Key Points
Question
Is high-flow nasal oxygen noninferior to noninvasive ventilation for rate of endotracheal intubation or death at 7 days in patients with acute respiratory failure due to a variety of causes?
Findings
In this randomized clinical trial (n = 1766 patients), a bayesian hierarchical model with dynamic borrowing across 5 patient groups found high-flow nasal oxygen was noninferior (defined by a posterior probability ≥0.992 for an odds ratio <1.55) to noninvasive ventilation for rates of endotracheal intubation or death at 7 days in 4 of the patient groups (nonimmunocompromised with hypoxemic acute respiratory failure, chronic obstructive pulmonary disease exacerbation with respiratory acidosis, acute cardiogenic pulmonary edema, and hypoxemic COVID-19). One patient group (immunocompromised with hypoxemic acute respiratory failure) was stopped for futility.
Meaning
Compared with noninvasive ventilation, high-flow nasal oxygen met prespecified criteria for noninferiority for the outcome of endotracheal intubation or death within 7 days in 4 of the 5 patient groups with acute respiratory failure.
Abstract
Importance
High-flow nasal oxygen (HFNO) and noninvasive ventilation (NIV) are commonly used respiratory support therapies for patients with acute respiratory failure (ARF).
Objective
To assess whether HFNO is noninferior to NIV on the rates of endotracheal intubation or death at 7 days in 5 patient groups with ARF.
Design, Setting, and Participants
This noninferiority, randomized clinical trial enrolled hospitalized adults (aged ≥18 years; classified as 5 patient groups with ARF: nonimmunocompromised with hypoxemia, immunocompromised with hypoxemia, chronic obstructive pulmonary disease [COPD] exacerbation with respiratory acidosis, acute cardiogenic pulmonary edema [ACPE], or hypoxemic COVID-19, which was added as a separate group on June 26, 2023) at 33 hospitals in Brazil between November 2019 and November 2023 (final follow-up: April 26, 2024).
Interventions
High-flow nasal oxygen (n = 883) or NIV (n = 883).
Main Outcomes and Measures
The primary outcome was endotracheal intubation or death within 7 days assessed using a bayesian hierarchical model with dynamic borrowing across patient groups. Noninferiority was defined by a posterior probability of 0.992 or greater for an odds ratio (OR) less than 1.55.
Results
Among 1800 patients, 1766 completed the study (mean age, 64 [SD, 17] years; 707 [40%] were women). The primary outcome of endotracheal intubation or death at 7 days occurred in 39% (344/883) in the HFNO group vs 38% (336/883) in the NIV group. In the immunocompromised with hypoxemia patient group, the primary outcome occurred in 57.1% (16/28) in the HFNO group vs 36.4% (8/22) in the NIV group; enrollment was stopped for futility (final OR, 1.07; 95% credible interval [CrI], 0.81-1.39; noninferiority posterior probability [NPP], 0.989). In the nonimmunocompromised with hypoxemia group, the primary outcome occurred in 32.5% (81/249) in the HFNO group vs 33.1% (78/236) in the NIV group (OR, 1.02 [95% CrI, 0.81-1.26]; NPP, 0.999). In the ACPE group, the primary outcome occurred in 10.3% (14/136) in the HFNO group vs 21.3% (29/136) in the NIV group (OR, 0.97 [95% CrI, 0.73-1.23]; NPP, 0.997). In the hypoxemic COVID-19 group, the primary outcome occurred in 51.3% (223/435) in the HFNO group vs 47.0% (210/447) in the NIV group (OR, 1.13 [95% CrI, 0.94-1.38]; NPP, 0.997). In the COPD exacerbation with respiratory acidosis group, the primary outcome occurred in 28.6% (10/35) in the HFNO group vs 26.2% (11/42) in the NIV group (OR, 1.05 [95% CrI, 0.79-1.36]; NPP, 0.992). However, a post hoc analysis without dynamic borrowing across the 5 ARF patient groups revealed some qualitatively different results in patients with COPD, immunocompromised patients, and patients with ACPE. The incidence of serious adverse events was similar (9.4% of patients in HFNO group vs 9.9% in NIV group).
Conclusions and Relevance
Compared with NIV, HFNO met prespecified criteria for noninferiority for the primary outcome of endotracheal intubation or death within 7 days in 4 of the 5 patient groups with ARF. However, the small sample sizes in some patient groups and the sensitivity of the findings to the choice of analysis model suggests the need for further study in patients with COPD, immunocompromised patients, and patients with ACPE.
Trial Registration
ClinicalTrials.gov Identifier: NCT03643939
This randomized clinical trial compares the use of high-flow nasal oxygen vs noninvasive ventilation on the rates of endotracheal intubation or death at 7 days across 5 patient groups with acute respiratory failure.
Introduction
Clinicians use high-flow nasal oxygen and noninvasive ventilation (pressure support ventilation with positive end-expiratory pressure) to treat acute respiratory failure associated with several conditions, including hypoxemic acute respiratory failure in both nonimmunocompromised and immunocompromised patients, exacerbation of chronic obstructive pulmonary disease (COPD), acute cardiogenic pulmonary edema, and COVID-19.1 However, patients may not always tolerate noninvasive ventilation due to discomfort.2 Compared with low-flow oxygen, high-flow nasal oxygen offers physiological advantages, including improved oxygenation, reduced dead space, alveolar recruitment, humidification, heating, and enhanced secretion clearance.3,4 Moreover, high-flow nasal oxygen has been shown to effectively reduce Paco2 levels in patients experiencing an exacerbation of COPD,5,6 decrease cardiac preload, and improve signs of respiratory failure for patients with acute heart failure.7,8
Compared with noninvasive ventilation, high-flow nasal oxygen is easier to use and more comfortable for the patient.9 Patients using a high-flow nasal cannula can eat, drink, and talk more easily than with noninvasive ventilation. However, high-flow nasal oxygen may be less effective than noninvasive ventilation in reducing the workload on respiratory muscles during acute respiratory failure.10 Evidence on the most appropriate form of noninvasive respiratory support across different causes of acute respiratory failure is limited. Guidelines recommend noninvasive ventilation for acute respiratory failure caused by COPD exacerbation and acute cardiogenic pulmonary edema; however, the evidence11,12 supporting the use of noninvasive ventilation is based on comparison with low-flow oxygen not high-flow nasal oxygen. High-flow nasal oxygen is preferred over low-flow oxygen for treating patients with hypoxemic acute respiratory failure, including those with immunosuppression or COVID-19.13,14 However, considerable uncertainty remains regarding the comparative effectiveness of high-flow nasal oxygen vs noninvasive ventilation.9,15,16,17,18,19
We conducted a multicenter, adaptive, randomized clinical trial to evaluate the noninferiority of high-flow nasal oxygen compared with noninvasive ventilation and assess potential superiority in reducing the rates of endotracheal intubation or death across 5 patient groups hospitalized with acute respiratory failure (nonimmunocompromised with hypoxemia, immunocompromised with hypoxemia, COPD exacerbation with respiratory acidosis, acute cardiogenic pulmonary edema, or hypoxemic COVID-19).
Methods
Study Design and Oversight
The High-Flow Nasal Oxygen Cannula Compared to Non-Invasive Ventilation in Adult Patients With Acute Respiratory Failure (RENOVATE) was a multicenter, adaptive, noninferiority randomized clinical trial conducted at 33 hospitals in Brazil. The main adaptation in this trial was the use of frequent interim analyses that allowed for the stopping of individual patient groups with acute respiratory failure based on futility, noninferiority, or superiority (additional details appear in the eResults in Supplement 1). The trial protocol (Supplement 2) and statistical analysis plan (Supplement 3) were published.20,21 The trial was overseen by an international steering committee and an independent data and safety monitoring committee whose members were unaware of the treatment group assignment (additional details appear in the eMethods in Supplement 1).
The bayesian adaptive trial design, analysis of the primary outcome, and 28-day mortality assessments were performed by Berry Consultants, LLC. The Hcor Research Institute coordinated the trial and conducted all other statistical analyses. Additional details regarding trial governance and responsibilities appear in the eMethods in Supplement 1. The trial was conducted in accordance with principles of the Declaration of Helsinki,22 and the trial protocol (Supplement 2) was approved by the institutional review boards at each site and a national ethics review board. Due to the urgency of clinical decisions regarding respiratory support and based on the recommendation of the national ethics review board, written consent was sought from all patients or their surrogate decision-makers after enrollment in the trial.
Patients
Adult patients (aged ≥18 years) were eligible if they were admitted to intensive care units (ICUs), emergency departments, or hospital wards because of acute respiratory failure, which was defined by presence of hypoxemia (oxygen saturation as measured by pulse oximetry [Spo2] <90% or Pao2 <60 mm Hg at room air) and either respiratory effort (use of accessory musculature, paradoxical breathing, or thoracoabdominal asynchrony) or tachypnea (respiratory rate >25 breaths/min). Patients were initially classified into 1 of the following 4 mutually exclusive patient groups with acute respiratory failure: nonimmunocompromised with hypoxemia, immunocompromised with hypoxemia, COPD exacerbation with respiratory acidosis, or acute cardiogenic pulmonary edema (additional eligibility criteria appear in the eMethods in Supplement 1). Hypoxemic COVID-19 was added later and is further described below. Before randomization, the use of low-flow oxygen was allowed for all patients; the use of noninvasive ventilation was allowed for most patients, but not for those with acute cardiogenic pulmonary edema.
