Abstract
Epilepsy is one of the most common neurological disorders in children. Diagnosing epilepsy in children can be very challenging, especially as it often coexists with neurodevelopmental conditions like autism and ADHD. Functional brain networks obtained from neuroimaging and electrophysiological data in wakefulness and sleep have been shown to contain signatures of neurological disorders, and can potentially support the diagnosis and management of co-occurring neurodevelopmental conditions. In this work, we use electroencephalography (EEG) recordings from children, in restful wakefulness and sleep, to extract functional connectivity networks in different frequency bands. We explore the relationship of these networks with epilepsy diagnosis and with measures of neurodevelopmental traits, obtained from questionnaires used as screening tools for autism and ADHD. We explore differences in network markers between children with and without epilepsy in wake and sleep, and quantify the correlation between such markers and measures of neurodevelopmental traits. Our findings highlight the importance of considering the interplay between epilepsy and neurodevelopmental traits when exploring network markers of epilepsy.
Introduction
Epilepsy is estimated to impact nearly 10.5 million children worldwide [1]. According to the World Health Organization, approximately 80% of people with epilepsy live in low- and middle-income countries (LMICs), where epilepsy presents an increased risk of premature mortality, and where the highest prevalence peaks in children [2]. In addition to the personal, social, and economic impact of epilepsy on children and their families, seizures have been shown to be detrimental to brain development [3], potentially leading to cognitive dysfunction. The condition is often associated with lifelong disabilities, poor quality of life and even death [4, 5]. Therefore, early and accurate diagnosis of epilepsy is paramount. Early management can significantly reduce the adverse effects of seizures, improving quality of life, reducing the risks of harm and death, and improving the social and economic status of the families affected by epilepsy, especially in LMICs. Unfortunately, epilepsy diagnosis can be very challenging. The rate of epilepsy misdiagnosis is estimated to be near 20% generally [6]. Due to a wide range of non-epileptic paroxysmal disorders affecting children [7], as well as the practical hurdles in implementing EEG in children (especially those with challenging behaviour) and the difficulties associated to interpretation of paediatric EEG [8], misdiagnosis in children is believed to be even greater than for adults [9].
Diagnosis and management of neurological and neurodevelopmental conditions are made more challenging when they coexist. This is frequently the case with epilepsy, where its prevalence in children with Autism Spectrum Disorder and ADHD is 20% and 15%, respectively, which is significantly higher than in neurotypical children (~1%) [10]. The complex relationship between epilepsy and co-occurring neurodevelopmental conditions remains an important open question, the resolution of which could improve clinical outcomes and provide optimal and individualised care.
Epilepsy is increasingly conceptualised as a condition of aberrant brain networks [11, 12]. Scalp electroencephalography (EEG) is one of the most widespread methods used to quantify these networks. Functional networks obtained from scalp EEG have shown fundamental differences between people with epilepsy and healthy controls, for both adults [13–15] and children [16, 17]. Neurodevelopmental conditions, such as autism and ADHD, have also been investigated using the framework of network science [18], although these methods are less well stablished in this context. Moreover, very few studies have investigated the joint effect of epilepsy and co-occurring neurodevelopmental conditions on functional brain networks [19]. This is necessary to understand network signatures that are specific either to epilepsy, or to neurodevelopmental conditions, rather than being sensitive to their co-occurrences. Network markers of epilepsy may be influenced by the presence of neurodevelopmental traits, potentially leading to erroneous interpretations of the relationship between these markers and seizure propensity.
Another important factor when exploring network markers of co-occurring neurological and neurodevelopmental conditions is the influence of sleep. A growing number of studies support the association between poor sleep and both epilepsy and neurodevelopmental conditions [20–25]. This relationship tends to be bidirectional, where sleep disruption can increase seizure propensity and presentation of neurodevelopmental conditions, which can in turn result in poor sleep [26]. At the same time, graph metric analysis has shown that several aspects of sleep, such as the wake-sleep transition itself as well as sleep deprivation, are associated with connectivity changes in functional brain networks [27–32]. Markers of epilepsy might also be influenced by stages of awareness (wake and sleep), given the known changes to the propensity for epileptic discharges with sleep stage [33, 34].
In this work we explore the combined effects of epilepsy and neurodevelopmental traits on functional connectivity networks obtained from EEG recordings from children in waking restfulness and sleep. We identify differences in functional connectivity between subjects with and without epilepsy, which are consistent across frequency bands. We also show that such differences are less pronounced during sleep. Finally, we quantify the correlation between neurodevelopmental traits and network measures, identifying similar effects as seen for epilepsy. These results highlight the importance of considering the co-occurrence of neurodevelopmental traits when a graph metric approach is implemented in this context.
