Abstract
The nervous system regulates tissue stem and precursor populations throughout life. Parallel to roles in development, the nervous system is emerging as a critical regulator of cancer, from oncogenesis to malignant growth and metastatic spread. Various preclinical models in a range of malignancies have demonstrated that nervous system activity can control cancer initiation and powerfully influence cancer progression and metastasis. Just as the nervous system can regulate cancer progression, cancer also remodels and hijacks nervous system structure and function. Interactions between the nervous system and cancer occur both in the local tumour microenvironment and systemically. Neurons and glial cells communicate directly with malignant cells in the tumour microenvironment through paracrine factors and, in some cases, through neuron-to-cancer cell synapses. Additionally, indirect interactions occur at a distance through circulating signals and through influences on immune cell trafficking and function. Such cross-talk among the nervous system, immune system and cancer—both systemically and in the local tumour microenvironment—regulates pro-tumour inflammation and anti-cancer immunity. Elucidating the neuroscience of cancer, which calls for interdisciplinary collaboration among the fields of neuroscience, developmental biology, immunology and cancer biology, may advance effective therapies for many of the most difficult to treat malignancies.
The nervous system is emerging as an important regulator of both healthy and malignant cellular niches throughout the body. Governing organ development, tissue homeostasis and regeneration throughout life, the nervous system is central to the physiological function of all organ systems, including the immune system. It is therefore not surprising that the nervous system has a similarly crucial role in a broad range of cancers. Comprising diverse neuronal and glial populations, the nervous system branches extensively throughout the body, typically travelling together with the microvasculature, to reach all tissue microenvironments except keratinized structures. This expansive network of nerves similarly reaches tumour microenvironments and can contribute in critical ways to cancer pathogenesis, from tumour initiation to progression and metastasis. In turn, the nervous system is often remodelled by the cancer it regulates. In this Review, we consider interactions with the nervous system in both central nervous system (CNS) cancers and cancers outside the CNS. This emerging field of ‘cancer neuroscience’ encompasses local and systemic interactions between cancer cells and the fundamental components of the nervous system—neurons, astrocytes, oligodendrocytes, microglia, Schwann cells and peripheral nerves—and the effects of these interactions on cancer initiation, progression, the tumour immune microenvironment and metastasis. At this intersection between neurobiology and cancer biology, new neuroscience-based targets for cancer therapy are now emerging.
CNS: activity-regulated development and cellular plasticity
The role of neural activity in organ development and plasticity is best studied in the nervous system itself, and for this reason we focus the beginning of this Review on activity-regulated neurodevelopment. Nervous system development is influenced by electrical activity from the earliest stages of neurodevelopment. Ongoing refinement and plasticity of neural circuits—from synaptic plasticity to plasticity of myelin structure—is further modulated by the activity of the circuit. Cancers in the brain ultimately hijack these activity-regulated mechanisms, and many important lessons can be gleaned about cancer through a deep understanding of activity-regulated neural development and the ongoing adaptive plasticity of neural circuit form and function.
Nervous system development
Nervous system development involves the generation of neurons and glial cells from neural stem and progenitor cells, diversification and maturation of neural cells, migration to the appropriate anatomical position, assembly of functional circuits between neurons—a process that involves axon pathfinding, synapse formation, circuit refinement and establishment of the myelinated infrastructure that supports fast communication in the nervous system. Astrocytes have critical roles in the establishment of synapses during development1–4 and subsequently in support of synaptic function throughout life4. Electrical activity influences all stages of nervous system development, from neural induction during embryogenesis5–7 to adaptive plasticity of complex neural circuits in the adult brain (for a review, please see ref. 8). Canonical neuronal activity—involving neurotransmitter release at mature electrochemical synapses and consequent membrane depolarization—results in voltage-dependent calcium influx that can affect a range of cellular functions9–11.
At the earliest developmental stages, patterned waves of electrical activity trigger calcium transients in developing neural tissues that are critical for both cellular and synaptic patterning. In the embryonic cerebrum, neural stem cells are coupled by gap junctions to one another and membrane depolarization-induced calcium transients propagate synchronously through the germinal zone to regulate stem cell proliferation12,13. Neurotransmitters—secreted by a variety of cell types in a non-synaptic manner during developmental stages that precede synapse formation—generally promote neurogenesis14–17. The migration of developing neurons is influenced by voltage-dependent mechanisms, including through transient synapses from subplate neurons onto migrating neocortical neuroblasts18, and neuronal activity also instructs pathfinding and targeting of axons from nascent neurons19–21.
The gap junctional coupling that occurs between stem and progenitor cells also takes place among various populations of neuroblasts and neurons during later stages of neurodevelopment22–26. This coupling, together with mechanisms such as extrasynaptic glutamate and ‘pacemaker’ neurons that oscillate spontaneously (see ref. 27 for a review), enables synchronized, action potential-dependent calcium transients to spread through the developing nervous system26,28–34. Such experience-independent, coordinated waves of activity are thought to promote the assembly of functional neural circuits through the Hebbian principle35, and these circuits are later refined in an experience-dependent manner (for a review, see refs. 36,37).
Postnatal neurodevelopment and ongoing neuroplasticity
In the postnatal brain, neuronal activity and neurotransmitter-mediated mechanisms continue to govern new cell generation from neural stem and precursor cells in neurogenic niches38–43. As in neuron generation, the generation of myelinating oligodendrocytes is regulated by neuronal activity throughout life. Myelination of the CNS—the process by which oligodendrocytes ensheath axons to facilitate fast saltatory neural conduction44 and to provide metabolic support to axons45—requires the proliferation and differentiation of oligodendrocyte precursor cells (OPCs), which are distributed throughout the nervous system across the lifespan46. Myelin development extends over a protracted postnatal period spanning at least three decades in humans47–49 and continues throughout life in certain regions such as the neocortex48,50–54. Extrinsic cues driven by neuronal activity and experience modulate developmental myelination and drive ongoing myelin plasticity (for a review, please see ref. 55). Neuronal activity can elicit proliferation of oligodendroglial precursor cells that generate new oligodendrocytes and alter myelination in an activity-regulated manner51,56–59. Such adaptive plasticity of myelin tunes neural circuit function59,60 and contributes to cognition57,59,61,62.
Activity-regulated communication between neurons and OPCs involves paracrine mechanisms such as secretion of neurotrophic factors. In addition to its numerous roles in neurodevelopment and plasticity, brain-derived neurotrophic factor (BDNF) promotes both developmental myelination63,64 and ongoing myelin plasticity57. Also mediating neuron–glial communication are bona fide glutamatergic and GABAergic neuron-to-OPC synapses65–68. While much remains to be learned about the function of these well-characterized but still somewhat enigmatic neuron-to-glial synapses, present evidence indicates that AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptor-dependent synapses on OPCs regulate cell survival69.
Neuronal activity drives glioma initiation and progression
It is on this backdrop of neuronal activity-regulated brain development and plasticity that we must consider the interactions of neurons with brain cancers. The most common primary brain cancers in both children and adults are gliomas, which contain heterogeneous tumour cells including subpopulations of oligodendroglial-like and astrocyte-like cells70–72. Although these cancers were originally named ‘astrocytomas’ because of histological characteristics reminiscent of astrocytes, it is now understood that most forms of glioma arise from stem and precursor cells in the oligodendrocyte lineage71–80. The robust mitogenic effect of neuronal activity on OPCs discussed above suggests that dysregulated or ‘hijacked’ mechanisms of myelin plasticity might similarly promote malignant cell proliferation in this devastating group of primary brain cancers. Indeed, neuronal activity promotes proliferation and growth of both high-grade81,82 and low-grade83 glioma subtypes in preclinical models (Fig. 1). Optogenetic stimulation of cortical projection neurons drives the growth of patient-derived high-grade glioma xenografts within the stimulated circuit81. Similarly, optogenetic stimulation of retinal ganglion cell axons in the optic nerve increases low-grade glioma growth in a genetically engineered mouse model of neurofibromatosis 1 (NF1)-associated optic pathway glioma83. Conversely, chemogenetic suppression of olfactory neuronal activity, or reduced olfactory experience through mechanical naris occlusion, decreases tumour growth in a genetically engineered mouse model of olfactory bulb high-grade glioma82.
Fig. 1 |. Neuron–glioma interactions in the CNS.

Neuronal activity promotes glioma progression that through activity-regulated secretion of paracrine growth factors, including NLGN3, IGF-1 and BDNF, and by electrochemical communication mediated by synapses between neurons and glioma cells, as well as through potassium-evoked glioma currents. Such electrochemical signals are amplified in a glioma-to-glioma gap junction-coupled network that serves–among other functions–to amplify and synchronize depolarizing currents in the tumour cell network. Membrane depolarization alone is sufficient to promote glioma cell proliferation through voltage-dependent mechanisms that remain to be fully elucidated. ‘Hub’ cells autonomously generate currents that spread through the gap junction-connected tumour network to drive a tumour-intrinsic rhythm of periodic depolarization and consequent calcium transients important for tumour growth. AMPA receptor-mediated synaptic signalling between neurons and glioma cells promotes both tumour cell proliferation and invasion. In turn, glioma cell secretion of factors such as glutamate and synaptogenic proteins (for example, glypican-3 and TSP-1) promotes neuronal hyperexcitability and functional remodelling of neural circuits, thereby increasing neuronal activity in the tumour microenvironment. Glioma-induced increases in excitatory neuronal activity enhance activity-regulated influences on glioma progression. Original figure created with BioRender.com.
Neuronal activity not only promotes growth of established glioma tumours, but also regulates glioma initiation and maintenance. In a mouse model of optic pathway glioma driven by mutations in the Nf1 gene, visual experience-induced stimulation of optic nerve activity during a developmental period of tumour susceptibility was necessary for optic pathway glioma tumour formation and maintenance. Despite the genetic predisposition to and high penetrance of optic pathway gliomas in this model, tumours did not form when optic pathway activity was reduced by dark-rearing the mice just before the time tumours typically form, in stark contrast to universal tumour formation in littermate control animals raised with normal visual experience83. Decreasing visual experience just after the time that tumours typically form also blocked optic pathway tumour maintenance83. Similarly, in a mouse model of IDH-wild-type high-grade glioma that preferentially forms in the olfactory bulb, modulating olfactory experience and olfactory neuronal activity through unilateral naris occlusion regulates tumour formation ipsilateral to the occluded naris82. These two studies underscore an emerging principle that sensory experience can modulate cancer progression in cancers located within that sensory circuit (Fig. 2), further discussed below with respect to immune modulation and for cancers of the skin and pancreas.
Fig. 2 |. Sensory experience and cancers.