Immunocompromised patients were defined as those with (1) regular use of immunosuppressive drugs for more than 3 months or high doses of corticosteroids (>0.5 mg/kg/d); (2) presence of solid organ transplants, solid tumor treated with chemotherapy within the past 5 years, or hematologic malignancy treated within the past 5 years; or (3) diagnosed with AIDS or a primary immunodeficiency.23 A COPD exacerbation with respiratory acidosis was defined as being present in patients with (1) high clinical suspicion of COPD or previous COPD diagnosis; (2) a respiratory rate greater than 25 breaths/min or use of accessory musculature, paradoxical breathing, or thoracoabdominal asynchrony; or (3) an arterial blood pH level lower than 7.35 and a Paco2 level greater than 45 mm Hg. Acute cardiogenic pulmonary edema was defined as being present in patients (1) with sudden-onset dyspnea and diffuse crackling rales with or without the third audible heart sound; (2) without a history of pulmonary aspiration, infection, or pulmonary fibrosis; (3) with acute cardiogenic pulmonary edema as the primary clinical hypothesis; or (4) with a chest x-ray with bilateral alveolar infiltrates suggestive of pulmonary edema, respiratory rate greater than 25 breaths/min, and Spo2 level less than 95%.
The main exclusion criteria were (1) an urgent need for endotracheal intubation (prolonged respiratory pauses, cardiorespiratory arrest, heart rate <50 beats/min with decreased level of consciousness, arterial blood pH level <7.15, irrespective of the cause), (2) hemodynamic instability, and (3) contraindications to noninvasive ventilation (eg, uncontrolled vomiting, copious oral secretions, facial deformities, a Glasgow Coma Scale score ≤12 points, presence of pneumothorax, or a do-not-intubate order).
After March 2020, patients with COVID-19 inevitably entered the trial in 1 of the 4 patient groups with acute respiratory failure described above. All analyses were conducted without separating patients with COVID-19 into a distinct group until the fifth interim analysis was conducted in March 2023. After March 2023, patients with COVID-19 were separated out for the purposes of the statistical analysis (described in an amendment to the trial protocol in June 2023). This decision was made by the trial steering committee while still blinded to any outcome data and as a good faith effort to match the aims of the original design while addressing the emergence of the COVID-19 pandemic. This decision was based on several critical factors, including that COVID-19 represented a novel disease with an anticipated intubation rate that could differ significantly from other causes of acute respiratory failure.
In addition, there was a rapidly evolving learning curve regarding the optimal management of patients with COVID-19, particularly in relation to noninvasive respiratory support and endotracheal intubation. This learning curve led to substantial shifts in intubation rates and mortality over time as clinical practices adapted. Consequently, the original assumptions about endotracheal intubation and mortality rates in the noninvasive ventilation (control) group during the trial’s design phase may have been overly optimistic to accurately represent patients with COVID-19 (eMethods in Supplement 1).
Randomization
The randomization list was generated electronically with permuted block sizes that were unknown to the investigators. The allocation concealment was maintained via an online central automated system available 24 hours daily, stratified by center and acute respiratory failure patient group. Patients were randomly assigned in a 1:1 ratio to high-flow nasal oxygen or noninvasive ventilation (Figure 1). The blinding of patients and clinicians was not possible because of the nature of the intervention.
Figure 1. Enrollment, Randomization, and Follow-Up in the Study.
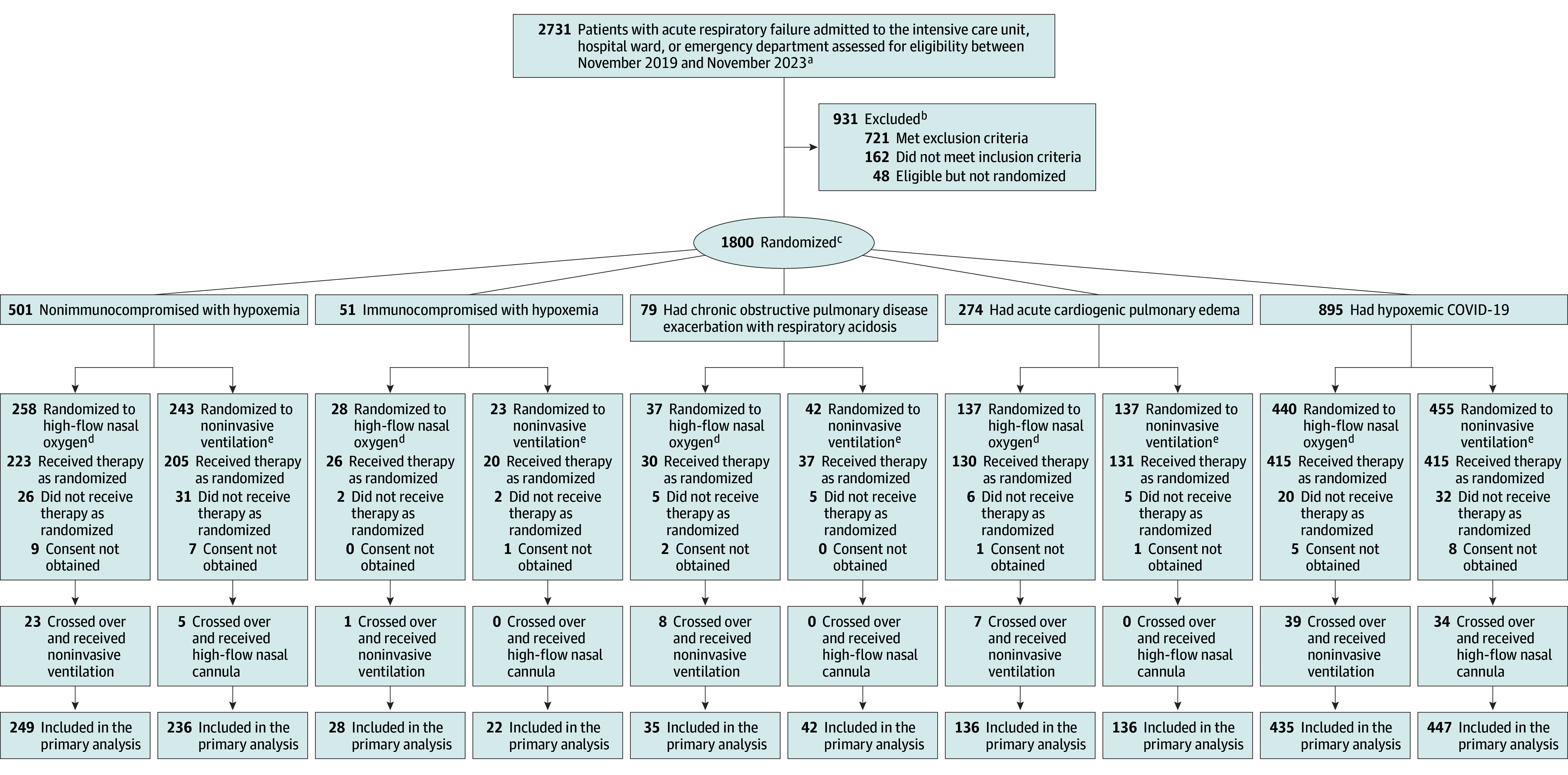
aInvestigators registered all patients assessed for eligibility during the trial period; however, they did not collect data on the total number of potentially eligible patients.
bPatients may have met more than 1 reason for exclusion. Detailed counts of individual exclusions stratified by the selected patient groups with acute respiratory failure appear in the eMethods and in eFigures 2-5 in Supplement 1.
cPatients or next of kin were asked to provide consent after randomization. The reason for postrandomization exclusion was death before their next of kin could provide consent and lack of authorization from the ethics committee to include the data.
dDelivered through a nasal cannula with larger-bore tubing.
eNoninvasive positive-pressure ventilation delivered through a face mask using either a ventilator in the intensive care unit or a bilevel device.
Interventions
High-flow nasal oxygen was delivered continuously (Airvo-2, Fisher & Paykel Healthcare) with a flow starting at 30 L/min for patients with COPD exacerbation with respiratory acidosis and at 45 L/min for those in the 4 other patient groups with acute respiratory failure, titrated gradually toward 60 L/min or the highest flow tolerated. Fraction of inspired oxygen (Fio2) started at 50% and was titrated to maintain SpO2 within 88% to 92% in the COPD exacerbation with respiratory acidosis patient group and within 92% to 98% in the other patient groups. After 24 hours, weaning from high-flow nasal oxygen therapy could begin if clinical improvement had been achieved (eMethods in Supplement 1). Noninvasive ventilation rescue therapy was allowed for the COPD exacerbation with respiratory acidosis and the acute cardiogenic pulmonary edema patient groups at the discretion of the treating physician (eFigure 1 in Supplement 1).
In the noninvasive ventilation group, the therapy was delivered through a face mask using either a ventilator designed primarily for invasive or noninvasive ventilation. In the COPD exacerbation with respiratory acidosis patient group, the settings for inspiratory positive airway pressure were between 12 and 16 cm of H2O; for all the other patient groups, the settings were between 12 and 14 cm of H2O. In the COPD exacerbation patient group, the expiratory positive airway pressure setting was 4 cm of H2O; for all the other patient groups, the setting was 8 cm of H2O. In the COPD exacerbation patient group, the Fio2 level was titrated to maintain an Spo2 within 88% and 92%; for all the other patient groups, Fio2 level was titrated to maintain an Spo2 within 92% and 98%.
The inspiratory positive airway pressure or the expiratory positive airway pressure could be increased independently by 1 or 2 cm of H2O until (1) a maximum of 20 cm of H2O for inspiratory positive airway pressure and a maximum of 12 cm of H2O for expiratory positive airway pressure or (2) patient tolerability and signs of clinical improvement (defined as a respiratory rate ≤25 breaths/min and no signs of accessory respiratory muscle activity). The tidal volume target was 6 to 9 mL/kg of ideal body weight.
