Abstract
Psychiatric disorders are highly prevalent, often devastating diseases that negatively impact the lives of millions of people worldwide. Although their etiological and diagnostic heterogeneity has long challenged drug discovery, an emerging circuit-based understanding of psychiatric illness is offering an important alternative to the current reliance on trial and error, both in the development and in the clinical application of treatments. Here we review new and emerging treatment approaches, with a particular emphasis on the revolutionary potential of brain-circuit-based interventions for precision psychiatry. Limitations of circuit models, challenges of bringing precision therapeutics to market and the crucial advances needed to overcome these obstacles are presented.
An estimated 970 million people worldwide are living with a mental health disorder1. Unlike many other chronic diseases, mental disorders often arise in childhood, adolescence or young adulthood and persist throughout life, impairing function and quality of life for many decades2. As a result, mental illness is cumulatively the leading cause of disability with global costs in the trillions3,4. Although many somatic and psychological treatments are available, access to quality care is woefully inadequate, and the effect sizes for most therapeutic approaches are small5. The general lack of an actionable understanding of disease pathophysiology and the paucity of reliable diagnostic or therapeutic biomarkers also limit clinical practice to a trial-and-error approach that can be a lengthy, frustrating process for patients and clinicians and still leaves up to 60% of individuals experiencing treatment resistance6.
Historically, the most common ‘biological’ conceptualization of psychiatric illness has emphasized molecular and cellular mechanisms and therapeutic targets; more recently, however, brain circuit organization and function have emerged as both an explanatory model and as a basis for designing interventions. The rapid development of technologies and advanced analytical tools (both in the laboratory and in the clinic) and collaborations across neuroscience disciplines, along with a decade of success in psychiatric genetics and genomics, are challenging traditional diagnostic boundaries and the notion of therapeutic specificity. What is emerging is a viable path forward in linking an individual’s symptoms and symptom clusters to mechanisms and treatments in a personalized manner. In this review, we outline how brain-stimulation techniques are advancing our understanding of psychiatric disease. We discuss new and emerging approaches to treatment based on a circuit understanding of psychiatric illness and consider the factors impeding delivery of precision therapeutics to the clinical market.
Circuit-level understanding of psychiatric disease
In the past few decades, the concept of psychiatric illness has transitioned from the notion of a ‘chemical imbalance’ to one that focuses on genetic risks, altered molecular and cellular development and function and disordered circuits. Traditionally, the term ‘neural circuit’ has referred to neuronal communication through synaptic connections and neurotransmission7. Here, we use the term to describe interconnections of vast numbers of neurons that make up the ‘connectome’ of the brain8. Techniques such as functional magnetic resonance imaging (fMRI) and electroencephalography (EEG) can quantify the functionally correlated regions of activation that define such circuits (Table 1). With the use of these tools, a set of core brain circuits have been identified that are consistently observed in the task-free state or when engaged by affective or cognitive tasks. These circuits include the default mode network (DMN), involved in task-free self-reflective processes; the negative affect circuit, engaged by negative emotional stimuli such as threat or sadness; the positive affect circuit (also known as the ‘reward’ circuit), engaged by responsiveness to social and learned rewards; and the cognitive control circuit, engaged by tasks that require inhibition and selective control (Fig. 1).
Table 1 |.
Tools to measure, modulate and model brain function
| Tools to measure brain function | |
| fMRI | Measures changes in blood flow that occur with brain activity. Identifies brain regions and circuits activated during emotions and behaviors. |
| Diffusion tensor imaging | Measures diffusion of water in tissues to identify white matter tract architecture and connectivity. |
| Magnetic resonance spectroscopy | Measures brain metabolism and can identify the concentrations of chemical components within brain regions. |
| EEG | Measures spontaneous electrical activity of the brain from electrodes placed across the scalp. Can identify and characterize brain circuits in terms of their spectral content with high temporal resolution. |
| Magnetoencephalography | Measures the magnetic field produced by electrical currents inside neurons. Can be combined with an anatomical image of the brain to evaluate circuit function during emotions and behaviors. |
| Intracranial EEG | Measures spontaneous electrical activity of the brain from electrodes surgically placed within the brain. Provides direct measurement of brain activity with high spatiotemporal resolution. |
| Tools to manipulate brain function | |
| Intracranial brain mapping | Procedure where direct electrical stimulation of brain tissue is used to map the function of the brain. Behavior: brief electrical stimulation of cortical and subcortical regions at varying stimulation parameters elicits specific motor, cognitive or emotional responses. Links brain site to a particular behavior. Evoked potential: single pulses of stimulation are delivered to one region of the brain, and the response (evoked potential) is recorded in a distal region. Characteristics of the evoked potential indicate the strength and direction of brain connections. |
| TMS | A non-invasive form of brain stimulation that uses an alternating magnetic field to induce electric current in the underlying cortical tissue. Single TMS pulses can be used to map cortical activity and plasticity when paired with EEG. |
| Low-intensity focused ultrasound | A non-invasive and reversible treatment under study for psychiatric disorders. Neuronal activity is modulated through low-frequency sound waves focally concentrated on a deep brain structure. |
| Optogenetics | A preclinical research tool that uses light to control activity of neurons. Through genetic engineering, specific populations of neurons are modified to express light-sensitive ion channels (opsins). These neurons can then be optically activated in different behavioral settings to understand their function. |
| Tools to model brain function | |
| Machine learning | A field of mathematics that derives latent structure from associations within data. Supervised: prediction models trained to associate one set of inputs (for example, features of brain connectivity) with a desired set of labeled outputs (for example, treatment response). Robustness of findings are tested by splitting data into subsets for training and testing to quantify the extent to which hypotheses may generalize to unseen samples in the real world. Unsupervised: identification of patterns in data without a label. May be used to uncover subtypes of a psychiatric disorder. |
| Graph theory | A field of mathematics that studies the relationships between nodes (for example, brain regions) and their connections. Can identify and quantify connectivity patterns in brain circuits. |
Fig. 1 |. Brain targets for selective pharmacotherapeutics.
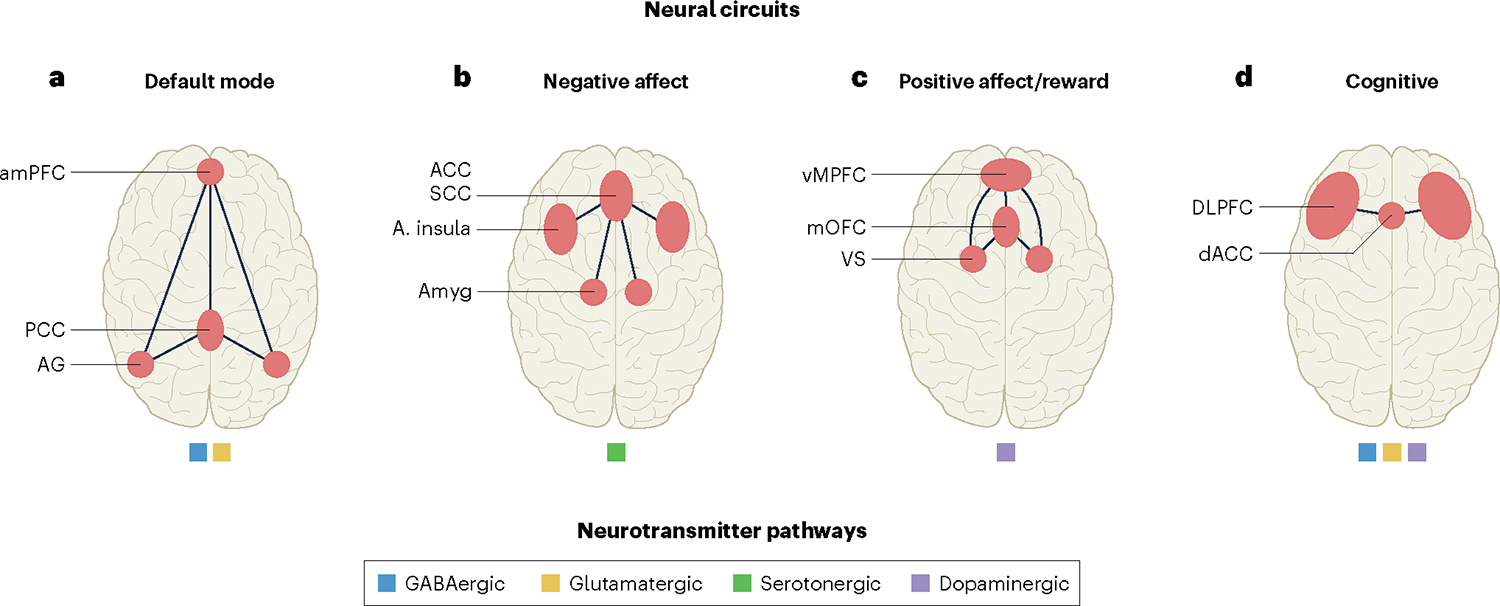
Major circuits and neurotransmitter pathways that are disrupted in a wide range of psychiatric disorders and that represent targets for treatment, based on extant knowledge. a, The DMN is a major intrinsic circuit involved in self-reflective processes and defined by functional connectivity between regions in the anterior medial prefrontal cortex (amPFC), the posterior cingulate cortex (PCC) and the angular gyrus (AG) within the parietal cortex. Default mode connectivity implicates excitatory glutamatergic (yellow) and inhibitory GABAergic (blue) neurotransmitter pathways. b, The negative affect circuit is engaged by negative emotional stimuli such as threat and sadness and is involved in reactions to these stimuli, the experience of the emotional states that they induce and regulation of these reactions and experiences. Key regions are the amygdala (Amyg), the anterior insula (A. insula) and the ACC, encompassing the subcallosal cingulate cortex (SCC), also referred to as the subgenual anterior cingulate cortex. It is modulated by serotonergic neurotransmitter pathways (green). c, The positive affect circuit, also known as the reward circuit, is engaged by responsiveness to socially rewarding stimuli, learned rewards, anticipation of these stimuli and the motivation to expend effort to obtain such rewards. Key regions are the VS, encompassing the nucleus accumbens, the medial orbitofrontal cortex (mOFC) and the ventral medial prefrontal cortex (vMPFC). These corticostriatal regions of positive affect circuitry interdigitate with mesolimbic dopamine pathways (purple). d, The cognitive control circuit is engaged by higher cognitive functions such as working memory and is required to inhibit task-irrelevant responses under task demands. Cognitive control circuitry is centered on regions of the DLPFC and the dorsal ACC (dACC) and implicates, among others, dopaminergic, GABAergic, glutamatergic and noradrenergic (not shown) neurotransmitter pathways.
Studies analyzing dysfunction within these core brain circuits as well as countless well-powered genomic studies (focused on either common alleles or rare large-effect mutations) do not support crisp diagnostic boundaries between psychiatric conditions, as codified by the Diagnostic and Statistical Manual (DSM). Partly in response to these types of findings, the US National Institute of Mental Health developed the Research Domain Criteria (RDoC)9, which emphasize multiple levels of analysis, including neural circuits, behaviors, self-report measures, physiology, cells, molecules and genes, with the hope of improving pathophysiological understanding and disentangling circuit dimensions underlying the heterogeneity of psychiatric disorders. An overarching goal of RDoC is to facilitate efforts to translate a circuit-level understanding of psychiatric diseases across basic science through clinical application and, ultimately, to inform neural targets for a more mechanistic and selective approach to developing treatments. Some argue that the way in which RDoC models psychiatric disease as a continuum of ‘normal’ and the fact that it does not include the natural history of any disorder (just a static view) moves away from a ‘disease’ model of psychiatric illness and may be a flaw of this paradigm10. Progress toward using RDoC-informed neural targets for more selective mechanistic treatment strategies has been slow.
As datasets increase in size, new analytical approaches such as machine learning and graph theory (Table 1) are enabling the discovery and quantification of new circuit patterns in psychiatric patient populations and are also beginning to uncover new disease phenotypes. Research consortia are using imaging and other modalities to develop brain-based markers that help select among treatments and predict response outcomes for depression and other disorders. RDoC and RDoC-‘themed’ constructs are being used to enrich trials for emerging treatments with patients more suited to the mechanism of action of the new compound and are also being used as endpoints for trial outcomes. To date, the most consistent or cross-validated predictive markers of treatment outcomes are specifically defined neural circuit targets, such as regions of interest implicated in the disorder and/or drug action.
