Abstract
Lactic acid bacteria has been extensively used in food industry because of widespread properties and Pediococcus is among one of them. This study aims to conduct a comprehensive genomic analysis of Pediococcus acidilactici strain BCB1H to elucidate its genetic composition, functional elements, and potential biotechnological applications. The objectives include identifying key genomic features such as coding sequences, tRNA and rRNA genes, antibiotic resistance genes, and secondary metabolite biosynthetic gene clusters, which will highlight the adaptability and potential of P. acidilactici strain BCB1H for use in a variety of industrial and therapeutic applications. P. acidilactici strain BCB1H was analyzed using whole-genome sequencing, which used advanced sequencing technologies to obtain comprehensive genomic data. Key genomic features, such as coding sequences, tRNA and rRNA genes, antibiotic resistance genes, and secondary metabolite biosynthetic gene clusters, were identified through bioinformatics analyses. The genomic analysis of P. acidilactici strain BCB1H revealed a genome size of approximately 1.92 million base pairs with a GC content of 42.4%. The annotation identified 1,895 genes across 192 subsystems, highlighting the metabolic pathways and functional categories. Notably, specialty genes associated with carbohydrate metabolism, stress response, pathogenicity, and amino acid synthesis were identified, underscoring the versatility and potential applications in food technology and medicine. These findings shed light on the genetic makeup and functional potential of P. acidilactici strain BCB1H, highlighting its flexibility and industrial importance. The genetic traits discovered suggest its prospective use in probiotics, food preservation, and biotechnological advancements.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-90633-9.
Keywords: Pediococcus acidilactici, BCB1H, tRNA genes, rRNA genes, Antibiotic resistance genes, Biosynthetic gene clusters, Metabolic pathways
Subject terms: Applied microbiology, Microbial genetics, Genetics, Functional genomics, Genome, Industrial microbiology, Genomics, Comparative genomics, Metagenomics, Microbiology, Symbiosis
Introduction
Pediococcus is a genus of lactic acid bacteria (LAB) that live in a diverse range of biological sites, including fermented foods, plant materials, and animal gastrointestinal systems1. These bacteria are well-known for their capacity to create lactic acid, which has several benefits, including food preservation and probiotic characteristics. P. acidilactici, one of several species of Pediococcus, has received a lot of interest due to its diversity and prospective uses in food fermentation, animal health, and biotechnology2. Recent advances in high-throughput sequencing technology have created an unparalleled chance to investigate these microbe’s genetic landscape, providing information about their functional potential, metabolic pathways, and ecological functions3,4. The complete annotation of these genomic traits improves our understanding of the organism’s genetic architecture, which is critical for biotechnological and industrial applications. In this study, the genomic analysis of P. acidilactici strain BCB1H is presented, focusing on its genetic content, functional elements, and potential applications in a variety of biotechnological fields, including antibiotic resistance, bioactive compound production, and horizontal gene transfer5,6.
In addition to core genomic studies, comparative genomics is critical in determining the genetic diversity and relatedness of P. acidilactici strains. The genetic links between strain BCB1H and other P. acidilactici strains was investigated using average nucleotide identity (ANI) and TETRA analysis7,8. These methods give a strong foundation for finding conserved and divergent genomic characteristics, shedding light on the evolutionary processes of this bacterial species. Furthermore, the work used pan-genome analysis to investigate common and unique genetic components among strains, yielding critical information about the genomic plasticity and adaptability of P. acidilactici BCB1H9,10. This study also emphasized the finding of specific genomic characteristics, including as antibiotic resistance genes, secondary metabolite biosynthetic gene clusters, and CRISPR-Cas systems, which help the organism survive and compete in a variety of ecological niches. The existence of these genetic components highlights P. acidilactici’s potential as a probiotic candidate, food preservation agent, and biotechnological application9,11. Finally, the thorough genomic investigation of P. acidilactici strain BCB1H revealed important information about its genetic composition, functional features, and prospective biotechnological uses. The study successfully identified key genomic features such as coding sequences, tRNA, rRNA genes, antibiotic resistance genes, and secondary metabolite biosynthetic gene clusters, highlighting the organism’s adaptability and potential for use in a variety of industrial and therapeutic applications. However, the functional validation of certain discovered genomic elements, notably those associated to bioactive chemical synthesis and antibiotic resistance, was not conducted experimentally. Future research should concentrate on experimental confirmation of projected bioactive chemicals and antibacterial capabilities, as well as investigating the function of horizontal gene transfer in molding the genetic diversity and adaptability of P. acidilactici BCB1H.
Materials and methods
Bacterial strain isolation and culturing
The P. acidilactici BCB1H strain was isolated from Chinese sauerkraut and preserved at -80°C in the Dairy Laboratory at Beijing Technology and Business University, China. To activate the strain, BCB1H was grown in MRS medium (Beijing Aoboxing Co., Ltd., Beijing, China) at 37°C for 12 hours. Two generations were employed to establish the seed culture. The activated strain was then moved to fresh MRS medium and cultured for 20 hours before bacterial DNA was extracted. DNA extraction was performed according to the instructions supplied by Beijing Tiangen’s “Bacterial Genomic DNA Extraction Kit. To extract DNA, 0.5-4.0 ml of cells (up to 2 × 10^9 cells) were collected by centrifugation for 1 minute at maximum speed. The supernatant was discarded to the extent practicable12.
Genomic DNA extraction and whole genome sequencing
The quality of isolated DNA were determined using a Nanodrop spectrophotometer and agarose gel electrophoresis. High-quality DNA was then sequenced using the Illumina NovaSeq 6000 platform, which offers high throughput and accuracy for whole genome sequencing. Libraries were created with the TruSeq DNA PCR-Free Library Preparation Kit (Illumina, USA) to ensure high-quality sequencing reads. The sequencing run produced paired end reads, which were then processed and combined with bioinformatics tools like SPAdes for de novo assembly. The raw reads were processed by FastQC and Trimmomatic. The sequence was submitted to NCBI (National Center for Biotechnology Information) under the specially allocated Accession Number GCF_021568595.112.
Genome exploration & visualization
The genome of P. acidilactici strain BCB1H was sequenced using a high-throughput sequencing technology, and the raw data was assembled. Prokka-BVBRC (https://www.bv-brc.org) annotation tools were used to identify coding sequences (CDS), tRNA, rRNA, and other genomic components13. The CDS, tRNA genes, rRNA genes, functional protein-coding genes and hypothetical proteins were found. It also determined the genome completeness, contamination and other genomic characteristics14.