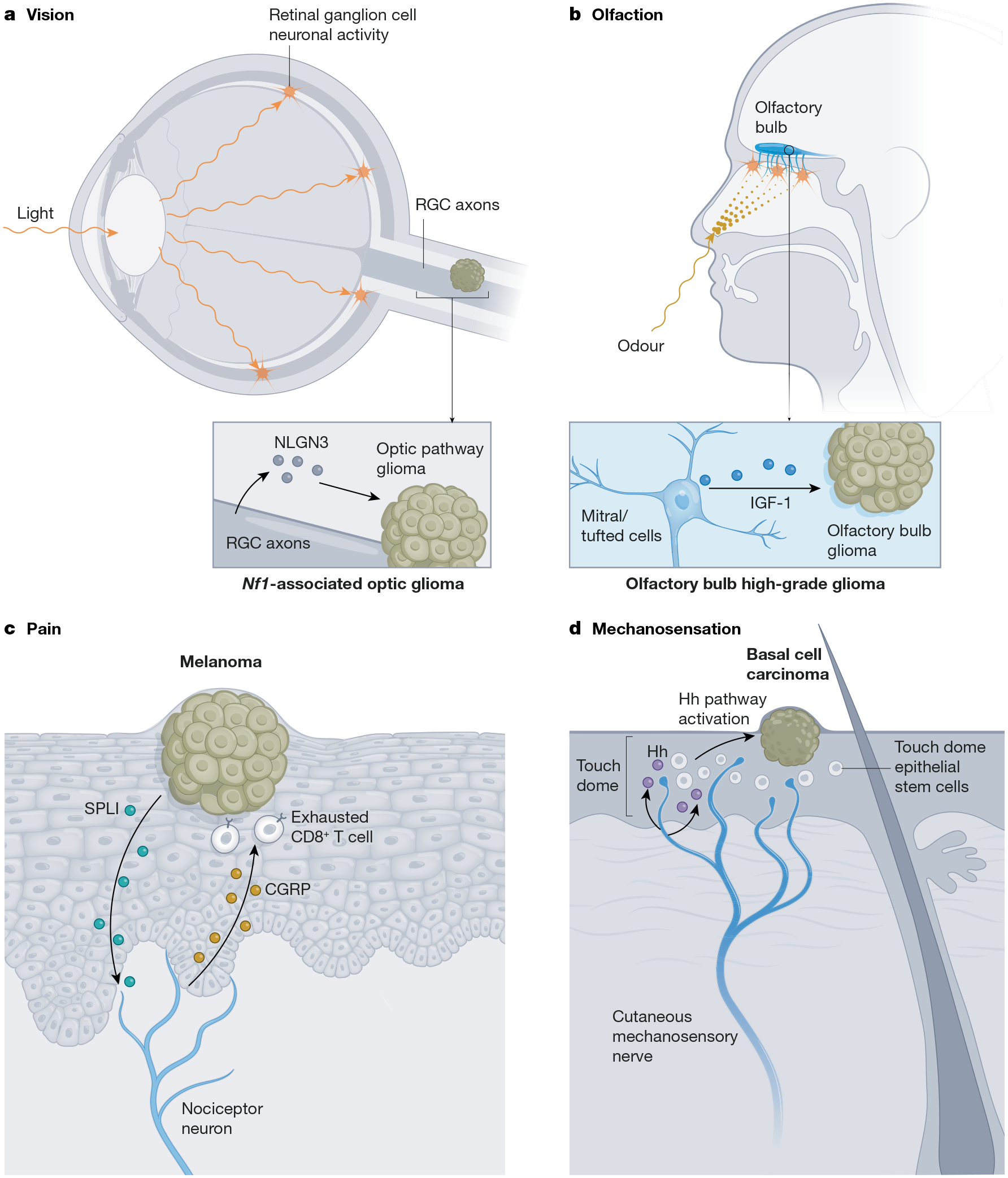
a, Visual experience, for example, light, induces activity in retinal ganglion cell (RGC) neurons, whose axons comprise the optic nerve. Optic nerve activity promotes shedding of NLGN3, thereby regulating the initiation, growth and maintenance of low-grade gliomas that occur in the optic pathway in association with the tumour predisposition syndrome NF1. b, Odorants stimulate olfactory receptor neurons to signal to mitral/tufted cells (a neuronal subtype) in the olfactory bulb, which secrete IGF-1 in an olfactory experience- and neuronal activity-dependent manner, contributing to olfactory bulb high-grade glioma initiation and growth in a mouse model. c, Pain mediated by cutaneous nociceptor nerves in the melanoma tumour microenvironment, stimulated by secretory leukocyte protease inhibitor (SLP1) secreted by cancer cells, results in nerve-derived release of the neuropeptide CGRP. CGRP acts on T lymphocytes to promote T cell exhaustion, thereby limiting anti-cancer immunity and permitting melanoma growth. d, Cutaneous mechanosensory nerves innervate the touch dome epithelium, providing Hedgehog (Hh) ligand to touch dome epithelial stem cells, which can give rise to basal cell carcinoma. Mechanosensory nerve-derived Hedgehog ligand induces Hedgehog pathway activity in basal cell carcinoma cells, promoting tumour initiation and growth. Original figure created with BioRender.com.
Paracrine mechanisms of neuronal activity-regulated glioma progression
Paracrine signalling mechanisms are one crucial way that neuronal activity regulates glioma growth (Fig. 1). Activity-regulated paracrine secretion of both BDNF and the synaptic protein neuroligin-3 (NLGN3) promotes the proliferation of high-grade81,84 and low-grade83 gliomas. In the genetically engineered mouse model of olfactory bulb high-grade glioma discussed above, insulin-like growth factor-1 (IGF-1) was identified as the key neuronal activity-regulated paracrine factor driving olfactory bulb glioma growth, suggesting region- or circuit-specific mechanisms of neuron–glioma interactions82. Given the many different subtypes of neurons that exist, such circuit-specific—and neuron subtype-specific—mechanisms are not surprising. Not all activity-regulated paracrine signalling mechanisms involve direct neuron–glioma interactions. In an example of neuron–immune–cancer cell cross-talk (Fig. 3) leading to tumour growth-promoting inflammation, retinal ganglion cell neurons secrete midkine, which stimulates CD8+ lymphocytes to secrete the chemokine CCL4, which in turn stimulates glioma-associated microglia/macrophages to secrete CCL5 that acts on NF1-associated optic pathway low-grade glioma cells to promote tumour maintenance and progression85,86. Such neuron–immune cell–glioma interactions highlight the influence of the nervous system in regulating the tumour immune microenvironment in ways that can influence both pro-tumour inflammation and anti-cancer immunity, an emerging principle probably relevant to numerous forms of cancer and to cancer immunotherapy, as discussed in more detail below.
Fig. 3 |. Neuronal mechanisms regulating the tumour immune microenvironment.
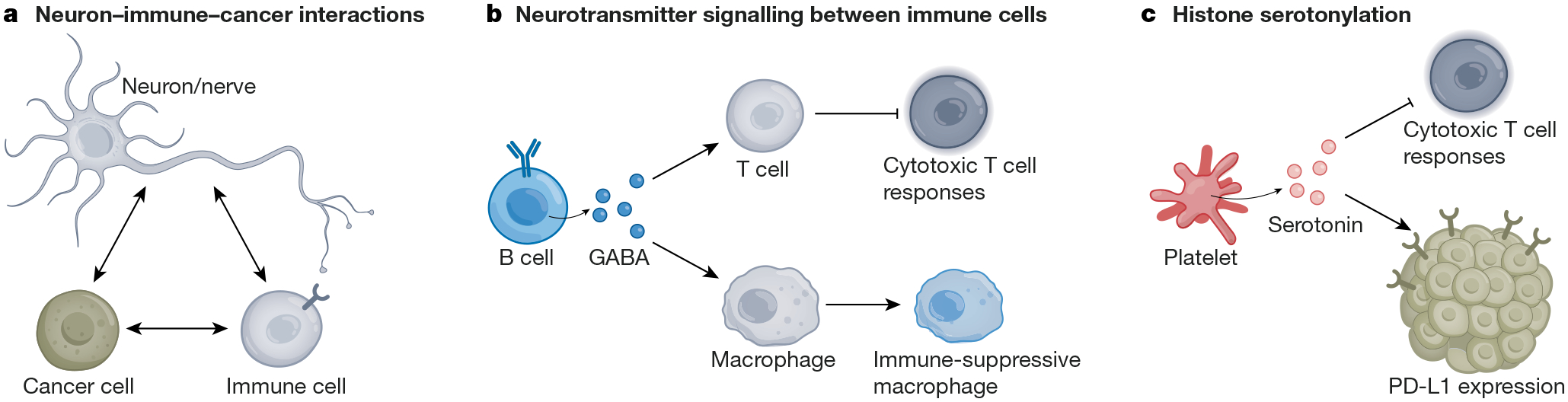
a, Extensive cross-talk occurs between neurons/nerves, immune cells and cancer cells. These interactions can influence anti-cancer immunity and pro-cancer inflammation. b, B cell-derived GABA drives immunosuppression in colon adenocarcinoma. GABA secreted by B lymphocytes binds to GABAA receptors on T cells to suppress cytotoxic T cell responses and promote an immune-suppressive state in tumour-associated macrophages. c, Serotonin produced by platelets in pancreatic and gastric cancer drives the upregulation of PD-L1 on cancer cells, suppressing cytotoxic T cell responses. Original figure created with BioRender.com.
The role for activity-regulated BDNF signalling in glioma81 parallels its role in promoting activity-regulated proliferation of healthy OPCs discussed above. More unexpectedly, the synaptic adhesion molecule NLGN3 has emerged as a critically important player in neuron–glioma interactions. Progression of high-grade and low-grade glial malignancies stalls in the absence of microenvironmental NLGN3 (refs. 83,84). Concordantly, mRNA81 and protein87 expression levels of NLGN3 inversely correlate with survival in human patients with adult glioblastoma. Genetic knockout and pharmacological blockade of activity-regulated NLGN3 shedding—mediated by the ADAM10 sheddase with shedding chiefly coming from OPCs, probably from neuron-to-OPC synapses84—demonstrate that NLGN3 is necessary for the growth of gliomas in a range of preclinical high-grade glioma models84. In an NF1-associated optic pathway low-grade glioma mouse model, the NF1 mutation—present in all microenvironmental cells in the NF1 tumour predisposition syndrome—results in excessive NLGN3 shedding in the optic nerve83 due to the hyperexcitability of NF1-mutant retinal ganglion cell neurons88. Similar to the dependency of high-grade glioma xenograft growth on microenvironmental NLGN3, optic pathway gliomas did not form in an NF1-associated optic pathway glioma mouse model deficient in NLGN3 or in which NLGN3 shedding was pharmacologically blocked83. On the basis of these preclinical findings, therapeutic targeting of NLGN3 for paediatric high-grade gliomas is presently in an early-phase clinical trial (NCT04295759).
This unexpected dependency of glioma progression focuses a spotlight on the mechanisms by which NLGN3 influences glioma pathophysiology. NLGN3 binding induces multiple oncogenic signalling pathways (upstream, focal adhesion kinase pathway; downstream, PI3K–mTOR, SRC and RAS pathways) in glioma cells84. Although this NLGN3-regulated stimulation of oncogenic signalling pathways helps to explain the sufficiency of NLGN3 in promoting glioma proliferation and growth, it does not explain the unexpected dependency on this shed synaptic adhesion protein84. Subsequent experiments revealed that NLGN3 upregulates the expression of synapse-associated genes in glioma cells84, raising the subsequently confirmed possibility that synapses may form between neurons and glioma cells.
Synaptic integration of glioma networks into neural circuits
A combination of electron microscopy, electrophysiology and calcium imaging studies have clearly demonstrated that glutamatergic synapses form between neurons and glioma cells in both paediatric and adult high-grade gliomas89,90.
Neuron-to-glioma synapses
NLGN3 in the tumour microenvironment promotes these neuron-to-glioma synapses, suggesting that regulating synaptic integration is a key role of activity-regulated NLGN3 signalling89. Like the neuron-to-OPC synapses that form in the healthy brain, neuron-to-glioma synapses are mediated by calcium-permeable AMPA receptors89,90. Whole-cell patch clamp electrophysiology of glioma cells has shown that a subset (~5–10%) of glioma cells within a given tumour exhibit glutamatergic, AMPA receptor-mediated synapses89,90, and single-cell transcriptomic studies have indicated that this subpopulation of synaptically integrated glioma cells corresponds to the malignant cells that most resemble OPCs in paediatric high-grade glioma89 and that most resemble OPCs or neuronal precursor cells (NPCs) in adult glioblastoma91. Activity-regulated secretion of BDNF from neurons increases the strength of these glutamatergic currents in glioma cells, a finding that recapitulates the adaptive plasticity operant in healthy synapses and underscores the way that neuron–glioma interactions can be reinforced through activity-regulated mechanisms92.
