Abstract
Purpose
We examined the effect of a high-target mean arterial pressure (MAP) on septic shock in a previously underrepresented region.
Methods
A multicentre, pragmatic, open-label, randomised controlled trial was conducted in 29 hospitals in Japan, where the prevalence of chronic hypertension among older individuals is 66.9%. Patients who were diagnosed with septic shock, aged ≥ 65 years, and admitted to an intensive care unit were randomised 1:1 to the high (target MAP = 80–85 mmHg) or control (target MAP = 65–70 mmHg) groups from 1 July 2021 to 12 December 2023. The target MAP was maintained for 72 h or until vasopressors were no longer required. The primary outcome was the 90-day all-cause mortality. Secondary outcomes included organ support-free days and adverse events.
Results
The trial was terminated early on the basis of the interim analysis results, suggesting the harm of the high-target strategy. Of the 518 patients, 258 were in the high-target group, and 260 were in the control group. By 90 days after randomisation, 101 patients (39.3%) in the high-target group and 74 (28.6%) in the control group had died from any cause (risk difference = 10.7; 95% confidence interval, 2.6–18.9). Renal replacement therapy-free days at 28 days were shorter in the high-target group. No clinical benefits for any outcome were observed in any subpopulation, including those with known chronic hypertension.
Conclusion
Among older patients with septic shock, high-target MAP significantly increased mortality compared with standard care.
Trial registration
UMIN Clinical Trials Registry; UMIN000041775; 13 September 2020.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-025-07910-4.
Keywords: Clinical trial, Critical care, Emergency medicine, Geriatrics, Septic shock
Take-home message
| Targeting a MAP of 80–85 mmHg increased the 90-day mortality, compared to usual care, among 518 Japanese patients with septic shock aged ≥ 65 years. While this RCT targeted a population with a 66.9% prevalence of chronic hypertension and employed the protocol of early concomitant vasopressin use, the clinical benefit of the high-MAP strategy was not observed in any outcome or subpopulation. |
Introduction
Sepsis is a major health care challenge worldwide. Septic shock is a subset of sepsis with a high mortality rate of approximately 38.5% even in developed countries [1]; it is characterised by sustained hypotension despite adequate fluid infusion [2] and requires immediate stabilisation of patient haemodynamics. However, the optimal target blood pressure, a key factor in circulatory management for septic shock, remains debatable [3].
Recent randomised trials in Europe and North America showed that a target MAP of > 65 mmHg in vasodilatory shock is not always beneficial [4–7]. However, targeting an MAP of 80–85 mmHg was suggested to benefit septic shock patients with chronic hypertension in some studies [8–10]. A previous trial [6] suggested that a lower target MAP of 60–65 mmHg is preferable in older patients; fewer than half of the participants in the trial had septic shock, and subgroup analysis reported that the effect of target blood pressure varied by disease [6]. Since autoregulation controlling organ perfusion varies depending on patient demographics, disease type, and severity [11, 12], the effect of the target MAP requires further examination in the population that has not been examined in previous trials regarding race, disease specificity, or prevalence of chronic hypertension. Additionally, since the results of previous trials might have been affected by catecholamine-related adverse events, the effect of a high-target MAP should be examined using a protocol minimising catecholamine dosage.
Therefore, we conducted a randomised trial evaluating the effects of high-target MAP, using a protocol of early concomitant use of vasopressin, in patients with septic shock aged ≥ 65 years in Japan in those the reported prevalence of chronic hypertension was 66.9%, according to a national survey [13].
Methods
Study design
The Optimal Target Blood Pressure in Elderly with Septic Shock (OPTPRESS) trial was a multicentre, pragmatic, open-label, randomised controlled trial conducted at 29 Japanese centres. Patients aged ≥ 65 years with septic shock were screened and randomised. Written informed consent was obtained from all the patients or their relatives. In cases of emergency, the trial procedures were applied, and informed consent was obtained subsequently. The trial protocol was published elsewhere [14]. This trial was pre-registered to the UMIN-Clinical Trials Registry (UMIN000041775).
Participants
Eligible patients were individuals aged ≥ 65 years, clinically diagnosed with septic shock, and admitted to an intensive care unit. Septic shock was diagnosed based on the Sepsis-3 definition [2]. Fluid resuscitation for the diagnosis of septic shock was not based on the uniform approach of “at least 30 mL/kg of a crystalloid solution within 3 h of the diagnosis” [15] but instead left to the clinician’s discretion because of recent findings suggesting the harmful effects of excessive fluid, especially in older populations [16, 17]. Patients who had been on vasopressors for ≥ 3 h, including settings before admission, were excluded, as a previous meta-analysis [18] suggested the potential risk of targeting high MAP after 6 h of norepinephrine administration. Details of the exclusion criteria are provided in ESM 1.