Comparative genomics & average nucleotide identity
The average nucleotide identity (ANI) of P. acidilactici strain BCB1H and other P. acidilactici strains was determined using the JSpeciesWS web server (https://jspecies.ribohost.com), which compares genomes based on nucleotide sequence alignments15. ANIb values were calculated, and aligned base pairs were discovered for each comparison. The HeatMapper Tool (http://www.heatmapper.ca) was used to create the heatmap displaying the comparison of ANI data16.
TETRA analysis for genomic relatedness
The JSpeciesWS Web Server (https://jspecies.ribohost.com), was used to conduct TETRA (Tetra nucleotide frequency-based) analysis to determine the genomic similarity of P. acidilactici strains. The Pearson correlation coefficient (PCC) values, which indicate the strain’s average nucleotide composition, were used to compute pairwise nucleotide composition similarity15.
Subsystem classification and functional annotation
The Prokka-BVBRC (https://www.bv-brc.org) pipeline was used to determine the full genomic subsystem distribution of P. acidilactici strain BCB1H. This annotation technique allowed the genome to be classified into several subsystems, giving an overview of the functional elements present. The Prokka output was then processed to establish how these subsystems were distributed across the genome, providing a complete understanding of the strain’s metabolic and functional capabilities13.
Identification of the specialty & transporter genes
P. acidilactici strain BCB1H’s specialized and transporter genes were identified using the Prokka-BVBRC (https://www.bv-brc.org) annotation pipeline. Transporter genes, which are necessary for the passage of substances across cellular membranes, were explicitly classified using the Transporter Classification Database (TCDB) and other relevant databases incorporated into Prokka. In addition, genes implicated in antibiotic resistance, virulence factors, and other specific pathways were annotated13.
Pan & core genome analysis
P. acidilactici strain’s pan and core genomes were analyzed using the Integrated Prokaryotic Genome and Pan-genome Analysis (IPGA) v1.09 (https://nmdc.cn/ipga) online tool. It compared the genomes of various P. acidilactici strains to uncover conserved core and accessory genes. The core genome was defined as genes shared by all strains, whereas the pan-genome included both core and accessory genes, reflecting the entire genetic material. The study results were then utilized to investigate the genetic variety and evolution of the P. acidilactici species, as well as to determine the presence of strain-specific genes that might contribute to functional diversity and adaptation17.
Genetic linkage and divergence analysis by SNP-Based phylogenetics
Genetic linkage and divergence among P. acidilactici strains were determined using SNP-based phylogenetic analysis with the KSNP tool (https://sourceforge.net/projects/ksnp). This approach entails identifying single nucleotide polymorphisms (SNPs) across strain genomes and then building a phylogenetic tree using these SNPs to infer genetic connections and evolutionary divergence. KSNP analyzed the SNP data to produce a matrix of SNP variations, which were then used to construct a phylogenetic tree18. The resultant tree was displayed with the Interactive Tree of Life (iTOL) program (https://itol.embl.de), which provided an intuitive graphical depiction of the strains’ genetic links and divergence patterns19.
Identification of horizontal gene transfer regions
Horizontal gene transfer (HGT) regions in the genomes of P. acidilactici strains were identified using the AllerHunter program (https://www.sanger.ac.uk/tool/alien_hunter), which detects possible HGT events based on sequence composition and similarity to known mobile genetic elements. The analysis was carried out by entering the strain’s genomic data into the AllerHunter program, which identified locations with unusual genomic fingerprints indicative of horizontal gene transfers20. These regions were then visualized by ProkSee (https://proksee.ca), a circular genome map generator, to illustrate the location and distribution of probable HGT sites throughout the genome14.
BLAST-based genomic comparison
A BLAST-based genomic analysis of P. acidilactici strains was performed to assess sequence similarity and identify conserved areas in the genomes. The BLAST tool (https://blast.ncbi.nlm.nih.gov) was used to match the strain’s genomic sequences against one another, calculate the percentage of sequence similarity, and identify homologs21. The results of the BLAST comparison were then shown using ProkSee (https://proksee.ca), a software tool for creating circular genome maps. The BLAST-based analysis revealed genetic connections and evolutionary diversity among the P. acidilactici strains14.
Profiling for antibiotic resistance genes
The Comprehensive Antibiotic Resistance Database (CARD) (https://card.mcmaster.ca) was used to detect and categorize resistance genes found in P. acidilactici BCB1H genome. The genomic sequences were examined for the presence of known antibiotic resistance genes using the CARD tool, which gave thorough annotations of resistance gene types and processes22. The found antibiotic resistance genes were then displayed using ProkSee (https://proksee.ca), a tool for graphically representing genomic characteristics. A circular genome map was created that highlighted the positions of these resistance genes within the genome, allowing for better viewing of their distribution and potential relationships with other genomic features14.
Identification of Type-II CRISPR-Cas systems
The Type-II CRISPR-Cas systems in P. acidilactici strains were identified using CRISPR Cas Finder (https://crisprcas.i2bc.paris-saclay.fr/CrisprCasFinder/Index), a bioinformatics tool for detecting and annotating CRISPR loci and cas genes. The genomic sequences were submitted to the CRISPR Cas Finder website23. These genomic characteristics were then displayed by ProkSee (https://proksee.ca), which produced a circular depiction of the genome, displaying the CRISPR loci and related genes with other genomic components14.
Identification of bioactive secondary metabolites
P. acidilactici BCB1H’s bioactive secondary metabolites were identified utilizing the antiSMASH (antibiotics and Secondary Metabolite Analysis Shell) program (https://antismash.secondarymetabolites.org). The strain’s genome sequences were uploaded to the antiSMASH online platform, which searched for biosynthetic gene clusters (BGCs) related with secondary metabolites. The results, which included anticipated secondary metabolite clusters and gene annotations, were displayed and annotated to offer a full perspective of the genome’s bioactive compounds24.
Results
Genomic architecture & structural insights
The comprehensive genomic analysis yielded thorough annotations of coding sequences, tRNA, rRNA, and other genomic characteristics. The genome of Pediococcus acidilactici strain BCB1H was assembled into four contigs comprising 1,915,620 bp with a GC content of 42.37%. Prokka annotation located 1,905 coding sequences, which included 1,817 functional genes, 379 putative proteins, and 7 incomplete CDS. The genome has high completeness (99.48%), negligible contamination (0.85%), and excellent assembly metrics, including a N50 of 1,820,736 bp. A circular image of the annotated genome was created that clearly depicted the genome’s structural arrangement. Table 1 gives a complete review of genomic properties.
Table 1.
