Abstract
The assessment of cardiac function using echocardiography has gained a strong foothold in clinical practice. Cardiac magnetic resonance (CMR) imaging harbors distinct advantages over echocardiography, as it is not affected by limitations of acoustic windows and operator dependence. CMR is also designed to non-invasively assess cardiac morphology, ventricular geometry, myocardial wall motion, and intra-cardiac flow quantification without the use of ionizing radiation. These inherent features make CMR appropriate for diagnosing cardiovascular diseases, monitoring patients after treatment, and providing longitudinal follow-up. In this paper, the state-of-the-art work that has demonstrated the aspects of cardiac function by CMR is reviewed, and acquisition techniques and clinical applications are covered.
Keywords: Heart function tests, Magnetic resonance imaging, Strain, Phase-contrast imaging
1. Introduction
The quantification of cardiac morphology and function is pivotal in cardiac imaging, in that it plays a significant role in the prediction, diagnosis, and treatment of patients with almost all types of cardiovascular disease including cardiomyopathies, coronary artery disease, valvular heart disease, congenital heart disease, and heart failure. The unique ability of echocardiography to provide real-time images of the cardiac cycle, coupled with its cost-effectiveness, portability, and widespread availability, makes it the most extensively employed modality for non-invasive assessment of cardiac function.[1] However, operator dependence and a limited acoustic window are some of the well-known technical limitations of echocardiography in clinical applications. To fill these gaps, other imaging modalities such as computed tomography, nuclear medicine, and cardiac magnetic resonance (CMR) are utilized. Nuclear medicine and computed tomography are known to provide accurate assessments of cardiac function with good reproducibility despite their relatively low spatial and temporal resolution, respectively.[2,3] Additionally, repeated measurements resulting in exposure to ionizing radiation make them unsuitable alternatives.
Owing to its excellent tissue characterization and endocardium definition, CMR imaging is widely acknowledged as the gold standard for evaluating cardiac function.[4] There have been numerous recent advances in the CMR assessment of cardiac function that warrant review. We will begin with the most applied and impactful cine technology, namely a traditional method to investigate ventricular and atrial geometry. Next, techniques to detect myocardial stiffness by strain CMR are discussed, given their value for detecting subtle deformations in the underlying substrate. This will be followed by an exploration of phase-contrast CMR imaging of blood flow, which is a relatively new technique but holds promise for a better understanding of blood-tissue interaction. Finally, we will elucidate their utility in diverse clinical scenarios where CMR imaging could prove valuable for cardiovascular diseases, encompassing diagnosis, risk stratification, therapy management, and monitoring [Figure 1].
Figure 1.

Representation and application of function parameters that can be obtained by cardiac magnetic resonance.
2. Conventional cardiac function assessment
2.1. Acquisition methods
Considering that the anatomical evaluation of the heart depends on high spatial resolution, while its functional evaluation requires sufficient temporal resolution, CMR cine imaging usually employs a balanced, steady-state, free precession technique combined with breath holding and electrocardiography gating. With the emergence of new technologies like compressed sensing which overcomes the constraints of traditional Nyquist sampling theorem, the acquisition time for cine imaging would be drastically shortened. It operates on the assumption that the k-space data is randomly undersampled, the image has a sparse representation in some pre-defined basis or dictionary, and a non-linear reconstruction is performed to enforce the sparsity of the image and consistency with the acquired MR data. Thus, the compressed sensing technique offers the advantage of acquiring functional information in at least one plane for patients who are unable to maintain breath-holding for multiple heartbeats or those with arrhythmias. Cine imaging is referred to as “bright blood imaging” because it produces images where the blood pool exhibits a higher brightness than the intermediate signal intensity observed in the adjacent myocardial region. By combining improved edge definition between intraventricular blood and the endocardium with its highly reproducible metrics and larger field of view than 2-dimensional (2D) echocardiography, cine imaging is considered a standard reference for evaluating chamber volumes and function against which other modalities are validated.[4] Nevertheless, there are some challenges in distinguishing certain pathologies or subtle tissue variations using this technique.
2.2. Quantification of chamber volume and function
The application of CMR has demonstrated its accuracy and reproducibility in the estimation of left ventricular volumes, mass, and ejection fraction [Table 1].[5,6] The technique provides a precise and easily traceable volume calculation by offering a spatially defined 3D dataset at multiple levels and phases, with excellent myocardium-to-blood contrast and without geometric assumptions. Distinct from echocardiography, which relies on the biplane method of disks summation (modified Simpson’s rule),[7] CMR is not limited by the assumption about left ventricular shape in deformed hearts. Therefore, CMR provides more accurate and reliable left ventricular volume measurements than transthoracic echocardiography.[8] Left ventricular ejection fraction (LVEF) is derived from volumetric data and calculated as the ratio of stroke volume (the difference between end-systolic and end-diastolic cavity volumes) to the end-diastolic volume (EDV). Despite its limitations, LVEF remains a well-established measure of systolic function given its simplicity and extensive validation. The calculation of left ventricular mass involves multiplying the total volume of the myocardium by the myocardial density which is taken as 1.05 g/mL.[9] High spatial-resolution imaging allows for accurate delineation of wall thickness in all segments, including improved recognition of the crista supraventricularis muscle, which may lead to an overestimation of left ventricular wall thickness. Furthermore, most volumetric and functional parameters used to assess the left ventricle can also be applied to the other chambers. For example, left atrial volume and emptying fraction assessed by CMR are markers of subclinical atrial fibrillation as detected by continuous monitoring; right ventricular ejection fraction is an important determinant of outcomes in heart failure cohorts.
Table 1.
Parameters of biventricular and bi-atrial size and function in healthy Chinese adults according to sex based on CMR.[5,6]
| Items | Men | Women | P |
|---|---|---|---|
| LV | |||
| LVEDVi (mL/m2)* | 77.7 (53.8, 101.6) | 72.7 (52.4, 93.0) | <0.001 |
| LVESVi (mL/m2)* | 30.0 (17.8, 42.2) | 26.8 (16.3, 37.3) | <0.001 |
| LVMi (g/m2)* | 49.1 (35.0, 63.2) | 41.8 (30.7, 52.9) | <0.001 |
| LVEF (%)* | 61.4 (52.1, 70.7) | 63.2 (54.0, 72.4) | <0.001 |
| RV | |||
| RVEDVi (mL/m2)* | 79.8 (53.1, 106.5) | 71.2 (49.4, 93.0) | <0.001 |
| RVESVi (mL/m2)* | 31.3 (18.2, 44.3) | 26.3 (15.5, 37.2) | <0.001 |
| RVEF (%)* | 60.9 (51.8, 69.9) | 63.2 (54.1, 72.3) | <0.001 |
| LA | |||
| LAV total (mL) | 39.1 (20.9–57.3) | 37.7 (23.2–52.2) | 0.109 |
| LAV passive (mL) | 23.2 (8.7–37.7) | 21.8 (10.2–33.4) | 0.041 |
| LAV active (mL) | 15.9 (5.1–26.7) | 15.9 (5.9–25.9) | 0.404 |
| LAEF total (%) | 60.0 (47.9–72.2) | 62.5 (50.9–74.1) | <0.001 |
| LAEF passive (%) | 35.6 (19.1–52.1) | 36.3 (20.2–52.4) | 0.968 |
| LAEF active (%) | 37.6 (22.1–53.1) | 40.9 (26.0–55.8) | <0.001 |
| RA | |||
| RAV total (mL) | 29.9 (11.5–48.3) | 27.5 (10.1–44.9) | 0.008 |
| RAV passive (mL) | 13.9 (1.8–26.1) | 13.3 (1.2–25.5) | 0.368 |
| RAV active (mL) | 16.1 (2.6–29.6) | 14.2 (1.5–26.9) | 0.005 |
| RAEF total (%) | 47.2 (30.7–63.7) | 52.6 (34.4–70.8) | <0.001 |
| RAEF passive (%) | 21.9 (5.4–38.4) | 25.6 (5.6–45.6) | <0.001 |
| RAEF active (%) | 32.3 (14.5–50.1) | 36.0 (15.2–56.8) | 0.001 |
Data are presented as mean (95% confidence interval). *Data are presented as mean (mean–2SD, mean+2SD). CMR: Cardiac magnetic resonance; LA: Left atrium; LAEF/RAEF: Left/right atrial ejection fraction; LAV/RAV: Left/right atrial volume; LV: Left ventricle; LVEDVi/RVEDVi: Left/right ventricular end-diastolic index; LVEF/RVEF: Left/right ventricular ejection fraction; LVESVi/RVESVi: Left/right ventricular end-systolic index; LVMi: Left ventricular mass index; RA: Right atrium; RV: Right ventricle; SD: Standard deviation.
2.3. Clinical applications
2.3.1. Left ventricle
The presence of left ventricular diastolic dysfunction is closely associated with left ventricular hypertrophy, which can be accurately quantified by precise measurements of the left ventricular wall thickness. Notably, compared to 2D echocardiography, CMR has demonstrated superior precision in assessing left ventricular wall thickness in some patients with hypertrophic cardiomyopathy, such as when 64.5% apical aneurysm detected by CMR was not reliably identifiable using 2D echocardiography.[10] Thus, the European Society of Cardiology has recommended performing CMR for the diagnosis of hypertrophic cardiomyopathy when acoustic windows are suboptimal or certain regions are poorly visualized—such as the anterolateral wall, left ventricular apex, or right ventricle.[11] This further solidifies the role of CMR as an indispensable adjunct to echocardiography.
After an acute myocardial infarction, increased pressure and volume load can lead to asymmetrical hypertrophy of the non-infarcted myocardium to preserve cardiac function. Over time, this causes significant increase in left ventricular wall tension, EDV and end-systolic volume, eventually impairing cardiac function by causing mitral or tricuspid regurgitation as well as reducing LVEF. In a contemporary cohort of patients presenting with ST-elevation myocardial infarction (STEMI), CMR imaging observations strongly suggested that an increase in left ventricular EDV index ≥12% might be considered an “adverse” phenomenon linked to decreased LVEF; an increase in end-systolic volume index ≥12% might not necessarily indicate an “adverse” outcome, rather a “compensatory” response associated with improved LVEF at the follow-up.[12] Another study showed that ≥10% EDV reduction at 4 months is strongly correlated with clinical outcome in the same cohort.[13] While the adverse remodeling, defined as a combination >15% increase in EDV with >3% ejection fraction reduction at 6 months, could be the best predictor of 6-year cardiovascular events.[14] Given the variations in the baseline and the period of follow-up, studies on left ventricular remodeling cut-off values have yielded diverse results, indicating a lack of consensus regarding volume thresholds for defining adverse left ventricular remodeling. However, it is widely acknowledged that the degree of structural remodeling observed through CMR is useful to guide therapeutic interventions or assess treatment response.[15,16,17,18] Moreover, the left atrioventricular coupling index has demonstrated incremental prognostic value in predicting incident heart failure, new onset atrial fibrillation, and cardiovascular death beyond traditional risk factors by measuring the ratio of left atrial to left ventricular volumes at left ventricular end-diastole.[19,20,21] Still, it remains unclear whether this index can be incorporated into algorithms to assess left ventricular diastolic function owing to a lack of studies linking it with invasive measurements of left ventricular filling pressure (LVFP) to establish a cut-off value. Thus, LVEF is still widely accepted as a functional surrogate for evaluation of disease progression, therapy response, cardiovascular outcome prediction, or determination of the next step in the treatment algorithm. In patients post-myocardial infarction and those with heart failure, accurate and reliable assessment of LVEF is crucial, because once it exceeds 45%, ejection fraction no longer contributes significantly to cardiovascular risk assessment.[22] Meanwhile, it is recognized that there may be some discrepancy between LVEF derived from CMR and echocardiography.[8] If a specific cut-off value for ejection fraction is used to stratify risk or guide treatment decisions, the diverse techniques will have considerable impact on who is treated, with both clinical and financial consequences. Therefore, caution should be exercised when interpreting LVEF findings in light of locally available techniques.