Methods
Data acquisition and participants
The data used in this study were acquired at Birmingham Children’s Hospital and Worcestershire Royal Hospital. Written informed consent and assent were obtained from parents and children and the study received NHS ethical approval from the Northwest—Preston Research Ethics Committee (REC reference 19/NW/0337). Methods were performed in accordance with relevant guidelines and regulations. EEG recordings were collected in “nap sleep EEG” sessions (i.e., recordings taken during a short period where the child falls asleep) from children suspected of having epilepsy, as part of the diagnostic process. Sixty-two recordings, collected between September 2019 and December 2021, were retrieved. EEG data were acquired from 19 electrodes positioned according to the 10–20 system and sampled at 512Hz. In some participants, melatonin or mild sleep deprivation were used to encourage sleep, according to clinical protocols. Families also completed the Social Communication Questionnaire (SCQ) [35] and the Conners’ 3AI Questionnaires [36], which are standard tools to describe autism and ADHD characteristics, respectively. All questionnaires were evaluated by experienced psychologists (AW and CR) to provide continuous indices associated with autism/ADHD traits. Raw scales of the SCQ and Conners’ questionnaires can have values in the ranges of [0,40] and [0,20], respectively.
In order to define a quantity that represented overall neurodevelopmental traits (NT), we combined SCQ and Conners’ raw scores as:
With this definition, NT ranges in [0,1], where 0 means a null score in both tests while 1 means maximum scores in both tests. This index allows us to quantify the overall level of neurodevelopmental traits in a single dimension [37]. It is important to clarify here that NT should not be interpreted as a detailed quantification of autism and ADHD diagnosis. These conditions have complex diagnostic pathways, which go beyond the interpretation of these questionnaires. However, despite its limitations, these questionnaires (and therefore NT) constitute an accessible and informative marker for the characteristics associated with these conditions.
EEG analysis
EEG annotation was performed by two experienced electrophysiologists (Neuronostics Ltd). For each participant, electrophysiologists were provided with the complete EEG recording from the nap sleep session (recording duration between 00:28:00 and 03:48:49 [hh:mm:ss]) and asked to identify the cleanest and most “uneventful” 30-second long EEG segments (avoiding major artifacts or clear epileptiform activity) in wakefulness, and sleep stages N1—N3, when available. Sleep stages were defined according to AASM guidelines [38]. Very few epochs were identified in sleep stage N3, so those were not considered in this analysis. Electrophysiologists were blind to epilepsy diagnosis and to any metadata associated with neurodevelopmental traits.
Final cohort
From the original 62 participants, 34 had at least one EEG epoch identified and complete metadata available (age, sex, epilepsy diagnosis, SCQ score and Conners’ score) and were included in this study. These participants were aged between 4 and 15 years old (median 9 y) and included 13 females and 21 males. 24 participants were diagnosed with epilepsy (11 focal, 7 generalised, 4 Rolandic, and 2 Encephalopathy) while 10 were not. These groups will be referred to as “epilepsy” and “controls”, respectively. Raw scores for the SCQ ranged between 0 and 27 (median 9), while Conners’ raw score ranged between 0 and 20 (median 9.5). Tables with detailed metadata are provided in the S1 File.
Functional networks
We derived weighted undirected functional networks from each EEG epoch using the phase locking factor (PLF). To do this, we first downsampled the data to 256 Hz and band-pass filtered between the desired frequencies. A 4th order Butterworth filter was used with forward and backward filtering to minimise phase distortions. Functional networks were calculated in five frequency bands: delta (1Hz-4Hz), theta (4Hz-7Hz), alpha (7Hz-13Hz) and beta (13Hz-30Hz), as well as low alpha (6Hz-9Hz). Low alpha was used as networks calculated in this frequency band in adults have shown different properties in healthy individuals and those with generalized epilepsy [13].
For a pair of signals k and l, the PLFkl is given by , where T is the number of equally-spaced time samples in an epoch and θk is the phase of the Hilbert transform of signal k. We also calculated the time-averaged lag, . Only nonzero time lags (|τkl|>0) were considered to avoid spurious connections due to volume conduction. We then computed 99 surrogate epochs from each of the EEG signals using a univariate iterated amplitude adjusted Fourier transform (iAAFT). Functional networks were then calculated for the EEG epochs and for the surrogates. For each epoch, we rejected connections that did not exceed a 95% significance level compared to the same connection weights computed from the surrogates calculated for that epoch. This method results in a weighted, undirected network akl, which we used to calculate graph metrics. The PLF framework has been extensively used to explore biomarkers of epilepsy from EEG data in different contexts [13, 15, 39–42]. Details about the methods used to calculate the network mean degree (MD), degree standard deviation (DStd), average local clustering coefficient (ALCC) and global efficiency (GE) can be found in the S1 File.