Another subpopulation of tumour cells, representing the majority of the cells in some patient-derived glioma models, instead exhibit potassium-evoked depolarizing currents in response to neuronal activity89,90. As extracellular potassium levels increase with neuronal action potentials, these glioma potassium-evoked currents scale with field potential—the more neurons are active, the larger and longer these glioma currents become89. Furthermore, glioma cells connect to each other by gap junctions93 in both adult93,94 and paediatric78,89 glial malignancies. Gap junctional coupling of glioma cells serves to amplify the potassium-evoked currents and to promote synchrony of neuronal activity-evoked calcium transients propagating through this neuron-to-glioma network of cells89,90. A subset of the gap junction-coupled cells also receive synaptic inputs from neurons91, and thus this malignant glioma-to-glioma network is integrated synaptically and electrically into the brain (Fig. 1).
Evolution of malignant circuity
How this malignant circuitry develops and evolves over the course of disease remains to be fully elucidated. Astrocytes enable the establishment of heathy neural circuits during development3 through the secretion of synaptogenic factors4, but the role of astrocytes in the tumour microenvironment, or of astrocyte-like glioma cells, as discussed below, in the establishment of neuron-to-glioma synapses, remains to be determined. Early insights indicate that activity-regulated signals, including NLGN3 (ref. 89) and BDNF92, promote malignant synapse establishment and/or maintenance, predicting that neuron-to-glioma synaptic connectivity may increase over time. Concordantly, single-cell transcriptomic examination of primary and recurrent biopsy samples from adult patients with glioblastoma demonstrates an increase in synaptic gene expression by the time of tumour recurrence95. In adult glioblastoma, neuron-to-glioma synapses facilitate glioma invasion at the invasive edge of the tumour in a calcium signalling-dependent manner91, mirroring the role of transient synaptic inputs in migration of neocortical neuroblasts in normal brain development18 discussed above. These synaptically connected, invasive glioblastoma cells transition to gap junction-coupled, interconnected tumour cells over time91. The extent to which activity-regulated paracrine factors promote invasion remains to be determined, but such activity-regulated invasion mechanisms would help to explain the circuit-specific and sometimes expertise-specific patterns of glioma invasion and progression observed in patients.
Cancer cell membrane depolarization
The synaptic and electrical integration of glioma into neural circuits is central to tumour progression in preclinical models; blockade of glioma AMPA receptors or gap junctions impedes glioma growth89,90, whereas increasing AMPA receptor signalling accelerates glioma progression89. Given the roles for membrane voltage changes in brain development discussed above, and given that glioma cells exhibit at least two mechanisms of activity-evoked membrane depolarization, the hypothesis that membrane depolarization itself may drive glioma proliferation was tested using an in vivo optogenetic strategy, in which channelrhodopsin-2 was expressed in xenografted patient-derived glioma cells. Reminiscent of the roles that synchronized waves of membrane depolarization have in normal neurodevelopment, in vivo optogenetic depolarization of the glioma xenografts robustly increased glioma proliferation89 through voltage-sensitive mechanisms that remain to be defined. Underscoring the importance of these depolarizing waves and consequent calcium transients, tumour cells exhibiting autonomous, oscillatory calcium transients have been identified in both paediatric89 and adult96 high-grade gliomas that serve as ‘hub’ cells, driving autonomous waves of depolarization and calcium transients through the gap junction-connected network of tumour cells. These tumour cells, reminiscent of ‘pacemaker’ neurons in the developing brain, achieve these autonomous, oscillatory calcium transients through expression of the calcium-activated potassium channel KCa3.1, which has a similar role in sinoatrial cardiac cells96. These glioma cells exhibiting periodic depolarization are extensively connected to the tumour network and thus serve as a ‘hub’ of depolarizing current, with important functional consequences for glioma growth96. Further emphasizing membrane voltage-dependent mechanisms in brain cancer, voltage-gated sodium channel expression in a Drosophila brain tumour model regulates tumour growth just as it does Drosophila neuroblast development97. These mechanistic parallels between normal and malignant neuron–glial interactions underscore the extent to which mechanisms of neurodevelopment and plasticity are subverted by malignant gliomas and the importance of understanding the neuroscience of brain cancers. Given the many different subtypes of neurons in the CNS, much remains to be uncovered about the interactions between various neuronal types and brain cancer cells that may inform patterns of cancer growth and invasion in the CNS and elucidate important therapeutic targets.
Synaptic pathophysiology in brain metastases
Synaptic pathophysiology is not restricted to primary brain cancer. In the context of breast-to-brain metastases (B2BMs), tumour cells can co-opt neuronal programmes to promote metastatic growth in the brain. Recent immunohistochemical analysis of primary human breast cancer samples, along with brain metastasis samples, demonstrated substantial expression of glutamatergic NMDA receptors, which are involved in excitatory synaptic transmission98. Further electron microscopy analysis showed that these NMDA receptor-expressing B2BM cells localize at glutamatergic synapses, forming pseudo-tripartite synapses at glutamatergic terminals where glutamate released at neuron-to-neuron synapses can stimulate the perisynaptic breast cancer cell. Knockdown of these NMDA receptors in human B2BMs substantially reduced colonization and growth in the brain following intracardiac injection of human B2BM cells in an immunodeficient mouse model98. Taken together, these results demonstrate a mechanism by which cancer cells can express neuronal machinery to enhance their metastatic growth potential in the brain.
Effects of glioma on neurons
Just as neuronal activity drives glioma initiation, growth and progression, glioma cells increase neuronal excitability and thus neuronal activity89,99–103. Gliomas have long been associated with seizures, and pioneering work from the Sontheimer group demonstrated that gliomas directly increase the excitability of neurons100. This occurs through numerous mechanisms that include non-synaptic secretion of glutamate from adult glioblastoma cells through the glutamate–cysteine exchanger system Xc (refs. 99,104), loss of inhibitory neurons in the tumour microenvironment101,104, changes in the neuronal response to GABA (γ-aminobutyric acid)101 and glioma secretion of synaptogenic factors such as glypican-3 (refs. 102,103) and thrombospondin-1 (TSP-1)105. Synaptogenic factor secretion is an important function of normal astrocytes, and astrocyte-like glioma cell subtypes are thought to be responsible for the synaptogenic factor secretion driving glioma-associated seizures102. Fascinatingly, different point mutations in the same oncogene (PIK3CA) influence the extent to which glioma cells induce hyperexcitability through differential glypican-3 secretion in adult IDH-wild-type glioblastoma models103, indicating that the molecular characteristics of the glioma may account for heterogeneity in the mechanisms and robustness of neuron–glioma interactions. In an immunocompetent mouse model of adult glioblastoma, early microglial infiltration was observed before the onset of seizures and microgliosis increased as tumour-associated seizures worsened over the disease course, suggesting a possible role for tumour-associated microglia in remodelling cortical network excitability104.
The hyperexcitability of the glioma-infiltrated cortex first described in mice has now been confirmed using intraoperative electrocorticography in awake, resting adults with glioblastoma89 and those executing cognitive tasks105. As a measure of oscillatory brain activity, broadband power measurements obtained using magnetic encephalography (MEG) in adults with glioma indicate an inverse relationship between global and peritumoral broadband power and progression-free survival87,106. Glioma-induced neuronal hyperexcitability is thought to contribute to glioma-associated seizures99,100,102,103 and to augment the tumour-promoting effects of neuronal activity. These feed-forward, bidirectional neuron–cancer interactions represent a principle found in numerous types of cancer, as discussed below (Figs. 1 and 4).
Fig. 4 |. PNS interactions with cancer.

a, Local paracrine signalling from nerves to the tumour or to stromal cells in the tumour microenvironment regulates cancer growth and invasion, while tumour-derived factors remodel peripheral nerves, promoting further nerve ingrowth into the tumour microenvironment. b, Nerve-derived factors such as neurotransmitters and neuropeptides can modulate immune cell trafficking and function. Consequently, altered immune function can influence anti-cancer immunity and tumour growth-promoting inflammation. c, Systemic interactions between the nervous system and cancers can be mediated by systemic paracrine signalling, for example, circulating catecholamines (including adrenaline), signalling directly to tumour cells or to other cell types in the tumour microenvironment. Reciprocally, tumour cells can influence the nervous system through circulating factors that may in turn regulate such systemic nervous system–cancer interactions (for example, altering the function of the hypothalamic–pituitary–adrenal axis). Original figure created with BioRender.com.
Glioma-derived synaptogenic factors not only influence neuronal excitability, but also can promote functional remodelling of neural circuits105. Intraoperative electrophysiological studies have shown that adults with IDH-wild-type glioblastoma involving the language-associated cortical areas in the left hemisphere exhibit remodelling of language circuitry such that naming tasks recruit not only the usual cortical regions involved in expressive language, but also the entirety of the glioma-infiltrated cortex105. Secretion of the synaptogenic factor TSP-1 by glioma cells was identified as a key mechanism mediating this remodelling of normal functional circuity and increasing the responsiveness of glioma cells to neurons105. Synaptogenic factor secretion is expected to influence not only neuron-to-neuron synaptic connections but also neuron-to-glioma synaptic connectivity. Accordingly, and highlighting the functional importance of neuron–glioma interactions, the degree of functional connectivity between normal brain and adult glioblastoma at the time of diagnosis influences language task performance and is also a powerful predictor of patient survival, with markedly shorter overall survival for patients with glioblastomas that exhibit high functional connectivity105. Gliomas thus functionally remodel and hijack the neural circuits underlying cognitive domains such as language function105.
Peripheral nervous system
The peripheral nervous system (PNS), including motor, sensory and autonomic components, innervates organs and tissues throughout the body, releasing neurotransmitters and trophic signals to maintain tissue homeostasis throughout life. The autonomic PNS mediates both adrenergic (sympathetic) and cholinergic (parasympathetic) autonomic responses, producing critical muscle contractions and gland secretions to enable proper visceral organ function107. Function of the gastrointestinal system is robustly regulated by enteric nervous system (ENS) innervation, including extensive autonomic innervation of the gastrointestinal tract in addition to 500 million enteric neurons located in myenteric and submucosal plexi108. Peripheral nerves are critical components of the stem cell niches across multiple organ systems and tissue types109–114. For example, fundamental studies by Knox and colleagues have demonstrated the importance of parasympathetic innervation for glandular organogenesis and regeneration109, including for tubular network and lumen formation during salivary gland organogenesis110. Just as the PNS regulates tissue development, homeostasis and regeneration, recent studies have shown the critical role of peripheral innervation in the progression of various cancers, including prostate, gastric, pancreatic and breast cancers, discussed in detail below. PNS–tumour cross-talk regulates cancer initiation and progression in ways that closely mirror the principles discussed above in CNS cancers (Figs. 4 and 5).
Fig. 5 |. Autonomic nervous system regulation of cancer.