New brain-stimulation and recording techniques represent a remarkable approach that may directly probe disease etiology at the circuit level. These techniques enable direct access to the living human brain, coupled with the ability to modulate circuit activity, and have already identified new principles of circuit–behavior relationships and demonstrated the feasibility of personalized circuit-targeted therapy. The approach could further inform biomarkers of disease severity and treatment prediction and could help uncover disease mechanisms to develop new treatments.
A brain circuit model can advance our understanding of types of disease etiology that may benefit from particular treatments, but it may not be universally applicable to all diseases or therapies at this time. In the sections that follow, we discuss promising new treatment approaches with a particular focus on those that fit the circuit-based framework; we also discuss emerging approaches for which this model has not yet been sufficiently investigated but which are of widespread interest in the field.
Brain stimulation as a research tool and therapy
Brain stimulation is an effective alternative to pharmacological agents for the treatment of psychiatric disorders; several common approaches are shown in Fig. 2. While all these approaches use electrical activity to modulate brain function (connectivity, activity, oscillatory activity), they vary in the strength and focality of the electric fields. Repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) are non-invasive approaches that target the cortex. Deep brain stimulation (DBS) enables the direct modulation of deeper (subcortical) structures with the highest spatial and temporal precision and holds tremendous potential for the treatment of psychiatric disorders because it enables precise modulation of activity at any node along a dysfunctional circuit.
Fig. 2 |. Brain-stimulation techniques and mechanism of action.
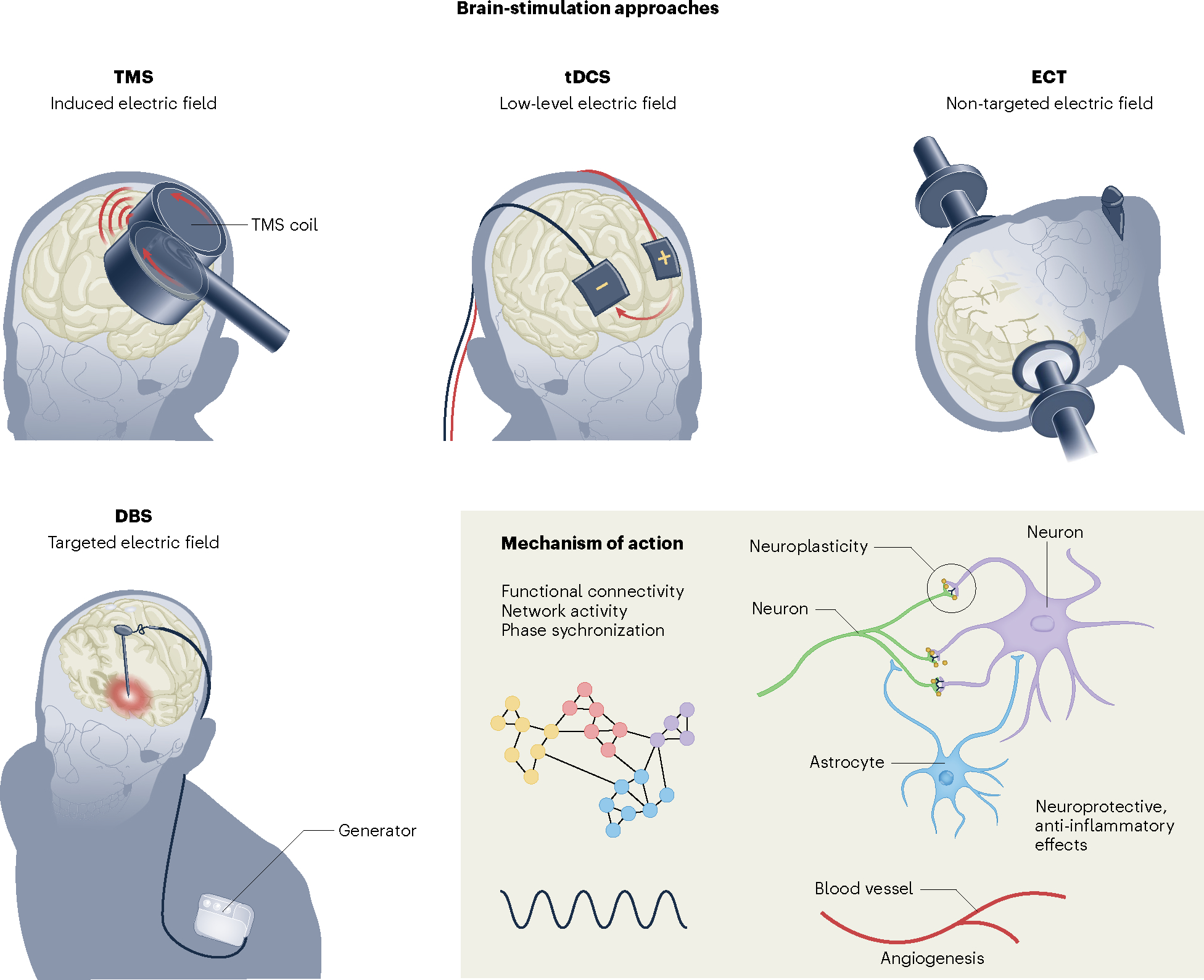
In rTMS, a rapidly alternating magnetic field is used to induce a secondary electric field 2–4 cm deep in the cortical tissue, directly activating cortical neurons195. The standard ‘figure eight’ coil is shown. In tDCS, a weak current (1–2 mA) is applied continuously to the scalp for 20–30 min, increasing (anodal tDCS) or decreasing (cathodal tDCS) excitability of the underlying cortical tissue196. In ECT, an electric current is passed through the brain to safely induce a seizure while a patient is under general anesthesia. A standard course may include 12 ECT sessions over the course of 1–2 months54. In DBS, electrodes are surgically implanted directly into the target brain structure, and stimulation is regulated by a pulse generator placed under the skin in the chest wall11. Stimulation intensity, pulse width, frequency and polarity can be programmed to optimize clinical response. Standard continuous high-frequency stimulation may inhibit neuronal activity locally while activating inhibitory presynaptic terminals and axonal activity. The mechanism of action for brain stimulation (inset) remains incompletely understood but is hypothesized to be similar across modalities. It may change network activity, connectivity and phase-amplitude coupling, disrupt pathologic oscillations, induce neuroplasticity, neurogenesis and angiogenesis and alter inflammatory mediators.
In DBS, an electrode is surgically implanted directly into a disease-specific target brain structure and stimulation is regulated by a pulse generator typically placed under the skin in the chest wall11. Stimulation is delivered continuously at an intensity and frequency selected in the clinic by testing several parameters and observing the effect on a patient’s symptoms. This procedure is commonly used to treat Parkinson’s disease and can dramatically restore motor function in this context. In 2009, the US Food and Drug Administration (FDA) granted humanitarian device exemption to DBS treatment of obsessive–compulsive disorder (OCD) after a series of studies showed substantial reduction of symptoms12. The anterior capsule was first targeted for DBS in patients with OCD based on its surgical corollary, the anterior capsulotomy (creating a lesion within this region was shown to alleviate symptoms). In 2003, Helen Mayberg showed that DBS targeting the subcallosal cingulate cortex (SCC), a region involved in emotional behavior, could treat major depression, even in patients who were resistant to other treatments13. Additional open-label trials targeting the SCC14 or ventral capsule–ventral striatum (VC/VS)15 (a region innervated with dopaminergic neurons known to be involved in reward processing and error prediction) showed similar effects and led to excitement at the prospect of treating major depressive disorder (MDD) by reversing dysfunctional brain activity. However, several disappointing randomized controlled trials followed. A trial of SCC DBS for MDD was halted prematurely after a 6-month futility analysis in 2012, and a second randomized trial of VC/VS DBS failed to show a significant improvement in symptoms compared to sham stimulation16,17. The reasons for the failures are likely in part due to the lack of precision targeting or personalized treatment.
Several compelling advances have since emerged and promise improvement over traditional DBS. One is the emerging concept of a connectomic target, where white matter tracts or their intersections (rather than isolated brain areas) are stimulated with electric current to target disease-related brain circuits. Imaging is used to identify the correct location based on the anatomy of each individual18. For MDD, it has been shown that stimulation at the intersection of white matter tracts traveling through the SCC best predict treatment response19, and prospectively targeting this region has yielded a 73% response rate at 6 months and an 82% response rate at 1 year20. Similarly, in OCD, multiple targets along the corticostriatal–thalamocortical loop that projects from the anterior capsule have shown benefit, and a retrospective analysis demonstrated that stimulation of a subsection of the anterior capsule that directly connects areas of the prefrontal cortex with the subthalamic nucleus predicted clinical improvement, regardless of lead location21.
Yet preclinical and clinical research have shown that stimulation in different brain regions elicits a wide range of emotions, suggesting that there is unlikely to be a single brain target sufficient to treat heterogeneous psychiatric disorders. Frustration with a lack of reproducibility of small-scale imaging investigations focused on anatomical regions of interest and disappointment with an attendant failure of clinical psychiatric research to provide definitive insights into mechanisms underlying psychiatric disorders have reinvigorated interest in using experimental manipulation of brain circuits. The use of electrical stimulation to understand brain structure–function relationships is not new; Wilder Penfield used this approach to map the somatosensory homunculus many decades ago22, but complex and varied behavioral effects and the invasive nature of this procedure limited its use for a time23. Nonetheless, it has now been shown by multiple groups that stimulating different parts of the brain elicits different affective experiences in animal models24–27 and human studies28–36.
One recent study performed electrical stimulation mapping of brain function (Table 1) before DBS implantation to uncover dysfunctional circuit activity underlying core depression symptoms at the individual level. In doing so, key principles of the relationship between depression symptoms, mood state and neural stimulation were uncovered and suggested the importance of linking the timing of stimulation to the presence of a negative symptom state34. Multi-site stimulation across the corticolimbic circuitry elicited a wide range of rapid-onset subjective experiences (that is, anxiety, pleasure, energy) and was found to be ‘dose’ responsive, sustained beyond the stimulation period itself and context dependent. The emotional state of the patient at the time of stimulation determined the clinical response in a reproducible manner, suggesting that there is an important interplay between a patient’s current symptom profile and the therapeutic effect of stimulation. How a region is stimulated also plays a role in its effect. Another ongoing study is using stimulation–response mapping to identify optimal stimulation parameters based on both their network and clinical effects37.
During electrical stimulation mapping, intracranial recordings provide feedback on network connectivity and response to stimulation. Intracranial EEG activity marks the presence of some neurological symptoms; for example, pathological beta frequency oscillations are correlated with motor symptoms in Parkinson’s disease38. While there is evidence that scalp EEG captures states of arousal or attention39, biomarkers of dynamic symptom states and targeted deep sources of these signals have remained elusive in psychiatry. In one study, a multi-day brain-mapping period enabled researchers to correlate intracranial EEG activity with symptom severity and identify an electrographic marker of a negative mood state in a single individual: namely, high-frequency activity in the amygdala, a brain region involved in emotion processing40. A structurally and functionally connected depression subnetwork composed of the amygdala and regions of the VC/VS was identified; stimulation in the VC/VS node consistently improved core depressive symptoms and reduced high-frequency activity in the amygdala.
These findings led researchers to rationally design personalized DBS treatment. Using a device capable of both sensing neural activity and delivering stimulation, neural activity was continuously recorded from the amygdala and focal stimulation was automatically delivered to the VC/VS only when the electrographic biomarker of a severe symptom state was detected40. This real-time, biomarker-driven therapy, referred to as closed-loop DBS, led to rapid remission in a pilot patient with only 30 min of stimulation per day. Further work in larger patient groups will be needed to determine interindividual biomarker variability and therapeutic effects of personalized closed-loop DBS. Nonetheless, the concept of treating a chronic disorder by temporally targeting symptom states defined by objective brain markers offers a new framework for circuit-targeted precision therapeutics in psychiatry. A new clinically available DBS system that enables sensing and recording of neural activity (Medtronic’s Percept) is an exciting advance that may reveal new biomarkers of symptom severity in larger populations41. Furthermore, the ability to explore cellular populations and projections underlying such biomarkers and circuits using optogenetics (Table 1) in animal models promises a realm of exciting experimental discoveries42,43.
