Abstract
Background
Creatinine-to-albumin ratio (CAR) has been recognized as a predictive indicator in the postoperative setting. However, its relationship with outcomes in patients receiving cardiac surgery remains elusive. This study aimed to discuss the link between CAR and 28-day mortality in patients admitted to intensive care unit (ICU) following cardiac surgery, hoping to provide some insights for targeted interventions for improvement of patient outcomes.
Methods
MIMIC-IV database was searched to obtain data of patients admitted to ICU following cardiac surgery. Retrieved patients were split into three groups based on CAR levels. The 28-day ICU mortality in each group was evaluated and compared using Kaplan—Meier analysis. Subgroup analysis, multivariate Cox regression and restricted cubic spline (RCS) analysis were used to further examine the relationship between CAR and outcomes. Receiver operating characteristic (ROC) curves were used to assess the predictive ability of CAR. Mediation analysis was conducted to investigate the potential mechanism by which CAR affects 28-day ICU mortality.
Results
A total of 5,670 patients were included and divided into three groups. Patients with high CAR values (CAR ≥ 0.31) had a significantly increased rate of 28-day ICU mortality (11.4%), as compared to those with low CAR levels (CAR < 0.23, 1.83%). In addition, patients with high CAR values (CAR ≥ 0.31) had a lowest survival rate than the other two groups (p < 0.0001). ROC curve analysis showed that CAR exhibited a moderate predictive power (AUC = 0.748). Moreover, CAR was identified as a strong risk factor for 28-day ICU mortality, and a significant dose–response association was presented. Further subgroup analysis revealed pronounced mortality risks in females and patients without chronic conditions such as chronic kidney disease (CKD) and type 2 diabetes mellitus (T2DM). Mediation analysis indicated that CAR affected 28-day ICU mortality through biomarkers like chloride (39.8%), glucose (11.8%), potassium (24.4%), and sodium (28.3%).
Conclusion
CAR served as a risk factor for 28-day ICU mortality in patients receiving cardiac surgery, and it showed a complex dose–response and subgroup-specific association with 28-day ICU mortality. Additionally, CAR affected 28-day ICU mortality through multiple key biomarkers, providing some insights for targeted interventions.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-025-04505-1.
Keywords: Cardiac Surgery, Creatinine to Albumin Ratio, MIMIC-IV Database, 28-Day Mortality
Introduction
It is estimated that approximately 2 million people worldwide undergo cardiac surgery each year [1]. Cardiovascular diseases are becoming increasingly prevalent due to population aging, which leads to an increased requirement for cardiac surgery [2]. Postoperative patients are particularly vulnerable to adverse outcomes during the perioperative period [3]. Recent advances in surgical procedures and perioperative management have decreased the mortality associated with cardiac surgery, but how to minimize postoperative complications and deaths remains a major issue [4, 5]. As reported, low-risk procedures like aortic valve surgery can carry a mortality of 2% [6]. In this context, identifying factors associated with postoperative mortality is critical to improve risk stratification and guide personalized treatment strategies.
Creatinine-to-albumin ratio (CAR) is a sensitive biomarker for monitoring vital signs, and it is preferred in early detection of renal damage. Serum creatinine (Cr) is considered a reliable renal function predictor, and test for serum Cr is cost-effective and easily accessible [7, 8]. A decrease in admission album (Alb) level is often linked to increased rates of morbidity and mortality, and longer intensive care unit (ICU) stays [9].
The Intermountain Risk Score (IMRS) is a risk assessment tool that uses routine blood test results, with Cr being a key factor. IMRS is important for predicting mortality risks in patients with ST-segment elevation myocardial infarction (STEMI) and those with cardiogenic shock induced by STEMI [10, 11]. Additionally, the Naples Score (NS), based on Alb levels, can be used to predict the prognosis of patients with STEMI, while the Naples Prognostic Score (NPS) serves as a predictor of long-term mortality risk in patients with pulmonary embolism [12, 13]. Additionally, the albumin-to-C-reactive protein ratio provides a more accurate prediction of all-cause mortality after interventional procedures [14]. Nitrogenous and non-nitrogenous metabolites accumulate in the kidneys during cardiopulmonary bypass, potentially causing acute kidney injury (AKI) within hours or days postoperatively [15]. Cr levels often increase after surgery, while Alb levels decrease to varying degrees. Studies have highlighted the significance of serum Cr in combination with Alb in prognosis [16]. CAR is closely associated with pathological conditions such as hypertension and coronary heart disease (CHD) [17, 18]. Zhao et al. found that the creatinine-albumin ratio (CAR) may serve as a practical and easily measurable biochemical indicator for evaluating outcomes following debridement of pancreatic necrosis [19]. The relationship between CAR and 28-day mortality following cardiac surgery remains unclear.
In this study, MIMIC-IV database was searched to collect data of patients who were admitted to ICU after cardiac surgery between 2008 and 2019. This study aimed to discuss any potential connections between CAR and 28-day ICU mortality in patients receiving cardiac surgery, which will help for better diagnosis and management in this population.
Methods
Study population
This retrospective analysis was performed based on MIMIC-IV database (version 3.1), which was developed and is maintained by the Massachusetts Institute of Technology (MIT). MIMIC-IV database provides systematically collected clinical data from Beth Israel Deaconess Medical Center in Boston, Massachusetts, spanning the period from 2008 to 2019. This database is now managed by a MIT’s Laboratory for Computational Physiology [20]. Shi Pengtao, a member of the research team, was authorized to access the website and responsible for data collecting (Certification number: 13939446). The study followed established guidelines for observational research [21]. As the data were from a publicly available source, informed consent was not obtained. Based on MIMIC-IV database and the International Classification of Diseases (ICD) code (Supplementary Table 1), the date of first ICU admission of patients with at least one ICU admission following cardiac surgery was used, and 223,452 patients were identified initially [22]. Patients aged < 18 or > 120 years and those previous had no less than one ICU admission (n = 200,371) were excluded from the study. Of the remaining 23,081 patients, 9,224 patients were excluded due to missing survival information, and 8,187 patients were excluded because of missing data on Cr or Alb. Finally, 5,670 patients were included in the analysis (Fig. 1). We identified every adult patient who was hospitalized and underwent cardiac surgery between January 2014 and December 2019. The following were the exclusion criteria: (1) Under the age of 18; (2) Non-first ICU admission; (3) Missing Cr or Alb data; (4) Missing survival data.