Statistical analysis
We explored the weighted mean degree of different classes (controls and epilepsy types) using boxplots (see Fig 1), where the median (red line), 25th - 75th percentiles (blue box), non-outlier extremes (black dashed lines) and outliers (red crosses) of the distributions are presented. Effect size was quantified using the rank-biserial correlation [43] (|r|∈[0,1], where 0 means no rank correlation and 1 means perfect separation between groups), and significance was calculated using the Wilcoxon rank-sum and Kruskal-Wallis tests. To further quantify the differences between classes, receiver operating characteristic (ROC) curves were calculated for all frequency bands. The area under the ROC curve (AUC) was calculated and uncertainty (error bars) was quantified using a leave-one-out approach. To quantify the relationship between mean degree and neurodevelopmental traits (continuous index), we used the nonparametric Spearman rank correlation measure.
Fig 1. Summary statistics of the functional connectivity networks’ mean degree (corrected for sex), calculated for different frequency bands and using wake epochs.
The first two boxes in each plot (“Cont” and “Epi”) indicate the comparison between subjects without and with epilepsy, respectively. Subsequent boxes show the breakdown of different epilepsy types (Ge: generalised, Ro: Rolandic, Fo: focal, and EE: encephalopathy). The rank-biserial correlation and p-value (two-tailed Wilcoxon rank sum test, uncorrected for multiple comparisons) for the difference between “Cont” and “Epi” are also shown.
When comparing controls and epilepsy groups, the age distributions were not significantly different (p-value: 0.79), however there was a clear sex imbalance (controls: 60% female, epilepsy: 29% female), so we corrected the marker values for sex in all comparisons presented below by subtracting the mean over the respective sex. Regarding the correction for confounding factors for the NT index, no significant differences were observed between epilepsy and controls, or between males and females. Also, no significant correlation was observed between the NT index and age. Nevertheless, to avoid cumulative effects of potential confounding factors, when considering relationships between NT and mean degree, we corrected this network marker for age, sex, and epilepsy diagnosis using linear regression.
Results
Mean degree is smaller in epilepsy compared to controls
The mean node degree calculated using functional connectivity networks obtained from EEG epochs during wakefulness is presented in Fig 1. Each plot describes the summary statistics of the mean degree distribution for the different frequency bands of interest. The first two boxes in each plot indicate the mean degree distribution for control and epilepsy subjects, respectively. The subsequent boxes, in faded colours, indicate results for the sub-groups of epilepsy types (Ge: generalised, Ro: Rolandic, Fo: focal, and EE: encephalopathy). For all frequency bands, the median mean degree calculated for subjects with epilepsy was lower than for controls. This result was not only consistent across frequency bands, but also held when controls were compared with most epilepsy types individually. Rolandic epilepsy presented mean degree values similar to controls in the low-alpha and alpha bands. However, it is important to note that this group consisted of only 4 subjects, so any comparison for this group in isolation has to be considered carefully. The rank-biserial correlations presented in each plot indicate that the difference between the mean degree for controls and subjects with epilepsy was clearer in the beta band. We also quantified the differences between controls and epilepsy in degree standard deviation (DStd), average weighted clustering coefficient (AWCC), and global efficiency (GE). We observed trends that were consistent over all frequency bands (elevated DStd and GE for controls and elevated AWCC for children with epilepsy). However, the effect sizes were small (see S1 Fig in S1 File) and these metrics were not considered further.
To further quantify the differences between the mean degree for controls and epilepsy, and to estimate its classification power as a marker, we calculated the receiver operating characteristic (ROC) curve, presented in Fig 2. The area under the ROC curve (AUC) varied between 0.66 and 0.84, depending on the frequency band used to calculate the networks, reflecting the consistent difference observed in the mean degree for controls and epilepsy in Fig 1.
Fig 2. Receiver operating characteristic (ROC) curve calculated using the mean degree to classify subjects without and with epilepsy (wake epochs).
Differences in mean degree are smaller in sleep compared to wakefulness
As sleep has been shown to be an important factor impacting seizure susceptibility in different types of epilepsy [20, 26], one important question is how it impacts functional brain networks of children with epilepsy. To answer this question, we calculated differences in mean degree between controls and children with epilepsy for epochs obtained from sleep stages N1 and N2. Following the calculation of the area under the ROC curve for epochs obtained from wakefulness, presented in Fig 2, we used the AUC to quantify the differences between mean degree for controls and children with epilepsy in sleep (Fig 3). As subjects transition from wakefulness into sleep (N1 and N2), the differences in mean degree between cases and controls decrease, as evidenced by the decrease in the AUC from wake to N1 and N2 in Fig 3. For the delta, theta and beta bands, significant differences were observed between wake and N1/N2, while no significant differences were observed between N1 and N2. In the low alpha and alpha bands, no significant differences were observed between wake and N1, while both stages have significantly different AUC than N2 (Kruskal-Wallis test, Bonferroni correction for multiple comparisons).
Fig 3. Area under the ROC curve for the mean degree, calculated in different stages of awareness (wake, N1 and N2).
