ABSTRACT
Background
The growing participation of women in competitive sports necessitates a comprehensive understanding of sex‐specific cardiovascular adaptations and risks. Historically, research has predominantly focused on male athletes, leaving a gap in knowledge about the unique cardiovascular dynamics of female peers.
Hypothesis
we hypothesized that female athletes exhibit distinct cardiovascular adaptations and face different risks, influenced by physiological, hormonal, and structural differences.
Methods
A systematic review of the literature was conducted, analyzing studies on cardiovascular responses and adaptations in athletes. Data were extracted on hemodynamic changes, autonomic and neural reflex regulation, cardiac remodeling, and arrhythmias. Comparative analyses were performed to identify sex‐specific patterns and discrepancies in cardiovascular health outcomes.
Results
We revealed considerable sex differences in cardiovascular adaptations to athletic training. Female athletes generally have longer QT intervals, greater sinoatrial node automaticity, and enhanced atrioventricular node function compared to males. They also exhibit lower sympathetic activity, lower maximal stroke volumes, and a tendency toward eccentric cardiac remodeling. Conversely, male athletes are more prone to concentric hypertrophy and higher incidences of bradyarrhythmia and accessory pathway arrhythmias. Female athletes are more likely to experience symptomatic atrial fibrillation and face higher procedural complications during catheter ablation.
Conclusions
Our findings underscore the necessity for sex‐specific approaches in sports cardiology. Recognizing and addressing these differences could enhance performance and reduce adverse cardiac events in athletes. Future research should focus on developing tailored screening, prevention, and treatment strategies to bridge the knowledge gap and promote cardiovascular health in both male and female athletes.
Keywords: arrhythmia, athletes, cardiac remodeling, cardiovascular adaptations, sex differences
The current study examines sex‐specific cardiovascular adaptations and risks among elite athletes, revealing distinct differences between male and female responses to training. Key findings include cardiac remodeling variations, arrhythmia risks, and exercise‐induced hemodynamic compliance, highlighting necessities for tailored sex‐specified approaches in sports cardiology to enhance performance and reduce adverse cardiac events.
Abbreviations
- AF
atrial fibrillation
- AV
atrioventricular
- AVO2
arteriovenous oxygen difference
- BP
blood pressure
- CO
cardiac output
- EDV
end‐diastolic volume
- ESV
end‐systolic volume
- ERP
early repolarization pattern
- HCM
hypertrophic cardiomyopathy
- HR
heart rate
- JWI
J‐wave interval
- LA
left atrium
- LV
left ventricle
- LVH
left ventricular hypertrophy
- MVP
mitral valve prolapse
- NICM
non‐ischemic cardiomyopathy
- PVC
premature ventricular contraction
- RV
right ventricle
- SBP
systolic blood pressure
- SCD
sudden cardiac death
- SV
stroke volume
- SVR
systemic vascular resistance
- TWI
T‐wave inversion
- VA
ventricular arrhythmias
- VO2
oxygen consumption
- VO2max
maximal oxygen uptake
1. Introduction
In recent decades, there has been a consistent rise in the number of adults worldwide engaging in physical activity, observed across both developed and developing countries [1]. This trend is part of a larger global movement aimed at incorporating fitness into daily routines to mitigate the risk of metabolic‐related disorders and consequent cardiovascular disease, while also enhancing mental health, cognitive function, and overall quality of life [2]. Concurrently, the number of elite athletes has surged, catalyzing the development of a specialized field within cardiovascular medicine known as “sports cardiology” [3]. This discipline is dedicated to addressing the unique cardiovascular requirements and challenges faced by this population [3].
Notably, the participation of female athletes in competitive sports has seen a remarkable increase over the years. For instance, the proportion of women athletes in the Olympic Games has escalated from 23% in 1984 in Los Angeles to 49% in 2021 in Tokyo [3]. Despite this, the extant body of research investigating the cardiovascular impacts of exercise has predominantly concentrated on male athletes, with female athletes receiving comparatively limited attention.
Moreover, despite burgeoning interest in the biological underpinnings of sex differences in cardiovascular events, there remains a paucity of definitive evidence elucidating how physical activity may differentially influence cardiovascular health outcomes between genders [4]. Disparities in anthropometrics, physiological parameters, sex hormones, biochemical profiles, and psychological factors between genders contribute to a complex interplay of variables that affect cardiovascular adaptation to athletic training [4, 5].
Given these complexities, our review aims to provide an examination of sex‐specific characteristics in hemodynamic adaptation, autonomic and neurological reflexes, cardiac diagnostics, cardiac remodeling, and sport‐related cardiac pathologies. We also elucidate the mechanisms underlying these differences and identify critical gaps in the current knowledge that warrant further investigation. Addressing these gaps will foster a more equitable approach to cardiovascular health in athletes, ultimately enhancing performance and reducing the risk of adverse cardiac events.