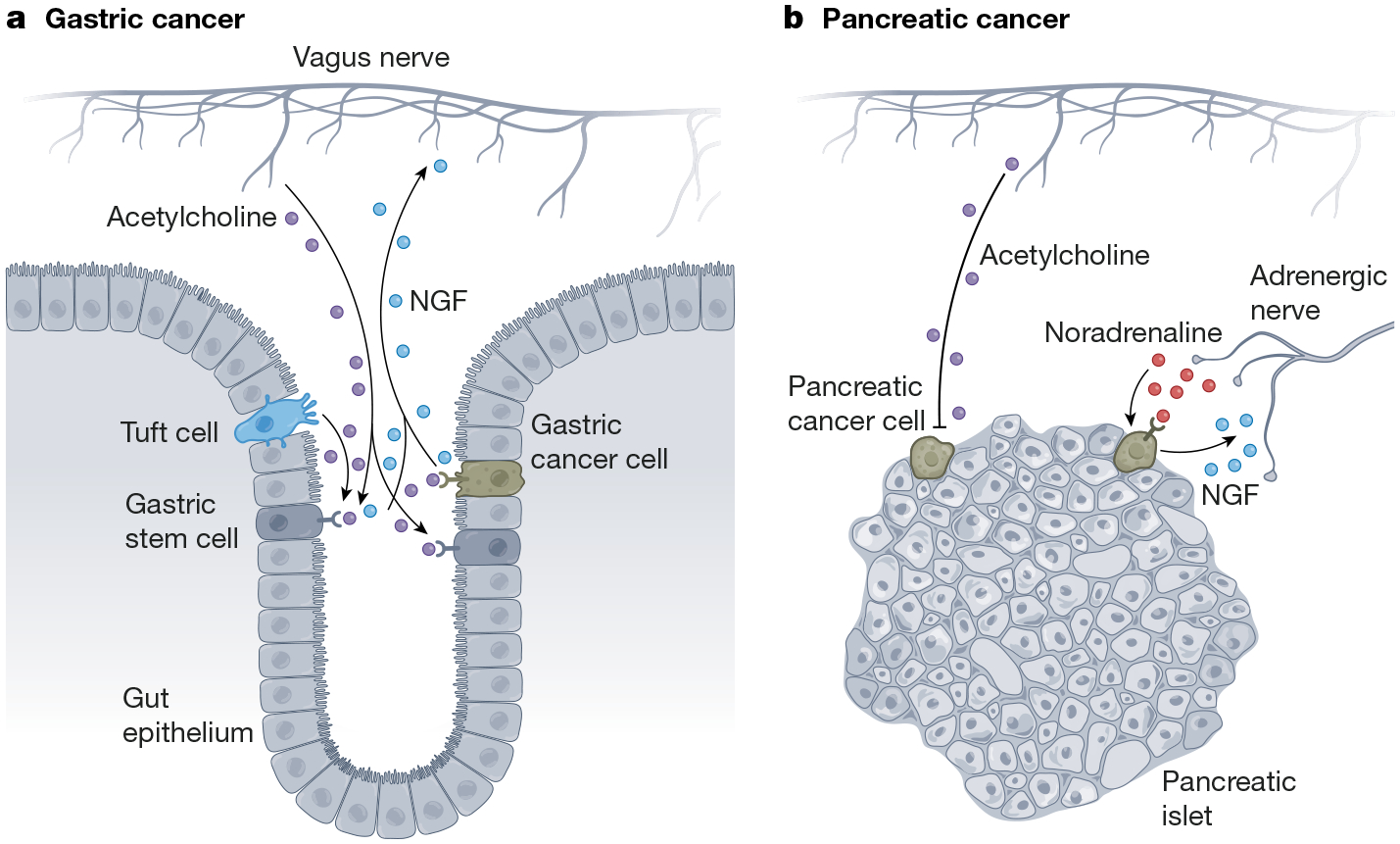
a, Nerve–cancer cross-talk in gastric and intestinal cancers. In gastric cancer, acetylcholine signals to tumour cell muscarinic acetylcholine receptors to promote tumour cell proliferation, while tumour cells secrete axonogenic factors such as NGF to increase nerve ingrowth in the tumour microenvironment. b, Nerve-cancer cross-talk in pancreatic cancer. In contrast to its role in gastric cancer, acetylcholine can suppress pancreatic tumorigenesis. By contrast, β-adrenergic signalling (noradrenaline) promotes pancreatic cancer growth, and pancreatic cancer cells secrete NGF to increase sympathetic innervation of the tumour microenvironment. Original figure created with BioRender.com.
Prostate cancer
The prostate is a densely innervated organ for which both parasympathetic and sympathetic inputs regulate normal prostate growth, homeostasis and function. Early studies using surgical denervation of the prostate demonstrated prominent prostatic atrophy115. Conversely, adrenergic agonists cause prostatic hyperplasia116. These roles for the autonomic nervous system in the regulation of prostate homeostasis and growth suggested that neural elements could have parallel roles in prostate cancer. Concordantly, histopathological analyses of prostate adenocarcinoma identified a phenomenon known as perineural invasion, in which tumour cells invade into and grow along nerves117,118. Retrospective studies examining the clinical and pathological features of patients with prostate cancer identified increased perineural invasion and diameter of the invaded nerve as an indicator of poor prognosis118. Important early in vitro experiments using a dorsal root ganglion and human prostate cancer cell co-culture system showed that neurons increase prostate cancer cell proliferation, providing some of the first evidence for a growth-promoting effect of neurons on cancer cells119.
A seminal study by Frenette and colleagues provided direct evidence for neural regulation of prostate cancer by both sympathetic and parasympathetic nerves120. Ablating adrenergic (sympathetic) nerves chemically or surgically in mouse prostate prevented growth of patient-derived orthotopic prostate cancer xenografts. Further, genetic ablation of β2- and β3-adrenergic receptors in recipient mice substantially reduced both growth of patient-derived orthotopic prostate cancer xenografts and tumour cell dissemination into the lymph nodes and distant organs. Parasympathetic nerves also influence prostate cancer progression. Muscarinic acetylcholine receptor 1 (Chrm1) signalling in the tumour stroma was found to enhance the dissemination and metastasis of orthotopically xenografted prostate cancer cells. Taken together, these ground-breaking findings from the Frenette group demonstrated the crucial and complex relationship between prostate cancer and the nervous system120.
Follow-up studies have implicated additional cellular and molecular components of the nervous system in prostate cancer growth and progression. β2-adrenergic signalling promotes vascularization in orthotopic xenograft and MYC-driven genetic mouse models of prostate cancer, in which expression of β2-adrenergic receptors on endothelial cells in the tumour stroma decreases oxidative phosphorylation to enhance tumour-promoting angiogenesis121. Unexpectedly, recent work in a MYC-driven genetic mouse model of prostate cancer demonstrated that doublecortin-positive NPCs migrate from the brain to the prostate cancer niche and generate new adrenergic neurons that then promote prostate cancer initiation and progression122.
Gastrointestinal tract cancers
Similar to the prostate and other glandular organs, the gastrointestinal stem cell niche is regulated by extensive innervation, and autonomic nervous system signalling has been implicated as an essential regulator of stem and progenitor cells in the liver111,123 and intestinal tract112. The ENS in the gut coordinates with the CNS to regulate gastrointestinal development, homeostasis and function, maintaining a delicate biochemical and immunological balance112,124,125. Underscoring this point, denervation of the stomach leads to profound changes in critical functions such as acid secretion126 and gastric mucosal cell proliferation127–129. Nervous system regulation of the gastrointestinal system becomes particularly important in gastrointestinal cancers, in which local nerves affect gastrointestinal tumorigenesis and growth.
Studies have shown enhanced nerve ingrowth in human and murine models of colon adenocarcinoma130, further supported by the correlation between nerve density and overall survival in human patients131. Surgical denervation (vagotomy) of the stomach reduced both the number and size of gastric tumours in a rat model of gastric cancer induced by carcinogen exposure132. Pioneering work by Wang and colleagues has illuminated the mechanisms driving this tumour-promoting innervation in a genetic mouse model of spontaneous gastric cancer. Surgical or pharmacological cholinergic denervation in the pre-neoplastic stage of gastric tumorigenesis clearly demonstrated a critical role for cholinergic innervation in both gastric tumour initiation and progression. Differential gene expression analyses in a vagotomized genetic mouse model of spontaneous gastric cancer showed that Wnt and Notch signalling pathway activity was markedly reduced following vagotomy. Concordantly, cholinergic neuronal signalling to muscarinic acetylcholine receptor 3 (Chrm3) in gastrointestinal cancer stem cells enhances the activity of the downstream Wnt signalling pathway to promote tumorigenesis. The gastrointestinal epithelial tuft cell subtype is the initial source of tumour-promoting acetylcholine in the gastric mucosa133. Production of acetylcholine by these tuft cells drives nerve growth factor (NGF) production in gastric stem cells to promote axonogenesis and further cholinergic nerve ingrowth, with increased acetylcholine release from nerves in the tumour microenvironment. Acetylcholine promotes LGR5+ stem cell proliferation through YAP-dependent modulation of Wnt signalling and further increases secretion of NGF in gastric cancer mouse models133 (Fig. 5). Strikingly, increased NGF expression and consequently increased acetylcholine in the gastric stem cell niche is sufficient to induce tumours, whereas NGF–Trk signalling antagonism is robustly therapeutic133. These seminal studies elucidate a direct role for the PNS in shaping the cancer stem cell niche to drive tumour progression in a feed-forward loop of nerve–cancer interactions and represent a clear example of the bidirectional signalling relationships between nerves and cancer cells, as discussed above in glioma (Fig. 1), in which neuronal signalling to cancer cells promotes progression and cancer cells remodel the nervous system to further enhance the pro-tumorigenic signals from neurons (Fig. 4).
In the uppermost part of the gastrointestinal tract, oral squamous cell carcinomas are densely innervated by nociceptive nerves134. Oral squamous cell carcinoma cells secrete NGF to promote this innervation of the tumour microenvironment, and nociceptive nerves secrete the neuropeptide calcitonin gene-related peptide (CGRP) to promote cancer growth. The growth-promoting effects of nociceptor-secreted CGRP are mediated by effects on cancer cell metabolism, inducing cytoprotective autophagy in low-glucose environments such as the oral cavity134. In preclinical models, the CGRP-blocking migraine medication rimegepant reduced cancer cell autophagy and augmented the therapeutic effects of nutrient-starvation therapies such as anti-angiogenic medications134.
Pancreatic cancer
The pancreas is an organ densely innervated by both sympathetic and parasympathetic nerves, coordinating closely with the nervous system to adapt exocrine and endocrine secretions in response to physiological changes135,136. Pancreatic cancer frequently presents with pain, hinting at the connection between pancreatic cancers and the nervous system. Early histological analyses of human pancreatic cancer samples documented prominent perineural invasion in pancreatic ductal adenocarcinoma (PDAC)137–139. Initial correlative studies in human patients also highlighted an association between NGF expression and this perineural invasion, with NGF expression correlating with pancreatic cancer progression and prognosis140,141. More recent studies have identified the critical biological mechanisms that facilitate malignant cross-talk between the nervous system and pancreas in cancer.
Pancreatitis, characterized by increased inflammation in the pancreas, can promote initiation and development of PDAC142,143. Mouse models of pancreatic cancer recapitulate the neuroplastic changes observed in human patients with PDAC and have been used to identify many important transcriptional and neuro-anatomical features present in the early stages of pancreatitis and tumour formation. For example, enhanced expression of TRPV1 and TRPA1 channels, which traditionally have been implicated in neurogenic inflammation, was observed in the sensory neurons of genetic mouse models of PDAC144. Elevated expression of neurotrophic factors such as NGF and neurotrophic receptors such as TrkB was also observed in the pancreas during these early pathogenic stages of PDAC formation. Further studies in genetic mouse models of PDAC have shown that precancerous inflammation in the pancreas, induced either by repeated injections of cerulein or by Kras-driven precancerous inflammatory neoplastic disease (PanIN), causes a substantial increase in CNS glial cell activation and neuronal injury responses145. Strikingly, capsaicin-induced sensory neuron ablation in these mouse models of pancreatitis abrogated CNS inflammation and reduced pancreatic cancer initiation and progression145, identifying a role for sensory innervation in pancreatic cancer and demonstrating how peripheral inflammatory signals can be transmitted from the periphery to the CNS through afferent nerves.