Circuit targeting with the non-invasive brain-stimulation technique rTMS is also accelerating (Fig. 2). This technique does not have the spatial or temporal resolution of DBS but can provide targeted treatment non-invasively in a standard psychiatry clinic and is now approved by the FDA for treatment-resistant MDD and OCD and anxious depression44,45. In this treatment, a time-varying magnetic field is applied to the scalp through a transcranial magnetic stimulation (TMS) coil and induces an electric field in underlying tissue that can depolarize cortical neurons. The type of coil, its positioning on the brain and the frequency of magnetic pulses lead to different clinical and circuit-level effects46–48. A standard treatment course consists of 20–30 sessions over 4–6 weeks with a session duration of 30–40 min for standard 10-Hz rTMS. A new stimulation paradigm, intermittent theta burst stimulation, was found to have to have similar efficacy in about one-tenth of the treatment session time49. The dorsolateral prefrontal cortex (DLPFC), a region involved in higher cognitive functions, is targeted in MDD and has traditionally been identified through scalp measurements that can be inaccurate. Recent evidence suggests that treatment outcome can be enhanced by using imaging to target the circuit comprising the region in the DLPFC that has the highest negative connectivity with the anterior cingulate cortex (ACC), a region with a key role in cognition and emotion50. In one study, the entire TMS treatment course was compressed into 5 d by delivering ten high-dose intermittent theta burst stimulation sessions per day with individual functional connectivity MRI-guided targeting, and the authors reported an astonishing 86.4% remission rate51. Advances that may further improve circuit-targeted rational treatment design include new coils that can reach deeper brain structures47, EEG-synchronized therapy52 and multi-target TMS53.
An electrical intervention in which circuit targeting does not apply is electroconvulsive therapy (ECT), a treatment developed in the 1930s that involves applying electric current to the scalp to intentionally trigger a brief seizure in a safe and controlled manner (Fig. 2). Although its mechanism remains incompletely understood, it may induce neuroplastic changes, allowing the brain to form new connections54. Despite its lack of circuit specificity, it remains the most effective therapy for MDD with a remission rate greater than 70% (compared to 30% with typical antidepressants)55. Side effects and stigma have limited its use, however, and there is substantial interest in developing new, non-invasive treatments that can target deep brain structures without the requirement for anesthesia. One emerging treatment is low-intensity focused ultrasound (Table 1), which uses pulses of sound to reversibly alter neural activity and can target deep brain circuit structures with high precision56. A pilot study in depression demonstrated a reduction in worry and improved mood57, and further studies are underway.
Big data to map the human brain and identify diagnostic and susceptibility markers
A parallel and complimentary approach to identifying dysfunctional circuits at the individual level is to search for disease or symptom-related pathologic circuit patterns in large neuroimaging datasets at the population level. Taking many brain-based features, such as the connectivity of each voxel (a three-dimensional ‘pixel’ that represents a tiny cube of brain tissue) of an fMRI with every other voxel, and deriving latent structure from the associations between them has been approached by mathematics through the field of machine learning. The push to collaborate to generate large datasets and apply these analytic approaches was felt first in the field of psychiatric genomics and was met with considerable success (Box 1). The goal of these efforts was to identify markers of diagnostic susceptibility. The frequency of small-effect common alleles in the population and successful application of large-scale genome-wide association studies allow for the calculation of polygenic risk scores, a cumulative measure of individual genetic risk. While still aspirational in the clinic, this type of genetic profiling has the potential to drive genetically informed, clinically relevant research into differential diagnosis, natural history of disorders and treatment response58.
Box 1. Advances in understanding psychiatric genetics.
Rigorous large-scale genome-wide association studies, enome-wide studies of copy number variation and whole-genome and whole-exome sequencing studies emerged in the first decade of the new millennium and, together with new statistical approaches, have led to major advances in the understanding of psychiatric genetics. A key finding has been the varying contribution of common (greater than 1% of the population) versus rare mutations across categorical diagnoses. For example, progress in early-onset neurodevelopmental disorders (including autism spectrum disorders and intellectual disability) has mainly come from the identification of rare, often spontaneous (de novo) copy number variants197–200 and protein-damaging sequence mutations200–206 carrying large biological effects. By contrast, for many later-onset disorders (such as schizophrenia or bipolar disorder), progress has primarily involved the identification of common polymorphisms, mainly in non-protein-coding regions, contributing individually small effects, with hundreds to thousands of genetic variations conspiring to determine individual risk207–211. Overall, the move to collaborative large-scale studies has identified definitive risk genes and loci and clarified the genetic architecture of a wide range of categorical diagnoses. At the same time, this work has directly challenged the notion that genes ‘breed true’ by demonstrating tremendous overlap in common genetic risks across disparate diagnoses and widely differing outcomes, in contrast to what is seen with identical, large-effect rare mutations found in unrelated individuals.
A critical lesson learned from the replication failure of small candidate gene association studies is that very large neuroimaging datasets may be critical to identifying diagnostic or susceptibility markers. Reproducibility, or lack thereof, is a well-known concern in neuroimaging for group-averaged data due to low statistical power, software errors, the lack of appropriate correction for multiple comparisons and incomplete standardization of analysis methods59. Multiple large-scale meta-analyses of task activation and resting-state or structural imaging have found shared pathology across disorders but no circuit-specific effect of diagnosis or RDoC domain60–62. A recent analysis combining multiple datasets of structural imaging and resting functional connectivity interrogated correlations between these imaging measures and individual variation in subclinical ‘symptoms’ in healthy participants, revealing much smaller effect sizes than expected63. These results highlight the need for caution when seeking to identify individual differences in behavior and both resting-state and structural imaging64.
Multimodal quantitative meta-analyses that combine data from several MRI modalities may help distinguish true disease-specific circuit pathology from the numerous false positives in the literature. One such study was able to successfully identify MDD-specific findings from pooled, spatially normalized structural and functional data65. Further integration of data across additional MRI modalities and EEG using similar coordinate-based meta-analytic methods holds promise for increasing reproducibility66.
Emerging therapies and circuit-based biomarkers
The use of predictive or response biomarkers for emerging treatments may help to identify which individuals and mechanisms are targeted by each treatment and thereby improve statistical power and enhance effect sizes in clinical studies. According to National Institutes of Health (NIH)–FDA guidelines, a predictive biomarker identifies individuals likely to experience a benefit from treatment and a response biomarker indicates the biologic effect of a treatment without necessarily drawing conclusions about links between these biological mechanisms and clinical efficacy67. Consortia focused on predictive and response markers of treatment outcomes include PReDicT (Predicting Response to Depression Treatment), EMBARC (Establishing Moderators and Biosignatures of Antidepressant Response in Clinical Care), iSPOT-D (international Study to Predict Optimized Treatment in Depression) and FAST-MAS (Fast-Fail Trials in Mood and Anxiety Spectrum Disorders). One emerging focus is the use of fMRI treatment response targets as clinical trial endpoints (relevant to targeting more selective drug mechanisms), rather than classically defined DSM diagnoses. Another is illustrated in machine learning clustering approaches, with EEG datasets or combined imaging and treatment data for68 identification of new disease subgroups69–71. Across studies, cross-validated predictive models based on dysfunction within neural circuits have demonstrated the promise of increasing the current trial-and-error chance of responding to available treatments72–74. Below, we provide several illustrative examples that highlight the relationship of four core brain circuits with treatment outcomes, measured using fMRI, and the associated efforts to identify circuit-based predictive and response biomarkers (Fig. 1). Table 2 lists treatments associated with specific circuits that have resulted in positive phase 2 trials or are further along toward FDA approval.
Table 2 |.
Emerging therapies and their putative circuit effects
| FDA-approved indication | Fast-track or breakthrough therapy designation | Indications in phase 3 trials | Additional indications (completed positive phase 2 trials) | Circuit effects | |
|---|---|---|---|---|---|
| Selective dopamine 3 receptor modulation of reward circuit processes 178,179 | |||||
| D3R agonists | Restless leg syndrome, PD | MDD, TRD | Illness-related MDD, anhedonia in MDD and BD | Reward circuit including the VS, NAc, putamen, caudate, striatal interaction with the anterior insula180,181 | |
| Selective κ-opioid receptor modulation of reward circuit processes 182 | |||||
| JNJ-67953964; BTRX-335140 (NMRA-140) | In development | MDD with anhedonia | MDD, smoking cessation | Reward circuit, including the VS82 | |
| KCNQ2–KCNQ3-selective potassium channel opener modulation of reward circuit processes 83,84 | |||||
| Retigabine (ezogabine) | In development | MDD | Reward circuitry including the VS83 | ||
| Selective inhibitor of TRPC4 and TRPC5 ion channels implicating negative affect circuit processes 89 | |||||
| BI 135889 | In development | MDD, PTSD, borderline personality disorder | Negative affect circuit, specifically the AMY90 | ||
| Selective orexin 2 receptor antagonism modulating negative affect circuit processes 91,92 | |||||
| Seltorexant (JNJ-42847922, MIN-202) | In development | MDD with insomnia | MDD, insomnia | Negative affect circuit, specifically the central AMY via orexin-mediated activation of 5-HT pathways92 | |
| Negative-allosteric modulator of the α-7 nicotinic acetylcholine receptor modulating negative affect circuit processes 183 | |||||
| BNC210 | PTSD, fast-track designation (2019) | In development | PTSD, agitation in elderly | Negative affect circuit, including the AMY and AMY–ACC connectivity183 | |
| Glycine transporter 1 inhibitor modulating glutamatergic pathways through NMDA receptors and cognitive circuits and processes 184 | |||||
| BI 425809 | Cognitive impairment associated with SCZ, breakthrough therapy designation (2021) | SCZ, cognitive impairment in SCZ | Cognitive impairment in AD | Cognitive control circuitry | |
| NMDA glycine-site partial agonist modulating glutamatergic pathways through NMDA receptors 185 | |||||
| Rapastinel (zelquistinel) | In development | MDD | OCD | PFC, HPC186 | |
| Apimostinel | MDD | Unknown | |||
| Neuroactive steroid and GABAA receptor agonist modulating excitatory–inhibitory GABAergic pathways and default mode connectivity187 | |||||
| Brexanolone | PPD | DMN connectivity involving PCC102 and excitatory/inhibitory, GABAergic pathways | |||
| Ganaxolone | Seizures (CDKL5) | PPD | |||
| Zuranalone (SAGE-217) | Episodic MDD, breakthrough therapy designation (2021) | MDD, PPD | BD I/II | ||
| Selective vasopressin V1a receptor antagonist modulating prefrontal circuits involved in social cognitive processes and in regulating negative affective processes 188 | |||||
| SRX246 | Intermittent explosive disorder | Social cognitive and threat regulation circuit, including mPFC, ACC and SCC188 | |||
| NMDA receptor antagonist modulating DMN connectivity and negative affect circuitry 117,118 | |||||
| Ketamine, esketamine | MDD in conjunction with oral antidepressant, MDD with acute SI | SI, PTSD, late-life MDD, PPD, SI in BD | MDD with alcohol use, OCD, PTSD, agitation, alcohol-use disorder | During treatment, disrupts hyperconnectivity of the DMN, decreases connectivity between the HPC and other limbic structures; may increase connectivity within the frontostriatal network117,118. | |
| Dextromethorphan, AXS-05 (dextromethorphan–bupropion) | Breakthrough therapy designation for dextromethorphan–bupropion treatment of MDD (2019) | MDD, BD, SCZ, agitation in AD, irritability in HD, stimulant-use disorder | PTSD, smoking cessation | ||
| Nitrous oxide | In development | PPD | MDD, PTSD | ||
| Serotonergic hallucinogens modulating global internetwork connectivity and social reward and negative affect processes 130–132,135 | |||||
| Psilocybin | Breakthrough therapy designation for MDD (2019) | MDD | Illness-related anxiety/depression | Global increase in internetwork connectivity during treatment, increased connectivity within the DMN following treatment | |
| LSD | Illness-related anxiety | ||||
| Ayahuasca/DMT | MDD | ||||
| Entactogen with serotonergic activity modulating fear memory extinction and reconsolidation 136 | |||||
| MDMA | MDMA-assisted psychotherapy for PTSD, breakthrough therapy designation (2017) | MDMA-assisted psychotherapy for PTSD, PTSD | Illness-related anxiety, SAD in autistic adults | Facilitates fear memory extinction, modulates fear memory reconsolidation, altered activity in frontolimbic regions136 | |
| Brain stimulation with target-specific network effects mediated by direct or indirect activation of neural activity | |||||
| rTMS | MDD, OCD, chronic migraine pain, smoking cessation, anxious depression | Alcohol-use disorder, SCZ, GAD, TBI, BD, SI | Panic disorder and MDD, PPD, Tourette’s syndrome, PTSD, cocaine dependence | Decrease in SCC activity, change in SCC-DLPFC and DLPFC–VMPFC connectivity, decrease in SCC–DMN connectivity189 | |
| tDCS | In development | MDD, SCZ, BD, gambling disorder | Cocaine dependence, alcohol-use disorder | Increase in activation of VAN, increase in VAN–FPN connectivity during working memory task and DMN–FPN connectivity during language task190 | |
| DBS | OCD, humanitarian device exemption (2009) | MDD | Tourette’s syndrome | Stimulation-site-specific network modulation (OCD18) | |
| ECT | Labeled class II for catatonia, severe depressive episode of MDD or BD, 2018); class III (premarket approval) for SCZ, schizoaffective disorder, mania | SCZ | Hippocampal plasticity, modulation of corticolimbic circuitry via interaction with thalamocortical, cerebellar networks191 | ||
| Anti-inflammatory therapies targeting cognitive, reward and DMNs 192,193 | |||||
| Rapamycin | MDD (with ketamine) | May modulate corticostriatal circuits and reward pathway | |||
| Celecoxib | In development | BD, MDD | SCZ | Alters HPC circuitry involved in spatial learning | |
| Minocycline | In development | BD, SCZ, MDD | Alters HPC circuitry involved in spatial learning | ||
| Omega 3 fatty acids | In development | MDD, ADHD, SCZ, PTSD, alcohol dependence | Lower DMN and FPN hyperconnectivity | ||
| Anti-cytokine treatment (infliximab, losmapimod) | MDD, BD | Decrease in limbic activation (insula, cingulate cortex) | |||
| Pioglitazome | MDD, cocaine dependence, autism | Alters HPC circuitry involved in memory | |||
| Digital therapy 194 | |||||
| Digital pill | Substance-use disorder | MDD, SCZ, BD | Unknown | ||
| Digital biomarker-based detection and intervention system | Substance-use disorder | MDD | Unknown | ||
| Psychoeducation and intervention apps | SI | MDD | Unknown | ||
| Gaming/virtual reality | ADHD (marketing authorization) | MDD, autism | Unknown | ||
TRD, treatment-resistant depression; BD, bipolar disorder; SCZ, schizophrenia; PD, Parkinson’s disease; AD, Alzheimer’s disease; SI, suicidal ideation; HD, Huntington’s disease; SAD, social anxiety disorder; GAD, generalized anxiety disorder; TBI, traumatic brain injury; ADHD, attention-deficit hyperactivity disorder; D3R, dopamine receptor 3; CDKL5, cyclin-dependent kinase-like 5; V1a, vasopressin receptor 1A; AMY, amygdala; HPC, hippocampus; PFC, prefrontal cortex; mPFC, medial prefrontal cortex; VMPFC, ventromedial prefrontal cortex; NAc, nucleus accumbens; VAN, ventral attention network; FPN, frontoparietal network.
