Abstract
Background and Objectives
Fremanezumab is an effective treatment for episodic (EM) and chronic migraine (CM) patients in Japan, but its cost effectiveness remains unknown. The objective of this study was to determine the cost effectiveness of fremanezumab compared with standard of care (SOC) in previously treated EM and CM patients from a Japanese healthcare perspective.
Methods
Estimated regression models were implemented in a probabilistic Markov model to inform effectiveness and health-related quality-of-life data for fremanezumab and SOC. The model was further populated with data from the literature. The adjusted Japanese healthcare perspective included productivity losses. The main model outcomes were quality-adjusted life-years (QALYs), costs (2022 Japanese Yen [¥]), and incremental outcomes including the incremental cost-effectiveness ratio (ICER). Analyses were performed separately for the EM and CM patients and combined. Costs and effects were discounted at an annual rate of 2.0%.
Results
The mean QALYs over a 25-year time horizon for the EM and CM populations combined were 13.03 for SOC and 13.15 for fremanezumab. The associated costs were ¥27,550,292 for SOC and ¥28,371,048 for fremanezumab. QALYs were higher and costs lower for EM patients compared with CM patients for both fremanezumab and SOC. The deterministic ICERs of fremanezumab versus SOC were ¥6,334,861 for EM, ¥7,393,824 for CM, and ¥6,530,398 for EM and CM combined. Indirect costs and choice of mean migraine days model distribution had a substantial impact on the ICER.
Conclusion
Using fremanezumab in a heterogeneous mixture of Japanese EM and CM patients resulted in a reduction of monthly migraine days and thus more QALYs compared with SOC. The cost effectiveness of fremanezumab versus SOC in EM and CM patients resulted in an ICER of ¥6,530,398, from an adjusted Japanese public healthcare perspective.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-024-01380-0.
Plain Language Summary
Fremanezumab is an effective treatment for episodic and chronic migraine patients in Japan, but it is unknown how the costs relate to the health benefits. The current research determined the relation between costs and effects of fremanezumab compared with the current standard of care in Japanese clinical practice, to see if the costs are justified by the health benefits. A model was used to inform the treatment effect of fremanezumab and standard of care. Data on costs, the frequency in which health care was used, and impairment of work due to migraine were also included in the model and obtained from the literature. The main outcomes were the number of years that patients were alive while taking their quality of life into account, costs, and the difference in these outcomes between patients who were treated with fremanezumab and those receiving standard of care. Subsequently, it was estimated how costs and effects related to one another and whether the costs were justified by the health benefits. The outcomes showed that patients treated with fremanezumab had a better quality of life compared with those receiving standard of care, while the costs associated with fremanezumab were higher. Compared with standard of care, the health benefits of treating patients with fremanezumab were justified by the costs within an acceptable range. Taking the absence from work due to illness into account had a substantial impact on the model outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-024-01380-0.
Key Points for Decision Makers
| This cost-effectiveness analysis found that treating episodic (EM) and chronic migraine (CM) patients resulted in an incremental cost-effectiveness ratio (ICER) of ¥6,530,398. Results indicate that fremanezumab resulted in more QALY gain and a lower ICER for EM patients compared with CM. |
| Indirect costs for migraine patients such as productivity losses should be explored in cost-effectiveness analysis in Japan, as they are strongly related to the severity of the disease and have a substantial impact on the ICER. |
| The choice of distribution for modeling mean monthly migraine days can have a substantial impact on the ICER and should be carefully informed by statistical fit and experts’ opinions. |
Introduction
Migraine is a neurological condition, characterized by a throbbing headache, nausea, vomiting, photophobia and phonophobia [1]. The disease disproportionately occurs more in women than men, affecting 6–8% of men compared with 15–18% of women each year [2–4]. Different from a headache, a migraine attack is associated with at least one of the symptoms mentioned above [1]. Furthermore, migraine can be classified into episodic migraine (EM; < 15 monthly headache days [MHDs] including ≥ 4 monthly migraine days [MMDs]) and chronic migraine (CM; ≥ 15 MHDs including ≥ 8 MMDs over the preceding 3 months) [1, 5].
In Japan, the United States (US), and Europe, nearly 75 million people have EM and CM [6]. In 2016, migraine was reported to be the fourth leading cause of years lived with disease in Japan [7]. Migraine is associated with personal pain, disability, a reduced health-related quality-of-life (HRQOL), and financial cost to society [8, 9]. The high economic burden of migraine is especially due to its costs associated with productivity loss [4, 8, 10–12], as migraine prevents people from going to work and is most common between the age of 25 and 55 years [10]. In Japan, an increasing amount of real-world data suggests that both EM and CM poses a notable disease burden and highlights high levels of unmet need with prescribed acute and preventative medication among people with migraine [13, 14].
Previously, migraine was treated with acute treatment such as painkillers (e.g., triptans and non-steroidal anti-inflammatory drugs) to relieve symptoms during a migraine attack and prophylactic treatment (e.g., β-blockers, anticonvulsant drugs, antidepressant drugs, selective serotonin and selective serotonin-norepinephrine reuptake inhibitors, calcium channel antagonists and neurotoxins) to reduce the severity, duration and frequency of attacks [15]. Until recently, preventive therapies have been repurposed from other indications, often with unknown working mechanisms, leading to a poor tolerability and lack of efficacy [16–18]. This in turn will lead to increasing discontinuation rates and dissatisfaction amongst patients [16–18].
Recently introduced monoclonal antibodies against calcitonin gene-related peptide (CGRP) and the CGRP receptor for preventive treatment of migraine have shown an improved tolerability, efficacy, safety and ease of use compared with conventional treatments [16, 17]. Fremanezumab is one of the available anti-CGRPs to treat migraine and has been proven effective in preventing EM and CM in two Japanese-Korean phase IIb/III clinical trials [19, 20]. Anti-CGRP monoclonal antibodies are more expensive compared with conventional treatment and should therefore only be prescribed if the improvement in effectiveness is high enough considering the costs [16, 17].