Although LVEF has been the primary metric to assess systolic left ventricular dysfunction, it is influenced by cardiac load and therefore may be insensitive to detect subtle changes in contractile reserve. As a result, this measure can significantly underestimate or misdiagnose asymptomatic patients with slowly progressing myocardial disorders. To address this limitation, novel parameters based on volume data have been proposed which may play a promising role in quantifying or predicting left ventricular dysfunction. For example, simultaneous measurements of volume from cine imaging and invasive left ventricular pressures could be used to quantitatively evaluate contractility and compliance through pressure-volume loops during dynamic preload reduction.[23]
2.3.2. Left atrium
The left atrial performance can be divided into 3 distinct phases throughout the cardiac cycle: an atrial systole filling phase, an early diastolic rapid ventricular filling conduit phase, and a late diastole active contraction phase. These phases serve 3 functions, namely reservoir, conduit, and pumping that can be evaluated by utilizing CMR-based volumes at different stages. Total emptying, assessed as a cyclic change, refers to the disparity between the maximum and minimum volumes of the left atrium. Passive emptying, represented as left atrial passive volume, can be quantified by calculating the difference between maximum left atrial and pre-atrial contraction volumes. Active left atrial emptying, indicated by left atrial stroke volume, is defined as the difference between pre-atrial contraction and the minimum left atrial volume. The filling function can be further represented by an ejection fraction in each distinct phase, encompassing total left atrial ejection fraction (LAEF), passive LAEF, and active LAEF. Reference values for phasic left atrial volumes and ejection fraction according to sex are shown in Table 1. Left atrial dilation is a compensatory mechanism in response to elevated LVFP resulting from left ventricular diastolic dysfunction. Garg et al[24] proved the diagnostic accuracy of CMR-modeled LVFP estimated from left atrial volume and left ventricular mass. In patients with suspected heart failure, this method can correctly reclassify cases where echocardiology is inconclusive and identify individuals at an elevated risk of mortality. In addition, its predictive accuracy for survival prognosis is comparable to, if not non-inferior to, that of pulmonary capillary wedge pressure derived from right heart catheterizationd. Furthermore, superior to left atrial size enlargement, LAEF may also be a classifier for left ventricular dysfunction as it deteriorates before atrial volumes are measurably affected.[13,25] In patients diagnosed with systemic light-chain amyloidosis, CMR-assessed LAEF shows association with Mayo Clinic stage, New York Heart Association functional class, myocardial late gadolinium enhancement (LGE), and 2-year mortality.[26]
Traditionally, left atrial dysfunction has been regarded as an indicator or consequence of other cardiac conditions rather than the primary etiological factor itself, but recent evidence has indicated that left atrial failure may be an early driver or a crucial pathogenic factor in cardiac dysfunction. For example, LAEF and left atrial volume are strongly linked to prevalent and incident cardiovascular outcomes, regardless of left ventricular metrics.[27] Furthermore, up to 45% patients presenting with heart failure with preserved ejection fraction (HFpEF) onset have underlying left atrial dysfunction as the mechanism.[28] Therefore, gaining a comprehensive understanding of left atrial mechanics is imperative.
2.3.3. Right atrium and ventricle
The use of CMR is particularly advantageous for assessing the morphology and function of the right ventricle and right atrium, overcoming the challenges posed by the unique anatomy of the right chamber and anterior location beneath the sternum within the chest. For example, one of the diagnostic criteria for arrhythmogenic right ventricular cardiomyopathy is severe dilation of the right ventricle and a reduction in its ejection fraction, which can be assessed using CMR.[29] Reference values for right atrial and ventricular volumes and ejection fraction according to sex are shown in Table 1. Given the independent association between right ventricular ejection fraction and heart failure hospitalization as well as mortality, it serves as a valuable tool for selecting candidates for transcatheter aortic valve replacement,[30] or revising the stratification of prognostic assessment in patients with heart failure.[31]
Although there is a scarcity of data on right atrial remodeling and shape alterations, recent advance of artificial intelligence has enabled automated analysis of right atrial size and function with high efficiency and moderate associations compared to invasive hemodynamics.[32] Men demonstrate greater right atrial volume, while women have higher right atrial ejection fraction; for both sexes, aging is associated with decreased right atrial ejection fraction.[5] Although recent studies have suggested a frequent occurrence of bi-atrial remodeling in atrial fibrillation, an interesting finding was that the presence of right atrial remodeling does not predict atrial fibrillation recurrence after atrial fibrillation ablation.[33]
Overall, while CMR cine imaging allows for a comprehensive evaluation of cardiac morphology and function, the measurable indices like ejection fraction or volume primarily reflect manifestations of advanced disease progression. However, it is important to undertake the arduous task of identifying early disease development indicators or subtle regional information, thereby shifting the emphasis from LVEF to an alternative metric such as strain.
3. Advanced myocardial deformation imaging with strain
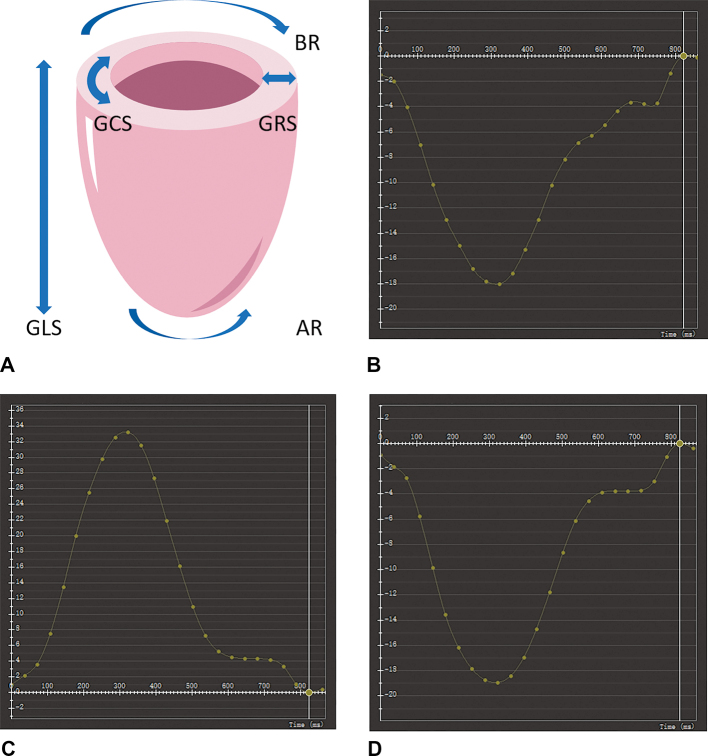
Myocardial deformation is considered complementary to standard measures of ejection fraction and is sometimes more effective in detecting alterations in left ventricular function, which can be precursors to overt structural and functional deficits. To gain a comprehensive understanding of deformation, it is imperative to possess a profound comprehension of myocardial architecture and the distinct contributions made by various layers of the myocardium to left ventricular strain. The myocytes are arranged in planes that run parallel to the longitudinal axis of the heart, forming local average “fiber” direction. In proximity to the midwall of the ventricle, this orientation becomes predominantly circumferential (resembling a “hoop”). However, it undergoes clockwise rotation resulting in a left-handed helix with an angle greater than or equal to 60° in the subepicardium and counterclockwise rotation leading to a right-handed helix with an angle less than or equal to 60° in the subendocardium. Contraction induces strains and corresponding shears across these layers with varying orientations. Radial strain arises from the contraction of fibers occurring in all layers, accompanied by thickening and inward displacement of the myocardium. Longitudinal strain refers to the contraction of myocardial fibers from the base toward the apex, whereas circumferential strain signifies fiber shortening along the circular perimeter [Figure 2].[34] The intricate architecture of the left ventricle enables only 15% fiber shortening to translate into a substantial 60% alteration in the left ventricular volume.
Figure 2.
Components of LV myocardial deformation. (A) Myocardial strain can be projected into a cylindrical system of reference centered around the left ventricle. A longitudinal strain curve (B) is derived from the long-axis four chamber cine image, while the short-axis image is utilized for calculating radial (C) and circumferential strain (D) curves in a healthy volunteer. AR: Apical rotation; BR: Basal rotation; GCS: Global circumferential strain; GLS: Global longitudinal strain; GRS: Global radial strain; LV: Left ventricular.
3.1. Acquisition methods
Speckle-tracking echocardiography is well established as a modality for assessing myocardial stiffness,[35] but its reproducibility is heavily dependent on image quality.[36] Strain imaging using CMR has generated considerable attention as a promising development in this field. It can provide valuable clinical contributions to the appraisement of myocardial deformation through either tailored image acquisition or post-processing software [Table 2].
Table 2.
Characteristics of CMR techniques to assess myocardial strain with main advantages and disadvantages.
| CMR technique | How to work | Advantages | Disadvantages |
|---|---|---|---|
| Dedicated acquisition | |||
| CMR tagging | Track planes of saturated myocardial magnetization tags | •Good tags tracking •Higher reproducibility of plane acquisitions •Extensive validation |
•Low spatial and temporal resolution •Fading tags •Long post-processing and acquisition time |
| DENSE | Encode tissue displacement with intrinsic phase correction | •High spatial resolution •Good-quality strain in short acquisition time |
•Low signal-to-noise ratio •Fading tags •Modest clinical experience |
| SENC | Use magnetization tags parallelly overlaid on the myocardium | •High spatial resolution •Fast post-processing •Better reproducibility |
•Measures only through plane strain •No radial strain •Fading tags •Modest clinical experience |
| PVM | Encode myocardial velocity in 3 directions | •High spatial resolution | •Low temporal resolution •Time-consuming and cumbersome |
| Post-processing method | |||
| FT-CMR | Track features along the myocardial wall detailing endocardial and epicardial contour | •Regional and global strain measurements •No additional image acquisition |
•Low spatial and temporal resolution •Less performance for regional strain •No standardization •Inter-vendor differences •No rotation/twist |
CMR: Cardiac magnetic resonance; DENSE: Displacement encoding with stimulated echoes; FT-CMR: Feature-tracking CMR; PVM: Phase-velocity mapping; SENC: Strain-encoded imaging.
The CMR tagging technique, initially introduced by Zerhouni et al,[37] has been extensively employed in numerous clinical studies and is widely acknowledged as the gold standard for validating other strain measurement techniques including speckle-tracking echocardiography.[38,39] This methodology entails an initial pre-acquisition phase wherein magnetic labels (black lines, tags) are precisely positioned over the myocardium. Subsequently, by tracking the motion of these tags that move simultaneously with the myocardium, a direct quantification of myocardial relaxation and strain throughout the cardiac cycle can be obtained.[40] Despite being the most extensively validated CMR technique for assessing myocardial strain, tagging is limited in terms of low spatial resolution and its ability to evaluate thin myocardial layers. Furthermore, dedicated acquisition and long post-processing time are what mainly limit its use as predominant research tools. Subsequent methods based on the principles of tagging have been introduced for strain evaluation, including displacement encoding with stimulated echoes and strain-encoded imaging.[41,42] These techniques enable rapid examination through a single-shot acquisition in one heartbeat, facilitating faster post-processing and ensuring excellent reproducibility. However, similar to tags, displacement encoding with stimulated echoes is unable to fully assess the entire cardiac cycle because of the tendency for encoding to disappear. The main limitation of strain-encoded imaging is its inability to evaluate radial deformation; whereas, longitudinal strain on short-axis images and circumferential strain on long-axis images can be assessed. Likewise, phase-velocity mapping CMR offers an alternative approach for evaluating myocardial deformation by allowing calculations of myocardial velocities in 3 directions with enhanced spatial resolution, albeit at the expense of reduced temporal resolution.[43] Feature-tracking CMR is a post-processing technique that can be applied to routinely acquired cine images.[44,45] The endocardium features are tracked over time in successive imaging frames, allowing for the measurement of deformation. Specifically, this method entails the delineation of a small patch surrounding the pixel within a single frame, followed by an extensive search for a corresponding patch of pixels in the subsequent image frame. The distance between these 2 identified regions determines the local tissue displacement, providing measurements independent of ventricular size or shape and without requiring dedicated acquisition.[44,45] However, it should be noted that cine images used in feature-tracking CMR exhibit significantly reduced spatial and temporal resolution when compared to tagging.[45]
Despite the presence of various strain techniques based on CMR, standardization remains a distant objective considering their research progress, technological challenges, or variations in clinical practices. The reported normal ranges exhibit significant variabilities attributed to multiple factors, including those associated with the imaging modality, software and vendor used, operator expertise, patient demographics, and hemodynamic characteristics.[46,47,48] As shown in Table 3, longitudinal and circumferential strains demonstrate negative values indicating shortening, thinning, and/or contraction; whereas, radial strain shows positive values suggesting lengthening, thickening, and/or relaxation. An abnormality is determined by a mathematically higher threshold for negative-strain variables and a lower threshold for positive-strain variables. Longitudinal strain is among the most frequently utilized deformation components given its (almost) uniform distribution along the wall and its ability to evaluate the entire left ventricle in an easy and reliable manner.
