Abstract
Background
Osteoporosis and sarcopenia frequently occur in patients with end-stage renal disease undergoing hemodialysis (HD), and depression is also a common mental health issue in this population. Despite the prevalence of these conditions, the interrelationships among them remain poorly understood in HD patients.
Methods
In this multicenter cross-sectional study, 858 HD patients from 7 dialysis centers were recruited. Bone mineral density (BMD) was assessed using dual-energy X-ray absorptiometry. Skeletal muscle mass index (SMI) was calculated from body composition data obtained through multifrequency bioimpedance analysis (BIA), while handgrip strength (HGS) was measured with a dynamometer. Gait speed was evaluated with a 4-meter walk test, and depression was assessed using the Patient Health Questionnaire-9 (PHQ-9).
Results
Among the 858 participants (524 men, 334 women), 39.2% had osteoporosis. The prevalence of sarcopenia and depression was 18.9% and 42.1%, respectively. Logistic regression analysis showed that SMI was significantly associated with a decreased risk of osteoporosis (OR = 0.638, 95% CI = 0.494–0.823, P = 0.001), while HGS was not(OR = 0.990, 95% CI = 0.963–1.017, P = 0.449). HD patients with sarcopenia were 1.92 times more likely to have osteoporosis than those without sarcopenia. Most notably, after adjusting for both sarcopenia and SMI, the risk of osteoporosis in HD patients with depression was 1.45 times higher than in those without depression (OR = 1.452, 95% CI = 1.060–1.989, P = 0.020).
Conclusions
In HD patients, increased muscle mass, rather than muscle strength, is linked to a lower risk of osteoporosis. Notably, depression emerges as a significant risk factor for osteoporosis in this population, highlighting the need for mental health considerations in managing bone health.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-025-03963-1.
Keywords: Sarcopenia, Depression, Osteoporosis, Hemodialysis, Bone mineral density
Introduction
Chronic kidney disease (CKD) is a progressive disease with high morbidity and mortality. Low bone mineral density (BMD) is common in patients with end-stage renal disease (ESRD), and CKD-mineral bone disorder (MBD) has been reported to contribute to low BMD in patients with ESRD [1]. Osteoporosis is a condition characterized by low bone mass or qualitative bone deterioration that results in decreased bone strength and an increased risk of fracture [2]. According to research, the prevalence of osteoporosis ranges from 10 to 64% at the femoral site and 4 to 47% at the lumbar spine [3]. Traditional risk factors for osteoporosis include increasing age, female sex, long dialysis duration, history of fractures, low bone mass, smoking, CKD-MBD, and the use of prednisone or other medications [4]. Sarcopenia is a condition characterized by reduced muscle mass and limited mobility and function, and it is one of the important comorbidities in HD patients [5]. The incidence of sarcopenia in dialysis patients varies widely from 4–68% [6, 7]. Sarcopenia is associated with worse clinical outcomes, including worse quality of life and higher hospitalization and mortality [8]. Previous studies have suggested that sarcopenia is associated with osteoporosis [9, 10], Muscle mechanical loading is a crucial component in BMD maintenance. BMD is correlated with muscular strength and muscle mass, which are sources of mechanical loading [11]. There are few reports on the association between muscle mass, muscle strength, and osteoporosis in HD patients. Several studies have suggested a positive correlation between handgrip strength (HGS) and BMD in HD patients [12, 13] whereas some studies have suggested the opposite [14, 15].
Depression is a type of mood disorder caused by a variety of causes, often manifested as disproportionate depression and loss of interest, sometimes accompanied by anxiety, agitation, even hallucinations, delusions, and other psychotic symptoms. The prevalence of depression in HD patients is 20–47% [16]. An observational cross-sectional study of 414 HD patients from 24 dialysis centers in Greece found higher rates of depression in female patients than in males [17]. Depression increases the likelihood of cardiovascular disease, malnutrition, and inflammatory response, and affects the prognosis of HD patients [16]. Depression, being the most common psychological disorder among HD patients, may exacerbate the development and progression of osteoporosis by influencing lifestyle, dietary habits, and levels of physical activity. Existing studies suggest that depression is a risk factor for osteoporosis in the general population. Depression induces bone loss and osteoporotic fractures, primarily via specific immune and endocrine mechanisms, with poor lifestyle habits and use of specific antidepressants also potential contributory factors [18], but the specific mechanisms in HD patients are unclear. Although previous studies have explored the relationship between muscle mass, muscle strength, and osteoporosis, research on the connection between depression and osteoporosis remains limited, especially in HD patients. Therefore, this study aims to (1)investigate the relationship between depression and osteoporosis in HD patients, with a focus on muscle mass and muscle strength, offering new insights and evidence for clinical interventions and management, (2) investigate the relationship between muscle mass, muscle strength, depression, and osteoporosis in males and females HD patients, offering gender-specific interventions and management.
Methods
Study subjects
This cross-sectional multicenter study enrolled patients with ESRD undergoing HD from seven HD centers between July 2020 and April 2021. Adult patients on maintenance HD for at least 3 months who were able to provide informed consent were eligible to enroll, while the exclusion criteria included: (1) acute infection or acute cardiovascular and cerebrovascular events occurring one month before enrollment; (2) use of hormones or immunosuppressive therapy for more than 3 months before inclusion; (3) connective tissue disease or tumor; (4) bone diseases such as primary bone disorder. The study was approved by the Ethics Committee of the Shanghai University of Medicine and Health Sciences, and the methods were carried out following the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients before enrollment in the study. The study followed the STROBE guidelines [19].
Baseline variables
All participants were invited to a face-to-face interview to answer a standardized questionnaire. Baseline data of sociodemographic characteristics, health behaviors, and chronic disease conditions were considered. Demographic characteristics, including age, sex, height, and post-dialysis weight, were used to calculate body mass index (BMI, kg/m2) and dialysis duration. Health behaviors included smoking and drinking habits. Physical activity was assessed using the short form of the International Physical Activity Questionnaire (IPAQ). Nutritional status was assessed using the Malnutrition Inflammation Score (MIS). A valid method used to assess the risk of death from comorbid disease was the Charlson comorbidity index (CCI). In addition, all blood samples were drawn before HD. Blood samples were analyzed for the following markers: hemoglobin, albumin, C-reactive protein (CRP), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-cholesterol), low-density lipoprotein cholesterol (LDL-cholesterol), serum phosphate, serum calcium, parathyroid hormone (PTH), vitamin D and Kt/V (fractional clearance index for urea).
