi: 10.1016/j.fertnstert.2008.04.069. [DOI] [PubMed] [Google Scholar]
- 60.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 61.Chegini N, Roberts M, Ripps B. Differential expression of interleukins (IL)-13 and IL-15 in ectopic and eutopic endometrium of women with endometriosis and normal fertile women. Am J Reprod Immunol. 2003;49(2):75–83. doi: 10.1034/j.1600-0897.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 62.Lee YH, Cui L, Fang J, et al. Limited value of pro-inflammatory oxylipins and cytokines as circulating biomarkers in endometriosis—a targeted ‘omics study. Sci Rep. 2016;6:26117. doi: 10.1038/srep26117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jørgensen H, Hill AS, Beste MT, et al. Peritoneal fluid cytokines related to endometriosis in patients evaluated for infertility. Fertil Steril. 2017;107(5):1191. doi: 10.1016/j.fertnstert.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 64.Han G, Li F, Singh TP, et al. The pro-inflammatory role of TGFβ1: a paradox? Int J Biol Sci. 2012;8(2):228–235. doi: 10.7150/ijbs.8.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shull MM, Ormsby I, Kier AB, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li MO, Wan YY, Sanjabi S, et al. Transforming growth factor-β regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 67.Lee HJ, Kim H, Ku SY, et al. Transforming growth factor-β1 gene polymorphisms in Korean women with endometriosis. Am J Reprod Immunol. 2011;66(5):428–434. doi: 10.1111/j.1600-0897.2011.01009.x. [DOI] [PubMed] [Google Scholar]
- 68.Komiyama S, Aoki D, Komiyama M, et al. Local activation of TGF-beta1 at endometriosis sites. J Reprod Med. 2007;52(4):306–312. [PubMed] [Google Scholar]
- 69.Meng Q, Sun W, Jiang J, et al. Identification of common mechanisms between endometriosis and ovarian cancer. J Assist Reprod Genet. 2011;28(10):917–923. doi: 10.1007/s10815-011-9573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu YX, Xiu YL, Chen X, et al. Transforming growth factor-beta 1 involved in the pathogenesis of endometriosis through regulating expression of vascular endothelial growth factor under hypoxia. Chin Med J (Engl) 2017;130(8):950–956. doi: 10.4103/0366-6999.204112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi LB, Zhou F, Zhu HY, et al. Transforming growth factor beta1 from endometriomas promotes fibrosis in surrounding ovarian tissues via Smad2/3 signaling. Biol Reprod. 2017;97(6):873–882. doi: 10.1093/biolre/iox140. [DOI] [PubMed] [Google Scholar]
- 72.Choi HJ, Park MJ, Kim BS, et al. Transforming growth factor β1 enhances adhesion of endometrial cells to mesothelium by regulating integrin expression. BMB Rep. 2017;50(8):429–434. doi: 10.5483/BMBRep.2017.50.8.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cai X, Shen M, Liu X, et al. Reduced expression of eukaryotic translation initiation factor 3 subunit e and its possible involvement in the epithelial-mesenchymal transition in endometriosis. Reprod Sci. 2018;25(1):102–109. doi: 10.1177/1933719117702248. [DOI] [PubMed] [Google Scholar]
- 74.Podgaec S, Rizzo LV, Fernandes LF, et al. CD4(+) CD25(high) Foxp3(+) cells increased in the peritoneal fluid of patients with endometriosis. Am J Reprod Immunol. 2012;68(4):301–308. doi: 10.1111/j.1600-0897.2012.01173.x. [DOI] [PubMed] [Google Scholar]
- 75.Young VJ, Brown JK, Maybin J, et al. Transforming growth factor-β induced Warburg-like metabolic reprogramming may underpin the development of peritoneal endometriosis. J Clin Endocrinol Metab. 2014;99(9):3450–3459. doi: 10.1210/jc.2014-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Young VJ, Brown JK, Saunders PT, et al. The peritoneum is both a source and target of TGF-β in women with endometriosis. PLoS One. 2014;9(9):e106773. doi: 10.1371/journal.pone.0106773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hanada T, Tsuji S, Nakayama M, et al. Suppressive regulatory T cells and latent transforming growth factor-β-expressing macrophages are altered in the peritoneal fluid of patients with endometriosis. Reprod Biol Endocrinol. 2018;16(1):9. doi: 10.1186/s12958-018-0325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaiko GE, Phipps S, Angkasekwinai P, et al. NK cell deficiency predisposes to viral-induced Th2-type allergic inflammation via epithelial-derived IL-25. J Immunol. 2010;185(8):4681–4690. doi: 10.4049/jimmunol.1001758. [DOI] [PubMed] [Google Scholar]
- 79.Gregory LG, Mathie SA, Walker SA, et al. Overexpression of Smad2 drives house dust mite-mediated airway remodeling and airway hyperresponsiveness via activin and IL-25. Am J Respir Crit Care Med. 2010;182(2):143–154. doi: 10.1164/rccm.200905-0725OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Y, Wang Y, Li MQ, et al. IL-25 promotes Th2 bias by upregulating IL-4 and IL-10 expression of decidual γδT cells in early pregnancy. Exp Ther Med. 2018;15(2):1855–1862. doi: 10.3892/etm.2017.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rank MA, Kobayashi T, Kozaki H, et al. IL-33-activated dendritic cells induce an atypical TH2-type response. J Allergy Clin Immunol. 2009;123(5):1047–1054. doi: 10.1016/j.jaci.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu WT, Huang LL, Li MQ, et al. Decidual stromal cell-derived IL-33 contributes to Th2 bias and inhibits decidual NK cell cytotoxicity through NF-κB signaling in human early pregnancy. J Reprod Immunol. 2015;109:52–65. doi: 10.1016/j.jri.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 83.Guo PF, Du MR, Wu HX, et al. Thymic stromal lymphopoietin from trophoblasts induces dendritic cell-mediated regulatory TH2 bias in the decidua during early gestation in humans. Blood. 2010;16(12):2061–2069. doi: 10.1182/blood-2009-11-252940. [DOI] [PubMed] [Google Scholar]