2. Search Strategy
The search strategy of the current review involved a comprehensive search of electronic databases including PubMed, Scopus, Web of Science, and Embase. The search was limited to studies published between January 2000 and March 2024. A broad range of keywords and MeSH terms were used to capture relevant studies. These terms were categorized into two main groups: 1. Sex and Gender Differences Keywords, including “sex differences,” “gender differences,” “male vs female,” “sex‐specific,” “gender‐specific,” “biological sex,” “hormonal differences,” “gender disparities,” “female athlete,” “male athlete,” “sex‐based analysis,” “gender‐based analysis”; and 2. Cardiovascular Keywords, including “cardiovascular adaptations,” “cardiac remodeling,” “arrhythmia,” “hemodynamic response,” “left ventricular hypertrophy,” “sudden cardiac death,” “electrocardiogram variations,” “cardiac output,” “stroke volume,” “QT interval,” “sinoatrial node,” “atrioventricular node,” “cardiac electrophysiology,” “ventricular function,” “autonomic nervous system,” “sympathetic activity,” “baroreflex,” “myocardial strain,” “exercise‐induced cardiac changes.” Boolean operators (AND, OR, and NOT) were employed to combine search terms effectively and ensure the retrieval of a comprehensive set of relevant articles. Selection criteria studies were included if they met the following criteria: (1) original research articles or systematic reviews focusing on cardiovascular adaptations or risks in athletes; (2) studies that included both male and female athletes, allowing for direct comparison of sex‐specific cardiovascular responses; (3) studies that involved elite or competitive athletes, defined as individuals who participate in sports at a national or international level; (4) studies published in peer‐reviewed journals in English; and (5) studies providing quantitative data on cardiac function, structure, or incidence of cardiovascular events. Further, articles were excluded if they: (1) focused exclusively on non‐athlete populations; (2) included participants with known cardiovascular diseases or pre‐existing conditions that could confound the results; (3) were case reports, editorials, conference abstracts, or expert opinions without original data; (4) did not provide sex‐specific analysis or failed to report data separately for male and female athletes; (5) were published in languages other than English, where translation was not feasible; and (6) studies with insufficient methodological quality.
2.1. Study Selection Process
The initial search yielded a total of 645 articles. After removing duplicates, 452 articles were screened based on titles and abstracts. Two independent reviewers performed the screening, with discrepancies resolved by a third reviewer. A total of 167 articles were selected for full‐text review. Each article was evaluated for eligibility based on the predefined inclusion and exclusion criteria. Following the full‐text review, 50 articles were deemed suitable for inclusion in the current review.
3. Sex Differences in Hemodynamic Adaption
In human physiology, the increased energy demand and oxygen consumption (VO2) by muscles during exercise lead to a proportional increase in cardiac output (CO) [6]. Specifically, for every 1 L/min increase in VO2 during exercise, CO increases by approximately 1 L/min, reaching up to 6 L/min (average CO at rest is about 5 L/min) [7]. CO can be determined by two interrelated contexts: first, CO is expressed as heart rate (HR) multiplied by stroke volume (SV), where SV is the difference between end‐diastolic volume (EDV) and end‐systolic volume (ESV) [6]. EDV and ESV represent preload and afterload, respectively. Thus, CO can be re‐expressed as HR × (EDV − ESV). Favorably, increases in HR, SV, and CO during exercise prevent a decline in arterial blood flow due to systemic vasodilation, which occurs in response to metabolic‐induced vasodilators (e.g., lactate and carbon dioxide) [6, 7]. Blood pressure (BP) stabilization is modulated by neuromuscular and neurovascular pathways, including arterial baroreflex and the autonomic nervous system. Second, according to the Fick principle, CO equals total oxygen consumption divided by the arteriovenous oxygen difference (AVO2) [8]. AVO2 is the difference between oxygen content in arterial blood and mixed venous blood and depends on both the concentration and oxygen affinity of hemoglobin: AVO2 = hemoglobin × 1.34 × hemoglobin oxygen saturation [8]. Therefore, CO can be interpreted as total oxygen consumption divided by (hemoglobin × oxygen saturation) [8, 9]. Using these formulas, cardiovascular workload adjustment during physical activity relies on HR, preload, afterload, hemoglobin concentration, and oxygen saturation. Additionally, the rate of oxygen utilization by target muscles, termed “oxygen extraction fraction,” varies by exercise type (aerobic vs. endurance) and can further drive increases in CO [10].
Accumulating evidence indicates that women have lower sympathetic activity and peripheral artery resistance compared to men [11]. Accordingly, women exhibit lower resting systolic BP (SBP) levels and a milder response to baroreflex buffering compared to age‐matched men [3, 11]. This pattern is also observed among female athletes compared to their male counterparts [12]. Furthermore, during recovery from dynamic exercise, women demonstrate a lower capacity for vasoconstriction in the arteriolar bed compared to men, resulting in a greater risk of postexercise orthostatic hypotension due to a lower increase in systemic vascular resistance (SVR) [13]. This effect is particularly noticeable during inactive recovery, where female athletes exhibit a greater reduction in CO without adequate arteriolar constriction compared to men [8, 13]. However, these sex differences may not be extrapolated to elite athletes [14]. The lower sympathetic tone and vasodilatory tendency in women are even more pronounced during their reproductive lifespan compared to the postmenopausal state [15]. It is believed that sex hormones partially account for the lifelong sex differences in BP adaptation [13]. The arterial relaxant effect of estrogen is well established in prior studies [15]. However, sex disparities in BP response appear to be multifactorial rather than solely hormone dependent. For instance, most of the previous trials did not show promising results for hormone replacement therapy in attenuating arterial stiffness or prehypertension during the postmenopausal state [16]. Additionally, residual confounders, such as socioenvironmental factors and exercise type/duration, can affect sex differences in BP response to physical stress [17].