Table 3.
| CMR technique | LVGLS | LVGCS | LVGRS | RVGLS | LAtotal | RAtotal |
|---|---|---|---|---|---|---|
| CMR tagging | −14.6 (−16.2 to −12.9) | −19.9 (−21.1 to −17.7) | 16.2 (11.2–21.2) | ND | ND | ND |
| DENSE | ND | −19.0 (−19.7 to −18.3) | 24.3 (16.2–32.2) | ND | ND | ND |
| SENC | −20.0 (−22.5 to −17.4) | −20.9 (−22.4 to −19.3) | ND | −18.7 (−19.5 to −17.9) | ND | ND |
| FT-CMR | −18.4 (−19.2 to −17.6) | −21.4 (−22.3 to −20.6) | 43.7 (40–47.4) | −24.0 (−25.8 to −22.1) | 34.9 (29.6–40.2) | 36.3 (15.5–57.0) |
Data are presented as mean (95% confidence interval). CMR: Cardiac magnetic resonance; DENSE: Displacement encoding with stimulated echoes; FT-CMR: Feature-tracking CMR; GCS: Global circumferential strain; GLS: Global longitudinal strain; GRS: Global radial strain; LA: Left atrium; LV: Left ventricle; ND: No data; RA: Right atrium; RV: Right ventricle; SENC: Strain-encoded imaging.
3.2. Strain parameters and factors affecting strain values
The normal motion of the heart wall and its associated strain patterns are influenced by a complex interplay of forces among various structures. Myocardial deformation, as a compensatory mechanism, develops in response to changes in various conditions within the cardiac muscle. Previous studies have confirmed that preload positively affects strain through the Frank-Starling mechanism when contractile function is preserved.[49] In patients with acute volume overload caused by mitral or aortic regurgitation, early-stage disease is characterized by increased deformation while maintaining intact myocardial contractility.[50,51] The impact of chronic increases in afterload, such as systemic hypertension, pulmonary hypertension, and aortic stenosis, has been consistently observed to result in the reduction of left ventricular global longitudinal strain (GLS) according to several studies.[52,53] In addition, the afterload on myofibers is determined by the pressure within the cavity, while ventricular geometry (such as diameter and wall thickness) determines how cavity pressure translates into the relevant parameter of “wall stress.” For example, in later stages of severe regurgitation, prolonged volume overload leads to progressive chamber enlargement accompanied by escalating wall stress and ultimately irreversible contractile dysfunction characterized by reduced strain values.[51] To account for abnormal volume compensation, it might be worth considering correcting strain values based on left ventricular volumes in future analyses. Furthermore, for a given pre- and afterload, in addition to intrinsic contractility, the shortening of myofibers can be further influenced by various other tissue characteristics. One such characteristic is fibrosis and depositions within the myocardium that can alter the contractile properties of the muscle fibers. The heterogeneity of myocardial function, either regionally or temporally, as well as alterations in passive properties of the myocardium can also affect myofiber shortening. Illustrative examples include ischemic disease, which leads to reduced contractility due to scar formation following inadequate blood supply over a prolonged period; various infiltrative and storage diseases such as amyloidosis or hemochromatosis; toxic effects resulting from chemotherapy; and other conditions that induce irreversible damage to myocytes. Finally, pathologic interactions occur between the left ventricular walls owing to conduction delays like left bundle branch block, resulting in heterogeneous myocardial activation.[54]
3.3. Clinical applications
Strain measurements have been recommended as essential functional parameters in routine echocardiography guidelines. Thanks to ongoing technological advancements, strain derived from CMR is poised to offer valuable supplementary insights in the domains of diagnosis, pathophysiology, and risk stratification [Table 4]. Given these advantages, the incorporation of CMR-based strain analysis into routine clinical evaluations is eagerly anticipated and expected to become a standard practice in the near future.
Table 4.
Clinical application of LV myocardial strain by cardiac disease.
| Disease | Factors affecting strain | Recent clinical application |
|---|---|---|
| Ischemic disease | •Regional inhomogeneity in contractility | •Predict outcomes and stratify risks in ischemic cardiomyopathy •Monitor cardiac function and validate novel therapies •Identify infarct tissue and segments that will recover function |
| Cardiomyopathy | •Chamber geometry •Myocardial deposits and fibrosis •Reduced contractility •Regionally inhomogeneous function |
•Understand the pathophysiology of non-ischemic cardiomyopathies •Distinguish cardiac amyloidosis from other forms of LV hypertrophy •Detect early cardiac dysfunction before LVEF •Predict outcomes in DCM, HCM, amyloidosis, and myocarditis |
| Valvular heart diseases and congenital heart disease | •Preload and afterload •Chamber geometry (remodeling) •Contractility |
•Identify asymptomatic patients who may benefit from surgery •Select optimal surgical time and approach •Identify early dysfunction after surgery |
| Cardiotoxicity | •Globally impaired contractility | •Identify and monitor for subclinical cardiac dysfunction after cancer treatment |
| Cardiac resynchronization therapy | •Inhomogeneous timing of contraction •Inhomogeneous regional remodeling |
•Deliver insights into the complexities of cardiac mechanical dyssynchrony •Guide LV lead deployment •Identify responders for cardiac resynchronization therapy |
DCM: Dilated cardiomyopathy; HCM: Hypertrophic cardiomyopathy; LV: Left ventricle; LVEF: Left ventricular ejection fraction.
3.3.1. Left ventricular strain
3.3.1.1. Ischemic heart disease
The subendocardially located longitudinally orientated fibers are particularly vulnerable to ischemia, making GLS a more sensitive metric for risk stratification and an independent and superior predictor of outcomes compared to well-known risk markers such as LVEF, severity of infarction, or microvascular obstruction in ischemic heart disease.[55] When it comes to monitoring cardiac function and validating novel therapies among STEMI patients, both GLS and global circumferential strain (GCS) have shown robustness and potential accuracy.[56] Aditionally, deterioration of GCS has discriminative value between normal and stenotic segments as well as between stenotic and neighboring segments.[57] However, the impact of most pathologies on the heart is not uniform, necessitating the identification of regional dysfunction which may be concealed by seemingly “normal” values of global function. For instance, the circumferential strain in regions of infarcted tissue, as highlighted by LGE, is markedly different from that of healthy myocardium. This distinction provides clinically valuable insights into the contractile function and its potential for recovery in patients with myocardial infarction.[58]
3.3.1.2. Non-ischemic heart disease
Strain analysis offers valuable insights into the pathophysiology of various non-ischemic heart diseases. Specifically, in isolated left ventricular non-compaction, the apical subendocardium is predominantly affected because of the compaction process occurring from the basal segment to the apex and from the epicardium to the endocardium.[59] This phenomenon accounts for why middle and apical longitudinal strain decrease more prominently in left ventricular non-compaction than in dilated cardiomyopathy, which primarily affects disordered myocardial fibers in the midwall layer, that is circumferentially arranged myocardium.[60] “Apical sparing” is a phenomenon observed in patients with cardiac amyloidosis, where the apex of the heart is preserved or relatively spared from significant strain and thickening compared to other regions. The coexistence of a base-to-apex gradient in quantitative LGE burden among these patients suggests that apex sparing may be partially attributed to the extent of amyloid deposition and fibrosis. Therefore, one potential significance of strain measurements lies in their ability to eliminate the need for intravenous contrast material if a strong linear correlation between impaired strain and the presence of LGE is observed.[61] Meanwhile, several studies have utilized CMR to differentiate cardiac amyloidosis from hypertensive heart disease and hypertrophic cardiomyopathy by examining the presence of apical sparing.[62,63] Overall, strain measurement sheds lights on the pathophysiology of various non-ischemic heart diseases and thus provides a new approach for differential diagnosis; however, further research in these directions is still needed.
CMR strain imaging has demonstrated its significance across a broad spectrum of cardiovascular diseases, not only allowing for early detection of cardiac dysfunction before changes in LVEF occur,[64,65] but also contributing to improved risk stratification and provides incremental/independent prognostic value. For instance, impaired GLS demonstrated significant independent predictive value for mortality or major adverse cardiac events (MACE) in patients with dilated cardiomyopathy, surpassing routine and dedicated functional parameters such as ejection fraction and LGE.[66,67] In patients with hypertrophic cardiomyopathy, reduced longitudinal deformation among strains in 3 directions remains a significant independent predictor of MACE, including sudden cardiac death, resuscitated cardiac arrest due to ventricular fibrillation or hemodynamically unstable ventricular tachycardia, and hospitalization for heart failure.[68] Strain as an independent predictor of adverse outcomes is similarly validated against in myocarditis.[69,70]
3.3.1.3. Valvular heart disease and congenital heart disease
Strain may potentially contribute to patient selection for surgery in valvular heart disease. For example, patients with severe aortic stenosis who are asymptomatic or have only mild symptoms demonstrate a significant reduction in both circumferential and longitudinal strain, comparable to those with pronounced symptoms necessitating aortic valve replacement.[71] Strain is also a valuable tool for clinicians and researchers to understand and compare the immediate effects of different treatments after aortic valve replacement: transcatheter aortic valve implantation leads to an immediate improvement in GLS, and early after surgical aortic valve replacement, there is a notable worsening of GLS. In the longer-term follow-up, both interventions are found to be associated with an improvement in left ventricular myocardial deformation and a reduction in left ventricular hypertrophy.[72] Additionally, several pieces of evidence have provided further information, paving the way toward validating strain as an indicator for early identification of dysfunction after surgery in several congenital heart disease and asymptomatic heart transplant patients.[73]
3.3.1.4. Cardiotoxicity
The advantage of strain measurement, which exhibits less dependency compared to ejection fraction, is particularly valuable in clinical scenarios where subtle changes in myocardial function are of critical importance, such as the detection of chemotherapy-induced cardiotoxicity. The American Society of Echocardiography and the European Society of Cardiology recommend a relative drop in GLS exceeding 15% compared to baseline as clinically significant.[74] Although fairly under developed, clinical trials are currently underway to evaluate the efficacy of initiating cardioprotective medication based on CMR strain parameters. CMR has shown that LVEF and GCS exhibit early deterioration and persistently abnormal levels even after 6 months of initiating low-to-moderate doses of anthracyclines.[75] Breast cancer patients undergoing trastuzumab treatment exhibit a significant decrease in GLS and GCS, which corresponds with a decline in LVEF at 6 months and 12 months.[76] Previous studies have demonstrated that left ventricular diastolic impairment precedes the reduction of LVEF in the context of anthracycline-related cardiotoxicity.[77] However, it appears that systolic strain rates may be more pertinent than diastolic strain rates in monitoring subclinical trastuzumab-related myocardial impairment, as evidenced by the sustained stability of diastolic strain rates for 18 months following therapy initiation in a study involving cancer patients receiving trastuzumab.[78] Therefore, investigating whether strain-guided therapy could provide enhanced cardiac protection to survivors of potentially cardiotoxic chemotherapy is an area of research interest.[79,80]
3.3.1.5. Cardiac resynchronization therapy
Considerable efforts have been made to the imaging-based identification of dyssynchrony to select optimal candidates for cardiac resynchronization therapy. More recently, the feasibility of feature-tracking CMR has been demonstrated for evaluating left ventricular dyssynchrony.[81,82] In patients undergoing cardiac resynchronization therapy implantation, deploying a lead over non-scarred segments with the latest mechanical activation has demonstrated significant reverse remodeling and improved clinical outcomes compared to deploying the lead over scarred and/or earlier activated segments.[83] Emerging evidence suggests that identifying specific patterns of mechanical dyssynchrony can greatly increase the response rates to cardiac resynchronization therapy, while relying solely on simplistic measures such as time to peak strain or peak velocity differences may lead to suboptimal patient selection for cardiac resynchronization therapy.[81,84]
3.3.2. Left atrial strain
Atrial strain, which indirectly reflects left ventricular relaxation ability and compliance, has increasingly played a significant role in cardiac performance. Given the unique orientations of fibers and the thinness of the atrial wall, only longitudinal strain is typically assessed at the atrial level. Left atrium strain comprises 3 components, namely passive strain (εE, corresponding to conduit function), active strain (εA, related to booster pump function), and total strain (εS, representing reservoir function). Normal values for left atrium-global strain are 34.9% (29.6%–40.2%) for the reservoir function, 21.3% (16.6%–26.1%) for the conduit function, and 14.3% (11.8%–16.8%) for the booster pump phases, respectively. The left atrial strain can vary with age, sex, and ethnicity. Female patients tend to have higher values of left atrial strain parameters than male patients. As individuals age, the active strain and strain rate gradually increase, while passive strain gradually decreases. This phenomenon may serve as a compensatory mechanism in response to impaired ventricular diastolic function. The variation in active strain was influenced by the regional distribution of the population, with Asian subjects exhibiting the highest strain values. Other factors also include post-processing vendors.