Roles have also been clearly demonstrated for the sympathetic and parasympathetic nervous systems in pancreatic cancer (Fig. 5). Catecholamines produced by the sympathetic nervous system, which can be released by local nerves or systemically by the adrenal glands during stress responses, have been linked to PDAC growth in vitro and in vivo146–148, and systemic stress correlates with cancer progression in human patients with cancers of various tissue types, including the bladder, liver, pancreas, stomach, ovary, kidney, breast and lung149. When chronic stress was induced through a chronic restraint paradigm in a genetic mouse model of early-stage PDAC, levels of β2-adrenergic receptors, nerve infiltration and tumorigenesis were enhanced150. Catecholamines were ultimately shown to directly promote NGF secretion from PDAC cells, leading to increased neurite outgrowth, increased nerve density and accelerated tumour formation.
In contrast to adrenergic sympathetic signalling, cholinergic parasympathetic signalling can suppress pancreatic tumorigenesis. Parasympathetic denervation (vagotomy) increased the incidence of pancreatic tumorigenesis in genetic mouse models of PDAC through expansion of malignant CD44+ epithelial cells in the tumour stroma150. Further experiments in these mouse models of PDAC demonstrated that cholinergic signalling agonists could suppress pancreatic tumorigenesis and prolong survival, primarily through the anti-proliferative effects of Chrm1 signalling in PDAC cells. The role of cholinergic signalling in PDAC stands in stark contrast to its role in gastric and intestinal cancers and underscores the concept that, although the nervous system consistently has important roles in modulating cancer, the role of a particular nerve type or neurotransmitter can vary among cancer types.
Breast cancer
The nervous system similarly has key roles in breast development and cancer. Studies using immunohistochemistry151 and retrograde transneuronal viral tracing152,153 have identified prominent adrenergic sympathetic innervation in the mammary gland. Signalling between mammary gland-derived BDNF and its receptor TrkB on sensory neurons is necessary to establish sensory innervation of the female mammary gland. In males, androgen hormones prevent this BDNF–TrkB signalling axis in sensory neurons, demonstrating a sexually dimorphic mechanism whereby the nervous system directs organ development154. This cross-talk between breast tissue and the nervous system is maintained in the context of cancer, regulating breast cancer progression.
Analysis of breast cancer specimens from human patients has shown that perineural invasion is positively correlated with disease progression, metastasis and clinical staging155. Early in vitro studies demonstrated that co-culture of human breast cancer cells and rat neurons resulted in increased NGF production by breast cancer cells and subsequent enhanced neurite outgrowth by the associated nerves156. Further in vivo work identified sympathetic innervation of breast tumours in humans and in preclinical mouse models of spontaneous mammary tumours157. Chemical ablation of these sympathetic nerve fibres leads to a decrease in tumour burden157. Recent work using a retrograde viral vector-based genetic approach manipulated local autonomic nerves in a tumour- and nerve-specific manner to better characterize innervation of the breast cancer microenvironment158. Specific stimulation of local sympathetic nerve fibres in an orthotopic human breast cancer xenograft model promoted primary breast tumour growth and distant metastasis through locally released noradrenaline. Chemical ablation of the sympathetic nerve fibres in these breast cancer models was shown to reduce immune-suppressive mechanisms, including reduced expression of immune checkpoint signals such as PD-1 and PD-L1 and a decrease in tumour-infiltrating regulatory T lymphocytes. In contrast to local sympathetic nerves, stimulation of parasympathetic nerve fibres in the breast cancer microenvironment decreased primary tumour growth and distant metastasis, as well as suppressed expression of immune checkpoint molecules. Like the role of parasympathetic nerves, destruction of sensory neurons using high-dose capsaicin enhanced breast cancer metastases and promoted a more aggressive gene expression phenotype159. Taken together, these findings suggest that sympathetic nerves promote breast cancer growth, whereas parasympathetic and sensory nerve mechanisms suppress breast cancer growth, again underscoring the complex regulatory roles that different nerve types can have in specific tumours and highlighting nervous system regulation of the tumour immune microenvironment.
The nervous system also has important roles in regulating breast cancer metastasis. Inducing chronic stress through restraint paradigms substantially increased colonization and metastasis to distant tissues in systemically injected and orthotopic murine models of breast cancer, respectively160. Blocking β-adrenergic receptors abrogated this stress-induced pro-metastatic switch in orthotopic breast cancer models. Concordantly, pharmacological β-blockade in human patients with breast cancer has been linked to increased immune cell infiltration and a reduction in metastasis-related biomarkers161. Strikingly, macrophages and myeloid cells were found to mediate this stress-enhanced metastatic spread, with stress-exposed breast cancer-bearing mice treated with CSF-1 receptor antagonists to deplete macrophages and myeloid cells no longer exhibiting enhanced metastasis160.
Basal cell carcinoma
Not all interactions between the PNS and cancer cells hinge on neurotransmitter signalling. Basal cell carcinoma, classically driven by Hedgehog signalling, can arise from various populations of Hedgehog-responsive epithelial stem cells, especially stem cells in the touch dome epithelium162. Mechanosensory nerves are a major source of Hedgehog ligand in the skin, and nerve-derived Hedgehog ligand drives oncogenic signalling in basal cell carcinoma models (Fig. 2). Concordantly, denervation of the skin strongly abrogates basal cell carcinoma growth in mouse models162.
Neural regulation of the immune system and tumour immune microenvironment
Neural–immune cross-talk in the local tumour microenvironment
Neural–immune interactions regulate the functions of both systems (for a review, see ref. 163), and cross-talk among immune cells, tumour cells and neurons or neuronal mechanisms can profoundly influence cancer progression (Fig. 3). Prominent examples include the immunomodulatory influence of a range of neurotransmitters that can affect pro-tumour inflammation, anti-tumour immunity or immunotherapeutic effects. For example, adrenergic signalling, derived from local sympathetic nerves or from circulating catecholamines, can exert wide-ranging effects on the tumour immune microenvironment. Noradrenaline signalling, chiefly to the β2-adrenergic receptor in immune cells, regulates myeloid-derived suppressor cell states164,165, upregulation of checkpoint molecules such as PD-1 (ref. 166) and T cell exhaustion167. Adrenergic signalling also limits migration of immune cells in tissues through the effects of sympathetic nerves on vasculature168. Similar to the effects of noradrenaline from sympathetic nerves, nociceptor sensory nerves promote CD8+ T cell exhaustion in the tumour microenvironment of melanoma through release of the neuropeptide CGRP, enabling melanoma progression by impairing anti-tumour immunity (Fig. 2)169.
In some cases, neuronal mechanisms contribute to cancer pathophysiology in contexts that do not involve neural structures or cells. For example, the neurotransmitter GABA appears to have important roles in multiple cancer types: in primary tumour samples from patients with non-small-cell lung adenocarcinoma or colon cancer, GABA levels increased with tumour stage and inversely correlated with patient survival170. Autocrine GABA signalling through the GABAB receptor in colon cancer cells promotes cancer cell Wnt signalling and proliferation while decreasing colon cancer cell expression and secretion of the CCL4 and CCL5 chemokines, thereby reducing tumour infiltration by T lymphocytes and dendritic cells170. Further implicating GABA in the suppression of anti-cancer immunity, B lymphocyte-derived GABA signals through GABAA receptors on CD8+ T lymphocytes to reduce cytotoxic T cell responses and promote tumour growth in a mouse model of subcutaneous colon adenocarcinoma171. This B cell-derived GABA concomitantly promoted an immune-suppressive phenotype of tumour-associated macrophages in the colon adenocarcinoma tumour microenvironment (Fig. 3). Strikingly, B cell-specific deletion of GABA synthesis through Gad67 knockout significantly reduced colon adenocarcinoma growth in this model, demonstrating the important role of neurotransmitters in shaping the tumour immune microenvironment to affect tumour progression.
Serotonin—best known for its roles in the CNS—also has an important immune-modulatory role. Peripheral serotonin is taken up by platelets to influence inflammatory processes and cell proliferation. In the context of orthotopic xenograft mouse models of both gastric and pancreatic cancers, platelet-derived serotonin enhanced tumour growth through PD-L1 upregulation on cancer cells by transglutaminase 2 (TGM2)-mediated serotonylation of histones and consequent epigenetic regulation of immune checkpoint molecule expression172. In this way, peripheral, platelet-delivered serotonin directly influences CD8+ T cell infiltration in these glandular tumours to affect cancer progression (Fig. 3) in a fascinating mechanism at the crossroads of neuroscience, immunology and epigenetics.
Systemic neural–immune interactions
In addition to the examples discussed above in which the nervous system or neuronal mechanisms influence cancer progression through effects on immune cells in the local tumour microenvironment, immune system function can be profoundly influenced at the systemic level by the nervous system (Fig. 4). Such systemic effects, including effects on leukocyte trafficking and function, may influence the efficacy of anti-cancer immunity or immunotherapeutic strategies. The vagus nerve has crucial immunomodulatory roles, not only conveying information about peripheral immune challenges to the brain, but also mitigating inflammatory responses through efferent cholinergic pathways, such as limiting pro-inflammatory cytokine release in experimental lipopolysaccharide (LPS)-induced sepsis173. Distinct brain regions and neuronal subtypes orchestrate systemic immune cell trafficking during physiological stress, with the activity of paraventricular hypothalamus corticotropin-releasing hormone neurons and consequent stimulation of the hypothalamic–pituitary–adrenal axis responsible for driving lymphocytes and monocytes from peripheral tissues into bone marrow174. In health as well as in times of stress, adrenergic signalling and sympathetic innervation of the haematopoietic stem cell niche in bone marrow controls the egress of haematopoietic stem cells into the circulation175,176. Adrenergic signalling also strongly influences immune cell trafficking through lymph nodes177. Direct innervation of lymph nodes by peptidergic nociceptor sensory nerves modulates leukocyte gene expression in a manner that may influence immune function178.
Beyond regulating broad shifts in leukocyte population dynamics, the nervous system can encode and retrieve specific immune responses179. Ensembles of cortical neurons in the insula become active during specific immune challenges, and subsequent stimulation of these specific insular cortex neurons—which project to autonomic nervous system regulatory sites—recreates the immune response operant during the initial inflammatory challenge179. In this way, the brain can recapitulate a specific immune response through stored neuronal representations of immunological information. How such complex, newly recognized mechanisms of CNS control of immune system function may influence cancer progression or cancer immunotherapy remains to be investigated and may represent important therapeutic opportunities at the intersection of neuroscience, immunology and oncology (Fig. 3).
Conclusions and future directions
Although the field of cancer neuroscience is still in its infancy, pioneering studies have clearly demonstrated the importance of elucidating nervous system–cancer interactions. Building on the foundational discoveries discussed here, progress in cancer neuroscience will benefit from interdisciplinary collaboration among the fields of neuroscience, developmental biology, immunology and cancer biology. As cancers hijack and subvert mechanisms operant during healthy development, insights gleaned from nervous system–cancer interactions are likely to both inform and be informed by mechanisms of development and regeneration.
Targeting nervous system–cancer interactions has the potential to become a pillar of oncological therapy, joining traditional approaches such as surgery, radiation and chemotherapy and the recently codified pillar of immunotherapy. Although targeting nervous system–cancer interactions may not by itself be sufficient to eradicate a tumour, this may be a necessary component of effective therapeutic regimens for currently intractable cancers such as high-grade gliomas and pancreatic cancers.