Another sex difference in hemodynamic adaptation is the lower maximal SV observed in women compared to men. The increase in SV during exercise occurs due to an increase in preload and a decrease in afterload, driven by increased cardiac inotropism [13]. The magnitude of changes in preload and afterload during exercise is reported to be similar between sexes [15]. Thus, the lower maximal SV in women could be attributed to a lower baseline volume of cardiac chambers rather than dynamic changes in ESV and EDV [7]. Specifically, women have a lower left ventricular (LV) capacity compared to men, which also explains the lower CO observed in women based on the CO = SV × HR formula [7]. Notably, HR does not exhibit sex‐related differences, as maximal HR during exercise is generally age‐dependent rather than sex‐dependent [14, 18]. Some studies suggest that SV, even when adjusted for body surface area, is lower in women compared to men, indicating that the lower overall SV in women may be independent of their smaller body size [19]. Differences in body composition (e.g., higher muscle mass in men compared to higher adiposity in women) may also affect maximal SV and CO during dynamic activity [19]. Furthermore, there appears to be a sex‐related difference in the SV increment slope during physical activity. Male athletes exhibit a gradual linear increase in SV without a significant plateau until maximal workload is reached, whereas female athletes, even at the elite level, reach a plateau in SV increment during submaximal workload [13, 20].
Recent research indicates that female athletes exhibit greater LV twist mechanics, potentially compensating for smaller ventricular volumes and aiding efficient CO during exercise. This compensation mechanism may partly explain why female athletes can achieve high‐performance levels despite smaller cardiac dimensions [21].
Another notable sex disparity is the lower AVO2 in women compared to men [19]. As previously mentioned, AVO2 depends on hemoglobin concentration, which is significantly lower in women [11]. Consequently, female athletes have a lower oxygen supply for the same blood flow compared to their male counterparts. Additionally, since CO, a major factor in oxygen transport, is lower in women, maximal oxygen uptake by muscles (VO2max) during exercise is lower in women, even after adjusting for age, fitness levels, and body surface area [13, 14]. Despite these differences, studies have shown that both sexes exhibit similar changes in the VO2max to CO ratio, suggesting a similar response to oxygen demand during exercise [3, 20]. With increasing age, the decline in HR and SV leads to a decrease in maximal CO and subsequent VO2max during exercise. This age‐related phenomenon does not appear to be significantly influenced by sex, although some evidence suggests that the age‐associated reduction in CO and VO2max occurs with greater intensity in men compared to women [22].
Additionally, recent studies have highlighted that hormonal fluctuations throughout the menstrual cycle can influence cardiovascular responses during exercise in women, with variations in VO2max, HR, and CO observed across different phases of the cycle [21]. This underscores the importance of considering menstrual cycle phases in research and training programs for female athletes to optimize performance and cardiovascular health. Figure 1 illustrates the overview of the mentioned findings.
Figure 1.

The sex differences in physiological response to physical activity.
4. Sex Differences in Neural Reflex Regulation
Three neural adaptations regulate the hemodynamic response to physical activity: the “central command reflex,” responsible for activating the sympathetic nervous system and recruiting motor units; the “pressor reflex,” which adjusts mean arterial pressure (MAP) and HR based on peripheral mechanical and metabolic triggers from working muscles; and the “arterial baroreflex,” which maintains the balance between the sympathetic and parasympathetic nervous systems modulated by prior reflexes [23, 24].
Several studies have explored potential sex differences in these regulatory mechanisms. Recent research indicates distinct patterns of activation in the central command reflex within the insular cortex between sexes [13]. Specifically, women exhibit more right‐sided insular activation, while men show more left‐sided activation during exercise [23]. This lateralization may have implications for how each sex processes and responds to physical exertion [18, 25].
Studies also suggest that both metabolic‐reflex and mechano‐reflex components of the exercise pressor reflex are reduced in women compared to men, resulting in a lower increase in BP and sympathetic nerve activity during exercise. Recent findings indicate that postmenopausal women exhibit an augmented exercise pressor reflex, which can impair muscle blood flow due to excessive sympathetic vasoconstriction. Estrogen replacement therapy has been shown to attenuate these effects, highlighting the role of sex hormones in modulating this reflex [26].
Research on the arterial baroreflex has produced mixed results, with some studies reporting reduced cardiac baroreflex sensitivity at rest in women, while others have found no significant sex differences [18]. Current studies emphasize the need to consider the interaction between aging and sex hormones in understanding these differences. For instance, testosterone levels in men have been identified as predictors of the exercise pressor response, suggesting that both male and female hormones significantly influence neural cardiovascular control during exercise [26].
Overall, understanding these sex‐specific differences in neural reflex regulation is crucial for optimizing cardiovascular health and performance in both male and female athletes, especially given the potential implications for personalized training and therapeutic strategies. Further investigations are warranted to provide a more comprehensive understanding of these mechanisms and to inform targeted interventions.
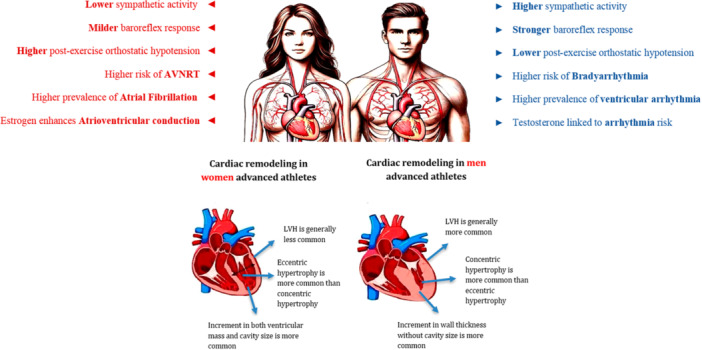
5. Sex Differences in Cardiac Remodeling
Cardiac adaptation to regular exercise is influenced by a variety of factors including the type and intensity of exercise, age, ethnicity, and sex. Studies have indicated that most athletes exhibit normal LV geometry, with a higher prevalence among female athletes compared to their male counterparts (Figure 2). Recent research underscores the importance of differentiating between physiological and pathological changes in cardiac structure for both sexes [27, 28].