On the first day, 24 hours of noninvasive ventilation use was encouraged. Thereafter, weaning from noninvasive ventilation could be started in patients without signs of respiratory distress and included decreasing the duration of the noninvasive ventilation sessions, decreasing Fio2 level, and decreasing pressure levels each day at the discretion of the clinical team. A patient was considered weaned when Fio2 level was less than or equal to 30%, and both the expiratory positive airway pressure and pressure support were less than or equal to 6 cm of H2O (eMethods in Supplement 1). The criteria for endotracheal intubation appear in the eMethods in Supplement 1. The final decision about endotracheal intubation was made by the attending physician.
Primary Outcome
The primary outcome was endotracheal intubation or death within 7 days of randomization. The primary outcome was initially defined as endotracheal intubation within 7 days. However, we expanded the definition in the fifth version of the protocol (April 2021) to include both endotracheal intubation and death within 7 days to also consider eventual patients with acute respiratory failure who died without being intubated.
To maintain consistency in the indication for endotracheal intubation across sites and to minimize the risk of delayed intubation, predefined criteria were applied: (1) respiratory or cardiac arrest; (2) hemodynamic instability with mean arterial pressure lower than 65 mm Hg, systolic arterial pressure lower than 90 mm Hg after proper fluid resuscitation, or need for increasing doses of vasopressors (>0.3 g/kg/min of norepinephrine); (3) cognitive impairment and agitation that prevents medical or nursing care without full sedation; (4) a Glasgow Coma Scale score of less than 11; (5) failure to maintain an Spo2 level greater than 92% (or >88% in patients with COPD) despite having an Fio2 level of 60%; (6) a progressive increase in Paco2 that is greater than 10 mm Hg and a concurrent drop in arterial blood pH level despite attempts of improving ventilation; (7) an intolerability to high-flow nasal oxygen or noninvasive ventilation; (8) development of hypersecretion and inability to eliminate such secretion; (9) require frequent discontinuation of noninvasive ventilation therapy; (10) severe arrhythmia with hemodynamic instability; (11) persistent respiratory acidosis with arterial blood pH level lower than 7.2 after 60 minutes of optimal treatment; or (12) physician discretion.
Secondary and Tertiary Outcomes
The secondary outcomes were 28-day mortality, 90-day mortality, mechanical ventilation–free days at 28 days, and ICU-free days at 28 days. The tertiary outcomes were hospital and ICU length of stay within 90 days, vasopressor-free days within 28 days, the proportion of patients who received a do-not-intubate order within 7 days after randomization, and patient comfort score.
Statistical Analysis
The trial used bayesian adaptive statistical methods to assess the primary outcome (statistical analysis plan appears in Supplement 3). We hypothesized that high-flow nasal oxygen would be noninferior or superior to noninvasive ventilation in all 5 patient groups with acute respiratory failure. The noninferiority margin was established based on an estimated absolute effect of 36% for noninvasive ventilation on the rate of endotracheal intubation.24 Considering that the noninferiority margin should not exceed half of this effect, we selected an absolute difference of 10%.25 Next, we proportionately extrapolated the 10% absolute difference on a 30% event rate (derived from a population with hypoxemic acute respiratory failure) to the other patient groups through the conversion to an odds ratio (OR) of 1.55 (the noninferiority margin).
The noninferiority and superiority hypotheses were tested in order with gatekeeping, thus without the need for a multiplicity correction. Noninferiority was declared if the noninferiority posterior probability was higher than 0.992. If noninferiority was demonstrated, then superiority was declared if the superiority posterior probability was also higher than 0.992 for an OR less than 1. If noninferiority was not demonstrated, the final result was futility. Stopping for futility, superiority, and noninferiority was allowed and conditioned on thresholds defined for each interim analysis (eMethods in Supplement 1 and Supplements 2-3). The interim and final values for the posterior probability thresholds for noninferiority and superiority were set to control for type I error at less than 0.025 in all 5 patient groups with acute respiratory failure (eTable 1 in Supplement 1). Up to 6 interim analyses were planned, starting after the 500th patient was randomized, with a maximum total sample size of 2000 patients. Simulations showed that, in a scenario of similar treatment effects, the power of our trial to demonstrate noninferiority would be 84.9% for nonimmunocompromised patients with hypoxemia, 76.1% for immunocompromised patients with hypoxemia, 68.5% for patients with COPD exacerbation with respiratory acidosis, 5.5% for patients with acute cardiogenic pulmonary edema, and 92.5% for patients with hypoxemic COVID-19 (eTable 2 in Supplement 1).
The primary analysis considered an intention-to-treat population that consisted of all randomized patients with informed consent. The primary outcome was analyzed using bayesian hierarchical modeling with dynamic borrowing across the 5 patient groups with acute respiratory failure, which shrinks the posterior distributions of the effects in each group toward the overall estimate. We used modestly informative prior distribution for the primary outcome rate for the noninvasive ventilation (control) group for each of the 5 patient groups with acute respiratory failure (eMethods in Supplement 1 and Supplement 3). A time-varying effect was considered for patients with hypoxemic COVID-19 to account for potential changes in the risk of the primary outcome across time.26 The results are presented as rates, model-fitted median ORs, 95% credible intervals (CrIs), and posterior probabilities of noninferiority and superiority.
Mortality within 28 days was analyzed using the same bayesian model structure as the primary outcome, with adjustments to the prior distributions to account for different rates of 28-day mortality. For the other secondary outcomes, we used bayesian linear and logistic regression models to calculate the posterior probability of the intervention’s superiority, using uninformative prior distributions. Specifically, we applied flat priors for the model coefficients, allowing the data to drive the estimates without influence from prior assumptions. Posterior probabilities of superiority and the 95% CrIs for the secondary outcomes did not account for multiplicity, and, therefore, should not be used to infer definitive differences in treatment effects. There were no missing data for the primary outcome. The secondary outcomes were analyzed using only patients with available data, without imputation of missing data.
The sensitivity analyses included (1) a per-protocol analysis; (2) an analysis only including the COPD exacerbation with respiratory acidosis and the acute cardiogenic pulmonary edema patient groups and using escalation of ventilatory support as the outcome (escalation from noninvasive ventilation to endotracheal intubation and escalation from high-flow nasal oxygen therapy to noninvasive ventilation or endotracheal intubation); (3) a post hoc analysis with weaker informative priors for all 5 patient groups with acute respiratory failure; and (4) a post hoc analysis of the primary outcome using the same bayesian model structure as the main analysis, but without borrowing.
The cmdstanr version 0.6.1 (Stan Development Team) was used in conjunction with R version 4.3.1 (R Foundation for Statistical Computing).27,28
Results
Patients
Enrollment began in November 2019. A total of 2731 patients with acute respiratory failure were screened, and 1800 were enrolled at 33 sites in Brazil and randomized (Figure 1). Thirty-four patients were excluded after randomization because informed consent was not obtained.
In April 2021 (first interim analysis), enrollment was stopped for futility (defined as a posterior probability of noninferiority <0.30) in the immunocompromised with hypoxemia patient group (eTable 3 in Supplement 1). Enrollment was stopped when the predetermined noninferiority threshold was met in March 2023 (fifth interim analysis) for the hypoxemic COVID-19 patient group and in October 2023 (sixth interim analysis) for the nonimmunocompromised with hypoxemia and the acute cardiogenic pulmonary edema patient groups. Enrollment continued as planned until the final analysis for the COPD exacerbation with respiratory acidosis patient group.
Informed consent was not obtained from 49 patients because they died before their next of kin could provide it. However, the ethics committees at the sites permitted the use of data from 15 of these patients, resulting in a total of 1766 patients (mean age, 63.7 years [SD, 16.5 years]; 40% were women) included in the final analysis (eFigures 2-5 in Supplement 1).
The characteristics at enrollment were comparable across all 5 patient groups with acute respiratory failure (Table 1 and eTables 4-5 in Supplement 1). There were 485 nonimmunocompromised patients (27.5%) with hypoxemia, 50 immunocompromised patients (2.8%) with hypoxemia, 77 patients (4.4%) with COPD exacerbation with respiratory acidosis, 272 patients (15.4%) with acute cardiogenic pulmonary edema, and 882 patients (49.9%) with hypoxemic COVID-19. Forty percent of the patients were randomized and treatment was initiated while they were being treated in the emergency department or hospital ward.
Table 1. Characteristics of the Patients at Baseline.
