Abstract
Surgical management paradigms of spinal pathologies in the aging population carry inherent substantial risks, with surgical complications being more prevalent among patients with osteoporosis compared to those with normal bone mineral density. In this narrative review, we aim to highlight important clinical understanding and considerations in perioperative evaluation and management of patients elected to undergo spinal surgery. Osteoporosis is a well-defined risk factor for mechanical complications following spinal surgery, and as such, perioperative optimization of bone health in the setting of surgery for geriatric patients remains a critical research area alongside intraoperative surgical augmentation techniques. Surgical techniques to circumvent challenges with instrumentation of poor bone mineral density have included augmentation of pedicle screw fixation, including segmental bicortical screw fixation techniques, cement augmentation with fenestrated screws, or use of expandable pedicle screws to improve bone-implant interface. Judicious selection of treatment modalities and subsequent perioperative optimization is paramount to minimize surgical complications. Contemporary guidelines and evolving paradigms in perioperative evaluation, optimization, and management of the aging spine include the advent of quantitatively evaluating computed tomography (CT) via assessment of the magnitude of Hounsfield units. Prescribing pharmacotherapeutic agents and monitoring bone health requires a multidisciplinary team approach, including endocrinologists and geriatricians to coordinate high-quality care for advanced-age patients who require surgical management of their spinal disorders.
Keywords: Osteoporosis, Bone density, Geriatric, Fragility fracture, T-score, Kyphosis, Fixation
Osteoporosis epidemiology and clinical implications
The epidemiologic profile of the general population has changed dramatically as modern technology and advances in medicine impact life expectancy and disease outcomes. In the United States in the early eighteenth century, the average life expectancy was about 47 years of age (1). The present-day average life expectancy is about 77.5 years with the U.S. population being older than it has ever been. To be considered geriatric, by definition, one must be over the age of 65 (2). According to U.S. census projections, the number of Americans 65 years of age and older will increase by 47% from 58 million as measured back in 2022 to 82 million by 2050 (2). This means almost ¼ of the entire American population’s age is over 65 (2). Globally, this population group was projected to outnumber persons under 15 by 2024 (3). Improvements in healthcare services and access have changed factors influencing the morbidity and mortality of the elderly. Processes affecting the quality of life and geriatric health are changing, bringing to the forefront new health concerns associated with increased age.
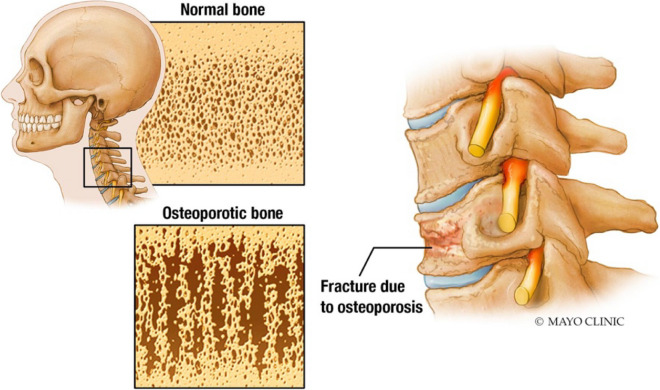
One such ailment is the development of osteoporosis. This condition is characterized by low bone mass, skeletal fragility, and microarchitectural disruption, leading to an increased risk for bone fracture (Fig. 1). It occurs in both genders; however, it is more commonly diagnosed in women. Management of osteoporotic fractures and other related complications can be significant contributors to healthcare costs (4, 5). In the United States, historical data suggest that there are approximately 1.5 million fractures associated with osteoporosis occurring each year, costing the healthcare system nearly 18 billion dollars annually (5). These injuries lead to more than 800,000 trips to the emergency room, 500,000 hospitalizations, and 2.5 million physician clinic visits (6). Given the significant impact and risk these fractures have on the geriatric population [1–3], the Fracture Risk Assessment Tool (FRAX) is commonly used to predict the 10-year risk of hip or other osteoporotic fracture. The FRAX screening tool uses patient bone mineral density, age, demographic information, and associated comorbidities to assess the risk of future fractures (Table 1) [4].
Fig. 1.
Normal bone mineral density compared to “porous bone” seen with osteoporosis
Table 1.
The fracture risk and assessment tool (FRAX)
| Fracture Risk Assessment Tool Questionnaire (FRAX) | |
|---|---|
| Age between 40 and 90 years | Glucocorticoid use |
| Sex | Rheumatoid arthritis |
| Weight | History of secondary osteoporosis |
| Height | Alcohol of three or more units per day |
| Previous fracture | Femoral neck bone mineral density |
| Parent fractured hip | |
| Current smoking status | |
Whether due to fracture or degenerative disease, spine surgery in the geriatric population is often complicated by osteoporosis. Osteoporosis increases the risk of mechanical complications, including pseudoarthrosis, instrumentation failure, screw pullout, progressive kyphosis, compression fracture, and adjacent-level degeneration [5]. Studies show the axial pull-out strength of pedicle screws in the lumbar spine in osteoporotic patients to be 61.1 to 78.9% that of a normal patient [6]. Further studies have expanded upon this, demonstrating a 2-year re-operation rate of 30.4% in patients with osteoporosis compared to 22.9% in patients without osteoporosis when looking at Medicare patients who underwent instrumentation for adult spinal deformity [7]. The increased risk of re-operation and subsequent complications are not insignificant. Reoperation, longer instrumentation constructs, and the need for combined anterior/posterior approaches increase the cost of these surgeries in the osteoporotic population, adding more weight to the risk profile in these patients [8, 9] These variables add an additional average cost of over $20,000 in patients who have an osteoporosis-related complication after spinal fusion [10]. Thorough evaluation, planning, and treatment are required in the osteoporotic geriatric population.
Evaluation, diagnostic modalities, and radiographic findings
Perioperative workup of osteoporosis must include definitive criteria for diagnosis. Osteoporosis is defined as “a skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture.” Bone strength reflects the integration of two main features: Bone density and bone quality. Bone quality is difficult to quantify but has been associated with fragility fractures. These fractures result from low-energy trauma that would not cause a fracture in an otherwise normal individual. Fragility fractures themselves are a diagnostic criterion for osteoporosis; however, the other half of the equation, bone density, is often assessed via dual-energy X-ray of absorptiometry [11]. Current general guidelines for osteoporosis screening recommend workups in women over the age of 65 and men who are over the age of 70 per the International Society for Densitometry (ISCD) [12]. Similarly, current recommendations for patients undergoing spine surgery recommend the screening of both men and women over the age of 65 to reduce the risk of underdiagnosis in men [13]. Patients under the age of 65 should be screened if they have associated risk factors (Table 2).