Randomisation and masking
The patients were randomly assigned via an online-based system to the high-target group (target MAP = 80–85 mmHg) or the control group (target MAP = 65–70 mmHg) in a 1:1 ratio, using a centralised, computer-generated allocation sequence prepared by a programmer, independent of the conduct of the trial. Stratified block randomisation was performed according to the presence or absence of a history of chronic hypertension and age (< 80 years or older). Chronic hypertension was determined by previous diagnosis or history of antihypertensive medication. The block size was ten which was not disclosed to all study members, to reduce the predictability of the random sequence. Given the pragmatic character of the trial, a history of chronic hypertension for stratification was determined according to the information at enrolment, regardless of the actual morbidity. The treating physicians were not blinded to the assigned groups owing to the nature of the trial. However, the statisticians were masked to the group allocation until all analyses were completed.
Procedures
The target MAP was maintained for 72 h after randomisation or until vasopressors were no longer needed, owing to improved patient conditions. In the high-target group, the target MAP was changed to 65 mmHg if an adverse event potentially related to vasopressors occurred. The target MAP after 72 h was determined at the physician’s discretion. Since invasive intra-arterial blood pressure can only be monitored in intensive care units, considering the generalisability, blood pressure was measured non-invasively on the upper arm in principle.
In this trial, concurrent use of vasopressin from the early stage was protocolised to minimise the potential adverse effects of catecholamine [19, 20]. If a norepinephrine dose of ≥ 0.1 µg/kg/min was needed to achieve the target MAP, vasopressin was initiated, and the dose was increased to 0.04 U/min. If the target MAP was still not achieved, the treating physician could add another vasopressor, increase the norepinephrine dose, or add dobutamine or hydrocortisone. Similarly, the treating physician determined the volume and speed of fluid administration. In principle, physicians were required to follow the latest clinical practice guidelines for the sepsis management [15, 21]. The decision on whether to reduce or discontinue vasopressors, the initial choice of empiric antibacterial agents, the introduction of mechanical ventilation or renal replacement therapy (RRT), and the use of other adjunctive medications was left to the treating physician. The treating physician also determined the type and dose of analgesics and sedatives, in principle, targeting a Richmond Agitation-Sedation Scale [22] score of − 3 to 0.
Outcomes
The primary endpoint was all-cause mortality 90 days after randomisation. The secondary outcomes included all-cause mortality at 28 days; mortality from sepsis; lactate clearance at 24 h; ventilator-free, RRT-free, and catecholamine-free days at 28 days; and safety endpoints. Details of the secondary outcomes described in ESM 1. The cause of death was judged by the treating physician according to the patient’s clinical course. The lactate clearance at 24 h was defined by “100 × (lactate level after the 24 h of ICU admission − lactate level at ICU admission)/lactate level at ICU admission”. Support-free days at 28 days of patients who died within 28 days were treated as zero. Major adverse kidney events (MAKE) [23] at hospital discharge were also collected retrospectively.
Statistical analyses
The estimated incidence of the primary endpoint in the control group was 45% based on the previous studies involving a similar population. The anticipated absolute difference from the intervention was 10%. Thus, an estimated 376 patients per group were required to have an 80% power to achieve a two-sided alpha level of 0.05. Assuming that approximately 10% of the patients dropped out for any reason, this trial planned to enrol 836 patients. All analyses were performed by statisticians who were independent of the trial implementation and masked to patient allocation based on a prespecified statistical analysis plan (ESM 2).
The intention-to-treat population was examined in the primary analysis based on randomisation results. A secondary per-protocol analysis was performed on participants who received any treatment for the disease without deviation until the end of the intervention or withdrawal from the trial. Differences in 90-day mortality were compared using Fisher exact test. Secondary analyses included logistic regression adjusted for the factors used for the stratification at registration and survival time analysis by estimating Kaplan–Meier curves, the log-rank test, and other methods. The same analyses were performed for the secondary endpoints of mortality and the incidence of adverse events. A safety analysis was conducted on participants who received any treatment for the disease, regardless of withdrawal from the trial. For the endpoints of lactate clearance and each organ support-free days, summary statistics were compared using the Student's t test, Wilcoxon rank sum test, and other methods, as appropriate. Post-hoc sensitivity analysis using a multivariate logistic regression model was performed to adjust for potential imbalances in the patient background between the trial arms.
Interim analysis was conducted after confirming the 90-day survival outcomes of the 300 patients. Participant registration continued during the interim analysis. Bayesian predictive power was calculated when the planned number of patients was registered. When it declined by approximately ≤ 5%, the safety monitoring board recommended trial discontinuation. As no decision on continuation owing to efficacy was made, the significance level was not adjusted for in the final analysis. Statistical significance was set at a P value of < 0.05. Statistical Analysis Software (SAS) version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for all analyses.