| Patient groups with acute respiratory failure (ARF) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nonimmunocompromised with hypoxemia |
Immunocompromised with hypoxemia |
COPD exacerbation with respiratory acidosis | Acute cardiogenic pulmonary edema | Hypoxemic COVID-19 | ||||||
| High-flow nasal oxygen (n = 249)a | Noninvasive ventilation (n = 236)b | High-flow nasal oxygen (n = 28)a | Noninvasive ventilation (n = 22)b | High-flow nasal oxygen (n = 35)a | Noninvasive ventilation (n = 42)b | High-flow nasal oxygen (n = 136)a | Noninvasive ventilation (n = 136)b | High-flow nasal oxygen (n = 435)a | Noninvasive ventilation (n = 447)b | |
| Age, mean (SD), y | 66 (17) | 70 (63) | 54 (16) | 53 (13) | 74 (10) | 71 (11) | 71 (13) | 70 (14) | 60 (16) | 60 (16) |
| Sex, No. (%) | ||||||||||
| Female | 115 (46.2) | 105 (44.7) | 12 (42.9) | 7 (31.9) | 15 (42.9) | 27 (64.3) | 66 (48.5) | 68 (50.0) | 140 (32.2) | 152 (34.0) |
| Male | 134 (53.8) | 131 (55.3) | 16 (57.1) | 15 (68.1) | 20 (57.1) | 15 (37.7) | 70 (51.5) | 68 (50.0) | 295 (67.8) | 295 (66.0) |
| Site of inclusion, No. (%)c | ||||||||||
| Intensive care unit | 135 (54.2) | 125 (53.0) | 26 (92.9) | 22 (100.0) | 12 (34.3) | 22 (52.4) | 14 (10.3) | 13 (9.6) | 347 (79.8) | 348 (77.9) |
| Emergency department | 100 (40.2) | 94 (39.8) | 0 | 0 | 20 (57.1) | 19 (45.2) | 120 (88.2) | 120 (88.2) | 46 (10.6) | 53 (11.9) |
| Hospital ward | 14 (5.6) | 17 (7.2) | 2 (7.1) | 0 | 3 (8.6) | 1 (2.4) | 2 (1.5) | 3 (2.2) | 42 (9.7) | 46 (10.3) |
| SAPS 3, median (IQR)d | 60 (54-69) | 60 (53-66) | 65 (60-71) | 63 (57-68) | 63 (57-67) | 58 (51-64) | 58 (54-62) | 59 (53-64) | 55 (50-61) | 55 (50-62) |
| ARF diagnosis to randomization, median (IQR), h | 1 (0-4) | 2 (0-4) | 7 (4-14) | 2 (1-11) | 2 (0-4) | 1 (0-3) | 1 (0-2) | 1 (0-2) | 1 (0-9) | 1 (0-6) |
| Respiratory support before randomization, No. (%)e | ||||||||||
| Low-flow oxygen | 230 (92.4) | 218 (92.4) | 23 (82.1) | 18 (85.7) | 31 (88.6) | 38 (92.7) | 110 (80.9) | 120 (88.2) | 388 (89.4) | 383 (85.7) |
| Noninvasive ventilation | 96 (39.0) | 81 (34.3) | 5 (17.9) | 4 (18.2) | 13 (37.1) | 16 (38.1) | 0 | 1 (0.7) | 111 (25.7) | 129 (28.9) |
| Heart rate, median (IQR), beats/min | 90 (75-107) | 90 (79-108) | 101 (84-115) | 97 (87-110) | 93 (84-104) | 97 (79-109) | 90 (73-105) | 88 (73-107) | 84 (75-96) | 82 (73-95) |
| Mean arterial pressure, median (IQR), mm Hg | 93 (83-103) | 91 (81-102) | 93 (85-101) | 85 (80-94) | 97 (84-104) | 95 (88-107) | 99 (85-112) | 99 (89-115) | 94 (85-103) | 97 (87-104) |
| Respiratory rate, median (IQR), breaths/min | 28 (26-31) | 28 (26-30) | 29 (28-33) | 29 (26-31) | 27 (26-29) | 27 (25-30) | 28 (26-29) | 28 (26-30) | 30 (27-33) | 29 (26-32) |
| Spo2, median (IQR), %f | 86 (82-88) | 86 (82-88) | 86 (83-91) | 88 (84-93) | 83 (80-88) | 85 (81-88) | 88 (85-90) | 88 (85-90) | 85 (80-88) | 85 (81-88) |
| Arterial blood gas levels | ||||||||||
| pH, median (IQR) | 7.41 (7.35-7.45) | 7.41 (7.37-7.46) | 7.42 (7.40-7.47) | 7.42 (7.40-7.44) | 7.32 (7.28-7.34) | 7.30 (7.24-7.36) | 7.39 (7.34-7.44) | 7.38 (7.31-7.42) | 7.44 (7.40-7.46) | 7.44 (7.41-7.46) |
| Pao2, median (IQR), mm Hgf | 72 (58-90) | 71 (60-86) | 82 (55-106) | 68 (62-89) | 61 (52-93) | 80 (60-114) | 80 (62-106) | 79 (60-107) | 72 (61-91) | 74 (60-92) |
| Paco2, median (IQR), mm Hg | 37 (31-45) | 38 (31-44) | 31 (28-38) | 32 (28-38) | 55 (46-73) | 64 (51-73) | 37 (31-43) | 40 (34-47) | 36 (32-39) | 35 (31-39) |
| Pao2:Fio2, median (IQR)g | 191 (127-262) | 194 (136-261) | 181 (115-220) | 212 (150-281) | 189 (143-290) | 209 (163-308) | 247 (167-340) | 225 (156-346) | 150 (108-208) | 153 (112-217) |
| Comorbidities, No. (%)c | ||||||||||
| Arterial hypertension | 127 (51.0) | 122 (51.7) | 11 (39.3) | 5 (22.7) | 21 (60.0) | 22 (52.4) | 104 (76.5) | 117 (86.0) | 190 (43.7) | 195 (43.6) |
| Diabetes | 81 (32.5) | 68 (28.8) | 3 (10.7) | 2 (9.1) | 12 (34.3) | 13 (31.0) | 64 (47.1) | 69 (50.7) | 111 (25.5) | 108 (24.2) |
| COPD | 70 (28.1) | 65 (27.5) | 2 (7.1) | 3 (13.6) | 35 (100.0) | 42 (100.0) | 26 (19.1) | 33 (24.3) | 14 (3.2) | 22 (4.9) |
| Heart failure | 44 (17.7) | 53 (22.5) | 0 | 2 (9.1) | 9 (25.7) | 8 (19.0) | 72 (52.9) | 80 (58.8) | 26 (6.0) | 22 (4.9) |
| Chronic kidney failure | 29 (11.6) | 19 (8.1) | 1 (3.6) | 3 (13.6) | 2 (5.7) | 2 (4.8) | 23 (16.9) | 37 (27.2) | 29 (6.7) | 36 (8.1) |
| Solid tumors | 21 (8.4) | 15 (6.4) | 9 (32.1) | 11 (50.0) | 0 | 2 (4.8) | 3 (2.2) | 3 (2.2) | 15 (3.4) | 12 (2.7) |
| Hematologic malignancies | 2 (0.8) | 1 (0.4) | 5 (17.9) | 3 (13.6) | 1 (2.9) | 0 | 0 | 0 | 6 (1.4) | 4 (0.9) |
Abbreviations: COPD, chronic obstructive pulmonary disease; Fio2, fraction of inspired oxygen; SAPS 3, Simplified Acute Physiology Score 3; Spo2, oxygen saturation as measured by pulse oximetry.
Delivered through a nasal cannula with larger-bore tubing.
Noninvasive positive-pressure ventilation delivered through a face mask using either a ventilator in the intensive care unit or a bilevel device.
Percentages may not total 100 because of rounding.
Calculated from 20 variables at enrollment, information about previous health status, and information obtained at admission. Scores range from 0 to 217 points; higher scores indicate a greater severity of illness. A score between 55 and 65 indicates a moderate to high severity of illness, suggesting a mortality risk between 30% and 40%.
A patient may have received both types.
The timing of these measurements was different. The level of Spo2 was measured at room air and arterial blood gas was collected under oxygen delivered via low-flow oxygen cannula, nonrebreathing face mask, or noninvasive ventilation.
The level of Fio2 was determined using the following calculation: (oxygen flow in L/min) × 0.03 + 0.21.
Interventions
The allocated intervention was received by 93.3% of patients (824/883) in the high-flow nasal oxygen treatment group and by 91.5% of patients (808/883) in the noninvasive ventilation treatment group. There was a lower duration of therapy use within the first 48 hours in the noninvasive ventilation group (Table 2). Among patients assigned to high-flow nasal oxygen, the use of noninvasive ventilation was less than 10% in 4 of the 5 patient groups with acute respiratory failure; however, the use of noninvasive ventilation was 22.9% in the COPD exacerbation with respiratory acidosis patient group. The ventilatory settings for both high-flow nasal oxygen and noninvasive ventilation are described in eTable 6 in Supplement 1.
Table 2. Adherence to Trial Therapy and Co-Interventions.