Anhedonia and the reward circuit
Anhedonia, a reduced ability to experience pleasure, is a symptom observed in multiple mental disorders75,76 and implicates dysfunction within the corticostriatal circuits, interplay with mesolimbic dopamine systems and dysfunction in constructs described within the RDoC positive valence system9,75. In patients treated with currently approved antidepressants, higher resting-state connectivity between reward circuit regions of interest (measured by fMRI) and greater behavioral sensitivity (measured by computational modeling of performance on a reward task) has been found to identify individuals who respond to the norepinephrine- and dopamine-reuptake inhibitor bupropion, following non-response to the selective serotonin receptor inhibitor (SSRI) sertraline77. Machine learning using task-evoked reward circuit activation has been found to predict response specifically to bupropion versus placebo78. These findings indicate the potential promise of reward circuit measures as predictive biomarkers. Changes in activation of the striatum, a key region in the brain’s positive affect–reward circuit, also show potential as a response biomarker of new selective treatments. Treatment with pramipexole, a selective dopamine 3 receptor antagonist, has been associated with changes in both striatal activation and accompanying improvements in anhedonia79,80. Changes in both striatal activation and anhedonia have also been observed within a study design that links the striatal response biomarker to the putative mechanism of action using a κ-opioid receptor antagonist (that stimulates striatal dopamine release)81,82 and a potassium channel opener (KCNQ2–KCNQ3 subtype)83,84.
Negative affect and the corticolimbic circuit
Alterations in amygdala reactivity and negative affect circuit connectivity cut across multiple mental disorders and involve an interplay with serotonergic systems85. During the processing of negative affect stimuli, hyporeactivity of the amygdala, quantified using a standardized task-based fMRI method86, has been found to predict differential response to SSRIs versus serotonin–norepinephrine receptor inhibitors in patients with MDD72,87. Amygdala reactivity also shows early changes following treatment with the SSRI escitalopram, showing its promise as a response biomarker88. Several emerging treatments target changes in negative affect circuitry as a response biomarker. BI 1358894, an inhibitor of the transient receptor potential canonical (TRPC)4 and TRPC5 ion channels89, has completed phase 1 evaluation using fMRI response biomarkers of amygdala activation and emotion processing and is in phase 2 trials for MDD90, post-traumatic stress disorder (PTSD) and borderline personality disorder. An inability to downregulate negative affect circuitry during sleep may contribute to a subtype of MDD with sleep–wake problems, excessive arousal and stress-related physical symptoms. The orexin 2 antagonist seltorexant is being developed for sleep–wake and arousal features of depression91, and orexin may help downregulate central amygdala reactivity that underlies these features via mediation of serotonergic pathways92.
Executive function and cognitive control circuitry
Disruption of the cognitive control circuit and executive processes has been reported across schizophrenia, MDD, PTSD, anxiety and attention disorders and may be another neural hallmark and treatment target93,94. Noradrenergic and dopaminergic neurotransmitter systems are implicated in the modulatory interplay with cognitive control circuitry, among others. Emerging treatments designed to enhance cognitive processes and underlying cognitive circuit function, assessed using behavioral performance on cognitive tests and fMRI include iclepertin (also known as BI 425809). Iclepertin is a glycine transporter 1 (GlyT1) inhibitor that has FDA breakthrough therapy designation for cognitive impairment in schizophrenia95.
Depression and the DMN
Another prominent circuit is the DMN. There is a mixed pattern of findings for DMN and medication outcomes; consistency has been challenged by different study-design strategies and analytic methods96. DMN hypoconnectivity has been found to characterize persistent depressive disorder and to normalize with duloxetine, a commonly prescribed serotonin–norepinephrine receptor inhibitor, compared to placebo97. Frontal nodes of the DMN have been found to normalize with standard antidepressants in late-life depression comorbid with anxiety98, and the posterior-node DMN has been found to correlate with symptom improvement following treatment with the SSRI escitalopram in geriatric depression99, indicating the potential of DMN node features as response biomarkers. Pretreatment task-free DMN hypoconnectivity has been found to predict subsequent non-remission across commonly prescribed antidepressants, while intact DMN connectivity predicts remission to antidepressant treatment73. DMN connectivity has also been studied as a putative circuit biomarker for new and emerging therapeutics. Baseline SCC connectivity within the DMN has consistently been implicated in response to TMS96,100, with responders tending to show lower pre-TMS connectivity and a greater inverse relationship of the SCC to frontal regions of cognitive control circuits. Zuranolone (SAGE-217)101,102, currently in phase 3 development for MDD and post-partum depression (PPD), was found to modulate resting DMN connectivity, and the putative mechanism of action is alteration of the γ-aminobutyric acid (GABA)ergic excitatory–inhibitory balance96,100.
Emerging therapies that lack predictive, response and mechanistic biomarkers
Despite the appeal of applying a circuit-based model to all psychiatric disorders and treatments, we are challenged by our lack of mechanistic understanding of these. While it may turn out that all brain disorders can be treated at the level of the neural circuit, neural circuit dysfunction is unlikely to be the etiology of every disease phenotype and therefore may not be the only or the optimal treatment target. No review of new and emerging approaches to treatment in psychiatry would be complete without the mention of rapidly acting therapeutics, hallucinogens or immunotherapeutics. We know that these treatments affect the brain, but they are not circuit specific and no clear circuit markers of disease have yet predicted treatment response, although such studies are underway. In the following sections, we discuss these treatments and the surrounding literature involving circuit effects while acknowledging where the circuit model falls short.
Rapidly acting therapies
Rapidly acting therapies such as ketamine represent the first new class of pharmacotherapies in decades (Table 2). Similar to some forms of brain stimulation, clinical effects of these pharmacotherapies are observed within hours. This incredible near-immediate reversal of severe pathology suggests that circuit reorganization or other mechanisms of action may occur on multiple timescales and both rapid-onset and sustained changes could be targeted by new treatments. Ketamine is a synthetic dissociative anesthetic and N-methyl-d-aspartate (NMDA) receptor antagonist103. A placebo-controlled study published in 2006 demonstrated that a single 40-min intravenous ketamine infusion significantly improved treatment-resistant MDD compared to placebo, beginning just 2 h after infusion104. Subsequent studies have confirmed this finding for MDD105 and explored efficacy for suicidal ideation106, PTSD107,108, OCD109 and PPD110. The intranasal formulation of the S-enantiomer, esketamine (Spravato), was approved by the FDA for treatment-resistant MDD (in conjunction with an oral antidepressant) and for MDD with acute suicidal behavior in 2019 and 2020, respectively111–114. Neuroplastic processes, mediated through a surge in glutamate, are critical for ketamine’s antidepressant effects, although recent evidence has implicated agonism of opioid receptors as well115,116. Although the clinical effects of ketamine are promising for treatment-resistant disorders, we currently lack knowledge about circuit-based predictive and response biomarkers and the mechanisms that mediate clinical response. Neuroimaging studies of ketamine have revealed mixed results. In MDD, lower pre-infusion resting-state connectivity involving the SCC and frontal regions of the DMN, followed by relative normalization of connectivity after ketamine infusion, has been found to characterize responders (for reviews, see refs. 117,118). However, pretreatment differences have not been replicated across region-of-interest and whole-brain data-driven analyses117. Connectivity between the posterior region of the DMN and the insula, which is a core region in negative affect and other circuits, has also been positively associated with post-ketamine clinical response117. By contrast, in task (as opposed to resting) fMRI, higher baseline SCC activity elicited by positive incentive trials is correlated with anhedonia improvement119. In studies of task fMRI with negative emotion tasks, ketamine has been found to reduce pre-infusion amygdala overactivity within the negative affect circuit, correlating with a change in anhedonia and overall depression improvement117,118. Other dissociative anesthetics including dextromethorphan120 and nitrous oxide121 have also been shown to rapidly and significantly reduce MDD severity in randomized trials and are thought to have similar circuit effects. Together, these findings hold promise for identifying circuit-based response biomarkers that also shed light on the mechanisms by which ketamine exerts its clinical effects. More research is needed to disentangle systematic effects across studies, especially given that work to date has varied widely in terms of the timing of imaging relative to ketamine administration, imaging conditions, analysis methodology and population characteristics. It is also unknown whether mixed results reflect different subtypes of disorder or interactive effects of differing oral antidepressants in patients.
Positive allosteric modulation of GABA type A (GABAA) (an effect that enhances GABA inhibition) represents a new mechanism of action that also has rapid-onset effects122. In 2019, brexanolone received FDA approval for PPD, the first drug approved specifically for this indication123. While it must be administered by intravenous infusion over a 60-h period, newer formulations are under study, including the orally administered compound zelquistenel124.