Dysfunction of the left atrium is commonly observed in patients with atrial fibrillation. The strain on the left atrial reservoir and conduit is significantly reduced in patients with atrial fibrillation who exhibit extensive atrial fibrosis.[85] Therefore, apart from predicting the onset of new atrial fibrillation, left atrial strain can also serve as a predictor for atrial fibrillation recurrence after ablation.[86,87] Left atrial remodeling, characterized by decreased peak reservoir strain even in the presence of normal left atrial size, has been observed in cardiometabolic conditions such as diabetes.[88] These patients exhibited a heightened risk of MACE in the presence of elevated filling pressure and diastolic dysfunction diseases such as STEMI and heart failure.[89,90] In patients with hypertrophic cardiomyopathy, impairments of both left atrial reservoir and conduit precede the enlargement of the left atrium,[91] suggesting that left atrial strain is a more sensitive indicator than left atrial volume for detecting involvement of the left atrium in hypertrophic cardiomyopathy. While they are both strongly associated with outcomes,[92] left atrial conduit strain appears to be a stronger predictor of outcome than reservoir strain. It outperforms left ventricular GLS, LVEF, and left atrial volume index in predicting all-cause mortality and hospitalization due to heart failure.[93] In summary, the utilization of left atrial strain analysis holds significant potential in various clinical scenarios primarily involving the left atrium, including atrial fibrillation prediction, post-ablation atrial fibrillation recurrence evaluation, and risk stratification in cardiomyopathy.
3.3.3. Right atrial and ventricular strain
Similarly, extensive research has been conducted on right ventricular free wall strain and right atrial strain. In contrast to the observed left ventricular strain patterns, the right ventricular myofibers exhibit a predominant inner longitudinal and outer oblique orientation.[94] As a result, right ventricular strain and strain rates demonstrate a more heterogeneous distribution compared to the left ventricle, displaying a reverse base-apical gradient. However, there is currently a relative lack of validation in large samples for reference ranges of right ventricular strain [Table 3].
The latest research findings have demonstrated that in healthy volunteers, right ventricular longitudinal shortening plays a more significant role in determining right ventricular systolic function than circumferential shortening. Moreover, right ventricular free wall longitudinal strain provides prognostic insights for patients with severe functional tricuspid regurgitation beyond common clinical and CMR risk factors such as right ventricular function, size, and the presence of LGE.[95] However, in patients with pulmonary hypertension, the rate of GCS was superior than GLS in identifying patients at heightened risk of combined events of death, lung transplantation, or functional class deterioration.[96] In addition to its diagnostic value in cases of arrhythmogenic right ventricular cardiomyopathy,[97] biventricular myocardial deformation represents a significant advancement in risk stratification over traditional measures such as right ventricle size and clinical variables when assessing prognosis in patients with repaired tetralogy of Fallot.[98] Interestingly enough, right ventricular longitudinal strain emerges as an independent predictor of ventricular tachycardia in hypertrophic cardiomyopathy[99]; however, its diagnostic value is limited in the context of myocarditis.[100] Taken together, recent studies have suggested that CMR-derived right ventricular strain could serve as a reliable prognostic indicator for patients with tricuspid regurgitation, hypertensive heart disease, arrhythmogenic right ventricular cardiomyopathy, tetralogy of Fallot, or certain types of cardiomyopathy.
The right atrium is now being recognized as more than a passive chamber and can be divided, similar to the left atrial strain, into 3 phases (reservoir, conduit, and booster). However, accurately assessing right atrial deformation using speckle-tracking echocardiography poses significant challenges due to its extremely thin atrial wall, the retrosternal location, and the presence of the caval venous system. Many studies have focused on the crucial role of right atrial strain in pulmonary hypertension.[101,102] Moreover, it is interesting to note that altered phasic function of the right atrium in response to chronic right ventricular pressure overload highlights the importance of paying attention to right atrial function when managing patients with pulmonary hypertension and systemic congestion caused by backward venous flow.[103] While the incremental deterioration of bi-atrium strain indicates bi-atrial dysfunction caused by increasing left ventricular diastolic dysfunction and atrial fibrillation,[104] it has been found that right atrial parameters could not predict atrial fibrillation recurrence after atrial fibrillation ablation.[33] Furthermore, analysis of right atrial strain may also have clinical utility and prognostic value in conditions such as dilated cardiomyopathy,[105] HFpEF,[106] and myocardial infarction.[107]
4. Flow imaging
To maintain efficient cardiac output, the intra-cardiac blood flow should be adjusted in response to structural changes in the heart. Hence, flow analysis may likely unveil preclinical manifestations of disease or physiologically unstable conditions that may initiate progressive left ventricular remodeling and eventually lead to clinically overt heart failure. While wall motion abnormalities like hypokinesia or asynchrony directly indicate the presence of overt clinical disease, abnormal flow patterns could suggest maladaptive function even prior to detectable structural changes. The utilization of phase-contrast imaging techniques or cine imaging techniques has provided valuable clinical insights particularly into physiological and pathophysiological processes of cardiovascular diseases, otherwise unachievable with conventional cardiovascular imaging.
4.1. The utilization of phase-contrast imaging
4.1.1. 2D CMR phase-contrast imaging
Although Doppler echocardiography is commonly selected as the initial test for assessing flow in valvular heart disease, its precision heavily relies on the operator. Challenges such as poor acoustic windows, inappropriate probe placement, and misalignment of the left ventricle often compromise its reliability. In contrast, the 2D CMR phase-contrast imaging overcomes many limitations associated with echocardiography by utilizing its inherent flow sensitivity to generate phase images, in addition to acquiring magnitude images similar to conventional cardiac gated cine MR.[108] This technique operates on the principle of velocity-encoding, where 2 opposing gradient pulses are added to the imaging sequence. In pixels with stationary tissue, the effects of the 2 pulses cancel each other out. However, if there is tissue motion between the pulses, a phase shift proportional to the velocity along the gradient’s direction is observed in that pixel. Typically, single-direction (through-plane) velocity encoding is used for image acquisition during breath-holding, as it is less affected by motion outside of the imaging plane. Consequently, if flow is required at different locations, it is necessary to repeat the acquisition process at each site to ensure perpendicular intersection with the vessel of interest. However, this technical and time-consuming procedure may pose difficulties for patients, particularly those with complex congenital heart disease. In addition, precise setting of the sensitivity of velocity encoding (Venc) before image acquisition is crucial to phase-contrast imaging, and it is determined by the disparity in flow sensitivities between the 2 acquisitions. High Venc causes a decreased velocity-to-noise ratio, while low Venc leads to aliasing artifacts. After acquiring the image data, the resulting phase is calculated and subtracted from 2 separate images to generate a velocity image, in which the gray scale of each pixel represents the velocity of blood flow. Generally, the stationary tissue appears as mid-gray, while forward (positive) and reverse (negative) velocities are indicated by higher and lower pixel intensities, respectively. Phase-contrast imaging can assess various aspects of complex blood flow in the heart and vessels: volume flow, peak blood and pulse-wave velocity, patterns and timings of velocity waveforms, and wall shear stress (mainly in the great vessels). The current clinical applications of phase-contrast imaging in cardiac assessment will be discussed later. More comprehensive applications pertaining to the great vessels are beyond the scope of this review, but have been covered elsewhere.[109]
In patients with valvular heart disease, it is important to identify valvular stenosis and/or regurgitation, assess their severity, as well as any associated anatomical abnormalities. Historically, the severity of aortic stenosis has been assessed by evaluating the transvalvular pressure gradient and valve area calculated through phase-contrast-derived measures that have demonstrated excellent concordance with color Doppler echocardiography measurements.[110,111] However, there is often disagreement between Doppler echocardiography and CMR in grading the severity of regurgitation, which affects intervention timing and surgical planning in patients with aortic regurgitation.[112] The slight superiority (difference) of CMR over echocardiography can be attributed to its direct measurement of valve flow, including regurgitant volume, as opposed to relying on secondary estimates like vena contracta or proximal isovelocity surface area, which compromise the reliability of echocardiography. In the evaluation of left-to-right shunts, 2D phase-contrast imaging not only provides precise characterization of the defect in terms of its presence, location, and size but also enables simultaneous computation of the pulmonary-to-systemic ratio by evaluating both the pulmonary trunk and ascending aorta. This approach exhibits substantial concordance when compared to catheter oximetry.[113] Therefore, phase-contrast-CMR is highly suitable for the pre- and post-surgical evaluation of patients with congenital heart disease owing to its capability in providing reliable evaluation of intricate cardiac and vascular anatomy as well as hemodynamics. This enables distinct management approaches and facilitates routine follow-up tests.[114] However, before its practical clinical usefulness can be confirmed, further studies involving larger populations are needed.
4.1.2. 4D flow CMR
4D flow CMR encodes the velocity in all 3 directions within a defined volume over time. This technique provides a time-resolved, three-directional phase-contrast imaging where “4D” denotes the combination of 3D plus time, thereby overcoming previous limitations in capturing multi-dimensional and multi-directional flow characteristics. Just as in 2D phase-contrast CMR, Venc sensitivity is required for optimal control, which is usually close to the maximum velocity (<25% above), but this value must be adjusted as guidance when stenosis or abnormal structure is suspected.[115] Similar to 2D phase-contrast imaging, variations in gradient fields give rise to spatial and temporal phase offsets, leading to inaccuracies in velocity measurements. The primary sources of errors that necessitate correction prior to flow visualization and quantification include Maxwell terms, Eddy current effects, gradient field distortions, and phase wraps. Optimal corrections for background phase offsets may differ depending on CMR systems, sequences, protocols, and regions of interest.
Despite the time-consuming and intricate nature of data processing, the utilization of diverse visualization modalities offers unparalleled capabilities to present distinct and complementary representations of the same flow field. Visualization techniques commonly employed in 4D flow CMR encompass velocity-based color coding, instantaneous streamlines, and pathlines [Figure 3]. The magnitude of velocity can be visually depicted through color coding or by adjusting vector sizes, or a combination thereof. Instantaneous streamlines are traces that run parallel to velocity vectors at all spatial points along their length in a 3D velocity field, rendering them suitable for illustrating a specific moment within an evolving flow field. This enables characterization of blood flow patterns such as laminar flow, helical flow, and vortical flow. In contrast to streamlines, pathlines illustrate the trajectory of a particle (ie, voxel) over time by performing backward/forward particle tracing using time integration methods for displacement calculation based on velocity data.
Figure 3.
Visualization of blood flow using 4-dimensional flow cardiac magnetic resonance. The blood flow in panel (A) is visualized using a color-coded vector glyph representation, which accurately depicts both the direction and magnitude of velocity. In contrast, panel (B) utilizes a pathline representation, while panel (C) employs a streamline representation.
The quantification of flow volume using CMR is commonly employed in numerous institutions for the estimation of mean/peak velocity and flow volume, among other parameters.[116] The 4D flow measurements of blood flow exhibit strong agreement with those obtained in 2D CMR and demonstrate excellent scan-rescan repeatability.[117] In addition to the above-mentioned conventional intra-cardiac flow parameters, an increasing number of novel hemodynamic parameters are being investigated, including kinetic energy, turbulent kinetic energy (TKE), pressure gradient, and differential flow analysis. With significant advancements in CMR acceleration, 4D flow CMR has transitioned from being solely a research tool to becoming an essential resource for clinicians. Consequently, there has been an exponential increase in the number of publications on this topic in recent years.
4.1.2.1. 4D flow CMR vs. 4D color Doppler echocardiography
To enhance the clinical assessment of cardiac function by incorporating flow-mediated hemodynamic forces (HDFs), the application of 4D color Doppler echocardiography has been extensively investigated in clinical research, such as the direct quantification of mitral regurgitant flow volume using dealiasing of color Doppler flow at the vena contracta or imaging the mitral annulus and the left ventricular outflow tract to quantify mitral inflow and aortic stroke volumes within the same cardiac cycle.[118,119] However, several challenges such as the difficulty in configuring echo window settings remain unresolved, making it impractical for cases involving complex 3D flow or high-velocity jets. In comparison, besides offering a comprehensive evaluation of flow in any direction within the acquired volume of interest, 4D flow CMR also enables the generation of numerous novel hemodynamic parameters that have the potential to unlock new avenues for managing cardiovascular diseases. Given the significance of ensuring consistency, reliability, and comparability across different clinical settings, it is imperative to establish standardized protocols for integrating sophisticated 4D CMR techniques into clinical practice.
