Abstract
Abstract
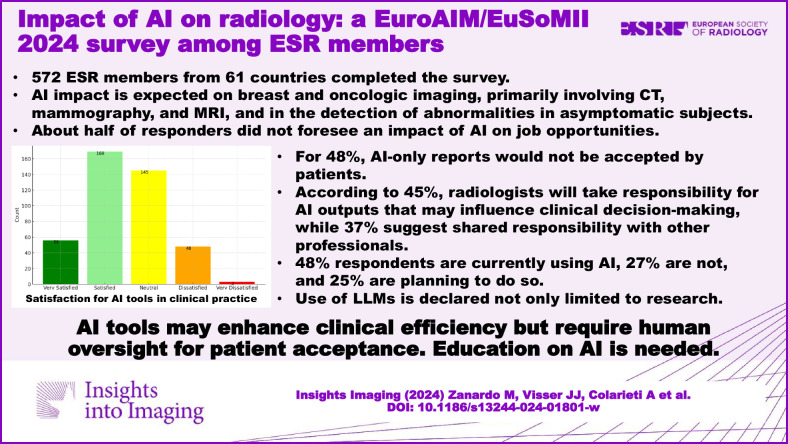
In order to assess the perceptions and expectations of the radiology staff about artificial intelligence (AI), we conducted an online survey among ESR members (January–March 2024). It was designed considering that conducted in 2018, updated according to recent advancements and emerging topics, consisting of seven questions regarding demographics and professional background and 28 AI questions. Of 28,000 members contacted, 572 (2%) completed the survey. AI impact was predominantly expected on breast and oncologic imaging, primarily involving CT, mammography, and MRI, and in the detection of abnormalities in asymptomatic subjects. About half of responders did not foresee an impact of AI on job opportunities. For 273/572 respondents (48%), AI-only reports would not be accepted by patients; and 242/572 respondents (42%) think that the use of AI systems will not change the relationship between the radiological team and the patient. According to 255/572 respondents (45%), radiologists will take responsibility for any AI output that may influence clinical decision-making. Of 572 respondents, 274 (48%) are currently using AI, 153 (27%) are not, and 145 (25%) are planning to do so. In conclusion, ESR members declare familiarity with AI technologies, as well as recognition of their potential benefits and challenges. Compared to the 2018 survey, the perception of AI's impact on job opportunities is in general slightly less optimistic (more positive from AI users/researchers), while the radiologist’s responsibility for AI outputs is confirmed. The use of large language models is declared not only limited to research, highlighting the need for education in AI and its regulations.
Critical relevance statement
This study critically evaluates the current impact of AI on radiology, revealing significant usage patterns and clinical implications, thereby guiding future integration strategies to enhance efficiency and patient care in clinical radiology.
Key Points
The survey examines ESR member's views about the impact of AI on radiology practice.
AI use is relevant in CT and MRI, with varying impacts on job roles.
AI tools enhance clinical efficiency but require radiologist oversight for patient acceptance.
Graphical Abstract
Keywords: Artificial intelligence, Radiology, Diagnostic imaging, Surveys and questionnaires
Background
In 2019, the European Society of Radiology (ESR)/European Network for the Assessment of Imaging in Medicine (EuroAIM) published the first survey on the impact of artificial intelligence (AI) on radiology [1]. Since then, AI has gained remarkable interest for its applications to radiology. AI systems, based on machine learning or deep learning are showing potential for image acquisition, pre- and post-processing, and, ultimately, disease risk stratification and clinical decision-making [2–4], as also happened with the recent COVID-19 pandemic [5]. A notable increase in AI usage was also reported between 2018 and 2022 among members of the ESR [6].
The debate about AI’s impact on biomedicine and healthcare has grown, with an obvious focus on radiology. In fact, in addition to the interest in radiomics (“images are more than pictures, they are data” [7]), machine/deep learning was early applied to medical images from reconstruction to clinical prediction and diagnosis [8, 9]. Such a transformative innovation raised hopes and fears within the radiological community and beyond [6, 10]. In the last couple of years, a substantial step forward has been the advent of generative AI, with large language models (LLMs) and foundation models beyond language, enabling the automatic creation of images and videos, as well as image interpretation [11].
When applied to radiology, AI tools are considered equivalent to medical devices, with related ethical and regulatory issues [12], as recently defined also by the AI Act from the European Union Parliament (https://artificialintelligenceact.eu). Many efforts were dedicated to the standardization of reporting of AI studies, with guidelines and checklists available [13–17]. Of note is that the editors of the ESR journals published a statement about the use of LLMs by authors, reviewers, and editors [18].
Radiologists’ views and perceptions about AI applications to medical imaging have been reported [3, 19–24]. Our aim was to capture the current opinions and experiences of ESR members regarding the impact of AI on radiology with a new online survey. This study seeks to provide insights into how perceptions and uses of AI have changed over the past six years. By comparing the findings from 2018 [1] and 2024, we aim to understand the evolving impact of AI on radiology practices, job roles, and the professional landscape within the ESR community. This longitudinal analysis is crucial for identifying trends, assessing the progress of AI integration, and informing future strategies for AI implementation and educational needs to be addressed by the ESR.
Methods
Survey design
The survey design was defined taking into account that previously conducted in 2018 [1], updated to reflect recent advancements and emerging topics. To facilitate a meaningful comparison with the 2018 survey, we retained 15 core questions that addressed fundamental aspects of AI’s impact on radiology. Thirteen new questions were introduced to address recent advancements in AI technology, including the rise of generative AI, LLMs, and updated regulatory frameworks. The new questions were proposed by the EuroAIM working group and reviewed and integrated by the European Society of Medical Imaging Informatics (EuSoMII) working group, as well as, by the dedicated group of the ESR. The survey consisted of three parts: seven questions about demographics and professional background (I–VII); and 28 multiple-choice questions on AI issues; of them, 15 were identical to those posed in the 2018 survey (1–15) and 13 were new questions (16–28). The survey mandated that all questions be answered before submission. The full questionnaire is reported in the Supplementary material.