Neurodevelopmental traits correlate with decrease in mean degree
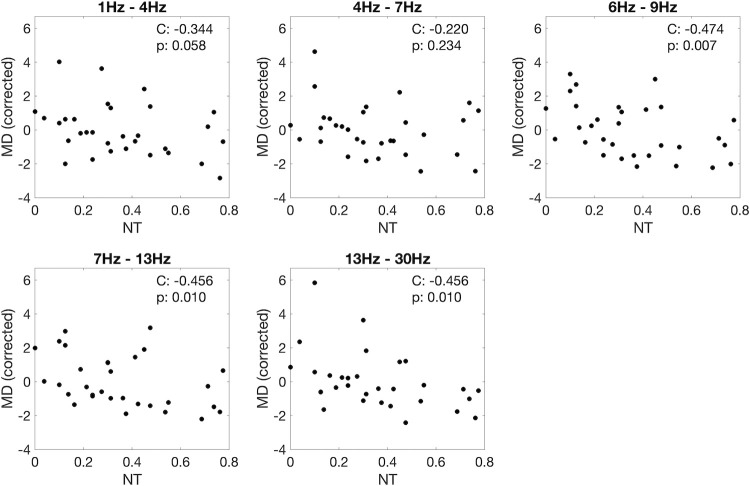
The effect of autism and ADHD traits on functional brain networks is explored in Fig 4. In this figure, the network mean degree (corrected for age, sex and epilepsy diagnosis), calculated for wake epochs, was plotted as a function of the neurodevelopmental trait index (see Methods), for all frequency bands. Fig 4 shows a negative correlation between neurodevelopmental traits and mean degree, for all frequency bands. The correlation is clearer for higher frequencies, and remains significant when corrected for multiple comparisons in the low alpha, alpha and beta bands. It is important to note that the mean degree values here are corrected for epilepsy diagnosis (see Methods), so the correlation between mean degree and neurodevelopmental trait index is independent of epilepsy diagnosis. When we consider N1 and N2 epochs, the correlation was generally less clear but followed a similar trend (see S2 and S3 Figs in S1 File).
Fig 4. Spearman correlation and p-value (C and p) between neurodevelopmental trait index (NT) and mean degree (MD) corrected for age, sex, and epilepsy diagnosis.
MD calculated using wake epochs.
The influence of the neurodevelopmental trait index (NT) on the mean degree affects the classification of controls and epilepsy subjects using this marker. Fig 5 (left) shows NT and mean degree (corrected for sex), calculated for controls and epilepsy in the low alpha band (which had the highest correlation with NT). For the MD threshold of maximum balanced accuracy (dashed line), some subjects were misclassified (blue dots below the dashed line, and red dots above it). When we analysed the NT of the misclassified subjects (Fig 5 - right), we noticed that controls misclassified as epilepsy have a larger median NT than controls correctly classified. The opposite effect was seen for epilepsy. The number of misclassified subjects was small, but the trend was clear and consistent across all frequency bands (see S4 Fig in S1 File). When estimating the classification power of MD through the calculation of the AUC, if instead of only correcting this marker for sex imbalance (as in Fig 3) we also correct it for NT, the AUC generally improves, especially for wake epochs, as shown in Fig 6.
Fig 5.
(Left) mean degree corrected for age, calculated using wake epochs, as a function of neurodevelopmental traits. Cont (Epi) are shown in blue (red). The dashed line represents the threshold of optimal balanced accuracy for the separation between Cont and Epi. (Right) Comparison between neurodevelopmental traits of subjects classified correctly (Cont > threshold / Epi < threshold) and incorrectly (Cont < threshold / Epi > threshold). Shown here only for low alpha band (6Hz– 9Hz). See Supporting Information for the same calculation in other frequency bands.
Fig 6. Comparison between AUC calculated using MD only corrected for sex (as in Fig 3) and corrected for sex and neurodevelopmental traits index (NT).
Discussion
In this work, we investigated how paediatric epilepsy and co-occurring traits of neurodevelopmental conditions impact functional brain networks obtained from EEG in wakeful rest and sleep. We showed that, for networks obtained from wake resting-state epochs, epilepsy diagnosis correlates with a decreased mean degree within different frequency bands, with this effect being most apparent in the beta band. For epochs obtained in sleep stages N1 and N2, this effect is generally less pronounced. We have also shown that a marker associated with autism and ADHD characteristics (NT) has a negative correlation with mean degree, which is consistent across frequency bands and stages of awareness. We also quantified how neurodevelopmental traits can influence the classification power of mean degree when separating controls and epilepsy subjects. We showed that children without epilepsy and with high NT have a higher risk of being misclassified than those with low NT. Conversely, children with epilepsy with low NT might have a higher risk of being classified as not having epilepsy if the influence of NT is not accounted for when identifying optimal classification thresholds.