Psychedelics
In the last 10 years, interest in psychedelic medicine has surged. On the one hand, the rise of psychedelics clashes with the goal of circuit-targeted precision and rational treatment design. These plant-based compounds, in use for thousands of years, affect the brain globally. Yet, the renewed excitement regarding their use may in part be due to rise of circuit-based understanding of psychiatric disease and the role of neuroplasticity in reshaping circuitry. Psilocybin, a classic hallucinogen derived from the Psilocybe mushroom, received FDA breakthrough designation for the treatment of MDD in 2019 (ref. 125). In therapeutic trials, two doses spaced 3 weeks apart were shown to be safe and to have comparable efficacy to escitalopram treatment for 6 weeks126, with antidepressant activity observed within 24 h of administration. Active metabolites of classic hallucinogens act primarily as full or partial agonists of serotonin (5-HT2A) receptors127. Downstream activation of glutamate receptors, NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid promote rapid neurotrophic and neuroplastic effects128,129. During the hyperplastic period, increased global connectivity with specific connectivity of sensory cortices has been reported130–132, followed by context-dependent reorganization after exposure132. Evidence suggests that therapeutic efficacy requires psychotherapy before and after exposure and is correlated with the positive quality of the psychedelic experience133,134. Similar network restructuring after exposure has also been observed for lysergic acid diethylamide (LSD)130, N,N-dimethyltryptamine (DMT, N,N-DMT)135, ketamine118 and 3,4-methylenedioxy-methamphetamine (MDMA)136. Clinical trials for psychedelics are challenged by the lack of an adequate placebo, cost of on-site monitoring by medical professionals, schedule 1 substance designation and the reality-altering and potentially addictive properties137,138. Identification of non-hallucinogenic plasticity-promoting antidepressants with greater circuit specificity are under investigation and may be critical for a scalable path to the clinical market139.
Immunotherapeutics
Immunomodulatory therapies for psychiatric diseases are receiving increasing attention based on an emerging literature elucidating the impact of systemic inflammation on the brain and behavior140,141 (Box 2 and Fig. 3). Inflammatory biomarkers are observed in a host of psychiatric diseases including mood and anxiety disorders, PTSD, psychotic disorders and OCD140,142–145. They have been associated with non-response to conventional antidepressants and antipsychotics and can predict response to ECT and ketamine146–149. Aided by biomarkers of inflammation that can identify relevant subgroups of psychiatric patients, the opportunity now exists to target inflammatory pathways related to pathology of psychiatric disorders.
Box 2. The role of inflammation in psychiatric disorders.
Early studies in laboratory animals indicated that systemic inflammation induces a prototypical behavioral response referred to as ‘sickness behavior’, characterized by symptoms of psychiatric diseases including apathy, anxiety, anorexia, hypersomnia and cognitive impairment212.
Increased inflammation in psychiatric disorders is believed to arise from factors such as obesity, diabetes, cardiovascular disease, cancer and infectious diseases; childhood trauma; and disruption of homeostasis of the gut microbiota140.
Chronic stress and inflammation may disrupt integrity of the blood–brain barrier, allowing direct access of peripheral inflammatory cytokines to neuronal systems in specific brain regions including the VS213. These findings complement data demonstrating immune cell trafficking to the brain, where local release of cytokines can lead to these entering the brain parenchyma and cerebrospinal fluid, as well as the glymphatic pathways that support immune regulation of brain function214–216.
The effect of inflammation on neural circuitry may arise from changes in neurotransmitter levels or their metabolism. Decreased dopamine availability in the striatum and increased glutamate in the basal ganglia may in part be related to the induction of the kynurenine pathway and the production of quinolinic acid, which increases release and decreases reuptake of glutamate from astrocytes while binding to excitotoxic extrasynaptic glutamate receptors217.
A shift from energy-efficient oxidative phosphorylation to the energy-expedient glycolysis in activated immune cells, referred to as the Warburg effect, may activate several signaling pathways associated with reward circuit connectivity and symptoms of anhedonia and psychomotor slowing192,193 including mammalian target of rapamycin (mTOR).
Fig. 3 |. Inflammation as a therapeutic target in psychiatric disease.
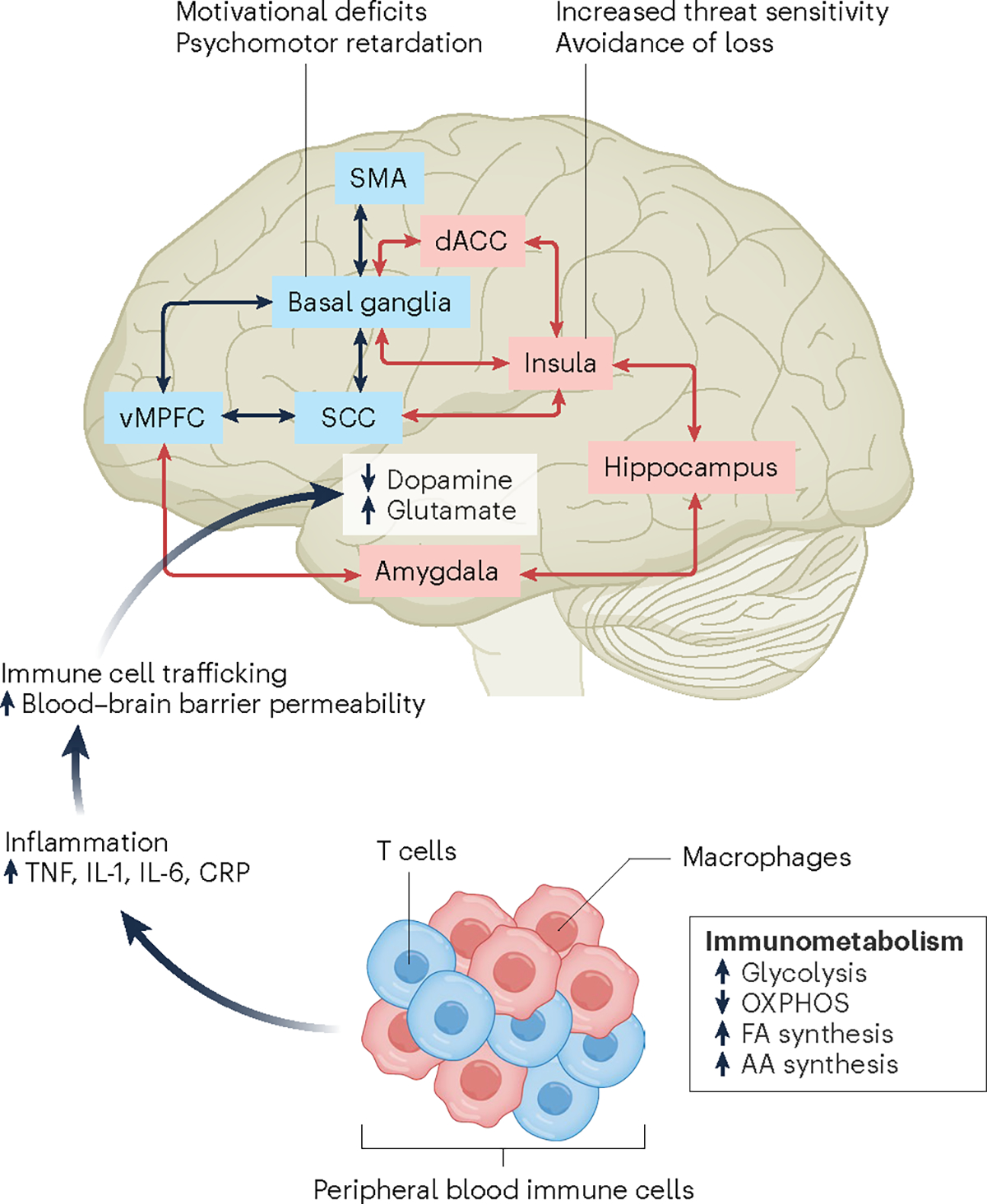
There are many points along the pathway from the immune system to the brain that can serve as therapeutic targets. External and internal environmental factors can activate peripheral blood immune cells, which then undergo metabolic reprogramming that involves a shift from energy-efficient oxidative phosphorylation (OXPHOS) to energy-expedient glycolysis, allowing rapid growth and proliferation, supported by increased fatty acid (FA) and amino acid (AA) synthesis. Activated immune cells in turn produce inflammatory cytokines including tumor necrosis factor (TNF) that can increase permeability of the blood–brain barrier by inhibiting production of claudin 5, a key protein in blood–brain barrier integrity, thus allowing direct access of inflammatory cytokines to the brain, produced in part by immune cells trafficking to perivascular spaces in the brain and the meninges. Once in the brain, inflammatory cytokines can decrease availability and release of dopamine in basal ganglia (for example, the striatum), while increasing the excitatory amino acid glutamate, whose activity may be further enhanced by activation of the kynurenine pathway. Altered neurotransmitter metabolism affects multiple brain regions, leading to disruption of neurocircuits that regulate motivation and motor activity (black arrows) as well as sensitivity to threat and loss (red arrows). These circuit-based behavioral biases in turn contribute to symptoms of anhedonia, psychomotor slowing and anxiety, arousal and alarm. IL, interleukin; CRP, C-reactive protein; SMA, supplementary motor area.
Based on studies administering inflammatory stimuli to humans, inflammation reliably engages neurocircuits that regulate motivational processing, psychomotor speed and threat sensitivity150–156. Changes in these neurocircuits map onto behaviors of anhedonia, psychomotor retardation and anxiety and therefore represent attractive targets for circuit-based therapeutics across psychiatric disorders140. For example, exogenous administration of the inflammatory cytokine interferon α to patients with infectious diseases and cancer as well as administration of typhoid vaccination or endotoxin to healthy volunteers have shown inflammation-induced decreases in activity within reward-related brain regions including the ventral striatum, as well as increases in activity within threat-related regions, including the dorsal ACC, the insula and the amygdala150–153,157. In addition, increased endogenous inflammation has been shown to affect functional connectivity in corticostriatal and corticolimbic circuits related to motivational processing and psychomotor speed, as well threat sensitivity and avoidance of loss150,154,156,158.
Studies in humans and laboratory animals have also revealed neurotransmitter changes related to the effect of inflammation on neurocircuitry. Indeed, inflammation-induced decreases in dopamine availability and release in the striatum have been associated with decreased effort-based motivation153,159. Moreover, increases in glutamate in the basal ganglia impact local coherence of neuronal activity and ultimately decrease functional connectivity in multiple neural networks, including those involved in reward processing160.
Studies have examined the efficacy of compounds with anti-inflammatory properties including inhibitors of cyclo-oxygenase 2 (for example, celecoxib), minocycline, omega 3 fatty acids and cytokine-targeted therapies as well as drugs that target immunometabolism (such as pioglitazone in patients with mood disorders and schizophrenia)161,162 (Table 2). Although meta-analyses of this literature have suggested modest efficacy, results have been underwhelming. Moreover, two recent studies failed to find separation of either celecoxib or minocycline from placebo in the treatment of depression163,164. Nevertheless, the results to date should be interpreted with caution because virtually all studies failed to stratify patients on the basis of inflammation, there was no assessment of target engagement in the immune system or brain, and primary outcome variables were not aligned with the known impact of inflammation on specific brain circuits and behaviors140. Inclusion of these design elements in future clinical trials will help realize the potential of immunomodulatory strategies to treat inflammation-induced transdiagnostic symptoms in psychiatric diseases.
Precision psychiatry: challenges and future outlook
Drug discovery for central nervous system disorders in general is plagued by higher failure rates, longer development times and higher costs than other areas of medicine, which has led many pharmaceutical companies to abandon the effort entirely165. While precision psychiatry may be a solution, there are challenges with implementing the approach. The emerging genetics and neuroimaging findings that do not support crisp DSM diagnostic boundaries are misaligned with the current diagnostic indication system used by payors and regulatory agencies. The FDA and the European Medicines Agency are actively developing a framework for the use of symptom domains and circuits as drug targets and clinical trial endpoints, placing emphasis on phase 1–2 proof-of-concept biomarker studies and studies that demonstrate subtype specificity. Further validation or qualification of circuit targets is needed for their acceptance by these regulatory agencies. Focusing on well-defined brain regions that tie to behaviors of interest offers one means to develop tractable patient-level measures suited to surrogate endpoints. Continuing to work together as a scientific community to build and extend collaborative consortia and define methodological standards to improve replicability will be critical. Integration of multimodal data including genetics, EEG, fMRI, tasks of cognitive function, clinical data and patient history using computational approaches may also help capture the full heterogeneity of symptom clusters and identify subgroups that respond preferentially to one treatment option or another.