Survey promotion and administration
The survey was promoted by the EuroAIM, the EuSoMII, and the EFRS - European Federation of Radiographer Societies, under the umbrella of the ESR. Participation was voluntary, and no personal identifying information was collected, ensuring respondent anonymity. The survey was conducted in accordance with the ESR ethical standards and adhered to GDPR regulations for data protection and privacy. It was administered between January and March 2024, and intended to be completed in about 10 min. Invitation to participate was emailed to ESR members, reaching approximately 28,000 individuals. The e-mail contained a URL link to the survey, hosted on the Google form platform. Two reminder emails were sent on January 26 and March 18, 2024. The survey was closed on March 31, 2024.
Data collection and analysis
Survey responses were automatically recorded and processed using Excel® (Microsoft, RedMond, WA, USA). Descriptive statistics, including frequencies and percentages, were calculated for all categorical variables. Association analysis was performed using the χ2 test. For the comparison of answers on AI given in 2024 to those in 2018, 95% confidence intervals (CI) were calculated using Excel® (Microsoft, RedMond, WA, USA).
Results
Demographics and professional profiles (questions from I to VII)
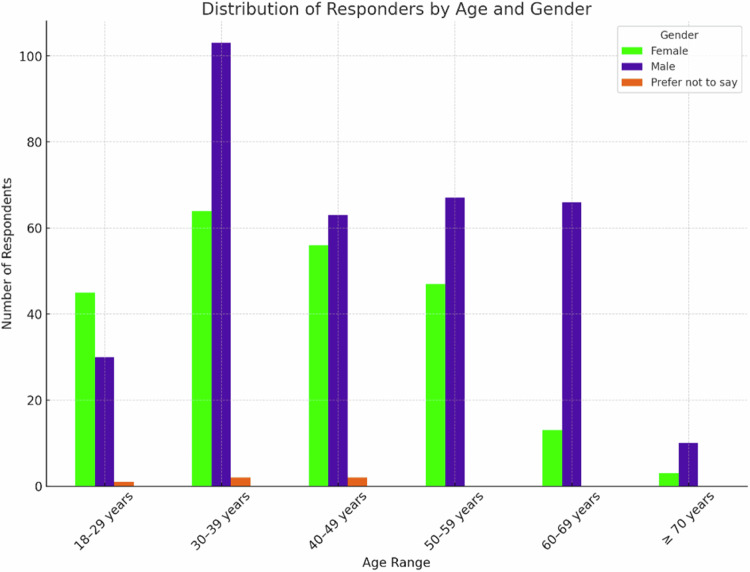
A total of 572 responders out of 28,000 sent e-mails completed the whole survey (2.0%). Among 572 survey responders, 350 (61.2%) were radiologists, 87 (15.2%) were radiology residents, 84 (14.7%) were radiographers, 19 (3.3%) physicists, 32 (5.6%) other categories (mainly physicians with a non-radiological specialization and medical students). The working places were: university/teaching hospital for 314 (54.9%) responders; hospital for 189 (33.0%); private practice for 37 (6.5%); private company for 20 (3.5%); and private research center for 7 (1.2%). Information about gender distribution according to age classes is reported in Fig. 1. Of 572 respondents, males were 339 (59.3%), females 228 (39.9%), while 5 (0.8%) answered: “Prefer not to say”.
Fig. 1.
Distribution of responders by age and gender (“Prefer not to say” answers, n = 5, 0.8%)
Responses came from 61 countries with the following distribution per continent: Europe: 535 (93.5%); Asia: 29 (5.1%); Africa: 3 (0.5%); North America: 3 (0.5%); South America: 1 (0.2%); and Australia: 1 (0.2%). Data on country distribution is detailed in the Supplementary File.
AI and medical imaging (questions from 1 to 3)
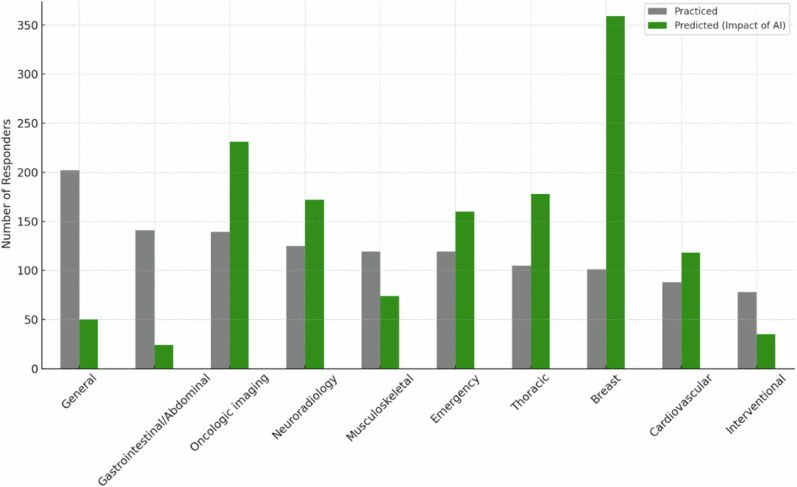
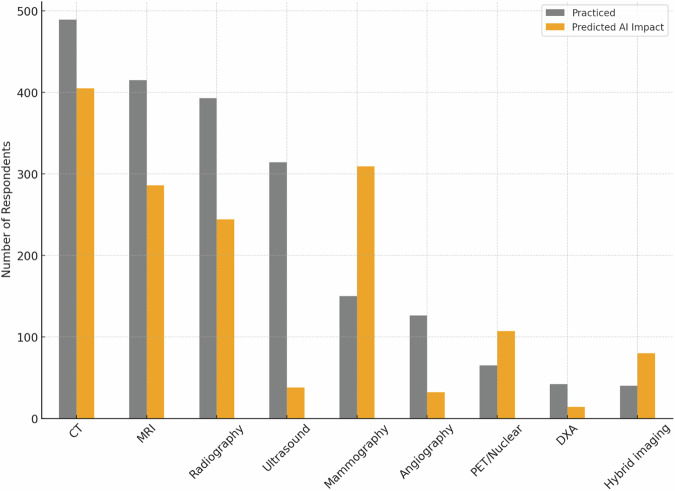
The distribution of responders according to radiology subspecialty and their opinion about which subspecialties will be mostly influenced by the introduction of AI systems is reported in Fig. 2. Responders’ distribution according to practised imaging modalities and their opinion about which imaging modality will be used most to provide input data for AI systems is shown in Fig. 3. Table 1 shows AI application judged by the responders as most relevant in radiology and their corresponding rates by responders.