Results
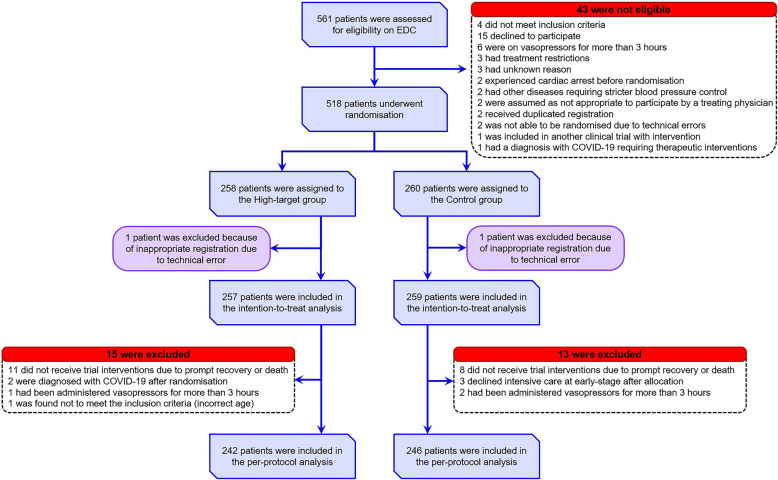
The interim analysis results indicated that the high-target MAP strategy met the predefined termination criteria for ineffectiveness and even suggested harm; therefore, the Safety Monitoring Committee recommended early trial termination, and patient enrolment was discontinued on 12 December 2023. It took approximately eight months from the enrolment of the 300th participant to the interim analysis due to delays in data entry and validation. Finally, between 1 July 2021 and 12 December 2023 among the 561 patients were screened, and 518 were allocated to the high-target (258 patients) and control (260 patients) groups (Fig. 1). After excluding two patients with registration errors (one from each group), 516 patients were included in the analysis. All participants were Japanese. The median age (interquartile range) of the overall population was 78 years (73–85). The most common source of infection was the abdomen (30.2%), followed by the urinary tract (26.4%) and lungs (25.0%). The median duration from the start of norepinephrine administration to randomisation was 60 min in both groups. The baseline characteristics were similar between the two groups (Table 1; ESM 3, Table E1). Approximately 53.3% of the high-target group and 52.9% of the control group had a history of chronic hypertension.
Fig. 1.
Patient flow diagram. Patients aged ≥ 65 years who were clinically diagnosed with septic shock based on the Sepsis-3 criteria were assessed for eligibility. A total of 518 patients were randomly assigned to the high-target or control group in a 1:1 ratio. One patient from each group was excluded from the primary analysis because of inappropriate registration owing to technical errors. EDC electronic data capture, MAP mean arterial pressure
Table 1.
Patient baseline characteristics (intention-to-treat population)
| High-target group (N = 257) | Control group (N = 259) | |
|---|---|---|
| Median age (IQR)—yr | 79 (74–84) | 77 (73–85) |
| Male sex—no. (%) | 143 (55.6) | 137 (52.9) |
| Japanese ethnicity—no. (%) | 251 (97.7) | 255 (98.5) |
| Median body mass index (IQR) | 21.2 (18.6–24.3) | 21.5 (18.3–24.6) |
| Median clinical frailty scale (IQR) | 5 (3–6) | 4 (3–6) |
| Transfer from another hospital—no. (%) | 59 (23.0) | 69 (26.6) |
| In-hospital onset—no. (%) | 47 (18.3) | 37 (14.3) |
| Comorbidities—no. (%) | ||
| Chronic hypertension | 137 (53.3) | 137 (52.9) |
| Ischaemic heart disease | 29 (11.3) | 24 (9.3) |
| Chronic heart failure | 46 (17.9) | 43 (16.6) |
| Chronic obstructive pulmonary disease | 12 (4.7) | 10 (3.9) |
| Chronic kidney disease | 41 (16.0) | 37 (14.3) |
| Liver cirrhosis | 5 (1.9) | 6 (2.3) |
| Diabetes mellitus | 66 (25.7) | 74 (28.6) |
| Cancer | 43 (16.7) | 33 (12.7) |
| None | 42 (16.3) | 63 (24.3) |
| Source of infection—no. (%) | ||
| Lung | 65 (25.3) | 64 (24.7) |
| Abdomen | 80 (31.1) | 76 (29.3) |
| Urinary tract | 61 (23.7) | 75 (29.0) |
| Soft tissue | 21 (8.2) | 27 (10.4) |
| Blood | 9 (3.5) | 8 (3.1) |
| Nervous system | 2 (0.8) | 0 (0) |
| Other | 1 (0.4) | 4 (1.5) |
| Unknown | 23 (8.9) | 12 (4.6) |
| Median mean blood pressure (IQR) at randomisation—mmHg | 58 (53–65) | 56 (50–64) |
| Median lactate level (IQR) at randomisation—mmol/L | 4.0 (2.7–6.3) | 3.9 (2.5–7.0) |
| Median P/F ratio (IQR) at randomisation | 241 (152–352) | 265 (160–385) |
| Median SOFA score (IQR) at randomisation | 10 (8–12) | 9 (7–12) |
| Median APACHEII score (IQR) at randomisation | 27 (21–32) | 25 (20–32) |
| Median duration (IQR) from the start of norepinephrine administration to randomisation—min | 60 (20–120) | 60 (15–107) |