Fig. 1.
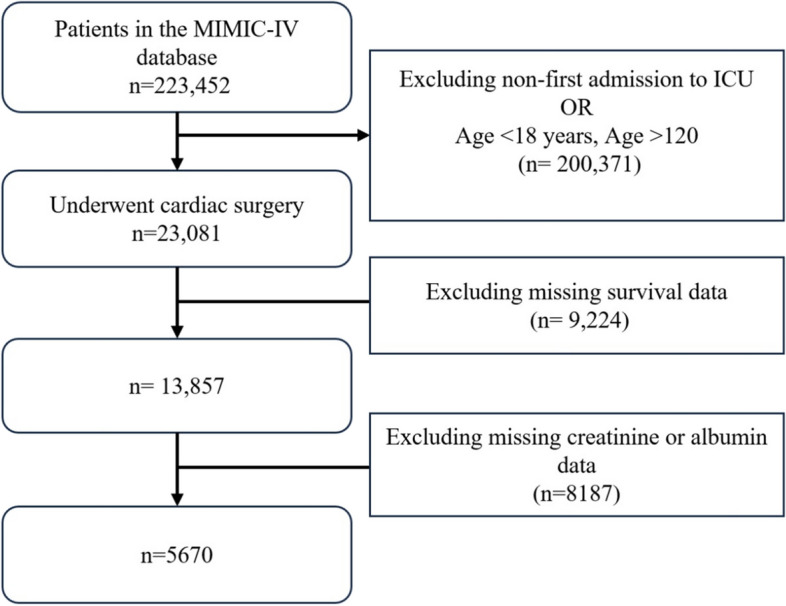
Flowchart of the study population selection process
Clinical outcome
The main outcome was 28-days ICU all cause mortality following cardiac surgery.
Variable extraction
CAR served as the primary variable in this study, and it was calculated as: CAR = Cr/Alb. Participants were categorized into three groups based on their CAR levels: < 0.23, 0.23—0.31, and > 0.31. Key variables included demographic characteristics and comorbidities. Demographic variables comprised age, gender, marital status, and insurance status. Comorbidities included heart failure (HF), myocardial infarction (MI), ischemic heart disease (IHD), hypertension, stroke, type 2 diabetes mellitus (T2DM), cancer, chronic kidney disease (CKD), and chronic obstructive pulmonary disease (COPD). Mean values of laboratory indicators (hemoglobin, red blood cell count, sodium, chloride, glucose, and potassium) and vital signs (heart rate, respiratory rate, and SpO2) were recorded within 48 h of ICU admission. In the meantime, treatment-related data, including use of mechanical ventilation, nonsteroidal anti-inflammatory drugs (NSAIDs), antihypertensive drugs, antibiotics, and corticosteroids, were also recorded. To mitigate potential bias from sample exclusion, variables with more than 20% missing data were excluded, while those with less than 20% missing values were addressed using multiple imputation with the "mice" package in R [23, 24].
Statistical analysis
Non-normally distributed variables were reported as medians and interquartile ranges (IQR) and analyzed using Mann—Whitney U test. Normally distributed data were expressed by means ± standard deviations (SD) and compared using independent t-tests. Categorical variables were reported as percentages, and group comparisons were made with χ2 test.
The relationship between CAR and 28-day ICU mortality was determined with Kaplan–Meier survival curves. Receiver operating characteristic (ROC) curves were constructed to evaluate the predictive performance of CAR. Cox proportional hazards regression models were built to evaluate the role of CAR in survival outcomes. The models incorporated CAR as both a continuous variable and a categorical variable. Four models were developed with progressive adjustment for covariates. The unadjusted model included no covariates. The first adjusted model (model 1) included demographic characteristics; the second adjusted model (model 2) included comorbidities; and the third adjusted model (model 3) included treatment data, vital signs, and laboratory results within 48 h of ICU admission. When proportional hazards assumption was not met, hazard ratios (HRs) were interpreted as a time-weighted average across the follow-up period [25, 26] (Supplementary Table 2). Variance inflation factors (VIF) were used to assess multicollinearity, and a VIF > 10 indicated potential collinearity issues [27] (Supplementary Table 3). Restricted cubic spline (RCS) analysis with four knots was used to study the non-linear relationship between CAR and survival outcomes. Subgroup analysis was devised to explore the effect of CAR on patient survival with the following stratification strategies: age, gender, CKD, HF, MI, IHD, hypertension, stroke, COPD, T2DM, use of corticosteroids, NSAIDs, antihypertensive drugs, and antibiotics. Subgroups with insufficient sample sizes were excluded to ensure result stability.
Mediation analysis was performed to investigate the potential mechanism by which CAR influences 28-day ICU mortality in patients. The average causal mediation effect (ACME) and average direct effect (ADE) were reported to reflect CAR impact [28]. The statistical significance of both the relationships between CAR and the mediator and between the mediator and outcome was deemed crucial for mediation to be considered significant. R software (version 4.3.0) was used for each analysis, and significance was determined by a two-sided p-value less than 0.05.
Results
Baseline characteristics
Following inclusion and exclusion, 5,670 patients undergoing cardiac surgery were retrieved from MIMIC-IV database. Three tertiles were created using the baseline CAR data: tertile 1 (n = 1,967, CAR < 0.23), tertile 2 (n = 1,844, 0.23 ≤ CAR < 0.31), and tertile 3 (n = 1,859, CAR ≥ 0.31). The 28-day ICU mortality for the three groups was 1.83%, 3.94%, and 11.4%, respectively. Patents in tertile 3 were dominated by males and elderly people, Asians or African Americans, widowed individuals, and people covered by Medicare. Patients in this category had higher levels of urea nitrogen, platelet count, glucose, potassium, and respiratory rate, but lower levels of hemoglobin, red blood cells, and SpO2. The use of NSAIDs, antihypertensive drugs, antibiotics, and glucocorticoids was less frequent in this group of patients. Additionally, the incidence of comorbidities including HF, MI, hypertension, stroke, CKD, IHD, COPD, T2DM, and cancer was high in these patients (Table 1).