Intervening on neural mechanisms of cancer growth, spread and therapeutic resistance may leverage existing drugs used in neurology and psychiatry that modulate neurotransmitter receptors, ion channels and other neurophysiological targets. Repurposing existing medication such as anti-epileptic drugs that target neuron–glioma interactions may provide powerful new tools for cancer management. For example, parampanel, which targets AMPA receptors, has demonstrated some promise in preclinical models89,90 and early clinical studies180. However, caution is warranted and preclinical evidence is required before testing a seemingly safe drug in patients with cancer because, whereas some neurophysiological medications may exhibit anti-cancer effects, others may conversely promote progression of a particular cancer. Elucidating the precise neuroscience mechanisms operant in the tumour and tumour microenvironment of each molecularly defined malignancy is of paramount importance to choose helpful medications and avoid potentially deleterious ones.
A number of potential therapeutic interventions have already emerged from preclinical work that may, in clinical studies, be shown to improve cancer outcomes. Strategies to block nervous system–cancer interactions that increase the rate of growth and/or tumour spread may be useful on their own and also complementary to traditional anti-cancer therapies targeting cancer cell-intrinsic vulnerabilities. For each tumour type, it will be important to understand whether nervous system–cancer interactions promote tumour cell survival as well as growth and invasion/metastasis. If it is the case that certain neural–cancer interactions promote therapeutic resistance, combining therapies to block these neural–cancer interactions with cytotoxic therapies such as radiation and cytotoxic chemotherapy may be powerfully synergistic. Blocking neural–cancer interactions that promote tumour growth also may be particularly synergistic with immunotherapies, such as chimeric antigen receptor T cell therapies, for which the rate of immune-based tumour cell killing must outpace the rate of cancer cell proliferation. Furthermore, the recognition that neurotransmitters and other signalling molecules classically associated with the nervous system can directly influence immune cell function highlights the need to study the influence of neurophysiological medications commonly used to manage seizures, pain, sleep, nausea, anxiety and depression on cancer immunotherapies. Modulating the influence of neurotransmitters and neuropeptides on immune cell function may prove invaluable in overcoming immune-suppressive tumour microenvironments and developing effective immunotherapeutic strategies.
A great deal of work remains to be done in cancer neuroscience, but this avenue of inquiry holds enormous promise to improve outcomes for a wide range of malignancies. At the same time, such inquiry may provide new insights into nervous system regulation of normal organ and immune development, homeostasis and plasticity/regeneration, viewed through the magnifying lens of cancer.
Acknowledgements
M.M. is grateful for support from the US National Institutes of Health, including from the National Institute of Neurological Disorders and Stroke (R01NS092597), an NIH Director’s Pioneer Award (DP1NS111132) and the National Cancer Institute (P50CA165962, R01CA258384, R01CA263500, U19CA264504); Cancer Research UK; the Waxman Family Fund; the McKenna Claire Foundation; the Will Irwin Research Fund; and the Virginia and D.K. Ludwig Fund for Cancer Research.
Footnotes
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at https://doi.org/10.1038/s41586-023-05968-y.
Competing interests M.M. holds equity in MapLight Therapeutics.
References
- 1.Mauch DH et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science 294, 1354–1357 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Nagler K, Mauch DH & Pfrieger FW Glia-derived signals induce synapse formation in neurones of the rat central nervous system. J. Physiol 533, 665–679 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ullian EM, Sapperstein SK, Christopherson KS & Barres BA Control of synapse number by glia. Science 291, 657–661 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Christopherson KS et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120, 421–433 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Leclerc C et al. L-type calcium channel activation controls the in vivo transduction of the neuralizing signal in the amphibian embryos. Mech. Dev 64, 105–110 (1997). [DOI] [PubMed] [Google Scholar]
- 6.Leclerc C et al. Neural determination in Xenopus laevis embryos: control of early neural gene expression by calcium. J. Soc. Biol 195, 327–337 (2001). [PubMed] [Google Scholar]
- 7.Webb SE, Moreau M, Leclerc C & Miller AL Calcium transients and neural induction in vertebrates. Cell Calcium 37, 375–385 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Pan Y & Monje M Activity shapes neural circuit form and function: a historical perspective. J. Neurosci 40, 944–954 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deisseroth K, Bito H & Tsien RW Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron 16, 89–101 (1996). [DOI] [PubMed] [Google Scholar]
- 10.Bito H, Deisseroth K & Tsien RW CREB phosphorylation and dephosphorylation: a Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell 87, 1203–1214 (1996). [DOI] [PubMed] [Google Scholar]
- 11.Deisseroth K, Heist EK & Tsien RW Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature 392, 198–202 (1998). [DOI] [PubMed] [Google Scholar]
- 12.Bittman K, Owens DF, Kriegstein AR & LoTurco JJ Cell coupling and uncoupling in the ventricular zone of developing neocortex. J. Neurosci 17, 7037–7044 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weissman TA, Riquelme PA, Ivic L, Flint AC & Kriegstein AR Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron 43, 647–661 (2004). [DOI] [PubMed] [Google Scholar]
- 14.LoTurco JJ, Owens DF, Heath MJ, Davis MB & Kriegstein AR GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron 15, 1287–1298 (1995). [DOI] [PubMed] [Google Scholar]
- 15.Luk KC & Sadikot AF Glutamate and regulation of proliferation in the developing mammalian telencephalon. Dev. Neurosci 26, 218–228 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Canudas AM et al. PHCCC, a specific enhancer of type 4 metabotropic glutamate receptors, reduces proliferation and promotes differentiation of cerebellar granule cell neuroprecursors. J. Neurosci 24, 10343–10352 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Platel JC et al. NMDA receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network. Neuron 65, 859–872 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohtaka-Maruyama C et al. Synaptic transmission from subplate neurons controls radial migration of neocortical neurons. Science 360, 313–317 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Ming G, Henley J, Tessier-Lavigne M, Song H & Poo M Electrical activity modulates growth cone guidance by diffusible factors. Neuron 29, 441–452 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Catalano SM & Shatz CJ Activity-dependent cortical target selection by thalamic axons. Science 281, 559–562 (1998). [DOI] [PubMed] [Google Scholar]
- 21.Dantzker JL & Callaway EM The development of local, layer-specific visual cortical axons in the absence of extrinsic influences and intrinsic activity. J. Neurosci 18, 4145–4154 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marins M et al. Gap junctions are involved in cell migration in the early postnatal subventricular zone. Dev. Neurobiol 69, 715–730 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Penn AA, Wong RO & Shatz CJ Neuronal coupling in the developing mammalian retina. J. Neurosci 14, 3805–3815 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peinado A, Yuste R & Katz LC Extensive dye coupling between rat neocortical neurons during the period of circuit formation. Neuron 10, 103–114 (1993). [DOI] [PubMed] [Google Scholar]
- 25.Picken Bahrey HL & Moody WJ Early development of voltage-gated ion currents and firing properties in neurons of the mouse cerebral cortex. J. Neurophysiol 89, 1761–1773 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Tritsch NX, Yi E, Gale JE, Glowatzki E & Bergles DE The origin of spontaneous activity in the developing auditory system. Nature 450, 50–55 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Blankenship AG & Feller MB Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat. Rev. Neurosci 11, 18–29 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corlew R, Bosma MM & Moody WJ Spontaneous, synchronous electrical activity in neonatal mouse cortical neurones. J. Physiol 560, 377–390 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meister M, Wong RO, Baylor DA & Shatz CJ Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science 252, 939–943 (1991). [DOI] [PubMed] [Google Scholar]
- 30.Wong RO, Chernjavsky A, Smith SJ & Shatz CJ Early functional neural networks in the developing retina. Nature 374, 716–718 (1995). [DOI] [PubMed] [Google Scholar]
- 31.Garaschuk O, Hanse E & Konnerth A Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J. Physiol 507, 219–236 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leinekugel X et al. Correlated bursts of activity in the neonatal hippocampus in vivo. Science 296, 2049–2052 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Watt AJ et al. Traveling waves in developing cerebellar cortex mediated by asymmetrical Purkinje cell connectivity. Nat. Neurosci 12, 463–473 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lippe WR Rhythmic spontaneous activity in the developing avian auditory system. J. Neurosci 14, 1486–1495 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hebb D The Organization of Behavior (Wiley, 1949). [Google Scholar]
- 36.Katz LC & Shatz CJ Synaptic activity and the construction of cortical circuits. Science 274, 1133–1138 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Kirkby LA, Sack GS, Firl A & Feller MB A role for correlated spontaneous activity in the assembly of neural circuits. Neuron 80, 1129–1144 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deisseroth K et al. Excitation–neurogenesis coupling in adult neural stem/progenitor cells. Neuron 42, 535–552 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Tozuka Y, Fukuda S, Namba T, Seki T & Hisatsune T GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron 47, 803–815 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Wang Q, Haydar TF & Bordey A Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat. Neurosci 8, 1179–1187 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Keeffe GC et al. Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proc. Natl Acad. Sci. USA 106, 8754–8759 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banasr M, Hery M, Printemps R & Daszuta A Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology 29, 450–460 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Paez-Gonzalez P, Asrican B, Rodriguez E & Kuo CT Identification of distinct ChAT+ neurons and activity-dependent control of postnatal SVZ neurogenesis. Nat. Neurosci 17, 934–942 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huxley AF & Stämpeli R Evidence for saltatory conduction in peripheral myelinated nerve fibres. J. Physiol 108, 315–339 (1949). [PubMed] [Google Scholar]
- 45.Funfschilling U et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hughes E, Kang S, Fukaya M & Bergles D Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci 16, 668–676 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flechsig P Anatomie des Menschlichen Gehirns und Rückenmarks auf Myelogenetischer Grundlage (Thieme, 1920). [Google Scholar]
- 48.Yakovlev PI in Regional Development of the Brain in Early Life (ed. Minkowski A) 3–70 (Blackwell Scientific Publications, 1967). [Google Scholar]
- 49.Lebel C et al. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage 60, 340–352 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Hill RA, Li AM & Grutzendler J Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat. Neurosci 21, 683–695 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hughes EG, Orthmann-Murphy JL, Langseth AJ & Bergles DE Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat. Neurosci 21, 696–706 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peters A & Sethares C Oligodendrocytes, their progenitors and other neuroglial cells in the aging primate cerebral cortex. Cereb. Cortex 14, 995–1007 (2004). [DOI] [PubMed] [Google Scholar]
- 53.Young KM et al. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873–885 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yeung M et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 159, 766–774 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Yalcin B & Monje M Microenvironmental interactions of oligodendroglial cells. Dev. Cell 56, 1821–1832 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gibson EM et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geraghty AC et al. Loss of adaptive myelination contributes to methotrexate chemotherapy-related cognitive impairment. Neuron 103, 250–265 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mitew S et al. Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat. Commun 9, 306 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steadman PE et al. Disruption of oligodendrogenesis impairs memory consolidation in adult mice. Neuron 105, 150–164 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noori R et al. Activity-dependent myelination: a glial mechanism of oscillatory self-organization in large-scale brain networks. Proc. Natl Acad. Sci. USA 117, 13227–13237 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McKenzie I et al. Motor skill learning requires active central myelination. Science 346, 318–322 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pan S, Mayoral SR, Choi HS, Chan JR & Kheirbek MA Preservation of a remote fear memory requires new myelin formation. Nat. Neurosci 23, 487–499 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vondran MW, Clinton-Luke P, Honeywell JZ & Dreyfus CF BDNF+/– mice exhibit deficits in oligodendrocyte lineage cells of the basal forebrain. Glia 58, 848–856 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong AW, Xiao J, Kemper D, Kilpatrick TJ & Murray SS Oligodendroglial expression of TrkB independently regulates myelination and progenitor cell proliferation. J. Neurosci 33, 4947–4957 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bergles DE, Roberts JD, Somogyi P & Jahr CE Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405, 187–191 (2000). [DOI] [PubMed] [Google Scholar]; This report demonstrated that bona fide synapses form between neurons and OPCs, an interaction later shown to be hijacked in gliomas.