- 84.Xie F, Liu LB, Shang WQ, et al. The infiltration and functional regulation of eosinophils induced by TSLP promote the proliferation of cervical cancer cell. Cancer Lett. 2015;364(2):106–117. doi: 10.1016/j.canlet.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 85.Pattarini L, Trichot C, Bogiatzi S, et al. TSLP-activated dendritic cells induce human T follicular helper cell differentiation through OX40-ligand. J Exp Med. 2017;214(5):1529–1546. doi: 10.1084/jem.20150402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ochiai S, Jagot F, Kyle RL, et al. Thymic stromal lymphopoietin drives the development of IL-13+ Th2 cells. Proc Natl Acad Sci USA. 2018;115(5):1033–1038. doi: 10.1073/pnas.1714348115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saenz SA, Siracusa MC, Perrigoue JG, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464(7293):1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mjösberg JM, Trifari S, Crellin NK, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12(11):1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 89.Han M, Rajput C, Hong JY, et al. The innate cytokines IL-25, IL-33, and TSLP cooperate in the induction of type 2 innate lymphoid cell expansion and mucous metaplasia in rhinovirus-infected immature mice. J Immunol. 2017;199(4):1308–1318. doi: 10.4049/jimmunol.1700216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Halim TY, Krauss RH, Sun AC, et al. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36(3):451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 91.Matalliotakis I, Cakmak H, Matalliotakis M, et al. High rate of allergies among women with endometriosis. J Obstet Gynaecol. 2012;32(3):291–293. doi: 10.3109/01443615.2011.644358. [DOI] [PubMed] [Google Scholar]
- 92.Bungum HF, Vestergaard C, Knudsen UB. Endometriosis and type 1 allergies/immediate type hypersensitivity: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2014;179:209–215. doi: 10.1016/j.ejogrb.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 93.Santulli P, Borghese B, Chouzenoux S, et al. Serum and peritoneal interleukin-33 levels are elevated in deeply infiltrating endometriosis. Hum Reprod. 2012;27(7):2001–2009. doi: 10.1093/humrep/des154. [DOI] [PubMed] [Google Scholar]
- 94.Urata Y, Osuga Y, Izumi G, et al. Interleukin-1β stimulates the secretion of thymic stromal lymphopoietin (TSLP) from endometrioma stromal cells: possible involvement of TSLP in endometriosis. Hum Reprod. 2012;27(10):3028–3035. doi: 10.1093/humrep/des291. [DOI] [PubMed] [Google Scholar]
- 95.Mbarik M, Kaabachi W, Henidi B, et al. Soluble ST2 and IL-33: Potential markers of endometriosis in the Tunisian population. Immunol Lett. 2015;166(1):1–5. doi: 10.1016/j.imlet.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 96.Miller JE, Monsanto SP, Ahn SH, et al. Interleukin-33 modulates inflammation in endometriosis. Sci Rep. 2017;7(1):17903. doi: 10.1038/s41598-017-18224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bungum HF, Nygaard U, Vestergaard C, et al. Increased IL-25 levels in the peritoneal fluid of patients with endometriosis. J Reprod Immunol. 2016;114:6–9. doi: 10.1016/j.jri.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 98.Kondera-Anasz Z, Sikora J, Mielczarek-Palacz A, et al. Concentrations of interleukin (IL)-1alpha, IL-1 soluble receptor type II (IL-1 sRII) and IL-1 receptor antagonist (IL-1 Ra) in the peritoneal fluid and serum of infertile women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2005;123(2):198–203. doi: 10.1016/j.ejogrb.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 99.Zhang X, Wen J, Deng L, et al. Decreased levels of peritoneal interleukin-1 receptor antagonist in patients with endometriosis and disease-related dysmenorrhea. Fertil Steril. 2007;88(3):594–599. doi: 10.1016/j.fertnstert.2006.11.155. [DOI] [PubMed] [Google Scholar]
- 100.Nold MF, Nold-Petry CA, Zepp JA, et al. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11(11):1014–1022. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bulau AM, Nold MF, Li S, Nold-Petry CA, et al. Role of caspase-1 in nuclear translocation of IL-37, release of the cytokine, and IL-37 inhibition of innate immune responses. Proc Natl Acad Sci USA. 2014;111(7):2650–2655. doi: 10.1073/pnas.1324140111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xie Y, Li Y, Cai X, et al. Interleukin-37 suppresses ICAM-1 expression in parallel with NF-κB down-regulation following TLR2 activation of human coronary artery endothelial cells. Int Immunopharmacol. 2016;38:26–30. doi: 10.1016/j.intimp.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 103.Jiang JF, Deng Y, Xue W, et al. Increased expression of interleukin 37 in the eutopic and ectopic endometrium of patients with ovarian endometriosis. Reprod Sci. 2016;23(2):244–248. doi: 10.1177/1933719115602775. [DOI] [PubMed] [Google Scholar]
- 104.Kaabachi W, Kacem O, Belhaj R, et al. Interleukin-37 in endometriosis. Immunol Lett. 2017;185:52–55. doi: 10.1016/j.imlet.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 105.Pizzo A, Salmeri FM, Ardita FV, et al. Behaviour of cytokine levels in serum and peritoneal fluid of women with endometriosis. Gynecol Obstet Investig. 2002;54(2):82–87. doi: 10.1159/000067717. [DOI] [PubMed] [Google Scholar]
- 106.Laganà AS, Vitale SG, Salmeri FM, et al. Unus pro omnibus, omnes pro uno: a novel, evidence-based, unifying theory for the pathogenesis of endometriosis. Med Hypotheses. 2017;103:10–20. doi: 10.1016/j.mehy.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 107.Paul Dmowski W, Braun DP. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):245–263. doi: 10.1016/j.bpobgyn.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 108.Bos PD, Plitas G, Rudra D, et al. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J Exp Med. 2013;210(11):2435–2466. doi: 10.1084/jem.20130762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Qiao YC, Pan YH, Ling W, et al. The Yin and Yang of regulatory T cell and therapy progress in autoimmune disease. Autoimmun Rev. 2017;16(10):1058–1070. doi: 10.1016/j.autrev.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 110.Pereira LMS, Gomes STM, Ishak R, et al. Regulatory T cell and forkhead box protein 3 as modulators of immune homeostasis. Front Immunol. 2017;8:605. doi: 10.3389/fimmu.2017.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Basta P, Majka M, Jozwicki W, et al. The frequency of CD25+CD4+ and FOXP3+ regulatory T cells in ectopic endometrium and ectopic decidua. Reprod Biol Endocrinol. 2010;8:116. doi: 10.1186/1477-7827-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Olkowska-Truchanowicz J, Bocian K, Maksym RB, et al. CD4+ CD25+ FOXP3+ regulatory T cells in peripheral blood and peritoneal fluid of patients with endometriosis. Hum Reprod. 2013;28(1):119–124. doi: 10.1093/humrep/des346. [DOI] [PubMed] [Google Scholar]