Comprehensive overview of the annotated genomic features of P. acidilactici strain BCB1H, including coding sequences, tRNA, rRNA, and other functional elements.
| Feature | Value |
|---|---|
| Genome Length | 1,915,620 bp |
| Contigs | 4 |
| GC Content | 42.37% |
| Contig L50 | 1 |
| Contig N50 | 1,820,736 bp |
| tRNA Genes | 56 |
| rRNA Genes | 12 |
| Coding Sequences (CDS) | 1,905 |
| Hypothetical CDS | 379 |
| Partial CDS | 7 |
| PLFAM CDS | 1,817 |
| Genome Quality (Completeness) | 99.48% |
| Genome Quality (Contamination) | 0.85% |
| Antibiotic Resistance Genes (CARD) | 1 |
| Antibiotic Resistance Genes (PATRIC) | 23 |
| Antibiotic Resistance Genes (NDARO) | 1 |
| Transporter Genes | 8 |
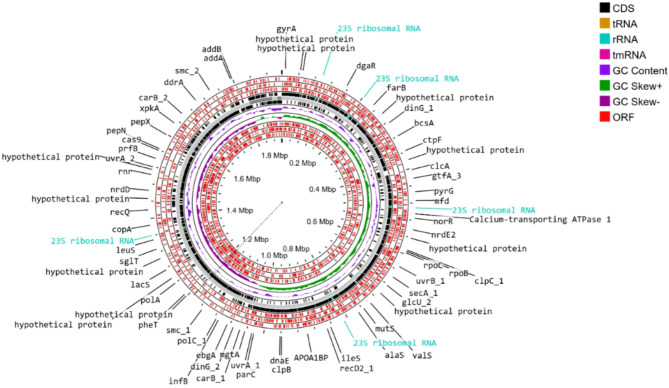
Figure 1 depicts the circular genome, emphasizing critical aspects such as gene distribution, CDS, and tRNA, rRNA, tmRNA, GC Content and ORFs. These findings provide a detailed insight of the genetic landscape of the P. acidilactici strain BCB1H.
Fig. 1.
Circular representation of the genome of P. acidilactici strain BCB1H, visualized using ProkSee, highlighting annotated features such as such as gene distribution, CDS, and tRNA, rRNA, tmRNA, GC Content and ORFs.
Comparative analysis & average nucleotide identity (ANI)
When compared to strain BCB1H, the P. acidilactici strains exhibited high average nucleotide identity (ANI). The compared species along with their accession numbers are given in the Table 2. The ANIb values ranged from 96.86 to 99.39%, with the aligned fractions used for ANI calculation varying from 81.79 to 92.42%. P. acidilactici FDAARGOS_1007 showed the highest similarity (ANIb: 99.39%, aligned fraction: 92.42%), while P. acidilactici CCUG32235 had the lowest similarity (ANIb: 96.87%, aligned fraction: 81.79%). The aligned base pairs ranged from 1,671,844 to 1,889,126 bp, contributing to a total genome size of 2,044,083 bp. The results are summarized in Table 3.
Table 2.
The specie name, strain and NCBI accession numbers.
| Specie Name | Strain | Accession Numbers |
|---|---|---|
| Pediococcus_acidactici | FDAARGOS_1007 | NZ_CP066046.1 |
| Pediococcus_acidactici | CITKHZ7 | NZ_CP096031.1 |
| Pediococcus_acidactici | BCB1H | NZ_JAKGSE000000000.1 |
| Pediococcus_acidactici | A1575 | NZ_JAUJES000000000.1 |
| Pediococcus_acidactici | PA_ZFRA1 | NZ_CP159760.1 |
| Pediococcus_acidactici | SRCM101189 | NZ_CP021529.1 |
| Pediococcus_acidactici | ZPA017 | NZ_CP015206.1 |
| Pediococcus_acidactici | SRCM103289 | NZ_SBJJ00000000.1 |
| Pediococcus_acidactici | AF2019 | NZ_CP138505.1 |
| Pediococcus_acidactici | 13 − 7 | NZ_CP156997.1 |
| Pediococcus_acidactici | GLP06 | NZ_CP118436.1 |
| Pediococcus_acidactici | CLP03 | NZ_CP118924.1 |
| Pediococcus_acidactici | CCUG32235 | KC767944.1 |
| Pediococcus_acidactici | SRCM102732 | NZ_CP028250.1 |
Table 3.
ANI results for P. acidilactici strains, showing ANIb percentages, aligned percentages, aligned base pairs, and total genome size.
| Genome | ANIb [%] | Aligned [%] | Aligned [bp] | Total [bp] |
|---|---|---|---|---|
| Pediococcus_acidactici_FDAARGOS_1007 | 99.39 | 92.42 | 1,889,126 | 2,044,083 |
| Pediococcus_acidactici_CITKHZ7 | 99.26 | 90.56 | 1,851,121 | 2,044,083 |
| Pediococcus_acidactici_BCB1H | 98.54 | 83.99 | 1,716,795 | 2,044,083 |
| Pediococcus_acidactici_A1575 | 98.48 | 84.74 | 1,732,100 | 2,044,083 |
| Pediococcus_acidactici_PA_ZFRA1 | 98.38 | 86.68 | 1,771,787 | 2,044,083 |
| Pediococcus_acidactici_SRCM101189 | 98.29 | 88 | 1,798,767 | 2,044,083 |
| Pediococcus_acidactici_ZPA017 | 98.26 | 88.87 | 1,816,483 | 2,044,083 |
| Pediococcus_acidactici_SRCM103289 | 98.23 | 86.77 | 1,773,559 | 2,044,083 |
| Pediococcus_acidactici_AF2019 | 98.08 | 87.19 | 1,782,314 | 2,044,083 |
| Pediococcus_acidactici_13 − 7 | 97.99 | 88.45 | 1,807,960 | 2,044,083 |
| Pediococcus_acidactici_GLP06 | 96.88 | 83.63 | 1,709,543 | 2,044,083 |
| Pediococcus_acidactici_CLP03 | 96.88 | 83.63 | 1,709,543 | 2,044,083 |
| Pediococcus_acidactici_CCUG32235 | 96.87 | 81.79 | 1,671,844 | 2,044,083 |
| Pediococcus_acidactici_SRCM102732 | 96.86 | 83.7 | 1,710,870 | 2,044,083 |
Figure 2 shows a heatmap that graphically illustrates the genetic similarity data. Higher ANIb values indicate tighter links between strains, whereas lower ANIb values indicate less similarity. This gradient clearly shows the grouping of strains that have a strong genetic similarity to P. acidilactici BCB1H.
Fig. 2.