To our knowledge, only limited data exists on the cost effectiveness of fremanezumab compared with conventional treatment, consisting of acute treatment and prophylactics [21, 22]. For Japan, where conventional treatment primarily consists of acute treatment, no available evidence exists on the cost effectiveness of fremanezumab to inform treatment selection from a health economic perspective [23]. Also, there is no formal willingness-to-pay (WTP) threshold to determine whether a treatment is cost effective in Japan, and cost effectiveness is applied for price adjustment after reimbursement under current pricing rules. A range of 5–15 million Japanese Yen (¥) per quality-adjusted life-year (QALY) will be used for price adjustment [24].
The objective of the current research is to determine the cost effectiveness of fremanezumab compared with standard of care (SOC) in previously treated EM and CM patients from a Japanese healthcare perspective.
Methods
Overview
To estimate the effectiveness and cost effectiveness of fremanezumab in migraine patients, a decision analytic model is needed. The effectiveness of fremanezumab and placebo on the number of MMDs and HRQOL in EM and CM patients was captured in a previous study by Wang et al. using regression models [25]. For the current research, these regression models were implemented in a probabilistic Markov model that was developed in Microsoft Excel. This model was further populated with healthcare resource use (HCRU) and cost data and used to estimate the cost effectiveness of fremanezumab compared with SOC. An adjusted Japanese public healthcare perspective was adopted in which productivity losses were included, and the main model outcomes were life-years (LY), QALYs, costs in Japanese Yen (¥) inflated to 2022, and an incremental cost-effectiveness ratio (ICER). The current cost-effectiveness model (CEM) performed separate analyses for the EM and CM patients and merged the results for combined analyses.
Model Population
The model population was based on a cohort of Japanese EM (n = 357) and CM (n = 571) patients whose data was obtained from Japanese-Korean clinical trials [19, 20, 26]. The population consisted of patients who had a history of migraine for ≥ 12 months prior to trial admission. EM patients had 6–14 headache days with ≥ 4 migraine days (MDs), and CM patients ≥ 15 headache days with ≥ 8 MDs per 28 days. For the base-case analysis, only data from the Japanese patients was used. More details on the clinical trials are listed in the electronic supplementary material (ESM).
Treatments for Episodic and Chronic Migraine
In the clinical trials [19, 20, 26] that were used to estimate MMDs and HRQOL [25], patients were treated with fremanezumab 675 mg in the first month, followed by two doses of placebo in the second and third months. Alternatively, patients could also be treated with monthly administered fremanezumab 275 mg. Patients who were not treated with fremanezumab were treated with monthly administered placebo. Patients that were treated with both dosing schedules for fremanezumab were in the same intervention arm of the current CEM. The patients who received placebo were considered as the control arm (i.e., SOC) in the current CEM.
Model Structure
The disease course was simulated in the Markov model with three health states: alive (on treatment), alive (off treatment), and death (Fig. 1). Within each ‘alive’ health state, patients were partitioned over 29 MMDs per month (assuming 28-day months and 0 MMDs included) using the regression models from the previous modeling study [25]. Patients begin treatment, and after 3 months, either respond to and remain on treatment, or do not respond and discontinue (i.e. off treatment). ‘No response’ was defined as patients who did not achieve a 30% reduction in mean MMDs for CM and 50% for EM at 3 months. These definitions for no treatment response are considered clinically meaningful in the respective patient populations and were validated with Japanese clinical experts [27, 28]. The model population was simulated with 28-day cycles over a 25-years’ time horizon. Migraine is most common in younger and middle-aged people aged 25 years and older, and predominately effects women [2]. Since the course of the disease changes significantly after menopause, which on average occurs around the age of 50 years, the 25-year time horizon was deemed appropriate [29]. The Markov model was developed in Microsoft Excel.
Fig. 1.
Model structure. Patients could either be on treatment, off treatment or die. The arrow between on and off treatment represented the discontinuation rate and the arrows to the death state the general mortality rate. In each ‘alive’ health state the distribution of patients across based on mean MMDs was determined, which was different for patients that were treated with fremanezumab and SOC (i.e., off treatment). MMD monthly migraine day, SOC standard of care
Model Input
Treatment Efficacy
Model input parameters are listed in Table 1. Results from the previous modeling study by Wang et al. [25] were used to inform treatment efficacy and utility data (for utilities see Sect. 2.5.3) in the current model. Parametric regression models were used to model mean MMD distributions for EM and CM patients treated with fremanezumab or SOC over a period of 3 months to inform health state transitions. For the base case, the zero-inflated beta binomial (ZIBB) distribution was used with only trial data from the Japanese participants [25]. This approach effectively modeled all 29 potential health states based on the number of MMDs during each model cycle. Due to a lack of long-term efficacy data, the model assumed that the treatment effect at 3 months was maintained as long as patients remained on treatment. This assumption was validated by clinical experts within the field of neurology (TT and FS). More details on estimating the MMDs can be found in the previous modeling study [25].
Table 1.