- 113.Wei C, Mei J, Tang L, et al. 1-Methyl-tryptophan attenuates regulatory T cells differentiation due to the inhibition of estrogen-IDO1-MRC2 axis in endometriosis. Cell Death Dis. 2016;7(12):e2489. doi: 10.1038/cddis.2016.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gazvani R, Templeton A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction. 2002;123(2):217–226. doi: 10.1530/rep.0.1230217. [DOI] [PubMed] [Google Scholar]
- 115.Oral E, Olive DL, Arici A. The peritoneal environment in endometriosis. Hum Reprod Update. 1996;2(5):385–398. doi: 10.1093/humupd/2.5.385. [DOI] [PubMed] [Google Scholar]
- 116.Wu MY, Ho HN, Chen SU, et al. Increase in the production of interleukin-6, interleukin-10, and interleukin-12 by lipopolysaccharide-stimulated peritoneal macrophages from women with endometriosis. Am J Reprod Immunol. 1999;41(1):106–111. doi: 10.1111/j.1600-0897.1999.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 117.Wang XQ, Yu J, Luo XZ, et al. The high level of RANTES in the ectopic milieu recruits macrophages and induces their tolerance in progression of endometriosis. J Mol Endocrinol. 2010;45(5):291–299. doi: 10.1677/JME-09-0177. [DOI] [PubMed] [Google Scholar]
- 118.Mei J, Xie XX, Li MQ, et al. Indoleamine 2,3-dioxygenase-1 (IDO1) in human endometrial stromal cells induces macrophage tolerance through interleukin-33 in the progression of endometriosis. Int J Clin Exp Pathol. 2014;7(6):2743–2757. [PMC free article] [PubMed] [Google Scholar]
- 119.Yang HL, Zhou WJ, Chang KK, et al. The crosstalk between endometrial stromal cells and macrophages impairs cytotoxicity of NK cells in endometriosis by secreting IL-10 and TGF-β. Reproduction. 2017;154(6):815–825. doi: 10.1530/REP-17-0342. [DOI] [PubMed] [Google Scholar]
- 120.Wang XQ, Zhou WJ, Luo XZ, et al. Synergistic effect of regulatory T cells and proinflammatory cytokines in angiogenesis in the endometriotic milieu. Hum Reprod. 2017;32(6):1304–1317. doi: 10.1093/humrep/dex067. [DOI] [PubMed] [Google Scholar]
- 121.Sugamata M, Ihara T, Uchiide I. Increase of activated mast cells in human endometriosis. Am J Reprod Immunol. 2005;53(3):120–125. doi: 10.1111/j.1600-0897.2005.00254.x. [DOI] [PubMed] [Google Scholar]
- 122.Berbic M, Fraser IS. Regulatory T cells and other leukocytes in the pathogenesis of endometriosis. J Reprod Immunol. 2011;88(2):149–155. doi: 10.1016/j.jri.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 123.Menzies FM, Shepherd MC, Nibbs RJ, et al. The role of mast cells and their mediators in reproduction, pregnancy and labour. Hum Reprod Update. 2011;17(3):383–396. doi: 10.1093/humupd/dmq053. [DOI] [PubMed] [Google Scholar]
- 124.Mariuzzi L, Domenis R, Orsaria M, et al. Functional expression of aryl hydrocarbon receptor on mast cells populating human endometriotic tissues. Lab Investig. 2016;96(9):959–971. doi: 10.1038/labinvest.2016.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4(12):941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 127.Chen H, Qin S, Lei A, et al. Expansion of monocytic myeloid-derived suppressor cells in endometriosis patients: a pilot study. Int Immunopharmacol. 2017;47:150–158. doi: 10.1016/j.intimp.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 128.Zhang T, Zhou J, Man GCW, et al. MDSCs drive the process of endometriosis by enhancing angiogenesis and are a new potential therapeutic target. Eur J Immunol. 2018;48(6):1059–1073. doi: 10.1002/eji.201747417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sun Y, Shao J, Jiang F, et al. CD33+ CD14+ CD11b+ HLA-DR− monocytic myeloid-derived suppressor cells recruited and activated by CCR9/CCL25 are crucial for the pathogenic progression of endometriosis. Am J Reprod Immunol. 2018;30:e13067. doi: 10.1111/aji.13067. [DOI] [PubMed] [Google Scholar]
- 130.Chen Y, Wang K, Xu Y, et al. Alteration of myeloid-derived suppressor cells, chronic inflammatory cytokines, and exosomal miRNA contribute to the peritoneal immune disorder of patients with endometriosis. Reprod Sci. 2018;19:1933719118808923. doi: 10.1177/1933719118808923. [DOI] [PubMed] [Google Scholar]
- 131.Ouyang Z, Osuga Y, Hirota Y, et al. Interleukin-4 induces expression of eotaxin in endometriotic stromal cells. Fertil Steril. 2010;94(1):58–62. doi: 10.1016/j.fertnstert.2009.01.129. [DOI] [PubMed] [Google Scholar]
- 132.Sui C, Mecha E, Omwandho CO, et al. PAI-1 secretion of endometrial and endometriotic cells is Smad2/3- and ERK1/2-dependent and influences cell adhesion. Am J Transl Res. 2016;8(5):2394–2402. [PMC free article] [PubMed] [Google Scholar]
- 133.Roberts M, Luo X, Chegini N. Differential regulation of interleukins IL-13 and IL-15 by ovarian steroids, TNF-alpha and TGF-beta in human endometrial epithelial and stromal cells. Mol Hum Reprod. 2005;11(10):751–760. doi: 10.1093/molehr/gah233. [DOI] [PubMed] [Google Scholar]
- 134.Chang KK, Liu LB, Li H, et al. TSLP induced by estrogen stimulates secretion of MCP-1 and IL-8 and growth of human endometrial stromal cells through JNK and NF-κB signal pathways. Int J Clin Exp Pathol. 2014;7(5):1889–1899. [PMC free article] [PubMed] [Google Scholar]
- 135.Yang HL, Chang KK, Mei J, et al. Estrogen restricts the apoptosis of endometrial stromal cells by promoting TSLP secretion. Mol Med Rep. 2018;18(5):4410–4416. doi: 10.3892/mmr.2018.9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gargett CE. Identification and characterisation of human endometrial stem/progenitor cells. Aust N Z J Obstet Gynaecol. 2006;46(3):250–253. doi: 10.1111/j.1479-828X.2006.00582.x. [DOI] [PubMed] [Google Scholar]
- 137.Gargett CE, Schwab KE, Zillwood RM, et al. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80(6):1136–1145. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nikoo S, Ebtekar M, Jeddi-Tehrani M, et al. Menstrual blood-derived stromal stem cells from women with and without endometriosis reveal different phenotypic and functional characteristics. Mol Hum Reprod. 2014;20(9):905–918. doi: 10.1093/molehr/gau044. [DOI] [PubMed] [Google Scholar]