Heatmap representing the genomic similarity of P. acidilactici strains based on ANIb values. Red indicates high similarity, while blue denotes lower similarity.
Genomic relatedness based on TETRA analysis
The TETRA analysis of P. acidilactici strains demonstrated a strong sequence similarity based on average nucleotide composition. The PCC values for the strains varied from 0.99472 to 0.99925, showing extremely tight genetic connections. The maximum PCC value was detected with P. acidilactici FDAARGOS_1007 (0.99925), while the lowest PCC value was found with P. acidilactici ZPA017 (0.99472). These findings indicate that the strains are closely linked at the nucleotide composition level (Table 4).
Table 4.
TETRA results for P. acidilactici strains, showing the percent of genomic sequence similarity based on the average nucleotide composition (PCC).
| Genome | PCC |
|---|---|
| Pediococcus_acidactici_FDAARGOS_1007 | 0.99925 |
| Pediococcus_acidactici_CITKHZ7 | 0.99908 |
| Pediococcus_acidactici_A1575 | 0.99894 |
| Pediococcus_acidactici_PA_ZFRA1 | 0.99831 |
| Pediococcus_acidactici_SRCM101189 | 0.99797 |
| Pediococcus_acidactici_BCB1H | 0.9978 |
| Pediococcus_acidactici_SRCM103289 | 0.99759 |
| Pediococcus_acidactici_13 − 7 | 0.99756 |
| Pediococcus_acidactici_CCUG32235 | 0.99707 |
| Pediococcus_acidactici_SRCM102732 | 0.997 |
| Pediococcus_acidactici_GLP06 | 0.99692 |
| Pediococcus_acidactici_CLP03 | 0.99692 |
| Pediococcus_acidactici_AF2019 | 0.99622 |
| Pediococcus_acidactici_ZPA017 | 0.99472 |
Comprehensive genomic subsystem distribution
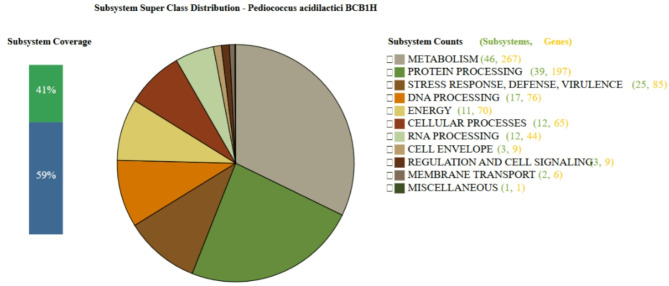
The genome of P. acidilactici BCB1H was evaluated for subsystem coverage and gene distribution using Prokka, indicating a thorough functional classification of the genome. A total of 41% of the genome was classified into specified subsystems, demonstrating a high level of functional annotation. The bulk of the genes were connected with Metabolism (46 subsystems, 267 genes), followed by Protein Processing (39 subsystems, 197 genes), indicating that a considerable component of the genome is involved in basic biological operations such as energy generation and protein synthesis. Notably, Stress Response, Defense, and Virulence (25 subsystems, 85 genes) and DNA Processing (17 subsystems, 76 genes) were well represented, demonstrating the organism’s potential resistance to environmental stressors and genetic regulatory mechanisms.
Smaller but significant groupings included Energy (11 subsystems, 70 genes), Cellular Processes (12 subsystems, 65 genes), and RNA Processing (12 subsystems, 44 genes), demonstrating the organism’s capacity to adapt and maintain cellular homeostasis. The Cell Envelope (3 subsystems, 9 genes), Regulation and Cell Signaling (3 subsystems, 9 genes), and Membrane Transport (2 subsystems, 6 genes) were underrepresented, indicating that these domains have fewer specialized responsibilities. A single subsystem was classified as Miscellaneous (1 subsystem, 1 gene), which highlighted genes with less specific functional annotation. Overall, this subsystem study gives a complete picture of the functional architecture of P. acidilactici BCB1H, focusing on its ability for metabolic activity, protein handling, and stress responses. The Fig. 3 below represents the subsystem coverage and classification of genes in the genome of P. acidilactici BCB1H as determined by Prokka – BVBRC.
Fig. 3.
The subsystem coverage and classification of genes in the genome of P. acidilactici BCB1H as determined by Prokka. The analysis reveals that 41% of the genome is covered by well-defined subsystems.
Discovery of specialized transport and functional genes
Using Prokka and BVBRC annotations, The specialized transporter genes were predicted in the P. acidilactici BCB1H genome. Several transporter genes were found, each connected with a different protein family, indicating specific transport capabilities. Notably, genes linked to holin-like proteins (LrgA, hypothetical proteins with a potential holin-like toxin function) were discovered, indicating a role in cell membrane disruption or antimicrobial action. Furthermore, PTS system components (mannose-specific IID and IIC components) were discovered, demonstrating the bacterium’s ability to transport sugars, notably mannose and related sugars.
The ABC transporter family was represented by genes such as the competence-stimulating peptide ABC transporter ATP-binding protein (ComA), which plays a function in bacterial communication and DNA absorption. Other transporters involved in glycerol uptake (glycerol uptake facilitator protein) and bacteriocin export (pediocin and bacteriocin exporter auxiliary protein) were predicted, indicating the possibility of antimicrobial peptide synthesis and export. These findings highlight the numerous and specific transport systems found in P. acidilactici BCB1H, which may contribute to the species’ adaptability and ecological niche in a variety of settings. The Table 5 below represents the predicted specialty genes by Prokka – BVBRC in the P. acidilactici BCB1H Genome.
Table 5.
Prediction of Specialty genes in P. Acidilactici BCB1H Genome.