Table 2.
Best practice screening parameters described by Sardar et al. [2]. Asterisk (*) indicates chronic glucocorticoid use defined as > 3 months of prednisone use, minimum 5 mg per day. Asterisks (**) indicate fracture, pseudarthrosis, and instrumentation failure
| > 65 years of age | 50–64 years of age | < 50 years of age |
|---|---|---|
| Always | Chronic glucocorticoid use* | Chronic glucocorticoid use* |
| Low-energy fracture of the hip or spine | Low-energy fracture of the hip or spine | |
| History of metabolic bone disease | History of metabolic bone disease | |
| Chronic kidney disease stage 3 or greater | Currently undergoing cancer treatment | |
| High risk of fracture calculated with FRAX | Chronic kidney disease stage 3 or greater | |
| Prior failed spine surgery** | ||
| Alcohol use > 3 units per day | ||
| Vitamin D deficiency | ||
| Current smoker | ||
| Wheelchair use | ||
| Currently undergoing cancer treatment | ||
| Diabetes mellitus |
Dual-energy X-ray absorptiometry
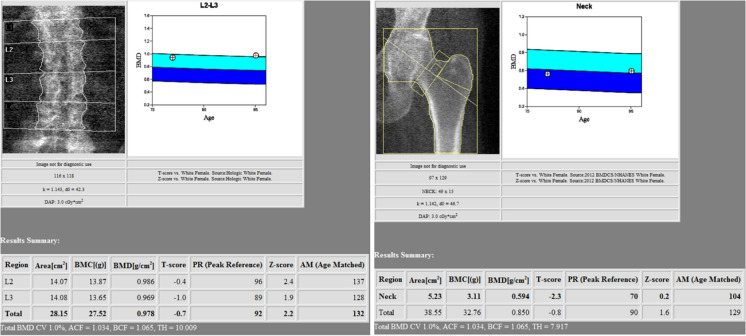
Once patients meet screening criteria, the World Health Organization (WHO) recommends initial screening/evaluation be completed via dual-energy X-ray absorptiometry (DEXA). DEXA scans (Fig. 2) are a subset of X-ray imaging. This technology uses X-rays of two different photon energy levels (high and low) that are used to calculate the areal densities of two different tissue types: hydroxyapatite (bone) and soft tissue [14]. As the X-rays pass through the body, they are absorbed by the internal structures. Higher-density objects (such as a bone) absorb more of the X-rays, resulting in less signal in those regions as detected by the detector plate. These results are output as a bone mineral density (g/cm2). Evaluation of osteoporosis uses DEXA scans of the hip and spine, as these anatomical locations are commonly associated with osteoporotic fractures. In addition, studies have demonstrated higher fracture predictability for bone mineral density testing completed at the femoral neck and in the lumbar spine (Fig. 3) [15]. In the pre-operative setting, DEXA scans at the femoral neck are preferred, as sclerosis and spondylosis of the spine can artificially inflate the DEXA scan value (Table 3) [16].
Fig. 2.
DEXA scanner
Fig. 3.
Sample DEXA scans of the lumbar spine and femoral neck
Table 3.
WHO defined characteristics and effectiveness results of dual-energy X-ray absorptiometry scans
| Patient and scan characteristics that can limit the accuracy of a dual-energy X-ray absorptiometry test (DEXA) |
|---|
| Severe spinal curvature, deformity, or scoliosis |
| Overlying or nearby metal objects causing interference |
| Recent contrast injection |
| Previous fractures with subsequent healing and sclerosis |
| Ascites or obesity |
| Osteomalacia |
| Osteoarthritis particularly in the spine causing sclerosis of endplates and cortical bone |
| Soft tissue calcifications |
| Improper machine calibration and/or improper reference ranges when performing the scan |
T-score
Bone mineral density (BMD) presents as a normal distribution and remains constant until the age of 50, as such, an individual’s BMD testing results can be compared to that of a standardized reference population. In the United States, the NHANES III Study and its associated database are used as a reference point for a healthy non-osteoporotic patient [17]. The patient’s T-score is the BMD results calculated as the standard deviation above or below that of a healthy individual of the same sex measured at the femoral neck. Osteoporosis is defined as a male or female over the age of 50 with decreased bone mineral density and a T-score of less than − 2.5 (Table 4) [16].
Table 4.
The WHO T-score osteoporosis cutoffs
| T-score | |
|---|---|
| Normal | 0 to − 1 |
| Osteopenia | − 1 to − 2.5 |
| Osteoporosis | < − 2.5 |
| Severe osteoporosis | < − 2.5 with associated fragility fracture |
Z-score
The Z-score is the output standard deviation of the patient’s bone mineral density when compared to a standardized age and sex-matched cohort. This contrasts with a T-score, which compares the patient’s bone mineral density to that of a healthy same-sex individual with normal bone mineral density. A low Z-score indicates an unusually low bone mineral density when compared to that of the patient of a similar demographic, indicating there may be a secondary cause to the patient’s osteoporosis, including hyperparathyroidism, excessive alcohol intake, celiac disease, or excessive steroid use [18]. Investigation into secondary causes of osteoporosis should be routinely considered in the management of patients with osteoporosis.
Other imaging modalities
Currently, the DEXA scan is the gold standard for the evaluation of osteoporosis in the aging population in the preoperative setting. Other imaging modalities that can be used to assess bone strength and quality defined by the American College of Radiology include quantitative computer tomography, digital X-ray radiogrammetry, radiographic absorptiometry, and quantitative ultrasound techniques (Table 5) [19]. These techniques are used if DEXA scans are non-diagnostic, patients are unable to have a DEXA scan due to resource limitations, or if there is a need to decrease radiation exposure.
Table 5.