Figure 2.

The sex differences in cardiac remodeling as a response to physical activity among advanced athletes.
Exercise‐induced cardiac remodeling can result in changes to cavity size and wall thickness, manifesting as either eccentric or concentric hypertrophy. Eccentric hypertrophy involves an increase in both LV mass and cavity size due to volume overload and elevated diastolic wall stress, whereas concentric hypertrophy is characterized by increased wall thickness without significant changes in cavity size [29, 30]. In female athletes, eccentric hypertrophy is more common in response to dynamic exercises, while male athletes are more likely to develop concentric hypertrophy [13, 30]. LV wall thickness greater than 12 mm and LV cavity enlargement are rare among female athletes. Additionally, left atrial (LA) enlargement, common among elite athletes, is less frequently observed in female athletes compared to males. Concentric changes in female athletes may suggest pathological cardiomyopathies rather than physiological adaptations [30].
The pattern of LV remodeling in male athletes can be partially attributed to higher testosterone levels [23]. Male athletes also tend to experience higher peak BP during exercise, which contributes to LV hypertrophy, despite similar peak HRs between sexes. Female athletes, on the other hand, generally exhibit lower BP, CO, and absolute peak workload [4, 21].
Right ventricular (RV) remodeling, like LV changes, also occurs in response to regular exercise. Echocardiographic studies have shown that both RV size and wall thickness increase in athletes of both sexes, though the extent of these changes is typically less in female athletes. However, the lack of standardized indices for RV remodeling evaluation in athletes necessitates further research to confirm these findings [31].
Overall, cardiac remodeling is more pronounced in male athletes, particularly in the LV. When adjusting for body surface area, both LV and RV sizes are lower in male athletes compared to female athletes, highlighting the importance of considering body composition in these assessments. Factors such as differences in training patterns, body surface area, height, lean body mass, and allometric scaling significantly influence right and left heart remodeling [14, 31].
6. Sex Differences in Electrocardiogram
Regular exercise induces normal variations in the 12‐lead ECG of athletes. If accompanied by other pathological symptoms or ECG patterns, these variations require further evaluation [32]. These normal variations result from both structural adaptations of the heart—discussed earlier as cardiac remodeling—and the nerve conduction system of the heart, prominently the vagus nerve, in response to regular physical activity [23]. A list of normal and pathological ECG patterns has been described in previous studies, but the differences between male and female athletes are still under investigation [33].
Early repolarization pattern (ERP), defined as J‐point elevation of 0.1 mV or more in lateral and/or inferior leads, is a common finding in the ECGs of healthy young individuals and has been positively correlated with physical activity and male sex. Recent studies have confirmed that the prevalence of ERP is higher in male athletes compared to female athletes [34]. Individuals with ERP in their ECGs are at increased risk of sudden cardiac death (SCD) and ventricular and atrial arrhythmias [35].
Cardiac arrhythmias such as ventricular arrhythmias (VAs) (couplets, triplets, and non‐sustained ventricular tachycardia), atrial tachyarrhythmias (supraventricular tachycardia, atrial fibrillation [AF], atrial flutter), and frequent premature ventricular contractions (PVCs) are common abnormal findings in athletes' ECGs [36]. However, the difference between sexes in these arrhythmias is not well documented. Vagotonia, more prevalent in physically active individuals than in the general population, contributes to the higher prevalence of J‐point elevation in athletes [37]. Endocrine and transmembrane voltage gradient differences between sexes have been suggested as reasons for the higher prevalence of ERP, but data on these mechanisms in physically active individuals are lacking [29, 36].
Another common finding in the ECGs of male athletes compared to female athletes is widened or deep Q waves [38]. Abnormal Q waves in the lateral and inferior leads are often not considered pathological variations but are attributed to physiological LVH and technical factors such as lead misplacement. However, they can sometimes indicate structural heart diseases such as hypertrophic cardiomyopathy (HCM) [11, 36, 38]. Studies have shown that female athletes exhibit a lower increase in LV wall thickness and rarely exceed the maximum wall thickness defined for physiological LVH [14]. The upper limit of LV wall thickness in athletes, known as physiological LVH, is reported to be 16 mm. While a thickness of 13 mm or higher, suggestive of HCM, is rare among male athletes compared to non‐athletes, recent studies report that a wall thickness of 12 mm or higher is even less common among female athletes, which could explain the pathological Q waves more commonly seen in male athletes [38, 39].
Evidence of RV and LV hypertrophy is more frequently found in the ECGs of male athletes [21, 29]. Anterior T wave inversion (TWI) is more common in female athletes, while deep or lateral TWI and a combination of anterior TWI and J‐point elevation of more than 0.1 mV are more prevalent in male athletes [37, 38]. Female athletes also tend to have shorter PR intervals and QRS durations compared to male athletes [37].
These findings underscore the necessity for sex‐specific criteria when interpreting ECGs in athletes to accurately differentiate between physiological adaptations and potential pathologies.
7. Sex Differences in Cardiac Arrhythmias
Sex differences in electrophysiological properties are well documented. Table 1 compares an overview of male and female athletes regarding arrhythmia risks, types, and treatment outcomes. Women typically exhibit greater sinoatrial node automaticity, enhanced atrioventricular node function, longer infra‐Hisian conduction times, and prolonged ventricular action potential durations [31, 37, 38]. Conversely, studies indicate that male athletes have a higher susceptibility to bradyarrhythmia and accessory pathway arrhythmias, such as high‐degree atrioventricular block, while female athletes are more prone to atrioventricular nodal re‐entry tachycardias (AVNRT) [40].