| Patient groups with acute respiratory failure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nonimmunocompromised with hypoxemia |
Immunocompromised with hypoxemia |
COPD exacerbation with respiratory acidosis | Acute cardiogenic pulmonary edema | Hypoxemic COVID-19 | ||||||
| High-flow nasal oxygen (n = 249)a | Noninvasive ventilation (n = 236)b | High-flow nasal oxygen (n = 28)a | Noninvasive ventilation (n = 22)b | High-flow nasal oxygen (n = 35)a | Noninvasive ventilation (n = 42)b | High-flow nasal oxygen (n = 136)a | Noninvasive ventilation (n = 136)b | High-flow nasal oxygen (n = 435)a | Noninvasive ventilation (n = 447)b | |
| Trial respiratory support during first 3 d | ||||||||||
| Received assigned therapy, No. (%) | 223 (89.6) | 205 (86.9) | 26 (92.9) | 20 (90.9) | 30 (85.7) | 37 (88.1) | 130 (95.6) | 131 (96.3) | 415 (95.4) | 415 (92.8) |
| Time receiving therapy, median (IQR), d | 2 (1-3) | 1 (1-3) | 3 (1-3) | 2 (1-3) | 1 (1-3) | 1.5 (1-3) | 1 (1-2) | 1 (1-2) | 3 (1-3) | 2 (1-3) |
| Crossed over to another therapy, No. (%)c | 23 (9.2) | 5 (2.1) | 1 (3.6) | 0 | 8 (22.9) | 0 | 7 (5.1) | 0 | 39 (9.0) | 34 (7.6) |
| Trial respiratory support on day 1 | ||||||||||
| Received assigned therapy, No. (%) | 221 (88.9) | 203 (86.0) | 26 (92.9) | 20 (90.9) | 30 (85.7) | 37 (88.1) | 129 (94.9) | 130 (95.6) | 415 (95.4) | 409 (91.5) |
| Duration of use, median (IQR), h | 24 (9-24) | 10 (4-17) | 19 (12-24) | 10 (3-21) | 21 (7-24) | 12 (6-17) | 13 (7-24) | 11 (6-16) | 24 (12-24) | 13 (6-22) |
| Trial respiratory support on day 2 | ||||||||||
| Received assigned therapy, No./total (%) | 138/191 (72.3) | 114/184 (62.0) | 20/21 (95.2) | 14/18 (77.8) | 17/25 (68.0) | 21/35 (60) | 46/121 (38) | 50/115 (43.5) | 309/331 (93.4) | 289/354 (81.6) |
| Duration of use, median (IQR), h | 24 (22-24) | 6 (2-13) | 24 (23-24) | 10 (4-12) | 24 (18-24) | 4 (2-14) | 24 (10-24) | 2 (1-10) | 24 (22-24) | 11 (5-16) |
| Trial respiratory support on day 3 | ||||||||||
| Received assigned therapy, No./total (%) | 108/178 (60.7) | 82/161 (50.9) | 16/17 (94.1) | 8/14 (57.1) | 13/24 (54.2) | 13/30 (43.0) | 28/107 (26.2) | 30/106 (28.3) | 246/288 (85.0) | 212/291 (72.9) |
| Duration of use, median (IQR), h | 24 (20-24) | 3.2 (2.0-8.1) | 24.0 (22.5-24.0) | 5.5 (2.0-7.2) | 21.9 (4.2-24.0) | 3 (1.7-6.0) | 24.0 (19.2-24.0) | 2.0 (1.5-3.0) | 24.0 (24.0-24.0) | 8.4 (3.3-13.3) |
| Type of co-intervention within 7 d, No. (%) | ||||||||||
| Vasopressors or inotropes | 79 (31.7) | 63 (26.7) | 12 (42.9) | 6 (27.3) | 7 (20.0) | 10 (23.8) | 65 (47.8) | 75 (55.1) | 144 (33.1) | 155 (34.7) |
| Dexmedetomidine | 32 (12.9) | 44 (18.6) | 3 (10.7) | 4 (18.2) | 4 (11.4) | 9 (21.4) | 13 (9.6) | 11 (8.1) | 57 (13.1) | 117 (26.2) |
Abbreviation: COPD, chronic obstructive pulmonary disease.
Delivered through a nasal cannula with larger-bore tubing.
Noninvasive positive-pressure ventilation delivered through a face mask using either a ventilator in the intensive care unit or a bilevel device. If needed, standard oxygen therapy could be given through a nonrebreather face mask or low-flow nasal cannula between sessions in patients with device intolerance or agitation at a flow rate varying from 2 to 10 L/min.
If there was a worsening of respiratory failure, the trial protocol recommended noninvasive ventilation for patients with COPD exacerbation and for those with acute cardiogenic pulmonary edema. The use of noninvasive ventilation was not a protocol violation in these patients.
Primary Outcome
The primary outcome of endotracheal intubation or death within 7 days occurred in 39.0% of patients (344 of 883) in the high-flow nasal oxygen group vs 38.1% of patients (336 of 883) in the noninvasive ventilation group. High-flow nasal oxygen was noninferior to noninvasive ventilation in the patient groups of nonimmunocompromised with hypoxemia, COPD exacerbation with respiratory acidosis, acute cardiogenic pulmonary edema, and hypoxemic COVID-19 (Figure 2 and eFigure 6 in Supplement 1). Among nonimmunocompromised patients with hypoxemia, the primary outcome occurred in 32.5% of patients in the high-flow nasal oxygen group vs 33.1% of patients in the noninvasive ventilation group (OR, 1.02 [95% CrI, 0.81-1.26]; noninferiority posterior probability, 0.999).
Figure 2. Primary Outcome of Endotracheal Intubation or Death Within 7 Days.
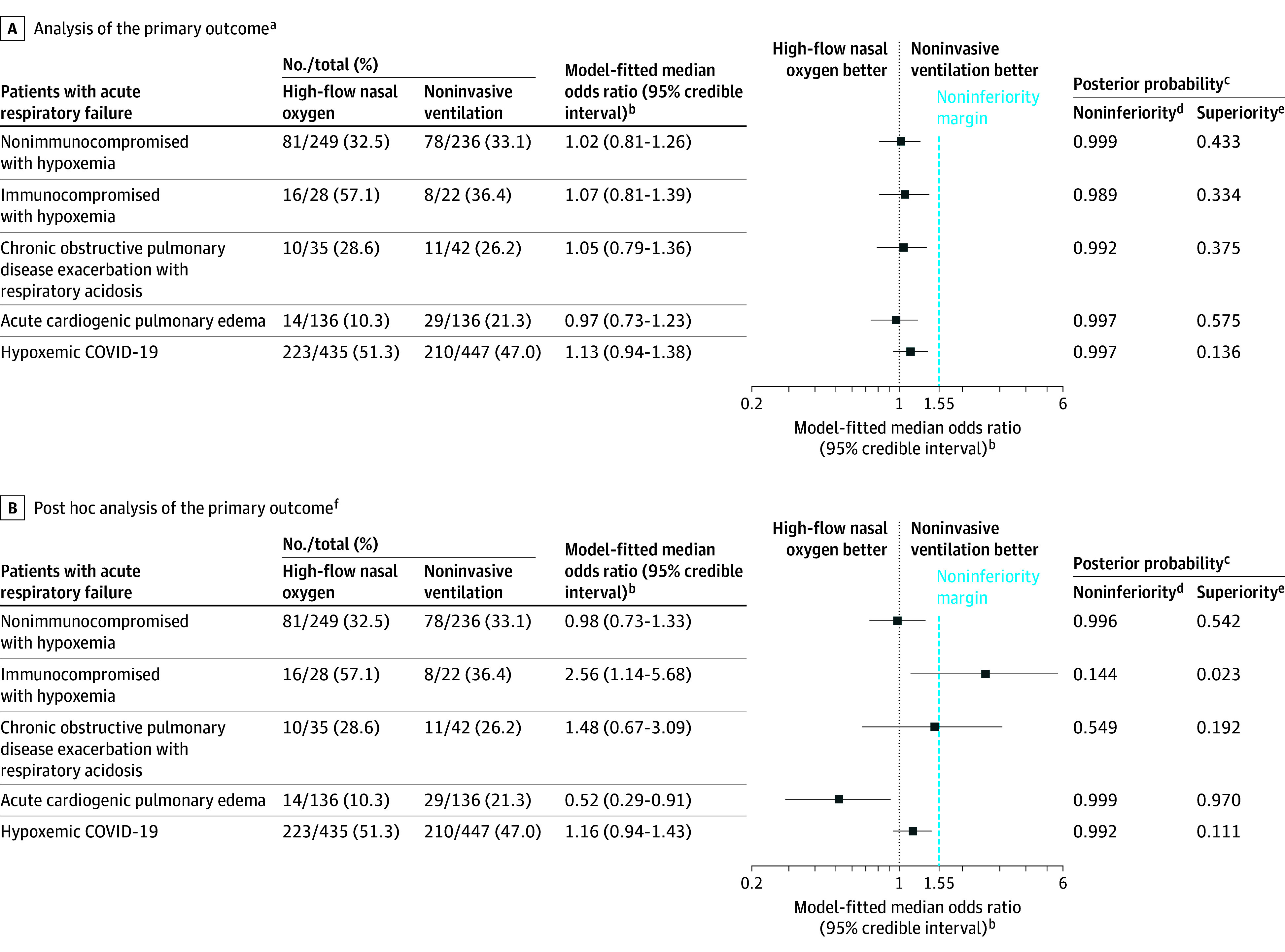
aIncludes all randomized patients with informed consent. The primary outcome was analyzed with a bayesian hierarchical modeling with dynamic borrowing across the 5 patient groups with acute respiratory failure. More borrowing occurs when the groups are consistent, and less borrowing occurs when the groups differ. Borrowing via a hierarchical model is a type of shrinkage estimation (it provides a formal mechanism by which extreme observations are shrunk toward the mean). The model is a compromise between the extremes of a completely pooled analysis as opposed to a separate analysis in each group.
bOdds of requiring endotracheal intubation or dying within 7 days in the high-flow nasal oxygen group vs the noninvasive ventilation group.
cA bayesian approach based on posterior probabilities was used to test the noninferiority and superiority hypotheses with predefined thresholds.
dDefined as a posterior probability greater than 0.992 that the odds ratio was less than 1.55. For each patient group with acute respiratory failure, noninferiority was declared if the posterior probability was greater than 0.992. If noninferiority was not demonstrated, the final result was futility.
eDefined as a posterior probability greater than 0.992 that the odds ratio was less than 1. If noninferiority was demonstrated, then superiority was declared if the superiority posterior probability was also higher than 0.992.
fThe same bayesian model structure was used as in the primary analysis, but without borrowing. Although borrowing can improve precision under the assumption of similar treatment effects, it could also produce biased estimates when there is heterogeneity across groups.
Among patients with COPD exacerbation with respiratory acidosis, the primary outcome occurred in 28.6% of patients in the high-flow nasal oxygen group vs 26.2% of patients in the noninvasive ventilation group (OR, 1.05 [95% CrI, 0.79-1.36]; noninferiority posterior probability, 0.992). Among patients with acute cardiogenic pulmonary edema, the primary outcome occurred in 10.3% of patients in the high-flow nasal oxygen group vs 21.3% of patients in the noninvasive ventilation group (OR, 0.97 [95% CrI, 0.73-1.23]; noninferiority posterior probability, 0.997).