Summary statistics were calculated excluding missing values. The numbers and proportions of missing values for each variable are described in Table E1
IQR interquartile range, P/F ratio the ratio of partial pressure of oxygen in arterial blood (PaO2) to the fraction of inspiratory oxygen concentration (FiO2), SOFA Sequential Organ Failure Assessment, APACHE Acute Physiology Assessment and Chronic Health Evaluation
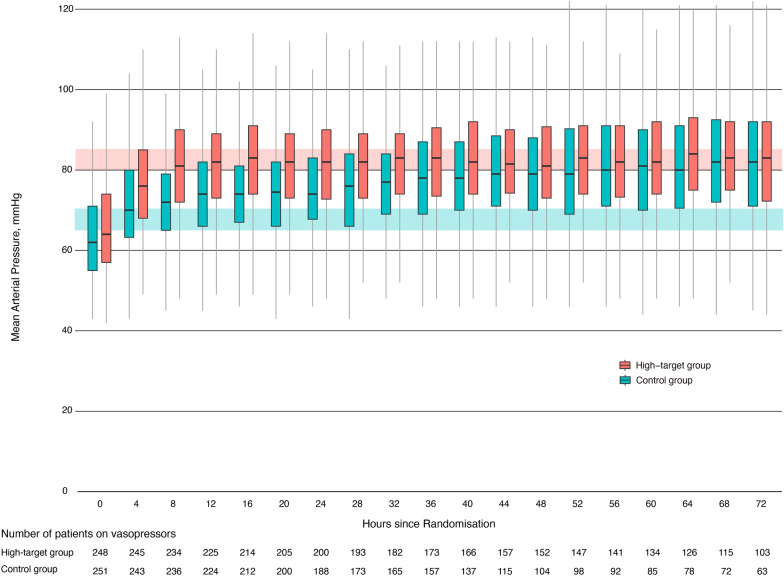
MAP during and after resuscitation was significantly higher in the high-target group than in the control group in the first 64 h (Fig. 2; ESM 3, Table E2–E3 and Figure E1). The median time to reach the target MAP (interquartile range) for the first time was 3 h (1–7) in the high-target group and 1 h (0–3) in the control group. Sixteen patients in the high-target group and five patients in the control group died before reaching the target MAP. The median vasopressor administration period in the high-target and the control groups was 65 h and 42 h, respectively (Fig. 2; ESM, Table E4). The target MAP was reduced to 65–70 mmHg in two patients in the high-target group due to adverse events. Clinical management during the 72 h after randomisation is summarised in Table E5 in ESM 3. The administered fluid volume was similar between the arms. The median norepinephrine doses used during the first 72 h were 15.6 mg in the high-target group and 9.6 mg in the control group, and those for vasopressin were 34.0 U and 11.8 U, respectively. Corticosteroid supplementation was performed in 60.3% of the patients in the high-target group and in 56.8% of those in the control group.
Fig. 2.
Blood pressure management according to mean arterial pressure target. The box and whisker plots show transition of median MAPs and interquartile ranges according to the target MAP. The red shaded region shows the target range of the high-MAP group, while blue shaded region shows the target range of the control group. Outliers are not shown. The target MAP was achieved 8 h after randomisation in majority of patients in the high-target group and 4 h after randomisation in the control group. Number of patients receiving vasopressors is also shown. The median vasopressor administration period in the high-target group and the control group was 65 h and 42 h, respectively. MAP mean arterial pressure
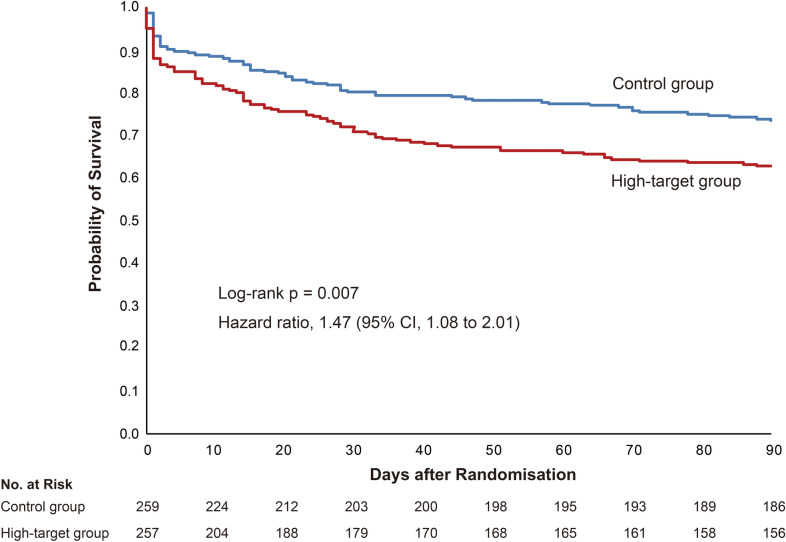
By 90 days after randomisation, 101 patients (39.3%) in the high-target group and 74 patients (28.6%) in the control group had died from any cause (risk difference = 10.7; 95% CI, 2.6 to 18.9) (Table 2). There were no differences between two groups regarding causes of death other than primary sepsis, including the death due to ischemic events (ESM, Table E6). The cumulative number of patients who had died within 90 days is shown in Fig. 3. Death due to sepsis within 90 days of randomisation occurred in 77 patients (30.0%) in the high-target group and 46 patients (17.8%) in the control group (risk difference = 12.2; 95% CI, 4.9 to 19.5). Cumulative number of deaths according to the cause of death is shown in Figure E2 in ESM 3. The mean RRT-free days at 28 days (standard deviation) were 18 (13.1) days in the high-target group and 20 (12.1) days in the control group (difference = 2.5 days; 95% CI − 4.7 to − 0.3). All main safety outcomes occurred more frequently in the high target group, including arrhythmia, thromboembolism, ischaemia not caused by thromboembolism, and haemorrhagic events requiring blood transfusion.