Another challenge is the evolutionary distance between commonly used animal model systems and human brains. Preclinical models of psychiatric disorders lack predictive validity166. Recent advances are expanding the armamentarium to study circuit-level biology in the human brain, including the ability to implant human brain-like collections of cells, called organoids, into rodent circuits to control behavior167 and the ability to perform ex vivo studies of circuit function in intact post-mortem human brains168. The experimental manipulation of circuit function in humans with focal electrical stimulation or other emerging forms of neuromodulation and direct assessment of changes in symptoms could provide tremendous potential for precision psychiatry, especially if the established circuit–symptom findings can then be backtranslated into animal models to identify causal molecular and cellular effects.
Another recent advance that may improve the success rate of candidate molecules is the ability to focus drug discovery on behavioral outcomes of circuit dysfunction rather than specific mechanisms of action. A time series analysis approach called motion sequencing derives a ‘language’ of natural segments or ‘syllables’ of drug-induced behavior in the rodent, using thousands of hours of recorded video from hundreds of animals. These can be used to discern linkages of drug-induced behavioral ‘units’ to specific neural circuits underlying clinical symptoms, such as hyperactivity and anxiety, and are capable of identifying drug class and dose169.
Currently accepted outcome measures in clinical trials of psychiatric disorders are self-reported measures or clinician rating scales that are subjective accounts of a patient’s disease state, require a look-back period of a week or more and are known to lack coherence with behavioral measures170. They may obscure relevant symptom patterns that occur on a finer temporal scale or behavioral dynamics critical for identification of diagnostic or treatment response biomarkers170. Validated, standardized measures that examine symptom severity on shorter timescales would also benefit the development of new brain-stimulation and rapid-acting therapeutics. With advances in sensor technology and artificial intelligence, digitally collected behavioral measures may provide one solution for this challenge and are already showing promise for predicting DBS treatment parameters at the individual level171–174 and symptom severity at the population level175. A next step will be to determine to what extent the digital signals might act as surrogates of underlying brain circuits, particularly given the host of interpretability issues around distinguishing clinically significant variance from variance due to measurement or contextual factors176. Indeed, the NIH’s recent Brain Behavior Quantification and Synchronization effort seeks to bring together researchers across scientific disciplines to quantify high-density behavioral data with simultaneously recorded brain activity and contextual data from the environment (https://event.roseliassociates.com/bbqs-workshop/). Managing ethical and privacy questions surrounding deep dynamic phenotyping of healthcare data will be critical177.
Given the remarkable complexity of the human brain and the nature of psychiatric symptoms involving the most distinctively human aspects of function, it is not surprising that precision psychiatry has been slow to emerge. Yet recent progress in technologies, treatments and biomarkers is bringing us closer to this goal at a time when increasing the therapeutic armamentarium in psychiatry could not be of greater need. This review is not comprehensive; we have focused on new and upcoming therapeutic approaches that emerge from a deeper understanding of neural circuits, but foundational advances in other fields of psychiatry such as data-driven mining of medical records, computational psychiatry models and therapeutic approaches combining mechanistic with digital interventions are also moving the field forward. Together, these advances promise an auspicious future for psychiatry and propel the field forward toward a better quality of life for patients and their families.
Footnotes
Competing interests
K.W.S. receives salary and equity options from Neumora Therapeutics. L.M.W. has received advisory board fees from One Mind PsyberGuide and the Laureate Institute for Brain Research and declares US patent applications 10/034,645 and 15/820,338: systems and methods for detecting complex networks in MRI image data. J.T.B. has received consulting fees and equity options from Mindstrong Health as well as consulting fees from Verily Life Sciences. A.H.M. has received consulting fees from Cerevel Therapeutics and Sirtsei Pharmaceuticals.
Peer review information Nature Medicine thanks Edward Bullmore, James Murrough and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Karen O’Leary, in collaboration with the Nature Medicine team.
Reprints and permissions information is available at www.nature.com/reprints.
References
- 1.GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 9, 137–150 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erskine HE et al. A heavy burden on young minds: the global burden of mental and substance use disorders in children and youth. Psychol. Med. 45, 1551–1563 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel V et al. The Lancet Commission on global mental health and sustainable development. Lancet 392, 1553–1598 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Whiteford HA et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382, 1575–1586 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Leichsenring F, Steinert C, Rabung S & Ioannidis JPA The efficacy of psychotherapies and pharmacotherapies for mental disorders in adults: an umbrella review and meta-analytic evaluation of recent meta-analyses. World Psychiatry 21, 133–145 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howes OD, Thase ME & Pillinger T Treatment resistance in psychiatry: state of the art and new directions. Mol. Psychiatry 27, 58–72 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuste R From the neuron doctrine to neural networks. Nat. Rev. Neurosci. 16, 487–497 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Bullmore E & Sporns O Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Insel T et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry 167, 748–751 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Ross CA & Margolis RL Research domain criteria: strengths, weaknesses, and potential alternatives for future psychiatric research. Mol. Neuropsychiatry 5, 218–236 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lozano AM et al. Deep brain stimulation: current challenges and future directions. Nat. Rev. Neurol. 15, 148–160 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alonso P et al. Deep brain stimulation for obsessive–compulsive disorder: a meta-analysis of treatment outcome and predictors of response. PLoS ONE 10, e0133591 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayberg HS et al. Deep brain stimulation for treatment-resistant depression. Neuron 45, 651–660 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Holtzheimer PE et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch. Gen. Psychiatry 69, 150–158 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malone DA et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol. Psychiatry 65, 267–275 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dougherty DD et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol. Psychiatry 78, 240–248 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Holtzheimer PE et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry 4, 839–849 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Baldermann JC et al. Connectomic deep brain stimulation for obsessive–compulsive disorder. Biol. Psychiatry 90, 678–688 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Riva-Posse P et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol. Psychiatry 76, 963–969 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riva-Posse P et al. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol. Psychiatry 23, 843–849 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li N et al. A unified connectomic target for deep brain stimulation in obsessive–compulsive disorder. Nat. Commun. 11, 3364 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penfield W & Boldrey E Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60, 389–443 (1937). [Google Scholar]
- 23.Borchers S, Himmelbach M, Logothetis N & Karnath H-O Direct electrical stimulation of human cortex—the gold standard for mapping brain functions? Nat. Rev. Neurosci. 13, 63–70 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Lim LW et al. Electrical stimulation alleviates depressive-like behaviors of rats: investigation of brain targets and potential mechanisms. Transl. Psychiatry 5, e535 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim LW, Janssen MLF, Kocabicak E & Temel Y The antidepressant effects of ventromedial prefrontal cortex stimulation is associated with neural activation in the medial part of the subthalamic nucleus. Behav. Brain Res. 279, 17–21 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Srejic LR, Hamani C & Hutchison WD High-frequency stimulation of the medial prefrontal cortex decreases cellular firing in the dorsal raphe. Eur. J. Neurosci. 41, 1219–1226 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luyck K et al. Electrical stimulation of the bed nucleus of the stria terminalis reduces anxiety in a rat model. Transl. Psychiatry 7, e1033 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parvizi J et al. Complex negative emotions induced by electrical stimulation of the human hypothalamus. Brain Stimul. 15, 615–623 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Caruana F et al. Motor and emotional behaviours elicited by electrical stimulation of the human cingulate cortex. Brain 141, 3035–3051 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Inman CS et al. Human amygdala stimulation effects on emotion physiology and emotional experience. Neuropsychologia 145, 106722 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi KS, Riva-Posse P, Gross RE & Mayberg HS Mapping the ‘depression switch’ during intraoperative testing of subcallosal cingulate deep brain stimulation. JAMA Neurol. 72, 1252–1260 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riva-Posse P et al. Autonomic arousal elicited by subcallosal cingulate stimulation is explained by white matter connectivity. Brain Stimul. 12, 743–751 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Haq IU et al. Smile and laughter induction and intraoperative predictors of response to deep brain stimulation for obsessive–compulsive disorder. Neuroimage 54, S247–S255 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scangos KW, Makhoul GS, Sugrue LP, Chang EF & Krystal AD State-dependent responses to intracranial brain stimulation in a patient with depression. Nat. Med. 27, 229–231 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guillory SA & Bujarski KA Exploring emotions using invasive methods: review of 60 years of human intracranial electrophysiology. Soc. Cogn. Affect. Neurosci. 9, 1880–1889 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao VR et al. Direct electrical stimulation of lateral orbitofrontal cortex acutely improves mood in individuals with symptoms of depression. Curr. Biol. 28, 3893–3902 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Sheth SA et al. Deep brain stimulation for depression informed by intracranial recordings. Biol. Psychiatry 92, 246–251 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quinn EJ et al. Beta oscillations in freely moving Parkinson’s subjects are attenuated during deep brain stimulation. Mov. Disord. 30, 1750–1758 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Nguyen G & Postnova S Progress in modelling of brain dynamics during anaesthesia and the role of sleep–wake circuitry. Biochem. Pharmacol. 191, 114388 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Scangos KW et al. Closed-loop neuromodulation in an individual with treatment-resistant depression. Nat. Med. 27, 1696–1700 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waltz E Green light for deep brain stimulator incorporating neurofeedback. Nat. Biotechnol. 38, 1014–1015 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Deisseroth K Optogenetics. Nat. Methods 8, 26–29 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyden ES, Zhang F, Bamberg E, Nagel G & Deisseroth K Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Hyde J et al. Efficacy of neurostimulation across mental disorders: systematic review and meta-analysis of 208 randomized controlled trials. Mol. Psychiatry 27, 2709–2719 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossi S et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin. Neurophysiol. 132, 269–306 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klooster DCW, Ferguson MA, Boon PAJM & Baeken C Personalizing repetitive transcranial magnetic stimulation parameters for depression treatment using multimodal neuroimaging. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 7, 536–545 (2022). [DOI] [PubMed] [Google Scholar]
- 47.Deng Z-D, Lisanby SH & Peterchev AV Coil design considerations for deep transcranial magnetic stimulation. Clin. Neurophysiol. 125, 1202–1212 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Philip NS, Barredo J, Aiken E & Carpenter LL Neuroimaging mechanisms of therapeutic transcranial magnetic stimulation for major depressive disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 211–222 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blumberger DM et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet 391, 1683–1692 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Weigand A et al. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol. Psychiatry 84, 28–37 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cole EJ et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am. J. Psychiatry 177, 716–726 (2020). [DOI] [PubMed] [Google Scholar]
- 52.Leuchter AF et al. Efficacy and safety of low-field synchronized transcranial magnetic stimulation (sTMS) for treatment of major depression. Brain Stimul. 8, 787–794 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Koponen LM, Nieminen JO & Ilmoniemi RJ Multi-locus transcranial magnetic stimulation—theory and implementation. Brain Stimul. 11, 849–855 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Lisanby SH Electroconvulsive therapy for depression. N. Engl. J. Med. 357, 1939–1945 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Husain MM et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J. Clin. Psychiatry 65, 485–491 (2004). [DOI] [PubMed] [Google Scholar]