Functional networks extracted from EEG have been studied in the context of epilepsy previously [44]. Adebimpe et al. [39] used high-density scalp EEG to explore differences in functional connectivity networks between children with Rolandic epilepsy and healthy controls. The authors show that networks from subjects with epilepsy present significantly lower mean degree than healthy controls in the delta and beta bands. This agrees with the results presented in this work. Chowdhury et al. [13] compared functional networks from adult controls and adults diagnosed with idiopathic generalised epilepsy. They showed that, in the low-alpha band, network mean degree and degree variance are elevated in epilepsy, while clustering coefficient is lower in epilepsy. These results differ from what has been observed in paediatric cases. However, it is important to point out that changes in the pre-processing and calculation of functional networks can have a significant effect on network markers, as can type of epilepsy, so comparisons across different studies need to be interpreted carefully. Potential differences between the effects of epilepsy on network markers in children and adults can result from the intricate influence of brain maturation in the paediatric brain. Resting-state functional EEG networks have been shown to present complex band-specific changes during the maturation period (e.g., positive correlation between network segregation and age in the upper alpha band) [45]. These results evidence the importance of considering the influence of brain maturation in the study of epileptogenic brain networks in children. The effects of age were accounted for in the present study, but comparisons were made considering a relatively broad age range (4 to 15 years old). Further studies with larger sample sizes, clustering participants in narrower age ranges, are needed to clarify the influence of brain maturation on EEG networks in the context of epilepsy and neurodevelopmental disorders.
The results described above, observed in networks derived from wakeful rest, were also consistent with those from epochs from sleep stages N1 and N2, however the effect size was generally smaller during sleep. This result is interesting since NREM sleep has been shown to activate interictal epileptiform discharges (IED) in many types of epilepsies [46], which actually underpins the use of nap studies to support epilepsy diagnosis. However, it is important to notice that smaller control-epilepsy differences for markers in sleep than in wake does not imply that ictal or interictal activity should be less frequent in sleep. The relationship between IEDs and seizure susceptibility is still unclear, with some works suggesting that IEDs can have anti-seizure effects, depending on the underlying physiological mechanisms leading to seizures [47, 48]. In this scenario, states where IEDs are more frequent could lead to network representations with features associated to low ictogenicity. The detailed relationship between IEDs and network markers would require long wake and sleep recordings, rich in IEDs, and is beyond the scope of this work.
Some limitations of this work need to be considered when interpreting the results presented above. Our analysis was implemented considering a relatively small number of subjects, especially in the control group. Despite this limitation, significant differences and clear trends were observed in the relationship between network markers and epilepsy diagnosis and/or neurodevelopmental traits. These results will serve as a basis for further validation studies using larger cohorts. Another important consideration is that different epilepsy syndromes were grouped together in part of the analysis presented above. This is justified by the observation, in this and other works [15], of shared network characteristics in different epilepsies when compared to controls. Future studies with a larger number of subjects should also focus on stratifying the analyses above in different epilepsy types, presenting a more detailed quantification of the influence of each epilepsy syndrome in network markers. One factor potentially influencing the results presented above is the administration of melatonin and/or sleep deprivation to encourage sleep. Previous works have suggested that sleep deprivation can alter functional brain networks obtained from EEG [30, 49], but a comprehensive understanding of these effects (especially in addition to the use of melatonin) is still an open question. Detailed information about individual sleep facilitation protocols was not available at the time of data gathering for this study and therefore its effects on functional networks could not be quantified. Despite the difficulties to access such data in clinical scenarios, future studies should try to disentangle those effects, or attempt to include data only from individuals who were able to achieve sleep without any additional protocols. Finally, autism and ADHD traits were not different between the control and epilepsy groups. Previous works suggest that both conditions have a higher prevalence in epilepsy than in typically developing children [10], indicating that the data used in this study might not be representative of the general population. However, it is important to point out that the “control” group in this work represents children suspected of having epilepsy who had a differential diagnosis. To the best of our knowledge, the expected prevalence of autism and/or ADHD in such a group is unknown.
The use of network-based biomarkers in the context of epilepsy and other neurodevelopmental disorders has the potential to significantly improve the diagnostic journey. Future studies in this area should focus on exploring local changes in functional brain networks associated to these conditions (e.g., altered connectivity patterns in sub-networks associated to specific brain functions which are strongly implicated in the epilepsy). Another important factor to be explored is the influence of age in brain networks, and how different conditions alter functional connectivity patterns at different stages of brain maturation [50]. A comprehensive quantification of biomarkers along developmental trajectories would result in more accurate and personalized frameworks to support diagnosis.
Conclusion
The influence of neurodevelopmental conditions, like autism and ADHD, on functional networks extracted from EEG data is still an open question. Evidence suggests that autism is characterised by long-range underconnectivity [51], but this has been challenged and the diversity in methodology makes it difficult to evaluate and compare across studies [52]. In this study we have shown that network mean degree presents a negative correlation with the neurodevelopmental trait index NT (autism and ADHD characteristics). This relationship does not comprehensively describe the effect of autism and/or ADHD on functional brain networks, but it shows how the traits associated with these conditions can influence network-based biomarkers and, therefore, their potential clinical value. In order to disentangle the influences of autism and ADHD on network markers, future studies should extend the analysis presented here by considering cases with confirmed clinical diagnoses of these conditions, and focus on the main characteristics that differentiate their classification.