Fig. 2.
Distribution of responders according to subspecialties, practised and predicted to be impacted by AI. The gray bars represent the number of responders that practice each subspecialty (sorted in decreasing order), while the green bars represent those who foresaw an impact of AI on each subspecialty
Fig. 3.
Distribution of responders according to imaging modalities, practised and predicted to be impacted by AI. Gray bars represent the number of responders practising each modality (sorted in decreasing order), while the orange bars represent those who believe that that modality will be impacted by AI applications. CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography; DXA, dual-energy X-ray absorptiometry
Table 1.
Relevant applications of AI in radiology by 572 responders
| AI application | Respondents | |
|---|---|---|
| Number | Percentage | |
| Detection in asymptomatic subjects (screening) | 298 | 52.1% |
| Image postprocessing | 271 | 47.4% |
| Imaging protocols optimization | 227 | 39.7% |
| Staging/restaging in oncology | 208 | 36.4% |
| Support for structured reporting | 161 | 28.1% |
| Quantitative measure of imaging biomarkers | 157 | 27.5% |
| Detection of incidental findings | 150 | 26.2% |
| Lesion characterization/diagnosis in symptomatic subjects | 134 | 23.4% |
| Prognosis | 31 | 5.4% |
The sum of the percentages is superior to 100% due to the possibility of responders selecting up to three options
AI impact on radiological work (questions from 4 to 10)
About half of 572 responders do not foresee an impact of AI in terms of job opportunities (295, 51.6%), while 162 (28.3%) expect a reduction in job opportunities, and the remaining 115 (20.1%) expect an increase. Tables 2 and 3 show the subgroup analyses according to being/not being AI users or researchers.
Table 2.
Association between expected variations in job positions due to AI with being/not being AI users
| Expected job positions increase | Excepted job positions unchanged | Expected job position reduction | Total | p-value* | |
|---|---|---|---|---|---|
| Users of AI-based products or services in clinical practice | 65 (23.7%) | 140 (51.1%) | 69 (25.2%) | 274 (100%) | 0.071 |
| Nonusers of AI-based products or services in clinical practice | 50 (16.8%) | 155 (52.0%) | 93 (31.2%) | 298 (100%) | |
| Total | 115 (20.1%) | 295 (51.6%) | 162 (28.3%) | 572 (100%) |
* χ2 test
Table 3.
Association between expected variations of job positions due to AI with being/not being AI researchers
| Expected job positions increase | Excepted job positions unchanged | Expected job position reduction | Total | p-value* | |
|---|---|---|---|---|---|
| Researchers on AI-based products or services in clinical practice | 49 (22.8%) | 114 (53.0%) | 52 (24.2%) | 215 (100%) | 0.178 |
| Not researchers on AI-based products or services in clinical practice | 66 (18.5%) | 181 (50.7%) | 110 (30.8%) | 357 (100%) | |
| Total | 115 (20.1%) | 295 (51.6%) | 162 (28.3%) | 572 (100%) |
* χ2 test
The use of AI-based applications will make the radiological team’s duties more clinical according to 256 (44.8%) responders, more technical for 208 (36.4%), unchanged for 92 (16.1%), and both more technical and clinical for 16 (2.8%). For the relative majority of the responders (229, 40.0%), radiologists will be more focused on radiology subspecialties and therefore AI-based applications will not help to report examinations outside the field of subspecialization or will not change the current practice (207, 36.2%), while 136 (23.8%) think that radiologists will be less focused on radiology subspecialties. Regarding reporting workload, 270 (47.2%) expect an impact in terms of increased total reporting workload, while 184 (32.2%) expect a reduced reporting workload, and 118 (20.6%) expect no impact.
The legal responsibility of AI systems outcome will be taken by radiologists alone for 255 (44.6%) responders, will be “shared” for 211 (36.8%), and will be taken by AI developers for 68 (11.9%), insurance companies for 21 (3.7%), other physicians (e.g. clinicians asking for the exam) for 13 (2.3%) (four other answers, 0.7%). Of the 572 responders, 273 (47.7%) believe that patients will not accept a report made by an AI application alone without supervision and approval by a physician, while 92 (16.1%) believe that an AI alone report will be accepted; on the other hand, 207 (36.2%) believe it is now too early to estimate patients’ reaction to this scenario.
Among responders, 242 (42.3%) think that the use of AI systems will not change the relationship between the radiological team and the patient, while 191 (33.4%) believe it will become more interactive and 139 (24.3%) expect that it will become more impersonal.
Radiological team involvement in AI tools development (questions from 11 to 15)
Among 572 responders, 559 (97.7%) believe that the radiological team will play a role in the development and validation of AI applications to medical imaging, in particular, the relative majority believes that they should supervise all development stages of an AI system applied to radiology (355, 62.1%). Specific tasks were rated as follows: helping in task definition (256, 44.8%), providing labelled images (181, 31.6%), and developing AI-based applications (133, 23.3%).
The relative majority of the responders would like to be educated on advantages and limitations of AI applications (410, 71.7%), on the clinical use of AI applications (357, 62.4%), on technical methods (142, 24.8%), on how to get into the driver’s seat in using AI (134, 23.4%), on how to survive to the AI revolution (48, 8.4%), and on how to avoid the use of AI applications (14, 2.4%).
Moreover, responders believe that if AI systems will allow to save working/reporting time, they should be used to interact with: other clinicians (228, 39.9%), patients (206, 36.0%), AI developers (e.g. engineers, computer scientists) (83, 14.5%), other radiologists (53, 9.3%), or administrators (2, 0.3%).
Of 572 responders, 274 (47.9%) are currently using AI systems in their clinical practice, while 153 (26.8%) do not use them, and 145 (25.3%) do not use them at present but are planning to do it. Meanwhile, 239 of 572 responders (41.8%) are currently not involved in research projects on AI applications development, 118 (20.6%) are planning to be involved, 113 (19.8%) are currently involved in AI systems development, and 102 (17.8%) in their testing.