American College of Radiology imaging appropriateness criteria for assessing BMD in patients with spinal degenerative disease
| Appropriateness of imaging modality | Advantages | Disadvantages | |
|---|---|---|---|
| DEXA SCAN | Appropriate |
Low radiation dosage Ease of use |
Altered bone mineral density values due to spondylosis and degenerative arthritis of the spine |
| QCT | Appropriate |
Increased accuracy when compared to DEXA scan when assessing patients with significant spondylosis Only reports bone mineral density of cancellous bone Provides adjunctive surgical information when evaluating patients in the preoperative setting |
High radiation dosage |
| QUS | Usually not appropriate | ||
| RADIOGRAPHY APPENDICULAR SKELETON | Usually not appropriate | ||
| RADIOGRAPHY AXIAL SKELETON | Usually not appropriate | ||
| SINGLE X-RAY | Usually not appropriate |
Quantitative computed tomography
When it comes to evaluating a surgical population preoperatively, there has been increased interest and utilization of quantitative computed tomography (QCT). QCT results are measured in Hounsfield units (HU), which represent a standardized index focusing on X-ray attenuation using air as a numerical baseline at − 1000 and water at 0. The standard Hounsfield unit for bone ranges from 300 to 3000, with metal implants surpassing 5000 [20]. This modality is especially useful as patients being evaluated for spine surgery often undergo CT imaging, eliminating the need for additional imaging modalities. Recent meta-analyses have determined optimal cutoffs for defining normal bone, osteoporosis, and osteopenia. Further, results suggest 97.9 HU as the cutoff for osteoporosis [21, 22]. Studies have shown some advantages in the technology when evaluating patients with deformity, degenerative disease, and spondylolytic disorders, as they may have higher accuracy when assessing bone mineral density when compared to that of DEXA scans [23]. This modality serves as a valuable adjunctive imaging evaluation as it provides local information on bone mineral density, which may help assess the quality of the bone-screw interface [24]. The technology can be incorporated into surgical planning (i.e., the number of instrumented levels included or in the selection of upper instrumented vertebra) in an effort to minimize surgical complications related to instrumentation failure in poor bone quality.
Perioperative management and modifiable risk factors
Management of the geriatric osteoporotic population in the preoperative setting is a multi-faceted issue, often due to declining patient health, associated comorbidities, and socioeconomic problems. Treatment revolves around managing and strengthening bone health while limiting the effect of modifiable risk factors. The preoperative status of patients with preventable and treatable causes of osteoporosis (including vitamin D deficiency, smoking, and alcohol use) should be optimized. Adequate intake of calcium and vitamin D is quintessential, as studies have shown that secondary hyperparathyroidism (due to negative calcium balance) and low levels of vitamin D may result in reduced bone strength and bone loss [25]. Alcohol use has been linked as an independent risk factor for osteoporosis. The pathomechanism is multifactorial and includes both direct and indirect pathways. Studies have demonstrated that greater than three drinks per day significantly increases a patient’s risk for developing osteoporosis [26]. Smoking has also been shown to decrease bone mineral density and calcium absorption [27]. Other secondary medical causes of osteoporosis and weakened bone include prolonged steroid use, hypogonadism, hyperparathyroidism, chronic liver disease, rheumatoid arthritis, chronic renal disease, cardiovascular disease, diabetes mellitus, dementia, and sub-optimal nutrition status [28]. These risk factors should be addressed prior to any surgical intervention and may require a multidisciplinary care team including the surgeon, primary care physician, geriatrician, endocrinologist, and other associated medical sub-specialties.
Prevention of osteoporosis should be emphasized beyond its treatment. Identifying risk factors may serve as focus points for targeting non-medical preventative measures, which could enhance postoperative outcomes. Diet, including both caloric amount as well as vitamin and mineral consumption, has been identified as a proven modifiable risk factor that impacts bone quality [29]. Calcium and vitamin D are known to directly impact bone health and its supplementation is promoted in the medical optimization of bone health. The Food and Drug Administration (FDA) and the National Osteoporosis Foundation have made recommendations ranging from 800 to 1500 IU of calcium, and 400 to 1000IU of vitamin D daily [29]. Primary care and endocrinology providers are commonly at the forefront to provide comprehensive management that ensures adequate calcium and vitamin D levels known to optimize bone health [30].
Medication
Medication therapy should be individually tailored to a patient based off their clinical status and patient condition. Agents targeting increasing bone mineral density are particularly important to discuss when considering perioperative optimization of patients undergoing spine surgery. The strength of any hardware construct or fixation ultimately depends on bone quality—iatrogenic instability or fracture could otherwise result if BMD is not sufficient. The Congress of Neurological Surgeons (CNS) task force and review published that a T-score < − 2.5, qCT < 97.9 HUs, or serum vitamin D3 level < 20 ng/ml is associated with an increased risk of post-operative adverse events after spinal instrumentation (grade B recommendation) [31]. These results demonstrate the need for pre- and postoperative evaluation for early identification and preoperative treatment in the osteoporotic population.
A number of medications are approved by the FDA for the treatment of osteoporosis (Table 6) [32].
Table 6.
Current FDA-approved drugs for osteoporosis
| Bisphosphonates | SCLEROSTIN INHIBITOR |
|---|---|
| Estrogen-related therapy | Calcitonin salmon |
| Parathyroid hormone analogs | |
| Rank ligand inhibitor |
Bisphosphonate medications concentrate on blocking bone resorption. Two different classes of this family of drugs exist based on their nitrogen content. Nitrogen-containing medications include alendronate and risedronate that work by inhibiting farnesyl pyrophosphate synthase (FPPS) which facilitates osteoclast binding to bone, thus blocking osteoclastic bone resorption [30]. The other, non-nitrogen-containing drugs like tiludronate and clodronate, form an ATP sister molecule that is toxic to osteoclasts and promotes their apoptosis [33]. Biphosphates are considered a second-line agent in the treatment of osteoporosis in the spine surgery population. Ohtori et al. failed to demonstrate that bisphosphonates were inferior to teriparatide when assessing time to fusion and fusion rate. The authors observed that bisphosphonates did not demonstrate a significant difference in pedicle screw rate loosening postoperatively when compared to control groups [33, 34]. Theoretically, the results may be attributed to the underlying mechanism of the drug. Via biochemical analysis, Nagahama et al. found that alendronate inhibits bone resorption in the early phase of the bony fusion process, while also suppressing bone formation at the 6-month mark. This could be due to the osteoclast inhibition or inhibiting osteoblasts directly [35]. This, in conjunction with the side effect profile (osteonecrosis of the jaw, disruption of calcium equilibrium), deems the usage of these drugs for the management of osteoporosis controversial [29].
Newer medications used in the treatment of osteoporosis include recombinant parathyroid hormone or teriparatide [33]. Teriparatide is a synthetic version of human parathyroid hormone. It has an osteoanabolic effect that promotes increased BMD. The drug has been associated with significant reductions in the rates of vertebral and nonvertebral fractures compared with the use of bisphosphonates [30, 33]. Studies matching these two medications found teriparatide to have statistically significant higher rates of bony fusion as compared to patients on bisphosphonates, as well as lower rates of pedicle screw loosening [33, 34, 36]. The drug stimulates osteoblasts and increases lumbar spine, femoral neck, and hip BMD by ∼10%. The cost of teriparatide is high at approximately $850 per week of treatment [36, 37] (wholesale cost, $3426.50 for a 4-week supply). The high price of these agents such as teriparatide must be weighed against the benefits due to the subsequent risk of complications and reoperation [37].
Denosumab is a RANKL inhibitor that blocks the activation of osteoclasts by inhibiting osteoblast binding, thereby preventing the resorption of bone [29]. It has been shown to increase BMD in postmenopausal women and has been documented to have a less severe side-effect profile as compared to other cannon medications like bisphosphonates [29].