- 56.Arulpragasam AR et al. Low intensity focused ultrasound for non-invasive and reversible deep brain neuromodulation—a paradigm shift in psychiatric research. Front. Psychiatry 13, 825802 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanguinetti JL et al. Transcranial focused ultrasound to the right prefrontal cortex improves mood and alters functional connectivity in humans. Front. Hum. Neurosci. 14, 52 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin AR, Daly MJ, Robinson EB, Hyman SE & Neale BM Predicting polygenic risk of psychiatric disorders. Biol. Psychiatry 86, 97–109 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poldrack RA et al. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat. Rev. Neurosci. 18, 115–126 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Müller VI et al. Altered brain activity in unipolar depression revisited: meta-analyses of neuroimaging studies. JAMA Psychiatry 74, 47–55 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sprooten E et al. Addressing reverse inference in psychiatric neuroimaging: meta-analyses of task-related brain activation in common mental disorders. Hum. Brain Mapp. 38, 1846–1864 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goodkind M et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 72, 305–315 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marek S et al. Reproducible brain-wide association studies require thousands of individuals. Nature 603, 654–660 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goldstein-Piekarski AN et al. Mapping neural circuit biotypes to symptoms and behavioral dimensions of depression and anxiety. Biol. Psychiatry 91, 561–571 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gray JP, Müller VI, Eickhoff SB & Fox PT Multimodal abnormalities of brain structure and function in major depressive disorder: a meta-analysis of neuroimaging studies. Am. J. Psychiatry 177, 422–434 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fox PT, Lancaster JL, Laird AR & Eickhoff SB Meta-analysis in human neuroimaging: computational modeling of large-scale databases. Annu. Rev. Neurosci. 37, 409–434 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.FDA–NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource (Food and Drug Administration (US), 2016). [PubMed] [Google Scholar]
- 68.Webb CA et al. Personalized prediction of antidepressant v. placebo response: evidence from the EMBARC study. Psychol. Med. 49, 1118–1127 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Drysdale AT et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 23, 28–38 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grosenick L et al. Functional and optogenetic approaches to discovering stable subtype-specific circuit mechanisms in depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4, 554–566 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scangos KW et al. Distributed subnetworks of depression defined by direct intracranial neurophysiology. Front. Hum. Neurosci. 15, 746499 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Williams LM et al. Amygdala reactivity to emotional faces in the prediction of general and medication-specific responses to antidepressant treatment in the randomized iSPOT-D trial. Neuropsychopharmacology 40, 2398–2408 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goldstein-Piekarski AN et al. Intrinsic functional connectivity predicts remission on antidepressants: a randomized controlled trial to identify clinically applicable imaging biomarkers. Transl. Psychiatry 8, 57 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dunlop BW et al. Functional connectivity of the subcallosal cingulate cortex and differential outcomes to treatment with cognitive-behavioral therapy or antidepressant medication for major depressive disorder. Am. J. Psychiatry 174, 533–545 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dichter GS, Damiano CA & Allen JA Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J. Neurodev. Disord. 4, 19 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Treadway MT & Zald DH Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci. Biobehav. Rev. 35, 537–555 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ang Y-S et al. Pretreatment reward sensitivity and frontostriatal resting-state functional connectivity are associated with response to bupropion after sertraline nonresponse. Biol. Psychiatry 88, 657–667 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nguyen KP et al. Patterns of pretreatment reward task brain activation predict individual antidepressant response: key results from the EMBARC randomized clinical trial. Biol. Psychiatry 91, 550–560 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fawcett J et al. Clinical experience with high-dosage pramipexole in patients with treatment-resistant depressive episodes in unipolar and bipolar depression. Am. J. Psychiatry 173, 107–111 (2016). [DOI] [PubMed] [Google Scholar]
- 80.Ventorp F Preliminary evidence of efficacy and target engagement of pramipexole in anhedonic depression. Psychiatr. Res. Clin. Pract. 4, 42–47 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bruijnzeel AW κ-opioid receptor signaling and brain reward function. Brain Res. Rev. 62, 127–146 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krystal AD et al. A randomized proof-of-mechanism trial applying the ‘fast-fail’ approach to evaluating κ-opioid antagonism as a treatment for anhedonia. Nat. Med. 26, 760–768 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Costi S et al. Impact of the KCNQ2/3 channel opener ezogabine on reward circuit activity and clinical symptoms in depression: results from a randomized controlled trial. Am. J. Psychiatry 178, 437–446 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Costi S, Han M-H & Murrough JW The potential of KCNQ potassium channel openers as novel antidepressants. CNS Drugs 36, 207–216 (2022). [DOI] [PubMed] [Google Scholar]
- 85.Williams LM Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry 3, 472–480 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Korgaonkar MS, Grieve SM, Etkin A, Koslow SH & Williams LM Using standardized fMRI protocols to identify patterns of prefrontal circuit dysregulation that are common and specific to cognitive and emotional tasks in major depressive disorder: first wave results from the iSPOT-D study. Neuropsychopharmacology 38, 863–871 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ruhé HG, Booij J, Veltman DJ, Michel MC & Schene AH Successful pharmacologic treatment of major depressive disorder attenuates amygdala activation to negative facial expressions: a functional magnetic resonance imaging study. J. Clin. Psychiatry 73, 451–459 (2012). [DOI] [PubMed] [Google Scholar]
- 88.Godlewska BR, Browning M, Norbury R, Cowen PJ & Harmer CJ Early changes in emotional processing as a marker of clinical response to SSRI treatment in depression. Transl. Psychiatry 6, e957 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Minard A et al. Remarkable progress with small-molecule modulation of TRPC1/4/5 channels: implications for understanding the channels in health and disease. Cells 7, 52 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grimm S et al. The effects of transient receptor potential cation channel inhibition by BI 1358894 on cortico–limbic brain reactivity to negative emotional stimuli in major depressive disorder. Eur. Neuropsychopharmacol. 65, 44–51 (2022). [DOI] [PubMed] [Google Scholar]
- 91.Recourt K et al. The selective orexin-2 antagonist seltorexant (JNJ-42847922/MIN-202) shows antidepressant and sleep-promoting effects in patients with major depressive disorder. Transl. Psychiatry 9, 216 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Soya S & Sakurai T Orexin as a modulator of fear-related behavior: hypothalamic control of noradrenaline circuit. Brain Res. 1731, 146037 (2020). [DOI] [PubMed] [Google Scholar]
- 93.Wang X et al. Shared and distinct brain fMRI response during performance of working memory tasks in adult patients with schizophrenia and major depressive disorder. Hum. Brain Mapp. 42, 5458–5476 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bandelow B et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J. Biol. Psychiatry 18, 162–214 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fleischhacker WW et al. Efficacy and safety of the novel glycine transporter inhibitor BI 425809 once daily in patients with schizophrenia: a double-blind, randomised, placebo-controlled phase 2 study. Lancet Psychiatry 8, 191–201 (2021). [DOI] [PubMed] [Google Scholar]
- 96.Dichter GS, Gibbs D & Smoski MJ A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J. Affect. Disord. 172, 8–17 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Posner J et al. Antidepressants normalize the default mode network in patients with dysthymia. JAMA Psychiatry 70, 373–382 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Andreescu C et al. Resting state functional connectivity and treatment response in late-life depression. Psychiatry Res. 214, 313–321 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kilpatrick LA, Krause-Sorio B, Siddarth P, Narr KL & Lavretsky H Default mode network connectivity and treatment response in geriatric depression. Brain Behav. 12, e2475 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liston C et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol. Psychiatry 76, 517–526 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Deligiannidis KM et al. Effect of zuranolone vs placebo in postpartum depression: a randomized clinical trial. JAMA Psychiatry 78, 951–959 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deligiannidis KM et al. Resting-state functional connectivity, cortical GABA, and neuroactive steroids in peripartum and peripartum depressed women: a functional magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology 44, 546–554 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Henter ID, Park LT & Zarate CA Novel glutamatergic modulators for the treatment of mood disorders: current status. CNS Drugs 35, 527–543 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zarate CA et al. A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 63, 856–864 (2006). [DOI] [PubMed] [Google Scholar]
- 105.McIntyre RS et al. The effect of intravenous, intranasal, and oral ketamine in mood disorders: a meta-analysis. J. Affect. Disord. 276, 576–584 (2020). [DOI] [PubMed] [Google Scholar]
- 106.Wilkinson ST et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am. J. Psychiatry 175, 150–158 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Abdallah CG et al. Dose-related effects of ketamine for antidepressant-resistant symptoms of posttraumatic stress disorder in veterans and active duty military: a double-blind, randomized, placebo-controlled multi-center clinical trial. Neuropsychopharmacology 47, 1574–1581 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Feder A et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry 71, 681–688 (2014). [DOI] [PubMed] [Google Scholar]
- 109.Rodriguez CI et al. Randomized controlled crossover trial of ketamine in obsessive–compulsive disorder: proof-of-concept. Neuropsychopharmacology 38, 2475–2483 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Q, Wang S & Mei X A single intravenous administration of a sub-anesthetic ketamine dose during the perioperative period of cesarean section for preventing postpartum depression: a meta-analysis. Psychiatry Res. 310, 114396 (2022). [DOI] [PubMed] [Google Scholar]
- 111.Popova V et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am. J. Psychiatry 176, 428–438 (2019). [DOI] [PubMed] [Google Scholar]
- 112.Daly EJ et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry 76, 893–903 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ionescu DF et al. Esketamine nasal spray for rapid reduction of depressive symptoms in patients with major depressive disorder who have active suicide ideation with intent: results of a phase 3, double-blind, randomized study (ASPIRE II). Int. J. Neuropsychopharmacol. 24, 22–31 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim J, Farchione T, Potter A, Chen Q & Temple R Esketamine for treatment-resistant depression—first FDA-approved antidepressant in a new class. N. Engl. J. Med. 381, 1–4 (2019). [DOI] [PubMed] [Google Scholar]
- 115.Zanos P et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533, 481–486 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zarate CA et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am. J. Psychiatry 163, 153–155 (2006). [DOI] [PubMed] [Google Scholar]
- 117.Alario AA & Niciu MJ Biomarkers of ketamine’s antidepressant effect: a clinical review of genetics, functional connectivity, and neurophysiology. Chronic Stress 5, 24705470211014210 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ionescu DF et al. Ketamine-associated brain changes: a review of the neuroimaging literature. Harv. Rev. Psychiatry 26, 320–339 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Morris LS et al. Ketamine normalizes subgenual cingulate cortex hyper-activity in depression. Neuropsychopharmacology 45, 975–981 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tabuteau H, Jones A, Anderson A, Jacobson M & Iosifescu DV Effect of AXS-05 (dextromethorphan–bupropion) in major depressive disorder: a randomized double-blind controlled trial. Am. J. Psychiatry 179, 490–499 (2022). [DOI] [PubMed] [Google Scholar]
- 121.Nagele P et al. A phase 2 trial of inhaled nitrous oxide for treatment-resistant major depression. Sci. Transl. Med. 13, eabe1376 (2021). [DOI] [PubMed] [Google Scholar]
- 122.Edinoff AN et al. Brexanolone, a GABAA modulator, in the treatment of postpartum depression in adults: a comprehensive review. Front. Psychiatry 12, 699740 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dacarett-Galeano DJ & Diao XY Brexanolone: a novel therapeutic in the treatment of postpartum depression. Am. J. Psychiatry Resid. J. 15, 2–4 (2019). [Google Scholar]
- 124.Burgdorf JS, Zhang X-L, Stanton PK, Moskal JR & Donello JE Zelquistinel is an orally bioavailable novel NMDA receptor allosteric modulator that exhibits rapid and sustained antidepressant-like effects. Int. J. Neuropsychopharmacol. 25, 979–991 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Galvão-Coelho NL et al. Classic serotonergic psychedelics for mood and depressive symptoms: a meta-analysis of mood disorder patients and healthy participants. Psychopharmacology 238, 341–354 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Carhart-Harris R et al. Trial of psilocybin versus escitalopram for depression. N. Engl. J. Med. 384, 1402–1411 (2021). [DOI] [PubMed] [Google Scholar]
- 127.Ling S et al. Molecular mechanisms of psilocybin and implications for the treatment of depression. CNS Drugs 36, 17–30 (2022). [DOI] [PubMed] [Google Scholar]
- 128.Ly C et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. 23, 3170–3182 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shao L-X et al. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 109, 2535–2544 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Girn M et al. Serotonergic psychedelic drugs LSD and psilocybin reduce the hierarchical differentiation of unimodal and transmodal cortex. Neuroimage 256, 119220 (2022). [DOI] [PubMed] [Google Scholar]
- 131.Daws RE et al. Increased global integration in the brain after psilocybin therapy for depression. Nat. Med. 28, 844–851 (2022). [DOI] [PubMed] [Google Scholar]
- 132.Carhart-Harris RL et al. Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Sci. Rep. 7, 13187 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Johnson MW, Hendricks PS, Barrett FS & Griffiths RR Classic psychedelics: an integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol. Ther. 197, 83–102 (2019). [DOI] [PubMed] [Google Scholar]