- 139.Gargett CE, Masuda H. Adult stem cells in the endometrium. Mol Hum Reprod. 2010;16(11):818–834. doi: 10.1093/molehr/gaq061. [DOI] [PubMed] [Google Scholar]
- 140.Kao AP, Wang KH, Chang CC, et al. Comparative study of human eutopic and ectopic endometrial mesenchymal stem cells and the development of an in vivo endometriotic invasion model. Fertil Steril. 2011;95(4):1308–1315. doi: 10.1016/j.fertnstert.2010.09.064. [DOI] [PubMed] [Google Scholar]
- 141.Gurung S, Deane JA, Masuda H, et al. Stem cells in endometrial physiology. Semin Reprod Med. 2015;33(5):326–332. doi: 10.1055/s-0035-1558405. [DOI] [PubMed] [Google Scholar]
- 142.Li F, Alderman MH, 3rd, Tal A, et al. Hematogenous dissemination of mesenchymal stem cells from endometriosis. Stem Cells. 2018;36(6):881–890. doi: 10.1002/stem.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Koippallil Gopalakrishnan Nair AR, Pandit H, Warty N, et al. Endometriotic mesenchymal stem cells exhibit a distinct immune phenotype. Int Immunol. 2015;27(4):195–204. doi: 10.1093/intimm/dxu103. [DOI] [PubMed] [Google Scholar]
- 144.Li J, Dai Y, Zhu H, et al. Endometriotic mesenchymal stem cells significantly promote fibrogenesis in ovarian endometrioma through the Wnt/β-catenin pathway by paracrine production of TGF-β1 and Wnt1. Hum Reprod. 2016;31(6):1224–1235. doi: 10.1093/humrep/dew058. [DOI] [PubMed] [Google Scholar]
- 145.Kokoroishi K, Nakashima A, Doi S, et al. High glucose promotes TGF-β1 production by inducing FOS expression in human peritoneal mesothelial cells. Clin Exp Nephrol. 2016;20(1):30–38. doi: 10.1007/s10157-015-1128-9. [DOI] [PubMed] [Google Scholar]
- 146.Young VJ, Ahmad SF, Duncan WC, et al. The role of TGF-β in the pathophysiology of peritoneal endometriosis. Hum Reprod Update. 2017;23(5):548–559. doi: 10.1093/humupd/dmx016. [DOI] [PubMed] [Google Scholar]
- 147.Omwandho CO, Konrad L, Halis G, et al. Role of TGF-betas in normal human endometrium and endometriosis. Hum Reprod. 2010;25(1):101–109. doi: 10.1093/humrep/dep382. [DOI] [PubMed] [Google Scholar]
- 148.Brosens IA. Endometriosis–a disease because it is characterized by bleeding. Am J Obstet Gynecol. 1997;176(2):263–267. doi: 10.1016/S0002-9378(97)70482-4. [DOI] [PubMed] [Google Scholar]
- 149.Ding D, Liu X, Duan J, et al. Platelets are an unindicted culprit in the development of endometriosis: clinical and experimental evidence. Hum Reprod. 2015;30(4):812–832. doi: 10.1093/humrep/dev025. [DOI] [PubMed] [Google Scholar]
- 150.Guo SW, Ding D, Geng JG, et al. P-selectin as a potential therapeutic target for endometriosis. Fertil Steril. 2015;103(4):990–1000. doi: 10.1016/j.fertnstert.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 151.Zhang Q, Ding D, Liu X, et al. Activated platelets induce estrogen receptor β expression in endometriotic stromal cells. Gynecol Obstet Investig. 2015;80(3):187–192. doi: 10.1159/000377629. [DOI] [PubMed] [Google Scholar]
- 152.Smyth SS, McEver RP, Weyrich AS, et al. Platelet colloquium participants. Platelet functions beyond hemostasis. J Thromb Haemost. 2009;7(11):1759–1766. doi: 10.1111/j.1538-7836.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- 153.Wu Q, Ding D, Liu X, et al. Evidence for a hypercoagulable state in women with ovarian endometriomas. Reprod Sci. 2015;22(9):1107–1114. doi: 10.1177/1933719115572478. [DOI] [PubMed] [Google Scholar]
- 154.Li XJ, Zhou M, Li XH, et al. Effects of Tanshinone IIa on cytokines and platelets in immune vasculitis and its mechanism. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2009;17(1):188–192. [PubMed] [Google Scholar]
- 155.Shang Q, Xu H, Huang L. Tanshinone IIA: a promising natural cardioprotective agent. Evid Based Complement Alternat Med. 2012;2012:716459. doi: 10.1155/2012/716459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang Q, Liu X, Guo SW. Progressive development of endometriosis and its hindrance by anti-platelet treatment in mice with induced endometriosis. Reprod Biomed Online. 2017;34(2):124–136. doi: 10.1016/j.rbmo.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 157.Guo SW, Du Y, Liu X. Endometriosis-derived stromal cells secrete thrombin and thromboxane A2, inducing platelet activation. Reprod Sci. 2016;23(8):1044–1052. doi: 10.1177/1933719116630428. [DOI] [PubMed] [Google Scholar]
- 158.Guo SW, Du Y, Liu X. Platelet-derived TGF-β1 mediates the down-modulation of NKG2D expression and may be responsible for impaired natural killer (NK) cytotoxicity in women with endometriosis. Hum Reprod. 2016;31(7):1462–1474. doi: 10.1093/humrep/dew057. [DOI] [PubMed] [Google Scholar]
- 159.Du Y, Liu X, Guo SW. Platelets impair natural killer cell reactivity and function in endometriosis through multiple mechanisms. Hum Reprod. 2017;32(4):794–810. doi: 10.1093/humrep/dex014. [DOI] [PubMed] [Google Scholar]
- 160.Liu XT, Sun HT, Zhang ZF, et al. Indoleamine 2,3-dioxygenase suppresses the cytotoxicity of NK cells in response to ectopic endometrial stromal cells in endometriosis. Reproduction. 2018;156(5):397–404. doi: 10.1530/REP-18-0112. [DOI] [PubMed] [Google Scholar]
- 161.Jovanovic I, Radosavljevic G, Mitrovic M, et al. ST2 deletion enhances innate and acquired immunity to murine mammary carcinoma. Eur J Immunol. 2011;1(7):1902–1912. doi: 10.1002/eji.201141417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mancini F, Milardi D, Carfagna P, et al. Low-dose SKA Progesterone and Interleukin-10 modulate the inflammatory pathway in endometriotic cell lines. Int Immunopharmacol. 2018;55:223–230. doi: 10.1016/j.intimp.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 163.Jiang J, Yu K, Jiang Z, et al. IL-37 affects the occurrence and development of endometriosis by regulating the biological behavior of endometrial stromal cells through multiple signaling pathways. Biol Chem. 2018;399(11):1325–1337. doi: 10.1515/hsz-2018-0254. [DOI] [PubMed] [Google Scholar]