Model input parameters
| Parameter | Parameter estimate | SEa | Distribution | Sources |
|---|---|---|---|---|
| Model population | ||||
| EM patients, % | 70.0 | – | Fixed | [19, 20] |
| CM patients, % | 30.0 | – | Fixed | [19, 20] |
| Proportion female EM, % | 84.6 | 16.9 | Beta | [19, 20] |
| Proportion female CM, % | 86.0 | 17.2 | Beta | [19, 20] |
| Patient starting age EM, years | 44.0 | 8.8 | Normal | [19, 20] |
| Patient starting age CM, years | 43.0 | 8.6 | Normal | [19, 20] |
| Treatment effect on MMDs | ESM | ZIBB | [25] | |
| Discontinuation Log rate per model cycle | 7.716 | 0.154 | Exponential | [19, 20, 26] |
| Drug acquisition costs, ¥ | ||||
| Fremanezumab per kit (225 mg per month) | 39,090 | 7818 | Gamma | [35] |
| SOCb | 99 | 20 | Gamma | [33] |
| Drug administration costs, ¥ | 200 | 40 | Gamma | [32] |
| HCRU costs, ¥ | ||||
| Generalist visit | 3130 | 606 | Gamma | [32] |
| Acute treatment drug cost | 99 | 20 | Gamma | [33] |
| HCRU | ||||
| Generalist visit per MMD | 0.0711 | 0.0142 | Normal | [32] |
| SOC drug use per MMD | 1.0 | 0.2 | Normal | Assumption |
| Indirect cost parameters, % c | ||||
| Work time missed (0–3 MMDs) | 3.8 | 0.8 | Beta | [34] |
| Work time missed (4–7 MMDs) | 4.4 | 0.9 | Beta | [34] |
| Work time missed (8–14 MMDs) | 5.5 | 1.1 | Beta | [34] |
| Work time missed (15–28 MMDs) | 6.2 | 1.2 | Beta | [34] |
| Impairment while working (0–3 MMDs) | 29.8 | 6.0 | Beta | [34] |
| Impairment while working (4–7 MMDs) | 39.6 | 7.9 | Beta | [34] |
| Impairment while working (8–14 MMDs) | 44.1 | 8.8 | Beta | [34] |
| Impairment while working (15–28 MMDs) | 49.9 | 10.0 | Beta | [34] |
| Monthly salaries | ESM | [36] | ||
| Utilities | ESM | [25] |
CM chronic migraine, EM episodic migraine, ESM electronic supplementary material, HCRU health care resource use, MMDs monthly migraine days, SE standard error, SOC standard of care, ZIBB zero-inflated beta binomial
aAll SE were 20% of the mean because they were not available from the original source, except for the discontinuation rate, treatment effect on MMDs and utilities
bCosts for SOC consisted of acute and preventive treatment drug costs
cAbsenteeism and presenteeism differed per number of MMDs and was equally applied for EM and CM patients
Patients discontinued treatment when they did not adhere to the response criteria for fremanezumab in EM and CM and, therefore, a ‘negative’ stopping rule was applied. This concerned 50% of the EM and 25% of the CM patients that were treated with fremanezumab, which was based on clinical experts’ opinions (TT and FS). For each non-responder to fremanezumab, the treatment effect obtained by fremanezumab was replaced with the treatment effect for SOC. More details on treatment discontinuation can be found in the ESM.
Patients could also discontinue treatment for any reason [19, 20, 26]. An exponential parametric model was fitted to the observed data to estimate the discontinuation rate per model cycle (Table 1).
Migraine-related mortality was not modeled. However, given the 25-year time horizon, age-related mortality was included and modelled separately, using life tables from the Ministry of Health, Labor and Welfare in Japan [30].
Costs and Resource Use
Costs (2022 Japanese Yen [¥]), HCRU and discount rates were based on the Japanese C2H guidelines, clinical experts’ opinion and real-world patient survey data [31, 32]. Model costs and HCRU were chosen if they were expected to introduce any incremental differences between fremanezumab and SOC. The following cost categories were included in the model: treatment acquisition and administration costs, generalist visits, acute treatment costs, and productivity losses.
Treatment acquisition costs were applied to all patients that were on treatment with fremanezumab. The SOC drug acquisition costs were captured under resource use costs for patients in the fremanezumab arm that were off treatment and in the SOC arm. The SOC drug acquisition costs were calculated based on the JMDC database from Jan 2021 to Dec 2021 [33]. The ten most-sold medicines in the database were averaged (weighted by usage) and it was assumed that patients used these drugs once every migraine day in the model. An overview of these ten most-sold medicines is provided in the ESM. Treatment required a trained specialist to perform each administration. It was assumed that all patients had the injections administered in the base case. As such, all patients incurred an additional cost of ¥200 per administration [32]. Cost and HCRU for generalists visits were obtained from the literature [32]. The HCRU was obtained for 1 MMD and extrapolated linearly as the number of MMDs increased. Acute treatment costs were equal to SOC acquisition costs and applied to the fremanezumab arm. The HCRU of acute treatment costs was assumed to be 1 per MMD and also extrapolated linearly per MMD. Finally, productivity losses were included in the base-case analysis of the model because of the strong relationship between migraine and productivity [8]. The productivity losses were calculated using the annual salary for men and women in Japan multiplied with the percentage of work time missed (absenteeism) and impairment while working (presenteeism). The absenteeism and presenteeism differed between the number of MMDs based on the literature [34]. This calculation resulted in the annual productivity loss which was recalculated to monthly costs and applied to each model cycle. Costs and effects were discounted with an annual discount rate of 2% [31]. More details on productivity losses are listed in the ESM.
Utilities
MMD-specific utility values were used that were also estimated in the previous modeling study [25]. In that study, utility values were obtained from the Japanese-Korean clinical trials [19, 20, 26], with the migraine-specific quality-of-life (MSQ) questionnaire and mapped to EuroQol–five dimension–three level (EQ-5D-3L) values [25]. The MSQ-derived EQ-5D-3L values were modelled as a function of MMDs using a linear regression model, resulting in specific values for each health state in the current Markov model [25]. Different from the previous modeling study, treatment-specific utilities were assumed in the base case by incorporating treatment as a covariate in the regression model. This was advised by clinical experts within the field of neurology (TT and FS) and health technology assessment (AI) as they believed that the number of MMDs should not be the only factor that impacts HRQOL, but also factors such as the length and severity of migraine. More details on the utilities can be found in the ESM.
Analyses
Base-Case Analysis
A deterministic base-case analysis was performed using the point estimates of each model parameter. Incremental LY, QALY and costs were calculated between fremanezumab and SOC. The ICER was calculated using the incremental QALY and costs but there was no specific WTP threshold in Japan as a reference.
Sensitivity Analyses
Several sensitivity analyses were performed to test the robustness and uncertainty of the model. Deterministic one-way sensitivity analyses (OWSA) were performed to test the influence of each individual parameter on the model outcomes. Parameter values were varied on their standard error, which was estimated based on the input’s source, or set to 20% of the mean if not available.