Still, more research and future studies are needed to assess the cost-effectiveness and value of various pharmacotherapeutic treatments for perioperative bone health optimization in geriatric patients undergoing spine surgery. Prescribing pharmacotherapeutic agents and monitoring bone health requires a multidisciplinary team approach to coordinate high-quality care for our aging population with osteoporosis who undergo consideration for spine surgery.
Current best practice recommendations for preoperative screening and medical treatment
Given the high risk associated with spine surgery in the osteoporotic and geriatric population, screening and treatment guidelines for surgical candidates have been described in the literature by Sardar et al. [13] and the Congress of Neurological Surgeons [31]. Combined panelists included in these studies were orthopedic spine surgeons, rheumatologists, endocrinologists, neurologic spine surgeons, and family medicine providers. Screening guidelines are proposed in current best practice guidelines by Sardar et al. (Table 2). Once screening has been completed, results of a T-score < − 2.5, qCT < 78.5–97.8 HUs, or serum vitamin D3 level < 20 ng/ml indicate treatment [13, 31]. Regarding management recommendations from the expert panel, all patients undergoing spine surgery should be on a dose of 1000 and 1200 mg per day of calcium, unless contraindicated [level evidence—5] [13]. The preoperative status of patients with preventable and treatable causes of osteoporosis (such as vitamin D deficiency, smoking, and alcohol use) should also be optimized and treated.
Patients with continued poor BMD should be treated with bone-building agents such as teriparatide or abaloparatide as first-line management due to its effects on fusion rates and postoperative osteoporotic complications [33, 34, 34, 36]. If patients are unable to tolerate the medications or cannot afford them, they should be started on antiresorptive agents such as denosumab or bisphosphonate. These medications are considered a second-line treatment regimen due to their benefits regarding overall fusion rates but have limited benefit when compared to first-line agents [35, 38]. Patients should be treated for at least 2 months preoperatively on first-line agents, and 6 months if the elective surgery requires long-segment instrumentation. Postoperatively, patients should be treated for at least 8 months on a first-line agent if able, without holding/interrupting the dosing schedule for surgery. Patients may continue second-line agents after their course of bone-building agents is completed [13].
Surgical management
Management of osteoporotic fractures and deformity can include cement augmentation and/or instrumented fixation for spinal reconstruction. Patients who fail cement augmentation or require segmental instrumentation warrant additional considerations in construct design and fixation points to minimize complications in the setting of poor bone density. In patients with severe osteoporosis or who are medically high risk, treatment should be avoided or delayed until bone and medical health are optimized.
Vertebroplasty and kyphoplasty
Osteoporotic patients are prone to insufficiency fractures including compression fractures of the vertebral body. Studies have found that 21.8% of women over the age of 70 have a concomitant vertebral body fracture [39]. From a surgical standpoint, vertebroplasty and kyphoplasty are a less invasive and non-instrumented form of treatment. Both interventions are percutaneous and involve the use of polymethylmethacrylate (PMMA) to augment and strengthen the fractured body. The goal of treatment is to improve patient pain symptoms and prevent worsening fracture/deformity. The primary difference between kyphoplasty and vertebroplasty is that kyphoplasty involves inflating a balloon placed in the vertebral body prior to cement augmentation, which helps to restore height to the fractured body. Traditionally these procedures have been thought to be beneficial with the need for further study [40, 41]. The literature is currently mixed regarding the topic with some recent studies defining the number needed to save one life as 22.8 at 1 year and 23.8 patients at 5 years for vertebroplasty. Kyphoplasty has a number needed to treat 14.8 patients at 1 year and 11.9 at five years [42].
This contrasts with prior randomized studies such as the INVEST trial [43] and the study completed by Buchbinder et al. [44] which found no clinically significant difference between patients undergoing vertebroplasty and non-operative management. A meta-analysis published jointly by Kallmes and Buchbinder et al. in 2018 continued to show no clinically significant difference in the treatment of acute and subacute compression fractures with vertebroplasty [45]. Evans et al. also found no difference in outcomes when comparing kyphoplasty to vertebroplasty [46]. As it stands, there are numerous studies showing no significant advantage to the treatment of these fractures with cement augmentation, while other studies show some benefits. Given this, there is still significant debate in the literature regarding the use of vertebra/kyphoplasty. As the technology of intervention methods continues to advance and evolve, an increase in its potential benefits over non-operative management is expected, and further investigations are needed [47].
Optimizing fixation
Geriatric patients are at high risk for instrumentation failure with an increased rate of collapsed and delayed union after spinal fusion [48]. This is compounded by osteoporotic patients who may require deformity correction as these procedures inherently carry greater associated complication risk profiles. These complications include fracture, screw pullout, pseudoarthrosis, and adjacent segment degeneration [49–51]. Multiple surgical adjuvants have been described to help limit these complication risks.
The pedicle screws are the weakest point of a construct when surgically treating patients with osteoporosis, as they are the location of the bone-implant interface. Complications involving pedicle screws typically consist of screw pullout or fracture with a subsequent lack of fixation. Multiple techniques have been evaluated to combat complications at the bone-screw junction, including the use of longer screws, bicortical purchase, larger diameter screws, hydroxyapatite-coated screws, sub laminar hooks and wires, cross-linking, and the use of expandable screws [52].
When treating patients requiring long-segment instrumentation for instability or deformity, multiple points of fixation should be considered above and below the apex of the curvature due to the increased risk of graft subsidence and screw pullout. Hosogane et al. demonstrated that in patients with combined anterior and posterior instrumentation, the mean number of fixed vertebrae in patients with cage subsidence was 5.4 compared to that of 7.4 in patients without subsidence, thus demonstrating that a higher number of fixation points effects and decreases load at each segment [53]. Surgical instrumentation stopping at junctional levels and excessive correction of coronal and sagittal deformity should be avoided as it can lead to the overload of the construct and subsequent hardware and surgical failure. If possible, iliac and sacral instrumentation should be considered to help with construct strength [54]. Similarly, a combined approach including anterior and posterior fixation should be considered when able. Iwasaki et al. demonstrated that patients undergoing anterior-only fixation required more frequent reinforcement from a posterior approach in patients with bone mineral density below 0.60 g/cm2, and in patients with more than three levels of fixation [55].
Other surgical adjuncts include grafting products that facilitate bony fusion by providing scaffolding, osteogenic cells, or osteoinductive molecular signals that promote fusion. Options include local autograft from the surgical field, iliac crest bone grafting, Cellular bone allograft, demineralized bone matrix, synthetic bone graft, autogenous growth factors, and bone marrow aspirates [56, 57]. Bone growth stimulators should also be considered, as they use electromagnetic fields to encourage bone cell proliferation and osteogenic differentiation. These devices benefit the geriatric osteoporotic population as a noninvasive way to promote bony healing and fusion with minimal side effects [58, 59].