- 164.Yang WV, Au HK, Chang CW, et al. Matrix remodeling and endometriosis. Reprod Med Biol. 2005;4(2):93–99. doi: 10.1111/j.1447-0578.2005.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ulukus M, Cakmak H, Arici A. The role of endometrium in endometriosis. J Soc Gynecol Investig. 2006;13(7):467–476. doi: 10.1016/j.jsgi.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 166.Szymanowski K. Apoptosis pattern in human endometrium in women with pelvic endometriosis. Eur J Obstet Gynecol Reprod Biol. 2007;132(1):107–110. doi: 10.1016/j.ejogrb.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 167.Li MQ, Luo XZ, Meng YH, et al. CXCL8 enhances proliferation and growth and reduces apoptosis in endometrial stromal cells in an autocrine manner via a CXCR1-triggered PTEN/AKT signal pathway. Hum Reprod. 2012;27(7):2107–2116. doi: 10.1093/humrep/des132. [DOI] [PubMed] [Google Scholar]
- 168.Li MQ, Shao J, Meng YH, et al. NME1 suppression promotes growth, adhesion and implantation of endometrial stromal cells via Akt and MAPK/Erk1/2 signal pathways in the endometriotic milieu. Hum Reprod. 2013;28(10):2822–2831. doi: 10.1093/humrep/det248. [DOI] [PubMed] [Google Scholar]
- 169.Li X, Zhang Y, Zhao L, et al. Whole-exome sequencing of endometriosis identifies frequent alterations in genes involved in cell adhesion and chromatin-remodeling complexes. Hum Mol Genet. 2014;23(22):6008–6021. doi: 10.1093/hmg/ddu330. [DOI] [PubMed] [Google Scholar]
- 170.Mei J, Zhu XY, Jin LP, et al. Estrogen promotes the survival of human secretory phase endometrial stromal cells via CXCL12/CXCR4 up-regulation-mediated autophagy inhibition. Hum Reprod. 2015;30(7):1677–1689. doi: 10.1093/humrep/dev100. [DOI] [PubMed] [Google Scholar]
- 171.Yang HL, Mei J, Chang KK, et al. Autophagy in endometriosis. Am J Transl Res. 2017;9(11):4707–4725. [PMC free article] [PubMed] [Google Scholar]
- 172.OuYang Z, Hirota Y, Osuga Y, et al. Interleukin-4 stimulates proliferation of endometriotic stromal cells. Am J Pathol. 2008;173(2):463–469. doi: 10.2353/ajpath.2008.071044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Quattrone F, Sanchez AM, Pannese M, et al. The targeted delivery of interleukin 4 inhibits development of endometriotic lesions in a mouse model. Reprod Sci. 2015;22(9):1143–1152. doi: 10.1177/1933719115578930. [DOI] [PubMed] [Google Scholar]
- 174.Schwager K, Bootz F, Imesch P, et al. The antibody-mediated targeted delivery of interleukin-10 inhibits endometriosis in a syngeneic mouse model. Hum Reprod. 2011;26(9):2344–2352. doi: 10.1093/humrep/der195. [DOI] [PubMed] [Google Scholar]
- 175.Liu Q, Ma P, Liu L, et al. Evaluation of PLGA containing anti-CTLA4 inhibited endometriosis progression by regulating CD4+CD25+ Treg cells in peritoneal fluid of mouse endometriosis model. Eur J Pharm Sci. 2017;96:542–550. doi: 10.1016/j.ejps.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 176.Chegini N. TGF-beta system: the principal profibrotic mediator of peritoneal adhesion formation. Semin Reprod Med. 2008;26(4):298–312. doi: 10.1055/s-0028-1082388. [DOI] [PubMed] [Google Scholar]
- 177.Bristol-Gould SK, Hutten CG, Sturgis C, et al. The development of a mouse model of ovarian endosalpingiosis. Endocrinology. 2005;146(12):5228–5236. doi: 10.1210/en.2005-0697. [DOI] [PubMed] [Google Scholar]
- 178.Correa LF, Zheng Y, Delaney AA, et al. TGF-β induces endometriotic progression via a noncanonical, KLF11-mediated mechanism. Endocrinology. 2016;157(9):3332–3343. doi: 10.1210/en.2016-1194. [DOI] [PubMed] [Google Scholar]
- 179.Lin X, Dai Y, Xu W, et al. Hypoxia promotes ectopic adhesion ability of endometrial stromal cells via TGF-β1/Smad signaling in endometriosis. Endocrinology. 2018;159(4):1630–1641. doi: 10.1210/en.2017-03227. [DOI] [PubMed] [Google Scholar]
- 180.Tani H, Sato Y, Ueda M, et al. Role of versican in the pathogenesis of peritoneal endometriosis. J Clin Endocrinol Metab. 2016;101(11):4349–4356. doi: 10.1210/jc.2016-2391. [DOI] [PubMed] [Google Scholar]
- 181.Au HK, Chang JH, Wu YC, et al. TGF-βI regulates cell migration through pluripotent transcription factor OCT4 in endometriosis. PLoS One. 2015;10(12):e0145256. doi: 10.1371/journal.pone.0145256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang Q, Duan J, Liu X, et al. Platelets drive smooth muscle metaplasia and fibrogenesis in endometriosis through epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation. Mol Cell Endocrinol. 2016;428:1–16. doi: 10.1016/j.mce.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 183.Liu YG, Tekmal RR, Binkley PA, et al. Induction of endometrial epithelial cell invasion and c-fms expression by transforming growth factor beta. Mol Hum Reprod. 2009;15(10):665–673. doi: 10.1093/molehr/gap043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Stocks MM, Crispens MA, Ding T, et al. Therapeutically targeting the inflammasome product in a chimeric model of endometriosis-related surgical adhesions. Reprod Sci. 2017;24(8):1121–1128. doi: 10.1177/1933719117698584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yang YM, Yang WX. Epithelial-to-mesenchymal transition in the development of endometriosis. Oncotarget. 2017;8(25):41679–41689. doi: 10.18632/oncotarget.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bilyk O, Coatham M, Jewer M, et al. Epithelial-to-mesenchymal transition in the female reproductive tract: from normal functioning to disease pathology. Front Oncol. 2017;7:145. doi: 10.3389/fonc.2017.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Matsuzaki S, Darcha C, Pouly JL, et al. Effects of matrix stiffness on epithelial to mesenchymal transition-like processes of endometrial epithelial cells: implications for the pathogenesis of endometriosis. Sci Rep. 2017;7:44616. doi: 10.1038/srep44616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Matsuzaki S, Darcha C. Antifibrotic properties of epigallocatechin-3-gallate in endometriosis. Hum Reprod. 2014;29(8):1677–1687. doi: 10.1093/humrep/deu123. [DOI] [PubMed] [Google Scholar]
- 189.Matsuzaki S, Canis M, Pouly JL, et al. Soft matrices inhibit cell proliferation and inactivate the fibrotic phenotype of deep endometriotic stromal cells in vitro. Hum Reprod. 2016;31(3):541–553. doi: 10.1093/humrep/dev333. [DOI] [PubMed] [Google Scholar]
- 190.Zeng X, Yue Z, Gao Y, et al. NR4A1 is involved in fibrogenesis in ovarian endometriosis. Cell Physiol Biochem. 2018;46(3):1078–1090. doi: 10.1159/000488838. [DOI] [PubMed] [Google Scholar]