Table 1.
Baseline demographic and clinical characteristics of patients stratified by CAR Levels
| CAR (< 0.23) | CAR (0.23–0.31) | CAR (> 0.31) | P | |
|---|---|---|---|---|
| N = 1967 | N = 1844 | N = 1859 | ||
| Creatinine | 0.76 (0.14) | 1.00 (0.14) | 1.90 (1.61) | < 0.001 |
| Albumin | 3.98 (0.47) | 3.79 (0.49) | 3.31 (0.62) | < 0.001 |
| Age | 66.0 [59.0;74.0] | 69.0 [61.0;77.0] | 72.0 [63.0;80.0] | < 0.001 |
| Body Mass Index | 28.1 [24.6;32.1] | 28.3 [25.0;32.2] | 28.2 [25.1;32.1] | 0.371 |
| Insurance: | < 0.001 | |||
| Medicaid | 224 (11.4%) | 160 (8.68%) | 191 (10.3%) | |
| Medicare | 940 (47.8%) | 1062 (57.6%) | 1226 (65.9%) | |
| Other | 51 (2.59%) | 52 (2.82%) | 35 (1.88%) | |
| Private | 752 (38.2%) | 570 (30.9%) | 407 (21.9%) | |
| Race: | 0.060 | |||
| Asian | 39 (1.98%) | 28 (1.52%) | 38 (2.04%) | |
| Black | 51 (2.59%) | 65 (3.52%) | 84 (4.52%) | |
| Hispanic | 55 (2.80%) | 48 (2.60%) | 47 (2.53%) | |
| Other | 638 (32.4%) | 569 (30.9%) | 609 (32.8%) | |
| White | 1184 (60.2%) | 1134 (61.5%) | 1081 (58.1%) | |
| Marital status: | < 0.001 | |||
| Divorced | 165 (8.39%) | 156 (8.46%) | 125 (6.72%) | |
| Married | 1213 (61.7%) | 1165 (63.2%) | 1083 (58.3%) | |
| Single | 395 (20.1%) | 344 (18.7%) | 414 (22.3%) | |
| Widowed | 194 (9.86%) | 179 (9.71%) | 237 (12.7%) | |
| Gender: | < 0.001 | |||
| Female | 790 (40.2%) | 439 (23.8%) | 494 (26.6%) | |
| Male | 1177 (59.8%) | 1405 (76.2%) | 1365 (73.4%) | |
| Urea nitrogen | 13.3 [11.0;16.0] | 16.0 [13.5;19.7] | 25.8 [19.0;36.8] | < 0.001 |
| Hemoglobin | 10.7 [9.66;11.8] | 10.6 [9.60;11.8] | 10.0 [9.08;11.4] | < 0.001 |
| Red blood cells | 3.52 [3.17;3.95] | 3.52 [3.18;3.93] | 3.36 [3.01;3.85] | < 0.001 |
| Platelet count | 161 [133;202] | 162 [131;204] | 166 [127;220] | 0.203 |
| Sodium | 138 [136;139] | 138 [136;140] | 138 [136;140] | 0.003 |
| Chloride | 106 [104;108] | 106 [104;108] | 105 [102;108] | < 0.001 |
| Glucose | 119 [106;135] | 120 [106;137] | 129 [110;158] | < 0.001 |
| Potassium | 4.25 [4.00;4.49] | 4.33 [4.10;4.55] | 4.40 [4.07;4.80] | < 0.001 |
| White blood cells | 12.2 [9.71;15.3] | 12.4 [9.87;15.5] | 12.2 [9.40;16.0] | 0.595 |
| Heart rate | 80.9 [74.5;88.1] | 80.1 [73.7;87.5] | 80.7 [73.7;89.9] | 0.050 |
| Respiratory rate | 17.7 [16.1;19.5] | 17.8 [16.1;19.8] | 18.4 [16.4;21.0] | < 0.001 |
| Spo2 | 97.7 [96.5;98.6] | 97.6 [96.4;98.5] | 97.4 [96.1;98.6] | < 0.001 |
| CKD: | < 0.001 | |||
| No | 1960 (99.6%) | 1789 (97.0%) | 1453 (78.2%) | |
| Yes | 7 (0.36%) | 55 (2.98%) | 406 (21.8%) | |
| Heart failure: | < 0.001 | |||
| No | 1917 (97.5%) | 1720 (93.3%) | 1315 (70.7%) | |
| Yes | 50 (2.54%) | 124 (6.72%) | 544 (29.3%) | |
| Myocardial infarction: | < 0.001 | |||
| No | 1940 (98.6%) | 1759 (95.4%) | 1574 (84.7%) | |
| Yes | 27 (1.37%) | 85 (4.61%) | 285 (15.3%) | |
| IHD: | < 0.001 | |||
| No | 1896 (96.4%) | 1624 (88.1%) | 1220 (65.6%) | |
| Yes | 71 (3.61%) | 220 (11.9%) | 639 (34.4%) | |
| Hypertension: | < 0.001 | |||
| No | 1913 (97.3%) | 1714 (93.0%) | 1644 (88.4%) | |
| Yes | 54 (2.75%) | 130 (7.05%) | 215 (11.6%) | |
| Stroke: | < 0.001 | |||
| No | 1956 (99.4%) | 1826 (99.0%) | 1770 (95.2%) | |
| Yes | 11 (0.56%) | 18 (0.98%) | 89 (4.79%) | |
| COPD: | < 0.001 | |||
| No | 1947 (99.0%) | 1817 (98.5%) | 1732 (93.2%) | |
| Yes | 20 (1.02%) | 27 (1.46%) | 127 (6.83%) | |
| T2DM: | < 0.001 | |||
| No | 1929 (98.1%) | 1744 (94.6%) | 1493 (80.3%) | |
| Yes | 38 (1.93%) | 100 (5.42%) | 366 (19.7%) | |
| Cancer: | < 0.001 | |||
| No | 1957 (99.5%) | 1803 (97.8%) | 1766 (95.0%) | |
| Yes | 10 (0.51%) | 41 (2.22%) | 93 (5.00%) | |
| Ventilation: | 0.990 | |||
| No | 148 (7.52%) | 139 (7.54%) | 142 (7.64%) | |
| Yes | 1819 (92.5%) | 1705 (92.5%) | 1717 (92.4%) | |
| NSAID Use: | < 0.001 | |||
| No | 872 (44.3%) | 865 (46.9%) | 1214 (65.3%) | |
| Yes | 1095 (55.7%) | 979 (53.1%) | 645 (34.7%) | |
| Antihypertensive Use: | 0.020 | |||
| No | 730 (37.1%) | 667 (36.2%) | 751 (40.4%) | |
| Yes | 1237 (62.9%) | 1177 (63.8%) | 1108 (59.6%) | |
| Antibiotic Use: | < 0.001 | |||
| No | 818 (41.6%) | 765 (41.5%) | 886 (47.7%) | |
| Yes | 1149 (58.4%) | 1079 (58.5%) | 973 (52.3%) | |
| Glucocorticoid Use: | 0.004 | |||
| No | 1930 (98.1%) | 1797 (97.5%) | 1792 (96.4%) | |
| Yes | 37 (1.88%) | 47 (2.55%) | 67 (3.60%) | |