Most studies that explore network markers of epilepsy from EEG recordings tend to exclude subjects with co-occurring conditions from the analysis, especially neurodevelopmental conditions. However, it is often unclear how and to what extent subjects have been tested, especially when sub-clinical traits of neurodevelopmental conditions are considered. The results presented in this work show that ignoring this information can lead to skewed model calibration and inaccurate classification, especially for children with high NT. Such inaccuracies could lead to even longer diagnostic delays, misdiagnosis, and inappropriate treatment strategies.
Supporting information
(PDF)
Data Availability
Data cannot be shared publicly because participants did not give their consent to open sharing of their data and this was not covered in the ethical approval of this study. Data contain potentially sensitive patient information (restrictions imposed by North West - Preston Research Ethics Committee). For data availability please contact the North West - Preston Research Ethics Committee - Ref. 19/NW/0337 (preston.rec@hra.nhs.uk).
Funding Statement
L.J. acknowledges support from The Waterloo Foundation via a Child Development Fund Research Grant (grant ref. no. 1970-4687). A.W., C.R., and A.P.B. acknowledge support from The Waterloo Foundation (grant ref no. 1970/3346). L.J., D.G. and J.R.T. acknowledge support from the University of Birmingham Dynamic Investment Fund. J.R.T. acknowledges support from the EPSRC (grant ref. no. EP/T027703/1). S.J. acknowledges support from the Alan Turing Institute and the EPSRC (grant ref. EP/N510129/1).
References
- 1.Guerrini R. Epilepsy in children. Lancet. 2006;367: 499–524. doi: 10.1016/S0140-6736(06)68182-8 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO | Epilepsy: a public health imperative. Who. 2019. [Google Scholar]
- 3.Holmes GL. Effect of Seizures on the Developing Brain and Cognition. Semin Pediatr Neurol. 2016;23: 120–126. doi: 10.1016/j.spen.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nickels KC, Zaccariello MJ, Hamiwka LD, Wirrell EC. Cognitive and neurodevelopmental comorbidities in paediatric epilepsy. Nat Rev Neurol. 2016;12: 465–476. doi: 10.1038/nrneurol.2016.98 [DOI] [PubMed] [Google Scholar]
- 5.Menlove L, Reilly C. Memory in children with epilepsy: A systematic review. Seizure. 2015;25: 126–135. doi: 10.1016/j.seizure.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 6.Oto M (Meritxell). The misdiagnosis of epilepsy: Appraising risks and managing uncertainty. Seizure. 2017;44: 143–146. doi: 10.1016/j.seizure.2016.11.029 [DOI] [PubMed] [Google Scholar]
- 7.DiMario FJ. Non-epileptic Childhood Paroxysmal Disorders. Oxfor University Press; 2009. [Google Scholar]
- 8.Greenblatt AS, Beniczky S, Nascimento FA. Pitfalls in scalp EEG: Current obstacles and future directions. Epilepsy Behav. 2023;149: 109500. doi: 10.1016/j.yebeh.2023.109500 [DOI] [PubMed] [Google Scholar]
- 9.Chitre M. Pitfalls in the diagnosis and misdiagnosis of epilepsy. Paediatr Child Health (Oxford). 2013;23: 237–242. doi: 10.1016/j.paed.2012.11.004 [DOI] [Google Scholar]
- 10.Shankar R, Perera B, Thomas RH. Epilepsy, an orphan disorder within the neurodevelopmental family. J Neurol Neurosurg Psychiatry. 2020;91: 1245–1247. doi: 10.1136/jnnp-2020-324660 [DOI] [PubMed] [Google Scholar]
- 11.Kramer MA, Cash SS. Epilepsy as a Disorder of Cortical Network Organization. Neurosci. 2012;18: 360–372. doi: 10.1177/1073858411422754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis KA, Jirsa VK, Schevon CA. Wheels Within Wheels: Theory and Practice of Epileptic Networks. 2021;21: 243–247. doi: 10.1177/15357597211015663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowdhury FA, Woldman W, FitzGerald THB, Elwes RDC, Nashef L, Terry JR, et al. Revealing a Brain Network Endophenotype in Families with Idiopathic Generalised Epilepsy. Hayasaka S, editor. PLoS One. 2014;9: e110136. doi: 10.1371/journal.pone.0110136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petkov G, Goodfellow M, Richardson MP, Terry JR. A critical role for network structure in seizure onset: a computational modeling approach. Front Neurol. 2014;5: 261. doi: 10.3389/fneur.2014.00261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woldman W, Schmidt H, Abela E, Chowdhury FA, Pawley AD, Jewell S, et al. Dynamic network properties of the interictal brain determine whether seizures appear focal or generalised. Sci Rep. 2020;10: 7043. doi: 10.1038/s41598-020-63430-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Diessen E, Otte WM, Braun KPJ, Stam CJ, Jansen FE. Improved Diagnosis in Children with Partial Epilepsy Using a Multivariable Prediction Model Based on EEG Network Characteristics. Maurits NM, editor. PLoS One. 2013;8: e59764. doi: 10.1371/journal.pone.0059764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shrey DW, Kim McManus O, Rajaraman R, Ombao H, Hussain SA, Lopour BA. Strength and stability of EEG functional connectivity predict treatment response in infants with epileptic spasms. Clin Neurophysiol. 