4.1.3. Advanced hemodynamic analysis based on 4D flow
4.1.3.1. Kinetic energy
Kinetic energy is transferred to the blood as the left ventricle actively expands during diastole and draws blood in from the left atrium, which can be calculated based on local velocity and rate of blood flow. In normal hearts, there are 3 kinetic energy peaks corresponding to the velocity peaks observed on trans-mitral Doppler echocardiography, occurring in mid-systole, early diastole (also known as the “E wave”), and end/late diastole (also known as the “A wave”). Within the left ventricle, the highest kinetic energy peak generated in early diastole serves as a reminder that ventricular relaxation is an active process. Reportedly, early diastolic peak kinetic energy declines with aging. It can be attributed to age-associated increases in cardiac stiffness which are believed to contribute to HFpEF.[120] Therefore, diastolic kinetic energy could serve as a surrogate marker for ventricular compliance, and applying this technique to patients with HFpEF could provide further insights into the underlying pathophysiological mechanisms. A significant reduction in peak E wave kinetic energy is inversely associated with adverse remodeling observed in patients with STEMI, providing new hemodynamic insights for left ventricular remodeling.[121]
4.1.3.2. TKE and viscous energy loss
A portion of the blood flow’s kinetic energy is converted into TKE when standard cardiovascular blood flow transitions from laminar to turbulent due to high-velocity fluctuations caused by conditions such as valvular stenosis. Energy loss is considered a novel indicator of flow inefficiency, resulting from friction between blood and the ventricular wall.
In the normal, healthy heart, TKE ranges from 0 to 5 mJ. However, in patients with dilated cardiomyopathy and valve diseases, the left ventricular TKE is significantly higher than that in a normal heart.[122,123] Nevertheless, there is no current consensus on whether significant turbulence exists within the normal heart. Therefore, TKE is recommended as a complementary tool to echocardiography to provide new information about the pattern of blood flow. Similarly, the clinical implications of elevated levels of energy loss in the left ventricle require further investigation.
4.1.3.3. Flow component analysis
Quantification of 3D blood flow within the left ventricle has enabled separation into different functional flow components [Figure 4]: direct flow, the most efficient component that transits the heart in one cardiac cycle; retained inflow, which enters during diastole but does not leave until at least 1 cycle; delayed ejection flow, which resides inside the left ventricle during diastole and leaves during systole; and residual volume, which remains in the left ventricle for at least 2 cycles.
Figure 4.
Components of the left ventricular flow in a 50-year-old male patient with hypertrophic cardiomyopathy. The schematic representation in panel (A) illustrates the 4 components of left ventricular flow. Green: direct flow; Blue: delayed ejection; Yellow: retained inflow; Red: residual volume. Three time-points in the cardiac cycle are represented: diastole in panel (B), end diastole in panel (C), and systole in panel (D). AO: Aorta; LA: Left atrium; LV: Left ventricle.
The relative volumes and kinetic energy of each flow mentioned above can also be calculated to provide enhanced insights into left ventricular hemodynamics, alterations in which have been shown between normal left ventricles and under certain myopathic left ventricles.[124] Several studies have showed a direct correlation between the volume and diastolic kinetic energy of the flow component.[125] This is not surprising given that mass (where mass = mean density of blood × voxel volume) is a central component of kinetic energy. However, research on assessing left ventricular diastolic function using 4D flow CMR has so far been limited to small single-center studies.[124,125,126] To comprehensively assess cardiac conditions involving left ventricular diastolic dysfunction and make definitive comparisons of novel 4D parameters with contemporary diagnostic markers requires larger multicenter and multimodality studies. Furthermore, despite no change in flow component volume, patients with left bundle branch block exhibit lower pre-systolic kinetic energy of direct flow component than those without it. This intriguing research proposes that 4D flow can serve as markers of response to cardiac resynchronization therapy.[126]
4.1.3.4. Vortex flow analysis
A vortex is formed during diastole as blood flows into the left ventricle through the mitral valve, causing a redirection toward the left ventricular outflows. This process exhibits significant responsiveness to subsequent left ventricular maladaptation by amplifying even minor modifications in surrounding conditions like differences in energetic or dynamic properties. Relevant studies in echocardiography have previously characterized the phenomenon of vortex formation and its correlation with energy dissipation and impairment of diastolic function. Compared with echocardiography, vortex assessment based on 4D flow CMR, although relatively newer, can adequately detail vortex structures, dimensions, vorticity, and kinetic energy. The shape of mitral inflow to a significant extent determines the vortex shape and is hence of importance to vortex formation and energy efficiency of blood flow. This may explain why several groups have reported that advancements in blood flow analysis can be applied to regurgitant or stenotic valves. Vortex flow visualization techniques provide new options to quantify flow in atrioventricular septal defect and ischemic heart disease with reasonable accuracy.[127,128]
4.1.3.5. Cardiac fluid dynamic forces
HDFs represent the force exchange between ventricular blood and the endocardium, serving as a global measure of the interventricular pressure gradient (IVPG) integrated over the left ventricular volume.[129] HDFs changes have shown potential to assess cardiac function when volumetric and deformation cardiac measures remain intact[130] making them a promising candidate for detecting early alterations in cardiac function and predicting disease outcomes.
HDFs occur along 3 planes within the left ventricle: basal-apical, septal-lateral, and inferior-anterior; among these, the basal-apical force has emerged as the most reproducible and detectable force across all patients. Normalized with respect to left ventricular volume, HDFs are expressed as a percentage of gravity acceleration, encompassing amplitude, timing, and orientation parameters.
In the 1990s, non-invasive estimation of IVPG was investigated by the spatial-temporal velocity distribution derived from 4D flow CMR. This approach deviated from the need for invasive assessments by utilizing either the simplified Bernoulli equation or the Navier-Stokes equation.[131] The present literature provides characteristics for healthy subjects,[132] against which the distribution of HDFs in left ventricles of dilated cardiomyopathy patients is altered.[133] It has been reported that diastolic force patterns can differentiate between healthy individuals and patients with clinically compensated heart failure but dyssynchrony, thus indicating a potential role for this new approach in guiding cardiac resynchronization therapy treatment for patients with heart failure.[134] However, recent research suggests that base-to-apical HDFs derived from HDF analysis (see below) are not consistently abnormal,[135] implying that left ventricle HDFs are not yet ready for clinical trials assessing HFpEF.
4.2. The utilization of cine imaging
HDF analysis is an emerging technique that exploits endocardial edge tracking technology, requiring only manual drawing of valve diameters and endocardial contours on conventional 2-, 3-, and 4-chamber cine images [Figure 5].[136] HDFs can be estimated using a validated mathematical model based on feature tracking, which has shown high accuracy when compared to 4D flow MRI for both instantaneous values (mean correlation coefficient r = 0.77) and global values (r = 0.88).[137] Its straightforward application in existing datasets within minutes provides an opportunity to enhance the detection of early myocardial dysfunction beyond ejection fraction. Therefore, HDF assessment shifts the focus from wall mechanics to intracavitary fluid dynamics, which play a crucial role in inducing left ventricular remodeling during the early phase of myocardial infarction.[138] In patients with HFpEF compared to controls, progressive reduction of HDFs revealed impairment of left ventricular systolic function prior to ejection fraction and deformation parameters such as GLS,[135] and were superior to GLS in prediction of cardiovascular hospitalization.[139] Recently, 2 visually distinctive IVPG patterns have been introduced in the dilated cardiomyopathy population, of which the pressure reversal pattern was negatively affecting diastolic filling, independently associated with worse outcome.[140] Despite being considered a valuable novel tool for quantitative cardiac function assessment, further larger-scale studies are needed to validate the proposed techniques and explore the clinical advantages offered by HDF analysis over standard practices.
Figure 5.
Procedure of hemodynamic forces analysis based on cine imaging. 2C/3C/4C: 2/3/4 chambers; HDF: Hemodynamic force; RMS: Root mean square.
5. Limitations
Over the past decade, advances in CMR have rendered this imaging modality indispensable for managing patients with cardiovascular disease. However, it does possess certain limitations and contraindications that need to be considered. CMR requires a dedicated cardiac scanner and local expertise, along with longer acquisition times and higher costs than echocardiography. Other limitations include unreliable measurements in patients with tachyarrhythmias or breathing artifacts, while claustrophobia and commonly contraindicated magnetic devices such as left ventricular assist devices are important contraindications.
6. Conclusions
Although non-invasive volumetric estimation from cine imaging is the cornerstone of cardiac function assessment, contemporary CMR moves far beyond the simplistic evaluation of LVEF to encompass the intricate mechanics and pathophysiological changes of the ventricles. Strain imaging progressively and firmly integrates into daily clinical practice, providing an additional layer of knowledge on cardiac mechanics. Differently, flow imaging has evolved to provide novel physiological insights and the potential to detect functional alterations before evident or irreversible tissue modifications occur. Incorporating emerging cardiac imaging modalities into routine practice will significantly enhance cardiac function assessment. Despite gaps in knowledge, the future remains brimming with newer opportunities.
Funding
This work was supported by National Natural Science Foundation of China (Grant 81971588), Construction Research Project of Key Laboratory of Chinese Academy of Medical Sciences (2019PT310025), Youth Key Program of High-level Hospital Clinical Research (2022-GSPQZ-5), and Undergraduate Education Reform Project of Peking Union Medical College (2023zlgl 026).
Author contributions
Mengdi Jiang drafted the manuscript. Minjie Lu and Shihua Zhao read and edited the manuscript. All authors read and approved the final manuscript.
Conflicts of interest
None.
Editor note: Shihua Zhao is an Editorial Board Member of Cardiology Discovery. The article was subject to the journal’s standard procedures, with peer review handled independently of this editor and his research group.