Table 1.
Comparison of male and female athletes regarding arrhythmia risks, types, and treatment outcomes.
| Arrhythmia type and complications | Men | Women |
|---|---|---|
| General electrophysiological properties | Shorter QT interval postpuberty, faster repolarization, increased risk of ventricular arrhythmias in older age due to higher QT dispersion | Longer QT interval, greater sinoatrial node automaticity, enhanced AV node function, prolonged ventricular action potential durations |
| Atrial fibrillation (AF) | Higher incidence in general; vigorous endurance exercise increases risk; often asymptomatic | Higher prevalence in older age due to longer life expectancy; mitral valve prolapse increases risk; more symptomatic with palpitations, shortness of breath, fatigue; higher recurrence and complications post‐catheter ablation |
| AV nodal re‐entry tachycardias (AVNRT) | Less common | More common in female athletes |
| Idiopathic ventricular arrhythmias (VA) | Less common, more associated with structural heart disease | More common due to myocardial tissue properties and sex hormone influence |
| Risk factors (alcohol, hypertension, etc.) | A similar association with AF development as women | A similar association with AF development in men |
| Exercise‐related risk | Higher risk of AF with vigorous endurance exercise; prone to ventricular arrhythmias during intense exercise | Mitral valve prolapses linked to new‐onset AF; higher symptomatic AF; benefit from targeted interventions for AF and AVNRT |
| Hormonal influence | Testosterone accelerates ventricular repolarization, associated with adverse cardiac remodeling and increased arrhythmia risk | Estrogen reduces maladaptive cardiac remodeling and fibrosis, decreasing VA incidence in premenopausal women; progesterone is protective against severe ventricular arrhythmias and sudden cardiac death during pregnancy |
| Sudden cardiac death (SCD) | Higher incidence compared to women, especially in older age | Lower incidence overall, but risk increases postpartum due to a drop in progesterone levels |
| Supraventricular tachycardias (SVT) | Less common | More likely to experience SVT such as AVNRT, increased risk with structural changes in the heart during pregnancy and menopause |
| Long QT syndrome (LQTS) | Shorter QT interval due to higher testosterone levels; risk of arrhythmias increases with age | Longer QT interval; risk of cardiac events varies with menstrual cycle, lower during pregnancy but increases postpartum |
| Torsades de Pointes | Less common | More susceptible due to longer QT interval, especially in those with LQTS or during hormonal fluctuations |
| Implantable cardioverter‐defibrillator (ICD) outcomes | More likely to receive ICDs, better outcomes with fewer complications | Less likely to receive ICDs; higher complication rates postimplantation |
| Treatment of atrial fibrillation | Equally likely to receive antiarrhythmic drugs; more likely to undergo catheter ablation with better outcomes | Less likely to receive invasive treatments; higher risk of complications like cardiac tamponade; primary AV‐nodal ablation more common due to comorbidities and older age at diagnosis |
| Ventricular tachycardia (VT) | VT associated with structural heart disease more common; higher comorbidity burden | Idiopathic VT is more common, especially RVOT‐VT; worse VT–free survival despite favorable baseline characteristics |
| Management and outcomes | Better outcomes with fewer complications for catheter ablation; more often receive cardiac resynchronization devices | Higher recurrence rates post‐catheter ablation; more procedural complications; less likely to be referred for invasive procedures |
In terms of AF, a common arrhythmia with significant morbidity and mortality, there is substantial evidence of sex‐based differences in its mechanisms, treatment responses, and outcomes within the general population [41, 42]. However, it remains unclear whether these sex‐specific differences extend to the relationship between AF and long‐term exercise, as most studies have primarily focused on male athletes. Moderate regular exercise seems to mitigate AF risk in both sexes, while vigorous endurance exercise may increase it, especially in male athletes [43, 44]. Some research suggests a J‐shaped relationship between the risk of AF and the intensity of endurance exercise [45, 46].
In athletes who develop AF without significant cumulative exercise exposure, mitral valve prolapse (MVP) has been linked to new‐onset AF, with female athletes being at higher risk than males [47]. Classic risk factors for AF, such as alcohol consumption, hypertension, thyrotoxicosis, and ischemic heart disease, do not exhibit sex differences in their association with AF development [48]. Female athletes are more likely to experience symptomatic AF, presenting with palpitations, shortness of breath, and fatigue, whereas asymptomatic AF is more commonly reported in male athletes [45, 49].
Sex differences also extend to the management and outcomes of cardiac arrhythmias [45]. Women athletes undergoing catheter ablation for AF are typically older than men and show higher rates of AF recurrence and procedural complications, such as cardiac tamponade [9, 50]. This finding may be at least in part due to the higher prevalence of non‐pulmonary vein–mediated AF in women, which is more challenging to treat with standard pulmonary vein isolation techniques [51]. Additionally, VAs display significant sex differences [52]. For instance, idiopathic VAs are more common in women, whereas men are more likely to experience VA associated with structural heart disease. These variations are partly attributed to differences in myocardial tissue properties and the influence of sex hormones [37, 52].
Furthermore, sex hormones play a critical role in modulating arrhythmia risk. Estrogen provides cardioprotective effects by reducing maladaptive cardiac remodeling and fibrosis, thereby decreasing VA incidence in pre‐menopausal women, while testosterone has been associated with adverse cardiac remodeling and increased arrhythmia risk in men [13, 37].