Usage and perception of AI tools (questions from 16 to 23)
Radiological team members that never used any AI tools were 151/572 (26.4%); 56/421 (13.3%) were very satisfied, 169/421 (40.1%) were satisfied, 145/421 (34.5%) were neutral, 48/421 (11.4%) dissatisfied and 3/421 (0.7%) very dissatisfied (Fig. 4).
Fig. 4.
Bar chart representing the distribution of satisfaction levels regarding the performance of certified AI tools used by responders in clinical practice
The most common radiological modalities for certified AI tools used in clinical practice are CT (222, 38.8%) and radiography (137, 24.0%), with MRI (134, 23.4%) and mammography (75, 13.1%) also being frequently mentioned.
The survey results indicate that 169/572 (29.6%) of respondents are not familiar with the classification under the medical devices regulation and postmarket surveillance requirements for the certified AI tools they are using. Meanwhile, 110/572 (19.2%) are familiar with these requirements, while an equal 110/572 (19.2%) do not know the classification and 31/572 (5.4%) do not know the postmarket surveillance requirements;152/572 (26.6%) declared no current or previous use of these tools.1 Data on the association between being familiar/not familiar with medical devices regulation and postmarket surveillance requirements for certified AI tools among responders being/not being AI users are reported in Table 4.
Table 4.
Association between being familiar/not familiar with medical devices regulation and postmarket surveillance requirements for certified AI tools among responders being/not being AI users
| Being familiar | Being not familiar | Total | p-value* | |
|---|---|---|---|---|
| Users of AI-based products or services in clinical practice | 79 (28.8%) | 195 (71.2%) | 274 (100%) | < 0.001 |
| Nonusers of AI-based products or services in clinical practice | 31 (10.4%) | 267 (89.6%) | 298 (100%) | |
| Total | 110 (19.2%) | 462 (80.8%) | 572 (100%) |
* χ2 test
The survey results indicate that the most common sources for staying informed about AI are conferences and congresses (428, 74.8%), followed by scientific papers (382, 66.8%), and colleagues (242, 42.3%). Social media (206, 36.0%) and newsletters (197, 34.4%) are also significant sources, while books (69, 12.1%) and AI itself (64, 11.2%) are less common. Other sources like vendors, podcasts, and industry-related information are mentioned less frequently.
Among 572 respondents, 354 (61.9%) declare that their department does not have a dedicated budget for AI, while 164 (28.7%) of respondents are unsure. Only 54 (9.4%) of respondents indicated that their department has a dedicated budget for AI, mainly based in Italy, Germany, Netherlands and Switzerland.
The survey results indicate that 286/572 respondents (50%) are not involved in any AI research. The tasks/roles declared by those involved in AI research are reported in Table 5.
Table 5.
Tasks/roles declared by 286 responders involved in AI research
| Tasks/roles | Respondents | |
|---|---|---|
| Number | Percentage | |
| Image annotation/segmentation (and or provider of annotated datasets) | 135 | 47.2% |
| Cooperation in writing of scientific/clinical papers | 127 | 44.4% |
| Supervision training and internal validation | 126 | 44.1% |
| Proponent (identifier of clinical needs) | 113 | 39.5% |
| Developer (direct interaction and collaboration with data scientists and/or IT specialists to develop AI models) | 104 | 36.4% |
| Collaboration in external validation | 104 | 36.4% |
| Data selector and provider | 101 | 35.3% |
The sum of the percentages is superior to 100% due to the possibility of responders selecting up to three options
AI artificial intelligence, IT information technology
The relative majority of respondents (341/572, 59.6%) believe that the Covid-19 pandemic accelerated the development or implementation of AI research in medical imaging, while 129/572 (22.6%) are unsure, and 102/572 (17.8%) do not believe it had an accelerating effect.
The main potential barriers to AI implementation in clinical practice are costs/lack of budget opted by 283 (49.5%) responders, legal issues (250, 43.7%), lack of validation/scientific evidence (203, 35.5%), lack of vision/policy/ownership (157, 27.4%), and information technology and systems integration (137, 24.0%). Other barriers such as low efficacy, lack of reimbursement, and various less common concerns were mentioned by less than 2% of respondents each.
LLM perception and utilization (questions from 24 to 28)
The survey results indicate that 299/572 respondents (52.3%) do not currently use LLMs in their practice but are interested in doing so. Meanwhile, 124/572 (21.7%) use them occasionally, 122/572 (21.3%) are not interested, and 27/572 (4.7%) use them often. Among those who answered (151, 26.4%) positively, the most common settings for using LLMs in clinical practice are: research activities/scientific writing (98/151, 64.9%), literature review (80/151, 53.0%), risk assessment (19/151, 12.6%), image annotation and reporting (18/151, 11.9%), and screening/diagnosis (17/151, 11.2%). The main potential benefits of using LLMs in radiology are access to up-to-date medical literature and research (241/572, 42.1%), enhanced efficiency in image interpretation (183/572, 32.0%), improved diagnostic accuracy (101/572, 17.7%), cost savings (80/572, 14.0%), and enhanced efficiency in imaging procedure execution (78/572, 13.6%). Additionally, 199/572 (34.8%) respondents are unsure or have no opinion on the potential LLMs benefits.
The main concerns or reservations about incorporating LLMs into clinical practice are AI model reliability and bias (318/572, 55.6%), legal and ethical implications (266/572, 46.5%), and data privacy vulnerability (181/572, 31.6%). Additionally, 64/572 of respondents (11.2%) have no concerns or reservations, while 44/572 (7.7%) are worried about the negative impact on the radiological team.
Among the 572 respondents, 380 (66.4%) have not used AI imaging generative models (e.g. ChatGPT 4o, Midjourney, DALL-E, or Adobe Firefly) in their clinical practice or for academic purposes but are interested in doing so. Additionally, 119/572 (20.8%) have not used these models and are not interested, while 59/572 (10.3%) use them occasionally, and 14/572 (2.5%) use them often.