Table 2.
Clinical outcomes (intention-to-treat population)
| High-target group (N = 257) | Control group (N = 259) | Difference (95% CI) | P value | |
|---|---|---|---|---|
| Primary outcome | ||||
| All-cause mortality at 90 days—no. (%) | 101 (39.3) | 74 (28.6) | 10.7 (2.6 to 18.9)** | 0.012* |
| Secondary outcomes | ||||
| All-cause mortality at 28 days—no. (%) | 78 (30.4) | 56 (21.6) | 8.7 (1.2 to 16.3)** | 0.027* |
| Mortality from sepsis at 90 days—no. (%) | 77 (30.0) | 46 (17.8) | 12.2 (4.9 to 19.5)** | 0.001* |
| Mortality from sepsis at 28 days—no. (%) | 62 (24.1) | 42 (16.2) | 7.9 (1.0 to 14.8)** | 0.028* |
| Mean renal replacement therapy-free days at 28 days (SD)—days | 18 (13) | 20 (12) | − 2.5 (− 4.7 to − 0.3)†† | 0.024† |
| Mean ventilator-free days at 28 days (SD)—days | 15 (12) | 18 (12) | − 2.7 (− 4.8 to − 0.6)†† | 0.012† |
| Mean catecholamine-free days at 28 days (SD)—days | 16 (11) | 19 (10) | − 2.4 (− 4.3 to − 0.5)†† | 0.012† |
| Mean lactate clearance at 24 h (SD)—%‡ | 31.4 (74.2) | 39.7 (44.6) | − 8.4 (− 19.5 to 2.8)†† | 0.140† |
| Mean change in clinical frailty scale at 90 days (SD)§ | 1 (2) | 1 (2) | − 0.2 (− 0.5 to 0.2)†† | 0.355† |
| Major kidney adverse events at hospital discharge¶—no. (%) | 94 (37.8) | 79 (31.5) | 6.3 (− 2.0 to 14.6)** | 0.158* |
| Safety outcomes‖ | ||||
| Any arrhythmia—no. (%) | 34 (13.2) | 29 (11.2) | 2.0 (− 3.6 to 7.7)** | 0.504* |
| Ischaemic events—no. (%) | 23 (9.0) | 14 (5.4) | 3.5 (− 0.9 to 8.0)** | 0.128* |
| Haemorrhagic events—no. (%) | 14 (5.5) | 8 (3.1) | 2.4 (− 1.1 to 5.8)** | 0.199* |
CI confidence interval, SD standard deviation
*P values were calculated using the Fisher exact test
**Risk difference is provided
†P values were calculated using Student’s t test
††Mean difference is provided
‡Summary statistics were calculated based on 228 and 234 patients in the high-target group and the control group, respectively
§Summary statistics were calculated based on 192 and 161 patients in the high-target group and the control group, respectively
¶Major adverse kidney events include any of persistent kidney dysfunction defined by a ≥ 25% decline in eGFR from the reference, continued need for RRT, and death. This outcome was not defined a priori and collected retrospectively. Summary statistics were calculated based on 249 and 251 patients in the high-target group and the control group, respectively
‖Summary statistics were calculated in the safety analysis population in which at least one treatment intervention (fluid administration, norepinephrine use, vasopressin use, or epinephrine use) was provided. The safety analysis population included 247 and 251 patients in the high-target and the control groups, respectively
Fig. 3.
Kaplan–Meier curves for cumulative survival
The sensitivity analysis using a Wilcoxon rank sum test demonstrated similar results (ESM, Table E7). No clinical benefit of the high-MAP strategy was observed for any outcomes in any subpopulation, including patients with known chronic hypertension (ESM 3, Table E8 and Figures E3–E4). The secondary per-protocol analyses and the prespecified sensitivity analysis adjusted by the stratification factors demonstrated similar results (ESM 3, Tables E9–E10 and Figure E5). The results of the post-hoc multivariate analysis adjusted for potential confounders were similar to those of the primary analysis (ESM 3, Table E11).