| Death: | < 0.001 | |||
| No | 1931 (98.2%) | 1788 (97.0%) | 1647 (88.6%) | |
| Yes | 36 (1.83%) | 56 (3.04%) | 212 (11.4%) | |
| OS | 28.0 [28.0;28.0] | 28.0 [28.0;28.0] | 28.0 [28.0;28.0] | < 0.001 |
| CAR | 0.20 [0.17;0.21] | 0.26 [0.24;0.29] | 0.42 [0.35;0.58] | < 0.001 |
CAR Creatinine to albumin ratio, BMI Body mass index, CKD Chronic kidney disease, IHD Ischemic heart disease, COPD Chronic obstructive pulmonary disease, T2DM Type 2 diabetes mellitus, NSAID Non-steroidal anti-inflammatory drug, OS Overall survival
Kaplan–meier curve analysis
Survival probability was highest in tertile 1 (CAR < 0.23) and lowest in tertile 3 (CAR ≥ 0.31) (Fig. 2). The difference between groups was statistically significant (p < 0.0001). There was a reduction in survival in all three groups over time. Notably, tertile 3 showed the fastest decline, followed by tertile 2 (0.23 ≤ CAR < 0.31).
Fig. 2.
Kaplan–Meier survival curves for 28-day overall survival across CAR groups
Association between 28-day ICU mortality and CAR
Cox proportional hazards analysis demonstrated a strong positive association between CAR level and 28-day ICU mortality. CAR as a continuous variable was an important hazard factor for 28-day ICU mortality within each model. HR (95% CI) was 1.916 (1.728—2.224) for the unadjusted model, 2.221 (1.911—2.557) for Model 1, 2.121 (1.788—2.516) for Model 2, and 1.436 (1.139—1.811) for Model 3. The P-value for Model 3 was 0.002, while that for the other models was less than 0.001(Table 2). Patients in tertile 3 (CAR ≥ 0.31) had a higher risk of death than those in tertile 1 (CAR < 0.23) across all models [unadjusted model: HR (95% CI): 6.577 (4.620—9.365); model 1: HR (95% CI): 6.483 (4.524—9.291); model 2: HR (95% CI): 4.025 (2.748—5.895); model 3: HR (95% CI): 2.329 (1.573—3.449); all p < 0.001]. RCS analysis showed a non-linear association between CAR and mortality risk across all models, and a complex dose–response association was demonstrated (p < 0.05, Fig. 3).
Table 2.
Results of the Multiple Cox Regression for CAR
| Model | CAR | CAR (< 0.23) | CAR (0.23–0.31) | CAR (> 0.31) | |||
|---|---|---|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | ||
| Unadjusted Model | 1.961 (1.728–2.224) | < 0.001 | Reference | 1.667 (1.097–2.535) | 0.017 | 6.577 (4.620–9.365) | < 0.001 |
| Model 1 | 2.211 (1.911–2.557) | < 0.001 | Reference | 1.756 (1.151–2.678) | 0.009 | 6.483 (4.524–9.291) | < 0.001 |
| Model 2 | 2.121 (1.788–2.516) | < 0.001 | Reference | 1.541 (1.008–2.356) | 0.046 | 4.025 (2.748–5.895) | < 0.001 |
| Model 3 | 1.436 (1.139–1.811) | 0.002 | Reference | 1.304 (0.852–1.997) | 0.222 | 2.329 (1.573–3.449) | < 0.001 |
Unadjusted model: Includes no covariates
Model 1: Adjusts for baseline demographic characteristics, including age, body mass index, gender, race, marital status, and insurance type
Model 2: Further adjusts for comorbidities, including chronic kidney disease, acute kidney injury, heart failure, myocardial infarction, ischemic heart disease, hypertension, stroke, chronic obstructive pulmonary disease, type 2 diabetes mellitus, and cancer
Model 3: Further includes laboratory results, treatment and clinical scores within the first 48 h, such as mechanical ventilation, NSAID use, antihypertensive, antibiotic, glucocorticoid, heart rate, respiratory rate, oxygen saturation (SpO2), blood urea nitrogen, hemoglobin, red blood cell count, platelet count, sodium, chloride, glucose, potassium, and white blood cell count
Fig. 3.
RCS analysis illustrating the nonlinear association between CAR levels and mortality risk across different models
Subgroup analysis
Subgroup analysis revealed a significant relationship between elevated CAR and adverse outcomes [HR (95% CI): 1.333 (1.061—1.675), p = 0.014]. Significant associations were also noted in subgroups, including females [HR (95% CI): 1.420 (1.050—1.900)], individuals without CKD [HR (95% CI): 1.400 (1.103—1.777)], individuals without MI [(HR (95% CI): 1.352 (1.352)], individuals without T2DM [(HR (95% CI): 2.141 (1.214—3.774)], individuals without NSAIDs [(HR (95% CI): 1.356 (1.037—1.772)], individuals on antihypertensive drugs [(HR (95% CI): 1.957 (1.402—2.731)], and individuals on antibiotics [(HR (95% CI): 1.406 (1.025—1.929)] (Fig. 4).