Among patients with hypoxemic COVID-19, the primary outcome occurred in 51.3% of patients in the high-flow nasal oxygen group vs 47.0% of patients in the noninvasive ventilation group (OR, 1.13 [95% CrI, 0.94-1.38]; noninferiority posterior probability, 0.997). Among immunocompromised patients with hypoxemia, the primary outcome occurred in 57.1% of patients in high-flow nasal oxygen group vs 36.4% of patients in noninvasive ventilation group (OR, 1.07 [95% CrI, 0.81-1.39]; noninferiority posterior probability, 0.989), which met the criterion for futility. Summary of the individualized components of primary outcome and reasons for endotracheal intubation appear in Table 3 and in eTable 7 in Supplement 1.
Table 3. Primary and Secondary Outcomes.
| Patient groups with acute respiratory failure | No. of events/total No. of patients (%) | Effect estimate (95% credible interval) | Posterior probability of superioritya | |||
|---|---|---|---|---|---|---|
| High-flow nasal oxygen | Noninvasive ventilation | Absolute | Relative | |||
| Nonimmunocompromised with hypoxemia | ||||||
| Primary outcome of endotracheal intubation or death within 7 d | 81/250 (32.4) | 78/236 (33.1) | MD, 0.5 (−4.5 to 4.6) | MOR, 1.02 (0.81 to 1.26) | 0.687 | |
| Individual components of primary outcome | ||||||
| Endotracheal intubation within 7 d | 78/250 (31.2) | 69/236 (29.2) | ||||
| Death within 7 d | 30/250 (12.0) | 29/236 (12.3) | ||||
| Secondary outcomesb | ||||||
| 28-d mortality | 61/249 (24.5) | 67/236 (28.4) | MD, −1.1 (−5.5 to 2.9)c | MOR, 0.94 (0.74 to 1.17) | 0.687 | |
| 90-d mortality | 74/249 (29.7) | 80/236 (33.9) | MD, −4.1 (−12.3 to 3.9) | MHR, 0.86 (0.61 to 1.15) | 0.826 | |
| No. of ICU-free days at 28 d, median (IQR), d [No.]d | 19 (−1 to 24) [240] | 21 (−1 to 24) [231] | MD, 0 (−2 to 2.8) | MPOR,1.00 (0.69 to 1.34) | 0.496 | |
| No. of ventilator-free days at 28 d, median (IQR), d [No.]d | 28 (−1 to 28) [242] | 28 (−1 to 28) [231] | MD, 0.9 (−2.31 to 4.1) | MPOR, 1.16 (0.8 to 1.63) | 0.795 | |
| Immunocompromised with hypoxemia | ||||||
| Primary outcome of endotracheal intubation or death within 7 d | 16/28 (57.1) | 8/22 (36.4) | MD, 1.4 (−4.9 to 7.5) | MOR, 1.07 (0.81 to 1.39) | 0.526 | |
| Individual components of primary outcome | ||||||
| Endotracheal intubation within 7 d | 14/28 (50.0) | 7/22 (31.8) | ||||
| Death within 7 d | 5/28 (17.9) | 3/22 (13.6) | ||||
| Secondary outcomesb | ||||||
| 28-d mortality | 15/28 (53.6) | 10/22 (45.5) | MD, −0.2 (−6.3 to 6.0)c | MOR, 0.99 (0.76 to 1.30) | 0.523 | |
| 90-d mortality | 17/28 (60.7) | 13/22 (59.1) | MD, 1.6 (24.9 to 27.1) | MHR, 1.29 (0.47 to 2.41) | 0.242 | |
| No. of ICU-free days at 28 d, median (IQR), d [No.]d | −1 (−1 to 19) [27] | 13 (−1 to 24) [22] | MD, −4.2 (−12.4 to 4.6) | MPOR, 0.48 (0.11 to 1.25) | 0.102 | |
| No. of ventilator-free days at 28 d, median (IQR), d [No.]d | −1 (−1 to 28) [28] | 26 (−1 to 28) [22] | MD, −4.0 (−13.9 to 6.2) | MPOR, 0.56 (0.11 to 1.38) | 0.136 | |
| Chronic obstructive pulmonary disease exacerbation with respiratory acidosis | ||||||
| Primary outcome of endotracheal intubation or death within 7 d | 10/35 (28.6) | 11/42 (26.2) | MD, 0.8 (−4.0 to 5.3) | MOR, 1.05 (0.79 to 1.36) | 0.546 | |
| Individual components of primary outcome | ||||||
| Endotracheal intubation within 7 d | 10/35 (28.6) | 10/42 (23.8) | ||||
| Death within 7 d | 3/35 (8.6) | 1/42 (2.4) | ||||
| Secondary outcomesb | ||||||
| 28-d mortality | 8/35 (22.9) | 8/42 (19.0) | MD, −0.2 (−4.0 to 3.7)c | MOR, 0.98 (0.75 to 1.29) | 0.546 | |
| 90-d mortality | 11/35 (31.4) | 13/42 (31.0) | MD, 0.5 (−20.1 to 20.8) | MHR, 1.09 (0.39 to 2.24) | 0.416 | |
| No. of ICU-free days at 28 d, median (IQR), d [No.]d | 22 (0 to 28) [33] | 22 (12 to 25) [39] | MD, 0.2 (−6.6 to 7.1) | MPOR, 1.05 (0.33 to 2.23) | 0.541 | |
| No. of ventilator-free days at 28 d, median (IQR), d [No.]d | 28 (0 to 28) [33] | 28 (20 to 28) [39] | MD, −1.0 (−8.4 to 6.2) | MPOR, 0.82 (0.22 to 1.93) | 0.360 | |
| Acute cardiogenic pulmonary edema | ||||||
| Primary outcome of endotracheal intubation or death within 7 d | 14/136 (10.3) | 29/136 (21.3) | MD, −0.3 (−3.7 to 2.4) | MOR, 0.97 (0.73 to 1.23) | 0.608 | |
| Individual components of primary outcome | ||||||
| Endotracheal intubation within 7 d | 12/136 (8.8) | 27/136 (19.9) | ||||
| Death within 7 d | 7/136 (5.1) | 16/136 (11.8) | ||||
| Secondary outcomesb | ||||||
| 28-d mortality | 19/136 (14.0) | 24/136 (17.6) | MD, −0.5 (−4.0 to 3.2)c | MOR, 0.97 (0.74 to 1.40) | 0.608 | |
| 90-d mortality | 23/136 (16.9) | 30/136 (22.1) | MD, −5.1 (−14.7 to 4.3) | MHR, 0.73 (0.38 to 1.18) | 0.872 | |
| No. of ICU-free days at 28 d, median (IQR), d [No.]d | 28 (20 to 28) [131] | 28 (17 to 28) [132] | 0.7 (−2.0 to 3.3) | MPOR, 1.19 (0.68 to 1.80) | 0.749 | |
| No. of ventilator-free days at 28 d, median (IQR), d [No.]d | 28 (28 to 28) [132] | 28 (26 to 28) [132] | 2.2 (−0.8 to 5.5) | MPOR, 1.85 (0.95 to 3.17) | 0.983 | |
| Hypoxemic COVID-19 | ||||||
| Primary outcome of endotracheal intubation or death within 7 d | 223/435 (51.3) | 210/437 (48.1) | MD, 3.1 (−1.5 to 7.9) | MOR, 1.13 (0.94 to 1.38) | 0.568 | |
| Individual components of primary outcome | ||||||
| Endotracheal intubation within 7 d | 219/435 (50.3) | 209/437 (47.8) | ||||
| Death within 7 d | 34/435 (7.8) | 17/437 (3.9) | ||||
| Secondary outcomesb | ||||||
| 28-d mortality | 97/435 (22.3) | 102/447 (22.8) | MD, −0.4 (−4.0 to 3.3)e | MOR, 0.98 (0.79 to 1.21) | 0.568 | |
| 90-d mortality | 126/435 (29.0) | 129/447 (28.9) | MD, 0.2 (−5.7 to 6.1) | MHR, 0.98 (0.76 to 1.25) | 0.560 | |
| No. of ICU-free days at 28 d, median (IQR), d [No.]d | 15 (0 to 21) [434] | 17 (0 to 22) [439] | MD, −1.0 (−3.1 to 1.1) | MPOR, 0.85 (0.67 to 1.06) | 0.840 | |
| No. of ventilator-free days at 28 d, median (IQR), d [No.]d | 24 (1 to 28) [434] | 28 (0 to 28) [441] | MD, −0.4 (−2.8 to 1.9) | MPOR, 0.94 (0.75 to 1.19) | 0.310 | |
Abbreviations: ICU, intensive care unit; MD, median difference; MHR, median hazard ratio; MPOR, median proportional odds ratio.
Superiority is declared if the posterior probability is greater than 0.992.
The secondary outcomes are inferences based on a bayesian linear and logistic regression model; however, 28-day mortality is based on a bayesian model with the same structure as the primary outcome with some modification of the prior distributions to reflect the different rates of 28-day mortality within the groups without consideration of the noninferiority margin. Borrowing of information between groups was present.
The model-fitted rate differences were calculated by subtracting the rate for patients who received noninvasive ventilation from the rate for those who received high-flow nasal oxygen.
Defined as the number of days without invasive mechanical ventilation or days outside the ICU. For patients who died, a value of −1 day was assigned.
Time-averaged rate that was weighted by the number of COVID-19 patients enrolled over time.
Other Outcomes
The secondary outcomes, including median 28-day mortality rates, were not different between the 2 treatment groups for any of the 5 patient groups with acute respiratory failure (Table 3). The tertiary outcome of comfort was superior with high-flow nasal oxygen vs noninvasive ventilation (eTable 8 in Supplement 1). Other outcomes appear in eTable 9 in Supplement 1.
Sensitivity Analysis
The results for the posterior probabilities of noninferiority were similar to those of the main analysis for the per-protocol population and the post hoc sensitivity analysis with weakly informative priors (eTables 10-11 in Supplement 1).