BMD and osteoporosis
BMD was measured by dual-energy X-ray absorptiometry (DXA) using an EXA3000 (OsteoSys, Seoul, Korea). Bone mass density (g/cm2) was measured at the radius bone. T scores of radius bone densities were used. To reduce measurement differences, all scans and calculations were completed by a single radiologic technologist. Testing was done before HD. According to the guidelines of the World Health Organization, osteoporosis is defined as a reduced bone density of 2.5 standard deviations (SD) below the average values for bone density among young and normal individuals of a society (T score≤-2.5) [2].
Parameters for sarcopenia
Sarcopenia was defined according to the criteria of the Asia Working Group for Sarcopenia (AWGS) [20]. The cutoff values for skeletal muscle mass index (SMI) measured by bioimpedance analysis (BIA) were < 7.0 kg/m2 for men and < 5.7 kg/m2 for women. The cutoff points for decreased HGS were < 26 kg (male) and < 18 kg (female). Walking disability was defined as gait speed < 0.8 m/s. The sarcopenia stage was defined as low SMI plus low HGS or low gait speed. SMI was calculated using body composition measurements obtained with multifrequency BIA (InBody S10; InBody Japan, Tokyo, Japan). BIA has been validated in various populations, including old adults, Asians, Koreans, and HD patients. Compared to DXA, BIA is easily applicable in clinical practice, relatively inexpensive, and does not pose a radiation hazard [9]. HGS was evaluated using a handgrip dynamometer (GRIP-D; Takei Ltd, Niigata, Japan) by using the non-fistula arm of the subject. For patients with dialysis catheters, we used the dominant hand to test HGS. Participants were asked to exert maximum effort twice, and the result from the strongest handgrip strength was used for analysis. Gait speed was assessed with the 4-m walk test. Patients were instructed to stand with both feet touching the starting line and started walking at their usual speed after verbal commands. All testing was done before HD.
Determination of depression
The Patient Health Questionnaire-9 (PHQ-9) was administered to assess depressive symptoms over the last two weeks [21]. Using a 4-point scale (0 = not at all, 1 = several days, 2 = more than half the days, 3 = nearly every day), patients reported the frequency with which they had experienced the following nine symptoms of major depressive disorder: (1) anhedonia, (2) depressed mood, (3) sleep disturbance, (4) fatigue, (5) appetite changes, (6) low self-esteem, (7) concentration problems, (8) psychomotor disturbances, and (9) suicidal ideation. Total scores range from 0 to 27. The instrument has demonstrated excellent reliability, validity, and responsiveness. The cut-offs have been proposed as 0–4, 5–9, 10–14, 15–19, and 20- for no, mild, moderate, moderately severe and severe depression, respectively [21]. According to the PHQ-9 cut-offs, depression is defined as the total score > 4.
Statistical analysis
In this cross-sectional survey investigation, the estimated prevalence of osteoporosis among HD patients was determined to be about 35% based on a small sample, with a confidence level of 1-α = 0.95 and a tolerance of 3.5%. Using the PASS 11, the sample size to be surveyed was determined to be 740. Assuming a 10% dropout rate, a minimum sample size of 814 cases was needed. Baseline sociodemographic and health-related characteristics were compared between patients with and without osteoporosis. The normality test was performed. Data with a normal distribution were expressed as the mean ± SD, using an independent t-test for numeric variables. Data with a nonnormal distribution are expressed as the median M (P25, P75). Nonparametric tests were used for comparisons between groups. Categorical variables were expressed as proportions, and a chi-square test was used for categorical variables. Spearman correlation coefficients were calculated for the correlation analysis. To determine which variables were related to BMD, stepwise linear regression analysis was performed. Logistic regression analysis for the association between sarcopenia, SMI, HGS, and depression with osteoporosis with the adjusted models was performed. Because sarcopenia was diagnosed based on SMI, HGS, and gait speed, sarcopenia had covariance with SMI and HGS and therefore was adjusted separately in the logistic regression analysis. Statistical significance was indicated by P < 0.05. All statistical analyses were performed using IBM SPSS Statistics v26.0 (SPSS Inc., Chicago, Illinois, United States).
Results
Baseline characteristics
The final analytic sample consisted of 858 patients. A flow chart detailing the derivation of the sample was presented in Fig. 1. Among 858 participants (524 male, 334 female, mean age 61.4 ± 12.6 years) who were available for analysis, 336 cases (39.2%) had osteoporosis, of which 37.6% were male and 41.6% were female. The prevalence of sarcopenia in HD was 18.9% (162/858), with 18.1% for the male and 20.1% for the female. The prevalence of depression in HD was 42.1% (361/858), with 41.2% for the male and 43.4% for the female.
Fig. 1.
Flowchart of the study population selection process
Comparing the basic characteristics of HD patients with and without osteoporosis
The sociodemographic and health-related characteristics of patients undergoing HD with and without osteoporosis are presented in Table 1. Age, dialysis duration, HDL-cholesterol, and PTH of the osteoporosis group were significantly higher than those of the non-osteoporosis group, with significant differences between groups (P < 0.05). The prevalence of sarcopenia and depression in osteoporosis group was significantly higher than the non-osteoporosis group (χ2 = 27.907, P < 0.001;χ2 = 7.615, P = 0.006). BMI, SMI, BMD, TG, phosphate, and HGS of the osteoporosis group were significantly lower than those of the non-osteoporosis group (P < 0.05). BMD, SMI, HGS, gait speed, CCI, hemoglobin, and vitamin D were significantly higher in male patients than in female patients (P < 0.05), while dialysis duration, MIS, TC, HDL-cholesterol, LDL-cholesterol, PTH, and Kt/V were lower (Table S1).
Table 1.