Footnotes
How to cite this article: Jiang M, Lu M, Zhao S. Cardiac Functional Assessment by Magnetic Resonance Imaging. Cardiol Discov 2024;4(4):284–299. doi: 10.1097/CD9.0000000000000141
References
- [1].Nagueh SF Smiseth OA Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29(4):277–314. doi:10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- [2].Partington SL, Kwong RY, Dorbala S. Multimodality imaging in the assessment of myocardial viability. Heart Fail Rev 2011;16(4):381–395. doi:10.1007/s10741-010-9201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kwan AC Pourmorteza A Stutman D, et al. Next-Generation Hardware Advances in CT: Cardiac Applications. Radiology 2021;298(1):3–17. doi:10.1148/radiol.2020192791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].American College of Cardiology Foundation Task Force on Expert Consensus Documents, Hundley WG Bluemke DA, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation 2010;121(22):2462–2508. doi:10.1161/CIR.0b013e3181d44a8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gao Y Zhang Z Zhou S, et al. Reference values of left and right atrial volumes and phasic function based on a large sample of healthy Chinese adults: A cardiovascular magnetic resonance study. Int J Cardiol 2022;352:180–187. doi:10.1016/j.ijcard.2022.01.071. [DOI] [PubMed] [Google Scholar]
- [6].Xu Z Li W Wang J, et al. Reference Ranges of Ventricular Morphology and Function in Healthy Chinese Adults: A Multicenter 3 T MRI Study. J Magn Reson Imaging 2024;59(3):812–822. doi:10.1002/jmri.28903. [DOI] [PubMed] [Google Scholar]
- [7].Lang RM Badano LP Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16(3):233–270. doi:10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- [8].Bellenger NG Burgess MI Ray SG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable. Eur Heart J 2000;21(16):1387–1396. doi:10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- [9].Lang RM Bierig M Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18(12):1440–1463. doi:10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- [10].Yang K Song YY Chen XY, et al. Apical hypertrophic cardiomyopathy with left ventricular apical aneurysm: prevalence, cardiac magnetic resonance characteristics, and prognosis. Eur Heart J Cardiovasc Imaging 2020;21(12):1341–1350. doi:10.1093/ehjci/jeaa246. [DOI] [PubMed] [Google Scholar]
- [11].Authors/Task Force members, Elliott PM Anastasakis A, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35(39):2733–2779. doi:10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- [12].Pica S Crimi G Castelvecchio S, et al. Cardiac magnetic resonance predictors of left ventricular remodelling following acute ST elevation myocardial infarction: The VavirimS study. Int J Cardiol 2023;370:8–17. doi:10.1016/j.ijcard.2022.11.006. [DOI] [PubMed] [Google Scholar]
- [13].Reindl M Reinstadler SJ Tiller C, et al. Prognosis-based definition of left ventricular remodeling after ST-elevation myocardial infarction. Eur Radiol 2019;29(5):2330–2339. doi:10.1007/s00330-018-5875-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rodriguez-Palomares JF Gavara J Ferreira-González I, et al. Prognostic Value of Initial Left Ventricular Remodeling in Patients With Reperfused STEMI. JACC Cardiovasc Imaging 2019;12(12):2445–2456. doi:10.1016/j.jcmg.2019.02.025. [DOI] [PubMed] [Google Scholar]
- [15].Verma S Mazer CD Yan AT, et al. Effect of Empagliflozin on Left Ventricular Mass in Patients With Type 2 Diabetes Mellitus and Coronary Artery Disease: The EMPA-HEART CardioLink-6 Randomized Clinical Trial. Circulation 2019;140(21):1693–1702. doi:10.1161/CIRCULATIONAHA.119.042375. [DOI] [PubMed] [Google Scholar]
- [16].Santos-Gallego CG Vargas-Delgado AP Requena-Ibanez JA, et al. Randomized Trial of Empagliflozin in Nondiabetic Patients With Heart Failure and Reduced Ejection Fraction. J Am Coll Cardiol 2021;77(3):243–255. doi:10.1016/j.jacc.2020.11.008. [DOI] [PubMed] [Google Scholar]
- [17].Brown A Gandy S McCrimmon R, et al. A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type two diabetes: the DAPA-LVH trial. Eur Heart J 2020;41(36):3421–3432. doi:10.1093/eurheartj/ehaa419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee M Brooksbank K Wetherall K, et al. Effect of Empagliflozin on Left Ventricular Volumes in Patients With Type 2 Diabetes, or Prediabetes, and Heart Failure With Reduced Ejection Fraction (SUGAR-DM-HF). Circulation 2021;143(6):516–525. doi:10.1161/CIRCULATIONAHA.120.052186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pezel T Venkatesh BA De Vasconcellos HD, et al. Left Atrioventricular Coupling Index as a Prognostic Marker of Cardiovascular Events: The MESA Study. Hypertension 2021;78(3):661–671. doi:10.1161/HYPERTENSIONAHA.121.17339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pezel T Ambale-Venkatesh B Quinaglia T, et al. Change in Left Atrioventricular Coupling Index to Predict Incident Atrial Fibrillation: The Multi-Ethnic Study of Atherosclerosis (MESA). Radiology 2022;303(2):317–326. doi:10.1148/radiol.210315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pezel T Garot P Toupin S, et al. AI-Based Fully Automated Left Atrioventricular Coupling Index as a Prognostic Marker in Patients Undergoing Stress CMR. JACC Cardiovasc Imaging 2023;16(10):1288–1302. doi:10.1016/j.jcmg.2023.02.015. [DOI] [PubMed] [Google Scholar]
- [22].Curtis JP Sokol SI Wang Y, et al. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol 2003;42(4):736–742. doi:10.1016/s0735-1097(03)00789-7. [DOI] [PubMed] [Google Scholar]
- [23].Seemann F Bruce CG Khan JM, et al. Dynamic pressure-volume loop analysis by simultaneous real-time cardiovascular magnetic resonance and left heart catheterization. J Cardiovasc Magn Reson 2023;25(1):25. doi:10.1186/s12968-023-00913-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Garg P Gosling R Swoboda P, et al. Cardiac magnetic resonance identifies raised left ventricular filling pressure: prognostic implications. Eur Heart J 2022;43(26):2511–2522. doi:10.1093/eurheartj/ehac207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bertelsen L Diederichsen SZ Haugan KJ, et al. Left atrial volume and function assessed by cardiac magnetic resonance imaging are markers of subclinical atrial fibrillation as detected by continuous monitoring. Europace 2020;22(5):724–731. doi:10.1093/europace/euaa035. [DOI] [PubMed] [Google Scholar]
- [26].Mohty D Boulogne C Magne J, et al. Prognostic value of left atrial function in systemic light-chain amyloidosis: a cardiac magnetic resonance study. Eur Heart J Cardiovasc Imaging 2016;17(9):961–969. doi:10.1093/ehjci/jew100. [DOI] [PubMed] [Google Scholar]
- [27].Raisi-Estabragh Z McCracken C Condurache D, et al. Left atrial structure and function are associated with cardiovascular outcomes independent of left ventricular measures: a UK Biobank CMR study. Eur Heart J Cardiovasc Imaging 2022;23(9):1191–1200. doi:10.1093/ehjci/jeab266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sanchis L Gabrielli L Andrea R, et al. Left atrial dysfunction relates to symptom onset in patients with heart failure and preserved left ventricular ejection fraction. Eur Heart J Cardiovasc Imaging 2015;16(1):62–67. doi:10.1093/ehjci/jeu165. [DOI] [PubMed] [Google Scholar]
- [29].Marcus FI McKenna WJ Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010;121(13):1533–1541. doi:10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Koschutnik M Dannenberg V Nitsche C, et al. Right ventricular function and outcome in patients undergoing transcatheter aortic valve replacement. Eur Heart J Cardiovasc Imaging 2021;22(11):1295–1303. doi:10.1093/ehjci/jeaa342. [DOI] [PubMed] [Google Scholar]
- [31].Al’Aref SJ Altibi AM Malkawi A, et al. Cardiac magnetic resonance for prophylactic implantable-cardioverter defibrillator therapy international study: prognostic value of cardiac magnetic resonance-derived right ventricular parameters substudy. Eur Heart J Cardiovasc Imaging 2023;24(4):472–482. doi:10.1093/ehjci/jeac124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Alandejani F Alabed S Garg P, et al. Training and clinical testing of artificial intelligence derived right atrial cardiovascular magnetic resonance measurements. J Cardiovasc Magn Reson 2022;24(1):25. doi:10.1186/s12968-022-00855-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hopman L Visch JE Bhagirath P, et al. Right atrial function and fibrosis in relation to successful atrial fibrillation ablation. Eur Heart J Cardiovasc Imaging 2023;24(3):336–345. doi:10.1093/ehjci/jeac152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Thomas JD, Popović ZB. Assessment of left ventricular function by cardiac ultrasound. J Am Coll Cardiol 2006;48(10):2012–2025. doi:10.1016/j.jacc.2006.06.071. [DOI] [PubMed] [Google Scholar]
- [35].Stokke TM Hasselberg NE Smedsrud MK, et al. Geometry as a Confounder When Assessing Ventricular Systolic Function: Comparison Between Ejection Fraction and Strain. J Am Coll Cardiol 2017;70(8):942–954. doi:10.1016/j.jacc.2017.06.046. [DOI] [PubMed] [Google Scholar]
- [36].Shah AM, Solomon SD. Myocardial deformation imaging: current status and future directions. Circulation 2012;125(2):e244–248. doi:10.1161/CIRCULATIONAHA.111.086348. [DOI] [PubMed] [Google Scholar]
- [37].Zerhouni EA Parish DM Rogers WJ, et al. Human heart: tagging with MR imaging--a method for noninvasive assessment of myocardial motion. Radiology 1988;169(1):59–63. doi:10.1148/radiology.169.1.3420283. [DOI] [PubMed] [Google Scholar]
- [38].Young AA Axel L Dougherty L, et al. Validation of tagging with MR imaging to estimate material deformation. Radiology 1993;188(1):101–108. doi:10.1148/radiology.188.1.8511281. [DOI] [PubMed] [Google Scholar]
- [39].Yoneyama K Venkatesh BA Bluemke DA, et al. Cardiovascular magnetic resonance in an adult human population: serial observations from the multi-ethnic study of atherosclerosis. J Cardiovasc Magn Reson 2017;19(1):52. doi:10.1186/s12968-017-0367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Moore CC, Reeder SB, McVeigh ER. Tagged MR imaging in a deforming phantom: photographic validation. Radiology 1994;190(3):765–769. doi:10.1148/radiology.190.3.8115625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kim D Gilson WD Kramer CM, et al. Myocardial tissue tracking with two-dimensional cine displacement-encoded MR imaging: development and initial evaluation. Radiology 2004;230(3):862–871. doi:10.1148/radiol.2303021213. [DOI] [PubMed] [Google Scholar]
- [42].Osman NF Sampath S Atalar E, et al. Imaging longitudinal cardiac strain on short-axis images using strain-encoded MRI. Magn Reson Med 2001;46(2):324–334. doi:10.1002/mrm.1195. [DOI] [PubMed] [Google Scholar]
- [43].Markl M, Schneider B, Hennig J. Fast phase contrast cardiac magnetic resonance imaging: improved assessment and analysis of left ventricular wall motion. J Magn Reson Imaging 2002;15(6):642–653. doi:10.1002/jmri.10114. [DOI] [PubMed] [Google Scholar]
- [44].Claus P Omar A Pedrizzetti G, et al. Tissue Tracking Technology for Assessing Cardiac Mechanics: Principles, Normal Values, and Clinical Applications. JACC Cardiovasc Imaging 2015;8(12):1444–1460. doi:10.1016/j.jcmg.2015.11.001. [DOI] [PubMed] [Google Scholar]
- [45].Pedrizzetti G Claus P Kilner PJ, et al. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J Cardiovasc Magn Reson 2016;18(1):51. doi:10.1186/s12968-016-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yang W Xu J Zhu L, et al. Myocardial Strain Measurements Derived From MR Feature-Tracking: Influence of Sex, Age, Field Strength, and Vendor. JACC Cardiovasc Imaging 2024;17(4):364–379. doi:10.1016/j.jcmg.2023.05.019. [DOI] [PubMed] [Google Scholar]
- [47].Vo HQ, Marwick TH, Negishi K. MRI-Derived Myocardial Strain Measures in Normal Subjects. JACC Cardiovasc Imaging 2018;11(2 Pt 1):196–205. doi:10.1016/j.jcmg.2016.12.025. [DOI] [PubMed] [Google Scholar]
- [48].Wang T Kwon DH Griffin BP, et al. Defining the Reference Range for Left Ventricular Strain in Healthy Patients by Cardiac MRI Measurement Techniques: Systematic Review and Meta-Analysis. AJR Am J Roentgenol 2021;217(3):569–583. doi:10.2214/AJR.20.24264. [DOI] [PubMed] [Google Scholar]
- [49].Schick BM Dlugas H Czeiszperger TL, et al. Reduced preload increases Mechanical Control (strain-rate dependence) of Relaxation by modifying myosin kinetics. Arch Biochem Biophys 2021;707:108909. doi:10.1016/j.abb.2021.108909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mentias A Naji P Gillinov AM, et al. Strain Echocardiography and Functional Capacity in Asymptomatic Primary Mitral Regurgitation With Preserved Ejection Fraction. J Am Coll Cardiol 2016;68(18):1974–1986. doi:10.1016/j.jacc.2016.08.030. [DOI] [PubMed] [Google Scholar]
- [51].Marciniak A Claus P Sutherland GR, et al. Changes in systolic left ventricular function in isolated mitral regurgitation. A strain rate imaging study. Eur Heart J 2007;28(21):2627–2636. doi:10.1093/eurheartj/ehm072. [DOI] [PubMed] [Google Scholar]