Probabilistic sensitivity analyses were performed in Microsoft Excel Visual Basic for Applications (VBA) to evaluate uncertainty of all model parameters simultaneously. This was done by drawing random values for each model parameter from prespecified distributions using Monte Carlo simulations with 2000 iterations. Model convergence was tested to determine if this was enough iterations for the PSA to produce robust outcomes. More details on model convergence can be found in the ESM.
Scenario analyses were performed to explore the impact of certain model settings and assumptions on the results. Scenario 1 included both the Japanese and Korean clinical trial data to model the effectiveness of fremanezumab and SOC (both on mean MMDs and utilities) and explore the difference compared with using Japanese-specific data only. Scenario 2 removed productivity losses from the analysis, since the Japanese healthcare perspective does not typically include indirect costs. In Scenario 3 and Scenario 4, the zero-inflated negative-binomial (ZINBI) and zero-inflated gamma (ZAGA) models were used to model the mean MMD distributions over time, respectively, and explore the impact of the selected parametric model distribution. Scenario 5 and Scenario 6 were a combination of Scenario 2 and Scenario 3 and Scenario 2 and Scenario 4, respectively. In Scenario 7, a time horizon of 5 years was adopted to estimate the short-term impact of fremanezumab on costs and effects. Scenario 8 included a positive stopping rule for patients to discontinue treatment after 1 year if they have not experienced loss of response. It was assumed that this concerned 10% of the EM and CM patients and that the treatment effect linearly waned back to baseline (i.e., treatment effect at month 0) over a period of 1 year.
Model Validation
The model was checked for face validity and assumptions were validated by experts within the field of neurology (KY, FS) and health economic outcomes research (AI). Model input parameters, outcomes and scenarios analyses were also validated by the clinical physicians (KY, FS, AI). The model was also checked for validity by one of the authors (MS) using the Assessment of the Validation Status of Health Economic decision models (AdViSHE) checklist [37]. The completed checklist can be found in the ESM.
Results
Base Case
The model results showed that in the EM population, patients had 2624 MMDs in total while treated with fremanezumab compared with 2789 MMDs with SOC (increment of 83.3). For the CM population, the total MMDs amounted to 4882 for fremanezumab and 4965 for SOC (increment of 164.9). The total population (consisting of 70% EM and 30% CM patients) had in total 3301 MMDs while treated with fremanezumab and 3442 with SOC (increment of 140.4).
The main deterministic base-case results are listed in Table 2. The mean QALYs over the entire time horizon for the total population (consisting of 70% EM and 30% CM patients) were 13.03 for SOC and 13.15 for fremanezumab. The mean costs over the entire time horizon for the total population were ¥27,550,292 for SOC and ¥28,371,048 for fremanezumab. The total QALYs for EM patients were higher and the total costs lower compared with the CM patients for both fremanezumab and SOC (Table 2). The ICER was ¥6,334,861 for the EM and ¥7,393,824 for CM patients, while for the total population it amounted to ¥6,530,398. Additional intermediate base-case results are listed in the ESM.
Table 2.
Total mean outcomes per patients of the deterministic base-case analysis
| Treatment | LY | QALY | Costs, ¥ | Incremental LY | Incremental QALY | Incremental costs, ¥ | ICER, ¥ |
|---|---|---|---|---|---|---|---|
| EM patients | |||||||
| SOC | 19.21 | 13.38 | 26,364,783 | ||||
| Fremanezumab | 19.21 | 13.53 | 27,292,163 | 0a | 0.15 | 927,380 | 6,334,861 |
| CM patients | |||||||
| SOC | 19.25 | 12.21 | 30,316,481 | ||||
| Fremanezumab | 19.25 | 12.29 | 30,888,446 | 0a | 0.08 | 571,965 | 7,393,824 |
| EM (70%) and CM (30%) patients (weighted average) | |||||||
| SOC | 19.22 | 13.03 | 27,550,292 | ||||
| Fremanezumab | 19.22 | 13.15 | 28,371,048 | 0a | 0.13 | 820,755 | 6,530,398 |
CM chronic migraine, EM episodic migraine, ICER incremental cost-effectiveness ratio, INMB incremental net monetary benefit, LY life-years, QALY quality-adjusted life-year, SOC standard of care
aThe incremental life-years were 0, as there was no migraine-specific mortality included in the model and thus no difference in life expectancy between the fremanezumab and SOC arms
One-Way Sensitivity Analysis
In general, results of the OWSA showed that the model results were robust (Fig. 2). However, the percentage of work time missed for several different numbers of MMDs and for both on and off treatment had quite some impact on the ICER, especially in EM patients. Varying the percentage of work time missed for EM patients with 4–7 MMDs who were off treatment based on the SE, resulted in an ICER range of −¥1,482,707 to ¥14,010,238. Furthermore, varying the treatment acquisition costs of fremanezumab resulted in an ICER range of ¥2,934,538 to ¥10,896,186. Additional results on the OWSA can be found in the ESM.
Fig. 2.
Tornado plot of the one-way sensitivity analysis results of fremanezumab versus standard of care on the incremental cost-effectiveness ratio. ICER incremental cost-effectiveness ratio, MMDs monthly migraine days
Probabilistic Sensitivity Analysis
Results of the PSA are shown in Table 3, Fig. 3, and Fig. 4. The incremental outcomes showed that fremanezumab always resulted in more QALYs (0.12 [credible interval: 0.07–0.19]), however, in a few cases in less costs (¥961,080 [−¥301,302 to ¥2,210,494]) compared with SOC. The probabilistic ICER of fremanezumab versus SOC was ¥7,958,648. Based on the PSA results, fremanezumab had a 51.0% and 93.2% chance of being cost effective compared with SOC, if the WTP per QALY were ¥8,320,000 and ¥15,040,000, respectively (Fig. 4). Fremanezumab had about a 26.0% chance of being cost effective compared with SOC, if the WTP was ¥5,000,000.
Table 3.