Comparing the basic characteristics of HD patients with osteoporosis and non-osteoporosis
| Characteristics | All | Non-Osteoporosis | Osteoporosis | P value |
|---|---|---|---|---|
| (n = 858) | (n = 522) | (n = 336) | ||
| Age (years) | 61.45 ± 12.55 | 60.22 ± 12.84 | 63.36 ± 11.85 | < 0.001 |
| Sex (%) | 0.239 | |||
| Male | 524 (61.07) | 327 (62.64) | 197 (58.63) | |
| Female | 334 (38.93) | 195 (37.36) | 139 (41.37) | |
| BMI (kg/m2) | 23.38 ± 3.82 | 23.88 ± 3.82 | 22.60 ± 3.70 | < 0.001 |
| Dialysis duration (months) | 46.33 (23.87, 93.20) | 40.43 (21.13, 71.43) | 58.03 (29.41,120.28) | < 0.001 |
| Cause of ESRD (%) | 0.035 | |||
| Glomerulonephritis | 264 (30.77) | 150 (28.74) | 114 (33.93) | |
| Diabetic | 190 (22.14) | 132 (25.29) | 58 (17.26) | |
| Hypertensive | 130 (15.15) | 84 (16.09) | 46 (13.69) | |
| Polycystic kidney | 51 (5.94) | 30 (5.74) | 21 (6.25) | |
| Other | 223 (26.00) | 126 (24.14) | 97 (28.87) | |
| Drinking (%) | 10 (1.16) | 5 (0.96) | 5 (1.49) | 0.069 |
| Smoking (%) | 185 (21.56) | 117 (22.41) | 68 (20.24) | 0.180 |
| BMD (g/cm2) | 0.41 ± 0.09 | 0.46 ± 0.07 | 0.33 ± 0.06 | < 0.001 |
| Sarcopenia (%) | 162 (18.88) | 69 (13.22) | 93 (27.67) | < 0.001 |
| SMI (kg/m2) | 6.98 ± 1.21 | 7.20 ± 1.20 | 6.65 ± 1.14 | < 0.001 |
| Handgrip strength (kg) | 24.87 ± 8.74 | 25.94 ± 8.73 | 23.21 ± 8.50 | < 0.001 |
| Gait speed (m/s) | 0.98 ± 0.30 | 0.98 ± 0.31 | 0.97 ± 0.30 | 0.508 |
| IPAQ (Met/wk) | 1386 (594,3066) | 1386 (551,3066) | 1386 (608,3066) | 0.456 |
| MIS | 4.30 ± 2.84 | 4.25 ± 2.87 | 4.38 ± 2.79 | 0.537 |
| CCI | 3.87 ± 1.68 | 3.87 ± 1.65 | 3.88 ± 1.70 | 0.918 |
| Depression (%) | 361(42.1) | 201 (38.5) | 160 (47.6) | 0.006 |
| Laboratory parameters | ||||
| Hemoglobin (g/L) | 110.97 ± 15.79 | 110.73 ± 15.69 | 111.33 ± 15.96 | 0.592 |
| Albumin (g/L) | 39.80 (37.60, 41.90) | 39.80 (37.60,42.0) | 39.75 (37.72, 41.80) | 0.954 |
| CRP (mg/L) | 2.88 (1.29, 6.04) | 2.84 (1.31, 5.97) | 2.92 (1.22, 6.30) | 0.909 |
| Total cholesterol (mmol/L) | 3.92 ± 1.21 | 3.93 ± 1.30 | 3.89 ± 1.04 | 0.630 |
| Triglycerides (mmol/L) | 1.70 (1.19, 2.74) | 1.80 (1.23, 2.89) | 1.64 (1.06, 2.51) | 0.040 |
| HDL-cholesterol (mmol/L) | 0.97 ± 0.28 | 0.95 ± 0.27 | 1.01 ± 0.29 | 0.002 |
| LDL-cholesterol (mmol/L) | 2.30 ± 0.81 | 2.32 ± 0.81 | 2.27 ± 0.81 | 0.332 |
| Phosphate (mmol/L) | 1.90 (1.51,2.34) | 1.94 (1.55, 2.37) | 1.84 (1.46, 2.24) | 0.027 |
| Calcium (mmol/L) | 2.27 ± 0.26 | 2.27 ± 0.25 | 2.26 ± 0.27 | 0.421 |
| PTH (pg/mL) | 272.75 (143.15,469.17) | 258.45 (138.08,424.68) | 309.65 (155.30, 554.73) | 0.001 |
| Vitamin D (nmol/L) | 31.56 (24.41, 44.16) | 31.30 (24.32, 43.37) | 32.00 (24.88, 47.00) | 0.369 |
| Kt/V | 1.37 ± 0.33 | 1.33 ± 0.30 | 1.43 ± 0.36 | < 0.001 |
BMI, body mass index; SMI, skeletal mass index; ESRD, end stage renal disease; BMD, bone mineral density; IPAQ, international physical activity questionnaire; Met/wk, metabolic equivalent task minutes per week; MIS, malnutrition inflammation score; CCI, Charlson Comorbidity Index; CRP, C-reactive protein; HDL-cholesterol, high-density lipoprotein cholesterol; LDL-cholesterol, low-density lipoprotein cholesterol; PTH, parathyroid hormone; Kt/V, fractional clearance index for urea
Spearman correlation analysis of BMD and factors
BMI, SMI, HGS, CCI, and gait speed were positively correlated with BMD (P < 0.05). Age, female sex, dialysis duration, TC, HDL-cholesterol, LDL-cholesterol, phosphate, MIS, Kt/V, and PTH were negatively correlated with BMD (P < 0.05) (Table 2). SMI, HGS, dialysis duration, PTH, and Kt/V were correlated with BMD in HD patients whether male or female (P < 0.05) (Table S2).
Table 2.
The correlation analysis of BMD and factors
| Characteristics | Spearman correlation analysis | Multiple stepwise linear regression analysis | |||
|---|---|---|---|---|---|
| r | P value | Unstandardized coefficient | Standardized coefficient | P value | |
| Age (years) | -0.157 | < 0.001 | -0.001 | -0.096 | 0.010 |
| Female | -0.580 | < 0.001 | -0.059 | -0.305 | < 0.001 |
| BMI (kg/m2) | 0.218 | < 0.001 | |||
| Dialysis duration (months) | -0.300 | < 0.001 | -0.0002 | -0.154 | < 0.001 |
| SMI (kg/m2) | 0.570 | < 0.001 | 0.018 | 0.237 | < 0.001 |
| Handgrip strength (kg) | 0.483 | < 0.001 | 0.001 | 0.101 | 0.021 |
| Gait speed (m/s) | 0.102 | < 0.001 | |||
| IPAQ (Met/wk) | -0.016 | 0.649 | |||
| MIS | -0.129 | < 0.001 | |||
| CCI | 0.068 | 0.047 | |||
| Hemoglobin (g/L) | 0.026 | 0.443 | |||
| Albumin (g/L) | 0.056 | 0.1 | |||
| CRP (mg/L) | 0.036 | 0.327 | |||
| Total cholesterol (mmol/L) | -0.149 | < 0.001 | |||
| Triglycerides (mmol/L) | 0.054 | 0.112 | |||
| HDL-cholesterol (mmol/L) | -0.221 | < 0.001 | -0.027 | -0.079 | 0.015 |
| LDL-cholesterol (mmol/L) | -0.108 | 0.002 | |||
| Phosphate (mmol/L) | 0.079 | 0.022 | |||
| Calcium (mmol/L) | -0.034 | 0.319 | |||
| PTH (pg /mL) | -0.170 | < 0.001 | -0.00005 | -0.162 | < 0.001 |
| Vitamin D (nmol/L) | 0.001 | 0.98 | |||
| Kt/V | -0.357 | < 0.001 | |||
BMD, bone mineral density; BMI, body mass index; SMI, skeletal mass index; IPAQ, international physical activity questionnaire; Met/wk, metabolic equivalent task minutes per week; MIS, malnutrition inflammation score; CCI, Charlson Comorbidity Index; CRP, C-reactive protein; HDL cholesterol, high-density lipoprotein cholesterol; LDL- cholesterol, low-density lipoprotein cholesterol; PTH, parathyroid hormone; Kt/V, fractional clearance index for urea
Multiple linear regression analysis of BMD and factors
Table 2 showed that age, sex, dialysis duration, SMI, HDL-cholesterol, HGS, and PTH were independent influencing factors of BMD in HD patients. Dialysis duration, SMI, and PTH were independent influencing factors of BMD in HD patients whether male or female (P < 0.05), while age was an independent influencing factor of BMD in female patients (P < 0.05) (Table S3).