Fig. 4.
Subgroup analysis of the relationship between CAR levels and mortality risk
Predictive ability of CAR
Area under ROC curve (AUC) was calculated (Fig. 5). The AUC value for CAR was 0.748 (optimal cut-off point: 0.303, specificity: 67.2%, sensitivity: 73.1%), indicating a moderate ability of CAR to predict 28-day ICU mortality.
Fig. 5.

ROC curve showed that CAR had good predictive ability
Mediation analysis
Mediation analysis was performed to explore the potential mechanism by which CAR affects 28-day ICU mortality in postoperative patients. Chloride (mediation proportion: 39.8%, p < 0.001), glucose (mediation proportion: 11.8%, p = 0.004), potassium (mediation proportion: 24.4%, p = 0.004), and sodium (mediation proportion: 28.3%, p < 0.001) were identified as significant mediators. This finding suggested that CAR may affect the 28-day ICU mortality in postoperative patients indirectly through these biomarkers (Fig. 6).
Fig. 6.
Mediation analysis illustrating the indirect effects of CAR on ICU mortality through various clinical mediators
Discussion
This study identified CAR as a significant risk factor for 28-day ICU mortality in patients receiving cardiac surgery. It was found that higher CAR levels correlated to increased 28-day ICU mortality risks, and patients in tertile 3 (CAR ≥ 0.31) had worst outcomes. RCS analysis highlighted that there was a non-linear dose–response relationship between higher CAR levels and worse outcomes, and the extent of the increase in risk varied with different CAR ranges. Cox regression analysis further validated that CAR could be used as an independently predictor for 28-day ICU mortality in postoperative patients. Moreover, subgroup analysis revealed that no significant associations were found between CAR and most demographic or clinical data.
Cr is primarily filtered and excreted by the glomeruli, and it serves as an indicator of renal function. Cardiac surgeries, particularly those requiring extracorporeal circulation, can cause renal injury, leading to excess fluid state, electrolyte imbalance, and increased mortality. Elevation of Cr during cardiac surgery often suggests renal dysfunction, which can impede the clearance of drugs and metabolites and then affect recovery. Cardiopulmonary bypass can cause hemolysis. Free hemoglobin and reactive iron released during hemolysis generate hydroxyl radicals through Fenton-type Haber–Weiss reactions, in turn causing damages to renal function [29]. Kolli H et al. found that slight elevations of Cr postoperatively correlated to increased 90-day mortality rates and prolonged hospital stays in patients [30]. Moreover, elevated serum Cr and blood urea nitrogen concentrations also impair glucose, lipid, protein, and amino acid metabolisms, disrupting cellular energy utilization and metabolism [31]. Collectively, elevation of Cr adversely impacts fluid balance, electrolyte stability, drug metabolism, inflammatory response, and clearance of metabolic byproducts, thereby influencing renal function and then postoperative survival in patients.
Alb is known to be a marker of nutritional and inflammatory status, and it plays critical roles in preserving colloid osmotic pressure and delivering hormones and drugs [32, 33]. Alb is a negative acute-phase reactant associated with disease severity, prognosis, and mortality [34]. During cardiac procedures, Alb outperforms crystalloids in maintaining hemodynamics, mitigating platelet count reduction, and preventing fluid overload. Hypoalbuminemia is widely recognized as an indicator of poor outcomes in critically ill patients, including those receiving cardiac surgery. It is established that patients with hypoalbuminemia have poor nutritional reserves and heightened systemic inflammation. Moreover, hypoalbuminemia leads to a reduction in free radical clearance while an increase in renal tubular apoptosis, which makes it an important risk factor for AKI following cardiac surgery [35]. Alb is involved in phosphoinositide 3-kinase activation, nitric oxide synthesis, renal tubular cell proliferation, and renal blood flow [36, 37]. Hypoalbuminemia causes damage to the endothelial cell barrier, resulting in inadequate renal perfusion [38]. Furthermore, hypoalbuminemia is associated with fluid shifts and edema, which may pose heavy burdens on cardiac and respiratory systems post-surgery. A meta-analysis reported that the incidence of complications and all-cause mortality rose by 89% and 137% per 10 g/L drop in serum Alb, respectively [39]. Moguel-González et al. conducted a prospective study in 164 adult patients receiving cardiac surgery and found that low Alb, high blood urea nitrogen, and elevated Cr were major risk factors for mortality [40].
CAR is a combination of Cr and Alb that has much more significant implications for postoperative risk assessment. It was reported Alb alone was less effective than CAR in predicting mortality [41]. In addition, Alb in combination with Cr was reported to be superior to Alb or Cr alone in predicting 30-day all-cause mortality [42]. An elevated value of CAR indicates concurrent renal dysfunction and systemic inflammation, both of which exacerbate organ dysfunction and increase mortality risk. Research also shows that CAR is associated with glycemic control in T2DM patients [43]. These research findings demonstrate a clear dose–response association between CAR and short-term mortality in patients receiving cardiac surgery, highlighting the role of CAR as a marker of physiological stress. Consistently, the longitudinal study in 11,200 atherosclerosis patients made by Grams ME et al. reported a positive relationship between CAR and AKI risk [44]. Compared to similar studies in other populations, the predictive value of CAR has also been validated across various disease contexts. For instance, in patients with acute pancreatitis, CAR has been shown to be an independent predictor of both short-term and long-term all-cause mortality, demonstrating superior predictive performance over individual markers such as Cr and Alb [45]. This further supports the potential and reliability of CAR as a risk assessment tool in diverse clinical settings. The link between CAR and poor outcomes in patients receiving cardiac surgery may be attributed to several mechanisms. First, elevated Cr levels and decreased Alb levels are closely associated with inflammatory responses. Inflammation is triggered and maintained through cytokines such as TNF-α, IL-6, and IL-1β, which activate the NF-κB pathway [46]. Hypoalbuminemia limits the proliferation and differentiation of immune cells, impairing their function by suppressing the activity of T cells, B cells, and macrophages, ultimately weakening the intensity and duration of the immune response and negatively affecting patient survival [47]. Second, high creatinine levels induce oxidative stress, which promotes cell apoptosis and tissue damage through endoplasmic reticulum stress and mitochondrial dysfunction [48]. Additionally, reactive oxygen species (ROS) activate signaling molecules such as PKC and MAPK, further driving apoptosis and inflammation [49]. Oxidative stress may also impair Alb synthesis, creating a self-reinforcing vicious cycle that exacerbates the condition [50]. Third, inflammation and surgical trauma increase vascular endothelial permeability and cause the shedding of the glycocalyx, facilitating the leakage of fluid from blood vessels into the interstitial spaces [51]. This activates the renin–angiotensin–aldosterone system (RAAS) and the antidiuretic hormone (ADH) system, which aggravates fluid overload and contributes to postoperative cardiovascular and respiratory complications [52]. Fourth, elevated Cr and decreased Alb levels may also result in insufficient microvascular perfusion, worsening ischemia, and increasing tissue hypoxia and cell death, which ultimately impair wound healing. Alb levels influence the function of dermal fibroblasts, and low Alb levels promote ferroptosis, delaying ischemic wound healing [52]. Furthermore, persistent inflammation and alterations in the protein hydrolysis balance in chronic wounds hinder the healing process [53]. Taken together, CAR serves as a significant biomarker for adverse outcomes in patients following cardiac surgery.