- 191.Itoga T, Matsumoto T, Takeuchi H, et al. Fibrosis and smooth muscle metaplasia in rectovaginal endometriosis. Pathol Int. 2003;53:371–375. doi: 10.1046/j.1440-1827.2003.01483.x. [DOI] [PubMed] [Google Scholar]
- 192.Sandoval P, Jiménez-Heffernan JA, Guerra-Azcona G, et al. Mesothelial-to-mesenchymal transition in the pathogenesis of post-surgical peritoneal adhesions. J Pathol. 2016;239(1):48–59. doi: 10.1002/path.4695. [DOI] [PubMed] [Google Scholar]
- 193.Ji X, Li J, Xu L, et al. IL4 and IL-17A provide a Th2/Th17-polarized inflammatory milieu in favor of TGF-β1 to induce bronchial epithelial-mesenchymal transition (EMT) Int J Clin Exp Pathol. 2013;6(8):1481–1492. [PMC free article] [PubMed] [Google Scholar]
- 194.Liu CY, Xu JY, Shi XY, et al. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Investig. 2013;93(7):844–854. doi: 10.1038/labinvest.2013.69. [DOI] [PubMed] [Google Scholar]
- 195.Cao H, Zhang J, Liu H, et al. IL-13/STAT6 signaling plays a critical role in the epithelial-mesenchymal transition of colorectal cancer cells. Oncotarget. 2016;7(38):61183–61198. doi: 10.18632/oncotarget.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Xu Z, Zhao C, Wang Z, et al. Interleukin-33 levels are elevated in chronic allograft dysfunction of kidney transplant recipients and promotes epithelial to mesenchymal transition of human kidney (HK-2) cells. Gene. 2018;644:113–121. doi: 10.1016/j.gene.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 197.Jiang M, Wang Y, Zhang H, et al. IL-37 inhibits invasion and metastasis in non-small cell lung cancer by suppressing the IL-6/STAT3 signaling pathway. Thorac Cancer. 2018;9(5):621–629. doi: 10.1111/1759-7714.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang T, Liu Y, Zou JF, et al. Interleukin-17 induces human alveolar epithelial to mesenchymal cell transition via the TGF-β1 mediated Smad2/3 and ERK1/2 activation. PLoS One. 2017;12(9):e0183972. doi: 10.1371/journal.pone.0183972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Weng SY, Wang X, Vijayan S, et al. IL-4 receptor alpha signaling through macrophages differentially regulates liver fibrosis progression and reversal. EBioMedicine. 2018;29:92–103. doi: 10.1016/j.ebiom.2018.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Santulli P, Even M, Chouzenoux S, et al. Profibrotic interleukin-33 is correlated with uterine leiomyoma tumour burden. Hum Reprod. 2013;28(8):2126–2133. doi: 10.1093/humrep/det238. [DOI] [PubMed] [Google Scholar]
- 201.Tang J, Huang H, Ji X, et al. Involvement of IL-13 and tissue transglutaminase in liver granuloma and fibrosis after Schistosoma japonicum infection. Mediat Inflamm. 2014;2014:753483. doi: 10.1155/2014/753483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhang LJ, Zheng WD, Chen YX, et al. Antifibrotic effects of interleukin-10 on experimental hepatic fibrosis. Hepatogastroenterology. 2007;54(79):2092–2098. [PubMed] [Google Scholar]
- 203.Djokovic D, Calhaz-Jorge C. Angiogenesis as a therapeutic target in endometriosis. Acta Med Port. 2014;27(4):489–497. doi: 10.20344/amp.5244. [DOI] [PubMed] [Google Scholar]
- 204.Marí-Alexandre J, García-Oms J, Barceló-Molina M, et al. MicroRNAs and angiogenesis in endometriosis. Thromb Res. 2015;135(Suppl 1):S38–S40. doi: 10.1016/S0049-3848(15)50439-8. [DOI] [PubMed] [Google Scholar]
- 205.Salcedo R, Young HA, Ponce ML, et al. Eotaxin (CCL11) induces in vivo angiogenic responses by human CCR3+ endothelial cells. J Immunol. 2001;166(12):7571–7578. doi: 10.4049/jimmunol.166.12.7571. [DOI] [PubMed] [Google Scholar]
- 206.Na YJ, Yang SH, Baek DW, et al. Effects of peritoneal fluid from endometriosis patients on the release of vascular endothelial growth factor by neutrophils and monocytes. Hum Reprod. 2006;21(7):1846–1855. doi: 10.1093/humrep/del077. [DOI] [PubMed] [Google Scholar]
- 207.Young VJ, Ahmad SF, Brown JK, et al. Peritoneal VEGF-A expression is regulated by TGF-β1 through an ID1 pathway in women with endometriosis. Sci Rep. 2015;5:16859. doi: 10.1038/srep16859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Fujimoto J, Sakaguchi H, Aoki I, et al. Clinical implications of expression of interleukin-8 related to angiogenesis in uterine cervical cancers. Cancer Res. 2000;60:2632–2635. [PubMed] [Google Scholar]
- 209.Xie F, Meng YH, Liu LB, et al. Cervical carcinoma cells stimulate the angiogenesis through TSLP promoting growth and activation of vascular endothelial cells. Am J Reprod Immunol. 2013;70(1):69–79. doi: 10.1111/aji.12104. [DOI] [PubMed] [Google Scholar]
- 210.Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 211.Urata Y, Osuga Y, Akiyama I, et al. Interleukin-4 and prostaglandin E2 synergistically up-regulate 3β-hydroxysteroid dehydrogenase type 2 in endometrioma stromal cells. J Clin Endocrinol Metab. 2013;98(4):1583–1590. doi: 10.1210/jc.2012-3475. [DOI] [PubMed] [Google Scholar]
- 212.Wang Y, Yu J, Luo X, et al. Abnormal regulation of chemokine TECK and its receptor CCR9 in the endometriotic milieu is involved in pathogenesis of endometriosis by way of enhancing invasiveness of endometrial stromal cells. Cell Mol Immunol. 2010;7(1):51–60. doi: 10.1038/cmi.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Reis FM, Petraglia F, Taylor RN. Endometriosis: hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum Reprod Update. 2013;19(4):406–418. doi: 10.1093/humupd/dmt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Borrelli GM, Carvalho KI, Kallas EG, et al. Chemokines in the pathogenesis of endometriosis and infertility. J Reprod Immunol. 2013;98(1–2):1–9. doi: 10.1016/j.jri.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 215.Borrelli GM, Abrão MS, Mechsner S. Can chemokines be used as biomarkers for endometriosis? A systematic review. Hum Reprod. 2014;29(2):253–266. doi: 10.1093/humrep/det401. [DOI] [PubMed] [Google Scholar]
- 216.Wu RF, Yang HM, Zhou WD, et al. Effect of interleukin-1β and lipoxin A4 in human endometriotic stromal cells: proteomic analysis. J Obstet Gynaecol Res. 2017;43(2):308–319. doi: 10.1111/jog.13201. [DOI] [PubMed] [Google Scholar]