The comparisons between answers on AI given in the current survey with those given in 2018 judged to be relevant for outlining the current trends are provided in Table 6 with their 95% CI for the two surveys.
Table 6.
Comparison of answers to the survey about AI in radiology: 2024 vs 2018
| Variable/question | 2018 | 2024 | Δ | |
|---|---|---|---|---|
| Rate of responders |
675/24.000 2.8% (2.6–3.0%) |
572/28.000 2.0% (1.9–2.2%) |
−0.8% | |
| Do you foresee an AI impact on a professional radiologist’s life in terms of the number of job positions in the next 5–10 years? | Yes, job positions will increase |
218/675 32.3% (28.8–36.0%) |
115/572 20.1% (16.9–23.6%) |
−12.2% |
| Yes, job positions will be reduced |
157/675 23.3% (20.1–26.6%) |
162/572 28.3% (24.7–32.2%) |
+5.0% | |
| No |
300/675 44.4% (40.7–48.3%) |
295/572 51.6% (47.4–55.7%) |
+7.2% | |
| In the next 5–10 years, who will take the legal responsibility for AI-system output? | Radiologists |
277/675 41.0% (37.3–44.9%) |
255/572 44.6% (40.5–48.8%) |
+3.6% |
| Other physicians (e.g. clinicians requesting the imaging study) |
7/675 1.0% (0.4–2.1%) |
13/572 2.3% (1.2–3.9%) |
+1.3% | |
| Developers of AI applications |
69/675 10.2% (8.0–12.8%) |
68/572 11.9% (9.4–14.8%) |
+1.7% | |
| Insurance companies |
24/675 3.6% (2.3–5.2%) |
21/572 3.7% (2.3–5.6%) |
+0.1% | |
| Shared responsibility |
277/675 41.0% (37.3–44.9%) |
211/572 36.9% (32.9–41.0%) |
−4.1% | |
| Other |
21/675 3.1% (1.9–4.7%) |
4/572 0.7% (0.2–1.8%) |
−2.4% | |
| In the next 5–10 years, will patients mostly accept a report from AI applications without supervision and approval by a physician? | Yes |
79/675 11.7% (9.4–14.4%) |
92/572 16.1% (13.2–19.4%) |
+4.4% |
| No |
374/675 55.4% (51.6–59.2%) |
273/572 47.7% (43.6–51.9%) |
−7.7% | |
| Difficult to estimate at present |
222/675 32.9% (29.4–36.6%) |
207/572 36.2% (32.2–40.3%) |
+3.3% | |
| What will be the role of radiologists in developing/validating AI applications for medical imaging? | None |
0/675 0.0% (0.0–0.5%) |
13/572 2.3% (1.2–3.9%) |
+2.3% |
| Provide labelled images |
197/675 29% (25.8–32.8%) |
181/572 31.6% 27.8–35.6%) |
+2.6% | |
| Help in task definition |
359/675 53.2% (49.3–57.0%) |
256/572 44.8% (40.6–48.9%) |
−8.4% | |
| Develop AI-based applications |
188/675 27.9% (24.5–31.4%) |
133/572 23.3% (19.8–26.9%) |
−4.6% | |
| Supervise all stages needed to develop an AI-based application |
434/675 64.3% (60.6–67.9%) |
335/572 62.1% (57.9–66.1%) |
−2.2% | |
| Are you utilizing AI-based products or services in your clinical practice? | Yes |
138/675 20.4% (17.5–23.7%) |
274/572 47.9% (43.7–52.1%) |
+27.5% |
| No, but planning to utilise |
205/675 30.4% (26.9–34.0%) |
145/572 25.3% (21.8–29.1%) |
−5.1% | |
| No |
321/675 47.6% (43.7–51.4%) |
153/572 26.8% (23.2–30.6%) |
−20.8% | |
| Should radiologists be educated on: | Technical methods (e.g. machine/deep learning algorithms) |
119/675 17.6% (14.8–20.7%) |
142/572 24.8% (21.3–28.6%) |
+7.2% |
| Advantages and limitations of AI applications |
463/675 68.6% (64.9–72.1%) |
410/572 71.7% (67.8–75.3%) |
+3.1% | |
| Clinical use of AI applications |
392/675 58.1% (54.2–61.8%) |
357/572 62.4% (58.3–66.4%) |
+4.3% | |
| How to get into the driver’s seat using AI |
228/675 33.8% (30.2–37.5%) |
134/572 23.4% (20.0–27.1%) |
−10.4% | |
| How to avoid the use of AI applications |
6/675 0.9% (0.3–1.9%) |
14/572 2.4% (1.3–4.1%) |
+1.5% | |
| How to survive the AI revolution |
75/675 11.1% (8.8–13.7%) |
48/572 8.4% (6.3–11.0%) |
−2.7% | |
| Are you involved in research projects on AI-based application development? | Yes, testing |
61/675 9.0% (7.0–11.5%) |
102/572 17.8% (14.8–21.2%) |
+8.8% |
| Yes, developing |
74/675 11.0% (8.7–13.6%) |
113/572 19.8% (16.6–23.3%) |
+8.8% | |
| No, but planning to be involved |
158/675 23.4% (20.3–26.8%) |
118/572 20.6% (17.4–24.2%) |
−2.8% | |
| No |
390/675 57.8% (54.0–61.5%) |
239/572 41.8% (37.7–45.9%) |
−16% | |
Data are given as absolute ratio and percentage (95% CI). Differences are reported in bold characters when 95% CI are not overlapped
Discussion
This survey highlights promises and challenges regarding the integration of AI in radiology. The rate of responders (2.0%), slightly lower than what we had in 2018 (2.8%), may be attributable not only to the saturation of surveys, but also to the self-perception of lacking sufficient knowledge about AI. Radiographers were now included and non-radiologist/non-radiology residents reached 23.6%, compared to 1.3% in 2018. As of 2018, about 50% of the respondents work at universities/teaching hospitals. Similarly, the large majority of responders (over 90%) are based in Europe.