2018;129: 2137–2148. doi: 10.1016/j.clinph.2018.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rashid B, Calhoun V. Towards a brain‐based predictome of mental illness. Hum Brain Mapp. 2020;41: 3468–3535. doi: 10.1002/hbm.25013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckley AW, Holmes GL. Epilepsy and Autism. Cold Spring Harb Perspect Med. 2016;6: a022749. doi: 10.1101/cshperspect.a022749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winsor AA, Richards C, Bissell S, Seri S, Liew A, Bagshaw AP. Sleep disruption in children and adolescents with epilepsy: A systematic review and meta-analysis. Sleep Med Rev. 2021;57: 101416. doi: 10.1016/j.smrv.2021.101416 [DOI] [PubMed] [Google Scholar]
- 21.Cohen S, Conduit R, Lockley SW, Rajaratnam SMW, Cornish KM. The relationship between sleep and behavior in autism spectrum disorder (ASD): a review. J Neurodev Disord. 2014;6: 44. doi: 10.1186/1866-1955-6-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds AM, Malow BA. Sleep and Autism Spectrum Disorders. Pediatr Clin North Am. 2011;58: 685–698. doi: 10.1016/j.pcl.2011.03.009 [DOI] [PubMed] [Google Scholar]
- 23.Becker SP, Langberg JM, Eadeh H, Isaacson PA, Bourchtein E. Sleep and daytime sleepiness in adolescents with and without ADHD: differences across ratings, daily diary, and actigraphy. J Child Psychol Psychiatry. 2019;60: 1021–1031. doi: 10.1111/jcpp.13061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gregory AM, Agnew-Blais JC, Matthews T, Moffitt TE, Arseneault L. ADHD and Sleep Quality: Longitudinal Analyses From Childhood to Early Adulthood in a Twin Cohort. J Clin Child Adolesc Psychol. 2017;46: 284–294. doi: 10.1080/15374416.2016.1183499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winsor AA, Richards C, Seri S, Liew A, Bagshaw AP. The contribution of sleep and co-occurring neurodevelopmental conditions to quality of life in children with epilepsy. Epilepsy Res. 2023;194. doi: 10.1016/j.eplepsyres.2023.107188 [DOI] [PubMed] [Google Scholar]
- 26.Gibbon FM, Maccormac E, Gringras P. Sleep and epilepsy: unfortunate bedfellows. Arch Dis Child. 2019;104: 189–192. doi: 10.1136/archdischild-2017-313421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith RJ, Alipourjeddi E, Garner C, Maser AL, Shrey DW, Lopour BA. Infant functional networks are modulated by state of consciousness and circadian rhythm. Netw Neurosci. 2021. doi: 10.1162/netn_a_00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larson-Prior LJ, Power JD, Vincent JL, Nolan TS, Coalson RS, Zempel J, et al. Modulation of the brain’s functional network architecture in the transition from wake to sleep. Progress in Brain Research. 2011. pp. 277–294. doi: 10.1016/B978-0-444-53839-0.00018-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Baba M, Lewis DJ, Fang Z, Owen AM, Fogel SM, Morton JB. Functional connectivity dynamics slow with descent from wakefulness to sleep. PLoS One. 2019;14: e0224669. Available: doi: 10.1371/journal.pone.0224669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verweij IM, Romeijn N, Smit DJA, Piantoni G, Van Someren EJW, van der Werf YD. Sleep deprivation leads to a loss of functional connectivity in frontal brain regions. BMC Neurosci. 2014;15: 88. doi: 10.1186/1471-2202-15-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khalsa S, Mayhew SD, Przezdzik I, Wilson R, Hale J, Goldstone A, et al. Variability in Cumulative Habitual Sleep Duration Predicts Waking Functional Connectivity. Sleep. 2016;39: 87–95. doi: 10.5665/sleep.5324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Facer-Childs ER, Campos BM, Middleton B, Skene DJ, Bagshaw AP. Circadian phenotype impacts the brain’s resting-state functional connectivity, attentional performance, and sleepiness. Sleep. 2019;42. doi: 10.1093/sleep/zsz033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitt B. Sleep and epilepsy syndromes. Neuropediatrics. 2015;46. doi: 10.1055/s-0035-1551574 [DOI] [PubMed] [Google Scholar]
- 34.Bagshaw AP, Jacobs J, LeVan P, Dubeau F, Gotman J. Effect of sleep stage on interictal high-frequency oscillations recorded from depth macroelectrodes in patients with focal epilepsy. Epilepsia. 2009;50: 617–628. doi: 10.1111/j.1528-1167.2008.01784.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutter M, Bailey A, Lord C. The Social Communication Questionnaire. Los Angeles, CA. 2003.