Risk of sarcopenia, depression, SMI, and HGS on osteoporosis
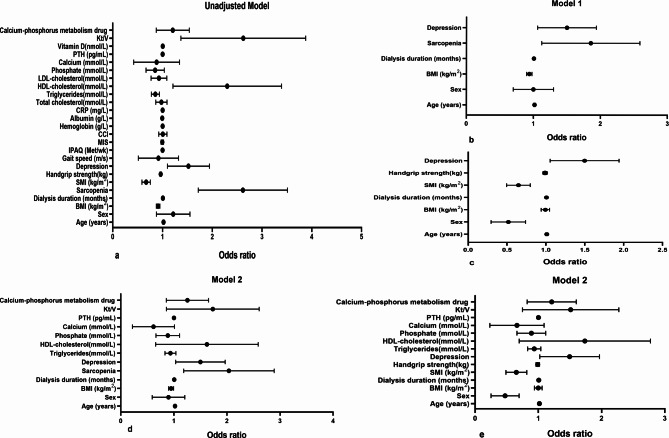
The risk of sarcopenia, depression, SMI, and HGS in osteoporosis was first assessed using logistic regression analysis in the univariate model (Table 3; Fig. 2). Depression showed a significantly increased risk of osteoporosis (Odds Ratio (OR) = 1.482, 95% Confidence Interval (CI) = 1.120–1.962, P = 0.006). Meanwhile, SMI and HGS were negatively correlated with osteoporosis (P < 0.001), while gait speed was not related to osteoporosis (P = 0.507). Subgroup analysis by gender found that osteoporosis was not associated with depression in male patients, while osteoporosis was associated with depression in female patients (P < 0.05). Multivariate logistic regression analysis was further adjusted for potential confounders (age, sex, BMI, dialysis duration) (Table 4; Fig. 2), and sarcopenia, SMI, and depression were significantly associated with osteoporosis. Subgroup analysis by gender found that sarcopenia and depression were not associated with osteoporosis in male patients, while sarcopenia, SMI, and depression were significantly associated with osteoporosis in female patients (P < 0.05). After adjusting for potential confounders such as age, sex, BMI, dialysis duration, TG, HDL-cholesterol, serum phosphate, serum calcium, PTH, calcium-phosphorus metabolism drugs, and Kt/V (Table 5; Fig. 2), which were statistically significant in the univariate analysis, as well as additional clinically relevant factors like calcium and phosphorus, the risk of osteoporosis in HD patients with sarcopenia was found to be about 2 times higher compared to those without sarcopenia(OR = 1.919, 95% CI = 1.251–2.943, P = 0.003). Additionally, higher SMI reduced the risk of osteoporosis (OR = 0.638, 95% CI = 0.494–0.823, P = 0.001). Most notably, after adjusting for both sarcopenia and SMI, the risk of osteoporosis in HD patients with depression was 1.45 times higher than in those without depression (OR = 1.452, 95% CI = 1.060–1.989, P = 0.020). Subgroup by gender found that depression was not associated with osteoporosis in male patients, while sarcopenia was not associated with osteoporosis in female patients. The risk of osteoporosis in female HD patients with depression was found to be 1.9 times higher compared to those without depression (OR = 1.942, 95% CI = 1.120–3.368, P = 0.018). Additionally, higher SMI reduced the risk of osteoporosis whether male or female (P < 0.05).
Table 3.
Associations of Sarcopenia, SMI, HGS, and depression with osteoporosis by univariate logistic regression analysis
| Variables | All | Male | Female | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Sex (male vs. female) | 1.183 (0.894, 1.566) | 0.240 | ||||
| Age (years) | 1.021 (1.009, 1.032) | < 0.001 | 1.004 (0.990, 1.018) | 0.602 | 1.051 (1.030, 1.073) | < 0.001 |
| BMI (kg/m2) | 0.910 (0.875, 0.946) | < 0.001 | 0.881 (0.833, 0.931) | < 0.001 | 0.945 (0.894, 0.999) | 0.045 |
| Duration of dialysis (months) | 1.008 (1.005, 1.010) | < 0.001 | 1.008 (1.005, 1.011) | < 0.001 | 1.007 (1.003, 1.011) | 0.001 |
| Sarcopenia | 2.513 (1.774, 3.559) | < 0.001 | 2.132 (1.360, 3.341) | 0.001 | 3.191 (1.826, 5.579) | < 0.001 |
| SMI (kg/m2) | 0.667 (0.589, 0.756) | < 0.001 | 0.605 (0.500, 0.732) | < 0.001 | 0.498 (0.371, 0.668) | < 0.001 |
| Handgrip strength(kg) | 0.963 (0.948, 0.979) | < 0.001 | 0.975 (0.953, 0.991) | 0.025 | 0.902 (0.866, 0.941) | < 0.001 |
| Depression | 1.482 (1.120, 1.962) | 0.006 | 1.228 (0.856, 1.761) | 0.264 | 1.961 (1.252, 3.073) | 0.003 |
| Gait speed (m/s) | 0.858 (0.546,1.349) | 0.507 | 1.333 (0.733,2.424) | 0.346 | 0.481 (0.236, 0.919) | 0.044 |
| IPAQ (Met/wk) | 1.000 (1.000, 1.000) | 0.457 | 1.000 (1.000, 1.000) | 0.862 | 1.000 (1.000, 1.000) | 0.231 |
| MIS | 1.015 (0.967, 1.006) | 0.536 | 1.012 (0.948, 1.079) | 0.727 | 1.011 (0.938, 1.090) | 0.768 |
| CCI | 1.004 (0.925, 1.090) | 0.918 | 0.983 (0.887, 1.089) | 0.739 | 1.067 (0.927, 1.228) | 0.365 |
| Hemoglobin (g/L) | 1.002 (0.994, 1.011) | 0.592 | 0.998 (0.988, 1.009) | 0.773 | 1.012 (0.996, 1.027) | 0.133 |