Case illustration highlighting surgical considerations
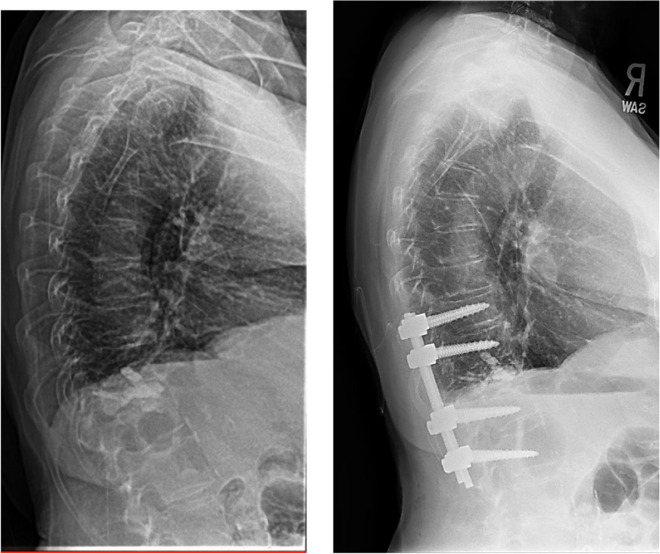
In this case, a 75-year-old female with a history significant for osteoporosis, diabetes, multiple myeloma, and breast cancer presented initially with a T12 compression fracture. Given her age and symptoms, the patient elected for kyphoplasty at the pathologic level. The patient subsequently developed a compression fracture at the level below her cement augmentation at the L1 vertebrae. This fracture caused the patient to develop a significant kyphotic deformity at the thoracolumbar junction with a sagittal vertical axis of 18 cm (Fig. 4). The patient underwent a T10–L3 posterior spinal fusion at an outside hospital for correction of her deformity. This construct was complicated by postoperative screw pullout due to her poor bone-implant interface and severe deformity (Fig. 5).
Fig. 4.
A 75-year-old female with a L1 compression fracture status post cement augmentation who continued to develop progressive thoracolumbar kyphosis and sagittal imbalance
Fig. 5.
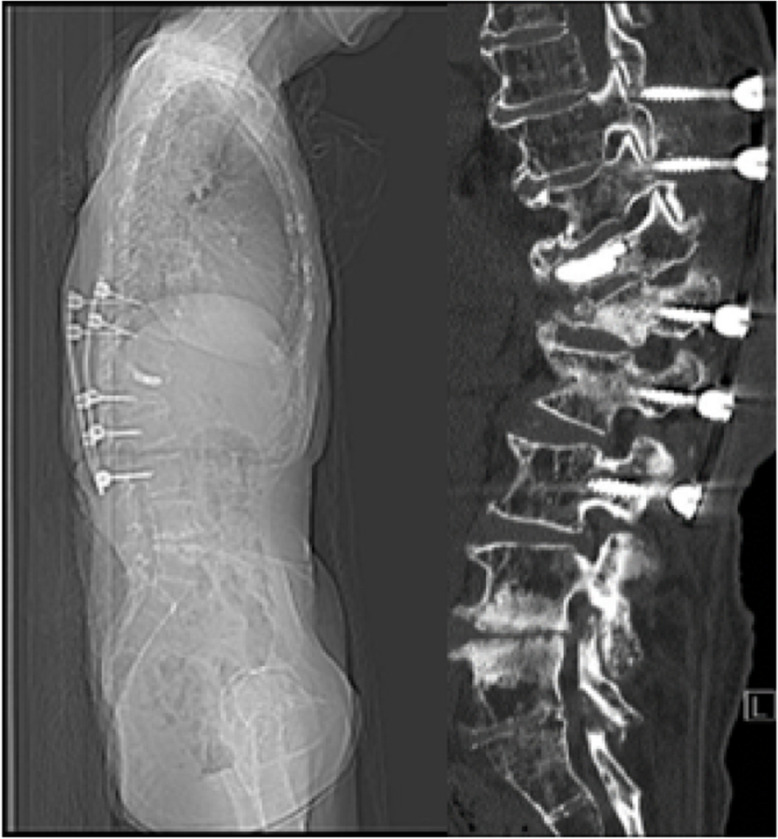
Lateral scout and CT scan demonstrating screw pullout and failure of the short segment instrumented construct due to poor bone mineral density at the screw interface
Positive sagittal balance biomechanically places excessive strain on posterior instrumentation and fusion mass, which contributes to instrumentation failure and pseudarthrosis, especially in the osteoporotic spine. As discussed in this review, long-segment instrumentation with large diameter and length pedicle screws is recommended to correct her sagittal balance and decrease the rate of instrumentation-related complications. Subsequently, the patient underwent revision of her prior instrumentation with extension from thoracic 2 through sacral 1 and pelvis with a 4-rod construct alongside a T12–L1 vertebral column resection with cage placement for anterior column support. The procedure corrected her sagittal imbalance the patient reported improved symptoms with stable alignment at 2 years (Fig. 6). This case highlights the utility of long-segment instrumentation and pelvic fixation in treating geriatric patients with osteoporosis while also addressing their spinal deformity. In contrast to the case in Fig. 6, targeting the lower magnitude kyphotic deformity at the thoracolumbar junction despite vertebral augmentation, careful level selection based on the magnitude of HU on QCT can allow for a shorter construct providing stable alignment and symptom relief (Fig. 7).
Fig. 6.
Thoracic 2 through sacral 1 and pelvic fixation, with thoracic 12 through lumbar 1 vertebral column resection. Allowing for correction of the kyphotic deformity after instrumentation failure of a thoracic 10 through lumbar 3 construct
Fig. 7.