Discussion
The OPTPRESS trial examined the effectiveness of high-target MAP in patients aged ≥ 65 years with septic shock in Japan. In addition, this trial used the strategy of early use of vasopressin, which has been proposed as a novel approach in recent years [19, 20], to minimise the adverse effects related to catecholamines. As a result, targeting a MAP of 80–85 mmHg significantly increased mortality compared with management targeting a MAP of 65–70 mmHg. All secondary outcomes regarding survival and organ support-free days were significantly lower in the high-target group. Potential effect modifiers, including chronic hypertension, had little effect on the outcomes. While cumulative evidence from Western countries suggested the futility of a higher MAP strategy in vasodilatory shock in general [4–7, 24], the generalisability has been limited because patterns of diseases that have a significant impact on systemic haemodynamics, including cardio and cerebrovascular diseases, vary depending on country, region, and ethnicity [25]. This trial could have added global relevance to the existing evidence on this topic by conducting trials in underrepresented regions.
Consistent with previous trials [4–6], the actual MAP in the control group exceeded the target range. Since more than half of patients no longer did not receive vasopressors after 42 h of randomization in the control group, it was considered that the excess was largely led by improved patient condition, not by the issue of protocol compliance. Meanwhile, some patients could not reach the target MAP even with high-dose vasopressor because of their poor condition. This difference in target and observed MAP is the reason why this topic can be appropriately examined only in randomized trials rather than analysis of existing observed data. Although the difference in MAP between the groups was smaller than trial protocol, clear separation in MAP between the arms from 8 h of randomisation means that it is reasonable to compare the effect.
In this trial, while the administered fluid volume was similar between the arms, the cumulative amount of vasopressors was higher in the high-target group. As suggested in a previous prospective study [26], the vasopressor-related risks might have outweighed the hypotension-related risks in the high-target MAP strategy with increased exposure to vasopressors. Theoretically, there is a risk of organ ischemia due to excessive vasoconstriction [27]. Increased risk of various comorbidities in older population may make them more vulnerable to vasopressors-induced adverse events. However, although adverse events were more frequent in the high-target group, there were no differences in causes of death other than sepsis, including the death due to ischemic events, between the arms (ESM 3, Table E6 and Figure E2). It was reported that vasopressors have immune effects potentially influencing the outcome of septic shock. Norepinephrine is suggested to have immunosuppressive and bacterial growth-promoting effects in preclinical models [28, 29]. Contrary to a previous trial [4] that suggested the benefits of a high MAP target for renal function in patients with chronic hypertension, such a trend was not observed regarding MAKE development and RRT-free days. The difference in baseline conditions, including age and prevalence of chronic kidney disease, might have caused these differences. Another trial that included patients aged ≥ 65 years and compared the effect of target MAP reported no difference in renal replacement days [6].
Recently, personalised resuscitation was proposed [30, 31]. A randomised trial [32] reported reduced postoperative organ dysfunction following personalised blood pressure management during general anaesthesia. However, information on blood pressure before illness onset is not always available in emergency settings, and undiagnosed chronic hypertension is common [33]. Additionally, the effectiveness of fluid resuscitation and vasoactive agents depends on pre-existing cardiovascular conditions and cardiac depression due to sepsis. Cardiac function monitoring and management were not protocolised in this trial, regardless of pre-existing cardiac conditions. The effectiveness of personalised haemodynamic management, considering a patient's cardiovascular status, using echocardiography or haemodynamic monitoring devices, should be evaluated in the future.
The present trial has some limitations. The open-label design might have affected the clinical management and have introduced bias such as outcome ascertainment. The use of fixed blocks could have led ineffective randomisation concealment. The status of chronic hypertension comorbidity was sometimes incorrect, owing to limited information on emergency settings and undiagnosed status. There was no strict titration or tapering protocol for vasopressors, which might had led to differences in the period to reach target MAP and the accuracy of MAP control. The pre-estimated mortality rate to calculate sample size was higher than observed mortality rate. While pre-estimation was made based on data from Europe and the United States given the scale of the study, previous epidemiological study suggested different mortality rates in sepsis between Western countries and Japan [34, 35]. Since information on the non-serious adverse events were not reported immediately, concern of under reporting existed. In addition, records of adverse events on detailed time of occurrence and onset after 72 h were lacked. While the result of this trial could provide insights from a previously unexplored region, generalisability was limited due to the nature of single-country trial and ethnic homogeneity.
Conclusions
Among older patients with septic shock in Japan, despite the use of a catecholamine-sparing protocol with vasopressin, management targeting a MAP of 80–85 mmHg did not reduce and instead significantly increased mortality compared with management targeting a MAP of 65–70 mmHg. It would be reasonable to be attentive to excessive vasopressor use in patients with septic shock unless novel evidence emerges in the future.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all patients and their relatives who agreed to participate in this trial. We appreciate the endeavours of physicians treating the participants as well as the trial collaborators, including the data management committee and the safety monitoring board. OPTPRESS trial investigators: Tomohiko Akutsu, Daiki Kaito, Hiromichi Ohsaka, Yohei Iwasaki, Fumino Taketazu, Tomoko Iguchi, Ryu Azumaguchi, Noritaka Ushio, Takahiro Okane, Toshiyuki Karumai, Ayaka Matsuoka, Chikashi Takeda, Toshiki Sera, Hiroko Okura, Yoichi Katayama, Keiichiro Shimoyama, Tadanaga Shimada, Ryuzo Abe, Tsuyoshi Nakashima, Tatsuhiko Anzai, Keisuke Suzuki, Takahiro Yamanaka, Youichi Yanagawa, Yuichi Araki.