- [52].Lin A Seale H Hamilton-Craig C, et al. Quantification of biventricular strain and assessment of ventriculo-ventricular interaction in pulmonary arterial hypertension using exercise cardiac magnetic resonance imaging and myocardial feature tracking. J Magn Reson Imaging 2019;49(5):1427–1436. doi:10.1002/jmri.26517. [DOI] [PubMed] [Google Scholar]
- [53].Carasso S Cohen O Mutlak D, et al. Relation of myocardial mechanics in severe aortic stenosis to left ventricular ejection fraction and response to aortic valve replacement. Am J Cardiol 2011;107(7):1052–1057. doi:10.1016/j.amjcard.2010.11.032. [DOI] [PubMed] [Google Scholar]
- [54].Risum N Jons C Olsen NT, et al. Simple regional strain pattern analysis to predict response to cardiac resynchronization therapy: rationale, initial results, and advantages. Am Heart J 2012;163(4):697–704. doi:10.1016/j.ahj.2012.01.025. [DOI] [PubMed] [Google Scholar]
- [55].Tantawy SW Mohammad SA Osman AM, et al. Strain analysis using feature tracking cardiac magnetic resonance (FT-CMR) in the assessment of myocardial viability in chronic ischemic patients. Int J Cardiovasc Imaging 2021;37(2):587–596. doi:10.1007/s10554-020-02018-w. [DOI] [PubMed] [Google Scholar]
- [56].Podlesnikar T Pizarro G Fernández-Jiménez R, et al. Effect of Early Metoprolol During ST-Segment Elevation Myocardial Infarction on Left Ventricular Strain: Feature-Tracking Cardiovascular Magnetic Resonance Substudy From the METOCARD-CNIC Trial. JACC Cardiovasc Imaging 2019;12(7 Pt 1):1188–1198. doi:10.1016/j.jcmg.2018.07.019. [DOI] [PubMed] [Google Scholar]
- [57].Khan JN Singh A Nazir SA, et al. Comparison of cardiovascular magnetic resonance feature tracking and tagging for the assessment of left ventricular systolic strain in acute myocardial infarction. Eur J Radiol 2015;84(5):840–848. doi:10.1016/j.ejrad.2015.02.002. [DOI] [PubMed] [Google Scholar]
- [58].McComb C Carrick D McClure JD, et al. Assessment of the relationships between myocardial contractility and infarct tissue revealed by serial magnetic resonance imaging in patients with acute myocardial infarction. Int J Cardiovasc Imaging 2015;31(6):1201–1209. doi:10.1007/s10554-015-0678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhu L Wu J Hao X, et al. Value of cardiac magnetic resonance feature tracking technology in the differential diagnosis of isolated left ventricular noncompaction and dilated cardiomyopathy. Quant Imaging Med Surg 2023;13(3):1453–1463. doi:10.21037/qims-22-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yu S Chen X Yang K, et al. Correlation between left ventricular fractal dimension and impaired strain assessed by cardiac MRI feature tracking in patients with left ventricular noncompaction and normal left ventricular ejection fraction. Eur Radiol 2022;32(4):2594–2603. doi:10.1007/s00330-021-08346-2. [DOI] [PubMed] [Google Scholar]
- [61].Oda S Utsunomiya D Nakaura T, et al. Identification and Assessment of Cardiac Amyloidosis by Myocardial Strain Analysis of Cardiac Magnetic Resonance Imaging. Circ J 2017;81(7):1014–1021. doi:10.1253/circj.CJ-16-1259. [DOI] [PubMed] [Google Scholar]
- [62].Wang F Xu X Wang Q, et al. Comparison of left ventricular global and segmental strain parameters by cardiovascular magnetic resonance tissue tracking in light-chain cardiac amyloidosis and hypertrophic cardiomyopathy. Quant Imaging Med Surg 2023;13(1):449–461. doi:10.21037/qims-22-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Williams LK Forero JF Popovic ZB, et al. Patterns of CMR measured longitudinal strain and its association with late gadolinium enhancement in patients with cardiac amyloidosis and its mimics. J Cardiovasc Magn Reson 2017;19(1):61. doi:10.1186/s12968-017-0376-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Cavus E Muellerleile K Schellert S, et al. CMR feature tracking strain patterns and their association with circulating cardiac biomarkers in patients with hypertrophic cardiomyopathy. Clin Res Cardiol 2021;110(11):1757–1769. doi:10.1007/s00392-021-01848-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Chen Y Sun Z Xu L, et al. Diagnostic and Prognostic Value of Cardiac Magnetic Resonance Strain in Suspected Myocarditis With Preserved LV-EF: A Comparison Between Patients With Negative and Positive Late Gadolinium Enhancement Findings. J Magn Reson Imaging 2022;55(4):1109–1119. doi:10.1002/jmri.27873. [DOI] [PubMed] [Google Scholar]
- [66].Ochs A Riffel J Ochs MM, et al. Myocardial mechanics in dilated cardiomyopathy: prognostic value of left ventricular torsion and strain. J Cardiovasc Magn Reson 2021;23(1):136. doi:10.1186/s12968-021-00829-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Romano S Judd RM Kim RJ, et al. Feature-Tracking Global Longitudinal Strain Predicts Death in a Multicenter Population of Patients With Ischemic and Nonischemic Dilated Cardiomyopathy Incremental to Ejection Fraction and Late Gadolinium Enhancement. JACC Cardiovasc Imaging 2018;11(10):1419–1429. doi:10.1016/j.jcmg.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Negri F Muser D Driussi M, et al. Prognostic role of global longitudinal strain by feature tracking in patients with hypertrophic cardiomyopathy: The STRAIN-HCM study. Int J Cardiol 2021;345:61–67. doi:10.1016/j.ijcard.2021.10.148. [DOI] [PubMed] [Google Scholar]
- [69].Fischer K Obrist SJ Erne SA, et al. Feature Tracking Myocardial Strain Incrementally Improves Prognostication in Myocarditis Beyond Traditional CMR Imaging Features. JACC Cardiovasc Imaging 2020;13(9):1891–1901. doi:10.1016/j.jcmg.2020.04.025. [DOI] [PubMed] [Google Scholar]
- [70].Awadalla M Mahmood SS Groarke JD, et al. Global Longitudinal Strain and Cardiac Events in Patients With Immune Checkpoint Inhibitor-Related Myocarditis. J Am Coll Cardiol 2020;75(5):467–478. doi:10.1016/j.jacc.2019.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Al Musa T Uddin A Swoboda PP, et al. Myocardial strain and symptom severity in severe aortic stenosis: insights from cardiovascular magnetic resonance. Quant Imaging Med Surg 2017;7(1):38–47. doi:10.21037/qims.2017.02.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Nucifora G Tantiongco JP Crouch G, et al. Changes of left ventricular mechanics after trans-catheter aortic valve implantation and surgical aortic valve replacement for severe aortic stenosis: A tissue-tracking cardiac magnetic resonance study. Int J Cardiol 2017;228:184–190. doi:10.1016/j.ijcard.2016.11.200. [DOI] [PubMed] [Google Scholar]
- [73].Shen X Yuan Y Yang M, et al. Cardiovascular magnetic resonance-derived myocardial strain in asymptomatic heart transplanted patients and its correlation with late gadolinium enhancement. Eur Radiol 2020;30(8):4337–4346. doi:10.1007/s00330-020-06763-3. [DOI] [PubMed] [Google Scholar]
- [74].Plana JC Galderisi M Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2014;15(10):1063–1093. doi:10.1093/ehjci/jeu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Drafts BC Twomley KM D’Agostino R Jr, et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging 2013;6(8):877–885. doi:10.1016/j.jcmg.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ong G Brezden-Masley C Dhir V, et al. Myocardial strain imaging by cardiac magnetic resonance for detection of subclinical myocardial dysfunction in breast cancer patients receiving trastuzumab and chemotherapy. Int J Cardiol 2018;261:228–233. doi:10.1016/j.ijcard.2018.03.041. [DOI] [PubMed] [Google Scholar]
- [77].Tassan-Mangina S Codorean D Metivier M, et al. Tissue Doppler imaging and conventional echocardiography after anthracycline treatment in adults: early and late alterations of left ventricular function during a prospective study. Eur J Echocardiogr 2006;7(2):141–146. doi:10.1016/j.euje.2005.04.009. [DOI] [PubMed] [Google Scholar]
- [78].Gong IY Ong G Brezden-Masley C, et al. Early diastolic strain rate measurements by cardiac MRI in breast cancer patients treated with trastuzumab: a longitudinal study. Int J Cardiovasc Imaging 2019;35(4):653–662. doi:10.1007/s10554-018-1482-2. [DOI] [PubMed] [Google Scholar]
- [79].Negishi T Thavendiranathan P Penicka M, et al. Cardioprotection Using Strain-Guided Management of Potentially Cardiotoxic Cancer Therapy: 3-Year Results of the SUCCOUR Trial. JACC Cardiovasc Imaging 2023;16(3):269–278. doi:10.1016/j.jcmg.2022.10.010. [DOI] [PubMed] [Google Scholar]
- [80].Thavendiranathan P Negishi T Somerset E, et al. Strain-Guided Management of Potentially Cardiotoxic Cancer Therapy. J Am Coll Cardiol 2021;77(4):392–401. doi:10.1016/j.jacc.2020.11.020. [DOI] [PubMed] [Google Scholar]
- [81].Onishi T Saha SK Ludwig DR, et al. Feature tracking measurement of dyssynchrony from cardiovascular magnetic resonance cine acquisitions: comparison with echocardiographic speckle tracking. J Cardiovasc Magn Reson 2013;15(1):95. doi:10.1186/1532-429X-15-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Sohal M Duckett SG Zhuang X, et al. A prospective evaluation of cardiovascular magnetic resonance measures of dyssynchrony in the prediction of response to cardiac resynchronization therapy. J Cardiovasc Magn Reson 2014;16(1):58. doi:10.1186/s12968-014-0058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Taylor RJ Umar F Panting JR, et al. Left ventricular lead position, mechanical activation, and myocardial scar in relation to left ventricular reverse remodeling and clinical outcomes after cardiac resynchronization therapy: A feature-tracking and contrast-enhanced cardiovascular magnetic resonance study. Heart Rhythm 2016;13(2):481–489. doi:10.1016/j.hrthm.2015.10.024. [DOI] [PubMed] [Google Scholar]
- [84].Rathi A Kanee L Limoges M, et al. Relationship Between Left Ventricular Strain Assessment by Cardiac Magnetic Resonance Imaging and Response to Cardiac Resynchronization Therapy. J Thorac Imaging 2022;37(4):W58–W59. doi:10.1097/RTI.0000000000000652. [DOI] [PubMed] [Google Scholar]
- [85].Hopman L Mulder MJ van der Laan AM, et al. Impaired left atrial reservoir and conduit strain in patients with atrial fibrillation and extensive left atrial fibrosis. J Cardiovasc Magn Reson 2021;23(1):131. doi:10.1186/s12968-021-00820-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hopman L Mulder MJ van der Laan AM, et al. Left atrial strain is associated with arrhythmia recurrence after atrial fibrillation ablation: Cardiac magnetic resonance rapid strain vs. feature tracking strain. Int J Cardiol 2023;378:23–31. doi:10.1016/j.ijcard.2023.02.019. [DOI] [PubMed] [Google Scholar]
- [87].Benjamin MM Moulki N Waqar A, et al. Association of left atrial strain by cardiovascular magnetic resonance with recurrence of atrial fibrillation following catheter ablation. J Cardiovasc Magn Reson 2022;24(1):3. doi:10.1186/s12968-021-00831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Evin M Broadhouse KM Callaghan FM, et al. Impact of obesity and epicardial fat on early left atrial dysfunction assessed by cardiac MRI strain analysis. Cardiovasc Diabetol 2016;15(1):164. doi:10.1186/s12933-016-0481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Leng S Ge H He J, et al. Long-term Prognostic Value of Cardiac MRI Left Atrial Strain in ST-Segment Elevation Myocardial Infarction. Radiology 2020;296(2):299–309. doi:10.1148/radiol.2020200176. [DOI] [PubMed] [Google Scholar]
- [90].Bo K Gao Y Zhou Z, et al. Incremental prognostic value of left atrial strain in patients with heart failure. ESC Heart Fail 2022;9(6):3942–3953. doi:10.1002/ehf2.14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Yang Y Yin G Jiang Y, et al. Quantification of left atrial function in patients with non-obstructive hypertrophic cardiomyopathy by cardiovascular magnetic resonance feature tracking imaging: a feasibility and reproducibility study. J Cardiovasc Magn Reson 2020;22(1):1. doi:10.1186/s12968-019-0589-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Li Y Xu Y Tang S, et al. Left Atrial Function Predicts Outcome in Dilated Cardiomyopathy: Fast Long-Axis Strain Analysis Derived from MRI. Radiology 2022;302(1):72–81. doi:10.1148/radiol.2021210801. [DOI] [PubMed] [Google Scholar]
- [93].Raafs AG Vos JL Henkens M, et al. Left Atrial Strain Has Superior Prognostic Value to Ventricular Function and Delayed-Enhancement in Dilated Cardiomyopathy. JACC Cardiovasc Imaging 2022;15(6):1015–1026. doi:10.1016/j.jcmg.2022.01.016. [DOI] [PubMed] [Google Scholar]