Lateral X-rays demonstrating a short segment construct providing adequate fixation. Planning based on quantitative computed tomography and associated thoracolumbar curvature
Conclusion
Osteoporosis in the geriatric population is a complex issue that contributes to the vulnerability of our geriatric population. Osteoporosis (defined as a T-score of less than 2.5 and/or fragility-associated fracture) decreases patient health-related quality of life and increases overall morbidity. Surgical management of the spine should include preoperative screening, assessment, and treatment of a patient’s bone health. While there is no uniform agreed guideline, evolving clinical evidence has identified risk factors that may warrant perioperative management with FDA-approved medical therapies as increasingly supported by societal guidelines. Spinal reconstruction should not be withheld solely based on low bone density; rather, practices are encouraged to collaborate with specialty care directed at promoting bone health. Surgical techniques to circumvent challenges with instrumentation of poor bone mineral density have included augmentation of pedicle screw fixation, including segmental bicortical screw fixation techniques, cement augmentation with fenestrated screws, or the use of anterior column support. Judicious selection of treatment modalities and subsequent perioperative optimization is paramount to minimize surgical complications and ultimately improve outcomes.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nguyen ND, Ahlborg HG, Center JR, Eisman JA, Nguyen TV. Residual lifetime risk of fractures in women and men. J Bone Miner Res. 2007;22(6):781–8. 10.1359/jbmr.070315. 10.1359/jbmr.070315 [DOI] [PubMed] [Google Scholar]
- 2.Randell AG, Nguyen TV, Bhalerao N, Silverman SL, Sambrook PN, Eisman JA. Deterioration in quality of life following hip fracture: a prospective study. Osteoporos Int. 2000;11(5):460–6. 10.1007/s001980070115. 10.1007/s001980070115 [DOI] [PubMed] [Google Scholar]
- 3.Block JE, Stubbs H. Hip fracture-associated mortality reconsidered. Calcif Tissue Int. 1997;61(1):84–84. 10.1007/s002239900300. 10.1007/s002239900300 [DOI] [PubMed] [Google Scholar]
- 4.Kanis JA, Oden A, Johansson H, Borgström F, Ström O, McCloskey E. FRAX and its applications to clinical practice. Bone. 2009;44(5):734–43. 10.1016/j.bone.2009.01.373. 10.1016/j.bone.2009.01.373 [DOI] [PubMed] [Google Scholar]
- 5.DeWald CJ, Stanley T. Instrumentation-related complications of multilevel fusions for adult spinal deformity patients over age 65: surgical considerations and treatment options in patients with poor bone quality. Spine (Phila Pa 1976). 2006;31(19 Suppl):S144-51. 10.1097/01.brs.0000236893.65878.39. 10.1097/01.brs.0000236893.65878.39 [DOI] [PubMed] [Google Scholar]
- 6.Mohanty S, Sardar ZM, Hassan FM, Lombardi JM, Lehman RA, Lenke LG. Impact of teriparatide on complications and patient-reported outcomes of patients undergoing long spinal fusion according to bone density. J Bone Joint Surg. 2024;106(3):206–17. 10.2106/JBJS.23.00272. 10.2106/JBJS.23.00272 [DOI] [PubMed] [Google Scholar]
- 7.Varshneya K, Bhattacharjya A, Jokhai RT, et al. The impact of osteoporosis on adult deformity surgery outcomes in Medicare patients. Eur Spine J. 2022;31(1):88–94. 10.1007/s00586-021-06985-z. 10.1007/s00586-021-06985-z [DOI] [PubMed] [Google Scholar]
- 8.Shahrestani S, Chen XT, Ballatori AM, et al. Complication trends and costs of surgical management in 11,086 osteoporotic patients receiving lumbar fusion. Spine (Phila Pa 1976). 2021;46(21):1478–84. 10.1097/BRS.0000000000004051. 10.1097/BRS.0000000000004051 [DOI] [PubMed] [Google Scholar]
- 9.Beckerman D, Esparza M, Lee SI, et al. Cost analysis of single-level lumbar fusions. Global Spine J. 2020;10(1):39–46. 10.1177/2192568219853251. 10.1177/2192568219853251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain N, Labaran L, Phillips FM, et al. Prevalence of osteoporosis treatment and its effect on post-operative complications, revision surgery and costs after multi-level spinal fusion. Global Spine J. 2022;12(6):1119–24. 10.1177/2192568220976560. 10.1177/2192568220976560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown JP, Josse RG, Scientific Advisory Council of the Osteoporosis Society of Canada. 2002 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. CMAJ. 2002;167(10 suppl):S1–34. [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson PA, Morgan SL, Krueger D, et al. Use of bone health evaluation in orthopedic surgery: 2019 ISCD official position. J Clin Densitom. 2019;22(4):517–43. 10.1016/j.jocd.2019.07.013. 10.1016/j.jocd.2019.07.013 [DOI] [PubMed] [Google Scholar]
- 13.Sardar ZM, Coury JR, Cerpa M, et al. Best practice guidelines for assessment and management of osteoporosis in adult patients undergoing elective spinal reconstruction. Spine (Phila Pa 1976). 2022;47(2):128–35. 10.1097/BRS.0000000000004268. 10.1097/BRS.0000000000004268 [DOI] [PubMed] [Google Scholar]
- 14.Blake GM, Fogelman I. Technical principles of dual energy x-ray absorptiometry. Semin Nucl Med. 1997;27(3):210–28. 10.1016/s0001-2998(97)80025-6. 10.1016/s0001-2998(97)80025-6 [DOI] [PubMed] [Google Scholar]
- 15.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312(7041):1254–9. 10.1136/bmj.312.7041.1254. 10.1136/bmj.312.7041.1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanis JA, Johnell O, Oden A, Jonsson B, De Laet C, Dawson A. Risk of hip fracture according to the World Health Organization criteria for osteopenia and osteoporosis. Bone. 2000;27(5):585–90. 10.1016/s8756-3282(00)00381-1. 10.1016/s8756-3282(00)00381-1 [DOI] [PubMed] [Google Scholar]
- 17.Looker AC, Orwoll ES, Johnston CC, et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997;12(11):1761–8. 10.1359/jbmr.1997.12.11.1761. 10.1359/jbmr.1997.12.11.1761 [DOI] [PubMed] [Google Scholar]
- 18.McKiernan FE, Berg RL, Linneman JG. The utility of BMD Z-score diagnostic thresholds for secondary causes of osteoporosis. Osteoporos Int. 2011;22(4):1069–77. 10.1007/s00198-010-1307-1. 10.1007/s00198-010-1307-1 [DOI] [PubMed] [Google Scholar]