| Property | RefSeq Locus Tag | Source ID | Product | Function |
|---|---|---|---|---|
| Transporter | L2V67_06040 | D2EIF4 | Antiholin-like protein LrgA | the cida/lrga holin (cida/lrga holin) family. |
| Transporter | L2V67_02420 | Q2QKM2 | PTS system, mannose-specific IID component | the pts mannose-fructose-sorbose (man) family. |
| Transporter | L2V67_02415 | Q2QKM3 | PTS system, mannose-specific IIC component | the pts mannose-fructose-sorbose (man) family. |
| Transporter | L2V67_09270 | P37249 | hypothetical protein | the abc bacteriocin exporter accessory protein (bea) family. |
| Transporter | L2V67_09280 | P29430 | hypothetical protein | the pediocin (pediocin) family. |
| Transporter | L2V67_01875 | D7V8E7 | Glycerol uptake facilitator protein | the major intrinsic protein (mip) family. |
| Transporter | L2V67_09090 | D6KVW0 | hypothetical protein | the putative holin-like toxin (hol-tox) family. |
| Transporter | L2V67_09265 | P36497 | Competence-stimulating peptide ABC transporter ATP-binding protein ComA | the atp-binding cassette (abc) superfamily. |
PanGenome analysis
Cluster of orthologous groups (COG) analysis
The results of the Cluster of Orthologous Groups (COG) analysis for P. acidilactici strain BCB1H, comparative to other strains including P. acidilactici PA_ZFRA1, 13.7, SRCM101189, SRCM103289, PMC65, CITKHZ7, FDAARGOS 1007, ZPA017, GLPOS, CLP3, SRCM102732, CCUG32235, BCB1H, A1575, and AF2019, are shown in this Fig. 4. The Integrated Prokaryotes Genome and Pan-genome Analysis (IPGA) service v1.09 was used for the analysis, which offers information on the strains’ gene content and functional classification. The figure displays significant genomic characteristics, such as the number of genes and genome lengths for each strain. These findings contribute to the identification of conserved and strain-specific clusters within the P. acidilactici species, showing common orthologous groups engaged in a variety of biological activities. The Fig. 5 depicts the genetic diversity of distinct strains of P. acidilactici.
Fig. 4.
Cluster Share Analysis of P. acidilactici Strains.
Fig. 5.
The genetic diversity of distinct strains of P. acidilactici by Cluster of Orthologous Groups (COG).
Core genome analysis
The core genome analysis of P. acidilactici strains revealed the amount of gene clusters present in the genomes studied. As the number of genomes raises, the number of core gene clusters stabilizes, with roughly 2200 identified. The number of common gene clusters among the 12 to 16 genomes varied from 1600 to 2000. This tendency suggests that the core genome of P. acidilactici is highly conserved, with most genes identified in many strains, implying that these strains share critical physiological functions. The core genome clusters emphasize the fundamental biological processes that are conserved across all P. acidilactici species, highlighting their critical metabolic and cellular activities. The figure S1 represents the cluster COG for the genomes of P. acidilactici.
Pan-genome diversity and accessory genes
The pan-genome analysis of P. acidilactici strains revealed the total number of gene clusters present in the genomes. As the number of genomes climbs from 12 to 16, so does the number of gene clusters in the pan genome, which now totals roughly 4000. The pan genome has both core and accessory gene clusters, with the number of accessory genes growing as additional genomes are studied, indicating the genetic diversity and strain-specific traits of the P. acidilactici species. The results of pan-genome analysis are given below in the figure S2. This figure illustrates the increase in the number of gene clusters in the pan genome of P. acidilactici as more genomes are included in the analysis. The total number of gene clusters rises from approximately 2500 to 4000 as the number of genomes increases from 12 to 16, highlighting the diversity and strain-specific genetic content within the species.
Cluster share analysis
The cluster share analysis for distinct P. acidilactici strains reveals the number of gene clusters shared by strain pairings. The numbers in parenthesis denote gene clusters shared by two strains, demonstrating their genetic similarities and common functional features. For example, P. acidilactici PA_ZFRA1 and 13.7 share 1876 and 2177 gene clusters, whereas P. acidilactici SRCM103289 and FDAARGOS 1007 share 1617 and 2405 gene clusters. These numbers represent the amount of conservation and genetic overlap within the species, representing both core and auxiliary gene clusters found in various strains. Variations in common gene clusters illustrate the genetic diversity and possibly strain-specific characteristics seen in P. acidilactici. Figure 4 represents the cluster sharing between various genomes of P. acidilactici, comparative to BCB1H.
Cluster upset analysis
The cluster upset analysis for P. acidilactici strains shows the overlap of gene clusters from distinct strains. The technique identifies the amount of gene clusters shared by several strains, showing both conserved and novel gene sets. For example, the gene clusters of P. acidilactici strains A1575, PMC65, FDAARGOS 1007, CLP3, and others are compared, with intersections indicating the level of similar genetic material. These intersections show the degree of genetic overlap and can assist identify significant clusters shared by multiple strains as well as those unique to individual strains, offering insight into strain-specific adaptations and diversity within the species. Figure 6 below represents the Cluster Upset Analysis of P. acidilactici Strains comparative to BCB1H.
Fig. 6.
The shared and unique gene clusters across different P. acidilactici strains. The intersections highlight the extent of genetic overlap, revealing both conserved and strain-specific gene clusters within the species.
Core-Pan refraction analysis
The core-pan refraction analysis of P. acidilactici strains reveals the number of gene clusters as the number of genomes grows from 12 to 16. Initially, when more genomes are added, the number of gene clusters increases, reaching around 4000 at 16 genomes. This shows that genetic variety is rising in the pan genome, with the core genome staying relatively constant while the accessory genome increases, suggesting the species’ capacity for strain-specific genetic variation and adaptability. Figure S3 represents the Core-Pan Genome Refraction of P. acidilactici Strains comparative to BCB1H.
Genetic linkage and divergence by SNP-Based phylogenetics
The SNP-based phylogenetic tree for P. acidilactici strains, including P. acidilactici BCB1H, was created to investigate the genetic links among strains. The tree size is set to 0.1, which represents the amount of genetic difference between the strains. The research provides the phylogenetic locations of P. acidilactici BCB1H in respect to other strains, shedding light on their evolutionary connections based on single nucleotide polymorphisms. This technique aids in the identification of closely related strains as well as the assessment of genetic diversity within species. Figure 7 represents the SNP based phylogenetic tree constructed by KSNP.
Fig. 7.
SNP-based phylogenetic tree of P. acidilactici strains, including P. acidilactici BCB1H. The tree scale is set at 0.1, with branches representing the genetic distances between strains based on single nucleotide polymorphisms (SNPs). The analysis shows the evolutionary relationships and genetic divergence among the strains.
Exploring horizontal gene transfer contributions to genetic diversity
Aller Hunter predicts numerous unique horizontal gene transfer (HGT) sites in the P. acidilactici BCB1H genome. These sections, which range in size from 0.2 Mbp to 1.8 Mbp, represent parts of the genome that may have been acquired by horizontal gene transfer. These HGT areas may contain genes involved in the strain’s adaptability, such as antibiotic resistance, pathogenicity, or metabolic capacities. The existence of several HGT areas shows that genetic material was exchanged from external sources, which may have an impact on P. acidilactici’s genetic diversity and functional capability. Figure 8 represents the circular view of the Horizontal Gene Transfer (HGT) Regions in P. acidilactici BCB1H Genome.
Fig. 8.