- [94].Tadic M. Multimodality Evaluation of the Right Ventricle: An Updated Review. Clin Cardiol 2015;38(12):770–776. doi:10.1002/clc.22443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Romano S Dell’atti D Judd RM, et al. Prognostic Value of Feature-Tracking Right Ventricular Longitudinal Strain in Severe Functional Tricuspid Regurgitation: A Multicenter Study. JACC Cardiovasc Imaging 2021;14(8):1561–1568. doi:10.1016/j.jcmg.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].de Siqueira ME Pozo E Fernandes VR, et al. Characterization and clinical significance of right ventricular mechanics in pulmonary hypertension evaluated with cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson 2016;18(1):39. doi:10.1186/s12968-016-0258-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Prati G Vitrella G Allocca G, et al. Right Ventricular Strain and Dyssynchrony Assessment in Arrhythmogenic Right Ventricular Cardiomyopathy: Cardiac Magnetic Resonance Feature-Tracking Study. Circ Cardiovasc Imaging 2015;8(11):e003647; discussion e003647. doi:10.1161/CIRCIMAGING.115.003647. [DOI] [PubMed] [Google Scholar]
- [98].Jiang L Guo YK Xu HY, et al. Incremental prognostic value of myocardial strain over ventricular volume in patients with repaired tetralogy of Fallot. Eur Radiol 2023;33(3):1992–2003. doi:10.1007/s00330-022-09166-8. [DOI] [PubMed] [Google Scholar]
- [99].Mahmod M Raman B Chan K, et al. Right ventricular function declines prior to left ventricular ejection fraction in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 2022;24(1):36. doi:10.1186/s12968-022-00868-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bernhard B Tanner G Garachemani D, et al. Predictive value of cardiac magnetic resonance right ventricular longitudinal strain in patients with suspected myocarditis. J Cardiovasc Magn Reson 2023;25(1):49. doi:10.1186/s12968-023-00957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Vos JL Leiner T van Dijk A, et al. Right atrial and ventricular strain detects subclinical changes in right ventricular function in precapillary pulmonary hypertension. Int J Cardiovasc Imaging 2022;38(8):1699–1710. doi:10.1007/s10554-022-02555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Kallifatidis A Mouratoglou SA Giannakoulas G, et al. Myocardial deformation assessment in patients with precapillary pulmonary hypertension: A cardiac magnetic resonance study. Diagn Interv Imaging 2021;102(3):153–161. doi:10.1016/j.diii.2020.08.001. [DOI] [PubMed] [Google Scholar]
- [103].Tello K Dalmer A Vanderpool R, et al. Right ventricular function correlates of right atrial strain in pulmonary hypertension: a combined cardiac magnetic resonance and conductance catheter study. Am J Physiol Heart Circ Physiol 2020;318(1):H156–H164. doi:10.1152/ajpheart.00485.2019. [DOI] [PubMed] [Google Scholar]
- [104].Guensch DP Kuganathan S Utz CD, et al. Analysis of bi-atrial function using CMR feature tracking and long-axis shortening approaches in patients with diastolic dysfunction and atrial fibrillation. Eur Radiol 2023;33(10):7226–7237. doi:10.1007/s00330-023-09663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Li Y Guo J Li W, et al. Prognostic value of right atrial strain derived from cardiovascular magnetic resonance in non-ischemic dilated cardiomyopathy. J Cardiovasc Magn Reson 2022;24(1):54. doi:10.1186/s12968-022-00894-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Jain S Kuriakose D Edelstein I, et al. Right Atrial Phasic Function in Heart Failure With Preserved and Reduced Ejection Fraction. JACC Cardiovasc Imaging 2019;12(8 Pt 1):1460–1470. doi:10.1016/j.jcmg.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Schuster A Backhaus SJ Stiermaier T, et al. Impact of Right Atrial Physiology on Heart Failure and Adverse Events after Myocardial Infarction. J Clin Med 2020;9(1):210. doi:10.3390/jcm9010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Moran PR. A flow velocity zeugmatographic interlace for NMR imaging in humans. Magn Reson Imaging 1982;1(4):197–203. doi:10.1016/0730-725x(82)90170-9. [DOI] [PubMed] [Google Scholar]
- [109].Jarral OA Tan M Salmasi MY, et al. Phase-contrast magnetic resonance imaging and computational fluid dynamics assessment of thoracic aorta blood flow: a literature review. Eur J Cardiothorac Surg 2020;57(3):438–446. doi:10.1093/ejcts/ezz280. [DOI] [PubMed] [Google Scholar]
- [110].Defrance C Bollache E Kachenoura N, et al. Evaluation of aortic valve stenosis using cardiovascular magnetic resonance: comparison of an original semiautomated analysis of phase-contrast cardiovascular magnetic resonance with Doppler echocardiography. Circ Cardiovasc Imaging 2012;5(5):604–612. doi:10.1161/CIRCIMAGING.111.971218. [DOI] [PubMed] [Google Scholar]
- [111].Troger F Lechner I Reindl M, et al. A novel approach to determine aortic valve area with phase-contrast cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2022;24(1):7. doi:10.1186/s12968-021-00838-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Neisius U Tsao CW Hauser TH, et al. Aortic regurgitation assessment by cardiovascular magnetic resonance imaging and transthoracic echocardiography: intermodality disagreement impacting on prediction of post-surgical left ventricular remodeling. Int J Cardiovasc Imaging 2020;36(1):91–100. doi:10.1007/s10554-019-01682-x. [DOI] [PubMed] [Google Scholar]
- [113].Yamasaki Y Kawanami S Kamitani T, et al. Noninvasive quantification of left-to-right shunt by phase contrast magnetic resonance imaging in secundum atrial septal defect: the effects of breath holding and comparison with invasive oximetry. Int J Cardiovasc Imaging 2018;34(6):931–937. doi:10.1007/s10554-018-1297-1. [DOI] [PubMed] [Google Scholar]
- [114].Dellas C Kammerer L Gravenhorst V, et al. Quantification of pulmonary regurgitation and prediction of pulmonary valve replacement by echocardiography in patients with congenital heart defects in comparison to cardiac magnetic resonance imaging. Int J Cardiovasc Imaging 2018;34(4):607–613. doi:10.1007/s10554-017-1267-z. [DOI] [PubMed] [Google Scholar]
- [115].Bissell MM Raimondi F Ait Ali L, et al. 4D Flow cardiovascular magnetic resonance consensus statement: 2023 update. J Cardiovasc Magn Reson 2023;25(1):40. doi:10.1186/s12968-023-00942-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Lee DC Markl M Ng J, et al. Three-dimensional left atrial blood flow characteristics in patients with atrial fibrillation assessed by 4D flow CMR. Eur Heart J Cardiovasc Imaging 2016;17(11):1259–1268. doi:10.1093/ehjci/jev304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Brix L Ringgaard S Rasmusson A, et al. Three dimensional three component whole heart cardiovascular magnetic resonance velocity mapping: comparison of flow measurements from 3D and 2D acquisitions. J Cardiovasc Magn Reson 2009;11(1):3. doi:10.1186/1532-429X-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Plicht B Kahlert P Goldwasser R, et al. Direct quantification of mitral regurgitant flow volume by real-time three-dimensional echocardiography using dealiasing of color Doppler flow at the vena contracta. J Am Soc Echocardiogr 2008;21(12):1337–1346. doi:10.1016/j.echo.2008.09.022. [DOI] [PubMed] [Google Scholar]
- [119].Thavendiranathan P Liu S Datta S, et al. Automated quantification of mitral inflow and aortic outflow stroke volumes by three-dimensional real-time volume color-flow Doppler transthoracic echocardiography: comparison with pulsed-wave Doppler and cardiac magnetic resonance imaging. J Am Soc Echocardiogr 2012;25(1):56–65. doi:10.1016/j.echo.2011.10.004. [DOI] [PubMed] [Google Scholar]
- [120].Wong J Chabiniok R deVecchi A, et al. Age-related changes in intraventricular kinetic energy: a physiological or pathological adaptation. Am J Physiol Heart Circ Physiol 2016;310(6):H747–755. doi:10.1152/ajpheart.00075.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Demirkiran A van der Geest RJ Hopman L, et al. Association of left ventricular flow energetics with remodeling after myocardial infarction: New hemodynamic insights for left ventricular remodeling. Int J Cardiol 2022;367:105–114. doi:10.1016/j.ijcard.2022.08.040. [DOI] [PubMed] [Google Scholar]
- [122].Zajac J Eriksson J Dyverfeldt P, et al. Turbulent kinetic energy in normal and myopathic left ventricles. J Magn Reson Imaging 2015;41(4):1021–1029. doi:10.1002/jmri.24633. [DOI] [PubMed] [Google Scholar]
- [123].Binter C Gotschy A Sündermann SH, et al. Turbulent kinetic energy assessed by multipoint 4-dimensional flow magnetic resonance imaging provides additional information relative to echocardiography for the determination of aortic stenosis Severity. Circ Cardiovasc Imaging 2017;10(6) :e003951. doi:10.1161/CIRCIMAGING.116.005486. [DOI] [PubMed] [Google Scholar]
- [124].Ashkir Z Johnson S Lewandowski AJ, et al. Novel insights into diminished cardiac reserve in non-obstructive hypertrophic cardiomyopathy from four-dimensional flow cardiac magnetic resonance component analysis. Eur Heart J Cardiovasc Imaging 2023;24(9):1192–1200. doi:10.1093/ehjci/jead074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Sundin J Engvall J Nylander E, et al. Improved Efficiency of Intraventricular Blood Flow Transit Under Cardiac Stress: A 4D Flow Dobutamine CMR Study. Front Cardiovasc Med 2020;7:581495. doi:10.3389/fcvm.2020.581495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Zajac J Eriksson J Alehagen U, et al. Mechanical dyssynchrony alters left ventricular flow energetics in failing hearts with LBBB: a 4D flow CMR pilot study. Int J Cardiovasc Imaging 2018;34(4):587–596. doi:10.1007/s10554-017-1261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Elbaz MS van der Geest RJ Calkoen EE, et al. Assessment of viscous energy loss and the association with three-dimensional vortex ring formation in left ventricular inflow: In vivo evaluation using four-dimensional flow MRI. Magn Reson Med 2017;77(2):794–805. doi:10.1002/mrm.26129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Kanski M Arvidsson PM Töger J, et al. Left ventricular fluid kinetic energy time curves in heart failure from cardiovascular magnetic resonance 4D flow data. J Cardiovasc Magn Reson 2015;17:111. doi:10.1186/s12968-015-0211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Vallelonga F Airale L Tonti G, et al. Introduction to Hemodynamic Forces Analysis: Moving Into the New Frontier of Cardiac Deformation Analysis. J Am Heart Assoc 2021;10(24):e023417. doi:10.1161/JAHA.121.023417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Daal M Strijkers GJ Hautemann DJ, et al. Longitudinal CMR assessment of cardiac global longitudinal strain and hemodynamic forces in a mouse model of heart failure. Int J Cardiovasc Imaging 2022;38(11):2385–2394. doi:10.1007/s10554-022-02631-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Yang GZ Kilner PJ Wood NB, et al. Computation of flow pressure fields from magnetic resonance velocity mapping. Magn Reson Med 1996;36(4):520–526. doi:10.1002/mrm.1910360404. [DOI] [PubMed] [Google Scholar]
- [132].Arvidsson PM Töger J Carlsson M, et al. Left and right ventricular hemodynamic forces in healthy volunteers and elite athletes assessed with 4D flow magnetic resonance imaging. Am J Physiol Heart Circ Physiol 2017;312(2):H314–H328. doi:10.1152/ajpheart.00583.2016. [DOI] [PubMed] [Google Scholar]
- [133].Eriksson J Bolger AF Ebbers T, et al. Left ventricular hemodynamic forces are altered in patients with dilated cardiomyopathy. J Cardiovasc Magn Reson 2015;17(S1):282. doi:10.1186/1532-429X-17-S1-P282. [Google Scholar]
- [134].Arvidsson PM Töger J Pedrizzetti G, et al. Hemodynamic forces using four-dimensional flow MRI: an independent biomarker of cardiac function in heart failure with left ventricular dyssynchrony. Am J Physiol Heart Circ Physiol 2018;315(6):H1627–H1639. doi:10.1152/ajpheart.00112.2018. [DOI] [PubMed] [Google Scholar]
- [135].Lapinskas T Pedrizzetti G Stoiber L, et al. The Intraventricular Hemodynamic Forces Estimated Using Routine CMR Cine Images: A New Marker of the Failing Heart. JACC Cardiovasc Imaging 2019;12(2):377–379. doi:10.1016/j.jcmg.2018.08.012. [DOI] [PubMed] [Google Scholar]
- [136].Pedrizzetti G. On the computation of hemodynamic forces in the heart chambers. J Biomech 2019;95:109323. doi:10.1016/j.jbiomech.2019.109323. [DOI] [PubMed] [Google Scholar]
- [137].Pedrizzetti G Arvidsson PM Töger J, et al. On estimating intraventricular hemodynamic forces from endocardial dynamics: A comparative study with 4D flow MRI. J Biomech 2017;60:203–210. doi:10.1016/j.jbiomech.2017.06.046. [DOI] [PubMed] [Google Scholar]
- [138].Filomena D Cimino S Monosilio S, et al. Impact of intraventricular haemodynamic forces misalignment on left ventricular remodelling after myocardial infarction. ESC Heart Fail 2022;9(1):496–505. doi:10.1002/ehf2.13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Backhaus SJ Uzun H Rösel SF, et al. Hemodynamic force assessment by cardiovascular magnetic resonance in HFpEF: A case-control substudy from the HFpEF stress trial. EBioMedicine 2022;86:104334. doi:10.1016/j.ebiom.2022.104334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Vos JL Raafs AG Henkens M, et al. CMR-derived left ventricular intraventricular pressure gradients identify different patterns associated with prognosis in dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging 2023;24(9):1231–1240. doi:10.1093/ehjci/jead083. [DOI] [PMC free article] [PubMed] [Google Scholar]