Some studies underscore the sex‐specific differences in athletes' cardiac arrhythmias for developing tailored screening, prevention, and treatment strategies for athletes. For instance, male athletes are more prone to ventricular arrhythmias during intense exercise, which necessitates regular monitoring and possibly more stringent cardiovascular screening protocols [37, 41]. Conversely, female athletes might benefit more from targeted interventions aimed at managing AF and AVNRT, emphasizing the need for personalized medical approaches [21, 41].
8. Sex Differences in SCD
Extensive epidemiological research indicates that women have a lower incidence of SCD during physical activity, irrespective of age and athletic proficiency [4, 31, 37]. Younger female athletes exhibit fewer cardiomyopathies linked to SCD compared to their male counterparts [4]. Additionally, females who experience SCD typically have structurally normal hearts [4, 31, 37]. Women with structural heart diseases are also generally at a lower risk of SCD than men, though conditions like MVP may significantly heighten this risk in females [33, 34, 48].
Studies on elite athletes showed that female athletes tend to have smaller LV wall thickness and larger LV chambers, indicating that eccentric hypertrophy is more common in women, whereas concentric hypertrophy predominates in men [27, 29]. This suggests that the type of cardiac remodeling seen in female athletes might offer protection against SCD. Hormonal differences, such as the contrasting effects of estrogen and testosterone on myocardial hypertrophy and adrenaline levels during intense exercise, likely contribute to this protective mechanism [18]. Moreover, the cardioprotective role of estrogen, which reduces maladaptive cardiac remodeling and fibrosis and stabilizes electrophysiological properties, further underscores the importance of hormonal influences in sports cardiology [37].
Recent research has also highlighted that women have more active enzymatic reactions related to energy substrates, which can prevent myocardial fibrosis [53]. This points to a complex interplay of biomechanical factors, including epigenetics, hormones, and biochemical pathways, contributing to the reduced risk of sport‐related SCD in women.
The implications of these sex‐specific differences are crucial for the field of sports cardiology. Understanding these protective mechanisms in women can guide the development of tailored screening and intervention strategies. For example, male athletes may benefit from more rigorous cardiovascular monitoring and screenings for conditions such as HCM and arrhythmogenic RV cardiomyopathy (ARVC), which are more frequently associated with SCD in men due to their tendency for structural heart abnormalities and arrhythmias under high physical stress. Conversely, female athletes might need focused screenings for conditions like MVP and AF. Given these conditions' contribution to increased SCD risk in women, particularly under intense physical activity, early identification and intervention are essential.
9. Conclusion
The increasing participation of women in elite sports has highlighted the critical need for a deeper understanding of sex‐specific cardiovascular adaptations and associated risks. Cumulating evidence underscores the importance of recognizing sex as a fundamental variable in sports cardiology, influencing not only athletic performance but also the risk of adverse cardiovascular events. The findings of this review emphasize the necessity for sex‐specific approaches in the evaluation, management, and treatment of athletes. It is clear that the one‐size‐fits‐all model in sports cardiology does not adequately address the unique physiological and hormonal differences between men and women. This gap in knowledge must be bridged to ensure optimal cardiovascular health and performance for all athletes, regardless of sex. To advance the field of sports cardiology and promote highly personalized medicine, we urge the scientific community to prioritize research that investigates sex‐specific cardiovascular dynamics in athletes. Future studies could aim to develop tailored screening protocols, prevention strategies, and plausible therapeutic interventions that account for the distinct cardiovascular profiles of male and female athletes. Enhanced research in this domain will not only contribute to safer athletic participation but also pave the way for innovations in personalized medicine, ultimately improving outcomes for all athletes.
Conflicts of Interest
The authors declare no conflicts of interest.
Ethics Statement
The authors have nothing to report.
Acknowledgments
The authors received no specific funding for this work.
Funding: The authors received no specific funding for this work.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Borodulin K. and Anderssen S., “Physical Activity: Associations With Health and Summary of Guidelines,” Food & Nutrition Research 67 (2023): 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roychowdhury D., “Using Physical Activity to Enhance Health Outcomes Across the Life Span,” Journal of Functional Morphology and Kinesiology 5, no. 1 (2020): 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. D'Ascenzi F., Ragazzoni G. L., Boncompagni A., and Cavigli L., “Sports Cardiology: A Glorious Past, a Well‐Defined Present, a Bright Future,” Clinical Cardiology 46, no. 9 (2023): 1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rao S. J. and Shah A. B., “Exercise and the Female Heart,” Clinical Therapeutics 44, no. 1 (2022): 41–49. [DOI] [PubMed] [Google Scholar]