| Albumin (g/L) | 0.994 (0.980, 1.008) | 0.408 | 0.996 (0.981, 1.011) | 0.600 | 0.990 (0.959, 1.021) | 0.529 |
| CRP (mg/L) | 1.002 (0.992, 1.012) | 0.717 | 1.008 (0.995, 1.020) | 0.243 | 0.988 (0.967, 1.010) | 0.285 |
| Total cholesterol(mmol/L) | 0.973 (0.866, 1.092) | 0.639 | 0.961 (0.804, 1.148) | 0.660 | 0.950 (0.808, 1.118) | 0.539 |
| Triglycerides(mmol/L) | 0.853 (0.776, 0.938) | 0.001 | 0.839 (0.740, 0.951) | 0.006 | 0.869 (0.752, 1.005) | 0.059 |
| HDL-cholesterol(mmol/L) | 2.128 (1.306, 3.468) | 0.002 | 2.549 (1.349, 4.819) | 0.004 | 1.479 (0.667, 3.282) | 0.335 |
| LDL-cholesterol(mmol/L) | 0.919 (0.774, 1.090) | 0.332 | 0.843 (0.664, 1.072) | 0.164 | 0.965 (0.748, 1.246) | 0.785 |
| Phosphate (mmol/L) | 0.838 (0.675, 1.041) | 0.110 | 0.849 (0.647, 1.114) | 0.239 | 0.817 (0.569, 1.174) | 0.274 |
| Calcium (mmol/L) | 0.801 (0.466, 1.376) | 0.421 | 0.670 (0.337, 1.332) | 0.253 | 1.018 (0.415, 2.498) | 0.969 |
| PTH (pg/mL) | 1.001 (1.001, 1.002) | < 0.001 | 1.001 (1.000, 1.001) | 0.005 | 1.002 (1.001, 1.002) | < 0.001 |
| Vitamin D(nmol/L) | 1.005 (0.997, 1.013) | 0.259 | 1.012 (1.002, 1.022) | 0.015 | 0.990 (0.975, 1.005) | 0.197 |
| Kt/V | 2.259 (1.591, 4.019) | < 0.001 | 2.908(1.504, 5.619) | 0.001 | 2.088 (0.981, 4.444) | 0.056 |
| Calcium-phosphorus metabolism drug | 1.175 (0.890, 1.552) | 0.256 | 1.247 (0.868, 1.792) | 0.232 | 1.103 (0.712, 1.709) | 0.660 |
BMI, body mass index; SMI, skeletal mass index; IPAQ, international physical activity questionnaire; Met/wk, metabolic equivalent task minutes per week; MIS, malnutrition inflammation score; CCI, Charlson Comorbidity Index; CRP, C-reactive protein; HDL-cholesterol, high-density lipoprotein cholesterol; LDL-cholesterol, low-density lipoprotein cholesterol; PTH, parathyroid hormone; Kt/V, fractional clearance index for urea
Fig. 2.
The relationships of sarcopenia, SMI, HGS, and depression with osteoporosis using logistic regression analysis. a: Unadjusted model; b: Model 1: the relationship of sarcopenia and depression with osteoporosis: adjusted for age, sex, BMI, dialysis duration; c: Model 1: the relationships of SMI, HGS, and depression with osteoporosis: adjusted for age, sex, BMI, dialysis duration; d: Model 2: the relationship of sarcopenia and depression with osteoporosis: adjusted for age, sex, BMI, dialysis duration, phosphate, calcium, PTH, calcium-phosphorus metabolism drug; triglycerides, HDL-cholesterol, Kt/V; e: Model 2: the relationships of SMI, HGS, and depression with osteoporosis: adjusted for age, sex, BMI, dialysis duration, phosphate, calcium, PTH, calcium-phosphorus metabolism drug; triglycerides, HDL-cholesterol, Kt/V
Table 4.
Associations of Sarcopenia, SMI, HGS, and depression with osteoporosis
| Variables | All | Male | Female | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Sex (male vs. female) | 0.970 (0.716, 1.315) | 0.846 | 0.485 (0.313, 0.753) | 0.001 | ||||||||
| Age (years) | 1.018 (1.005, 1.031) | 0.005 | 1.011 (0.997, 1.025) | 0.131 | 0.999 (0.983, 1.015) | 0.925 | 0.993 (0.996, 1.010) | 0.426 | 1.052 (1.028, 1.077) | <0.001 | 1.041 (1.015, 1.068) | 0.002 |
| BMI (kg/m2) | 0.939 (0.899, 0.979) | 0.004 | 0.992(0.940, 1.047) | 0.781 | 0.910 (0.859, 0.964) | 0.001 | 0.996(0.896, 1.041) | 0.366 | 0.950 (0.887, 1.018) | 0.145 | 0.992 (0.913, 1.077) | 0.840 |
| Dialysis duration (months) | 1.007 (1.005, 1.010) | <0.001 | 1.007 (1.004, 1.010) | <0.001 | 1.007 (1.003, 1.011) | <0.001 | 1.007 (1.003, 1.010) | <0.001 | 1.008 (1.004, 1.012) | <0.001 | 1.008 (1.003, 1.012) | 0.001 |
| Sarcopenia | 1.760 (1.177, 2.632) | 0.006 | 1.574 (0.932, 2.659) | 0.090 | 2.059 (1.070, 3.960) | 0.031 | ||||||
| SMI (kg/m2) | 0.635 (0.502, 0.804) | <0.001 | 0.678 (0.506, 0.908) | 0.009 | 0.583 (0.385, 0.884) | 0.011 | ||||||
| Handgrip strength(kg) | 0.989 (0.965, 1.015) | 0.407 | 0.992 (0.965, 1.021) | 0.586 | 0.968 (0.915, 1.023) | 0.249 | ||||||
| Depression | 1.458 (1.086, 1.958) | 0.012 | 1.454 (1.078, 1.962) | 0.014 | 1.220(0.836, 1.781) | 0.303 | 1.220(0.833, 1.787) | 0.308 | 1.839(1.129, 2.994) | 0.014 | 1.794(1.084, 2.970) | 0.023 |
BMI, body mass index; SMI, skeletal mass index;
Adjusted for age, sex, BMI, dialysis duration
Table 5.