- 66.Lin SC & Bergles DE Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat. Neurosci 7, 24–32 (2004). [DOI] [PubMed] [Google Scholar]
- 67.Karadottir R, Cavelier P, Bergersen L & Attwell D NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 438, 1162–1166 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mount CW, Yalcin B, Cunliffe-Koehler K, Sundaresh S & Monje M Monosynaptic tracing maps brain-wide afferent oligodendrocyte precursor cell connectivity. eLife 8, e49291 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kougioumtzidou E et al. Signalling through AMPA receptors on oligodendrocyte precursors promotes myelination by enhancing oligodendrocyte survival. eLife 6, e28080 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neftel C et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell 178, 835–849 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Filbin MG et al. Developmental and oncogenic programs in H3K27M gliomas dissected by single-cell RNA-seq. Science 360, 331–335 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu I et al. The landscape of tumor cell states and spatial organization in H3-K27M mutant diffuse midline glioma across age and location. Nat. Genet 54, 1881–1894 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu C et al. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell 146, 209–221 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study implicated OPCs as a cell of origin for adult glioblastoma.
- 74.Monje M et al. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proc. Natl Acad. Sci. USA 108, 4453–4458 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; This work implicated early OPCs as a cell of origin for diffuse intrinsic pontine glioma.
- 75.Galvao RP et al. Transformation of quiescent adult oligodendrocyte precursor cells into malignant glioma through a multistep reactivation process. Proc. Natl Acad. Sci. USA 10.1073/pnas.1414389111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alcantara Llaguno SR et al. Adult lineage-restricted CNS progenitors specify distinct glioblastoma subtypes. Cancer Cell 28, 429–440 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nagaraja S et al. Transcriptional dependencies in diffuse intrinsic pontine glioma. Cancer Cell 31, 635–652 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagaraja S et al. Histone variant and cell context determine H3K27M reprogramming of the enhancer landscape and oncogenic state. Mol. Cell 76, 965–980 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Z et al. Cell lineage-based stratification for glioblastoma. Cancer Cell 38, 366–379 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jessa S et al. K27M in canonical and noncanonical H3 variants occurs in distinct oligodendroglial cell lineages in brain midline gliomas. Nat. Genet 54, 1865–1880 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Venkatesh HS et al. Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell 161, 803–816 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provided direct evidence that neuronal activity can drive glioma proliferation and growth and identified activity-regulated paracrine factors (NLGN3 and BDNF) contributing to glioma growth.
- 82.Chen P et al. Olfactory sensory experience regulates gliomagenesis via neuronal IGF1. Nature 10.1038/s41586-022-04719-9 (2022). [DOI] [PubMed] [Google Scholar]
- 83.Pan Y et al. NF1 mutation drives neuronal activity-dependent initiation of optic glioma. Nature 594, 277–282 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; This work demonstrated that visual experience and optic nerve activity regulate not only glioma growth but also tumour initiation and maintence in NF1-associated low-grade optic glioma.
- 84.Venkatesh HS et al. Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma. Nature 549, 533–537 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo X et al. Midkine activation of CD8+ T cells establishes a neuron–immune–cancer axis responsible for low-grade glioma growth. Nat. Commun 11, 2177 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; This report established a three-way signalling relationship among neurons, immune cells (lymphocytes and microglia/macrophages) and glioma cells promoting tumour growth in NF1-associated low-grade glioma.
- 86.Pan Y et al. Athymic mice reveal a requirement for T-cell–microglia interactions in establishing a microenvironment supportive of Nf1 low-grade glioma growth. Genes Dev 32, 491–496 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Derks J et al. Oscillatory brain activity associates with neuroligin-3 expression and predicts progression free survival in patients with diffuse glioma. J. Neurooncol 140, 403–412 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anastasaki C et al. Neuronal hyperexcitability drives central and peripheral nervous system tumor progression in models of neurofibromatosis-1. Nat. Commun 13, 2785 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Venkatesh HS et al. Electrical and synaptic integration of glioma into neural circuits. Nature 573, 539–545 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study discovered bona fide synaptic communication between neurons and glioma cells mediated by AMPA receptors that robustly contributes to tumour growth (published back to back with ref. 90).
- 90.Venkataramani V et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 573, 532–538 (2019). [DOI] [PubMed] [Google Scholar]; This study discovered bona fide synaptic communication between neurons and glioma cells mediated by AMPA receptors that robustly contributes to tumour growth (published back to back with ref. 89).
- 91.Venkataramani VTK & Winkler F Glioblastoma hijacks neuronal mechanisms for brain invasion. Cell 85, 2899–2917 (2022). [DOI] [PubMed] [Google Scholar]; This report demonstrated that neuron-to-glioma synapses promote tumour cell invasion.
- 92.Taylor KR et al. Glioma synapses recruit mechanisms of adaptive plasticity. Preprint at bioRxiv 10.1101/2021.11.04.467325 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Osswald M et al. Brain tumour cells interconnect to a functional and resistant network. Nature 528, 93–98 (2015). [DOI] [PubMed] [Google Scholar]; This work illustrated gap junctional connectivity between glioma cells, forming a tumour network through long extensions called tumour microtubes.
- 94.Jung E et al. Tweety-homolog 1 drives brain colonization of gliomas. J. Neurosci 37, 6837–6850 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Varn FS et al. Glioma progression is shaped by genetic evolution and microenvironment interactions. Cell 10.1016/j.cell.2022.04.038 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hausmann D et al. Autonomous rhythmic activity in glioma networks drives brain tumour growth. Nature 10.1038/s41586-022-05520-4 (2022). [DOI] [PubMed] [Google Scholar]; This report identified highly gap junction-connected ‘hub’ cells with autonomous, periodic membrane depolarization driving synchronous calcium transients in the glioma network.
- 97.Piggott BJ et al. Paralytic, the Drosophila voltage-gated sodium channel, regulates proliferation of neural progenitors. Genes Dev 33, 1739–1750 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zeng Q et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 573, 526–531 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified a tumour growth-promoting role for glutamatergic signalling through the NMDA receptor in breast cancer brain metasteses; the metastatic breast cancer cells form an astrocyte-like perisynaptic process to usurp perisynaptic glutamate in this ‘pseudo-tripartite’ positon.
- 99.Buckingham SC et al. Glutamate release by primary brain tumors induces epileptic activity. Nat. Med 17, 1269–1274 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Campbell SL, Buckingham SC & Sontheimer H Human glioma cells induce hyperexcitability in cortical networks. Epilepsia 53, 1360–1370 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; This work demonstrated that gliomas induce neuronal hyperexcitability, thereby driving glioma-associated seizures.
- 101.Campbell SL et al. GABAergic disinhibition and impaired KCC2 cotransporter activity underlie tumor-associated epilepsy. Glia 63, 23–36 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.John Lin CC et al. Identification of diverse astrocyte populations and their malignant analogs. Nat. Neurosci 20, 396–405 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study discovered that an astrocyte-like subpopulation of glioma cells in adult glioblastoma, similar to normal astrocytes, secrete synaptogenic factors that promote synaptogenesis and contribute to neuronal hyperexcitability and glioma-associated seizures.
- 103.Yu K et al. PIK3CA variants selectively initiate brain hyperactivity during gliomagenesis. Nature 10.1038/s41586-020-1952-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; This report demonstrated that glioma cells with different point mutations in the same oncogene differentially contribute to neruonal hyperexcitability and seizures.
- 104.Hatcher A et al. Pathogenesis of peritumoral hyperexcitability in an immunocompetent CRISPR-based glioblastoma model. J. Clin. Invest 130, 2286–2300 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Krishna S et al. Glioblastoma remodeling of human neural circuits decreases survival. Nature 10.1038/s41586-023-06036-1 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]; This work demonstrated that glioblastoma remodels functional neural circuits in the human brain to promote neuronal activity in the tumour microenvironment, thereby impairing cognition and decreasing patient survival.
- 106.Belgers V et al. Postoperative oscillatory brain activity as an add-on prognostic marker in diffuse glioma. J. Neurooncol 147, 49–58 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kaucka M & Adameyko I Non-canonical functions of the peripheral nerve. Exp. Cell. Res 321, 17–24 (2014). [DOI] [PubMed] [Google Scholar]
- 108.Furness JB The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol 9, 286–294 (2012). [DOI] [PubMed] [Google Scholar]
- 109.Knox SM et al. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science 329, 1645–1647 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated that innervation of the salivary gland during development regulates glandular organogenesis, highlighting the crucial role for tissue stem cell niche innervation in development.
- 110.Nedvetsky PI et al. Parasympathetic innervation regulates tubulogenesis in the developing salivary gland. Dev. Cell 30, 449–462 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cassiman D et al. The vagal nerve stimulates activation of the hepatic progenitor cell compartment via muscarinic acetylcholine receptor type 3. Am. J. Pathol 161, 521–530 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gross ER et al. Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology 143, 408–417 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bower DV et al. Airway branching has conserved needs for local parasympathetic innervation but not neurotransmission. BMC Biol 12, 92 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weiner GA et al. Cholinergic neural activity directs retinal layer-specific angiogenesis and blood retinal barrier formation. Nat. Commun 10, 2477 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McVary KT et al. Growth of the rat prostate gland is facilitated by the autonomic nervous system. Biol. Reprod 51, 99–107 (1994). [DOI] [PubMed] [Google Scholar]
- 116.Golomb E, Kruglikova A, Dvir D, Parnes N & Abramovici A Induction of atypical prostatic hyperplasia in rats by sympathomimetic stimulation. Prostate 34, 214–221 (1998). [DOI] [PubMed] [Google Scholar]
- 117.Villers A, McNeal JE, Redwine EA, Freiha FS & Stamey TA The role of perineural space invasion in the local spread of prostatic adenocarcinoma. J. Urol 142, 763–768 (1989). [DOI] [PubMed] [Google Scholar]
- 118.Maru N, Ohori M, Kattan MW, Scardino PT & Wheeler TM Prognostic significance of the diameter of perineural invasion in radical prostatectomy specimens. Hum. Pathol 32, 828–833 (2001). [DOI] [PubMed] [Google Scholar]
- 119.Ayala GE et al. In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate 49, 213–223 (2001). [DOI] [PubMed] [Google Scholar]; This report provided early in vitro evidence that interactions between nerves and prostate cancer cells can influence cancer growth.
- 120.Magnon C et al. Autonomic nerve development contributes to prostate cancer progression. Science 341, 1236361 (2013). [DOI] [PubMed] [Google Scholar]; This study demonstrated that prostate innervation by autonomic nerves (sympathetic and parasympathetic) regulates prostate cancer progression in vivo.