- 5. Sobhani V., Rostamizadeh M., and Hosseini S. M., et al., “Anthropometric, Physiological, and Psychological Variables That Determine the Elite Pistol Performance of Women,” International Journal of Environmental Research and Public Health 19, no. 3 (2022): 1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Petek B. J., Gustus S. K., and Wasfy M. M., “Cardiopulmonary Exercise Testing in Athletes: Expect the Unexpected,” Current Treatment Options in Cardiovascular Medicine 23, no. 7 (2021): 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schierbauer J., Hoffmeister T., Treff G., Wachsmuth N. B., and Schmidt W. F. J., “Effect of Exercise‐Induced Reductions in Blood Volume on Cardiac Output and Oxygen Transport Capacity,” Frontiers in Physiology 12 (2021): 679232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fuller A., Okwose N., Scragg J., et al., “The Effect of Age on Mechanisms of Exercise Tolerance: Reduced Arteriovenous Oxygen Difference Causes Lower Oxygen Consumption in Older People,” Experimental Gerontology 149 (2021): 111340. [DOI] [PubMed] [Google Scholar]
- 9. Del Punta L., De Biase N., Balletti A., et al., “Arterial Hypertension and Cardiopulmonary Function: The Value of a Combined Cardiopulmonary and Echocardiography Stress Test,” High Blood Pressure & Cardiovascular Prevention 29, no. 2 (2022): 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Skattebo Ø., Calbet J. A. L., Rud B., Capelli C., and Hallén J., “Contribution of Oxygen Extraction Fraction to Maximal Oxygen Uptake in Healthy Young Men,” Acta Physiologica 230, no. 2 (2020): e13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kittnar O., “Selected Sex Related Differences in Pathophysiology of Cardiovascular System,” Physiological Research 69, no. 1 (2020): 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joyner M. J., Barnes J. N., Hart E. C., Wallin B. G., and Charkoudian N., “Neural Control of the Circulation: How Sex and Age Differences Interact in Humans,” Comprehensive Physiology 5, no. 1 (2015): 193–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. P.Bassareo P., “Gender Differences in Hemodynamic Regulation and Cardiovascular Adaptations to Dynamic Exercise,” Current Cardiology Reviews 16, no. 1 (2020): 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carter R., Watenpaugh D. E., and Smith M. L., “Selected Contribution: Gender Differences in Cardiovascular Regulation During Recovery From Exercise,” Journal of Applied Physiology 91, no. 4 (2001): 1902–1907. [DOI] [PubMed] [Google Scholar]
- 15. Duque C., Feske S., and Sorond F., “Cerebrovascular Hemodynamics in Women,” Seminars in Neurology 37, no. 6 (2017): 679–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu C. F., “Therapeutic Modification of Arterial Stiffness: An Update and Comprehensive Review,” World Journal of Cardiology 7, no. 11 (2015): 742–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naci H., Salcher‐Konrad M., Dias S., et al., “How Does Exercise Treatment Compare With Antihypertensive Medications? A Network Meta‐Analysis of 391 Randomised Controlled Trials Assessing Exercise and Medication Effects on Systolic Blood Pressure,” British Journal of Sports Medicine 53, no. 14 (2019): 859–869. [DOI] [PubMed] [Google Scholar]
- 18. Dart A., “Gender, Sex Hormones and Autonomic Nervous Control of the Cardiovascular System,” Cardiovascular Research 53, no. 3 (2002): 678–687. [DOI] [PubMed] [Google Scholar]
- 19. Wheatley C. M., Snyder E. M., Johnson B. D., and Olson T. P., “Sex Differences in Cardiovascular Function During Submaximal Exercise in Humans,” Springerplus 3 (2014): 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wiebe C. G., Gledhill N., Warburton D. E. R., Jamnik V. K., and Ferguson S., “Exercise Cardiac Function in Endurance‐Trained Males Versus Females,” Clinical Journal of Sport Medicine 8, no. 4 (1998): 272–279. [DOI] [PubMed] [Google Scholar]
- 21. Forså M. I., Bjerring A. W., Haugaa K. H., et al., “Young Athlete's Growing Heart: Sex Differences in Cardiac Adaptation to Exercise Training during Adolescence,” Open Heart 10, no. 1 (2023): e002155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carrick‐Ranson G., Hastings J. L., Bhella P. S., et al., “The Effect of Age‐Related Differences in Body Size and Composition on Cardiovascular Determinants of VO2max,” The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 68, no. 5 (2013): 608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Daniela M., Catalina L., Ilie O., Paula M., Daniel‐Andrei I., and Ioana B., “Effects of Exercise Training on the Autonomic Nervous System With a Focus on Anti‐Inflammatory and Antioxidants Effects,” Antioxidants 11, no. 2 (2022): 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weissman D. G. and Mendes W. B., “Correlation of Sympathetic and Parasympathetic Nervous System Activity During Rest and Acute Stress Tasks,” International Journal of Psychophysiology 162 (2021): 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crisafulli A., “The Impact of Cardiovascular Diseases on Cardiovascular Regulation During Exercise in Humans: Studies on Metaboreflex Activation Elicited by the Post‐Exercise Muscle Ischemia Method,” Current Cardiology Reviews 13, no. 4 (2017): 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grotle A. K., Langlo J. V., Holsbrekken E., Stone A. J., Tanaka H., and Fadel P. J., “Age‐Related Alterations in the Cardiovascular Responses to Acute Exercise in Males and Females: Role of the Exercise Pressor Reflex,” Frontiers in Physiology 14 (2023): 1287392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kessler E. L., Rivaud M. R., Vos M. A., and van Veen T. A. B., “Sex‐Specific Influence on Cardiac Structural Remodeling and Therapy in Cardiovascular Disease,” Biology of Sex Differences 10, no. 1 (2019): 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoon H. J., Kim K. H., Hornsby K., et al., “Gender Difference of Cardiac Remodeling in University Athletes: Results from 2015 Gwangju Summer Universiade,” Korean Circulation Journal 51, no. 5 (2021): 426–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaplan A., Abidi E., Diab R., et al., “Sex Differences in Cardiac Remodeling Post Myocardial Infarction With Acute Cigarette Smoking,” Biology of Sex Differences 13, no. 1 (2022): 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shim