Results of the probabilistic base-case analysis
| Treatment | QALY (95% CrI) | Costs, ¥ (95% CrI) | Incremental QALY (95% CrI) | Incremental costs, ¥ (95% CrI) | ICER, ¥ |
|---|---|---|---|---|---|
| EM patients | |||||
| SOC | 13.30 (11.91–14.53) | 23,572,184 (7,981,237–37,842,142) | |||
| Fremanezumab | 13.44 (12.06–14.69) | 24,611,488 (9,243,321–37,914,937) | 0.14 (0.07–0.22) | 1,039,303 (− 762,309 to 2,759,890) | 7,360,884 |
| CM patients | |||||
| SOC | 12.16 (10.98–13.22) | 26,968,587 (8,807,275–41,505,270) | |||
| Fremanezumab | 12.23 (11.03–13.32) | 27,595,564 (9,519,467–42,048,272) | 0.07 (0.03–0.14) | 626,976 (− 233,435 to 1,499,489) | 10,095,698 |
| EM (70%) and CM (30%) patients (weighted average) | |||||
| SOC | 13.00 (11.69–14.14) | 24,118,958 (12,479,408–35,816,903) | |||
| Fremanezumab | 13.12 (11.81–14.27) | 25,080,038 (13,728,683–36,341,852) | 0.12 (0.07–0.19) | 961,080 (− 301,302 to 2,210,494) | 7,958,648 |
CM chronic migraine, CrI credible interval, EM episodic migraine, ICER incremental cost-effectiveness ratio, QALY quality-adjusted life-year, SOC standard of care
Fig. 3.
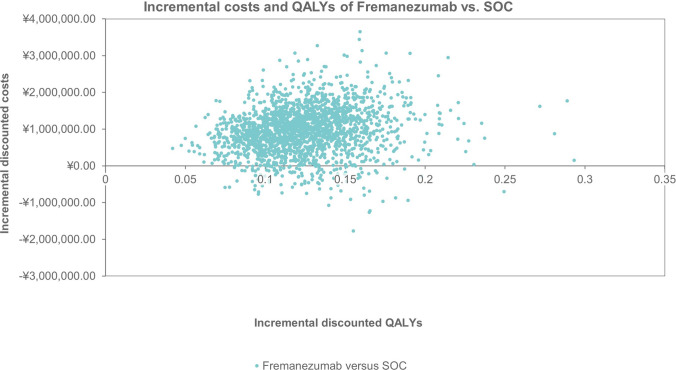
Incremental cost-effectiveness plane of fremanezumab versus standard of care. QALY quality-adjusted life-year, SOC standard of care
Fig. 4.
Incremental cost-effectiveness ratio of fremanezumab versus standard of care for an increasing willingness-to-pay threshold. QALY quality-adjusted life-year, SOC standard of care
Scenario Analysis
Results of the scenario analysis are listed in Table 4. Using both Japanese and Korean clinical trial data (Scenario 1) resulted in slightly more incremental costs and a comparable ICER compared with the base case, indicating that the data of both nationalities were comparable. Excluding the productivity losses (Scenario 2) resulted in an increase in incremental costs and a higher ICER, as more indirect costs were accrued by patients treated with SOC compared with fremanezumab. Using the ZINBI distribution to model the mean MMDs (Scenario 3) resulted in slightly lower incremental costs and a comparable ICER compared with the base case, as this model resulted in slightly more patients having fewer MMDs compared with the ZIBB model. Using the ZAGA distribution to model the mean MMDs (Scenario 4) resulted in less incremental costs and a lower ICER compared with the base case, as this model resulted in more patients having fewer MMDs compared with the ZIBB model. Combining Scenario 2 with 3 (Scenario 5) and Scenario 2 with 4 (Scenario 6) both resulted in incremental costs and ICERs that were higher than the base case and comparable with Scenario 2, indicating that the exclusion of productivity losses had the most influence on the outcomes. Shortening the time horizon to 5 years (Scenario 7) resulted in less incremental QALYs and costs and a comparable ICER compared with the base case. This indicates that the most gain in QALYs and costs of fremanezumab compared with SOC takes place in the first few years and differences between the two treatment arms become less as we move further in time. Applying a positive stopping rule to patients that still respond to treatment after a year (Scenario 8) resulted in less incremental costs and a lower ICER compared with the base case. This is because patients that discontinue fremanezumab because of this positive stopping rule slowly wane back to baseline in terms of treatment effect, while they immediately stop accruing treatment costs.
Table 4.