The post hoc sensitivity analysis of the primary outcome without borrowing appears in Figure 2. Among nonimmunocompromised patients with hypoxemia, the OR was 0.98 (95% CrI, 0.73-1.33; noninferiority posterior probability, 0.996). Among immunocompromised patients with hypoxemia, the OR was 2.56 (95% CrI, 1.14-5.68; noninferiority posterior probability, 0.144).
Among patients with COPD exacerbation with respiratory acidosis, the OR was 1.48 (95% CrI, 0.67-3.09; noninferiority posterior probability, 0.549). Among patients with acute cardiogenic pulmonary edema, the OR was 0.52 (95% CrI, 0.29-0.91; noninferiority posterior probability, 0.999; superiority posterior probability, 0.970). Among patients with hypoxemic COVID-19, the OR was 1.16 (95% CrI, 0.94-1.43; noninferiority posterior probability, 0.992). The need for respiratory support escalation among patients with COPD exacerbation or acute cardiogenic pulmonary edema appears in eTable 12 in Supplement 1.
Adverse Events
The incidence of serious adverse events was similar between treatment groups (9.4% of patients in the high-flow nasal oxygen group vs 9.9% of patients in the noninvasive ventilation group; eTable 13 in Supplement 1).
Discussion
This multicenter, adaptive, randomized clinical trial used a bayesian hierarchical model with dynamic borrowing across groups and found that high-flow nasal oxygen was noninferior to noninvasive ventilation with respect to endotracheal intubation or death within 7 days for the following patient populations with acute respiratory failure: nonimmunocompromised with hypoxemia, COPD exacerbation with respiratory acidosis, acute cardiogenic pulmonary edema, and hypoxemic COVID-19. Noninferiority of high-flow nasal oxygen compared with noninvasive ventilation was not shown among immunocompromised patients with hypoxemic acute respiratory failure.
In the FLORALI trial,16 there was no significant difference in intubation rates among patients with non–hypercapnic acute hypoxemic respiratory failure who were treated with high-flow nasal oxygen (n = 106) vs noninvasive ventilation (n = 110), although 90-day mortality was lower in the high-flow nasal oxygen group. However, for patients with hypoxemic acute respiratory failure due to COVID-19, the effectiveness of high-flow nasal oxygen was uncertain compared with noninvasive ventilation.9,29
The current study included 485 nonimmunocompromised patients with acute hypoxemic respiratory failure and 881 patients with acute hypoxemic respiratory failure due to COVID-19 and showed noninferiority of high-flow nasal oxygen compared with noninvasive ventilation in both these patient groups. The results of a post hoc analysis without borrowing were also consistent with noninferiority of high-flow nasal oxygen for these patient groups (nonimmunocompromised with hypoxemia and hypoxemic COVID-19). In addition, there were no significant differences in 90-day mortality or in any secondary outcomes.
Among patients with COPD exacerbation with respiratory acidosis or acute cardiogenic pulmonary edema, previous evidence supported noninvasive ventilation as the recommended respiratory support modality.12,30,31 However, these recommendations were based on studies comparing noninvasive ventilation with low-flow oxygen.12 Although the results from the primary analysis in the current study demonstrated noninferiority of high-flow nasal oxygen compared with noninvasive ventilation for the COPD exacerbation with respiratory acidosis patient group, the post hoc analysis without borrowing produced discrepant results; the 95% CrI included ORs as high as 3.09, suggesting potential harm with use of high-flow nasal oxygen.
Among the patients with COPD or acute cardiogenic pulmonary edema assigned to high-flow nasal oxygen who showed signs of worsening respiratory failure, the trial protocol recommended escalation to noninvasive ventilation. Notably, 23% of patients with COPD initially assigned to high-flow nasal oxygen required rescue noninvasive ventilation. Furthermore, the patients with acute cardiogenic pulmonary edema who needed noninvasive ventilation before randomization were excluded from the trial. Therefore, the results from the current trial may not be generalizable to patients with more severe forms of acute cardiogenic pulmonary edema.
There were no significant differences in 28-day or 90-day mortality between the 2 treatment groups within any of the 5 acute respiratory failure patient groups. However, overall mortality was higher compared with other studies.16,18,23,32 Several factors may explain this elevated mortality. First, mortality among critically ill patients has generally been higher in Brazil than in high-income countries.33,34,35 Second, during the COVID-19 pandemic, patients tended to avoid leaving home, so only more severe cases sought hospital care.36 Third, the inclusion criteria for patients with COPD exacerbation required respiratory acidosis, thereby selecting for more severe cases.
The strengths of the current trial include its generalizability because it included a broad population of patients with acute respiratory failure. At the same time, the results are specific to each of the 5 patient groups with acute respiratory failure studied. The adaptive bayesian design allowed dynamic borrowing of information across patient groups, which leverages the information that arises from the evaluation of the treatments in multiple and related disease categories. This dynamic borrowing increases the precision of the estimates and includes information not available with simpler models without borrowing, and may be useful when the assumption of similarity of treatment effects across groups is appropriate.
Limitations
The current trial has limitations. First, enrollment in the immunocompromised with hypoxemia patient group was stopped early for futility after the first interim analysis. The futility stopping criterion in this analysis may have been set too high (a posterior probability of noninferiority below 30%). If a lower threshold (eg, 10%) had been selected, enrollment in this group might have continued, potentially resulting in a different conclusion. As a result, the validity of effect estimates in the immunocompromised with hypoxemia patient group is limited by the small sample size. In addition, patients with COVID-19 were not classified into a separate group before March 2023, and some of the patients included in the interim analysis for the immunocompromised with hypoxemia patient group had COVID-19. Future trials comparing high-flow nasal oxygen vs noninvasive ventilation in immunocompromised patients with acute hypoxemic respiratory failure are warranted.
Second, although the COVID-19 pandemic led to a protocol amendment that categorized patients with COVID-19 as a separate group, a blinded steering committee made these adjustments. Third, the effect estimates relied on borrowing across patient groups, which assumes some degree of similarity in underlying treatment effects. A post hoc sensitivity analysis without borrowing showed substantial deviations from the main model, particularly in the COPD and the immunocompromised with hypoxemia patient groups. However, an analysis without borrowing loses information and results in a systematic overestimate of the variability of treatment effects across patient groups.
Fourth, the comparator intervention was noninvasive ventilation administered through a face mask; thus, our results do not address comparisons of high-flow nasal oxygen with continuous positive airway pressure or with helmet-delivered noninvasive ventilation.
Conclusions
Compared with noninvasive ventilation, high-flow nasal oxygen met prespecified criteria for noninferiority for the primary outcome of endotracheal intubation or death within 7 days in 4 of the 5 patient groups with acute respiratory failure. However, the small sample sizes in some patient groups and the sensitivity of the findings to the choice of analysis model suggests the need for further study in patients with COPD, immunocompromised patients, and patients with acute cardiogenic pulmonary edema.
Section Editor: Christopher Seymour, MD, Associate Editor, JAMA (christopher.seymour@jamanetwork.org).