Author contributions
All authors contributed to the trial design, interpretation of data, and critical revision of the final version of the report. AE acquired funding for this trial. AT and KT contributed to the statistical design and analysis. AE, KY, TT, and YU wrote the first draft of this manuscript. All authors had the opportunity to provide input into the study protocol, access the data in the study, and have the final responsibility for the decision to submit for publication. The corresponding author confirms that all listed authors meet the authorship criteria and that none meeting the criteria have been omitted.
Funding
This study was supported by the Japan Society for the Promotion of Science KAKENHI (Grant numbers 21H03197 and 23K21518). The funding body had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Data availability
After all ancillary analyses by the trial group, the datasets analysed in the present study will be available from the corresponding author on reasonable request and after the approval of the Steering Committee members.
Declarations
Conflicts of interest
The authors declare no conflicts of interest that could influence or bias the implementation and results of this study.
Footnotes
The members of OPTPRESS trial investigators in mentioned in “Acknowledgement” section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Akira Endo, Email: akira.endo.0112@gmail.com.
the OPTPRESS trial investigators:
Tomohiko Akutsu, Daiki Kaito, Hiromichi Ohsaka, Yohei Iwasaki, Fumino Taketazu, Tomoko Iguchi, Ryu Azumaguchi, Noritaka Ushio, Takahiro Okane, Toshiyuki Karumai, Ayaka Matsuoka, Chikashi Takeda, Toshiki Sera, Hiroko Okura, Yoichi Katayama, Keiichiro Shimoyama, Tadanaga Shimada, Ryuzo Abe, Tsuyoshi Nakashima, Tatsuhiko Anzai, Keisuke Suzuki, Takahiro Yamanaka, Youichi Yanagawa, and Yuichi Araki
References
- 1.Bauer M, Gerlach H, Vogelmann T, Preissing F, Stiefel J, Adam D (2020) Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019—results from a systematic review and meta-analysis. Crit Care 24:239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW et al (2016) The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315:801–810. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Backer D, Deutschman CS, Hellman J et al (2024) Surviving sepsis campaign research priorities 2023. Crit Care Med 52:268–296. 10.1097/CCM.0000000000006135 [DOI] [PubMed] [Google Scholar]
- 4.Asfar P, Meziani F, Hamel JF et al (2014) High versus low blood-pressure target in patients with septic shock. N Engl J Med 370:1583–1593. 10.1056/NEJMoa1312173 [DOI] [PubMed] [Google Scholar]
- 5.Lamontagne F, Meade MO, Hébert PC et al (2016) Higher versus lower blood pressure targets for vasopressor therapy in shock: a multicentre pilot randomized controlled trial. Intensive Care Med 42:542–550. 10.1007/s00134-016-4237-3 [DOI] [PubMed] [Google Scholar]
- 6.Lamontagne F, Richards-Belle A, Thomas K et al (2020) Effect of reduced exposure to vasopressors on 90-day mortality in older critically ill patients with vasodilatory hypotension: a randomized clinical trial. JAMA 323:938–949. 10.1001/jama.2020.0930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcucci M, Painter TW, Conen D et al (2023) Hypotension-avoidance versus hypertension-avoidance strategies in noncardiac surgery: an international randomized controlled trial. Ann Intern Med 176:605–614. 10.7326/M22-3157 [DOI] [PubMed] [Google Scholar]
- 8.Carayannopoulos KL, Pidutti A, Upadhyaya Y et al (2023) Mean arterial pressure targets and patient-important outcomes in critically ill adults: a systematic review and meta-analysis of randomized trials. Crit Care Med 51:241–253. 10.1097/CCM.0000000000005726 [DOI] [PubMed] [Google Scholar]
- 9.Tran PNT, Kusirisin P, Kaewdoungtien P, Phannajit J, Srisawat N (2022) Higher blood pressure versus normotension targets to prevent acute kidney injury: a systematic review and meta-regression of randomized controlled trials. Crit Care 26:364. 10.1186/s13054-022-04236-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leone M, Asfar P, Radermacher P, Vincent JL, Martin C (2015) Optimizing mean arterial pressure in septic shock: a critical reappraisal of the literature. Crit Care 19:101. 10.1186/s13054-015-0794-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato R, Pinsky MR (2015) Personalizing blood pressure management in septic shock. Ann Intensive Care 5:41. 10.1186/s13613-015-0085-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferlini L, Su F, Creteur J, Taccone FS, Gaspard N (2020) Cerebral autoregulation and neurovascular coupling are progressively impaired during septic shock: an experimental study. Intensive Care Med Exp 8:44. 10.1186/s40635-020-00332-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Japanese Ministry of Health, Labour and Welfare Result of National health and nutrition examination survey. https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00450171&tstat=000001041744&cycle=7&year=20190&month=0&tclass1=000001148507&stat_infid=000032041920&tclass2val=0. Accessed 8 Oct 2024