- 217.Mei J, Zhou WJ, Zhu XY, et al. Suppression of autophagy and HCK signaling promotes PTGS2high FCGR3- NK cell differentiation triggered by ectopic endometrial stromal cells. Autophagy. 2018;14(8):1376–1397. doi: 10.1080/15548627.2018.1476809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89(4):587–596. doi: 10.1016/S0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 220.Farrar JD, Asnagli H, Murphy KM. T helper subset development: roles of instruction, selection, and transcription. J Clin Investig. 2002;109(4):431–435. doi: 10.1172/JCI0215093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chen P, Wang DB, Liang YM. Evaluation of estrogen in endometriosis patients: regulation of GATA-3 in endometrial cells and effects on Th2 cytokines. J Obstet Gynaecol Res. 2016;42(6):669–677. doi: 10.1111/jog.12957. [DOI] [PubMed] [Google Scholar]
- 222.Saia RS, Bertozi G, Cunha FQ, et al. Estradiol and thermoregulation in adult endotoxemic rats exposed to lipopolysaccharide in neonatal life. Acta Physiol (Oxf) 2011;203(4):429–439. doi: 10.1111/j.1748-1716.2011.02334.x. [DOI] [PubMed] [Google Scholar]
- 223.Zhang Y, Jin LP. Effects of TSLP on obstetrical and gynecological diseases. Am J Reprod Immunol. 2017 doi: 10.1111/aji.12612. [DOI] [PubMed] [Google Scholar]
- 224.Wang G, Tokushige N, Russell P, et al. Neuroendocrine cells in eutopic endometrium of women with endometriosis. Hum Reprod. 2010;25(2):387–391. doi: 10.1093/humrep/dep379. [DOI] [PubMed] [Google Scholar]
- 225.Kempuraj D, Papadopoulou N, Stanford EJ, et al. Increased numbers of activated mast cells in endometriosis lesions positive for corticotropin-releasing hormone and urocortin. Am J Reprod Immunol. 2004;52(4):267–275. doi: 10.1111/j.1600-0897.2004.00224.x. [DOI] [PubMed] [Google Scholar]
- 226.Florio P, Reis FM, Torres PB, et al. Plasma urocortin levels in the diagnosis of ovarian endometriosis. Obstet Gynecol. 2007;110(3):594–600. doi: 10.1097/01.AOG.0000278572.86019.ae. [DOI] [PubMed] [Google Scholar]
- 227.Novembri R, Borges LE, Carrarelli P, et al. Impaired CRH and urocortin expression and function in eutopic endometrium of women with endometriosis. J Clin Endocrinol Metab. 2011;96(4):1145–1150. doi: 10.1210/jc.2010-2263. [DOI] [PubMed] [Google Scholar]
- 228.Novembri R, Carrarelli P, Toti P, et al. Urocortin 2 and urocortin 3 in endometriosis: evidence for a possible role in inflammatory response. Mol Hum Reprod. 2011;17(9):587–593. doi: 10.1093/molehr/gar020. [DOI] [PubMed] [Google Scholar]
- 229.Somigliana E, Vigano P, Barbara G, et al. Treatment of endometriosis-related pain: options and outcomes. Front Biosci (Elite Ed) 2009;1:455–465. doi: 10.2741/e41. [DOI] [PubMed] [Google Scholar]
- 230.Ferrero S, Alessandri F, Racca A, et al. Treatment of pain associated with deep endometriosis: alternatives and evidence. Fertil Steril. 2015;104(4):771–792. doi: 10.1016/j.fertnstert.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 231.Tosti C, Biscione A, Morgante G, et al. Hormonal therapy for endometriosis: from molecular research to bedside. Eur J Obstet Gynecol Reprod Biol. 2017;209:61–66. doi: 10.1016/j.ejogrb.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 232.Angioni S, Pontis A, Dessole M, et al. Pain control and quality of life after laparoscopic en-block resection of deep infiltrating endometriosis (DIE) vs. incomplete surgical treatment with or without GnRHa administration after surgery. Arch Gynecol Obstet. 2015;291(2):363–370. doi: 10.1007/s00404-014-3411-5. [DOI] [PubMed] [Google Scholar]
- 233.Velasco I, Campos A, Acién P. Changes in cytokine levels of patients with ovarian endometriosis after treatment with gonadotropin-releasing hormone analogue, ultrasound-guided drainage, and intracystic recombinant interleukin-2. Fertil Steril. 2005;83(4):873–877. doi: 10.1016/j.fertnstert.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 234.Selak V, Farquhar C, Prentice A, et al. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. 2007;4:CD000068. doi: 10.1002/14651858.CD000068.pub2. [DOI] [PubMed] [Google Scholar]
- 235.Uchiyama M, Jin X, Zhang Q, et al. Induction of regulatory CD4+ cells and prolongation of survival of fully allogeneic murine cardiac grafts by danazol. Transplant Proc. 2012;44(4):1067–1069. doi: 10.1016/j.transproceed.2012.01.103. [DOI] [PubMed] [Google Scholar]
- 236.Uchiyama M, Jin X, Zhang Q, et al. Danazol induces prolonged survival of fully allogeneic cardiac grafts and maintains the generation of regulatory CD4(+) cells in mice. Transpl Int. 2012;25(3):357–365. doi: 10.1111/j.1432-2277.2011.01427.x. [DOI] [PubMed] [Google Scholar]
- 237.Umezawa M, Tanaka N, Takeda K, et al. Clarithromycin and telithromycin increases interleukin-10 expression in the rat endometriosis model. Cytokine. 2011;55(3):339–342. doi: 10.1016/j.cyto.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 238.Maione F, De Feo V, Caiazzo E, et al. Tanshinone IIA, a major component of Salvia milthorriza Bunge, inhibits platelet activation via Erk-2 signaling pathway. J Ethnopharmacol. 2014;155(2):1236–1242. doi: 10.1016/j.jep.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 239.Jiang X, Chen Y, Zhu H, et al. Sodium Tanshinone IIA sulfonate ameliorates bladder fibrosis in a rat model of partial bladder outlet obstruction by inhibiting the TGF-β/Smad pathway activation. PLoS One. 2015;10(6):e0129655. doi: 10.1371/journal.pone.0129655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Tang H, He H, Ji H, et al. Tanshinone IIA ameliorates bleomycin-induced pulmonary fibrosis and inhibits transforming growth factor-beta-β-dependent epithelial to mesenchymal transition. J Surg Res. 2015;197(1):167–175. doi: 10.1016/j.jss.2015.02.062. [DOI] [PubMed] [Google Scholar]
- 241.Christensen LP. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 242.Wong AS, Che CM, Leung KW. Recent advances in ginseng as cancer therapeutics: a functional and mechanistic overview. Nat Prod Rep. 2015;32(2):256–272. doi: 10.1039/C4NP00080C. [DOI] [PubMed] [Google Scholar]
- 243.Xu FY, Shang WQ, Yu JJ, et al. The antitumor activity study of ginsenosides and metabolites in lung cancer cell. Am J Transl Res. 2016;8(4):1708–1718. [PMC free article] [PubMed] [Google Scholar]