Associations of Sarcopenia, SMI, HGS, and depression with osteoporosis by multivariate logistic regression analysis adjusted for age, sex, BMI, dialysis duration, phosphate, calcium, PTH, calcium-phosphorus metabolism drug; triglycerides, HDL-cholesterol, Kt/V
| Variables | All | Male | Female | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Sex (male vs. female) | 0.864 (0.611, 1.223) | 0.410 | 0.439 (0.271, 0.710) | 0.001 | ||||||||
| Age (years) | 1.022 (1.007, 1.036) | 0.003 | 1.016 (1.000, 1.031) | 0.046 | 0.999 (0.981, 1.017) | 0.918 | 0.993 (0.974, 1.012) | 0.485 | 1.059 (1.032, 1.087) | < 0.001 | 1.053 (1.023, 1.083) | < 0.001 |
| BMI (kg/m2) | 0.948 (0.902, 0.996) | 0.035 | 0.997 (0.938, 1.060) | 0.914 | 0.942 (0.882, 1.007) | 0.077 | 0.996 (0.915, 1.085) | 0.933 | 0.940 (0.856, 1.022) | 0.144 | 0.982 (0.890, 1.083) | 0.714 |
| Dialysis duration (months) | 1.007 (1.004, 1.010) | < 0.001 | 1.007 (1.004, 1.010) | < 0.001 | 1.007 (1.003, 1.011) | 0.001 | 1.006 (1.002, 1.010) | 0.002 | 1.007 (1.002, 1.012) | 0.003 | 1.008 (1.003, 1.013) | 0.003 |
| Sarcopenia | 1.919 (1.251, 2.943) | 0.003 | 1.859 (1.056, 3.272) | 0.032 | 1.901 (0.938, 3.849) | 0.075 | ||||||
| SMI (kg/m2) | 0.638 (0.494, 0.823) | 0.001 | 0.648 (0.499, 0.937) | 0.018 | 0.546 (0.339, 0.879) | 0.013 | ||||||
| Handgrip strength(kg) | 0.990 (0.964, 1.017) | 0.456 | 0.987 (0.958, 1.018) | 0.412 | 0.995 (0.937, 1.057) | 0.877 | ||||||
| Depression | 1.452 (1.060, 1.989) | 0.020 | 1.445 (1.050, 1.989) | 0.024 | 1.282(0.852, 1.927) | 0.233 | 1.267 (0.840, 1.910) | 0.26 | 1.886 (1.111, 3.204) | 0.019 | 1.942(1.120, 3.368) | 0.018 |
BMI, body mass index; SMI, skeletal mass index; HDL-cholesterol, high-density lipoprotein cholesterol PTH, parathyroid hormone; Kt/V, fractional clearance index for urea
Adjusted for age, sex, BMI, dialysis duration, phosphate, calcium, PTH, calcium-phosphorus metabolism drug; triglycerides, HDL-cholesterol, Kt/V
Discussion
This study investigated the associations between sarcopenia, muscle mass, muscle strength, depression, and osteoporosis in HD patients. We found that HD patients with sarcopenia were more likely to have osteoporosis. Muscle mass rather than muscle strength was associated with osteoporosis in HD patients. Depression was a significant risk factor for osteoporosis in HD patients. In addition, male HD patients had higher BMD, SMI, HGS, and gait speed than females. The prevalence of sarcopenia, depression, and osteoporosis was lower in males than females. Dialysis duration, SMI, and PTH were independent influencing factors of BMD in HD patients whether male or female, while older age was an independent risk factor of lower BMD in females. Depression was not associated with osteoporosis in male patients, while sarcopenia was not associated with osteoporosis in female patients.
Osteoporosis is common in dialysis patients [3]. The hip bone and vertebral density are commonly used to diagnose osteoporosis; however, the radius bone has recently attracted attention in terms of feasibility and accessibility, as it can be done with precision using portable technologies. The study specified that radius density had a statistically significant linear correlation with the hip or spine density final result on which the osteoporosis diagnosis was based [22]. In our study, we found that osteoporosis was common in dialysis patients, and it was indicated by radial BMD in 39.2% of patients. Lee et al. reported that 38.9% of HD patients had osteoporosis [9]. This reported result was similar to those of our study. Suggests that it was plausible to detect BMD through the radius for the diagnosis of osteoporosis.
Due to uremic toxins, oxidative stress, persistent inflammation, and starvation, sarcopenia is worsened by CKD [23]. It has been estimated that 20% of dialysis patients suffer sarcopenia. Our result was consistent with a previous result [5]. Our study found that HD patients with sarcopenia were 1.92 times more likely to have osteoporosis than patients without sarcopenia. There is evidence of a mechanistic link between muscle and bone, with sarcopenic people having a higher risk of osteoporosis [24]. It has been widely assumed that bone and skeletal muscle are interrelated tissues. Both bone and muscle share genetic, environmental, endocrine, and paracrine factors, several pathways and genes, such as androgen receptor, insulin-like growth factor I, myostatin, vitamin D receptor, and interleukin-6, appear to have a biologically plausible pleiotropic effect [25]. The sarcopenia stage was defined as a low SMI plus low HGS or low walking speed. Our results found that the SMI and HGS were positively associated with BMD. SMI and HGS showed a significantly negative correlation with osteoporosis in the univariate model, which was consistent with previous studies. Interestingly, after adjusting for potential confounders, we found that HGS was not associated with the risk of osteoporosis in HD patients, while SMI was associated with a lower risk of osteoporosis. This indicated that the effects of muscle strength on osteoporosis risk may be an indirect phenomenon, possibly mediated by muscle mass. Even though muscles and bones have a physiological connection, muscle mass, and muscle strength were independently linked to osteoporosis and should be considered separately in HD patients. The differential impact of SMI and HGS on osteoporosis risk in HD patients underscores the distinct physiological roles of muscle mass and muscle strength. Muscle mass, reflected by SMI, may have a more direct influence on osteoporosis through mechanical loading and the stimulation of bone formation. In contrast, HGS, a measure of muscle strength, might not directly affect osteoporosis but serves as an indicator of overall muscle function and health. Moreover, restricted activity during and fatigue after HD reduces physical activity, which causes a greater reduction in muscle mass than muscle strength [26, 27]. This distinction could explain why, after adjusting for confounders, SMI remains associated with a lower risk of osteoporosis, highlighting the primary role of muscle mass in maintaining bone health. The finding that muscle mass, rather than HGS, is more closely linked to osteoporosis risk in HD patients suggests that interventions aimed at preserving or increasing muscle mass could be more beneficial in preventing osteoporosis than those focusing solely on increasing muscle strength. Resistance training, known to enhance both muscle mass and strength, could be particularly effective if tailored to emphasize muscle hypertrophy. Clinicians should consider assessing muscle mass as part of routine care in HD patients and prioritize interventions that address muscle wasting.