- 36.Conners CK. Conners 3rd Edition (Conners 3). Journal of Psychoeducational Assessment. 2008. [Google Scholar]
- 37.Caspi A, Moffitt TE. All for One and One for All: Mental Disorders in One Dimension. Am J Psychiatry. 2018;175: 831–844. doi: 10.1176/appi.ajp.2018.17121383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richard B. Berry MD; Rita Brooks MEd, RST RPSGT; Charlene E. Gamaldo M, Susan M. Harding MD; Robin M Lloyd MCLM. American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications, Version 2.2. Am Acad Sleep. 2016;28: 391–397. [Google Scholar]
- 39.Adebimpe A, Aarabi A, Bourel-Ponchel E, Mahmoudzadeh M, Wallois F. Functional Brain Dysfunction in Patients with Benign Childhood Epilepsy as Revealed by Graph Theory. He B, editor. PLoS One. 2015;10: e0139228. doi: 10.1371/journal.pone.0139228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt H, Petkov G, Richardson MP, Terry JR. Dynamics on Networks: The Role of Local Dynamics and Global Networks on the Emergence of Hypersynchronous Neural Activity. PLoS Comput Biol. 2014;10. doi: 10.1371/journal.pcbi.1003947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt H, Woldman W, Goodfellow M, Chowdhury FA, Koutroumanidis M, Jewell S, et al. A computational biomarker of idiopathic generalized epilepsy from resting state EEG. Epilepsia. 2016;57: e200–e204. doi: 10.1111/epi.13481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopes MA, Junges L, Tait L, Terry JR, Abela E, Richardson MP, et al. Computational modelling in source space from scalp EEG to inform presurgical evaluation of epilepsy. Clin Neurophysiol. 2020;131: 225–234. doi: 10.1016/j.clinph.2019.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cureton EE. Rank-biserial correlation. Psychometrika. 1956;21: 287–290. doi: 10.1007/BF02289138 [DOI] [Google Scholar]
- 44.Falsaperla R, Vitaliti G, Marino SD, Praticò AD, Mailo J, Spatuzza M, et al. Graph theory in paediatric epilepsy: A systematic review. Dialogues Clin Neurosci. 2021;23: 3–13. doi: 10.1080/19585969.2022.2043128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chung YG, Jeon Y, Kim RG, Cho A, Kim H, Hwang H, et al. Variations of Resting-State EEG-Based Functional Networks in Brain Maturation From Early Childhood to Adolescence. J Clin Neurol. 2022;18: 581–593. Available: doi: 10.3988/jcn.2022.18.5.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin B, Aung T, Geng Y, Wang S. Epilepsy and Its Interaction With Sleep and Circadian Rhythm. Front Neurol. 2020;11. doi: 10.3389/fneur.2020.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Avoli M, de Curtis M, Köhling R. Does interictal synchronization influence ictogenesis? Neuropharmacology. 2013;69: 37–44. doi: 10.1016/j.neuropharm.2012.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chvojka J, Kudlacek J, Chang W-C, Novak O, Tomaska F, Otahal J, et al. The role of interictal discharges in ictogenesis—A dynamical perspective. Epilepsy Behav. 2021;121: 106591. doi: 10.1016/j.yebeh.2019.106591 [DOI] [PubMed] [Google Scholar]
- 49.Miraglia F, Tomino C, Vecchio F, Gorgoni M, De Gennaro L, Rossini PM. The brain network organization during sleep onset after deprivation. Clin Neurophysiol. 2021;132. doi: 10.1016/j.clinph.2020.10.016 [DOI] [PubMed] [Google Scholar]
- 50.Menon V. Developmental pathways to functional brain networks: Emerging principles. Trends in Cognitive Sciences. 2013. doi: 10.1016/j.tics.2013.09.015 [DOI] [PubMed] [Google Scholar]
- 51.O’Reilly C, Lewis JD, Elsabbagh M. Is functional brain connectivity atypical in autism? A systematic review of EEG and MEG studies. Gozzi A, editor. PLoS One. 2017;12: e0175870. doi: 10.1371/journal.pone.0175870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohammad-Rezazadeh I, Frohlich J, Loo SK, Jeste SS. Brain connectivity in autism spectrum disorder. Curr Opin Neurol. 2016;29: 137–147. doi: 10.1097/WCO.0000000000000301 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
Data cannot be shared publicly because participants did not give their consent to open sharing of their data and this was not covered in the ethical approval of this study. Data contain potentially sensitive patient information (restrictions imposed by North West - Preston Research Ethics Committee). For data availability please contact the North West - Preston Research Ethics Committee - Ref. 19/NW/0337 (preston.rec@hra.nhs.uk).