In this study, mediation analysis revealed that CAR affected the 28-day ICU mortality in postoperative patients through biomarkers such as chloride, glucose, potassium, and sodium. Changes in these biomarkers may reflect the indirect effect of CAR on patient prognosis. For example, chloride and sodium levels may indicate electrolyte and acid–base disorders, which is common in critically ill patients. Elevated glucose and potassium levels may signal stress response or metabolic disturbances, which affects outcomes in high-risk cases receiving cardiac surgery. These findings offer new perspectives on the relationship between CAR and mortality in postoperative patients, and they may guide the development of therapeutic strategies for improvement of patient outcomes. CAR is superior to other indicators in specificity and sensitivity, making it a more accurate indicator of postoperative mortality in patients receiving cardiac surgery.
As a biomarker for predicting 28-day ICU mortality, CAR exhibits several advantages. First, tests for both serum Cr and Alb are easily accessible and cost-effective, facilitating its widespread application in preoperative and perioperative settings. Second, in postoperative cardiac surgery patients in the ICU, an elevated CAR often indicates a higher risk of complications. This highlights the need for enhanced hemodynamic monitoring, incorporating dynamic parameters (e.g., pulse pressure variation or central venous oxygen saturation) to optimize fluid management and avoid overhydration or underhydration. Active nutritional support, including early enteral or parenteral nutrition and supplementation with specific nutrients, is essential for improving protein metabolism. For patients with elevated creatinine levels, early identification of AKI risk is crucial, necessitating the implementation of kidney-protective strategies such as restrictive fluid management and continuous renal replacement therapy [54]. Third, preoperative optimization of patient conditions can mitigate the risks associated with an elevated CAR. This includes albumin supplementation, medication adjustments, and anti-inflammatory treatment for patients with chronic inflammation. Perioperative management is equally critical, involving stringent control of cardiopulmonary bypass duration and cross-clamp time, as well as the adoption of renal-protective measures (e.g., temperature regulation and fluid strategies) to minimize intraoperative damage [55]. Postoperatively, early interventions with renal-protective medications and nutritional support can further reduce complication risks. Dynamic monitoring of inflammatory markers and renal function indicators enables timely adjustments to treatment plans based on observed changes. Forth, the clinical application of CAR demonstrates its potential as a risk stratification and decision-making tool. Dynamic CAR assessment helps clinicians identify high-risk patients, allocate them to higher-level monitoring units, and implement targeted multi-organ protective strategies. CAR trends also provide guidance for modifying treatment plans, such as intensifying renal protective measures or enhancing nutritional support when CAR levels remain elevated. Integrated with existing scoring systems (e.g., EuroSCORE), CAR complements these tools by addressing gaps in postoperative dynamic assessments, enhancing risk prediction accuracy, and optimizing postoperative ICU management. These measures underscore CAR's capacity to drive precision management in the ICU and deliver more comprehensive, individualized care for cardiac surgery patients.
This study also has several limitations. First, the causality between CAR and mortality was not involved due to the retrospective nature of the study, requiring prospective studies for confirmation. Second, electrolyte and glucose levels were identified as mediators in the mediation analysis, but the underlying biological mechanisms remain unclear and require further investigation. Third, Despite efforts to maximize sample collection from the MIMIC database, power analysis indicates the sample size remains insufficient for robust calculations, leaving the risk of a Type II error due to low statistical power. Fourth, although the MIMIC-IV database is comprehensive, it originates from a single center, which may not fully represent global or regional patient populations, as there are significant differences in medical practices, patient baseline characteristics, and surgical techniques across regions. Moreover, the data from a single center may be influenced by selection biases and specific treatment strategies, limiting the generalizability of the findings. Furthermore, although the CAR demonstrated potential predictive value for 28-day mortality in ICU patients following cardiac surgery, the exclusion of a large number of patients due to missing Cr or Alb data may have introduced selection bias. Despite employing appropriate statistical methods to enhance the reliability and generalizability of our results, future studies should aim to optimize data collection and handling techniques to minimize the impact of missing data on research outcomes. Future research should consider addressing these limitations, which may enhance the reliability and clinical utility of CAR as a prognostic biomarker.
Conclusions
This study identified CAR as a robust risk factor for 28-day ICU mortality in patients receiving cardiac surgery, and found that CAR affected patient mortality through factors such as chloride, glucose, potassium, and sodium. Findings of the study offer a method for identifying high-risk patients. Additional research is required to validate these results and explore how CAR-based strategies improve patient outcomes after cardiac surgery.
Supplementary Information
Acknowledgements
We express our profound appreciation to the diligent team at the helm of MIMIV database’s design and upkeep for their steadfast dedication and distinguished contributions to the field.