The predicted HGT regions in the P. acidilactici genome based on analysis by Aller Hunter. The identified HGT regions, ranging in size from 0.2 Mbp to 1.8 Mbp, highlight areas of the genome that may have been acquired via horizontal gene transfer, potentially contributing to the strain’s genetic diversity and adaptive traits.
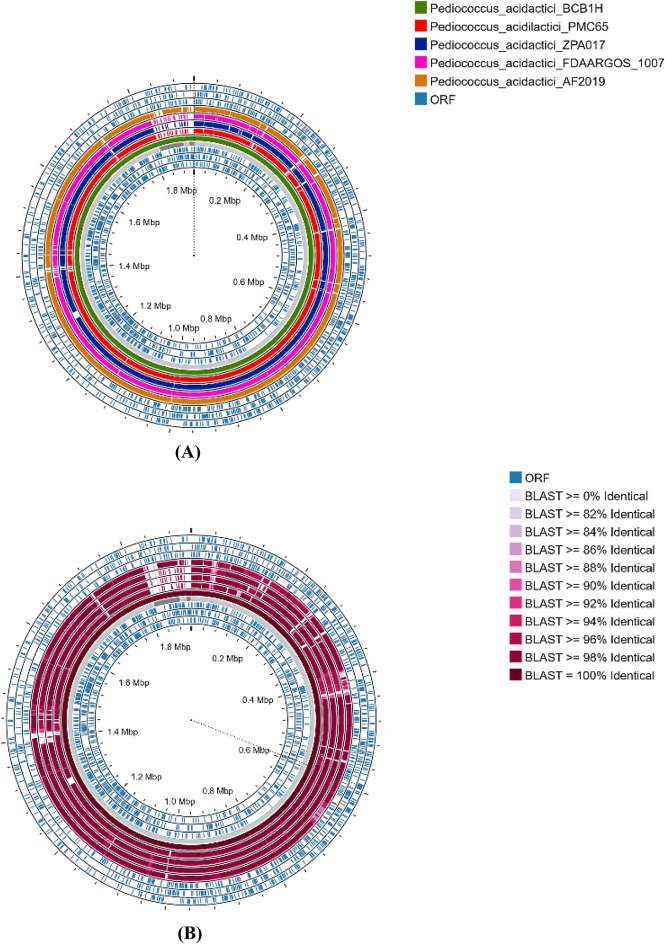
BLAST-Based genomic comparison
The BLAST comparison of multiple P. acidilactici genomes to the P. acidilactici BCB1H genome indicates variable levels of sequence identity across different sections of the genomes. The comparison was based on the percentage of identical sequences, which ranged from 82 to 100%. The technique finds areas with significant sequence conservation (more than 90% identity), indicating that genetic regions are substantially conserved across strains. Specific areas with identities more than 98% were discovered, indicating near-perfect homology, whilst other regions showed lower but still considerable similarity (82–86% identity). The Fig. 9 represents the percentage identity of four P. acidilactici genomes with BCB1H. The comparison shows the percentage of identical sequences across various genomic regions, ranging from 82 to 100% identity, indicating conserved and variable genetic regions among the strains.
Fig. 9.
(A) The comparison of P. acidilactici BCB1H Genome with other strains (B) The percentage identity on the basis of comparison for P. acidilactici BCB1H with other strains.
Antibiotic resistance genes profiles
The Comprehensive Antibiotic Resistance Database (CARD) identified antibiotic resistance genes (ARGs) in the genome of interest, which were then visualized with ProkSee. The study found a number of ARGs that impart antibiotic resistance, including fluoroquinolones (Clostridioides difficile gyrA), mupirocin (Staphylococcus aureus mupA/mupB), rifampicin (Staphylococcus aureus rpoB), and amoxicillin (Helicobacter pylori pbp3). Other ARGs, such as tet genes for tetracycline resistance and macB for macrolide resistance, were also discovered. The genomic sites of these ARGs were mapped, with clusters found between 0.2 Mbp and 1.8 Mbp, indicating the possibility of multidrug resistance mechanisms. Genes such as vanI (vancomycin resistance), mexy (efflux pump for multidrug resistance), and poxtA (oxazolidinone resistance) indicate a complex network of resistance features in the studied genome. Figure S4 represents the circular genome map of P. acidilactici BCB1H highlighting the regions for the presence of antibiotic resistance genes.
Mapping type II-A CRISPR-Cas systems
The study indicated the existence of Type II-A CRISPR-Cas systems, such as cas9, cas1, cas2 (Type I-II-III), and csn2 genes, grouped across the genome. These systems are essential for prokaryotes’ adaptive immunity, defending them against foreign genetic material like phages and plasmids. The genomic loci harboring these CRISPR-Cas components are spread over many areas, with unique CRISPR arrays and related open reading frames (ORFs) ranging from 0.2 Mbp to 1.8 Mbp (Figure S5). The discovery of these clusters demonstrates the potential for CRISPR-mediated genetic defense and biotechnological applications in the investigated organism.
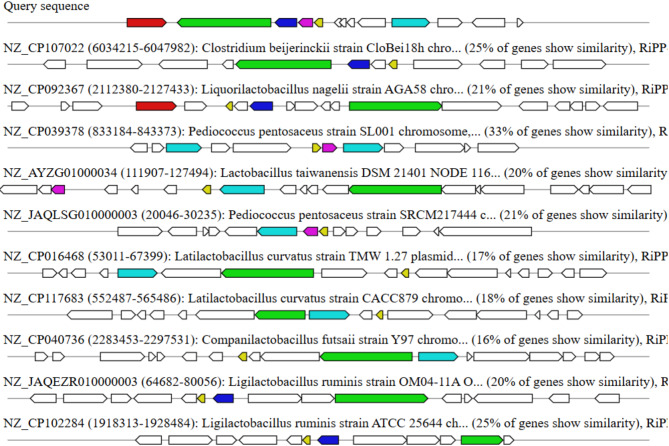
Genomic predictions of bioactive secondary metabolites
AntiSMASH predicted secondary metabolites and found several biosynthetic gene clusters (BGCs) in the target organism’s genome. These BGCs comprise a variety of RiPPs (ribosomally produced and post-translationally modified peptides) as well as various types of secondary metabolites. Significant similarities were discovered between sequences from Clostridium beijerinckii (25% similarity), Liquorilactobacillus nagelii (21% similarity), Pediococcus pentosaceus (33% similarity), and Latilactobacillus curvatus (17–18% similarity), among others. These findings emphasize the organism’s ability to produce bioactive molecules and its intimate interaction with other species that produce secondary metabolites of relevance. Figure 10 represents the visualization of predicted biosynthetic gene clusters (BGCs) using antiSMASH, showing diverse secondary metabolite categories such as RiPPs.