C. Y., “Sex Differences in Cardiac Remodeling in Athletes: Men on the Left, Women on the Right?,” Korean Circulation Journal 51, no. 5 (2021): 439–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sanz‐de la Garza M., Carro A., and Caselli S., “How to Interpret Right Ventricular Remodeling in Athletes,” Clinical Cardiology 43, no. 8 (2020): 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Katritsis G. D. and Katritsis D. G., “The Electrocardiogram in Athletes Revisited,” Arrhythmia & Electrophysiology Review 2, no. 2 (2013): 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grazioli G., Sanz M., Montserrat S., Vidal B., and Sitges M., “Echocardiography in the Evaluation of Athletes,” F1000Research 4 (2015): 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vecchiato M., Baioccato V., Adami P. E., et al., “Early Repolarization in Adolescent Athletes: A Gender Comparison of ECG and Echocardiographic Characteristics,” Scandinavian Journal of Medicine & Science in Sports 32, no. 11 (2022): 1581–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kocsis L., Pap Z., and Frigy A., “Cardiac Morphofunctional Characteristics of Individuals With Early Repolarization Pattern: A Literature Review,” Journal of Cardiovascular Development and Disease 10, no. 1 (2022): 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fan J., Yao F. J., Cheng Y. J., Ji C. C., Chen X. M., and Wu S. H., “Early Repolarization Pattern Associated With Coronary Artery Disease and Increased the Risk of Cardiac Death in Acute Myocardium Infarction,” Annals of Noninvasive Electrocardiology 25, no. 6 (2020): e12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Asatryan B. and Barth A. S., “Sex‐Related Differences in Incidence, Phenotype and Risk of Sudden Cardiac Death in Inherited Arrhythmia Syndromes,” Frontiers in Cardiovascular Medicine 9 (2022): 1010748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Corîci O. M., Mirea‐Munteanu O., Donoiu I., Istrătoaie O., Corîci C. A., and Iancău M., “Gender‐Related Electrocardiographic Changes in Athletes,” Current Health Sciences Journal 44, no. 1 (2018): 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pelliccia A., Maron B. J., Spataro A., Proschan M. A., and Spirito P., “The Upper Limit of Physiologic Cardiac Hypertrophy in Highly Trained Elite Athletes,” New England Journal of Medicine 324, no. 5 (1991): 295–301. [DOI] [PubMed] [Google Scholar]
- 40. Etaee F., Elayi C. S., Catanzarro J., et al., “Gender Associated Disparities in Atrioventricular Nodal Reentrant Tachycardia: A Review Article,” Journal of Cardiovascular Electrophysiology 32, no. 6 (2021): 1772–1777. [DOI] [PubMed] [Google Scholar]
- 41. Hunter S. K., S. Angadi S., Bhargava A., et al., “The Biological Basis of Sex Differences in Athletic Performance: Consensus Statement for the American College of Sports Medicine,” Medicine & Science in Sports & Exercise 55, no. 12 (2023): 2328–2360. [DOI] [PubMed] [Google Scholar]
- 42. Thibault V., Guillaume M., Berthelot G., et al., “Women and Men in Sport Performance: The Gender Gap Has Not Evolved Since 1983,” Journal of Sports Science & Medicine 9, no. 2 (2010): 214–223. [PMC free article] [PubMed] [Google Scholar]
- 43. Mozaffarian D., Furberg C. D., Psaty B. M., and Siscovick D., “Physical Activity and Incidence of Atrial Fibrillation in Older Adults: The Cardiovascular Health Study,” Circulation 118, no. 8 (2008): 800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jin M. N., Yang P. S., Song C., et al., “Physical Activity and Risk of Atrial Fibrillation: A Nationwide Cohort Study in General Population,” Scientific Reports 9, no. 1 (2019): 13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Centurión O. A., Candia J. C., Scavenius K. E., García L. B., Torales J. M., and Miño L. M., “The Association Between Atrial Fibrillation and Endurance Physical Activity: How Much Is Too Much?,” Journal of Atrial Fibrillation 12, no. 3 (2019): 2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Margolis G., Cohen O., and Roguin A., “Vigorous Physical Activity and Atrial Fibrillation in Healthy Individuals: What Is the Correct Approach?,” Clinical Cardiology 47, no. 3 (2024): e24237. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47. Newman W., Parry‐Williams G., Wiles J., et al., “Risk of Atrial Fibrillation in Athletes: A Systematic Review and Meta‐Analysis,” British Journal of Sports Medicine 55, no. 21 (2021): 1233–1238. [DOI] [PubMed] [Google Scholar]
- 48. Guasch E., Mont L., and Sitges M., “Mechanisms of Atrial Fibrillation in Athletes: What We Know and What We Do Not Know,” Netherlands Heart Journal 26, no. 3 (2018): 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Drca N., Larsson S. C., Grannas D., and Jensen‐Urstad M., “Elite Female Endurance Athletes Are at Increased Risk of Atrial Fibrillation Compared to the General Population: A Matched Cohort Study,” British Journal of Sports Medicine 57, no. 18 (2023): 1175–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ngo L., Ali A., Ganesan A., Woodman R., Adams R., and Ranasinghe I., “Gender Differences in Complications Following Catheter Ablation of Atrial Fibrillation,” European Heart Journal—Quality of Care and Clinical Outcomes 7, no. 5 (2021): 458–467. [DOI] [PubMed] [Google Scholar]
- 51. Farrell M., Yoneda Z., Montgomery J., et al., “Non‐Pulmonary Vein Mediated Atrial Fibrillation: A Novel Sub‐Phenotype,” PLoS One 12, no. 9 (2017): e0184354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tarantino N., Della Rocca D., De Leon De La Cruz N., et al., “Catheter Ablation of Life‐Threatening Ventricular Arrhythmias in Athletes,” Medicina 57, no. 3 (2021): 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ruberti O. M. and Rodrigues B., “Estrogen Deprivation and Myocardial Infarction: Role of Aerobic Exercise Training, Inflammation and Metabolomics,” Current Cardiology Reviews 16, no. 4 (2021): 292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