Clinical trial number
Not applicable.
Abbreviations
- Alb
Album
- Cr
Serum creatinine
- CAR
Creatinine-to-albumin ratio
- ICU
Intensive care unit
- MIMIC-IV
Medical Information Mart for Intensive Care IV
- CI
Confidence interval
- AKI
Acute kidney injury
- CHD
Coronary heart disease
- ICD
International Classification of Diseases
- HF
Comorbidities included heart failure
- MI
Myocardial infarction
- IHD
Ischemic heart disease
- T2DM
Hypertension, stroke, type 2 diabetes mellitus
- CKD
Cancer, chronic kidney disease
- COPD
Chronic obstructive pulmonary disease
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- SD
Standard deviations
- HR
Hazard ratio
- IMRS
Intermountain Risk Score
- STEMI
ST-segment elevation myocardial infarction
- NS
Naples Score
- NPS
Naples Prognostic Score
- LPS
Lipopolysaccharide
Authors’ contributions
S P designed the study, retrieved data from the database and wrote the manuscript, RS analyzed the data, MQ reviewed and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Data availability
The data that support the findings of this study are available from MIMIC-IV. The database is accessible via PhysioNet at https://mi mic.mit.edu/. To gain access to the MIMIC-IV database, researchers must finish the necessary training and accept the data usage agreement found on the PhysioNet website.
Declarations
Ethics approval and consent to participate
The MIMIC-IV database adheres to the principles outlined in the Declaration of Helsinki. The dataset was approved by the Institutional Review Boards of the Massachusetts Institute of Technology (MIT) and Beth Israel Deaconess Medical Center (approval number: 2001-P-001699/14). As the data is publicly available, this study was exempt from the requirement for ethical approval and informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shen Rui, Email: 2024110394@cmu.edu.cn.
Qingyou Meng, Email: mengqy@163.com.
References
- 1.Parikh CR, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22(9):1748–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicolini F, et al. The evolution of cardiovascular surgery in elderly patient: a review of current options and outcomes. Biomed Res Int. 2014;2014:736298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaborative CO: Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396(10243):27–38. 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed]
- 4.Crawford TC, et al. Complications after cardiac operations: all are not created equal. Ann Thorac Surg. 2017;103(1):32–40. [DOI] [PubMed] [Google Scholar]
- 5.Ball L, Costantino F, Pelosi P. Postoperative complications of patients undergoing cardiac surgery. Curr Opin Crit Care. 2016;22(4):386. [DOI] [PubMed] [Google Scholar]
- 6.Landoni G, et al. Nonsurgical strategies to reduce mortality in patients undergoing cardiac surgery: an updated consensus process. J Cardiothorac Vasc Anesth. 2018;32(1):225–35. [DOI] [PubMed] [Google Scholar]
- 7.Yang HT, et al. Assessment of biochemical markers in the early post-burn period for predicting acute kidney injury and mortality in patients with major burn injury: comparison of serum creatinine, serum cystatin-C, plasma and urine neutrophil gelatinase-associated lipocalin. Crit Care. 2014;18(4):R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim Y, et al. Diagnostic performance of plasma and urine neutrophil gelatinase-associated lipocalin, cystatin C, and creatinine for acute kidney injury in burn patients: a prospective cohort study. PLoS One. 2018;13(6):e0199600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thongprayoon C, et al. Impacts of admission serum albumin levels on short-term and long-term mortality in hospitalized patients. QJM. 2020;113(6):393–8. [DOI] [PubMed] [Google Scholar]
- 10.Çınar T, et al. Evaluation of intermountain risk score for short- and long-term mortality in ST elevation myocardial infarction patients. Angiology. 2023;74(4):357–64. [DOI] [PubMed] [Google Scholar]
- 11.Mert İlker H, et al. Prognostic value of Intermountain Risk Score for short- and long-term mortality in patients with cardiogenic shock. Coron Artery Dis. 2023;34(2):154–9. [DOI] [PubMed] [Google Scholar]
- 12.Şaylık F, et al. Evaluation of Naples score for long-term mortality in patients With ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Angiology. 2024;75(8):725–33. [DOI] [PubMed] [Google Scholar]
- 13.Pay L, et al. Evaluation of Naples prognostic score to predict long-term mortality in patients with pulmonary embolism. Biomark Med. 2024;18(6):253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Çinier G, et al. The value of C-reactive protein-to-albumin ratio in predicting long-term mortality among HFrEF patients with implantable cardiac defibrillators. Eur J Clin Invest. 2021;51(8):e13550. [DOI] [PubMed] [Google Scholar]
- 15.Gül I, et al. The negative effect of mean perfusion pressure on the development of acute kidney injury after transcatheter aortic valve implantation. Braz J Cardiovasc Surg. 2018;33(6):559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Z, et al. Prediction of severe acute pancreatitis using a decision tree model based on the revised atlanta classification of acute pancreatitis. PLoS One. 2015;10(11):e0143486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu S, et al. Urinary albumin-to-creatinine ratio levels are associated with subclinical atherosclerosis and predict CVD events and all-cause deaths: a prospective analysis. BMJ Open. 2021;11(3):e040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue K, et al. Urinary albumin-to-creatinine ratio within normal range and all-cause or cardiovascular mortality among U.S. adults enrolled in the NHANES during 1999–2015. Ann Epidemiol. 2021;55:15–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Z, et al. Prognostic value of the creatinine-albumin ratio in acute pancreatitis debridement. BMC Surg. 2020;20(1):322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson AEW, et al. Author Correction: MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. 2023;10(1):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Elm E, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Q, et al. Association between preadmission metformin use and outcomes in intensive care unit patients with sepsis and type 2 diabetes: a cohort study. Front Med (Lausanne). 2021;8:640785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Austin PC, et al. Missing data in clinical research: a tutorial on multiple imputation. Can J Cardiol. 2021;37(9):1322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blazek K, et al. A practical guide to multiple imputation of missing data in nephrology. Kidney Int. 2021;99(1):68–74. [DOI] [PubMed] [Google Scholar]