As of 2018, breast imaging is the subspecialty thought to be most impacted by AI, followed by oncologic imaging. Conversely, general, gastrointestinal/abdominal, musculoskeletal and interventional radiology are considered to be less AI-impacted (see Fig. 3). CT is mostly predicted to be impacted by AI, followed by mammography, in agreement with the perception about breast and oncologic imaging. In fact, screening detection is the first selected application, followed by image postprocessing, protocol optimization, and oncology staging/restaging; only 5% of responders selected “prognosis” as a relevant AI application, probably due to a minor propensity to prognostication.
The perceived AI impact on job opportunities (20% increase, 28% reduction, and 52% no change), when compared to the 2018 survey (32%, 23%, and 45%, respectively) shows no overlapped percentages only for the expectations of job opportunities increase, i.e. a less optimistic vision. The subdivision into AI users (48%) and AI nonusers (52%) shows the responders’ interest in AI; 145/298 nonusers (48%) declared to be planning to use AI. About 50% of both groups do not expect AI-induced changes in job positions, with a slightly more optimistic evaluation by AI users (see Table 2), with borderline significance (p = 0.071). A similar trend can be observed for the association between expected variations in job positions due to AI with being/not being AI researchers (see Table 3). These results suggest that direct experience in AI usage/research may reduce the fear of job position reduction. In 2018, the primary concerns were regarding the AI model's reliability and the potential for job displacement. Now, the focus has expanded to include legal and ethical implications (29%) and data privacy vulnerabilities (20%).
The future radiologists’ AI-driven profile is perceived as more clinical (45%) or more technical (36%); more subspecialized (40%) or more open to report examinations outside their own subspecialty (36%). A similarly balanced trend characterises the perception of the AI's impact on radiological workload. This reflects the unclear consequences of AI in radiology in the mid-long term.
Importantly, 45% of responders call for the radiologist’s responsibility for AI outputs influencing clinical decision-making, while 37% foresee a “shared” responsibility. This data is mirrored by the 45% of responders who believe that patients will not accept a report made by AI alone, even though 36% think that is now too early for this prediction, without substantial change in comparison to 2018. This remains a hot topic, considering the need to keep “machines in the loop”, and avoid machines taking “humans in the loop” [3, 19]. It should be noted that the recently approved EU AI Act (https://artificialintelligenceact.eu/) mandates human oversight for all high-risk AI applications, which include any medical device.
The radiological team hopes that the time saved using AI will be dedicated to a more interactive relationship with other clinicians (40%) and patients (36%). However, to realize this hope a strong effort is needed to counteract a strategy purely aiming to maximize the number of examinations performed/reported [3].
The role of the radiological team in the development and validation of AI tools is perceived by 98% of responders (100% in 2018); “supervision of all stages” and “developing AI-based applications” were stable (64% vs 62 and 28% vs 23%, respectively), while “helping in task definition” decreased from 53% to 45%. We here should consider the declared research involvement in AI systems development and AI testing that in five years increased from 11% to 20% and from 9% to 18%, respectively. Regarding the use of AI tools in clinical practice, 48% of responders declare they are current users, mainly for CT, radiography, MRI, or mammography (only 20% in 2018), and 25% are planning to become users (30% in 2018). An increase in AI research and/or clinical use in the last five years was associated with always relevant roles of the radiological team. A question remains about cross-fertilization between data/computer scientists and the radiological world, up to complete integration of these potential “new colleagues” in the radiological team [25].
Responders demand education in AI. They confirm the topics of advantages/limitations (72% vs 69%), clinical use (62% vs 58%), “how to survive the AI revolution” (8% vs 11%), and “how to avoid the use of AI applications” (2% vs 1%). The choice of technical methods (25% vs 18%) and “how to get into the driver’s seat in using AI” (23% vs 34%) changed, showing the need to learn how AI works when already using it.
About three-quarters of responders are previous/current AI users. Of them, 53% are satisfied/very satisfied with the tools, 35% are “neutral”, and 12% are dissatisfied/very dissatisfied, showing a good-to-moderate evaluation of AI tools implemented in practice. AI companies should investigate these opinions to get feedback for improvement.
We highlight that about 80% of responders are not familiar with medical device regulation and postmarket surveillance requirements for AI tools, topics that need education efforts by radiological societies and academic institutions [12, 26, 27]. Although there is a significant difference between AI users and nonusers regarding familiarity with these regulations—approximately 30% of users and 10% of non-users are familiar (p < 0.001)—it is noteworthy that over 70% of AI users are not familiar, highlighting the strong demand for education in this area.
The lack of a budget dedicated to AI is noted by a majority of responders (only 9% of responders are aware of a dedicated budget). Indeed, the available evidence about the added value of AI is limited. High-quality research with well-designed clinical studies is needed, including randomized controlled trials. Both the previous and the current surveys identified similar barriers to AI implementation, such as costs, lack of validation, and integration challenges, confirming what was reported by the ESR International Forum on AI at the ECR 2021 [28].
The COVID-19 pandemic could have accelerated the adoption of AI in radiology, as supposed by nearly 60% of respondents, even though no definitive evidence exists for this.
Finally, an interest in LLMs emerges, with about 25% of the responders declaring to use LLMs often or occasionally, mainly for scientific purposes. The rate of LLMs use for clinical purposes (about 11–13%) raises concerns considering model reliability and bias, ethical implications and privacy vulnerability. It must be understood that foundation models and LLMs are not present in any current product certified for medical use. The EU AI Act does allow the use of general AI systems for high-risk applications, but actual implementations have not yet become available to test the regulatory framework’s efficacy. Therefore, any current use of generative AI or LLMs in clinical practice is “unauthorized”, with liability falling entirely on the user.
Recent regulations in the EU and the United States address the integration of AI in healthcare. The EU’s AI Act [29] mandates rigorous evaluations for high-risk AI systems, particularly in medical devices, to ensure safety, transparency, and accountability. It also requires high-quality data processing in compliance with GDPR [30]. In the United States, the 2023 Executive Order sets standards for AI, focusing on data protection, non-discrimination, and ethical use, particularly in healthcare [31]. Radiological staff members must ensure AI applications are safe and ethical, maintaining the human element in patient care. Both regions aim to balance innovation with stringent regulatory frameworks to enhance patient outcomes [32].