- 244.Gu CJ, Cheng J, Zhang B, et al. Protopanaxadiol and metformin synergistically inhibit estrogen-mediated proliferation and anti-autophagy effects in endometrial cancer cells. Am J Transl Res. 2017;9(9):4071–4082. [PMC free article] [PubMed] [Google Scholar]
- 245.Kim MK, Lee SK, Park JH, et al. Ginsenoside Rg3 decreases fibrotic and invasive nature of endometriosis by modulating miRNA-27b: in vitro and in vivo studies. Sci Rep. 2017;7(1):17670. doi: 10.1038/s41598-017-17956-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhang B, Zhou WJ, Gu CJ, et al. The ginsenoside PPD exerts anti-endometriosis effects by suppressing estrogen receptor-mediated inhibition of endometrial stromal cell autophagy and NK cell cytotoxicity. Cell Death Dis. 2018;9(5):574. doi: 10.1038/s41419-018-0581-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Cao Y, Ye Q, Zhuang M, et al. Ginsenoside Rg3 inhibits angiogenesis in a rat model of endometriosis through the VEGFR-2-mediated PI3K/Akt/mTOR signaling pathway. PLoS One. 2017;12(11):e0186520. doi: 10.1371/journal.pone.0186520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Qin X, Liu Y, Feng Y, et al. Ginsenoside Rf alleviates dysmenorrhea and inflammation through the BDNF-TrkB-CREB pathway in a rat model of endometriosis. Food Funct. 2018 doi: 10.1039/c8fo01839a. [DOI] [PubMed] [Google Scholar]
- 249.Laschke MW, Schwender C, Scheuer C, et al. Epigallocatechin-3-gallate inhibits estrogen-induced activation of endometrial cells in vitro and causes regression of endometriotic lesions in vivo. Hum Reprod. 2008;23(10):2308–2318. doi: 10.1093/humrep/den245. [DOI] [PubMed] [Google Scholar]
- 250.Xu H, Lui WT, Chu CY, et al. Anti-angiogenic effects of green tea catechin on an experimental endometriosis mouse model. Hum Reprod. 2009;24(3):608–618. doi: 10.1093/humrep/den417. [DOI] [PubMed] [Google Scholar]
- 251.Xu H, Becker CM, Lui WT, et al. Green tea epigallocatechin-3-gallate inhibits angiogenesis and suppresses vascular endothelial growth factor C/vascular endothelial growth factor receptor 2 expression and signaling in experimental endometriosis in vivo. Fertil Steril. 2011;96(4):1021–1028. doi: 10.1016/j.fertnstert.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 252.Wang CC, Xu H, Man GC, et al. Prodrug of green tea epigallocatechin-3-gallate (Pro-EGCG) as a potent anti-angiogenesis agent for endometriosis in mice. Angiogenesis. 2013;16(1):59–69. doi: 10.1007/s10456-012-9299-4. [DOI] [PubMed] [Google Scholar]
- 253.Carvalho LF, Samadder AN, Agarwal A, et al. Oxidative stress biomarkers in patients with endometriosis: systematic review. Arch Gynecol Obstet. 2012;286(4):1033–1040. doi: 10.1007/s00404-012-2439-7. [DOI] [PubMed] [Google Scholar]
- 254.Rocha MG, Gomes VA, Tanus-Santos JE, et al. Reduction of blood nitric oxide levels is associated with clinical improvement of the chronic pelvic pain related to endometriosis. Braz J Med Biol Res. 2015;48(4):363–369. doi: 10.1590/1414-431x20143619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Chang RH, Feng MH, Liu WH, et al. Nitric oxide increased interleukin-4 expression in T lymphocytes. Immunology. 1997;90(3):364–369. doi: 10.1111/j.1365-2567.1997.00364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mier-Cabrera J, González-Gallardo S, Hernández-Guerrero C. Effect of nitric oxide and TH1/TH2 cytokine supplementation over ectopic endometrial tissue growth in a murine model of endometriosis. Reprod Sci. 2013;20(11):1332–1338. doi: 10.1177/1933719113485297. [DOI] [PubMed] [Google Scholar]
- 257.Mei J, Li MQ, Ding D, et al. Indoleamine 2,3-dioxygenase-1 (IDO1) enhances survival and invasiveness of endometrial stromal cells via the activation of JNK signaling pathway. Int J Clin Exp Pathol. 2013;6(3):431–444. [PMC free article] [PubMed] [Google Scholar]
- 258.Mei J, Chang KK, Sun HX. Immunosuppressive macrophages induced by IDO1 promote the growth of endometrial stromal cells in endometriosis. Mol Med Rep. 2017;15(4):2255–2260. doi: 10.3892/mmr.2017.6242. [DOI] [PubMed] [Google Scholar]
- 259.Weigert C, Brodbeck K, Sawadogo M, et al. Upstream stimulatory factor (USF) proteins induce human TGF-beta1 gene activation via the glucose-response element-1013/-1002 in mesangial cells: up-regulation of USF activity by the hexosamine biosynthetic pathway. J Biol Chem. 2004;279(16):15908–15915. doi: 10.1074/jbc.M313524200. [DOI] [PubMed] [Google Scholar]
- 260.Sinaii N, Clearly SD, Ballweg ML, et al. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715–2724. doi: 10.1093/humrep/17.10.2715. [DOI] [PubMed] [Google Scholar]
- 261.Wang CT, Wang DB, Liu KR, et al. Inducing malignant transformation of endometriosis in rats by long-term sustaining hyperestrogenemia and type II diabetes. Cancer Sci. 2015;106(1):43–50. doi: 10.1111/cas.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot Essent Fatty Acids. 2005;73(3–4):141–162. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 263.Mitchell S, Thomas G, Harvey K, et al. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol. 2002;13(10):2497–2507. doi: 10.1097/01.ASN.0000032417.73640.72. [DOI] [PubMed] [Google Scholar]
- 264.Kumar R, Clerc AC, Gori I, et al. Lipoxin A4 prevents the progression of de novo and established endometriosis in a mouse model by attenuating prostaglandin E2 production and estrogen signaling. PLoS One. 2014;9(2):e89742. doi: 10.1371/journal.pone.0089742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kyama CM, Overbergh L, Mihalyi A, et al. Effect of recombinant human TNF-binding protein-1 and GnRH antagonist on mRNA expression of inflammatory cytokines and adhesion and growth factors in endometrium and endometriosis tissues in baboons. Fertil Steril. 2008;89(5 Suppl):1306–1313. doi: 10.1016/j.fertnstert.2006.11.205. [DOI] [PubMed] [Google Scholar]
- 266.Daftary GS, Zheng Y, Tabbaa ZM, et al. A novel role of the Sp/KLF transcription factor KLF11 in arresting progression of endometriosis. PLoS One. 2013;8(3):e60165. doi: 10.1371/journal.pone.0060165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Falconer H, Mwenda JM, Chai DC, et al. Effects of anti-TNF-mAb treatment on pregnancy in baboons with induced endometriosis. Fertil Steril. 2008;89(5 Suppl):1537–1545. doi: 10.1016/j.fertnstert.2007.05.062. [DOI] [PubMed] [Google Scholar]