Depression is a chronic and recurrent illness that often coexists with physical conditions, such as those faced by HD patients, who must endure physical discomfort from their disease along with numerous psychological stressors, including social and familial issues [28]. This combination leads to a significantly increased risk of depression, with its prevalence being more than four times higher in HD patients compared to the general population [16]. The interplay between depression and osteoporosis is complex, as both conditions share common pathophysiological pathways, including chronic inflammation, hormonal imbalances, and lifestyle factors such as reduced physical activity. Although the relationship between depression and osteoporosis has been widely studied in the general population, few studies have examined this link specifically in HD patients [29]. Our findings suggest that depression is a significant risk factor for osteoporosis in HD patients, independent of sarcopenia and muscle mass. After adjusting for sarcopenia and SMI, HD patients with depression were still 1.45 times more likely to have osteoporosis. This underscores the need to consider depression as a potential contributing factor to bone health in HD patients, beyond the traditional focus on muscle mass and strength. Depression can exacerbate physical inactivity, increase cortisol levels [16], and impair nutrition, all of which may negatively impact bone metabolism and increase the risk of osteoporosis. Our study highlights that depression’s role in osteoporosis might be an overlooked but critical factor in the HD patient population. Addressing depression in HD patients could not only improve mental health but also help in preventing the deterioration of bone health, suggesting the importance of comprehensive care that includes mental health management as part of the strategy to mitigate osteoporosis risk. Therefore, early identification and treatment of depression should be integrated into routine osteoporosis prevention and management protocols in HD patients, as psychological well-being plays a crucial role in maintaining bone health.
There are many risk factors for osteoporosis in HD patients. Females as a greater risk of developing osteoporosis. In our study, we found that the prevalence of sarcopenia, depression, and osteoporosis was lower in males than in females. Male HD patients had higher BMD, SMI, HGS, and gait speed than those in females. This result was consistent with previous studies [9, 30]. This study found that depression was not associated with osteoporosis in male patients, while sarcopenia was not associated with osteoporosis in female patients. Additionally, higher SMI reduced the risk of osteoporosis whether male or female. Based on this result we should pay more attention to mental health in female patients and reduce the incidence of sarcopenia in male patients, as increasing muscle mass is effective in reducing the risk of osteoporosis.
This study had several limitations. First, being a cross-sectional study, it does not allow for establishing cause-and-effect relationships between sarcopenia, muscle mass, muscle strength, depression, and osteoporosis. Future research should focus on conducting longitudinal studies to clarify these relationships over time. Secondly, we assessed radial bone density for convenience, rather than measuring hip or vertebral density. Although previous research has suggested that radial BMD is comparable to BMD measurements from the hip or spine, this choice may have influenced the generalizability of our findings. Thirdly, we didn’t measure estrogen levels, which is an important factor in osteoporosis in female patients.
Conclusions
Overall, this study highlights that muscle mass, rather than muscle strength, is more closely associated with osteoporosis in HD patients, indicating distinct roles for muscle mass and strength in this condition. HD patients with sarcopenia were at greater risk of developing osteoporosis, suggesting that maintaining or increasing muscle mass could help reduce osteoporosis incidence in this population. However, our findings also emphasize the critical role of depression as an independent risk factor for osteoporosis in HD patients. Given the high prevalence and often underdiagnosed nature of depression in this group, addressing psychological well-being is essential for reducing osteoporosis risk. Depression may exacerbate bone loss through mechanisms such as decreased physical activity, hormonal imbalances, and poor nutrition. Therefore, comprehensive management of HD patients especially for females should include routine screening and treatment for depression as part of an integrated approach to preventing osteoporosis. Further research is needed to better understand how psychological factors like depression contribute to bone health in HD patients and to develop effective interventions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Table S1 S2 S3
Supplementary Material 2: STROBE Statement
Acknowledgements
We are grateful to all the medical workers at the multi-center dialysis for their generous technical assistance and guidance.
Abbreviations
- CKD
Chronic Kidney Disease
- BMD
Bone Mineral Density
- ESRD
End-Stage Renal Disease
- MBD
Mineral Bone Disorder
- HGS
Handgrip Strength
- STROBE
The Strengthening the Reporting of Observational Studies in Epidemiology
- BMI
Body Mass Index
- IPAQ
International Physical Activity Questionnaire
- CCI
Charlson Comorbidity Index
- CRP
C-Reactive Protein
- TC
Total Cholesterol
- TG
Triglycerides
- HDL
High-Density Lipoprotein
- LDL
Low-Density Lipoprotein
- PTH
Parathyroid Hormone
- Kt/V
Fractional Clearance Index for Urea
- DXA
Dual-energy X-ray Absorptiometry
- SD
Standard Deviations
- SMI
Skeletal Muscle Mass Index
- BIA
Bioimpedance Analysis
- PHQ-9
Patient Health Questionnaire-9
- Met/wk
Metabolic Equivalent Task Minutes Per Week
- OR
Odds Ratio
- CI
Confidence Interval
Author contributions
X. H. conceived and performed the study, analyzed the data, and contributed to the writing of the manuscript. X. Y. analyzed the data and contributed to the writing of the manuscript. H. C. revised the manuscript. C. X. and L. Z. designed and supervised the writing of the manuscript. B. W.and Q. G.and C. Y. and W. D. and J. N. and J. Z.and H. Q. and S. Z. performed the study. All authors reviewed the manuscript.
Funding
This work was supported by the Shanghai Jing’an District Health Commission Research Project (2022MS04) and Shanghai Science and Technology Innovation Action Plan of Scientific Instruments and Chemical Reagents Project (24142201800).
Data availability
The datasets generated for this study are available on request to the corresponding author.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Shanghai University of Medicine and Health Sciences. Informed consent was obtained from all subjects involved in the study.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaohua Hu, Xianwu Ye and Haimin Chen contributed equally to this work.
Contributor Information
Cheng Xue, Email: chengxia1568@126.com.
Liming Zhang, Email: zlm198291@163.com.