This survey has limitations. The low response rate and the probable self-selection imply that the herein presented data probably do not reflect the AI perceptions of the entire ESR community. Indeed, these data offer a view of the opinions and perceptions of those ESR members who currently have an interest in the impact of AI in radiology sufficient for deciding to participate in an online survey announced to require 10 min to be completed. Secondly, the survey’s voluntary nature means that individuals with strong views on AI, whether positive or negative, may be more likely to respond, potentially leading to an overrepresentation of these perspectives. Finally, the survey was administered online, which may exclude individuals who are less comfortable with digital technologies, thereby introducing a “digital” bias, even though this should not be so relevant for members of the radiological staff. Despite these limitations, the data collected provide valuable insights into the attitudes of ESR members who have a current interest in the impact of AI on radiology. Indirectly, we could hypothesize that there is no relevant fear of AI spreading within the ESR community. If such concerns existed, the opportunity to express worries and anxieties through an online survey would likely have been utilized.
Future research should focus on longitudinal studies to track AI’s evolving impact, methods to mitigate bias in AI algorithms, and exploring AI’s role in clinical decision-making. Importantly, starting from the viewpoint that AI tools are “medical devices”, the radiology community should try to evaluate their impact through randomized controlled trials. This top-level-of-evidence approach can be used for comparing the diagnostic/clinical outcome of patients randomized to AI-supported diagnoses with that of patients randomized to the traditional pathway. Additionally, ethical and legal implications such as data privacy and liability should be thoroughly investigated. For practical steps, radiology staff should engage in continuous education and training on AI advancements, adhere to ethical guidelines, collaborate with AI developers, communicate transparently with patients about AI usage, and ensure regulatory compliance. These efforts will help integrate AI effectively and ethically into radiological practice.
In conclusion, among the ESR members who participated in the survey, there is a substantial familiarity with AI technologies, as well as recognition of their potential benefits and challenges. Compared to the 2018 survey, there is no relevant change in terms of perception of AI's impact on job position opportunities, with a trend for a more optimistic evaluation from AI users/researchers. The vision of radiologists’ responsibility for AI outputs influencing clinical decision-making also remained unchanged. The use of LLMs is declared not only limited to research, highlighting the need for education in AI and its regulations.
Supplementary information
Acknowledgements
The authors thank the members of the Board of Directors of the ESR Prof. Regina Beets-Tan, Prof. Adrian Brady, Prof. Carlo Catalano, and Prof. Andrea Rockall, for their suggestions during the preparation of the questionnaire. In addition, the authors thank the ESR staff, Ms Stefanie Bolldorf and Ms Bettina Leimberger, and in particular, the communications department of the ESR Ms. Monika Hierath and Mr. Aidan Boyd-Thorpe, who helped in the implementation of the online survey. Finally, the authors thank Prof. Luca Maria Sconfienza, Milan, Italy, for manuscript reviewing as a prominent radiologist “external” to the authors’ panel. We would like to express our sincere gratitude to all the colleagues who took the time to complete the survey. Without their responses and valuable contributions, this important data would not have been possible.
Abbreviations
- AI
Artificial intelligence
- CI
Confidence intervals
- EuroAIM
European Network for the Assessment of Imaging in Medicine
- EuSoMII
European Society of Medical Imaging Informatics
- ESR
European Society of Radiology
- LLMs
Large language models
Authors contributions
All authors participated in the survey design and questionnaire draughting. M.Z., A.C., and F.S. worked on data processing and analysis, and the first manuscript draughting. J.J.V., R.C., M.E.K., and D.P.d.S. participated in data interpretation and contributed relevant intellectual content with specific requests of subgroup analysis and edited the manuscript. All authors read and approved the final version of the submitted manuscript.
Funding
This study received no specific grant from any funding agency in the public, commercial, or non-profit sectors.
Data availability
Data supporting the results reported in the article are available upon reasonable request to the corresponding author.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
J.J.V.: grant to the institution from Qure.ai/Enlitic; consulting fees from Tegus; payment to an institution for lectures from Roche; travel grant from Qure.ai; participation on advisory board from Contextflow, Noaber Foundation, and NLC Ventures; leadership role on the steering committee of the PINPOINT Project (payment to the institution from AstraZeneca) and RSNA Common Data Elements Steering Committee (unpaid); chair scientific committee EuSoMII (unpaid); chair ESR value-based radiology subcommittee (unpaid); phantom shares in Contextflow and Quibim; and member editorial Board European Journal of Radiology (unpaid). D.P.d.S.: consultation fees from Cook Medical, speaker honoraria Bayer AG, and author honoraria Amboss GmbH. Deputy editor for European Radiology member of the Scientific Editorial Board of Insights into Imaging. F.S.: Bayer Healthcare: research grant, ad hoc advisory board; Bracco Imaging: research grant, ad hoc advisory board; GE Healthcare: research grant, ad hoc advisory board; Siemens Healthineers: sponsored talk. Editor-in-Chief of European Radiology Experimental. M.Z. is a member of the Scientific Editorial Board of Insights into Imaging. The remaining authors have no competing interests to declare.
Footnotes
Compared to the same question asked above, one more responder answered: “Never used”.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1186/s13244-024-01801-w.