- 14.Endo A, Yamakawa K, Tagami T et al (2022) Optimal target blood pressure in elderly with septic shock (OPTPRESS) trial: study protocol for a randomized controlled trial. Trials 23:799. 10.1186/s13063-022-06732-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans L, Rhodes A, Alhazzani W et al (2021) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 47:1181–1247. 10.1007/s00134-021-06506-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Investigators ProCESS, Yealy DM, Kellum JA et al (2014) A randomized trial of protocol-based care for early septic shock. N Engl J Med 370:1683–1693. 10.1056/NEJMoa1401602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodacre S, Fuller G, Conroy S, Hendrikse C (2023) Diagnosis and management of sepsis in the older adult. BMJ 382:e075585. 10.1136/bmj-2023-075585 [DOI] [PubMed] [Google Scholar]
- 18.Lamontagne F, Day AG, Meade MO et al (2018) Pooled analysis of higher versus lower blood pressure targets for vasopressor therapy septic and vasodilatory shock. Intensive Care Med 44:12–21. 10.1007/s00134-017-5016-5 [DOI] [PubMed] [Google Scholar]
- 19.Russell JA, Walley KR, Singer J et al (2008) Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 358:877–887. 10.1056/NEJMoa067373 [DOI] [PubMed] [Google Scholar]
- 20.Sacha GL, Bauer SR (2023) Optimizing vasopressin use and initiation timing in septic shock: a narrative review. Chest 164:1216–1227. 10.1016/j.chest.2023.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egi M, Ogura H, Yatabe T et al (2021) The Japanese clinical practice guidelines for management of sepsis and septic shock 2020 (J-SSCG 2020). Acute Med Surg 8:e659. 10.1002/ams2.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sessler CN, Grap MJ, Brophy GM (2001) Multidisciplinary management of sedation and analgesia in critical care. Semin Respir Crit Care Med 22:211–226. 10.1055/s-2001-13834 [DOI] [PubMed] [Google Scholar]
- 23.Billings FT 4th, Shaw AD (2014) Clinical trial endpoints in acute kidney injury. Nephron Clin Pract 127:89–93. 10.1159/000363725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshimoto H, Fukui S, Higashio K, Endo A, Takasu A, Yamakawa K (2022) Optimal target blood pressure in critically ill adult patients with vasodilatory shock: a systematic review and meta-analysis. Front Physiol 13:962670. 10.3389/fphys.2022.962670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA (2022) The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol 80:2361–2371. 10.1016/j.jacc.2022.11.005 [DOI] [PubMed] [Google Scholar]
- 26.Roberts RJ, Miano TA, Hammond DA et al (2020) Evaluation of vasopressor exposure and mortality in patients with septic shock. Crit Care Med 48:1445–1453. 10.1097/CCM.0000000000004476 [DOI] [PubMed] [Google Scholar]
- 27.Dalimonte MA, DeGrado JR, Anger KE (2019) Vasoactive agents for adult septic shock: an update and review. J Pharm Pract 33:523–532. 10.1177/0897190019844124 [DOI] [PubMed] [Google Scholar]
- 28.Stolk RF, van der Poll T, Angus DC, van der Hoeven JG, Pickkers P, Kox M (2016) Potentially inadvertent immunomodulation: norepinephrine use in sepsis. Am J Respir Crit Care Med 194:550–558. 10.1164/rccm.201604-0862CP [DOI] [PubMed] [Google Scholar]
- 29.Russell JA, Walley KR (2010) Vasopressin and its immune effects in septic shock. J Innate Immun 2:446–460. 10.1159/000318531 [DOI] [PubMed] [Google Scholar]
- 30.De Backer D, Cecconi M, Chew MS et al (2022) A plea for personalization of the hemodynamic management of septic shock. Crit Care 26:372. 10.1186/s13054-022-04255-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zampieri FG, Bagshaw SM, Semler MW (2023) Fluid therapy for critically ill adults with sepsis: a review. JAMA 329:1967–1980. 10.1001/jama.2023.7560 [DOI] [PubMed] [Google Scholar]
- 32.Futier E, Lefrant JY, Guinot PG et al (2017) Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 318:1346–1357. 10.1001/jama.2017.14172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.NCD Risk Factor Collaboration (NCD-RisC) (2021) Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 398:957–980. 10.1016/S0140-6736(21)01330-1(NCDRisC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vincent JL, Jones G, David S, Olariu E, Cadwell KK (2019) Frequency and mortality of septic shock in Europe and North America: a systematic review and meta-analysis. Crit Care 23:196. 10.1186/s13054-019-2478-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abe T, Ogura H, Shiraishi A et al (2018) Characteristics, management, and in-hospital mortality among patients with severe sepsis in intensive care units in Japan: the FORECAST study. Crit Care 22:322. 10.1186/s13054-018-2186-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
After all ancillary analyses by the trial group, the datasets analysed in the present study will be available from the corresponding author on reasonable request and after the approval of the Steering Committee members.