- 19.Yu JS, Krishna NG, Fox MG, et al. ACR Appropriateness Criteria® osteoporosis and bone mineral density: 2022 update. J Am Coll Radiol. 2022;19(11):S417–32. 10.1016/j.jacr.2022.09.007. 10.1016/j.jacr.2022.09.007 [DOI] [PubMed] [Google Scholar]
- 20.Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG. Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg. 2011;93(11):1057–63. 10.2106/JBJS.J.00160. 10.2106/JBJS.J.00160 [DOI] [PubMed] [Google Scholar]
- 21.Ahern DP, McDonnell JM, Riffault M, et al. A meta-analysis of the diagnostic accuracy of Hounsfield units on computed topography relative to dual-energy X-ray absorptiometry for the diagnosis of osteoporosis in the spine surgery population. Spine J. 2021;21(10):1738–49. 10.1016/j.spinee.2021.03.008. 10.1016/j.spinee.2021.03.008 [DOI] [PubMed] [Google Scholar]
- 22.Patel SP, et al. Normative vertebral hounsfield unit values and correlation with bone mineral density. J Clin Exp Orthop. 2016;2:14. 10.4172/2471-8416.100014. 10.4172/2471-8416.100014 [DOI] [Google Scholar]
- 23.Gregson CL, Hardcastle SA, Cooper C, Tobias JH. Friend or foe: high bone mineral density on routine bone density scanning, a review of causes and management. Rheumatology (United Kingdom). 2013;52(6):968–85. 10.1093/rheumatology/ket007. 10.1093/rheumatology/ket007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wähnert D, Frank A, Ueberberg J, Heilmann LF, Sauzet O, Raschke MJ, Gehweiler D. Development and first biomechanical validation of a score to predict bone implant interface stability based on clinical qCT scans. Sci Rep. 2021;11(1):3273. 10.1038/s41598-021-82788-y. 10.1038/s41598-021-82788-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boonen S, Lips P, Bouillon R, Bischoff-Ferrari HA, Vanderschueren D, Haentjens P. Need for additional calcium to reduce the risk of hip fracture with vitamin D supplementation: evidence from a comparative metaanalysis of randomized controlled trials. J Clin Endocrinol Metab. 2007;92(4):1415–23. 10.1210/jc.2006-1404. 10.1210/jc.2006-1404 [DOI] [PubMed] [Google Scholar]
- 26.Mikosch P. Alcohol and bone. Wien Med Wochenschr. 2014;164(1–2):15–24. 10.1007/s10354-013-0258-5. 10.1007/s10354-013-0258-5 [DOI] [PubMed] [Google Scholar]
- 27.Krall EA, Dawson-Hughes B. Smoking increases bone loss and decreases intestinal calcium absorption. J Bone Miner Res. 1999;14(2):215–20. 10.1359/jbmr.1999.14.2.215. 10.1359/jbmr.1999.14.2.215 [DOI] [PubMed] [Google Scholar]
- 28.Pouresmaeili F, Kamalidehghan B, Kamarehei M, Goh YM. A comprehensive overview on osteoporosis and its risk factors. Ther Clin Risk Manag. 2018;14:2029–49. 10.2147/TCRM.S138000. 10.2147/TCRM.S138000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehman RA, Kang DG, Wagner SC. Management of osteoporosis in spine surgery. J Am Acad Orthop Surg. 2015;23(4):253–63. 10.5435/JAAOS-D-14-00042. 10.5435/JAAOS-D-14-00042 [DOI] [PubMed] [Google Scholar]
- 30.Miyauchi A, Matsumoto T, Sugimoto T, Tsujimoto M, Warner MR, Nakamura T. Effects of teriparatide on bone mineral density and bone turnover markers in Japanese subjects with osteoporosis at high risk of fracture in a 24-month clinical study: 12-month, randomized, placebo-controlled, double-blind and 12-month open-label phases. Bone. 2010;47(3):493–502. 10.1016/j.bone.2010.05.022. 10.1016/j.bone.2010.05.022 [DOI] [PubMed] [Google Scholar]
- 31.Dimar J, Bisson EF, Dhall S, et al. Congress of neurological surgeons systematic review and evidence-based guidelines for perioperative spine: preoperative osteoporosis assessment. Neurosurgery. 2021;89:S19–25. 10.1093/neuros/nyab317. 10.1093/neuros/nyab317 [DOI] [PubMed] [Google Scholar]
- 32.LeBoff MS, Greenspan SL, Insogna KL, et al. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2022;33(10):2049–102. 10.1007/s00198-021-05900-y. 10.1007/s00198-021-05900-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohtori S, Inoue G, Orita S, Yamauchi K, Eguchi Y, Ochiai N, Kishida S, Kuniyoshi K, Aoki Y, Nakamura J, Ishikawa T, Miyagi M, Kamoda H, Suzuki M, Kubota G, Sakuma Y, Oikawa Y, Inage K, Sainoh T, Takaso M, Ozawa T, Takahashi K, Toyone T. Teriparatide accelerates lumbar posterolateral fusion in women with postmenopausal osteoporosis: prospective study. Spine (Phila Pa 1976). 2012;37(23):E1464-8. 10.1097/BRS.0b013e31826ca2a8. 10.1097/BRS.0b013e31826ca2a8 [DOI] [PubMed] [Google Scholar]
- 34.Ohtori S, Inoue G, Orita S, Yamauchi K, Eguchi Y, Ochiai N, Kishida S, Kuniyoshi K, Aoki Y, Nakamura J, Ishikawa T, Miyagi M, Kamoda H, Suzuki M, Kubota G, Sakuma Y, Oikawa Y, Inage K, Sainoh T, Takaso M, Toyone T, Takahashi K. Comparison of teriparatide and bisphosphonate treatment to reduce pedicle screw loosening after lumbar spinal fusion surgery in postmenopausal women with osteoporosis from a bone quality perspective. Spine (Phila Pa 1976). 2013;38(8):E487–92. 10.1097/BRS.0b013e31828826dd. 10.1097/BRS.0b013e31828826dd [DOI] [PubMed] [Google Scholar]
- 35.Nagahama K, Kanayama M, Togawa D, Hashimoto T, Minami A. Does alendronate disturb the healing process of posterior lumbar interbody fusion? A prospective randomized trial. J Neurosurg Spine. 2011;14(4):500–7. 10.3171/2010.11.SPINE10245. 10.3171/2010.11.SPINE10245 [DOI] [PubMed] [Google Scholar]
- 36.Ebata S, Takahashi J, Hasegawa T, et al. Role of weekly teriparatide administration in osseous union enhancement within six months after posterior or transforaminal lumbar interbody fusion for osteoporosis-associated lumbar degenerative disorders: a multicenter, prospective randomized study. J Bone Joint Surg Am. 2017;99(5):365–72. 10.2106/JBJS.16.00230. 10.2106/JBJS.16.00230 [DOI] [PubMed] [Google Scholar]