Deterministic results of the scenario analysis of the pairwise comparison of fremanezumab versus placebo
| Scenario | EM patients | CM patients | EM (70%) and CM (30%) patients (weighted average) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Incr. QALY |
Incr. costs, ¥ |
ICER, ¥ | Incr. QALY |
Incr. costs, ¥ |
ICER, ¥ | Incr. QALY |
Incr. costs, ¥ |
ICER, ¥ | |
| Base case | 0.15 | 927,380 | 6,334,861 | 0.08 | 571,965 | 7,393,824 | 0.13 | 820,755 | 6,530,398 |
| Scenario 1: Using Japanese and Korean clinical trial data | 0.15 | 949,866 | 6,514,786 | 0.09 | 527,243 | 5,870,344 | 0.13 | 823,079 | 6,380,186 |
| Scenario 2: Excluding productivity losses | 0.15 | 1,473,079 | 10,062,489 | 0.08 | 732,120 | 9,464,154 | 0.13 | 1,250,791 | 9,952,007 |
| Scenario 3: Using the ZINBI distribution to model the mean MMD distributions | 0.15 | 906,652 | 6,051,094 | 0.09 | 554,708 | 6,350,115 | 0.13 | 801,069 | 6,110,871 |
| Scenario 4: Using the ZAGA distribution to model the mean MMD distributions | 0.15 | 871,173 | 5,958,304 | 0.10 | 480,039 | 4,826,120 | 0.13 | 753,833 | 5,702,725 |
| Scenario 5: Scenario 2 + Scenario 3 | 0.15 | 1,471,672 | 9,822,096 | 0.09 | 728,237 | 8,336,609 | 0.13 | 1,248,642 | 9,525,130 |
| Scenario 6: Scenario 2 + Scenario 4 | 0.15 | 1,473,186 | 10,075,714 | 0.10 | 723,371 | 7,272,490 | 0.13 | 1,248,242 | 9,442,917 |
| Scenario 7: Using a 5-year time horizon | 0.09 | 590,883 | 6,497,924 | 0.05 | 390,407 | 7,694,131 | 0.08 | 530,741 | 6,728,779 |
| Scenario 8: Including a positive stopping rule for treatment discontinuation | 0.15 | 796,023 | 5,437,570 | 0.08 | 514,161 | 6,646,585 | 0.13 | 711,464 | 5,660,814 |
CM chronic migraine, EM episodic migraine, ICER incremental cost-effectiveness ratio, Incr. incremental, MMDs monthly migraine days, QALY quality-adjusted life-years, ZAGA zero-inflated gamma, ZINBI zero-inflated negative binomial
Discussion
The current study determined the cost effectiveness of fremanezumab compared with SOC in Japanese EM and CM patients. Regression models were used to model the treatment effect of fremanezumab on MMDs and HRQOL [25]. The model used a Japanese public healthcare perspective but also included productivity losses in its base-case analysis. The deterministic ICER of fremanezumab compared with SOC was ¥6,334,861 for EM, ¥7,393,824 for CM and ¥6,530,398 for the total population. Our findings showed that fremanezumab compared with SOC always resulted in more QALYs but in a few cases in less costs with a probabilistic ICER of ¥7,360,884 for EM, ¥10,095,698 for CM and ¥7,958,648 for the total population. Using different parametric distributions to model the mean MMDs resulted in better ICERs compared with the base case (Scenario 3: ¥6,110,871 and Scenario 4: ¥5,702,725), as well as including a positive stopping rule (Scenario 8: ¥5,660,814). The highest ICERs were found in the scenarios in which productivity losses were excluded from the analysis (Scenario 2: ¥9,952,007, Scenario 5: ¥9,525,130, and Scenario 6: ¥9,442,917). Given the WTP threshold range that is used for price adjustment, all ICERs of the base-case analysis and scenario analyses were within the range of ¥5.0 to ¥15.0 million. Of course, depending on what threshold is used, these results could suggest that fremanezumab is cost effective compared with SOC. However, if a WTP threshold of ¥5.0 million were adopted, fremanezumab would only have about a 26% chance of being cost effective compared with SOC.
To our knowledge, fremanezumab has been evaluated and proven to have met the WTP threshold in numerous countries [21, 22]. However, it is hard to compare CE studies due to different assumptions, parameter inputs and comparators.
These results came with some assumptions regarding extrapolating the treatment effect on MMDs and utilities beyond the observed effectiveness data. We explored the impact of choosing different parametric distributions on the main outcomes in scenario analyses. The ZINBI distribution produced a slightly better ICER compared with the base case, while the ZAGA produced a substantially better ICER. This was because the ZINBI and ZAGA models estimated more patients with less MMDs compared with the ZIBB. The study by Wang et al. (2023) found that the ZINBI distribution had a comparable statistical fit to the ZIBB model for EM patients based on the Akaike information criterion (AIC) [25]. This was not the case for CM patients, nor for the ZAGA model for both EM and CM patients. Ultimately, the ZIBB was chosen as the best fitting model according to expert validation and therefore was selected for the base-case analysis of the current research [25]. However, due to convergence problems with our data in a few PSA iterations and the assumed uncertainty around the indirect costs (main driver of the ICER), the ZIBB model estimated a mean probabilistic ICER that was higher than the deterministic one.
According to a clinical expert within the field of neurology (FS), the effects of anti-CGRP are groundbreaking, as many patients experience a significant reduction in their disability in clinical practice. The rate of improvement in the degree of disability is comparable to reports from Europe and the US [21, 22, 38]. Many patients realized that they had a high level of disability only after the disability was improved.
A limitation of the current study was that we were not able to model HCRU as a function of MMDs as was done for HRQOL in the previous study by Wang et al. [25]. Evidence suggests that model parameters like medication use and productivity are non-linearly related to the number of MMDs [4, 8]. Capturing the relationship between these parameters and MMDs would require clinical trial data that includes data on effectiveness, HCRU and productivity. Also, there was no data available on long-term effectiveness of fremanezumab in EM and CM patients. Therefore, we assumed that the treatment effect at 3 months remained the same as long as patients stayed on treatment. This assumption was validated by clinical experts within the field of neurology but has to be confirmed when long-term data becomes available.
A strength of the current research is that, to our knowledge, we were the first to estimate the cost effectiveness of fremanezumab compared with SOC in EM and CM patients in Japan. Also, we were able to model the MMDs as a continuous variable using regression models. This made it easy to link model parameters to each number of MMDs and add granularity without having a computationally burdensome or unwieldy model with many health states and transition probabilities. This was proven by the fact that the sensitivity analysis only took a couple of minutes to run despite the fact that we were able to model a high level of detail regarding MMDs.
Our probabilistic results suggest that fremanezumab is cost effective compared with SOC in EM and CM patients if there is at least a WTP of ¥7,958,648 per QALY gained. Our deterministic results also showed that fremanezumab resulted in more QALYs in the EM patient population compared with CM and cost effectiveness is reached earlier in EM patients given the lower ICER. Also, productivity losses should not be negated in EM and CM patients as this is strongly connected to the disease [4, 8, 10–12]. Excluding it from the analyses has a substantial impact on the ICER, which makes it likely that the model results would be more removed from the reality whereby patients are impaired from work by migraine. Furthermore, our results suggest that modeling the effectiveness of fremanezumab on MMDs using different regression models enables the desired level of detail to be modeled while maintaining ease of use. While using different parametric distributions can have substantial impact on the ICER, it shows the importance of carefully informing the selection by statistical fit and expert opinions.