References
- 1.Tanaka S, Ito M. [Bone and Nutrition. Nutrition care of renal osteodystrophy]. Clin Calcium. 2015;25(7):1057–62. [PubMed] [Google Scholar]
- 2.NIH Consensus Development Panel on Osteoporosis, Prevention D. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285(6):785–95. [DOI] [PubMed] [Google Scholar]
- 3.Slouma M, Sahli H, Bahlous A, Laadhar L, Smaoui W, Rekik S, Gharsallah I, Sallami M, Moussa FB, Elleuch M, et al. Mineral bone disorder and osteoporosis in hemodialysis patients. Adv Rheumatol. 2020;60(1):15. [DOI] [PubMed] [Google Scholar]
- 4.Evenepoel P, Cunningham J, Ferrari S, Haarhaus M, Javaid MK, Lafage-Proust MH, Prieto-Alhambra D, Torres PU, Cannata-Andia J. European Consensus Statement on the diagnosis and management of osteoporosis in chronic kidney disease stages G4-G5D. Nephrol Dial Transpl. 2021;36(1):42–59. [DOI] [PubMed] [Google Scholar]
- 5.Isoyama N, Qureshi AR, Avesani CM, Lindholm B, Bàràny P, Heimbürger O, Cederholm T, Stenvinkel P, Carrero JJ. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol. 2014;9(10):1720–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.da Silva MZC, Vogt BP, Reis N, Caramori JCT. Update of the European consensus on Sarcopenia: what has changed in diagnosis and prevalence in peritoneal dialysis? Eur J Clin Nutr. 2019;73(8):1209–11. [DOI] [PubMed] [Google Scholar]
- 7.Matsuzawa R, Yamamoto S, Suzuki Y, Imamura K, Harada M, Matsunaga A, Tamaki A, Fukui T, Shimokado K. The clinical applicability of ultrasound technique for diagnosis of Sarcopenia in hemodialysis patients. Clin Nutr. 2021;40(3):1161–7. [DOI] [PubMed] [Google Scholar]
- 8.Sabatino A, Cuppari L, Stenvinkel P, Lindholm B, Avesani CM. Sarcopenia in chronic kidney disease: what have we learned so far? J Nephrol. 2021;34(4):1347–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee H, Kim K, Ahn J, Lee DR, Lee JH, Hwang SD. Association of nutritional status with osteoporosis, Sarcopenia, and cognitive impairment in patients on hemodialysis. Asia Pac J Clin Nutr. 2020;29(4):712–23. [DOI] [PubMed] [Google Scholar]
- 10.Xiang T, Fu P, Zhou L. Sarcopenia and Osteosarcopenia among patients undergoing hemodialysis. Front Endocrinol. 2023;14:1181139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tachiki T, Kouda K, Dongmei N, Tamaki J, Iki M, Kitagawa J, Takahira N, Sato Y, Kajita E, Fujita Y, et al. Muscle strength is associated with bone health independently of muscle mass in postmenopausal women: the Japanese population-based osteoporosis study. J Bone Min Metab. 2019;37(1):53–9. [DOI] [PubMed] [Google Scholar]
- 12.Ozawa M, Hirawa N, Haze T, Haruna A, Kawano R, Komiya S, Ohki Y, Suzuki S, Kobayashi Y, Fujiwara A, et al. The implication of calf circumference and grip strength in osteoporosis and bone mineral density among hemodialysis patients. Clin Exp Nephrol. 2023;27(4):365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen SC, Chung WS, Wu PY, Huang JC, Chiu YW, Chang JM, Chen HC. Associations among Geriatric Nutrition Risk Index, bone mineral density, body composition and handgrip strength in patients receiving hemodialysis. Nutrition. 2019;65:6–12. [DOI] [PubMed] [Google Scholar]
- 14.Ito K, Ookawara S, Hibino Y, Imai S, Fueki M, Bandai Y, Yasuda M, Kamimura T, Kakuda H, Kiryu S, et al. Skeletal muscle Mass Index is positively Associated with Bone Mineral density in Hemodialysis patients. Front Med (Lausanne). 2020;7:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tominaga H, Oku M, Arishima Y, Ikeda T, Ishidou Y, Nagano S, Minami M, Ido A, Komiya S, Setoguchi T. Association between bone mineral density, muscle volume, walking ability, and geriatric nutritional risk index in hemodialysis patients. Asia Pac J Clin Nutr. 2018;27(5):1062–6. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Zhu B, Shen J, Miao L. Depression in maintenance hemodialysis patients: what do we need to know? Heliyon. 2023;9(9):e19383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerogianni G, Lianos E, Kouzoupis A, Polikandrioti M, Grapsa E. The role of socio-demographic factors in depression and anxiety of patients on hemodialysis: an observational cross-sectional study. Int Urol Nephrol. 2018;50(1):143–54. [DOI] [PubMed] [Google Scholar]
- 18.Cizza G, Primma S, Csako G. Depression as a risk factor for osteoporosis. Trends Endocrinol Metab. 2009;20(8):367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(Suppl 1):S31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15(2):95–101. [DOI] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amiri L, Kheiltash A, Movassaghi S, Moghaddassi M, Seddigh L. Comparison of bone density of distal Radius with hip and spine using DXA. Acta Med Iran. 2017;55(2):92–6. [PubMed] [Google Scholar]
- 23.Moorthi RN, Avin KG. Clinical relevance of Sarcopenia in chronic kidney disease. Curr Opin Nephrol Hypertens 2017, 26(3):219–28. [DOI] [PMC free article] [PubMed]
- 24.Edwards MH, Dennison EM, Aihie Sayer A, Fielding R, Cooper C. Osteoporosis and sarcopenia in older age. Bone. 2015;80:126–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karasik D, Kiel DP. Evidence for pleiotropic factors in genetics of the musculoskeletal system. Bone. 2010;46(5):1226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noce A, Marrone G, Ottaviani E, Guerriero C, Di Daniele F. Pietroboni Zaitseva A, Di Daniele N: uremic Sarcopenia and its possible Nutritional Approach. Nutrients 2021, 13(1). [DOI] [PMC free article] [PubMed]
- 27.Shu X, Lin T, Wang H, Zhao Y, Jiang T, Peng X, Yue J. Diagnosis, prevalence, and mortality of Sarcopenia in dialysis patients: a systematic review and meta-analysis. J cachexia Sarcopenia Muscle. 2022;13(1):145–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al Zaben F, Sehlo MG, Khalifa DA, Al Shohaib S, Shaheen F, Alzaben L, Ahmad RG, Ashy JA, Felemban RG, Koenig HG. Prospective study of depression among dialysis patients in Saudi Arabia. Int Urol Nephrol. 2015;47(6):1001–10. [DOI] [PubMed] [Google Scholar]
- 29.Cizza G, Ravn P, Chrousos GP, Gold PW. Depression: a major, unrecognized risk factor for osteoporosis? Trends Endocrinol Metab. 2001;12(5):198–203. [DOI] [PubMed] [Google Scholar]
- 30.Abdala R, Elena Del Valle E, Negri AL, Bridoux P, Paganti LG, Bravo M, Sintado L, Di Rienzo P, Schiavelli OR, Zanchetta MB, et al. Sarcopenia in hemodialysis patients from Buenos Aires, Argentina. Osteoporos Sarcopenia. 2021;7(2):75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Table S1 S2 S3
Supplementary Material 2: STROBE Statement
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.