- 37.Murphy DR, Smolen LJ, Klein TM, Klein RW. The cost effectiveness of teriparatide as a first-line treatment for glucocorticoid-induced and postmenopausal osteoporosis patients in Sweden. BMC Musculoskelet Disord. 2012;13:213. 10.1186/1471-2474-13-213. 10.1186/1471-2474-13-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim SM, Rhee W, Ha S, Lim JH, Jang IT. Influence of alendronate and endplate degeneration to single level posterior lumbar spinal interbody fusion. Korean J Spine. 2014;11(4):221. 10.14245/kjs.2014.11.4.221. 10.14245/kjs.2014.11.4.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Yan L, Cai S, Wang P, Zhuang H, Yu H. The prevalence and under-diagnosis of vertebral fractures on chest radiograph. BMC Musculoskelet Disord. 2018;19(1):235. 10.1186/s12891-018-2171-y. 10.1186/s12891-018-2171-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watts NB, Harris ST, Genant HK. Treatment of painful osteoporotic vertebral fractures with percutaneous vertebroplasty or kyphoplasty. Osteoporos Int. 2001;12(6):429–37. 10.1007/s001980170086. 10.1007/s001980170086 [DOI] [PubMed] [Google Scholar]
- 41.Kallmes DF, Jensen ME. Percutaneous vertebroplasty. Radiology. 2003;229(1):27–36. 10.1148/radiol.2291020222. 10.1148/radiol.2291020222 [DOI] [PubMed] [Google Scholar]
- 42.Hirsch JA, Chandra RV, Carter NS, Beall D, Frohbergh M, Ong K. Number needed to treat with vertebral augmentation to save a life. Am J Neuroradiol. 2020;41(1):178–82. 10.3174/ajnr.A6367. 10.3174/ajnr.A6367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Comstock BA, Sitlani CM, Jarvik JG, Heagerty PJ, Turner JA, Kallmes DF. Investigational Vertebroplasty Safety and Efficacy Trial (INVEST): patient-reported outcomes through 1 year. Radiology. 2013;269(1):224–31. 10.1148/radiol.13120821. 10.1148/radiol.13120821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt C, Graves S, Staples MP, Murphy B. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361(6):557–68. 10.1056/NEJMoa0900429. 10.1056/NEJMoa0900429 [DOI] [PubMed] [Google Scholar]
- 45.Buchbinder R, Johnston RV, Rischin KJ, et al. Percutaneous vertebroplasty for osteoporotic vertebral compression fracture. Cochrane Database Syst Rev. 2018. 10.1002/14651858.CD006349.pub3. 10.1002/14651858.CD006349.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans AJ, Kip KE, Brinjikji W, et al. Randomized controlled trial of vertebroplasty versus kyphoplasty in the treatment of vertebral compression fractures. J Neurointerv Surg. 2016;8(7):756–63. 10.1136/neurintsurg-2015-011811. 10.1136/neurintsurg-2015-011811 [DOI] [PubMed] [Google Scholar]
- 47.Imamudeen N, Basheer A, Iqbal AM, Manjila N, Haroon NN, Manjila S. Management of osteoporosis and spinal fractures: contemporary guidelines and evolving paradigms. Clin Med Res. 2022;20(2):95–106. 10.3121/cmr.2021.1612. 10.3121/cmr.2021.1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okuda S, Oda T, Miyauchi A, Haku T, Yamamoto T, Iwasaki M. Surgical outcomes of posterior lumbar interbody fusion in elderly patients. J Bone Joint Surg Am. 2006;88(12):2714–20. 10.2106/JBJS.F.00186. 10.2106/JBJS.F.00186 [DOI] [PubMed] [Google Scholar]
- 49.Yagi M, Fujita N, Tsuji O, et al. Low bone-mineral density is a significant risk for proximal junctional failure after surgical correction of adult spinal deformity. Spine (Phila Pa 1976). 2018;43(7):485–91. 10.1097/BRS.0000000000002355. 10.1097/BRS.0000000000002355 [DOI] [PubMed] [Google Scholar]
- 50.Sakai Y, Takenaka S, Matsuo Y, et al. Hounsfield unit of screw trajectory as a predictor of pedicle screw loosening after single level lumbar interbody fusion. J Orthop Sci. 2018;23(5):734–8. 10.1016/j.jos.2018.04.006. 10.1016/j.jos.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 51.Cho JH, Hwang CJ, Kim H, Joo YS, Lee DH, Lee CS. Effect of osteoporosis on the clinical and radiological outcomes following one-level posterior lumbar interbody fusion. J Orthop Sci. 2018;23(6):870–7. 10.1016/j.jos.2018.06.009. 10.1016/j.jos.2018.06.009 [DOI] [PubMed] [Google Scholar]
- 52.Dodwad SNM, Khan SN. Surgical stabilization of the spine in the osteoporotic patient. Orthop Clin North Am. 2013;44(2):243–9. 10.1016/j.ocl.2013.01.008. 10.1016/j.ocl.2013.01.008 [DOI] [PubMed] [Google Scholar]
- 53.Takeuchi T, Yamagishi K, Konishi K, Sano H, Takahashi M, Ichimura S, Kono H, Hasegawa M, Hosogane N. Radiological evaluation of combined anteroposterior fusion with vertebral body replacement using a minimally invasive lateral approach for osteoporotic vertebral fractures: Verification of optimal surgical procedure. J Clin Med. 2022;11(3):629. 10.3390/jcm11030629. 10.3390/jcm11030629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu SS. Internal fixation in the osteoporotic spine. Spine (Phila Pa 1976). 1997;22(24 Suppl):43S-48S. 10.1097/00007632-199712151-00008. 10.1097/00007632-199712151-00008 [DOI] [PubMed] [Google Scholar]
- 55.Sudo H, Ito M, Kaneda K, et al. Anterior decompression and strut graft versus posterior decompression and pedicle screw fixation with vertebroplasty for osteoporotic thoracolumbar vertebral collapse with neurologic deficits. Spine J. 2013;13(12):1726–32. 10.1016/j.spinee.2013.05.041. 10.1016/j.spinee.2013.05.041 [DOI] [PubMed] [Google Scholar]
- 56.Wind J, Park D, Lansford T, et al. Twelve-month results from a prospective clinical study evaluating the efficacy and safety of cellular bone allograft in subjects undergoing lumbar spinal fusion. Neurol Int. 2022;14(4):875–83. 10.3390/neurolint14040070. 10.3390/neurolint14040070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park JJ, Hershman SH, Kim YH. Updates in the use of bone grafts in the lumbar spine. Bull Hosp Jt Dis. 2013;71(1):39–48. [PubMed] [Google Scholar]
- 58.Zhang W, Luo Y, Xu J, Guo C, Shi J, Li L, Sun X, Kong Q. The possible role of electrical stimulation in osteoporosis: A narrative review. Medicina (Kaunas). 2023;59(1):121. 10.3390/medicina59010121. 10.3390/medicina59010121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Androjna C, Fort B, Zborowski M, Midura RJ. Pulsed electromagnetic field treatment enhances healing callus biomechanical properties in an animal model of osteoporotic fracture. Bioelectromagnetics. 2014;35(6):396–405. 10.1002/bem.21855. 10.1002/bem.21855 [DOI] [PubMed] [Google Scholar]