Future research should be focused on long-term effectiveness of fremanezumab in EM and CM patients in Japan to better inform CEMs. It would also be interesting to estimate model parameters such as HCRU or productivity losses as a function of MMDs to capture the relationship of these parameters.
Conclusions
Using fremanezumab in a heterogeneous mixture of Japanese EM and CM patients resulted in a reduction of MMDs and thus more QALYs compared with SOC. Additionally, use of fremanezumab was associated with reductions in migraine-related HCRU and associated costs, and an improved work productivity. The cost-effectiveness ratio of fremanezumab versus SOC in EM and CM patients was ¥6,530,398, from an adjusted Japanese public healthcare perspective. Indirect costs are strongly related to diseases like migraine and should be explored in cost-effectiveness analysis in Japan.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Prof. Ataru Ignarashi from the Yokohama City University School of Medicine for validating the cost-effectiveness model of the current research.
Declarations
Funding
This work was supported by Otsuka Pharmaceuticals Co., Ltd. The funding source had a supervising role in conducting the study and in preparing the manuscript. The open access fee was paid by Otsuka Pharmaceuticals Co., Ltd.
Conflict of Interest
Takao Takeshima reports consulting fees or honorarium by Otsuka, Amgen, Eli-Lilly, Daiichi Sankyo, Shionogi, Bioheaven, and Lundbeck and being an advisor of Hedgehog MedTech and Sawai. Fumihiko Sakai reports consulting fees or honorarium by Otsuka, Amgen, Eli-Lilly, and Daiichi Sankyo. Xinyu Wang was employed by Otsuka Pharmaceutical Co., Ltd. during the conduct of the study and reports no conflicts of interest. Kentaro Yamato, Yoshitsugu Kojima, and Yilong Zhang are employed by Otsuka Pharmaceutical Co., Ltd. and report no conflicts of interest. Otsuka Pharmaceutical Co., Ltd. launched fremanezumab in Japan. Craig Bennison and Martijn Simons are employed by OPEN Health and report consulting fees from Otsuka. OPEN Health is a consultancy firm that was contracted by Otsuka to conduct the study and write the manuscript.
Availability of Data and Materials
The model developed during the current study is not publicly available due to confidentiality. The input data supporting the model are from previously reported publications and reports, which have been cited. No new data were generated or analyzed during the current study.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Data analyses were performed in R statistical software and the model was created and analyzed in Excel.
Authors’ Contributions
Conceptualization and design: XW, KY, YK, YZ, CB, MS. Data acquisition: XW, YZ. Methodology: TT, FS, XW, KY, YK, YZ, CB, MS. Formal analysis and interpretation of the data: XW, KY, YK, YZ, CB, MS. Validation: TT, FS, XW, KY, YK, YZ, CB, MS. Writing original draft: XW, YZ, CB, MS. Writing review and editing: TT, FS, XW, KY, YK, YZ, CB, MS. Critical revision of the article for important intellectual content: TT, FS, XW, KY, YK, YZ, CB, MS.
References
- 1.Katsarava Z, Buse DC, Manack AN, et al. Defining the differences between episodic migraine and chronic migraine. Curr Pain Headache Rep. 2012;16(1):86–92. doi: 10.1007/s11916-011-0233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Japanese Society of Neurology and the Japanese Headache Society. Clinical practice guideline for chronic headache. https://www.neurology-jp.org/guidelinem/ch/index.html. Accessed 22 Apr 22 2020. 2013.
- 3.Stovner LJ, Hagen K, Linde M, et al. The global prevalence of headache: an update, with analysis of the influences of methodological factors on prevalence estimates. J Headache Pain. 2022;23(1):34. doi: 10.1186/s10194-022-01402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosignoli C, Ornello R, Onofri A, et al. Applying a biopsychosocial model to migraine: rationale and clinical implications. J Headache Pain. 2022;23(1):100. doi: 10.1186/s10194-022-01471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buse DC, Manack AN, Fanning KM, et al. Chronic migraine prevalence, disability, and sociodemographic factors: results from the American Migraine Prevalence and Prevention Study. Headache. 2012;52(10):1456–1470. doi: 10.1111/j.1526-4610.2012.02223.x. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Headache disorders. 2016. http://www.who.int/mediacentre/factsheets/fs277/en. 2017. Accessed 20 Nov 2023.
- 7.Vos T, Abajobir AA, Abbafati C, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390(10100):1211–59. [DOI] [PMC free article] [PubMed]
- 8.Porter JK, Di Tanna GL, Lipton RB, et al. Costs of acute headache medication use and productivity losses among patients with migraine: insights from three randomized controlled trials. Pharmacoecon Open. 2019;3(3):411–417. doi: 10.1007/s41669-018-0105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munakata J, Hazard E, Serrano D, et al. Economic burden of transformed migraine: results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2009;49(4):498–508. doi: 10.1111/j.1526-4610.2009.01369.x. [DOI] [PubMed] [Google Scholar]
- 10.Martelletti P, Leonardi M, Ashina M, et al. Rethinking headache as a global public health case model for reaching the SDG 3 HEALTH by 2030. J Headache Pain. 2023;24(1):140. doi: 10.1186/s10194-023-01666-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raffaelli B, Rubio-Beltrán E, Cho SJ, et al. Health equity, care access and quality in headache—part 2. J Headache Pain. 2023;24(1):167. doi: 10.1186/s10194-023-01699-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tana C, Raffaelli B, Souza MNP, et al. Health equity, care access and quality in headache—part 1. J Headache Pain. 2024;25(1):12. doi: 10.1186/s10194-024-01712-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueda K, Ye W, Lombard L, et al. Real-world treatment patterns and patient-reported outcomes in episodic and chronic migraine in Japan: analysis of data from the Adelphi migraine disease specific programme. J Headache Pain. 2019;20(1):68. doi: 10.1186/s10194-019-1012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirata K, Ueda K, Ye W, et al. Factors associated with insufficient response to acute treatment of migraine in Japan: analysis of real-world data from the Adelphi Migraine Disease Specific Programme. BMC Neurol. 2020;20(1):274. doi: 10.1186/s12883-020-01848-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gürsoy AE, Ertaş M. Prophylactic treatment of migraine. Noro Psikiyatr Ars. 2013;50(Suppl 1):S30–s35. doi: 10.4274/npa.y7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Negro A, Martelletti P. Patient selection for migraine preventive treatment with anti-CGRP(r) monoclonal antibodies. Expert Rev Neurother. 2019;19(8):769–776. doi: 10.1080/14737175.2019.1621749. [DOI] [PubMed] [Google Scholar]
- 17.Silvestro M, Orologio I, Siciliano M, et al. Emerging drugs for the preventive treatment of migraine: a review of CGRP monoclonal antibodies and gepants trials. Expert Opin Emerg Drugs. 2023;28(2):79–96. doi: 10.1080/14728214.2023.2207819. [DOI] [PubMed] [Google Scholar]
- 18.Silberstein SD. Preventive Migraine Treatment. Continuum (Minneap Minn) 2015;21(4 Headache):973–989. doi: 10.1212/CON.0000000000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakai F, Suzuki N, Kim BK, et al. Efficacy and safety of fremanezumab for episodic migraine prevention: Multicenter, randomized, double-blind, placebo-controlled, parallel-group trial in Japanese and Korean patients. Headache. 2021;61(7):1102–1111. doi: 10.1111/head.14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakai F, Suzuki N, Kim BK, et al. Efficacy and safety of fremanezumab for chronic migraine prevention: Multicenter, randomized, double-blind, placebo-controlled, parallel-group trial in Japanese and Korean patients. Headache. 2021;61(7):1092–1101. doi: 10.1111/head.14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National institute for Health and Care Excellence. Fremanezumab for preventing migraine. 2020.