References
- 1.Codari M, Melazzini L, Morozov SP, van Kuijk CC, Sconfienza LM, Sardanelli F (2019) Impact of artificial intelligence on radiology: a EuroAIM survey among members of the European Society of Radiology. Insights Imaging 10:105. 10.1186/s13244-019-0798-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castiglioni I, Rundo L, Codari M et al (2021) AI applications to medical images: from machine learning to deep learning. Phys Med 83:9–24. 10.1016/j.ejmp.2021.02.006 [DOI] [PubMed] [Google Scholar]
- 3.Pesapane F, Codari M, Sardanelli F (2018) Artificial intelligence in medical imaging: Threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp 2:35. 10.1186/s41747-018-0061-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ESR (2019) What the radiologist should know about artificial intelligence—an ESR white paper. Insights Imaging 10:44. 10.1186/s13244-019-0738-2 [DOI] [PMC free article] [PubMed]
- 5.Shi F, Wang J, Shi J et al (2021) Review of artificial intelligence techniques in imaging data acquisition, segmentation, and diagnosis for COVID-19. IEEE Rev Biomed Eng 14:4–15. 10.1109/RBME.2020.2987975 [DOI] [PubMed] [Google Scholar]
- 6.Becker CD, Kotter E, Fournier L, Martí-Bonmatí L (2022) Current practical experience with artificial intelligence in clinical radiology: a survey of the European Society of Radiology. Insights Imaging 13:107. 10.1186/s13244-022-01247-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577. 10.1148/radiol.2015151169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson BJ, Korfiatis P, Akkus Z, Kline TL (2017) Machine learning for medical imaging. Radiographics 37:505–515. 10.1148/rg.2017160130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohli M, Prevedello LM, Filice RW, Geis JR (2017) Implementing machine learning in radiology practice and research. AJR Am J Roentgenol 208:754–760. 10.2214/AJR.16.17224 [DOI] [PubMed] [Google Scholar]
- 10.Geis JR, Brady A, Wu CC et al (2019) Ethics of artificial intelligence in radiology: summary of the joint European and North American multisociety statement. Insights Imaging 10:101. 10.1186/s13244-019-0785-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhayana R (2024) Chatbots and large language models in radiology: a practical primer for clinical and research applications. Radiology. 10.1148/radiol.232756 [DOI] [PubMed]
- 12.Pesapane F, Volonté C, Codari M, Sardanelli F (2018) Artificial intelligence as a medical device in radiology: ethical and regulatory issues in Europe and the United States. Insights Imaging 9:745–753. 10.1007/s13244-018-0645-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott I, Carter S, Coiera E (2021) Clinician checklist for assessing suitability of machine learning applications in healthcare. BMJ Heal Care Inform 28:e100251. 10.1136/bmjhci-2020-100251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zrubka Z, Gulacsi L, Pentek M (2022) Time to start using checklists for reporting artificial intelligence in health care and biomedical research: a rapid review of available tools. In: 2022 IEEE 26th international conference on intelligent engineering systems (INES). IEEE, pp 000015–000020
- 15.Mongan J, Moy L, Kahn CE (2020) Checklist for artificial intelligence in medical imaging (CLAIM): a guide for authors and reviewers. Radiol Artif Intell 2:e200029. 10.1148/ryai.2020200029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kocak B, Akinci D’Antonoli T, Mercaldo N et al (2024) METhodological RadiomICs Score (METRICS): a quality scoring tool for radiomics research endorsed by EuSoMII. Insights Imaging 15:8. 10.1186/s13244-023-01572-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tejani AS, Klontzas ME, Gatti AA et al (2024) Checklist for artificial intelligence in medical imaging (CLAIM): 2024 update. Radiol Artif Intell. 10.1148/ryai.240300 [DOI] [PMC free article] [PubMed]
- 18.Hamm B, Marti-Bonmati L, Sardanelli F (2024) ESR Journals editors’ joint statement on guidelines for the use of large language models by authors, reviewers, and editors. Eur Radiol. 10.1007/s00330-023-10511-8 [DOI] [PubMed]
- 19.Sardanelli F, Castiglioni I, Colarieti A, Schiaffino S, Di Leo G (2023) Artificial intelligence (AI) in biomedical research: discussion on authors’ declaration of AI in their articles title. Eur Radiol Exp 7:2. 10.1186/s41747-022-00316-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinto dos Santos D, Baeßler B (2018) Big data, artificial intelligence, and structured reporting. Eur Radiol Exp 2:42. 10.1186/s41747-018-0071-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glielmo P, Fusco S, Gitto S et al (2024) Artificial intelligence in interventional radiology: state of the art. Eur Radiol Exp 8:62. 10.1186/s41747-024-00452-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondylakis H, Ciarrocchi E, Cerda-Alberich L et al (2022) Position of the AI for health imaging (AI4HI) network on metadata models for imaging biobanks. Eur Radiol Exp 6:29. 10.1186/s41747-022-00281-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baselli G, Codari M, Sardanelli F (2020) Opening the black box of machine learning in radiology: Can the proximity of annotated cases be a way? Eur Radiol Exp 4:30. 10.1186/s41747-020-00159-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auloge P, Garnon J, Robinson JM et al (2020) Interventional radiology and artificial intelligence in radiology: Is it time to enhance the vision of our medical students? Insights Imaging 11:127. 10.1186/s13244-020-00942-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sardanelli F, Colarieti A (2022) Open issues for education in radiological research: data integrity, study reproducibility, peer-review, levels of evidence, and cross-fertilization with data scientists. Radiol Med 128:133–135. 10.1007/s11547-022-01582-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirvonen J, Becker M, Aronen HJ (2023) Resident education in radiology in Europe including entrustable professional activities: results of an ESR survey. Insights Imaging 14:139. 10.1186/s13244-023-01489-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brady AP, Visser J, Frija G et al (2021) Value-based radiology: What is the ESR doing, and what should we do in the future? Insights Imaging 12:108. 10.1186/s13244-021-01056-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahoney MC, McGinty G, Sanchez GMF et al (2022) Summary of the proceedings of the International Forum 2021: “A more visible radiologist can never be replaced by AI.” Insights Imaging 13:43. 10.1186/s13244-022-01182-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The AI Act: What will the impact be on the medical device industry? https://portolano.it/en/blog/lifesciences/ai-act-impact-medical-device-industry
- 30.EU (2017) The European Parliament and the Council of The European Union Regulation (EU) 2017/746 of the European Parliament and of the Council on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU. https://artificialintelligenceact.eu/
- 31.Biden Jr (2023) Executive order on the safe, secure, and trustworthy development and use of artificial intelligence. https://www.whitehouse.gov/briefing-room/presidential-actions/2023/10/30/executive-order-on-the-safe-secure-and-trustworthy-development-and-use-of-artificial-intelligence/
- 32.Pesapane F, Cuocolo R, Sardanelli F (2024) The Picasso’s skepticism on computer science and the dawn of generative AI: questions after the answers to keep “machines-in-the-loop.” Eur Radiol Exp 8:81. 10.1186/s41747-024-00485-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the results reported in the article are available upon reasonable request to the corresponding author.