Fig. 10.
Visualization of predicted biosynthetic gene clusters (BGCs) using antiSMASH, showing diverse secondary metabolite categories such as RiPPs.
Discussion
The complete genome investigation of P. acidilactici strain BCB1H revealed important information about its genetic composition, functional features, and prospective biotechnological uses25. The study successfully identified key genomic features such as coding sequences, tRNA, rRNA genes, antibiotic resistance genes, and secondary metabolite biosynthetic gene clusters, highlighting the organism’s adaptability and potential for use in a variety of industrial and therapeutic applications26,27. These findings are consistent with earlier study showing the genetic variety and flexibility of P. acidilactici strains, particularly in terms of probiotic characteristics and resilience to environmental stresses27,28. However, the functional validation of certain discovered genomic elements, notably those associated to bioactive chemical synthesis and antibiotic resistance, was not conducted experimentally29,30. Furthermore, additional research is needed to properly understand the possible linkages between the reported CRISPR-Cas systems, horizontal gene transfer sites, and other genomic elements in the organism’s ecology and applications7. These limitations are comparable with issues identified in previous genome investigations of P. acidilactici, where the functional implications of certain genetic characteristics have yet to be determined31–33.
Future studies should concentrate on experimental confirmation of the expected bioactive chemicals and antibacterial characteristics, as well as investigating the function of horizontal gene transfer in sculpting the genetic diversity and adaptation of P. acidilactici34. Furthermore, broadening the comparative research to encompass a larger spectrum of Pediococcus species may give more thorough insights into the genus genetic and functional development. Such techniques have been proposed in recent literature to improve our understanding of P. acidilactici’s genetic landscape and prospective uses35–37. In the future, the use of synthetic biology and genome editing techniques may allow for targeted modification of P. acidilactici strains to increase bioactive chemical and probiotic production31. Furthermore, investigating its genetic potential for environmental and industrial applications, such as bioremediation or the development of new medicinal compounds, shows promising results. Further study into the interactions between P. acidilactici and its environment, particularly its potential as a probiotic, would aid in realizing its full potential for biotechnological and health-care applications38,39. Future research that addresses present constraints and focuses on experimental validation and functional investigations might pave the road for the practical application of P. acidilactici in a variety of sectors, including food technology, medicine, and environmental biotechnology40,41.
Conclusion
Finally, the thorough genomic investigation of P. acidilactici strain BCB1H revealed important information about its genetic composition, functional features, and prospective biotechnological uses. The study successfully identified key genomic features such as coding sequences, tRNA, rRNA genes, antibiotic resistance genes, and secondary metabolite biosynthetic gene clusters, highlighting the organism’s adaptability and potential for use in a variety of industrial and therapeutic applications. These findings are consistent with earlier study showing the genetic variety and flexibility of P. acidilactici strains, particularly in terms of probiotic characteristics and resilience to environmental stresses. Furthermore, broadening the comparative research to encompass a larger spectrum of Pediococcus species may give more thorough insights into the genus’ genetic and functional development. Such techniques have been proposed in recent literature to improve our understanding of P. acidilactici’s genetic landscape and prospective uses. P. acidilactici strain BCB1H’s future uses in the food sector include probiotics and natural preservatives to improve food safety and shelf life. In medicine, its potential for producing antimicrobial peptides and improving gut health opens the door to new therapeutic uses. Further research will improve its industrial-scale use for sustainable and health-promoting advances.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Conceptualization, Tariq Aziz; methodology, Muhammad Naveed and Muhammad Aqib Shabbir; software, Abid Sarwar; validation, Ashwag Shami and Fahad Al Asmari; formal analysis, Jasra Naseeb, investigation, Tariq Aziz; resources, Zhennai Yang and Liqing Zhao; data curation, Cui Haiying.; writing—original draft preparation, Muhammad Aqib Shabbir.; writing—review and editing, Lin Lin; visualization, Tariq Aziz; Supervision, Liqing Zhao and Zhennai Yang; project administration, Lin Lin.
Funding
This research work was financially supported by the National Natural Science Foundation of China (Project No. 32272296), the National Key R&D Program of China (2021YFA0910800 and 2023YFF1103402), and the Natural Science Foundation of Guangdong Province (Grant No. 2022A1515012043). The authors are thankful to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R31), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors express their gratitude to the Deanship of Scientific Research (DSR) at King Faisal University under project no. [KFU243005].
Data availability
Availability of data and materials: All the data has been included in the manuscript. The whole genome sequence of this strain can be accessed on the NCBI database using accession number https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_021568595.1/.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. Therefore, as an observational study, it doesn’t require any ethical approval.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Liqing Zhao, Email: liqing.zhao@szu.edu.cn.
Zhennai Yang, Email: yangzhennai@th.btbu.edu.cn.
Lin Lin, Email: linl@ujs.edu.cn.