eResults
eMethods
Committee information
eFigure 1. Rescue respiratory support strategy for all acute respiratory failure groups
eFigure 2. Hypoxemic non-immunocompromised acute respiratory failure group flowchart
eFigure 3. Hypoxemic immunocompromised acute respiratory failure group flowchart
eFigure 4. Acute cardiogenic pulmonary edema group flowchart
eFigure 5. COPD exacerbation group flowchart
eFigure 6. Model-fitted median rate differences of endotracheal intubation or death in 7 days in the intention to treat population
eTable 1. Simulation Results for Futile, Noninferiority and Superiority for the Null Noninferiority Scenario
eTable 2. Simulation results for Futile, Noninferiority and Superiority for the Null Superiority Scenario eResults
eTable 3. Results of posterior probabilities for superiority and noninferiority in the interim and final analysis
eTable 4. Patients’ baseline characteristics – without segregation into acute respiratory groups
eTable 5. Causes of immunosuppression in hypoxemic immunocompromised group
eTable 6. High-flow nasal oxygen and noninvasive ventilation settings
eTable 7. Summary of reason for intubation within 7 days according to protocol
eTable 8. Patient Comfort Score in the First 12 hours after Randomization in the Intention-to-Treat Population
eTable 9. Tertiary Outcomes
eTable 10. Sensitivity analysis of treatment effects on the primary in the per protocol population
eTable 11. Sensitivity Analysis using less informative priors for the primary outcome in the intention-to-treat population
eTable 12. Sensitivity Analysis of respiratory support escalation rates in COPD and in acute cardiogenic pulmonary edema
eTable 13. Adverse events
Trial protocol
Statistical analysis plan
Nonauthor collaborators
Data sharing statement
References
- 1.Munshi L, Mancebo J, Brochard LJ. Noninvasive respiratory support for adults with acute respiratory failure. N Engl J Med. 2022;387(18):1688-1698. doi: 10.1056/NEJMra2204556 [DOI] [PubMed] [Google Scholar]
- 2.Carron M, Freo U, BaHammam AS, et al. Complications of non-invasive ventilation techniques: a comprehensive qualitative review of randomized trials. Br J Anaesth. 2013;110(6):896-914. doi: 10.1093/bja/aet070 [DOI] [PubMed] [Google Scholar]
- 3.Mauri T, Turrini C, Eronia N, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017;195(9):1207-1215. doi: 10.1164/rccm.201605-0916OC [DOI] [PubMed] [Google Scholar]
- 4.Cortegiani A, Crimi C, Noto A, et al. Effect of high-flow nasal therapy on dyspnea, comfort, and respiratory rate. Crit Care. 2019;23(1):201. doi: 10.1186/s13054-019-2473-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortegiani A, Longhini F, Madotto F, et al. ; H. F.-AECOPD study investigators . High flow nasal therapy versus noninvasive ventilation as initial ventilatory strategy in COPD exacerbation: a multicenter non-inferiority randomized trial. Crit Care. 2020;24(1):692. doi: 10.1186/s13054-020-03409-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan D, Wang B, Cao P, et al. High flow nasal cannula oxygen therapy versus non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease with acute-moderate hypercapnic respiratory failure: a randomized controlled non-inferiority trial. Crit Care. 2024;28(1):250. doi: 10.1186/s13054-024-05040-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roca O, Pérez-Terán P, Masclans JR, et al. Patients with New York Heart Association class III heart failure may benefit with high flow nasal cannula supportive therapy: high flow nasal cannula in heart failure. J Crit Care. 2013;28(5):741-746. doi: 10.1016/j.jcrc.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 8.Marjanovic N, Piton M, Lamarre J, et al. High-flow nasal cannula oxygen versus noninvasive ventilation for the management of acute cardiogenic pulmonary edema: a randomized controlled pilot study. Eur J Emerg Med. 2024;31(4):267-275. doi: 10.1097/MEJ.0000000000001128 [DOI] [PubMed] [Google Scholar]
- 9.Oczkowski S, Ergan B, Bos L, et al. ERS clinical practice guidelines: high-flow nasal cannula in acute respiratory failure. Eur Respir J. 2022;59(4):2101574. doi: 10.1183/13993003.01574-2021 [DOI] [PubMed] [Google Scholar]
- 10.Telias I, Brochard LJ, Gattarello S, et al. The physiological underpinnings of life-saving respiratory support. Intensive Care Med. 2022;48(10):1274-1286. doi: 10.1007/s00134-022-06749-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berbenetz N, Wang Y, Brown J, et al. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev. 2019;4(4):CD005351. doi: 10.1002/14651858.CD005351.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50(2):1602426. doi: 10.1183/13993003.02426-2016 [DOI] [PubMed] [Google Scholar]
- 13.Grasselli G, Calfee CS, Camporota L, et al. ; European Society of Intensive Care Medicine Taskforce on ARDS . ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023;49(7):727-759. doi: 10.1007/s00134-023-07050-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ospina-Tascón GA, Calderón-Tapia LE, García AF, et al. ; HiFLo-Covid Investigators . Effect of high-flow oxygen therapy vs conventional oxygen therapy on invasive mechanical ventilation and clinical recovery in patients with severe COVID-19: a randomized clinical trial. JAMA. 2021;326(21):2161-2171. doi: 10.1001/jama.2021.20714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreyro BL, Angriman F, Munshi L, et al. Noninvasive oxygenation strategies in adult patients with acute respiratory failure: a protocol for a systematic review and network meta-analysis. Syst Rev. 2020;9(1):95. doi: 10.1186/s13643-020-01363-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frat JP, Thille AW, Mercat A, et al. ; FLORALI Study Group; REVA Network . High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185-2196. doi: 10.1056/NEJMoa1503326 [DOI] [PubMed] [Google Scholar]
- 17.Lewis SR, Baker PE, Parker R, Smith AF. High-flow nasal cannulae for respiratory support in adult intensive care patients. Cochrane Database Syst Rev. 2021;3(3):CD010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perkins GD, Ji C, Connolly BA, et al. ; RECOVERY-RS Collaborators . Effect of noninvasive respiratory strategies on intubation or mortality among patients with acute hypoxemic respiratory failure and COVID-19: the RECOVERY-RS randomized clinical trial. JAMA. 2022;327(6):546-558. doi: 10.1001/jama.2022.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rochwerg B, Einav S, Chaudhuri D, et al. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med. 2020;46(12):2226-2237. doi: 10.1007/s00134-020-06312-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maia IS, Kawano-Dourado L, Zampieri FG, et al. ; RENOVATE Investigators and the BRICNet . High flow nasal catheter therapy versus non-invasive positive pressure ventilation in acute respiratory failure (RENOVATE trial): protocol and statistical analysis plan. Crit Care Resusc. 2023;24(1):61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maia IS, Kawano-Dourado L, Damiani LP, Fitzgerald M, Lewis RJ, Cavalcanti AB. Update in statistical analysis plan of the RENOVATE trial. Crit Care Resusc. 2023;25(3):113-114. doi: 10.1016/j.ccrj.2023.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human participants. JAMA. Published online October 19, 2024. doi: 10.1001/jama.2024.21972 [DOI] [PubMed] [Google Scholar]
- 23.Azoulay E, Lemiale V, Mokart D, et al. Effect of high-flow nasal oxygen vs standard oxygen on 28-day mortality in immunocompromised patients with acute respiratory failure: the HIGH randomized clinical trial. JAMA. 2018;320(20):2099-2107. doi: 10.1001/jama.2018.14282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu YJ, Zhao J, Tang H. Non-invasive ventilation in acute respiratory failure: a meta-analysis. Clin Med (Lond). 2016;16(6):514-523. doi: 10.7861/clinmedicine.16-6-514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones B, Jarvis P, Lewis JA, Ebbutt AF. Trials to assess equivalence: the importance of rigorous methods. BMJ. 1996;313(7048):36-39. doi: 10.1136/bmj.313.7048.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saville BR, Berry DA, Berry NS, Viele K, Berry SM. The Bayesian time machine: accounting for temporal drift in multi-arm platform trials. Clin Trials. 2022;19(5):490-501. [DOI] [PubMed] [Google Scholar]
- 27.R Foundation for Statistical Computing . A Language and Environment for Statistical Computing [computer program]. R Foundation for Statistical Computing; 2024.
- 28.Stan Development Team . Stan Modeling Language Users Guide and Reference Manual version 2.35 [computer program]. Accessed November 25, 2024. https://mc-stan.org
- 29.Pisciotta W, Passannante A, Arina P, Alotaibi K, Ambler G, Arulkumaran N. High-flow nasal oxygen versus conventional oxygen therapy and noninvasive ventilation in COVID-19 respiratory failure: a systematic review and network meta-analysis of randomised controlled trials. Br J Anaesth. 2024;132(5):936-944. doi: 10.1016/j.bja.2023.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weng CL, Zhao YT, Liu QH, et al. Meta-analysis: noninvasive ventilation in acute cardiogenic pulmonary edema. Ann Intern Med. 2010;152(9):590-600. doi: 10.7326/0003-4819-152-9-201005040-00009 [DOI] [PubMed] [Google Scholar]
- 31.Osadnik CR, Tee VS, Carson-Chahhoud KV, Picot J, Wedzicha JA, Smith BJ. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;7(7):CD004104. doi: 10.1002/14651858.CD004104.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plant PK, Owen JL, Elliott MW. Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000;355(9219):1931-1935. doi: 10.1016/S0140-6736(00)02323-0 [DOI] [PubMed] [Google Scholar]
- 33.Azevedo LCP, Park M, Salluh JIF, et al. ; ERICC (Epidemiology of Respiratory Insufficiency in Critical Care) investigators . Clinical outcomes of patients requiring ventilatory support in Brazilian intensive care units: a multicenter, prospective, cohort study. Crit Care. 2013;17(2):R63. doi: 10.1186/cc12594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santa Cruz R, Matesa A, Gómez A, et al. Mortality due to acute respiratory distress syndrome in Latin America. Crit Care Med. 2024;52(8):1275-1284. doi: 10.1097/CCM.0000000000006312 [DOI] [PubMed] [Google Scholar]
- 35.Kurtz P, Bastos LSL, Dantas LF, et al. Evolving changes in mortality of 13,301 critically ill adult patients with COVID-19 over 8 months. Intensive Care Med. 2021;47(5):538-548. doi: 10.1007/s00134-021-06388-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lau VI, Dhanoa S, Cheema H, et al. Non-COVID outcomes associated with the coronavirus disease-2019 (COVID-19) pandemic effects study (COPES): a systematic review and meta-analysis. PLoS One. 2022;17(6):e0269871. doi: 10.1371/journal.pone.0269871 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eResults
eMethods
Committee information
eFigure 1. Rescue respiratory support strategy for all acute respiratory failure groups
eFigure 2. Hypoxemic non-immunocompromised acute respiratory failure group flowchart
eFigure 3. Hypoxemic immunocompromised acute respiratory failure group flowchart
eFigure 4. Acute cardiogenic pulmonary edema group flowchart
eFigure 5. COPD exacerbation group flowchart
eFigure 6. Model-fitted median rate differences of endotracheal intubation or death in 7 days in the intention to treat population
eTable 1. Simulation Results for Futile, Noninferiority and Superiority for the Null Noninferiority Scenario
eTable 2. Simulation results for Futile, Noninferiority and Superiority for the Null Superiority Scenario eResults
eTable 3. Results of posterior probabilities for superiority and noninferiority in the interim and final analysis
eTable 4. Patients’ baseline characteristics – without segregation into acute respiratory groups
eTable 5. Causes of immunosuppression in hypoxemic immunocompromised group
eTable 6. High-flow nasal oxygen and noninvasive ventilation settings
eTable 7. Summary of reason for intubation within 7 days according to protocol
eTable 8. Patient Comfort Score in the First 12 hours after Randomization in the Intention-to-Treat Population
eTable 9. Tertiary Outcomes
eTable 10. Sensitivity analysis of treatment effects on the primary in the per protocol population
eTable 11. Sensitivity Analysis using less informative priors for the primary outcome in the intention-to-treat population
eTable 12. Sensitivity Analysis of respiratory support escalation rates in COPD and in acute cardiogenic pulmonary edema
eTable 13. Adverse events
Trial protocol
Statistical analysis plan
Nonauthor collaborators
Data sharing statement