- 25.Sjölander A, Dickman PW. Why test for proportional hazards-or any other model assumptions? Am J Epidemiol. 2024;193(6):926–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stensrud MJ, Hernán MA. Why test for proportional hazards? JAMA. 2020;323(14):1401–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Núñez ER, et al. Adherence to follow-up testing recommendations in US veterans screened for lung cancer, 2015–2019. JAMA Netw Open. 2021;4(7):e2116233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychol Methods. 2010;15(4):309–34. [DOI] [PubMed] [Google Scholar]
- 29.Ko S-W, et al. Hemojuvelin predicts acute kidney injury and poor outcomes following cardiac surgery. Sci Rep. 2018;8(1):1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolli H, et al. Mild acute kidney injury is associated with increased mortality after cardiac surgery in patients with eGFR < 60 mL/min/1.73 m2. Renal Failure. 2010;32(9):1066–72. [DOI] [PubMed] [Google Scholar]
- 31.Guerrero RB, Salazar D, Tanpaiboon P. Laboratory diagnostic approaches in metabolic disorders. Ann Transl Med. 2018;6(24):470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Attieh RM, H.M. Wadei Acute Kidney Injury in Liver Cirrhosis. Diagnostics. 2023;13. 10.3390/diagnostics13142361. [DOI] [PMC free article] [PubMed]
- 33.Xia M, et al. Impact of serum albumin levels on long-term all-cause, cardiovascular, and cardiac mortality in patients with first-onset acute myocardial infarction. Clin Chim Acta. 2018;477:89–93. [DOI] [PubMed] [Google Scholar]
- 34.Liu Q, et al. Association between lactate-to-albumin ratio and 28-days all-cause mortality in patients with acute pancreatitis: A retrospective analysis of the MIMIC-IV database. Front Immunol. 2022;13:1076121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao L, et al. Cordycepin inhibits albumin-induced epithelial-mesenchymal transition of renal tubular epithelial cells by reducing reactive oxygen species production. Free Radic Res. 2012;46(2):174–83. [DOI] [PubMed] [Google Scholar]
- 36.Peruchetti DB, et al. (Na+ + K+)-ATPase is a target for phosphoinositide 3-kinase/protein kinase B and protein kinase C pathways triggered by albumin. J Biol Chem. 2011;286(52):45041–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curry S. Lessons from the crystallographic analysis of small molecule binding to human serum albumin. Drug Metab Pharmacokinet. 2009;24(4):342–57. [DOI] [PubMed] [Google Scholar]
- 38.Becker BF, et al. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res. 2010;87(2):300–10. [DOI] [PubMed] [Google Scholar]
- 39.Finfer S, et al. Effect of baseline serum albumin concentration on outcome of resuscitation with albumin or saline in patients in intensive care units: analysis of data from the saline versus albumin fluid evaluation (SAFE) study. BMJ. 2006;333(7577):1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ostermann M, Lumlertgul N, Wilson FP. Predictive models for acute kidney injury following cardiac surgery: the importance of accurate and actionable prediction. JAMA. 2022;327(10):927–9. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, et al. Predictive model of risk factors for 28-day mortality in patients with sepsis or sepsis-associated delirium based on the MIMIC-IV database. Sci Rep. 2024;14(1):18751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schupp T, Behnes M, Rusnak J, Ruka M, Dudda J, Forner J, Egner-Walter S, Barre M, Abumayyaleh M, Bertsch T, et al. Does Albumin Predict the Risk of Mortality in Patients with Cardiogenic Shock? Int J Mol Sci. 2023;24(8). 10.3390/ijms24087375. [DOI] [PMC free article] [PubMed]
- 43.Comellas M, et al. Age and glycemic control among adults with type 2 diabetes in the United States: An assessment from the National Health and Nutrition Examination Survey (NHANES) 2013–2014. Diabetes Metab Syndr. 2019;13(6):3069–73. [DOI] [PubMed] [Google Scholar]
- 44.Moguel-González B, et al. Acute kidney injury in cardiac surgery. Rev Invest Clin. 2013;65(6):467–75. [PubMed] [Google Scholar]
- 45.Wang J, et al. Association between serum creatinine to albumin ratio and short- and long-term all-cause mortality in patients with acute pancreatitis admitted to the intensive care unit: a retrospective analysis based on the MIMIC-IV database. Front Immunol. 2024;15:1373371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu T, et al. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2(1):17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiedermann CJ. Hypoalbuminemia as Surrogate and Culprit of Infections. Int J Mol Sci. 2021;22(9). 10.3390/ijms22094496. [DOI] [PMC free article] [PubMed]
- 48.Huang Y, et al. Kaempferol attenuates hyperuricemia combined with gouty arthritis via urate transporters and NLRP3/NF-κB pathway modulation. iScience. 2024;27(11):111186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forrester SJ, et al. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018;122(6):877–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jubaidi FF, Zainalabidin S, Taib IS, Abdul Hamid Z, Mohamad Anuar NN, Jalil J, Mohd Nor NA, Budin SB. The Role of PKC-MAPK Signalling Pathways in the Development of Hyperglycemia-Induced Cardiovascular Complications. Int J Mol Sci. 2022;23(15). 10.3390/ijms23158582. [DOI] [PMC free article] [PubMed]
- 51.Rahbar E, et al. Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients. J Transl Med. 2015;13:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ames MK, Atkins CE, Pitt B. The renin-angiotensin-aldosterone system and its suppression. J Vet Intern Med. 2019;33(2):363–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schilrreff P, Alexiev U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. Int J Mol Sci. 2022;23(9). 10.3390/ijms23094928. [DOI] [PMC free article] [PubMed]
- 54.Kanbay M, et al. Intravenous fluid therapy in accordance with kidney injury risk: when to prescribe what volume of which solution. Clin Kidney J. 2023;16(4):684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barbu M, et al. Cardiopulmonary bypass management and acute kidney injury in cardiac surgery patients. Acta Anaesthesiol Scand. 2024;68(3):328–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from MIMIC-IV. The database is accessible via PhysioNet at https://mi mic.mit.edu/. To gain access to the MIMIC-IV database, researchers must finish the necessary training and accept the data usage agreement found on the PhysioNet website.