References
- 1.Porto, M. C. W. et al. Pediococcus spp.: an important genus of lactic acid bacteria and pediocin producers. Biotechnol. Adv.35 (3), 361–374 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Wade, M. et al. Role of Pediococcus in winemaking. Aust. J. Grape Wine Res.25 (1), 7–24 (2019). [Google Scholar]
- 3.Holzapfel, W. H. et al. The genera pediococcus and tetragenococcus. Prokaryotes4 (1), 229–266 (2006). [Google Scholar]
- 4.Lin, Y. et al. Comparative genomics reveals key molecular targets for mutant Pediococcus pentosaceus C23221 producing pediocin. Int. J. Biol. Macromol.242, 125006 (2023). [DOI] [PubMed] [Google Scholar]
- 5.Hu G et al. Revolutionizing the probiotic functionality, biochemical activity, antibiotic resistance and specialty genes of Pediococcus acidilactici BCB1H via in-vitro and in-silico approaches. Z Naturforsch. C J. Biosci. 1–16 (2024). [DOI] [PubMed]
- 6.Zhao, M. et al. Complete Genome Sequence and Probiotic Properties of Pediococcus acidilactici CLP03 Isolated from Healthy Felis catusp. 1–15 (Probiotics and Antimicrobial Proteins, 2023). [DOI] [PubMed]
- 7.Li, Z. et al. Comparative genomics analysis of Pediococcus acidilactici species. J. Microbiol.59, 573–583 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Jiang, J. et al. Comparative genomics of Pediococcus pentosaceus isolated from different niches reveals genetic diversity in carbohydrate metabolism and immune system. Front. Microbiol.11, 253 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanwal, H. et al. Probiotic characterization and population diversity analysis of gut-associated Pediococcus acidilactici for its potential use in the dairy industry. Appl. Sci.11 (20), 9586 (2021). [Google Scholar]
- 10.Garmyn, D. et al. Pediococcus acidilactici ldhD gene: cloning, nucleotide sequence, and transcriptional analysis. J. Bacteriol.177 (12), 3427–3437 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang, W. et al. Genomic and proteomic analyses reveal the reduction mechanism of hexavalent chromium by the culturing supernatant of strain Pediococcus acidilactici 13 – 7. J. Hazard. Mater.477, 135161 (2024). [DOI] [PubMed] [Google Scholar]
- 12.Hu, G. et al. Depiction of the dairy product supplemented with the exopolysaccharide from Pediococcus acidilactici BCB1H by metabolomics analysis. J. Food Meas. Charact.18 (3), 1690–1704 (2024). [Google Scholar]
- 13.Seemann, T. Prokka: rapid prokaryotic genome annotation. Bioinformatics30 (14), 2068–2069 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Grant, J. R. et al. Proksee: in-depth characterization and visualization of bacterial genomes. Nucleic Acids Res.51 (W1), W484–W492 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richter, M. et al. JSpeciesWS: a web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics32 (6), 929–931 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babicki, S. et al. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res.44 (W1), W147–W153 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu, D. et al. IPGA: a handy integrated prokaryotes genome and pan-genome analysis web service. iMeta1 (4), e55 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner, S. N. & Hall, B. G. When whole-genome alignments just won’t work: kSNP v2 software for alignment-free SNP discovery and phylogenetics of hundreds of microbial genomes. PloS One. 8 (12), e81760 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Letunic, I. & Bork, P. Interactive tree of life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res.49 (W1), W293–W296 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vernikos, G. S. & Parkhill, J. Interpolated variable order motifs for identification of horizontally acquired DNA: revisiting the Salmonella pathogenicity islands. Bioinformatics22 (18), 2196–2203 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Ye, J., McGinnis, S. & Madden, T. L. BLAST: improvements for better sequence analysis. Nucleic Acids Res.34 (suppl_2), W6–W9 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alcock, B. P. et al. CARD 2023: expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res.51 (D1), D690–D699 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couvin, D. et al. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res.46 (W1), W246–W251 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blin, K. et al. antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res.47 (W1), W81–W87 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aziz, T. et al. Unveiling the whole genomic features and potential probiotic characteristics of novel lactiplantibacillus plantarum HMX2. Front. Microbiol.15, 1504625 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aziz, T. et al. Comparative genomics of food-derived probiotic lactiplantibacillus plantarum K25 reveals its hidden potential, compactness, and efficiency. Front. Microbiol.14, 1214478 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao, M. et al. Probiotic characteristics and whole-genome sequence analysis of Pediococcus acidilactici isolated from the feces of adult beagles. Front. Microbiol.14, 1179953 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aziz, T. et al. Integrated genome based evaluation of safety and probiotic characteristics of lactiplantibacillus plantarum YW11 isolated from tibetan kefir. Front. Microbiol.14, 1157615 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aziz T et al. Whole Genome Analysis of Tibetan Kefir-Derived Lactiplantibacillus Plantarum 12-3 Elucidates Its Genomic Architecture, Antimicrobial and Drug Resistance, Potential Probiotic Functionality and Safety. Front Biosci (Landmark Ed). 1–13, 29(4), 147 (2024). [DOI] [PubMed]
- 30.Todorov, S. D., Holzapfel, W. H. & Nero, L. A. Safety evaluation and bacteriocinogenic potential of Pediococcus acidilactici strains isolated from artisanal cheeses. LWT139, 110550 (2021). [Google Scholar]
- 31.Al-Emran, H. M. et al. Genomic analysis and in vivo efficacy of Pediococcus acidilactici as a potential probiotic to prevent hyperglycemia, hypercholesterolemia and gastrointestinal infections. Sci. Rep.12 (1), 20429 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elkalla, W. S. et al. The complete genome sequences of the two novel probiotics were isolated from the human gut microbiota: Pediococcus acidilactici WNYM01 and Pediococcus acidilactici WNYM02, vitamin B9, and B2-producers. Egypt. Pharm. J.23(4), 104103 (2024).
- 33.Blanco, I. R. et al. Pan-genomic and comparative analysis of Pediococcus pentosaceus focused on the in silico assessment of pediocin-like bacteriocins. Comput. Struct. Biotechnol. J.20, 5595–5606 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snauwaert, I. et al. Comparative genome analysis of Pediococcus damnosus LMG 28219, a strain well-adapted to the beer environment. BMC Genom.16, 1–12 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen, H. et al. Metagenome-assembled genome reveals species and functional composition of Jianghan chicken gut microbiota and isolation of Pediococcus acidilactic with probiotic properties. Microbiome12 (1), 25 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lv, L. X. et al. Whole-genome sequence assembly of Pediococcus pentosaceus LI05 (CGMCC 7049) from the human gastrointestinal tract and comparative analysis with representative sequences from three food-borne strains. Gut Pathogens. 6, 1–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliveira, F. S. et al. Genomic analyses of Pediococcus pentosaceus ST65ACC, a bacteriocinogenic strain isolated from artisanal raw-milk cheese. Probiotics Antimicrob. Proteins. 15 (3), 630–645 (2023). [DOI] [PubMed] [Google Scholar]
- 38.Cho, S. W. et al. Complete genome sequence of lactic acid bacterium Pediococcus acidilactici strain ATCC 8042, an autolytic anti-bacterial peptidoglycan hydrolase producer. Biotechnol. Bioprocess Eng.24, 483–487 (2019). [Google Scholar]
- 39.Wang, S. et al. Pediococcus acidilactici strains as silage inoculants for improving the fermentation quality, nutritive value and in vitro ruminal digestibility in different forages. J. Appl. Microbiol.126 (2), 424–434 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Bansal, P., Kumar, R. & Dhanda, S. Characterization of starter cultures and nutritional properties of Pediococcus acidilactici NCDC 252: a potential probiotic of dairy origin. J. Food Process. Preserv.46 (10), e16817 (2022). [Google Scholar]
- 41.Ranjan, R. et al. Genome sequencing of Pediococcus acidilactici (NRCC1), a novel isolate from dromedary camel (Camelus dromedarius) rumen fluid. Ann. Microbiol.68, 103–110 (2018). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Availability of data and materials: All the data has been included in the manuscript. The whole genome sequence of this strain can be accessed on the NCBI database using accession number https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_021568595.1/.