- 121.Zahalka AH et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 358, 321–326 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mauffrey P et al. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 569, 672–678 (2019). [DOI] [PubMed] [Google Scholar]
- 123.Kotaka M et al. Adrenergic receptor agonists induce the differentiation of pluripotent stem cell-derived hepatoblasts into hepatocyte-like cells. Sci. Rep 7, 16734 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lundgren O, Jodal M, Jansson M, Ryberg AT & Svensson L Intestinal epithelial stem/progenitor cells are controlled by mucosal afferent nerves. PLoS ONE 6, e16295 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Raufman JP et al. Genetic ablation of M3 muscarinic receptors attenuates murine colon epithelial cell proliferation and neoplasia. Cancer Res 68, 3573–3578 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aihara T et al. Impaired gastric secretion and lack of trophic responses to hypergastrinemia in M3 muscarinic receptor knockout mice. Gastroenterology 125, 1774–1784 (2003). [DOI] [PubMed] [Google Scholar]
- 127.Hakanson R, Vallgren S, Ekelund M, Rehfeld JF & Sundler F The vagus exerts trophic control of the stomach in the rat. Gastroenterology 86, 28–32 (1984). [PubMed] [Google Scholar]
- 128.Axelson J, Ekelund M, Hakanson R & Sundler F Gastrin and the vagus interact in the trophic control of the rat oxyntic mucosa. Regul. Pept 22, 237–243 (1988). [DOI] [PubMed] [Google Scholar]
- 129.Raufman JP et al. Muscarinic receptor subtype-3 gene ablation and scopolamine butylbromide treatment attenuate small intestinal neoplasia in Apcmin/+ mice. Carcinogenesis 32, 1396–1402 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Albo D et al. Neurogenesis in colorectal cancer is a marker of aggressive tumor behavior and poor outcomes. Cancer 117, 4834–4845 (2011). [DOI] [PubMed] [Google Scholar]
- 131.Liebig C et al. Perineural invasion is an independent predictor of outcome in colorectal cancer. J. Clin. Oncol 27, 5131–5137 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhao CM et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med 6, 250ra115 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This report provided evidence that innervation of the stomach is crucial for gastric cancer progression.
- 133.Hayakawa Y et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell 31, 21–34 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This work identified a signalling loop in which parasympathetic nerve-derived acetylcholine stimulates gastrointestinal cancer oncogenic Wnt signalling and tumour growth and tumour cell-derived NGF promotes further nerve ingrowth into the tumour microenvironment.
- 134.Zhang Y et al. Cancer cells co-opt nociceptive nerves to thrive in nutrient-poor environments and upon nutrient-starvation therapies. Cell Metab 34, 1999–2017 (2022). [DOI] [PubMed] [Google Scholar]
- 135.Won MH, Park HS, Jeong YG & Park HJ Afferent innervation of the rat pancreas: retrograde tracing and immunohistochemistry in the dorsal root ganglia. Pancreas 16, 80–87 (1998). [DOI] [PubMed] [Google Scholar]
- 136.Fasanella KE, Christianson JA, Chanthaphavong RS & Davis BM Distribution and neurochemical identification of pancreatic afferents in the mouse. J. Comp. Neurol 509, 42–52 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kaneko T et al. Extrapancreatic nerve plexus invasion by carcinoma of the head of the pancreas. Diagnosis with intraportal endovascular ultrasonography. Int. J. Pancreatol 19, 1–7 (1996). [DOI] [PubMed] [Google Scholar]
- 138.Takahashi T et al. Perineural invasion by ductal adenocarcinoma of the pancreas. J. Surg. Oncol 65, 164–170 (1997). [DOI] [PubMed] [Google Scholar]
- 139.Mitsunaga S et al. Detail histologic analysis of nerve plexus invasion in invasive ductal carcinoma of the pancreas and its prognostic impact. Am. J. Surg. Pathol 31, 1636–1644 (2007). [DOI] [PubMed] [Google Scholar]
- 140.Zhu Z et al. Nerve growth factor expression correlates with perineural invasion and pain in human pancreatic cancer. J. Clin. Oncol 17, 2419–2428 (1999). [DOI] [PubMed] [Google Scholar]
- 141.Dang C, Zhang Y, Ma Q & Shimahara Y Expression of nerve growth factor receptors is correlated with progression and prognosis of human pancreatic cancer. J. Gastroenterol. Hepatol 21, 850–858 (2006). [DOI] [PubMed] [Google Scholar]
- 142.Guerra C et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 11, 291–302 (2007). [DOI] [PubMed] [Google Scholar]
- 143.Guerra C et al. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell 19, 728–739 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stopczynski RE et al. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res 74, 1718–1727 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saloman JL et al. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc. Natl Acad. Sci. USA 113, 3078–3083 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that sensory innervation of pancreatic cancer, which often presents with pain as an early symptom, promotes PDAC tumorigenesis.
- 146.Zhang D, Ma QY, Hu HT & Zhang M β2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NFκB and AP-1. Cancer Biol. Ther 10, 19–29 (2010). [DOI] [PubMed] [Google Scholar]
- 147.Guo K et al. Interaction of the sympathetic nerve with pancreatic cancer cells promotes perineural invasion through the activation of STAT3 signaling. Mol. Cancer Ther 12, 264–273 (2013). [DOI] [PubMed] [Google Scholar]
- 148.Kim-Fuchs C et al. Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for β-adrenergic signaling in the pancreatic microenvironment. Brain Behav. Immun 40, 40–47 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Batty GD, Russ TC, Stamatakis E & Kivimaki M Psychological distress in relation to site specific cancer mortality: pooling of unpublished data from 16 prospective cohort studies. Br. Med. J 356, j108 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Renz BW et al. β2 adrenergic–neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell 33, 75–90 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study elucidated a crucial role for sympathetic nervous system signalling through β2-adrenergic receptors on PDAC cells in tumour progression.
- 151.Hebb C & Linzell JL Innervation of the mammary gland. A histochemical study in the rabbit. Histochem. J 2, 491–505 (1970). [DOI] [PubMed] [Google Scholar]
- 152.Gerendai I et al. Transneuronal labelling of nerve cells in the CNS of female rat from the mammary gland by viral tracing technique. Neuroscience 108, 103–118 (2001). [DOI] [PubMed] [Google Scholar]
- 153.Koves K, Gyorgyi Z, Szabo FK & Boldogkoi Z Characterization of the autonomic innervation of mammary gland in lactating rats studied by retrograde transynaptic virus labeling and immunohistochemistry. Acta Physiol. Hung 99, 148–158 (2012). [DOI] [PubMed] [Google Scholar]
- 154.Liu Y et al. Sexually dimorphic BDNF signaling directs sensory innervation of the mammary gland. Science 338, 1357–1360 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Huang D et al. Nerve fibers in breast cancer tissues indicate aggressive tumor progression. Medicine 93, e172 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pundavela J et al. Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer. Mol. Oncol 9, 1626–1635 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Szpunar MJ, Belcher EK, Dawes RP & Madden KS Sympathetic innervation, norepinephrine content, and norepinephrine turnover in orthotopic and spontaneous models of breast cancer. Brain Behav. Immun 53, 223–233 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kamiya A et al. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat. Neurosci 22, 1289–1305 (2019). [DOI] [PubMed] [Google Scholar]; This work demonstrated that sympathetic innervation of breast cancer increases tumour progression whereas parasympathetic innervation decreases breast cancer progression in rodent models; in a human patient cohort, increased sympathetic innervation and decreased parasympathetic innervation of breast tumours correlated with poor clinical outcomes.
- 159.Erin N, Zhao W, Bylander J, Chase G & Clawson G Capsaicin-induced inactivation of sensory neurons promotes a more aggressive gene expression phenotype in breast cancer cells. Breast Cancer Res. Treat 99, 351–364 (2006). [DOI] [PubMed] [Google Scholar]
- 160.Sloan EK et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res 70, 7042–7052 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]; This report demonstrated that β-adrenergic signalling in the breast cancer microenvironment promotes metastatic spread through adrenergic effects on tumour-associated macrophages.
- 161.Hiller JG et al. Preoperative β-blockade with propranolol reduces biomarkers of metastasis in breast cancer: a phase II randomized trial. Clin. Cancer Res 26, 1803–1811 (2020). [DOI] [PubMed] [Google Scholar]
- 162.Peterson SC et al. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell 16, 400–412 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study discovered that cutaneous mechanosensory nerves release Hedgehog ligand, signalling to the touch dome epithelial stem cells that give rise to basal cell carcinoma and thereby providing an oncogenic signal required for basal cell carcinoma initiation and growth.
- 163.Salvador AF, de Lima KA & Kipnis J Neuromodulation by the immune system: a focus on cytokines. Nat. Rev. Immunol 21, 526–541 (2021). [DOI] [PubMed] [Google Scholar]
- 164.Mohammadpour H et al. β2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J. Clin. Invest 129, 5537–5552 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jiang SH et al. GABRP regulates chemokine signalling, macrophage recruitment and tumour progression in pancreatic cancer through tuning KCNN4-mediated Ca2+ signalling in a GABA-independent manner. Gut 68, 1994–2006 (2019). [DOI] [PubMed] [Google Scholar]
- 166.Yang H et al. Stress–glucocorticoid–TSC22D3 axis compromises therapy-induced antitumor immunity. Nat. Med 25, 1428–1441 (2019). [DOI] [PubMed] [Google Scholar]
- 167.Qiao G, Chen M, Bucsek MJ, Repasky EA & Hylander BL Adrenergic signaling: a targetable checkpoint limiting development of the antitumor immune response. Front. Immunol 9, 164 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Devi S et al. Adrenergic regulation of the vasculature impairs leukocyte interstitial migration and suppresses immune responses. Immunity 54, 1219–1230 (2021). [DOI] [PubMed] [Google Scholar]
- 169.Balood M et al. Nociceptor neurons affect cancer immunosurveillance. Nature 611, 405–412 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; This work showed that cutaneous nociceptor (pain-sensing) nerves promote CD8+ T cell exhaustion in the tumour microenvironment of melanoma through release of the CGRP neuropeptide.
- 170.Huang D et al. Cancer-cell-derived GABA promotes β-catenin-mediated tumour growth and immunosuppression. Nat. Cell Biol 24, 230–241 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang B et al. B cell-derived GABA elicits IL-10+ macrophages to limit anti-tumour immunity. Nature 599, 471–476 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that B lymphocytes secrete the neurotransmitter GABA, which signals to CD8+ T lymphocytes through GABAA receptors and reduces T cell function; B cell-derived GABA also induces an immune-suppressive phenotype in tumour-associated macrophages, blocking anti-cancer immunity and permitting increased tumour growth in a colon cancer model.
- 172.Schneider MA et al. Attenuation of peripheral serotonin inhibits tumor growth and enhances immune checkpoint blockade therapy in murine tumor models. Sci. Transl. Med 13, eabc8188 (2021). [DOI] [PubMed] [Google Scholar]
- 173.Borovikova LV et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 (2000). [DOI] [PubMed] [Google Scholar]
- 174.Poller WC et al. Brain motor and fear circuits regulate leukocytes during acute stress. Nature 10.1038/s41586-022-04890-z (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Katayama Y et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124, 407–421 (2006). [DOI] [PubMed] [Google Scholar]
- 176.Mendez-Ferrer S, Lucas D, Battista M & Frenette PS Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452, 442–447 (2008). [DOI] [PubMed] [Google Scholar]
- 177.Suzuki K, Hayano Y, Nakai A, Furuta F & Noda M Adrenergic control of the adaptive immune response by diurnal lymphocyte recirculation through lymph nodes. J. Exp. Med 213, 2567–2574 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Huang S et al. Lymph nodes are innervated by a unique population of sensory neurons with immunomodulatory potential. Cell 184, 441–459 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Koren T et al. Insular cortex neurons encode and retrieve specific immune responses. Cell 184, 5902–5915 (2021). [DOI] [PubMed] [Google Scholar]; This work identified neuronal representation of immune reactions in the insular cortex, showing that reactivation of the neurons involved in encoding ‘immunological memory’ can recapitulate the immune response.
- 180.Izumoto S et al. Seizures and tumor progression in glioma patients with uncontrollable epilepsy treated with perampanel. Anticancer Res 38, 4361–4366 (2018). [DOI] [PubMed] [Google Scholar]