- 134.Roseman L, Nutt DJ & Carhart-Harris RL Quality of acute psychedelic experience predicts therapeutic efficacy of psilocybin for treatment-resistant depression. Front. Pharmacol. 8, 974 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pasquini L, Palhano-Fontes F & Araujo DB Subacute effects of the psychedelic ayahuasca on the salience and default mode networks. J. Psychopharmacol. 34, 623–635 (2020). [DOI] [PubMed] [Google Scholar]
- 136.Müller F, Brändle R, Liechti ME & Borgwardt S Neuroimaging of chronic MDMA (‘ecstasy’) effects: a meta-analysis. Neurosci. Biobehav. Rev. 96, 10–20 (2019). [DOI] [PubMed] [Google Scholar]
- 137.Sanacora G & Schatzberg AF Ketamine: promising path or false prophecy in the development of novel therapeutics for mood disorders? Neuropsychopharmacology 40, 259–267 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nutt D & Carhart-Harris R The current status of psychedelics in psychiatry. JAMA Psychiatry 78, 121–122 (2021). [DOI] [PubMed] [Google Scholar]
- 139.Vargas MV, Meyer R, Avanes AA, Rus M & Olson DE Psychedelics and other psychoplastogens for treating mental illness. Front. Psychiatry 12, 727117 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Miller AH & Raison CL The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 16, 22–34 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Drevets WC, Wittenberg GM, Bullmore ET & Manji HK Immune targets for therapeutic development in depression: towards precision medicine. Nat. Rev. Drug Discov. 21, 224–244 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goldsmith DR, Rapaport MH & Miller BJ A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry 21, 1696–1709 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang J-J & Jiang W Immune biomarkers alterations in post-traumatic stress disorder: a systematic review and meta-analysis. J. Affect. Disord. 268, 39–46 (2020). [DOI] [PubMed] [Google Scholar]
- 144.Osimo EF et al. Inflammatory markers in depression: a meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav. Immun. 87, 901–909 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Konuk N et al. Plasma levels of tumor necrosis factor-α and interleukin-6 in obsessive compulsive disorder. Mediators Inflamm. 2007, 65704 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Haroon E et al. Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology 95, 43–49 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Enache D et al. Peripheral immune markers and antipsychotic non-response in psychosis. Schizophr. Res. 230, 1–8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kruse JL et al. Depression treatment response to ketamine: sex-specific role of interleukin-8, but not other inflammatory markers. Transl. Psychiatry 11, 167 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kruse JL et al. Inflammation and improvement of depression following electroconvulsive therapy in treatment-resistant depression. J. Clin. Psychiatry 79, 17m11597 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Harrison NA et al. A neurocomputational account of how inflammation enhances sensitivity to punishments versus rewards. Biol. Psychiatry 80, 73–81 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Eisenberger NI et al. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol. Psychiatry 68, 748–754 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Harrison NA et al. Neural origins of human sickness in interoceptive responses to inflammation. Biol. Psychiatry 66, 415–422 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Capuron L et al. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch. Gen. Psychiatry 69, 1044–1053 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Felger JC et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry 21, 1358–1365 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mehta D et al. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proc. Natl Acad. Sci. USA 110, 8302–8307 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Harrison NA et al. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol. Psychiatry 66, 407–414 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Felger JC et al. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol. Psychiatry 25, 1301–1311 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mehta ND et al. Inflammation negatively correlates with amygdala–ventromedial prefrontal functional connectivity in association with anxiety in patients with depression: preliminary results. Brain Behav. Immun. 73, 725–730 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Felger JC et al. Chronic interferon-α decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology 38, 2179–2187 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Haroon E et al. Increased inflammation and brain glutamate define a subtype of depression with decreased regional homogeneity, impaired network integrity, and anhedonia. Transl. Psychiatry 8, 189 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Çakici N, van Beveren NJM, Judge-Hundal G, Koola MM & Sommer IEC An update on the efficacy of anti-inflammatory agents for patients with schizophrenia: a meta-analysis. Psychol. Med. 49, 2307–2319 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Köhler-Forsberg O et al. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr. Scand. 139, 404–419 (2019). [DOI] [PubMed] [Google Scholar]
- 163.Husain MI et al. Minocycline and celecoxib as adjunctive treatments for bipolar depression: a multicentre, factorial design randomised controlled trial. Lancet Psychiatry 7, 515–527 (2020). [DOI] [PubMed] [Google Scholar]
- 164.Hellmann-Regen J et al. Effect of minocycline on depressive symptoms in patients with treatment-resistant depression: a randomized clinical trial. JAMA Netw. Open 5, e2230367 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gribkoff VK & Kaczmarek LK The need for new approaches in CNS drug discovery: why drugs have failed, and what can be done to improve outcomes. Neuropharmacology 120, 11–19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tricklebank MD, Robbins TW, Simmons C & Wong EHF Time to re-engage psychiatric drug discovery by strengthening confidence in preclinical psychopharmacology. Psychopharmacology 238, 1417–1436 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Revah O et al. Maturation and circuit integration of transplanted human cortical organoids. Nature 610, 319–326 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vrselja Z et al. Restoration of brain circulation and cellular functions hours post-mortem. Nature 568, 336–343 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wiltschko AB et al. Revealing the structure of pharmacobehavioral space through motion sequencing. Nat. Neurosci. 23, 1433–1443 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dang J, King KM & Inzlicht M Why are self-report and behavioral measures weakly correlated? Trends Cogn. Sci. 24, 267–269 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cohn JF et al. Automated affect detection in deep brain stimulation for obsessive–compulsive disorder: a pilot study. Proc. ACM Int. Conf. Multimodal Interact. 2018, 40–44 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Darzi A et al. Facial action units and head dynamics in longitudinal interviews reveal OCD and depression severity and DBS energy. In 2021 16th IEEE International Conference on Automatic Face and Gesture Recognition 10.1109/FG52635.2021.9667028 (IEEE, 2021). [DOI] [Google Scholar]
- 173.Ertugrul IO, Jeni LA, Ding W & Cohn JF AFAR: a deep learning based tool for automated facial affect recognition. Proc. Int. Conf. Autom. Face Gesture Recognit. 10.1109/fg.2019.8756623 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vijay S, Pennant L, Öngür D, Baker J & Morency L-P Computational study of psychosis symptoms and facial expressions. (2016).
- 175.Depp CA et al. GPS mobility as a digital biomarker of negative symptoms in schizophrenia: a case control study. NPJ Digit. Med. 2, 108 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Baker JT, Germine LT, Ressler KJ, Rauch SL & Carlezon WA Digital devices and continuous telemetry: opportunities for aligning psychiatry and neuroscience. Neuropsychopharmacology 43, 2499–2503 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shen FX et al. An ethics checklist for digital health research in psychiatry: viewpoint. J. Med. Internet Res. 24, e31146 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dell’Osso B & Ketter TA Assessing efficacy/effectiveness and safety/tolerability profiles of adjunctive pramipexole in bipolar depression: acute versus long-term data. Int. Clin. Psychopharmacol. 28, 297–304 (2013). [DOI] [PubMed] [Google Scholar]
- 179.Tundo A, de Filippis R & De Crescenzo F Pramipexole in the treatment of unipolar and bipolar depression. a systematic review and meta-analysis. Acta Psychiatr. Scand. 140, 116–125 (2019). [DOI] [PubMed] [Google Scholar]
- 180.Whitton AE et al. Baseline reward processing and ventrostriatal dopamine function are associated with pramipexole response in depression. Brain 143, 701–710 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ye Z, Hammer A, Camara E & Münte TF Pramipexole modulates the neural network of reward anticipation. Hum. Brain Mapp. 32, 800–811 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Carlezon WA & Krystal AD κ-opioid antagonists for psychiatric disorders: from bench to clinical trials. Depress. Anxiety 33, 895–906 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wise T et al. Cholinergic modulation of disorder-relevant neural circuits in generalized anxiety disorder. Biol. Psychiatry 87, 908–915 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Javitt DC Glycine transport inhibitors for the treatment of schizophrenia: symptom and disease modification. Curr. Opin. Drug Discov. Devel. 12, 468–478 (2009). [PubMed] [Google Scholar]
- 185.Wilkinson ST & Sanacora G A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discov. Today 24, 606–615 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Burgdorf J et al. The long-lasting antidepressant effects of rapastinel (GLYX-13) are associated with a metaplasticity process in the medial prefrontal cortex and hippocampus. Neuroscience 308, 202–211 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Walkery A, Leader LD, Cooke E & VandenBerg A Review of allopregnanolone agonist therapy for the treatment of depressive disorders. Drug Des. Devel. Ther. 15, 3017–3026 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zink CF & Meyer-Lindenberg A Human neuroimaging of oxytocin and vasopressin in social cognition. Horm. Behav. 61, 400–409 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schiena G et al. Connectivity changes in major depressive disorder after rTMS: a review of functional and structural connectivity data. Epidemiol. Psychiatr. Sci. 30, e59 (2021). [Google Scholar]
- 190.Chan MMY, Yau SSY & Han YMY The neurobiology of prefrontal transcranial direct current stimulation (tDCS) in promoting brain plasticity: a systematic review and meta-analyses of human and rodent studies. Neurosci. Biobehav. Rev. 125, 392–416 (2021). [DOI] [PubMed] [Google Scholar]
- 191.Leaver AM, Espinoza R, Wade B & Narr KL Parsing the network mechanisms of electroconvulsive therapy. Biol. Psychiatry 92, 193–203 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lucido MJ et al. Aiding and abetting anhedonia: impact of inflammation on the brain and pharmacological implications. Pharmacol. Rev. 73, 1084–1117 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Goldsmith DR et al. Protein and gene markers of metabolic dysfunction and inflammation together associate with functional connectivity in reward and motor circuits in depression. Brain Behav. Immun. 88, 193–202 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Torous J et al. Characterizing the clinical relevance of digital phenotyping data quality with applications to a cohort with schizophrenia. NPJ Digit. Med. 1, 15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lefaucheur J-P et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin. Neurophysiol. 131, 474–528 (2020). [DOI] [PubMed] [Google Scholar]
- 196.Stagg CJ, Antal A & Nitsche MA Physiology of transcranial direct current stimulation. J. ECT 34, 144–152 (2018). [DOI] [PubMed] [Google Scholar]
- 197.Sanders SJ et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron 70, 863–885 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Levy D et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron 70, 886–897 (2011). [DOI] [PubMed] [Google Scholar]
- 199.Sebat J et al. Strong association of de novo copy number mutations with autism. Science 316, 445–449 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sanders SJ et al. Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron 87, 1215–1233 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Neale BM et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485, 242–245 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.De Rubeis S et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515, 209–215 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Iossifov I et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 515, 216–221 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sanders SJ et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485, 237–241 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.O’Roak BJ et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485, 246–250 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Satterstrom FK et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell 180, 568–584 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Trubetskoy V et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 604, 502–508 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Coleman JRI et al. The genetics of the mood disorder spectrum: genome-wide association analyses of more than 185,000 cases and 439,000 controls. Biol. Psychiatry 88, 169–184 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Stahl EA et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat. Genet. 51, 793–803 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ruderfer DM et al. Genomic dissection of bipolar disorder and schizophrenia, including 28 subphenotypes. Cell 173, 1705–1715 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Psychiatric GWAS Consortium Bipolar Disorder Working Group. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat. Genet. 43, 977–983 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dantzer R, O’Connor JC, Freund GG, Johnson RW & Kelley KW From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Menard C et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 20, 1752–1760 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wohleb ES et al. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol. Psychiatry 75, 970–981 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.McKim DB et al. Microglial recruitment of IL-1β-producing monocytes to brain endothelium causes stress-induced anxiety. Mol. Psychiatry 23, 1421–1431 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Da Mesquita S, Fu Z & Kipnis J The meningeal lymphatic system: a new player in neurophysiology. Neuron 100, 375–388 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chen X et al. Kynurenines increase MRS metabolites in basal ganglia and decrease resting-state connectivity in frontostriatal reward circuitry in depression. Transl. Psychiatry 11, 456 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]