- 22.ICER. Calcitonin Gene-Related Peptide (CGRP) Inhibitors as Preventive Treatments for Patients with Episodic or Chronic Migraine: Effectiveness and Value - Evidence Report. 2018.
- 23.Khanal S, Underwood M, Naghdi S, et al. A systematic review of economic evaluations of pharmacological treatments for adults with chronic migraine. J Headache Pain. 2022;23(1):122. doi: 10.1186/s10194-022-01492-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Core-2-Health. Full Scale Introduction of Cost-Effectiveness Evaluations in Japan - Overview (2/20/2019). Available at: https://c2h.niph.go.jp/tools/system/overview_en.pdf. 2019. Accessed 18 Dec 2023.
- 25.Wang X, Yamato K, Kojima Y, et al. Modeling monthly migraine-day distributions using longitudinal regression models and linking quality of life to inform cost-effectiveness analysis: a case study of fremanezumab in Japanese-Korean Migraine Patients. Pharmacoeconomics 2023;41(10):1263–74. [DOI] [PMC free article] [PubMed]
- 26.Sakai F, Suzuki N, Ning X, et al. Long-term safety and tolerability of fremanezumab for migraine preventive treatment in japanese outpatients: a multicenter, randomized. Open-Label Study. Drug Saf. 2021;44(12):1355–1364. doi: 10.1007/s40264-021-01119-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silberstein S, Tfelt-Hansen P, Dodick DW, et al. Guidelines for controlled trials of prophylactic treatment of chronic migraine in adults. Cephalalgia. 2008;28(5):484–495. doi: 10.1111/j.1468-2982.2008.01555.x. [DOI] [PubMed] [Google Scholar]
- 28.Tfelt-Hansen P, Pascual J, Ramadan N, et al. Guidelines for controlled trials of drugs in migraine: third edition. A guide for investigators. Cephalalgia. 2012;32(1):6–38. doi: 10.1177/0333102411417901. [DOI] [PubMed] [Google Scholar]
- 29.James SL, Abate D, Abate HK, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858. [DOI] [PMC free article] [PubMed]
- 30.Mhlw.go.jp. Abridged Life Tables for Japan 2019. [online] Available at: https://www.mhlw.go.jp/english/database/db-hw/lifetb19/index.html. 2021.
- 31.Core-2-Health. Guideline for Preparing Cost-Effectiveness Evaluation to the Central Social Insurance Medical Council. [online] Available at: https://c2h.niph.go.jp/tools/guideline/guideline_en.pdf. 2022; Version 3.0. Accessed 28 Mar 2024.
- 32.Kikui S, Chen Y, Todaka H, et al. Burden of migraine among Japanese patients: a cross-sectional National Health and Wellness Survey. J Headache Pain. 2020;21(1):110. doi: 10.1186/s10194-020-01180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.JMDC. Database. https://www.jmdc.co.jp/bigdata/. 2021. Accessed 28 Sep 2023.
- 34.Matsumori Y, Ueda K, Komori M, et al. Burden of migraine in Japan: results of the ObserVational Survey of the Epidemiology, tReatment, and Care Of MigrainE (OVERCOME [Japan]) Study. Neurol Ther. 2022;11(1):205–222. doi: 10.1007/s40120-021-00305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ministry of Health Labour and Welfare. Drug price fremanezumab https://www.mhlw.go.jp/content/12404000/001068211.pdf. 2022. Accessed 31 Oct 2023.
- 36.Ministry of Health Labour and Welfare. Overview of the 2020 Wage Structure Basic Statistical Survey. Available at: https://www.mhlw.go.jp/toukei/itiran/roudou/chingin/kouzou/z2022/dl/13.pdf. 2022. Accessed 28 Mar 2024.
- 37.Vemer P, Corro Ramos I, van Voorn GA, et al. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. Pharmacoeconomics. 2016;34(4):349–361. doi: 10.1007/s40273-015-0327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McAllister P, Lamerato L, Krasenbaum LJ, et al. Real-world impact of fremanezumab on migraine symptoms and resource utilization in the United States. J Headache Pain. 2021;22(1):156. doi: 10.1186/s10194-021-01358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The model developed during the current study is not publicly available due to confidentiality. The input data supporting the model are from previously reported publications and reports, which have been cited. No new data were generated or analyzed during the current study.